- 1Key Laboratory of National Health and Family Planning Commission on Parasitic Disease Control and Prevention, Jiangsu Provincial Key Laboratory on Parasite and Vector Control Technology, Jiangsu Institute of Parasitic Diseases, Wuxi, JS, China
- 2Wujing Disease Control and Prevention Center, Changzhou, JS, China
- 3Jiangning Disease Control and Prevention Center, Nanjing, JS, China
- 4NTD Office Pemba, Ministry of Health of Zanzibar, Zanzibar, Tanzania
- 5School of Public Health, Nanjing Medical University, Nanjing, JS, China
Background: Bulinus globosus snail was the intermediate host of schistosome hematobium and hard to be found during the dry season. This study aimed to understand the vertical distribution of B. globosus in desiccated and re-hydrated soils and provide evidence on whether snails can drill into the soil for summer.
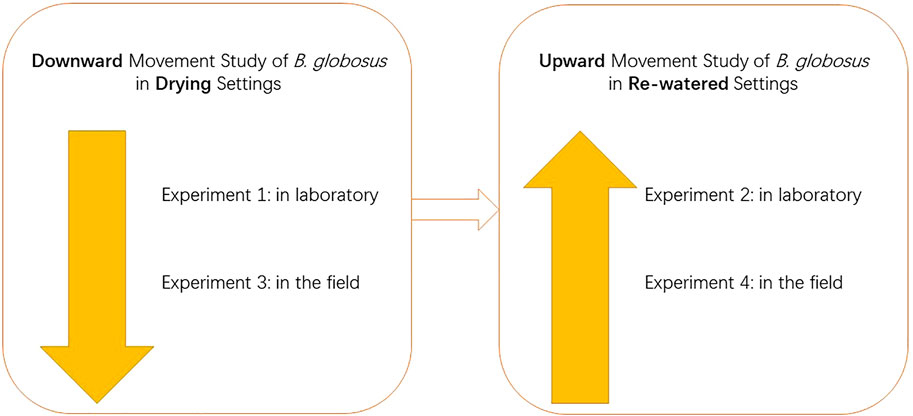
Methods: Four laboratory/field experiments were designed to study the downward movement of the snails in desiccated soils or upward movement in re-watered soils. In Experiment 1, aquaria containing snails on the soil surface were placed in an outdoor environment to desiccate naturally. Then, snails were retrieved from different soil layers. In Experiment 2, snails on the soil surface were covered with an extra 5 cm layer of soil and 4 cm layer of water. The snail positions and survival rates were checked on the first, third, fifth, seventh, and ninth day. In Experiment 3, a seasonal ditch was selected in the field. After the soil in the ditch was naturally desiccated, 1cm, 1–3cm, 3–5cm, and 5–10 cm depth of soil layers were screened to retrieve snails. In Experiment 4, after the above ditch was naturally re-watered, snails were checked in the surface water once a week for 5 weeks.
Results: At the end of Experiment 1, all the snails were only found at the soil surface. At the end of Experiment 2, snails were found neither within nor on top of the 4 cm layer of extra mud. At the end of Experiment 3, 96.92% of snails remained on the soil surface. Next, 2.77% of living snails were found in the mud crack within 1–5 cm depth. In Experiment 4, after the experimental field was naturally re-watered, no snails were found above the soil surface.
Conclusion: B. globosus snails do not initially move downward into the soil during the dry season or upward to the soil surface after being re-watered.
Introduction
Schistosomiasis is one of the tropical infectious diseases related to poverty, which seriously harms human health and socio-economic development. Until recently, the prevalence of schistosomiasis remains at a high level in many places in sub-Saharan Africa, which brings a large disease burden to the local community (Ren and Liang, 2015; GBD and HALE Collaborators, 2017). Zanzibar, including Unguja Island and Pemba Island, is located off the coast of Tanzania (Olsen et al., 2015). It is a heavily endemic area of schistosomiasis haematobium with a prevalence of 2.7–5.5% in adults and 4.3–8.9% in children (Knopp et al., 2013). Since 1986, a mass drug administration (MDA) focused intervention strategy has been implemented to reduce the incidence of schistosomiasis. However, a project study from 2012 to 2014 showed that the prevalence remained the same or even higher 2 years after all interventions were done in some communities in Zanzibar, with prevalence as high as 20% in schoolchildren, which suggested a high transmission or re-transmission level (Knopp et al., 2013; Pennance et al., 2016). Next, despite the convenient etiological treatment of praziquantel, the thorough block of schistosomiasis transmission could not be easily achieved by MDA strategy alone due to the Bulinus snails, the widely existing intermediate hosts, which would lead to frequent re-infection (Colley et al., 2014; Knopp et al., 2019a; He et al., 2019). A snail infected by a single miracidium could generate hundreds and thousands of cercariae and release them into the water to infect people through contact (Allan et al., 2020). Therefore, snail control could be the key to blocking schistosomiasis transmission (Liang et al., 2012; King et al., 2015; Sokolow et al., 2016; Li et al., 2019). In a 5-year intervention study, focal mollusciciding, acting as part of the combined interventions, helped reduce the schistosomiasis prevalence in the study group in Zanzibar, but the transmission was not interrupted (Knopp et al., 2019b).
