
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 12 December 2023
Sec. Toxicology, Pollution and the Environment
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1326194
To evaluate the effects of polyurethane sponge biocarriers with precultured biofilms (PSBF) on water quality, shrimp performance and bacterial communities, zero water exchange systems for Litopenaeus vannamei were constructed. The experiment consisted of four treatments: 1) NBF (control, PSB (polyurethane sponge biocarriers) 5% (v/v) + aeration); 2) PSBF2.5a (PSBF 2.5% (v/v) + aeration); 3) PSBF5a (PSBF 5% (v/v) + aeration); and 4) PSBF5 (PSBF 5% (v/v)). The results showed that the systems with PSBFs had low levels of NH4+-N, NO2−-N, and turbidity, and there was zero water exchange throughout the entire culture process. The mean final weight, survival rate and final biomass of Litopenaeus vannamei in the PSBFs treatments were significantly higher, while the feed conversion ratio was lower than in the NBF treatments. The high-throughput sequencing results showed that the bacterial community abundance and diversity of biofilms in the PSBF systems were higher than those in the NBF systems. Four main genera of bacteria related to nitrification, Nitrosococcus, Nitrosomonas, Nitrococcus and Nitrospira, were identified. The total relative abundances of Nitrospira and Nitrosomonas in the PSBF systems were significantly higher than those in the NBF system. Meanwhile, better removal effects of NH4+-N and NO2−-N could be achieved and were positively correlated with the abundances of nitrifying microbial communities in PSBs, further indicating that nitrifying microbial communities in PSBs had positive effects on water quality and shrimp productive performance. This study provides data to support the application of polyurethane sponge biocarriers with precultured biofilms in zero water exchange systems for L. vannamei culture.
With the development of intensive and factory-scale production in the aquaculture of Litopenaeus vannamei, the accumulation of pollutants within aquaculture systems, deterioration of water quality, and outbreaks of diseases have become increasingly severe (Defoirdt et al., 2011). The accumulation of inorganic nitrogen, especially NH4+-N and NO2−-N, is poisonous to L. vannamei during the breeding process (Piedrahita, 2003; Blancheton et al., 2013). Controlling the nitrogen content in culture water is significant for decreasing the damage caused by NH4+-N and NO2−-N to L. vannamei (Xu et al., 2020).
Biofilms attached to biocarriers in aquaculture systems allow microorganisms that carry out nitrification to adhere to the films (Ferreira et al., 2016; Ahmad et al., 2017; Fan et al., 2019), and these microorganisms can effectively eliminate nitrogen-containing compounds (Blancheton et al., 2013), particularly ammonia and nitrite, which have high toxicity. This enhances the survival rate and growth rate of L. vannamei. Biofilms can also serve as additional food sources for L. vannamei, providing necessary nutrients, such as unsaturated fatty acids, amino acids and vitamins. The microorganisms that carry out nitrification are autotrophic aerobiotic bacteria (Song et al., 2023). They have a low rate of growth and reproduction, have long generation cycles and are easily influenced by the environment and the properties of the biofilms to which they are attached. Therefore, it is difficult for them to compete with other bacteria in water to reach relatively high concentrations and maintain relatively high levels of activity, so it is necessary to choose proper biocarriers to which they can attach and form biofilms (Wang et al., 2023). An ideal carrier should have features such as a large specific surface area, high porosity, low density, ready bacterial attachment, and low cost. Performance is estimated by the biofilm formation time, processing efficiency, biofilm stability and other indicators. Polyurethane sponge biocarriers (PSBs) have advantages such as large specific surface areas, high porosity, low density, ready bacterial attachment, ease of recycling, etc., and are one kind of biocarrier that should be prioritized in biological treatment systems (Han et al., 2022).
In this study, a precultured biofilm system was adopted to allow the microorganisms to dominate the bacterial community present on the surface of PSBs. However, the matrix of the aquaculture system for L. vannamei is complex, and the bacterial community structure is affected by water quality, the bacterial community of L. vannamei, management measures and other factors (Satanwat et al., 2020). Therefore, investigating the bacterial community structure in biofilms is valuable for understanding water purification processes in aquaculture systems and determining related management and maintenance procedures.
In this study, zero water exchange systems for L. vannamei were constructed by using polyurethane sponge biocarriers with precultured biofilms (PSBFs), and the role of PSBFs in controlling inorganic nitrogen and suspended solids and on the performance of L. vannamei was determined. High-throughput sequencing was adopted to further explore changes in bacterial community structure and the relationship between the bacterial community structure of biofilms and adsorbates in a zero water exchange system with in situ water purification, the results of which elucidate the active mechanisms and processes regulating aquaculture systems.
The aquaculture system for L. vannamei consisted of a plastic bucket, polyurethane sponge biocarriers, a temperature-controlling device and an aeration device. First, 80.00 L of artificial seawater was added to the plastic bucket, the temperature and DO in the plastic bucket were monitored every day, the temperature-controlling device was adjusted to maintain a temperature of 26°C–28°C, and the aeration device was adjusted to maintain DO levels of 6.50–8.50 mg/L. Artificial seawater (Qingdao Haizhixuan Aquarium Supplies Co., Ltd.) was artificially prepared, with a salinity of 18‰.
