
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 19 July 2024
Sec. Conservation and Restoration Ecology
Volume 12 - 2024 | https://doi.org/10.3389/fenvs.2024.1291265
This article is part of the Research TopicLarge-Scale Dam Removal and Ecosystem RestorationView all 25 articles
 Roger J. Peters1*
Roger J. Peters1* Joseph H. Anderson2
Joseph H. Anderson2 Jeffrey J. Duda3
Jeffrey J. Duda3 Michael McHenry4
Michael McHenry4 George R. Pess5
George R. Pess5 Samuel J. Brenkman6
Samuel J. Brenkman6 Jeffery R. Johnson1
Jeffery R. Johnson1 Martin C. Liermann5
Martin C. Liermann5 Keith P. Denton7
Keith P. Denton7 Matt M. Beirne4
Matt M. Beirne4 Pat Crain6
Pat Crain6 Heidi A. Connor6
Heidi A. Connor6Adaptive management, a process of planning, implementing, and evaluating management strategies, is often recommended for monitoring ecological systems. However, few examples of successful implementation and retrospective case studies exist. We provide a case study of adaptively managing hatchery-assisted protection and recovery for Chinook salmon (Oncorhynchus tshawytscha) and winter steelhead trout (O. mykiss) during and after the removal of two large mainstem dams in the Elwha River, WA. We summarize key aspects of the monitoring and adaptive management plan over the last decade and highlight successes, challenges, and complications during the plan’s implementation. The Elwha Monitoring and Adaptive Management Guidelines included a trigger-based system for moving through four phases of recovery that included preservation, recolonization, local adaptation, and viable natural population, each with differing levels of hatchery production as the management actions. The monitoring component of the plan has been very successful, providing critical data to guide management actions that otherwise may not have occurred and, opportunistically, provided data for other native species in the Elwha River. Implementing adaptive management provided mixed results and was at times hindered by divergent management goals among project partners, the inflexibility of the Endangered Species Act regulatory requirements as implemented for this project, and conflicting information among guidance documents. We learned that some metrics and triggers in the plan were ill-defined or too difficult to measure in the field. In some cases, the performance indicators and/or triggers were successfully modified to incorporate what was learned; however, in other cases, we were unable to revise the values due to differing opinions among partners. The ability to reach consensus on revised triggers appeared to be influenced by the recovery trajectory of the species involved. The implemented adaptive management strategy resulted in substantial collaboration and learning, which resulted in revised management strategies, but was imperfect. Sufficient long-term funding is necessary to implement a well-designed monitoring program and could benefit from including a defined leadership position to shepherd and facilitate a multi-stakeholder adaptive management program. Additionally, incorporating adaptive management into legally binding conditions under the Endangered Species Act is feasible, but requires substantial pre-planning in close coordination with regulatory agencies.
Adaptive management (AM), a special case of structured decision-making, is an iterative data evaluation and response framework often implemented for the management of dynamic ecological systems (Williams, 2011; Westgate et al., 2013; Deitch et al., 2021). The approach has been widely used across a range of ecological management scenarios, from small-scale single species projects to large-scale ecosystem management (Roux and Foxcroft, 2011; Melis et al., 2015). AM consists of a series of steps that include developing objectives, identifying and assessing options, and learning from monitoring and evaluation, and adjusting management as necessary (Argent, 2009). In theory, monitoring and evaluation information drives management actions through achieving targets which may produce subsequent iterations of a plan (Nie and Schultz, 2012). Examples of AM frameworks are abundant in the literature and provide a robust conceptual knowledge base for planning and implementing a new plan (Gillson et al., 2019). Forms of AM involving natural resources have existed for at least 65 years (Williams, 2011), across a diverse range of disciplines including climate change (Galappaththi et al., 2022), environmental flows (Wineland et al., 2022), landscape management (McCord and Pilliod, 2022), stream restoration (Bradford et al., 2023), and fisheries (Walters, 2007).
Several reviews have identified barriers and common pitfalls to effective AM implementation (Halbert, 1993; Keith, 2000; Walters, 2007; Runge, 2011; Williams, 2011; Williams and Brown, 2014). These issues range widely from intrinsic and institutional to purely technical (Williams, 2011). Elements of intrinsic and institutional issues are often grounded in unstable or dysfunctional working groups, or an inability to embrace uncertainty and alternative perspectives to achieve participatory decision making (Gunderson, 1999; Stankey et al., 2005), which may result in conflict that result in failed AM (Westgate et al., 2013). Technical issues often stem from a difficulty or inability to monitor changes, or ineffective monitoring protocols that fail to collect relevant information with tenable levels of precision to inform policy (McDonald-Madden et al., 2010; Runge et al., 2011). Despite these issues, AM is still preferred over other alternate management paradigms such as ad hoc, wait-and-see, and steady state (Westgate et al., 2013).
Several key elements for successful AM have also been identified (Keith et al., 2011; Gillson et al., 2019). One fundamental requirement is identifying variables that can be monitored and/or managed for a dynamic system (Williams, 2011). Effective monitoring and management actions require an engaged community of managers and researchers (Keith et al., 2011). Similarly, the AM process often applies a substantial temporal and fiscal burden to researchers and managers, where funding for involvement is repeatedly identified as imperative (Wilhere, 2002). Therefore, developing an approach to secure substantive long-term funding, such as integration of a plan into legal documents (e.g., Congressional acts, listed species reviews), can promote success (Doremus, 2001). However, codifying management plans into a legal framework can limit progress (Benson and Schultz, 2015). For example, a 2011 survey found over 70% of AM practitioners felt hampered by legal and institutional constraints (Benson and Stone, 2013).
Although a large body of literature exists surrounding AM plans, retrospective case studies of AM implementation are useful but uncommon (Roux et al., 2022). To address this issue, we provide a case study of monitoring and adaptively managing hatchery-aided protection and recovery of two U.S. Endangered Species Act (ESA) listed species after large-scale dam removal in the Elwha River. This retrospective analysis of the Elwha Monitoring and Adaptive Management Guidelines (hereafter EMAM, Peters et al., 2014) process provides an example for future AM planners to consider, particularly for dam removal projects. The EMAM includes performance indicators, with associated empirical trigger values that guide movement through four recovery phases (preservation, recolonization, local adaptation, and viable natural population), each with differing levels of hatchery intervention (details below). The EMAM also includes detailed monitoring protocols for the performance indicators. This plan was developed with considerations of the best available guidance, including incorporating monitoring and AM into regulatory documents. The objectives of this paper are to: 1) describe our monitoring and AM process, 2) describe the monitoring results for Chinook salmon (Oncorhynchus tshawytscha) and winter steelhead trout (O. mykiss) following dam removal and how these data were used for AM, 3) identify factors leading to success, challenges, and unforeseen issues, and 4) provide recommendations to address these challenges.
Understanding the historical context of the Elwha River, its fish populations, and complicated management regime resulting from multiple agencies with management authority (Box 1 for details) is critical to understanding restoration strategies employed during and after dam removal. Throughout the decades-long planning period leading up to the start of dam removal, several institutional processes occurred which set the context for potential management options considered for the Elwha River AM program (Supplementary Table S1). Dam construction led to significant habitat degradation upstream and downstream of the two dams (Pess et al., 2008) and associated salmon population declines. This in turn resulted in intermittent (1911–2022, (Johnson, 2013) and then continuous hatchery production for Chinook (1930’s to present) and winter steelhead (1976 to present). Although Chinook salmon spawned naturally while their numbers declined, contemporary data indicate that a low proportion of the adults were progeny of natural-origin spawners, meaning the population was essentially sustained by hatchery production (Pess et al., 2024).
The license application for Elwha Dam (1968) and re-licensing application for Glines Canyon Dam (1973) by the dam owners to the Federal Energy Regulatory Commission, prompted Federal and State agencies (hereafter Agencies) and Lower Elwha Klallam Tribe (hereafter Tribe) to lobby for modifications to the projects to abate the degradation to commercially and culturally important anadromous fish and their habitat (see Winter and Crain, 2008 for detailed history). This was followed by years of administrative and legal challenges as the Tribe and Agencies argued that recommendations to restore fish passage and habitat conditions, including dam removal, should be considered. This resulted in a negotiated settlement among parties to lawsuits which was enshrined by passage of the Elwha River Ecosystem and Fisheries Restoration Act of 1992 (PL 102-495). The goal was restoration of the Elwha River’s anadromous fisheries and ecosystem, and it essentially set the boundaries for AM development for the Elwha River dam removal project by establishing goals for the project through the production of several legal documents (Supplementary Table S1). These documents culminated in the development of the Elwha Fish Restoration Plan (Ward et al., 2008), multiple Biological Opinions (BiOps), EMAM (Peters et al., 2014), and ultimately the evaluation and recommendations determination document (NMFS, 2015) that governs Elwha Recovery, which is largely guided by the EMAM. A BiOp is a process of analyzing the effects of proposed activities to species listed under the ESA and their critical habitat. Three primary BiOps were completed for the Elwha restoration project, one governing dam removal (NMFS, 2012a) and two governing hatchery operations (NMFS, 2012b, 2015). The EMAM addresses all three BiOps, but management actions are largely focused on hatchery operations.
The primary goal of dam removal on the Elwha River was to eliminate migration barriers and restoration of native anadromous fish populations and the ecosystem that supports them (Wunderlich et al., 1994; Duda et al., 2008; Pess et al., 2008; Winter and Crain, 2008). The project was unique due to the height of the dams (64 m and 32 m), the massive amount of sediment stored in the reservoirs (21 million m3), and the potential to restore connectivity for nine species of migratory fish into pristine spawning and rearing waters protected within Olympic National Park. This special opportunity to restore salmonid populations and their river ecosystem also presented management challenges ideally suited to an AM approach. A primary challenge was controlling the release of nearly a century worth of sediment accumulation into the river downstream (Randle et al., 2015), while protecting four fish species: Chinook salmon, steelhead trout, bull trout (Salvelinus confluentus), and eulachon (Thaleichthys pacificus) listed as threatened under the ESA. For these protected species, dam removal presented an interesting paradox (Stanley and Doyle, 2003). In the long term, dam removal could provide a tremendous benefit by providing access to approximately 187 km of mainstem, floodplain channel, and tributary habitats, mostly protected as wilderness inside the boundaries of Olympic National Park (Pess et al., 2008), and restoring natural processes to the lower river. However, in the short term, dam removal was expected to be a major disturbance, as nearly a century’s accumulation of the river’s annual sediment load was to be released during the two to four years of dam removal. This onslaught of sediment would increase channel instability and water column turbidity downstream of the two dams (East et al., 2015; Magirl et al., 2015), representing a significant threat to ESA-listed salmonids that depend on clean and stable spawning gravels, delivery of oxygen rich water for incubating eggs, and productive juvenile rearing habitats. This prompted a management strategy focused on the use of hatcheries to protect and restore salmon and winter steelhead trout during and following dam removal (Ward et al., 2008).
