- 1Guangxi Key Laboratory of Environmental Processes and Remediation in Ecologically Fragile Regions (Guangxi Normal University), Ministry of Education, Guilin, China
- 2Key Laboratory of Ecology of Rare and Endangered Species and Environmental Protection (Guangxi Normal University), Ministry of Education, Guilin, China
- 3Agricultural Resources and Environmental Research Institute, Guangxi Academy of Agricultural Sciences/Guangxi Key Laboratory of Arable Land Conservation, Nanning, China
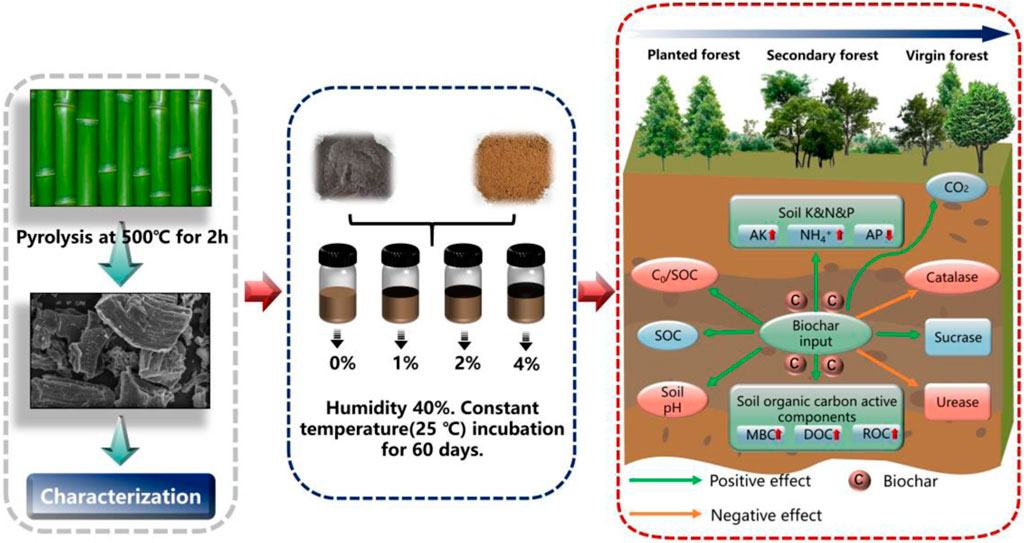
Introduction: As a soil amendment, Bamboo charcoal helps to contributes to the improvement of soil carbon sequestration, but its effect on the accumulation and transformation of different soil organic carbon in soil of karst forests is not clear.
Methods: The research focused on three distinct forest land succession stages: virgin forest, secondary forest, and planted forest. A 60-day indoor constant temperature culture experiment was conducted, applying bamboo charcoal to the soil of the three forest lands at four different addition ratios: 0%, 1.0%, 2.0%, and 4.0%. The analysis aimed to study the characteristics of SOC mineralization, different carbon fractions of organic carbon, and soil enzyme activity.
Results: The findings revealed that bamboo charcoal application led to an increase in the organic carbon (SOC) content within the three forest soils. Moreover, the organic carbon content showed an increase corresponding to the increased proportion of bamboo charcoal, with the highest SOC content observed in the planted forest land with 4.0% bamboo charcoal. The overall performance of the C0/SOC value in the three forest soils was ranked as follows: planted forest < secondary forest < virgin forest (C0: the mineralization potential of soil organic carbon). In both planted and secondary forest soils, the C0/SOC value increased after the application of bamboo charcoal. However, in the virgin forest soil, the application of 1.0% and 4.0% bamboo charcoal reduced the C0/SOC value, while the application of 2.0% bamboo charcoal increased the C0/SOC value. Particularly the C0/SOC value of the planted forest soil without bamboo charcoal was the smallest at 0.047, whereas that in the virgin forest soil with 2.0% bamboo charcoal had the largest value at 0.161.
Discussion: Herein, appropriate human intervention can enhance the carbon sequestration potential of forest soil, in different succession stages within the karst area. However, the external application of bamboo charcoal does not significantly improve the carbon sequestration potential in the planted and secondary forest. Notably, applying a higher proportion (4.0%) of bamboo charcoal can enhance the organic carbon sequestration potential, particularly in the virgin forest soil, representing the climax community of forest succession.
1 Introduction
The karst areas in China are the largest in the world (Song et al., 2014), and the southwestern region of China is the most concentrated distribution areas of karst (Teng et al., 2020). Currently, the contradiction between population and land is very prominent, and the predatory activities of destroying the vegetation cover of the mountains occur occasionally (Zhang et al., 2013). However, the different intensities of anthropogenic disturbances can change the soil carbon sequestration capacity, which affects the carbon cycle in soil ecosystems (Chen et al., 2015). Accordingly, further understanding the effect of anthropogenic disturbance intensity on forest soil carbon sequestration at different successional stages in karst areas is of great significance for evaluating the carbon sink function of forest soils.
China is one of the world’s largest producers of bamboo, the current research on bamboo is mostly focused on medicinal food, construction and other aspects (Yang, 2022). However, a considerable proportion of bamboo is either discarded in forests or utilized as fuel, leading to both resource wastage and severe environmental pollution (Zheng et al., 2021). Biochar mitigates climate change and improves soil quality due to its high carbon content and its stability (Pei et al., 2021). Therefore, bamboo was selected as the raw material and was prepared to generate bamboo charcoal by high temperature cracking under oxygen-limited conditions, which is characterized by fine pores, large specific surface area and excellent adsorption properties (Yang et al., 2011). The current research shows that bamboo charcoal added to agricultural soils can improve soil physicochemical properties, increase soil carbon storage, and increase the carbon sink function of soils (Marris, 2006; Theis and Rillig, 2009; Zwieten et al., 2010). Bamboo charcoal has been shown to have a positive impact on soil quality, but further research is necessary to investigate its application effect on forest soil carbon sequestration at different successional stages in karst areas. Therefore, we hypothesized that the application of bamboo charcoal to karst forestland soils could increase the organic carbon content of soils and improve the carbon sequestration capacity of forestland soils.
