
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 01 February 2024
Sec. Conservation and Restoration Ecology
Volume 12 - 2024 | https://doi.org/10.3389/fenvs.2024.1299139
Coastal wetlands play an irreplaceable role in the global ecosystem, and both human activities and natural factors may lead to the contamination of Tiaozini coastal wetland with heavy metals. The study was conducted to determine the contents of eight heavy metals, Hg, Cd, Cr, As, Pb, Cu, Ni, and Zn, in the above-ground and below-ground parts of the plants and in the rhizosphere sediment, using the invasive species S. alterniflora and the native plant S. glauca, calculating the geoaccumulation index (Igeo), bioaccumulation factor, transfer factor, total target risk quotient (TTHQ), and carcinogenicity risk (CR), to analyze the transfer characteristics and potential health risks to human beings of the heavy metals in plants. This study aims to investigate the enrichment characteristics of the dominant plant, S. alterniflora Loisel. (S. alterniflora) and Suaeda salsa (L.) Pall. (S. glauca). Regarding heavy metals, eight common heavy metal elements were selected, including Hg, Cd, Cr, As, Pb, Cu, Ni, and Zn, and examined their content in surface sediments and different parts of the two plants. The transfer characteristics of heavy metals in the plant body and their potential health risks to humans were also analyzed. These findings suggest that both plants accumulate higher concentrations of heavy metals in their below-ground parts. Cr, Cu, and Zn had the highest average concentrations in both plants. Geoaccumulation index (Igeo) indicated that the Tiaozini Wetland is not yet contaminated. S. alterniflora had transfer factors less than 1 for all heavy metals, while S. glauca had transfer factors greater than 1. Both plants had a certain purifying effect on heavy metal pollution in wetlands, including Cr, Cd, Cu, and Zn. However, Cr and As in the below-ground part of S. alterniflora and Cr in the above-ground part of S. glauca had a target hazard quotient (THQ) greater than 1, indicating a potential health risk to humans, but the carcinogenic risk is low. For other heavy metals, THQ was less than 1, indicating no health risk. The total target hazard quotient (TTHQ) of different parts of both plants was greater than 1, which must be taken into account when considering their suitability as edible resources.
Coastal wetlands are crucial components of interlinked land and sea ecosystems and perform vital ecological roles such as carbon sequestration (Chen and Lee, 2022), pollution remediation, improvement of coastal water quality, protection of biodiversity, migratory bird habitats, mitigating oceanic disasters (Hongyi et al., 2009), and regulating the climate. They also bring significant environmental and economic benefits to coastal regions (Sun et al., 2018). However, under the multiple impacts of land, ocean, and human activities, coastal waters often become the most polluted parts of marine areas, and coastal wetlands have become sources and sinks of various pollutants such as heavy metals (Cui et al., 2016; Ahmed et al., 2018). Heavy metals exist in various environmental media, with high concealment, long persistence, difficult biodegradation, and difficult remediation characteristics (Li and Zhang, 2010; Varol, 2011). Heavy metal elements enter sediments through various physical, chemical, and biological processes, and some are released back into the ocean under the action of waves and tides, or absorbed by plants, etc., affecting marine organisms, marine ecosystems, and water quality safety (Yin et al., 2016), and further affecting human health under the enrichment of the food chain (Habib et al., 2016).
Dongtai is located in the southern part of Yancheng, Jiangsu Province. It boasts an extensive coastline of 85 km and a near-shore and coastal wetland area of 2116.7 km2. It is estimated to be expanding into the sea at a rate of approximately 150 m per year, providing a significant natural resource for the ongoing ecological development of new land each year (Bai et al., 2022; Tian et al., 2022). The Tiaozini coastal wetland in Dongtai is the first of its kind and the second coastal wetland to be recognized as a World Natural Heritage Site [China’s Yellow (Bohai) SeaBird Habitat (Phase I)]. However, both anthropogenic activities (agriculture, reclamation (Jing et al., 2012), etc.) and natural factors (migratory bird roosting activities (Berglund, 2018), phytoplankton (Lin et al., 2002), and hydrodynamic conditions (Cai et al., 2023), etc.) may lead to heavy metal pollution in Tiaozini wetland. The vegetation in Tiaozini wetland spans from the sea to the land and exhibits different ecological successions, with the dominant species being S. alterniflora, S. glauca and reeds. S. alterniflora is mainly found in the intertidal zone beyond the embankment. It is a perennial salt marsh plant belonging to the Poaceae family that was introduced to China for stabilizing beaches, preventing waves and wind. However, due to its strong reproductive and propagation ability, S. alterniflora has now become an invasive alien plant, posing several negative impacts on the local natural ecosystem (Zhao et al., 2022; Zhang et al., 2023). In recent years, various researchers have explored the resource utilization of S. alterniflora and identified its nutritional and medicinal properties, particularly its flavonoid compounds, which possess the potential to reduce blood glucose and lipid levels, exhibit anti-inflammatory effects, and enhance immune function (Xu et al., 2019). In addition, S. alterniflora possesses enrichment capabilities for heavy metals in wetland areas (Zhang et al., 2020; Zhang et al., 2022). S. glauca is primarily found in the breeding grounds of black-headed gulls within the embankment. It is an annual herbaceous plant that belongs to the Chenopodiaceae Suaeda genus. This plant is capable of improving the soil’s ecological environment in wetlands, as it effectively reduces soil salinity. The metabolic activity and litter produced by S. glauca can enhance soil microorganism activity, while also having the ability to enrich certain heavy metal elements (Joshi et al., 2020; He et al., 2022).
In this study, the dominant plants in the coastal wetland of Tiaozini coastal wetland, S. alterniflora and S. glauca, were used to determine the contents of eight common heavy metals (Hg, Cd, Cr, As, Pb, Cu, Ni, and Zn) in the surface sediments and in different parts of the plant body, calculating the geoaccumulation index (Igeo), bioaccumulation factors, transfer factors, total target hazard quotient (TTHQ) and the carcinogenic risk (CR) of the different heavy metals in the two plants, to analyze the transfer characteristics and potential health risks to human beings of the heavy metals in plants.
The research area is situated in Tiaozini coastal wetland, Dongtai, Jiangsu, facing the Yellow Sea. Its geographic coordinates are between 32°33′N-32°85′N and 120°07′E-120°96′E. It falls under the transitional zone between the North Subtropical and Warm Temperate Zones and exhibits characteristics of a marine monsoon climate, with an average annual precipitation of 900–1,068 mm and an average annual temperature of 13.5°C–14.6°C. The soil in the region is alkaline sediment loamy coastal saline soil. Tiaozini is situated at the intersection of a radial underwater sand ridge group and the middle part of Jiangsu coast. It is the largest nearshore tidal flat wetland found in the middle of Jiangsu coast. The formation and development of the radial sand ridge group are facilitated under the influence of the water-sand dynamic environment (Hu et al., 2022). The transportation mechanisms of tidal currents and sediment have shaped the landscape and demonstrated the developmental direction of the tidal flats. Its coverage extends from the initial embankment of Tiaozini Phase I towards the east to Dongdagang, and from the outer harbor passageway of Liangduo River entrance to the south to the outer harbor passageway of Fangtang River entrance.
