- 1Heilongjiang River Fisheries Research Institute, Chinese Academy of Fishery Sciences, Harbin, China
- 2Scientific Observing and Experimental Station of Fishery Resources and Environment in Heilongjiang River Basin, Ministry of Agriculture and Rural Affairs, Harbin, China
- 3National Agricultural Experimental Station for Fishery Resources and Environment, Fuyuan, China
To study their feeding habits, Coregonus ussuriensis samples were collected seasonally in the Amur River, China. A total of 574 samples were collected, including 200 individuals with empty stomachs. The stomach contents of the remaining 374 samples were identified, counted, weighed, and analyzed. The results showed that the vacuity rate of Coregonus ussuriensis was 34.84% in total; the rates in summer and autumn were higher than in spring and winter. Prey items could be divided into three categories: fish, benthos, and mammals, with 62 taxonomic units. Of these, fish had the highest relative importance index (IRI), followed by benthos and mammals. Specifically, Exopalaemon modestus was the dominant species among the food species of Coregonus ussuriensis, and unidentified fish, Hydropsyche sp., Hemiculter leucisculus, Abbottina rivularis, and Saurogobio dabryi were important species. The average repletion index (RI, %) of Coregonus ussuriensis was highest in autumn (1.86), followed by winter (1.40), summer (1.26), and spring (1.02). The main food of Coregonus ussuriensis was benthos in spring and autumn, and fish in summer and winter. Cluster analysis showed that, according to the similarity level of the bait biological composition, the fork length group samples of Coregonus ussuriensis could be divided into three groups: 210–330 mm, 330–450 mm, and >450 mm. The highest IRI% of feed organisms in these three groups of samples were fish (73.67%), benthos (75.12%), and benthos (94.46%), respectively. It can be observed that with the growth of the fork length of Coregonus ussuriensis, the importance of benthos in its diet increases. The results of an RDA analysis on the relationship between main bait organisms and various factors indicated that season, river level, river width, and fish size have a significant impact on bait organisms and a positive or negative impact on the quality scores of some bait species. This study filled the gap in biological research on the feeding ecology of Coregonus ussuriensis and laid a research foundation for ecological research on this species and its resource protection and aquaculture.
Introduction
The Amur River in China is 4,440 km long, flows into the Okhotsk Strait in Russia, and has a main stem and tributaries (the Songhua River and the Ussuri River). It is located in the northeast region of China, with a latitude range of N 43.4–53.5°. It is a coldwater river, of which the ice cover period lasts for over 5 months, and is rich in fishery resources, among which Coregonus ussuriensis is an important species.
Coregonus ussuriensis Berg, a salmonid Amur whitefish, is a coldwater species with a long life span, slow growth, high fecundity, and late maturity and is distributed in the waters of the Amur River and southern Sea of Okhotsk (Nikolskiy, 1960; Zhang, 1995; Li et al., 2015; Wang et al., 2022). Coregonus ussuriensis migrates to the cold-water tributaries or estuary of the Amur River in summer and to the main stream of the Amur River waters such as the Songhua River and the Ussuri River in winter (Nikolskiy, 1960; Wang et al., 2019a). The construction of the Dadingzishan Navigation Power Junction in the Songhua River blocked the migration channel of Coregonus ussuriensis, resulting in a loss of habitat in the upstream waters (Wang et al., 2019b). In addition, the impact of fishing and other human activities has jointly led to a decline in the resources of Coregonus ussuriensis, which was listed as a “vulnerable” species in the China Red Data Book of Endangered Animals (Pisces) (Yue and Chen, 1998). Only by fundamentally understanding the ecological needs of fish can scientific and effective protection measures be formulated (Sánchez and Cobo, 2012). Previous studies on Coregonus ussuriensis focused on life history, age, growth, fecundity, and genetic structure (Ma et al., 2003; Liang et al., 2004; Li et al., 2015; Wang et al., 2019b). Previous studies have shown that Coregonus ussuriensis is characterized by slow growth and late sexual maturity, which indicates that it will not easily recover from human interference. However, there is currently insufficient research on its biology; in particular, there has been a lack of systematic research on feeding ecology. Fish feeding ecology is an important aspect of fish ecology research. Fish obtain energy and nutrition through feeding activities, providing a material basis for individual survival, growth, development, and reproduction, as well as population reproduction. The feeding habits of fish have important practical significance for studying their ecological characteristics, protecting fish resources, and promoting fish growth and aquaculture. Owing to a series of impacts from human activities, issues such as the decline of fishery resources have emerged, and key issues in aquatic ecosystems, including feeding ecology, have also attracted widespread attention (Yan et al., 2011). The stomach content analysis is a traditional method for studying fish feeding, and it is also the most direct and effective. It can accurately determine specific types of diet and is widely used in studies of fish feeding ecology (Yan et al., 2011; Huo et al., 2014; Ma et al., 2014; Sui et al., 2021; Sarker et al., 2023).
