- College of Plant Protection, Shanxi Agricultural University, Taiyuan, China
Oxadixyl and cymoxanil are widely used for controlling downy mildew in cucumber; however, there are few systematic studies on monitoring residue levels of these two pesticides in cucumber under greenhouse and open field conditions. In this study, a simplified quick, easy, cheap, effective, rugged, and safe (QuEChERS) method was applied to analyze target compounds in cucumber. The average recoveries of oxadixyl and cymoxanil in cucumber ranged from 96% to 102%, with relative standard deviations (RSDs) of 1.8%–4.0%. The limits of quantification (LOQs) for two pesticides were both 0.01 mg/kg. The dissipation of oxadixyl was in accordance with a first-order kinetics equation, with half-lives ranging from 1.8 to 3.1 days. At the pre-harvest interval (PHI) of 3 or 5 days, the residue levels of oxadixyl in cucumber under open field conditions were higher than those under greenhouse conditions. Compared to oxadixyl, the cymoxanil degraded quickly, and its residues were below LOQ on the 3rd or 5th day after the last application. The terminal residues of oxadixyl and cymoxanil in the cucumber were both lower than the maximum residue limits (MRLs) in China. The risk quotient (RQ) used for dietary risk assessment was 1.8%–3.5% and 0.26%–0.51% for oxadixyl and cymoxanil, respectively. The results showed that the risks of these two pesticides used on cucumber at the experimental dosages are comparably acceptable for Chinese consumers of different gender and age groups. This study provides a reference data to use oxadixyl and cymoxanil scientifically and rationally.
Introduction
Cucumber (Cucumis sativus L.), one of the most popular and widely cultivated fruiting vegetables in China, is rich in nutrients such as minerals, vitamins, and sugars, which are beneficial for human health (Shi et al., 2015; Li et al., 2021). Cucumber can be eaten raw or processed, which increases people’s preference for it (Feng et al., 2021). As one of the most economically valuable vegetables, the output of cucumber reached 56 million tons in 2018 (Bian et al., 2020; FAOSTAT, 2020). However, cucumbers are susceptible to a variety of fungal diseases during their cultivation, such as gray mold, downy mildew, and powdery mildew, among which cucumber downy mildew is one of the most serious epidemic and devastating diseases (Granke et al., 2014; Wang et al., 2015; Shirley et al., 2021). Consequently, fungicides are commonly used during cucumber cultivation for increasing cucumber output and improving quality. The unreasonable and excessive application of fungicides resulted in pesticide residues in agricultural products and threatened human health, which have been widely concerned and repeatedly reported (Benbrook and Davis, 2020; Heshmati et al., 2020; Chai et al., 2021; Wang et al., 2021). Hence, it is necessary to study the residues and dissipation of fungicides in cucumbers to ensure food safety as well as human health.
It is well known that the use of a single fungicide may cause resistance to the target, and mixed use of two or three pesticides with different modes of action has been developed in production. Oxadixyl is a systemic phenylamide fungicide with fungistatic and fungitoxic functions and is often applied to control diseases caused by downy mildew and phytophthora on many crops (Mirzoian and Ammann, 2014; Kwon et al., 2015; Liu et al., 2022), while cymoxanil is a new systemic fungicide with the characteristics of high efficiency (to Peronosporales fungi, especially Phytophthora and Peronospora) and low toxicity and is registered to control downy mildew and late blight in tomatoes, cucumbers, potatoes, and grapes (Gisi and Sierotzki, 2008; Cespedes et al., 2013; D'Arcangelo et al., 2021). Cymoxanil is often mixed with other protective fungicides to achieve better control effects. As one of these mixtures, a combination of oxadixyl and cymoxanil is used to control the cucumber downy mildew with good effects. They are both systematic fungicides with protective and curative functions, which could be absorbed by plants through roots and leaves after application, transferred to the edible parts (Pullagurala et al., 2018), and enriched in the food chains (Pirsaheb et al., 2019). However, few studies have been conducted on the residual behavior and dissipation of oxadixyl and cymoxanil in cucumber, which is insufficient to comprehensively evaluate the dietary risk.
In this study, an analytical method was developed for the determination of oxadixyl and cymoxanil, and the terminal residues and dissipation of these two pesticides in cucumbers under field or greenhouse conditions were obtained at 12 sites in China. Combined with the dietary data of different genders and ages and toxicological data, the risks were assessed. The results of this study would be helpful in providing guidance for the rational and safe use of oxadixyl and cymoxanil in cucumber.
Materials and methods
Chemicals and reagents
Standards of oxadixyl (97.69%) and cymoxanil (99.60%) were purchased from Dr. Ehrenstorfer GmbH (Augsburg, Germany). Acetonitrile (HPLC grade) and mass spectrometry-grade formic acid were obtained from Fisher Chemical Co., Ltd. (Waltham, MA, United States). Analytical-grade sodium chloride (NaCl) was purchased from Beijing Tongguang Fine Chemical Co. (Beijing, China). The pesticides 38% oxadixyl + cymoxanil water-dispersible granule (oxadixyl 8% and cymoxanil 30%) was used. The centrifuge tube (50 mL) and syringe filter (0.22 μm) were provided by ANPEL Laboratory Technologies Inc. (Shanghai, China). In addition, ultrapure water was prepared using a Milli-Q Advantage AW system (Millipore, United States).
