- 1Department of Environmental and Prevention Sciences, University of Ferrara, Ferrara, Italy
- 2Forest Research Centre (CEF) and Associate Laboratory TERRA, School of Agriculture, University of Lisbon, Lisbon, Portugal
- 3Southern Indian Ocean Fisheries Agreement (SIOFA) c/o DAAF Bâtiment B Parc de la Providence, Saint-Denis, France
Protected areas (PAs) are the cornerstones of global biodiversity conservation efforts, but to fulfil this role they must be effective at conserving both habitat and species. Among protected taxa, freshwater fish are exposed to multiple disturbances and are considered one of the most endangered. The Natura 2000 reserves network was established with the aim of preserving biodiversity across Europe, but few assessments have been made on its effectiveness on the conservation of freshwater fish species. We tested the hypothesis that fish community is exposed to less anthropogenic pressures within the Natura 2000 sites than outside, hosting a higher number of native species and maintain lower number of non-native species. We tested these hypotheses considering 3,777 sampling sites, found across the entire Italian territory. Results showed that PAs did not guarantee less anthropogenic impacts and higher fish species richness than outside PAs, suggesting that PAs are not a panacea for anthropogenic pressures and safeguarding fish diversity. Nevertheless, more caution should be applied to the management measures and the design of new PAs due to the limitations of the protection of a single stretch within a whole river ecosystem. Moreover, the impossibility to operate any management of invasive fish species on the broad scale of a whole river basin is likely the most limiting factor to fish biodiversity conservation in Italy. Finally, it is also necessary to extend the analysis to other basins and Natura 2000 sites in Europe.
1 Introduction
Biodiversity decline is a worldwide trend affecting almost all taxa and ecosystems (Butchart et al., 2010; Cardinale et al., 2012; Dirzo, 2014). From eight million of total estimated number of animal and plant species on Earth (including 5.5 million insect species), up to one million of species are threatened with extinction, with a rate of extinction higher than the rate to average over the last 10 million years, which is accelerating in the last 50 years (IPBES (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services), 2019).
In the last decades, the interest and awareness on the importance of biodiversity and on the effects on its loss lead to the increase of states’ commitments and laws to protect it. Another effort to halt and reverse the decrease in biodiversity resulted in the establishment of protected areas (PAs; e.g., Rands et al., 2010). Although evidence exists about the positive effects of PAs on species conservation (Cazalis et al., 2020), opposite evidence also exists. For example, Laurance et al. (2012) revealed a decrease of biodiversity inside tropical PAs mainly due to habitat disruption, hunting and resources exploitation which damage reserve health and boundary areas outside reserves. Other authors pointed out the ineffectiveness of PAs in maintaining biodiversity due to the lack of specific threat management (e.g., Chessman, 2013).
The European Union has strong legislation to protect nature and biodiversity, which revolves around a network of nature protection areas (Natura 2000 network). This network is the largest nature protection network in the world, which includes more than 26,000 protected sites and covers one-fifth of the Europe Union’s area. The Natura 2000 network comprises Special Areas of Conservation (SACs) focusing on habitat and species protection, designated under the Habitats Directive (Council Directive 92/43/EEC), and Special Protection Areas (SPAs) focusing on bird protection, designated under the 1979 Birds Directive (Council Directive 79/409/EEC). Due to the Natura 2000 network extension and severe legislation, it is expected that biodiversity would be adequately represented and preserved in those areas. However, some incertitude exists on the efficacy of the Natura 2000 network in maintaining biodiversity, especially for freshwater fish (Hermoso et al., 2015; Splendiani et al., 2019; Rico-Sánchez et al., 2020). Moreover, large predators in exotic communities pose a substantial threat not only to native fish, but also to protected birds, especially during nesting season. This effect has been recently evidenced in Milardi et al. (2022a) who found that waterbird reproductive performance was negatively affected by high densities of invasive wels catfish (Silurus glanis) by predating on chicks of waterfowl, coots, and grebes.
Freshwater ecosystems appear more vulnerable to biodiversity loss due to many and heterogeneous pressures such as overexploitation, water pollution, habitat degradation, flow modification and exotic species introductions (Vörösmarty et al., 2010; Carpenter et al., 2011; Dudgeon, 2019), and due to the high number of endemic and rare species in a limited spatial extension (Gleick, 1998; Balian et al., 2008; Collen et al., 2014). Indeed, reserve networks were not primarily designed to protect freshwaters, and therefore may not adequately address its biodiversity and related threats, such as, non-native species impacts, water flow changes, habitat alterations, contaminants from upstream stream stretches, among others (Chessman, 2013; Hermoso et al., 2015).
This general study aims to investigate the adequacy of the Natura 2000 reserves network in maintaining freshwater fish diversity. We focused on Italy as a study case of the Mediterranean region and on fish in inland waters as model taxa, since freshwater fish diversity has been highlighted as one of the most endangered taxa in this region, so far (Crivelli, 1995). To address this aim, we hypothesized that sites included in PAs showed less anthropogenic pressures than outside sites, and that sites included in PAs also showed higher fish richness with higher number of native species and lower number of non-native species.
2 Materials and methods
2.1 Study area
The study area has Mediterranean climate and includes freshwater watercourses from north to south across the Italian peninsula, including the rivers on the main islands (Figure 1).
