
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 26 October 2023
Sec. Toxicology, Pollution and the Environment
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1248373
This article is part of the Research TopicRemediation and Health Risks of Heavy Metal Contaminated SoilsView all 14 articles
 Shraddha Mohanty1*
Shraddha Mohanty1* Rabindra Kumar Nayak1
Rabindra Kumar Nayak1 Bandita Jena1
Bandita Jena1 Kshitipati Padhan1
Kshitipati Padhan1 Kiran Kumar Mohapatra1
Kiran Kumar Mohapatra1 Sanjib Kumar Sahoo1
Sanjib Kumar Sahoo1 Prava Kiran Dash1
Prava Kiran Dash1 Jyotirmayee Das1
Jyotirmayee Das1 Sujit Kumar Behera2
Sujit Kumar Behera2 Anukiran Sahu2
Anukiran Sahu2 Jitendra Kumar Nayak3
Jitendra Kumar Nayak3 Sudipta Padhan4
Sudipta Padhan4 Diptanu Datta5
Diptanu Datta5Introduction: Chronic kidney disease of unknown aetiology (CKDu) is an emerging public health concern in India. The present study was carried out to investigate the concentrations of potentially toxic heavy metals (Cd, Pb, Ni, Cr, Hg, and As) in locally grown food crops (rice, pulses, and vegetables) in CKDu prevalent areas of Cuttack district, India.
Methods: Exposure risks from food crops were analysed, including estimated daily intake, hazard quotient, hazard index, and carcinogenic risk.
Result: The overall heavy metal concentrations in the crop samples were in the following order: Pb>Ni>Cd>Cr>As>Hg. The mean concentration of heavy metals in different crops were as follows, ranked from highest to lowest: spinach, rice, okra, mustard, potato, carrot, tomato, green gram, black gram. A statistical multivariate analysis revealed that the primary sources of Cd, Pb, Ni, Cr, Hg, and As in crop samples were both natural and human activities. For lead, target hazard quotient (THQ) values in rice were greater than 1, indicating significant noncarcinogenic health risks to both adults and children.
Discussion: While the majority of the crop samples had Pb levels below the permissible level (10−5), the target carcinogenic risk of Cd was higher than the USEPA threshold value (10−4), showing a cancer risk to adults and children. This study concluded that long-term intake of locally grown food crops may produce a significant health risk to the local inhabitants, and that of regular heavy metal monitoring is strongly recommended in this region.
The contamination of soil and crops (grains, fruits, and vegetables) with hazardous metals, viz., cadmium (Cd), lead (Pb), chromium (Cr), nickel (Ni), copper (Cu), and zinc (Zn), is detrimental to the environment owing to their persistent and non-biodegradable nature and is primarily caused by natural and anthropogenic processes (Radwan and Salama, 2006; Shah et al., 2010; Muhammad et al., 2011; Sekomo et al., 2011). Heavy metals are taken up by crops together with other necessary soil nutrients, and the buildup of these metals is typically greater in crops cultivated in polluted soils than in those grown in uncontaminated soils (Jan et al., 2010; Yang et al., 2011; Ratul et al., 2018). Weathering of metal-bearing minerals and volcanic eruptions are natural or geological sources of heavy metals in the environment. Nevertheless, the use of wastewater for irrigation purposes has been found to have a significant effect on the buildup of both inorganic and organic contaminants within the soil (Arora et al., 2008; Rehman et al., 2019). Consequently, these contaminants can be taken up by plants that are subjected to irrigation with such wastewater (Singh et al., 2004; Rashid et al., 2022). Undoubtedly, the utilisation of wastewater for irrigation has resulted in a reduction in the demand on freshwater resources. Heavy metals deposited in food crops have been shown to reach the human body by inhalation and consumption (Mamat et al., 2014; Abuduwaili et al., 2015). These metals, once inside the body, interfere with enzymes, slowing or stopping essential physiological processes. Over time, they can lead to serious health issues, including anaemia, kidney failure, and brain damage (Pappas et al., 2006; Mitra et al., 2022). For example, the consumption of food contaminated with Cd has been linked to both acute and chronic health effects, including kidney damage, poor bone development, hypertension, and even cancer (Raknuzzaman et al., 2016).
Chronic kidney disease of unknown aetiology (CKDu) is a kidney disease that progresses very slowly and is almost asymptomatic until severe and cannot be attributed to diabetes, hypertension, or any other known causes (Gooneratne et al., 2008; Jayasumana et al., 2013). In Cuttack district, India, the disease was identified for the first time in the early 2000s. The male population of the area is more susceptible to the disease compared to the women by a ratio of 3:2. However, young men under the age of 50 who participated in agricultural activities had a higher prevalence of the disease (Varma, 2015; Senapati et al., 2018; ICMR, 2020; Mohanty et al., 2020). Almost every household in the area has been diagnosed with the sickness. The cause of CKDu being endemic to a particular region is still unclear, and many researchers are striving to uncover it as thousands of people suffer from this illness. There are 697.5 million people with chronic kidney disease worldwide, with 115.1 million of them being Indian (Cockwell and lori-Ann, 2020). The key histological characteristics of CKDu are interstitial fibrosis, interstitial mononuclear cell infiltration, and tubular atrophy. These histological changes suggest a role for nephrotoxins in the origins of CKDu (Nanayakkara et al., 2012).
At high exposure levels (more than prescribed limit set by FAO/WHO, 2019; Table 2), cadmium (Cd), lead (Pb), and mercury (Hg) are regarded as nephrotoxins (Ekong et al., 2006; Johri et al., 2010; Soderland et al., 2010; Evans and Elinder, 2011). Metals progressively accumulate in the body through chronic low-level exposure, especially in industrialising countries. Pb, Hg, and Cd are found in air, food, petrol, polluted crops, and seafood (Jarup, 2003; Soderland et al., 2010). Phosphate fertilisers are the main source of heavy metal contamination in soil, as the phosphate minerals contain Cd as a natural impurity. Cd accumulation in plants beyond its permissible limit (0.05 mg/kg) can inhibit nutrient assimilation, carbohydrate metabolism, photosynthesis, and enzymatic activities, which in turn reduce yield (Nazar et al., 2012; Bakhshayesh et al., 2014). Cd deposits irreversibly in the human lungs, liver, and kidneys (Sobukola et al., 2010). It can trigger organ oxidative stress, inflammation, and lipid peroxidation (Prozialeck et al., 2006; Johri et al., 2010). Chronic low Cd exposure can damage renal proximal tubules and lower the glomerular filtration rate (GFR) in animal models (Thijssen et al., 2007). Also it may worsen diabetic kidney disease in humans and animals (Edwards and Prozialeck, 2009). Kidneys and liver produce a specialised protein called metallothionein, which protects the cells from Cd by binding tightly to it. Cancers of the prostate, kidneys, and ovaries have been linked to chronic Cd exposure (Nazar et al., 2012). Lead (Pb) is a hazardous metal that can enter the body via air, food, and water and cannot be eliminated by washing contaminated fruits and vegetables (Abbas et al., 2010). It is added to fuel as an anti-knocking agent; therefore, it is possible that this is another source of the high Pb levels seen in some leafy crops (Zamor et al., 2012). Pb poisoning causes mitochondrial enlargement in renal tubular cells and reduces energy generation (Evans and Elinder, 2011). Occupational exposure to mercury vapour may trigger albuminuria and acute membranous nephropathy (Li et al., 2010). The interrelation between chronic kidney disease (CKDu) and exposure to environmental toxins has been investigated primarily in western nations, with few data available for Asian countries.
No efforts have been made to detect heavy metals in the edible parts of locally grown crops that are consumed frequently by the inhabitants of the study area. Thus, this study examined the risk of six harmful heavy metals (Cd, Pb, Ni, Cr, Hg, and As) in CKDu affected areas of Cuttack district, Odisha. The quantities of heavy metals in rice, pulse, and vegetable crops were measured, and the health risk to nearby populations was estimated based on potential carcinogenic and noncarcinogenic risk factors.
This study region in Cuttack, India, extended from 21°24′29″N to 81°40′19″E in latitude and longitude. The study area encompasses 3,432 km2. The majority of the area falls under Narasinghpur block. The Narsinghpur block consisted of 157 villages and 98,000 residents. The average altitude was between 50 and 100 m, and the maximum was 337 m. In the highlands, red soils were found, whereas around the Mahanadi River, younger alluvial soil can be found. Potash and lime are present, but nitrogen, phosphorus, and humus are absent from these recent alluvial soils. The area under study comprises granitic rocks along with khondalite and charnokite minerals, which cover a nearly equal area in hard rock terrain (CGWB, 2013). The details of the sampling site are given in Figure 1.
