- Department of Agricultural, Food, Environmental and Animal Sciences, University of Udine, Udine, Italy
Soil salinization caused by sea level rise threatens coastal agricultural soils and geochemically important wetlands worldwide. The aim of this review is to outline expected changes in soil biological activity by discussing the combined effects of salt stress and flooding on plants productivity and soil microbial communities, which determine consequences on fluxes of C, N and P. Finally, it outlines the expected repercussions on greenhouse gases emissions. The prediction of outcomes is made difficult by the concomitant and sometimes contrasting actions of flooding and seawater intrusion on partly acclimated and non-acclimated environments. Non-salt acclimated plants suffer from osmotic stress, but also from reduced O2 solubility. Microbial biomass declines with increasing salinity and microbial communities shift in composition. Large concentrations of Cl− inhibit nitrification, but salinity stimulates N2O fluxes. Impacts on C mineralisation rates is variable but enhanced by the larger availability of terminal electron acceptors. The reduction of Fe combined with that of SO42− could enhance P mobility. Salinization affects methanogenesis which is constrained in favour of SO42− reduction. Consequences are largely site specific and difficult to predict because of the complex network of processes occurring simultaneously in different compartments (i.e., soil, microbiome, vegetation). The distinction between short and long term effects is also important. A reliable prediction of outcomes at a planetary scale will only result from more precise inventories and monitoring of areas displaying specific similarities and from the implementation from these well-defined data sets of specifically devised models whose results can be finally combined on a weighted basis.
Introduction
Salinization has occurred throughout the Earth’s history via the natural accumulation of salts, released by the weathering of rocks. This process, nowadays intensified by rising temperature, is known as primary salinization. Associated changes in salinity occur over a time scale of approximately 100,000 years, though further variations arise over shorter orbital cycles of 23,000–41,000 years (Neukom et al., 2014). In contrast, much faster increases in salt concentrations caused by anthropogenic actions are known as secondary salinization. Since the onset of civilization, anthropogenic manipulations of the hydrologic cycle have artificially altered the balance between salt accumulation and water inputs, leading to increased salinity in some wetlands, inland aquatic systems and upland soils. Secondary salinization can take place over time scales as short as decades, or even more rapidly (Herbert et al., 2015).
Another threat, associated with climatic changes and the salinization of coastal agricultural soils and wetlands, is due to the worldwide sea level rise (SLR), which also endangers biodiversity, with coastal freshwater wetlands being among the most biodiverse environments on Earth. Wicke et al. (2011) estimated that, globally, 1.1 × 109 ha of land was salt-affected and 14% (1.5 × 108 ha) of this area is classified as forest, wetlands, or other legally protected areas. Blankespoor et al. (2012) projected that, following a 1 m rise in sea level, 64% of freshwater coastal wetlands would be lost and converted to saline systems, with the higher regional loss rates in the Middle East and North Africa (100%), Latin America and the Caribbean (74%), Sub-Saharan Africa (72.5%), East Asia and the Pacific (62.2%). Henman and Poulter (2008) estimated that, worldwide, there are approximately 15 × 106 ha of coastal wetlands below 5 m elevation above the mean sea level (MSL), and thus vulnerable to projected SLR. The Australian and New Zealand Environmental and Conservation Council predicts significantly high salt concentrations in 40,000 km of their waterways and associated wetlands by 2050 (Nielsen et al., 2003).
This review starts with a brief outline of the driving factors that determine the diversity of situations that arise from the combined action of the unprecedentedly rapid rise of sea level and the augmented probability of coast inundation events associated with the increased frequency of storms stemming from climatic changes. It then examines threats to coastal agricultural land and natural wetlands before focusing on the consequences of salinization on soil biological properties that regulate the contribution of coastal soils main to the biogeochemical cycles. The aim is to outline and clarify the complexity of affecting factors and feedbacks that arise from the often-contrasting actions of submergence, availability of electron acceptors and osmotic stress on acclimated (salt marshes) or non-acclimated (coastal freshwater wetlands and agricultural land) ecosystems. A specific discussion is devoted to the consequences on greenhouse gases (GHG) emissions.
Sea level rise and forecasted increases in sea water salinity
The fact that SLR is amongst the most important consequences of global climate change and a major threat to many countries on Earth is widely acknowledged. Habitat and infrastructures of the people living in coastal areas, which host about 10% of the total population of our planet, are likely to face increasingly frequent inundation events and eventually become permanently flooded (Carrasco et al., 2016). The global MSL was calculated to have increased, from 1993 to 2018, at a rate of 3.34 mm per year (based on near real-time satellite altimetry data, Aviso, 2003). As a consequence of global climate change, MSL is projected to increase by 0.26–0.82 m by 2100 (IPCC, 2013; IPCC, 2014), with some models projecting an upsurge of more than 1 m by 2100 (Richardson et al., 2009; Vermeer and Rahmstorf, 2009; Rignot et al., 2011). In addition to the melting of polar caps, the warming of the oceans will also contribute to the global SLR, because of the decrease in density of water with temperature. Vermeer and Rahmstorf (2009) used a semi-empirical method linking temperature changes to SLR, and the resulting projected global SLR by 2100 of 0.75–1.90 m is significantly higher than the IPCC projections (0.26–0.81 m, IPCC, 2013; IPCC, 2014).
Variations caused by gravitational effects resulting from land ice mass changes, thermal expansion and ocean dynamics will be observed at the regional scale. In fact, SLR does not manifest itself as a smooth, linear increase, rather its rates vary over time and between regions, complicating predictions for seawater intrusion. Mediterranean coasts and lands facing the Red Sea, the north-eastern part of the Indian Ocean, are potentially more at risk because of the high salinity of marine waters (Figure 1). Valjarević et al. (2020) updated the world maps of sea-surface salinity and calculated the expected increase in salinity (levels and distribution) caused by a 2°C increase in global mean temperature (by the CMIP5 climatological model). According to their projections, salinity levels above 39‰ will be experienced in the area of the Red Sea, the African countries in the Mediterranean, the countries in the Persian Gulf and the Bengal Gulf and Indochina, as well as the Victoria state and the Northern Territory in Australia (Figure 1). In fact, salinization prompted by the SLR varies geographically, because of the spatial variability of regional trends caused by smaller-scale alterations in water temperature, surface winds and geologic activity (IPCC, 2013). Heterogeneity is driven, among other factors, by the salinity of nearby sea surface layers. Climatic factors concur to exacerbate the phenomenon in some regions, e.g. a negative balance between precipitation and soil evapotranspiration causes salts accumulation in the presence of saline groundwater (Rose et al., 2005).
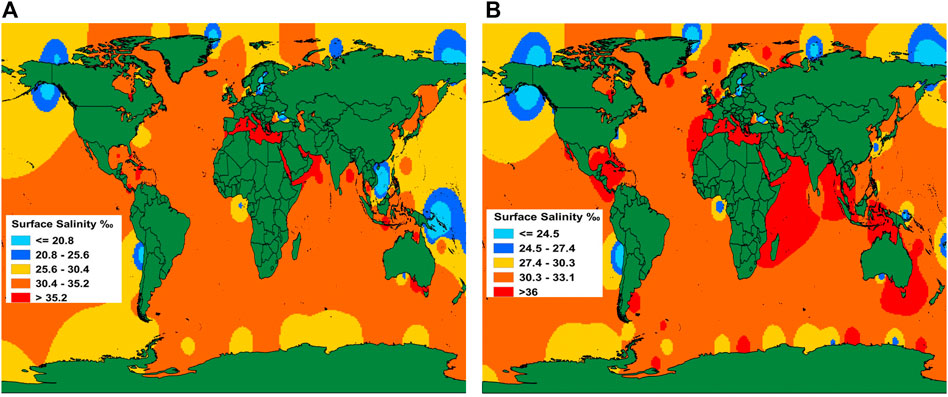
FIGURE 1. World map of sea surface salinity (A) shows current salinity distribution according to measured data from the world seas. A global mean temperature increase of 2°C will affect salinity levels and distribution in the world (B). Modified from Valjarević et al. (2020).