The use of hatcheries was identified as a significant component of stock preservation and recovery during and following dam removal (Department of the Interior et al., 1994; Ward et al., 2008). This approach was deemed necessary due to low population abundances of Elwha River salmonid stocks and uncertainties of the magnitude and duration of physical environment alterations resulting from dam removal. The use of hatcheries was also generally acceptable given the extensive history of hatchery intervention in the Elwha River. Hatchery managers generally avoided releasing non-local Chinook salmon into the Elwha River over the years (Brannon and Hershberger, 1984), and the steelhead hatchery program was established recently, in 2012, with native broodstock (Lower Elwha Klallam Tribe, 2011). However, the use of hatcheries also presented risk and uncertainty, related to documented genetic and ecological impacts of hatchery propagation (Naish et al., 2007; Anderson et al., 2020; McMillan et al., 2023), especially for stocks, like those in the Elwha, that had been reared in hatcheries for decades. Therefore, we developed the EMAM for monitoring and adaptively managing Chinook salmon and steelhead trout recovery following dam removal (Peters et al., 2014). The EMAM focused on Chinook salmon and winter steelhead trout because of their protected status and proposed management alternatives (i.e., hatchery conservation) required regulatory review that drove ongoing multi-agency efforts to monitor their populations during and following dam removal. The goal of hatchery intervention was to reduce extinction risk from high sediment loads in the short-term, when turbidity levels were expected to far exceed those known to be lethal to salmonids, and facilitate the colonization of newly accessible habitats upstream of the former dams (Ward et al., 2008). The strategy contained within EMAM was to phase out hatchery production incrementally as the stock’s population progressed through the recovery phase after dam removal.
Five entities have management and decision-making authority for fish populations in the Elwha River. Olympic National Park was the lead agency for planning and implementing dam removal and manages fisheries for the Elwha River within the park. Washington Department of Fish and Wildlife and Lower Elwha Klallam Tribe co-manage hatchery production in the Elwha River, and fisheries for Elwha populations in both the marine and river environment outside of the park. These three managing entities are subject to oversight of actions that may impact ESA listed fish by National Marine Fisheries Service (NMFS) (Chinook salmon, steelhead trout) and U. S. Fish and Wildlife Service (bull trout). Thus, each entity approached Elwha fish recovery with a different management authority and responsibility. As a result, the group has generally worked through consensus, towards the goal of ensuring any decisions would not violate the decision-making authority of another entity. In some cases, the diversity of management and legal obligations among agencies led to the disagreements and challenges to implementing AM described below.
The overall AM strategy for listed Elwha River Chinook salmon and steelhead trout (Peters et al., 2014) mirrored the AM framework described by Roux and Foxcroft (2011). This framework included adaptive planning, implementation, and evaluation. Adaptive planning consists of vision development, objective setting, and the development of management options (Roux and Foxcroft, 2011). Adaptive implementation includes development of a detailed action plan, implementing the plan, developing monitoring protocols linked to measurable targets, and developing a strategy for regularly evaluating monitoring results. Adaptive evaluation is the process of evaluation and learning that occurs continuously throughout the process and is facilitated by addressing pertinent questions developed within the AM process (Roux and Foxcroft, 2011). Much of the adaptive planning portion of the project largely occurred during the Federal Energy Regulatory Commission and Environmental impact statement processes described above (Supplementary Table S1); however, some aspects were completed during EMAM development. The adaptive implementation and evaluation components were developed and described in the EMAM (Peters et al., 2014).
The EMAM was developed to promote informed, shared decision making, with each agency retaining management authority according to jurisdiction and legal obligations. This included management of fisheries (Lower Elwha Klallam Tribe, Washington Department of Fish and Wildlife, National Park Service), management of hatcheries (Lower Elwha Klallam Tribe, Washington Department of Fish and Wildlife), implementation of the dam removal project (National Park Service), and ESA oversight of these activities (National Oceanic and Atmospheric Association, U.S. Fish and Wildlife Service). A recognition of the inter-connectedness of these decisions inspired the development of the EMAM and heightened the sense of collaboration. The creators of the EMAM were agency (State and Federal) and Tribal biologists with varying responsibilities related to administration, management, and monitoring (Box 1). The development of the EMAM was largely a technical exercise conducted by fishery professionals, as public involvement and comment were incorporated during the Federal Energy Regulatory Commission and Environmental Impact Statement process related specifically to dam removal. Staff members from each agency updated the appropriate executive staff within their agency about progress and potential issues as necessary. The group generally worked through consensus, while ensuring legal authorities were not violated. Conflicts were typically addressed with respectful, sincere debate though none of the participants were trained facilitators, and agreement was not always reached. By soliciting, accepting, and considering input on issues pertaining to their management authority, each agency implicitly acknowledged the shared responsibility of promoting the recovery of Elwha River fish populations.
The EMAM works from broad to specific levels in a hierarchical manner. The main elements included setting goals, objectives, performance indicators, decision rules, triggers, and finally decisions (i.e., management/policy response), which was completed individually for Chinook salmon and steelhead trout (Table 1). Performance indicators, triggers, and management responses were developed for each objective and help determine the outcome of management strategies implemented.
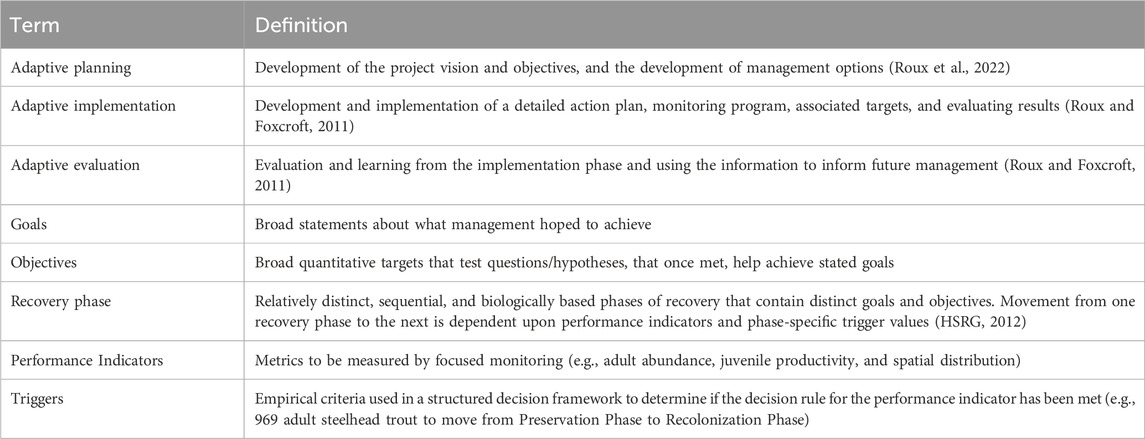
Table 1. Definition of terms related to Elwha Monitoring and Adaptive Management guidelines developed by Peters et al. (2014).
Given the scope of the Elwha River AM project—dam removal with hatchery intervention—the EMAM prescribes a passive AM approach. In contrast to an active AM approach which is explicitly experimental in nature, a passive AM approach implements a single ‘best’ management strategy and evaluates the outcome (Walters and Holling, 1990). The passive approach was selected since the project involved dam removal in a single system, thereby limiting the range of management options that could be applied and evaluated. Because a passive approach was used, we implemented a structured decision-making process (Gregory and Long, 2009; Runge et al., 2013) along with intensive monitoring to collect data for evaluating fish recovery and the influential mechanisms. The structured decision-making process, of which AM is a special case (Gregory and Long, 2009; Runge et al., 2013), compensated for the passive approach by providing periodic decision points throughout the AM process when performance indicators were evaluated. This evaluation included a simple decision-tree process to determine the next course of action (see below and Figure 1). Monitoring was focused on data collection to evaluate empirical triggers for the selected performance indicators, as well as exogenous variables outside of management control that could influence overall recovery. The purpose of this intensive monitoring program was to ensure data were available to understand observed recovery response and test hypotheses that may arise regarding why recovery was progressing in the observed manner.
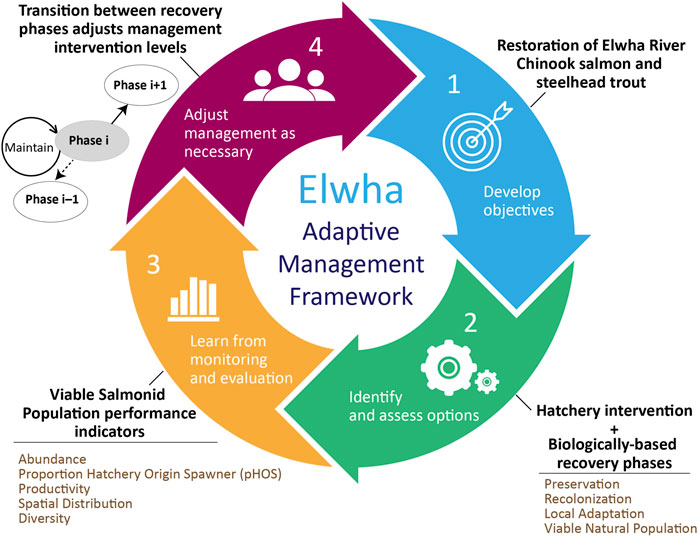
Figure 1. Conceptual diagram describing the adaptive management framework implemented for Elwha River Chinook salmon (Oncorhynchus tshawytscha) and steelhead trout (O. mykiss). The colored arrows show the four main elements of the framework, based on the adaptive management principles described in (Roux and Foxcroft, 2011). After developing objectives, it was determined that hatchery intervention would be required to mitigate the effects of the dams and their removal. The framework used biologically based recovery phases, which differed in levels of management intervention. Progress through the phases was determined by performance indicators exceeding “trigger values” derived for each species and based on viable salmonid population principles (McElhany et al., 2000). On an annual basis, monitoring data was used to assess whether the program would transition out of the current recovery phase.
Based on recommendations from the Hatchery Scientific Review Group (HSRG, 2012), the EMAM defined four recovery phases for both Chinook salmon and steelhead trout tailored to the specifics of dam removal and hatchery intervention, as well as the pre-dam removal status of Elwha River fish populations. These recovery phases—preservation, recolonization, local adaptation, and viable natural population—each had different goals and management strategies (Supplementary Tables S2, S3). Once the objectives, performance indicators, and associated triggers for a particular recovery phase were met in the same year, the AM program would move into the next recovery phase.
The preservation phase describes the period during and after dam removal when elevated suspended sediment concentrations were expected, at times, to be lethal to all fish in the river. This represented a high risk for complete or significant loss of extant fish populations or year-classes. The goal of the preservation phase is to protect the existing genetic and life history diversity of native salmonid populations until fish passage is restored and water quality impacted by the dam removal project returned to background levels. Hatchery management during this phase is maximum production and smolt releases directly from the hatcheries (Lower Elwha Klallam Tribe, 2011; Washington Department of Fish and Wildlife, 2012). In addition, adults volitionally entering the hatchery in excess of hatchery production needs were to be transported upstream of Elwha Dam and released into clean water refuges (i.e., tributaries unimpacted by dam removal) until turbidity returned to background levels (detailed in Liermann et al., 2017).
The recolonization phase describes the period following dam removal when passage was restored and access to refugia from lethal suspended sediment concentrations had been restored or suspended sediment concentrations were no longer lethal. The goal of the recolonization phase is to ensure that salmonids (hatchery-origin and natural-origin) are continually accessing habitats upstream of the former dam sites, with some fish spawning successfully and producing smolts. The EMAM proposed reduced hatchery production during this recovery phase based on adult abundance.
The local adaptation phase was the period when the already reduced releases of hatchery fish would be eliminated and the spawning of naturally produced adults would result in population growth. The goal is to maintain or increase life history diversity of natural spawning populations through their local adaptation to the Elwha River ecosystem. Hatchery production is eliminated at the end of this recovery phase when the triggers for final recovery phase (viable natural population phase) are met. This is the period when all aspects of the previous phases are met, and a viable natural population exists that can sustain recreational, commercial, and Tribal harvest without hatchery augmentation.
Performance indicators are specific metrics to be measured by focused monitoring and are used to define how recovery is progressing through the four recovery phases. Each performance indicator has an associated trigger representing target values for the phase being assessed (Supplementary Tables S2, S3). Performance indicator triggers were empirical targets based on published information, available active monitoring results from the Elwha River, and comparable watersheds that could be used as a potential reference (Peters et al., 2014). The performance indicators represented four viable salmonid population metrics (McElhany et al., 2000), including abundance, productivity, distribution, and diversity, plus managing for the proportion of hatchery-origin spawners (pHOS). We used a geometric mean calculated over a four-year period, representing the dominant age at maturity for both Chinook salmon and steelhead trout, to evaluate the status of each performance indicator.