Nine crops, i.e., one cereal (rice), two pulses (green gram and black gram), and six vegetables belonging to three different groups of vegetable crops: root, fruit, and leafy vegetables (potato, carrot, tomato, okra, and spinach), were selected for the study, as these crops were mostly consumed and frequently grown in the study area according to our socioeconomic survey. The description of the examined crops is shown in Table 1. Samples from each site were collected in triplicate. A total of 118 crop samples were collected between January and March 2022. These samples were collected from the fields and taken to the laboratory for further analysis in polythene bags with proper tags. Each sample was washed with double-distilled water to eliminate grime and grease from the surface of the crop samples. Then the samples were cut into small pieces with a stainless steel knife and air-dried for a week to reduce their moisture content. After air drying, samples were stored for 7 days in an oven at 65°C. The moisture content of samples was also determined by using the gravimetric method. After dehydrating the plant samples in an oven, they were grounded to a fine powder using a wooden mortar and pestle. Finally, the samples were stored at room temperature in airtight containers for further analysis (Sharma et al., 2009; Bhatia et al., 2015).
Plant samples were weighed precisely at 0.5 g and pre-digested overnight in 5 ml of nitric acid (HNO3) before being digested with 5 ml of diacid (NHO3+HClO4) mixture on a heated plate under a hood until a clear solution was obtained. The recovery of Cd, Pb, Ni, Cr, Hg, As were 96%, 95%, 97%, 95%, 94%, 97%, respectively after digestion. The volume of the digested sample solution was increased to 50 ml with double-distilled water. Inductively coupled plasma optical emission spectroscopy (ICP-OES) (Model Avio 200) was utilised to determine the concentration of heavy metals (Cd, Pb, Ni, Cr, Hg, and As) in the digested and filtered samples by using a standard solution of each metal. Standard solutions were prepared for each element under study. The blank solution, which lacked the crop sample material, was made to calibrate the initial reading and minimise metal contamination in crop samples. The value of metals in unknown sample solutions (ppm) was multiplied by the dilution factor to determine the actual metal concentration in dried plant samples. Metal analysis of plant samples was conducted in accordance with standard procedures and methodology (Singh et al., 1999).
The examination of specimens were conducted for the purposes of ensuring and maintaining quality control. Throughout the analytical procedures, the chemicals and reagents utilised were of analytical-grade quality. Double distilled water (DDW) was utilised in the preparation of the necessary reagents, standards, and analytical samples for processing and dilution purposes. Calibration curves were generated for each heavy metal under investigation. The analysis of blanks was conducted with regular frequency in order to maintain the analytical quality. To prevent any potential contamination in the equipment, procedural cleaning was performed at regular intervals using DDW during the whole analysis. The instrumental detection limit (IDL) values were found to be lower than both the method detection limit (MDL) and method quantification limit (MQL) values, indicating the high sensitivity of the inductively coupled plasma optical emission spectroscopy instrument for estimating heavy metals. Samples prepared for metal analysis included procedural blanks, replicate analyses, standard solutions and certified reference material (CRM) of white cabbage (BCR-679, European Commission Joint Research Centre, Institute for Reference Materials and Measurements). The certified values for Cd, Pb, Ni, Cr, Hg, and As are 1.66 ± 0.07, 37.21 ± 0.12, 27.0 ± 0.8, 11.23 ± 0.2, 6.17 ± 1.4, 3.21 ± 0.5 mg/kg, respectively. The values obtained for different metals viz., Cd, Pb, Cr, Ni, As, Hg were 94%, 92%, 88%, 86%, 84%, 89% of the certified value, respectively.
Estimated daily intakes (EDIs) of heavy metals (Cd, Pb, Ni, Cr, Hg, and As) (mg/day) were determined by multiplying the average concentration of heavy metals in crops by the weight of these foods consumed by a person. They are computed according to the following formula:
Where, Cm represents crop metal concentration (mg/kg), Cf represents the conversion factor of crops into dry weight (0.085) (Arora et al., 2008), FIR represents the average food consumption rate that was determined through a questionnaire survey in the study area (total of 378 individuals from the Narasinghpur block were surveyed), and then the data were used to compute the average food consumption rate shown in Table 5, Ef represents the exposure frequency (365 days), De represents the exposure duration (70 years), Tav represents the average time of exposure (365 days × 70) and Wb represents the average body weight of an individual (70 kg for adults and 15 kg for children) (WHO, 1985; USEPA, 2010).
The United States Environmental Protection Agency’s (USEPA) risk-based concentration table (USEPA, 2010) was used as the basis for the calculation of non-carcinogenic risks. Using the THQ, which is the ratio of single metal exposure over a given time to a reference dose (Df) for that metal over the same time period, the noncarcinogenic risk of ingesting metals through food crops were evaluated. The THQ can be estimated with the following equation:
Where, EDI is the estimated daily intake and Df is the reference dose for metals. Df for Cd, Pb, Ni, Cr, Hg, and As is 0.001, 0.0035, 0.02, 0.003, 0.0003, and 0.0003 mg/kg/day, respectively (USEPA, 2006). THQ values more than one indicates harmful noncarcinogenic effect on human health. However, THQ value less than one considered to be safe for consumption (Antoine et al., 2017).
The hazard index (HI) was developed to quantify the potential noncarcinogenic impacts of multiple heavy metals, and it is based on the risk assessment standards established by the USEPA. (1999). It is the sum of the hazard quotients (USEPA, 2010). The following equation is used to calculate the hazard index:
If HI is greater than one, then exposure to multiple elements has a negative impact on human health. The extent of the negative effect is considered to be proportional to the total of multiple metal exposures (Proshad et al., 2020).
The equation described in USEPA risk-based concentration (USEPA, 2006) can be used to estimate the target carcinogenic hazards associated with metals like Cd and Pb, which have been shown to cause cancer in humans. It can be calculated using the following formula:
Here, CR represents the carcinogenic risk, EDI represents the estimated daily intake, and CSo represents the carcinogenic slope factor for metals (ATSDR, 2010; Feed, 2013; Wei et al., 2020). Cd and Pb have oral cancer slope factors (CPSo) of 0.38 and 0.0085 mg kg-1 day-1, respectively. TCR values below 10−6 correspond to low cancer-causing risks; between 10−5 and 10−4 correspond to moderate cancer-causing risks; and between 10−3 and 10−1 correspond to high cancer-causing risks (Demirezen and Aksoy, 2006; Liu et al., 2006; USEPA, 2015).
IBM SPSS 22.0 software was used to perform statistical analysis of the data. Standard deviations and mean concentrations of metals in rice, pulses, and vegetables were calculated. The potential source of metals in food samples can be interpreted through a multivariate analysis using principal components. KMO values for this particular study is found to be 0.839 indicating the sampling is adequate for conducting factor analysis (Dodge, 2008). In the principal component analysis (PCA), eigen values were extracted to determine the principal components (PC). A dendrogram was constructed using Ward’s method to categorize crops into different groups. From the cluster analysis, similarities and differences between samples with respect to metal content were identified. The rest of the calculations were done in Microsoft Excel 2013.
The total concentration of Cd, Pb, Ni, Cr, Hg, and As in rice, pulses, and vegetables (mg/kg) was assessed, and their values were presented in Table 2 and Figure 2. The overall heavy metal concentrations in the samples were as follows: Pb>Ni>Cd>Cr>As>Hg. Among crop species, the average concentration of metals was as follows: spinach > rice > okra > mustard > potato > carrot > tomato > green gram > black gram. When comparing the metal concentration in crop samples with the permitted limit given by FAO/WHO (2019), it was found that the metal concentration in 89.91%, 100%, 0%, 0%, 10.08%, and 2.52% of samples, respectively, was above the permissible limit for Cd, Pb, Ni, Cr, Hg, and As (Table 2; Figure 2). Heavy metal concentrations were observed to vary significantly between samples due to environmental factors such as temperature, rainfall, crop growth stage, and metal accumulation and absorption capacities (Liu et al., 2006; Pandey and Pandey, 2009; Saha and Zaman, 2013; Garg et al., 2014). Phyto-accumulation of heavy metals, for instance, was shown to be greater in cassava tubers than in leaves (Harrison et al., 2018).

FIGURE 2. Heavy metals distribution in crop samples (n = 118) collected from CKDu hotspot of Cuttack district, India.
The average concentration of Pb (mg/kg) in the crop sample followed the decreasing order of spinach (10.51), paddy (7.88), potato (4.45), carrot (3.41), black gram (1.99), mustard (1.93), okra (1.78), tomato (1.68), and green gram (1.31) (Table 2). It was observed that the lead concentration in all samples was higher than the standard value (0.1 mg/kg) (FAO/WHO, 2019), suggesting excessive lead contamination in food crops grown in the study area and potential health risks to consumers. It was clear that spinach, paddy, potato, carrot, black gram, mustard, okra, tomato, and green gram contain 105, 79, 44.5, 34, 20, 19, 18, 17, and 13 times more concentrations of Pb than the MAC (Table 2). Cd and Pb accumulate in the renal cortex and bone, respectively. Metals have decades-long biological half-lives. Due to these long half-lives (Cd > 30 years), sustained, low-level exposure might produce excess accumulation in organs, especially in the kidney, which can disrupt the physiological function of the organ (Sharma et al., 2007; Navas-Acien et al., 2009).