In planning schistosomiasis control, understanding the interaction between the environment and the ecological features of local intermediate host snails is vital. In Zanzibar, the major intermediate host of schistosome haematobium is Bulinus globosus (Stothard et al., 2000). B. globosus is a water-borne snail. Rivers, streams, and seasonal ponds are typical habitats of Bulinus snails (Darby et al., 2008; Whitehead et al., 2009). Their reproduction could be done through either self-copulation or external mating, depending on the environmental conditions (Jarne et al., 1992). The water level change or drought due to the seasonal cycle, climate, or hydrological variations could change the distribution and abundance of Bulinus snails (Kalinda et al., 2018a). In Zanzibar, fully understanding the behavior of B. globosus in the dry season (June–October) and the rainy season (November–May) is essential to design a targeted snail control strategy. The density of B. globosus was found to be at the lowest level during the dry season in Zanzibar while at a much higher level during the rainy season in a snail survey (Marti, 1986). One possible reason could be the increased fecundity and hatching rate of snails in water bodies like streams at the end of the dry season (Marti, 1986). Another assumed reason was that B. globosus could drill into the soil, survive the whole dry season, and burrow out of the soil when the rainy season started. The former suggested a perfect molluscicidal time window at the end of the dry season, but the latter could make all molluscicide spay on the soil surface ineffective. However, in what way the vertical movement and distribution of Bulinus snails are affected by desiccation and rain is still not clear. Betterton et al. found, in a field observation in Nigeria, that B. globosus could be found buried at the bottom of its dried habitats at depths up to 3 cm (Betterton et al., 1988). Researchers also reported that B. globosus and B. truncates could burrow in soil but only in very soft soil and within the depth of 2 cm (Chu et al., 1967a; Kalinda et al., 2018b). Researchers claimed that B. truncates could not only burrow into the soil but also burrow upward to the soil surface after re-watering (Chu et al., 1967B). However, McCullough’s study led to a contradictory conclusion that no evidence showed Bulinus snails were able to burrow in the mud (McCullough, 1962). The mud-burrowing behaviors in dry and rainy seasons directly decide the vertical distribution of the snail and will make an impact on the effects of snail control activities like molluscicide spray. Therefore, we aimed to study the vertical distribution of B. globosus, by evaluating their downward movement in desiccating soils and upward movement in re-hydrated soils in both laboratory and field environments, to provide evidence for precise and effective vector control in schistosomiasis projects in Africa.
Materials and methods
This study was composed of four experiments. Experiments 1 and 3 were designed to study the downward mud-burrowing behavior of B. globosus in drying soils, in the laboratory and field settings, respectively. Experiments 2 and 4 were designed to research the upward movement of B. globosus in re-watered soils, respectively, in the laboratory and field settings (Figure 1).
Experiment preparation and data process
The snails used in the experiments were the laboratory-bred first filial generation of B. globosus collected from Kiwani Shehia in Kusini Pemba (5°21′15.96″ South, 39°44′2.55″ East). Adult snails 7–10 mm in length were grouped as small-sized snails; snails over 10 mm in length were grouped as large-sized snails (Marti, 1986; Kalinda et al., 2018a). Next, snails were placed in water with good aeration at 25°C after being thoroughly washed and were observed dead or alive for 24 h. Those with soft body reactions to mechanical irritation of needles were recorded as “alive.” If the reactions were not evident, microscopic identification would be used after gently crushing the snail. If the snail’s soft body showed no reaction, it would be recorded as “dead.”
Mucky soils and sandy soils were collected from natural breeding ponds of B. globosus in South Pemba (5°21′15.96″ South, 39°44′2.55″ East) and North Pemba (5°11′20.47″ South, 39°48′43.21″ East), respectively. The soils were dried, smashed, and filtered by stainless steel mesh with an aperture diameter of 7 mm, to remove snails and other objects, and then well stirred.
Prepared soils were well mixed with de-chlorinated water in Polypropylene (PP) cubical aquaria (0.490 m × 0.348 m × 0.292 m). To prevent snails from climbing out, aquaria were covered with gauzes. Then, the aquaria were placed in an outdoor environment sheltered from direct sunlight and rain for 24 h until the soil was saturated with water. The depth of soil was adjusted to 20 cm for snails to burrow.
Soil surface temperature and humidity were monitored using Wangyunshan WTHOT1-N-0.5: N6 temperature-humidity recorder (Wangyunshan, Fuzhou); Soil volume moisture content was measured using TR-80 soil recorder (Shunkeda, Beijing); Soil Ph value was measured using a Z-06 soil acidity meter (Taizhou, Jiangsu).
Experiment 1: Laboratory experiment on the downward movement of B. globosus in the drying environment
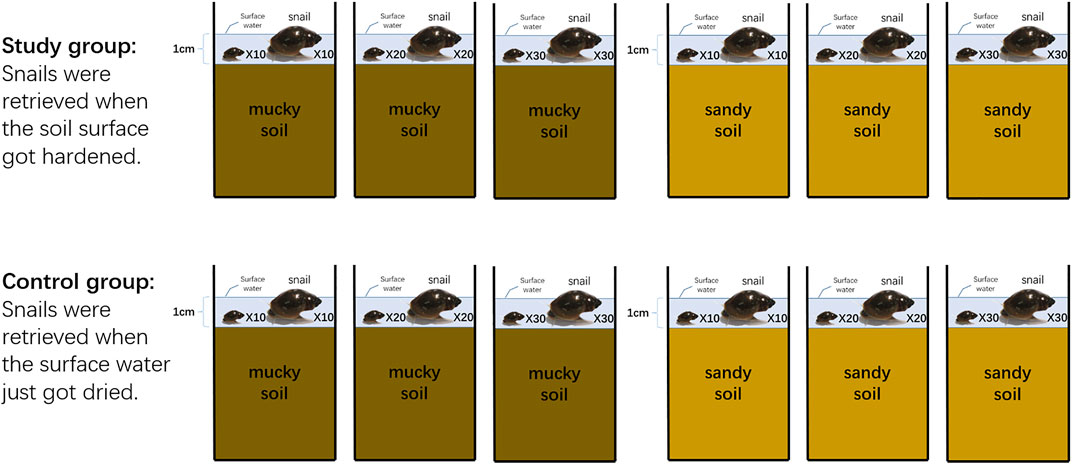
This experiment targeted to observe whether B. globosus with different sizes and distribution densities would initially move downward into the soil from the surface in different types of desiccating soils (Figure 2).
