- 1Fish Nutrition Research Laboratory, Department of Zoology, University of Kashmir, Srinagar, India
- 2Department of Veterinary Sciences, Polo Universitario Annunziata, University of Messina, Messina, Italy
Environmental pollution caused due to the presence of heavy metals has become a great concern as it has an adverse effect on almost all types of ecosystem. In this sense, these pollutants have a tendency to pollute the aquatic ecosystem, thus badly affecting the health of aquatic organisms. As a result, toxicological studies believe them to be the most harmful pollutants in the aquatic environment. Among all the aquatic organisms, fish—being a chief organism in this system—become the easiest victim of these pollutants. Heavy metals enter fish bodies through the alimentary system by consumption of polluted food, or through the gills, and skin. They are finally delivered by the bloodstream to the organs and tissues where they accumulate after absorption. Ultimately, in this way heavy metals make their way into humans through the food chain. The fluctuations in the hematological values may serve as an initial indicator of the toxicant’s impact on fish health. It has been observed that when pollutants impact the quality of the aquatic medium, the first consequence is apparent in the form of physiological changes in fish, which are reflected in one or more hematological parameters, such as hemoglobin, hematocrit, red blood cell count, white blood cell count, etc. As a result of these alterations, fish become weak, anemic, and more susceptible to diseases. Over the past several decades, a vast number of studies have been reported on the qualitative and quantitative variations in hematological parameters due to the presence of heavy metal intoxication. Heavy metal contamination of water resources not only degrades the water quality but also negatively impacts the quality of food in the form of fish proteins. Therefore, this article sheds light on the effects of heavy metals on hemoglobin and hematocrit of fish hematology and calls for more attention to the protection and preservation of aquatic ecosystems, particularly those contaminated with heavy metals.
1 Introduction
Aquaculture is a growing part of the world’s agricultural economy in general, and of India’s agricultural economy in particular, where fisheries are now considered a fast-growing sector with significant medicinal, nutritional, economic, aesthetic, industrial, and religious values, as well as offering employment to a large number of people in the country. Fish are among the most significant vertebrate groups, supplying a valuable supplement in the form of diversified, nutritious diets to the populace. The essentiality of fish in the form of a nutritious diet due to the presence of healthy protein, fats, vitamins, and minerals is well understood. It also has a good source of essential fatty acids, including long-chain omega-3, and other micronutrients important to support healthy life (Skonberg and Perkins, 2002; Nurullah et al., 2003; Deckelbaum and Torrejon, 2012; Thilsted et al., 2016; Hicks et al., 2019; Mohanty et al., 2019; Tacon, 2020). About 20 percent of animal proteins are provided to the world’s population by fish, and over 3.3 billion people depend on fish as their main source of animal protein (FAO, 2020). Sustainable, productive fisheries and aquaculture enhance revenue and alleviate poverty, boost the economy, and safeguard the environment and natural resources, ensuring food and nutrition security. As a result, productivity improvement of fisheries and aquaculture in developing countries is crucial to reducing food insecurity. However, due to their delicacy, fish are very sensitive to any environmental impacts triggered by various factors such as physical, chemical, and biological that can immediately affect the ecology and survival of species.
Fish live in close proximity to their surroundings and are thus vulnerable to chemical and physical variations, which could be mirrored in their blood and its components (LeaMaster et al., 1990; Luskova, 1997; Singh et al., 2008; Sheikh and Ahmed, 2016; Witeska, 2021). Abiotic and biotic factors such as parasitic invasions, predator pressure, or strong competition, variations in temperature, dissolved oxygen content, pH, aquatic pollutants namely insecticides, pesticides, and heavy metals, as well as human activities related to fish rearing and harvesting, such as manipulation, transport, and crowding, can all cause stress in fish (Meier et al., 1983; Witeska, 2005; Dekar and Magoulick, 2013; Gebrekiros, 2016; Mateo-Sagasta et al., 2017; Teunen et al., 2021; Seibel et al., 2021). The analysis of hematological indices is an important tool that provides reliable information for monitoring and assessing the alterations in fish physiology and pathology under adverse conditions (Svetina et al., 2002; Pavlidis et al., 2007; Prado et al., 2014; Witeska, 2021). In toxicological investigations and environmental monitoring, fish blood is increasingly being used as a potential indicator of physio-pathological changes in fish populations and disease investigations (Sancho et al., 2000; Barcellos et al., 2003; Fazio, 2019; Lawrence et al., 2020; Witeska, 2021). Hematological parameters can give a reliable evaluation in non-lethal ways, which is significant because there are many ethical concerns about using animals in investigations (Satheeshkumar et al., 2012; Lawrence et al., 2020; Witeska, 2021). Therefore, hematological indices have been used as crucial diagnostic tools to assess the health status of fish (Bhaskar and Rao, 1989; Gabriel et al., 2004; Pradhan et al., 2012; Fazio, 2019; Lawrence et al., 2020; Seibel et al., 2021). Alterations in hematological parameters have been viewed as special signs of the health of fish, and are strongly associated with the way fish respond to environmental variations. Thus, they’re useful for monitoring the health of fish exposed to different types of toxicity in aquatic habitats (Omoregie and Oyebanji, 2002; Adhikari et al., 2004; Al-Akel and Shamsi, 2000; Cicero et al., 2014; Hamidipoor et al., 2015; Fazio, 2019; Lawrence et al., 2020).
The term hematological analysis refers to the assessment of the cellular components of blood, including the measurement of hemoglobin (Hb), red blood and white blood cell counts, hematocrit (Hct), mean corpuscular volume (MCV), mean cell hemoglobin (MCH), mean cell hemoglobin concentration (MCHC), and erythrocyte sedimentation rate (ESR), which are considered as primary diagnostic methods for the analysis of fish health (Fazio, 2019). Fish hematological indices have been used to track metal contamination in the aquatic environment for years (Shah and Altindag, 2005; Authman et al., 2015). There is no doubt that some blood parameters serve as reliable indicators of the health of fish. So, in the present review, we focus on the Hct and Hb among the hematological parameters as an indicator for evaluation of the stress. The Hct is the percentage of blood that is made up of red blood cells in terms of volume or the ratio of red blood cell volume to whole blood volume. Gallaugher (1994) defined Hct as a measure of the capacity for blood to carry oxygen. Then theoretically, the higher the Hct, the greater will be the capacity for oxygen transport. The Hct is expressed in percentage. The micro-hematocrit constitutes the parameters frequently studied, probably because it is easily undertaken and interpreted. Since the environmental toxicants mainly targeted the fish among aquatic organisms, due to these pollutants, the health of fish deteriorates with the passage of time, therefore in the present review, an attempt has been made to correlate the impact of these pollutants on the fish’s initial health markers i.e., selected hematological parameters.
2 General aspects of heavy metals and fish hematology
Aquatic pollution is considered a major load/burden facing both freshwater and marine environments; the main sources of this great problem include expansion in population density, heavy industrialization, and agricultural activities, as a result, more and more waste finds its way towards the aquatic bodies. These wastewaters contain large quantities of heavy metals which are consequently harmful to the health of aquatic animals (Al–Masri et al., 2002; Karbassi et al., 2006), besides affecting the health of fish (Vutukuru, 2005; Vinodhini and Narayanan, 2008; Authman et al., 2015). Heavy metals are now posing a threat to living beings due to their widespread use in agricultural, chemical, and industrial operations. Heavy metals are defined as metals with a density of at least 5 g/cm3 and an atomic number of at least 11 that pose a concern to the environment and/or humans (Tchounwou et al., 2012). The major source of heavy metal pollution in the aquatic ecosystem is anthropogenic activity, principally smelting, mining, and foundries, as well as the leaching of metals from different sources, including waste dumps, excrement, runoffs, livestock droppings, and the use of pesticides, insecticides, fertilizers, and other agricultural chemicals (Figure 1). Heavy metal pollution can also be caused by natural causes (Figure 2) such as corrosion of metals, volcanic activity, sediment re-suspension, metal evaporation from soil and water, soil erosion, and geological weathering (Herawati et al., 2000; Tchounwou et al., 2012; Gautam et al., 2016; Masindi and Muedi, 2018). Their presences pose a disastrous effect on both ecological balance and the diversity of aquatic organisms. Many heavy metal ions are toxic or carcinogenic in nature, making them dangerous to human health and the environment (Farombi et al., 2007; Briffa et al., 2020; Balali-Mood et al., 2021). Excessive use of heavy metals in recent decades has probably resulted in increasing metallic component discharge into the aquatic environment (Yang and Rose, 2003; Li et al., 2011). Researchers from all around the world have been drawn to the poisoning of the aquatic environment by heavy metals and pesticides (Dutta and Dalal, 2008).
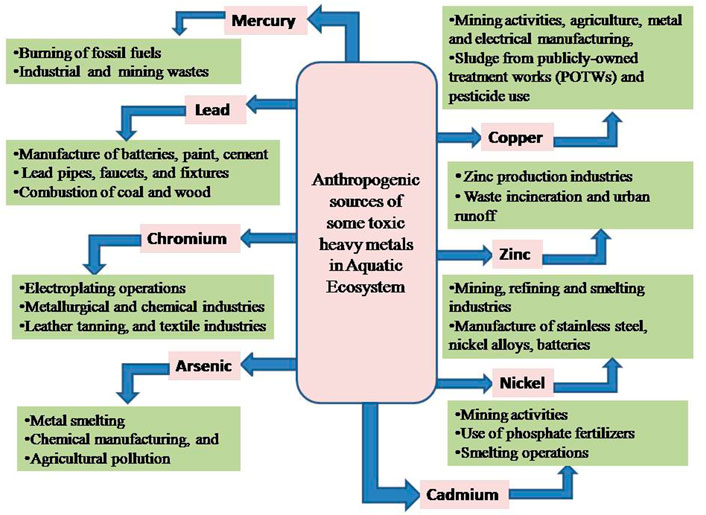
FIGURE 1. Illustrative depiction of anthropogenic sources of some toxic heavy metals in the Aquatic Ecosystem.
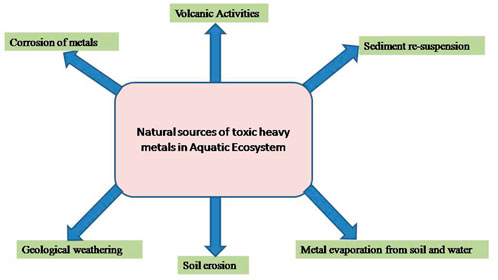
FIGURE 2. Illustrative depictions of natural sources of toxic heavy metals in the Aquatic Ecosystem.
Heavy metals are absorbed by fish either through their gills or their guts, after which they move into the bloodstream and are eventually deposited in various organs (Oliveira Ribeiro et al., 2005; Javed and Usmani, 2012; AL Taee et al., 2020). Consequently, fish accumulate large amounts of metals from the water, and their accumulation rate is influenced by both their absorption and elimination rates (Mansour and Sidky, 2002; Ahmed et al., 2016). Thus, metal accumulation in fish has been studied and monitored in several countries around the globe (Ahmed et al., 2016). The intake of aquatic food contaminated with toxic heavy metals has increased the risk of human health problems globally; particularly in underdeveloped countries such as India (Figure 3). As a result, fish are considered one of the most important indicators in aquatic systems for monitoring trace metal pollution and its possible health impacts on humans (Vieira et al., 2011; Pan and Wang, 2012; Ahmed et al., 2016). Since heavy metals first manifested themselves in the bloodstream, it is critical to investigate the hematological parameters that are used to determine the fish’s health and the state of the environment (Shah and Altindag, 2005; Javed and Usmani, 2015; Lawrence et al., 2020). The poisonous impact of excessive metals on both metabolic and hemopoietic processes in the animal was demonstrated by the relative up/down in hematological parameters, as previously reported (Hanan et al., 2013). Therefore, blood indices are considered pathophysiological indicators of the whole body, and therefore are helpful in determining whether fish have suffered structural and functional damage as a result of toxic exposure (Adhikari et al., 2004; Fazio, 2019). Hematological parameters can also be a valuable pointer for exposure to chemical contaminants and predict the deadly impacts of toxicants within the aquatic medium. Identically as for non-aquatic life forms, the hematological data of a fish have been used in evaluating the effect of heavy metals. According to studies, when pollutants alter the quality of the aquatic medium, any physiological changes in aquatic animals are reflected in the values of one or more hematological parameters (Gabriel et al., 2004; Akinrotimi et al., 2007; Adewumi et al., 2018). Different workers have done lots of work to designate the consequence of heavy metal intoxication on numerous hematological variables counting Hb, Hct, Rbc’s, and Wbc’s of particular fish species from various corners of the globe. Consequently, the present review paper has been made to collate all the data about the major impacts of heavy metals on the level of Hb and Hct of a variety of fish species inhabiting different parts of the world. This survey ought to be valuable for a variety of investigators counting fishery researchers and controllers, and may offer assistance to characterize the magnitude of heavy metal-induced hematological modification in exposed piscine populations.
FIGURE 3. Illustrative depictions of bioaccumulation of heavy metals in fish and its transmission through the food chain.
3 Impacts
3.1. Impacts of different heavy metals on the hematocrit(Hct) and hemoglobin (Hb)of fishes
It is evident that each heavy metal has its own properties that set it apart from the others. Because of this, the level of their toxicity to fish also varies. In addition, the toxicity of metals in various water sources also varies significantly. In toxicological research, certain elements like lead (Pb), mercury (Hg), cadmium (Cd), copper (Cu), nickel (Ni), iron (Fe), cobalt (Co), manganese (Mn), chromium (Cr), zinc (Zn), and arsenic (As) are considered as the deadliest at high concentrations for aquatic ecosystems due to their low biodegradability and long-lasting qualities, as well as their toxicity to ichthyofauna and humans (Yousif et al., 2016; Rajeshkumar and Li, 2018; Hong et al., 2020; Balali-Mood et al., 2021). Meanwhile, copper (Cu), nickel (Ni), iron (Fe), cobalt (Co), manganese (Mn), chromium (Cr), and zinc (Zn) are essential micronutrients/metals included in the active centers of enzymes and are essential regulators of a wide range of biological functions, but at high concentrations they become toxic. Therefore, it is also important to monitor the concentrations of these essential metals in aquatic environments. Heavy metals can be absorbed and accumulated by fish either directly by ingestion of polluted water or indirectly via polluted food such as phytoplankton, zooplankton, and crustaceans (Polat et al., 2015). Fish have the ability to tolerate stress before developing a major physiological change. In the case of minor stress, the inner equilibrium is normally restored but in the case of severe or protracted stress, the organism’s compensating skills may be overwhelmed, resulting in physiological abnormalities or even mortality. The various impacts of heavy metals on the above-selected blood parameters of fish have been documented by several authors in different countries around the world in the past several decades (Jaishankar et al., 2014; Authman et al., 2015; Rajeshkumar and Li, 2018; Hong et al., 2020; Naz et al., 2021).
