
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 06 March 2024
Sec. Toxicology, Pollution and the Environment
Volume 12 - 2024 | https://doi.org/10.3389/fenvs.2024.1328313
 Juan Manuel Gutierrez-Villagomez1†
Juan Manuel Gutierrez-Villagomez1† Linda Ramona Lara-Jacobo1†
Linda Ramona Lara-Jacobo1† Charles Gauthier1
Charles Gauthier1 Geraldine Patey1
Geraldine Patey1 Qin Xin2
Qin Xin2 Gaëlle Triffault-Bouchet3
Gaëlle Triffault-Bouchet3 Heather D. Dettman2
Heather D. Dettman2 Valerie S. Langlois1*
Valerie S. Langlois1*Canada is one of the main petroleum producers in the world. Through its oil sands exploitation, a viscous bitumen mixed with sand, water, and clay is being produced. This bitumen is so viscous that approximatively 20%–30% of diluent needs to be added to ease transportation, resulting in a mixture called diluted bitumen (dilbit). The transport of dilbit through North America comes with a potential risk for oil spills in freshwater ecosystems at any time of the year. In this study, a mesoscale spill tank was used to study dilbit spills in freshwater to understand the effect of cold (winter-like) vs. warmer (spring- and fall-like) water temperatures on its natural weathering and their toxicity to fathead minnow (Pimephales promelas) embryos. Water samples were collected weekly during two consecutive 35-day experiments ran at either 2 or 15 °C. Each week, fish larvae were exposed for 7 days, and water analysis was performed. Chemical analysis showed that the volatile organic compound, total organic carbon, and polycyclic aromatic hydrocarbon concentrations decreased in both experiments with time, while fish larvae exposed to both temperature settings yielded increased abnormalities, EROD activity, CYP1A, and glutathione S-transferase mRNA expression levels, and decreased heart rate. Importantly, there were no major differences between the temperature regimes on dilbit weathering, highlighting that if a spill occurs in colder waters, it would be equally toxic to organisms. This work provides new data on the potential risk of oil spill for use during response planning and modelling.
Canada is a major petroleum producer and as of January 2019, Canada ranked third in the world because of its proven oil reserves (Energy Information Administration, 2022). This was mainly due to the exploitation of Canada’s oil sands, where oil is in the form of bitumen mixed with sand, water, and clay. Bitumen is a complex crude oil composed of approximately 6,000 different high molecular weight compounds (Strausz et al., 2011) and contains high concentrations of nitrogen and oxygen, heavier hydrocarbons (Barber et al., 2020), naphthenic acids (Gutierrez-Villagomez et al., 2019b; Gutierrez-Villagomez et al., 2020), complex sulphur compounds, and metals (Attanasi and Meyer, 2010), and is deficient in hydrogen (National Energy Board, 2004). Bitumen is viscous and therefore it is mixed with approximately 20%–30% diluent for transportation, resulting in a mixture called diluted bitumen, or dilbit (Crosby et al., 2013). In 2019, Canada produced 4.7 MMb/d of oil transported through Canada either by truck, rail, or pipeline (Natural Resources Canada, 2019). There is more than 840,000 km of pipelines across Canada transporting dilbit, crude oil, and natural gas (Natural Resources Canada, 2020). These pipelines are an extensive network that crosses freshwater, marine, and terrestrial ecosystems that could pose a potential risk for oil spills.
The study of dilbit spills is highly complex since the diluent and bitumen have a variable composition. Some factors that contribute to the behaviour of an oil spill include the oil’s chemical composition (Fingas, 2007) and the natural conditions, such as weather and water temperature (Office of Emergency and Remedial Response, 1999). The dilbit chemical composition and the environmental conditions will impact the rate of evaporation, photo-oxidation, mixing, and biodegradation of the oil (National Academies of Sciences Engineering and Medicine, 2016). Also, dilbit can weather through physical (evaporation, dispersion, spreading, and emulsification), chemical (dissolution and UV-oxidation), and biological (microbial degradation) processes (Faksness et al., 2008; Bagi et al., 2013). After an oil spill, dilbit weathering can increase the oil density, viscosity, flash point, and adhesion characteristics and on some occasions, some oil components can sink to the bottom of the affected water body (National Academies of Sciences Engineering and Medicine, 2016). The weathered dilbit chemical profile could contain more hydrophilic molecules, thus polar polycyclic aromatic hydrocarbons (PAHs), such as oxygenated PAHs (oxy-PAHs), nitrated PAHs (N-PAHs), and N/S/O-heterocyclic PAHs (Idowu et al., 2019). Therefore, it is crucial to understand the consequences of a spill in representative conditions of North American ecosystems and in the living organisms it contains. However, just a few studies are available on the weathering of dilbit in freshwater systems and more information regarding oil spills in freshwater ecosystems is recommended (Department of Fisheries and Oceans Canada, 2018).
In this work, we used a mesoscale spill tank to study a Cold Lake Blend (CLB) dilbit spill in two North American seasonal settings (spring vs. fall) to observe the effect of temperature on the water’s chemical composition and the changing toxicity to aquatic organisms through time. For this, we used water that has been in contact with oil and contains hydrocarbons or water accommodated fraction (WAF). We assessed the toxicity evolution through time using fathead minnows (Pimephales promelas) embryos and measured physiological, morphological, and molecular parameters. We used P. promelas embryos as these North American fish have been shown to be in good model for aquatic toxicology, especially in oil studies (e.g., Bérubé et al., 2021).
This work is important given the high volume of dilbit being transported nationally and internationally, the risk factors associated with dilbit spills, the possible detrimental effects of the spill, and other key variables, such as temperature increases in Northern regions that could potentially augment dilbit’s toxicity to wildlife if a spill were to occur.
The CLB dilbit winter sample was obtained directly from a pipeline (Edmonton, AB, Canada). North Saskatchewan River water was collected from the Edmonton water facility before the potable water treatment process on 25 September 2018, for the “warm-temperature spill run” to mimic Fall- and Spring-like temperatures and on 21 January 2019, for the “cold-temperature spill run” to mimic Winter-like temperatures. All other chemicals and solvents were purchased from Sigma Aldrich as reagent grade and were used as received.
The experiment setup was similar to that described in Lara-Jacobo et al. (2021). The controlled spill tank measured 3 × 1 × 1.5 m (L × W × H) and was made of 316-stainless steel. One end of the tank inclined to simulate a shoreline. The tank had a motor at 105 rpm connected to a paddle-style wave-generating flap to generate breaking waves at a wavelength of 60 cm, an amplitude of approximately 8 cm, and a wave period of 0.5 s. Three temperature transmitters (TT-1001, TT-1002, and TT-1003; WIKA Instruments Ltd, Alberta, Canada.) were located at different heights to assess the temperature profile in the tank. The TT-1001 was above the water surface and recorded the ambient air temperature throughout the experiment, whereas the TT-1002 and TT-1003 recorded the water temperature (Supplementary Figure S1A). The water temperature was set to 2 and 15 ± 1°C; these temperatures were chosen to simulate the North Saskatchewan River (NSR) temperature regime in the spring and fall seasons, respectively. An external heater unit was used to maintain the water temperature at 2 and 15 ± 1 °C using heat-exchange coils located along the bottom and sides of the spill tank; the ambient air temperature was an average of 6 and 14 ± 2 °C for the cold and warm test, respectively. Light-emitting diode (LED) lights were installed to meet the Canadian electrical code for the potentially explosive environment above the tank. It was expected to have a low rate of photo-oxidation because LED lights tended to have low or no emissions in the ultraviolet wavelength range (Ticleanu and Littlefair, 2015).