Solution preparation
The stock solutions were prepared by accurately weighing the standards of oxadixyl (24.5 mg) and cymoxanil (25.0 mg) in 25-mL flasks separately and dissolving them in acetonitrile. A volume of 100 mg/L mixed standard solutions of oxadixyl and cymoxanil was prepared by diluting stock solutions with acetonitrile. All standard solutions were kept at 4°C in the dark before use. The working and matrix-matched standard solutions were prepared freshly when used at the concentrations of 0.005, 0.01, 0.025, 0.05, 0.1, 0.25, 0.50, 1.0, and 2.5 mg/L using acetonitrile or blank sample extracts.
Field trials and sample collection
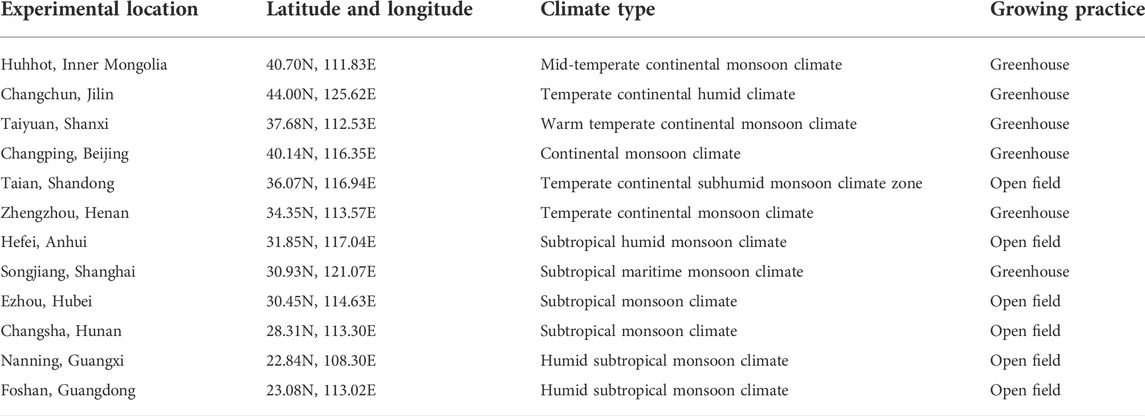
The field experiments were designed according to the pesticide registration information and the Guideline on Pesticide Residue Trials (NY/T 788-2018) issued by the Ministry of Agriculture and Rural Affairs, P. R. of China (Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2018). Field trials were conducted on 12 different production areas, which represented different climates and environmental conditions in China, while six sites were under open field conditions and the others in greenhouses. The latitude and longitude, climate type, and growing practices of 12 locations are shown in Table 1.
In order to test the dissipation and terminal residues of oxadixyl and cymoxanil in cucumber, a treatment area and a control area were set up in the experiment, and each plot was 50 m2 with a 2 m separated area between plots. In the terminal residue experiments, the cucumber plants were sprayed with the recommended high dosage (342 g a.i.·ha−1) three times with an interval of 7 days. Cucumber samples were randomly collected from each plot at 3 and 5 days after the last application. At least 12 normally growing cucumbers with a total weight of at least 2 kg were collected from each plot, and the sample collection was carried out in duplicate according to the guidelines. The cucumber samples from the field trials were cut into small pieces and put into plastic bags.
The dissipation tests were conducted on four sites, including Inner Mongolia, Beijing, Anhui, and Guangxi, under open field or greenhouse conditions, respectively. For the dissipation tests, cucumber samples were collected from treatment plot on 0, 1, 3, 5, and 7 days after the last application using the same sampling method. All test samples were transported to the laboratory within 8 h and kept at −20°C until analysis.
Storage stability test
In our study, the storage stability of oxadixyl and cymoxanil was tested according to the Guideline for the Stability Testing of Pesticide Residues in Stored Commodities of Plant Origin (NY/T 3094-2017, issued by the Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2017). Aliquots of 10 g blank cucumber samples were accurately weighed into a 50-mL centrifuge tube, then a 100 μL standard solution of oxadixyl or cymoxanil was added individually into the tubes with a spiked level of 0.10 mg/kg, and the samples were quickly mixed and stored at ≤ −18°C. The samples were analyzed at the intervals of 0, 30, 66, and 130 days.
Sample preparation
A volume of 10 g homogenized cucumber sample was accurately weighed into a 50-mL centrifuge tube, and then 10 mL of chromatographic-grade acetonitrile was added and well mixed. After ultrasonic extraction for 15 min, 6 g (± 0.05 g) of NaCl was added and then shaken vigorously for 1 min. It was centrifuged at 3,000 rpm for 5 min, and 1 mL of the supernatant was passed through a 0.22-μm membrane and put into the sample vial for UPLC-MS/MS analysis.