The experiment took place in October 2022, during the time of peak plant biomass and maximum absorption and accumulation of elements in the intertidal zone and S. glauca area of the study site.
Plant sampling: due to the irregularity of the growth area of the S. glauca, six representative sampling sites (JP1-JP6) were randomly selected, and 3-4 complete S. glauca plants were dug out with a plastic shovel in each sample site, divided into above-ground parts (SSU) and below-ground parts (SSD), and then put into polyethylene self-sealing bags and sealed for low-temperature storage. Two more evenly distributed sampling areas with the largest biomass were selected (CP1 and CP2) in the S. alterniflora zone. In CP1 and CP2 two sample zones were set up evenly distributed, larger biomass of 1 m × 1 m sample squares, each sample square using the plum blossom method to collect 4-5 complete plants of S. alterniflora, which were divided into above-ground parts (SAU) and below-ground parts (SAD), then put into polyethylene self-sealing bags and sealed and preserved at low temperatures.
Sediment sampling: in the sampling points JP1-JP6 in the S. glauca area, the rhizosphere sediment of S. glauca samples was collected by using a plastic shovel, and the sediment samples were put into polyethylene self-sealing bags, sealed and preserved at low temperatures. In the S. alterniflora area CP1 and CP2 two sample zones set up in the sample square to collect the rhizosphere sediment samples of S. alterniflora samples, into the polyethylene self-sealing bags sealing, and finally all the samples will be preserved at low temperature and transported back to the laboratory.
Figure 1 shows the specific locations of the sampling points.
The sediment samples were freeze-dried until a constant weight was achieved. They were then purified to remove any impurities and ground to a fine consistency before being sifted through a 100-mesh (150 μm) nylon screen and stored at 4°C for future experiments. The plant samples were washed thoroughly with tab water to remove large particles. Three rinses with deionized water were performed before the plants were air-dried. The plants were separated into above-ground and below-ground parts, each of which was placed in a drying oven at 45°C for 10 min. After cooling, the samples were ground into powder and sifted through a 40-mesh (0.425 mm) nylon screen. Finally, the samples were stored in labeled bags until needed.
0.2 g of sediment and plant samples were weighed separately and placed in digestion tubes. For sediment samples, a mixture of 6 mL nitric acid (65%–68%), 2 mL hydrofluoric acid (40%), and 2 mL hydrochloric acid (37%) was added for digestion. For plant samples, 6 mL concentrated nitric acid and 2 mL hydrogen peroxide (30%) were added and digestion was performed in a microwave according to standard procedures Take 0.2 g–0.5 g of solid rest sample and add 5 mL–10 mL, nitric acid, cover and leave it for 1 h or overnight, screw the lid of the jar tightly. The digestion is carried out in three steps, the first 120°C heating 5 min, constant temperature 5 min, followed by 150°C heating 5 min, constant temperature 10 min, and finally 180°C heating 5 min, constant temperature 20 min. After cooling, remove, slowly open the lid of the tank exhaust, with a small amount of water to rinse the inner lid, the digestion tank on a temperature-controlled hot plate or ultrasonic water bath, heating at T00°C for 30 min or ultrasonic Degas for 2 min–5 min, and then volume to 25 mL or 50 mL with water, mix well and standby, and do blank test at the same time. After cooling, the samples were transferred to an acid washing device and 1 mL perchloric acid was added to make a total volume of 10 mL with pure water. The processed samples were used to determine the concentrations of Cd, Cr, Pb, Cu, Ni, and Zn. For determination of Hg and As concentrations, 0.2 g of sediment and plant samples were respectively added with 2 mL nitric acid and 6 mL hydrochloric acid for digestion. Inductively coupled plasma atomic emission spectroscopy (ICP-OES: Thermo Fisher ICAP PRO) was used to determine the concentrations of Cd, Cr, As, Pb, Cu, Ni, and Zn, following the China National Standards (HJ781-2016). The operating conditions and measurement parameters of ICP-OES are as follows: after power on, start to evacuate the vacuum so that the vacuum degree is below 6.6e-6 Torr, and the temperature is set to 25°C. Then start to torch, and after the torch is finished, put the injection tube into about 2% nitric acid solution or ultrapure water to rinse the injection system. Edit the analytical method in advance and call the method for sample testing. Atomic fluorescence spectrophotometry was used to determine the concentration of Hg, following China National Standards (HJ680-2013). The LOD and LOQ values for studied heavy metals were displayed in Supplementary Table S1. To validate the accuracy and precision of the analysis method, each element was repeated four times, and one standard sample was analyzed. The experimental analysis error was below 20%, and the recovery rate was between 85% and 115%. Standard samples [GBW07387 (GSS-31), GBW07386 (GSS-30) and GBW07408 (GSS-08)] were provided by Institute of Geophysical and Geochemical Exploration, Chinese Academy of Geological Sciences, China (IGGE).
The geoaccumulation index, a method used by Muller to assess metal contamination in sediments (Prveena et al., 2008), not only takes into account the effects of natural factors such as geological changes, but also pays attention to anthropogenic effects, and is able to differentiate anthropogenic impacts with a high degree of accuracy. The formula is as follows (Prveena et al., 2008; Rabee et al., 2011):
In the equation, C0 represents the content of heavy metals in a certain part of the plant (mg/kg); 1.5 represents the background matrix correction factor due to lithogenic effects, and Bn represents the geochemical background value in the average shale of element n, where Hg, Cd, Cr, As, Pb, Cu, Ni, and Zn are 0.022, 0.089, 72, 9.5, 19.5, 21, 30.7, 67, respectively.
According to Çevik et al. (2009), the contamination level may be classified in a scale ranging from 1 to 6 (Igeo ≤ 0 = unpolluted, Igeo < 1 = unpolluted to moderately polluted, Igeo < 2 = moderately polluted, Igeo < 3 = moderately to strongly, Igeo < 4 = Strongly polluted, Igeo < 5 = strongly to very strongly polluted, Igeo > 5 = very strongly polluted).
The bioaccumulation factor (BAF) quantifies the capacity of organisms to absorb and amass heavy metals from soil ecosystems. This indicator is commonly stated as the proportion of heavy metal content in a specific organ of a plant to the corresponding element concentration in its growing soil. The BAF calculation formula is as follows (Luc et al., 2022):
In the equation, BAF represents the biotic accumulation factor of a certain heavy metal in plants,
The transfer factor (TF) is the ratio of the concentration of an element in the aerial parts (such as stems, leaves, flowers, and fruits) of a plant to its concentration in the below-ground parts (roots). It is commonly used to assess the transport ability of a plant for that element from roots to aerial parts. The calculation formula is as follows (Nurtjahya et al., 2023):
In the equation, TF represents the transfer coefficient of plants for a certain heavy metal,
The Target Hazard Quotient (THQ) is a method for assessing health risks associated with single and combined heavy metal exposure in plants. THQ assesses the hazard of a single heavy metal element by dividing the daily human intake by the reference dose, while TTHQ evaluates the hazard of multiple heavy metal elements by summing the THQ values for each element (EPA, 2000). The health risk assessment method for coastal plant products was used in this calculation. The formula is as follows (Kouali et al., 2022; Xing et al., 2022):
In the equation,
When the THQ and TTHQ indices are <1.0, it indicates that there is no significant health risk to the human body; when the TTHQ and THQ indices are >1.0, it indicates a greater risk to human health.