Approximately 1,200 L of river water was filtered to remove debris and was added to the tank to a height of 0.7 m and 2.4 kg of North Saskatchewan River sediment was added to the water and mixed with waves for 5 min to achieve 2000 ppm of suspended sediment. River sediment was collected from a floodplain of the North Saskatchewan River. The river sediment was composed of four major mineral components, 20 ± 6 wt% clay, 20 ± 2 wt% carbonates, 8 ± 2 wt% feldspars, and 52 ± 2 wt% quartz. Nearly 100% of the sediment consisted of particles having a diameter range of less than 250 μm, with over half of the sample consisting of particles having a diameter range of less than 45 µm (referred to as “fines”). Immediately afterward, 8.255 kg of mixed CLB (∼10 L) was poured onto the water surface using a pouring device attached to the edge of the tank and time was recorded as time zero (T0). Waves were applied continuously for the first 2 d and then turned off for the next 2 d; this on-off cycle was repeated over the 35-d experiment. During the experiment, the waves were on for half the time to induce surface mixing, and off to simulate low-wind settings. When the change-over from off to on, or on to off occurred on a weekend, the conditions were maintained until the Monday for a third day. Water evaporation was compensated using reverse osmosis water to maintain a constant water height of 70 cm.
Water samples were collected from the tank sample port (Supplementary Figure S1A), for chemical and toxicity analysis. The undiluted water samples were tested for benzene, toluene, ethylbenzene, and xylene (BTEX) using the US-EPA methods 5021A, 8015C and 8260D for gas chromatography-mass spectrometry (GC-MS) and flame ionization detection (United States Environmental Protection Agency; US-EPA, 2018a; US-EPA, 2014; US-EPA, 2007), as previously reported (Lara-Jacobo et al., 2021). The concentrations of PAHs and alkylated PAHs in undiluted water samples were analyzed using US-EPA methods 3510C and 8270E for GC-MS (United States Environmental Protection Agency; US-EPA, 2018b; US-EPA, 2004). The total organic carbon (TOC) of undiluted water samples was determined using a TOC V CPH (Shimadzu, Jiangsu, China) according to a modified version of the ASTM International method D7573-09 (ASTM, 2009).
The diluted samples were analyzed for 134 PAHs. Briefly, approximately 800 mL of pH 2 acidified water sample was extracted by liquid-liquid extraction using dichloromethane to a final volume of 100 mL of dichloromethane. A 10-mL aliquot of this extract was solvent-exchanged to hexane, treated with silica gel, and concentrated down to 1 mL. The prepared sample was analyzed without further purification using GC/single-quadrupole mass spectrometry (models 7890B GC and 5977A MSD, Agilent Technologies, Mississauga, Canada) using a protocol developed by the Centre d’expertise en analyse environnementale du Québec (2008). VOCs and C10-C50 were also measured according to established protocols by the Centre d’expertise en analyse environnementale du Québec (2016, 2014), as reported previously (Lara-Jacobo et al., 2021). All water chemistry analyses were performed at the by the Centre d’expertise en analyse environnementale du Québec (Québec, Canada). Pictures were taken to document the tank contents at the various sampling time points (Supplementary Figure S1B). For toxicity testing, five 4-L pails of water were collected in amber glass bottles once a week (day 1, 6, 14, 28, and 35). Please refer to the Supplementary Material S1 for a detailed description of the controlled oil spill.
P. promelas eggs were collected from approximately 30 adult couples from the INRS’ P. promelas colony and randomized before use. The embryos were exposed from the fertilized egg stage (stage 1) to hatching time (stage 32; approx. 5 days). The testing dilutions were prepared daily from a 100% CLB-WAF kept at 4 °C and covered from light until use. The nominal dilutions tested at each time point were 0, 12.5, 25, 50, and 100% v/v from the CLB-WAF stock. The dilutions were prepared with NSR water and a reconstituted water control was also included to observe potential NSR water effects on P. promelas embryonic development. The reconstituted water was prepared according to the standard protocol (ASTM International, 2004; per litre of deionized water: 0.55 mg NaBr, 51.2 mg NaHCO3, 2.1 mg KCl, 35.3 mg CaCl2, 3.4 mg CaSO4.2H2O, and 32.8 mg MgSO4 at pH 7.6-7.9). The dilutions were made in 1-L glass jars and 150 mL of the dilutions were divided equally into 250 mL glass jars each containing 25 embryos, with five replicates for each treatment. The toxicity tests were conducted in a room with a controlled temperature (25°C ± 1 °C) and a 16 h light and 8 h dark cycle. The jars were observed every 24 h, data was recorded, and dead embryos were removed. At the end of the 7-day exposures, larvae were randomly selected from the five replicate jars to assess malformation, heart rate, gene expression, and/or ethoxyresorufin-O-deethylase (EROD) in vivo. Experimental design set-up is found in the Supplementary Figure S2.
The malformation analysis and heartbeat rate were performed using a Nikon SMZ18 stereomicroscope (Nikon Instruments, Mississauga, Canada). For malformation analysis, stereoscopic observations of each individual larvae were made and were scored for malformations of the heart tube, craniofacial, spinal deformity, pericardial, and yolk sac edema (40 larvae per treatment). Embryos were considered “malformed” if they exhibited at least one malformation (Lara-Jacobo et al., 2021). For the heartbeat rate, embryos were placed individually in a well of a clear 24-well plate and recorded the number of heartbeats for 60 s (15 larvae per treatment) (Lara-Jacobo et al., 2021). All animal work was conducted following the INRS Animal Care Committee and the Canadian Council for Animal Care guidelines (protocol #Langlois-2009-03).
For EROD in vivo analysis, embryos were collected in pools of 3–6 for six replicates (n = 6) and immediately analyzed. Larvae were transferred to 2-mL Eppendorf tubes. A stock solution of 1 mM 7-ethoxyresorufin (7-ER; ≥98%; Millipore Sigma, Burlington, USA) was prepared in DMSO. From the 7-ER stock solution, a 1 µM 7-ER solution was prepared with reconstituted water and 600 μL of 7-ER 1 µM was added to each tube (modified from Schiano Di Lombo et al., 2021; Le Bihanic et al., 2013). A standard curve with resorufin (95%; Millipore Sigma, Burlington, USA) was prepared in reconstituted water (0–10 nM). After 4 h of incubation at 25 ± °C, 100-μL aliquots were transferred from each tube to a Microwell 96-well microplate (Thermo Scientific, Saint-Laurent, Canada). The standard curve was run with three technical replicates, while the samples were run with two technical duplicates. The fluorescence was determined using a FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices, San José, USA) at an excitation wavelength of 544 nm and emission at 590 nm. At the end of the assay, the larvae were sacrificed and stored at −80 °C. The amount of protein was assessed using the Coomassie (Bradford) protein assay kit (Thermo Scientific, Saint-Laurent, Canada) following the manufacturer’s protocol. Briefly, 500 mL of Phosphate-buffered saline (PBS) (“Phosphate-buffered saline (PBS),” Phosphate-buffered saline et al., 2006) was added to the samples and they were homogenized using a stainless-steel ball (5 mm) per sample and a Mixer mill MM 400 (Retsch, Fisher Scientific, Mississauga, Canada) for 2 min at 20 Hz. A standard curve of 0–2,000 μg/mL was included in each plate using albumin (Thermo Scientific, Saint-Laurent, Canada) as the standard. Five µL of the standard curve and the samples were aliquoted in a transparent 96-well microplate. Later, 250 µL of the Coomassie dye was added to each well. The absorbance was assessed at 595 nm in a FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices, San José, USA). The EROD activity is expressed as picomole of resorufin per milligram of protein.