UPLC-MS/MS analysis
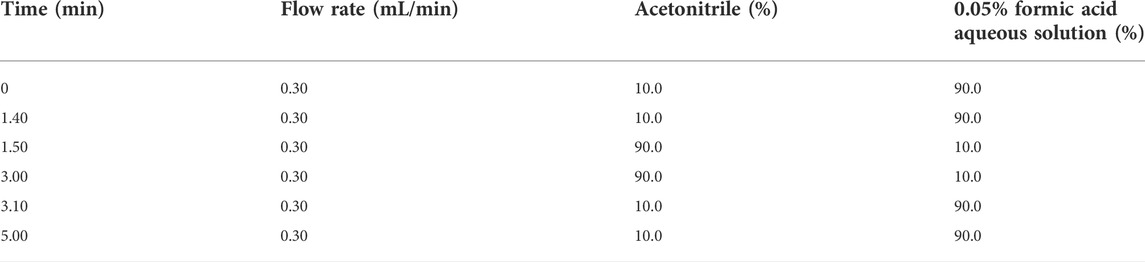
Oxadixyl and cymoxanil were analyzed by ultra-high performance liquid chromatography (Waters ACQUITY UPLC H-Class, Milford, MA, United States) and tandem triple quadrupole mass spectrometry (Waters Corp., Milford, MA, United States) with an electrospray ionization (ESI) source operated in the positive ion mode (ESI+). An ACQUITY UPLC BEH C18 column (100 mm × 2.1 mm, 1.7 μm) was used for chromatographic separation at a temperature of 30°C. The mobile phases consisted of A: acetonitrile and B: 0.05% formic acid in aqueous solution. The gradient elution procedure is shown in Table 2. The flow rate was 0.30 mL/min, and the injection volume was 1 µL. The analysis was finished within 5 min. The capillary voltages were 3,500 V, the taper hole voltages were 15 V, and the ion source temperature was set at 150°C under the positive ion detection mode. For MS detection working conditions, the desolvent gas temperature was set at 500°C, the desolvent gas flow rate at 1,000 L/h, and cone hole gas flow was 5 L/h. Analytes were determined in the multiple reaction monitoring (MRM) mode. The MS/MS parameters of oxadixyl and cymoxanil are listed in Table 3. The representative spectrums of oxadixyl and cymoxanil are shown in Figure 1. MassLynx version 4.1 SCN 9.4 (Waters Corp., Milford, MA, United States) was used for data acquisition and processing.
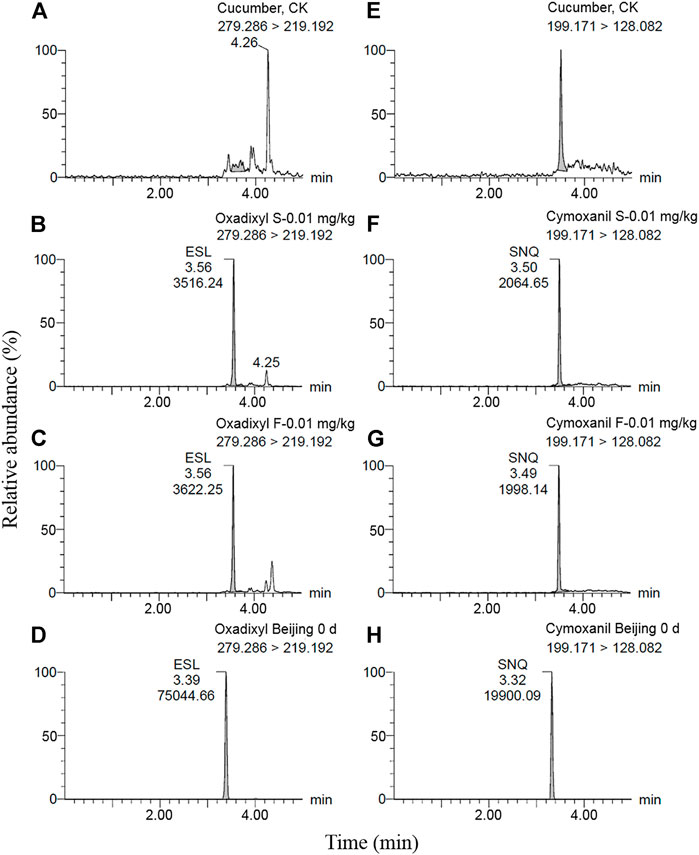
FIGURE 1. LC-MS/MS chromatograms of oxadixyl (A–D) and cymoxanil (E–H) in cucumber, control sample, matrix-matched standard solution (0.01 mg/kg), fortified level (0.01 mg/kg), and cucumber sample (S indicates matrix-matched standard solution, and F indicates the fortified level).
Method validation
According to the EU guidelines (European Commission, 2020), the analytical method was validated by the linearity, matrix effect, limit of quantification (LOQ), recovery, and relative standard deviations (RSDs). The linearity of this method was studied using the solvent standard solution and matrix-matched calibration. The ME was evaluated using the following equation (Ferrer et al., 2011):
A percentage of the matrix effect (ME) between −20% and 20% was considered no matrix effect. A medium matrix effect occurred when the values were between −50% and −20% or 20% and 50%, and a strong matrix effect would be below −50% or above 50%. The LOQ was defined as the lowest spiked level of oxadixyl and cymoxanil in cucumber (Vial et al., 2003; Burns and Valdivia, 2008). According to NY/T 788-2018, the accuracy and precision of this method were evaluated through recovery studies at three spiked levels with five replicates.