The carcinogenic risk (CR) and total carcinogenic risk (TCR) can be calculated by following formula (Liu et al., 2021):
In the equation, CR represents the potential carcinogenic risk through consumption of carcinogenic heavy metals; SF is the slope factor of carcinogenic heavy metals (This study is considering the toxic effects of the two plants when used as edible resources, so SF is the carcinogenicity slope factor for oral ingestion); TCR is the sum of the potential carcinogenic risk of each heavy metal. The heavy metals for which SF is currently available in this study are Cd, Cr, As, Pb, and Ni, with 6.1, 0.5, 8.5 × 10−3, and 1.7 respectively (Lin et al., 2023).
The carcinogenic risks were classified as no risk (TCR/CR < 10–6), a risk that humans can tolerate (10–6 ≤ TCR/CR < 10–4), and a risk that humans cannot tolerate (10–4 ≤ TCR/CR) (Rao et al., 2022; Lin et al., 2023).
Statistical software SPSS 22.0 (SPSS, Inc., Chicago, IL) and Excel 2019 were used to preprocess the data, and the single-factor analysis of variance (ANOVA) was used to measure the difference between treatments (n ≥ 3). The difference was considered to be statistically significant at p < 0.05. All data were processed using Origin 2023 (Origin Lap Corp, United States).
The levels of heavy metals present in various regions of S. alterniflora and the adjacent rhizosphere soil located within the intertidal zones were displayed in Figure 2A. The distribution and accumulation of distinct elements within different sections of S. alterniflora principally rely on the bioaccumulation of biomass and the concentration of the element present in various components (Feng et al., 2018). The concentrations of Hg, Pb, and Ni in S. alterniflora in the study area were significantly lower than those found in the rhizosphere soil. The order of heavy metal content was: soil > SAD > SAU, indicating that S. alterniflora had a minimal accumulation of Hg, Pb, and Ni and negligibly affected the concentration of these elements in the rhizosphere soil. The concentration of Cd, Cr, and Zn in S. alterniflora was mainly exhibited as SAD > soil > SAU, suggesting that S. alterniflora has a strong ability to absorb Cd, Cr, and Zn in the below-ground part. The concentration of As and Cu in S. alterniflora was mainly exhibited in the order: soil > SAD > SAU, and unlike Hg, Pb, and Ni, S. alterniflora below-ground part had a certain accumulation ability for As and Cu in the soil. The results indicated that the below-ground part of S. alterniflora had higher element accumulation than the above-ground part. The element accumulation of S. alterniflora was similar in sampling points CP1 and CP2, which may be due to the similar invasion age of S. alterniflora in the two areas.
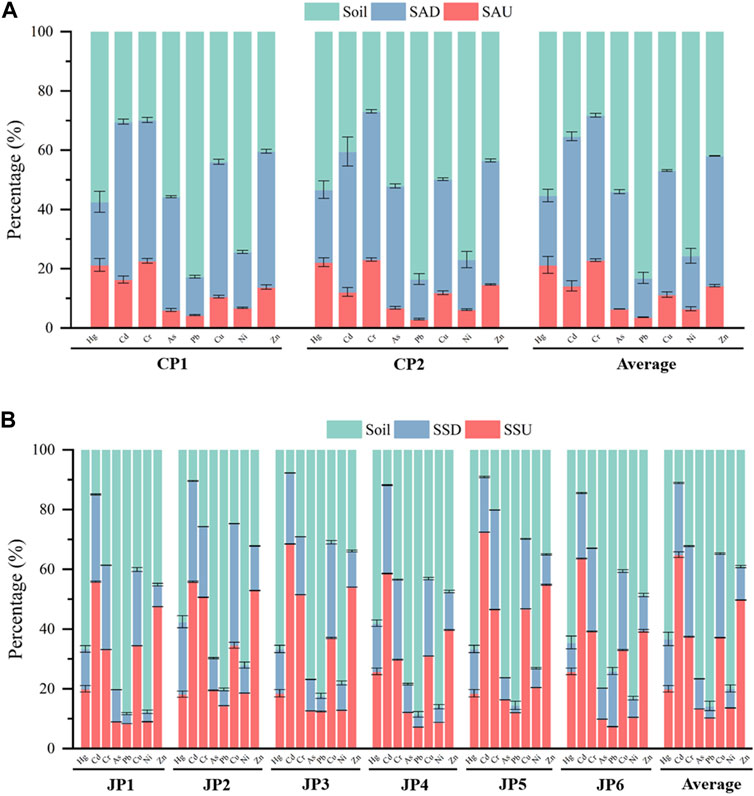
FIGURE 2. Percentage of heavy metal content in S. Alterniflora, S. glauca and their rhizosphere soil. (A) S. Alterniflora; (B) S. glauca.
Based on Figure 2B, the heavy metal concentration of Hg, As, Pb, and Ni in various parts of S. glauca is significantly lower than the rhizosphere soil in the study area. The order of heavy metal concentration in terms of quantity is: soil > SSU > SSD, indicating that S. glauca has a minimal accumulation of Hg, Pb, and Ni. Moreover, it has almost no impact on the concentration of Hg, As, Pb, and Ni in the rhizosphere soil. The heavy metal content in the above-ground parts of S. glauca is considerably higher compared to the corresponding elements in the below-ground parts. In the accumulation of Cd, S. glauca demonstrates a strong ability, with the concentration order mainly as SSU > SSD > soil. Furthermore, its above-ground parts hold a higher tendency for Cd accumulation. The order of Cr, Zn, and Cu concentration in different parts of S. glauca as mainly SSU > soil > SSD. This shows that S. glauca has a strong ability to enrich the soil with Cr, Zn, and Cu, transferring most of it to above-ground parts. This ability increases the accumulation of above-ground parts. The results also reveal that the above-ground parts’ accumulation of S. glauca is lower than the below-ground parts. In some sampling points, the abnormal high values in above-ground parts of the plant and abnormal low values in below-ground parts are related to environmental factors, such as the interactions between heavy metals and other elements in the soil (Song and Sun, 2014). At the same time, the growth periods of S. glauca vary across different sampling points, and the accumulation capacity and selectivity of S. glauca for various heavy metals also vary at different growth stages (Song et al., 2022).