The relationship between each performance indicator and associated triggers were typically evaluated annually. Once all trigger values within each phase were met in the same year, by one of the species, that species could proceed to the next recovery phase. Feedback mechanisms existed within each recovery phase, allowing for the regression to the previous recovery phase if an indicator’s geometric mean dipped below the trigger value for the previous recovery phase. Thus, during each annual assessment, there were four potential management responses (Figure 1). Importantly, our approach allowed for the re-evaluation of triggers for each phase because they were based on a set of assumptions, with unknown accuracy.
Monitoring protocols were developed and are detailed in Pess et al. (2024). In general, timing of river entry and adult abundance were estimated using sonar estimates, weekly tangle net sampling, and carcass surveys to assess species composition and hatchery-origin return percentage, depending on the species (Denton et al., 2023). Productivity was assessed using sonar estimates of adult returns to the river, adult scale samples used to apportion adult estimates to brood year (i.e., adult productivity; Weinheimer et al., 2018), and both mainstem and tributary screw traps (i.e., juvenile productivity; McHenry et al., 2023b). Spatial extent was estimated using spawning ground surveys (McHenry et al., 2023a). The EMAM recognized that monitoring methods could change over time and recommended that revisions not occur until after the new methods had been evaluated and, if applicable, calibrated with the previous method to allow the development of comparable datasets. Finally, data standards were developed for monitoring data based on Crawford and Rumsey (2011) to ensure data quality was sufficiently accurate and precise to guide management decisions (see section 4.3; (Peters et al., 2014)).
The monitoring component of the EMAM was deemed successful because monitoring data allowed for informed decisions based upon the recovery trajectory of Chinook salmon and steelhead trout through the EMAM recovery phases. The data also facilitated development and evaluation of hypotheses regarding the mechanisms influencing recovery.
The Elwha River Chinook salmon population consistently exceeded the preservation trigger value for abundance of naturally spawning adults and the recolonization trigger value for spatial distribution since 2016 (Figure 2). Productivity triggers are the same across all recovery phases, and while juvenile productivity has exceeded the trigger value since 2021, the trigger value for adult productivity (hatchery plus natural spawner-to-spawner) has not yet exceeded the preservation trigger value. In this case, the last assessment of the geometric mean of adult productivity was 0.96 for hatchery and natural-origin spawners, which was slightly less than the trigger value of 1.0. Thus, although the Chinook population exceeded all trigger values for the preservation phase at least once, they were not all met during the same assessment year as required.
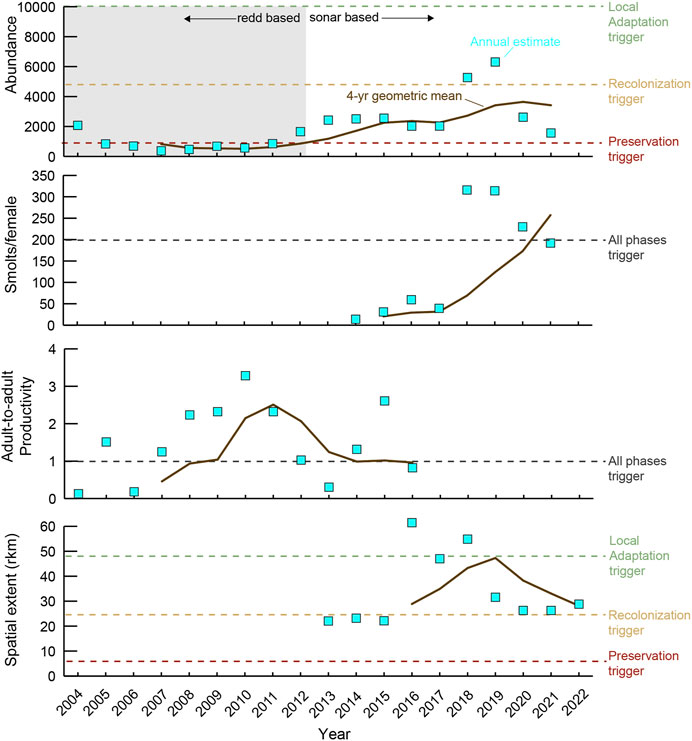
Figure 2. Quantitative assessment of performance indicators (abundance, smolts per female, adult-to-adult productivity, and spatial extent) for Chinook Salmon (Oncorhynchus tshawytscha) in the Elwha River from 2004 to 2022. Time period includes data before (2004–2010), during (2011–2014), and after (2015–2022) dam removal. Dashed lines represent performance indicator “trigger values” that when exceeded (4-year geometric mean) for all performance indicators during the same year represent completion of the current recovery phase. Note that abundance shifted from redd-based to sonar-based assessment at the start of dam removal (details in text). Data from Pess et al. (2024).
Elwha steelhead trout also exceeded the preservation phase trigger values that could be measured (Figure 3). Adult abundance exceeded the trigger value for both the preservation and recolonization phases (since 2016). Adult productivity exceeded the trigger value (same value across all recovery phases) during the first potential assessment for the 2016 brood year (Figure 3). Winter steelhead trout migrated upstream of both former dam sites and exceeded the distribution trigger for the recolonization phase, with some individual years surpassing the local adaptation trigger value. Finally, adult steelhead trout have been entering in January, exceeding the recolonization trigger value for run timing. The juvenile productivity trigger value was not met due to an inability to consistently capture enough steelhead trout smolts to estimate steelhead trout smolt abundance (McHenry et al., 2023b). Without this estimate, juvenile productivity could not be calculated, making it the only performance indicator whose trigger value was not exceeded for the preservation phase (but see below).
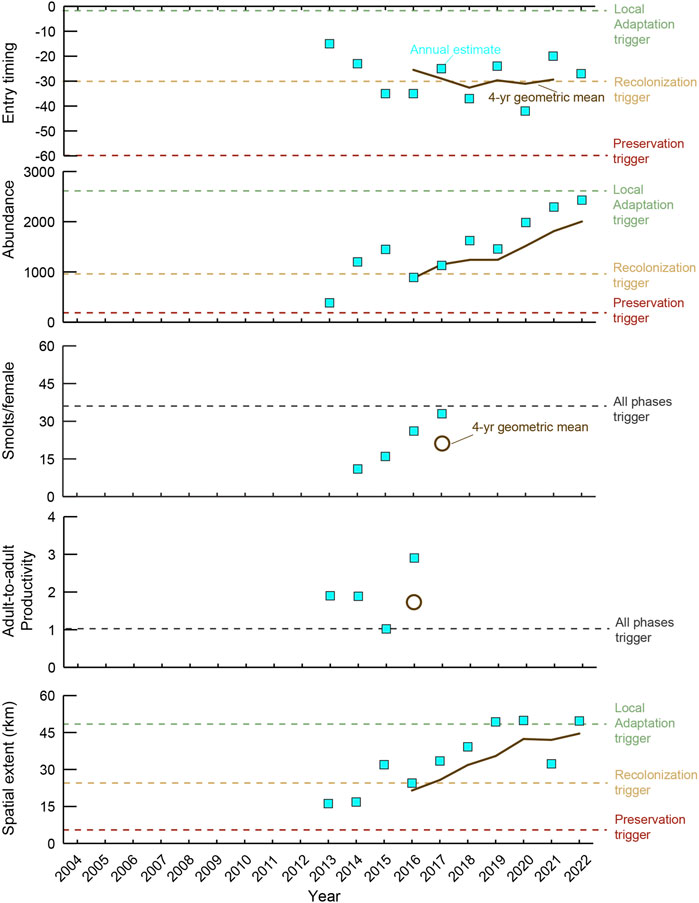
Figure 3. Quantitative assessment of performance indicators (entry timing, abundance, smolts per female, adult-to-adult productivity, and spatial extent) for steelhead trout (Oncorhynchus mykiss) in the Elwha River from 2004 to 2022. Time period includes data before (2004–2010), during (2011–2014), and after (2015–2022) dam removal. Dashed lines represent performance indicator “trigger values” that when exceeded (4-year geometric mean) for all performance indicators during the same year represent completion of the current recovery phase. Data from (Pess et al., 2024).
In addition to providing data to assess triggers, the collaborative interagency monitoring program guided by the EMAM has provided sufficient data to identify when monitoring methods needed to be adjusted. When problems were identified, scientists and managers worked together to make changes to the monitoring program. For example, at the inception of the EMAM, a channel spanning floating weir was a foundational method for estimating abundance, measuring pHOS, and describing run timing. However, river conditions during dam removal created high sediment and woody debris loads making it too difficult and labor intensive to safely and efficiently operate the weir, resulting in a low capture efficiency and insufficient data (Anderson et al., 2013). Recognizing the weir limitations, researchers pivoted towards using sonar methodology, which proved to be successful at estimating abundance and run timing despite challenging dam removal conditions. As a result, the weir was abandoned, and the sonar program expanded to include species composition netting, improving the precision of allocating image targets to species that overlapped in both size and run timing. Another example of adaptive monitoring included expanding the range and frequency of upper river summer snorkel surveys in response to the rapid expansion of summer steelhead trout (see Pess et al., 2024).
The EMAM monitoring program provided sufficient data to identify several modifications to recovery actions for Elwha River Chinook salmon and steelhead trout and to evaluate the quality of the performance indicators. The first case was removing a newly created fish passage barrier that might have gone unnoticed (or possibly delayed recognition) without intensive monitoring. After the complete removal of the Glines Canyon Dam in 2014, several species of anadromous fish were detected upstream of this site (Duda et al., 2021a), but data from a radio-telemetry study and spawning ground surveys in 2015 identified a fish passage barrier a short distance downstream of the Glines Canyon dam site. This prompted further investigation, which revealed that the barrier was the result of large boulders, likely portions of the canyon wall weakened during dam construction, falling into the channel shortly after dam removal was completed. In response, partnering federal agencies (National Marine Fisheries Service, Olympic National Park and the U.S. Army Corps of Engineers) conducted selective rock blasting to remove the boulders and reopen fish passage, which was completed by the autumn of 2016 (Ertle et al., 2019). The management action was successful in reestablishing fish passage upstream of Glines Canyon, with subsequent upstream detections and expanding spatial distribution of Chinook salmon and bull trout documented in 2016 and subsequent years (Duda et al., 2021b; Pess et al., 2024).
The second case of AM arising in the Elwha River EMAM project was adjusting the suite of performance indicators for steelhead trout. During the initial years of monitoring, during and following dam removal, insufficient numbers of steelhead trout smolts were captured and/or trap efficiency was too low to estimate entire basin steelhead trout smolt abundance in the mainstem, despite accurate estimates from two tributaries. The lack of data affected our ability to estimate the juvenile productivity performance indicator and to evaluate whether the trigger value had been met (Figure 3). Consequently, the interagency team recommended eliminating this performance indicator, a recommendation further informed by the observation that adult-to-adult productivity exceeded the trigger value throughout the monitoring period (Figure 3). The interagency team (i.e., stakeholders) concluded from adult productivity that the juvenile productivity trigger value had likely been exceeded. This recommendation has been evaluated for regulatory approval resulting in steelhead trout moving to the recolonization phase despite a lack of data to evaluate the juvenile productivity trigger, providing a prime example of AM.