Heavy metal exposure can induce an increase or reduction in the levels of blood parameters in a fish. Heavy metals affect the oxygen-carrying capacity of the blood by affecting the Hb and Hct levels in different ways depending on the metal type, the species, and the duration of exposure. In aquatic environments, several hematological indices, including Hb, Hct, RBCs, etc., have been used as indicators of metal pollution and are often used to assess the functional status of the oxygen-carrying capacity of the bloodstream (Fazio, 2019). In the present review, we will summarize the present knowledge on the toxic effects of heavy metals on the hematological parameters of various fish species, which is shown in Tables 1‐7.
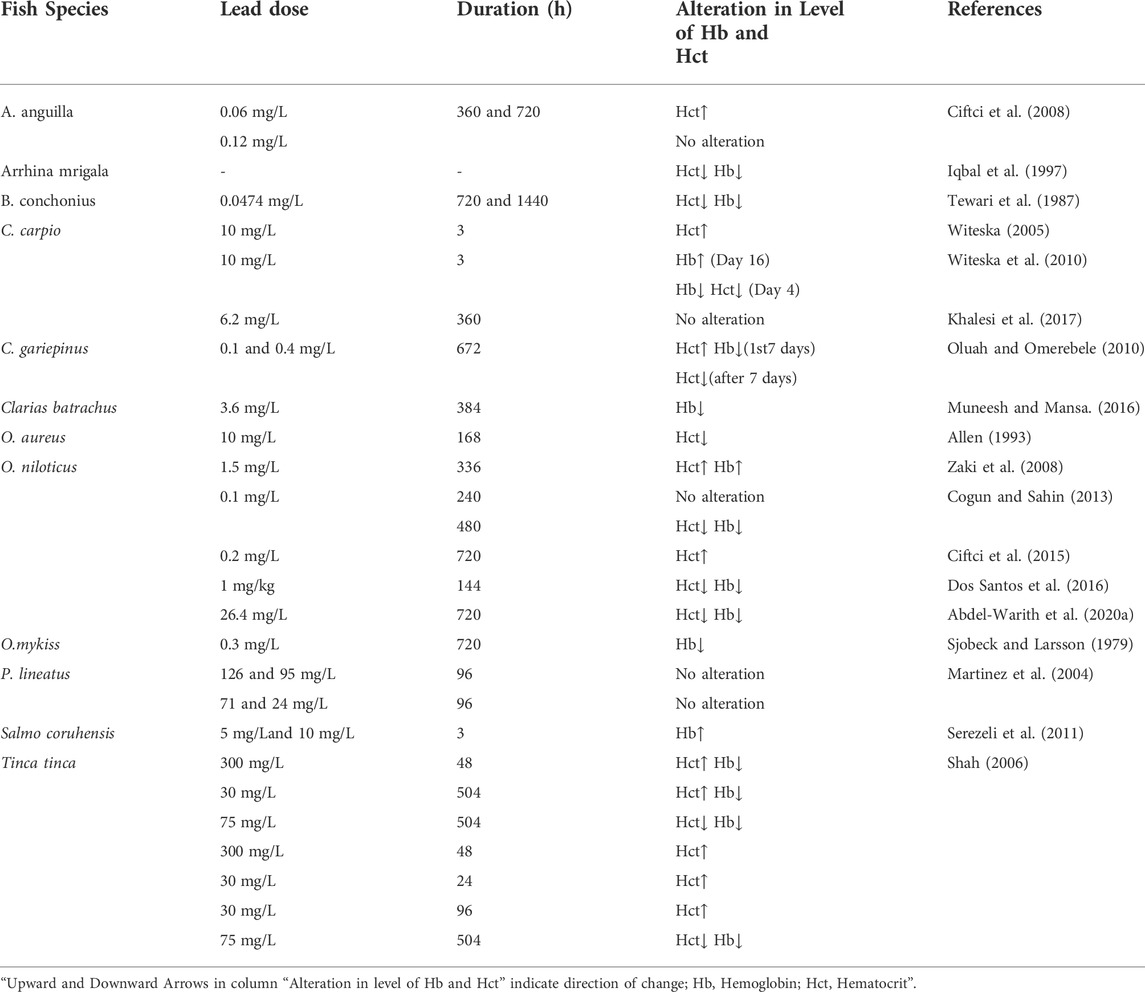
3.1.1 Effect of lead (Pb) on the level of Hct and Hb in fishes
Lead (Pb) is one of the heavy metals that are non-essential, non-beneficial, and highly toxic to several organisms at relatively low concentrations (Tchounwou et al., 2012; Rajeshkumar and Li, 2018; Balali-Mood et al., 2021). This unfavorable factor has been shown to change the hematologic system of hosts by blocking the activity of several heme production enzymes (Agency for Toxic Substances and disease Registry ATSDR, 2005). Pb is highly persistent in the environment and has been classified as an important hazardous substance (Sfakianakis et al., 2015). Additionally, several authors also agree that contaminants such as Pb accumulate in many fish species and cause harm to their physiology, behavior, and biochemistry, as well as causing damage to their central nervous system (CNS), peripheral nervous system (PNS), hematopoietic system, cardiovascular system, and organs such as the liver and kidney (Guo and Hsu, 2002).
Exposure to Pb can cause many effects depending on its level and duration (Table 1). As recoded in Oncorhynchus mykiss (Johansson-Sjobeck and Larsson, 1979) and Barbus conchonius (Tewari et al., 1987), chronic Pb exposure causes a reduction in Hb content and Hct values. Ciftci et al. (2008) examined the impact of two different Pb doses of 0.06 mg/L and 0.12 mg/L, which is 5 and 10% of the 96 h LC50 value (1.17 mg/L), on the Hct level of Anguilla anguilla for 360 and 720 h. They reported that a Pb concentration of 0.12 mg/L had no effect on Hct levels, however, 0.06 mg/L of Pb elevates the level of Hct. Cogun and Sahin (2013) conducted a toxicological study in Oreochromis niloticus for 240 and 480 h exposure respectively at 0.1 mg/L Pb, however, no significant changes in hematological parameters such as Hct and Hb count were observed after 240 h of exposure. On the other hand, the levels of these hematological indicators exhibited a downward trend at the end of the 480 h exposure period. Allen (1993) also noted a decrease in Hct values in Oreochromis aureus administered with 19 mg/L Pb. Martinez et al. (2004) did not observe any hematological changes in Prochilodus lineatus after acute Pb exposure to LC50 for 96 h. Khalesi et al. (2017) reported similar findings in carp, Cyprinus carpio, when exposed to sub-lethal Pb (6.2 mg/L) over a 360 h period. No significant change in the Hct level of P. lineatus and C. carpio exposed to Pb concentrations indicated that despite gill lesions, the fish were not exposed to internal hypoxia. Abdel-Warith et al. (2020a) found that the Hct and Hb values of O. niloticus exposed to sub-lethal concentrations of Pb during 720 h were higher than in the control group. They finally observed that the values of Hb and Hct showed a decreasing trend with respect to the increase of Pb concentration. The same trend has also been exhibited for Clarias gariepinus (Abdel-Warith et al., 2020b). The reason for the decrease in the values of Hb and Hct might be hemodilution, hemorrhagic anemia (blood loss), hemolytic anemia (erythrocyte destruction), and hypoplastic anemia (poor erythropoiesis) which resulted due to the exposure of fish species to heavy metal. Increased hematocrit levels are due to the release of erythrocytes from erythropoietic tissues following Pb stimulation. Zainuddin et al. (2017) investigated the effects of sub-lethal Pb levels on the hematological parameters of Nile tilapia, Oreochromis niloticus at different salinity levels and found that fish exposed to Pb had lower levels of Hb than fish raised without Pb. Whereas at salinity levels of 0, 5, and 10 ppt, the level of Hct in the Pb-exposed fish was lower than that in the control fish.
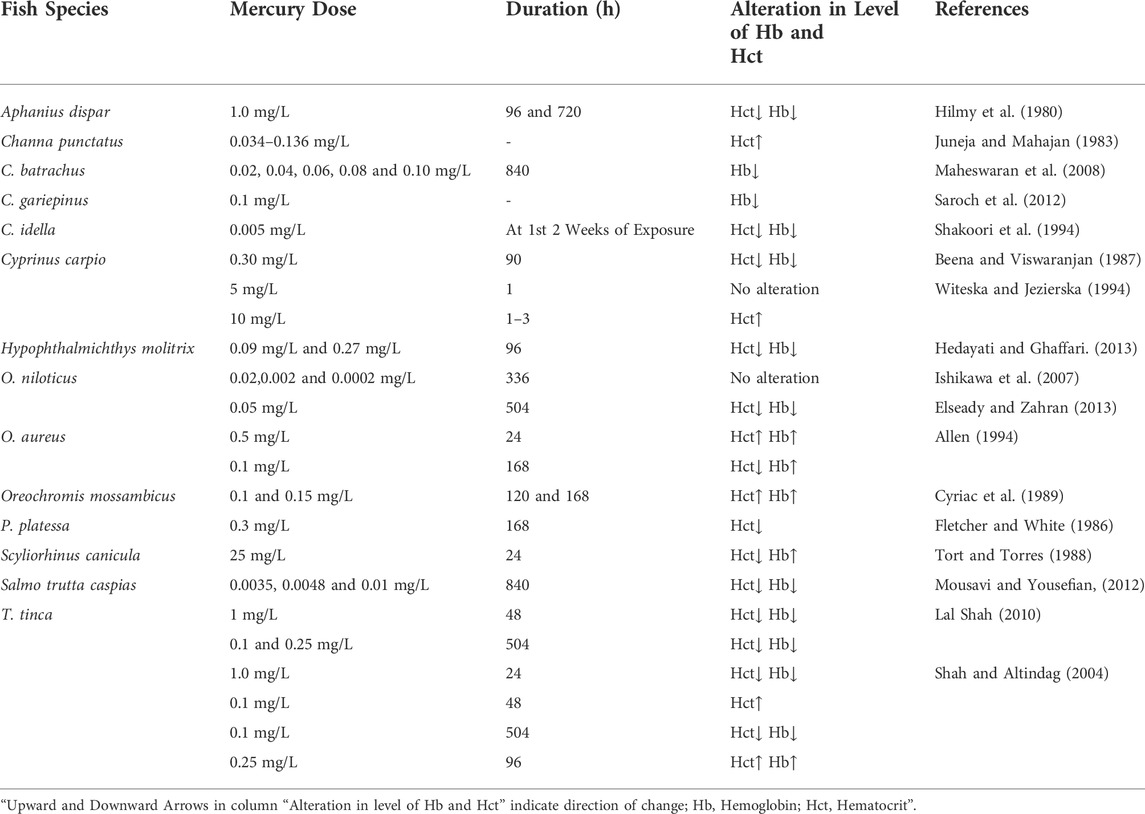
3.1.2 Effect of mercury (Hg) on the level of Hct and Hb in fishes
Mercury (Hg) can be toxic to both humans and animals, depending on its chemical form. It has been shown that organic methylated mercury is more toxic than organic mercury (Sorenson, 1991; Hong et al., 2012). In fish, Hg is mainly present as methylmercury (CH3Hg) (Spry and Wiener, 1991; Li et al., 2010). Fish can get CH3Hg in two ways: directly from the water (sulfate-reducing bacteria convert first-hand Hg into an organic form) or by eating other creatures (biomagnification of the food chain). The conversion of inorganic mercury by anaerobic bacteria to CH3Hg occurs most efficiently in aquatic ecosystems (Ullrich et al., 2001; Bravo and Cosio, 2019). Mercury serves no biological purpose in the body and has major metabolic and physiological consequences (Calabrese et al., 1975; Jarup, 2003; Rajeshkumar and Li, 2018). Several hematological parameters of fish have been affected by exposure to different levels of mercury.
Several studies have been conducted to observe the influence of different concentrations of Hg on various hematological indices in different fishes across the world (Table 2). Shah and Altindag (2004) investigated the effect of acute lethal (1.0 mg/L Hg/48 h) and chronic sub-lethal (0.25 mg/L Hg/3 weeks) Hg doses on the hematological parameters of Tench (Tinca tinca). These results observed a sharp decline in Hb and Hct levels at both higher acute lethal (1.0 mg/L Hg/48 h) and higher chronic sublethal (0.25 mg/L Hg/3 weeks) treatments, but at lower acute sublethal (0.1 mg/L Hg/24 h) treatment, a significant increase in Hct value was observed. Maheswaran et al. (2008) reported a drop in Hb content of Clarias batrachus when administered to various concentrations of mercuric chloride (HgCl2) for 840 h. Hedayati and Ghaffari (2013) observed reductions in Hb content and Hct value of Silver Carp, Hypophthalmichthys molitrix treated with low (10% LC50) and high (50% LC50) concentrations of HgCl2 for 96 h. Cyriac et al. (1989) studied the short-term influence of Hg on the Hb and Hct level of the fish Oreochromis mossambicus and revealed a considerable increase in the Hb and Hct values in Hg-exposed fish at 120 and 168 h respectively. Following exposure to three mercury treatments (one acute lethal and two chronic sub-lethal), Lal Shah (2010) found a significantly lower Hb and Hct level in fish Tinca tinca. Contrary to this, no significant variation in Hb and Hct has been observed at different Hg concentrations (0.02, 0.002, 0.0002 mg/L Hg) in Oreochromis niloticus (Ishikawa et al., 2007). Observations by Shakoori et al. (1994) demonstrated that the content of Hb and Hct in grass carp, Ctenopharyngodon idella, decreased in the first 2 weeks after exposure to HgCl2. The same results were also reported for carp, Cyprinus carpio exposed to HgCl2 (0.30 mg/L) for 90 h (Beena and Viswaranjan, 1987), Aphanius dispar exposed to the same product (1.0 mg/L) for 96 and 720 h (Hilmy et al., 1980), Channa punctatus (Juneja and Mahajan, 1983) and O. aureus (Allen, 1994) exposed to sub-lethal concentrations of HgCl2 (0.034–0.136 and 0.5 mg/L, respectively).
