- 1Department of Fisheries and Marine Science, Faculty of Science, Noakhali Science and Technology University, Noakhali, Bangladesh
- 2School of Engineering and Built Environment, Griffith University, Southport, QLD, Australia
- 3Faculty of Food Engineering and Biotechnology, Tianjin University of Science and Technology University, Tianjin, China
- 4Laboratory of Environmental Health and Ecotoxicology, Department of Environmental Sciences, Jahangirnagar University, Dhaka, Bangladesh
- 5School of Ecology and Environment Studies, Nalanda University, Bihar, India
An estuary represents a transition point between freshwater and saltwater and has a complex but productive environment due to a strong interplay between geological, physical, chemical, and biological processes. In Bangladesh, the ecological factors and biodiversity of different estuaries have been investigated for the last 35 years. However, the data is widely scattered, not easily accessible, unpublished, and/or in the form of grey literature. In this study, an attempt has been made to aggregate information available on the geo-environmental and biodiversity status of estuaries for their sustainable management. The biological and environmental data of 21 estuaries along the Bangladesh coast were collected from previously published literature and analyzed. The analyses revealed that the estuarine environment of Bangladesh is very dynamic and diverse like other tropical estuaries. The physico-chemical and geological parameters in estuaries significantly varied due to monsoon patterns, nutrient influx, salinity intrusion, riverine discharge, siltation, and human interventions in estuaries. Among the key environmental variables, such as salinity (3.7–30 ppt), pH (7.04–8), dissolved oxygen (3.30–13.63 mg/L), and water temperature (21–30°C) varied. Over 830 faunal and floral species of 273 genera were recorded from the estuarine environment, including 208 fishes, 87 species of phytoplankton, and 67 species of zooplankton in this region. This study suggests the development of an appropriate policy to protect valuable, productive, and diverse ecosystems, especially for erosion control, pollution abatement, and habitat destruction, particularly in the mangrove forests and their associated habitats of Bangladesh.
1 Introduction
Estuary, a transition point between fresh and salt water, is the world’s most diverse, dynamic, and productive ecosystem (Gray, 2004; Eick and Thiel, 2014). Estuary combines unique physical, chemical, biological, and geological features (Mann, 1982; Elliott and Quintino, 2007). As per a recent estimate, estuaries are four times more productive than ryegrass pastures and 20-times more productive than the open sea, with production occurring through active photosynthesis both throughout the water column and on the sediment surface (Boicourt et al., 2012). The estuarine organism uses productive ecosystems for spawning, feeding, nursing, and migration (Blaber et al., 2000). The coastline of Bangladesh stretches across 710 km of the Bay of Bengal (Hussain, 2013) and contains several river-based estuaries along their tidal zones (Hussain, 2013). The margins of estuaries support important primary producers, e.g., algae, seagrass, and mangroves, which provide large quantities of organic matter (Hoque et al., 1999). Marshes and mangroves may produce up to ten tones of plant detritus per hectare per year (Kamal and Khan, 2009). Thus, in addition to high productivity, estuaries are extremely rich in organic matter and nutrients, transported and trapped through freshwater flow, wind, waves, and tidal action (Mahmood et al., 1978; Ketchum, 1983). As a result of productivity and high nutrient loads, many species of fish and shellfish use estuaries as nursery grounds to spawn and allow juveniles to grow (Chowdhury et al., 2011). Additionally, birds, mammals, fish, and other wildlife depend on estuaries to complete their life cycle (Amin and Mahmood, 1979; Ketchum, 1983; Zafar et al., 1999).
Bangladesh coastal area with 21 major estuaries along the Bay of Bengal has a strong potential impact on fishing and the livelihoods of the local people (Ahmed, 2004; Islam and Wahab, 2005). Realizing the importance of diverse ecohydrological and biodiversity importance, significant studies have been conducted which highlighted the exclusive importance of estuaries and coastal areas for the economic development of Bangladesh as well (Hossain et al., 2012; Ahmed et al., 2019; Siddique et al., 2021). However, accessing these coastal resources is much more difficult for its marginal geography because of the lack of detailed, comprehensive studies and reviews on the total resource tabulation and their possibilities for the economic growth of Bangladesh. Therefore, this review paper summarizes available data on the physico-chemical, geological, and biological aspects of estuaries in Bangladesh along the Bay of Bengal coast. In addition to this, we have also discussed the diversities of various species and established hydrobiological correlations with correlation coefficients in estuary rivers. Future research priorities are identified, and recommendations are put forward.
2 Methods and materials
2.1 Study area
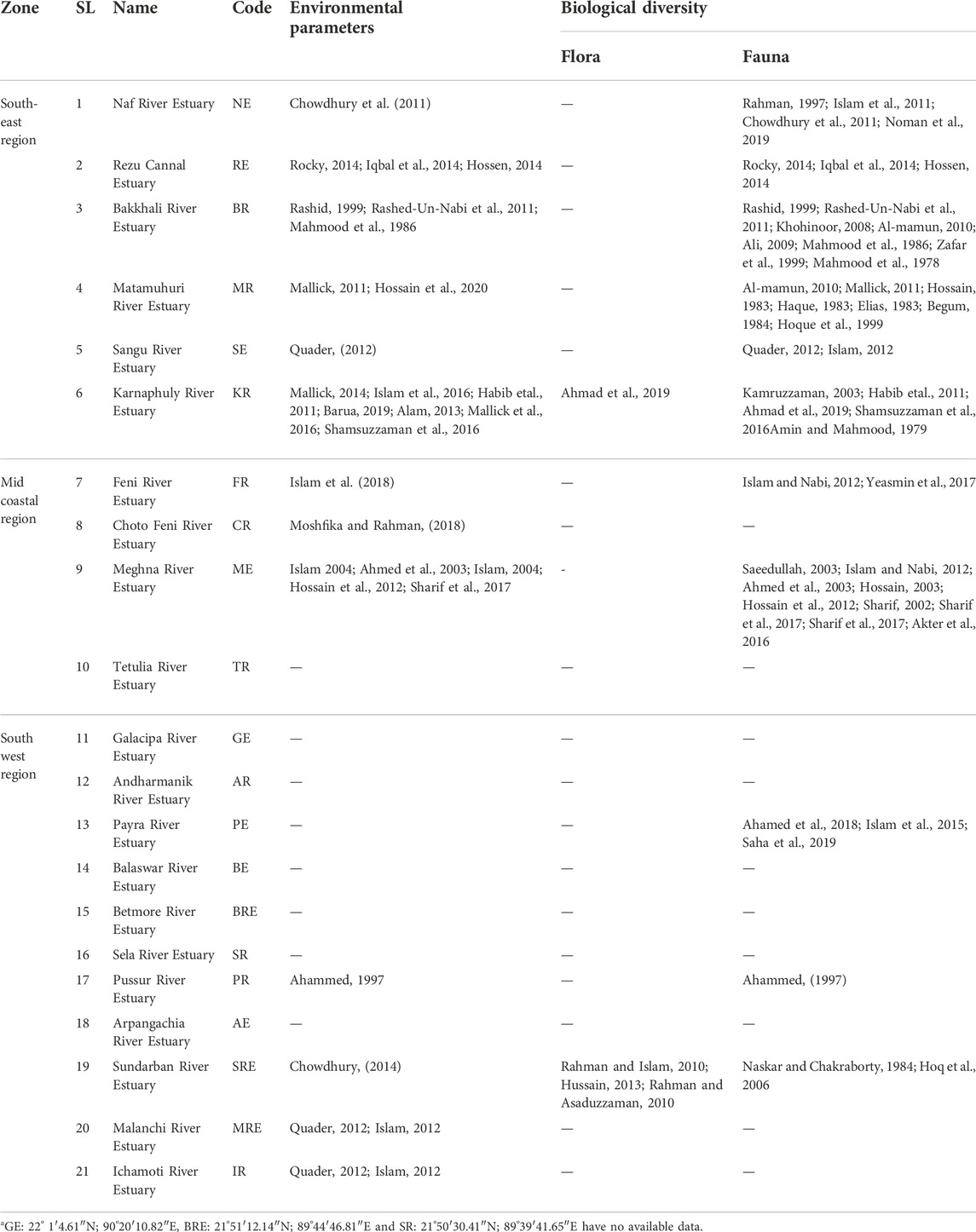
In this study, we examined a meta dataset obtained from 21 estuaries, namely Naf River Estuary, Rezu Cannal Estuary, Bakkhali River Estuary, Matamuhuri River Estuary, Sangu River Estuary, Karnaphuly River Estuary, Feni River Estuary, Choto Feni River Estuary, Meghna River Estuary, Tetulia River Estuary, Galacipa River Estuary, Andharmanik River Estuary, Payra River Estuary, Balaswar River Estuary, Betmore River Estuary, Sela River Estuary, Pussur River Estuary, Arpangachia River Estuary, Sundarban River Estuary, Malanchi River Estuary, Ichamoti River Estuary distributed along the coastal belt of the deltaic plain; the entire coastline of Bangladesh lies in the Bay of Bengal region (Figure 1). Based on the geographical locations, all the estuaries were divided into the south-east, south-west, and mid-coastal regions (Figure 1).
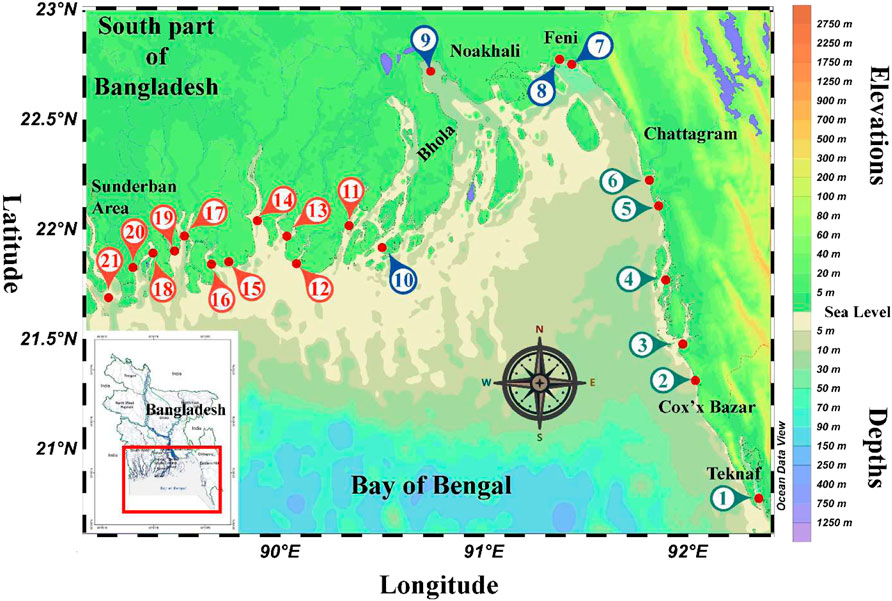
FIGURE 1. Location of each investigated estuaries in this review, where, 1: Naf River Estuary; 2: Rezu Cannal Estuary; 3: Bakkhali River Estuary; 4: Matamuhuri River Estuary; 5: Sangu River Estuary; 6: Karnaphuly River Estuary; 7: Feni River Estuary; 8: Choto Feni River Estuary; 9: Meghna River Estuary; 10: Tetulia River Estuary; 11: Galacipa River Estuary; 12: Andharmanik River Estuary; 13: Payra River Estuary; 14: Balaswar River Estuary; 15: Betmore River Estuary; 16: Sela River Estuary; 17: Pussur River Estuary; 18: Arpangachia River Estuary; 19: Sundarban River Estuary; 20: Malanchi River Estuary; 21: Ichamoti River Estuary.
2.2 Systematic data collection and analysis
The data was divided into biotic and abiotic parameters to better understand the processes, concentrations (heavy metals, nutrients, dissolved/suspended solids, trace metals, etc.), variabilities, and relationships. The abiotic parameters include physical, chemical, and geological variables. All the data were collected from previous studies conducted during the last 35 years (Table 1), including published manuscripts, theses, books, and other secondary sources, i.e., Marine resource inspector, Upazila fisheries officer, etc. All are sorted according to our targeted variables and excluded unnecessary findings. Available data were extracted from these sources, and key parameters associated with each estuary were compiled. Ocean data view (2016) software was used to visualize the concentration of parameters. Sunburst graphs were generated to portray species compositions for each estuary. Cluster analysis was performed using Pearson Coefficient in the Multivariate Statistical Package Software (MVSP 4). Stacked columns were used to present the data for physical properties.
