
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 20 October 2023
Sec. Drylands
Volume 11 - 2023 | https://doi.org/10.3389/fenvs.2023.1162930
Studying the interspecific relationships of exotic species can provide an important theoretical basis for revealing the invasion processes of exotic species, predicting the scope and harm of proliferation, and, subsequently, suggesting reasonable prevention and control measures. Buffalobur (Solanum rostratum Dunal.) is a typical alien invasive plant that causes significant harm in the oasis of the arid region of Xinjiang, being primarily distributed on both sides of the Toutun River and irrigated farmland. Parasitic dodders (Cuscuta australis R. Br.), in addition to phytophagous insects, such as potato beetles (Leptinotarsa decemlineata (Say)), and cotton bollworms (Helicoverpa armigera (Hubner)), that fed on the buffalobur plant were observed to be distributed in the field. In order to explore the impact of dodder parasitism and insect feeding on buffalobur invasion, buffalobur was selected as the main research material in this study. The effects of different degrees of parasitism (non-parasitism, mild parasitism, and severe parasitism), different stages of parasitism (non-parasitism, seedling parasitism, flowering parasitism, and fruit parasitism), and different levels of simulated insect feeding (non-parasitism, mild feeding, moderate feeding, and severe feeding) on the growth, development, and competitiveness of buffalobur were studied. The results showed that parasitism from dodders and feeding by phytophagous insects significantly reduced the biomass of buffalobur (p < 0.05), thus inhibiting its growth and development. In addition, the root–shoot ratio of the buffalobur was increased, which influenced its growth–defense strategy. At different degrees of parasitism from dodders and different degrees of feeding by phytophagous insects, the biomass of the buffalobur was decreased. However, parasitism from dodders at different stages reduced the biomass and competitive ability of buffalobur (p < 0.05). Considering that the dodders and phytophagous insects could parasitize and feed not only on buffalobur but also on other crops, they cannot be used for the control of buffalobur.
Natural enemies (such as parasitic plants and phytophagous animals) are one of the key factors affecting plant life history (Duncan and Williams, 2020; Huang et al., 2021). In the process of plant growth, natural enemies obtain nutrients and water by directly parasitizing or feeding on the stems, leaves, flowers, fruits, and other organs of the plant, subsequently impacting the photosynthetic rate of the host plant, thereby affecting the growth and development of the host plant, among other life history characteristics (Zhong et al., 2021; Min-Yao et al., 2022; Velasco Cuervo et al., 2022). The growth–defense balance hypothesis related to the relationship between plants and their natural enemies states that there is a trade-off between plant growth and defense; in other words, under adverse conditions (such as plant parasitism, insect herbivory, and abiotic factor stress), plants will increase their investment in defense by reducing investment in growth (Schooler et al., 2006; Pan et al., 2012; Pan et al., 2013). Therefore, research into the impact of natural enemies on host plants has important ecological significance in terms of understanding the growth and reproduction of individual plants, as well as the composition and distribution of species in natural communities. Furthermore, this can improve understanding of the structure and functioning of ecosystems while providing a theoretical basis for the use of biological control to reduce the harm of invasive plants (Guo, 1991; Iqbal et al., 2021).
As an important regulator of exotic plant populations, natural enemies play a decisive role in the invasion of exotic plants (Lin, 2019). The main hypotheses related to exotic plants and their natural enemies include the enemy release hypothesis, the biological resistance to enemies hypothesis, the evolution of increased competitive ability hypothesis, the evolution of reduced competitive ability hypothesis, and the new associations hypothesis. Among them, the enemy release hypothesis and the biological resistance to natural enemies hypothesis both believe that in the new environment of the invasion site, local or alien natural enemies may hinder invasion by eating exotic plants as food or using them as hosts and may also have a relatively strong adverse impact on exotic species by inhibiting or delaying the settlement, domestication, and persistence of exotic plants (Alpert, 2006; Heger and Jeschke, 2018; Paula et al., 2021). In contrast, in the invasive area, considering that there are no natural enemies, invasive plants do not need to defend and can thus use resources originally used for defense to grow and reproduce instead, ultimately improving their competitiveness (Blossey and Notzold, 1995; Felker-Quinn et al., 2013; Rotter and Holeski, 2018). Some studies have shown that the invasive plant Alternanthera philoxeroides increases its biomass due to a lack of natural enemy control after entering a new habitat, reducing defense investment and increasing investment in growth and reproduction, thereby enhancing its competitiveness (Geng, 2013). Furthermore, the evolutionary reduced competitive ability hypothesis proposes that if there is less competition within the scope of invasion and the competition involves the characteristics of adaptive cost, the invasive species will choose the aspects that may have adverse effects on them to evolve, thus reducing intraspecific interaction (Wolfe et al., 2004; Ren, 2020). The new association hypothesis states that the invasive species forms a new relationship with the species in the community. The impact of this relationship on the invasive species is generally manifested through the promotion or prevention of the invasive species from successfully invading the new habitat.
In the vicinity of Toutun River, Urumqi City, Xinjiang Uyghur Autonomous Region (hereafter Xinjiang) of China, we observed that buffalobur plants (Solanum rostratum Dunal.) were parasitized by dodders (Cuscuta australis R. Br.) and phytophagous insects, such as potato beetles (Leptinotarsa decemlineata (Say)), and cotton bollworms (Helicoverpa armigera (Hubner)). Buffalobur is an annual invasive weed belonging to the family Solanaceae, which is mainly distributed across both sides of rivers or canals as well as around irrigated farmland in Xinjiang, mainly being dispersed by irrigation water media (Eminniyaz et al., 2013). In recent years, buffalobur has spread rapidly in the oasis of the arid region of Xinjiang, impacting local agricultural production and ecological balance (Hasimu et al., 2017). Dodder is a parasitic plant of the Cuscuta genus belonging to the family Convolvulaceae (Sheng et al., 2006; Shen et al., 2020). It is widely recognized as a harmful weed in agriculture and forestry (Press and Phoenix, 2005; Marvier and Smith, 2010; Han et al., 2020). Potato beetles and cotton bollworms are both important pests worldwide, mainly causing harm to agricultural production (Abdala-Roberts et al., 2022).