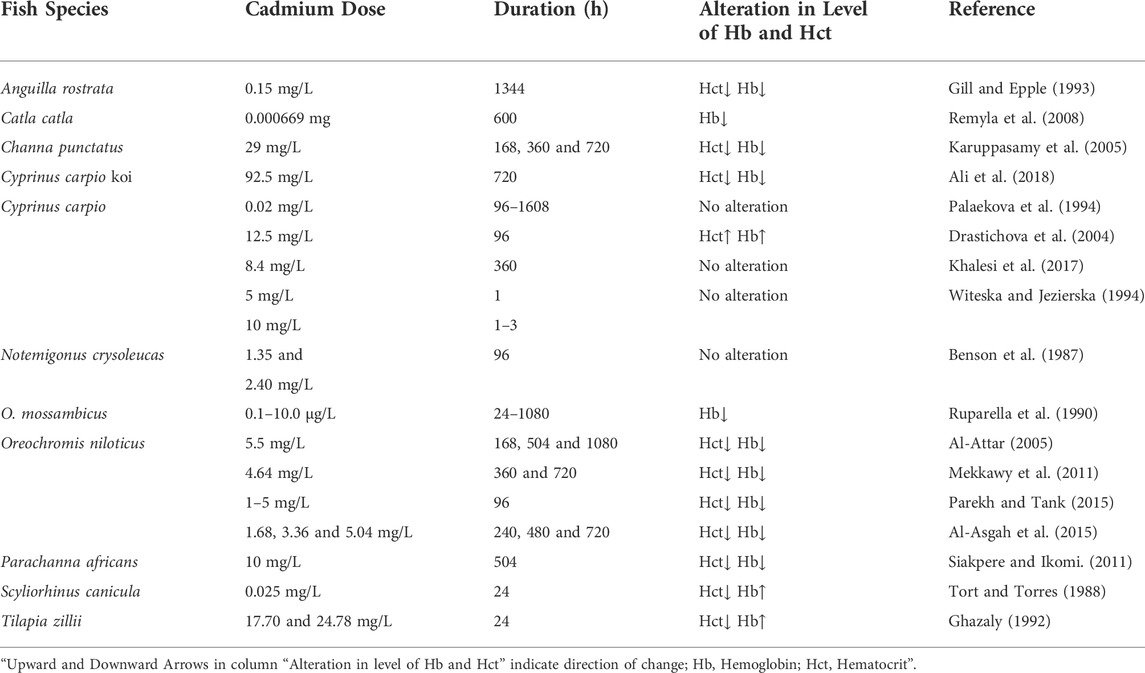
3.1.3 Effect of cadmium (Cd) on the level of Hct and Hb in fishes
Cadmium (Cd) is not a dietary essential element in fish nutrition. It is one of the most toxic heavy metals because of its non-biodegradable nature and non-beneficial effect and causes toxicity in aquatic organisms even at very low levels (Cicik and Engin, 2005). The average natural concentration of Cd in unpolluted natural waters is below 0.001 mg/L (Nordberg et al., 2007). Researchers have studied a variety of fish species from around the world and found that the exposure of a fish to Cd may alter their Hb and Hct values (Table 3). Mekkawy et al. (2011) observed a remarkable decline in the Hb content and Hct value of Oreochromis niloticus after exposure to Cd for 360–720 h. However, when Cd-exposed fish were treated with vitamin E or tomato paste, they saw a considerable rise in the falling value of Hb and Hct. Finally, they concluded that supplementing Cd-exposed fish with vitamin E and tomato paste phytonutrients counteracts the negative effects of Cd on the examined features. A decrease in blood Hb and Hct values has also been reported in Channa punctatus exposed to Cd (29 mg/L) for 168, 360, and 720 h (Karuppasamy et al., 2005). Drastichova et al. (2004) expose the fish Cyprinus carpio to Cd (12.5 mg/L) for 96 h and found higher Hb content and Hct value than the control group and concluded that the rise in Hb and Hct values in Cd-exposed fish might be due to a response to impairment of gas exchange in Cd affected gills. Similarly, Ghazaly (1992) observed a significant rise in Hb content and a sharp decline in Hct value in Tilapia zillii after exposure to 17.70 and 24.78 mg/L for 24 h Cd concentrations. Haux and Larsson, (1984) revealed a sharp decline in the value of Hct in Onchorhynchus mykiss with the increasing concentrations of Cd (0.01 mg/L). After being exposed to 0.05–0.5 mg/L of Cd, the amount of Hb and Hct in Pleuronectes flesus (flounder) fell dramatically (Johansson-Sjobeck and Larsson, 1978). Contrary to these findings, it has been reported that acute Cd exposure of 5 mg/L for 1 h had almost no influence on Hct value in C. carpio, but Cd concentration of 10 mg/L for 24h resulted in a reduction in Hct levels of C. carpio (Witeska and Jiezierska, 1994). O. niloticus was subjected to various doses of cadmium chloride (CdCl2) for a period of 240, 480, and 720 h and found that the Hb and Hct values were decreased in exposed fish to all periods (Al-Asgah et al., 2015). Naz et al., 2021 have reported that the Hb and Hct show a significant decrease upon exposure to Cd.
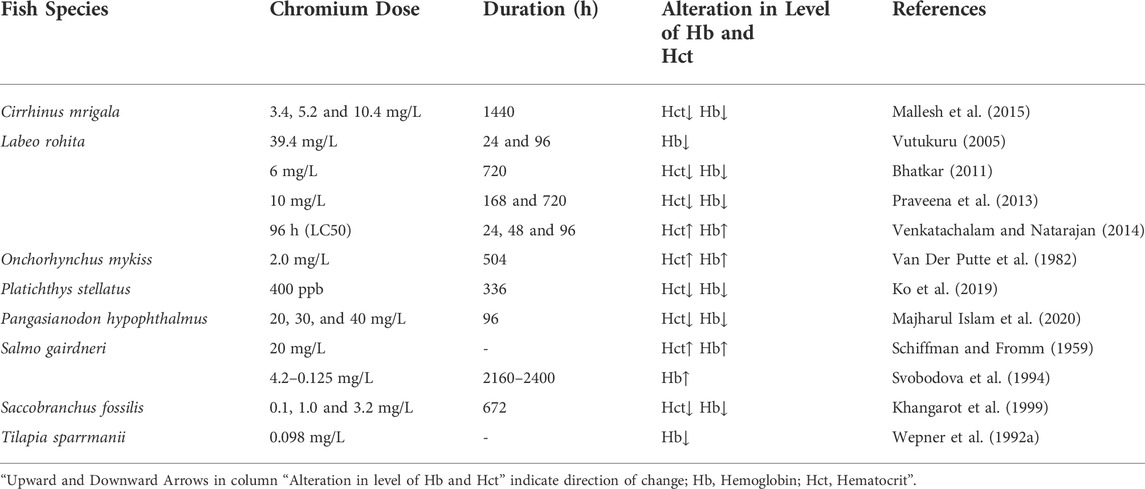
3.1.4 Effect of chromium (Cr) on the level of Hct and Hb in fishes
In the environment, Chromium (Cr) occurs naturally as trivalent (CrIII) and hexavalent (CrVI). Hexavalent Chromium(CrVI) is considered the most hazardous among them, as it easily penetrates through biological membranes before being reduced to trivalent form, which then combines with several macromolecules, such as genetic material inside the cytosol, exposing the toxic and mutagenic variations owing to the presence of toxicity (Velma et al., 2009; Ahmed et al., 2013). In an aquatic ecosystem, Cr makes its way through effluents mostly discharged from various sources such as metal finishing, dyeing and printing industries, leather tanneries, mining, photographic, pharmaceutical, electroplating, ceramic, and textile industries, etc. (Arunkumar et al., 2000; Tadesse and Guya, 2017; Grace Pavithra et al., 2019; Ukhurebor et al., 2021). Poor treatment of these effluents, on the other hand, might result in the presence of heavy metals like CrVI in nearby water bodies, where it is regularly detected at levels that are potentially dangerous to fish (Li et al., 2011; Bhatia, 2017; Lellis et al., 2019). The concentration of Cr ranges from 0.005 to 0.8 mg/L in sea water, and from 0.026 to 5.2 mg/L in rivers and lakes (Jacobs and Testa, 2005). According to the EPA, the total Cr including CrVI in drinking water must be less than 0.1 mg/L. In general, both biotic and abiotic variables influence Cr toxicity in aquatic ecosystems. Age, species type, and developmental stage of an individual are all biological factors. Abiotic parameters include pH, temperature, Cr concentration and oxidation state, water hardness, and alkalinity. Hematological abnormalities, morphological and histological changes, formation of reactive oxygen species (ROS), inhibition/reduction of growth, and reduced immunological function are all toxic effects of Cr in fish (Reid, 2011; Vera-Candioti et al., 2011).
Several variations in the hematologic indices of numerous fish species subjected to the presence of Cr are well documented (Table 4). Labeo rohita showed a significant decrease in Hb and the total erythrocyte count after 24 and 96 h of exposure to 39.4 mg/L CrVI (Vutukuru, 2005). Wepener et al. (1992a) recorded a marked decline in the blood Hb concentration of Tilapia sparrmanii exposed to 0.098 mg/L of CrVI. After rainbow trout were exposed to CrVI of 2.0 mg/L for 504 h, the levels of Hb and Hct in their blood dramatically increased, according to Van Der Putte et al. (1982). Venkatachalam and Natarajan (2014) investigate the hematologic alteration in the blood of a freshwater teleost L. rohita after being exposed to both CrIII and CrVI for 96h, and their finding revealed a significant rise in the value of Hb and Hct values. Similarly, Gill and Pant (1987) observed significant polycythemia in the acutely intoxicated fish Barbus conchonius with a marked increase in Hb and Hct. Furthermore, chronic exposure led to significant erythropenia with a reduction in Hb and Hct values. A similar trend in the Hb content and Hct value was also found in Salmo gairdneri exposed to Cr (20 mg/L) (Schiffman and Fromm, 1959). Various fish species have also been reported to have higher hematological indices after being exposed to 10 mg/L of CrVI for 360 h (Strik et al., 1975). Even after short-term (96 h), as well as long-term (2160–2400 h), treatment with potassium bichromate, the levels of Hb and Hct in the blood of C. carpio remain the same, whereas long-term exposure to Cr concentrations of 4.2–0.125 mg/L increased Hb content in Oncorhynchus mykiss (Svobodova et al., 1994). Moreover, Saccobranchus fossilis, a freshwater fish, was subjected to CrIV concentrations of 0.1, 1.0, and 3.2 mg/L for 672 h and demonstrated a dose-dependent drop in Hb and Hct levels (Khangarot et al., 1999). Majharul Islam et al. (2020) investigated the effects of Cr on the hematological indexes of the striped catfish, Pangasianodon hypophthalmus, and discovered that Cr concentrations of 30, 40, and 60 mg/L dramatically reduced Hb and Hct levels after 96 h. Mallesh et al. (2015) exposed the fish Cirrhinus mrigala to sub-lethal concentrations of Cr (3.4, 5.2, and 10.4 mg/L) for 1440 h and observed a marked drop in Hb and Hct values, which leads to anemia in fishes. Praveena et al. (2013) studied the impact of sub-lethal Cr concentrations on the hematological indices of L. rohita. They found considerably decreased values of Hb and Hct after 168 and 720 h of exposure. Similarly, after Cr exposure, Hb and Hct values in C. carpio were also found to be much lower (Shaheen and Akhtar, 2012). It is clear from the decreased hematological parameters that heavy metal exposure leads to anemic fish with erythropenia. The decline in hematological values could also be ascribed to heavy metals (pollutants) damaging the structure of RBCs, causing fewer oxygen molecules to bind to Hb, reducing RBC oxygen carrying capacity, lowering Hb levels, and even resulting in anemia (Javed et al., 2016).
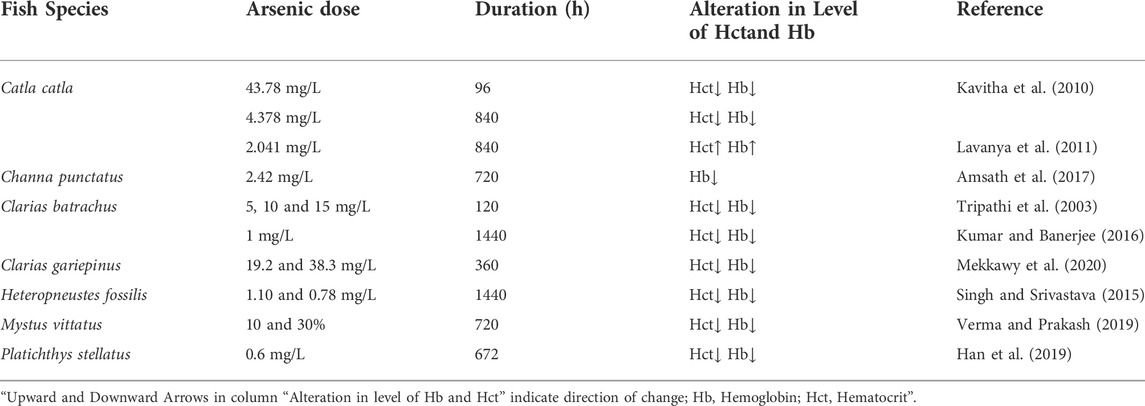
3.1.5 Effect of copper (Cu) and zinc (Zn) on the level of Hct and Hb in fishes
Copper (Cu) and zinc (Zn) are critical trace elements for fish and are necessary in small amounts for the normal development and metabolism of aquatic species, including fish. However, if the concentrations of Cu or Zn exceeds the physiological limits, they become toxicant and can even be lethal for almost all organisms, including fish (Heath, 1995). The US EPA (2009) issued water quality criteria for Zn, which stipulated that the criteria for freshwater aquatic organism protection were 0.12 mg/L (short-term hazardous concentration) and 0.12 mg/L (long-term hazardous concentration), while the criteria for human health were 7.4 mg/L (ingested potable water + edible aquatic organisms grown in the water) and 26 mg/L (only edible aquatic organisms). The toxicity of Zn and Cu to fishes is well documented and investigation has found that elevated doses of Zn and Cu may become acutely or chronically toxic to aquatic life, including fish, affecting growth, survival, and development, accruing structural damage, having an adverse effect on tissue respiration leading to death by oxygen deprivation, changes in heart and ventilator physiology as well as having a negative impact on fish hematological indices. The present intensive industrial developments have increased the concentration of Zn and Cu in aquatic ecosystems and make them one of the most common pollutants in natural waters. In the last few decades, the usage of these metals has increased significantly, by virtue of which the probability of accumulation of these elements increased in various aquatic environments. Because of these concerns, it is crucial to understand the toxicity of these metals on aquatic organisms before they lead to harmful effects on them.