This study analyzed the stomach contents of Coregonus ussuriensis individuals randomly collected in different seasons in China to systematically understand their feeding characteristics and fill the gap in ecological research. The objectives of this study were as follows: 1) analyze the feeding intensity and diet composition of Coregonus ussuriensis, 2) evaluate the effects of size and seasonal and environmental factors on the diet, and 3) determine the feeding strategy.
Materials and methods
Study area and sample collection
Coregonus ussuriensis samples were collected from sites that were interconnected in the waters of the Amur River, including the Amur River, the Songhua River, the Ussuri River, and the Tangwang River, the latter of which is a tributary of the Songhua River (Figure 1). These waters are located in the northeast of China and have typical seasonal characteristics, with ice cover from late November to mid-April of the following year.
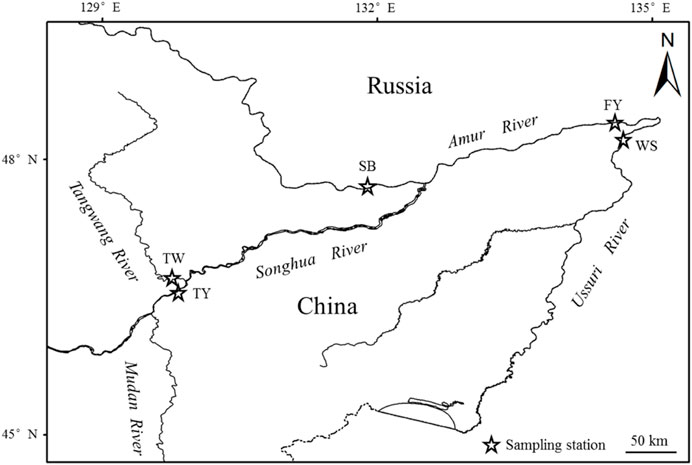
FIGURE 1. Sampling station of Coregonus ussuriensis at the Suibin (SB) and Fuyuan (FY) sections of the Amur River, the Wusuzhen (WS) section of the Ussuri River, and the Tangyuan (TY) section of the Songhua River and Tangwang River (TW). The fish were sampled seasonally from July 2016 to June 2018.
The fish were sampled using floating gill nets (mesh size 4, 8, and 10 cm) seasonally from July 2016 to June 2018. The samples were immediately transported to the laboratory in an icebox after fishing, where fork length (FL) and total length (TL) were measured to the nearest 1 mm, and body weight (BW) was weighed to the nearest 0.1 g. The fish body was then dissected so that the gonads could be observed and the contents of the stomach could be extracted. Sex was identified and the maturity stage was determined using a visual evaluation according to six scales (Wang et al., 2022). The stomach contents of each specimen were washed out into a Petri dish. Each prey item was sorted and identified to the lowest feasible taxon under a stereomicroscope, then counted and weighed to the nearest 0.1 mg individually, after absorbing excess water with blotting paper. All animal experiments were conducted in accordance with the guidelines and approval of the Animal Research and Ethics Committees of the Heilongjiang River Fisheries Research Institute.
Data analysis
To analyze the feeding intensity rhythm of Coregonus ussuriensis, the vacuity rate (V) and repletion index (RI, %) were computed (Morato et al., 2000; Figueiredo et al., 2005).
V=Ne/Ns×100%, where Ne is the number of empty stomachs and Ns is the total number of stomachs sampled.
Where F%, N%, and W% are the percentage of occurrence, number, and weight, respectively.
To study size-related diet variations, the samples were divided into nine groups by fork length from 215 mm to 588 mm with 30 mm intervals (210–240 mm, 240–270 mm, 270–300 mm, 300–330 mm, 330–360 mm, 360–390 mm, 390–420 mm, 420–450 mm, and >450 mm). Hierarchical cluster analysis based on the Bray–Curtis similarity index and the IRI% were used for the classification of size classes into groups (Clarke and Warwick, 2001). The RDA (redundancy analysis) was used to analyze the impacts of environmental factors (season, river width, sediment, river grade, longitude and latitude, water temperature, fish size, and fish sexual maturity ratio) on the W% of the prey items of Coregonus ussuriensis. Prey items with a low W% (<5%) were removed in the process of the analysis. River width was measured using range finder (Rasger S1500BE); river sediment was classified based on mud, sand, and stones of different particle sizes; river grade was divided by the main stem and tributary level of the river; fish size was reflected by fork length (FL); longitude and latitude were recorded by GPS (GARMIN 66S); water temperature was measured using a temperature recorder (HOBO U22-001); and the fish sexual maturity ratio is the proportion of mature fish individuals to the total number of individuals. Data and images were analyzed using Microsoft Excel 2010 and ArcGIS 10.5. All statistical analyses were performed using PRIMER 6.0 and Cannoco 5 software.