Previous relevant studies have found that local parasitic plants can reduce the biomass and other growth indicators of invasive plants, with inhibitory effects on invasive plants (Těšitel et al., 2020; Wan et al., 2022). Therefore, based on past field observations and relevant theories and studies, it was hypothesized that the growth, development, and competitive ability of buffalobur may vary when it is parasitized by dodders or fed upon by phytophagous insects. Therefore, in order to verify this hypothesis, this paper studies the effects of different degrees of parasitism (non-parasitism, mild parasitism, and severe parasitism), different stages of parasitism (non-parasitism, seedling parasitism, flowering parasitism, and fruit parasitism), and different degrees of simulated insect feeding (non-parasitism, mild feeding, moderate feeding, and severe feeding) on the growth, development, and competitiveness of buffalobur. This study addresses the following questions: 1) Verification of the natural enemy escape hypothesis and the biological resistance to natural enemies hypothesis: do parasitism from dodders and feeding by phytophagous insects affect the growth and development of buffalobur? If so, what is the impact? 2) Verification of the growth–defense trade-off hypothesis: Will parasitism from dodders and feeding by phytophagous insects influence the trade-off between the growth and defense of buffalobur? 3) Verification of the evolutionary increased/reduced competitive ability hypothesis: How does the competitive ability of buffalobur change under the conditions of parasitism from dodders and feeding by phytophagous insects?
Between March and April in 2020, the seeds of dodders and buffalobur were both collected near the farmland in the San Ping farm, a practice base of Xinjiang Agricultural University (43°56′N, 87°20′E, 790 m above sea level), and stored in a low-temperature and low-humidity storage box (DWS-150). In mid-April, dodders and buffalobur were planted in outdoor plastic pots (D = 38 cm and h = 40.5 cm), and the outdoor routine management was subsequently carried out. The soil used was collected from the farmland of the practice base.
The experimental observation site was located at the aforementioned practice base. This area belongs to the alluvial plain in the southern margin of the Junggar Basin, with a typical continental temperate desert climate. The annual average temperature is 7.2°C, with an annual average precipitation of 194.3 mm, and the soil type is desert clay soil (Chen et al., 2022).
In order to determine the effect of the degree of parasitism and parasitism time of dodders and feeding by phytophagous insects on the growth, development, and competitiveness of buffalobur, the following three outdoor controlled experiments were designed. Throughout the experiment, no pesticides or fertilizers were applied, and regular weeding was carried out to ensure that only experimental materials grew in the pots used in the experiment.
This experiment adhered to the method outlined by El-Enany and Zayed (2019). The parasitism degree of dodders on buffalobur was divided into three treatments: no parasitism (dodder coverage 0%, no dodder), mild parasitism (dodder coverage 30%, one successfully parasitized dodder retained), and severe parasitism (dodder coverage 60%, three successfully parasitized dodders retained) (Figure 1). Dodders and buffalobur were seeded in the pot (2 groups × 3 treatments × 30 replicates = 180 pots). Group A signified the growth and development experiment, while one buffalobur plant was reserved in each pot. Group B signified the competitive experiment, with two buffalobur plants being reserved in each pot, while the competitive treatment group was only used to treat one of the two buffalobur plants in each pot.
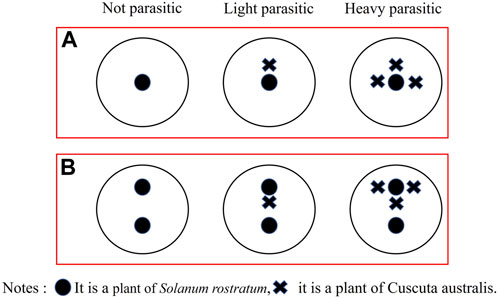
FIGURE 1. Influence of different degrees of parasitism of C. australis on the growth and development (A) and competitiveness (B) of S. rostratum.
To investigate the parasitism time of dodders on buffalobur, the methods by Qi (2010) and Guo et al. (2021) were followed in this study. The parasitism of dodders during the different growth stages of buffalobur was divided into four treatments: non-parasitism, seedling parasitism, flowering parasitism, and fruit parasitism (Figure 2). Buffalobur was then sown into the pots (2 groups × 4 treatments × 30 repetitions = 240 pots). For the parasitism treatment group, dodders at the seedling stage were sown into the pots of buffalobur at the seedling stage and retained one successfully parasitized dodder after emergence, while for the flowering parasitism treatment group, dodders were sown into pots 1 week before the flowering of buffalobur and subsequently retained a successfully parasitized dodder after emergence. Meanwhile, for the fruit parasitism treatment group, dodders were sown into the pots 1 week before the fruit period of buffalobur and retained a successfully parasitized dodder after emergence. For the competitive treatment group, only one of the two buffalobur plants in each pot was treated.
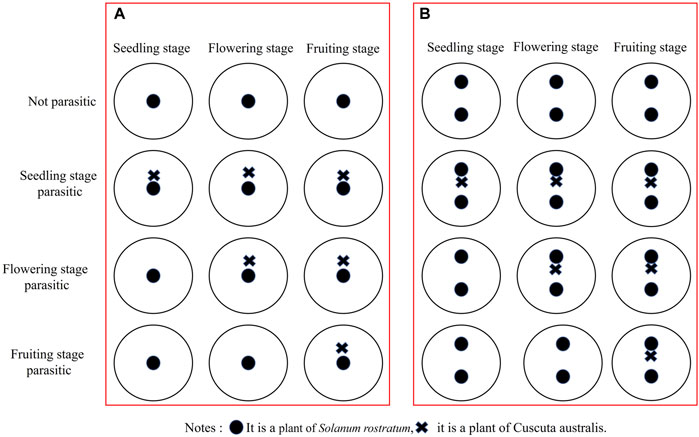
FIGURE 2. Influence of the parasitism of C. australis in different periods on the growth and development (A) and competitiveness (B) of S. rostratum.