The average Ni concentration in crops varied from 0.29 (tomato) to 1 mg/kg (rice) (Table 2). In crop samples, the concentration of Ni was as follows: rice > green gram > potato > spinach > mustard > carrot > black gram > okra > tomato. The concentration of nickel in all samples was less than the standard amount (10 mg/kg) (JECFA, 2003; FAO/WHO, 2019; WHO, 2011), showing that nickel did not contaminate the food samples (Figure 2).
Cadmium is a hazardous heavy metal present in very low concentrations in the environment. Air and water are known to be the primary sources of Cd exposure (FSANZ, 2003). The average Cd content varied from 0.09 (green gram) to 1.37 (spinach) mg/kg. In crop samples, the average Cd concentration (mg/kg) was as follows: spinach (1.37), potato (1.22), mustard (0.94), carrot (0.58), paddy (0.52), tomato (0.39), okra (0.27), black gram (0.12), green gram (0.09). In accordance with the maximum allowable concentration (MAC), the cadmium concentration in 89.91% of samples was higher than the standard value (0.05 mg/kg) (FAO/WHO, 2019). It was found that spinach, potato, mustard, carrot, paddy, tomato, okra, black gram, and green gram contain Cd concentrations that are 27, 24, 19, 12, 10, 8, 5, 2.5, and 2 times more than the maximum allowable concentration (MAC) (Table 2).
The average Cr concentration varied from 0.12 (spinach) to 0.94 mg/kg (okra) (Table 2). In crop samples, the average Cr content were as follows: okra > green gram > carrot > paddy > black gram > mustard > potato > tomato > spinach. Chromium concentrations in all samples were less than the standard value (2.3 mg/kg) (FAO/WHO, 2019), showing no chromium contamination of food samples (Figure 2).
The mean As concentration (mg/kg) in crop samples was in descending order as follows: potato (0.037), tomato (0.020), paddy (0.019), okra (0.016), green gram, mustard, okra (0.013), and black gram (0.008). According to Table 2, the arsenic concentration of 2.52 percent of the samples were higher than the standard value (0.1 mg/kg) given by FAO/WHO. (2019), which may pose a health risk to consumers. Rapid transfer of As from soil to plant, irrational use of As rich fertilizers and pesticides, and use of contaminated groundwater for irrigation may contribute to the presence of arsenic in crops (Alam et al., 2003; Renner, 2004; Neumann et al., 2010; Bhuiyan et al., 2011; Roberts et al., 2011; Polizzotto et al., 2013).
The average Hg concentration varied from 0 (green gram, carrot, and mustard) to 0.019 mg/kg (okra) (Table 2). Average Hg concentrations in crop samples were as follows: okra > paddy > potato > tomato > spinach > black gram > green gram > carrot > mustard. According to MAC, the Hg concentration in 10% of samples was higher than the standard value (0.01 mg/kg) given by FAO/WHO (2019), indicating a low level of mercury contamination in food samples (Figure 2).
Long-term consumption of food crops contaminated with heavy metals may pose numerous health risks to humans. Therefore, routine monitoring is essential to avoid excess accumulation of these metals in the food chain (Sharma et al., 2009; Kananke et al., 2014; Noor et al., 2022). According to Yana et al. (2012), heavy metals in leafy vegetables come from both soil and smelting waste gases, while those in fruits and roots come from soil and may vary by season (Tani and Barrington, 2005). Chronic exposure to Cd, Pb, As, and Hg causes renal tubular alterations, especially in the proximal convoluted tubule, and, in rare cases, acute renal failure that leads to chronic kidney disease (Gunawardana et al., 2006; Ferraro et al., 2010; Rango et al., 2015; Asraf et al., 2021). Lead is linked to terminal stages, although more research is needed (Ekong et al., 2006; Garcia and Arceo, 2018). In CKDu affected areas of Sri Lanka, Ni, Cd, Cr, and Pb contamination levels in vegetables exceeded FAO/WHO standards for human consumption (Bandara et al., 2010; Kananke et al., 2014; Kananke et al., 2016). In sensitive individuals with hypertension or diabetes, consumption of foods with high Cd may synergistically develop and progress CKD (Kim et al., 2015). The main irrigation source in the study area is the Mahanadi River, which flows adjacent to it and contains contaminated water and detritus. In Mahanadi sediments, Pb and Cd enrichment factors were higher, indicating contamination from many external sources (Nayak et al., 2002; Swain et al., 2021; Samal et al., 2022).
To determine the relationships between the metals in food samples, statistical analyses were conducted. From the intermetallic interaction in a particulate medium, the source and migration routes of the metals can be predicted (Raknuzzaman et al., 2016; Muhammad et al., 2021). The correlation coefficient matrix of heavy metals in food samples taken from CKDu-endemic regions in the Cuttack district is presented in Table 3. Cd showed significant positive correlations with Pb and Ni (r = 0.277**, r = 0.229**), while significant negative correlations were seen between Cd and Cr (r = −363**). Pb and Cr both showed positive correlations with Ni and Hg, respectively, that were statistically significant (r = 0.280** and r = 0.202*, respectively). According to Abbasi et al. (2013) and Mohammed et al. (2003), the combinations exhibited a strong positive association, showing that the traits were related and may have derived from the same sources. Other connections between the components of the meal sample were not significant.
Principal component analysis (PCA) was performed to determine the possible sources of heavy metals in the food crop collected from the study area (Franco-Uria et al., 2009; Kikuchi et al., 2009; Manea et al., 2020). Table 4 and Figure 3 display the results of PCA. Three principal components were found, and together they accounted for 67.01% of the variations in food crops. The variances explained by the first three PCs for crop samples were 28.40%, 21.11%, and 17.48%, respectively. PC1 has a strong correlation with Cd and Pb, PC2 has a strong correlation with Ni and Cr, and PC3 has a strong correlation with Hg and As. Cd had accumulated in the analyzed crops as a result of the widespread use of phosphate (P) fertilizers in agricultural soils (Mortvedt, 1996; Nziguheba and Smoulders, 2008; Hove et al., 2020); hence, Pb, which was significantly related to Cd in PC1, may also be significantly influenced by irrational use of fertilizers and agrochemicals (Huang et al., 2007; Atafar et al., 2010; Iqbal et al., 2021). Decades of intensive cultivation in the agricultural region and long-term application of fertilizer may be a significant source of heavy metal accumulation in crops. The Mahanadi River, which borders the affected area, contains additional evidence of industrial and anthropogenic contamination in its water and sediments (Behera et al., 2013; Raj et al., 2013; Swain et al., 2021). In conclusion, PC1 and PC3 can be inferred as anthropogenic components, which are more significantly influenced by human actions (fertilizer application) than other components. A significant association between Cr and Ni in PC2 indicated their origin from lithogenic sources as they have been detected in the parent materials of rural soils across the globe, with minimal temporal and spatial variation (Facchinelli et al., 2001; Salonen and Korkka-Niemi, 2007; Spurgeon et al., 2008; Wu et al., 2010; Kladsomboon et al., 2020). Thus, metals in PC1, PC2, and PC3 may derived from a variety of natural and anthropogenic sources, including industrial effluents, agricultural activities, and the granitic bedrock underlying the research region. Also from the dendrogram constructed using Ward’s method, several cluster configurations were identified and food samples belonging to the same cluster shared similar characteristics with respect to their metal contamination level (Figure 4). Within a distance of five on the dendrogram scale, the main clusters of various food items developed, including potatoes, tomatoes, rice, spinach, carrots, mustard, black gram, okra, and green gram.