Soil organic carbon is the largest carbon pool in terrestrial ecosystems and can evaluate soil quality conditions (Groenigen et al., 2014). Reactive organic carbon (microbiomass carbon, water-soluble organic carbon, and readily oxidizable organic carbon) is an important participant in soil nutrient mineralization, cycling, and energy release (Franzluebbers et al., 2017), and is able to respond to the rate of decomposition and the level of microbial activity of soil organic carbon (Li et al., 2010). Soil organic carbon mineralization is the most important biochemical process in soil, which is directly related to the release and supply of soil nutrients and the maintenance of soil quality (Lü et al., 2019). Soil enzymes (sucrase, urease and catalase) are catalysts for most of the biochemical reactions in the soil and reflect the level of soil fertility and its metabolism of organic matter (Zhang P. P. et al., 2018). Therefore, the amount of organic carbon mineralization, organic carbon and its different carbon fractions, and soil enzyme activity were selected as the indicators measured in this study to reflect the effects of bamboo charcoal application on soil microbial activity in the forest floor as well as the stability of the soil carbon pool.
This study selected soil from three distinct forest succession stages—typically planted forests, secondary forests, and virgin forests in Hechi, Guangxi, China−as its focal point. Different proportions of bamboo charcoal were utilized to analyze the influence of bamboo charcoal on soil mineralization across these three forest succession stages. This analysis aimed to provide a theoretical foundation regarding the influence of exogenous carbon on soil carbon sequestration in karst forest land.
2 Materials and methods
2.1 Overview of the study area
The study area resides in Huanjiang County, Guangxi, a concentrated area of karst in southwest China. This area has cone-shaped mountains and an exposed pure limestone mountain environment, representing a mid-subtropical monsoon climate zone. Its average annual temperature is 15.7°C, with an average of 10.1°C and 28°C in January and July, respectively, and the minimum temperature reaches −5.2°C over the years. The frost-free period is 290 days, with an average annual sunshine duration of 145.1 h. The area experiences an average annual rainfall of 1389.1 mm, mainly concentrated from April to September, accounting for 70% of the annual rainfall. Moreover, the average evaporation is 1571.1 mm, with an average relative humidity of 70% (Song et al., 2010).
2.2 Sample collection and processing
This research particularly targeted three forest soil types−virgin forest (DV), secondary forest (DS), and planted forest (DP). The 0–20 cm surface soil of the sample plots was sampled by using the plum blossom five-point sampling method. Subsequently, the soil of the sampled stand was cleaned of plant and animal debris and gravel, following which it was air-dried and sieved for 2 mm for further testing. The basic physical and chemical properties as detailed in Supplementary Table S1. During field sampling, factors such as altitude, slope, and aspect remained similar among the soil samples collected from each forest land. The collection of forest soil samples was completed in November 2021.
2.3 Experimental design and methods
The study selected soil from karst areas within virgin forest, secondary forest, and planted forest, applying bamboo charcoal in proportions of 0%, 1.0%, 2.0%, and 4.0% (by mass). The bamboo charcoal utilized was sourced from Guilin Ruizhu Ecological Agriculture Co., Ltd. (temperature: 500°C, time: 2 h). The crushed bamboo charcoal was sieved to 0.25 mm and mixed with soil. Three replicates were set for each treatment, resulting in a total of 36 forest soil samples.
In the forest soil culture experiment, 1 kg of air-dried forest soil sample that was sieved using a 2-mm sieve was collected in a 2-L polyethylene bottle. Varying proportions of bamboo charcoal were applied to the forest soil according to the experimental design and were mixed evenly. Deionized water was then added to maintain the water-holding capacity of the forest soil at approximately 40%. The polyethylene bottles containing the soil-charcoal mixture were incubated at 25°C for 60 days, and soil samples were collected at intervals of 1, 3, 8, 15, 25, 40, and 60 days for analysis. Each time, the amount of dirt is approximately 100 g. Fresh soil samples were either kept in a refrigerator at −4°C (Measured within 48 h) or were promptly evaluated for microbial carbon, water-soluble organic carbon, and enzyme activity. To ascertain the physical and chemical characteristics of the soil, further soil samples are air-dried and sieved.
The soil samples was placed in a 500 mL polyethylene bottle with 50 g of air-dried woodland soil sieved through a 2 mm sieve. The same treatments were used as in the soil cultivation test. Simultaneously, the beaker containing 10-mL NaOH (0.5 mol·L-1) absorption solution was placed in a 500-mL culture flask, after which the flask was sealed and incubated in a constant temperature incubator at 25°C. CO2 release from each forest soil sample was determined by BaCl2-HCl titration on days 1, 3, 8, 15, 25, 40, and 60.
2.4 Assay method
2.4.1 Characterization determination of bamboo charcoal
The pH of bamboo charcoal was determined using the potentiometric method, while conductivity was measured through electrode methodology. Cation exchange capacity (CEC) was evaluated using the extraction-spectrophotometric method employing cobalt hexamine trichloride. The surface morphology of the bamboo charcoal was characterized using the Hitachi Regulus 8100 field-emission scanning electron microscope (SEM). The functional groups on the bamboo charcoal surface were analyzed using Brook ALPHALL infrared spectroscopy.
2.4.2 Determination of forest soil index
Physicochemical properties were assessed using the methodology outlined by Bao (2000). Volume weight was measured using a 100 cm3 ring knife. pH was determined using the pH meter (water-to-soil ratio = 2.5:1). Conductivity was measured using the electrode method. CEC was evaluated using the BaCl2-H2SO4 exchange method. Available potassium content was determined using the 1 mol·L-1 ammonium acetate extraction-flame photometer method. Available phosphorus content was determined using the sodium carbonate extraction–molybdenum antimony anti-spectrophotometric method. Ammonia nitrogen content was assessed using the potassium chloride solution extraction–spectrophotometry method.
The determination of active carbon components included the assessment of microbial biomass carbon (MBC) content using the chloroform fumigation extraction method. Soil organic carbon (SOC) content was determined using the potassium dichromate volumetric external heating method. Dissolved organic carbon (DOC) content was evaluated by weighing 2 g of fresh soil sample mixed at a water-to-soil ratio of 5:1, after which the sample was subjected to agitation at 25°C and 250 r·min-1 for 1 h, and passed through a 0.45-μm filter membrane. The DOC content was determined using a carbon automatic analyzer. The content of readily oxidizable organic carbon (ROC) was assessed using the potassium permanganate oxidation (333 mmol·L-1) process. Soil organic carbon mineralization was determined by alkali absorption (BaCl2-HCl titration) (Lu, 2000).
Soil enzyme activity determination primarily followed the methods outlined by Guan (1986). Catalase activity was determined by potassium permanganate titration, urease activity through the phenol sodium-sodium hypochlorite colorimetric method, and sucrase activity using the 3,5-dinitrosalicylic acid method.
2.5 Data processin
The experimental data were initially analyzed and organized in Excel 2019 and plotted using Origin Pro2022 software. Statistical analyses were performed using IBM SPSS Statistics 22 with one-way ANOVA to test the significance of differences between microbial amount carbon and water soluble organic carbon at the level of significance (p < 0.05). Correlation analysis was performed with Pearson’s coefficient. The data in the chart are presented as mean ± standard deviation.