Results
Coregonus ussuriensis samples
A total of 574 fish samples were included in the feeding analysis of Coregonus ussuriensis in this study, including those with 200 empty stomachs and 374 stomachs with prey. The numbers of stomach samples with food collected in spring, summer, autumn, and winter were 46, 28, 172, and 128, respectively. Except for the summer samples collected only from the Tangwang River, samples were collected from several of the above stations for other seasons (Figure 1; Table 1). The fork length range of the samples was 215–588 mm and the age range was two to nine.
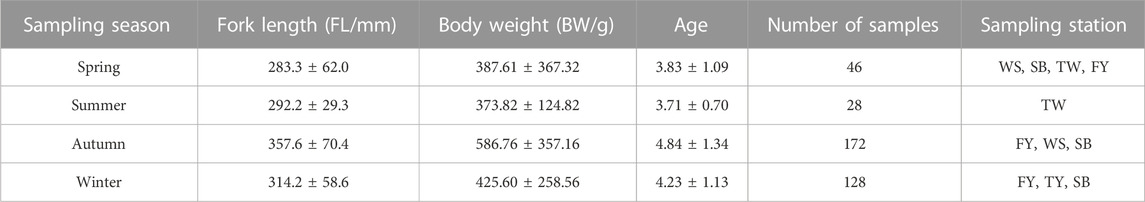
TABLE 1. Coregonus ussuriensis sample details from the Amur River waters from July 2016 to June 2018.
Diet composition of Coregonus ussuriensis
In general, the vacuity rate of Coregonus ussuriensis was 34.8%. Prey items were divided into three categories: fish, benthos, and mammals, with a total of 62 taxonomic units identified (Table 2). Among them, there were 30 taxonomic units for fish (including unidentified fish), 31 taxonomic units for benthos (including unidentified species), and 1 mammal species. In terms of quantity, benthos are the most abundant, accounting for 74.36% of the total, followed by fish (25.61%) and mammals (0.03%). In terms of weight, fish were the most abundant, accounting for 70.91%, followed by benthos (28.88%) and mammals (0.21%). In terms of the relative importance of IRI, fish and benthos were the dominant species, with higher levels, whereas mammals were the lowest and were occasional species.
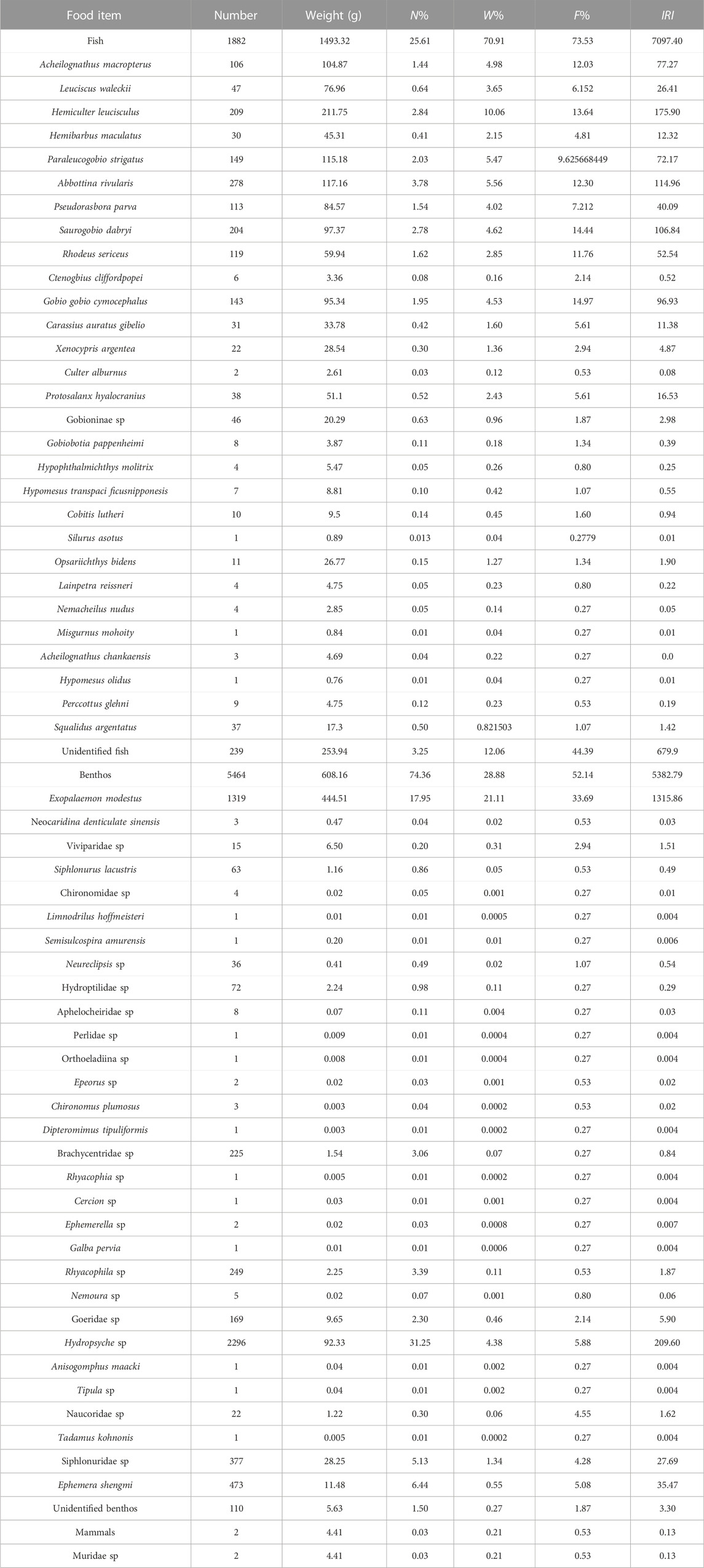
TABLE 2. Diet composition of Coregonus ussuriensis expressed as the percentage of occurrence (F%), percentage of number (N%), percentage of weight (W%), and relative importance index (IRI), (n = 374).