It is estimated that global soil salinization will continue spreading at a rate of up to 2 Mha yr−1 (Abbas et al., 2013). Case studies showing the influence of SLR on lagoons and/or estuaries include Lagoa dos Patos, Brazil (Toldo et al., 2000), Lake Illawarra and St. Georges Basin, New South Wales, Australia (Sloss et al., 2006), Venice Lagoon, Italy (Ferla et al., 2007), Pamlico–Albemarle Sound, North Carolina, United States (Pilkey et al., 2009), Wadden Sea, Netherlands/Germany (Dissanayake et al., 2012), Ria Formosa, Portugal (Andrade et al., 2004), Vistula Lagoon, Baltic Sea (Navrotskaya and Chubarenko, 2013), and Manzala Lagoon, Egypt (Frihy and El-Sayed, 2013). Several aquifers along the densely populated Mediterranean Sea coasts are already suffering seawater intrusion. Speed up of this phenomenon could be particularly intense in this part of the world, because the Mediterranean region, and especially its semi-arid areas, are likely to be seriously affected by a decline in freshwater resources (Kundzewicz and Döll, 2009).
Spatio-temporal drivers of salinization
As sea levels rise, seawater intrusion into freshwater ecosystems increases in frequency, duration and spatial extent (Weissman and Tully, 2020). The risk of salinization of coastal soils is related to the frequency of inundation, which increases with proximity to the seashore and decreases with ground height above the MSL. Tides of unusual elevation are becoming more frequent due to a combination of SLR and extreme weather conditions fostered by climate change (IPCC, 2014), and exacerbate the situation of submerging coastal soils and their salinization.
Strong winds may push the waves of high tides further inland and long drought periods will cause salts to accumulate in surface soil horizons. Once the soils have become submerged, salinity is expected to approach a steady-state equilibrium with brackish waters (Abbas et al., 2013), but even before this happens, evaporation from periodically inundated soils will cause soil salinity to increase at levels much higher than those of the recurrently invading waters. Geographical characteristics contribute to differentiate the effects: within a given tidal range, the frequency of inundation at a particular soil elevation in salt marshes is generally influenced by landscape characteristics, such as length and tortuosity of tidal creeks or distance from the shore. These factors may combine with local climate features, such as the direction of prevailing winds, and lead to locally different inundation patterns (Yang et al., 2015).
The main spatial drivers of variability in salinization intensity are local geomorphology, soil hydrology, textural composition and vegetation cover, which alter pathways and speed of the submarine water discharge, as well as the effects of tidal range and pore water exchange that determine the rates of pentration and drainage of sea water (Guimond and Tamborski, 2021).
Soil texture determines the thickness of the capillary fringe and the potential maximum flux of solutes to the soil surface (Fiola et al., 2020). The capillary rise is, in fact, lower in sandy layers and can retard the building up of salt accumulation. In the soils of estuarine and former floodplain areas, the succession and textural composition of soil horizons impact the height at which groundwater can rise. Textural discontinuities affect the hydrologic connectivity of the soil and reduce the flow of water and solutes to the surface (Gardner, 2005). Stratigraphic constraints, such as mud layers, hamper the discharge flow leading to smaller and denser salt fingers where the effect is exacerbated by the eventual presence of buried sand lenses (Wu et al., 2022). These factors are however important only in low-lying soils where the depth of the water table is sufficiently shallow, so the steepness of the banks may zonally reduce the impact of infiltration of salty ground water.
Soil morphology influences the type of vegetation cover that, in turn, affects the flow of water and solutes towards the surface as the suction exerted by plant water uptake and transpiration can greatly accelerate the process (Carmona et al., 2021). Plants affect the distribution of salinity both quantitatively and qualitatively. In fact, Cl− tends to be excluded from plant nutrient uptake and to accumulate in soil and groundwater as a function of evapotraspiration, with a decreasing gradient of Cl− concentration with depth (Grimaldi et al., 2009). Presence of roots of neighbour trees exploring the soil, increase the complexity of the spatial variability of Cl−, enhancing the risk of strong salinization during periods of drought concomitant to the growing season (Humphries et al., 2011). Salt reduces plant evaportraspiration of riparian species but, rising seawaters can lead to the die back of the riparian vegetation in favour of the establishment of halophytes and salt-tolerant woody species (e.g., Tamarix spp.). Salt-tolerant plants can take up water at very low soil water potential, thanks to the production of soluble osmolytes stored in the cell vacuoles to regulate the osmotic stress (Flowers et al., 2015). The consequent increase of evapotraspiration further enhances salt accumulation (with the exception of the very dry season where leaf stomata close and traspiration is reduced), exacerbating the process of soil salinization and increasing especially Na+ levels in the soil. Moreover, vegetation cover is reduced in halophytic compared to riparian communities, entailing an increase in soil temperature that further boosts salt accumulation due to evaporation (Liu et al., 2019) and causes changes in bacterial community structure (Zhao et al., 2022). Finally, plants can not avoid the passive accumulation of salt in their tissues: this salt is returned to the soil at the end of the growing season, during leaf/root renewal or plant death, supplying salt-enriched litter to the soil and altering OM decomposition.
The natural drivers of coastal soils salinization act through widely differentiated time and spatial spans (Figure 2). They may exacerbate soil salinity for less than a year affecting relatively limited areas or act across much longer periods and at a nearly planetary scale, such as the present climate change and past climate oscillations (White and Kaplan, 2017).
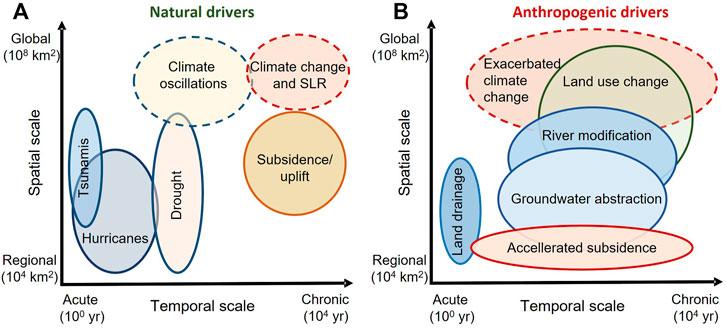
FIGURE 2. Time scales and affected surface areas of natural (A) and anthropogenic (B) drivers of salinization in coastal soils. Modified from White and Kaplan (2017).
Transient drivers, such as tsunamis or hurricanes, cause direct salinization of groundwater and soils in coastal areas, but previous salt levels may be restored within a year in many geographical regions, as salts can be leached away by intense seasonal rains (Kume et al., 2009). However, in combination with dry climates or low soil permeability, the effects of marine inundation caused by transient exceptional events are expected to last longer. All these natural actions are amplified by anthropogenic drivers such as land drainage (Valipour, 2014), accelerated subsidence (Daliakopoulos et al., 2016), groundwater abstraction (White and Kaplan, 2017), land use changes (Gopalakrishnan et al., 2019), and by all those activities that fuel climatic changes (Figure 2). Anthropogenic drivers may act faster and cause, at the same time, effects that may persist over very long time spans. For instance, the low-lying coastal area near Ravenna (north-eastern Italy) is affected by groundwater salinization from seawater infiltrations fostered by an increase in pumping that will cause threats to freshwater availability (Giambastiani et al., 2020). Gonneea et al. (2013) showed that seasonally enhanced SLR leads to increases in the amount and salt concentrations of submarine ground discharge (in summer). Wood and Harrington (2015) further illustrated, by a two-dimensional variable density model, that sea level fluctuations push saline water into inland groundwater-fed wetlands through the movement of the subterranean freshwater-saltwater interface.
It is therefore evident that the variety of drivers acting on coastal soils is matched by the large diversity of their spatio-temporal effects. Local factors and their combinations may greatly accelerate the process in specific areas or dampen consequences in others. A holistic approach is therefore necessary to understand the often contrasting outcomes of salinization from SLR on soil biological properties.
Salinization of coastal agricultural lands
Secondary salinization of agricultural soils is one of the most pressing environmental challenges that humankind will face in the current century (UNESCO, 2016). The Food and Agriculture Organization of the United Nations (FAO) estimates that, globally, over 830 M ha of arable land are affected by salinization (Martínez-Antonioand Collado-Vides, 2003). Salinization affects the 17 western states of the United States, up to 3 Mha in Europe, more than 5% of the arable land in Africa, about a fifth in West Asia and 30% in Australia (Chhabra, 1996; Rengasamy, 2006; IPCC, 2007; Ladeiro, 2012).