Dissipation study
Based on the previous reports, the dissipation rates of oxadixyl and cymoxanil were expressed using the first-order kinetic model and half-life values (Xie et al., 2019). The calculation formulas were as follows:
where Ct (mg/kg) is the residual concentration of the compound at time t (d), C0 (mg/kg) is the initial concentration of the compound, k is the dissipation rate constant, and t1/2 is the half-time of compound degradation.
Chronic dietary risk assessment
The national estimated daily intake (NEDI) and risk quotient (RQ) were used to evaluate chronic dietary risk and calculated using the following formulas:
where STMRi (mg/kg) is the the supervised trials median residue, Fi (kg) is the average daily intake of a certain food in the general population, and bw is the average body weight of the population subgroups. ADI (mg/kg bw) is the acceptable daily intake, and the values were derived from GB 2763-2021 (National Health and Family Planning Commission, Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2021). The corresponding MRLs can be used for NEDI calculation if there is no STMRi data. If the RQ value is ≥100%, the risk would be considered unacceptable; otherwise, the risk was acceptable.
Results and discussion
Method validation
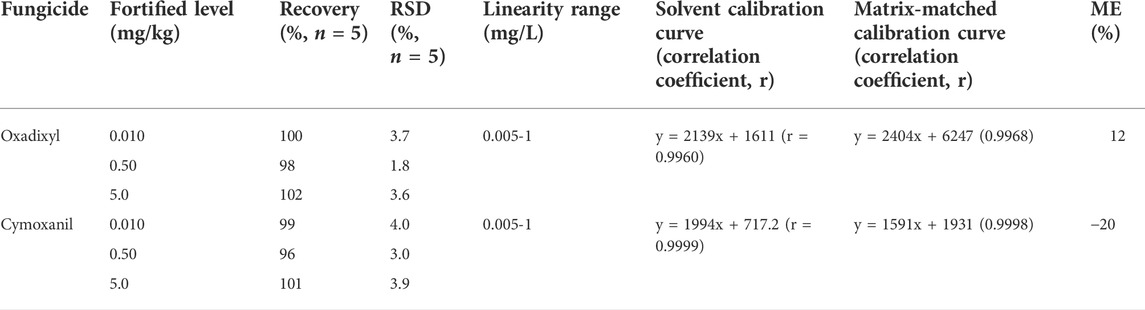
A simplified QuEChERS method was developed for the determination of oxadixyl and cymoxanil in cucumber. The linearity, limits of quantification (LOQs), matrix effect, precision, and accuracy were assessed to validate the performance of this method according to the EU guidelines (European Commission, 2020) and the NY/T 788-2018 (Ministry of Agriculture and Rural Affairs of the People’s Republic of China, 2018). As shown in Table 4, the linearity was obtained for two analytes in cucumber (r = 0.9968 for oxadixyl and cymoxanil r = 0.9998). The average recoveries of oxadixyl and cymoxanil in cucumber were 98%–102% and 96%–101% at spiked levels of 0.010, 0.50, and 5.0 mg/kg with RSDs of 1.8%–3.7% and 3.0%–4.0%, respectively. According to this definition and the recovery experiments, the LOQs of oxadixyl and cymoxanil in cucumber are both 0.01 mg/kg under the aforementioned analysis conditions. The ME values of oxadixyl and cymoxanil in cucumber were 12% and 20%, respectively, indicating that there existed matrix effect for these two pesticides. Hence, to eliminate the matrix effect, the matrix-matched calibration curves of oxadixyl and cymoxanil were implemented for quantitative analysis of test samples in this study.
Overall, these results indicated that the accuracy and precision of the method were acceptable and that it is suitable for the analysis of the residues oxadixyl and cymoxanil in cucumber.
Storage stability test
Field samples were stored in the laboratory for several weeks or even longer before analysis. The target compounds might change or degrade during storage, which might affect the accuracy of the detection. Therefore, the storage stability of pesticides is closely related to the reliability of pesticide residue detection results. In our study, the storage stability of oxadixyl and cymoxanil was evaluated under frozen conditions (≤−18°C) for 130 days. The results of the storage stability test are shown in Table 5. The degradation rates of oxadixyl and cymoxanil in the stored fortified samples were 11% and 13%, respectively, at 130 days of ≤ −18°C frozen storage. According to the Chinese standard NY/T 3094–2017 (Industrial Standard of the People’s Republic of China, 2017) and FAO regulations (Food and Agriculture Organization of the United Nations, 2016), the degradation rate was less than 30% during the storage before analysis, indicating that the target compounds were comparably stable.