Specifically, from the perspective of food quantity, Hydropsyche sp. Accounts for 31.25% at most, followed by Exopalaemon modestus (17.95%), Ephemera shengmi (6.44%), Siphlonuridae sp. (5.13%), Abbottina rivularis (3.78%), unidentified fish (3.25%), etc. In terms of weight, Exopalaemon modestus is the largest, accounting for 21.11%, followed by unidentified fish (12.06%), Hemiculter leucisculus (10.06%), Abbottina rivularis (5.56%), Paraleucogobio strigatus (5.47%), Acheilognathus macropterus (4.98%), and Saurogobio dabryi (4.62%). According to the analysis results of IRI, Exopalaemon modestus was the dominant species among the food species of Coregonus ussuriensis, and unidentified fish, Hydropsyche sp., Hemiculter leucisculus, Hemiculter leucisculus, Abbottina rivularis Abbottina rivularis, and Saurogobio dabryi are important species.
Seasonal feeding characteristics
As shown in Figure 2, the vacuity rates of Coregonus ussuriensis in spring, summer, autumn, and winter were 23.33%, 45.10%, 42.09%, and 22.89% respectively, which showed that the rates in summer and autumn are significantly higher than in spring and winter. The repletion index of Coregonus ussuriensis was highest in autumn (1.86), followed by winter (1.40), summer (1.26), and spring (1.02).
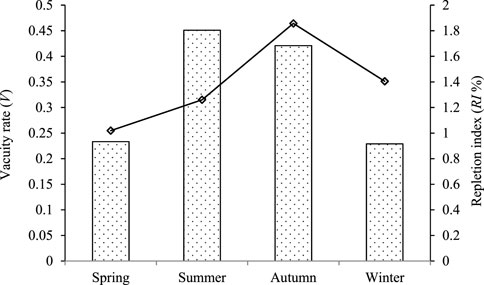
FIGURE 2. The vacuity rate (V) and repletion index (RI%) of Coregonus ussuriensis in spring, summer, autumn, and winter. The long bars represent the vacuity rate, and the diamond dots represent the repletion index.
Figure 3 shows the relative importance index percentage (IRI%) of food species of Coregonus ussuriensis. In general, fish was the highest, accounting for 56.87%, followed by benthos (43.13%) and mammals (0.01%). The prey of Coregonus ussuriensis in spring contained benthos and fish, with IRI% of 74.54% and 25.46%, respectively. Among them, Hydropsyche sp. was the dominant species in spring, with the highest IRI value, and Siphlonuridae sp., Exopalaemon modestus, and unidentified fish were important species. The food items in summer could be divided into three categories: fish, benthos, and mammals, with IRI% of 92.21%, 7.67%, and 0.1%, respectively. Among them, Abbottina rivularis and unidentified fish were the dominant species, and Exopalaemon modestus was an important species. In autumn, the food items consisted of benthos and fish, with IRI% of 53.23% and 46.77%, respectively. Among them, Exopalaemon modestus was the absolute advantage species, and unidentified fish, Paraleucogobio strigatus, Saurogobio dabryi, Gobio gobio cymocephalus, and Pseudorasbora parva were important species. The food items in winter contained fish and benthos, with IRI% of 84.01% and 15.99%, respectively. Among them, unidentified fish was the dominant species, and Hemiculter leucisculus, Hydropsyche sp., Acheilognathus macropterus, Saurogobio dabryi, Abbottina rivularis, Protosalanx hyalocranius, and Rhodeus sericeus were important species.
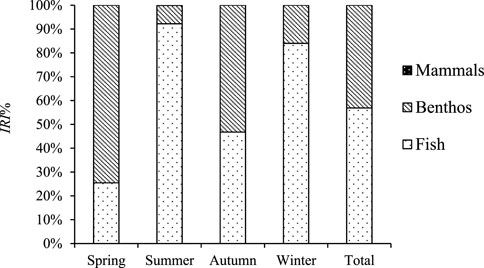
FIGURE 3. Relative importance index (IRI%) of Coregonus ussuriensis food items containing fish, benthos, and mammals in different seasons.