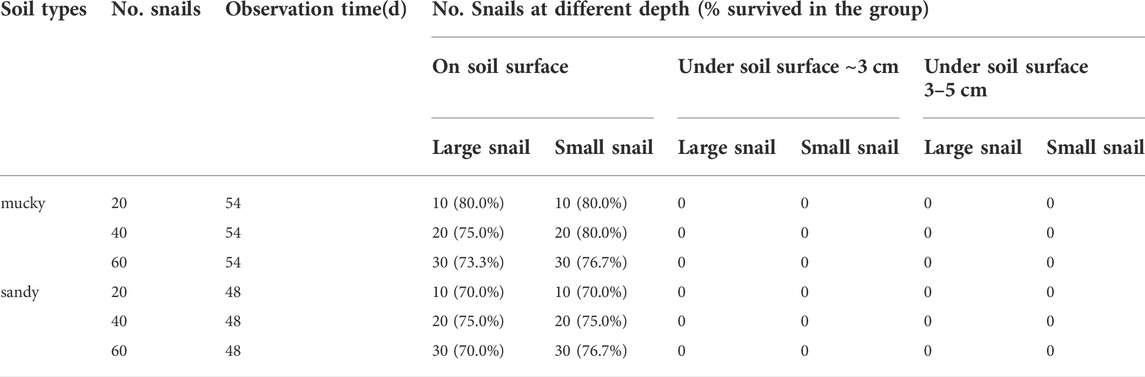
Snails were randomly designated into two groups: mucky soil group and sandy soil group. Each group contained six aquaria with correspondent soil and was further divided into the study and control groups. The surface water was adjusted to 1 cm in depth above the soil. In each group, 10, 20, and 30 respective large and small snails were put into each aquarium (this day was defined as Day 0). Next, the aquaria were placed in an outdoor environment sheltered from direct sunlight and rain and allowed to naturally desiccate. The survival of the snails in control groups was retrieved and checked when the surface water had just disappeared. The survival of the snails in the study group was not retrieved nor checked until the soil surface hardened, which meant the humidity recorder probe was no longer able to be inserted into the soil.
The surface water drying period, soil hardening period, continuous soil pH value, temperature and moisture, and soil volume moisture content were recorded. At the endpoint of each group, we dug and washed the soils to retrieve snails by layers, using spatula and forceps carefully to prevent shell damage. Snail quantity, survival status, and depth in the soil were recorded. The snails retrieved from the soil surface which could be seen by eye before digging were recorded as “on soil surface.” The other snails were recorded as “under soil surface” (∼3 cm from the surface) or (3∼5 cm from the surface) according to their depths in the soil.
Experiment 2: Laboratory experiment on the upward movement of B. globosus snails in the re-watered environment
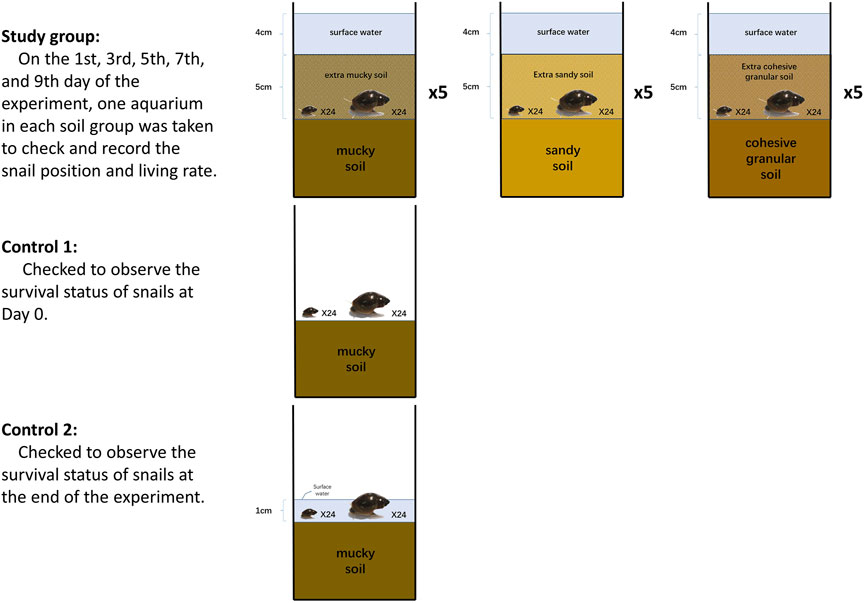
This experiment aimed to observe whether B. globosus snails would initially move upward in soil/mud which was changing to a water-saturated environment from dry status (Figure 3).
