
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci., 12 January 2024
Sec. Conservation and Restoration Ecology
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1254219
This article is part of the Research TopicThe Transition of Novel Genetic Control Strategies into Reality for Vector-Borne Tropical Disease Control and Prevention; Research Advances and next StepsView all 12 articles
Gene drives are genetic elements that in sexually reproducing organisms spread faster than those transmitted through a Mendelian fashion. Since gene drives can be engineered to modify different aspects of physiology and reproduction, they have been proposed as a new and revolutionary tool to control vector-borne diseases, particularly those transmitted by the genera Anopheles and Aedes (Culicidae), such as malaria, Dengue and Zika virus. This approach may impact on human health by lowering the transmission of such devastating diseases. However, the release of genetically modified mosquitos (or other species) into the environment raises a series of questions related to the still incipient technology and our present understanding of the complex structure and dynamics of terrestrial and aquatic ecosystems. Moreover, there are ethical concerns about human interventions in natural ecosystems that may eventually impact our way of living or the ecosystems themselves. This work is an interdisciplinary approach that analyzes from a biological, philosophical, and theological perspective the potential ecological impacts on natural environments of the release of genetically modified species, focusing on gene drive-modified mosquitos. It includes theological approach from a Catholic point of view (although it could be easily shared by other Christians) because we hold that world religions give valuable insights even though not everyone may share their groundings. We conclude that the focal problem is the relationship between humans and nature, and the release of genetically modified species may change this relationship unpredictably. However, given the complex interactions in ecosystems, new approaches such as Earth Stewardship principles could provide new and more widely accepted answers involving biological, philosophical, and theological concepts that will help engaging all relevant actors to make a better world.
According to the World Health Organization (WHO), vector-borne diseases (VBDs) account for over 17% of all infectious human diseases, causing over 700,000 deaths annually (World Health Organization, 2020). Malaria is a parasitic infection with a global estimate of 219 million cases and approximately 400,000 deaths per year, and Dengue is a viral infection associated with over 3.9 million cases per year in 219 countries. Among the vectors spreading these diseases are culid (Culidae) mosquitos and dipteran nematocerans (Harbach, 2023). These vectors include the genera Anopheles, Culex, Aedes and Hemagogus. Culidae includes 39 genera and 135 recognized subgenera with over 3,000 recognized species. Aedes mosquitos are vectors of Dengue, Yellow Fever, Zika, and Chikungunya viruses, while Anopheles carries malaria.
Local and governmental efforts to control or eradicate mosquitos as VBD carriers have been conducted for several decades and have mainly relied on human behavior changes and insecticide spreading (Gillies and Smith, 1960; Pugh, 2016). However, insecticides remain in the environment, and target populations develop resistance, making it more difficult to control VBDs.
Indeed, alternative, safer, and cheaper technologies are needed. For instance, biocontrol-based pests have been recognized as efficient and ecologically friendly (WHO, 2019; 2019; Shaw and Catteruccia, 2019). The sterile insect technique (SIT) was the first attempt to modify vector-borne biology to control different diseases in humans and crops.
The SIT relies on the release of males carrying dominant lethal mutations introduced by ionizing radiation in their sperm (Teem et al., 2020). SIT has been used successfully to eradicate pests such as the Mediterranean fruit fly (Medfly) Ceratitis capitata, different species of the tsetse fly (Glossina spp.), the screwworm fly Cochliomyia hominivorax, in various areas of the world and research has been perform in more than 125 species (Klanssen and Curtis, 2021). Trials with different mosquito species have given inconsistent results and STI decrease the fitness of males, so it has not been possible to use this technology efficiently for control population levels (Benedict MQ, 2003; Carvalho et al., 2015). In addition, imperfect pupal sexing in mosquitos, is another point that makes classical SIT not suitable to use it in mosquitos population control. Recent advances in genetic engineering have provided the basic tools for developing new methods to modify the genome of different species of public health and agricultural importance, with the aim of developing safer, more efficient, and cheaper technologies that are more environmentally friendly than pesticides. Today, the generation of genetically modified mosquitos (GMM), including those using gene drives based (GMGDM), mainly based on CRISPR/CAS9 technology, is a rising star that promises a revolutionary intervention in the environment due to its rapid penetrance in a target population and numerous ways to genetically control or suppress populations in a particular geographic area (Champer et al., 2016; Mitchell et al., 2018).
However, important questions and concerns have been raised regarding biosafety risks and unknown environmental impacts due to the nascent nature of this technique (Genewatch UK, 2012; World Health Organization WHO, 2014; Meghani and Kuzma, 2018). Thus, there is a dilemma. Should we use all the available technology to improve human health, despite potential harmful, and perhaps irreparable, environmental damage, or we should aim to maintain wild environments despite all the health benefits that technology can bring?
The possible answer–if any–to this dilemma rests upon, among other factors, scientific, ethical, and social analyses at different levels. We should fistly analize the risk, safety, and efficacy of the gene modification technique per se. Secondly, we need to address the ecological potential impacts of the release of GMMs into the wild (Ferguson et al., 2010). There are many ecological aspects to be considered, such as population dynamics, species niches or trophic food webs that have not been fully studied in mosquito populations and could severely impact ecosystems after the introduction of GMMs. Third, undoubtedly, there is an anthropocentric environmental vision underlying the use of GMMs carrying gene drives to modify environments. Do we consider the environment as a source of resources to satisfy our basic needs and desires, such as a machine or a factory, with interchangeable pieces that can be moved, replaced or even eliminated at will? Or, do we see the environment, particularly the biosphere components, plants, animals, fungi and microbes, as having an intrinsic value in themselves, thereby deserving moral or ethical consideration?
This complex biological and philosophical problem requires multilevel analyses to reach a conceptual framework for making guidelines that harmonize human and ecosystem health. In this work, we have undertaken an interdisciplinary methodology approach, including ecology, philosophy, and theology, to foresee which might be the fundamental concepts to be considered to take an ethical decision and allow sustainable environments without preventing human flourishing. For this purpose, we need to exply on some relevant ecological concepts and evidence. In the following sections, we will provide this information, in order to offer a better grounded ethical assessment of the topic at stake.
SIT was first used by E.F. Knipling, who produced and used X-ray-sterilized males, and as a pest control method (Klanssen and Curtis, 2021; Marec, Bloem, 2021), but it does not work in other relevant insect pests such as mosquitos, and therefore in recent years innovative strategies based on GMMs have been developed. One approach is the release of males mosquitos carrying a dominant lethal gene, a technique named RIDL, which stand for “release of insects carrying a dominant lethal” (Thomas et al., 2000). This procedure takes advantage of that organism carries a conditional dominat, sex-specific lethal gene, where the permissive condition, that is, its development and growth up to reproductive age, happens only in the presence of an additive, normally in the diet, not found in the wild.
Field trials in the Cayman Islands and Brazil with Aedes aegypti RIDL males (Oxitech OX153A GE line) showed that the local mosquito population decreased by 80% after several weeks of periodic release (Harris et al., 2012; Carvalho et al., 2015). The population decline is because the offspring inherit the conditional lethal sex-specific gene, along with a tetracycline-repressible trans-activator fusion protein, and in the wild, the absence of the additive, in this case thetracycline, causes the offsprings to die. Like in the SIT, periodic releases must be conducted until the necessary threshold for population suppression is achieved; then, the constant release of a small number of mosquitoes prevent the population from returning to the preintervention level (Figure 1).
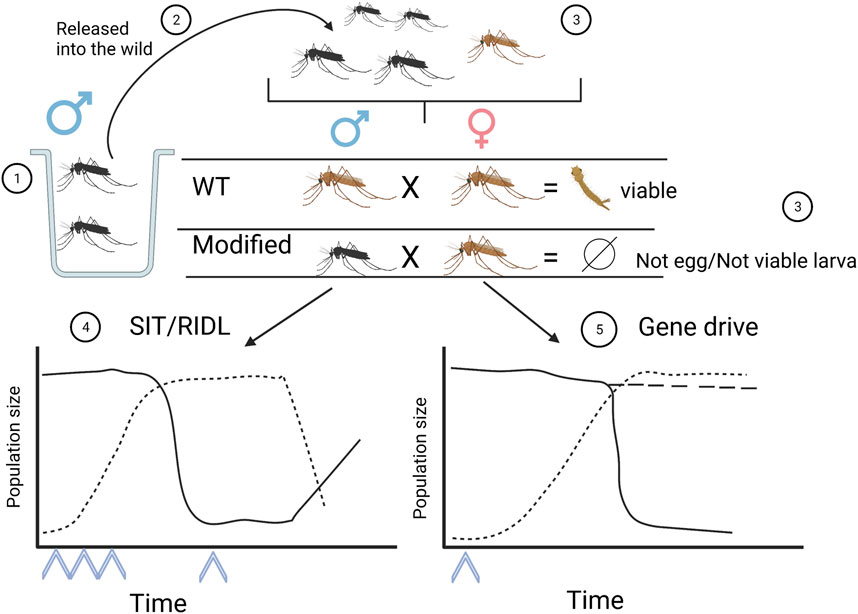
FIGURE 1. Schematic diagram of the production, release, and population levels of two types of modified mosquitoes. 1: Production of SIT, RIDL or gene drive mosquitoes. 2: Release of male mosquitoes into the environment; these mosquitoes will mate with wild females. The modified mosquitoes are assumed to compete equally with wild males for mating with wild females. 3: Crossing wild females with SIT, RIDL or gen drive mosquitoes does not produce embryos, and the larvae are not viable or are modified to not carry the parasite. 4: In the case of infertile (SIT or RIDL) males, multiple releases of individuals to the environment (arrowheads) must be conducted since their population does not expand. As the population increases and reaches the minimum threshold (dotted line), the wild population begins to decline (solid line). Subsequently, periodic releases must be made to keep the wild population at low levels; otherwise, the population levels will recover to those prior to the intervention. 5: In the case of Cas9-mediated gene drive, a single release into the environment is enough for the population to grow (dotted lines) and reach the threshold to suppress the target population within a few generations (solid line). Population levels would be maintained in the case of population replacement (segmented line). In both situations (4 and 5), it is expected that disease levels would begin to decline in the future. Made with Biorender.