3 Results and discussion
3.1 Geological properties of estuaries
Generally, an estuary is a dynamic and highly productive ecosystem where fresh and saltwater meet, resulting in diverse physico-chemical, biological, and geological features. Mangroves and the sandy deltaic plain lands are the common geological features of the estuaries near the coastal belt of Bangladesh, therefore, termed as Bangladesh estuarine complex (BEC). Deltaic estuaries possess huge sedimental loads through the Ganges-Brahmaputra-Meghna River system. Mid-coastal estuaries are relatable examples of these phenomena (Das et al., 2004). On the other hand, beach formations and hill ecosystems dominated all estuaries across the south-east of BEC (Mallick et al., 2016). Data on common geological features are included here, which helps to interpret the variation of local biodiversity.
3.1.1 Sediment texture
Sediment provides the necessary information about sessile biota, benthos, and water buffering capacity (Farhadinejad et al., 2014). It can also shape an estuary by its increment or removal (Dike and Agunwamba, 2012). Previous studies investigated the sediment texture of only four estuaries (BR, MR, KR, and CR) in Bangladesh (Table 1). It was observed that sediment load, especially the sand content (Figure 2A), was high in these estuaries (Sharif et al., 2017). MRE possessed the highest sand load (43%), including silt (16%) and clay (15%) concentrations. The lowest silt and clay concentrations were found in RE (0.08%) and SE (0.05%), respectively (Quader, 2012). Deltaic position of the river path may be responsible for this sand-loaded scenario across these rivers. Possessing the highest sediment load, RE also had moderate clay (10%) and silt (12%) concentration. It has been observed that hilly areas can provide clay increment towards these rivers (Islam, 2012). However, no studies were conducted on sediment pollutants in these estuaries of Bangladesh.
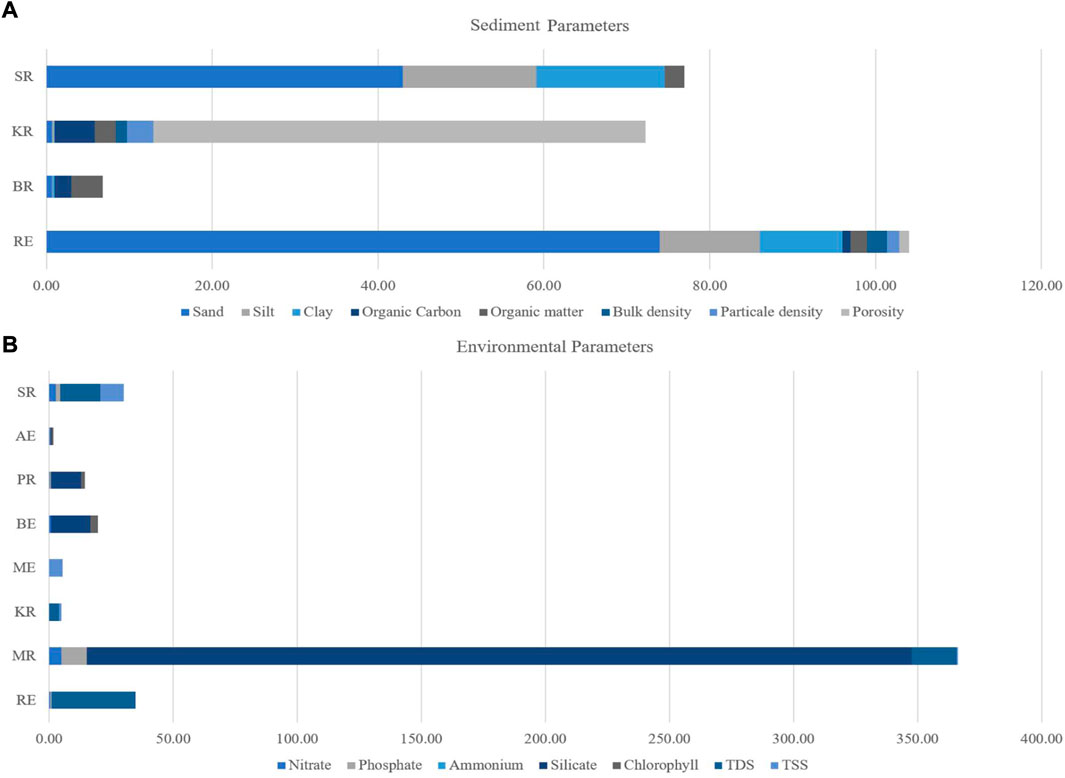
FIGURE 2. Comparative visualization of average sediment compositions (A) in various estuaries and comparative concentrations of different environmental parameters (B) in percentages for visualizing the enrichments among reported estuaries near Bay of Bengal (Estuary codes according to Table 1)
3.1.2 Bulk density, particle density, and porosity
Transparency of estuarine water depends on particle concentration in brackish water. It can also influence the concentration of other particles (Dike and Agunwamba, 2012). The present study collected available data in RE, BR, MR, KR, SE, and CR (Table 1). The highest bulk density (2.33 μg/g) was recorded in RE, while the lowest (1.34 μg/g) was found in SE (Figure 2A) (Islam, 2012; Quader, 2012). SE has higher particle density (3.19 g/cc) and porosity (59.30%) than IR (1.5 g/cc; 1.24% respectively). Hilly discharge around the year, especially during monsoon, may increase the level. Average bulk density, particle density, and porosity in estuaries were 1.84 μg/g, 2.35 g/cc, and 30.27%, respectively, across all estuaries.
3.1.3 Organic carbon (OC) and organic matter (OM)
Estuaries can potentially contribute to the organic carbon (OC) budget along the coastal belt (Khohinoor, 2008). The average OC level was 2.62% across the reported estuarine waters from only four estuaries, i.e., RE, BR, KR, and ME. The highest rate (4.85%) of OC was found in SE, while the lowest (1.01%) was in IR (Islam, 2012; Quader, 2012). The RE had 3.80% organic matter in water, the highest among all estuaries (Rocky, 2014). The lowest levels (2%) were found in IR. The average concentration of organic matter was 2.67%. The MRE (2.32%) and SE (2.55%) contained medium levels of organic matter. Organic plant matter, phytoplankton, and zooplankton contribute to hydrocarbon aggregations which affect the local carbon cycle in these areas (Das et al., 2002). For example, Indian estuaries transported an order of magnitude higher concentration of organic matters to the Bay of Bengal coast (×490 109 gC yr−1) than to the Arabian Sea (50 × 109 gC yr−1) (Kumar and Sarma, 2018). Since the Bangladeshi estuaries are connected to the northern Bay of Bengal (Figure 1), combined research on organic and inorganic matters is necessary for uncovering the levels and impacts of OC and OM in the Bay of Bengal region.
3.2 Physical parameters of estuaries
Physical parameters are the key factors influencing biodiversity as they directly/indirectly control other estuarine environmental conditions, i.e., biological interactions, chemical cycling, and geological aspirations (Mallick, 2014). Small changes in physical parameters can significantly affect species distribution within an estuary. Notably, though the atmospheric temperature of the North estuarine area was lower, the water temperature was warmer than the southern estuaries (Figures 3A, B). However, pH and salinity were higher in the southern estuaries compared to the northern estuaries (Figures 3D, F). These conditions are sensitive to seasonal changes around the year (Zafar et al., 1999).
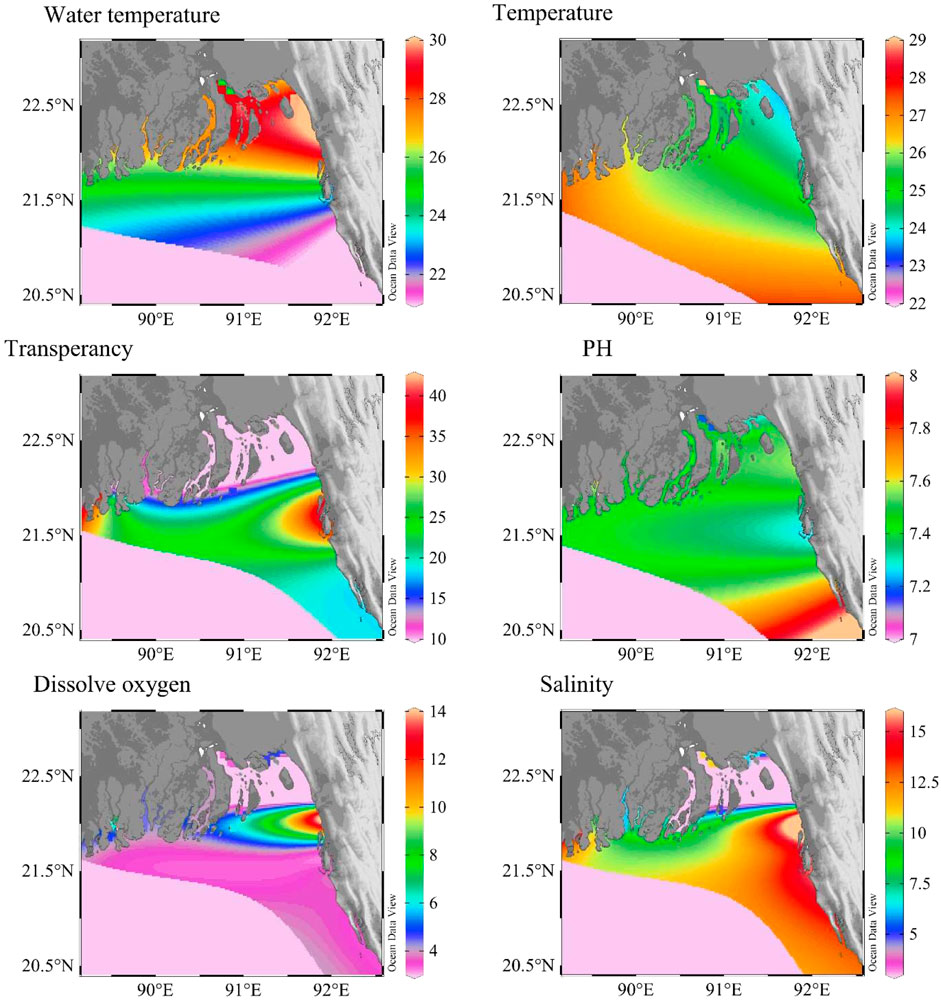
FIGURE 3. Average concentration of environmental parameters (A = water temperature, B = atmospheric temperature, C = transparency, D = pH, E = dissolved oxygen, and F = salinity) in the estuaries of Bangladesh (Greyish pink color indicated the data deficient area).
3.2.1 Temperature and salinity
Temperature and salinity are the major physical factors, among others. Temperature fluctuation has been seen in this environment for the past years (Varma et al., 2002; Basak et al., 2013). The salinity increase in the river water was gradually recorded from upstream to downstream. On Bangladeshi coasts, there is no exception broadly. The temperature data of 12 estuaries (NE, RE, BR, MR, SE, FR, ME, AR, BE, PR, AE and SRE) and salinity data of 13 estuaries (NE, RE, BR, MR, SE, KR, FR, ME, AR, BE, PR, AE and SRE) have been reported in the previous studies. The KR possessed the highest average water temperature (27 ± 25°C) (Figure 4B) (Mallick, 2014), and the lowest average temperature (21.50 ± 17°C) was recorded in the IR. Although temperature controls ecosystem diversity to a great extent (Menon et al., 2000), there are no reports on these issues.
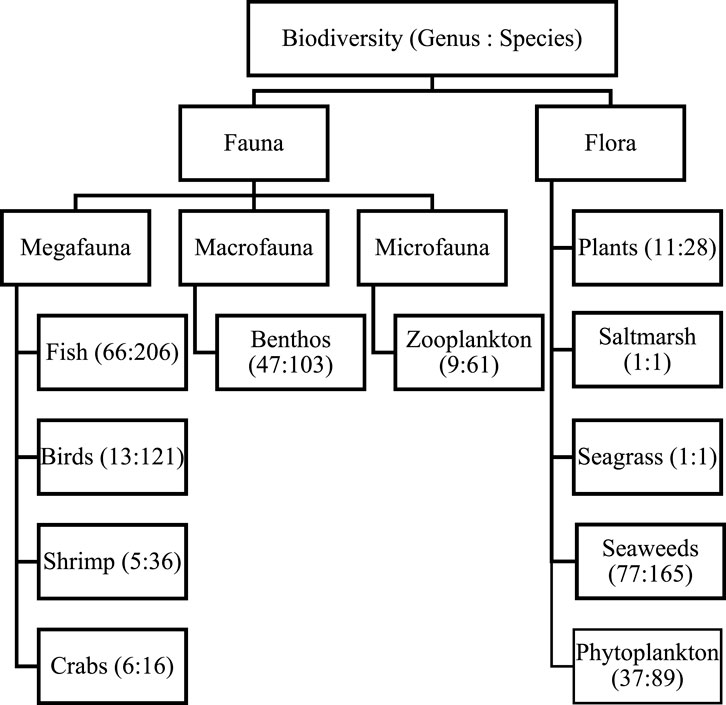
FIGURE 4. Genus and species count through all estuaries from available data (Microfauna: Microscopic organism (Size >1 mm), Macrofauna: Can see by open eye by not clearly (size >1 mm), Megafauna: Higher level species in taxonomy over benthos are considered).