The third example of AM actions was the hatchery production levels of steelhead trout. The removal of the juvenile productivity performance indicator from the EMAM for steelhead trout allowed steelhead trout to transition from the preservation phase to the recolonization phase of recovery. This led to management revisions in the spring of 2023 to reduce hatchery production of steelhead trout smolts from 175,000 to 30,000. This is an example of the goal of AM; using monitoring data to learn about a system and reduce uncertainty that results in updated management actions.
While the above are examples of successful implementation of AM principles, there were also missteps in application of AM for Elwha River Chinook salmon and steelhead trout. Differences existed in the timelines for development of the EMAM and drafting of the three BiOps for Chinook salmon and steelhead trout. As a result, draft values for performance indicators and triggers were incorporated into the BiOp (NMFS, 2012b), which were subsequently modified in the EMAM (Peters et al., 2014) (Supplementary Tables S2, S3). This conflict caused confusion among participants about which performance indicators and triggers should be used (Lower Elwha Klallam Tribe, 2011; Washington Department of Fish and Wildlife, 2012; Peters et al., 2014; NMFS, 2015). For example, the BiOp (NMFS, 2012b) list adult spawner escapement as natural-origin spawners, while the EMAM lists this as naturally spawning adults (i.e., hatchery- or natural-origin spawners). The EMAM was the final product of the scientists conducting the monitoring, yet it was not legally binding. NMFS has requested a memo to revise this language. However, the technical group has not reached consensus on all the trigger revisions for Chinook salmon and therefore have not submitted the memo, leaving this issue unresolved.
AM via the EMAM has also been hampered by some misguided performance indicators and triggers identified for the preservation phase. In retrospect, the established trigger values were too conservative in protecting fish from extirpation due to dam removal conditions. The river returned to levels where sediment levels were no longer a threat before the complete list of EMAM performance indicators could be assessed. Furthermore, performance indicators for river conditions directly impacted by dam removal, such as turbidity and channel stability, were not developed for the EMAM. This mismatch is highlighted by the recovery trajectory of Chinook salmon, which have fallen short of meeting the preservation phase triggers 10 years after dam removal (Figure 2). After about 4 years (2018), channel stability increased and turbidity no longer reached levels that were detrimental or lethal to fish (Magirl et al., 2015; East et al., 2018; Ritchie et al., 2018). Although Chinook salmon juvenile productivity has increased substantially in recent years, the adult productivity trigger value has not been met. Thus, despite reaching the overall conceptual goal for the preservation phase, Chinook salmon remain ‘stuck’ in the preservation phase, since adult-to-adult productivity did not exceed the trigger value during the last assessment, emphasizing the uncertainties of setting population benchmarks prior to a major, watershed-altering management action.
The issue of misguided performance indicators was recognized as early as 2017, and unsuccessful attempts to revise these triggers were made. The technical group developed draft performance indicators and triggers for Chinook salmon for the preservation phase. However, the technical group could not reach consensus on revised Chinook salmon performance indicators and triggers for the later recolonization and local adaptation phases. Thus, they have not submitted a request to the NMFS to revise the triggers in the BiOp. Since consensus could not be reached for Chinook salmon, no attempt was made to revise the performance indicators or triggers for steelhead trout.
Like many others have found (e.g., (Keith et al., 2011; Runge et al., 2011; Williams and Brown, 2014; Roux et al., 2022), we experienced both successes and challenges in implementing AM. The adaptive monitoring component was successful, providing the data to understand the status and response of Chinook salmon and steelhead trout to dam removal and the role of hatchery-assisted protection and mitigation from short-term negative effects associated with dam removal. Given the unprecedented nature of Elwha dam removal, challenges in implementing the EMAM were expected; responding to these challenges would require both flexibility and a commitment to learning. Below, we discuss factors affecting successes and challenges in the context of lessons learned, including topics such as funding, leadership, communication and legal frameworks (i.e., ESA, BiOp, as implemented here) that others have consistently identified as essential for successful implementation of AM (Walters, 2007; Westgate et al., 2013; Dreiss et al., 2017; Edmondson and Fanning, 2022; Månsson et al., 2023).
Ten years after dam removal, Chinook salmon and steelhead trout are still in the preservation (first phase) and recolonization (second phase) phases of recovery, respectively. Despite what is described as rapid recovery for steelhead trout by those monitoring this population, salmonid recovery takes time due to species-specific life history considerations such as age at maturity (i.e., 4 years for Elwha Chinook salmon and steelhead trout). However, this was also due to the performance indicators, triggers, and evaluation strategy initially selected requiring too much time before the first full evaluation could be completed. Therefore, performance indicators should be chosen that can be evaluated in as short of a timeframe as possible and be specific to the objectives for each recovery phase. This will allow progress to be assessed and hopefully observed more frequently throughout the AM program (Argent, 2009) to maintain momentum (i.e., progress). The EMAM included adult-to-adult productivity as a performance indicator for the preservation phase for both Chinook salmon and steelhead trout. Thus, the first potential progression from the preservation phase of recovery occurred 8 years (i.e., only two generations) after dam removal. However, the impacts from dam removal that raise concerns about the continued persistence of these two populations had largely subsided by this time (Warrick et al., 2015; East et al., 2018; Ritchie et al., 2018). In retrospect, the preservation phase should have been based on performance indicators with shorter evaluation periods that were more directly related to river conditions, allowing earlier evaluation and potentially more alternate management strategies that would have maintained observable progress through the recovery phases.
The monitoring component of the EMAM was very successful. In general, dedicated funding, a requirement for successful AM programs (Westgate et al., 2013; Newcomb et al., 2021; Edmondson and Fanning, 2022), allowed established teams to collect data to understand the status and response of Chinook salmon and steelhead trout to dam removal and hatchery production (Liermann et al., 2017; Munsch et al., 2023; Pess et al., 2024). Consistent and dedicated funding was made available for the project over 10 years by Olympic National Park to meet requirements in the BiOp (NMFS, 2012b). Although significant funding was provided, it was less than that requested for the program. In addition, no funding was provided after this 10-year period and the BiOP only addresses recovery through the recolonization phase. Thus, the future of the monitoring and AM program, which is necessary to determine when these two salmonid species have reached the local adaptation phase, is uncertain. Large projects requiring formal consultation or permit application could incorporate funding into legal documents to ensure dedicated funds are available (i.e., terms and conditions under Section 7, 10 of ESA, see Ruhl, 2004). Additional funding could be obtained by strategic and collaborative attempts to secure agency funding and/or agency staff dedicated to the program. The historic importance and visibility of the Elwha Dam removal project, coupled with the presence of species listed under the ESA, attracted funding support across a wide range of governmental, academic, and non-governmental organizations to conduct a variety of studies on the physical, biological, and ecological responses of the river and its freshwater and marine ecosystems (e.g., East et al., 2015; Ritchie et al., 2018; Brenkman et al., 2019; Duda et al., 2021a). We realize that the Elwha Dam removal project was unique in this regard, and future projects might not be able to attain funding to supply long-term data sets embedded within an AM framework. Yet, less than 10% of dam removals have been scientifically evaluated and most of these were of short duration or smaller dam removals (Bellmore et al., 2017). Thus, there is a scientific need for long-term evaluations of large dam removals. If funding is limited, attempting to implement an AM program would be more difficult and funding would be better spent implementing critical monitoring activities with an emphasis on data quality, since underfunded attempts to complete AM may be more costly over time to both financial and ecosystem resources (Rist et al., 2016).
In addition to understanding the response of Chinook salmon and steelhead trout to dam removal, the dedicated funds for monitoring enabled learning about unforeseen issues with fish passage and allowed collection of data for other species. One confirmed fish passage barrier, just downstream of former Glines Canyon Dam was identified and removed relatively quickly (Ertle et al., 2019). Finally, the monitoring program provided “value added” data for species and/or metrics that otherwise may not have been collected including key life history, abundance, and genetic information on coho (O. kisutch) (Liermann et al., 2017), chum (O. keta) and pink salmon (O. gorbuscha), Pacific lamprey (Entosphenus tridentatus) (Hess et al., 2021), bull trout (Quinn et al., 2017; Brenkman et al., 2019), and sockeye salmon (O. nerka) (Quinn et al., 2021).
Steelhead trout recovery has progressed more rapidly than recovery of Chinook salmon. Except for the juvenile productivity trigger value, which could not be calculated due to an inability to capture enough steelhead trout smolts to estimate smolt abundance, all the steelhead trout performance indicators have exceeded the recolonization trigger values. Although three of the four Chinook salmon performance indicator triggers have exceeded the recolonization trigger values, the adult productivity trigger did not meet the preservation trigger value during the last assessment. Factors influencing the different trajectories observed in these two species may include spawning season and habitats, which likely favored steelhead trout (i.e., more tributary use), more influence of ocean fisheries on Chinook salmon returns, more limited hatchery intervention in the native-steelhead trout program, and the contribution of resident fish in the upper basin that likely contributed to steelhead trout recovery (Fraik et al., 2021; Pess et al., 2024).
The successes and challenges associated with revising the EMAM process appear to be related to the recovery rate of Chinook salmon and steelhead trout. Chinook salmon recovery lagged steelhead trout recovery. This difference may have led to the success/challenge by the interagency team in revising triggers associated with these species and submitting requests for changes to the NMFS resulting in the inability (for Chinook salmon) or ability (for steelhead trout) to revise performance indicators and/or triggers. This supports the recommendation by (Argent, 2009) to seek ways to observe progress and maintain momentum of the AM program. We did see progress in the AM process for steelhead trout, which led to adaptive management actions, whereas progress in the AM process for Chinook salmon stalled (i.e., inability to revise triggers).
In contrast to the monitoring component, no funding was provided to fund a leadership position (and/or facilitator) to facilitate the adaptive implementation and evaluation components of the EMAM. Lacking directed funds, none of the agencies involved could afford to appoint someone to this leadership position, leaving the technical group to rely on several individuals to volunteer periodically (i.e., for annual meetings) to assume the ‘leaders’ role. This resulted in many of the challenges observed in implementing the EMAM. Strong and consistent leadership is a primary requirement for successful AM programs (Walters, 2007; Rist et al., 2016; Berkley and Beratan, 2021; Edmondson and Fanning, 2022). Lacking this steady leadership in the years following dam removal when monitoring from adaptive implementation was yielding actionable results, the EMAM suffered from a lack of communication among project partners, which negatively impacted collaboration, maintenance of common goals, and accurate implementation of selected management strategies. In addition, technical staff conducting the monitoring work were often not in decision making positions within regulatory agencies or the co-managers, which added another line of communication with regulatory and/or decision-making staff. Although significant learning occurred, that information has not been fully used to improve the adaptive evaluation component of the EMAM, including timely review and revision of performance indicators and associated triggers. Thus, an appointed leader to focus on continued communication and implementation of the adaptive evaluation component of an AM program would greatly enhance the AM success. The primary task would be communicating with participating agencies to ensure collaboration occurs to the extent possible, common goals are maintained, that issues identified through the AM process are addressed quickly, and that agreed upon management strategies are developed and implemented accurately. While there are no guarantees that having a dedicated leader would lead to success, it is hard to believe it would not have improved the situation since the group lacked a dedicated leader following dam removal. Although a decision charter would also solve these issues, this could not be accomplished in our case since it would be unlawful for the agencies involved to delegate their authorities.