It is speculated that due to the short lifespan of mosquitoes, the probability of GMMs escaping to areas without species release is very low and, in the event that this occurs, since GMMs result only in dead embryos, the genetic modification would not expand to mosquitos outside of the intervention area (Champer et al., 2016; James et al., 2018; Servick, 2019). However, citizens and nongovernmental organizations raised concerns due to breaches in biosafety measures in different field trials (Genewatch UK, 2012; Carvalho et al., 2015; Uk et al., 2017). Thus, it would be more advantageous to develop a system that would be self-sustaining over time, lowering the initial release threshold and cost and increasing the ease of implementation.
In a sexually reproducing organism, genes are usually transmitted in a Mendelian fashion, with 50% of the progeny receiving one copy of each parental allele. However, in certain organisms, some genes are transmitted to over 50% of the progeny, even at the cost of reducing the fitness of the organism, such a mechanism is usually defined as gene drive (Burt and Trivers, 2008). However, in this review we will use the term gene drive, or gene drive system, to address those genetic elements that have the ability to be transmitted over 50% of the progeny. One gene drive element that has recently attracted interest in the insect genetic modification field is that of homing endonuclease genes (HEG) that copy themselves into different genomic locations (Burt and Koufopanou, 2004). HEGs encode an endonuclease that recognizes a 15–30 bp sequence that normally occurs only once in the genome (Stoddard, 2011). The endonuclease recognizes and cuts the target sequence, and the homology-directed repair machinery (HDR) uses the HEG sequence as a template to fill the gap in a process called “homing” (Figure 2). However, the broken ends may be rejoined by NonHomologous End Joining (NHEJ), where some nucleotides are introduced to fill the gap, changing the original sequence and preventing this segment from being recognized by the nuclease (Figure 2) (Champer et al., 2016). In 2002, Burt first proposed HEGs as a tool to genetically modify target populations with the benefit of an autosustainable system that is transmitted to the progeny until it is spread throughout the whole population (Figure 1) (Burt, 2003; North et al., 2020). One of the restrictions imposed on this approximation is that the target species must have a short lifespan for the gene to spread rapidly in the population. Therefore, insects, particularly those considered pests or threatening to human health, such as mosquitos, are the first-choice target for this methodology. One possible application of homing gene drives is population replacement, where the vector (mosquito in the case of malaria or Dengue) is genetically modified to prevent parasite transmission or development; in this scenario, the disease incidence will decrease, but the mosquito population will not (Figure 2) (Shaw and Catteruccia, 2019). Another goal may be population suppression, similar to SIT and RIDL, where the release of male mosquitos may result in the demise of an entire population in a few generations, decreasing the disease incidence. In this case, the mosquito population will remain low enough to eradicate the disease or disappear entirely (Figure 1).
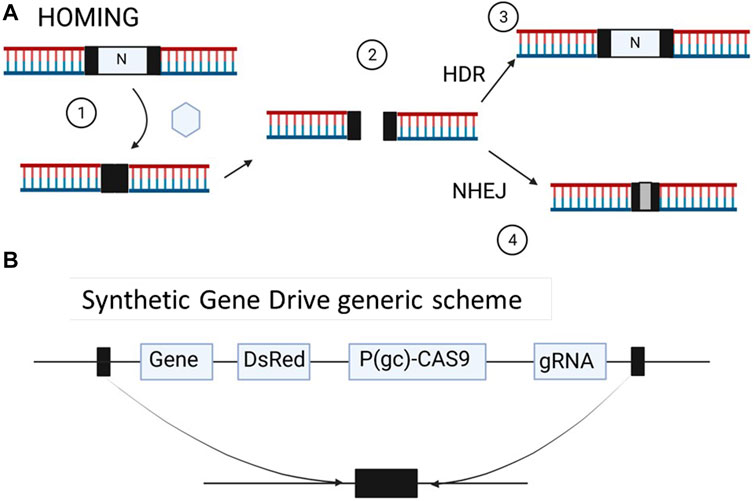
FIGURE 2. (A) 1: Homing gene drives are elements encoding an endonuclease that recognizes a sequence of 20–30 nucleotides typically present at only one genomic location. The cut (2) activates the (3) DNA repair machinery, and the gene drive will insert into the target sequence if the cell uses homology-directed repair machinery (HDR); alternatively, if the cell uses nonhomologous end joining (NHEJ), it will introduce one or more nucleotides, producing a new sequence no longer recognized by the endonuclease. (B) General scheme of a synthetic gene drive. The construct comprises the intended gene to be inserted, a marker gene (e.g.,. a gene encoding the red fluorescent protein, DsRed) and one or more guided RNAs (gRNA), the last two controlled by a general promoter. The CAS gene enzyme (CAS9) is under the control of germ cell-specific control (P (gc), e.g., VASA). Finally, the construct is flanked by homologous sequences to the target gene, which allows integration into the host genome. Made with Biorender.
Currently, CRISPR/CAS9 technology allows editing of any segment of the genome using sequences determined by guide RNAs (gRNAs) (Ressel and Charpentier, 2018). This procedure allows the insertion of any desired sequences flanked by homologous segments to those recognized by gRNA-mediated CAS9 in the target segment. Then, this construct is used as a template to repair the break as long as the cell uses the HDR machinery. Thus, with the aid of the CRISPR/cCAS9 system, the limitations imposed by HEG sequence recognition are solved, and it is possible to engineer gRNAs targeting any segment in the genome. This technology was first used in 2015 in Anopheles stephensi, aiming to design a system conferring resistance to Plasmodium falciparum as a method of population replacement (Gantz et al., 2015). After establishing a transgenic line of caged mosquitoes, the researchers showed that 99% of the third-generation larvae expressed the fluorescent marker gene. This work established the basis that nonmendelian transmission of an exogenous gene to an entire population of mosquitoes using a CAS9-mediated gene drive could be a successful tool to control wild-type mosquito populations. Later, the same authors showed that modified mosquitos released in large cages to mimic wild release at an initial frequency of 12.5% spread rapidly, and after 9–13 generations, the gene was present in almost the entire population (Hammond et al., 2021). A Cas9-based gene drive, also termed synthetic gene drive, usually comprises 5 basic elements: 1) a CAS9 gene controlled by a germ cell promoter (e.g., vasa or nanos); 2) one or more RNA guides (gRNA) under general promoter control; 3) a marker, usually DsRed under general promoter control; 4) the desired sequence to be inserted; and 5) flanking homologous sequences targeting the gene or segment to insert the whole synthetic gene (Figure 2). Due to the successful data obtained under controlled conditions, it is expected that the CAS9-mediated gene drive will be a new complement to the actions currently being taken to control or eradicate diseases such as malaria, Dengue or Zika virus, all of which are transmitted by mosquitoes (Shaw and Catteruccia, 2019; Courtier-Orgogozo et al., 2020; Nateghi Rostami, 2020; Dong et al., 2022). Since some mosquitoes transmit more than one disease (e.g., Aedes aegypti, which transmits the Zika, Yellow Fever and Dengue viruses), the elimination of a population in a particular area would mean the reduction not of one but all diseases transmitted by this type of mosquito (Shaw and Catteruccia, 2019).
Despite the aforementioned benefits, this technology has been questioned because several biosafety issues must be improved before its utilization in the wild. The efficacy and safety of the technique itself raise concerns. Another concern is refinement since it has been shown that mutations occur outside the target site (off-target), which could alter non-target biological functions (Ressel and Charpentier, 2018). Another concern is that germ cells use the HDR machinery as the main way to repair DNA, but they also use NHEJ, which introduces mutations in the target area and prevents construct insertion (Fuchs et al., 2021). Thus, these mutations could be transmitted to future generations, expanding resistance to modifications and generating unanticipated phenotypes that could eventually enhance pathogen transmission. Another methodological concern is that only genetically homogenous mosquito lines have been tested in published experiments, and these flies do not represent the genetically diverse wild population (Miles et al., 2017).
Another important issue is that these genes are self-sustaining, and various concerns have been raised about the substantial and largely unknown ecological impact that such genes may introduce into wild populations due to the possibility that the mosquitoes could interbreed with other wild species or acquire changes that place them outside the intended control area (Winskill et al., 2014; WHO, 2019; 2019; Dolezel et al., 2020; Devos et al., 2022). Thus, it is critical to conduct further research on the potential short- and long-term impact that GMGDMs could have on the local ecological network. This new technology could significantly improve human quality of life in VBD-rich areas; however, the environment has a significant impact on human health ad, thus, must also be healthy. There remain many aspects of mosquito biology that must be addressed to better define the best parameters to evaluate the efficacy and safety of GMMs before releasing them into the wild.