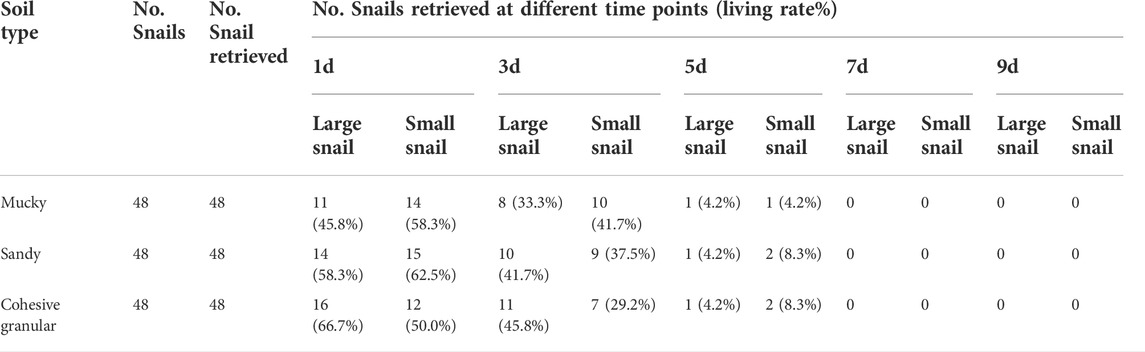
Seventeen prepared aquaria were used with surface water adjusted to 1 cm up the soil layer. Each aquarium was placed with 24 large-sized and 24 small-sized snails in the surface water. Aquaria were placed in an outdoor environment sheltered from direct sunlight and rain for 24 h until the soil was water-saturated for surface water to desiccate naturally. Next, snails were moved back to the soil surface from the edges and aquaria walls carefully every day. When the surface water was desiccated off, the aquaria were randomly categorized into the study group (15 aquaria) and the control group (2 aquaria). One aquarium in the control group was to observe the survival status of snails when the surface water simply dried away. Then, the other aquarium in the control group was covered with a 1 cm layer of water till the end of the experiment to check the survival status of its snails. The study group was categorized into three small groups (each comprising five aquaria). An extra 5 cm layer of muddy soil, sandy soil, and cohesive granular soil was covered on top of the snails, respectively (recorded as Day 0). In each aquarium, water was slowly sprayed onto the soil to reach 4 cm depth on top of the soil surface. Next, to attract snails, lettuce leaves were put in the water. On the first, third, fifth, seventh, and ninth day of the experiment, one aquarium in each group was taken to check and record the snail positions and living rates with the same method in Experiment 1. Water temperatures, soil moisture, and pH values were recorded.
Experiment 3: Field experiment on the downward movement of B. globosus snail in a desiccating stream
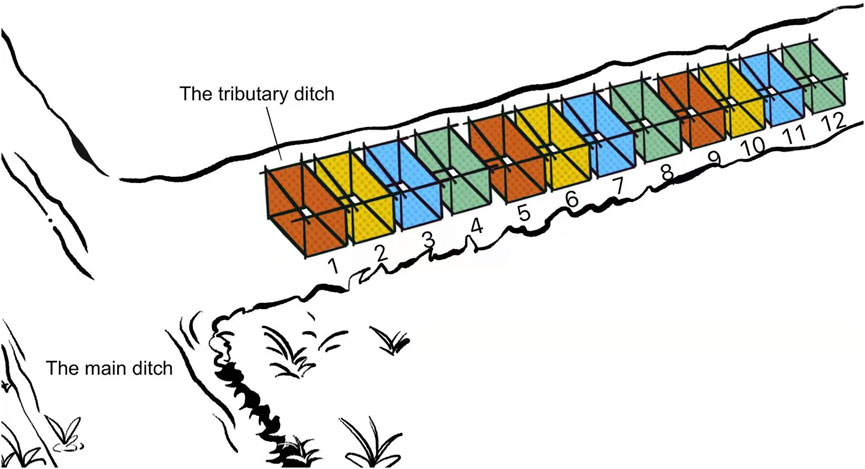
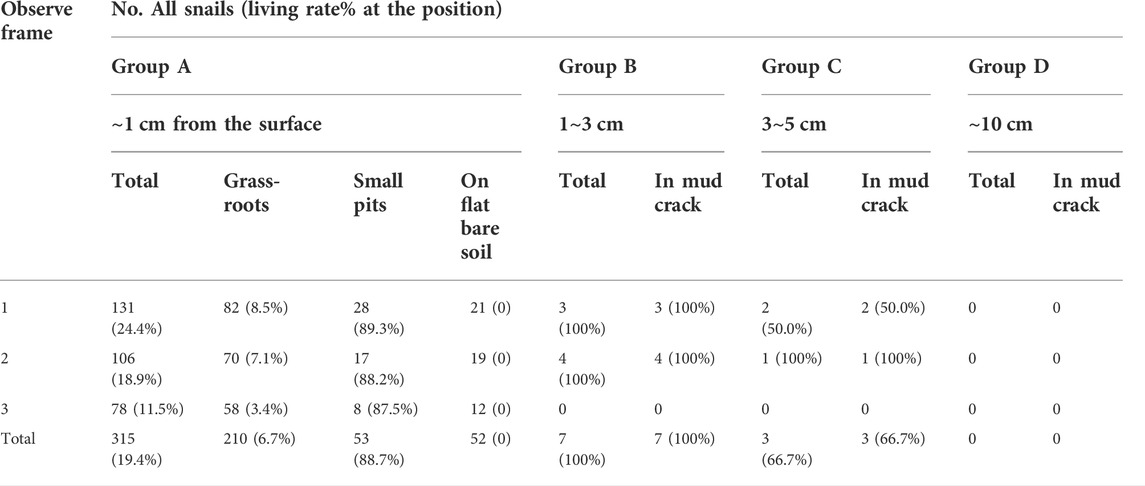
This experiment targeted to determine whether B. globosus would burrow downward in stream mud in the field environment. A ditch named Issa Moho Ussi (5°21′15.96″ South, 39°44′2.55″ East) with snail habitats and seasonal water level rise and fall, located in Kiwani of the southern province of Pemba Island was selected as the experimental site. This ditch was a branch of the main stream which received water throughout the year. The selected experimental section demonstrated a slope ratio of 0.5–1% and a width of 0.5–0.7 m. On Sept. 11th, 2018, when the ditch still received water with 5–10 cm depth, twelve 1 m × 0.5 m cuboid bottomless separation frames were set in the mud of the ditch with 0.3–0.5 m interval and were numbered sequentially from 1 to 12 (Figure 4). The separation frames were made of stainless steel mesh with apertures of 0.2 cm. The soil condition and surface humidity rate were checked weekly until it was naturally desiccated with cracks, which meant the humidity recorder could no longer insert its probe into the soil. Then, separation frames No. 1, 5, and 9 were categorized as Group A, in which the surface soil of 1 cm thick in the frame, including the grassroots and small pits, and were later dug, sieved, washed, and screened to retrieve snails. Group B (Separation frames No. 2, 6, 10), Group C (No. 3, 7, 11), and Group D (No. 4, 8, 12) screened soils with 1–3 cm, 3–5 cm, 5–10 cm depth from the ground surface to retrieve snails, respectively.