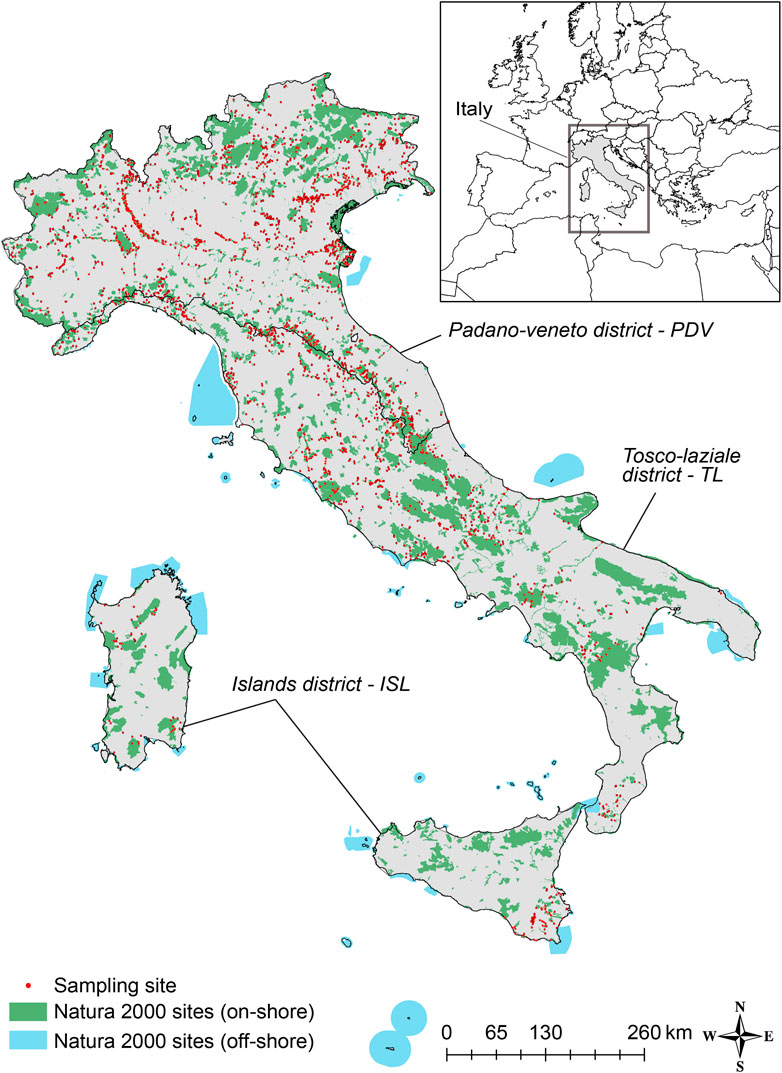
FIGURE 1. Map showing on-shore (green) and off-shore (cyan) protected areas of Natura 2000 network and 3,777 sampling sites (red dots). The solid black line shows the border of the Padano-Veneto (PDV), Tosco-Laziale (TL), and Islands (ISL) districts.
Italian rivers are divided in three fauna districts according to the established literature (Bianco, 1987; Bianco, 1998):the Padano-Veneto district (PDV), the Tosco-Laziale district (TL), and the islands district (ISL). The PDV district includes the largest river basin in Italy, the Po River basin (71,000 km2) within the Po River plain, limited in the north by the Alps and in the south by the Apennines. All rivers in this district flow ultimately into the Adriatic Sea. The TL district is characterized by the highest longitudinal extension; with Apennines separate the rivers that flow in the Tyrrenian Sea, in the west Italian coast, from rivers that flow in the Adriatic Sea and Ionian Sea. The ISL district encompasses the two major Italian islands (Sardinia and Sicily islands), completely isolated from the continental lands by sea stretches.
The Italian Natura 2000 network includes 2,636 protected areas, both SACs and SPAs, with areas ranging from 0.001 km2 to 3872.89 km2, covering more than 19% of the national inland territory and more than 13% of the marine one (ISPRA, 2020). In this study, only the on-shore inland Natura 2000 sites were considered (Figure 1). Overall, on-shore inland protected areas in PDV, TL, ISL districts showed similar shape complexity, although protected areas in ISL district are the largest (Supplementary Table S1).
2.2 Fish species data
We derived 3,777 sampling sites of freshwater fish community in Italian inland waters from Milardi et al., 2020b, covering most of the Italian peninsula and islands and spanning altitudes between -4 and 2,556 m above sea level, sampled through official monitoring programs. Fish sampling was mainly performed in the warm season by electrofishing, combined with nets in sites of higher water depth and high electrical conductivity as indicated in national monitoring guidelines (APAT, 2007).
Fish species were classified according to Kottelat and Freyhof (2007), taking into account recent taxonomic determinations and corresponding common names as listed in Eschmeyer’s Catalog of Fishes (Fricke and Eschmeyer, 2022) and FishBase (Froese and Pauly, 2019), respectively.
Species were categorized as native and non-native species according to their biogeographic origin, as established through the current scientific literature (e.g. IUCN, 2021). Hybrid specimens due to difficult identification in the field were excluded from this study.
For each sampling site the number of native species (i.e., Natives), the number of non-native species (i.e., NNS) and the species richness (i.e., the total amount of species) were calculated. Furthermore, the top 10 invasive species were selected based on their invasiveness rank which was defined through an index calculated by multiplying colonization (proportion of sites colonized) and prevalence (average relative abundance in the fish community) of each introduced species in Italy (Milardi et al., 2022b). Finally, the selected top 10 invasive species and the native species classified as Critically Endangered (CR) by IUCN (IUCN, 2014) were selected to investigate differences in their abundances among sites.
2.3 Geospatial features, land features and invasion degree
The geographical, climate and anthropogenic landscape factors used were derived from Milardi et al., 2022b (Table 1). Geographical and climate factors were used to investigate environmental differences across sites. Specifically, the mean slope of the sampling sites (derived from a seamless digital elevation model of the entire Italian territory at 10 m resolution) was considered to account for stream morphology (Tarquini et al., 2007). Among climate factors, the annual average of daily air temperature was considered as a proxy for temperature regimes and the mean annual basin precipitation was used as a proxy for hydrological regimes of the stream reaches (ISPRA, 2006). As a proxy for overall anthropogenic impact, the 2009 Human Footprint was used (Venter et al., 2018), with lower values indicating fewer anthropogenic impact. The intensity of animal farming in 2010 was also considered among anthropogenic impacts (numbers of animals reared, ISTAT, 2021).
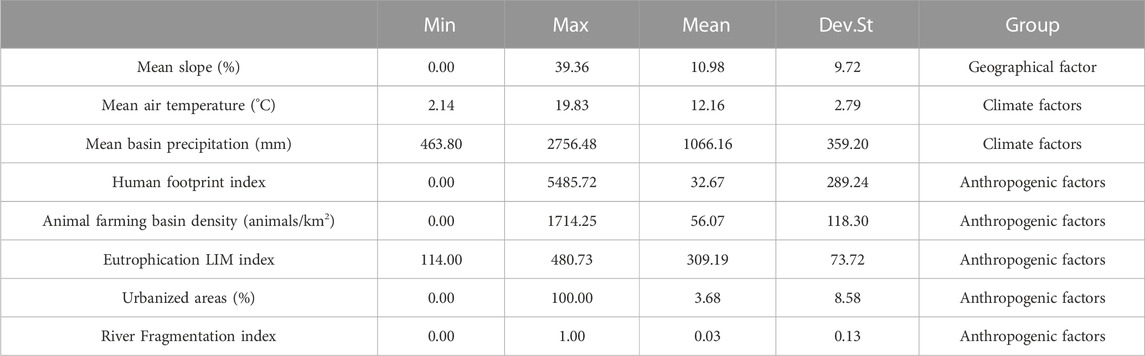
TABLE 1. Minimum, maximum, mean and standard deviation (Dev. St) of geographical, climate and anthropogenic landscape factors derived from Milardi et al., 2022b.