The WHO issued a document proposing 4 phase strategies to evaluate the safety, efficacy and risk of GMMs from controlled studies in the laboratory to eventual releases into the environment (World Health Organization, 2021). The proposal is based on the “go” and “no-go” criteria, i.e., moving to the next stage only if strict efficacy criteria are met in every previous phase (World Health Organization, 2021). Phase 1 refers to advances from laboratory studies to controlled cages, usually with a low number of individuals. Depending on the Phase 1 results, the experiments will move to Phase 2, involving physical containment in larger outdoor cages with similar ecological settings to the area intended for mosquito release. Then, GMM studies may proceed to Phase 3, designed to evaluate conditions such as efficacy in preventing infection or disease. Finally, Phase 4 is intended to make a public health intervention that must be accompanied by efficacy and safety monitoring. Each phase should evaluate the likelihood of specific harm at that step. The risk associated with GMGDM could be considered similar to that for GMMs, but because of their persistence and extremely high spreading capacity in the environment, the risk analysis must consider the possibility of higher levels of exposure (National Academies of Sciences, Engineering, and Medicine, 2016; Frieß et al., 2019). Thus, various laboratories are working on implementing built-in control tools that eventually could eliminate the released mosquito population in case biosafety barriers are broken putting in risk the environment (e.g., gene transfer to wild type species) (Vella et al., 2017; Webster et al., 2020; Zapletal et al., 2021; Bier, 2022). For instance, the elaboration of “CRISPR-base gene-drive arranged in a daisy-chain, such that each drives the next and where the spread capacity is limited by the successive loss of nondriving elements, from the end of the chain” therefore enables researchers or communities to decide whether and when to alter local ecological systems (Noble et al., 2019). The use of rescue drivers in relation to beneficial mutations in endangered populations “where the gene-driver is affected by different factors, that depend on the drive construct (e.g., fitness effect and timing of expression) or on the target species (e.g., mating system and population structure)” has connotations in conservation ecology and management (Rode et al., 2019).
Research investments in ecological and socioecological-related GMMs and the environmental implications of field releases, although lately making progress due to the focus on the control of VBDs in humans, are notoriously lagging behind scientific progress in areas such as molecular biology and genetic engineering, and this gap needs to be bridged.
The WHO and many technical documents strongly recommend considering ecological and biosafety implications and substantive engagement of communities before massive field releases of GMMs and GMGDMs (National Academies of Sciences, Engineering, and Medicine, 2016; World Health Organization, 2020). In our view, the ecological consequences of SIT releases reported by the authors apply not only to the eradication of species but also to GMGDM operations that might cause species eradication. Some of the ecological implications refer to the loss of genetic variability, the release of wildlife from diseases, pollinator-plant and host-vector interactions, effects on land use (since the control of disease vectors and parasites may increase domestic and wild herbivores), biodiversity (due to eradication of species and associated parasitoids) and some elements of conservation. For instance, according to (Feldmann and Hendrichs, 2001), in spite of controversies and arguments in favor or against, the hypothetical case of the eradication of tsetse flies would translate into a “more even distribution of livestock, thereby reducing overgrazing and erosion in the labile Sahel and highlands (Tanzania), and may also reduce poaching intensity in national parks and wilderness areas since eliminating wildlife reservoirs of trypanosomes would no longer be practical”. Therefore, there are several important ecological interactions that need to be address and studied before any decision is made that involves field intervention with GMMM. Some of these ecological interactions are:
In population and community ecology, species niche quantitative analysis appears critical, particularly where the release of GMGDMs may affect the population dynamics of targeted species. In summary, the niche of a species refers to the mapping of its population dynamics onto a space (Holt, 2009), where quantifications require a definition of the physical and biological environmental factors affecting the performance of individuals (birth-deaths) in the population. In the past 50 years, the niche concept has evolved from the Hutchinsonian fundamental and realized niche to the evolutionary niche (Holt, 2009). Wild mosquito ecological niches (e.g., niche partitioning and land covering) have been studied or modeled in different species around the world (e.g., Simard et al., 2009; Larson et al., 2010; Ochieng et al., 2016; Richman et al., 2018). Nevertheless, to the best of our knowledge, quantitative niche analysis of mosquito species targeted with GMMs has not been thoroughly conducted or maintained in locations where field GMM releases may occur.
In connection with ecological and risk assessments of field releases of GMGDMs, it would appear necessary to have information on the structure of the trophic web community where the targeted wild mosquito population resides before and after release. Knowledge of the position and trophic connections of the target species in the trophic web and its ecological guild would help to provide indications of possible community effects on the targeted population. As an example, some mosquitoes may be important pollinators, and it appears critical to know whether the target population is specific or generalist since insect-mediated pollination is a critical ecosystem service for humans.
Ecologically, a keystone species is one whose effect in the community is large and disproportionately large relative to its abundance. KS are critical in wild communities since their presence or absence will determine the community structure and dynamics; KS are present in marine, terrestrial and freshwater ecosystems (Power et al., 1996). Insects have not been described as KS, and we have not found mentions in the literature of mosquitoes as KS; however, regarding insects, the example given above of the tsetse fly (Feldmann and Hendrichs, 2001) might qualify as one referring to a KS.
Intra- or intercompetition only occurs when species resources are scarce, limited or in short supply. Intracompetition occurs among the individuals of a species in its niche, affecting individual species abundance. Intercompetition occurs within cooccurring niches and tends to constrain the abundance of one of the competing species (loser) and increase the abundance of the other (winner). For example, the field release of GMGDMs targeting the wild population of the same species might cause ecological interferences with the population dynamics, and competitive release might be an outcome. In the future, the analysis of intra- and intercompetition should form part of a basic protocol to evaluate the ecological implications of GMGDM releases.
Population dynamics is the study of the age structure of populations and their stability, densities, maintenance, declines and/or extinctions over time and is critical for understanding the relative importance of competition (Schowalter, 2006). Regarding mosquitoes as disease vectors and the use of insecticide as a vector control, several computerized population dynamics models have been published (e.g., (Molineaux et al., 1978; Gu and Novak, 2005; Ermert et al., 2011; White et al., 2011). As an example, White et al. used a population dynamics model for Anopheles gambiae, incorporating rainfall-dependent carrying capacity and density-dependent regulation for eggs, four larval instars, pupal stages, and female adult mosquitoes, in 8 villages in Nigeria (White et al., 2011). A density-dependent relationship between larval density and larval deaths (linear association) was found, and the mosquito reproduction number was dependent on seasonal rainfall. Insecticide applications (long-lasting and indoor) reduced oviposition, further reducing the adult mosquito density. The simulations show that selecting combinations of interventions targeting different stages of the life cycle of A. gamnbiae will result in maximum reductions in female mosquitoes. This and other kinds of population dynamics analyses in mosquitoes (and other species) can be adapted regarding GMGDM field releases.
Regarding any species, the increased knowledge of its life cycle, autecology, population dynamics, ecological interactions and genetics, plus environmental and socioecological data (e.g., those that are part of the niche of a species, e.g., Soberon and Peterson, 2005), will undoubtedly help in ecological modeling efforts regarding GMGDM field releases. For VBD species, including mosquitoes, it is also crucial to understand genetic structure, gene flow and, especially, dispersal. Pless et al. (2021) mapped the landscape genetic connectivity for Aedes aegypti in the southern tier of North America. Inputs to the model were genetic distances (response variable) and 29 environmental variables (including human density). The map shows the genetic connectivity of the species and discusses the environmental and anthropogenic variables that are most important for predicting gene flow in the context of vector control. This type of information will be of particular importance in the case of GMGDM field releases.
Conservation is a challenging concept (Castilla, 2012) that can be analyzed according to four historical phases: a) conservation for itself, b) despite people, c) for people, and d) for people and nature (Kareiva and Marvier, 2007; Mace, 2014). Although experience and proof-of-concept data are lacking (National Academies of Sciences, Engineering, and Medicine, 2016), gene-drive borne organisms associated with potential biodiversity risks and benefits. Harmful impacts include the possible modification of wild species genetic diversity, population dynamics and eventually ecosystem services or producing wild species that cause local or wider eradications, where biodiversity losses might occur in nontargeted species such as parasitoids and hyperparasitoids (Nagel and Paveling, 2005). Benefits include the conservation of endangered wild species and the management of invasive species, designed to swiftly propagate a desire mutation or transgene into a wild population (Rode et al., 2019); or with regards to species de-extinction (Shapiro, 2017). Undoubtedly, in the area of GMGDMs (and other engineered species), critical decisions must be made regarding the potential benefits and harms of the technology in terms of people, environmental impacts, conservation, biodiversity, and management when considering research or field releases (National Academies of Sciences, Engineering, and Medicine, 2016; World Health Organization, 2020).