On the whole, S. alterniflora and S. glauca contain relatively high concentrations of Cr, Cu, and Zn within their bodies. These heavy metals are vital elements for their growth, development, and reproduction. Appropriate levels of Cr can enhance net photosynthesis and promote healthy plant growth, but excessive Cr can have toxic effects on plants (Taufikurahman et al., 2019). Cu can be absorbed and utilized by plants to synthesize various enzymes and participate in numerous essential metabolic reactions within the plant’s body (Niyoifasha et al., 2023). The biosynthesis of several enzymes in plants necessitates the utilization of zinc, and these enzymes hold particular importance for the metabolism of carbon and nitrogen in plants (Liu et al., 2022).
Calculate the Igeo of heavy metals in plants according to Eq. 1. The results in Table 1 show that the Igeo of heavy metals in the plants were less than 0, indicating that the Tiaozini Wetland is clean in terms of heavy metals and is not yet contaminated. Compared with other wetlands in China, the Tiaozini Wetland is the least contaminated and uncontaminated, while all other wetlands are contaminated to a certain extent, and a common reason is the high concentration of Cd. The Tiaozini Wetland is not contaminated in terms of Igeo, but the Igeo of Cd is also the highest among the eight heavy metals, which is closely related to the intensity of anthropogenic activities (Wang et al., 2017; Lu et al., 2020; Hu et al., 2021). The Tiaozini wetland is China’s 14th World Natural Heritage Site, the core area of China’s Yellow (Bohai) Sea Migratory Bird Habitat (Phase I), and part of the area is not open to the public. In addition, the Tiaozini Wetland has been reclaimed and farmed, but the environmental protection is relatively strict.
The accumulation effect of plants on heavy metals is mainly measured by the bioaccumulation factors and transfer factor (Susarla et al., 2002).
Calculate the bioaccumulation factors of heavy metals in plants according to Eq. 2. The bioaccumulation factors (BAFs) of each heavy metal in S. Alterniflora and S. glauca were presented in Figure 3. The average BAFs of the eight heavy metal elements in above-ground parts of S. Alterniflora are ranked as follows: Cr (0.81)> Cd (0.42)> Hg (0.38)> Zn (0.34)> Cu (0.24)> As (0.12)> Ni (0.09)> Pb (0.04). It is observed that the above-ground parts of S. alterniflora have a weaker ability to accumulate heavy metals, as the average BAFs of all metals are not greater than 1. The highest accumulation ability is noted for Cr. Similarly, the average BAFs of S. alterniflora’s underground parts for each heavy metal are ranked as follows: Cr (1.73)> Cd (1.46)> Zn (1.05)> Cu (0.90)> As (0.74)> Hg (0.41)> Ni (0.24)> Pb (0.16), indicating a strong ability to accumulate Cr, Cd, and Zn. The order of accumulation ability of heavy metals in the underground parts is consistent with that in the above-ground parts except for Hg. The BAFs of heavy metals in the underground parts are more than twice that of the above-ground parts, suggesting that S. alterniflora mainly accumulates heavy metals in its underground parts. Overall, S. alterniflora has the potential for purifying and remediating soil contaminated with Cr, Cd, and Zn.
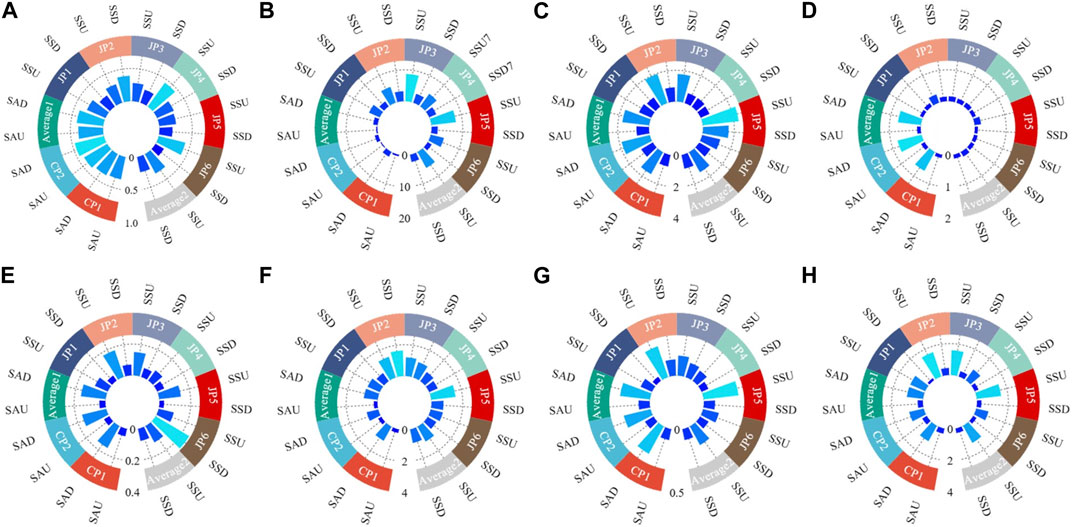
FIGURE 3. The bioaccumulation factors of each heavy metal in different parts of S. Alterniflora and S. glauca. (A) Hg; (B) Cd; (C) Cr; (D) As; (E) Pb; (F) Cu; (G) Ni; (H) Zn.
Based on the average BAFs of eight heavy metal elements found in the above-ground parts of S. glauca, the ranking is as follows: Cd (5.85)> Cr (1.46)> Zn (1.25)> Cu (1.09)> Hg (0.34)> As (0.17)> Ni (0.16)> Pb (0.12). It is evident that Cd, Cr, Zn, and Cu have average BAFs greater than 1 in the above-ground parts of S. glauca, with Cd showing a particularly strong accumulation ability with a BAF of 5.85, significantly higher than that of the other heavy metals. This indicates that S. glauca has a remarkably strong ability to accumulate Cd in the above-ground parts. On the other hand, the BAFs of Hg, As, Ni, and Pb are all less than 1, showcasing a relatively weak accumulation ability for these heavy metals in the above-ground parts. Moving on to the BAF rankings of heavy metal elements in the underground parts of S. glauca, this study finds that these rankings are consistent with those in the above-ground parts. This reveals that S. glauca has a strong ability to accumulate Cd in the underground parts, while the accumulation ability for Cr, Zn, Cu, As, Hg, Ni, and Pb is relatively weak. The BAF rankings across the six sampling points are generally consistent with the average BAF rankings. However, there is a considerable variation in the range of BAFs for some heavy metals among the different sampling points, which may be attributed to soil pollution from heavy metals in the local study area. Compared to the eight heavy metals analyzed, S. glauca has a stronger accumulation ability for Cd, Cr, Zn, and Cu, which is consistent with findings from previous studies on the accumulation ability of S. glauca for heavy metals (Shang et al., 2020; Cui et al., 2022; Quan et al., 2022). Meanwhile, S. glauca exhibits a high level of Cd accumulation, while its capacity to accumulate Pb is relatively weak. The findings suggest that the accumulation capabilities of both the above-ground and underground portions for each heavy metal are essentially consistent. The bioaccumulation factors (BAFs) of heavy metals in S. glauca’s above-ground sections are significantly greater than those in the underground parts, with the BAFs in the former being 0–2 times that of the latter. This suggests that S. glauca primarily absorbs and accumulates heavy metals in its above-ground portions, which may be influenced by factors such as soil pH, moisture levels, organic matter content, and microorganisms in the growing environment and plant growth cycles. Overall, S. glauca has the potential to purify and remediate Cr, Cd, Cu, and Zn in the soil to some extent.