Albeit the salinity of the seas varies, the elemental composition of marine salts is worldwide the same so that constant relative proportions are always maintained among the elements (Millero et al., 2008). On the contrary the composition of the salts accumulated by inland saline soils depends on the type of rocks from which they formed or that had been in contact with the waters entering the soil, as well as climatic and pedogenetic factors. This large variability of affecting factors is reflected by the variability of the composition of groundwaters, that in general contain a larger proportion of less mobile cations, whereas anions are dominated by the bicarbonate ion and not by chloride (Shvartsev, 2008). As a consequence, salinization of coastal soils, being imposed by sea water, modifies the composition of the soil exchange complex and of the soil solution in relatively similar ways throughout the globe, whereas the salinity affecting inland soils primarily reflects local situations.
Coastal agricultural practices are less resilient than upland agriculture because they need to cope with more frequent changes in ground water salinity, occasional sea inundations, water stresses and waterlogging (Awal, 2014). The sustainability of coastal agriculture is influenced by both climatic and non-climatic factors, of which SLR is the most influential. Apart from SLR, climate change has the potential to affect coastal areas in several ways, such as through increases in temperature and changes in the frequency and intensity of rainfall and storms (Gopalakrishnan et al., 2019).
The deterioration of soil fertility due to soil salt accumulation is a growing concern in coastal areas. Salinity reduces soil quality (by reducing nutrient content and enzymatic activities, Xian et al., 2019), limits the growth of crops, constrains agricultural productivity, and in severe cases, leads to the abandonment of agricultural lands (Diome and Tine, 2015; Sambou et al., 2016). High salt levels in agricultural soil or irrigation waters make water and nutrient uptake difficult for salt-sensitive plants, such as rice, thereby reducing plant growth and crop yields (Kaniewski et al., 2016). Moreover, saltwater inundation mobilizes nutrients that add to the loading in adjacent water bodies, reducing water quality (Herbert et al., 2015). Coastal farming systems result severely affected. In Bangladesh, for example, coastal soil salinity in agricultural lands has been found to dramatically affect crop revenue and the internal migration of farmers (Chen and Mueller, 2018). In Vietnam, more than 30% of the sugarcane plantations have been either destroyed or significantly damaged by the inundation and intrusion of saltwater into the Mekong Delta, resulting in a significant financial loss (Gopalakrishnan et al., 2019).
The productivity of coastal agriculture is increasingly hampered by the biophysical and biochemical constraints imposed by salinity on crop growth (Duarte et al., 2014). Novel perspectives will be, however, offered by studies on biostimulants and plant growth-promoting microorganisms isolated from the rhizosphere of salt tolerant plants (Pereira et al., 2019; Otlewska et al., 2020; Pereira et al., 2021). Literature shows that isolation and inoculation of specific bacterial strains, by soil amendment or foliar spread, can improve nutrient uptake in the plant and decrease plant sensitivity to Na+ (see, e.g., Etesami and Beattie, 2018).
Adoption of traditional amelioration practices on salt-affected coastal lands not only reducesthe direct impact of osmotic stress on crops, but also exerts a positive action on soil biological fertility by reverting the reduction in the biodiversity of the soil microbiome (Sun et al., 2022).
Impacts on freshwater coastal wetlands
The alteration of water chemistry caused by the ions brought in by marine water alters the chemical speciation of elements, their concentration in the soil solution and modifies habitat conditions and substrate availability for soil microorganisms (Shao et al., 2022), shifting the dominant biogeochemical processes within soil (Haywood et al., 2020) and ultimately altering the ability of wetlands to provide key ecosystem services (Wang et al., 2020).
Coastal wetlands are valuable transition environments, sensitive to changes in marine processes and freshwater flows from upstream catchments. Herbert et al. (2015) identified five mechanisms of salinization in coastal wetlands, including: 1) surface or subsurface seawater intrusion linked to SLR, 2) reduction of riverine freshwater flow, 3) alteration of subsurface freshwater, 4) anthropogenic alteration of coastal geomorphology, and 5) storm surges. The main driver of salinization of coastal freshwater wetlands is undoubtedly surface or subsurface seawater intrusion. Mechanisms 2, 3 and 5 could be probably better considered as climate change-related processes that locally can exasperate salinization.
The third mechanism responsible for the salinization of coastal freshwater wetlands relates to changes in groundwater recharge and discharge (Galliari et al., 2021; Wu et al., 2022). Aquifers are often exploited so intensively that their natural hydrological regime is strongly disturbed and may be thrown out of balance (Custodio, 2010; Dymond et al., 2019). In particular, changes in seawater intrusion are highly non-linear and exhibit important thresholds, or tipping points, beyond which full seawater intrusion into a coastal aquifer may occur in response to even small sea level and/or groundwater management changes (Elliott et al., 2016). Several aquifers along the densely populated Mediterranean coasts are already suffering seawater intrusion (Mazi et al., 2014).
Anthropogenic manipulations of coastal geomorphology, the fourth mechanism, principally affect coastal floodplain wetlands (Dymond et al., 2019; Mancuso et al., 2020). In the Netherlands, for example, the combination of lowland reclamation in the past centuries and ongoing SLR is expected to lead to strong salinization (Oude Essink, 2001). Similar impacts are expected due to the dredging of deep water channels in the Yangtze and Pearl River Deltas in China (Zhang et al., 2014).
The fifth mechanism, storm surges, can introduce saline water into coastal freshwater wetlands along the estuarine continuum, and in near-shore lagoons and depressional wetlands that have no permanent hydrologic connection to the sea (Guimond and Michael, 2021). The increased frequency of extreme weather events associated with climate change will proportionally increase its contribution to coastal salinization in the near future.
Effects of SLR on the biological activity of wetland soils are linked to O2 availability, which is not only related to flooding. Elevated ionic concentrations caused by salinity reduce the solubility of gases (Stumm and Morgan, 1996). This affects the diffusion of O2 in waterlogged soils, resulting in shallower penetration into the soil profile and more negative redox potential. Reduced gas solubility can accelerate GHG emissions by allowing a faster formation of bubbles, and reducing soil microorganisms accessibility to CO2 and N2O (McGinnis et al., 2006). In this way, CH4 oxidation and N2O reduction might become less efficient when soils are saturated with saline waters than similar soils saturated with fresh waters.
Saline water is denser than fresh water, and seawater intrusion via surface or groundwater movement can result in the establishment of strong stratification (and the formation of a halocline) in tidal rivers and depressional wetlands (Brock et al., 2005; Revsbech et al., 2005). Stratification is a barrier to the movement of O2 between the lighter superficial freshwater and the underlying saline strata. Salt loads of merely 2 g L−1 are enough to alter the density of water and produce a degree of stratification similar to the temperature-derived density stratification observed in holomictic freshwater lakes (Findlay and Kelly, 2011).
Ionic displacement has been suggested as a mechanism for desorption of plant nutrients and other chemical species from salinizing wetland soils, particularly inorganic nitrogen (e.g., ammonium) and phosphate (PO43−), with potential consequences for the fertility of these soils, but also eutrophication of down-stream waters. Therefore, the effects of salinization on wetland biogeochemistry typically include decreased inorganic N availability, decreased C inputs and increased generation of toxic sulfides. All this has negative effects on the biodiversity of wetlands, which include the direct loss of plant species diversity, reduced potential for plant population recruitment, reduction in primary production and subsequent loss of fauna diversity through the loss of habitat and food (Davis and Froend, 1999). Indeed, larger salt and sulfide concentrations induce physiological stress in wetland biota and ultimately can result in large shifts in wetland communities and their associated ecosystem functions (Herbert et al., 2015). Increased salinity is a stress that has been shown to reduce diversity in both terrestrial (Briggs and Taws, 2003; Hobbs et al., 2003) and freshwater aquatic systems (James et al., 2003; Brock et al., 2005). Ecological impacts are influenced by a range of factors including the sensitivity of a species to salt (for both sub-lethal and lethal effects), rates of salinity increase, length of exposure and the life stage at which a species is exposed to salt stress (Cocks, 2003). At soil conductivity above 1.5 dS m−1, reduced growth rates and reduced development of roots and leaves are observed in aquatic plants (James et al., 2003; Nielsen et al., 2003). Smith et al. (2009) found that a considerable loss of freshwater macrophytes may occur after a relatively small increase in salinity and with a severe loss of species already at conductivities of about 1 dS m−1.