The power, effectiveness, and ease of this new technology have raised several ethical, philosophical, and bioethical issues. Since this is a particularly simple tool with huge potential, it seems that the risks associated with its misuse are very high (Callies, 2019). Thus, international researchers (Resnik, 2014; Emerson et al., 2017; Bouyer et al., 2019; Capps, 2019) and international agencies such as WHO (WHO, 2009; WHO, 2020; WHO, 2021) have tried to face the ethical concerns associated with these technologies. To be clear, we will distinguish between two kinds of ethical problems: i. a priori ethical/philosophical concerns and ii. a posteriori ethical/philosophical concerns. The issues related to the first point mainly refer to the impact that the paradigm imposed by gene drive systems may have upon our life, together with the system of values that the paradigm implies. The second point refers to the the consequences that this technology may have on the environment (us and other living beings).
The issues we discuss in this section mainly refer to the philosophical and ethical background implied by this new technology. Since gene drive systems may involve major changes to our relationship with nature—as they provide new possibilities and challenges—it is worth considering three main relevant issues: 1. The role of technologies in our life; 2. The human potentialities and the possible (ethical) limits to this power; and 3. The value of nature.
Almost every technology has a twofold role as a possible solution to many problems created by humans and the cause of other problems. This is also true for genetic modification or editing technologies. In this sense, every technology—as a form of life—is not value-free since it always implies benefits (or values) and possible dangers (or disvalues) (Sandler, 2012; 2014). In this sense, Verbeek argues: “Maturity in our thinking about technology requires that we no longer exclude technologies from the realm of ethics” (Verbeek, 2014). In this regard, thinking at gene drive technologies, an appropriate assessment of both the use of these technologies and their role in our life should be made. Nevertheless, a point should be previously clarified: the use of these technologies is not something good in itself (per se). This argument implies avoiding the “techno-fix” (or “techno-optimism”) mentality, that is the idea that solutions to all problems can be found in better and new technologies–even in the case of gene drive technologies. This perspective is permeated by naïve optimism regarding “smarter technology” and strong beliefs that nature is “tough and resilient” (Sideris, 2017). A controversial example of this approach is the claim that anthropogenic global warming or climate change may be solved through gene drive systems: are we fixing a problem or creating new ones? Whatever is the answer, an historical outlook shows that technology can be claimed responsible for many problems in the natural world, but it is unlikely that from the mere technological viewpoint we can, in turn, address these difficulties (Piaggio et al., 2017). This argument has been the background of the critique against technocracy, which is “the worship of and domination by technology. Technocracy involves the appropriation and transformation of large portions of the earth by and for technology and the reign of the one best, most efficient, rational method over all cultural variegation. […] Technocracy especially means consideration only of technical solutions to problems–and sticking to technocracy instead of environmental concerns” (McDonald, 2014, 346–349). In this regard, the main critique of this approach is that technocracy reduces the environmental crisis to a problem that may be solved technically without understanding the core of the crisis, including the ethical and philosophical concerns. The paradigm opposite to “technocracy” (or “technophilia”) is “technophobia” (or “techno-fear”, or “techno-indifference”) (Brand and Fischer, 2013), which classifies any use of technology as improper intervention. Therefore, the debate on the ethical liability of gene drive systems oscillates between these two opposing poles, with no plausible middle (or third) option.
This second topic is intrinsically connected to the first, as it describes the anthropological dimension linked to technological developments. In this sense, the Promethean aspirations (Pugh, 2016) of contemporary human beings could be transformed into their true essence or image, modifying their place in the cosmos. The power implied in technologies like gene editing or gene drive systems may increase our “Promethean immodesty” (Jonas, 1984, 201), eroding our appreciation of the intrinsic “giftedness” of nature and pushing us to be the masters of nature (Sandel, 2007; Cohen, 2014; Sandler, 2014; 2019). While using these powerful technologies, “we are in a way playing God, thinking that we ought to have such power, regardless of whether such power would deliver benefits or disastrous side effects” (Callies, 2019). Thus, by using these powerful tools, the human being would be explicitly called to manage (or administer) natural resources, generating new cosmologies. The new potentialities provided by gene drive technologies raise significant ethical questions: should we limit our power? Is humility a virtue we should cultivate? Are we truly playing God or are we only assuming a necessary role for humanity in the current technological age? The response to all these preliminary questions may orient our relationship with both technology and nature, which is at the core of the debate on gene drive technologies.
A possible limitation to our impact on the environment would be the intrinsic value of nature. This is, obviously, a huge issue that we do not intend to solve in this paper. We focus only on value of nature in relation to gene drive systems and the “permissibility of eradicating a species” (Pugh, 2016). Nevertheless, it is worth at least considering its epistemological value. Here, we are necessarily faced with the classic distinction between instrumental and intrinsic values of the natural world (O’Neill, 2003; Chisholm, 2005; Sandler, 2012) and the possibility that nature may have inherent value. In this regard, Pugh (2016) asks: “Is there anything intrinsically wrong with bringing about the extinction of another species? In objecting to species eradication as a form of pest control, the entomologist R.L. Metcalf wrote that ‘…species should be regarded as sacred and man indeed has no right to destroy them’. Melanie Challenger echoed this appeal to the sanctity of life in the current controversy surrounding gene-driven technologies, in comments published in a Guardian newspaper article: ‘Is there a more intrinsic philosophical reason why we should not drive an animal to extinction? My instinct is: yes … the sanctity of life”. This last argument forces us to inquire into the significance and ethical usefulness of the concepts of “sanctity of life” and intrinsic value when referring to nature: are they truly used to assess our impact on nature, or are they needed only to refrain from the “unquestioned assumption” that “native ecosystems are better than changed ecosystems” (Marris, 2011)? It is also worth considering that “intrinsic value […] is a concept we struggle even to identify or understand, let alone operationalize” (Bouyer et al., 2019). In this sense, has the idea of inherent values any meaning? Bouyer et al. (2019) argue that it should not be the only element to assess to make informed decisions: “Although inherently ethical, species conservation and management are also conditioned by broader social, economic, and political contexts, in which knowledge of instrumental value and disvalue is essential to informed decision-making. However, if we are committed to the claim that species possess intrinsic value, ethical analysis cannot be reduced to a mere calculation of net benefits and costs for humans. […] Intrinsic value is a basic property of goodness in the world: When we acknowledge intrinsic value, we acknowledge its bearer as a good in itself and for its own sake”. Thus, intrinsic values have a heuristic function in environmental assessment. Obviously, the idea of the intrinsic value of nature does not exclude the possibility that instrumental values do exist; it only implies that human impact should have limits and that our actions that affect the environment are not morally indifferent or neutral. Furthermore, from the perspective of human interests, species may have values, but they “also arguably have disvalues or provide disservices that counteract the human good. This observation is exemplified by so-called pest species, such as tsetse flies, which actively detract from human wellbeing” (Bouyer et al., 2019, 129). In this sense, when talking about the ethics of the genetic modification of mosquitoes, it is worth considering that “VBDs [vector-borne diseases] cause more than 700 000 deaths annually and are responsible for 17% of the global burden of communicable diseases” (WHO, 2020; WHO, 2021). A realist assessment of the human impact on nature, thus, should start from these considerations that properly weigh the value of natural entities, privileging an “et-et” perspective more than an “aut-aut” one: nature has both an intrinsic value (i.e., we cannot significantly change nature if we have not got relevant reasons) and, at the same time, it is the source of human values (or disvalues).
When ethically assessing the possible gene modifications in species, the most common ethical concerns refer to the safety and effectiveness of technologies such as CRISPR‒Cas9 or gene drive systems. Very briefly, these assessments mainly focus on the short- or long-term consequences of these technologies on ecosystems (an a posteriori assessment). In this sense, avoiding any a priori consideration, Capps argues: “The debates about gene drives should be about whether, and to what degree, they create public goods and bads; not whether they satisfy a personal virtue or vice. In general, the optimist points to the opportunities of engineered nature and enhanced ecological services, and that is why releasing gene drives is worthy of consideration” (Capps, 2019). In our opinion, these last considerations should not be separated from the abovementioned a priori concerns, which may offer a more complex view on the issue at stake.
The effectiveness of these technologies is, more than an ethical issue, a precondition for their implementation. In this sense, without the certainty of their effective impact, their use would be senseless or absurd. The assessment of this point is not easy, since, as Pugh (2016, 580) states, “we cannot be certain that gene-drive technology will be successful in eradicating mosquitoes. For it to do so, GMM, including GMGDM, would have to mate with ‘natural’ mosquitoes in the wild to pass on the modified genes, and these genes would have to be passed down multiple generations; neither is certain to occur”. The pivotal point is here, obviously, the complexity that these technologies imply. This aspect concerns both the accumulative effects of technology (Jonas, 1984) and the feedback responses generated by the ecosystem.
This last consideration allows us to focus on the second a posteriori dimension, that is, safety, which is strongly connected to complexity. Due to the nature of these gene technologies, their implementation may have side effects, that is, secondary and possibly adverse effects (Capps, 2019). These adverse effects may negatively affect other species, such as nontarget predator species (Resnik, 2014), the food chain (Bouyer et al., 2019) or even the whole ecosystem. Indeed, GMM “may disrupt the ecosystem by interbreeding with closely related species to form hybrids” (Resnik, 2014). The extreme powerfulness of this tool requires a careful assessment, mostly because its use for population eradication may involve changes of ecological interaction (e.g., species niche, competition, population dynamics) of species other that the target one (Gillies and Smith, 1960). In this regard, “risks and benefits have impact at a collective level, and the impact on communities can persist and increase over time” (WHO, 2021, 91–92). A risk assessment is more than necessary when modifying species to anticipate possible side effects (WHO, 2020).
