
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 13 June 2022
Sec. Toxicology, Pollution and the Environment
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.914636
This article is part of the Research Topic Environmental Impacts of Pesticides: Environmental Fate, Ecotoxicology, Risk Assessment, and Remediation View all 17 articles
Bemisia tabaci, the tobacco whitefly, is one of the most notorious agricultural sucking insect pests that severely damage a series of crops worldwide. Throughout China, B. tabaci threatens agricultural production with increasing cases of resistance to commonly used insecticides, prompting the widespread use of cyantraniliprole as an alternative to control hemipteran pests. Here, we found overexpression of the CYP4G68 gene conferring cyantraniliprole resistance using quantitative real-time PCR (qPCR) and RNA interference (RNAi) in one lab-selected resistant strain CYAN-R (to about 80-fold higher than control). Furthermore, we measured levels of resistance to cyantraniliprole in whiteflies with 18 field-sampled populations across China and then confirmed that, among them, 14 field-sampled populations showed low-to-high resistance to cyantraniliprole compared with the susceptible strain. We measured CYP4G68 expression in the 14 field populations, and the results of qPCR and RNAi indicated that in two of these populations, Haikou and Wuhan, significant overexpression of CYP4G68 contributed to the development of field-evolved resistance to cyantraniliprole. These results indicate the need to facilitate strategies of management to delay the evolution of resistance to cyantraniliprole and control of whiteflies more sustainably, and to prevent overuse of insecticides in the environment through rational application practices.
Bemisia tabaci, the tobacco whitefly, is a destructive sucking pest that devastates the production of economically important and horticultural crops worldwide. The tobacco whitefly is invasive in many locations and reported to infect over 700 species of plants (Wang et al., 2017; Horowitz et al., 2020). In addition to damaging plants by sucking, B. tabaci transmits over 200 plant viruses in the process of feeding (Wei et al., 2017). In recent decades, B. tabaci has been controlled via the application of various widely used chemical agents including organophosphates, carbamates, pyrethroids, insect growth regulators (pyriproxyfen and buprofezin), and neonicotinoids. However, the management of whiteflies rests primarily on the extensive usage of chemical agents over the long-term, which has caused B. tabaci to develop a high or exceedingly high level of resistance to various popular chemical agents (Horowitz et al., 2020). Therefore, widely and heavily applied insecticides will not be effective for controlling B. tabaci in China.
It has been shown that anthranilic diamides can be used against a variety of agricultural insect pests efficiently since they have been introduced to markets around the world (Jeanguenat, 2013). Apart from excellent insecticidal functions with lethal concentrations of anthranilic diamides, they also exert influences on target insect pests at sublethal concentrations and then result in biological and physiological alterations in the pests (Huang et al., 2016; Nozad-Bonab et al., 2017; Meng et al., 2020). Among commercialized insecticides of anthranilic diamide, cyantraniliprole is a second-generation product and targets a wide range of agricultural pests from various orders of insects by acting on their ryanodine receptors (Lahm et al., 2005; Sattelle et al., 2008; Jeanguenat, 2013). Considering that cyantraniliprole can be absorbed via roots and stems of the plants, this chemical agent displays significant insecticidal effects against various orders of insects such as sucking and chewing pests, in comparison with first-generation products like flubendiamide and chlorantraniliprole, which are largely useful for controlling caterpillars (Foster et al., 2012; Barry et al., 2015; Bielza and Guillén, 2015; Grávalos et al., 2015; Moreno et al., 2018). Moreover, it was demonstrated to successfully manage immature stages and adults of B. tabaci and reduce the efficiency of transmitting plant viruses (Portillo et al., 2009; Schuster et al., 2009; (Stansly et al., 2010).
Due to long-term and excessive applications of insecticides, it has been well indicated that among most conventional chemical agents, a growing number of them became inefficacious for controlling agricultural insect pests (Palumbo et al., 2001). In the field applications in China, the heavy dependence on chemical agents for controlling insect pests contributed to more and more resistance cases to various classes of chemical agents that were significantly effective against B. tabaci. In particular, neonicotinoids are highly effective for whitefly control, but resistance to neonicotinoids has been widely reported in whiteflies from several geographic regions across China, which has led to serious control failures (Wang et al., 2010; Yang et al., 2013; Zheng et al., 2017; Zheng et al., 2021). Based on this situation, resistance to neonicotinoids has been investigated, and mechanisms of resistance were demonstrated gradually in China (Yang et al., 2020; Yang et al., 2021; Du et al., 2021; Liang et al., 2022). Not surprisingly, although it has been shown that cyantraniliprole could be one powerful alternative to popular chemical agents, field-evolved cyantraniliprole resistance in B. tabaci has been reported in China (Wang et al., 2018), and it has been reported that in Aphis gossypii, UGTs and P450s are possibly related with resistance to cyantraniliprole (Zeng et al., 2021).