Osmotic stress in salt-affected soils challenges the maintenance of turgor pressure in plants (Flowers et al., 2015). Acclimated species are able to accommodate high Na+ and Cl− concentrations by intracellular ion compartmentation and the production of osmolytes, i.e., organic solutes that contribute to the osmotic adjustment such as non-structural carbohydrates or amino acids (Gil et al., 2013). Mitochondrial enzymes are sensitive to Na+ and especially the cytochrome c, a crucial component of the electron transport chain (Figure 3). In fact, cell respiration was found to be inhibited in mitochondria isolated from salt-stressed plants compared to plants inhabiting non-saline soils, although the response of overall respiration is very variable (Jacoby et al., 2011).

FIGURE 3. Toxicity of chloride in plant cells. When Cl− accumulates in the apoplast (B), the osmotic potential of the apoplastic water film decreases. When phosphate and nitrate uptake proteins are exposed to high concentrations of chloride, they allow the entrance of Cl− in excess into the cell (A). This raises the ion strength in the cytosol, affecting the activity of cytosolic enzymes (B). When Cl− influx into chloroplasts or mitochondria exceeds its efflux, homeostatic control cannot be maintained. Destruction of electron acceptors triggers the formation of reactive oxygen species (ROS) which attack macromolecules causing cellular damage and de-pigmentation. Modified from Geilfus (2018).
Chloride is an essential micronutrient in plants and regulates enzyme activities in the cytoplasm: it is a co-factor in photosynthesis, acts as a counter anion to stabilize membrane potential and is involved in turgor and pH regulation (White and Broadley, 2001). However, it can be toxic to plants (Geilfus, 2018) and triggers the production of reactive oxygen species (ROS, Figure 3). Critical concentrations for toxicity are estimated to be 4–7 mg g−1 for Cl−-sensitive species and 15–50 mg g−1 for Cl−-tolerant species (White and Broadley, 2001). Reduced rhizodeposition of labile organic compounds and an overall decrease in C inputs (due to the alteration of the Calvincycle) can be expected to have detrimental effects on soil microbial biomass abundance and activity.
Glycophytes, i.e., non-tolerant species to salt, could hardly acclimate to increasing salinity. Therefore, salt stress directly impacts species diversity and composition of plant communities, leading to vegetation shifts in favour of the acclimated halophytic vegetation. Salt-tolerant species are capable to handle the reduced O2 solubility and mineral N availability (Flowers et al., 2015), osmotic stress and specific ion toxicity, determining the short and long term implications on the net primary productivity and, therefore, on the whole C and N inputs to soil.
Response of microbiota to salt toxicity
Soil contact with sea water is toxic for most soil organisms within 2 h and effects last for at least 48 h (Rath et al., 2016). Exposure to high concentrations of salts affects microorganisms in two ways: by osmotic stress and through specific ion toxicity (Serrano, 1996).
Similarly to plant cells, elevated concentrations of marine salts in the soil water phase cause abnormal Na+ and Cl− accumulation in microbial cells and disrupt the uptake of water and essential ions (Figure 3), which may ultimately lead to mortality (Serrano, 1996; Zhang et al., 2019). Toxicity levels inhibiting bacterial growth by about 50% range between 30 and 100 mM Na or between electrical conductivities of 3.0 and 10.7 dS m−1 (Rath et al., 2016).
Increasing salt concentrations in the surrounding medium elevate the osmolarity outside microbial cells and, as external salt concentrations rise, cells lose water. To maintain cell turgor and prevent dehydration, microorganisms produce and accumulate osmolytes in their cytoplasm (Empadinhas and Da Costa, 2008). They have evolved two different osmoadaptation strategies to achieve homeostasis with the surrounding environment, but both are energetically expensive (Gunde-Cimerman et al., 2018).
The first and more widespread strategy, adopted by microorganisms exposed to saline media, is the accumulation of low molecular weight organic compounds (osmolytes) such as amino acids and carbohydrates within the cell (Oren, 2008). The synthesis of organic osmolytes requires energy in the form of ATP. Oren (1999) calculated that heterotrophic microorganisms need to use between 23 and 79 ATP molecules to produce one molecule of an osmotic solute. Extrusion of Na+ and uptake of K+ also consume ATP equivalents.
The second survival strategy, demonstrated in many halophilic prokaryotes, minimizes osmotic potential differences by taking up ions (predominantly potassium) from the medium. This, however, requires the adaptation of intracellular enzymes to elevated ionic concentrations in the cytoplasm, since they should keep their original conformation to preserve enzymatic activity (Oren, 2008). This is not easily achieved because excessive salt concentrations may eventually denature proteins (Frankenberger and Bingham, 1982) and affect enzymes. Even when their solubility is not diminished, the spatial arrangement of amino acids at the active site, which is crucial for maintaining the catalytic efficiency, may be altered at high ionic strengths (Leprince and Quiquampoix, 1996) which favour hydrophobic interactions and de-stabilize electrostatic attraction forces.
Some studies indicate that changes both in microbial community composition and function occur with changes in salinity (Jackson and Vallaire, 2009). The resilience ability of soil fauna seems, however, a function of ecosystem complexity and site specific factors. Salt stress can differently impact the community based on its composition and some organisms might be impacted only marginally (Pereira et al., 2019). Bacteria appear to be more resilient to salinization than fungi, and fungal contribution to microbially derived C is negatively affected by salinity (Shao et al., 2022). Rath et al. (2019) investigated microbial salt tolerance in several saline soils and found that high salt concentrations could inhibit fungal growth by more than 90%. Sardinha et al. (2003) found a strong decrease in the ratio of the fungal biomarker ergosterol to microbial biomass C and that in the least saline site fungi made up 90% of the microbial biomass, but only 17% at the most saline site. The inoculation of salt-tolerant beneficial microorganisms (bacteria in particular) can reduce the salt stress by which the autochthonous microbial community is affected, with consequent positive repercussions to plant or crop growth (Pereira et al., 2016). The variety of microbial responses to salt stress and the existence of beneficial strains could possibly support future strategies to actively face soil salinization, representing a promising perspective in agriculture.
Lower microbial diversity was found in saline soils of the Yellow River delta (Zhao et al., 2020). However, the phyla Proteobacteria, Bacteroidetes, Chloroflexi, Acidobacteria and Planctomycetes, represented more than 70% of the bacterial community in the three different wetlands, indicating the wide adaption of these phyla to salinity changes. Specifically, Proteobacteria, which were recognized as the most dominant phylum (35–39%), appeared not to be affected by salinity. However, the bacterial composition was different among the wetlands, as revealed by β-diversity, and specific bacterial taxa were suggested to serve as bioindicators of soil salinization (Rath et al., 2019).
The species-specific response of soil microorganisms to increased Cl− concentration results in modifications of the soil microbial community. However, chlorine is naturally diffused in the environment because saltwater from the oceans is, with an average concentration of 19 g Cl L−1, one of the main sources of atmospheric Cl− (Stumm and Morgan, 1996). Inorganic chloride is a normal component of the soil solution which is more abundant in coastal regions, where it is carried by marine aerosols (Meira et al., 2006). Setia et al. (2010) suggested that, when chloride is the predominant anion of the soil solution, the nature of the accompanying cations is of little importance and that the osmotic effect plays an important role in reducing rates of organic matter decomposition. However, empirical data from a larger variety of habitats, range of scales and microbial functional groups are still needed to understand how the structure and function of microbial communities will be altered by salinization.
A substantial increase in Cl− concentration inhibits both nitrification and denitrification, but the associated microbial communities appear to be able to adapt to high Cl− concentrations over time (Hale and Groffman, 2006). Moreover, Soil anoxia tends to reduce the rate of chlorination of the soil organic matter (SOM) (Bastviken et al., 2007; Bastviken et al., 2009). By affecting the composition of soil microbial cenosis, the increase in chloride availability may even affect the chlorine flux from its inorganic form to organic compounds and vice versa (Gryndler et al., 2008) with consequences affecting the process of chlorination of SOM (Öberg et al., 2005).