In this sense, population replacement or population eradication raise important ethical questions, mainly due to the complexity of the consequences on the ecosystem and the effectiveness of such actions. It is not possible, in this case, to differentiate the ethical assessment regarding the two cases (i.e., replacing or eliminating a species). The moral issue, indeed, is mainly given by the same human impact on nature and the so called “heterogenesis of ends” (i.e., any action in a complex system could cause unpredictable and undesirable consequences, at least in the long run). In this case, a precautionary approach is mandatory: since we cannot predict the possible (positive or negative) consequences of a technological intervention on nature, it is worth acting cautiously–considering the possibility of not intervening.
The thoroughanalysis already conducted on the ecological and philosophical implications of the use of gene drive technology seems to leave little room for further speculation. Anyway, both the ongoing state of advancement of these gene-drive technologies and the challenging emergence of new values and cultures call for further reflections. Indeed, (Comradie, 2006), this same analysis has laid out that this kind of biotechnology not only has broad consequences for nature and humankind but specifically raises questions about how we relate to each other. A theological perspective can expand our categories for a deeper understanding of the way humankind is conceiving nature when we decide to heavily transform an ecosystem by modifying or wiping out a whole species.
Thus, we will focus on some questions that have already been addressed within theological thinking but for which a consensus has yet to be reached, which is unsurprising given that they are relatively recent questions in theological thinking, with eco-theology considered to originate in the nineties (Comradie, 2006).
In this section, we understand nature as the totality of beings other than God. It includes human beings, although some thinkers understand and still understand nature as “other than man” (Daston, 1995). In this sense, we would like to stress that there is a deep communality between beings precisely because they are all creatures. Creaturality is an understanding of being from a generative point of view. That is, a common origin. Big Bang theory, although not an account of creation, certainly stresses this common origin. Therefore, evolutionary theory affirms life, as we know it originated from a common ancestor. This fact shows that biocentric and anthropocentric perspectives are incomplete because they do not acknowledge this commonality. On the other hand, the Christian perspective is a rather theocentric approach (Hoffman and Sandelands, 2005). This does not mean that either humankind or the rest of living creatures are worthless; rather, they have a shared value inasmuch as they are created. The idea of man and woman created in God’s image, however, suggests that humans are different from the rest of creatures. In the rest of nature, we can find vestigia Dei, God’s work, or we can even conceive creation as a sacrament of God. These three categories mean we that can know something about God from creatures because God is, in some way, inner to them. The dialectics between immanence and transcendence is challenging to solve, although Christian theology is definitely not pantheistic and tends more to a desacralization of nature rather than an assimilation of it to the divine. The expression panen-theism is sometimes used as a way to underscore the need to keep together this polarity (Boff, 2015).
As a way to uphold the value of nature, it is often argued that nature has dignity (Huber, 1991). This is clearly an extensive use of a mainly anthropological concept. The endless debate on the nature of human dignity notwithstanding (Macklin, 2003), its extension to the rest of living beings is questionable. This is not only because it suggests a forced parity between humankind and the rest of living beings, which is precisely the distinction that the very concept of dignity tried to affirm but also because it does not consider the different ways of being that living beings have, although they are, in fact, all living. This does not mean that natural beings do not have an intrinsic value. They have it because they are creatures and are hence given to us, not produced by us. The human being, in fact, cannot produce life from nothing (and will probably never be able to) thus, at the very least, nature has intrinsic value because it cannot be replaced by humankind. Theologically speaking, the relation with God, rather than with humans, is what makes nature valuable. Humans, cannot, properly speaking, assign value, only acknowledge it. As we know, the classical legal distinction between persons and things (res et personae) has largely been outclassed by a differential approach to living beings and inanimate objects, natural or artificial. Inasmuch as we do not understand intrinsic value as equal value to humankind, there is no problem in speaking about nature’s intrinsic worth.
Acknowledging nature has intrinsic worth and does not provide all the answers needed to judge human intervention in nature. However, we can easily assume that humankind cannot do whatever he/she wants with nature. This is not unquestionable, though: there are people who assert the so-called technological imperative, which is “Whatever can be done, should (or at least is allowed to) be done” (Ozbekhan, 1968). Therefore, the question remains: What is the appropriate way for humankind to deal with nature? What are the limits of this intervention?
Although we can easily argue that care is the proper way of dealing with nature, since it is given rather than produced, this does not settle the question about the limits of human interventions on nature. There are basically two theological models that look to frame the answer to this question: humankind as steward or as cocreator.
The stewardship model is based on the mandate in Genesis 1,28. God puts creation on the human hands. As we know, this statement can and has been understood as illimited dominion; Lynn White’s argument is not totally outstretched (White, 1967). Nevertheless, the mainstream understanding of it implies a duty of care, which means at least do not harm (Berry, 2006). From this point of view, we can easily acknowledge that wiping out a whole species is not the kind of behavior God expects from human beings. That said, the question of what exactly we should care for is not easily answered. Is nature’s current state the one we need to care for?2 Is it its preindustrial state? How can we even think about a state if we do not have control of all the variables implied in the preservation of it? The issue is not more science to understand; it is about acknowledging that creation/nature is dynamic and evolving. It has directionality. Therefore, what is the human role regarding this directionality?
Building on the fact that humankind can influence nature, some theologians argue that the human being is a cocreator alongside God (Hefner, 1993). He collaborates and must collaborate with God in steering nature to its goal. This is mainly done through the human ability to transform nature, that is, technology. We all know modern technology is powerful enough to have a geological drive on Earth. That is what Anthropocene means: technology is changing parameters (such as carbon dioxide levels and, consequently, temperature) that were thought to be out of reach. Could it be that the human natural technical ability is a gift in the same way that nature is given? That is, assuming technical production is a natural human skill (theologically, a gift from the creator rather than a curse), could it be that we ought to use it to transform nature? This is the basic tenet that grounds the cocreator paradigm (Peterson, 2004). Thus, this paradigm basically affirms that the humans must transform nature, not in every possible way, but in collaboration with God’s Providence to help creation attain its goal3. This means that every time we evaluate a technical intervention targeting nature, we should ask not only if it is beneficial to humankind but also if it helps to fulfill Creation’s goal.
An obvious critique of this paradigm is that if we hardly know what it means to preserve nature, we cannot possibly know where to direct nature. It is well known that “from chance to choice” was one of the very first eugenics’ motto (Koch, 2011). Before thinking about which one is better, chance or choice, we certainly need to know what we are choosing exactly and that its outcome is better, not only for the individual, not even to humankind as a species, but to the whole of Creation. Therefore, the main problem with the cocreator paradigm is that we barely know how to “help” God in steering nature. Most likely, leaving nature alone as much as possible is the best choice or, at least, trying to insert our technological drive into nature’s directionality. Of course, some would argue that nature has no directionality; it is only outdated teleological thinking. Nevertheless, teleology and God’s providence are practically synonyms from a theological point of view; thus, this position is not difficult to accept from theological groundings (Aquinas, 1981).
As we can see, both paradigms presesnt serious challenges; thus, neither is likely better than the other. The appropriate way of thinking about the human role in nature is probably in the middle and should possibly be reactive rather than proactive, in the sense we need to address what we can clearly perceive as harm to nature rather than steering nature into a supposedly better path toward its goal (Jonas, 1984). Chance, or rather nature’s own dynamisms, which are providential rather than hazardous, are surely better for both nature and humans. Therefore, the very first principle of technological interventions in nature would probably be “first do not harm”, which is likely a safer way to align God’s action, often named creatio continua, with humankind’s free agency on nature.
Releasing GMGDMs in an ecosystem may represent either a significant advance for preventing human infections, such as malaria, Dengue, Zika, etc., or a major threat to the ecosystem balance and the species involved. In this sense, the gene drive technology based on CRISPR/CAS9 is quite different from other genetic modifications since the modification spreads in a higher-than-Mendelian ratio in the target population, representing a new way to regulate or even suppress a whole population. This technology still plays a twofold role since it may create or solve many concerns. As the WHO correctly highlights, “humans have a complex relationship with the environment, variably acting in ways that either instrumentalism nature or protect it” (WHO, 2021). Genetic engineering complicates this relationship by introducing the ability to do both things at once. What are, then, the criteria (or paradigms) to use when assessing the use of technology such as CRISPR/Cas9 and gene drive systems to release GMMs in a defined ecosystem? In the following section, we will point out some ethical considerations concerning GMGDM release.
It is important to follow a precautionary approach, which calls for more investigation and “asks (at least) four questions. First, is the technology already known to have intolerable risks? Second, does it have significant benefits? Third, do these benefits solve important problems? Fourth, could these problems be solved in some other, less risky way? The precautionary approach suggests that we should proceed only if the answers to these questions are as follows: no, we do not know it to have intolerable risks; yes, it has real benefits; yes, it solves real problems; and no, we cannot solve the problem in other ways” (Wolff, 2014). The precautionary approach provides a provisional guide for action: to prudential judgment (Marcos, 2014) Ultimately, it is a “modus operandi” (Valera and Marcos, 2017), that is, an attitude that would adequately complement the more conventional principles used for ethical assessment. Why is a precautionary approach necessary in this context? First, for epistemic reasons.