Two main challenges for estuarine communities, like phytoplankton, are high salinity and sedimentation rate variability in the estuaries of Bangladesh (Ahmed et al., 2003). Notably, salinity showed a significant variation across estuaries (Figure 3F). Due to the huge freshwater discharge through the GBM system, average salinity was reported as 11.66 ppt through all estuaries, varying from 0.5 to 30 ppt (Mallick, 2014). The highest salinity was recorded in MR (16 ppt), while the lowest average salinity was recorded in SE through different studies (3.70 ppt) (Islam, 2012; Quader, 2012). The IR, RE, and BR maintained similar approximate salinities of ∼15 ppt (Al-mamun, 2010; Rocky, 2014). The KR showed low salinity due to high freshwater influx from hilly areas (6.50 ppt). It regulates low productivity through estuaries and adjacent areas (Aziz and Paul, 2015).
3.2.2 Transparency, pH, and dissolved oxygen (DO)
Productivity inside the water depends on many factors, especially transparency/visibility, pH, and dissolved oxygen. Suitable condition of all these parameters drives proper estuarine vegetation along the coast. However, only a limited number of estuaries have been investigated for transparency (11 estuaries), pH (9 estuaries), and dissolved oxygen (13 estuaries) till this review. In Bangladesh, transparency and DO were higher in the mid estuaries due to larger sand-free freshwater input from rivers to estuaries, i.e., KR and BR possess a lower percentage of clay, silt, and sand than other estuaries (Figure 2A). The presence of sediment in larger amounts reduces transparency and dissolved oxygen (DO) in the river water (Aziz and Paul, 2015). For example, the transparency was nearly zero near the mouth of the ME estuary due to the huge sediment load in the water column (Islam, 2004).
Additionally, tontamination and ecological risk asseotal suspended solids (TSS) was higher in SRE (Figure 2B and discussed in the chemical section). So, siltation from the floodplains may explain low transparency at SRE (Aziz and Paul, 2015). The tidal influxes of ME suspend the huge sediment loads, which potentially reduces the transparency and DO along with mid-estuarine areas. SE has the lowest average water transparency (10 cm), and BR has the highest transparency (40.50 cm). 23.94 cm is the average transparency across all estuaries, as shown in Figure 3C (Al-mamun, 2010; Islam, 2012; Quader, 2012). Moderate transparency was observed in IR, BE, and MRE. Average pH levels (7.47) were recorded year-round (Figure 3D). For example, the lowest pH was observed in CR (pH = 7.20), and sea water level pH was observed (pH = 8) in MRE (Moshfika and Rahman, 2018). The lowest concentration of DO was in CR, which may be due to relatively low productivity and industrial pollution (Moshfika and Rahman, 2018). The average DO was 5.19 ± 2.50 mg L−1 across all estuaries. DO level in estuaries was low due to the mixing of saline water with fresh river water and high sedimental load from the Bengal fan (Figure 3E). The highest DO concentration was recorded in MR (13.63 mg L−1) (Mallick, 2011). Many animals burrow themselves in the sand to avoid predation and live in a more stable sediment environment. However, a plethora of bacterial diversity has been reported from the sediment with a very high oxygen demand (Islam, 2004). This reduces oxygen levels within the sediment, often resulting in partially anoxic conditions, which can be further exacerbated if the water flux is limited (Ahammed, 1997). It widely affects local biological production, effectively controlling the biodiversity of the estuary.
3.3 Chemical component: Trace metals, nutrients, dissolved solids
Chemical components are complex controlling variables in estuaries (Förstner, 2004). External environments, i.e., industrial sludge, domestic run-off, etc., may influence these parameters (Anilakumary et al., 2007). The chemical composition of these components is essential for living biota and their diversity (Wang et al., 2014). It controls the biodiversity pattern along the estuaries (Jiang-Qi et al., 2013). This review skimmed previously reported total dissolved solids (TDS), total suspended solids (TSS), nutrients (phosphates, silicates, nitrate, nitrite, ammonia, etc.), and particle concentrations as chemical components. Reports on seven estuaries (NE, RE, SE, KR, CR, BE, and BRE) have been found during this review (Table 1). Data deficiency has been found through several estuaries regarding chemical parameters. Future researchers should look at the data monitoring table for further research on the sectors.
3.3.1 Total dissolved solids (TDS) and total suspended solids (TSS)
Siltation and land erosion contribute to the concentration of TDS and TSS (Islam et al., 2016). These factors can help determine productivity and local water quality (Ali et al., 2013). A few reports on TDS (4 estuaries, i.e., RE, MR, KR and SRE) and TSS (5 estuaries, i.e., RE, MR, KR, ME and SRE) were found during this review. In Bangladesh, the highest TDS occurred in IR (33.88 g/L), and the lowest TDS was observed in SE (4.05 g/L) (Islam, 2012; Quader, 2012). The average TDS was 18.02 g/L across all estuaries. MRE showed 16 g/L TDS in its water body. MRE recorded the highest TSS (9.50 g/L), and SE had the lowest TSS (0.85 g/L) due to clean water flows from hilly areas (Islam, 2012; Quader, 2012). The average TSS was 4.05 g/L, and CR has 5.40 g/L in its adjacent areas (Moshfika and Rahman, 2018). Erosion of land margin increases these phenomena along the deltaic estuaries (Ali et al., 2013). It can potentially influence the particle aggregation in estuarine water and, subsequently, characteristics of estuarine bottom topography and siltation processes.
3.3.2 Nutrient concentration: Productivity
Nutrients, i.e., phosphates, silicates, nitrate, nitrite, ammonia, etc., are the key factors of any biological environment, and it drives fluctuations of potential in the water body (Nair, 1984). The present review collected nutrient data from six estuaries from secondary sources, i.e., RE, MR, BE, PR, AE, and SR (Table 1). Unfortunately, there has been no measurement of nutrients in other estuaries. The average phosphate (PO4-P) concentration was 4.14 μg/L across all estuaries. Heavy Gangetic siltation may be liable for this scenario. The highest concentration (13.34 ± 0.34 to 20.67 ± 0.11 μg/L) was found in the MR (Mallick, 2011), while the lowest concentration (Hussain, 2013) was recorded in SRE (1.72 ± 0.07 μg/L). The ammonium ion was higher in AE than in other estuaries. On the other hand, silicate was found high in ME (Figure 2B). The average nitrate concentration was 2.73 μg/L across all estuaries. At the same time, the lowest nitrate (0.15–0.79 μg/L) and phosphate (0.23–0.92 μg/L) were observed in Razu Khal River Estuary for high coastal vegetation (Iqbal et al., 2014). Thus, less primary production was reported due to low nutrient availability. Salinity and pollution influence the abundance of nutrients in these areas (Billah et al., 2016; Mallick et al., 2016; Alam et al., 2017), which are also similar factors in Indian estuaries (Nair et al., 1984a). However, the rest remain data deficient areas, except for six estuaries (RE, MR, ME, AE, SRE, and MRE). Deployment of nutrient profiling along coasts, especially estuaries, is vital for further scientific progress.
3.3.3 Metal concentrations in estuarine sediment and water
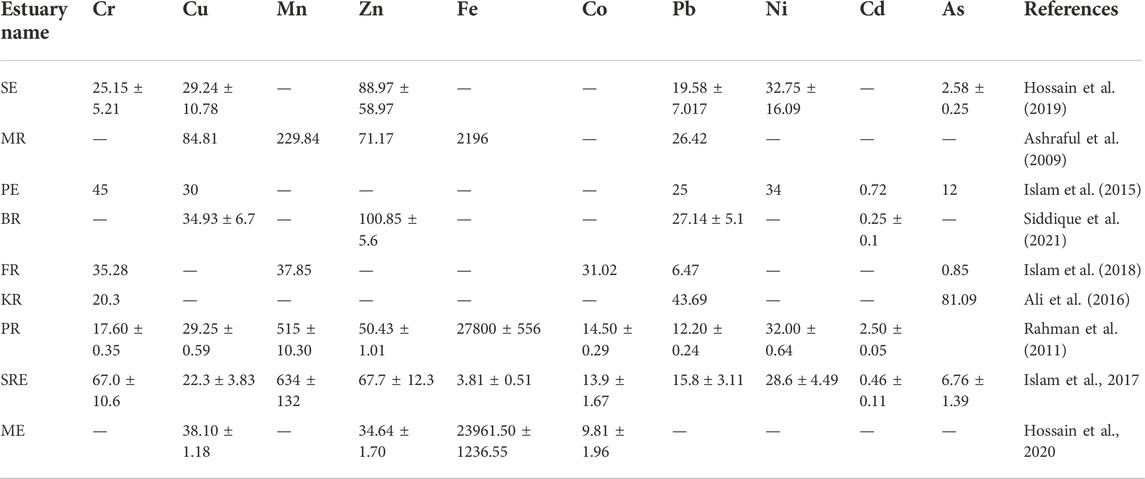
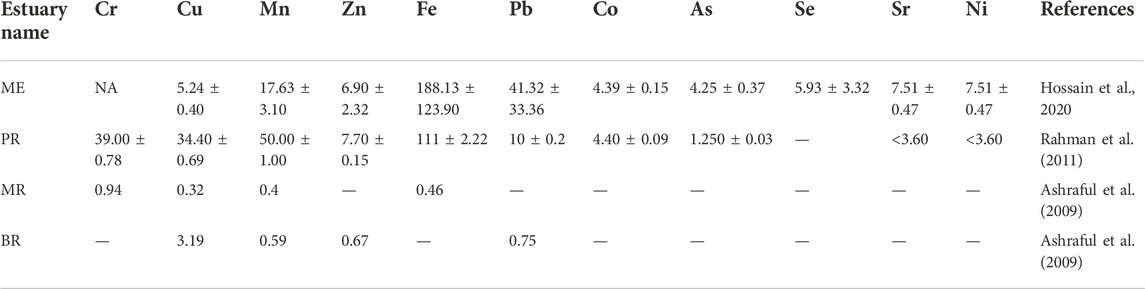
Estuaries demonstrated a spatial gradient in metal concentrations from river-to-river mouth to open BB (Rakib M. R. J. et al., 2021; Islam et al., 2021). Metal concentrations in estuarine sediment in nine estuaries (SE, MR, PE, BR, FR, KR, PR, SRE and ME) were reported (Table 1), whereas metal concentrations in adjacent water were available for four estuaries (ME, PR, MR and BR). In Bangladesh, sediments of FR showed low trace metal concentrations observed spatially and seasonally (Islam et al., 2018). Besides, the water of MR possessed low TMC as well for its dilution with open BB water (Ashraful et al., 2009). On the other hand, PR demonstrated the highest concentrations of trace metals both in sediment (Table 2) and water (Table 3), accordingly due to high pollution from land run-off (Rahman et al., 2011). It can help forming a coastal pollution order of metals (Fe > Ti > Zr > Rb > Zn > Sr > Pb > Y > Cu > Cr > As) from estuarine sampling (Rahman et al., 2019). Studies have reported that possible metal contamination sources are diesel and petrol from mechanized fishing trawlers and domestic disposals accordingly (Hossain et al., 2019; Rakib M. R. J. et al., 2021). Considering the sources of metals, land erosion by waves and tidal action, an influx of water and sediment from the surrounding rivers, agricultural waste, industrial effluent, and sewage are the most likely sources of metal pollution in the study area (Ashraful et al., 2009; Ali et al., 2016; Islam et al., 2018; Rakib M. R. J. et al., 2021). Even estuarine mangroves are reported risky for food and fodder due to accumulating heavy metal complexes (Rakib et al., 2021c). It has become a global estuarine problem. For example, Indian estuaries showed heavy metal enrichment to a hazardous level due to acidification through industrial disposals (Mitra, 2015). Ecosystem protection with riverine resource management was recommended in the available reports for minimizing metal concentration in brackish water and sediment as well (Bhuyan et al., 2017; Ali et al., 2021; Rakib M. R. J. et al., 2021; Rakib et al., 2021d; Jolly et al., 2021; Ali et al., 2022). Recently, Rakib et al. (2022) analyzed that microplastics have been found in sediments of Karnaphuli River Estuary, Bangladesh. Therefore, microplastics should be considered to be investigated in future studies across various estuaries of Bangladesh.