The most consistently observed alteration in bacterial communities caused by inundation or infiltration of sea water is a change in the number of methanogenic archaea and a coincident decrease in CH4 production (Baldwin et al., 2006). Observations suggest that Na+ and Cl− alone can inhibit methanogenesis in inland soils but, in coastal systems because of the concomitant increase in SO42−, the inhibition is fostered by a less efficient competition with facultative anaerobes (Pattnaik et al., 2000; Mishra et al., 2003; Baldwin et al., 2006; Chambers et al., 2011).
Extracellular enzyme activities are generally larger in non-saline soils compared to naturally saline soils (Rietz and Haynes, 2003; Ghollarata and Raiesi, 2007), even if halophytic microorganisms had the time to adapt and colonize the latter. Enzymatic activity is therefore often measured to assess biological activity in soils affected by salinization. This provides a convenient way to monitor the consequences of increased salt concentrations on the biological activity of soils (Lemanowicz et al., 2019), however this approach is not without drawbacks. Assays that measure enzyme activities in soil usually test the potential activity at substrate saturation, which is a condition very far from that normally encountered in the substrate starved soil environment (Hobbie and Hobbie, 2013). Moreover, conditions such as temperature or pH can either be optimized or kept close to natural conditions (German et al., 2011; German et al., 2012), which leads to a considerable difference in results (Burns et al., 2013). Current methods which estimate enzyme activities at saturated substrate levels and optimized conditions do not estimate actual in situ activities, but rather maximum activities related to the abundance of enzymes, which is largely determined by the size of the soil microbial community (Rath and Rousk, 2015). The observed decrease, therefore, reflects lower microbial biomass contents and only secondarily, a change in functionality of the microbial community.
On the other side, microbial communities faced with unfavourable osmotic potentials can allocate lower energy resources to protein production and therefore release fewer extra-cellular enzymes. To highlight this, the determination of specific activities (mg substrate transformed per mg of microbial biomass C) would be much a better index of induced functional changes, than activities per se. Unfortunately, this approach has scarcely been adopted, although it would also allow direct comparison among different situations and a better generalization of consequences (Moorhead et al., 2013).
Another problem with using enzyme activities as an indicator of overall microbial status is that results can vary considerably between different soils and different enzymes (Frankenberger and Bingham, 1982; Saviozzi et al., 2011). Therefore, it remains elusive to identify common general patterns from the assessments of extracellular enzyme activities in saline soils. This suggests that other metrics may be preferable to assess microbial functioning in saline soils, e.g., DNA/RNA-based molecular methods (Rath and Rousk, 2015).
Salinity effects on soil biogeochemical cycles
Increasing levels of chloride, sodium, potassium, magnesium and sulphates from sea water intrusion have major effects on biogeochemical processes (Weston et al., 2006; Setia et al., 2010) and the relationships between plants, soil and microbial communities (Nielsen et al., 2003; Munns and Tester, 2008) (Figure 4). SLR has important effects on soil biogeochemical cycles through the combined action of submergence and salinity on plant communities, in terms of productivity and type of vegetation (Hines et al., 2006). In fact, in addition to the anaerobic shift, imposed by the lack of O2 that accompanies soil submergence, microbial respiration is supported by the larger availability of terminal electron acceptors (TEAs) in sea water with respect to fresh water. TEAs support anaerobic respiration, which partly compensates for the low efficiency of anaerobic transformations. At the same time, changes in plant communities contribute to altering microbial activity due to the different quantity and quality of litter available as a substrate for microorganisms (Barry et al., 2022).
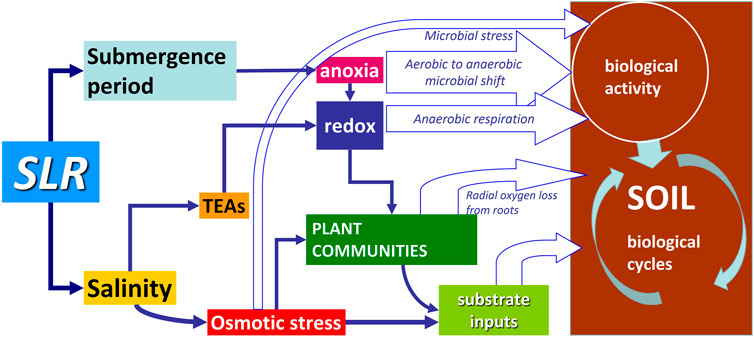
FIGURE 4. Conceptual map of direct and indirect effects of sea level rise (SLR) on soil biological cycles through increased submergence and salinity. TEAs stands for terminal electron acceptors.
In this paragraph, we will examine the complex interacting factors that drive the modifications in biological activities and govern the outcomes of SLR on the soil biogeochemical cycles of C, N and briefly on those of Fe, S and P.
Carbon cycle
Wetland soils have been estimated to contain from 45 to 70% of the terrestrial C pool (Mitra et al., 2005) and are assumed to play an important role in reducing atmospheric GHG concentrations, and mitigating climate change (McLeod et al., 2011). Coastal wetlands (i.e. salt marshes and mangrove swamps) sequester one order of magnitude more C than an equivalent area of terrestrial forests or peatlands (Chmura et al., 2003; McLeod et al., 2011). Considering that coastal wetland coverage will be reduced by 46–59% by the end of this century, based on current SLR predictions (Spencer et al., 2016), the reduction in C sequestration will have strong impacts on the terrestrial C cycle (Pfeffer et al., 2008; Vermeer and Rahmstorf., 2009), yet the overall effect is controversial and difficult to predict.
SLR will modify C cycling in coastal wetlands in two ways: 1) by increasing the length of submergence periods and, 2) by increasing salinity (Figure 4). The apparently contradictory results found in the literature may respond to the contrasting actions of these two concomitant factors that combine into a complex pattern. If sea rise per se will increase the incidence of soil anoxia, which is expected to favour C sequestration, salinity will increase the availability of TEAs and foster SOM mineralization (Stagg et al., 2017). Inundation by brackish waters may result, at least transiently in a negative C balance in freshwater wetlands, but is likely to have opposite effects in salt marsh sites with high elevation above the MSL. Effects of salinity on soil C depend on vegetation shifts (i.e., from non-halophytic to halophytic plant communities) that affect the quantity and quality of organic matter input to soil.
Salinity acts in contrasting ways on salt tolerant rather than salt sensitive plants. In a non-halophytic system inundated by saline waters, C inputs may dramatically diminish within a short period, because of the immediate anoxia and toxicity imposed by flooding and salt water, respectively (Figure 4). On the contrary, the same phenomenon may have a much lower impact on the productivity of the acclimated plant community (Glenn et al., 1992).
At the same time, in submerged soils, C mineralization efficiency depends on the availability of TEAs. Adverse effects on organic matter decomposition were found after the addition of salts to non-saline soils (Wichern et al., 2006; Wong et al., 2008), but sea salts composition and submergence interact in a complex pattern. Decomposition rates depend on the type of salt: for instance, Na2SO4 increased CO2 emission from submerged soils during laboratory incubation, while NaCl decreased it (Li et al., 2006). The addition of sulphate ions sustains anaerobic respiration and therefore a more efficient decomposition of SOM, compared to strictly anaerobic systems, where microorganisms are forced to derive their energy from fermentation. It also inhibits the reduction of CO2 to CH4. So, the increased availability of TEAs which are normal components of seawater is crucial. In tidal freshwater forests, salinity increased the amount of C mineralized (based on laboratory incubations) suggesting that even relatively low increases in salinity can alter the short-term C dynamics of coastal wetlands (Marton et al., 2012).
The primary consequence of increasing soil salinity on C dynamics of coastal freshwater wetlands is undoubtedly the decrease in plant productivity caused by osmotic stress, and hence, in the long term, the potentially diminished C inputs to the soil (Figures 3, 4). However, in the very short term or following transient pulses in salinity, caused by storms or tsunamis, a transient increase in C inputs to soil, e.g., caused by the more intense shredding of litter from salinity stressed plants, may also be observed. Over longer periods, when salt accumulates, stressed plants become less competitive, fostering changes in abundance and diversity of plant communities that can enforce feedbacks that affect soil formation processes (Ferronato et al., 2018; Pellegrini and Fernández, 2018). Neubauer et al. (2013) showed that the release of CO2 from soil increased in response to a short-term salinity pulse, but declined over time in response to long-term (3.5 years) exposure. This was attributed to slow indirect feedbacks on soil that affect the composition of SOM (e.g., higher C:N ratios) and are potentially driven by changes in plant productivity and species composition and abundance. In one of the few long-term studies reported in the literature, soils containing larger SOM stocks appear to lose more C in response to salinization, with respect to soils of low C content (Marton et al., 2012). Forecasting the consequences of SLR on organic matter decomposition in wetlands, therefore, requires the integration of biogeochemical dynamics along with vegetation patterns (Figure 5).