This simulated experiment adhered to the methods by Schooler et al. (2006) and Wang et al. (2021). The simulated feeding of different degrees was divided into four treatments: no feeding, light feeding (25% of the feeding area), moderate feeding (50% of the feeding area), and heavy feeding (75% of the feeding area) (Figure 3). Buffalobur was then seeded into the pots (2 groups × 4 treatments × 30 repetitions = 240 pots). The leaves, flowers, and fruits of buffalobur were then cut off according to the treatment, with jasmonic acid being applied on all incisions (Kallure et al., 2022). The competitive treatment group only used one of the two buffalobur plants in each pot.
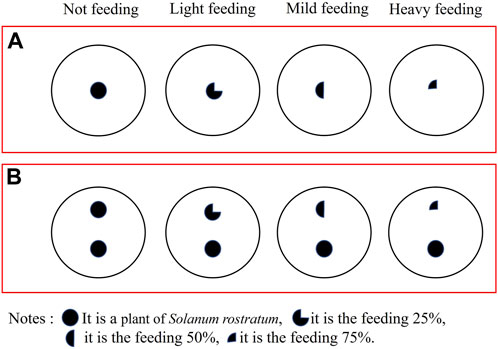
FIGURE 3. Effects of different simulated feeding degrees of herbivore insects on the growth and development (A) and competitiveness (B) of S. rostratum.
After the fruit of buffalobur was mature, dodders were separated from invasive plants, and buffalobur was separated according to the root, stem, leaf, and fruit. They were first dried at 105°C for 20 min and subsequently dried to a constant weight at 70°C. The biomass of each component of buffalobur was measured using the percentile electronic balance (J-SKY).
SPSS 23.0 was used for data analysis. The explore command was used to test the normality of the data, while Tukey’s multiple comparison tests were used to determine the differences between the average values of each data group. A one-way ANOVA was used to compare the difference between the buffalobur plants of same area under different degrees of parasitism by dodder and the difference between buffalobur plants of different areas under the same degree of parasitism by dodder. A two-way ANOVA was also used to determine whether the differences in growth, development, and competitiveness of buffalobur were affected by the interactions of different regions and degrees of parasitism. Excel 2019 was used to generate the two-way ANOVA table, while Origin 2018 software was used to construct the data processing analysis chart.
Varying degrees of dodder parasitism can significantly affect the growth and development of buffalobur (Figures 4A–F). Compared with the control, the biomass of buffalobur was significantly decreased in mild parasitism and severe parasitism (p < 0.05). The total biomass, aboveground biomass, and the biomass of root, stem, leaf, and fruit were decreased by 40%, 39%, 54%, 44%, 46%, and 34%, respectively, under mild parasitism. Under severe parasitism, these same parameters were decreased by 53%, 53%, 57%, 53%, 61%, and 50%, respectively. Furthermore, under different degrees of dodder parasitism, the root–shoot ratio of buffalobur showed an increasing trend; on the other hand, under severe parasitism, this was found to be significantly higher than that without parasitism (p < 0.05; Figure 4G), although there were no significant differences between other treatments. It can be observed that the dodder parasitism in the southern region will influence the biomass ratio of buffalobur. In terms of the influence of different degrees of parasitism from dodders on the competition ability of dodder, varying parasitism degrees can significantly affect the biomass of each dodder component (Figures 4H–M). Under mild and severe parasitism, the total biomass of buffalobur was decreased by 34% and 38%, respectively, significantly lower than that of the non-parasitism group (73%) (p < 0.05). Furthermore, the fruit biomass was decreased by 20% and 30%, respectively, which was significantly lower than that of the parasitism group (69%). The root biomass was also decreased by 13% and 49%, respectively, significantly lower than that of the non-parasitic reduction value (74%). In addition, the stem biomass was decreased by 29% and 30%, respectively, which was significantly lower than the non-parasitic reduction value (73%). The root shoot ratio of buffalobur was significantly higher when the dodder was mild parasitism than when it was not parasitic or severe parasitism (p < 0.05; Figure 4N). It was found that when dodders were parasitized to different degrees, the competitive ability of buffalobur was improved.
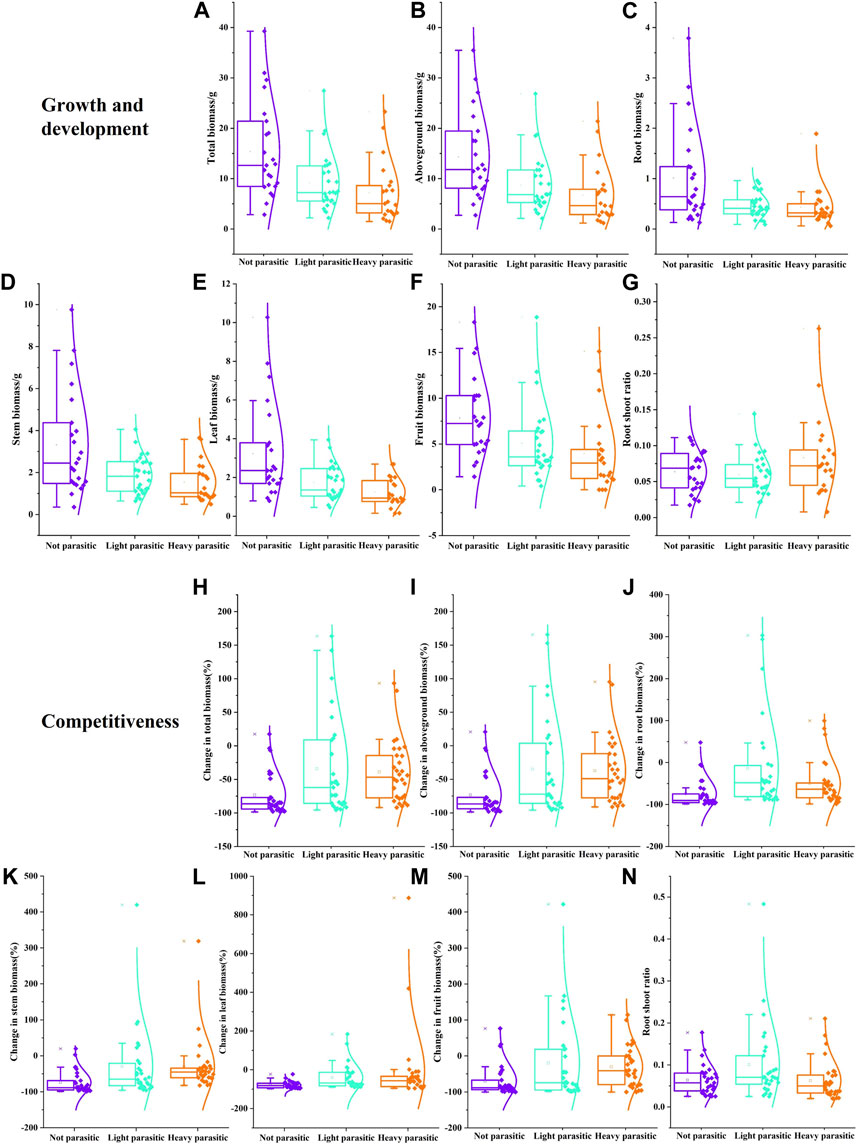
FIGURE 4. Influence of different degrees of parasitism of C. australis on the growth and development (A–G) and competitive ability (H–N) of S. rostratum. (A) Total biomass, (B) aboveground biomass, (C) root biomass, (D) stem biomass, (E) leaf biomass, (F) fruit biomass, and (G)root–shoot ratio in the growth experiment; (H) total biomass change rate, (I) aboveground biomass change rate, (J) root biomass change rate, (K) stem biomass change rate, (L) leaf biomass change rate, (M) fruit biomass change rate, and (N) root–shoot ratio in the competition experiment.