3 Result
3.1 Structural characteristics of bamboo charcoal
The basic properties and element content of bamboo charcoal are shown in Supplementary Table S2.
As shown in Supplementary Figure S1. The Supplementary Figure S1A shows the SEM-energy spectrum analysis of bamboo charcoal. The figure illustrates a surface structure displaying a complete block structure, smooth surface, evident tubular structure, and a uniformly dense void structure (Li et al., 2020). The Supplementary Figure S1B presents the Fourier transform infrared (FTIR) spectrum of bamboo charcoal. The absorption peaks observed in FTIR provide evidence of functional groups on the bamboo charcoal surface (Ting et al., 2017). The figure indicates that there are fewer functional groups on bamboo charcoal, with peaks appearing solely at 1579.68 cm−1 and 1045.03 cm−1. The absorption peak near 1579.68 cm−1 signifies the stretching vibration of C = O, indicating the presence of ring opening, ketones, or aldehydes (Li et al., 2020). The absorption peak around 1045.03 cm−1 belongs to the −CO stretching vibration of alcohol and aromatic compounds (Wang et al., 2013).
3.2 Effect of bamboo charcoal on the physical and chemical properties of forest land
As shown in Supplementary Table S3. The application of bamboo charcoal decreased the soil pH of the planted forest and virgin forest, and increased the soil pH of the secondary forest. The pH values of the two types of forestland soils, planted forest and secondary forest, were around 7.0 and overall neutral, while the pH values of the virgin forest forestland soils ranged from 5.5 to 6.5 and were overall weakly acidic. The application of bamboo charcoal decreased the CEC content of both virgin and planted forests soils, and the application of 2.0% bamboo charcoal decreased the CEC content of virgin and planted forests soils the least, by 1.12 cmol·kg-1 and 18.53 cmol·kg-1, respectively. The application of 4.0% bamboo charcoal increased the CEC content of forest soils in secondary forests, by 49.33 cmol·kg-1. The application of bamboo charcoal decreased the AP content of two types of forest soils, planted forest and secondary forest, with the AP content of forest soils in planted forest decreasing by 36.50%–46.00% and that of forest floor soils in secondary forest decreasing by 15.00%–24.00%. The application of 4.0% bamboo charcoal increased the AP content of the forest soil of virgin forest by 5.79 mg·kg-1. The AP content of the three forest soils showed the following pattern: virgin forest > secondary forest > planted forest. The application of bamboo charcoal increased the AK content and decreased the ammonia nitrogen content of the three forest soils. The AK content increased with the increase in bamboo charcoal application. Compared with DP, DS, and DV, the AK content of 4% DV increased the most with 258.7 mg·kg-1, and the AK content of 1% DS increased the least with 40.66 mg·kg-1. Compared with DP, DS, and DV, the ammonia nitrogen content of 2% DS decreased the least with 247.50 mg·kg-1, and the ammonia nitrogen content of 2% DS decreased the most with 800.69 mg·kg-1.
3.3 Mineralization characteristics of soil organic carbon in the forest land after the application of bamboo charcoal
3.3.1 Effect of bamboo charcoal application on soil mineralization rate in forest land
As shown in Supplementary Figure S2. The application of bamboo charcoal significantly increased the mineralization rate of forest soil, and the mineralization rate of forest soil showed a downward trend during the culture period. The mineralization rate of forest soil decreased rapidly in the early culture period and tended to be stable in the late culture period. After 1 day of cultivation with bamboo charcoal, the mineralization rate of the three forest soil types was the highest, and the mineralization rate of 1% DP was the highest, and the mineralization rate of 392.0 mg·kg-1·d-1, 2% DV was the lowest, and the mineralization rate was 227.7 mg·kg-1·d-1.
3.3.2 Effect of applying bamboo charcoal on the cumulative emission of carbon dioxide in forest land
As shown in Supplementary Figure S3. The application increased the cumulative mineralization, showing a significant increase in the early culture stage before stabilization. Soil treated with 4.0% bamboo charcoal displayed slightly higher cumulative mineralization than other treatments. At the end of cultivation, compared with DP, the cumulative mineralization of 1% DP, 2% DP, and 4% DP increased by 48.53%, 56.94%, and 52.34%, respectively. Similarly, compared with DS, the cumulative mineralization of 1% DS, 2% DS, and 4% DS increased by 2027.6 mg·kg-1, 2031.5 mg·kg-1, and 2404.7 mg·kg-1, respectively. Additionally, the cumulative mineralization of 1% DV, 2% DV, and 4% DV was 1.64, 1.63, and 1.69 times that of DS.
The first-order kinetic equation used for fitting is expressed as Ct = C0 (1−e-kt) (C0 denotes the amount of carbon dioxide released from organic carbon mineralization in an ideal state, and K denotes the rate constant of carbon mineralization in the soil). The kinetic parameters of soil carbon mineralization are presented in Supplementary Table S4. Apart from the simulation fitting correlation coefficient R2 = 0.84 for 1% DP and 2% DP, the simulation fitting correlation coefficient R2 > 0.95 for other treatments indicated a good fitting effect. The soil carbon mineralization rate of 1% DP, 2% DP, and 4% DP after bamboo charcoal application ranged from 0.049 to 0.069 days−1, which is lower than the DP (0.120). Similarly, the soil carbon mineralization rates of 1% DS, 2% DS, and 4% DS were 0.031–0.034 days−1, and the K values were less than that of DS (0.076). Moreover, the soil carbon mineralization rate of 1% DV, 2% DV, and 4% DV ranged from 0.024 to 0.036 days−1 after applying bamboo charcoal to virgin forest soil. Notably, the K value of 1% DV was greater than the DV (0.035), while 2% DV and 4% DV were lower than the DV (0.035). Bamboo charcoal application significantly increased the potential mineralization of C0/SOC in the three forest soils (the C0/SOC value indicates the proportion of soil organic carbon consumed by soil organic carbon mineralization degradation). In planted and secondary forest soils, bamboo charcoal augmented the C0/SOC of the forest soil. However, in virgin forest soil, the C0/SOC value of 4% DV was the smallest at 0.120.