Dissipation kinetics in cucumber
In our study, the residue level in the sample 2 h after application was defined as the initial residue level. According to the experimental results, the dissipation curve of oxadixyl in cucumbers from Inner Mongolia (greenhouse), Beijing (greenhouse), Anhui (open field), and Guangxi (open field) are shown in Figure 2. The initial residue of oxadixyl in cucumbers from Inner Mongolia, Beijing, Anhui, and Guangxi were 0.047, 0.17, 0.11, and 0.12 mg/kg after 2 h of spraying. The dissipation dynamics equations of oxadixyl were Ct = 0.0528 e−0.224x (Inner Mongolia), Ct = 0.2333 e−0.390x (Beijing), Ct = 0.1003 e−0.145x (Anhui), and Ct = 0.1191 e−0.041x (Beijing) in cucumber, with half-lives (t1/2) of 3.1, 1.8, 4.8, and 16.9 days, respectively. The maximum initial deposition (0.17 mg/kg) and the fastest dissipation rate (93% after 7 days) both occurred in the samples of Beijing. The minimum initial deposition (0.047 mg/kg) occurred in the samples of Inner Mongolia, and the slowest dissipation rate (27% after 7 days) occurred in the samples of Guangxi, with a half-life of 16.9 days.
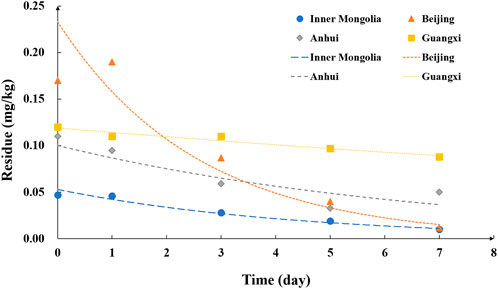
FIGURE 2. Dissipation of oxadixyl in cucumber samples in Inner Mongolia, Beijing, Anhui, and Guangxi.
In contrast to oxadixyl, cymoxanil dissipated rapidly in cucumber, and the kinetic equation was not available. The initial concentrations were 0.059, 0.27, 0.25, and 0.14 mg/kg in cucumbers from Inner Mongolia, Beijing, Anhui, and Guangxi, respectively. After 3 days, the residue of cymoxanil in samples of Beijing was 0.032 and <0.01 mg/kg than those in other three places. The initial residue level of oxadixyl and cymoxanil in cucumber was both below MRL in China, whether in open field or greenhouse. Until now, studies on the dissipation of oxadixyl were limited, while the half-lives of cymoxanil in different crops were reported previously, which were 6.3–6.7 days in the plant of ginseng, 0.5–0.7 days in grape, and 2.26 days in potato (He et al., 2007; Yan et al., 2016; Huang et al., 2019). In our study, cymoxanil dissipated rapidly in cucumber at three field sites, and the kinetic equation was not available for cymoxanil residues, which was consistent with the previous studies (Hong et al., 2018). The dissipation of pesticides in plants is usually related to the physicochemical properties of the pesticide, the climate and experimental conditions, and the growth dilution factor (Zhao et al., 2011). In our study, the environmental parameters, the crop, and the growth factor were all the same for oxadixyl and cymoxanil, and the difference in dissipation was correlated with the physical–chemical properties of these two pesticides.
Terminal residues in cucumber
The pesticide was sprayed three times with a dosage of 342 g a.i./ha at an interval of 7 days, and the terminal residue data of oxadixyl and cymoxanil in cucumber are shown in Table 6. The terminal residue levels of target compounds decreased with the increasing pre-harvest interval time. The PHI was 3 and 5 days; the terminal residues of oxadixyl in cucumber at harvest were <0.01–0.11 and <0.01–0.097 mg/kg, and the terminal residues of cymoxanil in cucumber at harvest were <0.01–0.032 and <0.01 mg/kg, respectively. Therefore, no matter on third or fifth day, the terminal residues of oxadixyl and cymoxanil in cucumbers were both lower than MRLs of China, which were 5 mg/kg for oxadixyl and 0.5 mg/kg for cymoxanil, respectively. The results of cymoxanil were consistent with those reported by Huang et al. (2019). The EU sets the MRL of oxadixyl at 0.01 mg/kg in vegetables (EFSA, 2021), which is much stricter than the limits in China.
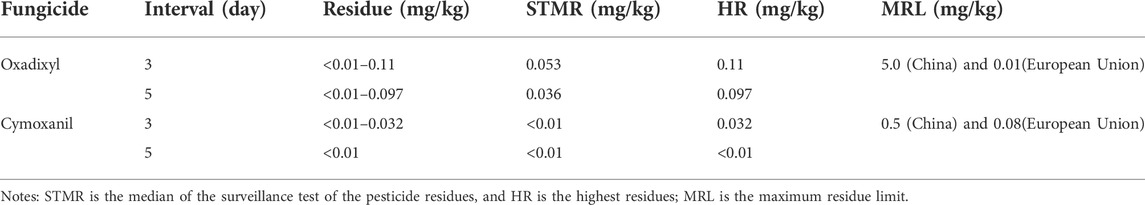
TABLE 6. Terminal residues, median residues, highest residues, and their corresponding MRLs on cucumbers at different intervals.