Size-related feeding characteristics
To analyze the difference in the food item composition of different sizes of Coregonus ussuriensis, cluster analysis was conducted on individual samples of different fork length groups according to the relative importance index percentage (IRI%) of stomach contents. Figure 4 shows that at a level of 58% similarity of food composition, the samples can be divided into three groups: 210–330 mm, 330–450 mm, and >450 mm. Among them, the IRI% of bait organisms in the 210–330 mm body fork length group was highest in fish, accounting for 73.67%, followed by benthos (26.32%) and mammals (0.01%). In the 330–450 mm fork length group, the IRI% of benthos was the largest (75.12%), followed by fish (24.87%) and mammals (0.01%). The prey of the >450 mm fork length group was composed of benthos and fish, of which benthos had the highest IRI% of 94.46% (fish only accounted for 5.54%). It can be observed that with the growth in the fork length of Coregonus ussuriensis, the importance of benthos in its diet increases.
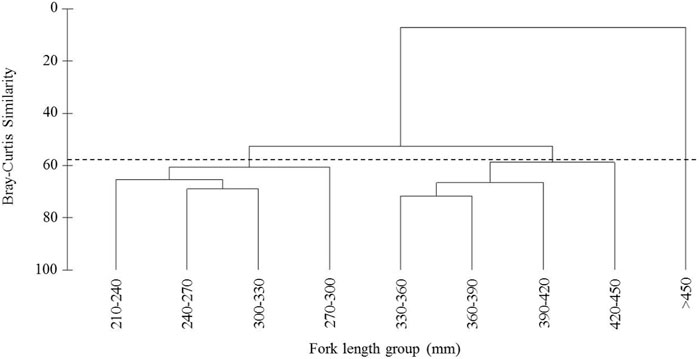
FIGURE 4. Hierarchical cluster analysis based on the relative importance index percentage (IRI%) of the nine size classes. The two groups defined at an arbitrary similarity level of 58% are indicated (dotted line).
The relationship between main bait organisms and environmental factors
RDA analysis was conducted on the mass fraction (>5%) of the main food of Coregonus ussuriensis in different factors, such as season, river width, sediment, river grade, longitude and latitude, water temperature, fish body size, and fish sexual maturity ratio (Figure 5). The results showed that season, river grade, river width, and fish body size had a greater impact on bait organisms. Of these, season, fish size, and sexual maturity ratio had a positive effect on the total mass fraction of bait organisms, such as Xenocypris argentea, Leuciscus waleckii, Paraleucogobio strigatus, Pseudorasbora parva, and Acheilognathus macropterus, but had a negative effect on Hydropsyche sp., Opsariichthys bidens, and Siphlonuridae sp. The level and width of rivers had a positive effect on Hemiculter leucisculus and Gobio cymocephalus but a negative effect on Ctenogbius cliffordpopei, Exopalaemon modestus, and Rhodeus sericeus. The sediment and water temperature of rivers had a significant impact on Opsariichthys bidens, Siphlonuridae sp., Gobio gobio cymocephalus, and Rhodeus sericeus.

FIGURE 5. Relationship between bait organisms and environmental factors determined by RDA analysis. The RDA analysis was conducted on the mass fraction (>5%) of the main food of Coregonus ussuriensis in different sampling locations and times, and factors such as season, river width, sediment, river grade, longitude and latitude, water temperature, fish body size, and fish sexual maturity ratio.
Discussion
The diets of fish were closely related to the environmental characteristics of their habitat, which often varies with spatiotemporal changes (Saikia, 2015). The seasonal changes in fish feeding can be found in most fish species (La et al., 2007; Ma et al., 2014; 2020). Compared with spring and winter, summer and autumn have higher water temperatures, faster digestion of prey items, and a shorter retention time of food in the stomach, resulting in higher vacuity rates in summer and autumn. Some studies have shown that the vacuity rate of fish increases during the spawning season, and the feeding intensity decreases (Hovde et al., 2002; Šantić et al., 2009). However, this study showed that the feeding intensity of Coregonus ussuriensis in spawning season (autumn and winter) has not been reduced, which is similar to the research results of Ma et al. (2020) and Yan et al. (2011). On the contrary, Coregonus ussuriensis has the highest RI in autumn, which may be due to it accumulating more energy for reproduction.