Calculate the transfer factors of heavy metals in plants according to Eq. 3. The transfer factors (TFs) of various heavy metals in the soil for S. Alterniflora and S. glauca were shown in Figure 4. The average values, from high to low, are as follows: Hg (0.92)> Cr (0.47)> Ni (0.36)> Zn (0.33)> Cd (0.28)> Pb (0.27)> Cu (0.26)> As (0.16) (Figure 4A). All values are below 1, with Hg having the highest TF of 0.92. This suggests that S. alterniflora primarily accumulates heavy metals in its underground parts and has lesser ability to transfer them upward. The plant appears to possess greater transportation capability for Hg. All values are below 1, with Hg having the highest TF of 0.92. This suggests that S. alterniflora primarily accumulates heavy metals in its underground parts and has lesser ability to transfer them upward. The plant appears to possess greater transportation capability for Hg. The average values, from high to low, are as follows: Zn (4.38)> Cd (2.54)> Pb (2.41)> Ni (2.09)> Cr (1.65)> Hg (1.54)> As (1.38)> Cu (1.30) (Figure 4B). All values are above 1 with Zn, Cd, Pb, and Ni having TFs above 2. This indicates that S. glauca possesses robust heavy metal TFs and relatively greater potential for the migration of Zn, Cd, Pb, and Ni. Consequently, these heavy metals can accumulate to high levels in the plant’s above-ground parts, and its remnants may reintroduce them into the soil (Weis and Weis, 2004). Compared to S. alterniflora, S. glauca exhibits much higher TFs for heavy metals, with some variations in the order of transfer ability for different elements. This difference can be attributed to the transfer mechanism of heavy metals in plants, as well as to the unique properties of different plant species and environmental conditions in various research locations. S. glauca grows in a wetland area of non-flooded zones within the embankment, characterized by low pH and high salinity, which facilitates the migration of heavy metals from the underground parts to the above-ground parts. Meanwhile, S. alterniflora grows in a wetland area of the intertidal zone outside the embankment, where flooding may weaken its ability to transport heavy metals within the plant (Weis and Weis, 2004; Yang et al., 2008).
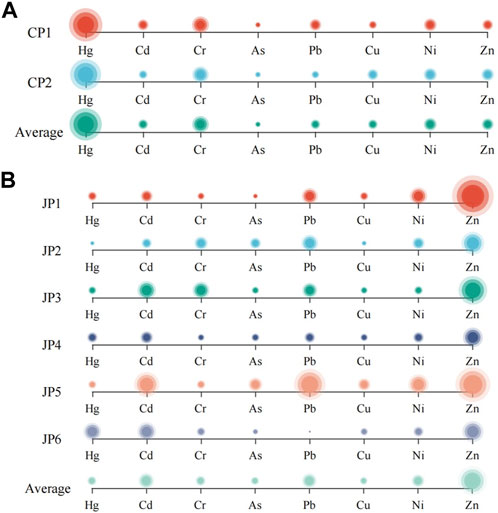
FIGURE 4. The transfer factors of S. Alterniflora and S. glauca. (A) S. Alterniflora; (B) S. glauca.
In summary, the comparative analysis of BAFs and TFs for S. alterniflora and S. glauca indicates that S. alterniflora exhibits superior enrichment effects for Cr, Cd, and Zn, while S. glauca possesses strong enrichment abilities for Cr, Cd, Cu, and Zn, suggesting that S. glauca could potentially become a hyperaccumulator for these four heavy metals. However, S. glauca exhibits lower accumulation abilities for other heavy metals and does not display hyperaccumulation characteristics. Generally, a vital feature in identifying hyperaccumulator plants is that their transfer factors are greater than or equal to 1. Additionally, a concentration factor greater than 1 is also a crucial criterion in determining hyperaccumulator plants (Siyar et al., 2022). Although there is no clearly defined limit for heavy metal content, it is generally believed that hyperaccumulator plants need to contain at least 100.0 mg/kg of Cd, 10,000 mg/kg of Zn, and 1,000 mg/kg of other heavy metals such as Cu, Pb, and Ni to exhibit hyperaccumulation properties. However, it is important to note that these reference values may vary depending on factors such as the plant species, the specific heavy metal, and the environmental conditions under which the plant is grown (Brooks et al., 1977). S. alterniflora exhibits a concentration factor that surpasses 1 for Cr, Cd, and Zn, while the transfer factors for these heavy metals remain less than 1. Similarly, S. glauca displays concentration and transfer factors greater than 1 for Cr, Cd, Cu, and Zn, but the concentrations of these heavy metals in the plant are beneath the hyperaccumulator reference values because the soils in the research area have relatively low concentrations of heavy metals. Thus, additional experiments are necessary to verify whether S. glauca can be categorized as a hyperaccumulator plant. Furthermore, both S. alterniflora and S. glauca have a relatively low capacity for Pb enrichment, rendering them unsuitable for ecological restoration in regions with high Pb concentrations. In general, both S. alterniflora and S. glauca exhibit a certain level of purification against heavy metals such as Cr, Cd, Cu, and Zn in wetlands. Additional practical applications are crucial to confirm their effectiveness in wetland heavy metal purification. S. alterniflora can prevent heavy metals from spreading to the ocean by accumulating specific heavy metals, hence reducing the ecological risk of heavy metal concentration in the ocean (Gao et al., 2016). S. alterniflora and S. glauca demonstrate a measure of effectiveness in purifying heavy metals such as Cr, Cd, Cu, and Zn in wetland environments. Additional practical applications are required to validate their efficacy in heavy metal purification. S. alterniflora may be employed to mitigate the spread of specific heavy metals to the ocean by accumulating them, ultimately decreasing the ecological risk posed to the marine environment.
Calculate the target hazard quotients and the total target hazard quotients of heavy metals in plants according to Eqs 4, 5. The health risk evaluation of S. alterniflora and S. glauca analyzed the individual risks for different parts, which are listed in Table 2. The target hazard quotients for eight heavy metals in the above-ground part of S. alterniflora (SAU) were arranged in descending order as Cr > As > Pb > Ni > Cu > Zn > Cd > Hg, while the below-ground part (SAD) ranked as Cr > As > Pb > Cu > Ni > Zn > Cd > Hg. The target hazard quotients for each heavy metal were similar in both parts. The THQ values for all eight heavy metals in the above-ground part were less than 1, with Cr having the highest value of 0.846. The results suggest that heavy metal exposure in the above-ground part of S. alterniflora does not pose a risk to human health. However, the THQ value for Cr was 1.808 and for As was 1.143 in the below-ground part, indicating that only Cr and As in the below-ground part of S. alterniflora may potentially be harmful to human health.