For gene expression analysis, larvae were collected in pools of six and then flash-frozen in liquid nitrogen and stored at −80 °C until analysis. Total RNA was isolated from larvae tissue using the RNeasy Micro kit (Qiagen, Toronto, Canada) with on-column DNase treatment following the manufacturer’s protocol. The purity and concentration of isolated RNA were assessed using a Nanodrop-2000 spectrophotometer (Fisher Scientific, Saint-Laurent, Canada). The QuantiTect Reverse Transcription kit (Qiagen, Toronto, Canada) was used to convert the RNA to complementary DNA (cDNA) with 1 μg RNA input including a no-reverse transcriptase (NRT) control. Real-time quantitative polymerase chain reaction (RT-qPCR) with BRYT Green® dye technology was used to assess the relative abundance of Cytochrome P450 Family 1 Subfamily A (cyp1a) and glutathione S-transferase (gst) using ribosomal protein L8 (rpl8) and elongation factor 1 alpha (ef1a) as reference transcripts. The transcripts cyp1a and gst were selected because they are known to be responsive to oil exposure (F. Alsaadi et al., 2018). The primers for the gene expression analysis were reported in previous articles (Supplementary Table S1) (F. M. Alsaadi et al., 2018; Mager et al., 2008; Martyniuk et al., 2012). The GoTaq qPCR Mastermix kit (Promega, Madison, USA) and the CFX96 Touch Real-Time PCR Detection System (Bio-Rad, Saint-Laurent, Canada) were used to amplify and detect the transcripts of interest. The relative standard curve method was used (Gutierrez-Villagomez et al., 2019a; Lebordais et al., 2021) following the Minimum Information for Publication of Quantitative Real-Time PCR experiments (MIQE) guidelines (Bustin et al., 2009). The thermal cycling parameters were as follows; an activation step at 95 °C for 30 s, followed by 40 cycles of 95 °C denaturation step for 15 s and one primer annealing and elongation temperature of 58 °C for 30 s. After 40 cycles, a melt curve was performed over a range of 65°C–95°C to verify a single amplified product. A standard curve was prepared on each plate with a serial-diluted pooled cDNA to create a calibration curve with a dilution factor of four (0.0048–50 ng). Each cDNA sample was diluted to 1:40 and 1:80 for optimized quantitative polymerase chain reaction (qPCR) analysis. The efficiency of all qPCR reactions was 97.3%–110.7%, and the coefficient of determination (R2) values was 0.989–0.999. Each plate contained an NRT and a no-template control (NTC). Gene fold changes were normalized to both reference genes, calculated relative to the control treatment and averaged by replicates (n = 6, essayed in duplicates). Please refer to the Supplementary Material Table S1 for a detailed description of the gene expression analysis.
Normalized fold change values were considered outliers if they were 1.5 times outside of the interquartile range and removed from the analysis. As previously reported by Gutierrez-Villagomez et al. (2021) to assess data normality and homogeneity of variance, Shapiro–Wilk’s test and Levene’s test were performed, respectively. Data that failed the normality and/or the equal variance tests were Log10 transformed to respect the assumptions of normality. One-way ANOVA and post hoc Student–Newman–Keuls (SNK) analyses were performed on normally distributed data. Non-parametric Kruskal–Wallis tests were performed on data that did not pass normality and/or equal variances tests followed by Dunn’s method for unequal sample sizes and SNK for equal sample sizes. The significance level was set at α = 0.05. One-way ANOVA analyses were performed using Sigma Plot 12.0.
The water chemical analysis showed that the BTEX concentration in water diminished to close to the detection limit after 24 h in both experiments (Figures 1A, B). Similarly, the TOC decrease rapidly and stayed constant afterwards (Figures 1C, D). Moreover, the highest concentration of PAHs and alkyl-PAHs in water was at 3 h in both temperatures; however, at 15 °C the concentration was two times higher than that of the 2 °C test (Figures 1E,F). The data suggest that the early evaporation of volatile compounds in fresh dilbit resulted in a quick decrease of the TOC and BTEX content in the water column, while the dispersion contributed to the highest of TPAHs in water columns in the early hours.
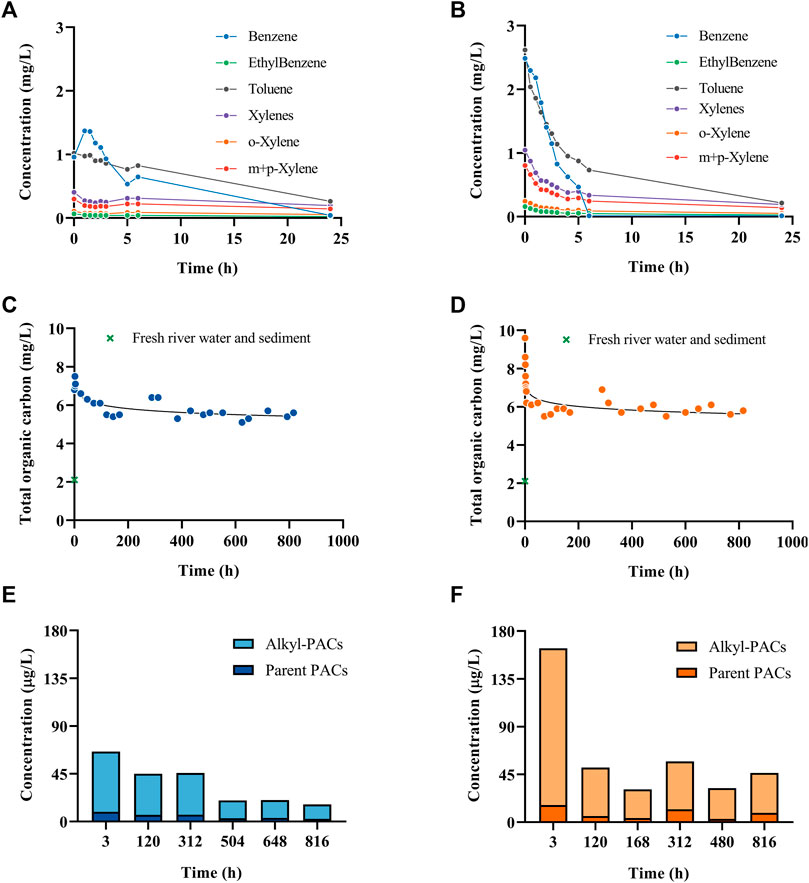
FIGURE 1. BTEX concentrations in water sampled at different times over 24 h at 2°C (A) and 15°C (B). Total organic carbon in water at different time points over 35 days (816 h) at 2°C (C) and 15°C (D). Total PAH and alkylated-PAH concentrations in water at different time points over 816 h at 2°C (E) and 15°C (F). More chemistry details can be found in Xin et al., 2023.
There have been studies comparing the oil weathering at different temperatures in seawater showing contradictory effects. For example, Payne et al. (1991, 1983) evaluated the temporal changes in the physical and chemical properties of crude oil (Exxon Valdez) in ice-free subarctic marine environments and a series of summer and winter outdoor flow-through seawater wave-tank experiments conducted at the National Oceanic and Atmospheric Administration (NOAA) and found different chemical profiles in the different environmental temperatures. However, Delille et al. (2009) studied the effects of temperature on the degradation of petroleum hydrocarbons (Arabian light crude oil) in sub-Antarctic coastal seawater at three temperatures (4, 10, and 20 °C) and they concluded that water temperature had little effects on biodegradation efficiency of PAHs. The latter data are similar to the data obtained in the present study, as both 2°C and 15 °C trials exhibited a similar PAH concentration over time. For more details on the chemistry characterization related to this study, please refer to Xin et al., 2023.
There were no significant differences in mortalities or hatching time, compared with the WAF treatments over time in any of the two temperatures (Supplementary Tables S2, 3). However, the abnormality analysis showed that in both temperature regimes and over time, the occurrence of abnormalities reflected a dilution-response effect and all the P. promelas larvae were affected with at least one type of abnormality in the 100% WAF treatment (Supplementary Figure S3). The most common type of malformations observed included pericardial edema, followed by heart tube, spinal and craniofacial abnormalities, and yolk sac edema (Supplementary Tables S2, 3). However, the relative malformation frequency pattern was different in both temperature regimes (Supplementary Figure S3F, L). For example, in the first time point in the 2°C regime, the frequency of abnormalities increased up to 8 times (Supplementary Figure S3F), while in the 15°C regime the frequency of abnormalities increased up to 20 times relative to the control (Supplementary Figure S3L). This difference could be because of the higher concentration of parent and alkyl PAHs, and BTEX in the WAF at 15°C, as shown in the chemical analysis (Figure 1). The observation that PAHs induce abnormalities in fish has been reported in several studies (Carls et al., 2008; Dubansky et al., 2013; Incardona et al., 2013; Incardona et al., 2014; Mager et al., 2014; Madison et al., 2015; Madison et al., 2017), but there is still a lack of data on the embryotoxicity of weathered oil and the associated chemical changes that oil undergoes in different environmental temperatures and how these chemical changes could modify toxicity. However, the changes in abnormality frequency patterns over time cannot be explained solely by the concentration of PAH because the concentration of PAHs stabilized and remained similar in both temperature regimes after 120 h (Figure 1), while the relative malformation frequency increased (Supplementary Figure S3). These data suggest that the continuous occurrence of fish malformations observed with time could be explained by the increased presence of the oxidized compounds as dilbit weathered, mainly as oxy-PAHs (Lara-Jacobo et al., 2021).