L. vannamei was purchased from Guangdong Haida Group Co., Ltd. (Guangzhou, China), with an average body length of 0.80 ± 0.10 cm and an average weight of 0.008 ± 0.001 g. They were used in the experiment after 18 days of breeding. The L. vannamei was adapted to the salinity range used in the experiment. The shrimp used in the experiment had an average body length of 1.60 ± 0.10 cm and an average weight of 0.10 ± 0.001 g. The initial density of shrimp was 800 shrimp/m3.
First, 80.00 L of artificial seawater at 18 practical salinity units (psu) and 24.00 L of PSBs (specification: 2 cm × 2 cm × 2 cm, porosity: 98%) were placed in a round bucket containing. The seawater contained 1% (v/v) nitrifying bacteria preparation (Qingdao Seadoctor Co., Ltd., Qingdao, China), 0.01% (w/v) yeast extract and 0.1% (v/v) trace element solution which consisted of MnCl2∙4H2O (14.85 mg/L), ZnSO4∙7H2O (0.287 mg/L), FeCl3 (5380 mg/L), CuSO4∙5H2O (7.5 mg/L), Na2MoO4∙2H2O (6.8 mg/L), CoCl2·6H2O (12 mg/L), EDTA (2.4 mg/L) and NiSO4 (2.4 mg/L). The PSBs floated freely in seawater. Second, 4.00 g of NaNO2 was added to make the concentration of NO2−-N reach 10.00 mg/L. When the concentration of NO2−-N was lower than 0.05 mg/L, 4.00 g of NaNO2 was added again, and the process was repeated 5 times. Finally, 0.31 g of NH4Cl was added to make the concentration of NH4+-N reach 10.00 mg/L. When the concentration of NH4+-N was lower than 0.05 mg/L, 0.31 g of NH4Cl was added again, and the process was repeated 5 times. The temperature and DO were controlled at 27–29°C and 4.50–6.00 mg/L in the process of biofilm cultivation, respectively (Wang Y. et al., 2022).
There were 4 aquaculture systems for L. vannamei, with three replicates and 80.00 L artificial seawater in each aquaculture system. The entire experiment lasted 66 days Table 1 shows the treatment details. For naturally formed biofilm treatment, PSBs were directly added to the aquaculture systems without pretreatment, but for the precultured biofilms, the PSBs were pretreated before use in the systems. Feed was provided 4 times every day at 8:00, 12:00, 16:00, and 20:00, and the amount of feed was based on 8.50% of the shrimp total weight. The mesh bag containing PSBs was removed every 10–15 days, and the materials adsorbed in the pores were cleared to recover the biocarrier adsorption capacity. The PSBs were gently squeezed during the cleaning process to avoid damaging the nitrifying biofilm. Volatilized water was added to the culture systems every day.
The parameters of all aquaculture systems were measured daily during the morning between 8:00 and 9:00 h for 66 days. The water temperature, pH, and salinity were measured directly using a Mercury thermometer, a digital pH meter (pH 610, Wiggens Company, Germany), and a Mercury pycnometer, respectively. Dissolved oxygen (DO) and turbidity were measured by a portable hand-held dissolved oxygen meter (HQ30D, Hach Company, USA) and portable turbidimetry (2100P, Hach Company, USA), respectively.
NH4+-N and NO2−-N were measured by Nessler’s reagent spectrophotometry and N-(1-naphthyl)-ethylenediamine spectrophotometry, respectively. NO3−-N was determined by ultraviolet spectrophotometry method and the total phosphorus (TP) was determined by the molybdenum-antimony antispectrophotometric method every 72 h.
Litopenaeus vannamei were harvested after draining the tanks. The mean final weight, survival rate, final biomass, and feed conversion ratio (FCR) were determined according to Hoang et al. (Hoang Manh et al., 2020). The specific growth rate (SGR) of shrimp was determined according to Powell et al. (Powell et al., 2020).
On day 66 of the experiment, PSBs were randomly selected from the NBF, PSBF2.5a, PSBF5a and PSBF5 systems and washed several times with sterilized water to obtain the suspension of adsorbates. The suspension was centrifuged, and the supernatant was removed to obtain adsorbate samples, which were successively denoted by NBF_A, PSBF25a_A, PSBF50a_A and PSBF50_A. The cleaned PSBs were cut and immersed in sterilized water again. Then, they were placed in ultrasonic cleaners (QTSXR20500, Tianjin Ruipu Electronic Instrument Company, China) for 15 min to obtain a biofilm suspension. The suspension was centrifuged, and the supernatant was removed to obtain the biofilm samples, which were successively denoted by NBF_M, PSBF25a_M, PSBF50a_M and PSBF50_M. Additionally, the initial biofilm sample of precultured polyurethane sponges was denoted by initial biofilms in sponges (IF).
Total DNA was extracted from the above samples using an E.Z.N.A. Soil DNA Kit (OMEGA Biotek, Norcross, USA) according to the kit instructions. 16S rRNA sequencing was performed using the MiSeq sequencing system of the Illumina platform (Shanghai Meiji Biotechnology Co., Ltd., Shanghai, China). QIIME software was used to eliminate sequences with a length of less than 150 bp and chimeras in the original sequence, and then high-quality sample sequences were obtained. The Uparse platform was used to cluster the high-quality sequences at the 97% similarity level to obtain the operational taxonomic units (OTUs) table. The sequence with the highest richness was selected as the representative sequence of the OTUs, and the ribosomal database project classifier Bayesian algorithm was used to count the community species composition in the OTU representative sequence. The comparison library was Silva138/16s_bacteria. The alpha and beta diversity analyses were carried out by the Shengxinyun platform (https://www.majorbio.com).