As presented in Figure 5, the target hazard quotient distribution for each heavy metal in the above-ground area of S. glauca (SSU) was arranged in a descending order as Cr>As>Cd>Pb>Cu>Zn>Ni>Hg. Meanwhile, in the below-ground part (SSD), the ranking order was Cr>As>Cu>Cd>Pb>Ni>Zn>Hg. The heavy metal ranking order in terms of the target hazard quotient was essentially the same in both the above-ground and below-ground areas. The THQ value of Cr was the highest in both the above-ground and below-ground parts of S. glauca, with a value of 1.124 for the above-ground area and less than 1 for the other seven heavy metals in both areas. These findings imply that only Cr in the above-ground area of S. glauca may present a potential health risk to humans, while other heavy metals do not appear to pose a risk.
Furthermore, the composite target hazard quotient for heavy metals in the above-ground and below-ground components of S. alterniflora and S. glauca was analyzed. The results indicated that the composite target hazard quotient for S. alterniflora was SAD (3.095) >SAU (1.074). Likewise, the composite target hazard index for S. glauca was SSU (1.479) >SSD (1.135). These findings demonstrate that the relationship between the composite target hazard quotient of heavy metals in different parts was not consistent across the two plant species. Additionally, all index values were greater than 1, and the contribution of Cr to the TTHQ value in all plant parts was the highest. This suggests that both plants may pose a potential health risk to humans due to the influence of Cr.
Calculate the carcinogenic risk and the total carcinogenic risk of heavy metals in plants according to Eqs 6, 7. The carcinogenic risk of the two plants as useable resources is shown in the Table 3. In terms of individual heavy metals, whether it is different plants or different parts of the same plant, he highest carcinogenic risk of the five heavy metals is Cr and the lowest is Pb, and only the CR of Cr is greater than 10–6, which is within the tolerable range of human beings (10–6 ≤ TCR/CR < 10–4), while the other heavy metals are less than 10–6, which is considered to be no risk (10–4 ≤ TCR/CR). In terms of TCR, the above-ground and below-ground parts of S. alterniflora and S. glauca had TCRs ranging from 4.5 × 10−6 to 1 × 10−5, with the largest being the below-ground part of S. alterniflora and the smallest being the above-ground part of S. alterniflora, which were within the tolerable range for human beings. These results indicate that both plants have some carcinogenic risk when used as edible resources, and that the carcinogenic risk is mainly caused by the heavy metal Cr, but is within the human tolerable range.
Therefore, the utilization of S. alterniflora and S. glauca cultivated in the study area as human food may pose potential health risks, but the carcinogenic risk is low. In particular, consumption of the underground part of S. alterniflora may have the highest health hazards for humans, mainly due to the significant contribution of Cr and as that pose high health risks. The composite target hazard quotient values of the above-ground and underground parts of S. alterniflora and S. glauca were slightly higher than 1, indicating that caution must be exercised in the development of food. The overall composite target hazard quotient of S. alterniflora was higher than that of S. glauca. The high single heavy metal health risk index values of Cr and as caused some plants to exceed the composite target hazard quotient. Since the Igeo indicates that the Tiaozini Wetland is not contaminated by human beings, these results are primarily due to the ability of these plants to accumulate heavy metals.
(1) The heavy metal accumulation in the below-ground part of S. alterniflora was higher than that in the above-ground part, while S. glauca is the opposite. The average concentrations of Cr, Cu, and Zn in both S. alterniflora and S. glauca were relatively high, which may be related to the essential elements required for their biological activities.
(2) Both plant species demonstrated a certain capacity to accumulate some heavy metals and had a certain purifying effect on heavy metal pollution in wetland environments, particularly Cr, Cd, Cu, and Zn. And S. alterniflora transported most of heavy metals to its below-ground part, while S. glauca accumulated the majority of heavy metals in its above-ground part.
(3) The composite target hazard quotient of both the above-ground and below-ground parts of both plants was greater than 1, which may pose a certain threat to human health if consumed in large amounts, but the carcinogenic risk is low.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
GL: Investigation, Conceptualization, Data curation, Formal Analysis, Writing–original draft, Writing–review and editing. ZC: Data curation, Investigation, Writing–original draft. SH: Conceptualization, Investigation, Writing–review and editing. ZS: Formal Analysis, Writing–review and editing. YZ: Methodology, Writing–review and editing. ZZ: Project administration, Writing–review and editing. RL: Investigation, Writing–review and editing. SW: Resources, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Research and development project of ecological restoration technology of Dongtai coastal wetland (SGH1829382). The authors declare that this study received funding from Dongtai Coastal Economic Zone. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article, or the decision to submit it for publication.
Thanks for the support of the Dongtai Coastal Economic Zone.
Author RL was employed by Yancheng Tiaozini Wetland Research Institute Co, Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1299139/full#supplementary-material
Ahmed, I., Mostefa, B., Bernard, A., and Olivier, R. (2018). Levels and ecological risk assessment of heavy metals in surface sediments of fishing grounds along Algerian coast. Mar. Pollut. Bull. 136, 322–333. doi:10.1016/j.marpolbul.2018.09.029
Bai, X., Xin, P., Li, F., and Xu, Z. (2022). Quantitative analysis of microplastics in coastal tidal-flat reclamation in Dongtai, China. Front. Environ. Sci. Eng. 16, 107–111. doi:10.1007/s11783-022-1528-5
Berglund, Å. M. (2018). Evaluating blood and excrement as bioindicators for metal accumulation in birds. Environ. Pollut. 233, 1198–1206. doi:10.1016/j.envpol.2017.10.031
Bortey-Sam, N., Nakayama, S. M., Ikenaka, Y., Akoto, O., Baidoo, E., Yohannes, Y. B., et al. (2015). Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: estimation of the daily intakes and target hazard quotients (THQs). Ecotoxicol. Environ. Saf. 111, 160–167. doi:10.1016/j.ecoenv.2014.09.008
Brooks, R. R., Lee, J., Reeves, R., and Jaffre, T. (1977). Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 7, 49–57. doi:10.1016/0375-6742(77)90074-7
Cai, Z., Li, G., Kuang, Z., and Wang, S. (2023). Content and ecological risk assessment of heavy metals in surface sediments of migratory bird habitat and its adjacent intertidal zones in Tiaozini Dongtai. J. Appl. Oceanogr. doi:10.3969/J.ISSN.2095-4972.20220330001
Çevik, F., Göksu, M. Z. L., Derici, O. B., and Fındık, Ö. (2009). An assessment of metal pollution in surface sediments of Seyhan dam by using enrichment factor, geoaccumulation index and statistical analyses. Environ. Monitoring&Assessment. 152, 309–317. doi:10.1007/s10661-008-0317-3
Chen, Z. L., and Lee, S. Y. (2022). Sediment carbon sequestration and sources in peri-urban tidal flats and adjacent wetlands in a megacity. Mar. Pollut. Bull. 185, 114368. doi:10.1016/j.marpolbul.2022.114368
Cui, S., Fu, Q., Guo, L., Li, Y. F., Li, T. x., Ma, W. l., et al. (2016). Spatial–temporal variation, possible source and ecological risk of PCBs in sediments from Songhua River, China: effects of PCB elimination policy and reverse management framework. Mar. Pollut. Bull. 106, 109–118. doi:10.1016/j.marpolbul.2016.03.018
Cui, X., Jia, B., Diao, F., Li, X., Xu, J., Zhang, Z., et al. (2022). Transcriptomic analysis reveals the molecular mechanisms of arbuscular mycorrhizal fungi and nitrilotriacetic acid on Suaeda salsa tolerance to combined stress of cadmium and salt. Process Saf. Environ. Prot. 160, 210–220. doi:10.1016/j.psep.2022.02.019
Epa, U. (2000). Risk-based concentration table. Philadelphia PA: United States Environmental Protection Agency, Washington DC.