The heart rate data showed that exposure to the WAF of the two temperature regimes significantly decreased the heart rate in P. promelas larvae in both regimes and all the time points in a dilution-response pattern (p < 0.05; Figure 2). On Day 1, both environmental conditions yielded the lowest heart rate of all the time points at 121 and 120 beats/min at 2°C and 15°C, respectively (Figure 2). The pattern showed that the effects on the heart rate stayed stable over the 35 days experiment in the 2 °C settings (Figure 2 F); however, in the 15°C, the effects decreased over time (Figure 2 L). The effects on the heart rate are not fully explained by the PAH concentration since the PAH level stayed stable over time in both temperature settings (Figure 1). In a similar setup but using conventional crude oil at 24°C, Heshka et al. (2022) observed that the concentration of oxidized species increased after 14 days. And thus, the increase in the relative malformation frequency and reduction of heart rate in P. promelas larvae could be due to a higher concentration of oxy-PAHs. Also, some PAHs have been identified to cause cardiotoxicity in fish (Incardona et al., 2006; Scott et al., 2011) and previous studies have found that cardiotoxicity can yield irregular heartbeats like bradycardia (Middaugh et al., 1996; Incardona et al., 2009; Shen et al., 2010; Incardona et al., 2012; Zhang and Yan, 2014; Tissier et al., 2015) or tachycardia (Philibert et al., 2016). Incardona et al. (2019) found cardiac abnormalities in embryonic Pacific Herring (Clupea pallasi) exposed to Alaska North Slope crude oil during weathering. Another study, conducted by Li et al. (2019), showed that exposed zebrafish to Oman crude oil and Merey crude oil-WAF depicted concentration-dependent cardiac toxicity, such as severe bradycardia, cardiac defects, and poor blood circulation. This is like the results obtained in the present study as the high concentrations of dilbit tested yielded significant decreases in the heart rate of dilbit-treated embryos compared to the controls and this was observed for the two temperature settings and all time points.

FIGURE 2. Heart rate in Pimephales promelas larvae exposed to the water accommodated fraction of a simulated controlled oil spill at 2°C (A–E) and 15°C (G–K) after 1, 6, 14, 28 and 35 days. Relative heart rate in Pimephales promelas larvae exposed to the water accommodated fraction of a simulated controlled oil spill over 35 days at 2°C (F) and 15°C (L).
EROD induction, which measures CYP1A activity, has been well documented in fish exposed to some PAHs such as B [a]P (Schnitz and O’Connor, 1992). EROD assays are often performed on the S9 microsomal fraction of larvae homogenate or directly in the fish liver (Peters and Livingstone, 1995; Koponen et al., 1998; Chen and Cooper, 1999; Billiard et al., 2004; Gagnon and Rawson, 2017), but at the larval stage it is not possible to perform it because of the small size of P. promelas; thus, we have optimized an in vivo EROD assay for this species. The EROD activity showed a different pattern over time between the 2°C and 15 °C trials (Figure 3F, L). In the 2°C, there was a greater induction of EROD activity compared to the 15 °C trial. The concentration of PAHs over time in the two spill tests was similar (Figure 1); however, the EROD activity data did not follow the same pattern (Figure 3F, L) suggesting that there are other components in the WAF besides PAHs that can induce EROD activity. Also, potentially the larvae’s capacity to maintain metabolic performance was compromised due to a higher proportion of malformations in the 15 °C trial. For the 2°C, the highest induction of EROD activity was measured on Day 1 in the 50% WAF treatment (Figure 3A; 239 pmol/µgprotein). Noteworthy, Day 14 showed the lowest induction of EROD activity of all the other time points, and there were no significant differences between the treatments (p > 0.05). At 15°C, all the time points showed a similar profile with the highest increase of EROD induction in the medium-range treatments and a decrease in the highest concentration (Figures 3G–K). Lin et al. (2020) exposed juvenile sockeye salmon (Oncorhynchus nerka) to unweathered CLB dilbit water-soluble fraction and showed a concentration-dependent induction of EROD activity. Similar results were observed by Alderman et al. (2017) when juvenile sockeye salmon was exposed to a summer blend dilbit from the Cold Lake region and found a significant interaction between time and concentration.
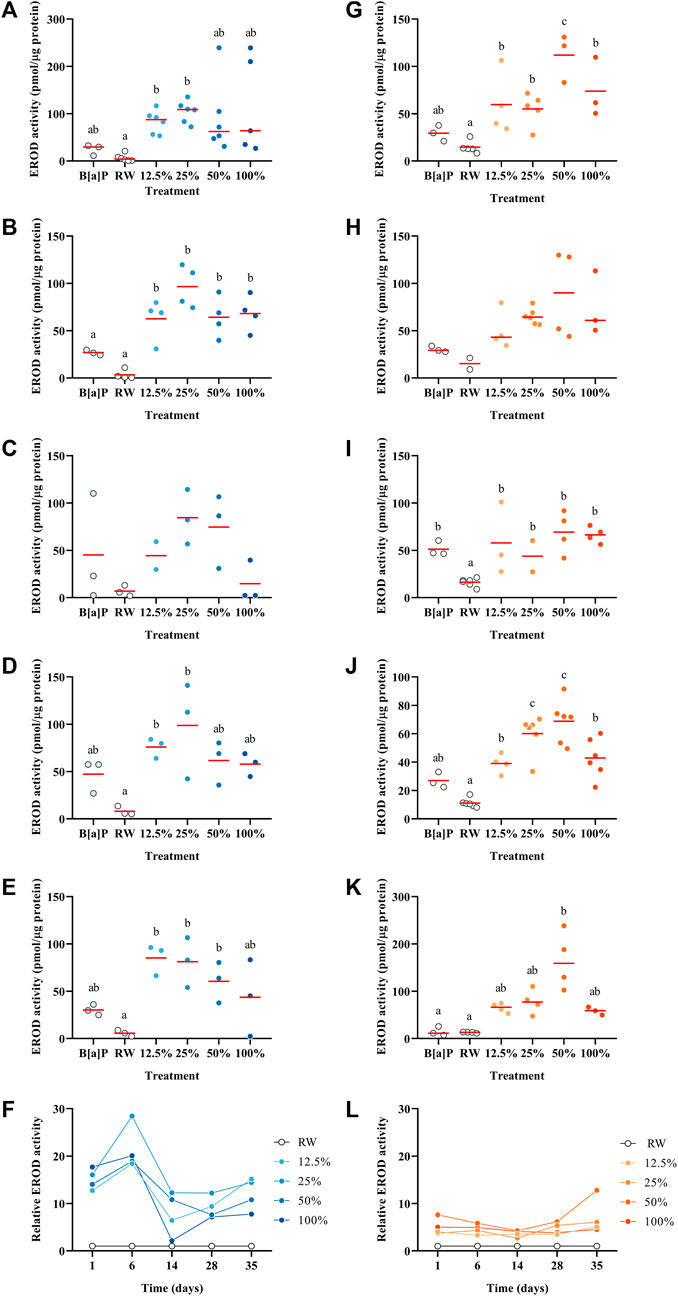
FIGURE 3. EROD activity in Pimephales promelas larvae exposed to the water accommodated fraction of a simulated controlled oil spill at 2°C (A–E) and 15°C (G–K) after 1, 6, 14, 28 and 35 days. Relative EROD activity in Pimephales promelas larvae exposed to the water accommodated fraction of a simulated controlled oil spill over 35 days at 2°C (F) and 15°C (L).