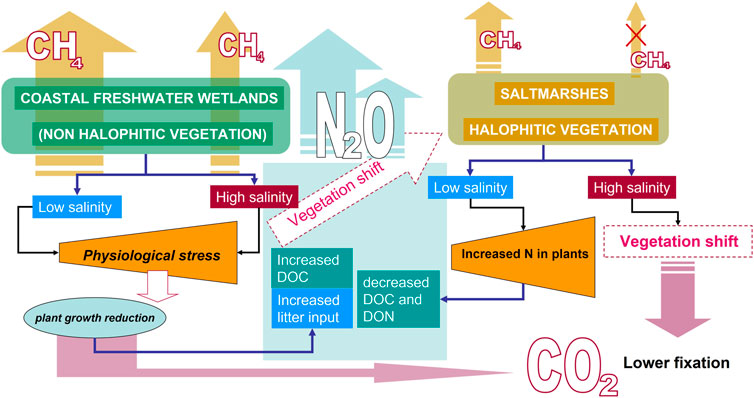
FIGURE 5. Contrasting effects of salinization caused by SLR on C and N cycles and GHG emissions in coastal freshwater wetlands and in salt marshes.
Besides governing the release of CO2 from soils, organic matter mineralization regulates the regeneration and availability of nutrients for plants, also in wetland systems (Reddy and DeLaune, 2020). In this respect, the ensuing decrease in soil microbial biomass and soil enzyme activities may reduce plant productivity in wetland soils, in which extracellular enzymes are crucial for the recycling of nutrients required to sustain plant growth (Pathak and Rao, 1998; Ghollarata and Raiesi, 2007).
Perduring submergence combined with salinity cause a decrease in soil microbial diversity: this reduction, besides being driven by a shift to anaerobic or facultative anaerobic microorganisms, is connected with the toxicity of sulfide and it eventually fosters slower SOM decomposition rates (Setia et al., 2013; Qu et al., 2019). Before this happens, any increase in salinity will be transiently accompanied by an increase in concentration in the soil solution of TEAs (such as iron, manganese and sulphate ions), that support anaerobic respiration and therefore stimulate CO2 production via increased efficiency in the mineralization of organic matter (Chambers et al., 2011; Meiggs and Taillefert, 2011; Weston et al., 2011; Marton et al., 2012; Neubauer et al., 2013). The larger availability of TEAs supports the shift of the dominant pathway of anaerobic metabolism from methanogenesis towards higher energy yielding pathways (e.g., SO42− reduction). This, combined with lower plant productivity, could eventually lead to a reduced carbon sequestration potential (Herbert et al., 2015).
The best studied effect of increased salinity on microbial C cycling in freshwater wetlands is the suppression of methanogenesis caused by the availability of SO42− (Weston et al., 2006; Chambers et al., 2011; Poffenbarger et al., 2011; Neubauer et al., 2013). This not only makes CO2 reduction to CH4 less advantageous, but even promotes CH4 oxidation. Recently, tidal freshwater sediments have been shown to support high rates of anaerobic CH4 oxidation coupled to SO42− (Segarra et al., 2015) and Fe (III), Mn (IV), and NO3− reduction (Segarra et al., 2013). The interactions between increased SO42− and methanogenesis and CH4 oxidation (aerobic and anaerobic) are likely to vary with site specific factors such as soil and water chemistry, O2 availability, vegetation type and water level fluctuations.
The balance between C inputs and mineralization rates does not only affect CO2 emissions and C sequestration potential, but is also crucial in determining the capability of coastal marshes to keep up with SLR. In fact, their resilience depends on the enhanced organic matter accretion and accelerated sediment deposition (Kirwan and Guntenspergen., 2010). The latter may be seriously hampered by anthropic actions that are the main causes of sediment erosion and loss of wetland surfaces (Fontolan et al., 2012).
Nitrogen cycle
Nitrogen often acts as a limiting nutrient for coastal salt marshes and N availability has considerable impacts on the structure and productivity of plant communities (Mitsch and Gosselink, 2015). Ammonification is the key process that converts organic N into inorganic N, but all the steps of the N cycle are impacted by inundation with marine waters. In tidal zones, increased sea level combined with tides may result in frequent alterations of the hydrologic status of soils, which stimulate decomposition of SOM and loss of N (Djaman et al., 2018). Bai et al. (2005), Bai et al. (2007) showed that flooding duration and frequencies could influence soil N distribution because submergence greatly influences soil properties.
A comprehensive meta-analysis of coastal soil salinization on N pools by Zhou et al. (2017) showed that soil salinization increased plant N content (+18%), soil NH4+ (+12%) and soil total N (+210%), although it decreased soil NO3− (−2%) and soil microbial biomass N (−74%). Therefore, we can expect the net primary production (NPP) of halophytic plant communities to benefit from this increased N availability, whereas effects on non-acclimated vegetation may be limited or even reversed by the action of osmotic stress.
Nitrate is the most efficient electron acceptor in submerged soils and helps to maintain the soil redox potential at more positive values. Nitrification, which may still go on in aerobic surface water and soil layers, provides a relatively low but continuous supply of this type of TEAs to the denitrifiers, facultative anaerobic bacteria, which produce the energy necessary for their metabolism by reducing NO3− to N2 using electrons derived from the oxidation of substrates. Denitrification has often been observed to decline along salinity gradients (Rysgaard et al., 1999; Craft et al., 2009; Giblin et al., 2010). However, this detrimental effect is limited to ammonium oxidizing bacteria and nitrite oxidizing bacteria: heterotrophic aerobic denitrifiers can thrive on the nitrate produced by heterotrophic nitrification and may successfully remove most of the N under different salinity conditions (Fu et al., 2019).
Isotopic analysis of N in ocean sediment layers covering the past 300 million years has shown that substantial variations in denitrification rates were associated with planetary climatic changes such as Quaternary glacial and interglacial periods. Enrichments in 15N, indicative of intensified denitrification, regularly occurred during the stages of rapid sea level rise and were followed by a decrease as sea level subsided again (Algeo et al., 2008). We can therefore expect a similar trend to occur during the early stages of inundation of coastal areas by sea water.
Large increases in salinity (16.5 dS m−1) ultimately inhibit soil nitrification (Ardón et al., 2018). In oxic zones, microbial nitrification converts NH4+ to nitrite (NO2−) and, finally, to nitrate (NO3−), but sulfide inhibits nitrifying bacteria (Joye and Hollibaugh, 1995). Therefore, with increased exposure to saltwater, nitrification rates may decrease and affect denitrification, because of the lower availability of substrate (Rysgaard et al., 1999; Noe et al., 2013). Sulfide ions inhibit denitrification directly by inhibiting the reductase enzymes that catalyze the final steps of denitrification, resulting in incomplete denitrification to NO2−, NO, or N2O (Brunet and Garcia-Gil, 1996), or indirectly by reducing NO3− availability via the inhibition of nitrification. Increased ionic strength can also interfere with the enzymes associated with denitrification (Glass and Silverstein, 1999). Dissolved salts alter the configuration of the active site of enzymes (non-competitive inhibition), modifying kinetic parameters such as rate and saturation constants (Dinçer and Kargi, 2001).
Increasing soil salinity stimulated soil N2O fluxes as well as hydrological NH4+ and NO2− fluxes more than threefold, although it decreased the hydrological dissolved organic nitrogen (DON) flux (−59%). Soil salinization also increased net N mineralization by 70%, although salinization effects were not observed on the net nitrification, denitrification and dissimilatory nitrate reduction to ammonium (Zhou et al., 2017).