In our previous work, a baseline of susceptibility to cyantraniliprole in China was established and five field-collected populations with moderate cyantraniliprole resistance were detected (Wang et al., 2018). Based on the above results, high cyantraniliprole resistance was observed in the SX population (138.4-fold) after successive selection (Wang et al., 2019). By crossing and successive backcrossing between SX and the susceptible population, one near-isogenic line of the CYAN-R strain was developed that showed 63.317-fold cyantraniliprole resistance compared to the control (Wang et al., 2020a). In the current work, we carried out lab experiments to select the CYAN-R strains with cyantraniliprole, generation by generation, to obtain stable cyantraniliprole resistance (80.8-fold) and found overexpression of the CYP4G68 gene conferring cyantraniliprole resistance in the use of qPCR and RNAi in the CYAN-R strain. Then, in 2021, we established the baseline of susceptibilities to cyantraniliprole in 18 field-sampled populations from China and demonstrated that most of the field-sampled populations of B. tabaci showed various levels of cyantraniliprole resistance. Furthermore, expression levels of CYP4G68 were measured in 14 field populations, and the results of qPCR and RNAi indicated that in two of the populations, Haikou and Wuhan, significant overexpression of CYP4G68 contributed to the development of field-evolved resistance to cyantraniliprole. These results provide new insights for the cognition of P450-associated insecticide resistance and are solid evidence for putting forward appropriate tactics for the sustainable management of whiteflies without the overuse of insecticides.
The CYAN-R strain of B. tabaci with cyantraniliprole resistance and the MED-S susceptible strain were recorded previously (Wang et al., 2020a), and all field-collected populations used in this work were recorded before (Wang et al., 2022). All used populations were raised on a plant of cotton without exposure to insecticides in the chamber with a temperature of 26 ± 1°C, relative humidity of 55 ± 5%, and photoperiod of 16 h light: 8 h dark. About 300 adults of B. tabaci were collected at random from each of the lab-reared and field-collected populations for identification of cryptic species according to the reported approach (Luo et al., 2002), and all of the tested ones were confirmed as Mediterranean cryptic species. All chemical agents utilized were analytically standardized, and cyantraniliprole (Sigma Aldrich, CAS# 736994-63-1, catalog# 32372-25MG), triton X-100 (Sigma Aldrich, CAS# 9002-93-1, catalog# 93443-100 ML), and dimethyl sulfoxide (Sigma Aldrich, CAS# 67-68-5, catalog# D8418-500 ML) were bought from Sigma Aldrich, Shanghai, China.
Based on our previously reported approach (Wang et al., 2018), leaf-dipping bioassays were carried out with whitefly adults from each of the tested populations. Cotton discs with a 2-cm diameter were soaked for about 20 s in the water (control) or the specific working concentration, and after air-drying, they were moved into test tubes with plug caps, respectively. After that, 25–35 whitefly adults were sampled and moved into each of the test tubes at random, and all the test tubes were kept in the chamber for 48 h and then mortality was checked. The CYAN-R cyantraniliprole resistance strain was screened with cyantraniliprole for 15 successive generations, every generation of B. tabaci adults were put through the selection with a median lethal concentration of cyantraniliprole. F1 offspring adults of 18 field-sampled populations from nine provinces of China were used for bioassay to monitor the levels of resistance to cyantraniliprole in China.
Based on previous publications concerning insecticide resistance associated with P450 genes in B. tabaci (Wang Q et al., 2020; Zhou et al., 2020), 12 candidate P450s (CYP6DZ4, CYP6CM1, CYP4G68, CYP6CX4, CYP6DW2, CYP303A1, CYP4C64, CYP6DZ7, CYP6CX1v1, CYP6CX3, CYP6CX5, and CYP6DW3) were picked out and set as candidates for the analysis of gene expression. For each tested population, total RNA was extracted from 100 adults of B. tabaci collected at random from each tested populations, and on the basis of our reported approach (Wang et al., 2020c), qPCR was carried out, and two reference genes, EF-1α and TUB1α, were selected for the normalization. All sequences of primers are listed in Supplementary Table S1.
Silencing of CYP6DW2 and CYP4G68 was conducted, respectively, to confirm the function of CYP6DW2 and CYP4G68 in whiteflies via RNA interference (RNAi) based on the method recorded before (Wei et al., 2018). The double-stranded RNA (dsRNA) was synthesized using a T7 RiboMAX Express RNAi kit (Promega, Madison, WI, United States), and the primer sequences are listed in Supplementary Table S1. Adult B. tabaci were fed dsRNAs targeting enhanced green fluorescent protein (dsEGFP), or CYP6DW2 (dsCYP6DW2), or CYP4G68 (dsCYP4G68) for 48 h, and concentrations of dsCYP6DW2, dsCYP4G68, and dsEGFP were 0.5 μg μL−1, and the artificial diet solution contained 30% sucrose (w/v) and 5% yeast extract.
Data of bioassays were analyzed using PoloPlus software (2003). Resistance ratio (RR) of each of the tested chemical agents was determined by dividing the median lethal concentration of the tested field-sampled population by the median lethal concentration of the susceptible population. Values of RR were utilized to display grades of insecticide resistance, and Student’s t-test and one-way ANOVA followed by Tukey’s HSD for multiple comparisons were performed to analyze statistical significance (p < 0.05) in SPSS software (SPSS Inc., Chicago, IL, United States).