Experiment 4: Field experiment on the upward movement of B. globosus snails in a re-hydrated stream
This experiment aimed to determine whether B. globosus would burrow upward in re-hydrated stream mud in the field environment. At the end of Experiment 3, each study group was left with three separated pits surrounded by bottomless frames. The depth of the pits was 1, 3, 5, and 10 cm for each group, respectively. After the separated pits were naturally re-watered in the rainy season, snails were checked in the surface water in each separation frame in all study groups once a week for 5 weeks.
Results
Experiment 1: The highest and lowest surface temperatures of the soil (± SD) varied from 24.59 ± 1.75°C to 28.98 ± 2.36°C. The surface humidity of the soil ranged from 48 to 84%. The pH values (±SD) for mucky soil and sandy soil were 6.72 ± 0.033 and 6.77 ± 0.025, respectively. It took 14 and 13 days for aquaria with mucky soil and sandy soil to desiccate off the surface water, respectively. 100% of the snails in the control group were found to be alive at this point in time. It took 54 and 48 days for the surface of the two soils to get hardened, with average soil surface humidity reaching 47.95 and 42.79%, respectively. All the snails could be identified by eye at the soil surface in different groups despite different snail sizes and densities (Table 1). For some snails, part of their shell was buried to different extents.
Experiment 2: The lowest and highest surface temperature of the soil (± SD) was 23.99 ± 1.03°C and 27.09 ± 1.99°C, respectively. The average pH values (± SD) for mucky soil, sandy soil, and cohesive granular soil were 6.73 ± 0.04, 6.77 ± 0.02, and 6.72 ± 0.02, respectively. After being placed with snails, it took 12 days for the aquaria to naturally desiccate off the surface water. The snails in the control group were found 100% alive at this point in time and at the end of the experiment. No snails were found within the 4 cm layer of added mud during the investigation. The snail survival period is shown in Table 2. The survival period of all snails was less than 7 days. Comparing the LT50 and LT90 of large-sized snails with small-sized ones, it showed no significant differences (p = 0.92 for LT50 and p = 0.515 for LT90). The contrast of LT50 and LT90 of snails between different soils indicated no significant differences (p = 0.960).
Experiment 3: On 11 October 2018, snails were retrieved by layers when the soil in the experimental sites was desiccated with cracks, and the humidity recorder could not insert for measurement. In total, 325 B. globosus snails were retrieved, 70 (21.54%) of which survived. Also, 315 (96.92%) of all snails remained on the soil surface (retrieved from Group A). Their depths and positions are shown in Table 3. Among them, 210 (66.67%) snails were found near grass roots and other vegetation roots. Another 53 (16.83%) snails were found in small pits with teir shells exposed in the air, most of these (51 snails) were buried by a quarter. The rest of the 52 (16.51%) snails were directly exposed on top of the soil. Nine (2.77%) living snails and one (0.31%) dead snail were found in mud cracks at a depth of 1–5 cm.
Experiment 4: The rainy days in September, October, and November in 2018 were 2, 10, and 19 days, respectively. Since 15 October 2018, Pemba Island had been in a minor rainy season. On 22 October 2018, all separation frames were naturally re-watered. From October 22nd to 26 November 2018, we conducted six checks on the separation frames in total, and no snails on the soil surface nor the water above during this period.
Discussion
The study of snail burrowing behavior in desiccating and re-watered soil is of growing importance though still controversial, as molluscicide-based snail control becomes part of the schistosomiasis control strategy in sub-Sahara African areas. With a comprehensive study design combining laboratory and field experiments, our study showed that B. globosus would not initiatively burrow into the mud in desiccating soil in Zanzibar, no matter whether the soil was sandy or mucky and no matter what the size of the snail was. In re-watered soil, the snails buried in mud would not move upward to the soil surface. Next, the field study further revealed that B. globosus mainly gathered around places with abundant vegetation like grass roots, in small pits within 1 cm depth, or in mud cracks when the natural habitats desiccated in the dry season.