The transcript abundance of cyp1a was affected by the exposure to the WAF in a dilution-response relationship in both temperature settings throughout all the five time points (p < 0.05; Figure 4). The highest effects on cyp1a were observed on day six in both experimental settings; however, at 15°C, cyp1a increased almost double (Figures 4F–L). A similar profile was observed for gst which on day six showed the highest effects compared to the control (Supplementary Figure S4). The expression of cyp1a is well known to be activated via the AhR-pathway by several PAHs (Wallace et al., 2020); and in this study, cyp1a abundance was induced at all the time points in a dose-response manner. However, cyp1a fold-change magnitude decreased over time. This could be explained either by the decrease of the PAHs capable of binding to the AhR over time or by the increased presence of oxidized compounds (oxy-PAHs, S-PAHs and/or S-PAHs), which are known as weak AhR activators (Machala et al., 2001; Misaki et al., 2007). Indeed, Wincent et al. (2015) exposed embryonic zebrafish (Danio rerio) to PAHs and oxy-PAHs and demonstrated that oxy-PAHs were weak inductors of AhR signalling, but still capable of inducing the expression of several genes involved in the AhR pathway, such as cyp1a. However, another study exposed zebrafish embryos until 5 days post-fertilization to 38 oxy-PAHs, where most of the oxy-PAHs tested did not induce cyp1a expression (Knecht et al., 2013). In the present study, we observed a significant induction of cyp1a abundance at the highest concentrations tested, but the magnitude of the induction decreased over time. Similarly, the mRNA profile of gst (also involved in the detoxification pathway) showed the same trends as cyp1a transcripts with concentrations and with time. Moreover, Avey et al. (2020) exposed juvenile Atlantic salmon to the dilbit Cold Lake Summer Blend and compared the relative abundance of cyp1a versus EROD activity in the liver noticing similar patterns, increasing both at higher concentrations. In our study, in the 2 °C settings similar patterns are observed in the relative abundance of cyp1a and EROD activity (Figure 3F; Figure 4F). However, this contrast with the results at 15 °C (Figure 3L; Figure 4L). These data highlight the importance of studying side-by-side the chemistry and toxicity profiles of complex oil mixtures as they weather in different environmental conditions.
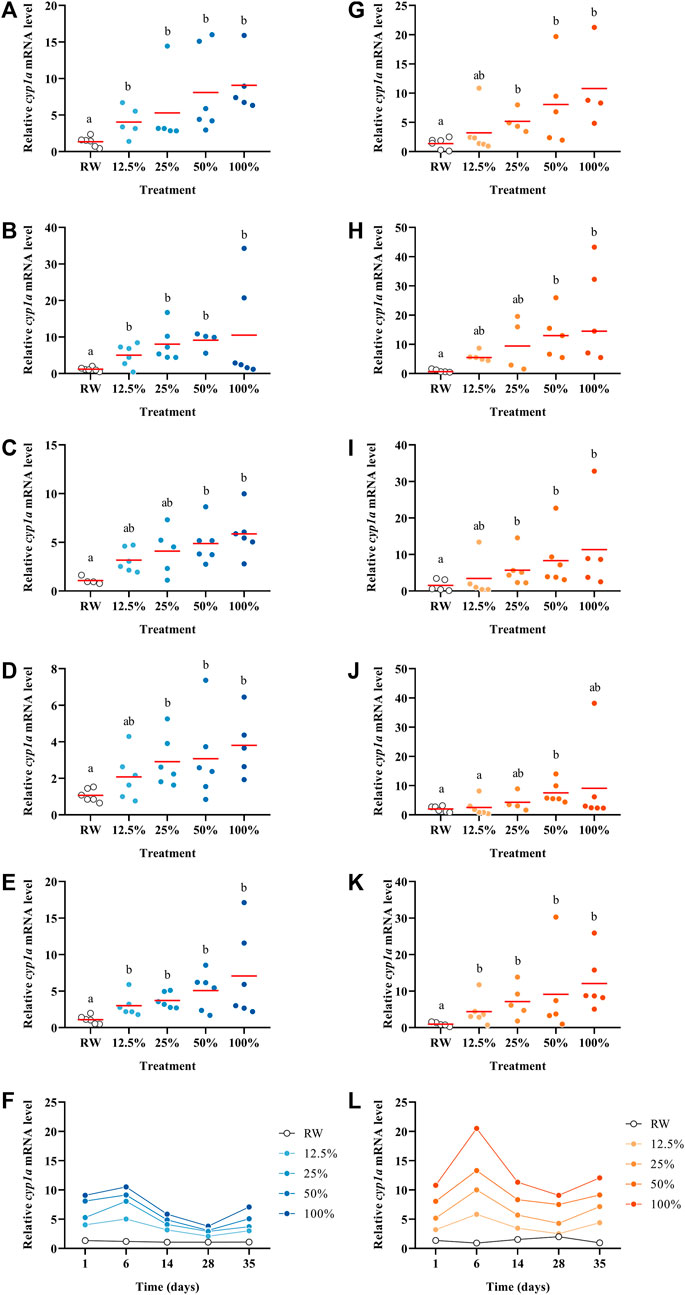
FIGURE 4. Relative cyp1a abundance in Pimephales promelas larvae exposed to the water accommodated fraction of a simulated controlled oil spill at 2°C (A–E) and 15°C (G–K) after 1, 6, 14, 28 and 35 days. Relative cyp1a abundance in Pimephales promelas larvae exposed to the water accommodated fraction of a simulated controlled oil spill over 35 days at 2°C (F) and 15°C (L).
Previously in a similar experimental design, Lara-Jacobo et al. (2021) reported on the weathering of conventional oil at 15°C. Similarly, to our results, the VOCs decreased right after the spill and at 24 h the VOC concentration was lower than 0.5 mg/L (Figure 1B), showing that VOCs are not the main long-term source of toxicity for aquatic organisms after a conventional or a non-conventional oil spill at 15 °C. The total organic carbon stayed relatively stable after 168 h (7 days) until the end of the experiment (Figure 1D) with dilbit; however, conventional oil produced a higher TOC concentration after 336 h (14 days), possibly due to the formation of oxy-PAHs (Heshka et al., 2022). The total PAH concentration was ∼20,000 μg/L with conventional oil on day three (Lara-Jacobo et al., 2021) while with dilbit was 164 μg/L (Figure 1F). This difference is likely due to dilbit having a much lower concentration of the identified PAHs when compared to conventional oil. Also, the low viscosity of conventional oil and the formation of oil droplets could have influenced the concentration of PAHs, while dilbit is less dispersible due to its higher viscosity and density. The spilled dilbit was mostly recoverable as floating water-in-oil emulsion, while this was poorly achievable with conventional oil (39%), likely due to viscosity differences between both types of oils. Conventional oil could also pose a higher danger to sediment-dwelling organisms since almost 10% of oil sank, while with dilbit only 1.2% sank. This data indicates that could be easier to clean dilbit than conventional oil in a freshwater oil spill. Similarly to dilbit (Supplementary Table S3), conventional oil induced yolk sac edema, heart tube, craniofacial, and spinal deformities in P. promelas larvae.
In this study, we report on the chemical and toxicity profiles of dilbit spills weathered at 2°C and 15°C using a mesoscale spill tank. Regardless of the tested temperature, the concentration of VOCs decreases almost to the detection limit within 24 h of the dilbit spill. Similarly, the TOC and PAH concentrations decrease rapidly but stabilize after the first 5 days. At both temperatures, the exposure to the WAF increases the presence of abnormalities in P. promelas larvae, including pericardial edema, heart tube, spinal and craniofacial abnormalities, and yolk sac edema. Also, the exposure decreases the heart rate, induces EROD activity, and increases cyp1a and gst abundance in P. promelas larvae. However, there are no major differences in the overall chemical and toxicological profiles of the modelled dilbit spills at 2°C and 15 °C. This is crucial information for first responders because the time to respond to an oil spill in a freshwater system should not depend on the season, since our results indicate that the temperature does not significantly change the chemical profile and its deleterious effects on fish. Also, cleaning and remediation protocols should be implemented as soon as the spill is detected in all seasons.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
The animal study was approved by the Institut national de la recherche scientifique (INRS)᾽s Animal Care Committee. The study was conducted in accordance with the local legislation and institutional requirements.