Cyantraniliprole resistance in the resistant CYAN-R strain of B. tabaci was continuously selected for 15 generations. According to the values of LC50, there was no considerable increase from G0 to G15 (Table 1), but the resistance ratio (RR) was increased from 50.0-fold at G0 to 80.8-fold at G15. Specifically, the resistant strain of B. tabaci developed resistance rapidly from G0 to G9 (RR from 55.0- to 70.2-fold) and then remained steady after G9, with RRs around 80-fold.
Baseline of susceptibilities to cyantraniliprole was constructed in the basis of 18 field-sampled populations from nine provinces across China in the year of 2021 (Table 2). Compared to the susceptible population MED-S, 14 of the 18 field-collected populations displayed low-to-high levels of resistance to cyantraniliprole with RRs ranging from 5.0- to 59.6-fold (LC50 from 8.521 to 101.474 mg L−1).
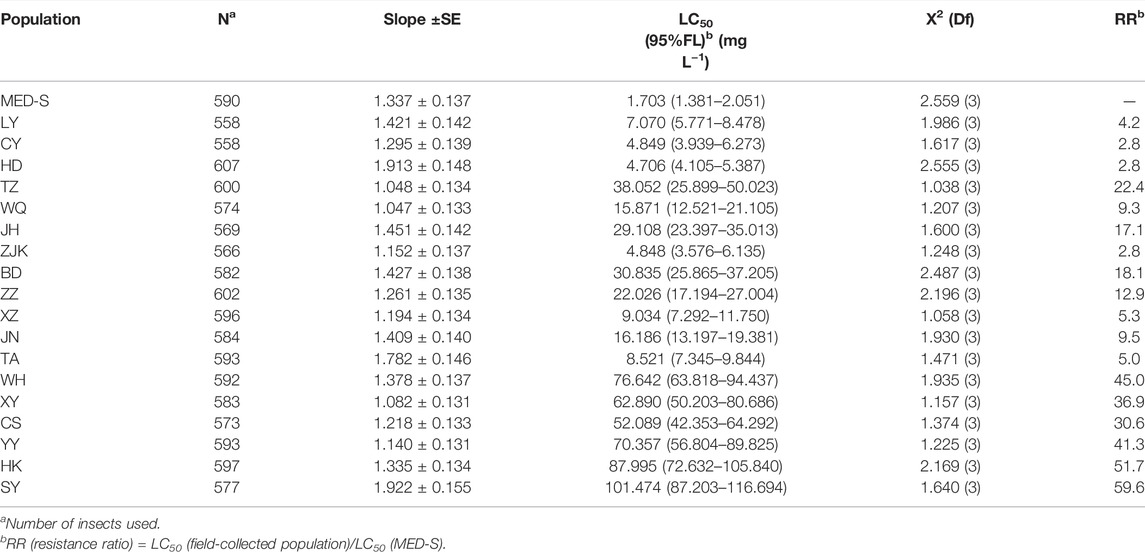
TABLE 2. Bioassays of 18 field populations and one susceptible strain of B. tabaci to cyantraniliprole.
Compared to the susceptible ones, expression patterns of the 12 candidates (CYP6DZ4, CYP6CM1, CYP4G68, CYP6CX4, CYP6DW2, CYP303A1, CYP4C64, CYP6DZ7, CYP6CX1v1, CYP6CX3, CYP6CX5, and CYP6DW3) in CYAN-R were measured using qPCR. Expressions of CYP6DW2 (increased 4.50-fold) and CYP4G68 (increased 6.52-fold) in CYAN-R were significantly elevated in comparison with the susceptible ones (Figure 1). To explore the functions of CYP6DW2 and CYP4G68 further, dsCYP6DW2 and dsCYP4G68 were made and fed to adult B. tabaci from the CYAN-R strain to knockdown the expression of CYP6DW2 and CYP4G68, respectively. After 48 h of feeding on dsCYP6DW2 and dsCYP4G68, the expression of CYP6DW2 and CYP4G68 in adult B. tabaci decreased by 41% (Figure 2A) and 47% (Figure 2C), respectively. After cyantraniliprole selection, feeding on dsCYP4G68 by adults resulted in considerably higher death rate compared to that of the dsEGFP control (Figure 2D), and little remarkable increase in death rate was found with the feeding on dsCYP6DW2 compared with the control (Figure 2B).