3.4 Estuarine biodiversity
Biodiversity can broadly divide into flora and fauna (Figure 5). For better understanding, this review will discuss major reported biodiversity groups from flora to fauna accordingly.
3.4.1 Phytoplankton diversity
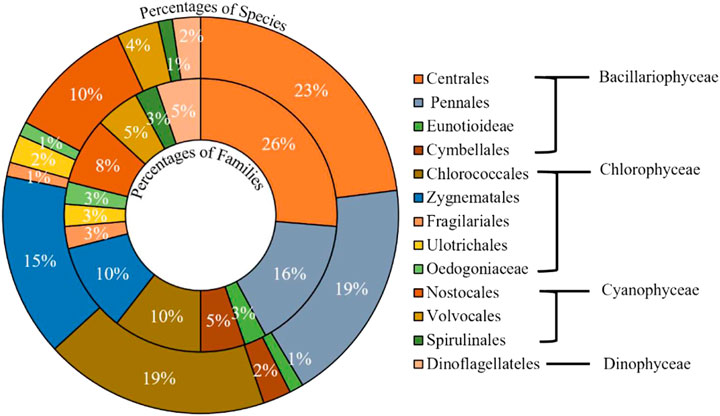
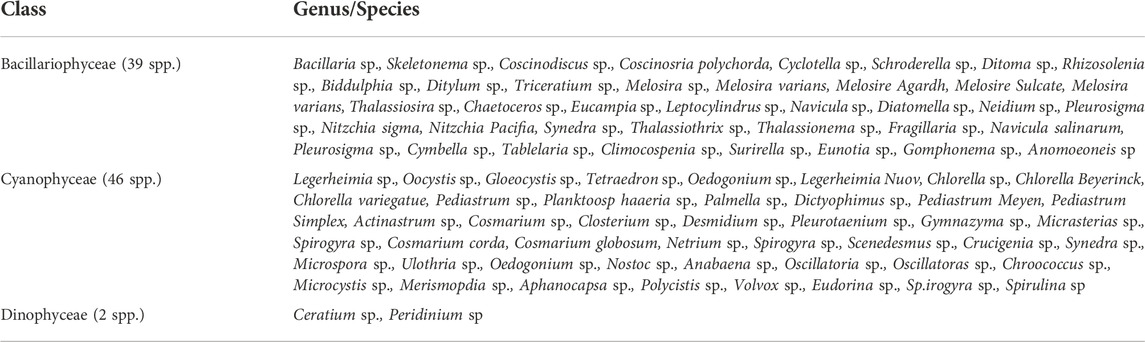
Estuaries provide some of the most productive habitats on Earth because of the accumulation and availability of nutrients and adequate light conditions (Hossain and Lin, 2001). Phytoplankton produces organic compounds by utilizing solar energy during photosynthesis and releases oxygen into the estuarine water (Ahsan et al., 2012). This system controls the oxygen balance in any aquatic environment, mainly near the surface (0.4–0.6 m), where productivity is high (Haque, 1983; Kamal and Khan, 2009). Their productivity largely depends on light, nutrients, and water turbidity (Sharif and Islam, 2017). Main phytoplankton presents diatoms and dinoflagellates, abundant in the water column and the sediment of estuaries (Rahman, 1997). In BEC, reports on five estuaries (NE, RE, MR, CR, and TR) have been found during this review (Table 1). Likely, 87 species representing phytoplankton genera were recorded from BEC estuaries, as shown in Figure 5 (Kamal and Khan, 2009). The most commonly reported species were Legerheimia sp., Oocystis sp., Gloeocystis sp., Tetraedron sp., Oedogonium sp., Legerheimia Nuov, Coscinodiscus sp., Coscinosria polychorda, Cyclotella sp., Schroderella sp., Ditoma sp., Synedra sp., Thalassiothrix sp., Thalassionema sp., Fragillaria sp., Navicula salinarum, and Pleurosigma sp. (Table 4). However, no relative reports were found about BEC during this review. Further designative research is recommended for understanding estuarine phytoplankton more clearly.
3.4.2 Seaweeds
Sub-tidal macroalgal beds, i.e., Sargassum, Dictyota, and Codium play an essential role in the life cycle of numerous economically important commercial fish species. Benthic forms of seaweeds are attached to the pneumatophores of mangroves in inter-tidal areas of the coast (Kamal and Khan, 2009) and rocky substratum, e.g., Saint Martin’s Island (Rakib et al., 2021d). Few estuarine and coast seaweed reports have been found in Bangladesh (Table 1). Here, about 165 species belonging to 77 genera of seaweeds have been recorded in the coastal and estuarine areas of Bangladesh (Figure 6B). Generic diversity of Rhodophyta was higher, but species diversity was higher in Phaeophyta (Figure 6B). Productive water with chlorophyll enriched environment derived these diversities along adjacent waters (Talukder, 2004). In India, nutrients were key marks for the number of seaweed assemblages along estuaries (Jansi and Ramadhas, 2009). However, seaweeds are not part of the traditional diet of Bangladesh, whereas they are often consumed elsewhere in the world. It can serve as a potential food source for cattle, poultry farms, and humans.
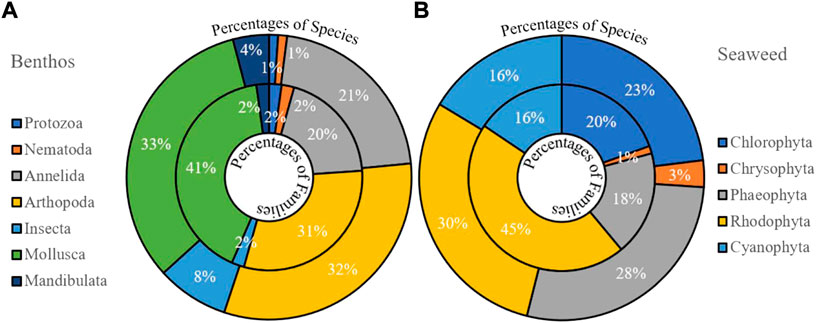
FIGURE 6. Representing available benthos (A) and seaweed (B) distribution in the coastal estuarine areas of Bangladesh.
3.4.3 Seagrass and salt marsh
Salt marshes and seagrass are recognized as essential components of coastal productivity worldwide (Kamal and Khan, 2009). These systems serve as feeding areas for various species, including avifauna (birds), and contribute considerable leaf detritus to the water column. The detritus from seagrass plays an active part in nitrogen and phosphorus cycles that provide essential elements to primary producers of all ecosystems. Seagrass also serves as a protective canopy, shielding the inhabitants of the bed from the effects of strong sunlight (Kamal and Khan, 2009). They also help to reduce sedimentation by trapping siltation locally (Neumeier and Ciavola, 2004). When seagrasses occur in the inter-tidal zone, the leaves may cover the substrate during low tide, protecting the inhabitants from desiccation. Although both salt marsh and seagrasses occur in Bangladesh’s estuaries, information on the diversity and distribution of these is lacking. Thus far, three species of salt marsh (spartina sp., Imperata cylindrical, Porteresia coarctata, and Porteresia sp.) and only five seagrasses (Halophila decipiens, Halophila beccarii, Halodule uninervis, Halodule pinifolia and Ruppia maritime) has been recorded (Hena et al., 2007; Billah et al., 2016; Hossain et al., 2021). Cultivating seagrasses has been encouraged worldwide (Neumeier and Ciavola, 2004). Besides, Indian researchers mentioned seagrass as a heavy metal consumer in coastal ecosystems, contributing to pollutant buffering and environmental health monitoring markers (Thangaradjou et al., 2010). In Bangladesh, some unpublished work was also found along the coastal zone of BB for investment, and research should be deployed in this sector to develop it for the betterment of renewable food sources for different biota. Very little research on estuarine salt marsh has been found in the coastal zone of Bangladesh. More investigation on the culture and management of estuarine salt marsh and sea grass should be deployed in the near future.
3.4.4 Mangroves forest
In BEC, mangrove covers six estuaries broadly, i.e., SR, PR, AE, SRE, MRE and IR. Total of 23 mangrove species representing 11 genera has been recorded till now. Some common species were Acanthus ilicifolius, Cynometra ramiflora, Acrosticum aureum, Phoenix paludosa, Rhizophora mucronata, R. apiculata, Bruguiera grymorrhiza, B. seangula, Ceriops decandra, C. tagal, Kandelia candel, Aegiceras corniculatum, Avicennia alba, A. marina, A. officinalis, Excoecaria agallocha, and E. indica (Table 5). Previous reports show that the Rhizophoraceae family is most common and Acanthaceae, Leguminosae, Pteridiaceae, Combretaceae, and Palmae are less diverse in this area. Along with providing an important coastal habitat for many species, a mangrove forest forms a community that helps stabilize riverbanks and coastlines. Mangroves also export large amounts of detritus and nutrients into nearby systems that form the basis of complex food webs (Rahman and Islam, 2010).
The plantation of mangroves was introduced in the BEC in 1964 to avoid natural disasters and is still carried out in the coastal belt of Cox’s Bazar, Chittagong, Barisal, and Patuakhali off-shore islands and now covers an area of 100,000 ha. Small patches of mangroves are also found along the belt of nearly all coastal sub-districts (Hossain and Lin, 2001). Additionally, salinity variation was reported to influence the mangrove species composition (Ahmed and Khurshid, 2011). It limits the growth of mangroves (Rahman and Islam, 2010). In Indian estuaries, it was already found as a looming danger to coastal biodiversity (Sandilyan et al., 2010). Increasing freshwater inputs by dredging coastal rivers may solve this problem periodically. Further steps should plan early in Bangladesh by deploying potential research projects to minimize these problems in the future.
3.5 Faunal biodiversity
3.5.1 Zooplankton communities
In estuaries, plankton plays a vital role in nutrient circulation and the transport cycle (Zhou et al., 2009). They followed the productivity line of phytoplankton accordingly (Iqbal et al., 2014). As a vital zooplankton taxa group, copepod, and crustacean larva were the most abundant zooplankton in estuaries (Ahmed et al., 2003). They acted principally as primary consumers in these estuarine food webs. Only six estuaries (NE, RE, BR, MR, FR, CR and TR) have conducted studies on this matter (Table 1). Previous reports show that the Rotifers family is the most common, and Decapoda larvae, Chaetognatha, and Ostracoda showed less diversity than BEC. So far, 67 species of zooplankton genera have been recorded from nearby areas of estuaries (Table 6) which is relatively less than a single estuary of India, i.e., Kaduviyar estuary (Vengadesh et al., 2009). Common zooplankton species were Calanus sp., Microsetella sp., Oncaea sp., Calanopia sp., Coryeacus sp., Oithona sp., Calanoid, Cyclops, Diaptomus, Nauplius, Mesocyclops edax, Cyclops sp., Diaptomus sp., Bryocvamptus sp., Penaeus monodon, P. merguiensis, Metapenaeus monoceros, M. brevicornis, Penaeus indicus, Macrobrachium rosenbergii, Acetes erythraeus, A. indicus, A. japonicas, Cladochera sp., M. Monoceros, and M. brevicornis. Suitable nutrients with active photosynthesis influence the phytoplankton community (Sharif, 2002; Aziz et al., 2012; Rahman et al., 2013; Mehedi Iqbal et al., 2017; Sharif et al., 2017), which directly increase the zooplankton diversity at these estuarine zones locally (Haque et al., 2015). Nutrient-rich estuaries amplify phytoplankton production, leading to higher growth of zooplankton (Iqbal et al., 2014). As a result, estuaries acted as suitable nursing grounds for fishes and crustaceans (Rahman et al., 2013).
3.5.2 Benthic communities
Benthic organisms are important for the ecology of estuaries both as consumers of plankton and as food for bottom-feeding fish (Chowdhury, 2014). They provide vital linkages between primary producers and higher trophic levels in estuarine food chains (Islam et al., 2013). For example, many clams and oysters feed on plankton in the water column (Hossain, 2003). Benthic organisms (i.e., polychaeta worms and crustaceans) form an important part of the diets of commercially important bottom-feeding fishes, such as Spot (Leiostomus xanthurus) and Croaker (Micropogonias undulatus) accordingly (Sharif et al., 2017) and commercially important invertebrate species such as Oysters (Ostrea edulis, Crassostrea belcheri) and Blue Crabs (Portunus pelagicus) (Kamruzzaman, 2003), are important commercially and recreationally.