3.4 The effect of bamboo charcoal on soil organic carbon and its carbon components in forest land
3.4.1 Effects of bamboo charcoal application on soil organic carbon (SOC) in forest land
As shown in Supplementary Figure S4. Bamboo charcoal application resulted in a notable increase in the organic carbon content within the forest soil. Among the three forest soil types, the organic carbon content in the planted forest soil surpassed that of the other two forest types. The effect of bamboo charcoal application on organic carbon content was in the following order: 4.0% bamboo charcoal >2.0% bamboo charcoal >1.0% bamboo charcoal >0% bamboo charcoal. The organic carbon levels in planted forest soil peaked on the 8th day of cultivation, measuring 68.782 g·kg-1 (1%DP), 70.616 g·kg-1 (2%DP), and 89.416 g·kg-1 (4%DP), respectively. The soil from virgin and secondary forests exhibited a fluctuating trend, initially decreasing and then ascending over the cultivation period, reaching its lowest point on the 40th day. On that day, the organic carbon contents of 1% DV, 2% DV, and 4% DV were 20.128 g·kg-1, 22.579 g·kg-1, and 35.223 g·kg-1, respectively. On the same day, the organic carbon contents of 1% DS, 2% DS, and 4% DS were 40.950 g·kg-1, 42.041 g·kg-1, and 41.314 g·kg-1, respectively.
3.4.2 Effects of bamboo charcoal application on soil microbial biomass carbon (MBC) in forest land
As shown in Supplementary Figure S5. The MBC content notably increased in planted forest soil after the application of bamboo charcoal. By the end of the cultivation period, the MBC content in 1% DP, 2% DP, and 4% DP was 4.7, 3.7, and 4.4 times higher than that of DP, respectively. In secondary and virgin forest soils, the MBC content displayed fluctuations after bamboo charcoal application. For instance, at 60 days of cultivation, compared to day one, the MBC content in DS, 1% DS, 2% DS, and 4% DS increased by −225.20 mg·kg-1, 493.85 mg·kg-1, 341.34 mg·kg-1, and 531.78 mg·kg-1, respectively. Notably, 1% DV and 4% DV had the lowest MBC content on day one, measuring 56.59 mg·kg-1 and 114.22 mg·kg-1, respectively, but reached maximum levels on the 60th day, measuring 704.67 mg·kg-1 and 582.89 mg·kg-1, respectively. The MBC content in 2% DV reached its minimum value (228.15 mg·kg-1) on the 8th day but peaked on the 40th day (684.00 mg·kg-1) of cultivation.
3.4.3 Effects of bamboo charcoal application on soil dissolved organic carbon (DOC) in forest land
As shown in Supplementary Figure S6. In both planted and secondary forest soils, the early application of bamboo charcoal (1–25 days) decreased the DOC content, while its later application (40–60 days) increased the DOC content. On the 25th day of cultivation, the maximum 1% DP value recorded was 487.27 mg·kg-1, whereas on the 40th day, the maximum 2% DP and 4% DP values were 607.00 mg·kg-1 and 581.70 mg·kg-1, respectively. The highest values for 1% DS, 2% DS, and 4% DS were observed on the 40th day, measuring 846.50 mg·kg-1, 835.60 mg·kg-1, and 715.73 mg·kg-1, respectively. After incubation, the DOC content of 1% DS, 2% DS, and 4% DS was 3.84, 3.31, and 2.81 times higher than that of DS, respectively. When bamboo charcoal was applied to virgin forest soil, the DOC content decreased in the initial 3 days but increased from days 8–60. Ultimately, the DOC content of DV, 1% DV, 2% DV, and 4% DV increased by −19.31%, 268.20%, 119.03%, and 76.41%, respectively.
3.4.4 Effects of bamboo charcoal application on readily oxidizable organic carbon (ROC) in forest land
As shown in Supplementary Figure S7. Overall, the application of bamboo charcoal increased the content of readily oxidizable organic carbon in the forest soil. In the planted forest, the trend of ROC content exhibited a decrease after bamboo charcoal application, reaching its peak on the 40th day, with values of 55.264 mg·kg-1 (4% DP), 57.349 mg·kg-1 (2% DP), and 62.562 mg·kg-1 (1% DP). However, the secondary forest soil showed different changes during the culture period. On the 40th day, 1% DS reached its maximum value at 67.775 mg·kg-1, while 4% DS reached its minimum value at 25.031 mg·kg-1. In the virgin forest soil, the ROC contents of 1% DV, 2% DV, and 4% DV reached their minimum on the 15th day of culture, measuring 21.584 mg·kg-1, 15.329 mg·kg-1, and 20.541 mg·kg-1, respectively.
3.5 Effect of bamboo charcoal on soil enzyme activity in forest land
As shown in Supplementary Figure S8. The effects of bamboo charcoal on urease, sucrase, and catalase activities in forest soil varied distinctly. Following the application of bamboo charcoal, the urease activity in the forest soil exhibited a declining trend during the incubation period, showcasing a reduction due to bamboo charcoal application. Across the three types of forest soil, urease activity was ranked as follows: planted forest > secondary forest > virgin forest. On the third day of incubation, the urease activity peaked at 1.742 mg·kg−1·d−1, 1.013 mg·kg−1·d−1, and 1.294 mg·kg−1·d−1 for 1% DS, 2% DS, and 4% DS, respectively. Conversely, the application of bamboo charcoal amplified sucrase activity in forest soil, indicating an upward trajectory during the incubation period. The peak values for 1% DP, 2% DP, and 4% DP were recorded on the 8th day of incubation, measuring 180.659 g·kg−1·d−1, 180.782 g·kg−1·d−1, and 196.487 g·kg−1·d−1, respectively. After the cultivation period, the sucrase activity for 1% DS, 2% DS, and 4% DS was 2.64, 1.87, and 2.15 times higher than that of DS, respectively. Similarly, for 1% DV, 2% DV, and 4% DV, the sucrase activity escalated to 337.581 g·kg−1·d−1, 342.427 g·kg−1·d−1, and 347.457 g·kg−1·d−1, respectively. The effect of bamboo charcoal on catalase activity within the forest soil exhibited varying patterns. Initially, catalase activity remained relatively stable during the early phase of incubation. However, substantial changes were observed during the later stages. By the end of the incubation period, catalase activity for 1% DV, 2% DV, and 4% DV surged by 1.35 mg·g−1·h−1 compared with DV. Moreover, the catalase activity for planted forest and secondary forest soil, after bamboo charcoal application increased by 1.77 times compared with the forest soil without bamboo charcoal. Among the increments, the smallest increase was observed in 1% DP, which was 1.26 times that of DP by the end of the incubation period.