According to some authors, ecology seems to have “abandoned predictions as a central focus and faces its own crisis of reproducibility” (Houlahan et al., 2017). The necessary uncertainty in ecological projections (Neupane et al., 2022), due to the complexity of the systems they are addressing, may imply the fact that we will try to imagine different possible scenarios depending on certain conditions. In this sense, unexpected events (e.g., surprises) play a central role in ecology (Doak et al., 2008): given their frequent occurrence, such events make certain predictions difficult. These dimensions are strictly connected to the very aim of ecology itself, that is, a pragmatic rather than speculative science (Pablo, 1997). In this sense, as we mentioned above, precaution is more than necessary: uncertain scenarios must be faced with extreme prudence, given the impossibility of balancing most of the more relevant consequences of our actions.
Second, whether or not to release GMGDMs into the wild implies considering that we are not truly facing a true dilemma (an “aut-aut”): between “conserving the environment” and “altering it”. First, “ecosystems are always changing, whether humans are involved or not” (Marris, 2011). In this sense, there is no ecosystem that always maintains its state and does not change. Human impact on Earth is an unavoidable starting point. Nevertheless, it is worth noting that we can mitigate our impact; the paradigm of responsible stewardship precisely argues that human beings have a relevant role in gardening our planet: “Responsible stewardship […] is our responsibility as humans and stewards of the natural world to avoid taking extreme stances regarding new technologies” (Piaggio et al., 2017). Stewarding or taking care of the Earth then does not mean preventing its development, nor does it imply that we must leave it as it is and not change it. Likewise, building does not mean destroying everything. Our responsibility is both directed toward nature as its end and toward technology as its object. The substantial human power endorsed by technology must simultaneously imply certain obligations for us. In this sense, the first duty for us is to foresee the possible consequences of the use of technologies such as CRISPR/Cas9 or gene drive systems, even though these predictions may be fallible or imprecise (Wolff, 2014). Thus, certain knowledge both of the possible short- and long-range consequences of the release of GMGDM in the ecosystem is mandatory.
Third, when considering possible GMGDM release in an ecosystem, we must consider that every decision may stem—more or less—from a shared culture and ethics. In this regard, community acceptance, for instance, of GMGDM operations, especially including steps of insect field release and community feedback information, will be critical elements for acceptance and success. Regarding GMGDM research and field releases, requirements for due governance must be considered at the international, national, and local scales. Many calls have been made to the community and stakeholders for effective engagement regarding combating VBDs through the use of genetically engineered species (WHO, 2020). True engagement, from the beginning of the process, increases the effectiveness of research, field implementation, follow-up, monitoring and reporting of results to communities.
Fourth, it is worth considering that—as many philosophers and environmental activists have pointed out—we not only share culture with our peers but we also intertwined with nonhuman living beings (Valera, 2018). There is a strong interconnection between humans and the environment. This is true at both the ontological and existential levels. When thinking of possible practical paradigms that may operatively translate this interconnection, One Health (OH) ethics (Capps, 2019) is particularly relevant and consistent with a theological approach that points out the unity of nature. Indeed, it “began as an aspiration to achieve improved health for people, animals and environment” (Capps, 2019), criticizing the dichotomy between human health and environmental health. This paradigm may help us focus both on human interests and ecological value since our health is strongly connected to the ecosystem, introducing environmental ethics in public health debates (Capps, 2019). It is about reconstituting the bridge between bioethics, public health ethics, and environmental ethics, just as Potter (Potter, 1971; Potter, 1988) envisioned at the very beginning of bioethics (Lee, 2017). This could help us assess with greater complexity the environmental, public health, and human health issues that the release of GDMs seek to combat, we should not forget that diverting funding (and profits) to technological solutions must not defund or weaken low-tech measures that have proven efficacy against those diseases. The choice is not between GMGDMs or nothing but between expensive, high-tech solutions and cheap but well-established efficacious ones.
RM: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Writing–original draft, Writing–review and editing. LV: Investigation, Writing–original draft, Writing–review and editing. CB: Conceptualization, Investigation, Methodology, Writing–review and editing. JC: Investigation, Methodology, Writing–review and editing. JR: Investigation, Writing–review and editing.
This work was funded by grant INTERDISCIPLINA II190084 from Vicerectoria de Investigación (VRI), Pontificia Universidad Católica de Chile, ANID/Fondecyt 1210081 and ANID/BASAL FB210018.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1We understand theology from a Catholic point of view, although, in these matters it is practically undistinguishable from other Christian theologies
2Earth was an iceball some million years ago, should we act to bring the iceball back?
3This is theologically said using the category of redemption of nature. Since the whole of creation is harmed by sin, its redemption is the action by which God takes her to its plenitude. Human being as co-creator means also that he/she is co-redeemer, in the sense that he/she has to collaborate, humanly, and thus technically, with God’s work. The building of something such as a Noah’s ark to save species could be a good example of this kind of co-creative action
Benedict Mq, R. A. (2003). The first releases of transgenic mosquitoes: an argument for the sterile insect technique. Trends Parasitol. 19, 349–355. doi:10.1016/s1471-4922(03)00144-2
Bier, E. (2022). Gene drives gaining speed. Nat. Rev. Genet. 23 (1), 5–22. doi:10.1038/s41576-021-00386-0
Bouyer, J., Carter, N. H., Batavia, C., and Nelson, M. P. (2019). The ethics of eliminating harmful species: the case of the tsetse fly. BioScience 69 (2), 125–135. doi:10.1093/BIOSCI/BIY155
Brand, R., and Fischer, J. (2013). Overcoming the technophilia/technophobia split in environmental discourse. Env. Polit. 22 (2), 235–254. doi:10.1080/09644016.2012.730264
Burt, A. (2003). Site-specific selfish genes as tools for the control and genetic engineering of natural populations. Proc. Biol. Sci. 270 (), 921–928. doi:10.1098/rspb.2002.2319
Burt, A., and Koufopanou, V. (2004). Homing endonuclease genes: the rise and fall and rise again of a selfish element. Curr. Opin. Cell Biol. 14, 609–615. doi:10.1016/j.gde.2004.09.010
Callies, D. E. (2019). The ethical landscape of gene drive research. Bioethics 33 (9), 1091–1097. doi:10.1111/BIOE.12640
Capps, B. (2019). Gene drive gone wild: exploring deliberative possibilities by developing One Health ethics. Law Innov. Technol. 11 (2), 231–256. doi:10.1080/17579961.2019.1665789
Carvalho, D. O., McKemey, A. R., Garziera, L., Lacroix, R., Donnelly, C. A., Alphey, L., et al. (2015). Suppression of a field population of Aedes aegypti in Brazil by sustained release of transgenic male mosquitoes. PLoS Neglected Trop. Dis. 9 (7)e0003864. doi:10.1371/journal.pntd.0003864
Castilla, J. C. (2012). Conservation and social-ecological systems in the 21st century of the Anthropocene era. CONTRIBUTIONS Sci. 8 (8), 11–21.
Champer, J., Buchman, A., and Akbari, O. S. (2016). Cheating evolution: engineering gene drives to manipulate the fate of wild populations. Nat. Rev. Genet. 17 (3), 146–159. doi:10.1038/nrg.2015.34
Chisholm, R. M. (2005). “Intrinsic value,” in Recent work on intrinsic value. Editors T. Rønnow-Rasmussen, and M. J. Zimmerman (Springer).
Cohen, S. (2014). The ethics of de-extinction. NanoEthics 8 (2), 165–178. doi:10.1007/S11569-014-0201-2
Courtier-Orgogozo, V., Danchin, A., Gouyon, P. H., and Boëte, C. (2020). Evaluating the probability of CRISPR-based gene drive contaminating another species. Evol. Appl. 13 (8), 1888–1905. doi:10.1111/eva.12939
Daston, L. (1995). “How nature became the other: anthropomorphism and anthropocentrism in early modern natural philosophy,” in Biology as society, society as biology: metaphors. Sociology of the sciences. Editors S. Maasen, E. Mendelsohn, and P. Weingart (Springer).
Devos, Y., Mumford, J. D., Bonsall, M. B., Glandorf, D. C. M., and Quemada, H. D. (2022). Risk management recommendations for environmental releases of gene drive modified insects. Biotechnol. Adv. 54 (), 107807. doi:10.1016/j.biotechadv.2021.107807
Doak, D. F., Estes, J. A., Halpern, B. S., Jacob, U., Lindberg, D. R., Lovvorn, J., et al. (2008). Understanding and predicting ecological dynamics: are major surprises inevitable? Ecology 89 (4), 952–961. doi:10.1890/07-0965.1
Dolezel, M., Lüthi, C., and Gaugitsch, H. (2020). Beyond limits – the pitfalls of global gene drives for environmental risk assessment in the European Union. BioRisk 15, 1–29. doi:10.3897/biorisk.15.49297
Dong, S., Dong, Y., Simões, M. L., and Dimopoulos, G. (2022). Mosquito transgenesis for malaria control. Trends Parasitol. 38 (1), 54–66. doi:10.1016/j.pt.2021.08.001
Emerson, C., James, S., Littler, K., and Randazzo, F. (2017). Principles for gene drive research. Science 358 (6367), 1135–1136. doi:10.1126/SCIENCE.AAP9026
Ermert, V., Fink, A. H., Jones, A. E., and Morse, A. P. (2011). Development of a new version of the liverpool malaria model. II. Calibration and validation for west africa. Malar. J. 10, 62. doi:10.1186/1475-2875-10-62
Feldmann, U., and Hendrichs, J. (2001). Integrating the sterile insect technique as a key component of area-wide tsetse and trypanosomiasis intervention. Available at: https://www.fao.org/3/Y2022E/y2022e01a.htm.