Sulphur, iron and phosphorus cycling
In wetlands, S cycling is tightly coupled to Fe cycling, both of which are driven by biotic and abiotic redox reactions (Smolders et al., 2007; Burgin et al., 2011). In oxic or sub-oxic soils, S and Fe are in their oxidized forms, i.e., SO42- and Fe3+, and are reduced to sulfide (S2-) and ferrous (Fe2+) ions during anaerobic microbial respiration. Sulphate reduction results in the formation of sulfide species (H2S, HS−, S2−), which are toxic to many organisms (Lamers et al., 2013). In many soils, this toxicity is controlled by the co-precipitation of insoluble forms. Released sulfide and Fe2+ ions combine abiotically to form, at first, highly insoluble iron monosulfide (FeS) (Schoepfer et al., 2014), regulating the concentration of sulfide in the soil solution. Salinization generally favours the formation of FeSx minerals in wetland soils such as mackinawite and pyrite (FeS2, Rickard and Morse, 2005; Tobias and Neubauer, 2019). Nevertheless, the formation of FeSx minerals retains S in sediments and can have potentially deleterious consequences. Reclamation of anaerobic soils with high concentrations of FeSx may generate acid sulphate soils. If drained, FeSx minerals react with O2 releasing a large amount of H+ (White et al., 1997), leading to soil acidification and the mobilization of aluminium (Al) and toxic metals (Johnston et al., 2003; Baldwin and Fraser, 2009; Lamers et al., 2013). These processes limit the reclamation of soils that have been submerged by sea water for a long time.
The interaction between S and Fe cycling indirectly also controls P cycling in periodically submerged soils. In fact, the reduction of Fe (III) to the more soluble ferrous Fe (II) form and its reaction in solution with sulfide results in the dissolution of Fe-PO4 minerals, releasing phosphate ions (Reddy and DeLaune, 2020). The increased availability of P freed from iron-sulphur complexes goes on for weeks to months following saltwater intrusion, and may contribute to the eutrophication of overlying and downstream waters (Lamers et al., 2001; Lamers et al., 2002; Weston et al., 2006). The concomitant increased potential for H2S build-up and toxicity, and for PO43− release, have important implications for overall ecosystem health and downstream eutrophication, though these effects may not be apparent with short-term salinization.
It is obvious that Fe, S, and P dynamics are highly complex and, albeit driven by biological reduction, they are controlled largely by abiotic factors such as water chemistry, SOM, soil type, hydrology and other site-specific factors.
Emission of greenhouse gases
Prediction of trends in the release of greenhouse gases (GHG) from soils is a complex issue where ecosystem type, soil hydrological status and quantity and composition of salts with respect to divalent cations combine in different ways (Figure 4).
Vegetated coastal ecosystems are net C sinks that fix and sequester large amounts of both locally produced and allochthonous organic C. In fact, from 428 to 681 g C m−2 y−1 are fixed by vascular plants and microalgae in North American salt marshes and rates of salt marsh C accumulation, due to sediment burial, reached on average 218 g C m−2 y−1 over the last 50–100 years (McLeod et al., 2011). In tidal salt marshes, CO2 emissions from soils (i.e., soil CO2 efflux) are highly variable ranging from 240 to 720 g C m−2 y−1 and this heterogeneity arises from a variety of processes, including autotrophic and heterotrophic respiration and fermentation (Tobias and Neubauer, 2019). Even if the global net C sequestration potential of vegetated coastal ecosystems is difficult to predict, mangrove swamps and salt marshes greatly contribute to the biological C pool (Chmura et al., 2003) and it would be important to predict changes brought by SLR.
An increase in sea level will obviously increase the length of submergence periods in tidal environments, eventually leading to permanent flooding of the lowest parts of coastland. As a consequence, the time allowed for oxygen diffusion will be restricted and anaerobic decomposition of SOMfostered by anoxia. In general, C mineralization is slower in submerged soils, because of thermodynamic impairments on decomposition, but submergence by sea water implies an increase in salinity, which drives a complex network of consequences.
Weston et al. (2006) observed a shift from methanogenesis to SO42− reduction in ocean water-amended sediments, though this biogeochemical shift was not accompanied by changes in microbial community dominance (Edmonds et al., 2009). Poffenbarger et al. (2011) performed a meta-analysis on CH4 fluxes along natural salinity gradients and showed that emissions decreased with increasing salinity. However, the decrease was significant only for salinity regimes above 18 g L−1 (polyhaline marshes), whereas for mesohaline marshes, about 52% of the positive GHG balance of the yearly sequestered C was offset by methane emissions that did not significantly differ from those of non-saline systems. This is indirectly confirmed by responses of GHG emissions to storm-surge reductions in salinity: Capooci et al. (2019) used a mixed effects models approach and found that a decrease in salinity can produce increased pulses up to 24% in GHG emissions.
Short-term laboratory incubations simulating saltwater intrusion in tidal forests (Marton et al., 2012) confirmed that CH4 production was inhibited by salinity, decreasing up to 90% in 5‰ salinity treatment, but variable responses were observed for N2O production. Tidal forest soils are capable of producing from 0.032 to 1.9 μg N2O g−1 soil h−1, but most of it is reduced to N2, unless the acidity of the freshwater inputs inhibits the N2O reductase activity. At high levels of seawater intrusion, however, the same enzyme may suffer from inhibition by sulfide ions caused by increased sulphate availability (Sorensen et al., 1980).
The production of GHG is regulated by the availability of TEAs that are preferentially used by microorganisms because of the greater thermodynamic yield compared to methanogenesis (Figure 4). Besides sulphate, the availability of other TEAs can also be influenced by SLR. For instance, humic substances are a widespread component of dissolved organic matter in rivers and can act as TEAs but, because of their poly-electrolytic nature, their solubility is suppressed in sea water. The normal trend can therefore be locally reversed with a lack of TEAs leading to increased methane production.
Conclusions and outlook on future investigation needs
Coastal soils feature some of the most densely inhabited and productive areas of the world and are natural hot spots of biodiversity. They also harbour biological processes that govern interchange between terrestrial and marine environments and between soils and the atmosphere. Because of the large number of effecting factors acting often in contrasting directions, the consequences of salinization of coastal soils are still difficult to predict on the global scale. In particular, the effects of SLR on C and N cycles and consequently on GHG emissions from coastal soils are highly variable, depending on the contrasting and concomitant effects of flooding and salinity. At present, it is unlikely that this variability can be handled by models aiming to predict consequences at a planetary scale. A reliable evaluation at a planetary scale should result from more precise inventories and monitoring of areas displaying specific similarities and from the implementation from these well-defined data sets, of specifically devised models whose results can be finally combined on a weighted basis.
Besides the collection of data, a deeper knowledge of the mechanisms and effecting factors that act, in both the short and long term on the biological activity of soils affected by SLR is certainly needed. In this way most of the apparently contradictory results that are nowadays reported in literature could be embedded into a holistic insight, allowing scientists and decision makers to devise ad hoc strategies and mitigate unavoidable impacts of SLR and salinization at local and regional scales.
Author contributions
MD conceptualized the project and, with SM, developed the structure for the review. MD compiled data and prepared the initial text. EP contributed to the writing of the text. SM, CB, and MC contributed to the editing and finalization of the manuscript. All authors approved the text for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, A., Khan, S., Hussain, N., Hanjra, M. A., and Akbar, S. (2013). Characterizing soil salinity in irrigated agriculture using a remote sensing approach. Phys. Chem. Earth Parts A/B/C 55, 43–52. doi:10.1016/j.pce.2010.12.004
Algeo, T., Rowe, H., Hower, J. C., Schwark, L., Herrmann, A., Heckel, P., et al. (2008). Changes in ocean denitrification during late carboniferous glacial-interglacial cycles. Nat. Geosci. 1 (10), 709–714. doi:10.1038/ngeo307
Andrade, C., Freitas, M. C., Moreno, J., and Craveiro, S. C. (2004). Stratigraphical evidence of Late Holocene barrier breaching and extreme storms in lagoonal sediments of Ria Formosa, Algarve, Portugal. Mar. Geol. 210, 339–362. doi:10.1016/j.margeo.2004.05.016
Ardón, M., Helton, A. M., and Bernhardt, E. S. (2018). Salinity effects on greenhouse gas emissions from wetland soils are contingent upon hydrologic setting: a microcosm experiment. Biogeochemistry 140 (2), 217–232. doi:10.1007/s10533-018-0486-2
AVISO (2003). AVISO and PODAAC user handbook—IGDR and GDR jason products. IGDR and GDR Jason Products. 2nm edn.