A series of one-way ANOVAs were performed, followed by Fisher’s LSD test to determine the differences among the systems for water quality parameters and the growth parameters of shrimp (p < 0.05). Data are expressed as the mean ± standard deviation. All statistical analyses were conducted using SPSS, version 26.0.
The water quality parameters of the experimental systems are presented in Table 2. The temperature, DO, and pH values were all within suitable ranges for the growth of L. vannamei (Chakravarty et al., 2016). Litopenaeus vannamei is a euryhaline species, which means the salinity range of the environment they can adapted to is wide (Li et al., 2017). The L. vannamei was adapted to the salinity in the experiment. The pH value of the NBF system was significantly higher than that of the PSBF system (p < 0.05), while among the PSBF2.5a, PSBF5a and PSBF5 systems, there were no significant differences. However, the TP concentration in the PSBF system was significantly higher than that in the NBF system (p < 0.05). The NBF system had the lowest turbidity, followed by the PSBF5a, PSBF2.5a and PSBF5 systems.
Among all the aquaculture systems, NH4+-N consistently remained at low concentrations (less than 0.35 mg/L), but the concentration in the NBF system was significantly higher than that in the PSBF system (p < 0.05). The NH4+-N concentration in the PSBF5a system was consistently lower than 0.08 mg/L. The peak concentration of NH4+-N in the PSBF5a system was 33.33% and 61.70% lower than that of the PSBF2.5a and PSBF5 systems, respectively. There were significant differences in NO2−-N concentration among the different aquaculture systems (p < 0.05), and the average concentration of NO2−-N in the NBF system was the highest (4.42 mg/L), while that in the PSBF5a system was lowest (0.81 mg/L). The average concentration of NO3−-N in the PSBF system was significantly higher than that in the NBF system (p < 0.05) and that in the PSBF5 system was significantly higher than that in the PSBF2.5a and PSBF5 systems, while there was no significant difference between the PSBF2.5a and PSBF5 systems.
There were significant differences in mean final weight, survival rate, final biomass, FCR and SGR among the treatments (Table 3). The survival rate, average weight, and yield of L. vannamei in the PSBF systems were significantly higher than those in the NBF system (p < 0.05), while there was no significant difference among the different PSBF systems. The PSPF5a system had the highest survival rate, average weight, and yield, followed by the PSBF2.5a, PSBF5 and NBF systems. The feed conversion ratio (FCR) of the PSBF systems was significantly lower than that of the NBF system (p < 0.05), while there was no significant difference among the different PSBF systems. The difference in the specific growth rate (SGR) between the PSBF and NBF systems was not significant. The average weight, survival rate and yield of the shrimp in the PSBF2.5a, PSBF5a and PSBF5 systems were higher than those in the NBF system. The average weight, survival rate and yield of the shrimp in the NBF system were 9.19%–16.21%, 17.95%–21.33% and 3.57%–11.86%, lower than those in the PSBF2.5a, PSBF5a and PSBF5 systems, respectively, while the FCR was 27.22%, 28.85% and 26.42% higher, respectively.
Table 4 shows the number of OTUs and alpha diversity index of the biofilm and adsorbate sample in different aquaculture systems. A total of 5711 OTUs were found in 9 samples, containing 406–839 per sample, and the coverage rate was 99.14%–99.81%. The ranges of the ACE index and Chao index were 463–903 and 458–893, respectively; among them, the bacterial community abundance of the IF sample was the lowest, while that of the PSBF50a_M sample was the highest. The ranges of the Shannon and Simpson indices were 3.11–4.50 and 0.024–0.141, respectively, and the bacterial community diversity of the PSBF50a_M sample was the highest, while that of the PSBF50_A sample was the lowest. The bacterial community abundance and diversity of the biofilm sample were both higher than those of the adsorbate sample in different aquaculture systems.
The PCoA results of the 9 samples are shown in Figure 1. The results show that at the OTU level, the contribution ratio of PC1 was 40.70%, while that of PC2 was 23.22%, which indicated that PC1 had a greater effect on the difference in bacterial community composition. There were significant differences in bacterial community composition between IF and other samples, while there were no differences among samples in the same aquaculture systems. The PSBF50a (PSBF50a_M, PSBF50a_A) and PSBF50 systems (PSBF50_M, PSBF50_A) had similar bacterial community compositions. The bacterial community structure of the PSBF2.5a system (PSBF25a_M, PSBF25a_A) was similar to that of the NBF system (NBF_M, NBF_A). There were significant differences in bacterial community structure between the PSBF2.5a system and the PSBF50a system. Therefore, the concentration of carrier has a great influence on the bacterial community structure in the L. vannamei culture system.
A Venn diagram can reflect the number of shared and special OTUs among different samples and is used to analyze similarities and differences in bacterial communities among samples (Figure 2). The number of shared OTUs among the 9 samples was 44, accounting for 2.34% of the total OTUs. The number of special OTUs in the IF sample was the largest (accounting for 31.90%), and that in the PSBF50_M sample was the smallest (accounting for 0.48%). The number of special OTUs in biofilms was larger than that of adsorbates in the aquaculture systems, except for the PSBF50 system.