The impact of these essential trace elements on the hematological parameters of fishes has been widely studied and a considerable number of experimental data are available (Table 5). Ciftci et al. (2015) examined the impact of Cu (4.0 mg/L) on the hematological parameters of O. niloticus and noticed that the value of Hct had raised significantly during the exposure periods of 168 and 360 h, later they also reported a decline in the value of Hct at 720 h exposure period. They revealed that the increase in Hct levels was reported to be a result of either hemoconcentration or stimulation of erythropoiesis by feedback mechanisms. A freshwater teleost, Colisa fasciatus showed significantly elevated Hb and Hct values after 96 h exposure to 3 mg/L of Cu (Mishra and Srivastava, 1980). Contrary to this, the same fish (C. fasciatus) showed a significantly lowered Hct value after 90 h exposure to 100 mg/L of zinc (Mishra and Srivastava, 1979). Hilmy et al. (1987) observed that the Hb and Hct contents of catfish, Clarias lazera, and tilapia, Tilapia zilli had significantly increased after 96 h exposure to 22 mg/L and 32 mg/L of Zinc. It was demonstrated by Celik et al. (2013) that Zn (1, 2.5, and 5 mg/L) negatively affected the hematology of Oreochromis mossambicus and their findings revealed that in all groups of exposure, the Hb content increases with the increase of exposure dose, while the Hct value decreases at high exposure dose but increases at low and medium doses of Zn. Cyriac et al. (1989) noted an increase in Hb and Hct concentrations of O. mossambicus after 24 h exposure to substantially lower Cu concentrations (100 and 200 mkg/L). Their findings demonstrated that the rise in Hb and Hct content in the blood of fish acutely exposed to Cu was generally triggered by erythrocyte enlargement or the spleen’s release of giant red blood cells. The Hb content of catfish (Clarias gariepinus) is significantly reduced at 48 h acute exposure to background copper concentrations, although after 48 h exposure the Hb content slightly increases (Van Vuren et al., 1994). The results of Dharam et al. (2008), who examined the hematological data in Channa punctatus following acute sublethal exposure to Cu (0.36 mg/L) revealed a substantial drop in Hb (down from 10.73 to 6.60%) and Hct levels (down from 31.00 to 23.33%), when compared to control, after 1080 h. Their study revealed that the reduction in blood Hb and Hct is primarily the result of increased destruction and decreased synthesis of Hb due to Cu (a heavy metal). The Hb an oxygen-carrying module serves as a marker for anemia in fish (Parekh and Tank, 2015). Therefore, the significant drop in Hb in C. punctatus reveals that the fish has anemia due to metal toxicity. Cu and Zn toxicity have shown detrimental effects on almost all physiological systems of fish, yet fish of different species had varying tolerance levels.
3.1.6 Effect of manganese (Mn), nickel (Ni), and cobalt (Co) on the level of Hct and Hb in fishes
Manganese (Mn) exists in the aquatic environment in two main forms: soluble MnII and insoluble MnIV oxidation states, with the latter being less bioavailable and toxic. In small amounts, Mn is essential for organisms, including fish, since it is a constituent of important enzymes and cofactors; however, at concentrations exceeding the threshold level, it is potentially harmful (Vieira et al., 2012). The acute toxicity of these elements has been recorded in fish ranging from 2.4 mg/L for coho salmon (Oncorhynchus kisutch) to 3350 mg/L for Indian catfish (Hippocampus fossilis) (Howe et al., 2004). The range of Mn concentrations that cause toxicity varies by species of fish, age group, and the composition of the surrounding water (Fish, 2009). Mn is considered to be less of an environmental hazard than other heavy metals, with evidence from the literature indicating that Mn’s acute and chronic toxicity to many freshwater biotas is low.
Many researchers have studied the influence of a high concentration of Mn on the hematological parameters of fish and found negative impacts on hematological indices (Table 6). A considerable drop in the Hb and Hct concentration was found in Tilapia sparmanii after exposure to Mn at pH5 (Wepener et al., 1992b). At 23°C, the freshwater fish Oreochromis mossambicus was exposed to both acute (345 mg/L for 96 h) and chronic (259 mg/L for 624 h) toxicity of Mn (Barnhoon, 1996). Acute Mn exposure increased Hb and Hct levels significantly. Whereas, an oxygen deficiency developed after chronic Mn exposure, resulting in hypoxia and increased hematologic data related to Hb and Hct contents.
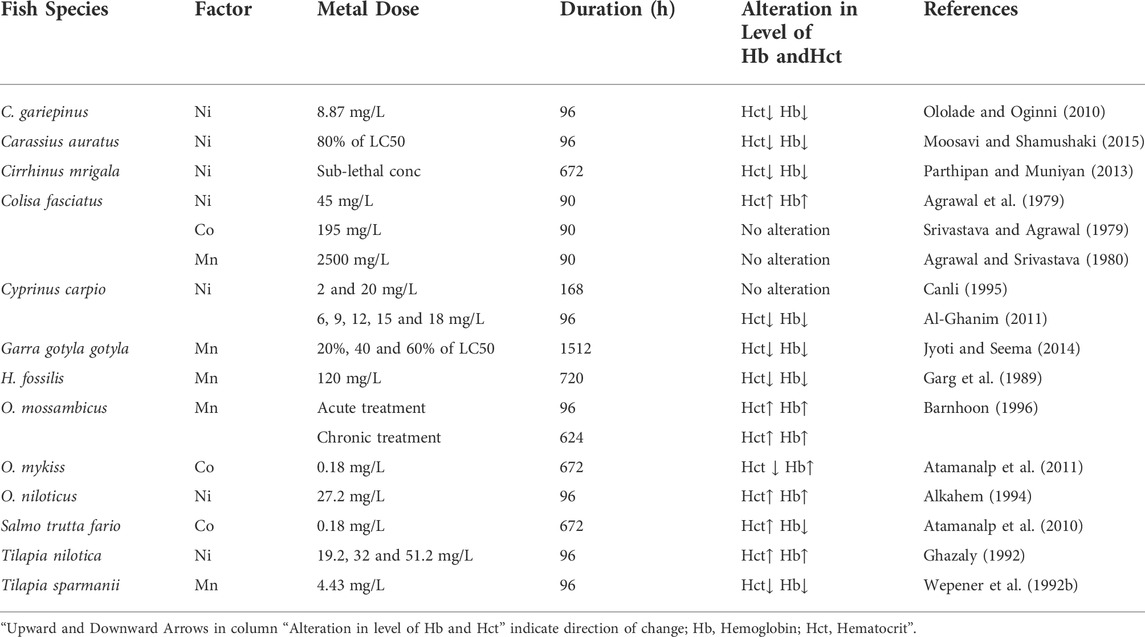
TABLE 6. Influence of Manganese (Mn), Nickel (Ni), and Cobalt (Co) on Hb and Hct. in different fish species.
In freshwaters, the dominant form of nickel (Ni) is soluble Ni2+, but other forms also exist, predominantly as complex forms with sulfate and chloride. In many aquatic ecosystems, Ni contamination results from various human processes, including extraction, casting, refining, and the production of steel materials and Ni-Cd batteries, etc. In unaffected water, Ni concentrations are normally below 10 g/L, but in heavily contaminated water, they can exceed several hundred g/L up to even 1000 g/L (Eisler, 1998). Despite the fact that Ni is an important trace element for a wide range of living creatures, at elevated concentrations, it can be very harmful to all living organisms. Ni and its compounds have high acute and chronic toxicity to aquatic life including fish.
Several studies reported Ni-related variations in the hematological indices of fishes (Table 6). Ololade and Oginni (2010) reported a significant decrease in the hematological indices such as Hb and Hct contents of C. gariepinus after exposure to 8.87 mg/L Ni for 96 h. Similarly, when Musa and Omoregie (1999) subjected C. gariepinus to a contaminated environment under laboratory conditions, the fish’s hematological profile was likewise reduced. The considerable fall in hematological markers indicates that experimental fish were exposed to Ni in the water, which produced severe anemia. After short-term exposure to high Ni concentrations in C. carpio, Al-Ghanim (2011) discovered a considerable drop in Hb and Hct levels.
Cobalt (Co) is a mineral that is necessary for the survival of fish and other species and plays a crucial role in the growth of all living organisms. Co is required by all animals, including fish, in trace amounts. Its high concentration, on the other hand, can be harmful to the health of the fish. As a result of their persistence and propensity to bio-magnify in the aquatic food chain, Co pollution poses a serious threat to aquatic life (Ubaidullah et al., 2004). Co, which is an integral part of vitamin B12 or cobalamin, has an important role in fish nutrition and aquaculture. Since fish and all other animals are unable to synthesize this vitamin, therefore, they must rely on bacterial production to meet their nutritional needs. Co compounds that dissolve easily in water are generally more dangerous than those that do not. Co is dispersed throughout the body, but especially in the liver, kidneys, and bones, once it enters the body (ATSDR, 2004). Several investigations have found Co-related variations in fish blood parameters (Table 6). Atamanalp et al. (2011) examined the hematological data in Co exposed fish, O. mykiss. Their findings revealed a significant rise in Hb concentration and a declining trend in Hct value after exposure to 0.18 mg/L for 672 h.
3.1.7 Effect of arsenic (As) on the level of Hct and Hb in fishes
Arsenic (As) is one of the major global environmental toxicants that is delivered either directly or indirectly into aquatic ecosystems (Agency for Toxic Substances and disease Registry ATSDR, 2002). The poisoning of aquatic ecosystems by this worldwide environmental contaminant, as well as its influence on aquatic creatures, has now become a major environmental issue all over the world (Allen and Rana, 2004). As is found in abundance in our environment and is known to come in different chemical forms. In general, As exists in both organic and inorganic forms; the latter exhibit the highest toxicity level, while the former are usually less toxic (Luh et al., 1973). Arsenate (AsV+), arsenite (AsIII+), arsenic (As0), and arsine (AsIII−) are the most common oxidation states (WHO, 2011). In most As-contaminated locations in India, arsenic trioxide (As2O3) was the dominating species, accounting for over half of the total As level (Chatterjee et al., 1993). Arsenical toxicity in animals varies according to inorganic or organic forms, exposure duration, exposure dose, sex, species, age, valence state, and other factors (Allen and Rana, 2004; Amsath et al., 2017). Fish can act as the most effective bioindicator of arsenic toxicity in the aquatic environment because their gills and diet are continuously exposed to As (Ahmed et al., 2008).
In general, As exposure in the aquatic environment induces bioaccumulation in aquatic fauna, which can lead to biochemical and physiological problems such as poisoning, tissue damage, liver lesions, cell death, and reduced fertility (Bears et al., 2006). As influences a number of parameters, including biochemical, hematological, and ionoregulatory, and their changes can be utilized to monitor As pollution in the environment (Lavanya et al., 2011). Fish have been regularly utilized as a model organism to assess the toxic burden of environmental pollutants for several decades. Blood in fish exhibits the early effects of As toxicity because it enters the blood primarily through the vast gill surface area, at which the barrier between the blood and the metal salt is much thinner, and also through the buccal cavity (Romeo et al., 1999; Kumar and Banerjee, 2012). Tripathi et al. (2003) reported a decreased level of Hb and Hct in C. batrachus exposed to water-borne arsenic (Table 7). Their findings revealed that a decline in Hb and Hct contents of C. batrachus is an indication of an anemic condition due to the poisoning effects of arsenic.
3.1.8 Effect of heavy metal mixture on the level of Hct and Hb in fishes
The effects of single metals on fish have been investigated in the majority of ecotoxicological investigations; however, studies evaluating the biological responses of fish to the influence of a blend of heavy metals are rare. Numerous reports have shown that the effects of metal mixtures on living life forms differ from the effects of single components in terms of toxicity. The toxicity of heavy metal complexes is determined by their quantities, composition, and duration of exposure to fish (Vosyliene and Jankaite, 2006). The impact of a model mixture of five heavy metals (copper, zinc, nickel, chromium, and iron) on Oncorhynchus mykiss (rainbow trout) during short-term exposure was investigated, and it was discovered that the most sensitive hematological parameters, such as Hb and Hct, changed depending on the concentration of the mixture and the duration of the exposure to it (Vosyliene, 1999). But the fish adapted to low concentrations of the mixture after prolonged exposure (from 10 days to 3 months), and variations in hematological markers could only be seen in blood smears. In perch, Perca fluviatilis was exposed for 288 and 648 h to the model heavy metal mixture (zinc, copper, lead, cadmium, and mercury) the Hct and Hb concentrations declined, according to the dosage of the metal mixture (Larsson et al., 1984)
4 Conclusion
In this review, an endeavor is made to understand the disparate evidence on the hazardous consequences of heavy metals to the selective hematological indices of various fish species. From the review of literature available, it is concluded that heavy metals impose a huge alteration in hematological parameters (specifically Hb and Hct) of fishes. Therefore, it is recommended that the assessment of heavy metals using hematological parameters might be helpful in the early analysis of fish health status before it becomes more severe. Thus, the scientific data discussed in this review provide a basis for understanding the potential effect of heavy metals on the hematological index (Hb and Hct) of a fish species and will aid the research community to assemble advanced information on the ecotoxicology and risk assessment of hazardous metal. It has been revealed that heavy metals can induce a variety of unfavorable impacts at histological, physiological, biochemical, enzymatic, hematological, and genetic levels in fish. These heavy metals will ultimately enter the food web via water and food, creating negative health impacts in humans and other animals, just as they do in indicator organisms. Therefore, one of the most important approaches to addressing the issue of heavy metal contamination in aquatic ecosystems is to prevent them from occurring in the future. Uncontrolled release of sewage and industrial wastes has seriously weakened the aquatic ecosystem in general and water quality in particular which ultimately influences the biochemical and physiological status of the aquatic flora and fauna including the fish. For maintaining ecological balance, it is therefore advisable that industrialists do not dispose of their waste unless it has been treated first. Ultimately, if the pollution from these point sources is reduced, the heavy metals in fish will be reduced as the degree of pollution in their habitat decreases. In order to provide a solution to this problem, such strategies or measures must be developed. Therefore, it is crucial to act now in order to ensure that future emissions and releases of heavy metals in aquatic ecosystems are minimized to the maximum extent possible in order to prevent contamination of aquatic ecosystems globally and to prevent hazardous impacts on fish and humans.
Author contributions
IA supervises the study and contributed in conceptualization, reviewing and editing. AZ wrote the original draft. FF contributed in reviewing and editing. All authors helped to revise the manuscript, read it, and approved the final version.
Acknowledgments
The authors are grateful to the Head of the Department of Zoology, University of Kashmir, India for providing essential facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel-Warith, A. A., Younis, E. M., Al-Asgah, N. A., Rady, A. M., and Allam, H. Y. (2020a). Bioaccumulation of lead nitrate in tissues and its effects on hematological and biochemical parameters of Clarias gariepinus. Saudi J. Biol. Sci. 27, 840–845. doi:10.1016/j.sjbs.2020.01.015
Abdel-Warith, A. W. A., Younis, E. M., Al-Asgah, N. A., Abd-Elkader, M. O., and Elsayed, E. A. (2020b). Effects of sub-lethal lead nitrate and copper sulfate concentrations on haematological parameters during long-term exposure in nile tilapia (Oreochromis niloticus). J. Sci. Ind. Res. 79, 437–441.