It can be observed that seasonal environmental conditions are the key factors affecting the feeding habits of Coregonus ussuriensis. Coregonus ussuriensis has migratory characteristics, which results in the habitat environment changing with migration seasonally. Habitat environment factors such as width, flow velocity, and sediment will affect the composition and distribution of prey items. Coregonus ussuriensis will make predation choices based on the abundance and availability of food. In summer and autumn, Coregonus ussuriensis generally lives in cold water tributaries, such as the Tangwang River. Fish and benthos are more active in summer and were easily preyed on by Coregonus ussuriensis, which prefers to prey on fish because of the higher energy. In autumn, the acquisition of fish and benthos is not as easy as in summer; therefore, the preference for food decreases, resulting in the similarity in the IRI% of fish and benthos. In winter and spring, Coregonus ussuriensis lives in the main stems of large rivers where its bait fish concentrate for winter, and the predation becomes easier and therefore Coregonus ussuriensis shows a preference for fish. In spring, the bait fish begin to disperse and forage due to the availability of food; therefore, benthos become the main food source.
During individual fish development, changes occur in its food intake and digestive organs (Wootton, 1998). Many studies have shown that as fish grow and develop, their feeding habits are generally transformed (Graeb et al., 2005; Yan et al., 2011; Huo et al., 2014; Su et al., 2015; Sarker et al., 2023). Different sizes of fish have different food preferences (different prey types and sizes), which are related to the strategy of predation (Graeb et al., 2005). Size-related diet variation was also found in this study, in which individuals in the 210–330 mm FL group, considered immature individuals (Wang et al., 2022), consumed more fish, whereas individuals in the 330–450 mm and >450 mm groups (mature individuals) preferred benthos. The relative importance index of benthos also increases with the increase of body fork length, mainly because benthos have higher accessibility and choose this food to meet their energy needs. Among benthos, the larvae of Hydropsyche sp., the protective shell of which has been removed in the stomach contents of Coregonus ussuriensis, are the main dominant species. This is related to its feeding strategy and mouth characteristics. Coregonus ussuriensis feeding on benthos with protective shells requires spitting them out, and the proficiency of this feeding technique may be related to its size. Large individual fish do not have significant restrictions on prey items, which is mainly related to the abundance of fish and benthos, whereas small individual fish seem to have some preferences, preferring to feed on fish more rather than undertaking the complex actions needed to prey on benthos. Different types of food with different sizes of fish may also be a strategy to reduce food competition (Schoener, 1974; Werner, 1979). With the growth of organisms, changes in diet may have evolved into a strategy to reduce intraspecific competition for food between the young and adults (Werner, 1979; Amundsen et al., 1996).
In this study, Hydropsyche sp. is the most abundant in the food of Coregonus ussuriensis but it is not the dominant species of benthos in the Amur River (Huo et al., 2013). Previous studies have shown that fish do not always consume the most abundant prey in their environment but rather have different preferences (Kati et al., 2015; Ma et al., 2020). According to the optimal foraging theory, fish should choose those prey taxa that maximize the net energetic gain in relation to the energetic cost of their capture, ingestion, and digestion (Gerking, 1994). In this study, the proportion of Hydropsyche sp. in foods is the highest, which is closely related to their easy capture and digestion. The weight proportion of Exopalaemon modestus in prey is the highest, which is not only related to its resource quantity but also the ease of catching benthos. Additionally, fish are the dominant species in food, which is mainly related to the high quality and energy of individual units. These findings are broadly in accordance with those of Huo et al. (2014) and Ma et al. (2020). This study showed the prey taxa and feeding intensity are various in seasons and it may be the opportunistic feeder which has the characteristics of seasonal and size-related variations in diet composition. These changes in diet indicated the adaptability of predators and the diversity of their diets (Zander, 1996).
In addition, this study provided a scientific basis for the further study of coldwater fish and their conservation in waters at high latitude. In this study, the smallest individual of Coregonus ussuriensis was 215 mm (2 years old) and there was little difference between its morphological characteristics and those of adults. Additionally, it could feed on fish and benthos. Smaller individuals (0+) were not captured, and their feeding habits could not be monitored. To further investigate its feeding ecology, small individual samples should be added for analysis in the future.
Conclusion
A total of 574 fish samples were included in the feeding analysis of Coregonus ussuriensis in this study, including individuals with 200 empty stomachs and 374 stomachs with prey. The vacuity rates of Coregonus ussuriensis in spring, summer, autumn, and winter were 23.33%, 45.10%, 42.09%, and 22.89% respectively, which showed that summer and autumn had significantly higher rates than spring and winter. The repletion index of Coregonus ussuriensis was highest in autumn. Prey items of Coregonus ussuriensis were divided into three categories, fish, benthos, and mammals, with a total of 62 taxonomic units identified. Among them, there were 30 taxonomic units for fish (including unidentified fish), 31 taxonomic units for benthos (including unidentified species), and 1 mammal species. Fish and benthos were the dominant species, whereas mammals were occasional species. With the growth of Coregonus ussuriensis, the importance of benthos in its diet increases. This study showed that prey taxa are various and there are seasonal trends in feeding intensity, and it may be the opportunistic feeder that has seasonal and size-related variations in diet composition. RDA analysis showed that season, river grade, river width, and fish body size had the greatest impact on the food items of Coregonus ussuriensis.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal studies were approved by the Animal Research and Ethics Committees of Heilongjiang River Fisheries Research Institute. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
JW: Formal Analysis, Funding acquisition, Investigation, Writing–original draft, Writing–review and editing. TH: Data curation, Project administration, Writing–review and editing. PL: Investigation, Resources, Writing–review and editing. WL: Data curation, Software, Writing–review and editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The research was financially supported by the Special project on agricultural financial fund from the Ministry of Agriculture and Rural Affairs of China entiled “Survey offishery resources and environment in key waters of Northeast China” and Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2020TD07).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Amundsen, P. A., Gabler, H. M., and Staldvik, F. J. (1996). A new approach to graphical analysis of feeding strategy from stomach contents data-modification of the Costello (1990) method. J. Fish. Biol. 48, 607–614. doi:10.1006/jfbi.1996.0060
Clarke, K. R., and Warwick, R. M. (2001). Change in marine communities: anapproach to statistical analysis and interpretation. Plymouth: 2ndedn. PRIMER-E.