Total of 44 species was found in the Naf estuary (Noman et al., 2019), but 109 species of the benthos genus were recorded from nearby estuaries (Kamal and Khan, 2009). Common species were Calanus sp., Microsetella sp., Oncaea sp., Calanopia sp., Coryeacus sp., Oithona sp., Calanoid, Cyclops, Diaptomus, Nauplius, Mesocyclops edax, Cyclops sp., Diaptomus sp., Bryocvamptus sp., Penaeus monodon, P. merguiensis, Metapenaeus monoceros, M. brevicornis, Penaeus indicus, Macrobrachium rosenbergii, Acetes erythraeus, A. indicus, A. japonicas, Cladochera sp., M. monoceros, M. brevicornis, Limnodrilus hoffmeister, Limnodrilus profundicola, Tubifex heterochaetus, Tubifex tubifex, Coleoptera, Unidentified Diptera, Hymenoptera, Hemiptera, Dubiraphia vittata, and Lutaria sp. (Hossain, 2003; Kamruzzaman, 2003; Islam et al., 2013) (Table 7; Figure 6A). Polychaetes were the dominant benthos reported from NE. Seasonal food availability may be liable behind these local diversities along the estuaries (Noman et al., 2019). Recently, benthos research accelerated into a new dimension, coupling with ocean acidification along these estuaries (Hossain and Rahman, 2017). New species (Nephtys Bangladeshi) discovered with the spatial and seasonal distribution of benthos under the tidal influence of estuaries have been started (Hossain and Hutchings, 2016). However, reports on seven estuaries (NE, BR, MR, KR, CR, TR, and BE) have been found during this review (Table 1). Even Indian estuaries have limited research on benthos (Nair et al., 1984b; Khan and Murugesan, 2005). Recently, Sivadas and Carvalho (2020) have reported the richness of marine annelid (727 species) belongings to 334 genera and 72 families. Of these, 152 marine annelid species are locally abundant in India, whereas 88 are endemic. Sukumaran et al. (2021) have also reported 2078 macrobenthic taxa in North-west India, belongings to 14 phyla, and the most abundant were Polychaeta, Gastropoda, and Bivalvia. Previous reports show that Mollusca species are common, and protozoa and nematodes are less diverse in this area. More innovations with life-focused techniques to develop sustainable management and conservation of the estuarine environment should be practiced in benthos communities (Mondal et al., 2018).
3.5.3 Crustaceans’ communities (shrimp and crab)
In BEC, 36 shrimps and 16 crab species were reported from the estuaries (Table 8). Common crabs species were Scylla serrate, Portunus rinolentus, Portunus pelagicus, Metopograpsus thukuhar, Metopograpsus messor, Prasesarma plicatum, Sesaema lanatum, Episesarma versicolor, Potamon wood-masoni, Potamon martensi, Paratelphusa lamellifrons, Uca urvillei, Uca annulipe,s Ocypode ceratophthalmus, Anapagurus laevis, and Ebalia cranchii. Among crabs, Grapsidae was abundant in estuaries of Bangladesh (Table 8). Fishing in the mangroves is one of the major activities in the coastal area (Kamal and Khan, 2009). Common shrimp species were Penaeus monodon, M.dobsoni, P. merguiensis, P. indicus., P. uncta, Metapenaeus monoceros, M. lysianass, M. spinulatus, M. brevicornis, M. affinis, Parapenaeopis sculptilis, P. stylifera, P. hardwickii, P. semisulcatus, P. Japonicus, M. villosimanus, M. mirabilis, P. melastigma, P. koelreuteri, M dobsoni, Exopalaemon Styliferus, Macrobrachium rosenbergii, M. lamarrei, M. rude, M. villosimanus, M. mirabile, M. birmanicum, Palaemon Styliferus, P (Nematopalaemon) tenuipes and P (N) karnafuliensis, etc. However, Penaeus monodon (black tiger shrimp), Metapenaeus monoceros, M. brevicornis, P. indicus, and Macrobrachium rosenbergii were diverse. Penaeus indicus, and P. monodon were the most abundant shrimp in estuaries. Besides, shrimp culture by collecting post larva from marine water is a seasonal practice along the south coast of Bangladesh (Rahman et al., 2008). However, expanding commercial culture of tiger shrimp has already led to the destruction of mangroves in Chakaria Sunderban, Moheskhali, Teknaf, and Sonadia Island on the south-east coast of Bangladesh (Akber et al., 2017; Saha, 2017). The government should take strict actions and implement laws under potential monitoring systems to mitigate this problem and preserve the biota at BEC and coastal areas.
3.5.4 Fish communities
Only 13 estuaries (NE, RE, BR, MR, SE, KR, FR, CR, TR, AR, BE, BRE, and AE) have been investigated previously on this aspect (Table 1). Previous reports show that the most common are Ariidae, Clupeidae, Gobiidae, and Sciaenidae families. In the present state of the investigation, proper classification of fish species based on their period of life and availability in the estuaries is difficult (Begum, 1984; Islam et al., 2015; Ahamed et al., 2018; Saha et al., 2019). The estuarine and adjacent coastal areas of BEC support a variety of economically important fishes. In BEC, 208 brackish water fish species were reported from the estuaries (Table 9). The most common species of fishes are found in the coastal and estuarine mangrove areas, such as mullet (Mugil sp. and Liza), marine catfish (Mystus sp.), seabass (Lates calcarifer) Eleutheronema tetradactylum, Polynemus paradiscus, Mugil cephalus, Liza tade, Rhlnomugli corsula, Mystus golio, Tenualosa toil, Gonialosa manminna, Tenualosa ilisha, Ilisha megalopetra, Setipina taty, Collia ramcarati, Septipinns phasa, and Trichurus Havmela. A tentative list of estuary fishes was reported, while the Food Agriculture Organization (FAO) gave a general list of the most common finfish for the estuaries (Kamal and Khan, 2009). The fishes spend all or a major part of their lifetime in the estuarine environment; marine or freshwater species migrate seasonally into or through the estuaries (Rashid, 1999). The diversity of fish depends on local environmental factors (Ahamed et al., 2018). So, habitat loss may potentially threaten fishes and other species along the brackish water (Barletta et al., 2010; Blaber and Barletta, 2016; Nanjo, 2020). Proper investigation of data deficient estuaries with intense monitoring of coastal habitat may resolve this problem actively.
3.5.5 Avifaunal biodiversity
Birds are important food chain members of food webs, especially in estuaries. The marshes, reeds, and mangroves provide sheltered breeding grounds for swamp birds such as the bittern, marsh crake, banded rail, fern bird, etc. A total of 690 avifauna species have been recorded from Bangladesh (Khan, 2008); however, only 121 species have been reported from BEC environments, as shown in Table 10 (Kamal and Khan, 2009). Common bird species were Dendrocygna bicolor, Dendrocygna javanica, Sarkidiornis melanotos, Gallinula chloropus Fulica atra, Tringa ochropus, Tringa glareola, Xenus cinereus, Actitis hypoleucos, Rynchops albicollis, Larus heuglini, Larus barabensis, Sterna hirundo, Sterna aurantia, Phalacrocorax carbo, Casmerodius albus, Anastomus oscitans, and Leptoptilos javanicus. It covered all estuaries of Bangladesh accordingly. Tidal flats hold a bounty of food for different aquatic birds (Table 10). Previous reports show that the Scolopacidae family is the most common, and Rostratulidae and Glareolidae are less diverse in this area (Kamal and Khan, 2009). For example, Mud probers, Wrybills, Herons, Caspian terns, Ducks, etc. (Hossain and Lin, 2001). In addition, mangroves of SRE acted as food sources for Avifauna (Gopal and Chauhan, 2006). Additionally, migratory birds travel thousands of kilometers from their Siberian and Alaskan breeding grounds each year, arriving at estuaries (Maheswaran and Rahmani, 2001; Albores and Siguenza, 2011). Tropical weather provided suitable cruising places (Gopal and Chauhan, 2006). Hunting down migratory birds in BEC was a potential threat to biodiversity, which the government mitigated through proper law and action during the winter. This model can apply worldwide for protecting avifaunal biodiversity as well.
3.5.6 Hydrobiological correlations of estuaries
A cluster analysis was used on available estuarine data by applying Pearson correlation after considering environmental and physical parameters (Figures 3A, B) as variables (Figure 7). Rezu and Naf River estuaries are situated in the southern part of Bangladesh. Considering environmental conditions (e.g., temperature, salinity, nutrients, etc.), Sundarban, Rezu, and Naf River estuaries clustered together in group 1. Temperature influenced by salinity and dissolved oxygen correlates with Sangu, Bakkhali, and KR in group 5, which were located near the hilly area of BEC (Figure 3). On the other hand, Balaswar and Possur river estuary demonstrated similarity in Group 2. Despite having different geographical positions, close water temperature acted as clustering variables for MRE and IR (Group 3), followed by Matamuhuri, Choto Feni, and AR too (Group 4) (Figure 7). This review found that no research is available to generalize the hydro-biographic conditions of these estuaries due to proper planning and research. All research is scattered with different purposes, which made a complex demography to understand the factual composition and their comparisons across these estuaries (Jiyu et al., 2001). A combined project on soil texture and nutrients of all main estuaries with their countable biodiversity tabulation is recommended to deploy soon for an in-depth assessment of the estuarine ecosystem and their interactions. Future researchers should concentrate on these to fulfill the dataset about all important estuaries of BEC for better scientific development and review.
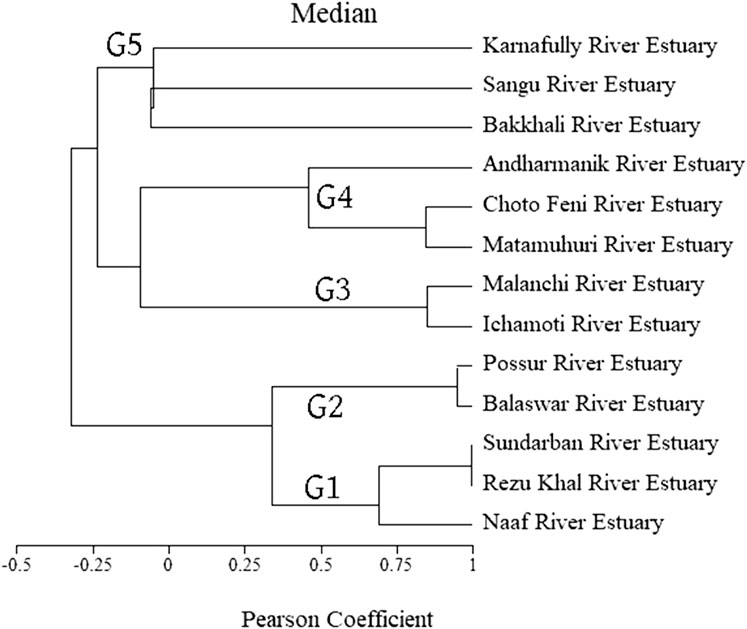
FIGURE 7. Cluster analysis of all estuaries in different groups (G) by considering different hydrobiological variables with Pearson coefficient.
Accordingly, possible recommendations should be considered by recording sand composition, angiosperm, birds, animals, fishes, weeds, and related environmental parameters. Data deficient areas, i.e., GE, BRE, and SR, need to be surveyed accordingly. Monitoring cells on wildlife conservation should be regulated to pursue existing biodiversity with proper protections. Strict laws, sync preservation, and maintenance of proper past records can help enhance the local biotic community. Explicit lab equipment to examine these data is also necessary at each bio-research institution. Fundamental regulations with modern technology can also develop such situations.
4 Concluding remarks and future recommendations
This is the first comprehensive review to unify all the available information on the ecological quality and biodiversity status of the estuarine environment in Bangladesh. The study demonstrates that the estuarine environment of Bangladesh is quite diverse and dynamic, like other tropical countries, due to the tropical climate and strong monsoonal influence. Ecological drivers varied daily and even hourly in Bangladesh. In these diverse and dynamic environments, for example, water temperature, salinity, and pH varied 15–34°C, 0.5–33 ppt, and 6.2–8.53, respectively. The total dissolved solids concentration was found to be very high, especially during monsoon, causing low water transparency/visibility (24 cm only) in the estuaries.
Additionally, higher concentrations of silicates and lower levels of ammonium ions were also observed. Despite having low chlorophyll assemblage caused by low dissolved oxygen (5.19 mg/L), 85 phytoplankton species were reported from the estuarine water of Bangladesh. Over 830 faunal and floral species of 273 genera were recorded from the estuaries. Considering the abundance of biota, Scylla serrate of crabs, Shrimp, Tubificidae and Nereidiae of benthos group, Copepoda of zooplankton, Zygnemataceae family from phytoplankton, Rhodophyta division from seaweed, and Scolopacidae family of birds were most abundant in these coastal estuaries. Among them, the International Union for Conservation of Nature (ICUN) red-listed organisms should be given the highest priorities for conservation, and close habitat monitoring should be deployed. To protect the estuarian ecosystems, the government, policymakers, scientists, non-governmental organizations (NGOs), researchers, etc., need to have an overall dataset to protect and manage the Bangladeshi estuarine ecosystem. This review reveals a paucity of available biotic data for major estuaries, and more research is needed on these aspects to record the current biodiversity status and understand interrelations among them. This research unraveled the need for the national database archiving system to closely monitor the subtle changes in this dynamic ecosystem.