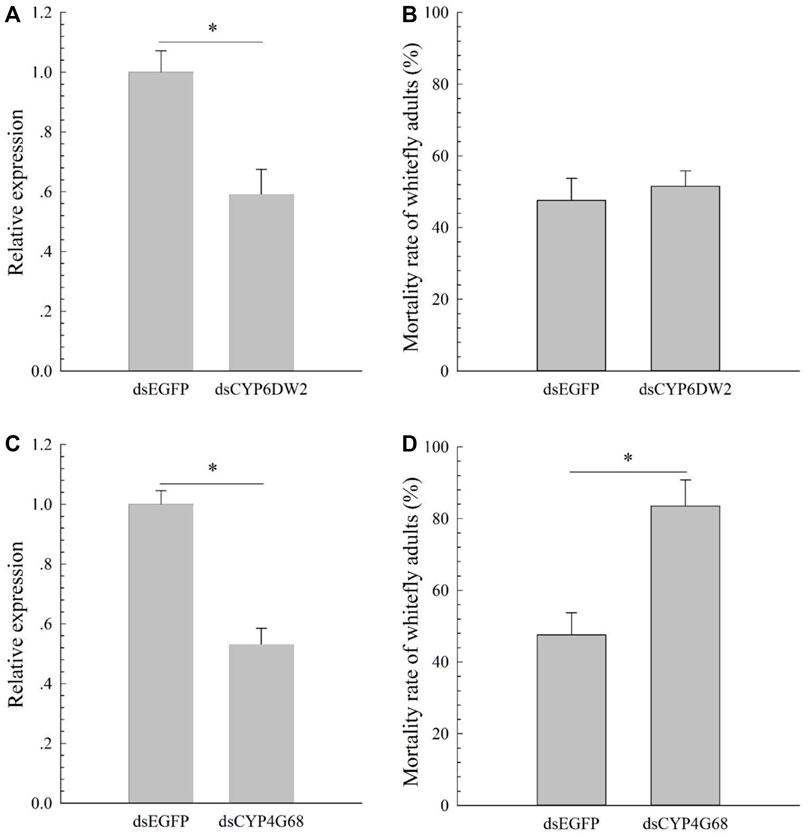
FIGURE 2. Effects of dsCYP6DW2 on the expression of CYP6DW2 (A) and effects of silencing CYP6DW2 (B) on resistance to cyantraniliprole, and effects of dsCYP4G68 on the expression of CYP4G68 (C) and effects of silencing CYP4G68 (D) on resistance to cyantraniliprole.
Relative expression profiles of CYP4G68 in 14 field-collected cyantraniliprole-resistant populations were established and compared to MED-S (Figure 3). Among the field-collected population and the susceptible strain, significant overexpression of CYP4G68 was observed in WH (3.52-fold), CS (3.41-fold), HK (4.55-fold), and SY (3.98-fold). In other field-resistant populations, significant overexpression of CYP4G68 was not observed.
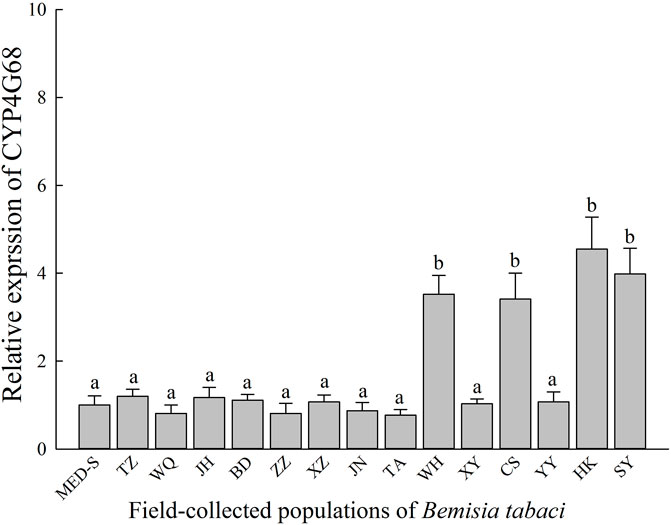
FIGURE 3. Expression profiles of CYP4G68 in 14 field-evolved cyantraniliprole-resistant B. tabaci populations from China.
Based on the above results, to further investigate the functions of CYP4G68 in the four field-resistant populations, dsCYP4G68 was synthesized and fed to the WH, CS, HK, and SY populations of adults to silence CYP4G68 in each of the populations. After 48 h of ingestion of dsCYP4G68, adult B. tabaci displayed decreased expression of CYP4G68 from 42 to 49% in the four tested populations (Figures 4A–D). After the cyantraniliprole treatment, feeding on dsCYP4G68 by adults resulted in a considerably elevated death rate compared to the dsEGFP control in WH and HK populations (Figure 4E and Figure 4G), but little remarkable increase in death rate was found with the feeding on dsCYP4G68 compared to that of the control in CS and SY populations (Figure 4F and Figure 4H).
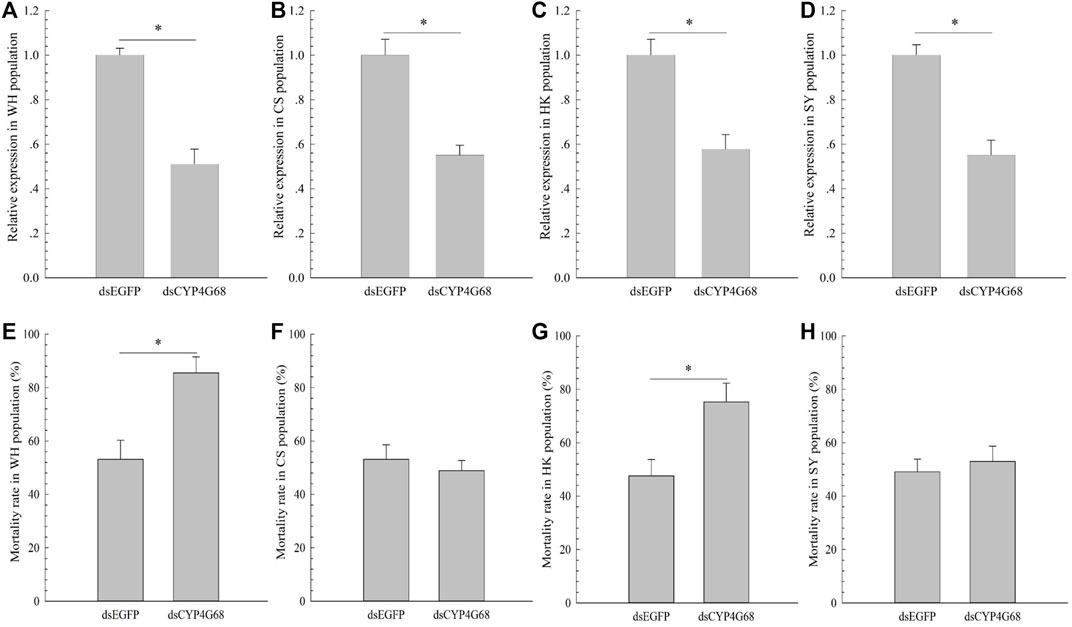
FIGURE 4. Effects of dsCYP4G68 on the expression of CYP4G68 in populations WH (A), CS (B), HK (C), and SY (D). Effects of silencing CYP4G68 on resistance to cyantraniliprole in populations WH (E), CS (F), HK (G), and SY (H).