Cortés, E. (1997). A critical review of methods of studying fish feeding based on analysis of stomach contents: application to elasmobranch fishes. Can. J. Fish. Aquat. Sci. 54, 726–738. doi:10.1139/f96-316
Figueiredo, M., Morato, T., Barreiros, J. P., Afonso, P., and Santos, R. S. (2005). Feeding ecology of the white seabream, Diplodus sargus, and the ballan wrasse, Labrus bergylta, in the Azores. Fish. Res. 75, 107–119. doi:10.1016/j.fishres.2005.04.013
Graeb, B. D. S., Galarowicz, T., Wahl, D. H., Dttmers, J. M., and Simpson, M. J. (2005). Foraging behavior, morphology, and life history variation determine the ontogeny of piscivory in two closely related predators. Can. J. Fish. Aquat. Scis 62, 2010–2020. doi:10.1139/f05-112
Hovde, S. C., Albert, O. T., and Nilssen, E. M. (2002). Spatial, seasonal and ontogenetic variation in diet of Northeast Arctic Greenland halibut (Reinhardtius hippoglossoides). ICES J. Mar. Sci. 59, 421–437. doi:10.1006/jmsc.2002.1171
Huo, B., Xie, C. X., Madenjian, C. P., Ma, B. S., Yang, X. F., and Huang, H. P. (2014). Feeding habits of an endemic fish, oxygymnocypris stewartii, in the yarlung zangbo River in tibet, China. Environ. Biol. Fish. 97, 1279–1293. doi:10.1007/s10641-013-0213-8
Huo, T. B., Li, Z., Jiang, Z. F., Ma, B., and Yu, H. X. (2013). Macrozoobenthos community structure and water quality bioassessment in the mid-reaches of the Heilongjiang River. J. Fish. Sci. China 20 (1), 177–188. doi:10.3724/sp.j.1118.2013.00177
Kati, S., Mozsár, A., Árva, D., Cozma, N. J., Czeglédi, I., Antal, L., et al. (2015). Feeding ecology of the invasive Amur sleeper (Perccottus glenii dybowski, 1877) in central europe. Int. Rev. Hydrobiol. 100, 116–128. doi:10.1002/iroh.201401784
La, M. G., La, M. M., and Tomassetti, P. (2007). Feeding habits of the Madeira rockfish Scorpaena maderensis from central Mediterranean Sea. Mar. Biol. 150, 1313–1320. doi:10.1007/s00227-006-0414-1
Li, P. L., Liu, W., Wang, J. L., Zhan, P. R., and Wang, C. (2015). Fecundity of Coregonus ussurinsis in the Heilongjiang River, China. J. Fish. Sci. China 22 (6), 1234–1242. doi:10.3724/SP.J.1118.2015.14488
Liang, L. Q., Chang, Y. M., and Dong, C. Z. (2004). Analysis of genetic diversity for Coregonus ussuriensis Berg in Heilongjiang River. J. Fish. Sci. China 11 (6), 501–505.
Ma, B., Shi, L. Y., and Dong, C. Z. (2003). Biochemical genetic structure in Coregonus ussuriensis Berg. J. Fish. Sci. China 10 (3), 195–200.