Our study found, in the laboratory and in the field, that all the snails after desiccation could be identified by eye at the soil surface, although some part of their shells was buried to different extents in Zanzibar. Researchers suggested that they may simply be passively buried by puddles of mud, which could probably be caused by intermittent rainfalls in the dry season. Some other studies found B. globosus 3 cm or 2 cm under the soil surface leading to the belief they would burrow in mud (Betterton et al., 1988; Kalinda et al., 2018b). The distinction could be due to the different soil types between Pemba Island and other study sites, as B. globosus could be temporarily or accidentally buried in mud if the soil is soft enough (McCullough, 1962). Also, these provided evidence for the selection of the molluscicide formulations. Niclosamide powder and granules could be applied as all snails were exposed on the soil surface in Zanzibar, particularly in the dry season when water for dissolving other formulations is rare (Xing et al., 2013).
Researchers reported that B. truncates could also burrow upward to the surface after re-watering and the maximum survival period was 11 days (Chu et al., 1967b), but our study found the buried B. globosus remained in the mud after re-watering, and the buried snails would not survive more than 7 days in mud saturated with water. It indicated that B. globosus was not capable of burrowing upward, and being buried in moist mud or sand is probably lethal to at least some species of Bulinus snails. Next, different snails influenced by various habitats may differ in their behaviors (McCullough, 1962; Chu et al., 1967c; Dagal et al., 1986), and the climate and ecology of some habitats could change due to human activities in the last decades (Rubaba et al., 2016). Therefore, further studies are required to explore relevant evidence.
As no B. globosus snails could hide under the earth during the dry season and then burrow up when the rainy season comes, this indicates an excellent choice to plan molluscicide spray at the end of the dry season in Pemba Island when the vegetation is sparse, the Bulinus snails are easy to identify, and the density of the snails would be lower compared to other times in the year (Marti et al., 1985). The mollusciciding could possibly fully cover the whole snail habitat as the water-body area reaches its lowest in the year. The cost could be relatively low, while the effect could be better compared to focal mollusciciding (Allan et al., 2020). Even for the accidentally buried B. globosus snails that were not killed by molluscicides, they may not be able to move upward, though they probably could survive for a long time in the dry mud (McCullough, 1962). Some aestivated snails could emerge after the dry season finished if they survived the desiccation and the first molluscicidal treatment. Therefore, a second molluscicidal window could be in the early beginning of the rainy season (Webbe, 1962). When heavy rain starts, it will not be easy to eliminate the spreading snails, as B. globosus could move upward and down in the water and reproduce all through the year in water (O’keeffe, 1985). The expansion of its population and habitats is mainly connected to the enlarging water body during the rainy season (Yang et al., 2005; Steinmann et al., 2006). To explore accurate molluscicidal timing, more studies on snail surviving time and aestivation are required in this area, and further field molluscicidal experiments are also needed.
Besides the timing for molluscicide spray, we also identified the key areas for snail control. In field observation, we further found that most survived B. globosus snails gathered around grassroots and other vegetation when the water body dried in their habitats during the dry season. Researchers reported that grass and other vegetation not only provided snails the food during the dry season but also played a role in their oviposition, or “breeding pocket” (Marti et al., 1985). Some surviving B. globosus snails were found in small pits and mud cracks. In Zanzibar, it may sometimes rain, even in the dry season. Next, the snails could climb into small pits or cracks by themselves and could also be passively pushed into them by rain. It is similar to the behavior of Biomphalaria glabrata and Biomphalaria straminea, the intermediate hosts of Schistosoma mansoni, which also seek protection under vegetation rather than burrow under the soil when the soil gets desiccated (Olivier, 1956; Rubaba et al., 2016). The full mollusciciding strategy at the end of desiccation could put particular emphasis on grass/vegetation, small pits, and mud cracks surrounding the residue water bodies to eliminate snails and their eggs, to hopefully at least slow down their population increase in the coming rainy season.
Our study was confined to the indoor laboratory environment, outdoor environment sheltered from direct sunlight and rain, and field environment of natural stream in Pemba Island, which did not consider all actual living environments of snail habitats. Moreover, Zanzibar was in an equatorial region where strong sunlight and rainy conditions may play an essential role in snail life, but they were not designed in the laboratory experiments (Cridland, 1967). Next, the raindrops and water flow may play a part in the passive movement of the snails (Woolhouse, 1988). Further, soil types were generally categorized in the study, but the quantified softness of the soil in the future study design may be more convincing. Multiple factors could demonstrate impacts on Bulinus snails; therefore, more in depth ecological studies are required to plan accurate snail control activities with increased effects.
Conclusion
B. globosus snails would not initially move downward into the soil during the dry season and upward to the soil surface after the soil was re-watered in both laboratory and field settings in Zanzibar. Researchers suggested that molluscicide-based snail control activities could be arranged at the end of the dry season and the early beginning of the rainy season, possibly fully covering the snail habitats with particular emphasis on the vegetation, small pits, and mud cracks.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding authors.
Author contributions
D-RH. designed and conducted the study. YF improved the study design and wrote the manuscript. D-RH and YF contributed equally to this study. KY and J-FZ conceptualized the study, provided resources, and improved the manuscript. Y-HW and D-RH analyzed the data. Y-RW, BZ, SJ, and MS assisted with the study conduction. All authors read and agreed to the final manuscript.