The Italian LIM index was considered as proxy for eutrophication levels in the watercourses. LIM index is calculated on the concentration of seven different parameters linked to nutrient levels (oxygen saturation, biochemical oxygen demand, chemical oxygen demand, NH4+, NO3−, total P and Escherichia coli levels (Spaggiari and Franceschini, 2000).
Among land use features, the total urbanized areas were considered as a proxy of human presence (European Environment Agency, 2012). Finally, the presence of barriers to fish migration (e.g., hydrological dams, weirs) was considered as a proxy for riverine habitat fragmentation (Milardi et al., 2022b).
2.4 Data analyses
To determine the protection status of each sampling site, ArcGIS Software (ESRI, 2011) was used by overlaying the GIS layer of the Natura 2000 network (European Environmental Agency, 2021) over the fish sampling sites layer. The 32% of the Natura 2000 areas contains sampling sites, these sampling sites within Natura 2000 areas were classified as Protected sites (n = 708 sampling sites), whereas sampling sites located more than 3 km away from a Natura 2000 area were defined as Unprotected sites (n = 1,291 sampling sites).
To identify diversity patterns at sampling sites outside the protected areas, but close to them, two buffer zones were defined. The first buffer zone included sampling sites closest to a Natura 2000 (i.e. site in the buffer zone of 1 km from the Natura 2000 site) which were classified as Contiguous sites (n = 832 sampling sites). The second buffer zone included sampling sites from 2 to 3 km away the Natura 2000 areas which were classifies as Nearby sites (n = 946 sampling sites). The assumptions of normality and homogeneity of variances were investigated through the shapiro.test and leveneTest functions in ‘car’ R package (Fox and Weisberg, 2020). As data did not satisfy these assumptions even after being transformed, non-parametric statistics were applied.
The Kruskal-Wallis test was used to test whether Protected, Contiguous, Nearby, and Unprotected sites differed in native, non-native species richness as also as geographical (i.e. mean slope), climate (i.e. mean air temperature and mean basin precipitation), and anthropogenic (i.e. human footprint mean density, animal farming basin density, LIM, urbanized areas, river fragmentation index) factors. Finally, the Kruskal-Wallis test was used to identify differences in the abundances of the top 10 invasive species and the CR native species among Protected, Contiguous, Nearby and Unprotected sites. The Dunn’s test of multiple comparisons with Bonferroni correction was used to determine which sites are different (Dunn, 1964). The Kruskal-Wallis test and the Dunn’s test were performed in FSA R package (Ogle et al., 2020).
3 Results
Overall, 98 fish species (of which 36 non-native) were found in Italian rivers (Supplementary Table S2).
Among geographical, climate and anthropogenic landscape factors, Protected, Contiguous, Nearby, and Unprotected sites showed no significant differences of human footprint levels (p > 0.05; Figure 2A) with mean ± SD of 30.13 ± 253.03, 49.95 ± 389.48, 19.38 ± 195.59 and 44.39 ± 515.32, respectively.
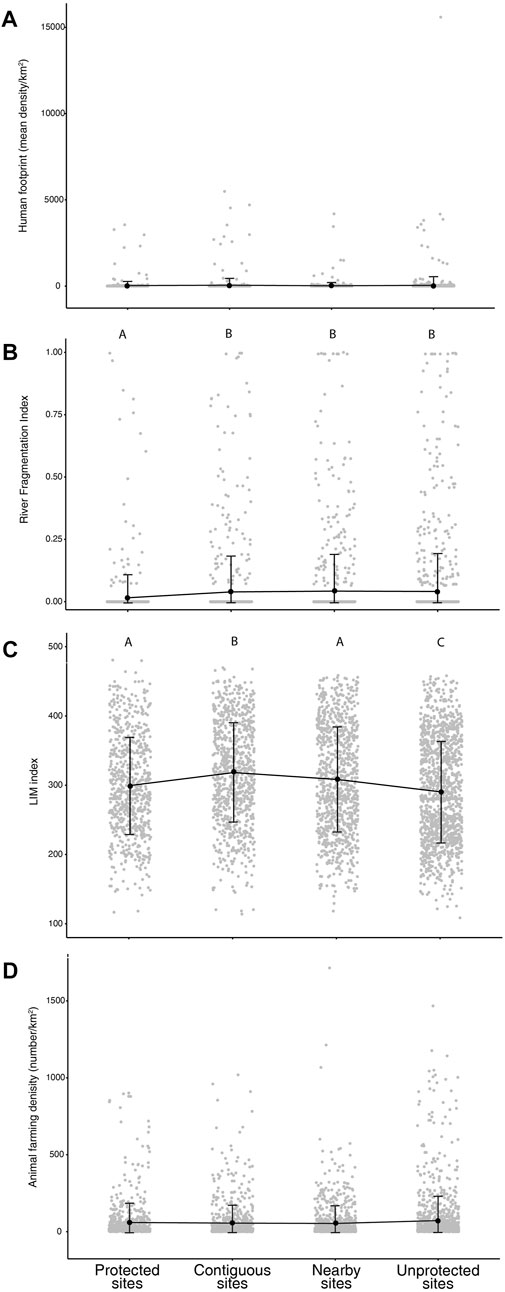
FIGURE 2. Mean values (black points) and standard deviations (vertical bars) of human footprint (A), river fragmentation index (B), LIM (C) and animal farming density (D) for Protected, Contiguous, Nearby, and Unprotected sites. Capital letters show the results of the Dunn’s test, with sites having different letters indicating significant difference (p < 0.05).
Among the four site types, significant differences in river fragmentation index and eutrophication LIM index were observed (KWχ2 = 26.13, df = 3, p < 0.001; KWχ2 = 82.97, df = 3, p < 0.001, respectively; Figures 2B, C). Protected sites showed the lower values of river fragmentation index (mean ± SD = 0.016 ± 0.093), whereas no differences in river fragmentation resulted between Contiguous (mean ± SD = 0.039 ± 0.144), Nearby (mean ± SD = 0.042 ± 0.148), and Unprotected sites (mean ± SD = 0.041 ± 0.152; Figure 2B). Contiguous sites showed the highest values of LIM index (i.e. lower levels of eutrophication) with mean value of 318.30 ± 115.89, whereas Unprotected sites showed the lowest values of LIM index (i.e. higher levels of eutrophication) with mean ± SD of 290.34 ± 73.31. In Protected and Nearby sites, LIM index showed mean ± SD of 299.60 ± 70.54 and 308.34 ± 76.21, respectively (Figure 2C). Furthermore, no differences were found in animal farming between Protected, Contiguous, Nearby, and Unprotected sites (p > 0.05; Figure 2D with mean values of 59.91 ± 126.75, 55.69 ± 115.89, 53.51 ± 113.82 and 72.33 ± 158.71 animal/km2, respectively).