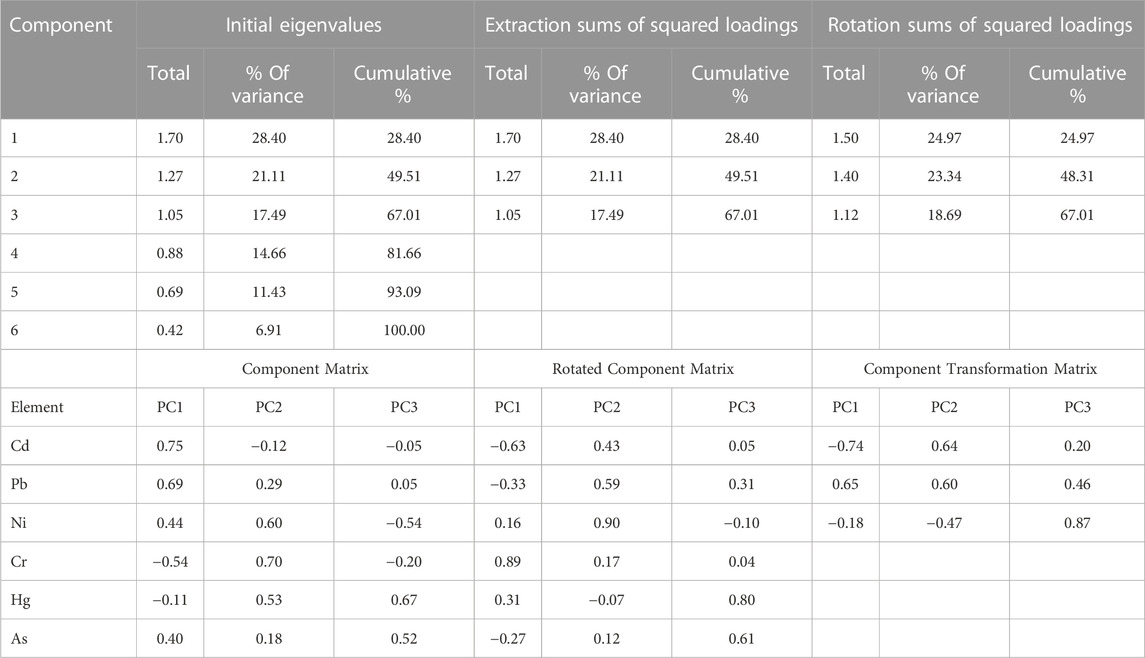
TABLE 4. Total variance explained and component matrices for the heavy metals in crops collected from Cuttack district, India.
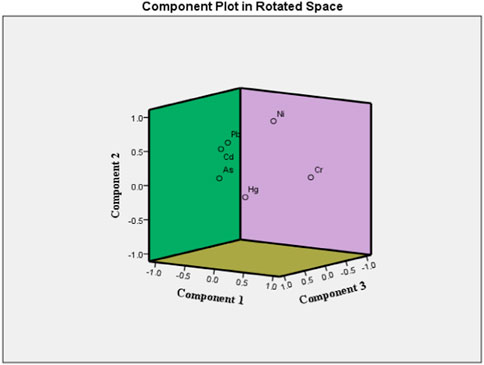
FIGURE 3. Principal component analysis (PCA) of heavy metals in food samples (n = 118) collected from different agricultural fields of Cuttack district, Odisha.
The USEPA has developed several indices (both non-carcinogenic and carcinogenic) to predict the potential health risk posed by long-term exposure to hazardous metals (Ali et al., 2019).
The most prevalent route of trace metal exposure for humans is through food (WHO, 1985; Kumar et al., 2019; Saraswat et al., 2023); however, inhalation and skin contact are also possible (ATSDR, 2010). Rice, pulses, and vegetables may make up a significant share of the Indian population’s total diet, and EDI is a significant method for assessing the health hazards associated with heavy metals through the consumption of these food items (Alam et al., 2003; Alsafran et al., 2021). Its calculation is based on the total metal concentration in food and their consumption rate in adults and children; the results are presented in Table 5. If the ratio of EDI to Df is less than Df, the health risk is minimal; if it is between 1 and 5 times the Df, there is a low health risk; five to ten times the Df, there is a moderate health risk; more than ten times the Df, there is a high health risk (Ali et al., 2019; Saxena et al., 2019). Based on the EDI/Df ratio, we determined that the metal content in pulses is between 5 and 10 times higher, posing a moderate health risk, while the metal content in rice and vegetables is greater than 10 times higher, posing a high health risk for consuming these crops grown in this region. Based on these results, we concluded that Cd, Pb, and Ni posed the greatest threat to the health of adults and children living in endemic regions of CKDu in the Cuttack district of India.
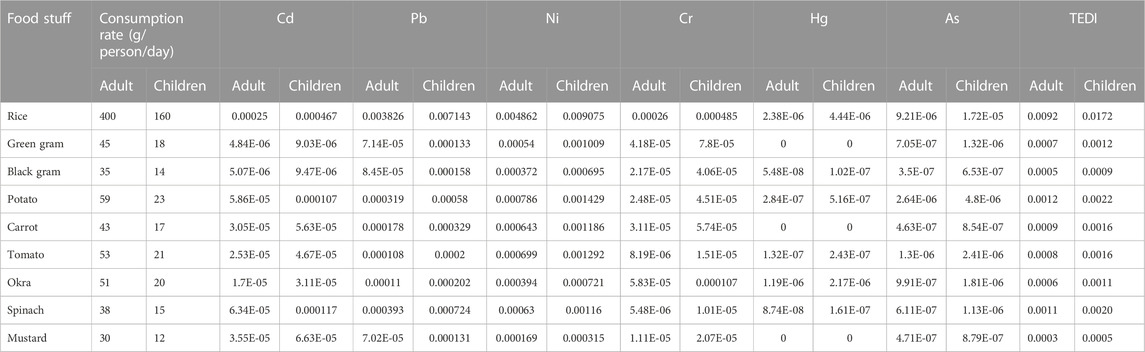
TABLE 5. Consumption rate (g/person/day), estimated daily intake (EDI) and total estimated daily intake (EDI) (mg/kg bw/day) of heavy metals from rice, pulse and vegetables for adult and children.
THQ is linked to a noncarcinogenic health risk, and its value less than 1 is considered as permissible (Rattan et al., 2005). If THQ levels exceed a certain threshold, it will pose a health risk (USEPA, 1989; Bounar et al., 2020). The calculated THQ value was presented in Table 6. In this investigation, for Pb, THQ values in rice were greater than 1. Therefore, their THQ levels may pose a noncarcinogenic risk to the population in this region. From these values, we determined that for Cd and Pb, the THQ values of spinach and mustard were higher than those of other vegetables and pulses under study (Gupta et al., 2019), whereas no such pattern was observed for other elements.
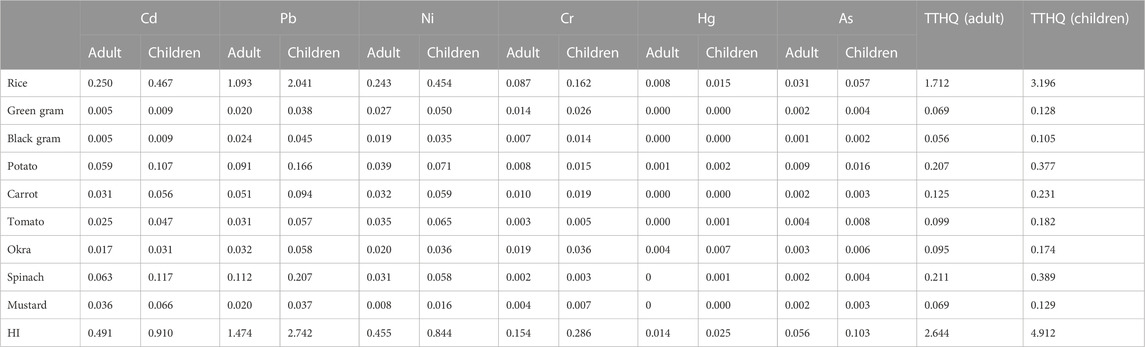
TABLE 6. Target hazard quotient (THQ) (noncarcinogenic risk), hazard index (HI) and total target hazard quotient (TTHQ) of heavy metals from rice, pulses and vegetables for adult and children.
HI provides a measure of the cumulative impact of harmful metals on human health through consumption of contaminated food crops (Filimon et al., 2021), and the data in Table 6 demonstrated that HI values for rice were higher than the allowed limit. Hence, consumption of rice grown in the study area may be associated with noncancerogenic health risks. The hazard index for adults and children was as follows: rice>spinach>potato>carrot>tomato>okra>mustard>green gram>black gram. In this study, only a limited number of food crops were evaluated to estimate the noncarcinogenic health risk. So, the results only considered a minor portion of the actual threat to the people in the study area.
Toxic metals are thought to have potential adverse effects on human health, and research suggests that exposure to certain carcinogenic metals, especially over extended periods of time, can raise the risk of developing cancer. TCR is an estimate of the expected malignancy. Then, it also indicates the possibility of cancer-causing hazards developing within an individual. TCR for Cd and Pb via consumption of contaminated food crops, as estimated by EDI and CPSo values, is displayed in Table 7. If TCR values are between 10−6 and 10−5, they are associated with low cancer risks; if they are between 10−5 and 10−4, they are associated with moderate risks; and if they are between 10−3 and 10−1, they are associated with high risks (Ali et al., 2019). The TCR values for Cd in the crop samples were 1.86E-04 and 3.46E-04 for adults and children, respectively (Table 7). Similarly, for Pb, it was 4.39E-05 for adults and 8.16E-05 for children. The cumulative carcinogenic risk of Cd from the foods was greater than 10−4, indicating cancer risk to both adults and children in the study area. In the current study, however, the TCR of Pb is between 10−5 and 10−4, posing a moderate cancer risk (USEPA, 1989; USEPA, 2015; Fonge et al., 2021). Due to the high cadmium content of some local foods, residents of the endemic area may consume more cadmium than is healthy on a daily basis. This can have serious consequences for the kidneys, especially in the young, the elderly, and those with underlying medical conditions. A cross-sectional investigation suggested cadmium as a risk factor for CKDu in Sri Lanka due to its greater urinary excretion and dose-effect connection with CKDu stages. When exposed to nephrotoxins, selenium deficiency and genetic vulnerability may predispose to CKDu (Jayatilake et al., 2013; Gupta et al., 2021). Therefore, the potential carcinogenic hazards posed by food consumption to residents of the CKDu endemic area must not be ignored. Thus, the present research demonstrates unequivocally that the Cuttack populace’s consumption of these foods poses a cancer risk.