Bacterial phyla with relative abundances higher than 1.00% were analyzed, and the results are shown in Figure 3. The dominant bacteria were Proteobacteria, Bacteroidetes, Desulfobacterota, Chloroflexi, and Acidobacteriota, and the relative abundance ranged from 75.81% to 96.05%. The dominant bacteria in NBF_A and PSBF50_M were Bacteroidetes (40.80%) and Chloroflexi (27.94%), respectively, and those in the other samples were Proteobacteria (40.12%–71.46%). The subdominant bacteria in NBF_A and PSBF50_M were Proteobacteria, and the relative abundance was 27.84%, which was slightly different from that of Chloroflexi. The subdominant bacteria in PSBF50_A was Desulfobacterota (35.79%), and those in the other 6 samples were Bacteroidetes (12.02%–40.80%). Nitrospirota was detected in PSBF50_M, PSBF25a_M, PSBF25a_A, NBF_M and NBF_A, and Nitrospirota had the highest relative abundance (5.61%) in PSBF25a_M.
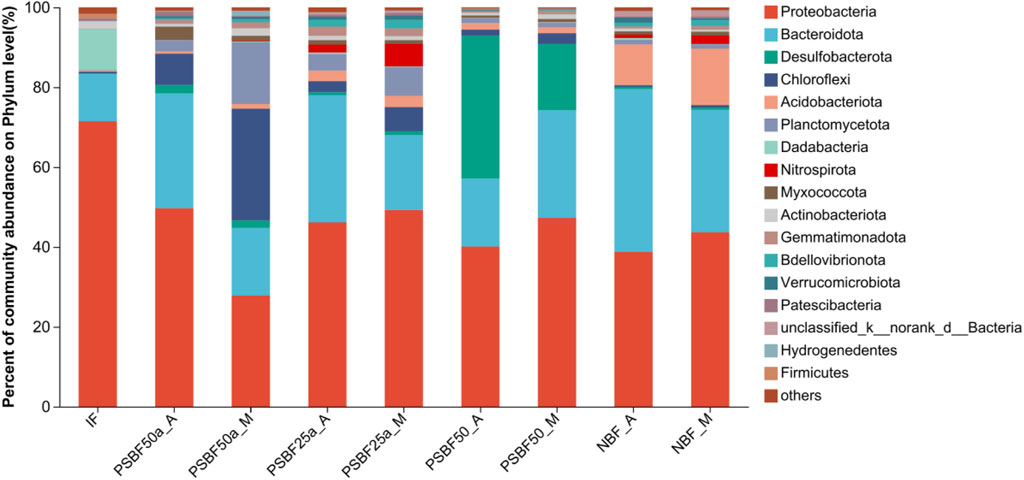
FIGURE 3. The diversity and relative abundance of the bacterial community in the biofilms and adsorbates at the phylum level.
The results of bacterial community class analysis in biofilm and adsorbent samples are shown in Figure 4. The figure shows that the relative abundances of Gammaproteobacteria, Bacteroidia and Alphaproteobacteria in all the samples were higher than 3%. Desulfuromonadia had a relative abundance higher than 10% in both PSBF50_M and PSBF50_A, but its relative abundance was lower than 2% in the other samples. Anaerolineae had a low relative abundance (<1.00%) in NBF_M and NBF_A but had a higher relative abundance (>1.00%) in the other samples. Desulfuromonadia and Anaerolineae were not detected in IF. The relative abundances of Nitrospiria in PSBF25a_M, PSBF25a_A and NBF_M were higher than 2.00%, while those in PSBF50a_M and NBF_A were lower than 1.00%.

FIGURE 4. The diversity and relative abundance of bacterial community in the biofilms and adsorbates at the class level.
The bacterial community in the biofilm and adsorbates belonged to a total of 629 genera. The top 50 genera by proportion were clustered based on their relative abundances and similarity among samples, as shown in Figure 5. The dominant genera included Hyphomicrobium, Halomonas, norank_f__A4b, SM1A02, Ruegeria, Acanthopleuribacter, Cellvibrio, Bowmanella, Pseudoalteromonas, Tenacibaculum, etc. Compared with those in the initial biofilm sample (IF), the relative abundances of norank_f__A4b, SM1A02, Ruegeria, and Acanthopleuribacter in the other samples increased, and the relative abundances of Halomonas and Hyphomicrobium significantly decreased (p < 0.05). The total relative abundance of Cellvibrio and Bowmanella in the biofilms of the PSBF systems increased by 58.5% compared with that in the biofilms of the NBF system, while the total relative abundances of Pseudoalteromonas and Tenacibaculum in the biofilms of the PSBF systems decreased by 59.3% compared with that in the biofilms of the NBF system.
In the present study, four genera of bacteria related to nitrification, Nitrosococcus, Nitrosomonas, Nitrococcus and Nitrospira, were identified. The relative abundances of Nitrosococcus and Nitrococcus in IF were more plentiful than those in the other samples. Moreover, the relative abundance of Nitrosococcus in IF was 4.16%, while that in the other samples was less than 0.01%. The relative abundances of Nitrospira and Nitrosomonas in the PSBF systems were 10.87% and 17.30%, respectively, which were significantly higher than those in the NBF system with values of 0.03% and 17.30% (p < 0.05).
FAPROTAX is a database that includes functional information for the classification of prokaryotic species to predict the functions of bacteria, including their roles in the carbon, nitrogen, hydrogen, and sulfur cycles. To investigate the distribution and relative abundance of the nitrifying bacterial community in the biofilm and adsorbate of the polyurethane sponges in different aquaculture systems, FAPROTAX functional analysis was performed to estimate the differences between the two communities, and the results are shown in Figure 6.