Author contributions
MJ: Conceptualization; data collection; Formal analysis; Investigation; Resources; Writing—original draft, editing and review. MI: Formal analysis; Writing-editing, and review. MH: Conceptualization, editing, and review. IH: Data collection. MR: Writing-editing, and review. RK: Formal analysis; Writing-editing, and review PS: Writing-editing, and review.
Acknowledgments
The authors gratefully acknowledge the Noakhali Science and Technology University for providing the facilities for this study. The authors are grateful to all researchers at the Institute of Marine Sciences (IMS), Chittagong University, Bangladesh, for giving access to collect their unpublished thesis with special permission.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, A. S., Rahman, M., Sultana, S., Babu, S. O. F., and Sarker, M. S. I. (2019). Bioaccumulation and heavy metal concentration in tissues of some commercial fishes from the Meghna River Estuary in Bangladesh and human health implications. Mar. Pollut. Bull. 145, 436–447. doi:10.1016/j.marpolbul.2019.06.035
Ahmed, A. S., Sultana, S., Habib, A., Ullah, H., Musa, N., Hossain, M. B., et al. (2019). Bioaccumulation of heavy metals in some commercially important fishes from a tropical river estuary suggests higher potential health risk in children than adults. Plos one 14 (10), e0219336. doi:10.1371/journal.pone.0219336
Ahmed, A. S. M. (2004). “Mathematical model investigation of the suitable countermeasure for the accretion problem at Rosetta estuary, Egypt,” in Proceeding of the Oceans' 04 MTS/IEEE Techno-Ocean'04 (IEEE Cat. No. 04CH37600), Kobe, Japan, November 2004 (IEEE), 78–82.
Ahamed, F., Saha, N., Ahmed, Z. F., Hossain, M. Y., and Ohtomi, J. (2018). Reproductive biology of Apocryptes bato (Gobiidae) in the Payra River, southern Bangladesh. J. Appl. Ichthyol. 34 (5), 1169–1175. doi:10.1111/jai.13781
Ahmed, K. K. U., Ahamed, S. U., Hossain, M. R. A., Ahmed, T., and Barman, S. (2003). Quantitative and qualitative assessment of plankton: Some ecological aspect and water quality parameters of the river Meghna, Bangladesh. Bangladesh. J. Fish. Res. 7 (2), 131–140.
Ahmed, M., and Khurshid, Y. (2011). Does silicon and irrigation have impact on drought tolerance mechanism of sorghum? Agric. Water Manag. 98 (12), 1808–1812. doi:10.1016/j.agwat.2011.07.003
Ahammed, S. S. (1997). Study of Trace metals in water, sediments and some commercially important fishes and shell fish of the Pusser River Estuary, Bangladesh. MS Thesis. Bangladesh: University of Chittagong.
Ahsan, D. A., Kabir, A. N., Rahman, M. M., Mahabub, S., Yesmin, R., Faruque, M. H., et al. (2012). Plankton composition, abundance and diversity in hilsa (Tenualosa ilisha) migratory rivers of Bangladesh during spawning season. Dhaka Univ. J. Biol. Sci. 21 (2), 177–189. doi:10.3329/dujbs.v21i2.11516
Akter, J., Sarker, M. H., Popescu, I., and Roelvink, D. (2016). Evolution of the bengal delta and its prevailing processes. J. Coast. Res. 32 (5), 1212–1226. doi:10.2112/jcoastres-d-14-00232.1
Akber, M. A., Islam, M. A., Ahmed, M., Rahman, M. M., and Rahman, M. R. (2017). Changes of shrimp farming in southwest coastal Bangladesh. Aquac. Int. 25 (5), 1883–1899. doi:10.1007/s10499-017-0159-5
Al-mamun, M. A. (2010). A comparative study on fin fish and shell fish assemblage structure between Bakkhali and Matamuhuri river estuary. MS Thesis. Bangladesh: University of Chittagong.
Alam, G. M., Alam, K., and Mushtaq, S. (2017). Climate change perceptions and local adaptation strategies of hazard-prone rural households in Bangladesh. Clim. Risk Manag. 17, 52–63. doi:10.1016/j.crm.2017.06.006
Alam, M. W. (2013). Microbial species diversity and hydrological effects on their occurrence at Karnaphuli River estuary. Agri Sci. Res. J. 3, 158–166.
Albores, J. E. R., and Sigüenza, A. G. N. (2011). “Relationships between bird species richness and natural and modified habitat in Southern Mexico,” in Changing diversity in changing environment. Editor O. Grillo, 932.
Ali, M. S., Haque, M. F., Rahman, S. M. M., Iqubal, K. F., Nazma, M., and Ahmed, A. (2013). Loss and gain of land of manpura island of bhola district: An integrated approach using remote sensing and GIS. Dhaka Univ. J. Biol. Sci. 22 (1), 29–37. doi:10.3329/dujbs.v22i1.46271
Ali, M. M., Ali, M. L., Islam, M. S., and Rahman, M. Z. (2016). Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 5, 27–35. doi:10.1016/j.enmm.2016.01.002
Ali, M. M., Ali, M. L., Rakib, M. R. J., Islam, M. S., Habib, A., Hossen, S., et al. (2021). Contamination and ecological risk assessment of heavy metals in water and sediment from hubs of fish resource river in a developing country. Toxin Rev., 1–16. doi:10.1080/15569543.2021.2001829
Ali, M. M., Rahman, S., Islam, M. S., Rakib, M. R. J., Hossen, S., Rahman, M. Z., et al. (2022). Distribution of heavy metals in water and sediment of an urban river in a developing country: A probabilistic risk assessment. Int. J. Sediment Res. 37 (2), 173–187. doi:10.1016/j.ijsrc.2021.09.002
Amin, M. N., and Mahmood, N. (1979). Seasonal occurrence of post larvae of penaeid shrimp in the Karnafull estuary. Bangladesh J. Agric. 4, 21–24.
Anilakumary, K. S., Abdul Aziz, P. K., and Natrajan, P. (2007). Water quality of the Adimalathma estuary, southwest coast of India. J. Mar. Biol. Ass India 49, 1–6.
Ashraful, M., Assim, Z., and Ismail, N. (2009). Monitoring and assessment of heavy metals levels in littoral sediments from the north eastern part of the Bay of Bengal coast. Bangladesh. J. Ind. Pollut. Contr 25, 105–111.
Aziz, A., and Paul, A. R. (2015). Bangladesh sundarbans: Present status of the environment and biota. Diversity 7 (3), 242–269. doi:10.3390/d7030242
Aziz, A., Rahman, M., and Ahmed, A. (2012). Diversity, distribution and density of estuarine phytoplankton in the Sundarban Mangrove Forests, Bangladesh. Bangladesh J. Bot. 41 (1), 87–95. doi:10.3329/bjb.v41i1.11086
Barletta, M., Jaureguizar, A. J., Baigun, C., Fontoura, N. F., Agostinho, A. A., Almeida-Val, V. M. F. D., et al. (2010). Fish and aquatic habitat conservation in south America: A continental overview with emphasis on neotropical systems. J. Fish. Biol. 76 (9), 2118–2176. doi:10.1111/j.1095-8649.2010.02684.x
Barua, P., Rahman, S. H., and Molla, M. H. (2019). Impact of river erosion on livelihood and coping strategies of displaced people in South-Eastern Bangladesh. Int. J. Migr. Resid. Mobil. 2 (1), 34–55. doi:10.1504/IJMRM.2019.103275
Basak, J. K., Titumir, R. A. M., and Dey, N. C. (2013). Climate change in Bangladesh: A historical analysis of temperature and rainfall data. J. Environ. Manage. 2 (2), 41–46.
Begum, S. (1984). Temporal and spatial distribution of ichthyoplankters and other zooplankters in the Mathamuhuri estuary, Bangladesh. MS Thesis. Chittagong (Bangladesh): Institute of Marine Science, University of Chittagong, 49.
Bhuyan, M. S., Bakar, M. A., Akhtar, A., Hossain, M. B., and Islam, M. S. (2017). Analysis of water quality of the Meghna River using multivariate analyses and RPI. J. Asiat. Soc. Bangladesh Sci. 43 (1), 23–35. doi:10.3329/jasbs.v43i1.46241
Billah, M. M., Kamal, A. H. M., Idris, M. H. B., and Ismail, J. B. (2016). Seasonal variation in the occurrence and abundance of mangrove macroalgae in a Malaysian estuary. Cryptogam. Algol. 37 (2), 109–120. doi:10.7872/crya/v37.iss2.2016.109
Blaber, S. J., Cyrus, D. P., Albaret, J. J., Ching, C. V., Day, J. W., Elliott, M., et al. (2000). Effects of fishing on the structure and functioning of estuarine and nearshore ecosystems. iCES J. Mar. Sci. 57 (3), 590–602. doi:10.1006/jmsc.2000.0723
Blaber, S. J. M., and Barletta, M. (2016). A review of estuarine fish research in south America: What has been achieved and what is the future for sustainability and conservation? J. Fish. Biol. 89 (1), 537–568. doi:10.1111/jfb.12875
Boicourt, W. C., Li, M., Nidzieko, N., Blumberg, A. F., Georgas, N., Kelly, E. J., et al. (2012). “Observing the urban estuary: Review and prospect,” in Proceeding of the 2012 Oceans, Hampton Roads, VA, USA, October 2012 (IEEE), 1–9.
Chowdhury, A. H. (2014). Draft report of the research impact of oil spillage on the environment of Sundarbans (World Largest Mangrove Forest) in Bangladesh, 1–16. Available at: https://ncbd.org/wp-content/uploads/2014/12/Impact-of-oil-spills-on-the-Sundarbans_AHC.pdf.
Chowdhury, M. S. N., Hossain, M. S., Das, N. G., and Barua, P. (2011). Environmental variables and fisheries diversity of the naaf river estuary, Bangladesh. J. Coast. Conserv. 15 (1), 163–180. doi:10.1007/s11852-010-0130-3
Das, B., Khan, Y. S. A., and Sarker, M. A. K. (2002). Trace metal concentration in water of the karnaphuli River estuary of the bay of bengal. Pak. J. Biol. Sci. 5, 607–608. doi:10.3923/pjbs.2002.607.608
Das, H. K., Mitra, A. K., Sengupta, P. K., Hossain, A., Islam, F., and Rabbani, G. H. (2004). Arsenic concentrations in rice, vegetables, and fish in Bangladesh: A preliminary study. Environ. Int. 30 (3), 383–387. doi:10.1016/j.envint.2003.09.005
Dike, C. C., and Agunwamba, J. C. (2012). A study on the effects of tide on sedimentation in estuaries of the Niger Delta, Nigeria. juee. 6 (2), 86–93. doi:10.4090/juee.2012.v6n2.086093
Eick, D., and Thiel, R. (2014). Fish assemblage patterns in the elbe estuary: Guild composition, spatial and temporal structure, and influence of environmental factors. Mar. Biodivers. 44 (4), 559–580. doi:10.1007/s12526-014-0225-4
Elias, S. (1983). Abundance of zooplankton of the matamuhuri River estuary with special reference to shrimp and larvae. Postgraduate dissertation. IMSF, Uni. Ctg, 172.
Elliott, M., and Quintino, V. (2007). The estuarine quality paradox, environmental homeostasis and the difficulty of detecting anthropogenic stress in naturally stressed areas. Mar. Pollut. Bull. 54 (6), 640–645. doi:10.1016/j.marpolbul.2007.02.003
Farhadinejad, T., Khakzad, A., Jafari, M., Shoaee, Z., Khosrotehrani, K., Nobari, R., et al. (2014). The study of environmental effects of chemical fertilizers and domestic sewage on water quality of Taft region, Central Iran. Arab. J. Geosci. 7 (1), 221–229. doi:10.1007/s12517-012-0717-0
Förstner, U. (2004). Sediment dynamics and pollutant mobility in rivers: An interdisciplinary approach. Lakes &. Reserv. 9 (1), 25–40. doi:10.1111/j.1440-1770.2004.00231.x
Gopal, B., and Chauhan, M. (2006). Biodiversity and its conservation in the sundarban mangrove ecosystem. Aquat. Sci. 68 (3), 338–354. doi:10.1007/s00027-006-0868-8
Habib, A., Hosokai, T., Mitsuo, N., Nakagawa, R., Nagamatsu, S., Aoki, M., et al. (2011). Observation and analysis of small inclination of thymine molecules on graphite. J. Phys. Chem. C 115 (2), 511–515. doi:10.1021/jp108869w
Haque, M. R., Islam, M. A., Rahman, M. M., Shirin, M. F., Wahab, M. A., and Azim, M. E. (2015). Effects of C/N ratio and periphyton substrates on pond ecology and production performance in giant freshwater prawn M acrobrachium rosenbergii (De Man, 1879) and tilapia O reochromis niloticus (Linnaeus, 1758) polyculture system. Aquac. Res. 46 (5), 1139–1155. doi:10.1111/are.12270
Haque, S. M. A. (1983). Study on phytoplankton of the Matamuhuri Estuary and the fish ponds in the vicinity. MS Thesis. Bangladesh: University of Chittagong.