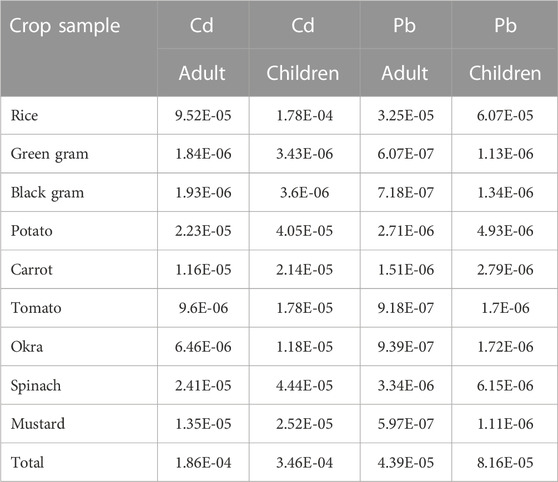
TABLE 7. Carcinogenic risks of Cd and Pb due to consumption rice, pulse and vegetables for adult and children.
The current study examined the heavy metal concentration of commonly consumed and locally grown food crops in CKDu-endemic areas of Cuttack district, India. It was found that the levels of Cd, Pb, Hg, and As in rice, pulses, and vegetables exceeded the WHO and FAO permissible levels. Metal concentrations in food samples were as follows: Pb>Ni>Cd>Cr>As>Hg. A multivariate study revealed that Cd, Pb, Ni, Cr, Hg, and As in dietary samples were primarily caused by both natural and anthropogenic activities. EDI for Cd and Pb was increased in both adults and children. THQ for Pb was over the permissible limit, putting consumers at high risk for non-cancerogenic risks associated with Pb. Based on the calculated hazard index for adults and children, it was evident that consuming rice was not safe. While the risk of Pb from the majority of meals was below the permitted level of 10−5, the total carcinogenic risk of Cd was greater than 10−4, indicating a cancer risk to both adults and children in the study area. Longitudinal studies are needed to assess the links between heavy metals like Cd and Pb and kidney impairment due to their extensive prevalence in the environment and the lack of treatment strategies to reduce their effects. Thus, chronic impacts might be determined and environmental monitoring increased to lower chemical concentrations. We recommend combining governmental and private sector efforts with research centres to further study into the disease’s causes and remedies.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
SM and RN: Conceptualization, Methodology, Software; SM and KM: Data curation, Writing–TableOriginal draft preparation. SM, KP, and BJ: Visualization, Investigation. RN and BJ: Supervision: SM, KKM, and SS: Software, Validation: PD, JD, AS, SB, JN, and SP: Writing–Reviewing and Editing. All authors contributed to the article and approved the submitted version.
Author SM has received financial support from University Grant Commission (UGC), New Delhi, India in form of Junior Research Fellowship (JRF) during her PhD tenure to conduct this research work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, M., Parveen, Z., Iqbal, M., Riazuddin, S., Ahmed, M., Bhutto, R., et al. (2010). Monitoring of toxic metals (cadmium, lead, arsenic and mercury) in vegetables of sindh, Pakistan. Kathmandu Univ. J. Sci. Eng. Technol. 6 (II), 60–65. doi:10.3126/kuset.v6i2.4013
Abbasi, A. M., Iqbal, J., Khan, M. A., and Shah, M. H. (2013). Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Pak. Ecotox. Environ. Saf. 92, 237–244. doi:10.1016/j.ecoenv.2013.02.011
Abuduwailil, J., Zhaoyong, Z., and Fengqing, J. (2015). Evaluation of the pollution and human health risks posed by heavy metals in the atmospheric dust in Ebinur Basin in Northwest China. Environ. Sci. Pollut. Res. 1–14, 14018–14031. doi:10.1007/s11356-015-4625-1
Alam, M. G. M., Snow, T., and Tanaka, A. (2003). Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total. Environ. 308, 83–96. doi:10.1016/s0048-9697(02)00651-4
Ali, H., Khan, E., and Ilahi, I. (2019) Environmental Chemistry and Ecotoxicology of Hazardous Heavy Metals: Environmental Persistence, Toxicity, and Bioaccumulation. Journal of Chemistry.
Alsafran, M., Usman, K., Rizwan, M., Ahmed, T., and Al Jabri, H. (2021). The carcinogenic and non-carcinogenic health risks of metal(oid)s bioaccumulation in leafy vegetables: a consumption advisory. Front. Environ. Sci. 9, 742269. doi:10.3389/fenvs.2021.742269
Antoine, J. M. R., Fung, L. A. H., and Grant, C. N. (2017). Assessment of the potential health risks associated with the aluminium, arsenic, cadmium and lead content in selected fruits and vegetables grown in Jamaica. Toxicol. Rep. 4, 181–187. doi:10.1016/j.toxrep.2017.03.006
Arora, M., Kiran, B., Rani, S., Rani, A., Kaur, B., and Mittal, N. (2008). Heavy metal accumulation in vegetables irrigated with water from different sources. Food. Chem. 111, 811–815. doi:10.1016/j.foodchem.2008.04.049
Ashraf, I., Ahmad, F., Sharif, A., Altaf, A. R., and Teng, H. (2021). Heavy metals assessment in water, soil, vegetables and their associated health risks via consumption of vegetables, district kasur, Pakistan. SN Appl. Sci. 3, 552. doi:10.1007/s42452-021-04547-y
Atafar, Z., Mesdaghinia, A., Nouri, J., Homaee, M., Yunesian, M., Ahmadimoghaddam, M., et al. (2010). Effect of fertilizer application on soil heavy metal concentration. Environ. Monit. Assess. 160 (1), 83–89. doi:10.1007/s10661-008-0659-x
ATSDR (Agency for toxic substance and disease registry) (2010). Public health assessment and health consultation. Washington, Quicy: CENEX supply and marketing. Avaliable At: https://www.atsdr.cdc.gov.
Bakhshayesh, B. E., Delkash, M., and Scholz, M. (2014). Response of vegetables to cadmium-enriched soil. Water 6, 1246–1256. doi:10.3390/w6051246
Bandara, J. M., Wijewardena, H. V., Liyanege, J., Upul, M. A., and Bandara, J. M. (2010). Chronic renal failure in Sri Lanka caused by elevated dietary cadmium: trojan horse of the green revolution. Toxicol. Lett. 198 (1), 33–39. doi:10.1016/j.toxlet.2010.04.016
Behera, B. C., Mishra, R. R., Patra, J. K., Sarangi, K., Dutta, S. K., and Thatoi, H. N. (2013). Impact of heavy metals on bacterial communities from mangrove soils of the Mahanadi Delta (India). Chem. Ecol. 29, 604–619. doi:10.1080/02757540.2013.810719
Bhatia, A., Singh, S., and Kumar, A. (2015). Heavy metal contamination of soil, irrigation water and vegetables in peri-urban agricultural areas and markets of Delhi. Water Environ. Res. 87 (11), 2027–2034. doi:10.2175/106143015x14362865226833
Bhuiyan, M. A. H., Suruvi, N. I., Dampare, S. B., Islam, M. A., Quraishi, S. B., Ganyaglo, S., et al. (2011). Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environ. Monit. Assess. 175 (1), 633–649. doi:10.1007/s10661-010-1557-6
Bounar, A., Boukaka, K., and Leghouchi, E. (2020). Determination of heavy metals in tomatoes cultivated under green houses and human health risk assessment. Qas 12 (1), 76–86. doi:10.15586/qas2019.639
CGWB (2013). Groundwater information booklet of Cuttack district, Odisha. Bhubaneswar, India: Central Ground Water Board, South eastern region.
Cockwell, P., and Lori-Ann, F. (2020). The global burden of chronic kidney disease. Lancet 395 (10225), 662–664. doi:10.1016/S0140-6736(19)32977-0
Demirezen, D., and Aksoy, A. (2006). Heavy metal levels in vegetables in Turkey are within safe limits for Cu, Zn, Ni and exceeded for Cd and Pb. J. Food Qual. 29 (3), 252–265. doi:10.1111/j.1745-4557.2006.00072.x
Edwards, J. R., and Prozialeck, W. C. (2009). Cadmium, diabetes and chronic kidney disease. Toxicol. Appl. Pharmacol. 238, 289–293. doi:10.1016/j.taap.2009.03.007
Ekong, E. B., Jaar, B. G., and Weaver, V. M. (2006). Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney. Int. 70, 2074–2084. doi:10.1038/sj.ki.5001809
Evans, M., and Elinder, C. G. (2011). Chronic renal failure from lead: myth or evidence-based fact? Kidney. Int 79, 272–279. doi:10.1038/ki.2010.394
Facchinelli, A., Sacchi, E., and Mallen, L. (2001). Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ. Pollut. 114 (3), 313–324. doi:10.1016/s0269-7491(00)00243-8
FAO/WHO (Food and Agriculture Organization/World Health Organization) (2019). General standard for contaminants and toxins in food and feed. Codex alimentarius commission. Available at: http://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B193-1995%252FCXS_193e.pdf.