3.6 Analysis of relationship
As shown in Supplementary Figure S9. Deeper shades of red indicate a stronger positive correlation between indicators, while deeper shades of blue signify a more significant negative correlation. In the soil of planted forest land, notable positive correlations were observed between pH, SOC with ammonia nitrogen, CEC. Additionally, AP demonstrated a significant positive correlation with CEC. Conversely, DOC, MBC exhibited significant negative correlations with CEC, ammonia nitrogen, while SOC showed a significant negative correlation with DOC, ROC. In the soil of the secondary forest, a significant negative correlation was observed between DOC and pH. SOC exhibited positive correlations with pH, AK, CEC, ammonia nitrogen, and ROC. In virgin forest land, SOC displayed a significant positive correlation with pH, AK. Moreover, DOC exhibited a significant positive correlation with sucrase activity, while ammonia nitrogen showed a significant negative correlation with DOC and sucrase.
4 Discussion
4.1 The effect of bamboo charcoal on the physical and chemical properties of forest land
Soil pH is a measure of the H+ ions in the soil solution (Seliskar, 1992) and plays a pivotal role in controlling SOC changes (Peng et al., 2018). The application of bamboo charcoal reduced the pH of the soil in two types of forestland soils: virgin and planted forests. This may be due to the fact that chemical oxidation and microbial decomposition of bamboo charcoal produce acidic compounds that contribute to lowering soil pH, and organic acids dissociate H+ from acid functional groups, thus lowering the pH of forest soils (Mihoub et al., 2022). CEC reflects the ability of soil to retain positively charged ions (Khaledian et al., 2017; Emamgholizadeh and Mohammadi, 2021). In this study, the application of bamboo charcoal reduced the CEC content of the soil in both forested and native forests, probably because the organic matter enriched the soil with humic and xanthic acids, which blocked the intrinsic voids of bamboo charcoal and prevented it from the next step of physical adsorption. The application of 4.0% bamboo charcoal increased the CEC content in the soil of secondary forest stands, which might be attributed to the transitional state of the secondary forest between the planted and virgin forests. The charged ions in the soil are more active, and the larger specific surface area and surface negative charge of more bamboo charcoal (4.0%) facilitate the adsorption of inorganic ions and polar or nonpolar organic compounds (Wu, 2015), consequently elevating the CEC content of the secondary forest soil.
The ability of soil to store and supply phosphorus and potassium can be gauged by the levels of available phosphorus and potassium (Jing et al., 2020). In this study, the application of bamboo charcoal decreased the overall available phosphorus content in the forest soil while increasing the available potassium content. Moreover, the proportion of bamboo charcoal correlated positively with the increase in available potassium content in the forest soil. The decline in the available phosphorus content due to bamboo charcoal application may be attributed to the high pyrolysis temperature, which encourages the accumulation of thermally stable carbon (predominantly aromatic C), or the elevated temperature causing reduced acidity due to increased levels of Ca2+ and Mg2+. This alteration promotes the adsorption of P (Ngatia et al., 2017). Across the three types of forest soil studied, the virgin forest soil exhibited higher levels of available phosphorus and potassium than the soil of planted and secondary forests. The undisturbed nature of virgin forest soil, untouched by human interference, accounted for its elevated background values of available potassium and available phosphorus. The application of bamboo charcoal reduced the ammonia nitrogen content of forest soils in three forest soils. Due to the application of bamboo charcoal, which increased the C/N ratio in the soil, reduced the rate of mineralization of nitrogen fertilizers in the soil at the rate of profit (Manirakiza et al., 2019), and reduced the ammonia nitrogen content of forest soils (Tammeorg et al., 2014).
4.2 The change of mineralization characteristics of soil organic carbon in forest land by applying bamboo charcoal
The rate of mineralization in bamboo charcoal was higher in both planted and secondary forests than in virgin forests. Virgin forests, undisturbed by human activities, typically maintain a conducive environment for SOC storage, resulting in lower mineralization rates. However, bamboo charcoal application increased the mineralization rate in forest soil. This increase could be attributed to bamboo charcoal, produced through pyrolysis, which contains easily decomposable organic carbon components. These components might offer available carbon sources for forest soil microorganisms, stimulating microbial activity and subsequently elevating the mineralization rate of forest soil. Notably, the highest mineralization rate of bamboo charcoal occurred on the first day in all three forest soils. This initial spike could be attributed to the interference of bamboo charcoal in the local carbon pool, triggering soil microorganisms’ activity, biodegrading biochar components, and intensifying carbon dioxide release (Blagodatskaya and Kuzyakov, 2008).
Soil with biochar demonstrated higher carbon dioxide emissions than soil without biochar (Amin, 2020). In this study, bamboo charcoal application significantly increased the cumulative mineralization in three forest soils. Moreover, the cumulative mineralization of forest soil increased with the percentage of bamboo charcoal application. This is mainly attributed to the applied bamboo charcoal being rich in fibers, which provide the microorganisms with nutrients and energetic substances needed for organic mineralization. Hence, a higher application of bamboo charcoal yields more nutrients.
In this study, the cumulative mineralization process of SOC in each treatment was fitted using first-order kinetics. The C0/SOC value represents the proportion of organic carbon consumed by mineralization and degradation. A smaller C0/SOC value indicates a stronger carbon sequestration capacity in soils (Nie et al., 2018). The C0/SOC of DP and DS was the smallest, 0.047 and 0.063, respectively, in both forest soils of planted and secondary forests, and the application of bamboo charcoal increased the carbon transformation in soils of both planted and secondary forests and decreased the carbon sequestration in forest soils. In the virgin forest soil, the forest soil applied with 4% bamboo charcoal exhibited the smallest C0/SOC value of 0.12, suggesting that a 4% bamboo charcoal application in the virgin forest benefited forest soil carbon sequestration.
4.3 The effect of bamboo charcoal on the active components of soil organic carbon in forest land
The application of bamboo charcoal led to an increase in the organic carbon content of forest soil. Additionally, the organic carbon content increased with higher proportions of bamboo charcoal, consistent with the findings reported Yuan et al. (2022) and Dong et al. (2018). This phenomenon may be due to bamboo charcoal’s inherent richness in carbon, rapidly becoming a source of forest SOC upon application, thereby directly elevating the SOC content (Ding et al., 2023). It can also be attributed to the formation of microaggregates between the porous structure of bamboo charcoal and soil minerals, which provide physical protection for soil organic carbon, thus increasing the content of soil organic carbon (Weng et al., 2017).