Adewumi, B., Germaine Akinola Ogunwole, G. A., Akingunsola, E., Falope, O. C., and Eniade, A. (2018). Effects of sub-lethal toxicity of chlorpyrifos and DD force pesticides on haematological parameters of Clarias gariepinus. Int. J. Environ. Res. Publ. Health. 5, 62–71. doi:10.15739/irjpeh.18.010
Adhikari, S., Sarkar, B., Chatterjee, A., Mahapatra, C. T., and Ayyappan, S. (2004). Effects of cypermethrin and carbofuran on certain hematological parameters and prediction of their recovery in a freshwater teleost, Labeo rohita (Hamilton). Ecotoxicol. Environ. Saf. 58, 220–226. doi:10.1016/j.ecoenv.2003.12.003
Agency for Toxic Substances and Disease Registry (ATSDR) (2004). Toxicological profile for cobalt. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service.
Agrawal, S. J., Srivastava, A. K., and Chaudhry, H. S. (1979). Haematological effects of nickel toxicity on a fresh water teleost Colisa fasciatus. Acta Pharmacol. Toxicol. (Copenh). 45, 215–217. doi:10.1111/j.1600-0773.1979.tb02384.x
Agrawal, S. J., and Srivastava, A. K. (1980). Haematological responses in a fresh water fish to experimental manganese poisoning. Toxicology 17, 97–100. doi:10.1016/0300-483x(80)90031-1
Ahmed, K., Anwaral, A., Hasan, M., Islam, M., and Hasan, A. (2008). Toxicity of arsenic (sodium arsenite) to Fresh water spotted snake head Channa punctatus (Bloch) on cellular death and DNA content. Am. Eu. J. Agric. Environ. Sci. 4, 18–22.
Ahmed, M. K., Baki, M. A., Kundu, G. K., Saiful Islam, M., Monirul Islam, M., and Muzammel Hossain, M. (2016). Human health risks from heavy metals in fish of Buriganga river, Bangladesh. SpringerPlus 5, 1697. doi:10.1186/s40064-016-3357-0
Ahmed, M. K., Kundu, G. K., Al-Mamun, M. H., Sarkar, S. K., Akter, M. S., and Khan, M. S. (2013). Chromium (VI) induced acute toxicity and genotoxicity in freshwater stinging catfish, Heteropneustes fossilis. Ecotoxicol. Environ. Saf. 92, 64–70. doi:10.1016/j.ecoenv.2013.02.008
Ajani, E. K., and Akpoilih, B. U. (2010). Effect of chronic dietary copper exposure on haematology and histology of common carp ( Cyprinus carpio L. ). J. Appl. Sci. Environ. Manage. 14, 39–45. doi:10.4314/jasem.v14i4.63254
Akbary, P., Yarahmadi, S. S., and Jahanbakhshi, A. (2018). Hematological, hepatic enzymes’ activity and oxidative stress responses of gray mullet (Mugil cephalus) after sub-acute exposure to copper oxide. Environ. Sci. Pollut. Res. 25, 1800–1808. doi:10.1007/s11356-017-0582-1
Akinrotimi, O. A., Gabriel, U. U., Anyanwu, P. E., and Anyanwu, A. O. (2007). Influence of sex, acclimation methods and period on haematology of Sarotherodon melanotheron. Res. J. Biol. Sci. 2, 348–352.
Al-Akel, A. S., and Shamsi, M. J. K. (2000). A comparative study of the toxicity of lead and its impact on the carbohydrate metabolism and some haematological parameters of cichlid fish, oreochromis niloticus and catfish, clarius gariepinus from saudi arabia. Toxicol. Environ. Chem. 74, 19–28. doi:10.1080/02772240009358867
Al – Masri, M., Aba, S., Khalil, A. H., and Al – Hares, Z. (2002). Sedimentation rates and pollution history of a dried lake: Al-oteibeh lake. Sci. Total Environ. 293, 177–189. doi:10.1016/s0048-9697(02)00013-x
Al-Asgah, N. A., Abdel-Warith, A. W. A., Younis, E. M., and Allam, H. Y. (2015). Haematological and biochemical parameters and tissue accumulations of cadmium in Oreochromis niloticus exposed to various concentrations of cadmium chloride. Saudi J. Biol. Sci. 22, 543–550. doi:10.1016/j.sjbs.2015.01.002
Al-Attar, A. M. (2005). Biochemical effects of short-term cadmium exposure on the freshwater fish, Oreochromis niloticus. J. Biol. Sci. 5, 260–265. doi:10.3923/jbs.2005.260.265
Al-Ghanim, K. A. (2011). Impact of nickel (Ni) on haematological parameters and behavioral changes in Cyprinus carpio (common carp). Afr. J. Biotechnol. 10, 13860–13866. doi:10.5897/AJB11.1893
Al-Taee, S. K., Karam, H., and Ismail, H. K. (2020). Review on some heavy metals toxicity on freshwater fishes. J. Appl. Vet. Sci. 5, 78–86. doi:10.21608/JAVS.2020.100157
Ali, A. J., Akbar, N. J., Arun Kumar, M. S., Vijayakumar, S., and Akbar John, B. (2018). Effect of cadmium chloride on the haematological profiles of the freshwater ornamental fish, Cyprinus carpio Koi (Linnaeus, 1758). J. CleanWAS 2, 10–15. doi:10.26480/jcleanwas.02.2018.10.15
Alkahem, H. F. (1994). The toxicity of nickel and the effects of sublethal levels on haematological parameters and behaviour of the fish, Orechromis niloticus. J. Univ. Kuwait. Sci. 21, 243–252.
Allen, P. (1993). Effects of acute exposure to cadmium (II) chloride and lead (II) chloride on the haematological profile of Oreochromis aureus (Steindachner). Comp. Biochem. Physiology Part C Comp. Pharmacol. 105, 213–217. doi:10.1016/0742-8413(93)90197-S
Allen, P. M. (1994). Changes in the haematological profile of the cichlid Oreochromis aureus (Steindachner) during acute inorganic mercury intoxication. Comp. Biochem. Physiology Part C Pharmacol. Toxicol. Endocrinol. 108, 117–121. doi:10.1016/1367-8280(94)90097-3
Allen, T., and Rana, S. V. S. (2004). Effect of arsenic (AsIII) on glutathione dependent enzymes in liver and kidney of the freshwater fish Channa punctatus. Biol. Trace Elem. Res. 100, 039–048. doi:10.1385/BTER:100:1:039
Amsath, A., Sugumaran, J., and Vanitha, S. (2017). Effect of arsenic (As2o3) on haemetological parameters of freshwater air breathing fish, Channa punctatus (bloch). Curr. Trends Biomed. Eng. Biosci. 7, 1–5. doi:10.19080/CTBEB.2017.07.555702
Arunkumar, R. I., Rajasekaran, P., and Michael, R. D. (2000). Differential effect of chromium compounds on the immune response of the African mouth breeder, Oreochromis mossambicus (Peters). Fish. Shellfish Immunol. 10, 667–676. doi:10.1006/fsim.2000.0281
Atamanalp, M., Aksakal, E., Kocaman, E. M., Ucar, A., Şisman, T., and Turkez, H. (2011). The alterations in the haematological parameters of rainbow trout, Oncorhynchus mykiss, exposed to cobalt chloride. Kafkas Univ. Vet. Fak. Derg. 17, S73–S76. doi:10.9775/kvfd.2010.3393
Atamanalp, M., Kocaman, E. M., Ucar, A., and Alak, G. (2010). The alterations in the hematological parameters of Brown trout Salmo trutta fario, exposed to cobalt chloride. J. Anim. Vet. Adv. 9, 2167–2170. doi:10.3923/javaa.2010.2167.2170
ATSDR (2005). Draft toxicological profile for lead. Atlanta, Georgia, USA: US Department of Health and Human Services, 102–225.
ATSDR (2002). Interaction profiles for toxic substances. Agency for toxic substances and disease Registry. Atlanta, GA: Agency for Toxic Substances and Disease Registry.
Authman, M. M., Zaki, M. S., Khallaf, E. A., and Abbas, H. H. (2015). Use of fish as bio-indicator of the effects of heavy metals pollution. J. Aquac. Res. Dev. 6, 1–13. doi:10.4172/2155-9546.1000328
Balali-Mood, M., Naseri, K., Tahergorabi, Z., Khazdair, M. R., and Sadeghi, M. (2021). Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 12, 643972. doi:10.3389/fphar.2021.643972
Barcellos, L. J. G., Kreutz, L. C., Rodrigues, L. B., Fioreze, I., Quevedo, R. M., Terra, S., et al. (2003). Haematological and biochemical characteristics of male jundia (Rhamdia quelen, quoy and gaimard, pimelodidae): Changes after acute stress. Aquac. Res. 34, 1465–1469. doi:10.1111/j.1365-2109.2003.00972.x
Barnhoon, I. E. J. (1996). “Effects of manganese on the haematology of Oreochromis mossambicus and the bioaccumulation of metals in Labeo umbratus,”. M.Sc. Thesis (Johannesburg, South Africa: Rand Afrikaans University).
Bears, H., Richards, J. G., and Schulte, P. M. (2006). Arsenic exposure alters hepatic arsenic species composition and stress-mediated gene expression in the common killifish (Fundulus heteroclitus). Aquat. Toxicol. 77, 257–266. doi:10.1016/j.aquatox.2005.12.008
Beena, S., and Viswaranjan, S. (1987). Effect of cadmium and mercury on the haematological parameter of the fish Cyprinus carpio. Environ. Ecol. 5, 726–732.
Benson, W. H., Baer, K. N., Stac Khouse, R. A., and Watson, C. F. (1987). Influence of cadmium exposure on selected hematological parameters in freshwater teleost, Notemigonus crysoleucas. Ecotoxicol. Environ. Saf. 13, 92–96. doi:10.1016/0147-6513(87)90046-7
Bhaskar, B. R., and Rao, K. S. (1989). Influence of environmental variables on haematological ranges of milkfish, Chanos chanos (Forskal), in brackishwater culture. Aquaculture 83, 123–136. doi:10.1016/0044-8486(89)90066-5
Bhatia, S. C. (2017). in Pollution control in textile industry. Editor S. Devraj. 1st ed. (Daryaganj, New Delhi: WPI Publishing). doi:10.1201/9781315148588
Bhatkar, N. V. (2011). Chromium (III) induced haematological alterations in Indian common carp, Labeo rohita (Ham.). J. Appl. Nat. Sci. 3, 258–263. doi:10.31018/jans.v3i2.192
Bravo, A. G., and Cosio, C. (2019). Biotic formation of methylmercury: A bio–physico–chemical conundrum. Limnol. Oceanogr. 65, 1010–1027. doi:10.1002/lno.11366
Briffa, J., Sinagra, E., and Blundell, R. (2020). Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 6, e04691. doi:10.1016/j.heliyon.2020.e04691
Calabrese, A., Thurberg, F. P., Dawson, M. A., and Wenzloff, D. R. (1975). Sublethal physiological stress induced by cadmium and mercury in the winter flounder, Pseudopleuronectes americanus. In Sublethal effects of toxic chemicals on aquatic animals (J. H. Koeman, and J. J. T. W. A. Strik, eds.), pp. 15–21. Elsevier. Amsterdam.
Canli, M. (1995). Effects of mercury, chromium and nickel on some blood parameters in the carp Cyprinus carpio. Tr. J Zoology. 19, 305–311.
Carvalho, C. S., and Fernandes, M. N. (2006). Effect of temperature on copper toxicity and hematological responses in the neotropical fish Prochilodus scrofa at low and high pH. Aquaculture 251, 109–117. doi:10.1016/j.aquaculture.2005.05.018
Celik, E. S., Kaya, H., Yılmaz, S., Akbulut, M., and Tulgar, A. (2013). Effects of zinc exposure on the accumulation, haematology and immunology of Mozambique tilapia, Oreochromis mossambicus. Afr. J. Biotechnol. 12, 744–753. doi:10.5897/AJB12.1408
Chatterjee, A., Das, D., and Chakraborti, D. (1993). A study of ground water contamination by arsenic in the residential area of behala, Calcutta due to industrial pollution. Environ. Pollut. 80, 57–65. doi:10.1016/0269-7491(93)90010-l
Christensen, G. M., McKim, J. M., Brungs, W. A., and Hunt, E. P. (1972). Changes in the blood of the brown bullhead (Ictalurus nebulosus (Lesueur)) following short and long term exposure to copper(II). Toxicol. Appl. Pharmacol. 23, 417–427. doi:10.1016/0041-008x(72)90044-0
Cicero, L. H., Barrella, W., and Rotundo, M. (2014). Haematological parameters of fish: Procedures for environmental analysis. Unisanta Biosci. 3, 5063.
Cicik, B., and Engin, K. (2005). The Effect of cadmium on levels of glucose in serum, glycogen reserves in the liver, muscle tissues of Cyprinus carpio (L. 1758). Turk. J. Veter. Anim. Sci. 29, 113–117.
Ciftci, N., Karayakar, F., Ay, Ö., Cicik, B., and Erdem, C. (2015). Effects of copper and lead on some haematological parameters of Oreochromis niloticus. Fresen. Environ. Bull. 24, 2771–2775.
Ciftci, N. S., Cicik, B., Erdem, C., and Ay, O. (2008). Effects of lead concentrations on sera parameters and hematocrit levels in Anguilla anguilla (Linnaeus, 1758). JFS. Com. 2, 616–622. doi:10.3153/jfscom.2008025
Ciji, P. P., and Bijoy Nandan, S. (2014). Toxicity of copper and zinc to Puntius parrah (Day, 1865). Mar. Environ. Res. 93, 38–46. doi:10.1016/j.marenvres.2013.11.006
Cogun, H. Y., and Sahin, M. (2013). The effect of lead and zeolite on hematological and some biochemical parameters in nile fish (Oreochromis niloticus). Curr. Prog. Biol. Res., 277–286. doi:10.5772/53076
Cyriac, P. J., Antony, A., and Nambisan, P. N. K. (1989). Hemoglobin and hematocrit values in the fish Oreochromis mossambicus (Peters) after short term exposure to copper and mercury. Bull. Environ. Contam. Toxicol. 43, 315–320. doi:10.1007/BF01701764
Deckelbaum, R. J., and Torrejon, C. (2012). The omega-3 fatty acid nutritional landscape: Health benefits and sources. J. Nutr. 142, 587S–591S. doi:10.3945/jn.111.148080
Dekar, M. P., and Magoulick, D. D. (2013). Effects of predators on fish and crayfish survival in intermittent streams. Southeast. Nat. 12, 197–208. doi:10.1656/058.012.0115
Dethloff, G. M., Schlenk, D., Khan, S., and Bailey, H. C. (1999). The effects of copper on blood and biochemical parameters of rainbow trout (Oncorhynchus mykiss). Arch. Environ. Contam. Toxicol. 36, 415–423. doi:10.1007/pl00006614
Dhanapakiam, P., and Ramasamy, V. (2001). Toxic effects of copper and zinc mixtures on some haematological and biochemical parameters in common carp, Cyprinus carpio (Linn). J. Environ. Biol. 22, 105–111.
Dharam, S., Kamlesh, N., Trivedi, S. P., and Sharma, Y. K. (2008). Impact of copper on haematological profile of freshwater fish, Channa punctatus. J. Environ. Biol. 29, 253–257.