Significant differences between sites were found in total species richness (KWχ2 = 56.41, df = 3, p < 0.001), native species richness (KWχ2 = 29.07, df = 3, p < 0.001), non-native species richness (KWχ2 = 46.28, df = 3, p < 0.001) and invasion degree (KWχ2 = 47.11, df = 3, p < 0.001; Figure 3).
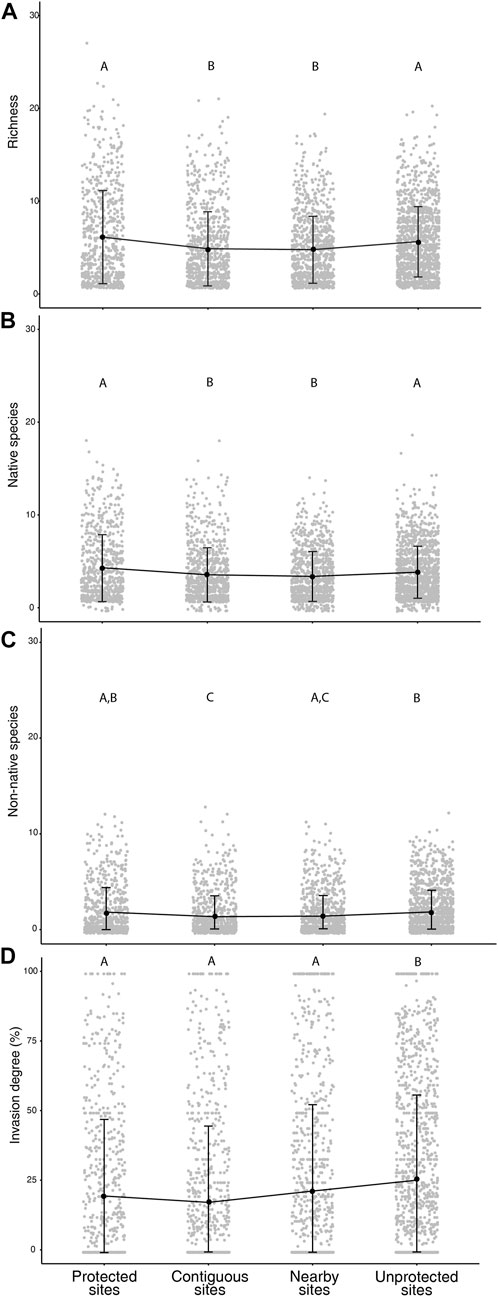
FIGURE 3. Mean values (black points) and standard deviations (vertical bars) of richness (A), number of native species (B), number of non-native species (C) and percentage of invasion degree (D) for Protected, Contiguous, Nearby, and Unprotected sites. Capital letters show the results of the Dunn’s test, having different letters indicating significant difference (p < 0.05).
Protected sites significantly differed from Contiguous and Nearby sites regarding total species richness, native and non-native species richness, but are similar to the Unprotected sites (Figures 3A–C). Unprotected sites were significantly different from Protected, Contiguous and Nearby sites regarding invasion degree, having higher values of invasion with mean ± SD of 25.91 ± 30.52%. Whereas Protected, Contiguous and Nearby sites did not differ in invasion degree with mean ± SD of 20.22 ± 27.52, 17.93 ± 27.54 and 22.00 ± 31.01, respectively (Figure 3D).
Mean abundance of non-natives Oncorhynchus mykiss, Gambusia holbrooki, Lepomis gibbosus, and Abramis brama did not differ significantly between Protected, Contiguous, Nearby, and Unprotected sites (Table 2).
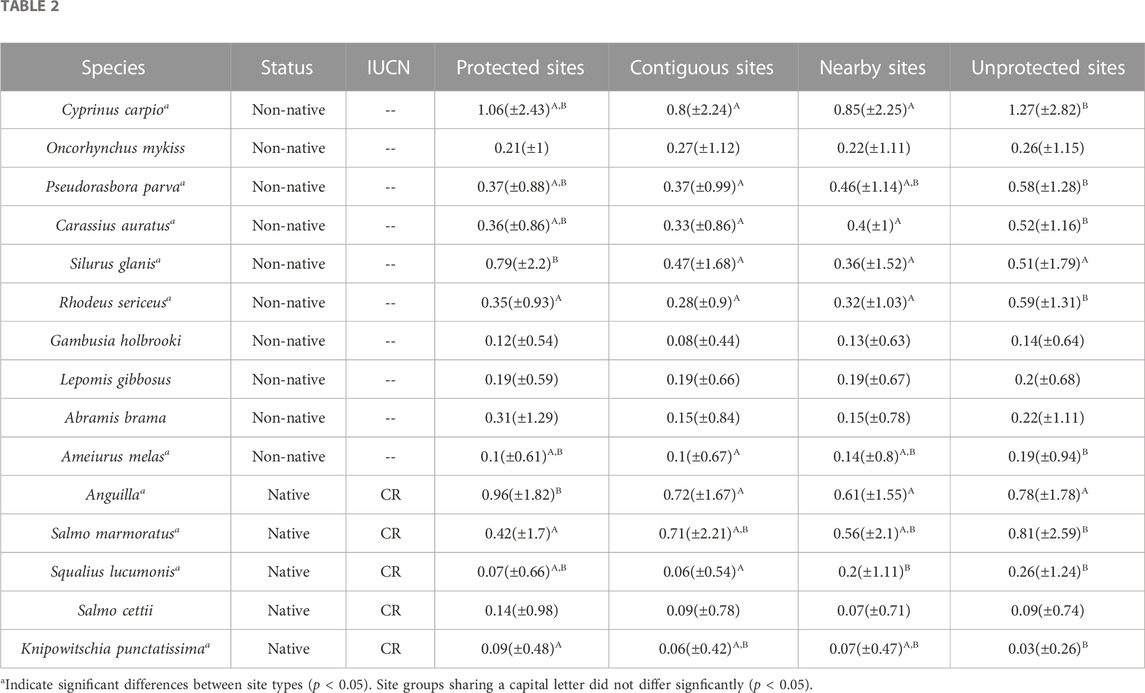
TABLE 2. Mean abundance (Moyle classes) and standard deviation of fish species in sites in Protected, Contiguous, Nearby and Unprotected sites. The status of non-native (i.e. top 10 invasive species) and native and the IUCN class (CR = Critically endangered) are reported for each species.