Cyantraniliprole has shown excellent efficacy against sucking insect pests worldwide, but field-evolved cyantraniliprole resistance in whiteflies has been recorded in China after several years of extensive application (Wang et al., 2019). Previously, we established the CYAN-R strain of B. tabaci on the basis of a field-developed cyantraniliprole-resistant population. After that, a series of biochemical assays were performed, and the results demonstrated that enhanced P450 activities functioned directly in the cyantraniliprole resistance of the CYAN-R strain (Wang et al., 2020a). Insect pests have been demonstrated to develop some resistance to other classes of chemical agents under chronic exposure of selection, prompting studies to explore mechanisms of resistance (Wang et al., 2020b; Wang et al., 2020d; Yang et al., 2020; Zeng et al., 2021). In the current work, continuous cyantraniliprole selections for 15 generations increased resistance from 55.0-fold to 80.9-fold in the CYAN-R strain.
The overexpression of P450s mediating metabolic resistance to various insecticides has been indicated in many species of insect pests worldwide (Nauen et al., 2022). In recent reports of insecticide resistance in B. tabaci, P450-mediated resistance was one of the most reported mechanisms underlying resistance to several insecticides including imidacloprid, thiamethoxam, acetamiprid, and flupyradifurone (Wang et al., 2020c; Wang Q et al., 2020; Yang et al., 2020; Zhou et al., 2020; Liang et al., 2022). In the current study, overexpression was detected for two P450 genes, CYP6DW2 and CYP4G68, in the CYAN-R strain compared with the susceptible strain. Similarly, a previous report showed that various levels of resistance to imidacloprid in field populations of B. tabaci resulted from the overexpression of CYP4C64 and CYP6CM1, two P450 genes (Yang et al., 2013). Furthermore, we found that silencing CYP4G68 resulted in a considerably increased death rate in adults treated with cyantraniliprole in the CYAN-R strain, yet silencing CYP6DW2 did not significantly increase the death rate of adults treated with cyantraniliprole in the CYAN-R strain. All the findings demonstrated that CYP4G68 can contribute to increased cyantraniliprole resistance in whiteflies. In addition to P450-mediated cyantraniliprole resistance, it has been demonstrated that the expression of calmodulin and 1,4,5-trisphosphate receptor can be associated with changed susceptibility to cyantraniliprole (Guo et al., 2017; Guo et al., 2021) and Ca2+-binding protein function in response to cyantraniliprole exposure through the stabilization of Ca2+ concentration (Guo et al., 2019).
Considering that the use of cyantraniliprole across China has facilitated reasonable solutions for solving underlying problems of resistance to popular chemical agent against B. tabaci, it is essential to understand whether whiteflies have already developed resistance to cyantraniliprole in the field. Previously, we monitored the resistance levels of cyantraniliprole against whiteflies throughout China from 2015 to 2016 and found only a few field-collected populations showing low resistance (Wang et al., 2018). In our current work, we monitored the levels of cyantraniliprole resistance in 18 field-sampled populations from nine provinces across China in 2021, and found that 14 of the populations showed low-to-high resistance to cyantraniliprole, which means field-evolved cyantraniliprole resistance has escalated in China. Furthermore, we found the overexpression of CYP4G68 in the cyantraniliprole-resistant populations, WH, CS, HK, and SY, and confirmed that this overexpression contributed to resistance in the WH and HK populations but not in the CS and SY populations. Considering that CYP4G68 functions in thiamethoxam and imidacloprid resistance in B. tabaci (Wang Q et al., 2020; Liang et al., 2022), we surmise that overuse and the long-term application of neonicotinoids and cyantraniliprole in China may give rise to the rapid development of resistance associated with overexpression of CYP4G68. Hence, CYP4G68 can be utilized for monitoring and managing cyantraniliprole resistance in field-developed resistant populations. Our current findings supply novel opinions and understandings concerning possible functions of P450 genes in cyantraniliprole resistance and provide more evidence for the further studies of P450s-associated resistance. Besides, our results could be instrumental in formulating strategies of pest management for controlling insect pests sustainably with more environment-friendly approaches.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RW, WC, and CL conceived and designed the study. RW and WC performed the experiments and analyzed the data with the help of CQ, JW, and CL. RW wrote the first draft of the manuscript. RW, WC, CQ, JW, and CL participated in manuscript drafting and modification.