Fifty-nine kinds of functions were found in the bacterial community of the biofilm and adsorbate of the polyurethane sponge, among which chemoheterotrophy and aerobic chemoheterotrophy had the highest relative abundance, and the sum of the relative abundance of these functions ranged from 46.42% to 76.99%. There were 11 functions related to nitrogen cycling, including nitrate reduction, nitrogen respiration, nitrate respiration, nitrite respiration, nitrate denitrification, nitrite denitrification, nitrous oxide denitrification, denitrification, nitrification, aerobic nitrite oxidation and aerobic ammonia oxidation. The relative abundances of bacteria with nitrification (5.32%), aerobic nitrite oxidation (2.64%) and aerobic ammonia oxidation (2.68%) functions in the IF sample were significantly higher than those in the other samples. The relative abundance of bacteria with functions related to nitrogen cycling in the PSBF systems (0.63%–3.08%) was higher than that in the NFB system (0.02%–1.91%), indicating that the precultured biofilm system had obvious advantages in nitrogen cycling. The relative abundances of nitrification and aerobic nitrite oxidation groups in the biofilms were higher than those in the adsorbates. The relative abundance of functions related to nitrogen cycling in PSBF50a_M increased by 76.19% compared with that in PSBF50_M, and the relative abundance of functions related to nitrogen cycling in PSBF25a_M decreased by 61.90% compared with that in PSBF50a_M.
Water quality is considered a major limiting factor for shrimp survival, especially in terms of pH and DO, NH4+-N and NO2−-N concentrations (Santacruz-Reyes and Chien, 2012). Ammonia and nitrite are the main targets for removal in aquaculture. In this study, the concentrations of NH4+-N in all systems remained within the recommended range for shrimp growth (Valencia-Castañeda et al., 2018). The PSBFs significantly improved the removal efficiency of NH4+-N and NO2−-N. The results showed that the concentration of NH4+-N in the PSBF systems decreased by 37.93%, 50.86% and 40.52%, respectively, compared with that in the NBF system. Moreover, the concentration of NO2−-N in the PSBF systems decreased by 67.19%, 81.67% and 74.43%, respectively, compared with that in the NBF system. Meanwhile, the shrimp survival rates and final biomass in the PSBF systems significantly increased compared with those in the NBF system. Furthermore, the survival rate and final biomass of shrimp were negatively correlated with NH4+-N, with correlation coefficients of 1.00 and 0.80, respectively. In addition, they were also negatively correlated with NO2−-N concentrations, with correlation coefficients of 1.00 and 0.82 (Figure 7A), which also demonstrated that the PSBF systems had an advantage of purifying water quality, and thus promoted the growth of shrimp.
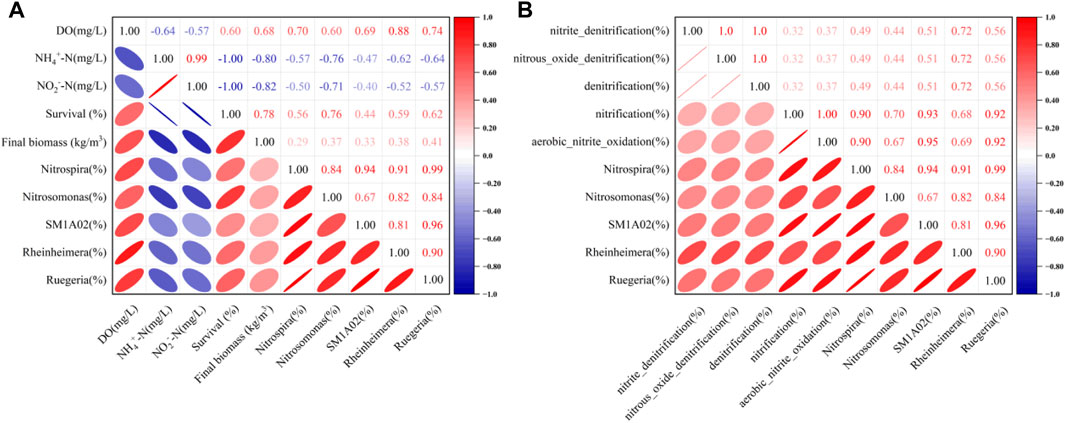
FIGURE 7. Relationship of bacterial communities, environmental factors and shrimp growth parameters (A) and relationship between bacterial communities and nitrogen functions (B).
The dominant phyla in the biofilms on the PSBs and those in the adsorbents in different aquaculture systems were Proteobacteria, Bacteroidetes, Desulfobacteria, Chloroflexi and Acidobacteria. Hou et al. (Hou et al., 2018) and Wang et al. (Wang et al., 2019) found that in L. vannamei culture, the major phyla were Proteobacteria, Bacteroidetes, Chloroflexi, Fusobacteria, Cyanobacteria, Firmicutes, Tenericutes, Acidobacteria, Planctomycetes, Actinobacteria, Verrucomicrobia, and Gemmatimonadetes. Meanwhile, there were significant differences in the relative abundance of phyla under different environmental factors of L. vannamei culture (Hou et al., 2018; Wang et al., 2019), which is consistent with the results of our study.