Ferguson, H. M., Dornhaus, A., Beeche, A., Borgemeister, C., Gottlieb, M., Mulla, M. S., et al. (2010). Ecology: a prerequisite for malaria elimination and eradication. PLoS Med. 7 (8)e1000303. doi:10.1371/journal.pmed.1000303
Frieß, J. L., Gleich, A. V., and Giese, B. (2019). Gene drives as a new quality in GMO releases — a comparative technology characterization. Peer J. 7, 6793. peerj. doi:10.7717/peerj.6793
Fuchs, S., Garrood, W. T., Beber, A., Hammond, A., Galizi, R., Gribble, M., et al. (2021). Resistance to a CRISPR-based gene drive at an evolutionarily conserved site is revealed by mimicking genotype fixation. PLoS Genet. 17 (10)e1009740. doi:10.1371/journal.pgen.1009740
Gantz, V. M., Jasinskiene, N., Tatarenkova, O., Fazekas, A., Macias, V. M., Bier, E., et al. (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. PNAS 112 (49), E6736–E6743. doi:10.1073/pnas.1521077112
Gillies, M. T., and Smith, A. (1960). The effect of a residual house-spraying campaign in east africa on species balance in the anopheles funestus group. the replacement of a. funestus giles by a. rivulorum leeson. Bull. Entomological Res. 51 (2), 243–252. doi:10.1017/S0007485300057953
Gu, W., and Novak, R. J. (2005). HABITAT-BASED MODELING OF IMPACTS OF MOSQUITO LARVAL INTERVENTIONS ON ENTOMOLOGICAL INOCULATION RATES, INCIDENCE, AND PREVALENCE OF MALARIA. Am. J. Trop. Med. Hyg. 73 (3), 546–552. doi:10.4269/AJTMH.2005.73.546
Hammond, A., Pollegioni, P., Persampieri, T., North, A., Minuz, R., Trusso, A., et al. (2021). Gene-drive suppression of mosquito populations in large cages as a bridge between lab and field. Nat. Commun. 12 (1), 4589–9. doi:10.1038/s41467-021-24790-6
Harbach, R. E. (2023). Mosquito taxonomic inventory. 1 B.C.E., from, Available at: https://mosquito-taxonomic-inventory.myspecies.info/valid-species-list (Accessed September 30).
Harris, A. F., McKemey, A. R., Nimmo, D., Curtis, Z., Black, I., Morgan, S. A., et al. (2012). Successful suppression of a field mosquito population by sustained release of engineered male mosquitoes. Nat. Biotechnol. 30 (9), 828–830. doi:10.1038/nbt.2350
Hoffman, A. J., and Sandelands, L. E. (2005). Getting right with nature: anthropocentrism, ecocentrism, and theocentrism. Organ. Environ. 18 (2), 141–162. doi:10.1177/1086026605276197
Holt, R. D. (2009). Bringing the Hutchinsonian niche into the 21st century: ecological and evolutionary perspectives. Proc. Natl. Acad. Sci. U. S. A. 106 (2), 19659–19665. doi:10.1073/PNAS.0905137106
Houlahan, J. E., McKinney, S. T., Anderson, T. M., and McGill, B. J. (2017). The priority of prediction in ecological understanding. Oikos 126 (1), 1–7. doi:10.1111/OIK.03726
Huber, W. (1991). Rights of nature or dignity of nature? Annu. Soc. Christian Ethics 11, 43–60. doi:10.5840/ASCE1991114
James, S., Collins, F. H., Welkhoff, P. A., Emerson, C., J Godfray, H. C., Gottlieb, M., et al. (2018). Pathway to deployment of gene drive mosquitoes as a potential biocontrol tool for elimination of malaria in sub-Saharan Africa: recommendations of a scientific working group. Am. J. Trop. Med. Hyg. 98 (6), 1–49. doi:10.4269/ajtmh.18-0083
Jonas, H. (1984). The imperative of responsibility: in search of an ethics for the technological age. University of Chicago Press.
Kareiva, P., and Marvier, M. (2007). Conservation for the people. Sci. Am. 297 (4), 50–57. doi:10.1038/SCIENTIFICAMERICAN1007-50
Klanssen, W., and Curtis, C. H. J. (2021). “History of the sterile insect technique,” in Sterile insect technique. Principles and practice in area-wide integrated pest managment. Editors R. Dyck VA, and A. Hendrichs (Boca Ratón: CRC Press), 1218.
Koch, T. (2011). Eugenics and the genetic challenge, again: all dressed up and just everywhere to go. Camb. Q. Healthc. Ethics 20 (2), 191–203. doi:10.1017/S0963180110000848
Larson, S. R., Degroote, J. P., Bartholomay, L. C., and Sugumaran, R. (2010). Ecological niche modeling of potential West Nile virus vector mosquito species in Iowa. J. Insect Sci. (Online) 10, 1–17. doi:10.1673/031.010.11001
Lee, L. M. (2017). A bridge back to the future: public health ethics, bioethics, and environmental ethics. Am. J. Bioeth. AJOB 17 (9), 5–12. doi:10.1080/15265161.2017.1353164
Macklin, R. (2003). Dignity is a useless concept. BMJ 327 (7429), 1419–1420. doi:10.1136/BMJ.327.7429.1419
Marcos, A. (2014). “Principio de precaución: un enfoque (neo)aristotélico,” in Man, culture, security. Editor P. Gondek (Lublin: Fundacja Szkola Filozofii Chrzescijanskiej), 43–58.
Marec, F., Bloem, S., and Carpenter, J. E. (2021). “Inherited sterility in insects,” in Sterile insect technique. Principles and practice in area-wide integrated pest managment. Editors A. S. ROBINSON, V. A. Dyck, and J. Hendriichs (Boca Ratón: CRC Press), 1218.
McDonald, H. P. (2014). Environmental philosophy. A revaluation of cosmopolitan ethics from an ecocentric standpoint. New York: Rodopi.
Meghani, Z., and Kuzma, J. (2018). Regulating animals with gene drive systems: lessons from the regulatory assessment of a genetically engineered mosquito. J. Responsible Innovation 5, S203–S222. doi:10.1080/23299460.2017.1407912
Miles, A., Harding, N. J., Bottà, G., Clarkson, C. S., Antão, T., Kozak, K., et al. (2017). Genetic diversity of the African malaria vector anopheles gambiae. Nature 552, 96–100. doi:10.1038/nature24995
Mitchell, P. D., Brown, Z., and McRoberts, N. (2018). Economic issues to consider for gene drives. J. Responsible Innovation 5, S180–S202. doi:10.1080/23299460.2017.1407914
Molineaux, L., Dietz, K., and Thomas, A. (1978). Further epidemiological evaluation of a malaria model. Bull. World Health Organ. 56 (4), 565–571. /pmc/articles/PMC2395644/?report=abstract.
Nagel, P., and Paveling, R. (2005). “Environment and the sterile insect technique,” in Principles and practice in area-wide integrated pest manageme. Editors V. A. Dick, J. Hendrichs, and A. S. Robinson (Springer), 499–524.
Nateghi Rostami, M. (2020). CRISPR/Cas9 gene drive technology to control transmission of vector-borne parasitic infections. Parasite Immunol. 42 (9), 127622–e12810. doi:10.1111/pim.12762
National Academies of Sciences, Engineering, and Medicine (2016). Gene drives on the horizon: advancing science, navigating uncertainty, and aligning research with public values. Washington DC: The National Academies Press. doi:10.17226/23405
Neupane, N., Zipkin, E. F., Saunders, S. P., and Ries, L. (2022). Grappling with uncertainty in ecological projections: a case study using the migratory monarch butterfly. Ecosphere 13 (1), e03874. doi:10.1002/ECS2.3874
Noble, C., Min, J., Olejarz, J., Buchthal, J., Chavez, A., Smidler, A. L., et al. (2019). Daisy-chain gene drives for the alteration of local populations. Proc. Natl. Acad. Sci. U. S. A. 116 (17), 8275–8282. doi:10.1073/pnas.1716358116
North, A. R., Burt, A., and Godfray, H. C. J. (2020). Modelling the suppression of a malaria vector using a CRISPR-Cas9 gene drive to reduce female fertility. BMC Biol. 18 (1), 98–15. doi:10.1186/s12915-020-00834-z
Ochieng, A. O., Nanyingi, M., Kipruto, E., Ondiba, I. M., Amimo, F. A., Oludhe, C., et al. (2016). Ecological niche modelling of Rift Valley fever virus vectors in Baringo, Kenya. Infect. Ecol. Epidemiol. 6 (1), 32322. doi:10.3402/IEE.V6.32322
O’Neill, J. (2003). “The varieties of intrinsic values,” in Environmental ethics. An anthology. Editors A. Light, and H. I. Rolston (Blackwell).