Effects of cultivation conditions on residues
According to the data of the National Bureau of Statistics, the planting scale of greenhouse vegetables in China is approximately 3.86 million hectares. Cucumber is mainly cultivated in greenhouses in northern China and open fields in southern China. The residue behavior of fungicides in greenhouse-cultivated vegetables is different from those in the open field (Bojac´a et al., 2013; Angioni et al., 2012). Residue levels of oxadixyl and cymoxanil in cucumber were determined in the greenhouse planting process, and the obtained results were compared with those in the open field conditions. Cymoxanil residues are not further considered in our study because the residue levels of cymoxanil were all <0.01 mg/kg except in Beijing (the residue value was 0.032 mg/kg with the PHI of 3 days). The comparison results of oxadixyl are presented in Figure 3 in the order of the smallest to largest in the residue level (residue levels below 0.01 mg/kg were expressed as 0.01 mg/kg). As shown in Figure 3, on PHI of 3 or 5 days, the residue levels of oxadixyl in cucumbers grown in open field are higher than those in greenhouse. This result showed that the greenhouse accelerated the dissipation of oxadixyl in cucumbers. Cultivation conditions and environmental factors could greatly influence the dissipation rate of fungicides in plants. Due to the better water and fertilizer conditions in the greenhouse, cucumbers grow faster than those in the open field, coupled with the fast growth dilution property of cucumbers (Wang et al., 2014), which may result in the quicker dissipation of oxadixyl in greenhouse cucumber than that in the open field.
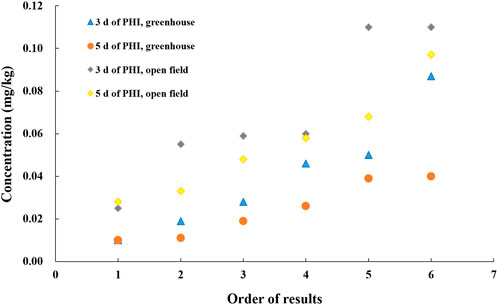
FIGURE 3. Residue levels of oxadixyl in greenhouse and open field cucumbers on different PHI (12 samples from different experiment sites, in the order of smallest to largest in the residue level).
Chronic dietary risk assessment
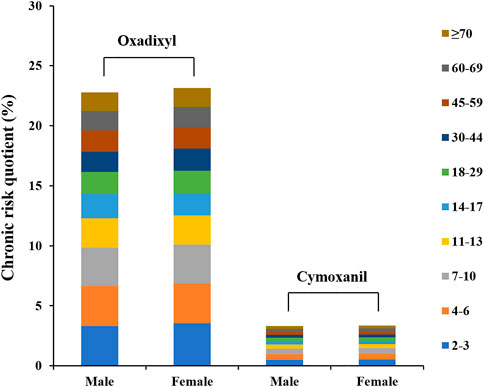
To evaluate the long-term dietary intake risk of oxadixyl and cymoxanil, the NEDI and RQ were used as important parameters. According to the experimental results, the STMR values of oxadixyl and cymoxanil were 0.053 and <0.01 mg/kg for the PHI of 3 days, respectively. The ADI values of oxadixyl and cymoxanil were 0.01 and 0.013 mg/kg bw, respectively. Combined with different dietary patterns of Chinese populations, the NEDI values of oxadixyl and cymoxanil across different age groups and different genders were estimated to be 0.0044–0.011 and 0.00082–0.0021 mg (kg bw·day)−1, while the RQ values were 1.8%–3.5% and 0.26%–0.51%, respectively. It can be seen intuitively from Figure 4 that the dietary risks of the two pesticides generally show a downward trend with age, and females had a greater risk than males with no significant difference. The RQ values were both less than 100%, indicating that the long-term dietary risk of oxadixyl and cymoxanil for Chinese residents of different age groups was low and acceptable.
Conclusion
In this study, the dissipation and terminal residues of oxadixyl and cymoxanil were analyzed in cucumber, and the long-term dietary intake risk was evaluated. In the dissipation study, cymoxanil dissipated faster than oxadixyl in cucumber. The terminal residues of oxadixyl and cymoxanil in cucumber were both lower than the maximum residue limit in China. The RQs of oxadixyl and cymoxanil in cucumber were both less than 100% in the dietary risk assessment, indicating that the long-term dietary risk for Chinese residents of different age groups was acceptable. Up to the present, there has been limited literature on the safety evaluation of oxadixyl, and the results will provide an important reference for the proper use of it in cucumbers.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
Investigation, LL; methodology, JF; supervision, LL; writing–original draft, JF and LL; and writing–review and editing, LL.