Proteobacteria is a dominant bacteria in many aquaculture systems, and it can decompose organic substances, degrade COD, and purify water (Shu et al., 2015). Gammaproteobacteria, Alphaproteobacteria, and Bacteroidia were dominant in the four aquaculture systems. Most Alphaproteobacteria are photoorganoheterotrophic bacteria that can decompose ammonia and organics containing carbon and sulfur and can live in various complex environments (Gérard et al., 2018; Degli Esposti et al., 2019). According to recent studies, ammonia-oxidizing bacteria and nitrite-oxidizing bacteria involved in nitrification mostly belong to Proteobacteria (Brenner et al., 2001; Wang et al., 2022). Bacteroidetes is a kind of chemoheterotrophic bacteria that can decompose carbohydrates, proteins, and other dissolved organics (Nie et al., 2019). It can also use NO2−-N and NO3−-N as electron acceptors for anaerobic respiration under anaerobic conditions (Wang et al., 2022). Many bacteria with nitrogen removal functions belong to Proteobacteria or Bacteroidetes, which play important roles in nitrogen removal from wastewater (Guo et al., 2017; Luan et al., 2023). In the PSBF systems, the relative abundance of Proteobacteria and Bacteroidetes was above 10.00%, and the sum of their relative abundance in the PSBF systems was higher than that in the NBF system, which was consistent with the results that the NH4+-N and NO2−-N concentrations in the NBF system were higher than those in the PSBF systems. In addition, the survival rates and final biomass of shrimp in the PSBF systems were significantly higher than those in the NBF system. The above results showed that Proteobacteria and Bacteroidetes could improve the quality of aquaculture water and the growth and survival rate of L. vannamei.
Genus-level analysis results showed that the bacterial genera were diverse in each sample, and there were significant differences in relative abundance among them. Dominant genera with functions related to nitrogen cycling include Nitrosomonas, Nitrospira, Ruegeria, Rheinheimera and SM1A02. According to the correlation coefficient between bacterial communities and nitrogen functions (Figure 7B), the dominant genera with nitrification functions were Nitrosomonas, Nitrospira and SM1A02, while the dominant genera with denitrification functions were Ruegeria and Rheinheimera. Nitrosococcus, Halomonas and Nitrococcus were the dominant bacteria in IF, but their relative abundances were less than 0.10%, which was greatly influenced by the environment of the aquaculture system (Gao et al., 2020). Nitrosomonas is a typical ammonia-oxidizing bacteria (AOB) responsible for the nitrification of NH4+-N and NO2−-N (He et al., 2020; Moschos et al., 2022). SM1A02 is involved in nitrification, denitrification, anammox and other nitrogen cycling processes (Vico et al., 2021; Huang et al., 2022). Ruegeria has the function of both nitrification and denitrification, which is a common denitrifier with a complete gene set for denitrification in saline conditions (Lin et al., 2022). In the present study, the relative abundances of Nitrosomonas Nitrospira, Ruegeria, Rheinheimera and SM1A02 in the PSBF systems significantly increased compared with those in the NBF system. The sum of the relative abundance of Nitrosomonas, Nitrospira, Ruegeria, Rheinheimera and SM1A02 was higher in the PSBF50a system (52.45%) than in the PSBF25a system (5.63%) or PSBF50 (17.88%).
In addition, the NH4+-N and NO2−-N concentrations were lower, while the survival rate and final biomass of L. vannamei were higher in the PSBF systems than in the NBF system. According to the results, the relative abundances of Nitrospira, Ruegeria, Rheinheimera and SM1A02 in the four systems positively influenced the survival rates and final biomass of L. vannamei, in contrast, the concentrations of NH4+-N and NO2−-N were negatively correlated with the relative abundances of Nitrospira, Ruegeria, Rheinheimera and SM1A02 (Figure 7A). Therefore, Nitrosomonas, Nitrospira, Ruegeria, Rheinheimera and SM1A02 could contribute to the growth of shrimp and the removal of NH4+-N and NO2−-N. Moreover, in the PSBF50a system, better removal effects of NH4+-N and NO2−-N and higher survival rate and biomass of L. vannamei could be achieved. The results indicated that the increase in PSB concentration and aeration were beneficial to the growth of nitrifying and denitrifying bacteria, as well as the removal of NH4+-N and NO2−-N in the zero water exchange system and the growth of shrimp.
In this study, PSBFs were used to construct zero water exchange systems for L. vannamei. The results demonstrated that high efficiency of NH4+-N and NO2−-N removal through nitrification could be achieved by supplementation with PSBFs. Increasing the biocarrier dosing ratio and installing built-in aeration were helpful for eliminating NH4+-N and NO2−-N in the aquaculture system. The final biomass, survival rate, mean final weight and feed conversion rate were higher in the PSBF systems (PSBF2.5a, PSBF5a and PSBF5) than in the NBF system. High-throughput sequencing results showed that the abundances of nitrifying microbial communities were higher in the PSBF systems than in the NBF system. Meanwhile, better removal effects of NH4+-N and NO2−-N could be achieved and were positively correlated with the abundances of nitrifying microbial communities in PSBs, further indicating that nitrifying microbial communities in PSBs had positive effects on water quality and shrimp productive performance.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
CL: Methodology, Writing–original draft, Writing–review and editing. LC: Writing–review and editing. AX: Resources, Writing–review and editing. ZS: Funding acquisition, Methodology, Supervision, Writing–review and editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Key R&D projects of Shandong Province (2018GSF117022), and the Natural Science Foundation of Shandong Province (ZR2020QC027).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmad, I., Babitha Rani, A. M., Verma, A. K., and Maqsood, M. (2017). Biofloc technology: an emerging avenue in aquatic animal healthcare and nutrition. Aquac. Int. 25, 1215–1226. doi:10.1007/s10499-016-0108-8
Blancheton, J. P., Attramadal, K. J. K., Michaud, L., d’Orbcastel, E. R., and Vadstein, O. (2013). Insight into bacterial population in aquaculture systems and its implication. Aquac. Eng. 53, 30–39. doi:10.1016/j.aquaeng.2012.11.009
Brenner, D. J., Krieg, N. R., Staley, J. T., et al. (2001). Bergey’s manual of systematic bacteriology. Boston, MA: Springer.