Soil MBC originates from the breakdown of soil organic matter, soil microorganisms, and their metabolites (Zhang et al., 2018). Bamboo charcoal application into the soil of planted forests notably increased the overall MBC content. This increase might be attributed to the bamboo charcoal application, which heightened the carbon content of the forest soil. Consequently, this influx of carbon could have provided a substantial carbon source, invigorating microbial activity (Elfstrand et al., 2007), and, consequently, enhancing the assimilation of more organic carbon. Observations from both secondary and virgin forest soils indicated a reduction in the organic carbon content following bamboo charcoal application, occurring on the 25th day (secondary forest) and the 15th day (virgin forest). This decrease might be due to the relatively minor human-induced disturbance in forest soil. Organic carbon stability tends to be higher in such conditions. However, the less stable organic carbon components could have been partially consumed or mineralized due to bamboo charcoal application, leading to a reduction in the MBC content of forest soil (Huang et al., 2021).
DOC is typically the most easily utilized organic carbon component by soil microorganisms (Santos et al., 2012). Initially, the DOC content in the three forest soils was higher than that of bamboo charcoal. Conversely, in the later stages of cultivation, the opposite was observed. The early decline in DOC content following bamboo charcoal application might be due to DOC adsorption onto the bamboo charcoal surface or its penetration into the microporous structure, where microorganisms degrade it (Li et al., 2017). These findings align with those reported by Lu et al. (2014). In the later stages, the absence of bamboo charcoal resulted in a lower DOC content in the forest soil without bamboo charcoal than in the forest soil with bamboo charcoal application. This could be attributed to the active organic carbon components present in bamboo charcoal, promoting the transformation of stable carbon components and leading to increased DOC content (Ye and Horwath, 2017).
ROC constitutes the fastest turnover component within the SOC (Yang et al., 2012). However, the effects of bamboo charcoal application on the ROC content in forest soil varied in this study. The discrepancy from the findings reported by Ke et al. (2014) might be due to differences in pyrolysis temperature and raw materials (Qi et al., 2022), resulting in distinct effects on the ROC content in forest soil following various proportions of bamboo charcoal application.
4.4 The effect of bamboo charcoal on soil enzyme activity in forest land
The study conducted by Chang et al. (2016) revealed that applying biochar reduced soil urease activity, aligning with the results observed in this study. Bamboo charcoal application suppressed urease activity in forest soil. On the one hand, this effect might be attributed to the numerous charges on the surface of bamboo charcoal and its large pore size, which could lead to enzyme adsorption, consequently reducing urease activity; on the other hand, the pH level of forest soil may have played a role; when the pH value is higher than the isoelectric point of the enzyme, bamboo charcoal tends to adsorb more enzymes (Foster et al., 2018). Soil organic matter profoundly affects sucrase activity (Li et al., 2005). In this research, bamboo charcoal application increased the organic carbon levels in forest soil. Throughout the later stage of the study, a consistent upward trend in the organic carbon content of the soil was observed. The application of bamboo charcoal also amplified the invertase activity within the forest soil. Even during the later phase of the study, invertase activity continued to show a positive incline. Overall, the application of bamboo charcoal had a positive effect on enhancing catalase activity. This enhancement might be attributed to the alteration of the forest soil’s microbial environment due to the bamboo charcoal application. This alteration likely increased the available nutrients for microorganisms, consequently heightening microbial activity and catalase levels, aligning with findings from Masto et al. (2013). However, on the 15th day of the study, distinct variations in catalase activity were observed within the disturbed forest soil that had been planted. This disparity might be due to the bamboo charcoal application’s influence on stabilizing soil organic carbon and elevating soil organic acid content. Notably, increased acidity in the soil might have an inhibiting effect on catalase activity (Wang et al., 2023).
5 Conclusion
(1) The application of bamboo charcoal increased the quick-acting potassium content and decreased the ammonia nitrogen and quick-acting phosphorus content of the forest soils as a whole. Among the three woodland soils, the application of 4.0% bamboo charcoal increased the quick-acting phosphorus content of the primary forest woodland soil by 5.79 mg·kg-1. The application of 4.0% bamboo charcoal promoted the fixation of cations in the secondary forest woodland soil and increased the CEC content of the woodland soil.
(2) Among the three forest soils, virgin forest soil exhibited the least cumulative mineralization. Bamboo charcoal application augmented the mineralization rate and cumulative mineralization of forest soil but did not alter the fact that virgin forest soil had the least cumulative mineralization. In planted and secondary forest soils, bamboo charcoal reduced the carbon sequestration potential, with greater application weakening this capacity. However, in virgin forest soil, the application of 1.0% and 4.0% bamboo charcoal increased the soil’s carbon sequestration potential.
In summary, bamboo charcoal was applied to the three types soils of forest lands: virgin forest, secondary forest and planted forest. The application of more bamboo charcoal (4.0%) was more favorable for carbon sequestration in the soil of virgin forest. The carbon sequestration capacity of the anthropogenically disturbed forest soils was weakened by the application of bamboo charcoal, and the carbon sequestration capacity decreased with the increase of the proportion of bamboo charcoal applied.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