Hena, M. A., Short, F. T., Sharifuzzaman, S. M., Hasan, M., Rezowan, M., and Ali, M. (2007). Salt marsh and seagrass communities of Bakkhali estuary, cox's bazar, Bangladesh. Estuar. Coast. Shelf Sci. 75 (1-2), 72–78. doi:10.1016/j.ecss.2007.01.022
Hoq, M. E., Wahab, M. A., and Islam, M. N. (2006). Hydrographic status of Sundarbans mangrove, Bangladesh with special reference to post-larvae and juveniles fish and shrimp abundance. Wetl. Ecol. Manag. 14 (1), 79–93. doi:10.1007/s11273-005-2569-9
Hoque, S. M. A., Zafar, M., and Mahmood, N. (1999). Temporal and spatial distribution of phytoplankton with emphasis on Skeletunema costatum in the Mathamuhuri river estuary (Chakharia mangrove ecosystem), Bangladesh. Pak. J. Mar. Sci. 8, 29–39.
Hossen, M. A. (2014). Water policy and governance for the empowerment of river basin communities in rural Bangladesh. Doctoral dissertation. University of British Columbia.
Hossain, M. B., and Hutchings, P. (2016). <strong><em>Nephtys Bangladeshi</em> n. sp., a new species of Nephtyidae (Annelida: Phyllodocida) from Bangladesh coastal waters </strong>. Zootaxa 4079 (1), 41. doi:10.11646/zootaxa.4079.1.3
Hossain, M. B. (2003). Macrozoobenthos of the Meghna River Estuarine bed with special reference to polychaete faunal biodiversity. MS Thesis. Bangladesh: University of Chittagong.
Hossain, M. B., Habib, S. B., Hossain, M. S., Jolly, Y. N., Kamal, A. H. M., Idris, M. H., et al. (2020). Data set on trace metals in surface sediment and water from a sub-tropical estuarine system, Bay of Bengal, Bangladesh. Data brief 31, 105911. doi:10.1016/j.dib.2020.105911
Hossain, M. B., and Rahman, M. (2017). ocean acidification: An impending disaster to benthic shelled invertebrates and ecosystem. J. Noakhali Sci. Technol. Univ. 1 (1), 19–30.
Hossain, M. B., Rakib, M. R. J., Jolly, Y. N., and Rahman, M. (2021). Metals uptake and translocation in salt marsh macrophytes, Porteresia sp. from Bangladesh coastal area. Sci. Total Environ. 764, 144637. doi:10.1016/j.scitotenv.2020.144637
Hossain, M. B., Shanta, T. B., Ahmed, A. S. S., Hossain, M. K., and Semme, S. A. (2019). Baseline study of heavy metal contamination in the Sangu River estuary, Chattogram, Bangladesh. Mar. Pollut. Bull. 140, 255–261. doi:10.1016/j.marpolbul.2019.01.058
Hossain, M. M. (1983). Pollution as revealed by macrobenthic organisms in the Karnafuli River estuary. Postgraduate dissertation. IMSF, Uni. Ctg., 96
Hossain, M. S., Das, N. G., Sarker, S., and Rahaman, M. Z. (2012). Fish diversity and habitat relationship with environmental variables at Meghna river estuary, Bangladesh. Egypt. J. Aquatic Res. 38 (3), 213–226. doi:10.1016/j.ejar.2012.12.006
Hossain, M. S., and Lin, C. K. (2001). Land use zoning for integrated coastal zone management. ITCZM Monogr. 3, 24.
Hussain, S. G. (2013). “An introduction to the coasts and the Sundarbans,” in Shores of tears. Editor P. Gain (Dhaka, Bangladesh: Society for Environment and Human Development), 01–19. ISBN: 978-984-8952-05-4©.
Islam, M., Idris, A. M., Islam, A. R. M., Ali, M. M., and Rakib, M. R. J. (2021). Hydrological distribution of physicochemical parameters and heavy metals in surface water and their ecotoxicological implications in the Bay of Bengal coast of Bangladesh. Environ. Sci. Pollut. Res. 28, 68585–68599. doi:10.1007/s11356-021-15353-9
Islam, M. A., Al-Mamun, A., Hossain, F., Quraishi, S. B., Naher, K., Khan, R., et al. (2017). Contamination and ecological risk assessment of trace elements in sediments of the rivers of Sundarban mangrove forest, Bangladesh. Mar. Pollut. Bull. 124 (1), 356–366. doi:10.1016/j.marpolbul.2017.07.059
Islam, M. A., Hasan, M., Peas, M. H., Naime, M. A., Gazi, M. Y., and Rahman, M. M. (2016). Shoreline vulnerability assessment in an offshore island (sandwip), Bangladesh-an appraisal of geospatial techniques. Dhaka Univ. J. Earth Env. Sci. 5, 51–60.
Islam, M. H. (2004). Study on bottom sediment organic carbon with emphass on environmental parameters of the lower Meghna Estuary, Bangladesh. MS Thesis. Bangladesh: University of Chittagong.
Islam, M. I., and Nabi, M. R. (2012). Temporal patterns of fish assemblages of feni River,Feni, Bangladesh: Fish biodiversity of feni river. LAP LAMBERT Academic Publishing, 80.
Islam, M. S., Ahmed, M. K., Habibullah-Al-Mamun, M., and Hoque, M. F. (2015). Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environ. Earth Sci. 73 (4), 1837–1848. doi:10.1007/s12665-014-3538-5
Islam, M. S., Hossain, M. B., Matin, A., and Sarker, M. S. (2018). Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere 202, 25–32. doi:10.1016/j.chemosphere.2018.03.077
Islam, M. S., Sikder, M. N. A., Al-Imran, M., Hossain, M. B., Mallick, D., and Morshed, M. M. (2013). Intertidal macrobenthic fauna of the karnafuli estuary: Relations with environmental variables. World Appl. Sci. J. 21 (9), 1366–1373. doi:10.5829/idosi.wasj.2013.21.9.72174
Islam, M. S., and Wahab, M. A. (2005). A review on the present status and management of mangrove wetland habitat resources in Bangladesh with emphasis on mangrove fisheries and aquaculture. Hydrobiologia II, 165–190. doi:10.1007/s10750-004-0756-y
Islam, S. N., Gnauck, A., and Voigt, H. J. (2011). Fourier polynomial approximation of estuaries water salinity in the Sundarbans region of Bangladesh. Int. J. Hydrol. Sci. Technol. 1 (3–4), 207–223. doi:10.1007/s10750-004-0756-y
Islam, Z. (2012). Seasonal variations of fish assemblages in Sangu River estuary, Chittagong Bangladesh. MS Thesis. Bangladesh: University of Chittagong.
Iqbal, M. M., Islam, M. S., and Haider, M. N. (2014). Heterogeneity of zooplankton of the Rezukhal Estuary, Cox's Bazar, Bangladesh with seasonal environmental effects. Int. J. Fish. Aqu. Stu 2 (2), 275–282.
Jansi, M., and Ramadhas, V. (2009). Effect of salinity and dissolved nutrients on the occurrence of some seaweeds in Manakkudy estuary. Indian J. Mar. Sci. 38 (4), 470–473.
Jiang-Qi, Q., Qing-Jing, Z., Pan, L., Cheng-Xia, J., and Mu, Y. (2013). Assessment of water quality using multivariate statistical methods: A case study of an urban landscape water, beijing. Int. J. Biosci. Biochem. Bioinforma. 3 (3), 196–200. doi:10.7763/ijbbb.2013.v3.195
Jiyu, C., Daoji, L., and Wenhua, J. (2001). Eco-engineering of jiuduansha island caused by pudong international airport construction. Eng. Sci. 3 (4), 1–8.
Jolly, Y. N., Rakib, M. R. J., Islam, M. S., Akter, S., Idris, A. M., and Phoungthong, K. (2021). Potential toxic elements in sediment and fishes of an important fish breeding river in Bangladesh: A preliminary study for ecological and health risks assessment. Toxin Rev., 1–14.
Kamal, A. H. M., and Khan, M. A. A. (2009). Coastal and estuarine resources of Bangladesh: Management and conservation issues. Maejo Int. J. Sci. Technol. 3 (2), 313–342.
Kamruzzaman, M. (2003). Macro benthos of Karnafully River Estuary receiving municipal and industrail effluents. MS Thesis. Bangladesh: University of Chittagong.
Khan, M. M. H. (2008). “Protected areas of Bangladesh- A. Guide to wildlife,” in Nishorgo support program, Bangladesh (Dhaka, Bangladesh: Bangladesh Forest Department), 304.
Khohinoor, S. M. S. (2008). Abundance and composition of macro bethos in the channel system of Bakkhali River estuary, Cox’s bazar. MS thesis. Bangladesh: University of Chittagong.
Kumar, B. S. K., and Sarma, V. V. S. S. (2018). Variations in concentrations and sources of bioavailable organic compounds in the Indian estuaries and their fluxes to coastal waters. Cont. Shelf Res. 166, 22–33. doi:10.1016/j.csr.2018.07.001
Maheswaran, G., and Rahmani, A. R. (2001). Effects of water level changes and wading bird abundance on the foraging behaviour of blacknecked storksEphippiorhynchus asiaticus in Dudwa National Park, India. J. Biosci. 26, 373–382. doi:10.1007/bf02703747
Mahmood, N. (1986). “Effects of shrimp farming and other impacts on man-groves of Bangladesh,” in Proceedings of the workshop of strategies for the management of fisheries and aquaculture in mangrove ecosystems (Thai-land, Bangkok), 46e66. FAO Fisheries Report No. 370.
Mahmood, N., Khan, Y. S. A., and Ahmed, M. K. (1978). Hydrology of the Karnafully estuary with special reference to prawn and other larvae of economic importance, Final Report. Research ProgramDhaka: University Grants Commission.
Mallick, D., Islam, M., Talukder, A., Mondal, S., Al-Imran, M., and Biswas, S. (2016). Seasonal variability in water chemistry and sediment characteristics of intertidal zone at Karnafully estuary, Bangladesh. Pollution 2 (4), 411–423. doi:10.7508/PJ.2016.04.004
Mallick, D. (2014). Monitoring trace/heavy metals in the Karnafually River Estuary and adjacent coastel ares of Chittagong, Bangladesh using innovative artificial mussel (am) technology. MS Thesis. Bangladesh: University of Chittagong.
Mallick, N. (2011). Fish assemblage of estuarine set bag net in Matamuhuri River estuary of Cox’s Bazar district with reference to some water quality parameters. MS Thesis. Bangladesh: University of Chittagong.
Mann, K. H. (1982). Ecology of coastal waters: A systems approach, 8. Berkeley: Univ of California Press.
Mehedi Iqbal, M., Masum Billah, M., Nurul Haider, M., Shafiqul Islam, M., Rajib Payel, H., Khurshid Alam Bhuiyan, M., et al. (2017). Seasonal distribution of phytoplankton community in a subtropical estuary of the south-eastern coast of Bangladesh. Zool. Ecol. 27 (3-4), 304–310. doi:10.1080/21658005.2017.1387728
Menon, N. N., Balchand, A. N., and Menon, N. R. (2000). Hydrobiology of the Cochin backwater system. Hydrobiologia 430, 149–183. doi:10.1023/a:1004033400255
Mitra, A. (2015). Health of estuaries in the east coast of the Indian SubContinent. Ann. Mar. Biol. Res. 2 (1), 1006.
Mondal, M. A. H., Islam, M. K., Islam, M. E., Barua, S., Hossen, S., Ali, M. M., et al. (2018). Pearson’s correlation and likert scale based investigation on livelihood status of the fishermen living around the Sundarban.