The parasitism of dodders at different stages can affect the growth and development of buffalobur (Figures 5A–F). The parasitism of dodders at the seedling, flowering, and fruit stages significantly reduced the biomass of buffalobur (p < 0.05), with parasitism at the seedling and flowering stages having the greatest impact on the biomass of buffalobur. The total biomass, aboveground biomass, and the biomass of root, stem, leaf, and fruit of buffalobur were all reduced by 97%, 97%, 94%, 95%, 93%, and 99%, respectively. Additionally, these same parameters of buffalobur were decreased by 91%, 92%, 87%, 87%, 79%, and 95%, respectively, due to parasitism at the flowering stage. Furthermore, parasitism at the fruit stage reduced these parameters by 26%, 25%, 37%, 29%, 34%, and 22%, respectively. With the parasitism of dodders at different stages, the root–shoot ratios of buffalobur at the seedling and flowering stages were significantly higher than those during the non-parasitism and fruit stages (p < 0.05; Figure 5G); on the other hand, parasitism at the seedling stage was also significantly higher than that at the flowering stage. It can be observed that parasitism from dodders at different stages can significantly affect the biomass ratios of buffalobur (Figure 5N). In terms of its impact on competitiveness, dodder parasitizing at different stages significantly affected the biomass of each component of buffalobur (p < 0.05) (Figures 5H–M). When parasitized during the fruit stage, the total biomass of buffalobur was decreased by 39%, which was significantly higher than that of the non-parasitized group (10%) (p < 0.05). The number of fruit organisms parasitized during the fruit stage decreased by 44%, which was also significantly higher than that of the non-parasitized group (16%). Furthermore, stem biomass decreased by 29% and 31% when parasitized during the flowering and fruit stages, respectively, while increasing by 9% when not parasitized. However, there were no significant differences among the other treatments.

FIGURE 5. Effect of parasitism of C. australis in different periods on the growth and development (A–G) and competitive ability (H–N) of S. rostratum. (A) Total biomass, (B) aboveground biomass, (C) root biomass, (D) stem biomass, (E) leaf biomass, (F) fruit biomass, and (G) root–shoot ratio in the growth experiment; (H) total biomass change rate, (I) aboveground biomass change rate, (J) root biomass change rate, (K) stem biomass change rate, (L) leaf biomass change rate, (M) fruit biomass change rate, and (N) root–shoot ratio in the competition experiment.
Feeding by herbivorous insects can significantly affect the growth and development of buffalobur (p < 0.05; Figures 6A–F). Compared with the control, light feeding reduced the total, aboveground, root, stem, and leaf biomass of buffalobur by 11%, 11%, 16%, 39%, and 42%, respectively, and light feeding increased fruit biomass of buffalobur by 10%. In comparison, moderate feeding reduced these same parameters of buffalobur by 26%, 25%, 37%, 48%, 52%, and 7%, respectively. Furthermore, heavy feeding reduced these parameters by 61%, 61%, 62%, 66%, 61%, and 58%, respectively. During different degrees of feeding treatment, with the increase in feeding degree, the inhibition of buffalobur was also found to be greater (p < 0.05; Figure 6G). When the feeding degree increases, the root–shoot ratios of buffalobur show a trend of an initial decrease before increasing. In addition, the root–shoot ratio of buffalobur was significantly higher than that for the light and moderate feeding groups (p < 0.05; Figure 6G). Among the effects of different feeding levels on the competitive ability of buffalobur, feeding significantly affected the biomass changes of each component (Figures 6H–M). Under mild, moderate, and severe feeding, the total biomass of buffalobur was decreased by 33%, 40%, and 20%, respectively, which was significantly lower than that of the non-feeding group (74%) (p < 0.05). Fruit biomass increased by 7% under conditions of heavy feeding, while it decreased by 53% with non-feeding. Additionally, root biomass decreased by 48%, 3%, and 23% under the respective conditions of mild, moderate, and severe feeding, which was significantly lower than that under non-feeding (79%). The stem biomass was decreased by 35%, 34%, and 43% under these three conditions, respectively, which was also significantly lower than that under non-feeding (78%). In the competition experiment, there was no significant change in the root shoot ratio of the parasitic buffalobur during different stages of the dodder (Figure 6N). Overall, it was found that phytophagous insects could improve the competitive ability of buffalobur.