Ozbekhan, H. (1968). “The triumph of technology: “Can implies ought.”,” in Planning for diversity and choice. Editor S. Anderson (MIT Press).
Pablo, A. L. (1997). Reconciling predictions of decision making under risk:Insights from a reconceptualized model of risk behaviour. J. Manag. Psychol. 12 (1), 4–20. doi:10.1108/02683949710164217
Peterson, G. R. (2004). THE CREATED CO-CREATOR: WHAT IT IS AND IS NOT. Zygon® 39 (4), 827–840. doi:10.1111/J.1467-9744.2004.00622.X
Piaggio, A. J., Segelbacher, G., Seddon, P. J., Alphey, L., Bennett, E. L., Carlson, R. H., et al. (2017). Is it time for synthetic biodiversity conservation? Trends Ecol. Evol. 32 (2), 97–107. doi:10.1016/J.TREE.2016.10.016
Pless, E., Saarman, N. P., Powell, J. R., Caccone, A., and Amatulli, G. (2021). A machine-learning approach to map landscape connectivity in Aedes aegypti with genetic and environmental data. Proc. Natl. Acad. Sci. U. S. A. 118 (9), e2003201118. doi:10.1073/PNAS.2003201118
Potter, V. R. (1988). Global bioethics. Building on the leopold legacy. East Lansing: Michigan State University Press.
Power, M. E., Tilman, D., Estes, J. A., Menge, B. A., Bond, W. J., Mills, L. S., et al. (1996). Challenges in the quest for keystones: identifying keystone species is difficult-but essential to understanding how loss of species will affect ecosystems. BioScience 46 (8), 609–620. doi:10.2307/1312990
Pugh, J. (2016). Driven to extinction? The ethics of eradicating mosquitoes with gene-drive technologies. J. Med. Ethics 42 (9), 578–581. doi:10.1136/medethics-2016-103462
Resnik, D. B. (2014). Ethical issues in field trials of genetically modified disease-resistant mosquitoes. Dev. World Bioeth. 14 (1), 37–46. doi:10.1111/dewb.12011
Ressel, S., Charpentier, E., Wong, S. P., and Bratovič, M. (2018). The biology of CRISPR-cas: backward and forward. Cell 172, 1239–1259. doi:10.1016/j.cell.2017.11.032
Richman, R., Diallo, D., Diallo, M., Sall, A. A., Faye, O., Diagne, C. T., et al. (2018). Ecological niche modeling of Aedes mosquito vectors of chikungunya virus in southeastern Senegal. Parasites Vectors 11 (1), 255. doi:10.1186/S13071-018-2832-6
Rode, N. O., Estoup, A., Bourguet, D., Courtier, V., and Florence, O. (2019). Population management using gene drive: molecular design, models of spread dynamics and assessment of ecological risks. Conserv. Genet. 20 (4), 671–690. doi:10.1007/s10592-019-01165-5
Sandel, M. J. (2007). The case against perfection: ethics in the age of genetic engineering. Harvard: Harvard University press.
Sandler, R. (2014). The ethics of reviving long extinct species. Conserv. Biol. 28 (2), 354–360. doi:10.1111/cobi.12198
Sandler, R. (2019). The ethics of genetic engineering and gene drives in conservation. Conserv. Biol. 34 (2), 378–385. doi:10.1111/cobi.13407
Schowalter, T. D. (2006). Insect ecology: an ecosystem approach. Insect Ecol. Ecosyst. Approach, 1–572. doi:10.1016/B978-0-12-088772-9.X5022-5
Servick, K. (2019). Doubts persist for claimed Alzheimer's drug. Science 366, 1298. doi:10.1126/science.366.6471.1298
Shapiro, B. (2017). Pathways to de-extinction: how close can we get to resurrection of an extinct species? Funct. Ecol. 31 (5), 996–1002. doi:10.1111/1365-2435.12705
Shaw, W. R., and Catteruccia, F. (2019). Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat. Microbiol. 4 (1), 20–34. doi:10.1038/s41564-018-0214-7
Sideris, L. H. (2017). Consecrating science: wonder, knowledge, and the natural world. Berkeley: University California press.
Simard, F., Ayala, D., Kamdem, G. C., Pombi, M., Etouna, J., Ose, K., et al. (2009). Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecol. 9, 17. doi:10.1186/1472-6785-9-17
Soberon, J., and Peterson, A. T. (2005). Interpretation of models of fundamental ecological niches and species’ distributional areas. Biodivers. Inf. 2 (0), 1–10. doi:10.17161/BI.V2I0.4
Stoddard, B. L. (2011). Homing endonucleases: from microbial genetic invaders to reagents for targeted DNA modification. Structure/Folding Des. 19 (1), 7–15. doi:10.1016/j.str.2010.12.003
Teem, J. L., Alphey, L., Descamps, S., Edgington, M. P., Edwards, O., Gemmell, N., et al. (2020). Genetic biocontrol for invasive species. Genet. Biocontrol Invasive Species 8, 452–518. doi:10.3389/fbioe.2020.00452
Thomas, D. D., Donnelly, C. A., Wood, R. J., and Alpheyl, L. S. (2000). Insect population control using a dominant, repressible, lethal genetic system. , 287, 2474–2476. doi:10.1126/science.287.5462.2474
Uk, T., Islands, C., Islands, C., Uk, G., Committee, N. C., Islands, C., et al. (2017). Oxitec ’ s genetically modified mosquitoes: failing in the field ? September, 1–21.
Valera, L. (2018). Home, ecological self and self-realization: understanding asymmetrical relationships through arne næss’s ecosophy. J. Agric. Environ. Ethics 31 (6), 661–675. doi:10.1007/S10806-018-9715-X
Valera, L., and Marcos, A. (2017). “Bioética, ética del medio ambiente y ecología humana: la prudencia como un medio de supervivencia para el ser humano,” in Ecología humana contemporánea. Apuntes y visiones en la complejidad del desarrollo. Editors A. Insfrán Ortiz, M. J. Aparecido Meza, and R. Gomes Alvim (San Lorenzo: Universidad Nacional de Asunción).
Vella, M. R., Gunning, C. E., Lloyd, A. L., and Gould, F. (2017). Evaluating strategies for reversing CRISPR-Cas9 gene drives. Sci. Rep. 7 (1), 11038–8. doi:10.1038/s41598-017-10633-2
Verbeek, P.-P. (2014). “Some misunderstandings about the moral significance of technology,” in The moral status of technical artefacts. Editors P. Kroes, and P.-P. Verbeek (Springer), 75–88.
Webster, S. H., Vella, M. R., and Scott, M. J. (2020). Development and testing of a novel killer–rescue self-limiting gene drive system in Drosophila melanogaster. Proc. R. Soc. B Biol. Sci. 287, 20192994. doi:10.1098/rspb.2019.2994
White, L. (1967). The historical roots of our ecologic crisis. Science 155 (3767), 1203–1207. doi:10.1126/SCIENCE.155.3767.1203
White, M. T., Griffin, J. T., Churcher, T. S., Ferguson, N. M., Basáñez, M. G., and Ghani, A. C. (2011). Modelling the impact of vector control interventions on Anopheles gambiae population dynamics. Parasites Vectors 4 (1), 153. doi:10.1186/1756-3305-4-153
Winskill, P., Harris, A. F., Morgan, S. A., Stevenson, J., Raduan, N., Alphey, L., et al. (2014). Genetic control of Aedes aegypti: data-driven modelling to assess the effect of releasing different life stages and the potential for long-term suppression. Parasites Vectors 7 (1), 68–11. doi:10.1186/1756-3305-7-68
Wolff, J. (2014). The precautionary attitude: asking preliminary questions. Hastings Cent. Rep. 44 (6), S27–S28. doi:10.1002/HAST.393
World Health Organization (WHO) (2020). Evaluation of genetically modified mosquitoes for the control of vector-borne diseases: position Statement. Geneva: WHO.
World Health Organization (WHO) (2021). Guidance framework for testing of genetically modified. Geneva: WHO.
World Health Organization (WHO) (2009). Progress and prospects for the use of genetically modified mosquitoes to inhibit disease transmission. Geneva: WHO.
World Health Organization (WHO) (2014). Guidance framework for testing of genetically modified mosquitoes, World Health Organization on Behalf of the Special Programme for Research and Training in Tropical Diseases. Geneva: WHO.
Keywords: mosquito, stewardship, ecology, malaria, CRISPR/Cas9, gene drives
Citation: Moreno RD, Valera L, Borgoño C, Castilla JC and Riveros JL (2024) Gene drives, mosquitoes, and ecosystems: an interdisciplinary approach to emerging ethical concerns. Front. Environ. Sci. 11:1254219. doi: 10.3389/fenvs.2023.1254219
Received: 06 July 2023; Accepted: 28 December 2023;
Published: 12 January 2024.
Edited by:
Adam E. Vorsino, United States Fish and Wildlife Service, United StatesReviewed by:
Irina Morozova, Institute for Globally Distributed Open Research and Education (IGDORE), RussiaCopyright © 2024 Moreno, Valera, Borgoño, Castilla and Riveros. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ricardo D. Moreno, cm1vcmVub0BiaW8ucHVjLmNs
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.