Funding
This research was funded by grants from Jiangsu Provincial Department of Science and Technology, grant number BZ2020003; Public Health Research Center, Jiangnan University, grant number JUPH201831.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Allan, F., Ame, S. M., Tian-Bi, Y. T., Hofkin, B. V., Webster, B. L., Diakite, N. R., et al. (2020). Snail-related contributions from the schistosomiasis consortium for operational research and evaluation program including xenomonitoring, focal mollusciciding, biological control and modelling. Am. J. Trop. Hyg. 00 (0), 1–14. doi:10.4269/ajtmh.19-0831
Betterton, C., Ndifon, G. T., and Tan, R. M. (1988). Schistosomiasis in kano state, Nigeria. Ann. Trop. Med. Parasitol. 82 (6), 571–579. doi:10.1080/00034983.1988.11812293
Chu, K. Y., Arfaa, F., and Massoud, J. (1967b). The survival of Bulinus truncates buried in mud under experimental outdoor conditions. Ann. Trop. Med. Parasitol. 61 (1), 6–10. doi:10.1080/00034983.1967.11686449
Chu, K. Y., Bijan, H., and Massoud, J. (1967a). The ability of Bulinus truncatus, Biomphalaria alexandrina and Lymnaea gedrosiana to survive out of water in the laboratory. Ann. Trop. Med. Parasitol. 61 (1), 1–5. doi:10.1080/00034983.1967.11686448
Chu, K. Y., Massoud, J., and Bijan, H. (1967c). The location of Bulinus truncates in dry habitats in Iran. Ann. Trop. Med. Parasitol. 61 (2), 134–138. doi:10.1080/00034983.1967.11686470
Colley, D. G., Bustinduy, A. L., Secor, W. E., and King, C. H. (2014). Human schistosomiasis. Lancet 383, 2253–2264. doi:10.1016/s0140-6736(13)61949-2
Cridland, C. C. (1967). Resistance of Bulinus (physopsis) globosus, Bulinus (Ph.) africanus, Biomphalaria pfeifferi and Lymnaea natalensis to experimental desiccation. Bull. World Health Organ. 36 (3), 507–513.
Dagal, M. A., Upatham, E. S., Kruatachue, M., and Viyant, V. (1986). Effects of some Physico-chemical factors on the hatching of egg masses and on the survival of juvenile and adult snails of Bulinus(Physopsis) abyssinicus. J. Sci. Soc. Thail. 12, 23–30. doi:10.1017/S0022149X00023300
Darby, P. C., Bennetts, R. E., and Percival, H. F. (2008). Dry down impacts on apple snail (Pomacea paludosa) demography: Implications for wetland water management. Wetlands 28 (1), 204–214. doi:10.1672/07-115.1
GBDHALE Collaborators (2017). Global, regional, and national disability-adjusted life-years (DALYs) for 333 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2016: A systematic analysis for the global burden of disease study 2016. Lancet 390, 1260–1344. doi:10.1016/S0140-6736(17)32130-X
He, M. Z., Li, W., Juma, S., Kabole, F., Xu, D. C., Wang, X. Y., et al. (2019). A Google Earth-based database management for schistosomiasis control in Zanzibar. Geospat. Health 14, 740. doi:10.4081/gh.2019.740
Jarne, P., Delay, B., Bellec, C., Roizes, G., and Cuny, G. (1992). Analysis of mating systems in the schistosome-vector hermaphrodite snail Bulinus globosus by DNA fingerprinting. Hered. (Edinb). 68 (2), 141–146. doi:10.1038/hdy.1992.22
Kalinda, C., Chimbari, M. J., Grant, W. E., Wang, H.-H., Odhiambo, J. N., Mukaratirwa, S., et al. (2018b). Simulation of population dynamics of Bulinus globosus: Effects of environmental temperature on production of Schistosoma haematobium cercariae. PLoS Negl. Trop. Dis. 12 (8), e0006651. doi:10.1371/journal.pntd.0006651
Kalinda, C., Chimbari, M. J., Malatji, M. P., and Mukaratirwa, S. (2018a). Influence of desiccation on the survival of Bulinus globosus under laboratory conditions. J. Freshw. Ecol. 33 (1), 461–473. doi:10.1080/02705060.2018.1520157
King, C. H., Sutherland, L. J., and Bertsch, D. (2015). Systematic review and meta-analysis of the impact of chemical-based mollusciciding for control of schistosoma mansoni and S. Haematobium transmission. PLoS Negl. Trop. Dis. 12, e0004290. doi:10.1371/journal.pntd.0004290
Knopp, S., Ame, S. M., Person, B., Hattendorf, J., Rabone, M., Juma, S., et al. (2019b). A 5-Year intervention study on elimination of urogenital schistosomiasis in Zanzibar: Parasitological results of annual cross-sectional surveys. PLoS Negl. Trop. Dis. 13 (5), e0007268. doi:10.1371/journal.pntd.0007268
Knopp, S., Person, B., Ame, S. M., Ali, S. M., Hattendorf, J., Juma, S., et al. (2019a). Evaluation of integrated interventions layered on mass drug administration for urogenital schistosomiasis elimination: A cluster-randomised trial. Lancet Glob. Health 7 (8), e1118–e1129. doi:10.1016/s2214-109x(19)30189-5
Knopp, S., Person, B., Ame, S. M., Mohammed, K. A., Ali, S. M., Khamis, I. S., et al. (2013). Elimination of schistosomiasis transmission in zanzibar: Baseline findings before the onset of a randomized intervention trial. PLoS Negl. Trop. Dis. 7, e2474. doi:10.1371/journal.pntd.0002474
Li, W., Zhang, J. F., Wu, F., Xiong, C. R., Yao, Y. Y., Zhao, S., et al. (2019). Progress of interruption of schistosomiasis transmission in Jiangsu Province. Chin. J. Schistosomiasis Control 31 (6), 583–590. doi:10.16250/j.32.1374.2019184
Liang, Y. S., Huang, Y. X., Hong, Q. B., Yang, K., Sun, L. P., Dai, J. R., et al. (2012). Novel strategies and technologies to achieve the transmission control of schistosomiasis in Jiangsu Province. Chin. J. Schistosomiasis Control 24 (2), 119–122.