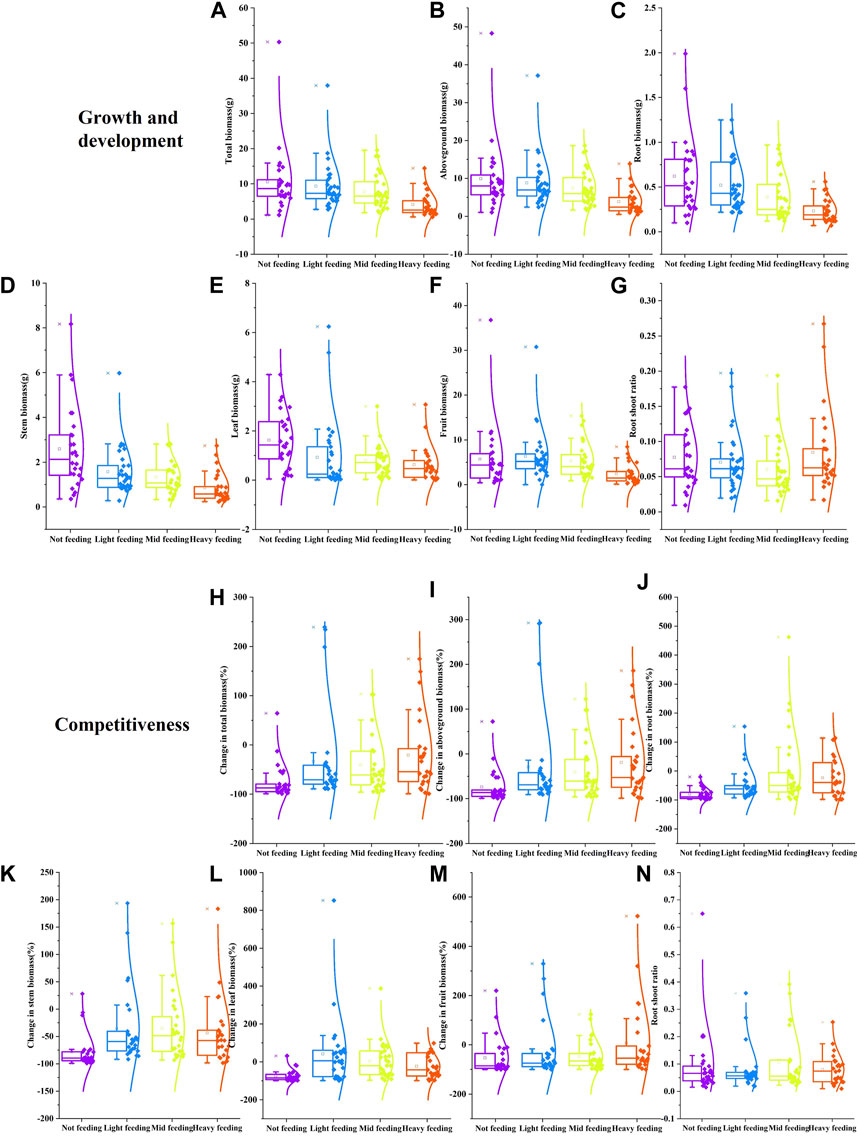
FIGURE 6. Effects of simulated insect feeding on the growth and development (A–G) and competitiveness (H–N) of S. rostratum. (A) Total biomass, (B) aboveground biomass, (C) root biomass, (D) stem biomass, (E) leaf biomass, (F) fruit biomass, and (G) root–shoot ratio in the growth experiment; (H) total biomass change rate, (I) aboveground biomass change rate, (J) root biomass change rate, (K) stem biomass change rate, (L) leaf biomass change rate, (M) fruit biomass change rate, and (N) root–shoot ratio in the competition experiment.
The results of this experiment showed that the biomass of buffalobur was reduced by 45%, 72%, and 33%, respectively, with different degrees of parasitism from dodder, parasitism at different stages, and feeding from phytophagous insects. The parasitism of dodders and the feeding of phytophagous insects increased the root–shoot ratio of buffalobur while also changing the growth–defense trade-off strategy of buffalobur. In the competition ability experiment, the relative decline of the total buffalobur biomass was significantly lower than that of the non-parasitic plants, demonstrating that the competition ability of the parasitized and fed upon buffalobur plants was higher than that of the non-parasitic plants. However, parasitism from dodders during the fruit stage led to the relative decline of the total biomass of buffalobur significantly higher than that of the non-parasitism group, which therefore reduced the competitiveness of buffalobur. These results were consistent with our expected assumptions, while their ecological significance is mainly shown in the following aspects.
Parasitism from dodders and insect feeding can both significantly affect the growth and development of buffalobur. Biomass was significantly inhibited as a result of dodder parasitizing in different degrees and different stages, among which dodder parasitizing during the seedling and flowering stages showed the most significant inhibition on the biomass of buffalobur. In addition, the biomass of buffalobur showed a downward trend under different degrees of insect feeding, while biomass significantly decreased under heavy ingestion. The results of this experiment showed that parasitism from dodders and insect feeding would both inhibit the biomass of buffalobur, thus inhibiting growth and development. This is consistent with Wang et al.’s (2012) research on the inhibition of growth and development of A. philoxeroides by dodder parasitism. In addition, this ultimately inhibits the propagation and diffusion of buffalobur throughout the invaded area. It can be seen that the parasitic relationship between dodders and buffalobur and the feeding relationship between phytophagous insects and buffalobur both supported the biological resistance to natural enemies hypothesis.
Biomass is one of the most important parameters of plant invasiveness, since plants with high biomass often have a strong reproductive capacity and high fitness (Stephane et al., 2017; Gao et al., 2021; Liu et al., 2021). Additionally, plants can change their morphology and respond to environmental conditions by adapting the competitiveness of their aboveground and underground parts. Considering that the distribution of the aboveground and underground parts will affect their rate of access to resources, this has become an important feature of plant growth and competitiveness (Aikio et al., 2009). The root–shoot ratio of the invasive plant buffalobur has previously been shown to increase when parasitized by dodder. There exist hypotheses such as growth–defense trade-off and resource availability concerning environmental change and plant biomass allocation. Additionally, the resource availability hypothesis proposes that the relationship between natural enemies and resource availability will lead to changes in the growth–defense trade-off in plants (Lonsdale, 1999; Shalimu et al., 2012; Gorgens et al., 2021). The research results indicate that the root–shoot ratio of buffalobur changes under dodder parasitism, indicating that buffalobur changes the growth–defense balance and increases energy input for defense when facing dodder parasitism. This is consistent with the research by Guo et al. (2014).