Dos Santos, C. R., Cavalcante, A. L. M., Hauser-Davis, R. A., Lopes, R. M., and Da Costa Mattos, R. D. C. O. (2016). Effects of sub-lethal and chronic lead concentrations on blood and liver ALA-D activity and hematological parameters in Nile tilapia. Ecotoxicol. Environ. Saf. 129, 250–256. doi:10.1016/j.ecoenv.2016.03.028
Drastichova, J., Svobodova, Z., Luskova, V., and Machova, J. (2004). Effect of cadmium on hematological indices of common carp (Cyprinus carpio L.). Bull. Environ. Contam. Toxicol. 72, 725–732. doi:10.1007/s00128-004-0305-4
Dutta, H. M., and Dalal, R. (2008). The effect of endosulfan on the ovary of bluegill sunfish: A histopathological study (Lepomis macrochirus sp). Int. J. Environ. Res. 2, 215–224.
Eisler, R. (1998). Nickel hazards to fish, wildlife, and invertebrates: A synoptic review. Report USGS/BRD/BSR-1998-0001. US: Geological Survey, Biological Science.
Elseady, Y., and Zahran, E. (2013). Ameliorating effect of β-carotene on antioxidant response and hematological parameters of mercuric chloride toxicity in Nile tilapia (Oreochromis niloticus). Fish. Physiol. Biochem. 39, 1031–1041. doi:10.1007/s10695-012-9760-8
Farombi, E. O., Adelowo, O. A., and Ajimoko, Y. R. (2007). Biomarkers of oxidative stress and heavy metal levels as indicators of environmental pollution in african cat fish (Clarias gariepinus) from Nigeria ogun river. Int. J. Environ. Res. Public Health 4, 158–165. doi:10.3390/ijerph2007040011
Fazio, F. (2019). Fish hematology analysis as an important tool of aquaculture: A review. Aquaculture 500, 237–242. doi:10.1016/J.AQUACULTURE.2018.10.030
Fish, J. T. (2009). Groundwater water treatment for iron and manganese reduction and fish rearing studies applied to the design of the Ruth Burnett Sport Fish Hatchery, Fairbanks, Alaska. Aquac. Eng. 41, 97–108. doi:10.1016/j.aquaeng.2009.06.005
Fletcher, T. C., and White, A. (1986). Nephrotoxic and haematological effects of mercuric chloride in the plaice (Pleuronectes platessa L.). Aquat. Toxicol. 8, 77–84. doi:10.1016/0166-445X(86)90054-8
Gabriel, U. U., Ezeri, G. N. O., and Opabunmi, O. O. (2004). Influence of sex, source, health status and acclimation on the haematology of Clarias gariepinus (Burch, 1822). Afr. J. Biotechnol. 3, 463–467. doi:10.5897/AJB2004.000-2090
Gallaugher, P. E. (1994). “The role of haematocrit in oxygen transport and swimming in salmonid fishes,”. PhD Thesis (UK: Simon Fraser University), 129.
Garg, V. K., Garg, S. K., and Tyagi, S. K. (1989). Manganese induced haematological and biochemical anomalies in Heteropneustes fossilis. J. Environ. Biol. 10, 349–353.
Gautam, P. K., Gautam, R. K., Banerjee, S., Chattopadhyaya, M. C., and Pandey, J. D. (2016). Heavy metals in the environment: Fate, transport, toxicity and remediation technologies, 60. Hauppauge, New York, United States: Nova Sci Publishers, 101–130.
Gebrekiros, S. T. (2016). Factors affecting stream fish community composition and habitat suitability. J. Aquac. Mar. Biol. 4, 00076. doi:10.15406/jamb.2016.04.00076
Ghazaly, K. S. (1992). Sublethal effects of nickel on carbohydrate metabolism, blood and mineral contents of Tilapia nilotica. Water Air Soil Pollut. 64, 525–532. doi:10.1007/bf00483362
Gill, T. S., and Epple, A. (1993). Stress-related changes in the hematological profile of the American eel (Anguilla rostrata). Ecotoxicol. Environ. Saf. 25, 227–235. doi:10.1006/eesa.1993.1021
Gill, T. S., and Pant, J. C. (1987). Hematological and pathological effects of chromium toxicosis in the freshwater fish, Barbus conchonius Ham. Water Air Soil Pollut. 35, 241–250. doi:10.1007/bf00290933
GracePavithra, K., Jaikumar, V., Kumar, P. S., and SundarRajan, P. (2019). A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 228, 580–593. doi:10.1016/J.JCLEPRO.2019.04.117
Guo, Y. L., and Hsu, P. C. (2002). Antioxidant nutrients and lead toxicity. Toxicology 180, 33–44. doi:10.1016/s0300-483x(02)00380-3
Hamidipoor, F., Pourkhabbaz, H. R., Banaee, M., and Javanmardi, S. (2015). Sub-lethal toxic effects of deltamethrin on blood biochemical parameters of Japanese quail, Coturnix japonica. Toxicol. Environ. Chem. 97, 1217–1225. doi:10.1080/02772248.2015.1093131
Han, J. M., Park, H. J., Kim, J. H., Jeong, D. S., and Kang, J. U. (2019). Toxic effects of arsenic on growth, hematological parameters, and plasma components of starry flounder, Platichthys stellatus, at two water temperature conditions. Fish. Aquat. Sci. 22, 3–8. doi:10.1186/s41240-019-0116-5
Hanan, S. G., El-Kasheif, M. A., Ibrahim, S. A., and Authman, M. M. N. (2013). Effect of water pollution in El-Rahawy drainage canal on hematology and organs of freshwater fish Clarias gariepinus. World Appl. Sci. J. 21, 329–341. doi:10.5829/idosi.wasj.2013.21.3.71192
Haux, C., and Larsson, A. (1984). Long-term sublethal physiological effects on rainbow trout, Salmo gairdneri, during exposure to cadmium and after subsequent recovery. Aquat. Toxicol. 5, 129–142. doi:10.1016/0166-445X(84)90004-3
Heath, A. G. (1995). Water pollution and fish physiology. 2nd Edn. New York and London: Lewis Publisher, 359.
Hedayati, A., and Ghaffari, Z. (2013). Effect of mercuric chloride on some hematological, biochemical parameters in silver carp (Hypophthalmichthys molitrix). Int. J. Vet. Med Res Rep. 20, 1–11. doi:10.5171/2013.183410
Herawati, N., Suzuki, S., Hayashi, K., Rivai, I. F., and Koyama, H. (2000). Cadmium, copper, and zinc levels in rice and soil of Japan, Indonesia, and China by soil type. Bull. Environ. Contam. Toxicol. 64, 33–39. doi:10.1007/s001289910006
Hicks, C. C., Cohen, P. J., Graham, N. A. J., Nash, K. L., Allison, E. H., D'lima, C., et al. (2019). Harnessing global fisheries to tackle micronutrient deficiencies. Nature 574, 95–98. doi:10.1038/s41586-019-1592-6
Hilmy, A. M., El-Domiaty, N. A., Daabees, A. Y., and Abdel-Latife, H. A. (1987). Some physiological and biochemical indices of zinc toxicity in two freshwater fishes, Clarias lazera and Tilapia zilli. Comp. Biochem. Physiology Part C Comp. Pharmacol. 87, 297–301. doi:10.1016/0742-8413(87)90011-9
Hilmy, A. M., Shabana, M. B., and Daabees, A. Y. (1985). Bioaccumulation of cadmium: Toxicity in Mugil cephalus. Comp. Biochem. Physiology Part C Comp. Pharmacol. 81, 139–144. doi:10.1016/0742-8413(85)90105-7
Hilmy, A. M., Shabana, M. B., and Said, M. M. (1980). Haematological responses to mercury toxicity in the marine teleost, Aphanius dispar (RÜPP). Comp. Biochem. Physiology Part C Comp. Pharmacol. 67C, 147–158. doi:10.1016/0306-4492(80)90010-6
Hong, Ya., Liao, W., Yan, Z. f., Bai, Y. c., Feng, C. l., Xu, Z. x., et al. (2020). Progress in the research of the toxicity effect mechanisms of heavy metals on freshwater organisms and their water quality criteria in China. J. Chem. 2020, 1–12. doi:10.1155/2020/9010348
Hong, Y. S., Kim, Y. M., and Lee, K. E. (2012). Methylmercury exposure and health effects. J. Prev. Med. Public Health 45, 353–363. doi:10.3961/jpmph.2012.45.6.353
Howe, P. D., Malcolm, H. M., and Dobson, S. (2004). Manganese and its compound: Environmental aspects. Concise international chemical assessment document. 63rd ed. NewYork: World Health Organization.
Iqbal, M. J., Ali, S. S., and Shakoori, A. R. (1997). Toxicity of lead in fresh water fish Arrhina mrigala: Haematological changes. J. Ecotoxicol. Environ. Monit. 7, 139–143.
Ishikawa, N. M., Ranzani-Paiva, M. J. T., Lombardi, J. V., and Ferreira, C. M. (2007). Haematological parameters in Nile Tilapia Oreochromis niloticus exposed to sublethal concentrations of mercury. Braz. J. Zool. 50, 619–626. doi:10.1590/S1516-89132007000400007
Jacobs, J. A., and Testa, S. M. (2005). “Overview of chromium(VI) in the environment: Background and history,” in Chromium (VI) handbook. Editors J. Guertin, J. A. Jacobs, and C. P. Avakian (Boca Raton, Fl: CRC Press), 1–22.
Jarup, L. (2003). Hazards of heavy metal contamination. Br. Med. Bull. 68, 167–182. doi:10.1093/bmb/ldg032
Jaishankar, M., Tseten, T., Anbalagan, N., Mathew, B., and Beeregowda, K. (2014). Toxicity, mechanism and health effects of some heavy metals. Interdisciplinary Toxicol. 7, 60–72. doi:10.2478/intox-2014-0009
Javed, M., Ahmad, I., Ahmad, A., Usmani, N., and Ahmad, M. (2016). Studies on the alterations in haemological indices, micronuclei induction and pathological marker enzyme activities in Channa punctatus (spotted snakehead) perciformes, channidae exposed to the thermal power plant efuent, 5. Berlin, Germany: Springer, 761. doi:10.1186/s40064-016-2478-9
Javed, M., and Usmani, N. (2015). Stress response of biomolecules (carbohydrate, protein and lipid profiles) in fish Channa punctatus inhabiting river polluted by Thermal Power Plant effluent. Saudi J. Biol. Sci. 22, 237–242. doi:10.1016/j.sjbs.2014.09.021
Javed, M., and Usmani, N. (2012). Toxic effects of heavy metals (Cu, Ni, Fe Co, Mn, Cr, Zn) to the haematology of Mastacembelus armatus thriving in Harduaganj Reservoir, Aligarh, India. Glob. J. Med. Res. 12, 59–64.
Johansson-Sjobeck, M., and Larsson, A. (1979). Effects of inorganic lead on delta-aminolevulinic acid dehydratase activity and hematological variables in the rainbow trout, Salmo gairdnerii. Arch. Environ. Contam. Toxicol. 8, 419–431. doi:10.1007/BF01056348
Johansson-Sjobeck, M., and Larsson, A. (1978). The effect of cadmium on the hematology and on the activity of delta-aminolevulinic acid dehydratase (ALA-D) in blood and hematopoietic tissues of the flounder, Pleuronectes flesus L. Environ. Res. 17, 191–204. doi:10.1016/0013-9351(78)90021-x
Juneja, C. J., and Mahajan, C. L. (1983). Haematological and haemopoietic changes in fish Channa punctatus due to mercury pollution in water. Indian J. Anim. Res. 17, 63–71.
Jyoti, S., and Seema, L. (2014). Effect of Manganese on haematological parameters of fish, Garra gotyla gotyla. J. Entomol. Zool. Stud. 2, 77–81.
Karbassi, R., Bayati, I., and Moattar, F. (2006). Origin and chemical partitioning of heavy metals in riverbed sediments. Int. J. Environ. Sci. Technol. (Tehran). 3, 35–42. doi:10.1007/BF03325905
Karuppasamy, R., Subathra, S., and Puvaneswari, S. (2005). Haematological responses to exposure to sublethal concentration of cadmium in air breathing fish, Channa punctatus (Bloch). J. Environ. Biol. 26, 123–128.
Kavitha, C., Malarvizhi, A., Senthil Kumaran, S., and Ramesh, M. (2010). Toxicological effects of arsenate exposure on hematological, biochemical and liver transaminases activity in an Indian major carp, Catla catla. Food Chem. Toxicol. 48, 2848–2854. doi:10.1016/j.fct.2010.07.017
Khalesi, K., Abedi, Z., Behrouzi, S., and Eskandari, S. K. (2017). Haematological, blood biochemical and histopathological effects of sublethal cadmium and lead concentrations in common carp. Bulg. J. Vet. Med. 20, 141–150. doi:10.15547/bjvm.965
Khangarot, B. S., Rathore, R. S., and Tripathi, D. M. (1999). Effects of chromium on humoral and cell-mediated immune responses and host resistance to disease in a Freshwater catfish, Saccobranchus fossilis (Bloch). Ecotoxicol. Environ. Saf. 43, 11–20. doi:10.1006/eesa.1998.1722
Ko, H. D., Park, H. J., and Kang, J. C. (2019). Change of growth performance, hematological parameters, and plasma component by hexavalent chromium exposure in starry flounder, Platichthys stellatus. Fish. Aquat. Sci. 22, 9. doi:10.1186/s41240-019-0124-5
Kori-Siakpere, O., and Ubogu, E. O. (2008). Sublethal haematological effects of zinc on the freshwater fish, Heteroclarias sp. (Osteichthyes: Clariidae). Afr. J. Biotechnol. 7, 2068–2073. doi:10.5897/AJB07.706
Kumar, R., and Banerjee, T. K. (2016). Arsenic induced hematological and biochemical responses in nutritionally important catfish Clarias batrachus (L.). Toxicol. Rep. 3, 148–152. doi:10.1016/j.toxrep.2016.01.001
Kumar, R., and Banerjee, T. K. (2012). Impact of sodium arsenite on certain biomolecules of nutritional importance of the edible components of the economically important catfish C. batrachus (Linn.). Ecol. Food Nutr. 51, 114–127. doi:10.1080/03670244.2012.661330
Lal Shah, S. (2010). Haematological changes in Tinca tinca after exposure to lethal and sublethal doses of Mercury, Cadmium and Lead. Iran. J. Fish. Sci. 9, 434–443.