Ma, B. S., Xie, C. X., Huo, B., and Yang, X. F. (2014). Feeding habits of Schizothorax oconnori lloyd, 1908 in the yarlung zangbo river, tibet. J. Appl. Ichthyol. 30, 286–293. doi:10.1111/jai.12283
Ma, B. S., Xu, B., Wei, K. J., Zhu, X. Y., Xu, J., Lu, J. C., et al. (2020). Feeding habits of the cyprinid gymnocypris firmispinatus in the anning river, China. Fish. Sci. 86, 749–758. doi:10.1007/s12562-020-01445-x
Morato, T., Santos, R. S., and Andrade, J. P. (2000). Feeding habits, seasonal and ontogenetic diet shift of blacktail comber, Serranus atricauda (Pisces: serranidae), from the Azores, north-eastern Atlantic. Fish. Res. 49 (1), 51–59. doi:10.1016/s0165-7836(00)00189-2
Saikia, S. K. (2015). Food and feeding of fishes. What do we need to know? Transylv. Rev. Syst. Ecol. Res. 17, 71–84. doi:10.1515/trser-2015-0049
Sánchez, H. J., and Cobo, F. (2012). Ontogenetic dietary shifts and food selection of endemic Squalius carolitertii (Actinopterygii: cypriniformes: Cyprinidae) in River Tormes, central Spain, in summer. Acta Ichthyol. Piscat. 42, 101–111. doi:10.3750/aip2011.42.2.03
Šantić, M., Podvinski, M., Pallaoro, A., Jardas, I., and Kirincic, M. (2009). Feeding habits of megrim, lepidorhombus whiffiagonis (walbaum, 1792), from the central adriatic Sea. J. Appl. Ichthyol. 25, 417–422. doi:10.1111/j.1439-0426.2009.01257.x
Sarker, M. J., Sarker, P. K., Cahoon, L. B., Dipty, A. K., Bashar, M. A., Hasan, M. M., et al. (2023). Seasonal variation in the epibenthic feeding habits of hilsa shad (tenualosa ilisha) in the upper meghna river estuary, Bangladesh. Fishes 8, 335. doi:10.3390/fishes8070335
Schoener, T. W. (1974). Resource partitioning in ecological communities. Sci 185, 27–39. doi:10.1126/science.185.4145.27
Su, X., Li, Y. D., He, X. B., Lu, H. S., and Yan, Y. R. (2015). Feeding habits and ontogenetic diet shifts of mackerel tuna (euthynnus affinis) in the beibu gulf, south China Sea. Prog. Fish. Sci. 36 (4), 65–72. doi:10.11758/yykxjz.20150409
Sui, H. Z., Xue, Y., Li, Y. K., Xu, B. D., Zhang, C. L., and Ren, Y. P. (2021). Feeding ecology of Japanese Spanish mackerel (Scomberomorus niphonius) along the eastern coastal waters of China. Acta Oceanol. Sin. 40 (8), 98–107. doi:10.1007/s13131-021-1796-0
Wang, J. L., Liu, W., Li, P. L., Tang, F. J., and Lu, W. Q. (2022). Estimation of Coregonus ussuriensis age, growth, and maturation in China’s Amur River. PeerJ.12817. doi:10.7717/peerj.12817
Wang, J. L., Liu, W., Lu, W. Q., Li, P. L., and Tang, F. J. (2019b). Assessment of the population resources of Coregonus ussuriensis in the middle reaches of Amur River. Chin. J. Ecol. 38 (6), 1824–1829.
Wang, J. L., Liu, W., Wang, C., Li, P. L., Tang, F. J., Jiang, T., et al. (2019a). Microchemistry analysis of Coregonus ussuriensis otoliths from the Heilong Rvier basin. Acta Hydrobiol. Sin. 43 (4), 825–831. doi:10.7541/2019.097
Werner, E. E. (1979). “Niche partitioning by food size in fish communi-ties,” in Predator–prey systems in fisheries management. Editors R. H. Stroud, and H. Clepper (Washington, DC: Sport Fishing Institute), 311–322.
Yan, Y. R., Yang, H. H., Lu, H. S., and Li, R. W. (2011). Feeding ecology of dorab wolf-herring, Chirocentrus dorab from the Beibu Gulf. Acta Ecol. Sin. 31 (3), 654–665.
Yue, P. Q., and Chen, Y. Y. (1998). China red data Book of endangered animals (pisces)[M]. Beijing:Science Press.
Zander, C. D. (1996). “The distribution and feeding ecology of small-size epibenthic fish in the coastal Mediterranean Sea,” in Biologyand ecology of shallow coastal waters. Editors A. A. EleftheriouA, and SmithCJ (Fredensborg: Olsen and Olsen), 369–376.
Keywords: Coregonus ussuriensis, feeding habits, stomach contents, Amur River, RDA
Citation: Wang J, Huo T, Li P and Lu W (2023) The feeding habits of the Amur whitefish Coregonus ussuriensis in the Amur River, China. Front. Environ. Sci. 11:1277815. doi: 10.3389/fenvs.2023.1277815
Received: 15 August 2023; Accepted: 14 December 2023;
Published: 29 December 2023.
Edited by:
Francesco Tiralongo, University of Catania, ItalyReviewed by:
Appukuttannair Biju Kumar, University of Kerala, IndiaYang Jin, Johns Hopkins University, United States
Copyright © 2023 Wang, Huo, Li and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jilong Wang, d2psMDMyMTIyNUAxNjMuY29t, d2FuZ2ppbG9uZ0BocmZyaS5hYy5jbg==
 Jilong Wang
Jilong Wang Tangbin Huo1,2,3
Tangbin Huo1,2,3 Peilun Li
Peilun Li Wanqiao Lu
Wanqiao Lu