Considering the change in biomass distribution of buffalobur, buffalobur reduced its investment in fruit while increasing its investment in roots. By balancing the energy distribution between growth and defense, the redistribution of resources from “growth” to “defense” enhanced its defense abilities. Therefore, the parasitic relationship between dodders and buffalobur supports both the growth–defense trade-off and resource availability hypotheses. This investment trade-off is conducive to the survival, expansion, and rapid evolution of buffalobur. However, the plant-eating insects reduced the root–shoot ratio of buffalobur while increasing fruit biomass under conditions of mild feeding. According to the habitat stability theory, when plants are under external pressure (such as natural enemy feeding), they will allocate more resources to growth and reproduction structures in order to obtain more light and other resources for reproduction in competition, such that under external pressure, this change will be conducive to high reproductive allocation (Evenson, 1983; Cao et al., 2005; Leihy and Chown, 2020). The feeding relationship between herbivorous insects and buffalobur increased the investment by buffalobur in breeding resources, which showed that this relationship supported the theory of habitat stability. This change will also be conducive to the spread of the population.
Competitive ability is one of the most significant determinants of whether invasive plants can successfully invade their new environment (Williamson and Fitter, 1996; Vilà et al., 2003). Strong competitiveness is conducive to resource competition between invasive plants and local or invasive plants in the invaded area while also accelerating the process of invasion. Weak competitiveness is unfavorable for the invasion of exotic plants (Carboni et al., 2021). In the experiments investigating the different degrees of parasitism of dodders and different degrees of feeding by phytophagous insects, the root, stem, leaf, and fruit biomass of buffalobur all increased significantly under the conditions of mild parasitism from dodders and feeding by phytophagous insects. As a result, it can be observed that under these conditions, the competitiveness of buffalobur will improve. However, the biomass of dodder parasitized at different stages decreased significantly, indicating that dodder parasitized at different stages reduced the competitiveness of buffalobur. Overall, the results of this experiment showed that the relationship between the different degrees of parasitism of dodders and the different degrees of feeding by phytophagous insects supported the evolutionary hypothesis of improving competitiveness, while the relationship between the different stages of parasitism of dodders supported the evolutionary hypothesis of reducing competitiveness.
Through the experiments investigating the growth, development, and competitive ability of buffalobur under dodder parasitism and herbivorous insect feeding, it can be clearly concluded that these conditions inhibited the growth and development of buffalobur. However, the competition experiment designed in this paper only included intraspecific competition and did not consider interspecific competition. In fact, in terms of wild communities and ecosystems, invasion is often accompanied by competition among similar species. At the same time, simulated feeding cannot fully reflect the impact of phytophagous insects on invasive plants. Therefore, the ability of competition to invade invasive plants and the propagation and diffusion in the invasive areas still require further research.
In summary, the biomass of buffalobur was significantly reduced by parasitism from dodders and feeding by phytophagous insects, which showed that the growth and development of buffalobur were both inhibited. Parasitism from dodders and feeding by phytophagous insects can change the biomass distribution of buffalobur. Among them, the fruit biomass of buffalobur was significantly reduced in the face of different degrees and different stages of parasitism from dodders, while the root–shoot ratio was significantly increased, indicating that buffalobur would use more resources to increase the underground biomass to resist parasitism by dodders (supporting the growth–defense trade-off hypothesis). However, under the feeding of phytophagous insects, the root–shoot ratio of buffalobur decreased while the fruit biomass increased significantly, indicating that this reduced the vegetative growth and transferred more resources to reproduction, which is beneficial for the growth and diffusion of buffalobur (supporting the theory of habitat stability). Parasitism from dodders and feeding by phytophagous insects both have significant impacts on the competitive ability of buffalobur. Under mild parasitism from dodders and feeding by phytophagous insects in South China, the competitive ability of buffalobur was significantly improved (which supports the evolutionary hypothesis of improving the competitive ability). However, when dodders were parasitized at different stages in the southern region, its competitive ability decreased significantly (which supports the evolutionary hypothesis of reducing competitive ability). This has affected the propagation and diffusion of buffalobur and put forward a relevant basis for the prevention and control of the invasive plant, buffalobur. However, since dodders and potato beetles also parasitize and feed on other plants, while the potato beetle itself is also an alien organism, this will cause potential ecological security problems. Therefore, further experimental demonstration is still necessary to prove whether dodders and potato beetles can control buffalobur.
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
J-XH, first author, performed the experiment and data analyses and wrote the manuscript. AY, second author and corresponding author, contributed to the conception of the study, contributed significantly to analysis and manuscript preparation, and helped perform the analysis with constructive discussions. All authors contributed to the article and approved the submitted version.
This work was supported in part by the National Natural Science Foundation of China (31760179), the General Survey of Exotic Invasive Wild Plants in Forest, Grassland, and Wetland Ecosystems in Xinjiang (XJLCRQSW-3), and the Third Xinjiang Comprehensive Scientific Expedition Project (2022xjkk0401).
Special thanks to Professor Tan Dunyan and Associate Professor Qiu Juan for their guidance on some experimental designs.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdala-Roberts, L., Vázquez-González, C., Rasmann, S., and Moreira, X. (2022). Test of communication between potato plants in response to herbivory by the Colorado potato beetle. Agric. For. Entomology 24 (2), 212–218. doi:10.1111/afe.12484
Aikio, S., Kaisa, R. K., and Mannin, S. (2009). Dynamics of biomass partitioning in two competing meadow plant species. Plant Ecol. 205, 129–137. doi:10.1007/s11258-009-9603-6
Alpert, P. (2006). The advantages and disadvantages of being introduced. Biol. Invasions 8 (7), 1523–1534. doi:10.1007/s10530-005-5844-z
Blossey, B., and Notzold, R. (1995). Evolution of increased competitive ability in invasive nonindigenous plants: a hypothesis. J. Ecol. 83 (5), 887–889. doi:10.2307/2261425
Cao, G. X., Zhong, Z. C., Xie, D. T., and Liu, Y. (2005). Study on the relationship between reproductive distribution and individual size of Chuan'elianrui tea in different communities. Chin. J. Plant Ecol. 3, 261–266.