A primary task of someone leading an AM program is goal setting, which itself is a primary activity identified in AM. Numerous authors recommend that all participants engage in this activity in a structured manner (Allen et al., 2001; Gregory and Long, 2009; Westgate et al., 2013; Berkley and Beratan, 2021; Edmondson and Fanning, 2022; Bamzai-Dodson et al., 2023). The value of common goals and the impacts of not maintaining/revising goals over time was highlighted while attempting to implement the EMAM. One individual led the planning and early implementation of this project, with a common goal existing throughout: remove the dams and develop a strategy to monitor and learn how fish respond to dam removal so that restoration actions beyond dam removal could be adjusted as needed. This common goal led to significant planning that resulted in the documents supporting dam removal (Department of the Interior et al., 1994; Department of the Interior, 1995; Department of the Interior, 1996) the fish restoration plan (Ward et al., 2008), and the EMAM (Peters et al., 2014). The common goal of learning has sustained an effective and collaborative monitoring program throughout the process, despite the lack of a single leader after the initial leader retired. However, divergent management goals and expectations among project partners developed after dam removal and with staff turnover at various agencies, leading to the emergence of different strategies for increasing Chinook salmon recolonization of the upper watershed. For example, the failure of Chinook salmon to consistently reach the upper watershed led to proposals to move adult Chinook salmon into the upper watershed (via helicopter). However, this view was not supported by all the technical team members and remains a point of contention. These divergent goals, expectations, and strategies impacted our ability to revise performance indicators and triggers for the recolonization phase for Chinook salmon, since no consensus could be reached. The group could not even agree upon a method for developing a distribution trigger value for Chinook salmon. As a result, Chinook salmon remain “stuck” in the preservation phase of recovery, although the consensus opinion among biologists involved in the project was that the lethal risk due to dam removal activities no longer exist. A primary leader or trained facilitator to facilitate communication and collaboration is necessary to maintain discussions that could result in the maintenance of common goals (Ebberts et al., 2018; Berkley and Beratan, 2021). It must also be recognized that the goals will evolve as projects progress and more information is obtained about the system being managed. Thus, the AM leader should maintain goal setting, regular review, and revision as a primary objective.
The legal framework imposed by the ESA, as implemented in this project, put some constraints on the EMAM process. This resulted from the overall legal process and the lack of understanding of the process among managers and researchers. Managers and researchers did not understand the processes (i.e., re-consultation under ESA) required if the management options changed from those evaluated in the BiOp (e.g., hatchery production alterations not described in the initial BiOp). These issues occurred in the planning and design phase of the EMAM and proved to be problematic later. The issues with the design stem from incongruence between guidance and regulatory documents described above and a lack of diversity in management options (i.e., generally maximum on-station releases of hatchery fish). Our lesson learned was to maintain a consistent schedule and coordination among all guiding documents, to the extent possible. For the Elwha River, the only clear resolution to the current incongruence is to re-initiate the ESA consultation process, which has not been a popular choice. This is due, in part, to the litigious environment regarding the use of hatcheries in Elwha River recovery. Although the lack of flexibility resulting from how the ESA framework was implemented in this project was an impediment, it does not have to be the case for all projects. The ESA was implemented to protect listed species from the environmental impacts of dam removal and resulting use of hatcheries to protect and restore Chinook salmon and steelhead trout. In other cases, such as species reintroductions, there are several provisions that provide flexibility under ESA (Dunham et al., 2016).
Another issue imposed by the legal framework was the lack of diverse management options. Although we followed recommendations to include AM within legal frameworks (Ruhl, 2004; Garmestani et al., 2009), we did not understand how this could be done effectively under the ESA framework. Thus, only hatchery management, with varying levels of production for specific recovery phases were incorporated into the BiOp (NMFS, 2012b). This was partially due to the long planning period for dam removal in the Elwha. The management options (i.e., hatchery intervention) were largely laid out in the environmental impact statement process (Department of the Interior et al., 1994; Department of the Interior, 1995; Department of the Interior, 1996; Department of the Interior, 2005) completed one to two decades before the development of the EMAM. In addition, to improve the likelihood of understanding factors influencing progression, the EMAM authors reduced the management options listed in the Elwha fisheries recovery plan (Ward et al., 2008). As a result, maximum on-station hatchery production was listed in the EMAM as the priority management option during the preservation phase even though the Elwha fisheries recovery plan and ESA documents list additional management strategies that could have been employed. For example, fish relocation was listed in the Elwha fisheries recovery plan and ESA documents and was completed for steelhead trout and Chinook salmon (Pess et al., 2024). The EMAM suggested prioritization of a reduced number of alternative strategies to improve learning associated with monitoring. Including more management options for the preservation and recolonization phases within the ESA framework, such as a range of hatchery production given different levels of turbidity and/or natural production, or triggers to initiate fish relocation, would have provided more flexibility during the early phases of recovery. We still recommend incorporating AM into the ESA framework, when possible, since this approach has been successfully applied elsewhere (Ebberts et al., 2018). Incorporating the EMAM into the legal documents also provided the framework to monitor and adaptively manage recovery. However, some revisions to the process used for the EMAM are recommended, particularly the inclusion of multiple management options within each recovery phase.
Careful consideration is necessary to incorporate the flexibility essential for AM into legal documents (i.e., ESA), which requires clear and concise language and lengthy review. Incorporating AM into legal documents requires defining all potential management options using a phased approach for implementing these options (McDonald and Styles, 2014) and triggers in order to progress through recovery phases (Nie and Schultz, 2012; Kingsford et al., 2021). Although this was completed for the EMAM, the steps between recovery phases were too long, and in some cases included too many or flawed performance indicators. We originally identified four viable salmonid population performance indicators for the preservation phase. In retrospect, this could have been reduced to three, including distribution, natural produced smolts, and hatchery produced smolts. Revised triggers were proposed for Chinook salmon for the preservation phase but were not formally submitted to the NMFS for review, due to failed attempts to reach agreement on revised recolonization triggers. The proposed preservation phase revisions essentially ensure Chinook salmon migrate upstream of the former dams and that both natural and hatchery-origin smolts are produced in sufficient numbers to prevent extinction of the population. Successfully meeting triggers for these performance indicators would indicate that river conditions have improved to the point where natural production is occurring and extirpation due to dam removal is no longer a threat. These revised triggers were an attempt to adjust trigger values for the preservation phase and incorporate smaller steps and/or alternatives within each recovery phase, thereby allowing adaptation within a phase that would likely maintain progress and momentum (Argent, 2009).
One limitation of the EMAM was the lack of monitoring specific to physical habitat and sediment, although these factors were a specific concern with respect to lethal conditions for fish during and immediately following dam removal. Turbidity, aggradation, and channel form monitoring occurred largely due to the requirement to maintain the City of Port Angeles domestic and industrial water supply (East et al., 2015; Magirl et al., 2015; Warrick et al., 2015; Ritchie et al., 2018). Physical monitoring was included in the EMAM to interpret recovery progress as observed but did not serve as performance indicators. In retrospect, performance indicators and associated triggers for physical variables including major habitat features, particularly at former dam sites, should have been included in the preservation phase since they are causally linked to the proposed performance indicators such as fish distribution and survival. Although this was lacking in the EMAM, fish passage was included in BiOps from the U.S. Fish and Wildlife Service and National Oceanic and Atmospheric Association covering dam removal (Crain and Brenkman, 2010; Olympic National Park, 2013) and the EMAM (Peters et al., 2014), while turbidity and geomorphic monitoring were identified within the sediment management plan (Bountry et al., 2018). Within the EMAM, viable salmonid population metrics served as proxies for physical monitoring and successfully identified a barrier (i.e., Ertle et al., 2019); however, the EMAM would have benefited from more direct inclusion of physical variables as performance indicators.
The EMAM recommended that the first assessment of trigger values and associated assumptions occur 8 years into recovery. This recommendation allowed for two full generations of Chinook salmon and steelhead trout to be completed prior to the assessment. In retrospect, the first assessment should have occurred sooner (i.e., 2–4 years) to better understand their utility for evaluating the rate of recovery. This likely would have increased the likelihood of identifying issues and successful revision of triggers that stalled progress through the AM process.
In the Elwha case study, the EMAM was designed specifically for Chinook salmon and steelhead trout and was required under ESA. However, it did not address other key fish species including bull trout, eulachon, sockeye salmon, coho salmon, chum salmon, and pink salmon. Of the species not addressed in the EMAM, hatchery intervention occurred for coho salmon, chum salmon (intermittently), and odd-year pink salmon (2 cycles only). However, management actions have been taken to benefit species addressed and not addressed by the EMAM. Thus, the progression of these different fish species through recovery offers an opportunity to compare monitoring data collected and associated changes in management through recovery. The amount of data collected and corresponding links to management varied among EMAM (legal requirements) and non-EMAM (no legal requirements) covered species. Although significant information was collected for non-EMAM covered species (e.g., coho salmon: (Liermann et al., 2017; Munsch et al., 2023); bull trout: (Quinn et al., 2017; Brenkman et al., 2019; Duda et al., 2021a, 2021b), only coho salmon have similar amounts of monitoring data collected as seen in EMAM covered species. However, much of the data for non-EMAM covered species was largely collected opportunistically during work focused on Chinook salmon and steelhead trout. The exception to this is fish passage data collected for bull trout (BiOp requirement) (NMFS, 2012a) and the recent addition of adult abundance estimates for coho salmon based on SONAR. Management actions taken in response to monitoring data also varied among EMAM and non-EMAM covered species. For EMAM covered species, the only management change has been reduced hatchery production for steelhead trout implemented in 2023. Rock blasting to restore passage through the 2014 rockfall in Glines Canyon benefitted both EMAM and non-EMAM covered species but was initiated in response to monitoring related to Chinook salmon (EMAM) and bull trout (non-EMAM, but FWS required monitoring) (Ertle et al., 2019). For non-EMAM covered species, management changes have been suggested (coho salmon) or made (coho and pink salmon) based on information gained. Recommendations were forwarded that adult hatchery coho relocation upstream of former Elwha dam were no long necessary every year (Mchenry et al., 2022), a ceremonial and subsistence fishery for coho salmon conducted by the Lower Elwha Klallam Tribe occurred during the fall of 2023, and the pink salmon captive brood program was terminated. Thus, when data were collected for non-EMAM covered species, the information was used to revise management strategies despite the lack of a formal AM program. These management changes were also much easier to complete than those described in the EMAM that were bound by regulatory/legal frameworks resulting from their listing under ESA.
Although few successes of AM are reported in the literature (Runge et al., 2011; Westgate et al., 2013; Gillson et al., 2019; Edmondson and Fanning, 2022; Månsson et al., 2023), the framework is still believed to be better than alternative management paradigms such as ad hoc, wait-and-see, and steady state (Westgate et al., 2013). AM has been implemented for decades for numerous natural resource disciplines (Walters, 2007; Wineland et al., 2022; Bradford et al., 2023) and at various scales (Roux and Foxcroft, 2011; Melis et al., 2015). Numerous pitfalls and factors have been identified that lead to success and failure of AM programs (Walters, 2007; Williams and Brown, 2014), including the lack of retrospective analysis of implemented AM programs (Roux et al., 2022). By examining the AM program implemented for the removal of two Elwha River dams and associated hatchery intervention for the recovery of two fish species listed under the ESA, we have been able to identify factors leading to successes and challenges and provide recommendations to facilitate the successful implementation of future AM programs. Many of the factors leading to success and failure were similar to prior AM case studies. Funding was the most important factor leading to learning through a well designed and implemented monitoring program. The lack of a leadership position likely impacted the AM process by reducing communication, collaboration, and maintenance of common goals. The inflexibility of the ESA framework, as implemented, along with manager and researcher lack of knowledge of this framework and the lack of management options incorporated into guiding legal documents (i.e., environmental impact statement process), limited the flexibility of the AM program. This impact could have potentially been minimized by incorporating more management options into the BiOp analysis. The rate of recovery also appeared to influence participants’ willingness to revise performance indicator triggers. To improve the likelihood of success, monitoring should be completed, and a leadership position should be established to shepherd the AM process through communication, collaboration, and maintenance of common goals. Finally, flexibility within the AM process should be maintained by identifying small attainable steps for the AM process that will require management actions that maintain momentum.