This work was supported by the National Natural Science Foundation of China (31972266, 32172438, 31801743).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.914636/full#supplementary-material
Barry, J. D., Portillo, H. E., Annan, I. B., Cameron, R. A., Clagg, D. G., Dietrich, R. F., et al. (2015). Movement of Cyantraniliprole in Plants after Foliar Applications and its Impact on the Control of Sucking and Chewing Insects. Pest. Manag. Sci. 71 (3), 395–403. doi:10.1002/ps.3816
Bielza, P., and Guillén, J. (2015). Cyantraniliprole: A Valuable Tool for Frankliniella occidentalis(Pergande) Management. Pest. Manag. Sci. 71 (8), 1068–1074. doi:10.1002/ps.3886
Du, T., Fu, B., Wei, X., Yin, C., Yang, J., Huang, M., et al. (2021). Knockdown of UGT352A5 Decreases the Thiamethoxam Resistance in Bemisia tabaci (Hemiptera: Gennadius). Int. J. Biol. Macromol. 186, 100–108. doi:10.1016/j.ijbiomac.2021.07.040
Foster, S. P., Denholm, I., Rison, J. L., Portillo, H. E., Margaritopoulis, J., and Slater, R. (2012). Susceptibility of Standard Clones and European Field Populations of the Green Peach Aphid, Myzus persicae, and the Cotton Aphid, Aphis gossypii (Hemiptera: Aphididae), to the Novel Anthranilic Diamide Insecticide Cyantraniliprole. Pest. Manag. Sci. 68 (4), 629–633. doi:10.1002/ps.2306
Grávalos, C., Fernández, E., Belando, A., Moreno, I., Ros, C., and Bielza, P. (2015). Cross-resistance and Baseline Susceptibility of Mediterranean Strains of Bemisia tabaci Cyantraniliprole. Pest. Manag. Sci. 71 (7), 1030–1036. doi:10.1002/ps.3885
Guo, L., Liang, P., Fang, K., and Chu, D. (2017). Silence of Inositol 1,4,5-trisphosphate Receptor Expression Decreases Cyantraniliprole Susceptibility in Bemisia tabaci. Pesticide Biochem. Physiol. 142, 162–169. doi:10.1016/j.pestbp.2017.07.005
Guo, L., Li, C., Liang, P., and Chu, D. (2019). Cloning and Functional Analysis of Two Ca2+-Binding Proteins (CaBPs) in Response to Cyantraniliprole Exposure in Bemisia tabaci (Hemiptera: Aleyrodidae). J. Agric. Food Chem. 67 (40), 11035–11043. doi:10.1021/acs.jafc.9b04028
Guo, L., Li, C., Coupland, G., Liang, P., and Chu, D. (2021). Up‐regulation of Calmodulin Involved in the Stress Response to Cyantraniliprole in the Whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). Insect Sci. 28 (6), 1745–1755. doi:10.1111/1744-7917.12887
Horowitz, A. R., Ghanim, M., Roditakis, E., Nauen, R., and Ishaaya, I. (2020). Insecticide Resistance and its Management in Bemisia tabaci Species. J. Pest Sci. 93, 893–910. doi:10.1007/s10340-020-01210-0
Huang, L., Lu, M., Han, G., Du, Y., and Wang, J. (2016). Sublethal Effects of Chlorantraniliprole on Development, Reproduction and Vitellogenin Gene (CsVg) Expression in the Rice Stem borer, Chilo suppressalis. Pest. Manag. Sci. 72 (12), 2280–2286. doi:10.1002/ps.4271
Jeanguenat, A. (2013). The Story of a New Insecticidal Chemistry Class: the Diamides. Pest. Manag. Sci. 69 (1), 7–14. doi:10.1002/ps.3406
Lahm, G. P., Selby, T. P., Freudenberger, J. H., Stevenson, T. M., Myers, B. J., Seburyamo, G., et al. (2005). Insecticidal Anthranilic Diamides: A New Class of Potent Ryanodine Receptor Activators. Bioorg. Med. Chem. Lett. 15 (22), 4898–4906. doi:10.1016/j.bmcl.2005.08.034
Liang, J., Yang, J., Hu, J., Fu, B., Gong, P., Du, T., et al. (2022). Cytpchrome P450 CYP4G68 is Associated with Imidacloprid and Thiamethoxam Resistance in Field Whitefly, Bemisia tabaci (Hemiptera: Gennadius). Agriculture 12 (4), 473. doi:10.3390/agriculture12040473
Luo, C., Yao, Y., Wang, R. J., Yan, F. M., Hu, D. X., and Zhang, Z. L. (2002). The Use of Mitochondrial Cytochrome Oxidase mt COI Gene Sequences for the Identification of Biotypes of Bemisia tabaci (Gennadius) in China. Acta Entomol. Sin. 45, 759–763.