Feed, F. (2013). Maximum limit of heavy metals. Iran: Iranian National Standard, Institute of Standard and Industrial Research of Iran.
Ferraro, P. M., Costanzi, S., Naticchia, A., Sturniolo, A., and Gambaro, G. (2010). Low-level exposure to cadmium increases the risk of chronic kidney disease: analysis of the NHANES 1999–2006. BMC Public Health 10, 304. doi:10.1186/1471-2458-10-304
Filimon, M. N., Caraba, I. V., Popescu, R., Dumitrescu, G., Verdes, D., Petculescu Ciochina, L., et al. (2021). Potential ecological and human health risks of heavy metals in soils in selected copper mining areas-A case study: the bor area. Ijerph 18 (4), 1516. doi:10.3390/ijerph18041516
Fonge, B. A., Larissa, M. T., Egbe, A. M., Afanga, Y. A., Frum, N. G., and Ngole-Jeme, V. M. (2021). An assessment of heavy metal exposure risk associated with consumption of cabbage and carrot grown in a tropical savannah region. Int. J. Environ. Health Sustain. 7 (1), 1–19. doi:10.1080/27658511.2021.1909860
Franco-Uria, A., Lopez-Mateo, C., Roca, E., and Fernández-Marcos, M. L. (2009). Source identification of heavy metals in pasture land by multivariate analysis in NW Spain. J. Hazard. Mater. 1651, 1008–1015. doi:10.1016/j.jhazmat.2008.10.118
FSANZ (2003). The 20th Australian Total Diet Survey: a total dietsurvey of pesticide residues and contaminants. Canberra, Australia: Food Standards Australia New Zealand.
Garcia, J. D. D., and Arceo, E. (2018). Renal damage associated with heavy metals: review work. Rev. Colomb. Nefrol. 5 (1), 43–53. doi:10.22265/acnef.5.2.254
Garg, V. K., Yadav, P., Mor, S., Singh, B., and Pulhani, V. (2014). Heavy metals bioconcentration from soil to vegetables and assessment of health risk caused by their ingestion. Biol. Trace Elem. Res. 157, 256–265. doi:10.1007/s12011-014-9892-z
Gooneratne, I. K., Ranaweera, A. K., Liyanarachchi, N. P., Gunawardane, N., and Lanerolle, R. D. (2008). Epidemiology of chronic kidney disease in a Sri Lankan population. Int. J. Diabetes Dev. Ctries. 28, 60–64. doi:10.4103/0973-3930.43101
Gunawardana, C. G., Martínez, R. E., Xiao, W., and Templeton, D. M. (2006). Cadmium inhibits both intrinsic and extrinsic apoptotic pathways in renal mesangial cells. Am. J. Physiol. Ren. Physiol. 290, 1074–1082. doi:10.1152/ajprenal.00067.2005
Gupta, N., Yadav, K. K., Kumar, V., Cabral-Pinto, M. M. S., Alam, M., Kumar, S., et al. (2021). Appraisal of contamination of heavy metals and health risk in agricultural soil of jhansi city, India. Environ. Toxicol. Pharmacol. 88, 103740. doi:10.1016/j.etap.2021.103740
Gupta, N., Yadav, K. K., Kumar, V., Kumar, S., Chadd, R. P., and Kumar, A. (2019). Trace elements in soil-vegetables interface: translocation, bioaccumulation, toxicity and amelioration - a review. Sci. Total Environ. 651, 2927–2942. doi:10.1016/j.scitotenv.2018.10.047
Harrison, U. E., Osu, S. R., and Ekanem, J. O. (2018). Heavy metals accumulation in leaves and tubers of cassava (<i>Manihot esculenta</i> Crantz) grown in crude oil contaminated soil at Ikot Ada Udo, Nigeria. J. Appl. Sci. Environ. Manag. 22 (6), 845–851. doi:10.4314/jasem.v22i6.1
Hove, G., Rathaha, T., and Mugiya, P. (2020). The impact of human activities on the environment, case of mhondongori in zvishavane, Zimbabwe. J. Geosci. Environ. Prot. 8, 330–349. doi:10.4236/gep.2020.810021
Huang, S., Liao, Q., Hua, M., Wu, X., Bi, K., Yan, C., et al. (2007). Survey of heavy metal pollution and assessment of agricultural soil in Yangzhong district, Jiangsu Province, China. Chemosphere 67 (11), 2148–2155. doi:10.1016/j.chemosphere.2006.12.043
ICMR (2020). Annual report (2019-2020). New Delhi: Division of Publication and Information on behalf of the Secretary DHR & DG, ICMR, 147–148. Avaliable At: https://main.icmr.nic.in/sites/default/files/annualrepoorts/ICMRAREnglish201920.
Iqbal, J., Su, C., Rashid, A., Yang, N., Baloch, M. Y. J., Talpur, S. A., et al. (2021). Hydrogeochemical assessment of groundwater and suitability analysis for domestic and agricultural utility in southern Punjab, Pakistan. Pak. Water. 13, 3589. doi:10.3390/w13243589
Jan, F. A., Ishaq, M., Khan, S., Ihsanullah, I., Ahmad, I., and Shakirullah, M. (2010). A comparative study of human health risks via consumption of food crops grown on wastewater irrigated soil (Peshawar) and relatively clean water irrigated soil (lower Dir). J. Hazard. Mater. 179, 612–621. doi:10.1016/j.jhazmat.2010.03.047
Jarup, L. (2003). Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182. doi:10.1093/bmb/ldg032
Jayasumana, M. A. C. S., Paranagama, P. A., Amarasinghe, M. D., Wijewardane, K. M. R. C., Dahanayake, K. S., Fonseka, S. I., et al. (2013). Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J. Nat. Sci. Res. 3 (1), 64–73.