LH: Conceptualization, Methodology, Writing–review and editing. XL: Conceptualization, Data curation, Methodology, Writing–original draft. YX: Methodology, Validation, Writing–original draft. YZ: Data curation, Methodology, Writing–original draft. HO: Resources, Software, Writing–review and editing. YY: Conceptualization, Data curation, Writing–review and editing. TH: Resources, Supervision, Writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (32160284), Guangxi Natural Science Foundation (2020GXNSFAA297092), and the fund of Guangxi Agricultural Science and Technology Innovation Alliance (202413).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1411122/full#supplementary-material
References
Amin, E. E. a.Z. (2020). Bagasse pith-vinasse biochar effects on carbon emission and nutrient release in calcareous sandy soil. J. Soil Sci. Plant Nutr. 20, 220–231. doi:10.1007/s42729-019-00125-9
Blagodatskaya, Е., and Kuzyakov, Y. (2008). Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol. Fertil. Soils 45, 115–131. doi:10.1007/s00374-008-0334-y
Chang, J., Luo, X., Li, M., Wang, Z., and Zheng, H. (2016). Short-Term influences of peanut-biochar addition on abandoned orchard soil organic N mineralization in north China. Pol. J. Environ. Stud. 25, 67–72. doi:10.15244/pjoes/60245
Chen, G., Fu, W., Shen, Y., Wu, Y., Hu, N., and Wen, Z. (2015). Effects of different land utilization on soil organic carbon and its fractions in karst areas. J. Soil Water Conservation 29, 123–129. doi:10.13870/j.cnki.stbcxb.2015.03.024
Ding, S., Ma, J., Qin, Y., Huang, F., Song, L., Liu, W., et al. (2023). Effects of biochar on soil organic carbon fractions and carbon pool management index in moso bamboo forest. Journal of Guangxi Normal University, 1–14. doi:10.16088/j.issn.1001-6600.2023020701
Dong, X., Singh, B. P., Li, G., Lin, Q., and Zhao, X. (2018). Biochar application constrained native soil organic carbon accumulation from wheat residue inputs in a long-term wheat-maize cropping system. Soil tillage Res. 252, 200–207. doi:10.1016/j.agee.2017.08.026
Elfstrand, S., Hedlund, K., and Mrtensson, A. (2007). Soil enzyme activities, microbial community composition and function after 47 years of continuous green manuring. Appl. Soil Ecol. 35, 610–621. doi:10.1016/j.apsoil.2006.09.011
Emamgholizadeh, S., and Mohammadi, B. (2021). New hybrid nature-based algorithm to integration support vector machine for prediction of soil cation exchange capacity. Soft Comput. 25, 13451–13464. doi:10.1007/s00500-021-06095-4
Foster, E., Fogle, E., and Cotrufo, M. (2018). Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture 8, 158. doi:10.3390/AGRICULTURE8100158
Franzluebbers, A. J., Haney, R. L., Honeycutt, C. W., Arshad, M. A., Schomberg, H. H., and Hons, F. M. (2017). Climatic influences on active fractions of soil organic matter, .33, 1103, 1111. doi:10.1016/s0038-0717(01)00016-5
Groenigen, V., Jan, K., Qi, X., Craig, W., Luo, Y., and Hungate, B. (2014). Faster decomposition under increased atmospheric CO2 limits soil carbon storage. Science 344, 508–509. doi:10.1126/science.1249534
Huang, R., Lan, T., Song, X., Li, J., Zeng, M., Deng, O., et al. (2021). Soil labile organic carbon impacts C:N:P stoichiometry in urban park green spaces depending on vegetation types and time after planting. Appl. Soil Ecol. 163, 103926. doi:10.1016/j.apsoil.2021.103926
Jing, Y., Zhang, Y., Han, I., Wang, P., Mei, Q., and Huang, Y. (2020). Effects of different straw biochars on soil organic carbon, nitrogen, available phosphorus, and enzyme activity in paddy soil. Sci. Rep. 10, 8837. doi:10.1038/s41598-020-65796-2
Ke, Y., Hu, X., Yi, Q., and Yu, Z. (2014). Effects of rice straw biochar on soil organic carbon and CO2 emission in cultivated land. Environ. Sci. 35, 7. doi:10.13227/j.hjkx.2014.01.015
Khaledian, Y., Brevik, E. C., Pereira, P., Cerdà, A., Fattah, M. A., and Tazikeh, H. (2017). Modeling soil cation exchange capacity in multiple countries. Catena 158, 194–200. doi:10.1016/j.catena.2017.07.002
Li, D., Wu, Z., Chen, L., Zhu, P., and Ren, J. (2005). Dynamic changes of soil sucrase activity and its influencing factors in long-term fertilization black soil. Chin. J. Eco-Agriculture 13 (4).
Li, J., Chen, H., Jiang, P., and Yu, G. (2020). Study on preparation and characterization of bamboo charcoal. Energy Environ., 24–38. doi:10.3969/j.issn.1672-9064.2020.02.009
Li, S., Qiu, L., and Zhang, X. J. E. S. (2010). Mineralization of soil organic carbon and its relations with soil physical and chemical properties on the Loess Plateau. 73, 37–52. doi:10.1016/j.marenvres.2011.10.009
Li, Y., Wei, Z., Li, H., Qiu, Y., Zhou, C., and Ma, X. (2017). Effects of biochar on soil carbon and nitrogen mineralization in Chinese fir plantation. J. Agric. Environ. Sci. 36, 314–321. doi:10.11654/jaes.2016-1086
Lu, W., Ding, W., Zhang, J., Li, Y., Luo, J., Bolan, N., et al. (2014). Biochar suppressed the decomposition of organic carbon in a cultivated sandy loam soil: a negative priming effect. Soil Biol. Biochem. 76, 12–21. doi:10.1016/j.soilbio.2014.04.029
Lü, Z., Liu, X., Zhong, J., Lan, X., Hou, H., Ji, J., et al. (2019). Effects of long-term fertilization on mineralization of soil organic carbon in red paddy soil. Sci. Agric. Sin. doi:10.3864/j.issn.0578-1752.2019.15.008
Manirakiza, E., Ziadi, N., Luce, M. S., Hamel, C., Antoun, H., and Karam, A. (2019). Nitrogen mineralization and microbial biomass carbon and nitrogen in response to co-application of biochar and paper mill biosolids. Appl. Soil Ecol. 142, 90–98. doi:10.1016/j.apsoil.2019.04.025
Masto, R. E., Kumar, S., Rout, T. K., Sarkar, P., George, J., and Ram, L. C. (2013). Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. CATENA 111, 64–71. doi:10.1016/j.catena.2013.06.025
Mihoub, A., Amin, E. E. Z., Motaghian, H. R., Saeed, M. F., and Naeem, A. (2022). Citric acid (CA)–Modified biochar improved available phosphorus concentration and its half-life in a P-fertilized calcareous sandy soil. J. Soil Sci. Plant Nutr. 22, 465–474. doi:10.1007/s42729-021-00662-2
Ngatia, L. W., Hsieh, Y. P., Nemours, D., Fu, R., and Taylor, R. W. (2017). Potential phosphorus eutrophication mitigation strategy: biochar carbon composition, thermal stability and pH influence phosphorus sorption. Chemosphere 180, 201–211. doi:10.1016/j.chemosphere.2017.04.012