Meng, X., Zhang, N., Yang, X., Miao, L., Jiang, H., Ji, C., et al. (2020). Sublethal Effects of Chlorantraniliprole on Molting Hormone Levels and mRNA Expressions of Three Halloween Genes in the Rice Stem Borer, Chilo suppressalis. Chemosphere 238, 124676. doi:10.1016/j.chemosphere.2019.124676
Moreno, I., Belando, A., Grávalos, C., and Bielza, P. (2018). Baseline Susceptibility of Mediterranean Strains of Trialeurodes vaporariorum (Westwood) to Cyantraniliprole. Pest. Manag. Sci. 74 (7), 1552–1557. doi:10.1002/ps.4869
Nauen, R., Bass, C., Feyereisen, R., and Vontas, J. (2022). The Role of Cytochrome P450s in Insect Toxicology and Resistance. Annu. Rev. Entomol. 67, 105–124. doi:10.1146/annurev-ento-070621-061328
Nozad-Bonab, Z., Hejazi, M. J., Iranipour, S., and Arzanlou, M. (2017). Lethal and Sublethal Effects of Some Chemical and Biological Insecticides on Tuta absoluta (Lepidoptera: Gelechiidae) Eggs and Neonates. J. Econ. Entomol. 110 (3), 1138–1144. doi:10.1093/jee/tox079
Palumbo, J. C., Horowitz, A. R., and Prabhaker, N. (2001). Insecticidal Control and Resistance Management for Bemisia tabaci. Crop Prot. 20 (9), 739–765. doi:10.1016/S0261-2194(01)00117-X
Portillo, H. E., Annan, I. B., and Marçon, G. Z. (2009). “Cyazypyr (DPX-HGW86, Cyantraniliprole): A Novel Anthranilic Diamide Insecticide for Control of Whiteflies and Other Important Arthropod Pests,” in 5th International Bemisia Workshop Abstracts, 60, Guangzhou, China. Available at: http://invasivespecies.org.cn/uploadfile/20091207/ 20091207104910715.pdf (Accessed September 8, 2011).
Sattelle, D. B., Cordova, D., and Cheek, T. R. (2008). Insect Ryanodine Receptors: Molecular Targets for Novel Pest Control Chemicals. Invert. Neurosci. 8 (3), 107–119. doi:10.1007/s10158-008-0076-4
Schuster, D. J., Perez, N. A., Portillo, H. E., Marçon, G. Z., and Annan, I. B. (2009). “Cyazypyr (DPX6HGW86, Cyantraniliprole): A Novel Anthranilic Diamide Insecticide for Managing Bemisia tabaci and Interfering With Transmission of Tomato Yellow Leaf Curl Virus on Tomato Transplants,” in 5th International Bemisia Workshop Abstracts, 59, Guangzhou, China. Available at: http://invasivespecies.org.cn/uploadfile/20091207/ 20091207104910715.pdf (Accessed September 8, 2011).
Stansly, P. A., Kostyk, B., and Riefer, R. (2010). Effect of Rate and Application Method of Cyazypyr (HGW86) on Control of Silverleaf Whitefly and Southern Armyworm in Staked Tomato. Arthropod. Manag. Tests 35, 1–3.
Wang, Z., Yan, H., Yang, Y., and Wu, Y. (2010). Biotype and Insecticide Resistance Status of the Whitefly Bemisia tabaci from China. Pest. Manag. Sci. 66 (12), 1360–1366. doi:10.1002/ps.2023
Wang, X. W., Li, P., and Liu, S. S. (2017). Whitefly Interactions with Plants. Curr. Opin. Insect Sci. 19, 70–75. doi:10.1016/j.cois.2017.02.001
Wang, R., Wang, J. D., Che, W. N., and Luo, C. (2018). First Report of Field Resistance to Cyantraniliprole, a New Anthranilic Diamide Insecticide, on Bemisia tabaci MED in China. J. Integr. Agric. 17 (1), 158–163. doi:10.1016/S2095-3119(16)61613-1
Wang, R., Wang, J. D., Che, W. N., Sun, Y., Li, W. X., and Luo, C. (2019). Characterization of Field-Evolved Resistance to Cyantraniliprole in Bemisia tabaci MED from China. J. Integr. Agric. 18 (11), 2571–2578. doi:10.1016/S2095-3119(19)62557-8
Wang, R., Che, W., Wang, J., Qu, C., and Luo, C. (2020a). Cross-resistance and Biochemical Mechanism of Resistance to Cyantraniliprole in a Near-Isogenic Line of Whitefly Bemisia tabaci Mediterranean (Q Biotype). Pesticide Biochem. Physiol. 167, 104590. doi:10.1016/j.pestbp.2020.104590
Wang, R., Qu, C., Wang, Z., and Yang, G. (2020b). Cross-resistance, Biochemical Mechanism and Fitness Costs of Laboratory-Selected Resistance to Pyridalyl in Diamondback Moth, Plutella xylostella. Pesticide Biochem. Physiol. 163, 8–13. doi:10.1016/j.pestbp.2019.10.008
Wang, R., Wang, J., Zhang, J., Che, W., Feng, H., and Luo, C. (2020c). Characterization of Flupyradifurone Resistance in the Whitefly Bemisia tabaci Mediterranean (Q Biotype). Pest Manag. Sci. 76 (12), 4286–4292. doi:10.1002/ps.5995