JG-V: Formal Analysis, Writing–original draft, Writing–review and editing. LL-J: Formal Analysis, Investigation, Methodology, Writing–original draft, Writing–review and editing. CG: Conceptualization, Formal Analysis, Investigation, Methodology, Writing–review and editing. GP: Methodology, Writing–review and editing. QX: Conceptualization, Methodology, Resources, Writing–review and editing. GT-B: Writing–review and editing. HD: Conceptualization, Funding acquisition, Resources, Writing–review and editing. VSL: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This project was funded by the Government of Canada’s Oceans Protection Plan Program of the Natural Resources Canada (including research contract to VSL), Canada’s Research Chair Program (to VSL), and the CONACYT-Mexico (to LL-J). Part of the analysis was funded by a program of the Quebec Government, the Stratégie maritime du Gouvernement du Québec, Plan d’action 2015-2020.
The authors would like to thank Hena Farooqi and Behnam Namsechi for conducting the spill tests, and the assistance from the Standard Analytical Laboratory, and Upstream and Environment Team at CanmetENERGY Devon, for performance of the oil physical and chemical analyses, and water analyses, respectively.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1328313/full#supplementary-material
PAH, polycyclic aromatic hydrocarbon; CLB, Cold Lake blend; WAF, water accommodated fraction; NSR, North Saskatchewan River; RT-qPCR, Real-time quantitative polymerase chain reaction; cyp1a, Cytochrome P450 Family 1 Subfamily A; gst, glutathione S-transferase; rpl8, ribosomal protein L8; ef1a, elongation factor 1 alpha; MIQE, Minimum Information for Publication of Quantitative Real-Time PCR experiments.
Alderman, S. L., Lin, F., Farrell, A. P., Kennedy, C. J., and Gillis, T. E. (2017). Effects of diluted bitumen exposure on juvenile sockeye salmon: from cells to performance. Environ. Toxicol. Chem. 36, 354–360. doi:10.1002/etc.3533
Alsaadi, F., Hodson, P. V., and Langlois, V. S. (2018a). An embryonic field of study: the aquatic fate and toxicity of diluted bitumen. Bull. Environ. Contam. Toxicol. 100, 8–13. doi:10.1007/s00128-017-2239-7
Alsaadi, F. M., Madison, B. N., Brown, R. S., Hodson, P. V., and Langlois, V. S. (2018b). Morphological and molecular effects of two diluted bitumens on developing fathead minnow (Pimephales promelas). Aquat. Toxicol. 204, 107–116. doi:10.1016/j.aquatox.2018.09.003
Astm, (2009). Standard test method for total carbon and organic carbon in water by high temperature catalytic combustion and infrared detection D7573. ASTM. West Conshohocken, PA, USA.
Attanasi, E. D., and Meyer, R. F. (2010). “Natural bitumen and extra-heavy oil,” in Survey of Energy resources. Editor I. Iancu (World Energy Council), Boston, MA, USA, 123–150.
Avey, S. R., Kennedy, C. J., Farrell, A. P., Gillis, T. E., and Alderman, S. L. (2020). Effects of diluted bitumen exposure on Atlantic salmon smolts: molecular and metabolic responses in relation to swimming performance. Aquat. Toxicol. 221, 105423. doi:10.1016/j.aquatox.2020.105423
Bagi, A., Pampanin, D. M., Brakstad, O. G., and Kommedal, R. (2013). Estimation of hydrocarbon biodegradation rates in marine environments: a critical review of the Q10 approach. Mar. Environ. Res. 89, 83–90. doi:10.1016/j.marenvres.2013.05.005
Barber, S., Candeal Duro, J., Caprio, E. D., Dyrssen, I., Eklund, J., Perez Guerra, F., et al. (2020). Hazard classification and labelling of petroleum substances in the European Economic Area- 2020. Concawe, Brussels. Belgium.
Bérubé, R., Gauthier, C., Bourdin, T., Bouffard, M., Triffault-Bouchet, G., Langlois, V. S., et al. (2021). Lethal and sublethal effects of diluted bitumen and conventional oil on fathead minnow (Pimephales promelas) larvae exposed during their early development. Aquat. Toxicol. 237, 105884. doi:10.1016/j.aquatox.2021.105884
Billiard, S. M., Bols, N. C., and Hodson, P. V. (2004). In vitro and in vivo comparisons of fish-specific CYP1A induction relative potency factors for selected polycyclic aromatic hydrocarbons. Ecotoxicol. Environ. Saf. 59, 292–299. doi:10.1016/j.ecoenv.2004.06.009
Bustin, S. A., Benes, V., Garson, J. A., Hellemans, J., Huggett, J., Kubista, M., et al. (2009). The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. doi:10.1373/clinchem.2008.112797
Carls, M. G., Holland, L., Larsen, M., Collier, T. K., Scholz, N. L., and Incardona, J. P. (2008). Fish embryos are damaged by dissolved PAHs, not oil particles. Aquat. Toxicol. 88, 121–127. doi:10.1016/j.aquatox.2008.03.014
Centre d’expertise en analyse environnementale du Québec, (2008). Détermination des hydrocarbures aromatiques polycycliques; Dosage par chromatographie en phase gazeuse couplée à un spectromètre de masse. Centre d’expertise en analyse environnementale du Québec, Laval, Canada, MA. 400 – HAP 1.1, Rév. 3
Centre d’expertise en analyse environnementale du Québec, (2014). Détermination des composés organiques volatils dans l’eau et les sols: dosage par « Purge and Trap » couplé à un chromatographe en phase gazeuse et à un spectromètre de masse. Centre d’expertise en analyse environnementale du Québec, Laval, Canada, MA. 400 – COV 2.0, Rév. 4
Centre d’expertise en analyse environnementale du Québec, (2016). Détermination des hydrocarbures pétroliers (C10 à C50): dosage par chromatographie en phase gazeuse couplée à un détecteur à ionisation de flamme. Centre d’expertise en analyse environnementale du Québec, Laval, Canada, MA. 400 – HYD. 1.1, Rév. 3
Chen, C. M., and Cooper, K. R. (1999). Developmental toxicity and EROD induction in the Japanese medaka (Oryzias latipes) treated with dioxin congeners. Bull. Environ. Contam. Toxicol. 63, 423–429. doi:10.1007/s001289900997
Crosby, S., Fay, R., Groark, C., Kani, A., Smith, J. R., Sullivan, T., et al. (2013). Transporting Alberta oil sands products: defining the issues and assessing the risks. National Ocean Service, Office of Response and Restoration. Silver Spring, MD, USA.
Delille, D., Pelletier, E., Rodriguez-Blanco, A., and Ghiglione, J.-F. (2009). Effects of nutrient and temperature on degradation of petroleum hydrocarbons in sub-Antarctic coastal seawater. Polar Biol. 32, 1521–1528. doi:10.1007/s00300-009-0652-z
Department of Fisheries and Oceans Canada, (2018). Status report on the knowledge of the fate and behaviour of diluted bitumen in the aquatic ecosystems. Department of Fisheries and Oceans Canada, Ontario, Canada.