Jayatilake, N., Mendis, S., Maheepala, P., and Mehta, R. (2013). Chronic kidney disease of uncertain aetiology: prevalence and causative factors in a developing country. BMC Nephrol. 14 (1), 180–189. doi:10.1186/1471-2369-14-180
JECFA (2003). Food additives and food contaminants. Rome: FAO procedural guidelines for the Joint FAO/WHO Expert Committee on Food Additives. Available at: http://apps.who.int/food-additives-contaminants-jecfadatabase/chemical.aspx?chemID=1376
Johri, N., Jacquillet, G., and Unwin, R. (2010). Heavy metal poisoning: the effects of cadmium on the kidney. Biometals 23, 783–792. doi:10.1007/s10534-010-9328-y
Kananke, T., Wansapala, J., and Gunaratne, A. (2016). Detection of Ni, Cd, and Cu in green leafy vegetables collected from different cultivation areas in and around Colombo District, Sri Lanka. Env. Moni. Assess. 188 (3), 187–212. doi:10.1007/s10661-016-5195-5
Kananke, T., Wansapala, J., and Gunaratne, A. (2014). Heavy metal contamination in green leafy vegetables collected from selected market sites of piliyandala area, colombo district, Sri Lanka. Am. J. Food Sci. Technol. 2 (5), 139–144. doi:10.12691/ajfst-2-5-1
Kikuchi, T., Furuichi, T., Hai, H. T., and Tanaka, S. (2009). Assessment of heavy metal pollution in river water of Hanoi, Vietnam using multivariate analyses. Bull. Environ. Contam. Toxicol. 83, 575–582. doi:10.1007/s00128-009-9815-4
Kim, N. H., Hyun, Y. Y., Lee, K. B., Chang, Y., Ryu, S., Oh, K. H., et al. (2015). Environmental heavy metal exposure and chronic kidney disease in the general population. J. Korean Med. Sci. 30 (3), 272–277. doi:10.3346/jkms.2015.30.3.272
Kladsomboon, S., Jaiyen, C., Choprathumma, C., Tusai, T., and Apilux, A. (2020). Heavy metals contamination in soil, surface water, crops, and resident blood in uthai district, phra nakhon Si ayutthaya, Thailand. Environ. Geochem. Health 42, 545–561. doi:10.1007/s10653-019-00388-2
Kumar, S., Prasad, S., Yadav, K. K., Shrivastava, M., Gupta, N., Nagar, S., et al. (2019). Hazardous heavy metals contamination of vegetables and food chain: role of sustainable remediation approaches - a review. Environ. Res. 179, 108792. doi:10.1016/j.envres.2019.108792
Li, S. J., Zhang, S. H., Chen, H. P., Zeng, C. H., Zheng, C. X., Li, L. S., et al. (2010). Mercury-induced membranous nephropathy: clinical and pathological features. Clin. J. Am. Soc. Nephrol. 5, 439–444. doi:10.2215/cjn.07571009
Liu, C. W., Liang, C. P., Huang, F. M., and Hsueh, Y. M. (2006). Assessing the human health risks from exposure of inorganic arsenic through oyster (Crassostrea gigas) consumption in Taiwan. Sci. Total. Environ. 361 (1–3), 57–66. doi:10.1016/j.scitotenv.2005.06.005
Mamat, Z., Yimit, H., Ji, R. Z. A., and Eziz, M. (2014). Source identification and hazardous risk delineation of heavy metal contamination in Yanqi basin, northwest China. Sci. Total. Environ. 493, 1098–1111. doi:10.1016/j.scitotenv.2014.03.087
Manea, D. N., Ienciu, A. A., Stef, R., Smuleac, I. L., Gergen, I. I., and Nica, D. V. (2020). Health risk assessment of dietary heavy metals intake from fruits and vegetables grown in selected old mining areas—a case study: the Banat area of Southern Carpathians. Int. J. Environ. Res. Public Health 17, 5172. doi:10.3390/ijerph17145172
Mitra, S., Chakraborty, A. J., Tareq, A. M., Emran, T. B., Nainu, F. Y., Khusro, A., et al. (2022). Impact of heavy metals on the environment and human health: novel therapeutic insights to counter the toxicity. J. King Saud Univ. Sci. 34 (3), 101865. doi:10.1016/j.jksus.2022.101865
Mohamed, A. E., Rashed, M. N., and Mofty, A. (2003). Assessment of essential and toxic elements in some kinds of vegetables. Ecotoxicol. Environ. Saf. 55, 251–260. doi:10.1016/s0147-6513(03)00026-5
Mohanty, N. K., Sahoo, K. C., Pati, S., Sahu, A. K., and Mohanty, R. (2020). Prevalence of chronic kidney disease in Cuttack district of Odisha, India. Int. J. Env. Res. Public Health. 17, 456. doi:10.3390/ijerph17020456
Mortvedt, J. (1996). Heavy metal contaminants in inorganic and organic fertilizers. Nutr. Cycl. Agroecosyst. 43 (1), 55–61. doi:10.1007/bf00747683
Muhammad, M., Habib, I. Y., Hamza, I., Mikail, T. A., Yunusa, A., Muhammad, I. A., et al. (2021). Heavy metals contamination of agricultural land and their impact on food safety. Ejnfs 13 (1), 104–111. doi:10.9734/ejnfs/2021/v13i130354
Muhammad, S., Shah, M. T., and Khan, S. (2011). Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J. 98, 334–343. doi:10.1016/j.microc.2011.03.003
Nanayakkara, S., Komiya, T., Ratnatunga, N., Senevirathna, S. T., Harada, K. H., Hitomi, T., et al. (2012). Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ. Health. Prev. Med. 17, 213–221. doi:10.1007/s12199-011-0243-9
Navas-Acien, A., Tellez-Plaza, M., Guallar, E., Muntner, P., Silbergeld, E., Jaar, B., et al. (2009). Blood cadmium and lead and chronic kidney disease in US adults: a joint analysis. Am. J. Epidemiol. 170, 1156–1164. doi:10.1093/aje/kwp248
Nayak, B. B., Das, J., Panda, U. C., and Acharya, B. C. (2002). “Industrial effluents and municipal sewage contamination of Mahanadi estuarine water, Orissa,” in Proceedings, published allied (New Delhi, India: Allied Publishers Pvt. Ltd), 77–86.
Nazar, R., Iqbal, N., Masood, A., Iqbal, M., Khan, R., Syeed, S., et al. (2012). Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am. J. Plant Sci. 3, 1476–1489. doi:10.4236/ajps.2012.310178
Neumann, R. B., Ashfaque, K., Badruzzaman, A. B. M., Ashraf Ali, M., Shoemaker, J. K., and Harvey, C. F. (2010). Anthropogenic influences on groundwater arsenic concentrations in Bangladesh. Nat. Geosci. 3, 46–52. doi:10.1038/ngeo685
Noor, S., Rashid, A., Javed, A., Khattak, J. A., and Farooqi, A. (2022). Hydrogeological properties, sources provenance, and health risk exposure of fluoride in the groundwater of Batkhela, Pakistan. Environ. Technol. Innovation 25, 102239. doi:10.1016/j.eti.2021.102239
Nziguheba, G., and Smolders, E. (2008). Inputs of trace elements in agricultural soils via phosphate fertilizers in European countries. Sci. Total Environ. 390 (1), 53–57. doi:10.1016/j.scitotenv.2007.09.031
Pandey, J., and Pandey, U. (2009). Accumulation of heavy metals in dietary vegetables and cultivated soil horizon in organic farming system in relation to atmospheric deposition in a seasonally dry tropical region of India. Environ. Monit. Assess. 148, 61–74. doi:10.1007/s10661-007-0139-8
Pappas, R., Polzin, G., Zhang, L., Watson, C., Paschal, D., and Ashley, D. (2006). Cadmium, lead, and thallium in mainstream tobacco smoke particulate. Food Chem. Toxicol. 44, 714–723. doi:10.1016/j.fct.2005.10.004
Polizzotto, M. L., Lineberger, E. M., Matteson, A. R., Neumann, R. B., Badruzzaman, A. B. M., and Ashraf Ali, M. (2013). Arsenic transport in irrigation water across rice-field soils in Bangladesh. Environ. Pollut. 179, 210–217. doi:10.1016/j.envpol.2013.04.025
Proshad, R., Islam, M. S., Tusher, T. R., Zhang, D., Khadka, S., Gao, J., et al. (2020). Appraisal of heavy metal toxicity in surface water with human health risk by a novel approach: a study on an urban river in vicinity to industrial areas of Bangladesh. Toxin Rev. 40, 803–819. doi:10.1080/15569543.2020.1780615
Prozialeck, W. C., Edwards, J. R., and Woods, J. M. (2006). The vascular endothelium as a target of cadmium toxicity. Life Sci. 79, 1493–1506. doi:10.1016/j.lfs.2006.05.007
Radwan, M. A., and Salama, A. K. (2006). Market basket survey for some heavy metals in Egyptian fruits and vegetables. Food Chem. Toxicol. 44 (8), 1273–1278. doi:10.1016/j.fct.2006.02.004
Raj, S., Jee, P. K., and Panda, C. R. (2013). Textural and heavy metal distribution in sediments of Mahanadi estuary, East coast of India. Indian J. geo-mar. Sci. 42, 370–374.
Raknuzzaman, M., Ahmed, M. K., Islam, M. S., Habibullah-Al-Mamun, M., Tokumura, M., Sekine, M., et al. (2016). Assessment of trace metals in surface water and sediment collected from polluted coastal areas of Bangladesh. J. Wat. Environ. Technol. 14, 247–259. doi:10.2965/jwet.15-038
Rango, T., Jeuland, M., Manthrithilake, H., and McCornick, P. (2015). Nephrotoxic contaminants in drinking water and urine, and chronic kidney disease in rural Sri Lanka. Sci. Total Environ. 518-519, 574–585. doi:10.1016/j.scitotenv.2015.02.097
Rashid, A., Ayub, M., Khan, S., Ullah, Z., Ali, L., Gao, X., et al. (2022). Hydrogeochemical assessment of carcinogenic and non-carcinogenic health risks of potentially toxic elements in aquifers of the Hindukush ranges, Pakistan: insights from groundwater pollution indexing, GIS-based, and multivariate statistical approaches. Environ. Sci. Pollut. Res. 29, 75744–75768. doi:10.1007/s11356-022-21172-3
Rattan, R. K., Datta, S. P., Chhonkar, P. K., Suribabu, K., and Singh, A. K. (2005). Long-term impact of irrigation with waste water effluents on heavy metal content in soils, crops and groundwater—a case study. Agric. Ecosyst. Environ. 109, 310–322. doi:10.1016/j.agee.2005.02.025
Ratul, A. K., Hassan, M., and Uddin, M. K. (2018). Potential health risk of heavy metals accumulation in vegetables irrigated with polluted river water. Int. Food Res. J. 25 (1), 329–338.