Wang, R., Wang, Z., Luo, C., and Yang, G. (2020d). Characterization of Pyridalyl Resistance in a Laboratory-Selected Strain of Frankliniella occidentalis. Pesticide Biochem. Physiol. 166, 104564. doi:10.1016/j.pestbp.2020.104564
Wang, R., Gao, B., Che, W., Qu, C., Zhou, X., and Luo, C. (2022). First Report of Field Resistance to Afidopyropen, the Novel Pyropene Insecticide, on Bemisia tabaci Mediterranean (Q Biotype) from China. Agronomy 12 (3), 724. doi:10.3390/agronomy12030724
Wang, Q., Wang, M. N., Jia, Z. Z., Ahmat, T., Xie, L. J., and Jiang, W. H. (2020). Resistance to Neonicotinoid Insecticides and Expression Changes of Eighteen Cytochrome P450 Genes in Field Populations of Bemisia tabaci from Xinjiang, China. Entomol. Res. 50 (4), 205–211. doi:10.1111/1748-5967.12427
Wei, J., He, Y. Z., Guo, Q., Guo, T., Liu, Y. Q., Zhou, X. P., et al. (2017). Vector Development and Vitellogenin Determine the Transovarial Transmission of Begomoviruses. Proc. Natl. Acad. Sci. U.S.A. 114 (26), 6746–6751. doi:10.1073/pnas.1701720114
Wei, P., Che, W., Wang, J., Xiao, D., Wang, R., and Luo, C. (2018). RNA Interference of Glutamate-Gated Chloride Channel Decreases Abamectin Susceptibility in Bemisia tabaci. Pesticide Biochem. Physiol. 145, 1–7. doi:10.1016/j.pestbp.2017.12.004
Yang, X., Xie, W., Wang, S. L., Wu, Q. J., Pan, H. P., Li, R. M., et al. (2013). Two Cytochrome P450 Genes are Involved in Imidacloprid Resistance in Field Populations of the Whitefly, Bemisia tabaci, in China. Pesticide Biochem. Physiol. 107 (3), 343–350. doi:10.1016/j.pestbp.2013.10.002
Yang, X., Deng, S., Wei, X., Yang, J., Zhao, Q., Yin, C., et al. (2020). MAPK-directed Activation of the Whitefly Transcription Factor CREB Leads to P450-Mediated Imidacloprid Resistance. Proc. Natl. Acad. Sci. U.S.A. 117 (19), 10246–10253. doi:10.1073/pnas.1913603117
Yang, X., Wei, X., Yang, J., Du, T., Yin, C., Fu, B., et al. (2021). Epitranscriptomic Regulation of Insecticide Resistance. Sci. Adv. 7 (19), eabe5903. doi:10.1126/sciadv.abe5903
Zeng, X., Pan, Y., Tian, F., Li, J., Xu, H., Liu, X., et al. (2021). Functional Validation of Key Cytochrome P450 Monooxygenase and UDP-Glycosyltransferase Genes Conferring Cyantraniliprole Resistance in Aphis gossypii Glover. Pesticide Biochem. Physiol. 176, 104879. doi:10.1016/j.pestbp.2021.104879
Zheng, H., Xie, W., Wang, S., Wu, Q., Zhou, X., and Zhang, Y. (2017). Dynamic Monitoring (B versus Q) and Further Resistance Status of Q-type Bemisia tabaci in China. Crop Prot. 94, 115–122. doi:10.1016/j.cropro.2016.11.035
Zheng, H., Xie, W., Fu, B., Xiao, S., Tan, X., Ji, Y., et al. (2021). Annual Analysis of Field‐evolved Insecticide Resistance in Bemisia tabaci across China. Pest Manag. Sci. 77 (6), 2990–3001. doi:10.1002/ps.6338
Zhou, C. S., Cao, Q., Li, G. Z., and Ma, D. Y. (2020). Role of Several Cytochrome P450s in the Resistance and Cross-Resistance against Imidacloprid and Acetamiprid of Bemisia tabaci (Hemiptera: Aleyrodidae) MEAM1 Cryptic Species in Xinjiang, China. Pesticide Biochem. Physiol. 163, 209–215. doi:10.1016/j.pestbp.2019.11.017
Keywords: Bemisia tabaci, cyantraniliprole, resistance management, field-evolved resistance, P450s, overexpression, RNA interference
Citation: Wang R, Che W, Qu C, Wang J and Luo C (2022) Identification and Detection of CYP4G68 Overexpression Associated With Cyantraniliprole Resistance in Bemisia tabaci From China. Front. Environ. Sci. 10:914636. doi: 10.3389/fenvs.2022.914636
Received: 07 April 2022; Accepted: 09 May 2022;
Published: 13 June 2022.
Edited by:
Liangang Mao, Institute of Plant Protection (CAAS), ChinaReviewed by:
Yu-Zhou Du, Yangzhou University, ChinaCopyright © 2022 Wang, Che, Qu, Wang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Luo, bHVvY2hlbkBpcGVwYmFhZnMuY24=; Ran Wang, cndhbmcxMTA1QDEyNi5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.