Dubansky, B., Whitehead, A., Miller, J. T., Rice, C. D., and Galvez, F. (2013). Multitissue molecular, genomic, and developmental effects of the Deepwater Horizon oil spill on resident Gulf killifish (Fundulus grandis). Environ. Sci. Technol. 47, 5074–5082. doi:10.1021/es400458p
Energy Information Administration, (2022). Country analysis executive summary: Canada [WWW document]. https://www.eia.gov/international/analysis/country/CAN (Accessed February 9, 22).URL
Faksness, L.-G., Brandvik, P. J., and Sydnes, L. K. (2008). Composition of the water accommodated fractions as a function of exposure times and temperatures. Mar. Pollut. Bull. 56, 1746–1754. doi:10.1016/j.marpolbul.2008.07.001
Fingas, M. F. (2007). “Estimation of oil spill behaviour parameters from readily-available oil properties,” in Arctic and marine oilspill program technical seminar (Edmonton, Canada: Environment Canada), 1.1999
Gagnon, M. M., and Rawson, C. A. (2017). Bioindicator species for EROD activity measurements: a review with Australian fish as a case study. Ecol. Indic. 73, 166–180. doi:10.1016/j.ecolind.2016.09.015
Gutierrez-Villagomez, J. M., Martyniuk, C. J., Xing, L., Langlois, V. S., Pauli, B. D., Blais, J. M., et al. (2019a). Transcriptome analysis reveals that naphthenic acids perturb gene networks related to metabolic processes, membrane integrity, and gut function in Silurana (Xenopus) tropicalis embryos. Front. Mar. Sci. 6, 533. doi:10.3389/fmars.2019.00533
Gutierrez-Villagomez, J. M., Patey, G., To, T. A., Lefebvre-Raine, M., Lara-Jacobo, L. R., Comte, J., et al. (2021). Frogs respond to commercial formulations of the biopesticide Bacillus thuringiensis var. israelensis, especially their intestine microbiota. Environ. Sci. Technol. 55, 12504–12516. doi:10.1021/acs.est.1c02322
Gutierrez-Villagomez, J. M., Peru, K. M., Edington, C., Headley, J. v., Pauli, B. D., and Trudeau, V. L. (2019b). Naphthenic acid mixtures and acid-extractable organics from oil sands process-affected water impair embryonic development of Silurana (Xenopus) tropicalis. Environ. Sci. Technol. 53, 2095–2104. doi:10.1021/acs.est.8b04461
Gutierrez-Villagomez, J. M., Vázquez-Martínez, J., Ramírez-Chávez, E., Molina-Torres, J., and Trudeau, V. L. V. L. (2020). Profiling low molecular weight organic compounds from naphthenic acids, acid extractable organic mixtures, and oil sands process-affected water by SPME-GC-EIMS. J. Hazard Mater 390, 122186. doi:10.1016/j.jhazmat.2020.122186
Heshka, N. E., Peru, K. M., Xin, Q., Dettman, H. D., and Headley, J. v. (2022). High resolution Orbitrap mass spectrometry analysis of oxidized hydrocarbons found in freshwater following a simulated spill of crude oil. Chemosphere 292, 133415. doi:10.1016/j.chemosphere.2021.133415
Idowu, O., Semple, K. T., Ramadass, K., O’Connor, W., Hansbro, P., and Thavamani, P. (2019). Beyond the obvious: environmental health implications of polar polycyclic aromatic hydrocarbons. Environ. Int. 123, 543–557. doi:10.1016/j.envint.2018.12.051
Incardona, J. P., Carls, M. G., Day, H. L., Sloan, C. A., Bolton, J. L., Collier, T. K., et al. (2009). Cardiac arrhythmia is the primary response of embryonic Pacific herring (Clupea pallasi) exposed to crude oil during weathering. Environ. Sci. Technol. 43, 201–207. doi:10.1021/es802270t
Incardona, J. P., Day, H. L., Collier, T. K., and Scholz, N. L. (2006). Developmental toxicity of 4-ring polycyclic aromatic hydrocarbons in zebrafish is differentially dependent on AH receptor isoforms and hepatic cytochrome P4501A metabolism. Toxicol. Appl. Pharmacol. 217, 308–321. doi:10.1016/j.taap.2006.09.018
Incardona, J. P., Gardner, L. D., Linbo, T. L., Brown, T. L., Esbaugh, A. J., Mager, E. M., et al. (2014). Deepwater Horizon crude oil impacts the developing hearts of large predatory pelagic fish. Proc. Natl. Acad. Sci. U. S. A. 111, E1510–E1518. doi:10.1073/pnas.1320950111
Incardona, J. P., Swarts, T. L., Edmunds, R. C., Linbo, T. L., Aquilina-Beck, A., Sloan, C. A., et al. (2013). Exxon Valdez to Deepwater Horizon: comparable toxicity of both crude oils to fish early life stages. Aquat. Toxicol. 142–143, 303–316. doi:10.1016/j.aquatox.2013.08.011
Incardona, J. P., Vines, C. A., Anulacion, B. F., Baldwin, D. H., Day, H. L., French, B. L., et al. (2012). Unexpectedly high mortality in Pacific herring embryos exposed to the 2007 Cosco Busan oil spill in San Francisco Bay. Proc. Natl. Acad. Sci. U. S. A. 109, E51–E58. doi:10.1073/pnas.1108884109
Knecht, A. L., Goodale, B. C., Truong, L., Simonich, M. T., Swanson, A. J., Matzke, M. M., et al. (2013). Comparative developmental toxicity of environmentally relevant oxygenated PAHs. Toxicol. Appl. Pharmacol. 271, 266–275. doi:10.1016/j.taap.2013.05.006
Koponen, K., Kukkonen, J. V. K., and Lindström-seppä, P. (1998). Chemical accumulation and biotransformation enzyme activities of rainbow trout embryos in waterborne exposure to PCB-77. Mar. Environ. Res. 46, 475–478. doi:10.1016/S0141-1136(97)00064-0
Lara-Jacobo, L. R., Gauthier, C., Xin, Q., Dupont, F., Couture, P., Triffault-Bouchet, G., et al. (2021). Fate and fathead minnow embryotoxicity of weathering crude oil in a pilot-scale spill tank. Environ. Toxicol. Chem. 40, 127–138. doi:10.1002/etc.4891
Le Bihanic, F., Couillard, C. M., Rigaud, C., and Légaré, B. (2013). A simple and reliable in vivo EROD activity measurement in single Fundulus heteroclitus embryo and larva. Mar. Environ. Res. 84, 17–23. doi:10.1016/j.marenvres.2012.11.003
Lebordais, M., Gutierrez-Villagomez, J. M., Gigault, J., Baudrimont, M., and Langlois, V. S. (2021). Molecular impacts of dietary exposure to nanoplastics combined with arsenic in Canadian oysters (Crassostrea virginica) and bioaccumulation comparison with Caribbean oysters (Isognomon alatus). Chemosphere 277, 130331. doi:10.1016/j.chemosphere.2021.130331
Li, X., Xiong, D., Ding, G., Fan, Y., Ma, X., Wang, C., et al. (2019). Exposure to water-accommodated fractions of two different crude oils alters morphology, cardiac function and swim bladder development in early-life stages of zebrafish. Chemosphere 235, 423–433. doi:10.1016/j.chemosphere.2019.06.199
Lin, F., Osachoff, H. L., and Kennedy, C. J. (2020). Physiological disturbances in juvenile sockeye salmon (Oncorhynchus nerka) exposed to the water-soluble fraction of diluted bitumen. Aquat. Toxicol. 220, 105383. doi:10.1016/j.aquatox.2019.105383
Machala, M., Ciganek, M., Bláha, L., Minksová, K., and Vondráck, J. (2001). Aryl hydrocarbon receptor-mediated and estrogenic activities of oxygenated polycyclic aromatic hydrocarbons and azaarenes originally identified in extracts of river sediments. Environ. Toxicol. Chem. 20, 2736–2743. doi:10.1002/etc.5620201212
Madison, B. N., Hodson, P. V., and Langlois, V. S. (2015). Diluted bitumen causes deformities and molecular responses indicative of oxidative stress in Japanese medaka embryos. Aquat. Toxicol. 165, 222–230. doi:10.1016/j.aquatox.2015.06.006
Madison, B. N., Hodson, P. V., and Langlois, V. S. (2017). Cold Lake Blend diluted bitumen toxicity to the early development of Japanese medaka. Environ. Pollut. 225, 579–586. doi:10.1016/j.envpol.2017.03.025
Mager, E. M., Esbaugh, A. J., Stieglitz, J. D., Hoenig, R., Bodinier, C., Incardona, J. P., et al. (2014). Acute embryonic or juvenile exposure to deepwater horizon crude oil impairs the swimming performance of mahi-mahi (Coryphaena hippurus). Environ. Sci. Technol. 48, 7053–7061. doi:10.1021/es501628k
Mager, E. M., Wintz, H., Vulpe, C. D., Brix, K. V., and Grosell, M. (2008). Toxicogenomics of water chemistry influence on chronic lead exposure to the fathead minnow (Pimephales promelas). Aquat. Toxicol. 87, 200–209. doi:10.1016/j.aquatox.2008.02.001
Martyniuk, C. J., Alvarez, S., Lo, B. P., Elphick, J. R., and Marlatt, V. L. (2012). Hepatic protein expression networks associated with masculinization in the female fathead minnow (Pimephales promelas). J. Proteome Res. 11, 4147–4161. doi:10.1021/pr3002468
Middaugh, D. P., Chapman, P. J., and Shelton, M. E. (1996). Responses of embryonic and larval inland silversides, Menidia beryllina, to a water-soluble fraction formed during biodegradation of artificially weathered Alaska North Slope crude oil. Arch. Environ. Contam. Toxicol. 31, 410–419. doi:10.1007/BF00212681
Misaki, K., Kawami, H., Tanaka, T., Handa, H., Nakamura, M., Matsui, S., et al. (2007). Aryl hydrocarbon receptor ligand activity of polycyclic aromatic ketones and polycyclic aromatic quinones. Environ. Toxicol. Chem. 26, 1370–1379. doi:10.1897/06-465r.1
National Academies of Sciences Engineering and Medicine, (2016). Spills of diluted bitumen from pipelines: a comparative study of environmental fate, effects, and response. National Academies Press. Washington, DC, USA.