Larsson, A., Haux, C., Sjobeck, M. L., and Lithner, G. (1984). Physiological effects of an additional stressor on fish exposed to a simulated heavy-metal-containing effluent from a sulfide ore smeltery. Ecotoxicol. Environ. Saf. 8, 118–128. doi:10.1016/0147-6513(84)90055-1
Lavanya, S., Mathan, R., Chokkalingam, K., and Annamalai, M. (2011). Hematological, biochemical and ionoregulatory responses of Indian major carp Catla catla during chronic sublethal exposure to inorganic arsenic. Chemosphere 82, 977–985. doi:10.1016/j.chemosphere.2010.10.071
Lawrence, M. J., Raby, G. D., Teffer, A. K., Jeffries, K. M., Danylchuk, A. J., Eliason, E. J., et al. (2020). Best practices for non-lethal blood sampling of fish via the caudal vasculature. J. Fish. Biol. 97, 4–15. doi:10.1111/jfb.14339
LeaMaster, B. R., Brock, J. A., Fujioka, R. S., and Nakamura, R. M. (1990). Hematologic and blood chemistry values for Sarotherodon melanotheron and a red hybrid tilapia in freshwater and seawater. Comp. Biochem. Physiology Part A Physiology 97, 525–529. doi:10.1016/0300-9629(90)90121-8
Lellis, B. C., Favaro-Polonio, C. Z., Pamphile, J. A., and Polonio, J. C. (2019). Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innovation 3, 275–290. doi:10.1016/j.biori.2019.09.001
Li, P., Feng, X., and Qiu, G. (2010). Methylmercury exposure and health effects from rice and fish consumption: A review. Int. J. Environ. Res. Public Health 7, 2666–2691. doi:10.3390/ijerph7062666
Li, Z. H., Li, P., and Randak, T. (2011). Evaluating the toxicity of environmental concentrations of waterborne chromium (VI) to a model teleost, Oncorhynchus mykiss: A comparative study of in vivo and in vitro. Comp. Biochem. Physiology Part C Toxicol. Pharmacol. 153C, 402–407. doi:10.1016/j.cbpc.2011.01.005
Luh, M. D., Baker, R. A., and Henley, D. E. (1973). Arsenic analysis and toxicity-a review. Sci. Total Environ. 2, 1–12. doi:10.1016/0048-9697(73)90002-8
Luskova, V. (1997). Annual cycles and normal values of haematological parameters in fishes. Acta Sc. Nat. brno. 31, 70–78.
Maheswaran, R., Devapaul, A., Muralidharan, S., Velmurugan, B., and Ignacimuthu, S. (2008). Haematological studies of freshwater fish, Clarias batrachus (L.) exposed to mercuric chloride. Int. J. Integr. Biol. 2, 49–54.
Majharul Islam, S. M., Rohani, M. F., Zabed, S. A., Islam, M. T., Jannat, R., Akter, Y., et al. (2020). Acute effects of chromium on hemato-biochemical parameters and morphology of erythrocytes in striped catfish Pangasianodon hypophthalmus. Toxicol. Rep. 7, 664–670. doi:10.1016/j.toxrep.2020.04.016
Mallesh, B., Pandey, P. K., Kumar, K., Vennila, A., and Kumar, S. (2015). Bioconcentration of hexavalent chromium in Cirrhinus mrigala (ham 1822): Effect on haematological parameters. J. Bio. Earth Sci. 5, 59–67.
Mansour, S. A., and Sidky, M. M. (2002). Ecotoxicological studies. 3. Heavy metals contaminating water and fish from Fayoum Governorate, Egypt. Food Chem. 78, 15–22. doi:10.1016/S0308-8146(01)00197-2
Martinez, C. B., Nagae, M. Y., Zaia, C. T., and Zaia, D. A. (2004). Acute morphological and physiological effects of lead in the neotropical fish Prochilodus lineatus. Braz. J. Biol. 64, 797–807. doi:10.1590/s1519-69842004000500009
Masindi, V., and Muedi, K. L. (2018). “Environmental contamination by heavy metals,” in Heavy metals. Editors H. E.-D. M. Saleh, and R. F. Aglan (london, UK: IntechOpen), 115–132.
Mateo-Sagasta, J., Zadeh, S. M., Turral, H., and Burke, J. (2017). Water pollution from agriculture: A global review. Colombo, Sri Lanka: Food and Agriculture Organization of the United Nations, Rome and the International Water Management Institute on behalf of the Water Land and Ecosystems Research Program.
Mazon, A. F., Monteiro, E. A. S., Pinheiro, G. H. D., and Fernandes, M. N. (2002). Hematological and physiological changes induced by short-term exposure to copper in the freshwater fish, Prochilodus scrofa. Braz. J. Biol. 62, 621–631. doi:10.1590/s1519-69842002000400010
McKim, J. M., Christensen, G. M., and Hunt, E. P. (1970). Changes in the blood of brook trout (Salvelinus fontinalis) after short-term and long-term exposure to copper. J. Fish. Res. Bd. Can. 27, 1883–1889. doi:10.1139/f70-210
Meier, P. G., Fook, D. C., and Lagler, K. F. (1983). Organochlorine pesticide residues in rice paddies in Malaysia 1981. Bull. Environ. Contam. Toxicol. 30, 351–357. doi:10.1007/bf01610144
Mekkawy, I. A. A., Mahmoud, U., Moneeb, R. H., and SayedAlaa El-Din, . H. (2020). Significance assessment of amphora coffeaeformis in arsenic-induced hemato- biochemical alterations of African catfish (Clarias gariepinus). Front. Mar. Sci. 7. doi:10.3389/fmars.2020.00191
Mekkawy, I. M. M., Mahmoud, U. M., Wassif, E. T., and Naguib, M. (2011). Effects of cadmium on some haematological and biochemical characteristics of Oreochromis niloticus(Linnaeus, 1758) dietary supplemented with tomato paste and vitamin E. Fish. Physiol. Biochem. 37, 71–84. doi:10.1007/s10695-010-9418-3
Mishra, S., and Srivastava, A. K. (1979). Hematology as index of sublethal toxicity of zinc in a freshwater teleost. Bull. Environ. Contam. Toxicol. 22, 695–698. doi:10.1007/bf02027009
Mishra, S., and Srivastava, A. K. (1980). The acute toxic effects of copper on the blood of a teleost. Ecotoxicol. Environ. Saf. 4, 191–194. doi:10.1016/0147-6513(80)90019-6
Mohanty, B. P., Mahanty, A., Ganguly, S., Mitra, T., Karunakaran, D., and Anandan, R. (2019). Nutritional composition of food fishes and their importance in providing food and nutritional security. Food Chem. 293, 561–570. doi:10.1016/j.foodchem.2017.11.039
Moosavi, M. J., and Shamushaki, V. A. J. (2015). Effect of sub-acute exposure to Nickel on haematological and biochemical indices in gold fish (Carassius auratus). J. Clin. Toxicol. 5, 1–5. doi:10.4172/2161-0495.1000228
Mousavi, S. E., and Yousefian, M. (2012). The alterations in the hematological parameters of endangered caspian Brown trout, Salmo trutta caspius, exposed to waterborne mercuric chloride. Asian J. Anim. Sci. 6, 154–163. doi:10.3923/ajas.2012.154.163
Muneesh, Kumar., and Mansa, Ram. (2016). Toxicity of some heavy metals on blood characteristics of freshwater fish Clarias batrachus. Int. J. Fish. Aquat. Stud. 4, 85–89.
Musa, S. O., and Omoregie, E. (1999). Haematological changes in the mud fish, Clarias gariepinus (burchell) exposed to malachite green. J. Aquat. Sci. 14, 37–42. doi:10.4314/jas.v14i1.19971
Naz, S., Hussain, R., Ullah, Q., Chatha, A. M. M., Shaheen, A., and Khan, R. U. (2021). Toxic effect of some heavy metals on hematology and histopathology of major carp (Catla catla). Environ. Sci. Pollut. Res. 28, 6533–6539. doi:10.1007/s11356-020-10980-0
Nordberg, G. F., Nogawa, K., Nordberg, M., and Friedmann, J. M. (2007). “Cadmium,” in Handbook on the toxicology of metals. Editors G. F. Nordberg, B. A. Fowler, M. Nordberg, and L. Friberg (Amsterdam: Elsevier), 445–486.
Nurullah, M., Kamal, M., Wahab, M. A., Islam, M. N., Ahasan, C. T., and Thilsted, S. H. (2003). “Nutritional quality of some small indigenous fish species of Bangladesh,” in Small indigenous species of fish in Bangladesh (MA Wahab. Editors S. H. Thilsted, and M. E. Hoq (Mymensingh, Bangladesh: Bangladesh Agricultural University), 151–158.
Oliveira Ribeiro, C. A., Vollaire, Y., Sanchez-Chardi, A., and Roche, H. (2005). Bioaccumulation and the effects of organochlorine pesticides PAH and heavy metals in the eel (Anguilla anguilla) at the Camargue Nature Reserve, France. Aquat. Toxicol. 74, 53–69. doi:10.1016/j.aquatox.2005.04.008
Ololade, I. A., and Ogini, O. (2009). Behavioural and haematological effect of zinc on African catfish, Clarias gariepinus. Int. J. Fish. Aquacult. 1, 022–027.
Ololade, I. A., and Oginni, O. (2010). Toxic stress and haematological effects of nickel on African catfish, Clarias gariepinus, fingerlings. J. Environ. Chem. Ecotoxicol. 2, 014–019.
Oluah, N. S., and Omerebele, U. A. M. (2010). Changes in haematological parameters of Clarias gariepinus exposed to lead poisoning. J. Fish. Inter. 5, 72–76. doi:10.3923/jfish.2010.72.76
Olurin, K. B., Olojo, E. A. A., and Tijani, O. B. (2012). Effect of zinc on haematological parameters of african catfish (Clarias gariepinus). Asian. J. Pharmacol. Health. Sci. 2, 266–272.
Omoregie, E., and Oyebanji, O. (2002). Oxytetracycline-induced blood disorder in juvenile nile Tilapia Oreochromis niloticus (trewavas). J. World Aquac. Soc. 33, 377–382. doi:10.1111/j.1749-7345.2002.tb00514.x
Palaekova, J., Pravda, D., Faoaic, K., and Eelechovska, O. (1994). “Sublethal effects of cadmium on carp (Cyprinus carpio) fingerlings,” in Sublethal chronic effects of pollutants on freshwater fish. Editors R. Muller, and R. Lloyd. Lugano, 53–58.
Pan, K., and Wang, W. X. (2012). Trace metal contamination in estuarine and coastal environments in China. Sci. Total Environ. 421, 3–16. doi:10.1016/j.scitotenv.2011.03.013
Parekh, H. M., and Tank, S. K. (2015). Studies of haematological parameters of Oreochromis niloticus exposed to cadmium chloride (cdcl2, 2h2o). Int. J. Environ. 4, 116–127. doi:10.3126/ije.v4i2.12631
Parthipan, P., and Muniyan, M. (2013). Effect of heavy metal Nickel on haematological parameters of fresh water fish, Cirrhinus mrigala. J. Environ. Curt. Life Sci. 1, 46–55.
Pavlidis, M., Futter, W. C., Katharios, P., and Divanach, P. (2007). Blood cell profile of six Mediterranean mariculture fish species. J. Appl. Ichthyol. 23, 70–73. doi:10.1111/j.1439-0426.2006.00771.x
Polat, F., Akın, S., Yıldırım, A., and Dal, T. (2015). The effects of point pollutants originated heavy metals (lead, copper, iron, and cadmium) on fish living in Yeşilırmak River, Turkey. Toxicol. Ind. Health 32, 1438–1449. doi:10.1177/0748233714565709
Pradhan, S. C., Patra, A. K., Sarkar, B., and Pal, A. (2012). Seasonal changes in hematological parameters of Catla catla (Hamilton 1822). Comp. Clin. Pathol. 21, 1473–1481. doi:10.1007/s00580-011-1316-2
Prado, L. R. G. B., Felix, C., Abessa, D. M. S., Buruaem, L. M., Abujamara, L. D., Kirschbaum, A. A., et al. (2014). Hematological parameters and nuclear abnormalities in peripheral erythrocytes of Achirus lineatus (Pleuronectiformes: Achiridae). Comp. Clin. Path. 24, 169–175. doi:10.1007/s00580-014-1880-3
Praveena, M., Sandeep, V., Kavitha, N., and Rao, J. K. (2013). Impact of tannery effluent, chromium on haematological parameters in a fresh water Fish, Labeo rohita (Hamilton). Res. J. Anim. Vet. Fish. Sci. 1, 1–5.
Rajeshkumar, S., and Li, X. (2018). Bioaccumulation of heavy metals in fish species from the meiliang bay, taihu lake, China. Toxicol. Rep. 5, 288–295. doi:10.1016/j.toxrep.2018.01.007
Remyla, S. R., Sajwan, K. S., Ramesh, M., and Kurunthachalam, S. K. (2008). Influence of zinc on cadmium-induced haematological and biochemical responses in a freshwater teleost fish Catla catla. Fish. Physiol. Biochem. 34, 169–174. doi:10.1007/s10695-007-9157-2
Roja, S. J. M., Abbas, B., Maryam, G., Seyed, M. V. F., Maryam, T., et al. (2013). Impact of copper sulphate on haematological and some biochemical parameters of common carp (Cyprinus carpio L., 1758) in different ph. World J. Fish Mar. Sci. 5, 486–491. doi:10.5829/idosi.wjfms.2013.05.05.73159
Romeo, M., Siau, Y., Sidoumou, Z., and Gnassia-Barelli, M. (1999). Heavy metal distribution in different fish species from the Mauritania coast. Sci. Total Environ. 232, 169–175. doi:10.1016/s0048-9697(99)00099-6
Ruparella, S. G., Verma, Y., Saiyed, S. R., and Rawal, U. M. (1990). Effect of cadmium on blood of tilapia Oreochromis mossambicus (Peters) during prolonged exposure. Bull. Environ. Contam. Toxicol. 45, 305–312. doi:10.1007/BF01700200
Sancho, E., Ceron, J. J., and Ferrando, M. D. (2000). Cholinesterase activity and hematological parameters as biomarkers of sublethal molinate exposure in Anguilla anguilla. Ecotoxicol. Environ. Saf. 46, 81–86. doi:10.1006/eesa.1999.1888
Saroch, J. D., Nisar, H., Shrivastav, R., Qureshi, T. A., and Manohar, S. (2012). Haematological studies of mercuric chloride affected freshwater Catfish Clarias gariepinus fed with Spirulina. J. Chem. Bio. Phys. Sci. B2, 1862–1869.
Satheeshkumar, P., Ananthan, G., Senthilkumar, D., Khan, A. B., and Jeevanantham, K. (2012). Comparative investigation on haematological and biochemical studies on wild marine teleost fishes from Vellar estuary, southeast coast of India. Comp. Clin. Pathol. 21, 275–281. doi:10.1007/s00580-010-1091-5
Schiffman, R. H., and Fromm, P. O. (1959). Chromium induced changes in the blood of rainbow trout (Salmo gairdneri). Sew. Ind. Wastes. 31, 205–211.