Moshfika, M., and Rahman, A. (2018). Hydrological and hydraulic consideration for design and construction of closure dam on little Feni River. Dhaka: Civil and Water Resources Engineering Conference. ISBN: 978-1-925488-52-4.
Nair, N. B., Dharmaraj, K., Azis, P. A., Arunachalam, M., Krishnakumar, K., and Balasubramanian, N, K. (1984a). Ecology of Indian estuaries: 8. Inorganic nutrients in the ashtamudi estuary. Mahasagar 17 (1), 19–32.
Nair, N. B., Azis, P. A., Arunachalam, M., Dharmaraj, K., and Krishnakumar, K. (1984b). Ecology of Indian estuaries: Ecology and distribution of benthic macrofauna in the Ashtamudi Estuary, Kerala. Mahasagar 17 (2), 89–101.
Nanjo, K. (2020). Effects of habitat degradation on fish production in a mangrove estuary. Impact 2020 (3), 32–33. doi:10.21820/23987073.2020.3.32
Naskar, K. R., and Chakraborty, N. M. (1984). Studies on the economic fauna from the sundarbans delta in West Bengal. J. Indian Soc. Coast Agric. Res. 2, 56–62.
Neumeier, U., and Ciavola, P. (2004). Flow resistance and associated sedimentary processes in a Spartina maritima salt-marsh. J. Coast. Res. 20 (2), 435–447. doi:10.2112/1551-5036(2004)020[0435:fraasp]2.0.co;2
Noman, M. A., Mamunur, R., Islam, M. S., and Hossain, M. B. (2019). Spatial and seasonal distribution of intertidal macrobenthos with their biomass and functional feeding guilds in the Naf River estuary, Bangladesh. J. Oceanol. Limnol. 37 (3), 1010–1023. doi:10.1007/s00343-019-8063-7
Quader, T. (2012). Comparison of fish and shrimp abundance between winter moonson season at Sangu River estuary with relation to some environment parameter. MS Thesis. Bangladesh: University of Chittagong.
Rahman, M. A., Zaher, M., Azimuddin, K. M., Yeasmine, S., Khan, M. M., and Arshad, A. (2013). Stocking density effects on growth and production of the threatened silurid catfish, Mystus cavasius (Hamilton) fingerlings in nursery ponds. Aquac. Res. 44 (7), 1132–1139. doi:10.1111/j.1365-2109.2012.03148.x
Rahman, M. M. (1997). Phytoplakton of the Naf River estuary during post monsoon near Teknaf coast. MS Thesis. Bangladesh: University of Chittagong.
Rahman, M. M., Flitner, M., Krause, G., and Maniruzzaman, M. (2008). Socioeconomic assessment of shrimp farming in relation to local livelihoods in the south-west coastal Bangladesh. Bangladesh J. Fish. Res. 12 (1), 109–120. Available at: https://aquadocs.org/bitstream/handle/1834/33370/BJFR12.1_109.pdf.
Rahman, M. M., and Islam, K. S. (2010). The causes of deterioration of Sundarban mangrove forest ecosystem of Bangladesh: Aquaculture, Aquarium, Conservation & Legislation. Intl J. Bioflux Soc. 3 (2), 77–90.
Rahman, M. R., and Asaduzzaman, M. (2010). Ecology of sundarban, Bangladesh. J. Sci. Found. 8 (1-2), 35–47. doi:10.3329/jsf.v8i1-2.14618
Rakib, M. R. J., Jolly, Y. N., Begum, B. A., Choudhury, T. R., Fatema, K. J., Islam, M. S., et al. (2021a). Assessment of trace element toxicity in surface water of a fish breeding river in Bangladesh: A novel approach for ecological and health risk evaluation. Toxin Rev. 41 (2), 1–17. doi:10.1080/15569543.2021.1891936
Rahman, M. S., Hossain, M. B., Rahman, M., Ahmed, A. S. S., Jolly, Y. N., Choudhury, T. R., et al. (2019). Source of metal contamination in sediment, their ecological risk, and phytoremediation ability of the studied mangrove plants in ship breaking area, Bangladesh. Mar. Pollut. Bull. 141, 137–146. doi:10.1016/j.marpolbul.2019.02.032
Rahman, M. T., Rahman, M. S., Quraishi, S. B., Ahmad, J. U., Choudhury, T. R., and Mottaleb, M. A. (2011). Distribution of heavy metals in water and sediments in passur river, sundarban mangrove forest, Bangladesh. Int. J. Environ. Sci. 6 (4), 537–546.
Rakib, M. R. J., Hossain, M. B., Jolly, Y. N., Akther, S., and Islam, S. (2021b). EDXRF detection of trace elements in salt marsh sediment of Bangladesh and probabilistic ecological risk assessment. Soil Sediment. Contam. 31 (2), 1–20. doi:10.1080/15320383.2021.1923644
Rakib, M. J. R., Jolly, Y. N., Enyoh, C. E., Khandaker, M. U., Hossain, M. B., Akther, S., et al. (2021c). Levels and health risk assessment of heavy metals in dried fish consumed in Bangladesh. Sci. Rep. 11 (1), 14642. doi:10.1038/s41598-021-93989-w
Rakib, M. J. R., Jolly, Y. N., Dioses-Salinas, D. C., Pizarro-Ortega, C. I., De-la-Torre, G. E., Bradley, D. A., et al. (2021d). Macroalgae in biomonitoring of metal pollution in the Bay of Bengal coastal waters of Cox’s Bazar and surrounding areas. Sci. Rep. 11 (1), 20999–21013. doi:10.1038/s41598-021-99750-7
Rakib, M., Jahan, R., Hossain, M. B., Kumar, R., Ullah, M., Al Nahian, S., et al. (2022). Spatial distribution and risk assessments due to the microplastics pollution in sediments of Karnaphuli River Estuary, Bangladesh. Sci. Rep. 12 (1), 8581–8615. doi:10.1038/s41598-022-12296-0
Rashed-Un-Nabi, M., Al-Mamun, M. A., Ullah, M. H., and Mustafa, M. G. (2011). Temporal and spatial distribution of fish and shrimp assemblage in the Bakkhali river estuary of Bangladesh in relation to some water quality parameters. Mar. Biol. Res. 7 (5), 436–452. doi:10.1080/17451000.2010.527988
Rashid, M. (1999). Study on water quality and commercial ichthyo fauna of the Bakkhali River Estuary. MS Thesis. Bangladesh: University of Chittagong.
Rocky, M. M. I. (2014). Phytoplankton species assemblages of the Rezu khal Estuary at Cox’s Bazar coast and their relationship to hydrological factors. MS Thesis. Bangladesh: University of Chittagong.
Saeedullah, M. (2003). Seasonal distribution of phytoplankton in the Meghna river estuary of Bangladesh with notes on biodiversity. Doctoral dissertation, M. Sc thesis. Institute of Marine Sciences and Fisheries, University of Chittagong.
Saha, N., Ullah, M. R., Islam, M. S., and Hossain, M. B. (2019). Morphometric relationships between length-weight and length-length and condition factor of four small indigenous fishes from the Payra River, southern Bangladesh. Arch. Agri. Environ. Sci. 4 (2), 230–234. doi:10.26832/24566632.2019.0402016
Saha, S. K. (2017). Socio-economic and environmental impacts of shrimp farming in the south-western coastal region of Bangladesh. Int J Res Land Use Sustain. 3, 128–137.
Sandilyan, S., Thiyagesan, K., Nagarajan, R., and Vencatesan, J. (2010). Salinity rise in Indian mangroves-a looming danger for coastal biodiversity. Curr. Sci. 98 (6), 754–756.
Shamsuzzaman, M. M., Barman, P. P., Hasan, A., and Rashed-Un-Nabi, M. (2016). Fish assemblage patterns: Temporal distribution structure and influence of environmental variables in the Karnafully River Estuary, Bangladesh. Int. J. Mar. Sci. 6. doi:10.5376/ijms.2016.06.0012
Sharif, A. S. M. (2002). A comparative study on plankton and benthos of the Meghna River-estuary during monsoon and post monsoon. Doctoral dissertation, MS Thesis. Bangladesh: Institute of Marine Sciences, University of Chittagong.
Sharif, A. S. M., and Islam, M. S. (2017). A preliminary taxonomic checklist of phytoplankton in the Lower Meghna River-Estuary. Discovery 53 (254), 117–132.
Sharif, A. S. M., Islam, S., and Islam, M. (2017). Occurrence and distribution of macrobenthos in relation to physico-chemical parameters in the lower Meghna River estuary, Bangladesh. Int. J. Mar. S. C. 7 (12), 102–113.
Siddique, M. A. M., Rahman, M., Rahman, S. M. A., Hassan, M. R., Fardous, Z., Chowdhury, M. A. Z., et al. (2021). Assessment of heavy metal contamination in the surficial sediments from the lower Meghna River estuary, Noakhali coast, Bangladesh. Int. J. Sediment Res. 36 (3), 384–391. doi:10.1016/j.ijsrc.2020.10.010
Sivadas, S. K., and Carvalho, R. (2020). Marine Annelida of India: Taxonomy and status evaluation and an updated checklist. J. Threat. Taxa 12, 16647–16714. doi:10.11609/jott.5357.12.12.16647-16714
Sukumaran, S., Vijapure, T., Mulik, J., and Ridha, H. (2021). Marine macrobenthos of NorthWest India-Reviewing the known and unknown. Front. Mar. Sci. 954, 671245. doi:10.3389/fmars.2021.671245
Talukder, M. A. U. (2004). Study on macrobenthic algae in the intertidal mangrove area of fauzdharhat coast, Chittagong. MSc thesis. Chittagong: Institute of Marine Sciences and Fisheries, University of Chittagong.
Thangaradjou, T., Nobi, E. P., Dilipan, E., Sivakumar, K., and Susila, S. (2010). Heavy metal enrichment in seagrasses of Andaman Islands and its implication to the health of the coastal ecosystem. Indian J. Mar. Sci. 39 (1), 85–91.
Varma, K. K., Cherian, C. J., Mrithunjayan, P. S., Raman, N. N., and Joseph, P. (2002). Characteristics of temperature and salinity fluctuations in a South Indian estuary. Earth Syst. Monit. 12 (4), 9–14.
Vengadesh, P. N., Rajkumar, M., Perumal, P., and Rajasekar, K. T. (2009). Seasonal variations of plankton diversity in the Kaduviyar estuary, Nagapattinam, southeast coast of India. J. Environ. Biol. 30 (6), 1035–1046.
Wang, Y. B., Liu, C. W., Liao, P. Y., and Lee, J. J. (2014). Spatial pattern assessment of river water quality: Implications of reducing the number of monitoring stations and chemical parameters. Environ. Monit. Assess. 186, 1781–1792. doi:10.1007/s10661-013-3492-9
Yeasmin, S., Latifa, G. A., and Chowdhury, G. W. (2017). Diversity of ichthyofauna of feni and muhuri rivers, feni, Bangladesh. Bangladesh J. Zool. 45 (1), 47–60. doi:10.3329/bjz.v45i1.34194
Zafar, M. K., Wouters, K., Belaluzzaman, K. M., and Islam, I. (1999). Occurrence, abundance and spawning of lingua anatina in the intertidal muddy beach of Bakkhali river estuary, Bangladesh. Pak. J. Mar. Bio 5, 41–47.
Keywords: estuary, biodiversity, ecology, water dynamics, soil texture, benthos, fish, mangroves
Citation: Rakib MRJ, Hossain MB, Islam MS, Hossain I, Rahman MM, Kumar R and Sharma P (2022) Ecohydrological features and biodiversity status of estuaries in Bengal delta, Bangladesh: A comprehensive review. Front. Environ. Sci. 10:990099. doi: 10.3389/fenvs.2022.990099
Received: 09 July 2022; Accepted: 17 October 2022;
Published: 16 November 2022.
Edited by:
Oladele Ogunseitan, University of California, Irvine, United StatesReviewed by:
Vineetha G, Central Marine Fisheries Research Institute (ICAR), IndiaRamanathan Alagappan, Jawaharlal Nehru University, India
Copyright © 2022 Rakib, Hossain, Islam, Hossain, Rahman, Kumar and Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Md. Refat Jahan Rakib, cmlmYXRqYWhhbnJha2liQGdtYWlsLmNvbQ==; Mohammad Belal Hossain, bWJobnN0dUBnbWFpbC5jb20=
 Md. Refat Jahan Rakib1*
Md. Refat Jahan Rakib1* Mohammad Belal Hossain
Mohammad Belal Hossain Mohammad Shahanul Islam
Mohammad Shahanul Islam Md. Mostafizur Rahman
Md. Mostafizur Rahman Rakesh Kumar
Rakesh Kumar Prabhakar Sharma
Prabhakar Sharma