Protected and Unprotected sites did not show differences in the abundances of non-natives Cyprinus carpio, Carassius auratus, Pseudorasbora parva, and Ameiurus melas species. The highest abbundance of Silurus glanis resulted in Protected sites, rather than Contiguous, Nearby, and Unprotected sites.
Among native species, Anguilla showed differences across site locations, with the highest abundance in sites located in protected areas. Salmo marmouratus showed the highest abundance in sites located outside protected areas (Table 2).
4 Discussion
Our results showed that the anthropogenic impacts (i.e., human footprint, animal farming and water eutrophication) within the PAs were not significantly lesser than outside areas where protection measures are not applied. Among the anthropogenic impacts considered, only the river fragmentation index showed lower impacts in PAs than outside areas. It was surprising to find that native, non-native, and total fish species richness were not significantly different between Protected and Unprotected sites, suggesting that sites located within PAs may not uniquely contribute to the species richness of the region, and also that native and non-native species showed similar level of richness despite the protection measures in the region.
Furthermore, Unprotected sites showed higher levels of invasion degree, but the abundance of some of the top 10 invasive species did not differ between Protected and Unprotected sites or, contrariwise, showed the higher abundance in Protected sites (e.g., the wels catfish S. glanis), suggesting that protection measures are not adequate to prevent non-native species presence in protected sites.
4.1 Anthropogenic pressures in protected areas
River fragmentation due to dams or barriers is a critical threat to the conservation of fish diversity (Poff et al., 2007; Anas and Mandrak, 2021). In our study, river interruptions are rarer in PAs than outside PAs, as a result of local protection measures. However, rivers which must be considered as continuous ecosystems, specifically for guaranteeing the longitudinal connectivity for fish fauna to complete their life cycles. Low river fragmentation restricted within PAs may be beneficial for short-range migrators for example stopping the non-native invasions (Gavioli et al., 2018), but it could be detrimental for long-range migrators (Hermoso et al., 2018).
Several studies documented the effects of dams along the river which can cause alteration of flow condition and the creation of lentic waters above the dam, and also obstruct the movement of fish along the river and alter the taxonomical diversity and the genetic patterns and the functional diversity of fish community (Oliveira et al., 2018; Machado et al., 2022). Unfortunately, in our study, long range migratory fish species, such as the Adriatic sturgeon (Acipenser naccarii), were not sampled in a sufficient number of sites to investigate fragmentation effects on these species and at widest scale. The European eel (A. anguilla) was the unique long range migratory species sufficiently sampled which resulted more present in PAs. This result was probably driven by the presence of vegetation and woody materials in these areas which are subjected to protection measures and which are used as refuge by eels (Acou et al., 2011; Harwood et al., 2022).
Water eutrophication showed lower values in protected areas and in the near buffer zones, suggesting that PAs showed better water quality conditions than unprotected sites. In PAs, limitations imposed to vegetation management by water authorities may favour self-purification capacity of eutrophication loads, thus allowing better water quality conditions. Several studies performed in the Po River basin have showcased this effect in a variety of water courses, from rivers to managed canals (e.g. Castaldelli et al., 2013a; Pierobon et al., 2013; Soana et al., 2018), highlighting also the exosystemic effect of grass carp introduction on the drop of water quality (Milardi et al., 2020a).
Human footprint and animal farming affected similarly all sites despite their degree of protection, suggesting that these kinds of anthropogenic pressures are widespread and their effects could not be highlighted at this scale (Milardi et al., 2022b; Schipper and Barbarossa, 2022).
4.2 Effectiveness to protect freshwater fish diversity in protected areas
The most striking result of this study is that protected and unprotected sites did not differ in terms of fish species richness both considering all fish community and separating native and non-native species. Although invasion degree was higher in unprotected sites, the occurrence of invasive species did not differ between Protected and Unprotected sites or, even, non-native species with detrimental effects on native fish diversity such as S. glanis (Castaldelli et al., 2013b) resulted more located in protected areas. Contrariwise, the marbled trout (S. marmuratus) showed the highest occurrence outside the PAs, suggesting that the protection measures are not effective in preserving this species inside PAs, and, most likely, outside PA areas were subject to restocking activities of marbled trout for angling purposes. These results suggest that the current PAs network, in Italy, is not effective in guaranteeing a higher level of fish species richness and protection to fish invasions compared to areas outside PAs.
The uncertainties on the usefulness of protected areas to preserve fish diversity has been previously highlighted in other European regions (Trochet and Schmeller, 2013; Hermoso et al., 2015), South Africa (Jordaan et al., 2020), Australia (Chessman, 2013), South America (Azevedo-Santos et al., 2019) and United States (Lawrence et al., 2011) where several anthropogenic factors were found to be responsible for the lack of effectiveness of the program, including fishing, water management (e.g., dams and flow restrictions), habitat degradation, and invasive non-native species.
In Italian rivers and canals, despite European, national, and regional regulations and the conservation measures, an ample majority of recreational fishermen have adopted the catch and release fishing technique both for native and non-native species. This implies that most of captured non-native species (included also the most invasives ones) are released right after being caught, not contributing to non-native biomass removal as desired. Due to the low number of fishermen active on large rivers, even professional fishing is unable to contrast the presence of non-native species. Also, the illegal activities targeting non-native species as wels catfish, common carp and other carp species are located outside PAs contributing in this way to remove non-native species only in the areas outside the PAs, in spite penalties and Police controls are less severe in such areas.
4.3 Management implications for conservation
The value of PAs for the protection of native biodiversity is undisputable but our results highlight the need to consider in detail the selective effect of protection measures on different taxa. In the case of native fish, the highly connected nature of freshwater ecosystems makes them intrinsically vulnerable to several threats from the basin and, in the specific case of invasive exotic species, from upstream and downstream reaches. In order to maintain the ecological and hydrological processes that support fish diversity, it is crucial to design conservation area networks considering these threats and their propagation dynamics (Tognelli et al., 2019).
For example, several actions were already proposed by Acreman et al. (2019) to enhance PA effectiveness, most of which are also applicable to the analysed situation in Italy, such as incorporating PA strategies into the conservation of aquatic habitats, including hydrological regime, water quality, and riparian vegetation and preventing, removing, or controlling invasive non-native species.