Rehman, K., Bukhari, S. M., Andleeb, S., Mahmood, A., Erinle, K. O., Naeem, M. M., et al. (2019). Ecological risk assessment of heavy metals in vegetables irrigated with groundwater and wastewater: the particular case of Sahiwal district in Pakistan. Agric. Water Manag. 226, 105816. doi:10.1016/j.agwat.2019.105816
Renner, R. (2004). Arsenic and lead leach out of popular fertilizer. Environ. Sci. Technol. 38, 382–383A. doi:10.1021/es040642c
Roberts, L. C., Hug, S. J., Voegelin, A., Dittmar, J., Kretzschmar, R., Wehrli, B., et al. (2011). Arsenic dynamics in pore water of an intermittently irrigated paddy field in Bangladesh. Environ. Sci. Tech. 45, 971–976. doi:10.1021/es102882q
Saha, N., and Zaman, M. R. (2013). Evaluation of possible health risks of heavy metals by consumption of food stuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 185, 3867–3878. doi:10.1007/s10661-012-2835-2
Salonen, V. P., and Korkka-Niemi, K. (2007). Influence of parent sediments on the concentration of heavy metals in urban and suburban soils in Turku, Finland. Appl. Geochem. 22 (5), 906–918. doi:10.1016/j.apgeochem.2007.02.003
Samal, P., Singarasubramanian, S. R., Manoj, M. C., Srivastava, J., Dsouza, N., Balakrishna, K., et al. (2022). Heavy metal contamination assessment and its associated human health risk evaluation in the Mahanadi River sediments, India. Int. J. Environ. Sci. Technol. 20, 10673–10694. doi:10.1007/s13762-022-04630-w
Saraswat, A., Ram, S., Raza, M. B., Islam, S., Sharma, S., Omeka, M. E., et al. (2023). Potentially toxic metals contamination, health risk, and source apportionment in the agricultural soils around industrial areas, Firozabad, Uttar Pradesh, India: a multivariate statistical approach. Environ. Monit. Assess. 195, 863. doi:10.1007/s10661-023-11476-3
Saxena, G., Purchase, D., Mulla, S. I., Saratale, G. D., and Bharagava, R. N. (2019). Phytoremediation of heavy metal-contaminated sites: eco-environmental concerns, field studies, sustainability issues, and future prospects. Rev. Environ. Contam. Toxicol. 249, 71–131. doi:10.1007/398_2019_24
Sekomo, C. B., Nkurang, E., Rousseau, D. P., and Lens, P. N. (2011). Fate of heavy metals in an urban natural wetland: the Nyabugogo Swamp (Rwanda). Water Air Soil Pollut. 214, 321–333. doi:10.1007/s11270-010-0426-9
Senapati, N. N., Lenka, D., Behera, S., Mahapatra, A., and Kar, C. (2018). An epidemiologic review of chronic kidney disease of unknown etiology (CKDu). Int. J. Sci. Res. 7 (1), 608–609.
Shah, M. T., Shaheen, B., and Khan, S. (2010). Pedo and biogeochemical studies of mafic and ultramfic rocks in the Mingora and Kabal areas, Swat, Pakistan. Environ. Earth Sci. 60, 1091–1102. doi:10.1007/s12665-009-0253-8
Sharma, R. K., Agrawal, M., and Marshall, F. M. (2007). Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 66, 258–266. doi:10.1016/j.ecoenv.2005.11.007
Sharma, R. K., Agrawal, M., and Marshall, F. M. (2009). Heavy metals in vegetables collected from production and market sites of a tropical urban area of India. Food chemi. Toxicol. 47 (3), 583–591. doi:10.1016/j.fct.2008.12.016
Singh, D., Chhonkar, P. K., and Pandey, R. N. (1999). Soil plant water analysis: a methods manual. New Delhi: IARI.
Singh, K. P., Mohan, D., Sinha, S., and Dalwani, R. (2004). Impact assessment of treated/untreated wastewater toxicants discharged by sewage treatment plants on health, agricultural, and environmental quality in the wastewater disposal area. Chemosphere 55, 227–255. doi:10.1016/j.chemosphere.2003.10.050
Sobukola, O. P., Adeniran, O. M., Odedairo, A. A., and Kajihausa, O. E. (2010). Heavy metal levels of some fruits and leafy vegetables from selected markets in Lagos, Nigeria. Afr. J. Food Sci. 4 (2), 389–393.
Soderland, P., Lovekar, S., Weiner, D. E., Brooks, D. R., and Kaufman, J. S. (2010). Chronic kidney disease associated with environmental toxins and exposures. Adv. Chronic Kidney Dis. 17, 254–264. doi:10.1053/j.ackd.2010.03.011
Spurgeon, D. J., Rowland, P., Ainsworth, G., Rothery, P., Long, S., and Black, H. I. J. (2008). Geographical and pedological drivers of distribution and risks to soil fauna of seven metals (Cd, Cu, Cr, Ni, 728 J Soils Sediments (2013) 13:720–729 Pb, V and Zn) in British soils. Environ. Pollut. 153 (2), 273–283. doi:10.1016/j.envpol.2007.08.027
Swain, S., Pattanayak, A. A., Sahu, B. K., Satapathy, D. R., and Panda, C. R. (2021). Time-series monitoring and ecological risk assessment of heavy metal pollution in Mahanadi estuary, east coast of India. Regional Stud. Mar. Sci. 47, 101923–104855. doi:10.1016/j.rsma.2021.101923
Tani, F. H., and Barrington, S. (2005). Zinc and copper uptake by plants under two transpiration ratios Part I. Wheat (Triticum aestivum L.). Environ. Pollut. 138, 548–558. doi:10.1016/j.envpol.2004.06.004
Thijssen, S., Maringwa, J., Faes, C., Lambrichts, I., and VanKerkhove, E. (2007). Chronic exposure of mice to environmentally relevant, low doses of cadmium leads to early renal damage, not predicted by blood or urine cadmium levels. Toxicol 229, 145–156. doi:10.1016/j.tox.2006.10.011
USEPA (2015). Regional Screening level (RSL) for chemical contaminant at superfound sites. Cincinnati, Ohio: U.S. Environmental Protection Agency, 176. Available at: http://www.epa.gov/reg3hwmd/risk/human/index.htm.
USEPA (2010). Risk based concentration table. Available at: http://www.epa.gov/reg3hwmd/risk/human/index.htm.
USEPA (1999). “Screening level ecological risks assessment protocol for hazardous waste combustion facilities,” in Appendix E: toxicity reference values (Cincinnati, Ohio: United States Environmental Protection Agency). Available at: https://archive.epa.gov/epawaste/hazard/tsd/td/web/pdf/appx-e.pdf
USEPA (US Environmental Protection Agency) (1989). Risk assessment guidance for superfund, human health evaluation manual (Part A). Washington, DC, USA: EPA.
USEPA (2006). USEPA Region III risk-based concentration table: technical back-ground information. Washington: Unites States Environmental Protection Agency.
Varma, P. P. (2015). Prevalence of chronic kidney disease in India: where are we heading? Indian J. Nephrol. 25 (3), 133–135.
Wei, R., Wang, X., Tang, W., Yang, Y., Gao, Y., Zhong, H., et al. (2020). Bioaccumulations and potential human health risks assessment of heavy metals in ppk-expressing transgenic rice. Sci. Total Environ. 710, 136496. doi:10.1016/j.scitotenv.2020.136496
WHO (1985). Guidelines for the study of dietary intakes of chemical contaminants WHO offset publication. Geneva: World Health Organization, 1–100.
Wu, S., Xia, X., Lin, C., Chen, X., and Zhou, C. (2010). Levels of arsenic and heavy metals in the rural soils of Beijing and their changes over the last two decades (1985–2008). J. Hazard Mater. 179 (1), 860–868. doi:10.1016/j.jhazmat.2010.03.084
Yana, C., Kelin, L., and Jian, Z. (2012). Analysis of heavy metal pollution in soil-vegetables at mining area in Hunan. Chin. Agric. Sci. Bull. 28, 226–232.
Yang, Q. W., Xu, Y., Liu, S. J., He, J. F., and Long, F. Y. (2011). Concentration and potential health risk of heavy metals in market vegetables in Chongqing, China. Ecotoxicol. Environ. Saf. 74, 1664–1669. doi:10.1016/j.ecoenv.2011.05.006
Keywords: rice, leafy vegetables, CKDu, noncarcinogenic risk, hazard index, carcinogenic risk
Citation: Mohanty S, Nayak RK, Jena B, Padhan K, Mohapatra KK, Sahoo SK, Dash PK, Das J, Behera SK, Sahu A, Nayak JK, Padhan S and Datta D (2023) Heavy metal contamination in rice, pulses, and vegetables from CKDu-endemic areas in Cuttack district, India: a health risk assessment. Front. Environ. Sci. 11:1248373. doi: 10.3389/fenvs.2023.1248373
Received: 27 June 2023; Accepted: 13 October 2023;
Published: 26 October 2023.
Edited by:
Weichun Yang, Central South University, ChinaReviewed by:
Jajati Mandal, University of Salford, United KingdomCopyright © 2023 Mohanty, Nayak, Jena, Padhan, Mohapatra, Sahoo, Dash, Das, Behera, Sahu, Nayak, Padhan and Datta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shraddha Mohanty, c2hyYWRkaGEubW9oYW50eTAwMUBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.