Box 1 Understanding goals, values and backgrounds for effective collaboration.
Our experience adaptively managing Elwha River Chinook salmon and steelhead trout emphasizes the importance of recognizing the different goals, values and backgrounds of Federal, State, and Tribal governments, their agencies, and their representatives collaborating on a large-scale restoration project. Recognizing different perspectives helps explain situations where two professionals offer divergent opinions at a given decision point. Even further, an understanding and sincere effort to “put yourself in another’s shoes” helps create a path toward finding common ground. Here we describe the diversity of goals, values and backgrounds in our Elwha working group, to help illustrate the challenges and critical importance of working collaboratively.
Our working group had a shared goal of restoring the health of the Elwha River ecosystem and increasing the abundance, productivity, and life history diversity of its fish populations. Individuals also had more focused goals aligned with agency and job responsibilities. Some were directly involved in the administration of dam removal and thus had a goal to manage the project to meet expectations and fulfill legal obligations. Others had a goal for dam removal to provide long-term sustainable fishing opportunities as a food source and cultural experience for local communities. Still others in the group were compelled to learn how the ecosystem and fish populations respond to dam removal to select, inform, and implement restoration projects elsewhere more effectively. Most everyone in the group identified with these various goals at some level, but individuals varied substantially in the extent to which they directly pursued them.
Divergent opinions on a given topic could often be traced to different value systems. One common source of debate was a spectrum of willingness for management intervention to support or accelerate fish reoccupying areas upstream of the former dams. We experienced divergent opinions over transplanting adult salmon to upstream areas with few salmon once water turbidity returned to pre-removal levels. Some felt that this action could only help recovery, with minimal risk if transplanted fish did not accelerate spatial expansion. Others emphasized the importance of fish expressing natural patterns of expansion past the former dams, allowing key ecological and evolutionary processes (e.g., habitat selection, life history diversity) to occur on a pathway towards the long-term sustainability of recovery. Our working group also had different views on the urgency of observing a fish response to dam removal. Some sought to observe shorter term increases in population status, whereas others expressed a willingness to wait longer. Often these differences were tied to professional roles and responsibilities, and the degree to which individuals were responsible for natural resources experiences in the communities they lead or represented.
A range of backgrounds in aquatic ecology, fish, and fisheries management were represented in our group. Some had extensive experience leading monitoring projects using a variety of methods. Our group also included leaders responsible for multiple natural resource management activities beyond Elwha dam removal, including managing lands, fishery harvest, and salmon recovery programs. For some, Elwha was one of many responsibilities, and they brought experiences from other watersheds elsewhere in Washington State and beyond. For others, most of their professional work was conducted in the Elwha watershed and adjacent aquatic systems on the North Olympic Peninsula. Some individuals were closer to regulatory requirements and legal obligations, some were involved in scholarly pursuits related to dam removal outcomes, whereas others were closer to day-to-day activities like monitoring in the Elwha River or producing fish in two hatcheries.
In summary, our group was most effective when we had open and regular lines of communication, which fostered the ability to learn from others doing different work and empathize with reasons behind divergent viewpoints. This was not always easy, and sometimes extended conversations were needed to reach mutual understanding. We did not always reach consensus or agreement on important issues. This may be due, in part, to the fact that we did not have a professional facilitator to lead our discussion. However, a shared respect for the relationships in our working group and sincerity in listening to alternative viewpoints laid the foundation for a healthy, productive collaboration.
The datasets presented in this study can be found in an online repository at https://ecos.fws.gov/ServCat/Reference/Profile/163653.
The animal study was approved by Washington Department of Fish and Wildlife sampling permits, U.S. Fish and Wildlife Service and National Marine Fisheries Service ESA sampling permits. The study was conducted in accordance with the local legislation and institutional requirements.
RP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. JA: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing–original draft, Writing–review and editing. JD: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. MM: Supervision, Validation, Writing–review and editing, Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources. GP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing–review and editing. SB: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing–review and editing. JJ: Formal Analysis, Validation, Writing–original draft, Writing–review and editing. ML: Data curation, Formal Analysis, Investigation, Methodology, Writing–review and editing. KD: Data curation, Formal Analysis, Investigation, Methodology, Validation, Writing–review and editing. MB: Supervision, Writing–review and editing. PC: Funding acquisition, Project administration, Resources, Supervision, Writing–review and editing. HC: Data curation, Writing–review and editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This project was funded through numerous sources including appropriations through the National Park Service for the administration of the Biological Opinions, through base funding of specific agencies, including through population assessment funding administered by the U.S. Fish and Wildlife Service, through the Western Washington Fish and Wildlife Conservation Office; Funding from outside sources resulting from competitive processes including EPA Performance Partnership Grant (66.605), BIA Operations (11 P.L. 93-638), BIA Mass Marking Program to the Lower Elwha Klallam Tribe, and Washington’s National Park Fund to Olympic National Park. Funding for these efforts, and loan of one SONAR unit to the Lower Elwha Klallam Tribe, was provided by the United States National Park Service—Olympic National Park to the Lower Elwha Klallam Tribe (Contract Nos. 1443PC00296, P14PC00364, and P16PC00005). The National Park Foundation also provided funds to the National Park Service and the Lower Elwha Klallam Tribe (Contract No. 140P2018R042). KD was funded by the Lower Elwha Klallam Tribe for all aspects of the SONAR and species composition work (Contract Nos. 1443PC00296, P14PC00364, and P16PC00005). Funding for general Chinook salmon and steelhead trout monitoring was also provided to the Lower Elwha Klallam Tribe by the Environmental Protection Agency/Puget Sound Partnership through the Northwest Indian Fisheries Commission (Contract No. PA-01J64601-1). Funding for the Elwha River native steelhead trout captive broodstock program was provided by the Pacific Coast Salmon Restoration Fund/Hatchery reform funds (Contract Nos. 09-1749, 11-1653, 12-1950, 13-1551, and 14-2247). The National Park Service—Olympic National Park provided funding to the Washington Department of Fish and Wildlife in support of the collection of biological data during carcass surveys from adult Chinook salmon returns (Contract Nos. P13PC00291, P14PX01377, P15PX02717, P16PX02828, and others). The National Parks Foundation provided additional funding to the Washington Department of Fish and Wildlife in support of the tagging of juvenile Chinook salmon with coded wire tags (CWTs), the collection of the tags during spawning operations and spawning ground surveys for carcasses, and the subsequent reading and interpretation of the CWTs and otoliths. The United States Fish and Wildlife Service provided funding for a portion of the labor associated with adult and juvenile enumeration and the proportion of hatchery-origin steelhead trout (pHOS), which received additional funding from the Lower Elwha Klallam Tribe and the Bureau of Indian Affairs. Funding, in part, was provided by the Lower Elwha Klallam Tribe to the National Oceanic and Atmospheric Administration—Northwest Fisheries Science Center for the contributions of George Pess, Todd Bennett, and Martin Liermann (Contract No. PA-01J64601-1). Oleksandr Stefankiv of AIS, Inc. (contractor to the Northwest Fisheries Science Center) provided assistance with SONAR enumeration. Funding for the weir project were obtained through the American Recovery and Reinvestment Act, agreement 13320RJ020 along with Modification No. 1 between U.S. Fish and Wildlife Service and the Lower Elwha Klallam Tribe) and the USEPA through the Puget Sound Partnership (EPA Agreement number DW-14-95792801-0). Additional funding for weir materials and staffing were provided by the USGS Ecosystems Mission Area funds.
We would like to thank the expert panel of Dr. Robert Bilby (Weyerhaeuser Company, retired), Dr. Peter Bisson (U.S. Forest Service, retired), Dr. Ray Hilborn (University of Washington), and Dr. Robert Hughes (Amnis Opes Institute) for taking time to review the original EMAM framework and participating in a workshop to further refine this monitoring and adaptive management framework. Brian Winter (Olympic National Park, retired) managed the Elwha project before and during dam removal and made significant contributions to the early documents that set the stage for Elwha restoration and adaptive management. Tim Tynan (NOAA, retired) participated in the development of the EMAM and the Elwha biological opinions. Mara Zimmerman (while with WDFW) made significant contributions to the EMAM. The partners involved would like to acknowledge the numerous technical staff that participated in data collection, highlighting those with significant contributions including Lower Elwha Klallam Tribe: Mel Elofson, Sonny Sampson, Randall McCoy; NOAA: Sarah Morley; Todd Bennett; NPS: Anna Geffre, Josh Geffre, Boone Jones, Phil Kennedy, Katie Kierczynski, Trevor Kumec, Kathryn Sutton; United States Fish and Wildlife Service: Dan Lantz; WDFW: Josh Weinheimer, Andrew Simmons, Chris O’Connell, Jeff Gufler, and Troy Tisdale. The manuscript was improved by reviews of previous drafts by Mara Zimmerman (Coastal Salmon Partnership and Foundation), Morgan Robinson (NMFS), Karrie Hanson (NOAA), Laurie Peterson and Kathryn Sutton, (WDFW), Ben Cross and Regan McNatt (both - United States Fish and Wildlife Service), Jason Dunham (U.S. Geological Survey), and peer-reviews from JW (USFWS) and RF (USDA).
Author KD owns and operates K. Denton and Associates, LLC and worked under contract with the Lower Elwha Klallam Tribe to collect data used in this study (SONAR).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The findings and conclusions in this document are those of the U.S. Fish and Wildlife Service author(s) and do not necessarily represent the views of the U.S. Fish and Wildlife Service, National Park Service, or NOAA Fisheries. This article has been peer reviewed and approved for publication consistent with USGS Fundamental Science Practices (https://pubs.usgs.gov/circ/1367/). Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2024.1291265/full#supplementary-material
Allen, W., Bosch, O., Kilvington, M., Harley, D., and Brown, I. (2001). Monitoring and adaptive management: resolving social and organisational issues to improve information sharing in natural resource management. Nat. Resour. Forum 25, 225–233. doi:10.1111/j.1477-8947.2001.tb00764.x
Anderson, J., Mizell, M., Ackley, M., Mayer, K., Zimmerman, M., and Crain, P. (2013). Elwha River weir project: 2013 operations and final summary report. Olympia, WA. Available at: https://wdfw.wa.gov/sites/default/files/publications/01706/wdfw01706.pdf.
Anderson, J. H., Warheit, K. I., Craig, B. E., Seamons, T. R., and Haukenes, A. H. (2020). A review of hatchery reform science in Washington State. Washington: Department of Fish and Wildlife.
Argent, R. M. (2009). “Components of adaptive management,” in Adaptive environmental management. Editors C Allan, and G H Stankey (Dordrecht: Springer International Publishing), 11–36. Available at:. doi:10.1007/978-1-4020-9632-7_2
Bamzai-Dodson, A., Cravens, A. E., and McPherson, R. A. (2023). Critical stakeholder engagement: the road to actionable science is paved with scientists’ good intentions. Ann. Am. Assoc. Geogr. 114, 1–20. doi:10.1080/24694452.2023.2242448
Bellmore, J. R., Duda, J. J., Craig, L. S., Greene, S. L., Torgersen, C. E., Collins, M. J., et al. (2017). Status and trends of dam removal research in the United States. Wiley Interdiscip. Rev. Water 4, e1164. doi:10.1002/wat2.1164
Benson, M. H., and Schultz, C. (2015). “Adaptive management and Law,” in Adaptive management of social-ecological systems. Editors C R Allen, and A S Garmestani (Dordrecht: Springer Netherlands), 39–59. doi:10.1007/978-94-017-9682-8_4
Benson, M. H., and Stone, A. B. (2013). Practitioner perceptions of adaptive management implementation in the United States. Ecol. Soc. 18, art32. doi:10.5751/es-05613-180332
Berkley, J., and Beratan, K. K. (2021). Capturing practitioners’ “how-to” knowledge in the form of recommendations for more effective planning of collaborative adaptive management projects. Ecol. Soc. 26, art24. doi:10.5751/ES-12840-260424
Bountry, J., Randle, T. J., and Ritchie, A. C. (2018). Adaptive sediment management program final report for the Elwha River restoration project. Denver, CO: Bureau of Reclamation. Available at: https://data.usbr.gov/catalog/8010/item/128610.