Funding
This work was supported by the Fundamental Research Program of Shanxi Province (No. 20210302124131).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Angioni, A., Porcu, L., and Dedola, F. (2012). Determination of famoxadone, fenamidone, fenhexamid and iprodione residues in greenhouse tomatoes. Pest Manag. Sci. 68, 543–547. doi:10.1002/ps.2287
Benbrook, C. M., and Davis, D. R. (2020). The dietary risk index system: A tool to track pesticide dietary risks. Environ. Health 19, 103. doi:10.1186/s12940-020-00657-z
Bian, Y., Guo, G., Liu, F., Chen, X., Wang, Z., and Hou, T. (2020). Meptyldinocap and azoxystrobin residue behaviors in different ecosystems under open field conditions and distribution on processed cucumber. J. Sci. Food Agric. 100, 648–655. doi:10.1002/jsfa.10059
Bojac´a, C. R., Arias, L. A., Ahumada, D. A., Casilimas, H. A., and Schrevens, E. (2013). Evaluation of pesticide residues in open field and greenhouse tomatoes from Colombia. Food control. 30, 400–403. doi:10.1016/j.foodcont.2012.08.015
Burns, M., and Valdivia, H. (2008). Modelling the limit of detection in real-time quantitative PCR. Eur. Food Res. Technol. 226, 1513–1524. doi:10.1007/s00217-007-0683-z
Cespedes, M. C., Cardenas, M. E., Vargas, A. M., Rojas, A., Morales, J. G., Jimenez, P., et al. (2013). Physiological and molecular characterization of phytophthora infestans isolates from the central Colombian andean region. Rev. Iberoam. Micol. 30, 81–87. doi:10.1016/j.riam.2012.09.005
Chai, Y. D., Liu, R., He, W., Xu, F. L., Chen, Z. L., Li, L., et al. (2021). Dissipation behavior, residue, and risk assessment of benziothiazolinone in apples. Int. J. Environ. Res. Public Health 18, 4478. doi:10.3390/ijerph18094478
D'arcangelo, K. N., Adams, M. L., Kerns, J. P., and Quesada-Ocampo, L. M. (2021). Assessment of fungicide product applications and program approaches for control of downy mildew on pickling cucumber in North Carolina, 140, 105412. doi:10.1016/j.cropro.2020.105412Crop Prot.
European Commission (2020). Guidance document on analytical quality control and method validation procedures for pesticide residues and analysis in food and feed (SANTE/12682/2019). Available at: https://www.eurl-pesticides.eu/userfiles/file/Eurl ALL/AqcGuidance_SANTE_2019_12682.pdf (Accessed August 20, 2021).
Faostat, (2020). The FAO (food and agriculture organization of the united Nations) compare data. Available at: http://www.fao.org/faostat/zh/#compare (Accessed August 5, 2021).
Feng, X., Pan, L., Jing, J., Zhang, J., Zhuang, M., Zhang, Y., et al. (2021). Dynamics and risk assessment of pesticides in cucumber through field experiments and model simulation. Sci. Total Environ. 773, 145615. doi:10.1016/j.scitotenv.2021.145615
Ferrer, C., Lozano, A., Aguera, A., Jiménez, G. A., and Fernández-Alba, A. R. (2011). Overcoming matrix effects using the dilution approach in multiresidue methods for fruits and vegetables. J. Chromatogr. A 1218 (42), 7634–7639. doi:10.1016/j.chroma.2011.07.033
Food and Agriculture Organization of the United Nations, (2016). Manual on the submission and evaluation of pesticide residues data for the estimation of maximum residue limits in food and feed. Rome: FAO.
Gisi, U., and Sierotzki, H. (2008). Fungicide modes of action and resistance in downy mildews. Eur. J. Plant Pathol. 122, 157–167. doi:10.1007/s10658-008-9290-5
Granke, L. L., Morrice, J. J., and Hausbeck, M. K. (2014). Relationships between airborne pseudoperonospora cubensis sporangia, environmental conditions, and cucumber downy mildew severity. Plant Dis. 98, 674–681. doi:10.1094/pdis-05-13-0567-re
He, L., Sun, Y., Huang, Y., Pei, R., and Zheng, S. (2007). Residue dynamics of cymoxanil in mixed formulation in potatoes and soil. J. Agro-Environment 2007, 322–325.
Heshmati, A., Nili-Ahmadabadi, A., Rahimi, A., Vahidinia, A., and Taheri, M. (2020). Dissipation behavior and risk assessment of fungicide and insecticide residues in grape under open-field, storage and washing conditions. J. Clean. Prod. 270, 122287. doi:10.1016/j.jclepro.2020.122287
Hong, S., S, Y., Zhang, C., Cao, X., Zheng, L., Wang, S., et al. (2018). Simultaneous detection and degradation of pyraclostrobin and cymoxanil in cucumber and soil. Food Sci. Biotechnol. 29, 262–266.
Huang, J., Ye, Q., Wan, K., and Wang, F. (2019). Residue behavior and risk assessment of cymoxanil in grape under field conditions and survey of market samples in Guangzhou. Environ. Sci. Pollut. Res. 26, 3465–3472. doi:10.1007/s11356-018-3890-1
Kwon, H., Kim, T. K., Hong, S. M., Se, E. K., Cho, N. J., and Kyung, K. S. (2015). Effect of household processing on pesticide residues in field-sprayed tomatoes. Food Sci. Biotechnol. 24, 1–6. doi:10.1007/s10068-015-0001-7
Li, C., Zhou, J., Yue, N., Wang, Y., Wang, J., and Jin, F. (2021). Dissipation and dietary risk assessment of tristyrylphenol ethoxylate homologues in cucumber after field application. Food Chem. x. 338, 127988. doi:10.1016/j.foodchem.2020.127988
Liu, J., X, X., Wu, A., Song, S., Kuang, H., Liu, L., et al. (2022). An immunochromatographic assay for the rapid detection of oxadixyl in cucumber, tomato and wine samples. Food Chem. 379, 132131. doi:10.1016/j.foodchem.2022.132131
EFSA (European Food Safety Authority) (2021). The 2019 European Union report on pesticide residues in food. EFSA J. 19 (4), e06491. doi:10.2903/j.efsa.2021.6491
Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2017). NY/T 3094-2017, guideline for the stability testing of pesticide residues in stored Commodities of plant Origin. Beijing: China agriculture press.