Rivers are among the most impacted ecosystems worldwide, being difficult to mitigate the multiple impacts on fish communities, and where the engagement of local communities and stakeholders in the application of protection measures is a key factor in managing the effectiveness of the protection in PAs (Fidler et al., 2022; Pereira et al., 2022). Additional management measures, such as removing barriers or building fish passes, are needed to allow the long-distance migratory fishes to complete their breeding cycle (Hermoso et al., 2015). However, removing barriers may also turn out in promoting invasions of fish species along the river continuum, since instream barriers restrict the movement of non-native species and thus may prevent localised extinctions of native fishes in headwater streams (Gavioli et al., 2018). This evidence supports that management measures (e.g., removing barriers) must be evaluated considering the entire ecosystem context (Jordaan et al., 2020).
All these management actions need to be framed into an ecological evaluation which goes beyond local and even national level, particularly in interstate river basins, to cite one, the Danube (e.g. Tóth et al., 2019).
PAs were historically developed and managed to protect terrestrial ecosystems and species (Abell and Harrison, 2020). In riverine ecosystems, a specific analysis is needed to increase the effectiveness of protection of fish diversity, in some cases to be performed at the basins scale or, at least, at the entire length of the river (Azevedo-Santos et al., 2019). Thus, our results highlight that the implementation of Natura 2000 network and its associated green infrastructure, within the Prioritised Action Framework (PAF), need a comprehensive overview of the measures, extended from the physical to the ecosystem and social levels, as requested to the Member States by the European Commission.
Our study highlights that in Italy the PAs network has not been effective to protect river stretches from multiple anthropogenic pressures and as real safeguard of fish diversity, according to other assessments at the global scale (Geldmann et al., 2019). As recently pointed out in an analysis of protected taxa inside Natura 2000 network, to increase the effectiveness of Natura 2000 network in Europe a general reconsideration of principles and criteria for PAs selection might be needed (Trochet and Schmeller, 2013). To achieve this aim, our results lead us to propose two operative levels, one general and one specific. To increase PAs’ effectiveness for endangered fish species, a general effort should be devoted, from local to national and international scale, to the development of public awareness and participation to improve a shared knowledge of the risk deriving from the introduction of new invaders, an updated knowledge of the conservation status of endangered fish species and a more deeply rooted consciousness of the cultural and historical value of iconic species on the brink of extinction as, to cite some, sturgeons, lampreys, and eels.
On the more specific level, technical and research institutions should increase management effectiveness by developing accurate species-specific analyses to define proper measures and interventions which, case by case, are addressed to restoring the specific ecological needs of each single protected species under consideration. This second operative level cannot be based on general knowledge but needs updates, accurate monitoring data and dedicated research from local to European scale.
All above cited actions need dedicated middle to long-term financing at national and international level which, in turn, is favoured by a more effective public engagement, bringing higher political attention on conservation. In a practical way, it remains a matter of debate what to start with first, if to increase social awareness/political support or to improve the technical base of knowledge. Surely, it is more certain that, to be effective, both political and monitoring programs should be discussed, financed, and enacted at the whole Natura 2000 network level.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding author.
Author contributions
AG and GC contributed to conception and design of the study. AG and MM provided the data curation and organization of the database. AG and KP performed the formal analysis. AG wrote the first draft of the manuscript and AF, KP, MM, and CG contributed to the writing of the original draft. CG provided resources and lead the funding acquisition.
Funding
AF was supported by Portuguese Foundation for Science and Technology (FCT, Portugal) under the third edition of Individual Stimulus of Scientific Employment (2020.03872.CEECIND).
Acknowledgments
The authors want to thank the Fisheries Bureau of the Emilia-Romagna Region (Italy) for the constructive long-lasting support on ecological research on fish communities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2023.1122464/full#supplementary-material
References
Abell, R., and Harrison, I. J. (2020). A boost for freshwater conservation. Science 370 (6512), 38–39. doi:10.1126/science.abe3887
Acou, A., Rivot, E., Van Gils, J. A., Legault, A., Ysnel, F., and Feunteun, E. (2011). Habitat carrying capacity is reached for the European eel in a small coastal catchment: Evidence and implications for managing eel stocks. Freshw. Biol. 56 (5), 952–968. doi:10.1111/j.1365-2427.2010.02540.x
Acreman, M., Hughes, K. A., Arthington, A. H., Tickner, D., and Duenas, M. (2019). Protected areas and freshwater biodiversity: A novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 13, 1–14. doi:10.1111/conl.12684
Anas, M. U. M., and Mandrak, N. E. (2021). Drivers of native and non-native freshwater fish richness across North America: Disentangling the roles of environmental, historical and anthropogenic factors. Glob. Ecol. Biogeogr. 30 (6), 1232–1244. doi:10.1111/geb.13298
Azevedo-Santos, V. M., Frederico, R. G., Fagundes, C. K., Pompeu, P. S., Pelicice, F. M., Padial, A. A., et al. (2019). Protected areas: A focus on Brazilian freshwater biodiversity. Divers. Distributions 25 (3), 442–448. doi:10.1111/ddi.12871
Balian, E. V., Segers, H., Leveque, C., and Martens, K. (2008). The freshwater animal diversity assessment: An overview of the results. Hydrobiologia 595 (1), 627–637. doi:10.1007/s10750-007-9246-3
Bianco, P. G. (1998). “Freshwater fish transfers in Italy: History, local changes in fish fauna and a prediction on the future of native populations,” in Stocking and introductions of fishes, 165–197. I. Cowx. B.
Bianco, P. G. (1987). “L’inquadramento zoogeografico dei pesci d’acqua dolce d’Italia e problemi determinati dalle falsificazioni faunistiche,” in Biologia e gestione dell’ittiofauna autoctona,” tti II Convegno dell’Associazione Italiana Ittiologi delle Acque Dolci Torino, 41–66.