Feng, H., Qian, Y., Cochran, J. K., Zhu, Q., Heilbrun, C., Li, L., et al. (2018). Seasonal differences in trace element concentrations and distribution in Spartina alterniflora root tissue. Chemosphere 204, 359–370. doi:10.1016/j.chemosphere.2018.04.058
Gao, W., Du, Y., Gao, S., Ingels, J., and Wang, D. (2016). Heavy metal accumulation reflecting natural sedimentary processes and anthropogenic activities in two contrasting coastal wetland ecosystems, eastern China. J. Soils Sediments 16, 1093–1108. doi:10.1007/s11368-015-1314-0
Habib, M. R., Mohamed, A. H., Osman, G. Y., Mossalem, H. S., Sharaf El-Din, A. T., and Croll, R. P. (2016). Biomphalaria alexandrina as a bioindicator of metal toxicity. Chemosphere 157, 97–106. doi:10.1016/j.chemosphere.2016.05.012
He, C., Zheng, L., Gao, W., Ding, J., Li, C., Xu, X., et al. (2022). Diversity and functions of quorum sensing bacteria in the root environment of the Suaeda glauca and Phragmites australis coastal wetlands. Environ. Sci. Pollut. Res. 29, 54619–54631. doi:10.1007/s11356-022-19564-6
Hongyi, N., Deng, W., Wu, Q., and Chen, X. (2009). Potential toxic risk of heavy metals from sediment of the Pearl River in South China. J. Environ. Sci. 21, 1053–1058. doi:10.1016/s1001-0742(08)62381-5
Hu, B., Guo, P., Wu, Y., Deng, J., Su, H., Li, Y., et al. (2021). Study of soil physicochemical properties and heavy metals of a mangrove restoration wetland. J. Clean. Prod. 291, 125965. doi:10.1016/j.jclepro.2021.125965
Hu, W., Chen, T., Xu, Z., Wu, D., and Lu, C. (2022). Occurrence dataset of waterbirds in the tiaozini wetland, a world nature heritage, China. Biodivers. Data J. 10, e90724. doi:10.3897/bdj.10.e90724
Jing, W., Min, X., and Yimin, Z. (2012). Ecological profit and loss analysis of tidal flat reclamation——tidal flat reclamation of tiaozini sand as a case study. J. Nanjing Normal Univ. Sci. Ed. 35, 113–119. doi:10.3969/j.issn.1001-4616.2012.02.022
Joshi, A., Kanthaliya, B., Rajput, V., Minkina, T., and Arora, J. (2020). Assessment of phytoremediation capacity of three halophytes: Suaeda monoica, Tamarix indica and Cressa critica. Biol. Futura 71, 301–312. doi:10.1007/s42977-020-00038-0
Kouali, H., Chaouti, A., Achtak, H., Elkalay, K., and Dahbi, A. (2022). Trace metal contents in the mussel Mytilus galloprovincialis from Atlantic coastal areas in northwestern Morocco: levels of contamination and assessment of potential risks to human health. Mar. Pollut. Bull. 179, 113680. doi:10.1016/j.marpolbul.2022.113680
Li, S., and Zhang, Q. (2010). Spatial characterization of dissolved trace elements and heavy metals in the upper Han River (China) using multivariate statistical techniques. J. Hazard. Mater. 176, 579–588. doi:10.1016/j.jhazmat.2009.11.069
Lin, C., Wang, W., Hu, G., Yu, R., Huang, H., and Liao, D. (2023). Incorporating source apportionment and bioaccessibility into human health risk assessment of heavy metals in a soil-rice system in the Jiulong River basin, southeast China. J. Food Compos. Analysis 124, 105692. doi:10.1016/j.jfca.2023.105692
Lin, S., Hsieh, I. J., Huang, K. M., and Wang, C. H. (2002). Influence of the Yangtze River and grain size on the spatial variations of heavy metals and organic carbon in the East China Sea continental shelf sediments. Chem. Geol. 182, 377–394. doi:10.1016/s0009-2541(01)00331-x
Liu, A., Wang, W., Zheng, X., Chen, X., Fu, W., Wang, G., et al. (2022). Improvement of the Cd and Zn phytoremediation efficiency of rice (Oryza sativa) through the inoculation of a metal-resistant PGPR strain. Chemosphere 302, 134900. doi:10.1016/j.chemosphere.2022.134900
Liu, X., Gu, S., Yang, S., Deng, J., and Xu, J. (2021). Heavy metals in soil-vegetable system around E-waste site and the health risk assessment. Sci. Total Environ. 779, 146438. doi:10.1016/j.scitotenv.2021.146438
Lu, Q., Bai, J., Zhang, G., and Wu, J. (2020). Effects of coastal reclamation history on heavy metals in different types of wetland soils in the Pearl River Delta: levels, sources and ecological risks. J. Clean. Prod. 272, 122668. doi:10.1016/j.jclepro.2020.122668
Luc, B. T., Karim, K., Moumouni, D., Alain, T. K., Cisse, O. I., and Zougmore, F. (2022). Assessment of heavy metal concentration in soil and plant and evaluation of bioconcentration factor at loumbila market gardening perimeters, Burkina Faso. Asian J. Phys. Chem. Sci. 10, 25–34. doi:10.9734/ajopacs/2022/v10i230153
Niyoifasha, C. J., Borena, B. M., Ukob, I. T., Minh, P. N., Al Azzawi, T. N. I., Imran, M., et al. (2023). Alleviation of Hg-Cr-Cu-and Zn-induced heavy metals stress by exogenous sodium nitroprusside in rice plants. Plants 12, 1299. doi:10.3390/plants12061299
Nurtjahya, E., Mellawati, J., Pratama, D., and Syahrir, S. (2023). Study of soil–to–plant transfer factors (TFs) of 226Ra, 232Th, and 40K on plants cultivated on ex–tin mining land in Bangka Belitung, Indonesia. J. Environ. Radioact. 261, 107144. doi:10.1016/j.jenvrad.2023.107144
Praveena, S. M., Ahmed, A., Radojevic, M., Abdullah, M. H., and Aris, A. Z. (2008). Multivariate and geoaccumulation index evaluation in mangrove surface sediment of mengkabong lagoon, sabah. Bull. Environ. Contam. Toxicol. 81 (1), 52–56. doi:10.1007/s00128-008-9460-3
Quan, L., Yi, T., Li, Q., Ju, H., and Wei, H. (2022). Bioaccumulation of heavy metals by Suaeda salsa in the tidal flat of the liaohe estuary. Separations 9, 374. doi:10.3390/separations9110374
Rabee, A., Faleh, Y., Al Fatlawy, Y. F., Najim, A.-A.-H, Own, A., and Nameer, M. (2011). Using pollution load index (PLI) and geoaccumulation index (I-geo) for the assessment of heavy metals pollution in tigris river sediment in baghdad region. Baghdad, Iraq: Al-Nahrain University.