Bradford, M. J., Korman, J., and Sneep, J. (2023). Adaptive management of flows in a regulated river: flow-ecology relationships revealed by a 26-year, five-treatment flow experiment. Environ. Manage. 71, 439–450. doi:10.1007/s00267-022-01750-4
Brannon, E. L., and Hershberger, W. K. (1984). “Elwha River fall Chinook salmon,” in Proceedings of Olympic Wildf Fish Conference, Port Angeles, WA, 169–172.
Brenkman, S. J., Peters, R. J., Tabor, R. A., Geffre, J. J., and Sutton, K. T. (2019). Rapid recolonization and life history responses of bull trout following dam removal in Washington’s Elwha River. North Am. J. Fish. Manag. 39, 560–573. doi:10.1002/nafm.10291
Crain, P., and Brenkman, S. (2010). Elwha River restoration project: bull trout protection and restoration plan. A plan completed in fulfillment of rpm 1 of the USFWS 2000 biological opinion for the Elwha River restoration project. Port Angeles, WA: Olympic National Park. Available at: https://www.nps.gov/olym/learn/nature/upload/Elwha-River-Bull-Trout-Recovery-Plan_Accepted-Final-_12-13-10.pdf.
Crawford, B. A., and Rumsey, S. (2011). Guidance for monitoring recovery of salmon and steelhead listed under the federal Endangered Species Act (Idaho, Oregon, and Washington). Northwest Region: NOAA, National Marine Fisheries Service.
Deitch, M. J., Gancel, H. N., Croteau, A. C., Caffrey, J. M., Scheffel, W., Underwood, B., et al. (2021). Adaptive management as a foundational framework for developing collaborative estuary management programs. J. Environ. Manage. 295, 113107. doi:10.1016/j.jenvman.2021.113107
Denton, K., McHenry, M., Moses, R., Ward, E., Stefankiv, O., Urnes, C., et al. (2023). 2023 Elwha River winter steelhead escapement estimate based on ARIS multi-beam SONAR data. Port Angeles, WA: Lower Elwha Klallam Tribe.
Department of the Interior (1995). Elwha River ecosystem restoration, final environmental impact statement. Olympic National Park: Department of the Interior, National Park Service.
Department of the Interior (1996). Elwha River ecosystem restoration implementation, final environmental impact statement. Port Angeles, WA: Department of the Interior, National Park Service, Olympic National Park.
Department of the Interior (2005). Elwha River ecosystem restoration implementation final supplement to the final environmental impact statement. Port Angeles, WA: Department of the Interior, National Park Service, Olympic National Park.
Department of the Interior, Department of Commerce, and Lower Elwha S’Klallam Tribe (1994). The Elwha report – restoration of the Elwha River ecosystem and native anadromous fisheries. Port Angeles, WA: Department of Interior, National Park Service, Olympic National Park.
Doremus, H. (2001). Adaptive management, the endangered species act, and the institutional challenges of “new age” environmental protection. Washburn Law J. 41, 50–89.
Dreiss, L. M., Hessenauer, J.-M., Nathan, L. R., O’Connor, K. M., Liberati, M. R., Kloster, D. P., et al. (2017). Adaptive management as an effective strategy: interdisciplinary perceptions for natural resources management. Environ. Manage. 59, 218–229. doi:10.1007/s00267-016-0785-0
Duda, J. J., Freilich, J. E., and Schreiner, E. G. (2008). Baseline studies in the Elwha River Ecosystem prior to dam removal: introduction to the special issue. Northwest Sci. 82 (SP1), 1–12. doi:10.3955/0029-344X-82.S.I.1
Duda, J. J., Hoy, M. S., Chase, D. M., Pess, G. R., Brenkman, S. J., McHenry, M. M., et al. (2021a). Environmental DNA is an effective tool to track recolonizing migratory fish following large-scale dam removal. Environ. DNA 3, 121–141. doi:10.1002/edn3.134
Duda, J. J., Torgersen, C. E., Brenkman, S. J., Peters, R. J., Sutton, K. T., Connor, H. A., et al. (2021b). Reconnecting the Elwha River: spatial patterns of fish response to dam removal. Front. Ecol. Evol. 9, 765488. doi:10.3389/fevo.2021.765488
Dunham, J. B., White, R., Allen, C., Marcot, B., and Shively, D. (2016). “The reintroduction landscape,” in Reintroduction of fish and wildlife populations (California: University of California Press).
East, A. E., Logan, J. B., Mastin, M. C., Ritchie, A. C., Bountry, J. A., Magirl, C. S., et al. (2018). Geomorphic evolution of a gravel-bed river under sediment-starved versus sediment-rich conditions: river response to the world's largest dam removal. J. Geophys. Res. Earth Surf. 123, 3338–3369. 2018JF004703. doi:10.1029/2018JF004703
East, A. E., Pess, G. R., Bountry, J. A., Magirl, C. S., Ritchie, A. C., Logan, J. B., et al. (2015). Large-scale dam removal on the Elwha River, Washington, USA: river channel and floodplain geomorphic change. Geomorphology 228, 765–786. doi:10.1016/j.geomorph.2014.08.028
Ebberts, B. D., Zelinsky, B. D., Karnezis, J. P., Studebaker, C. A., Lopez-Johnston, S., Creason, A. M., et al. (2018). Estuary ecosystem restoration: implementing and institutionalizing adaptive management. Restor. Ecol. 26, 360–369. doi:10.1111/rec.12562
Edmondson, E., and Fanning, L. (2022). Implementing adaptive management within a fisheries management context: a systematic literature review revealing gaps, challenges, and ways forward. Sustainability 14, 7249. doi:10.3390/su14127249
Ertle, C., Roth, M., Judson, J., and Vankirk, G. (2019). Rock demolition and hazardous debris removal for ecosystem restoration on the Elwha River. Vicksburg, MS: Engineer Research and Development Center.
Fraik, A. K., McMillan, J. R., Liermann, M., Bennett, T., McHenry, M. L., McKinney, G. J., et al. (2021). The impacts of dam construction and removal on the genetics of recovering steelhead (Oncorhynchus mykiss) populations across the Elwha River watershed. Genes 12, 89. doi:10.3390/genes12010089
Galappaththi, E. K., Susarla, V. B., Loutet, S. J. T., Ichien, S. T., Hyman, A. A., and Ford, J. D. (2022). Climate change adaptation in fisheries. Fish. Fish. 23, 4–21. doi:10.1111/faf.12595
Garmestani, A. S., Allen, C. R., and Cabezas, H. (2009). Panarchy, adaptive management and governance: policy options for building resilience. Neb. Law Rev. 87, 1036–1054.
Gillson, L., Biggs, H., Smit, I. P. J., Virah-Sawmy, M., and Rogers, K. (2019). Finding common ground between adaptive management and evidence-based approaches to biodiversity conservation. Trends Ecol. Evol. 34, 31–44. doi:10.1016/j.tree.2018.10.003
Gregory, R., and Long, G. (2009). Using structured decision making to help implement a precautionary approach to endangered species management. Risk Anal. 29, 518–532. doi:10.1111/j.1539-6924.2008.01182.x
Gunderson, L. (1999). Resilience, flexibility and adaptive management - antidotes for spurious certitude? Conserv. Ecol. 3, art7. doi:10.5751/es-00089-030107
Halbert, C. L. (1993). How adaptive is adaptive management? Implementing adaptive management in Washington State and British Columbia. Rev. Fish. Sci. 1, 261–283. doi:10.1080/10641269309388545
Hess, J. E., Paradis, R. L., Moser, M. L., Weitkamp, L. A., Delomas, T. A., and Narum, S. R. (2021). Robust recolonization of pacific lamprey following dam removals. Trans. Am. Fish. Soc. 150, 56–74. doi:10.1002/tafs.10273
HSRG (2012). Review of the Elwha River Fish restoration plan and accompanying hatchery and genetic management plans. Available at: http://www.hatcheryreform.us/.
Johnson, P. R. S. (2013). Elwha: value of a river. Managing risk in the Pacific Northwest. New Haven, CT: Yale.
Keith, D. A., Martin, T. G., McDonald-Madden, E., and Walters, C. (2011). Uncertainty and adaptive management for biodiversity conservation. Biol. Conserv. 144, 1175–1178. doi:10.1016/j.biocon.2010.11.022
Keith, M. (2000). Reservoir evolution following the removal of marmot dam on the sandy river, Oregon. M.S. Thesis. Portland, OR: Portland State University. doi:10.15760/etd.532
Kingsford, R. T., West, R. S., Pedler, R. D., Keith, D. A., Moseby, K. E., Read, J. L., et al. (2021). Strategic adaptive management planning—restoring a desert ecosystem by managing introduced species and native herbivores and reintroducing mammals. Conserv. Sci. Pract. 3. doi:10.1111/csp2.268
Liermann, M., Pess, G., McHenry, M., McMillan, J., Elofson, M., Bennett, T., et al. (2017). Relocation and recolonization of coho salmon in two tributaries to the Elwha River: implications for management and monitoring. Trans. Am. Fish. Soc. 146, 955–966. doi:10.1080/00028487.2017.1317664
Lower Elwha Klallam Tribe (2011). Hatchery and genetic management plan (HGMP) - Elwha River Steelhead Oncorhynchus mykiss late-timed native population. Port Angeles, WA: Lower Elwha Klallam Tribe.
Magirl, C. S., Hilldale, R. C., Curran, C. A., Duda, J. J., Straub, T. D., Domanski, M., et al. (2015). Large-scale dam removal on the Elwha River, Washington, USA: fluvial sediment load. Geomorphology 246, 669–686. doi:10.1016/j.geomorph.2014.12.032
Månsson, J., Eriksson, L., Hodgson, I., Elmberg, J., Bunnefeld, N., Hessel, R., et al. (2023). Understanding and overcoming obstacles in adaptive management. Trends Ecol. Evol. 38, 55–71. doi:10.1016/j.tree.2022.08.009
McCord, S. E., and Pilliod, D. S. (2022). Adaptive monitoring in support of adaptive management in rangelands. Rangelands 44, 1–7. doi:10.1016/j.rala.2021.07.003
McDonald, J., and Styles, M. C. (2014). Legal strategies for adaptive management under climate change. J. Environ. Law 26, 25–53. doi:10.1093/jel/equ003
McDonald-Madden, E., Baxter, P. W. J., Fuller, R. A., Martin, T. G., Game, E. T., Montambault, J., et al. (2010). Monitoring does not always count. Trends Ecol. Evol. 25, 547–550. doi:10.1016/j.tree.2010.07.002
McElhany, P., Rucklelshaus, M. H., Ford, M. J., and Wainwright, T. C. (2000). Viable salmonid populations and the recovery of evolutionarily significant units. National marine fisheries Service, Northwest Fisheries science center. Available at: https://repository.library.noaa.gov/view/noaa/3139/noaa_3139_DS1.pdf.
McHenry, M., Anderson, J., Connor, H., and Pess, G. (2023a). Spatial distribution of Chinook salmon (Oncorhynchus tshawytscha) spawning in the Elwha River, Washington State during dam removal and early stages of recolonization (2012-2022). Port Angeles, WA: Lower Elwha Klallam Tribe.