National Energy Board, (2004). Canada’s oil sands, opportunities and challenges to 2015. National Energy Board. Canada.
Natural Resources Canada, (2019). Crude oil facts [WWW document]. https://www.nrcan.gc.ca/science-data/data-analysis/energy-data-analysis/energy-facts/crude-oil-facts/20064 (Accessed January 7, 21).URL
Natural Resources Canada, (2020). Pipelines across Canada [WWW document]. https://www.nrcan.gc.ca/our-natural-resources/energy-sources-distribution/clean-fossil-fuels/pipelines/pipelines-across-canada/18856#shr-pg0 (Accessed February 5, 21).URL
Office of Emergency and Remedial Response, (1999). Understanding oil spills and oil spill response. United States Environmental Protection Agency. Washington, DC, USA.
Payne, J. R., Kirstein, B. E., McNabb, G. D., Lambach, J. L., de Oliveira, C., Jordan, R. E., et al. (1983). Multivariate analysis of petroleum hydrocarbon weathering in the subarctic marine environment1. Int. Oil Spill Conf. Proc. 1983 1983, 423–434. doi:10.7901/2169-3358-1983-1-423
Payne, J. R., Mcnabb, G. D., and Clayton, J. R. (1991). Oil-weathering behavior in Arctic environments. Polar Res. 10, 631–662. doi:10.3402/polar.v10i2.6774
Peters, L. D., and Livingstone, D. R. (1995). Studies on cytochrome P4501A in early and adult life stages of turbot (Scophthalmus maximus L.). Mar. Environ. Res. 39, 5–9. doi:10.1016/0141-1136(94)00064-V
Philibert, D. A., Philibert, C. P., Lewis, C., and Tierney, K. B. (2016). Comparison of diluted bitumen (dilbit) and conventional crude oil toxicity to developing zebrafish. Environ. Sci. Technol. 50, 6091–6098. doi:10.1021/acs.est.6b00949
Phosphate-buffered saline (Pbs), (2006). Cold spring harbor protocols 2006. Huntington: Cold Sping Harbour Press. pdb.rec8247
R Core Team, (2013). R: a language and environment for statistical computing. R Core Team. Vienna, Austria.
Rstudio Team, (2016). RStudio: integrated development environment for R. Rstudio Team. Boston, MA, USA.
Schiano Di Lombo, M., Weeks-Santos, S., Clérandeau, C., Triffault-Bouchet, G., Langlois, V. S., Couture, P., et al. (2021). Comparative developmental toxicity of conventional oils and diluted bitumen on early life stages of the rainbow trout (Oncorhynchus mykiss). Aq Toxicol. 239, 105937. doi:10.1016/j.aquatox.2021.105937ISSN 0166-445X
Schnitz, A. R., and O’Connor, J. M. (1992). In vivo DNA/RNA adduction of 7,12-dimethylbenz(a)anthracene (DMBA) and benzo(a)pyrene (BaP) in the liver of rainbow trout (Oncorhynchus mykiss). J. Environ. Pathol. Toxicol. Oncol. 11, 229–233.
Scott, J. A., Incardona, J. P., Pelkki, K., Shepardson, S., and Hodson, P. V. (2011). AhR2-mediated, CYP1A-independent cardiovascular toxicity in zebrafish (Danio rerio) embryos exposed to retene. Aquat. Toxicol. 101, 165–174. doi:10.1016/j.aquatox.2010.09.016
Shen, A., Tang, F., and Shen, X. (2010). “Toxicity of crude oil and fuel oil on the embryonic development of the Large Yellow Croaker (Larmichthys crocea),” in 2010 3rd International Conference on Biomedical Engineering and Informatics, 2138–2141. doi:10.1109/BMEI.2010.5639278
Strausz, O. P., Lown, E. M., Morales-Izquierdo, A., Kazmi, N., Montgomery, D. S., Payzant, J. D., et al. (2011). Chemical composition of athabasca bitumen: the distillable aromatic fraction. Energy and Fuels 25, 4552–4579. doi:10.1021/ef200833e
Ticleanu, C., and Littlefair, P. (2015). A summary of LED lighting impacts on health. Int. J. Sustain. Light. 17, 5–11. doi:10.17069/ijsl.2015.12.1.1.5
Tissier, F., Dussauze, M., Lefloch, N., Theron, M., Lemaire, P., Le Floch, S., et al. (2015). Effect of dispersed crude oil on cardiac function in seabass Dicentrarchus labrax. Chemosphere 134, 192–198. doi:10.1016/j.chemosphere.2015.04.026
United States Environmental Protection Agency; Us-Epa, (2004). Test method 3510C: separatory funnel liquid-liquid extraction. United States Environmental Protection Agency, Washington, DC, USA.
United States Environmental Protection Agency; Us-Epa, (2007). Test method 8015C: nonhalogenated organics by gas chromatography. United States Environmental Protection Agency, Washington, DC, USA.
United States Environmental Protection Agency; Us-Epa, (2014). Test method 5021A: volatile organic compounds (VOCs) in various sample matrices using equilibrium headspace analysis. United States Environmental Protection Agency, Washington, DC, USA.
United States Environmental Protection Agency; Us-Epa, (2018a). Method 8260D: volatile organic compounds by gas chromatography-mass spectrometry. GC/MS. United States Environmental Protection Agency, Washington, DC, USA.
United States Environmental Protection Agency; Us-Epa, (2018b). Method 8270E: semivolatile organic compounds by gas chromatography/mass spectrometry. GC-MS. United States Environmental Protection Agency, Washington, DC, USA.
Wallace, S. J., de Solla, S. R., Head, J. A., Hodson, P. V., Parrott, J. L., Thomas, P. J., et al. (2020). Polycyclic aromatic compounds (PACs) in the Canadian environment: exposure and effects on wildlife. Environ. Pollut. 265, 114863. doi:10.1016/j.envpol.2020.114863
Wincent, E., Jönsson, M. E., Bottai, M., Lundstedt, S., and Dreij, K. (2015). Aryl hydrocarbon receptor activation and developmental toxicity in zebrafish in response to soil extracts containing unsubstituted and oxygenated PAHs. Environ. Sci. Technol. 49, 3869–3877. doi:10.1021/es505588s
Xin, Q., Saborimanesh, N., Greer, C. W., Farooqi, H., and Dettman, H. D. (2023). The effect of temperature on hydrocarbon profiles and the microbial community composition in North Saskatchewan River water during mesoscale tank tests of diluted bitumen spills. Sci. Tot Environ. 859, 160161. doi:10.1016/j.scitotenv.2022.160161
Keywords: cold lake blend, PAHs, fish, malformations, heart rate, fathead minnow
Citation: Gutierrez-Villagomez JM, Lara-Jacobo LR, Gauthier C, Patey G, Xin Q, Triffault-Bouchet G, Dettman HD and Langlois VS (2024) Diluted bitumen weathered under warm or cold temperatures is equally toxic to freshwater fish. Front. Environ. Sci. 12:1328313. doi: 10.3389/fenvs.2024.1328313
Received: 26 October 2023; Accepted: 05 February 2024;
Published: 06 March 2024.
Edited by:
Christina Emmanouil, Aristotle University of Thessaloniki, GreeceReviewed by:
Abbas Güngördü, İnönü University, TürkiyeCopyright © His Majesty the King in Right of Canada as Represented by the Minister of Natural Resources, 2024. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Valerie S. Langlois, VmFsZXJpZS5MYW5nbG9pc0BpbnJzLmNh
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.