Butchart, S. H. M., Walpole, M., Collen, B., van Strien, A., Scharlemann, J. P. W., Almond, R. E. A., et al. (2010). Global biodiversity: Indicators of recent declines. Science 328, 1164–1168. doi:10.1126/science.1187512
Cardinale, B. J., Duffy, J. E., Gonzalez, A., Hooper, D. U., Perrings, C., Venail, P., et al. (2012). Biodiversity loss and its impact on humanity. Nature 486 (7401), 59–67. doi:10.1038/nature11148
Carpenter, S. R., Stanley, E. H., and Vander Zanden, M. J. (2011). State of the world’s freshwater ecosystems: Physical, chemical, and biological changes. Annu. Rev. Environ. Resour. 36 (1), 75–99. doi:10.1146/annurev-environ-021810-094524
Castaldelli, G., Pluchinotta, A., Milardi, M., Lanzoni, M., Giari, L., Rossi, R., et al. (2013b). Introduction of exotic fish species and decline of native species in the lower Po basin, north-eastern Italy. Aquatic Conservation Mar. Freshw. Ecosyst. 23 (3), 405–417. doi:10.1002/aqc.2345
Castaldelli, G., Soana, E., Racchetti, E., Pierobon, E., Mastrocicco, M., Tesini, E., et al. (2013a). Nitrogen budget in a lowland coastal area within the Po River Basin (Northern Italy): Multiple evidences of equilibrium between sources and internal sinks. Environ. Manag. 52 (3), 567–580. doi:10.1007/s00267-013-0052-6
Cazalis, V., Karine, P., Jean-Baptiste, M., Joseph, K., Stuart, H. M. B., and Ana, S. L. R. (2020). Effectiveness of protected areas in conserving tropical forest birds. Nat. Commun. 11 (1), 1–8. doi:10.1038/s41467-020-18230-0
Chessman, B. C. (2013). Do protected areas benefit freshwater species? A broad-scale assessment for fish in Australia’s murray-darling basin. J. Appl. Ecol. 50 (4), 969–976. doi:10.1111/1365-2664.12104
Collen, B., Whitton, F., Dyer, E. E., Baillie, J. E. M., Cumberlidge, N., Darwall, W. R. T., et al. (2014). Global patterns of freshwater species diversity, threat and endemism. Glob. Ecol. Biogeogr. 23 (1), 40–51. doi:10.1111/geb.12096
Crivelli, A. J. (1995). Are fish introductions a threat to endemic freshwater fishes in the northern Mediterranean region? Biol. Conserv. 72 (2), 311–319. doi:10.1016/0006-3207(94)00092-5
Dudgeon, D. (2019). Multiple threats imperil freshwater biodiversity in the Anthropocene,” Current Biology. Elsevier 29 (19), R960–R967. doi:10.1016/j.cub.2019.08.002
Dunn, O. J. (1964). Multiple comparisons using rank sums. Technometrics 6 (3), 241–252. doi:10.1080/00401706.1964.10490181
European Environment Agency (EEA) (2012). CORINE land cover (CLC) - copernicus land monitoring service. Available at: https://www.eea.europa.eu/data-and-maps/data/copernicus-land-monitoring-service-corine.
Fidler, R. Y., Ahmadia, G. N., Amkieltiela, , Awaludinnoer, , Cox, C., Estradivari, , et al. (2022). Participation, not penalties: Community involvement and equitable governance contribute to more effective multiuse protected areas. Sci. Adv. 8 (18), eabl8929. doi:10.1126/sciadv.abl8929
Fox, J., and Weisberg, S. (2020). An R companion to applied regression 3rd edition. Thousand Oaks, CA: Sage.
Fricke, R., Eschmeyer, W. N., and van der, R. L. (2022). ESCHMEYER’S catalog of fishes: GENERA, species, references. Available at: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp.
Froese, R., and Pauly, D. (2019). “FishBase,” in World wide web electronic publication, 04/2019. Available at: www.fishbase.org.
Gavioli, A., Marco, M., Marco, M., Vassilis, A., Ericae, R., Pierluigi, V., et al. (2018). Exotic species, rather than low flow, negatively affect native fish in the Oglio River, Northern Italy. River Res. Appl. 34, 887–897. doi:10.1002/rra.3324
Gleick, P. H. (1998). The human right to water. Water Policy 1, 487–503. Available at: http://webworld.unesco.org/Water/wwap/pccp/cd/pdf/educational_tools/course_modules/reference_documents/issues/thehumanrighttowater.pdf.
Harwood, A. J. P., Perrow, M. R., Sayer, C. D., Piper, A. T., Berridge, R. J., Patmore, I. R., et al. (2022). Catchment-scale distribution, abundance, habitat use, and movements of European eel ( Anguilla anguilla L.) in a small UK river: Implications for conservation management. Aquatic Conservation Mar. Freshw. Ecosyst. 32 (5), 797–816. doi:10.1002/aqc.3794
Hermoso, V., Filipe, A. F., Segurado, P., and Beja, P. (2015). Effectiveness of a large reserve network in protecting freshwater biodiversity: A test for the iberian peninsula. Freshw. Biol. 60 (4), 698–710. doi:10.1111/fwb.12519
Hermoso, V., Filipe, A. F., Segurado, P., and Beja, P. (2018). Freshwater conservation in a fragmented world: Dealing with barriers in a systematic planning framework. Aquatic Conservation Mar. Freshw. Ecosyst. 28 (1), 17–25. doi:10.1002/aqc.2826
IPBES (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services) (2019). Summary for policymakers of the global assessment report on biodiversity and ecosystem services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services.
ISPRA (2006). Sistema nazionale per l’elaborazione e diffusione di dati climatici (SCIA), 2006. Available at: http://www.scia.isprambiente.it/wwwrootscia/Home_new.html.
ISTAT (2021). Consistenze degli allevamenti. Available at: http://dati.istat.it/Index.aspx?DataSetCode=DCSP_CONSISTENZE.
IUCN (2014). International union for conservation of nature red list. Http://Discover.Iucnredlist.Org Available at:https://portals.iucn.org/library/sites/library/files/documents/2019-007-En.pdf.