Chakravarty, M. S., Ganesh, P. R. C., Amarnath, D., et al. (2016). Spatial variation of water quality parameters of shrimp (Litopenaeus vannamei) culture ponds at Narsapurapupeta, Kajuluru and Kaikavolu villages of East Godavari district, Andhra Pradesh. Int. J. Fish. Aquatic Stud. 4 (4), 390–395.
Defoirdt, T., Sorgeloos, P., and Bossier, P. (2011). Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 14 (3), 251–258. doi:10.1016/j.mib.2011.03.004
Degli Esposti, M., Mentel, M., Martin, W., and Sousa, F. L. (2019). Oxygen reductases in alphaproteobacterial genomes: physiological evolution from low to high oxygen environments. Front. Microbiol. 10, 499. doi:10.3389/fmicb.2019.00499
Fan, L., Wang, Z., Chen, M., Qu, Y., Li, J., Zhou, A., et al. (2019). Microbiota comparison of Pacific white shrimp intestine and sediment at freshwater and marine cultured environment. Sci. Total Environ. 657, 1194–1204. doi:10.1016/j.scitotenv.2018.12.069
Ferreira, L. M. H., Lara, G., Wasielesky, W., and Abreu, P. C. (2016). Biofilm versus biofloc: are artificial substrates for biofilm production necessary in the BFT system? Aquac. Int. 24, 921–930. doi:10.1007/s10499-015-9961-0
Gao, Y. S., Wang, X. P., Li, J. L., Lee, C. T., Ong, P. Y., Zhang, Z., et al. (2020). Effect of aquaculture salinity on nitrification and microbial community in moving bed bioreactors with immobilized microbial granules. Bioresour. Technol. 297, 122427. doi:10.1016/j.biortech.2019.122427
Gérard, E., Goeyse, S. D., Hugoni, M., Agogué, H., Richard, L., Milesi, V., et al. (2018). Key role of Alphaproteobacteria and Cyanobacteria in the formation of stromatolites of lake dziani dzaha (Mayotte, western Indian ocean). Front. Microbiol. 9, 796. doi:10.3389/fmicb.2018.00796
Guo, J., Ni, B. J., Han, X., Chen, X., Bond, P., Peng, Y., et al. (2017). Unraveling microbial structure and diversity of activated sludge in a full-scale simultaneous nitrogen and phosphorus removal plant using metagenomic sequencing. Enzyme Microb. Technol. 102, 16–25. doi:10.1016/j.enzmictec.2017.03.009
Han, D., Hu, Z., Li, D., and Tang, R. (2022). Nitrogen removal of water and sediment in grass carp aquaculture ponds by mixed nitrifying and denitrifying bacteria and its effects on bacterial community. Water 14 (12), 1855. doi:10.3390/w14121855
He, Q. L., Song, J. Y., Zhang, W., Gao, S., Wang, H., and Yu, J. (2020). Enhanced simultaneous nitrification, denitrification and phosphorus removal through mixed carbon source by aerobic granular sludge. J. Hazard. Mater. 382, 121043. doi:10.1016/j.jhazmat.2019.121043
Hoang Manh, N., Nguyen Phuoc, N., and Peter, B. (2020). Water quality, animal performance, nutrient budgets and microbial community in the biofloc-based polyculture system of white shrimp, Litopenaeus vannamei and gray mullet. Mugil cephalus. Aquaculture, 515. 734610. doi:10.1016/j.aquaculture.2019.734610
Hou, D. W., Huang, Z. J., Zeng, S. Z., Liu, J., Weng, S., and He, J. (2018). Comparative analysis of the bacterial community compositions of the shrimp intestine, surrounding water and sediment. J. Appl. Microbiol. 125 (3), 792–799. doi:10.1111/jam.13919
Huang, S., Zhang, J. R., Wang, C. Q., Zhu, G., and Hassan, M. (2022). Weak electric field effect of MFC biocathode on denitrification. J. Environ. Chem. Eng. 10 (6), 108596. doi:10.1016/j.jece.2022.108596
Li, E. C., Wang, X. D., Chen, K., Xu, C., Qin, J. G., and Chen, L. (2017). Physiological change and nutritional requirement of Pacific white shrimp Litopenaeus vannamei at low salinity. Rev. Aquac. 9 (1), 57–75. doi:10.1111/raq.12104
Lin, X., Mcnichol, J., Chu, X., Qian, Y., and Luo, H. (2022). Cryptic niche differentiation of novel sediment ecotypes of Rugeria pomeroyi correlates with nitrate respiration. Environ. Microbiol. 24, 390–403. doi:10.1111/1462-2920.15882
Luan, Y., Wang, Y., Liu, C., Lv, L., Xu, A., and Song, Z. (2023). Effects of potassium monopersulfate on nitrification activity and bacterial community structure of sponge biocarrier biofilm in Litopenaeus vannamei aquaculture system. Environ. Technol., 1–13. doi:10.1080/09593330.2023.2215455
Moschos, S., Kormas, K. A., and Karayanni, H. (2022). Prokaryotic diversity in marine and freshwater recirculating aquaculture systems. Rev. Aquac. 14 (4), 1861–1886. doi:10.1111/raq.12677