Carboni, M., Livingstone, S. W., Isaac, M. E., and Cadotte, M. W. (2021). Invasion drives plant diversity loss through competition and ecosystem modification. J. Ecol. 109, 3587–3601. doi:10.1111/1365-2745.13739
Chen, Y. X., Du, Y., and Wang, Y. X. (2022). Effects of habitat on young spike differentiation and reproductive pattern of Bromus inermis. Pratacultural Sci., 1–10.
Duncan, S. S., and Williams, J. L. (2020). Life history variation in an invasive plant is associated with climate and recent colonization of a specialist herbivore. Am. J. Bot. 107, 1366–1374. doi:10.1002/ajb2.1531
El-Enany, M. F., and Zayed, E. M. (2019). Performance of some egyptian clover cultivars and their tolerance to dodder infestation. Egypt. J. Agric. Sci. 4 (70), 437–451. doi:10.21608/ejarc.2019.211135
Eminniyaz, A., Qiu, J., Tan, D., Baskin, C. C., Baskin, J. M., and Nowak, R. S. (2013). Dispersal mechanisms of the invasive alien plant species Buffalobur (S. rostratum) in cold desert sites of Northwest China. Weed Sci. 61 (4), 557–563. doi:10.1614/ws-d-13-00011.1
Evenson, W. E. (1983). “Experimental studies of reproductive energy allocation in plants,” in Handbook of experimental pollination biology. Editors C. E. Jones, and R. J. Little (New York: Van Nostrand Reinhold), 249–275.
Felker-Quinn, E., Schweitzer, J. A., and Bailey, J. K. (2013). Meta-analysis reveals evolution in invasive plant species but little support for evolution of increased competitive ability (EICA). Ecol. Evol. 3 (3), 739–751. doi:10.1002/ece3.488
Gao, F. L., He, Q. S., Xie, R. Q., Hou, J. H., Shi, C. L., Li, J. M., et al. (2021). Interactive effects of nutrient availability, fluctuating supply, and plant parasitism on the post-invasion success of Bidens pilosa. Biol. Invasions 23, 3035–3046. doi:10.1007/s10530-021-02555-y
Geng, X. Y. (2013). The evolution of defense strategies and resource utilization efficiency of invasive species of arabidopsis philoxeroides. Shanghai: Fudan University.
Gorgens, E. B., Nunes, M. H., Jackson, T., Coomes, D., Keller, M., Reis, C. R., et al. (2021). Resource availability and disturbance shape maximum tree height across the Amazon. Glob. Change Biol. 27 (1), 177–189. doi:10.1111/gcb.15423
Guo, S. M., Li, J. M., Li, Y. H., and Yan, M. (2014). Growth defense trade-off of Alternanthera philoxeroides in response to Cuscuta australis parasitism. J. Ecol. 34 (17), 4866–4873.
Guo, Y. J. (1991). “Research on the relationship between natural enemies and pest host plants in biological control,” in Proceedings of the National Symposium on Biological Control.
Guo, Z. X. H., Wang, J. G., Zhang, R. M., Zhang, H., Xing, B., Naeem, M., et al. (2021). Effects of graphene oxide on tomato growth in different stages. Plant Physiology Biochem. 162 (1), 447–455. doi:10.1016/j.plaphy.2021.03.013
Han, C., Hu, A., Guo, W., Bai, B., Li, Y., and You, W. (2020). Effects of parasitism of C. australis on the growth performance of Alternanthera philoxeroides and its related specie.
Hasimu, D., Eminniyaz, A., Abuduwali, M., Abudukerimu, P., et al. (2017). The transmission characteristics of the fruit of the grassland poison grass, Clematis sativus. Seed 36 (6), 4.
Heger, T., and Jeschke, J. M. (2018). “Enemy release hypothesis,” in Invasion biology. Hypotheses and evidence. 1st ed. (Boston, MA: CABI), 92–102.
Huang, X., Yu, J., Guan, B., Xie, H., Liu, S., He, H., et al. (2021). Responses of morphological and physiological traits to herbivory by snails of three invasive and native submerged plants. J. Plant Ecol. 15 (3), 571–580. doi:10.1093/jpe/rtab107
Iqbal, M. F., Feng, Y. L., Feng, W. W., Liu, M. C., and Lu, X. R. (2021). Ecological impacts of the invasive plant Xanthium strumarium and the impacts of three aboveground herbivores on the invader. Ecol. Indic. 131 (108140), 108140. doi:10.1016/j.ecolind.2021.108140
Kallure, G. S., Kumari, A., Shinde, B. A., and Giri, A. P. (2022). Characterized constituents of insect herbivore oral secretions and their influence on the regulation of plant defenses. Phytochemistry 193, 113008. doi:10.1016/j.phytochem.2021.113008
Leihy, R. I., and Chown, S. L. (2020). Wind plays a major but not exclusive role in the prevalence of insect flight loss on remote islands. Proc. R. Soc. B 287 (1940), 20202121. doi:10.1098/rspb.2020.2121
Lin, C. G. (2019). Effects of short-term warming and natural enemy sources on competition between alien invasion and local clonal plants. Huazhong Agricultural University.
Liu, X. Y., Zhu, J. F., Li, F. F., and Zhao, C. F. (2021). Effects of ragweed invasion on the community structure of local herbaceous plants under the forests in the Ili Valley, Xinjiang. Acta Ecol. Sin. 41 (24), 9613–9620.