Jordaan, M. S., Chakona, A., and van der Colff, D. (2020). Protected areas and endemic freshwater fishes of the cape fold ecoregion: Missing the boat for fish conservation? Front. Environ. Sci. 8, 1–13. doi:10.3389/fenvs.2020.502042
Kottelat, M., and Freyhof, J. (2007). Handbook of European freshwater fishes. Copeia, 646. doi:10.1643/OT-08-098a.1
Laurance, W. F., Carolina Useche, D., Rendeiro, J., Kalka, M., Bradshaw, C. J. A., Sloan, S. P., et al. (2012). Averting biodiversity collapse in tropical forest protected areas. Nat. Publ. Group 489 (7415), 290–294. doi:10.1038/nature11318
Lawrence, D. J., Larson, E. R., Liermann, C. A. R., Mims, M. C., Pool, T. K., and Olden, J. D. (2011). National parks as protected areas for U.S. freshwater fish diversity. Conserv. Lett. 4 (5), 364–371. doi:10.1111/j.1755-263X.2011.00185.x
Machado, C. B., Braga-Silva, A., Freitas, P. D., and Galetti, P. M. (2022). Damming shapes genetic patterns and may affect the persistence of freshwater fish populations. Freshw. Biol. 67 (4), 603–618. doi:10.1111/fwb.13866
Milardi, M., Gavioli, A., Elisa, S., Mattia, L., Elisa Anna, F., and Giuseppe, C. (2020b). The role of species introduction in modifying the functional diversity of native communities. Sci. Total Environ. 699, 134364. doi:10.1016/j.scitotenv.2019.134364
Milardi, M., Green, A. J., Mancini, M., Trotti, P., Kiljunen, M., Torniainen, J., et al. (2022a). Invasive catfish in northern Italy and their impacts on waterbirds. NeoBiota 72, 109–128. doi:10.3897/neobiota.72.80500
Milardi, M., Iemma, A., Ian, R. W., Anna, G., Elisa, S., and Giuseppe, C. (2022b). Natural and anthropogenic factors drive large-scale freshwater fish invasions. Sci. Rep. 12 (1), 1–12. doi:10.1038/s41598-022-14556-5
Milardi, M., Soana, E., Duane, C., Elisa Anna, F., and Giuseppe, C. (2020a). Could a freshwater fish be at the root of dystrophic crises in a coastal lagoon? Sci. Total Environ. 711, 135093. doi:10.1016/j.scitotenv.2019.135093
Ogle, D., Wheeler, P., and Dinno, A. (2020). Fsa: Simple Fisheries stock assessment methods,” R package.
Oliveira, A. G., Baumgartner, M. T., Gomes, L. C., Dias, R. M., and Agostinho, A. A. (2018). Long-term effects of flow regulation by dams simplify fish functional diversity. Freshw. Biol. 63 (3), 293–305. doi:10.1111/fwb.13064
Pereira, P. H. C., Julia Caon, A., Gislaine, V. L., Luís, G. F. C., Erandy, G., and Rafael, A. M. (2022). Effectiveness of management zones for recovering parrotfish species within the largest coastal marine protected area in Brazil. Sci. Rep. 12, 12232. doi:10.1038/s41598-022-15990-1
Pierobon, E., Castaldelli, G., Mantovani, S., Vincenzi, F., and Fano, E. A. (2013). Nitrogen removal in vegetated and unvegetated drainage ditches impacted by diffuse and point sources of pollution. Clean. - Soil, Air, Water 41 (1), 24–31. doi:10.1002/clen.201100106
Poff, N. L., Olden, J. D., Merritt, D. M., and Pepin, D. M. (2007). Homogenization of regional river dynamics by dams and global biodiversity implications. Proc. Natl. Acad. Sci. U. S. A. 104 (14), 5732–5737. doi:10.1073/pnas.0609812104
Rands, M. R. W., William, M. A., Leon, B., Stuart, H. M. B., Andrew, C., David, C., et al. (2010). Biodivers. Conserv. Challenges Beyond 329, 1298–1304. doi:10.1126/science.118913
Rico-Sánchez, A. E., Sundermann, A., Lopez-Lopez, E., Torres-Olvera, M. J., Mueller, S. A., and Haubrock, P. J. (2020). Biological diversity in protected areas: Not yet known but already threatened. Glob. Ecol. Conservation 22, e01006. doi:10.1016/j.gecco.2020.e01006
Schipper, A. M., and Barbarossa, V. (2022). Global congruence of riverine fish species richness and human presence. Glob. Ecol. Biogeogr. 31, 1501–1512. doi:10.1111/geb.13519
Soana, E., Gavioli, A., Tamburini, E., Fano, E. A., and Castaldelli, G. (2018). To mow or not to mow: Reed biofilms as denitrification hotspots in drainage canals. Ecol. Eng. 113, 1–10. doi:10.1016/j.ecoleng.2017.12.029
Spaggiari, R., and Franceschini, S. (2000). Procedure di calcolo dello stato ecologico dei corsi d’acqua e di rappresentazione grafica delle informazioni. Biol. Ambient. 14, 1–6.
Splendiani, A., Massimo, G., Righi, T., and Tatiana, F. (2019). Introgression despite protection: The case of native Brown trout in Natura 2000 network in Italy. Conserv. Genet. 20 (2), 343–356. doi:10.1007/s10592-018-1135-y
Tarquini, S., Isola, I., Favalli, M., and Battistini, A. (2007). TINITALY, a digital elevation model of Italy with a 10 m-cell size (Version 1.0).
Tognelli, M. F., Anderson, E. P., Jimenez-Segura, L. F., Chuctaya, J., Chocano, L., Maldonado-Ocampo, J. A., et al. (2019). Assessing conservation priorities of endemic freshwater fishes in the Tropical Andes region. Aquatic Conservation Mar. Freshw. Ecosyst. 29 (7), 1123–1132. doi:10.1002/aqc.2971
Tóth, R., Czegledi, I., Kern, B., and Eros, T. (20192018). Land use effects in riverscapes: Diversity and environmental drivers of stream fish communities in protected, agricultural and urban landscapes. Agric. urban landscapes 101, 742–748. doi:10.1016/j.ecolind.2019.01.063
Trochet, A., and Schmeller, D. S. (2013). Effectiveness of the Natura 2000 network to cover threatened species. Nat. Conserv. 4, 35–53. doi:10.3897/natureconservation.4.3626
Keywords: non-native species, protected areas, river connectivity, human footprint, eutrophication, invasive species, biodiversity
Citation: Gavioli A, Filipe AF, Patonai K, Milardi M and Castaldelli G (2023) Effectiveness of the Natura 2000 network for freshwater fish conservation in a Mediterranean region. Front. Environ. Sci. 11:1122464. doi: 10.3389/fenvs.2023.1122464
Received: 12 December 2022; Accepted: 13 February 2023;
Published: 27 February 2023.
Edited by:
Rosana Mazzoni, Rio de Janeiro State University, BrazilReviewed by:
Jeyaraj Antony Johnson, Wildlife Institute of India, IndiaDoru Stelian Bănăduc, Lucian Blaga University of Sibiu, Romania
Copyright © 2023 Gavioli, Filipe, Patonai, Milardi and Castaldelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anna Gavioli, Z3Zsbm5hQHVuaWZlLml0
 Anna Gavioli
Anna Gavioli Ana Filipa Filipe
Ana Filipa Filipe Katalin Patonai
Katalin Patonai Marco Milardi
Marco Milardi Giuseppe Castaldelli1
Giuseppe Castaldelli1