Nie, Y., Chen, R., Tian, X., and Li, Y. Y. (2019). Characterization of the effect of surfactant on biomass adaptation and microbial community in sewage treatment by anaerobic membrane bioreactor. J. Industrial Eng. Chem. 76, 268–276. doi:10.1016/j.jiec.2019.03.051
Piedrahita, R. H. (2003). Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 226 (1), 35–44. doi:10.1016/s0044-8486(03)00465-4
Powell, C. D., Tansil, F., France, J., et al. (2020). Growth trajectory analysis of Pacific whiteleg shrimp (Litopenaeus vannamei): comparison of the specific growth rate, the thermal-unit growth coefficient and its adaptations. Aquaculture Research. 51. 480-489. doi:10.1111/are.14391
Santacruz-Reyes, R. A., and Chien, Y. H. (2012). The potential of Yucca schidigera extract to reduce the ammonia pollution from shrimp farming. Bioresour. Technol. 113, 311–314. doi:10.1016/j.biortech.2012.02.132
Satanwat, P., Tran, T. P., Hirakata, Y., Watari, T., Hatamoto, M., Yamaguchi, T., et al. (2020). Use of an internal fibrous biofilter for intermittentnitrification and denitrification treatments in a zero-discharge shrimp culture tank. Aquac. Eng. 88, 102041. doi:10.1016/j.aquaeng.2019.102041
Shu, D. T., He, Y. L., Yue, H., and Wang, Q. (2015). Microbial structures and community functions of anaerobic sludge in six full-scale wastewater treatment plants as revealed by 454 high-throughput pyrosequencing. Bioresour. Technol. 186, 163–172. doi:10.1016/j.biortech.2015.03.072
Song, Z., Liu, C., Luan, Y., Qi, Y., and Xu, A. (2023). Effect of zero water exchange systems for Litopenaeus vannamei using sponge biocarriers to control inorganic nitrogen and suspended solids simultaneously. Sustainability 15 (2), 1271. doi:10.3390/su15021271
Valencia-Castañeda, G., Frías-Espericueta, M. G., Vanegas-Pérez, R. C., Pérez-Ramírez, J. A., Chávez-Sánchez, M. C., and Páez-Osuna, F. (2018). Acute toxicity of ammonia, nitrite and nitrate to shrimp Litopenaeus vannamei postlarvae in low-salinity water. Bull. Environ. Contam. Toxicol. 101, 229–234. doi:10.1007/s00128-018-2355-z
Vico, P., Iriarte, A., Bonilla, S., and Piccini, C. (2021). Metagenomic analysis of Raphidiopsis raciborskii microbiome: beyond the individual. Biodivers. Data J. 9, e72514. doi:10.3897/bdj.9.e72514
Wang, J., Huang, Y. J., Xu, K. H., Zhang, X., Sun, H., Fan, L., et al. (2019). White spot syndrome virus (WSSV) infection impacts intestinal microbiota composition and function in Litopenaeus vannamei. Fish Shellfish Immunol. 84, 130–137. doi:10.1016/j.fsi.2018.09.076
Wang, L., Mao, X., Hamoud, Y. A., Zhu, N., Shao, X., Wang, Q., et al. (2023). Nitrogen removal for low concentration ammonium wastewater by adsorption, shortcut simultaneous nitrification and denitrification process in MBBR. Water 15 (7), 1334. doi:10.3390/w15071334
Wang, M., Fan, Z., Wang, R., Liu, Z., Gao, F., Zhang, Z., et al. (2022b). Nitrogen removal performance, and microbial community structure of water and its association with nitrogen metabolism of an ecological engineering pond aquaculture system. Aquac. Rep. 25, 101258. doi:10.1016/j.aqrep.2022.101258
Wang, Y., Sun, M., Tang, Y., Xu, A., Tang, J., and Song, Z. (2022a). Effects of Haematococcus pluvialis on the water quality and performance of Litopenaeus vannamei using artificial substrates and water exchange systems. Aquac. Int. 30 (4), 1779–1797. doi:10.1007/s10499-022-00872-0
Keywords: zero water exchange aquaculture systems, water quality, bacterial community structure, Litopenaeus vannamei, polyurethane sponge biocarrier
Citation: Liu C, Chen L, Xu A and Song Z (2023) Evaluation of polyurethane sponge biocarrier effects on Litopenaeus vannamei cultivation in zero water exchange systems based on water quality, shrimp performance and bacterial community analysis. Front. Environ. Sci. 11:1326194. doi: 10.3389/fenvs.2023.1326194
Received: 23 October 2023; Accepted: 22 November 2023;
Published: 12 December 2023.
Edited by:
Fayuan Wang, Qingdao University of Science and Technology, ChinaCopyright © 2023 Liu, Chen, Xu and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Liu, bGl1LmNoYW9AcXV0LmVkdS5jbg==; Zhiwen Song, c29uZ3poaXdlbkBxdXQuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.