McHenry, M., Elofson, M., Taylor, M., Liermann, M., Hinkley, L., Bennett, T., et al. (2023b). 2022 Elwha River smolt enumeration project report. Port Angeles, WA: Lower Elwha Klallam Tribe.
McHenry, M., McMillan, J., Moses, R., and Pess, G. (2022). Coho salmon relocations and redd surveys in the Elwha River 2011-2021: summary report. Port Angeles, WA: Lower Elwha Klallam Tribe.
McMillan, J. R., Morrison, B., Chambers, N., Ruggerone, G., Bernatchez, L., Stanford, J., et al. (2023). A global synthesis of peer-reviewed research on the effects of hatchery salmonids on wild salmonids. Fish. Manag. Ecol. fme 12643, 446–463. doi:10.1111/fme.12643
Melis, T. S., Walters, C. J., and Korman, J. (2015). Surprise and opportunity for learning in Grand Canyon: the Glen Canyon Dam adaptive management program. Ecol. Soc. 20, art22. doi:10.5751/ES-07621-200322
Munsch, S. H., McHenry, M., Liermann, M. C., Bennett, T. R., McMillan, J., Moses, R., et al. (2023). Dam removal enables diverse juvenile life histories to emerge in threatened salmonids repopulating a heterogeneous landscape. Front. Ecol. Evol. 11. doi:10.3389/fevo.2023.1188921
Naish, K. A., Taylor, J. E., Levin, P. S., Quinn, T. P., Winton, J. R., Huppert, D., et al. (2007). An evaluation of the effects of conservation and fishery enhancement hatcheries on wild populations of salmon. Adv. Mar. Biol. 53, 61–194. doi:10.1016/S0065-2881(07)53002-6
Newcomb, T. J., Simonin, P. W., Martinez, F. A., Chadderton, W. L., Bossenbroek, J. M., Cudmore, B., et al. (2021). A best practices case study for scientific collaboration between researchers and managers. Fisheries 46, 131–138. doi:10.1002/fsh.10536
Nie, M. A., and Schultz, C. A. (2012). Decision-making triggers in adaptive management. Conserv. Biol. 26, 1137–1144. doi:10.1111/j.1523-1739.2012.01915.x
NMFS, (National Marine Fisheries Service) (2012a). Endangered species act (ESA) section 7 biological opinion and Magnuson-Stevens fishery conservation and management act essential fish habitat (EFH) consultation: Elwha Channel Hatchery and Lower Elwha Fish Hatchery. Seattle, WA: National Marine Fisheries Service.
NMFS, (National Marine Fisheries Service) (2012b). Endangered species act (ESA) section 7(a)(2) biological opinion and, Magnuson-Stevens fishery conservation and management act essential fish habitat (EFH) consultation: reinitiation of the Elwha River ecosystem and fisheries restoration. Seattle, WA: National Marine Fisheries Service.
NMFS, (National Marine Fisheries Service) (2015). 4(d) rule limit 6 evaluation and recommended determination, Joint hatchery and genetic management plans for Elwha Channel Hatchery Elwha River summer/fall Chinook fingerlings and yearlings, Lower Elwha fish hatchery native steelhead, Lower Elwha fish hatchery coho salmon, Lower Elwha fish hatchery fall chum salmon, and Elwha River odd and even year pink salmon. Seattle, WA: National Marine Fisheries Service.
Olympic National Park (ONP) (2013). Elwha River restoration project: Chinook and steelhead monitoring plan. A plan completed in partial fulfillment of the amended incidental take statement for the Elwha River and fisheries restoration project biological opinion (NMFS 2011/03769). Port Angeles, WA: Olympic National Park. Available at: https://ecos.fws.gov/ServCat/DownloadFile/244704.
Pess, G. R., McHenry, M. L., Beechie, T. J., and Davies, J. (2008). Biological impacts of the Elwha River dams and potential salmonid responses to dam removal. Northwest Sci. 82 (SP1), 72–90. doi:10.3955/0029-344X-82.S.I.72
Pess, G. R., McHenry, M. L., Denton, K., Anderson, J. H., Liermann, M. C., Peters, R., et al. (2024). Initial responses of Chinook salmon (Oncorhynchus tshawytscha) and steelhead (Oncorhynchus mykiss) to removal of two dams on the Elwha River. Washington State, U.S.A: Frontiers in Ecology and Evolution.
Peters, R. J., Duda, J. J., Pess, G. R., Zimmerman, M., Crain, P., Hughes, Z., et al. (2014). Guidelines for monitoring and adaptively managing Chinook salmon (Oncorhynchus tshawytscha) and steelhead (O. mykiss) restoration in the Elwha River. Lacey, WA: U.S. Fish and Wildlife Service.
Quinn, T. P., Bond, M. H., Brenkman, S. J., Paradis, R., and Peters, R. J. (2017). Re-awakening dormant life history variation: stable isotopes indicate anadromy in bull trout following dam removal on the Elwha River, Washington. Environ. Biol. Fishes 100, 1659–1671. doi:10.1007/s10641-017-0676-0
Quinn, T. P., Pess, G. R., Sutherland, B. J. G., Brenkman, S. J., Withler, R. E., Flynn, K., et al. (2021). Resumption of anadromy or straying? Origins of sockeye salmon in the Elwha River. Trans. Am. Fish. Soc. 150, 452–464. doi:10.1002/tafs.10294
Randle, T. J., Bountry, J. A., Ritchie, A., and Wille, K. (2015). Large-scale dam removal on the Elwha River, Washington, USA: erosion of reservoir sediment. Geomorphology 246, 709–728. doi:10.1016/j.geomorph.2014.12.045
Rist, L., Felton, A., Mårald, E., Samuelsson, L., Lundmark, T., and Rosvall, O. (2016). Avoiding the pitfalls of adaptive management implementation in Swedish silviculture. Ambio 45, 140–151. doi:10.1007/s13280-015-0750-9
Ritchie, A. C., Warrick, J. A., East, A. E., Magirl, C. S., Stevens, A. W., Bountry, J. A., et al. (2018). Morphodynamic evolution following sediment release from the world’s largest dam removal. Sci. Rep. 8, 13279. doi:10.1038/s41598-018-30817-8
Roux, D. J., and Foxcroft, L. C. (2011). The development and application of strategic adaptive management within South African National Parks. Koedoe Afr. Prot. Area Conserv. Sci. 53, 1–5. doi:10.4102/koedoe.v53i2.1049
Roux, D. J., Novellie, P., Smit, I. P. J., De Kraker, J., Culloch-Jones, S. M., Dziba, L. E., et al. (2022). Appraising strategic adaptive management as a process of organizational learning. J. Environ. Manage. 301, 113920. doi:10.1016/j.jenvman.2021.113920
Runge, M. C. (2011). An introduction to adaptive management for threatened and endangered species. J. Fish. Wildl. Manag. 2, 220–233. doi:10.3996/082011-JFWM-045
Runge, M. C., Converse, S. J., and Lyons, J. E. (2011). Which uncertainty? Using expert elicitation and expected value of information to design an adaptive program. Biol. Conserv. 144, 1214–1223. doi:10.1016/j.biocon.2010.12.020
Runge, M. C., Grand, J. B., and Mitchell, M. S. (2013). “Structured decision making,” in Wildlife management and conservation: contemporary principles and practices (Baltimore, Maryland: The John Hopkins University Press), 51–73.
Stankey, G. H., Clark, R. N., and Bormann, B. T. (2005). Adaptive management of natural resources: theory, concepts, and management institutions. Portland, OR: U.S. Department of Agriculture. Gen. Tech. Rep. PNW-GTR-654. doi:10.2737/PNW-GTR-654
Stanley, E. H., and Doyle, M. W. (2003). Trading off: the ecological effects of dam removal. Front. Ecol. Environ. 1, 15–22. doi:10.1890/1540-9295(2003)001[0015:TOTEEO]2.0.CO;2
Walters, C. J. (2007). Is adaptive management helping to solve fisheries problems? AMBIO J. Hum. Environ. 36, 304–307. doi:10.1579/0044-7447(2007)36[304:IAMHTS]2.0.CO;2
Walters, C. J., and Holling, C. S. (1990). Large-scale management experiments and learning by doing. Ecology 71, 2060–2068. –2068. doi:10.2307/1938620
Ward, L., Crain, P., Freymond, B., McHenry, M., Morrill, D., Pess, G. R., et al. (2008). Elwha River fish restoration plan, developed pursuant to the Elwha River ecosystem and fisheries restoration act, public Law 102–495. Seattle, WA: U.S. Dept. of Commerce, NOAA.
Warrick, J. A., Bountry, J. A., East, A. E., Magirl, C. S., Randle, T. J., Gelfenbaum, G., et al. (2015). Large-scale dam removal on the Elwha River, Washington, USA: source-to-sink sediment budget and synthesis. Geomorphology 246, 729–750. doi:10.1016/j.geomorph.2015.01.010
Washington Department of Fish and Wildlife (2012). Hatchery and genetic management plan (HGMP) - Elwha River Summer/Fall Chinook hatchery program. Olympia, WA: Washington Department of Fish and Wildlife.
Weinheimer, J., Anderson, J., Cooper, R., Williams, S., McHenry, M., Crain, P., et al. (2018). Age structure and hatchery fraction of Elwha River Chinook Salmon: 2017 carcass survey report. Olympia, WA: Washington Department of Fish and Wildlife.
Westgate, M. J., Likens, G. E., and Lindenmayer, D. B. (2013). Adaptive management of biological systems: a review. Biol. Conserv. 158, 128–139. doi:10.1016/j.biocon.2012.08.016
Wilhere, G. F. (2002). Adaptive management in habitat conservation plans. Conserv. Biol. 16, 20–29. doi:10.1046/j.1523-1739.2002.00350.x
Williams, B. K. (2011). Adaptive management of natural resources—framework and issues. J. Environ. Manage. 92, 1346–1353. doi:10.1016/j.jenvman.2010.10.041
Williams, B. K., and Brown, E. D. (2014). Adaptive management: from more talk to real action. Environ. Manage. 53, 465–479. doi:10.1007/s00267-013-0205-7
Wineland, S. M., Bașağaoğlu, H., Fleming, J., Friedman, J., Garza-Diaz, L., Kellogg, W., et al. (2022). The environmental flows implementation challenge: insights and recommendations across water-limited systems. WIREs Water 9. doi:10.1002/wat2.1565
Winter, B. D., and Crain, P. (2008). Making the case for ecosystem restoration by dam removal in the Elwha River, Washington. Northwest Sci. 82 (SP1), 13–28. doi:10.3955/0029-344X-82.S.I.13
Keywords: adaptive management, dam removal, Elwha River, Chinook salmon, steelhead trout, restoration
Citation: Peters RJ, Anderson JH, Duda JJ, McHenry M, Pess GR, Brenkman SJ, Johnson JR, Liermann MC, Denton KP, Beirne MM, Crain P and Connor HA (2024) Challenges of implementing a multi-agency monitoring and adaptive management strategy for federally threatened Chinook salmon and steelhead trout during and after dam removal in the Elwha River. Front. Environ. Sci. 12:1291265. doi: 10.3389/fenvs.2024.1291265
Received: 19 October 2023; Accepted: 04 March 2024;
Published: 19 July 2024.
Edited by:
Arnaldo Marín, University of Murcia, SpainReviewed by:
Jennifer Wilkening, United States Fish and Wildlife Service (USFWS), United StatesCopyright © 2024 Peters, Anderson, Duda, McHenry, Pess, Brenkman, Johnson, Liermann, Denton, Beirne, Crain and Connor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Roger J. Peters, cm9nZXJfcGV0ZXJzQGZ3cy5nb3Y=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.