Nie, C., Yang, X., Niazi, N. K., Xu, X., Wen, Y., Rinklebe, J., et al. (2018). Impact of sugarcane bagasse-derived biochar on heavy metal availability and microbial activity: a field study. Chemosphere 200, 274–282. doi:10.1016/j.chemosphere.2018.02.134
Pei, J., Jinquanmia, S., Balwantwu, J., Feike, A., and Dijkstra, F. A. (2021). Biochar aging increased microbial carbon use efficiency but decreased biomass turnover time. Geoderma Int. J. Soil Sci. 382, 114710. doi:10.1016/j.geoderma.2020.114710
Peng, F., Xue, X., You, Q., Huang, C., Dong, S., Liao, J., et al. (2018). Changes of soil properties regulate the soil organic carbon loss with grassland degradation on the Qinghai-Tibet Plateau. Ecol. Indic., 93, 572–580. doi:10.1016/j.ecolind.2018.05.047
Qi, H., Xu, L., Wang, L., Li, Y., Sai, Z., Zhou, X., et al. (2022). Effects of biochar on soil carbon pool Heilongjiang Agricultural Science, 100–104. doi:10.11942/j.issn1002-2767.2022.11.0100
Santos, F., Torn, M. S., and Bird, J. (2012). Biological degradation of pyrogenic organic matter in temperate forest soils. Soil Biol. Biochem. 51, 115–124. doi:10.1016/j.soilbio.2012.04.005
Seliskar, D. M. (1992). Response of Ammophila breviligulata to acid rain and low soil pH. Water Air Soil Pollut. 63, 295–303. doi:10.1007/BF00475496
Song, T., Peng, E., Du, H., Wang, K., and Zeng, F. (2014). Characteristics of spatial and temporal evolution of karst desertification in southwestern China, occurrence mechanism and regulation countermeasures. Ecol. Lett. 34, 5328–5341. doi:10.5846/stxb201405090929
Song, T., Peng, W., Zeng, F., Liu, L., Du, H., and Lu, S. (2010). Spatial heterogeneity analysis of soil moisture in different vegetation types in karst peak-cluster depression area-Taking the southwest peak-cluster depression area of Huanjiang Maonan Autonomous County in Guangxi as an example karst in China. J. International Soc. Sports Nutrition 29, 6–11. doi:10.3969/j.issn.1001-4810.2010.01.002
Tammeorg, P., Simojoki, A., Mäkelä, P., Stoddard, F. L., Alakukku, L., and Helenius, J. (2014). Short-term effects of biochar on soil properties and wheat yield formation with meat bone meal and inorganic fertiliser on a boreal loamy sand. Agric. Ecosyst. Environ. Sci. 191, 108–116. doi:10.1016/j.agee.2014.01.007
Teng, Q., Shen, Y., Xu, G., Zhang, Z., Zhang, D., Zhou, L., et al. (2020). Characteristics of changes in soil carbon pool management indices of different vegetation types in the Karst mountainous areas of Guibei, China. J. Ecol. 39, 422–433. doi:10.13292/j.1000-4890.202002.007
Theis, J. E., and Rillig, M. C. (2009). “Characteristics of biochar: biological properties,” in Biochar for environmental management. Editors J. Lehmann, and S. Joseph (London, United Kingdom: Routledge), 976.
Ting, C., Wenfu, C., Tiexin, Y., Tianyi, H., Zunqi, L., and Jun, M. (2017). Surface characterization of aged biochar incubated in different types of soil. Bioresources 12, 6366–6377. doi:10.15376/biores.12.3.6366-6377
Wang, L., Liu, W., Wang, T., and Ni, J. (2013). Highly efficient adsorption of Cr(VI) from aqueous solutions by amino-functionalized titanate nanotubes. Chem. Eng. J. 225, 153–163. doi:10.1016/j.cej.2013.03.081
Wang, S., Wang, L., Sun, J., Yang, Z., Zhao, W., and Liu, F. (2023). Effects of exogenous organic carbon on soil fertility and soil enzyme activity of junzao orchard in xinjiang. J. Central South Univ. For. Technol. 43, 134–143. doi:10.14067/j.cnki.1673-923x.2023.04.014
Weng, Z., Van Zwieten, L., Singh, B. P., Tavakkoli, E., Joseph, S., Macdonald, L. M., et al. (2017). Biochar built soil carbon over a decade by stabilizing rhizodeposits. Nat. Clim. Change 7, 371–376. doi:10.1038/nclimate3276
Yang, L. (2022). Study on adsorption and degradation of organic pollutants by modified biomass bamboo charcoal. master. Zhengzhou university. doi:10.27466/d.cnki.gzzdu.2022.003666
Yang, L., Liu, H., Zhang, D., Liu, J., and He, Y. (2011). Electron microscopy study of the microstructure of bamboo charcoal. J. Electron Microsc. 30, 137–142. doi:10.3969/j.issn.1000-6281.2011.02.009
Yang, X., Ren, W., Sun, B., and Zhang, S. (2012). Effects of contrasting soil management regimes on total and labile soil organic carbon fractions in a loess soil in China. Geoderma 177-178, 49–56. doi:10.1016/j.geoderma.2012.01.033
Ye, R., and Horwath, W. R. (2017). Influence of rice straw on priming of soil C for dissolved organic C and CH4 production. Plant Soil 417, 231–241. doi:10.1007/s11104-017-3254-5
Yuan, J., Wang, Y., Zhao, X., Chen, H., Chen, G., and Wang, S.-Q. (2022). Seven years of biochar amendment has a negligible effect on soil available P and a progressive effect on organic C in paddy soils. Biochar 4, 1–13. doi:10.1007/s42773-021-00127-w
Zhang, E., Xu, E., and Li, M. (2013). Mining spatial information to investigate the evolution of karst rocky desertification and its human driving forces in Changshun, China. Sci. TOTAL Environ. 458, 419–426. doi:10.1016/j.scitotenv.2013.04.048
Zhang, P. P., Pu, X. Z., and Zhang, W. (2018a). Soil quality assessment under different cropping system and straw management in farmland of arid oasis region. Ying Yong Sheng Tai Yan Jiu Suo Zhu Ban. 29, 839–849. doi:10.13287/j.1001-9332.201803.030
Zhang, Z., Wang, W., Qi, J., Zhang, H., Tao, F., and Zhang, R. (2018b). Priming effects of soil organic matter decomposition with addition of different carbon substrates. J. Soil Sediments 19, 1171–1178. doi:10.1007/s11368-018-2103-3
Zheng, L., Wu, Y., and Zuo, Y. (2021). Research status and prospect of bamboo residues resource utilization. World For. Res. 34, 7. doi:10.13348/j.cnki.sjlyyj.2021.0002.y
Keywords: karst, soil organic carbon, forest land, bamboo charcoal, enzyme activity
Citation: Hu L, Liu X, Xie Y, Zeng Y, Ou H, Yu Y and He T (2024) Bamboo charcoal application altered the mineralization process of soil organic carbon in different succession stages of karst forest land. Front. Environ. Sci. 12:1411122. doi: 10.3389/fenvs.2024.1411122
Received: 02 April 2024; Accepted: 28 June 2024;
Published: 23 July 2024.
Edited by:
Kazem Zamanian, Leibniz University Hannover, GermanyReviewed by:
Yu Gong, Chinese Academy of Sciences (CAS), ChinaZhi Quan, Chinese Academy of Sciences (CAS), China
Copyright © 2024 Hu, Liu, Xie, Zeng, Ou, Yu and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tieguang He, dGdoZTExOEAxNjMuY29t
 Lening Hu
Lening Hu Xuehui Liu
Xuehui Liu Yaqi Xie1,2
Yaqi Xie1,2 Tieguang He
Tieguang He