Marti, H. (1986). Field observations on the population dynamics of Bulinus globosus, the intermediate host of schistosoma haematobium in the ifakara area, Tanzania. J. Parasitol. 72 (1), 119. doi:10.2307/3281803
Marti, H. P., Tanner, M., Degremont, A. A., and Freyvogel, T. A. (1985). Studies on the ecology of Bulinus globosus, the intermediate host of schistosoma haematobium in the Ifakara area, Tanzania. Acta Trop. 42 (2), 171–187.
McCullough, F. S. (1962). Further observations on Bulinus (Bulinus) truncatus rohlfsi (clessin) in Ghana seasonal population fluctuations and biology. Bull. World Health Organ. 27, 161–170.
O’keeffe, J. (1985). Population biology of the freshwater snail Bulinus globosus on the Kenya coast. I. Population fluctuations in relation to climate. J. Appl. Ecol. 22 (1), 73. doi:10.2307/2403328
Olivier, L. (1956). The location of the schistosome vectors, Australorbis glabratus and Tropicorbis centimetralis, on and in the soil on dry natural habitats. J. Parasitol. 42, 81. doi:10.2307/3274628
Olsen, A., Kinung’hi, S., and Kinung'hi, S. (2015). Schistosoma mansoni infection along the coast of lake victoria in mwanza region, Tanzania. Am. J. Trop. Med. Hyg. 92, 1240–1244. doi:10.4269/ajtmh.14-0676
Pennance, T., Person, B., Muhsin, M. A., Khamis, A. N., Muhsin, J., Khamis, I. S., et al. (2016). Urogenital schistosomiasis transmission on Unguja Island, Zanzibar, characterisation of persistent hot-spots. Parasit. Vectors 9, 646. doi:10.1186/s13071-016-1847-0
Ren, G. H., and Liang, Y. S. (2015). African schistosomiasis. Beijing: People's Medical Publishing House, 1–7.
Rubaba, O., Chimbari, M. J., and Mukaratirwa, S. (2016). The role of snail aestivation in transmission of schistosomiasis in changing climatic conditions. Afr. J. Aquat. Sci. 41 (2), 143–150. doi:10.2989/16085914.2016.1145103
Sokolow, S. H., Wood, C. L., Jones, I. J., Swartz, S. J., Lopez, M., and Hsieh, M. H. (2016). Global assessment of schistosomiasis control over the past century shows targeting the snail intermediate host works best. PLoS Negl. Trop. Dis. 10 (7), e0004794. doi:10.1371/journal.pntd.0004794
Steinmann, P., Keiser, J., Bos, R., Tanner, M., and Utzinger, J. (2006). Schistosomiasis and water resources development: Systematic review, meta-analysis, and estimates of people at risk. Lancet Infect. Dis. 6 (7), 411–425. doi:10.1016/s1473-3099(06)70521-7
Stothard, J. R., Loxton, N., Rollinson, D., Mgeni, A. F., Khamis, S., Ameri, H., et al. (2000). The transmission status of Bulinus on Zanzibar Island (Unguja), with implications for control of urinary schistosomiasis. Ann. Trop. Med. Parasitol. 94 (1), 87–94. doi:10.1080/00034980057653
Webbe, G. (1962). The transmission of schistosoma haematobium in an area of lake province, Tanganyika. Bull. World Health Organ. 27, 59–85.
Whitehead, P., Wilby, R., Battarbee, R., Kernan, M., and Wade, A. J. (2009). A review of the potential impacts of climate change on surface water quality. Hydrological Sci. J. 54 (1), 101–123. doi:10.1623/hysj.54.1.101
Woolhouse, M. E. J. (1988). Passive dispersal of Bulinus globosus. Ann. Trop. Med. Parasitol. 82 (3), 315–317. doi:10.1080/00034983.1988.11812250
Xing, Y. T., Dai, J. R., Dai, Y., Yang, Z. K., Wang, F., Liang, X. T., et al. (2013). Preparation and molluscicidal effect of 5% niclosamide ethanolamine granules. Chin. J. Schisto Control 25 (5), 473–476.
Keywords: Bulinus globosus, snail, vertical distribution, dry season, desiccation, re-hydration
Citation: Hang D-R, Feng Y, Zhang J-F, Wang Y-H, Zhang B, Juma S, Sleiman MM and Yang K (2022) Studies on the ecology of Bulinus globosus snails: Evidence against burrowing into the soil during the dry season. Front. Environ. Sci. 10:925065. doi: 10.3389/fenvs.2022.925065
Received: 10 May 2022; Accepted: 30 June 2022;
Published: 10 August 2022.
Edited by:
Jaan H. Pu, University of Bradford, United KingdomReviewed by:
Amir Ghaderi, Urmia University, IranSudipta Rakshit, Tennessee State University, United States
Prashanth Reddy Hanmaiahgari, Indian Institute of Technology Kharagpur, India
Copyright © 2022 Hang, Feng, Zhang, Wang, Zhang, Juma, Sleiman and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kun Yang, eWFuZ2t1bkBqaXBkLmNvbQ==; Jian-Feng Zhang, emhhbmdqaWFuZmVuZ0BqaXBkLmNvbQ==
†These authors share first authorship
 De-Rong Hang
De-Rong Hang Yun Feng
Yun Feng Jian-Feng Zhang1*
Jian-Feng Zhang1* Kun Yang
Kun Yang