Lonsdale, W. M. (1999). Global patterns of plant invasions and the concept of invasibility. Ecology 80 (5), 1522–1536. doi:10.1890/0012-9658(1999)080[1522:gpopia]2.0.co;2
Marvier, M. A., and Smith, D. L. (2010). Conservation Implications of Host Use for Rare Parasitic Plants. Implicaciones del Uso de Hospederos en la Conservacion de Plantas Parasiticas Raras. Conserv. Biol. 11 (4), 839–848. doi:10.1046/j.1523-1739.1997.96223.x
Min-Yao, J., Moran, F., Wang, L., Philbrook, R. N., Belcher, M. S., Nakayama, H., et al. (2022). Heinz-resistant tomato cultivars exhibit a lignin-based resistance to field dodder (Cuscuta campestris) parasitism. Plant Physiol. 189 (1), 129–151. doi:10.1093/plphys/kiac024
Pan, X. Y., Jia, X., Chen, J. K., and Li, B. (2012). For or against: the importance of variation in growth rate for testing the EICA hypothesis. Biol. Invasions 14 (1), 1–8. doi:10.1007/s10530-011-9941-x
Pan, X. Y., Jia, X., Fu, D. J., and Li, B. (2013). Geographical diversification of growth-defense strategies in an invasive plant. J. Syst. Evol. 51 (3), 308–317. doi:10.1111/j.1759-6831.2012.00239.x
Paula, D. P., Togni, P. H., Costa, V. A., Souza, L. M., Sousa, A. A. T. C., Tostes, G. M., et al. (2021). Scrutinizing the enemy release hypothesis: population effects of parasitoids on Harmonia axyridis and local host coccinellids in Brazil. BioControl 66 (1), 71–82. doi:10.1007/s10526-020-10041-y
Press, M. C., and Phoenix, G. K. (2005). Impacts of parasitic plants on natural communities. New Phytolgist 166, 737–751. doi:10.1111/j.1469-8137.2005.01358.x
Qi, L. L. (2010). Dynamic study on the parasitism of bat moth puccinella in different development stages by trichosporium chinensis. Sun Yat-sen University.
Ren, G. Q. (2020). Ecological adaptation and mechanism of invasive plant Solidago canadensis to nitrogen deposition under the background of global warming. Jiangsu: Jiangsu University.
Rotter, M. C., and Holeski, L. M. (2018). A meta-analysis of the evolution of increased competitive ability hypothesis: genetic-based trait variation and herbivory resistance trade-offs. Biol. Invasions 20 (9), 2647–2660. doi:10.1007/s10530-018-1724-1
Schooler, S., Baron, Z., and Julien, M. (2006). Effect of simulated and actual herbivory on alligator weed, Alternanthera philoxeroides, growth and reproduction. Biol. Control 36 (1), 74–79. doi:10.1016/j.biocontrol.2005.06.012
Shalimu, D., Juan, Q., Tan, D. Y., Baskin, C. C., and Baskin, J. M. (2012). Seed biology of the invasive species buffalobur (S. rostratum) in northwest China. Weed Sci. 60 (2), 219–224. doi:10.1614/ws-d-11-00148.1
Shen, G., Liu, N., Zhang, J., Xu, Y., Baldwin, I. T., and Wu, J. (2020). Cuscuta australis (dodder) parasite eavesdrops on the host plants’ FT signals to flower. Proc. Natl. Acad. Sci. 117 (37), 23125–23130. doi:10.1073/pnas.2009445117
Sheng, J. H., Zhang, X. J., Liu, H. Y., and Li, L. (2006). Summarization of parasttic plant. Bull. Biol. 41 (3), 9–13.
Stephane, A., Julien, P., Jean, C., and Muselli, A. (2017). Chemical composition of essential oils of Xanthium spinosum L., an invasive species of Corsica. Chem. Biodivers. 14 (1), 1–14. doi:10.1002/cbdv.201600148
Těšitel, J., Cirocco, R. M., Facelli, J. M., and Watling, J. R. (2020). Native parasitic plants: biological control for plant invasions? Appl. Veg. Sci. 23 (3), 464–469. doi:10.1111/avsc.12498
Velasco Cuervo, S. M., Galindo González, L., and Toro Perea, N. (2022). An omics evolutionary perspective on phytophagous insect–host plant interactions in Anastrepha obliqua: a review. Entomologia Exp. Appl. 171, 2–16. doi:10.1111/eea.13241
Vilà, M., Gómez, A., and Maron, J. L. (2003). Are alien plants more competitive than their native conspecifics?A test using Hypericum perforatum L. Oecologia 137, 211–215. doi:10.1007/s00442-003-1342-0
Wan, J., Yi, J., Tao, Z., Ren, Z., Otieno, E. O., Tian, B., et al. (2022). Species-specific plant-mediated effects between herbivores converge at high damage intensity. Ecology 103 (5), e3647. doi:10.1002/ecy.3647
Wang, R. K., Guan, M., Li, Y. H., Yang, P. F., and Li, J. M. (2012). The effect of parasitism of Cuscuta australis on the growth and community diversity of Alternanthera philoxeroides. J. Ecol. 32 (06), 1917–1923.
Wang, Y., Yan, J., Sun, J., Wangpeng, S., Harwood, J. D., Monticelli, L. S., et al. (2021). Effects of field simulated warming on feeding behavior of Sitobion avenae (Fabricius) and host defense systems. Entomol. Gen. 41 (6), 567–578. doi:10.1127/entomologia/2021/1271
Williamson, M. H., and Fitter, A. (1996). The characters of successful invaders. Biol. Conserv. 78, 163–170. doi:10.1016/0006-3207(96)00025-0
Wolfe, L. M., Elzinga, J. A., and Biere, A. (2004). Increased susceptibility to enemies following introduction in the invasive plant Silene latifolia. Ecol. Lett. 7 (9), 813–820. doi:10.1111/j.1461-0248.2004.00649.x
Keywords: arid oasis, buffalobur, interspecific relationship, growth, competitive ability
Citation: Jian-Xiao H and Yimingniyazi A (2023) Effect of natural enemies on the invasion of the exotic plant buffalobur (Solanum rostratum Dunal.) in the arid oasis of Urumqi. Front. Environ. Sci. 11:1162930. doi: 10.3389/fenvs.2023.1162930
Received: 10 February 2023; Accepted: 04 October 2023;
Published: 20 October 2023.
Edited by:
Jacinto Elías Sedeño-Díaz, Instituto Politécnico Nacional (IPN), MexicoReviewed by:
Renata Bažok, University of Zagreb, CroatiaCopyright © 2023 Jian-Xiao and Yimingniyazi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amanula Yimingniyazi, YW1hbnVsYS55QGVkdS54amF1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.