Ministry of Agriculture and Rural Affairs of the People’s Republic of China (2018). NY/T 788-2018, guideline for the testing of pesticide residues in crops. China: Agricultural Industry Standard of the People's Republic of China.
Mirzoian, A., and Ammann, J. R. (2014). Determination of oxadixyl in wines by liquid chromatography-tandem mass spectrometry: Single-laboratory and interlaboratory validation study. J. Aoac Int. 97, 1701–1706. doi:10.5740/jaoacint.13-359
National Health and Family Planning CommissionMinistry of Agriculture of the People’s Republic of China (2021). National food safety standard–maximum residue limits for pesticides in food (GB 2763–2019). Available at: http://www.nhc.gov.cn/sps/s7891/201702/ed7b47492d7a42359f839daf3f70eb4b.shtml (Accessed August 20, 2021).
Pirsaheb, M., Fakhri, Y., Karami, M., Akbarzadeh, R., Safaei, Z., Fatahi, N., et al. (2019). Measurement of permethrin, deltamethrin and malathion pesticide residues in the wheat flour and breads and probabilistic health risk assessment: A case study in kermanshah, Iran. Int. J. Environ. Anal. Chem. 99, 1353–1364. doi:10.1080/03067319.2019.1622009
Pullagurala, V. L. R., Rawat, S., Adisa, I. O., Hernandez-Viezcas, J. A., Peralta-Videa, J. R., and Gardea-Torresdey, J. L. (2018). Plant uptake and translocation of contaminants of emerging concern in soil. Sci. Total Environ. 636, 1585–1596. doi:10.1016/j.scitotenv.2018.04.375
Shi, J., Wang, J., Li, R., Li, D., Xu, F., Sun, Q., et al. (2015). Expression patterns of genes encoding plasma membrane aquaporins during fruit development in cucumber (Cucumis sativus L.). Plant Physiol. biochem. 96, 329–336. doi:10.1016/j.plaphy.2015.08.018
Shirley, A., Vallad, G. E., Dufault, N. S., Raid, R., and Quesada-Ocampo, L. (2021). Duration of downy mildew control achieved with fungicides on cucumber under Florida field conditions. Plant Dis. 106, 1167–1174. doi:10.1094/PDIS-03-21-0507-RE
Vial, J., Le Mapihan, K., and Jardy, A. (2003). What is the best means of estimating the detection and quantification limits of a chromatographic method? Chromatographia 57, 303–306. doi:10.1007/bf02492120
Wang, H., Li, M., Xu, J., Chen, M., Li, W., and Li, M. (2015). An early warning method of cucumber downy mildew in solar greenhouse based on canopy temperature and humidity modeling. Ying Yong Sheng Tai Xue Bao 26, 3027–3034.
Wang, M., Zhang, Q., Cong, L., Yin, W., and Wang, M. (2014). Enantioselective degradation of metalaxyl in cucumber, cabbage, spinach and pakchoi. Chemosphere 95, 241–246. doi:10.1016/j.chemosphere.2013.08.084
Wang, W., Gao, Z., Qiao, C., Liu, F., and Peng, Q. (2021). Residue analysis and removal of procymidone in cucumber after field application. Food control. 128, 108168. doi:10.1016/j.foodcont.2021.108168
Xie, J., Zheng, Y., Liu, X., Dong, F., Xu, J., Wu, X., et al. (2019). Human health safety studies of a new insecticide: Dissipation kinetics and dietary risk assessment of afidopyropen and one of its metabolites in cucumber and nectarine. Regul. Toxicol. Pharmacol. 103, 150–157. doi:10.1016/j.yrtph.2019.01.025
Yan, J., W, R., Xu, Y., Sun, G., Lu, B., Wang, Y., et al. (2016). The residual dynamics and final residue of cymoxanil in ginseng with the application of cymoxanil·mancozeb 72% WP. Agrochemicals 55, 275–277.
Keywords: oxadixyl, cymoxanil, cucumber, residue, dietary risk assessment
Citation: Fan J and Li L (2022) Residues, dissipation, and dietary risk assessment of oxadixyl and cymoxanil in cucumber. Front. Environ. Sci. 10:917334. doi: 10.3389/fenvs.2022.917334
Received: 11 April 2022; Accepted: 28 July 2022;
Published: 02 September 2022.
Edited by:
Liangang Mao, Institute of Plant Protection (CAAS), ChinaReviewed by:
Shuying Li, Zhejiang University, ChinaMiguel Ángel González-Curbelo, EAN University, Colombia
Kankan Zhang, Guizhou University, China
Copyright © 2022 Fan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Li, c3hhdWxpbGlAc3hhdS5lZHUuY24=
 Jiqiao Fan
Jiqiao Fan Li Li
Li Li