Rao, S., Fang, J., and Zhao, K. (2022). Health risks assessment of heavy metal pollution in the soil-crop system from an E-waste dismantling area. 国际实验植物学杂志(英文).
Shang, C., Wang, L., Tian, C., and Song, J. (2020). Heavy metal tolerance and potential for remediation of heavy metal-contaminated saline soils for the euhalophyte Suaeda salsa. Plant Signal. Behav. 15, 1805902. doi:10.1080/15592324.2020.1805902
Siyar, R., Doulati Ardejani, F., Norouzi, P., Maghsoudy, S., Yavarzadeh, M., Taherdangkoo, R., et al. (2022). Phytoremediation potential of native hyperaccumulator plants growing on heavy metal-contaminated soil of khatunabad copper smelter and refinery, Iran. Iran. Water 14, 3597. doi:10.3390/w14223597
Song, H., and Sun, Z. (2014). Temporal variations and bioaccumulation of heavy metals in different Suaeda salsa marshes of the Yellow River estuary, China. Environ. Sci. Pollut. Res. 21, 14174–14187. doi:10.1007/s11356-014-3296-7
Song, X., Yang, N., Su, Y., Lu, X., Liu, J., Liu, Y., et al. (2022). Suaeda glauca and Suaeda salsa employ different adaptive strategies to cope with saline–alkali environments. Agronomy 12, 2496. doi:10.3390/agronomy12102496
Sun, B., Cui, L., Li, W., Kang, X., Pan, X., and Lei, Y. (2018). A meta-analysis of coastal wetland ecosystem services in Liaoning Province, China. Coast. Shelf Sci. 200, 349–358. doi:10.1016/j.ecss.2017.11.006
Susarla, S., Medina, V. F., and McCutcheon, S. C. (2002). Phytoremediation: an ecological solution to organic chemical contamination. Ecol. Eng. 18, 647–658. doi:10.1016/s0925-8574(02)00026-5
Taufikurahman, T., Pradisa, M. A. S., Amalia, S. G., and Hutahaean, G. E. M. (2019). Phytoremediation of chromium (Cr) using Typha angustifolia L., Canna indica L. and Hydrocotyle umbellata L. in surface flow system of constructed wetland. IOP Conf. Ser. Earth Environ. Sci. 308, 012020. IOP Publishing. doi:10.1088/1755-1315/308/1/012020
Tian, P., Cao, L., Li, J., Pu, R., Liu, Y., Zhang, H., et al. (2022). Ecosystem stability assessment of Yancheng coastal wetlands, a world natural heritage site. Land 11, 564. doi:10.3390/land11040564
Varol, M. (2011). Assessment of heavy metal contamination in sediments of the Tigris River (Turkey) using pollution indices and multivariate statistical techniques. J. Hazard. Mater. 195, 355–364. doi:10.1016/j.jhazmat.2011.08.051
Wang, J., Ye, S., Laws, E. A., Yuan, H., Ding, X., and Zhao, G. (2017). Surface sediment properties and heavy metal pollution assessment in the Shallow Sea Wetland of the Liaodong Bay, China. Mar. Pollut. Bull. 120, 347–354. doi:10.1016/j.marpolbul.2017.05.051
Weis, J. S., and Weis, P. (2004). Metal uptake, transport and release by wetland plants: implications for phytoremediation and restoration. Environ. Int. 30, 685–700. doi:10.1016/j.envint.2003.11.002
Xing, H., Yu, X., Huang, J., Du, X., Wang, M., Sun, J., et al. (2022). Characteristics and health risks of phthalate ester contamination in soil and plants in coastal areas of South China. Int. J. Environ. Res. Public Health 19, 9516. doi:10.3390/ijerph19159516
Xu, C., Ge, Z., Wan, F., and Xiao, X. (2019). Inhibition of harmful algae Phaeocystis globosa and Prorocentrum donghaiense by extracts of coastal invasive plant Spartina alterniflora. Sci. Total Environ. 696, 133930. doi:10.1016/j.scitotenv.2019.133930
Yang, H., Shen, Z., Zhu, S., and Wang, W. (2008). Heavy metals in wetland plants and soil of Lake Taihu, China. Environ. Toxicol. Chem. Int. J. 27, 38–42. doi:10.1897/07-089.1
Yin, S., Wu, Y., Xu, W., Li, Y., Shen, Z., and Feng, C. (2016). Contribution of the upper river, the estuarine region, and the adjacent sea to the heavy metal pollution in the Yangtze Estuary. Chemosphere 155, 564–572. doi:10.1016/j.chemosphere.2016.04.095
Zhang, M., Schwarz, C., Lin, W., Naing, H., Cai, H., and Zhu, Z. (2023). A new perspective on the impacts of Spartina alterniflora invasion on Chinese wetlands in the context of climate change: a case study of the Jiuduansha Shoals, Yangtze Estuary. Sci. Total Environ. 868, 161477. doi:10.1016/j.scitotenv.2023.161477
Zhang, Q., Yan, Z., and Li, X. (2020). Ferrous iron facilitates the formation of iron plaque and enhances the tolerance of Spartina alterniflora to artificial sewage stress. Mar. Pollut. Bull. 157, 111379. doi:10.1016/j.marpolbul.2020.111379
Zhang, Z., Zhang, T., Yu, W., Xu, J., Li, J., Wu, T., et al. (2022). Heavy metal contamination in sediments from wetlands invaded by spartina alterniflora in the Yellow river delta. Toxics 10, 374. doi:10.3390/toxics10070374
Keywords: Tiaozini, coastal wetland, S. alterniflora Loisel., Suaeda salsa (L.) Pall., heavy metals and health risks
Citation: Li G, Cai Z, Huang S, Song Z, Zhang Y, Zheng Z, Luo R and Wang S (2024) Heavy metal accumulation and health risk assessment in S. alterniflora Loisel. and native plant Suaeda salsa (L.) Pall. in Dongtai Tiaozini wetland. Front. Environ. Sci. 12:1299139. doi: 10.3389/fenvs.2024.1299139
Received: 25 September 2023; Accepted: 12 January 2024;
Published: 01 February 2024.
Edited by:
Constantin Nechita, National Institute for Research and Development in Forestry Marin Dracea (INCDS), RomaniaReviewed by:
Basanta Kumar Das, Central Inland Fisheries Research Institute (ICAR), IndiaCopyright © 2024 Li, Cai, Huang, Song, Zhang, Zheng, Luo and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shoubing Wang, YnN3YW5nQGZ1ZGFuLmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.