Seibel, H., Baßmann, B., and Rebl, A. (2021). Blood will tell: What hematological analyses can reveal about fish welfare. Front. Vet. Sci. 8, 616955. doi:10.3389/fvets.2021.616955
Serezeli, R., Akhan, S., and Sonay, F. D. (2011). Acute effects of copper and lead on some blood parameters on Coruh trout (Salmo coruhensis). Afr. J. Biotechnol. 10, 3204–3209. doi:10.5897/AJB10.2505
Sfakianakis, D. G., Renieri, E., Kentouri, M., and Tsatsakis, A. M. (2015). Effect of heavy metals on fish larvae deformities: A review. Environ. Res. 137, 246–255. doi:10.1016/j.envres.2014.12.014
Shah, S. L., and Altindag, A. (2005). Alterations in the immunological parameters of Tench (Tinca tinca L. 1758) after acute and chronic exposure to lethal and sublethal treatments with mercury, cadmium and lead. Turk J. Vet. Anim. Sci. 29, 1163–1168.
Shah, S. L., and Altindag, A. (2004). Hematological parameters of Tench (Tinca tinca L.) after acute and chronic exposure to lethal and sublethal mercury treatments. Bull. Environ. Contam. Toxicol. 73, 911–918. doi:10.1007/s00128-004-0513-y
Shah, S. L. (2006). Hematological parameters in tench Tinca tinca after short term exposure to lead. J. Appl. Toxicol. 26, 223–228. doi:10.1002/jat.1129
Shaheen, T., and Akhtar, T. (2012). Assessment of chromium toxicity in Cyprinus carpio through hematological and biochemical blood markers. Turkish J. Zoology 36, 682–690. doi:10.3906/zoo-1102-21
Shakoori, A. R., Iqbal, M. J., Mughal, A. L., and Ali, S. S. (1994). Biochemical changes induced by inorganic mercury on the blood, liver and muscles of freshwater Chinese grass carp, Ctenopharyngodon idella. J. Ecotox. Environ. Mon. 4, 81–92.
Sheikh, Z. A., and Ahmed, I. (2016). Seasonal changes in haematological parameters of snow trout Schizothorax plagiostomus (Heckel 1838). Int. J. Fauna Biol. Stud. 3, 33–38.
Shokr, E. A. M. (2015). Effect of zinc on hematology and biochemistry of Nile Tilapia. J. Chem. Pharm. Res. 7, 1943–1950.
Siakpere, O. K., and Ikomi, U. (2011). Alterations in some haematological parameters of the african snakehead: Parachanna africans exposed to cadmium. Not. Sci. Biol. 3, 29–34. doi:10.15835/nsb346299
Singh, D., Nath, K., Trivedi, S. P., and Sharma, Y. K. (2008). Impact of copper on haematological profile of freshwater fish, Channa punctatus. J. Environ. Biol. 29, 253–257.
Singh, S., Srivastava, A. K., Kovacs, D., Benatar, D., Khosla, S., and Singh, H. (2015). A rare case of a intracardiac lipoma. Int. J. Surg. Case Rep. 15, 105–108. doi:10.1016/j.ijscr.2015.02.024
Skonberg, D. I., and Perkins, B. L. (2002). Nutrient composition of green crab (Carcinus maenus) leg meat and claw meat. Food Chem. 77, 401–404. doi:10.1016/s0308-8146(01)00364-8
Spry, D. J., and Wiener, J. G. (1991). Metal bioavailability and toxicity to fish in low-alkalinity lakes: A critical review. Environ. Pollut. 71, 243–304. doi:10.1016/0269-7491(91)90034-t
Srivastava, A. K., and Agrawal, S. J. (1979). Haematological anomalies in a fresh water teleost, Colisa fasciatus, on acute exposure to cobalt. Acta Pharmacol. Toxicol. (Copenh). 44, 197–199. doi:10.1111/j.1600-0773.1979.tb02317.x
Strik, J. J. T. W. A., De Iongh, H. H., Van Rijn van Alkemade, J. W. A., and Wuite, T. P. (1975). “Toxicity of cromium (VI) in fish, with special reference to organ weights, liver and plasma enzyme activities, blood parameters and histological alterations,” in Sublethal effects of toxic chemicals on aquatic animals. Editors J. H. Koeman, and J. J. T. W. A. Strik (Amsterdam, Netherlands: Elsiever), 31–41.
Svetina, A., Matasin, Z., Tofant, A., Vueemilo, M., and Fijan, N. (2002). Haematology and some blood chemical parameters of young carp till the age of three years. Acta Vet. hung. 50, 459–467. doi:10.1556/AVet.50.2002.4.8
Svobodova, Z., Vykusova, B., and Machova, J. (1994). in Sublethal chronic effects of pollutants on freshwater fish. Editors R. Muller ir, and R. L. Lugano, 39–52.
Tacon, A. G. J. (2020). Trends in global aquaculture and aquafeed production: 2000–2017. Rev. Fish. Sci. Aquac. 28, 43–56. doi:10.1080/23308249.2019.1649634
Tadesse, G. L., and Guya, T. K. (2017). Impacts of tannery effluent on environments and human health: A review article. Adv. Life Sci. Technol. 54, 58–67.
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., and Sutton, D. J. (2012). “Heavy metal toxicity and the environment,” in Molecular, clinical and environmental toxicology, volume 3 environmental Toxicology. Editor A. Luch (New York, USA: Springer), 133–164.
Tewari, H., Gill, T. S., and Pant, J. (1987). Impact of chronic lead poisoning on the hematological and biochemical profiles of a fish, Barbus conchonius (Ham). Bull. Environ. Contam. Toxicol. 38, 748–752. doi:10.1007/BF01616696
Teunen, L., De Jonge, M., Malarvannan, G., Covaci, A., Belpaire, C., Focant, J., et al. (2021). Effect of abiotic factors and environmental concentrations on the bioaccumulation of persistent organic and inorganic compounds to freshwater fish and mussels. Sci. Total Environ. 799. doi:10.1016/j.scitotenv.2021.149448
Thilsted, S. H., Thorne-Lyman, A., Webb, P., Bogard, J. R., Subasinghe, R., Phillips, M. J., et al. (2016). Sustaining healthy diets: The role of capture fisheries and aquaculture for improving nutrition in the post-2015 era. Food Policy 61, 126–131. doi:10.1016/j.foodpol.2016.02.005
Tishanova, V., and Ilieva, N. (1994). Joint influence of Zn and Pb on some haematological indices of the common carp (Cyprinus carpio L.). Annu. Sci. Stud. 83, 119–123.
Tort, L., and Torres, P. (1988). The effects of sublethal concentrations of cadmium on haematological parameters in the dogfish, Scyliorhinus canicula. J. Fish. Biol. 32, 277–282. doi:10.1111/j.1095-8649.1988.tb05361.x
Tripathi, S., Sahu, D. B., Kumar, R., and Kumar, A. (2003). Effect of acute exposure of sodium arsenite (Na3Aso3) on some haematological parameters of Clarias batrachus (common Indian cat fish) in vivo. Indian J. Environ. Health 45, 183–188.
Tyagi, A., and Srivastava, N. (2005). Haematological response of fish Channa punctatus (Bloch) to chronic zinc exposure. J. Environ. Biol. 26, 429–432.
Ubaidullah, M., Javed, M., and Abdullah, S. (2004). Metals toxicity of the river Ravi and tributary waters. Int. J. Biol. Sci. 1, 25–29.
Ukhurebor, K. E., Aigbe, U. O., Onyancha, R. B., Nwankwo, W., Osibote, O. A., Paumo, H. K., et al. (2021). Effect of hexavalent chromium on the environment and removal techniques: A review. J. Environ. Manage. 280, 111809–111825. doi:10.1016/j.jenvman.2020.111809
Ullrich, S. M., Tanton, T. W., and Abdrashitova, S. A. (2001). Mercury in the aquatic environment: A review of factors affecting methylation. Crit. Rev. Environ. Sci. Technol. 31, 241–293. doi:10.1080/20016491089226
US EPA (2009). National recommended water quality criteria. Washington DC: Office of Water, Office of Science and Technology.
Van Der Putte, I., Laurier, M. B. H. M., and Van Eijk, G. J. M. (1982). Respiration and osmoregulation in rainbow trout (Salmo gairdneri) exposed to hexavalent chromium at different pH values. Aquat. Toxicol. 2, 99–112. doi:10.1016/0166-445x(82)90009-1
Van Vuren, J. H. J., Van Der Merwe, M., and Du Preez, H. H. (1994). The effect of copper on the blood chemistry of Clarias gariepinus (Clariidae). Ecotoxicol. Environ. Saf. 29, 187–199. doi:10.1016/0147-6513(94)90019-1
Velma, V., Vutukuru, S. S., and Tchounwou, P. B. (2009). Ecotoxicology of hexavalent chromium in freshwater fish: A critical review. Rev. Environ. Health 24, 129–145. doi:10.1515/reveh.2009.24.2.129
Venkatachalam, T., and Natarajan, A. V. (2014). Haematological investigation on freshwater teleost Labeo rohita (Ham.) following aquatic toxicities of Cr (III) and Cr (VI). Int. J. Res. Biosci. 3, 1–14.
Vera-Candioti, J., Soloneski, S., and Larramendy, M. L. (2011). Acute toxicity of chromium on Cnesterodon decemmaculatus (pisces: Poeciliidae). Theoria 20, 81–88.
Verma, A. K., and Prakash, S. (2019). Impact of arsenic on haematology, condition factor, hepatosomatic and gastrosomatic index of a fresh water cat fish, Mystus vittatus. Int. J. Biol. Sci. 10, 49–54. doi:10.13140/RG.2.2.31567.10409
Vieira, C., Morais, S., Ramos, S., Delerue-Matos, C., and Oliveira, M. B. P. P. (2011). Mercury, cadmium, lead and arsenic levels in three pelagic fish species from the Atlantic Ocean: Intra- and inter-specific variability and human health risks for consumption. Food Chem. Toxicol. 49, 923–932. doi:10.1016/j.fct.2010.12.016
Vieira, M. C., Torronteras, R., Cordoba, F., and Canalejo, A. (2012). Acute toxicity of manganese in goldfish Carassius auratus is associated with oxidative stress and organ specific antioxidant responses. Ecotoxicol. Environ. Saf. 78, 212–217. doi:10.1016/j.ecoenv.2011.11.015
Vinodhini, R., and Narayanan, M. (2008). Bioaccumulation of heavy metals in organs of fresh water fish Cyprinus carpio (Common carp). Int. J. Environ. Sci. Technol. 5, 179–182. doi:10.1007/bf03326011
Vosyliene, M. Z. (1996). Haematological parameters of rainbow trout (Oncorhynchus mykiss) during short-term exposure to copper. Ecology 3, 12–18.
Vosyliene, M. Z., and Jankaite, A. (2006). Effect of heavy metal model mixture on rainbow trout biological parameters. Ekologija 4, 12–17.
Vosyliene, M. Z. (1999). “The effect of heavy metal mixture on haematological parameters of rainbow trout. Heavy metals in environment,” in An integrated approach. Editor D. A. Lovejoy, 295–298.
Vutukuru, S. S. (2005). Acute effects of hexavalent chromium on survival, oxygen consumption, hematological parameters and some biochemical profiles of the Indian major carp, Labeo rohita. Int. J. Environ. Res. Public Health 2, 456–462. doi:10.3390/ijerph2005030010
Wepener, V., Van Vaen, J. H. J., and Du Preez, H. H. (1992a). The effect of hexavalent chromium at different pH values on the haematology of Tilapia sparrmanii (Cichlidae). Comp. Biochem. Physiology Part C Comp. Pharmacol. 101, 375–381. doi:10.1016/0742-8413(92)90290-n
Wepener, V., Van Vuren, J. H. J., and Du Preez, H. H. (1992b). Effect of manganese and iron at a neutral and acidic pH on the hematology of the banded tilapia (Tilapia sparrmanii). Bull. Environ. Contam. Toxicol. 49, 613–619. doi:10.1007/BF00196307
WHO (2011). Guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization, 315–318.
Wilson, R. W., and Taylor, E. W. (1993). The physiological responses of freshwater rainbow trout, Oncorhynchus mykiss, during acutely lethal copper exposure. J. Comp. Physiol. B 163, 38–47. doi:10.1007/bf00309663
Witeska, M. (2021). Haematological methods in fish – Not only for beginners, 547. doi:10.1016/j.aquaculture.2021.737498Aquaculture
Witeska, M., and Jiezerska, B. (1994). The effect of cadmium and lead on selected blood parameters of common carp. Arch. Pol. Fish. 2, 123–132.
Witeska, M., Kondera, E., Szymanska, M., and Ostrysz, M. (2010). Haematological changes in common carp (Cyprinus carpio L.) after short-term lead (Pb) exposure. Pol. J. Environ. Stud. 19, 825–831.
Witeska, M. (2005). Stress in fish: Haematological and immunological effects of heavy metals. Electron. J. Ichthyol. 1, 35–41.
Yang, H., and Rose, N. L. (2003). Distribution of mercury in six lake sediment cores across the UK. Sci. Total Environ. 304, 391–404. doi:10.1016/S0048-9697(02)00584-3
Yousif, R. A., Masyamsir, , Dhahiyat, , Sunarto, , and Zahidah, (2016). Assessment the levels of heavy metals and water quality in Cikuda River, Indonesia. Glob. J. Bio-Sci Biotechnol. 5, 240–244.
Zainuddin, A., Putranto, T. W. C., Irawan, B., and Soegianto, A. (2017). Effect of sub-lethal lead exposure at different salinities on osmoregulation and haematological changes in tilapia, Oreochromis niloticus. Arch. Pol. Fish. 25, 173–185. doi:10.1515/aopf-2017-0017
Keywords: hemoglobin, hematocrit, heavy metal, environmental impacts, fish hematology
Citation: Ahmed I, Zakiya A and Fazio F (2022) Effects of aquatic heavy metal intoxication on the level of hematocrit and hemoglobin in fishes: A review. Front. Environ. Sci. 10:919204. doi: 10.3389/fenvs.2022.919204
Received: 13 April 2022; Accepted: 27 June 2022;
Published: 13 September 2022.
Edited by:
Joginder Singh, Lovely Professional University, IndiaReviewed by:
David Hernandez-Moreno, Spanish National Research Council (CSIC), SpainAhmet Regaib Oğuz, Yüzüncü Yıl University, Turkey
Andi Alijagic, Örebro University, Sweden
Copyright © 2022 Ahmed, Zakiya and Fazio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Imtiaz Ahmed, aW10aWF6YW11MUB5YWhvby5jb20=
†ORCID: orcid.org/0000-0003-4821-1707
 Imtiaz Ahmed
Imtiaz Ahmed Archo Zakiya
Archo Zakiya Francesco Fazio
Francesco Fazio