Awal, M. A. (2014). Water logging in south-western coastal region of Bangladesh: local adaptation and policy options. Sci. Postprint 1 (1), e00038. doi:10.14340/SPP.2014.12A0001
Brunet, R. C., and Garcia-Gil, L. J. (1996). Sulfide-induced dissimilatory nitrate reduction to ammonia in anaerobic freshwater sediments. FEMS Microbiol. Ecol. 21 (2), 131–138. doi:10.1111/j.1574-6941.1996.tb00340.x
Bai, J., Deng, W., Wang, Q., Cui, B., and Ding, Q. (2007). Spatial distribution of inorganic nitrogen contents of marsh soils in a river floodplain with different flood frequencies from soil-defrozen period. Environ. Monit. Assess. 134, 421–428. doi:10.1007/s10661-007-9633-2
Bai, J., Ouyang, H., Deng, W., Zhu, Y., Zhang, X., Wang, Q., et al. (2005). Spatial distribution characteristics of organic matter and total nitrogen of marsh soils in river marginal wetlands. Geoderma 124, 181–192. doi:10.1016/j.geoderma.2004.04.012
Baldwin, D. S., and Fraser, M. (2009). Rehabilitation options for inland waterways impacted by sulfidic sediments - a synthesis. J. Environ. Manage. 91 (2), 311–319. doi:10.1016/j.jenvman.2009.09.006
Baldwin, D. S., Rees, G. N., Mitchell, A. M., Watson, G., and Williams, J. (2006). The short-term effects of salinization on anaerobic nutrient cycling and microbial community structure in sediment from a freshwater wetland. Wetlands 26 (2), 455–464. doi:10.1672/0277-5212(2006)26[455:TSEOSO]2.0.CO;2
Barry, A., Ooi, S. K., Helton, A. M., Steven, B., Elphick, C. S., Lawrence, B. A., et al. (2022). Vegetation zonation predicts soil carbon mineralization and microbial communities in Southern New England salt marshes. Estuaries Coast. 45 (1), 168–180. doi:10.1007/s12237-021-00943-0
Bastviken, D., Svensson, T., Karlsson, S., Sandén, P., and Öberg, G. (2009). Temperature sensitivity indicates that chlorination of organic matter in forest soil is primarily biotic. Environ. Sci. Technol. 43 (10), 3569–3573. doi:10.1021/es8035779
Bastviken, D., Thomsen, F., Svensson, T., Karlsson, S., Sandén, P., Shaw, G., et al. (2007). Chloride retention in forest soil by microbial uptake and by natural chlorination of organic matter. Geochim. Cosmochim. Acta 71 (13), 3182–3192. doi:10.1016/J.GCA.2007.04.028
Blankespoor, B., Dasgupta, S., and Laplante, B. (2012). Sea-level rise and coastal wetlands: impacts and costs. Policy Research Working Paper; No. 6277. Available at: https://openknowledge.worldbank.org/handle/10986/16383 (Accessed May 1, 2021).
Briggs, S. V., and Taws, N. (2003). Impacts of salinity on biodiversity - clear understanding or muddy confusion? Aust. J. Bot. 51 (6), 609. doi:10.1071/BT02114
Brock, M. A., Nielsen, D. L., and Crosslé, K. (2005). Changes in biotic communities developing from freshwater wetland sediments under experimental salinity and water regimes. Freshw. Biol. 50 (8), 1376–1390. doi:10.1111/j.1365-2427.2005.01408.x
Burgin, A. J., Yang, W. H., Hamilton, S. K., and Silver, W. L. (2011). Beyond carbon and nitrogen: how the microbial energy economy couples elemental cycles in diverse ecosystems. Front. Ecol. Environ. 9 (1), 44–52. doi:10.1890/090227
Burns, R. G., DeForest, J. L., Marxsen, J., Sinsabaugh, R. L., Stromberger, M. E., Wallenstein, M., et al. (2013). Soil enzymes in a changing environment: current knowledge and future directions. Soil Biol. Biochem. 58, 216–234. doi:10.1016/j.soilbio.2012.11.009
Capooci, M., Barba, J., Seyfferth, A. L., and Vargas, R. (2019). Experimental influence of storm-surge salinity on soil greenhouse gas emissions from a tidal salt marsh. Sci. Total Environ. 686, 1164–1172. doi:10.1016/j.scitotenv.2019.06.032
Carmona, R., Muñoz, R., and Niell, F. X. (2021). Differential nutrient uptake by saltmarsh plants is modified by increasing salinity. Front. Plant Sci. 12, 709453. doi:10.3389/FPLS.2021.709453
Carrasco, A. R., Ferreira, O., and Roelvink, D. (2016). Coastal lagoons and rising sea level: a review. Earth. Sci. Rev. 154, 356–368. doi:10.1016/j.earscirev.2015.11.007
Chambers, L. G., Reddy, K. R., and Osborne, T. Z. (2011). Short-term response of carbon cycling to salinity pulses in a freshwater wetland. Soil Sci. Soc. Am. J. 75 (5), 2000–2007. doi:10.2136/sssaj2011.0026
Chen, J., and Mueller, V. (2018). Coastal climate change, soil salinity and human migration in Bangladesh. Nat. Clim. Chang. 8 (11), 981–985. doi:10.1038/s41558-018-0313-8
Chhabra, R. (1996). “Irrigation and salinity control,” in Soil salinity and water quality. Editor R. Chhabra (New Delhi, India: Oxford and IBH Publishing), 205–237.
Chmura, G. L., Anisfeld, S. C., Cahoon, D. R., and Lynch, J. C. (2003). Global carbon sequestration in tidal, saline wetland soils. Glob. Biogeochem. Cycles 17 (4), 1111. doi:10.1029/2002gb001917
Cocks, P. S. (2003). Land-use change is the key to protecting biodiversity in salinising landscapes. Aust. J. Bot. 51 (6), 627. doi:10.1071/BT03004
Craft, C., Clough, J., Ehman, J., Jove, S., Park, R., Pennings, S., et al. (2009). Forecasting the effects of accelerated sea-level rise on tidal marsh ecosystem services. Front. Ecol. Environ. 7 (2), 73–78. doi:10.1890/070219
Custodio, E. (2010). Coastal aquifers of Europe: an overview. Hydrogeol. J. 18 (1), 269–280. doi:10.1007/s10040-009-0496-1
Daliakopoulos, I. N., Tsanis, I. K., Koutroulis, A., Kourgialas, N. N., Varouchakis, A. E., Karatzas, G. P., et al. (2016). The threat of soil salinity: a European scale review. Sci. Total Environ. 573, 727–739. doi:10.1016/j.scitotenv.2016.08.177
Davis, J. A., and Froend, R. (1999). Loss and degradation of wetlands in southwestern Australia: underlying causes, consequences and solutions. Wetl. Ecol. Manag. 7, 13–23. doi:10.1023/A:1008400404021
Dinçer, A. R., and Kargi, F. (2001). Performance of rotating biological disc system treating saline wastewater. Process Biochem. 36, 901–906. doi:10.1016/S0032-9592(00)00287-9
Diome, F., and Tine, A. (2015). Impact of salinity on the physical soil properties in the groundnut basin of Senegal: case study of Ndiaffate. Int. J. Chem. 7 (2), 198. doi:10.5539/ijc.v7n2p198
Dissanayake, D. M. P. K., Ranasinghe, R., and Roelvink, J. A. (2012). The morphological response of large tidal inlet/basin systems to relative sea level rise. Clim. Change 113 (2), 253–276. doi:10.1007/s10584-012-0402-z
Djaman, K., Mel, V. C., Diop, L., Sow, A., El-Namaky, R., Manneh, B., et al. (2018). Effects of alternate wetting and drying irrigation regime and nitrogen fertilizer on yield and nitrogen use efficiency of irrigated rice in the Sahel. Water 10 (6), 711. doi:10.3390/w10060711
Duarte, B., Sleimi, N., and Cagador, I. (2014). Biophysical and biochemical constraints imposed by salt stress: learning from halophytes. Front. Plant Sci. 5, 746. doi:10.3389/FPLS.2014.00746
Dymond, S. F., Vadeboncoeur, M., Siegert, C. M., Minick, K. J., Mitra, B., Li, X., et al. (2019). Water table drawdown alters soil and microbial carbon pool size and isotope composition in coastal freshwater forested wetlands. Front. For. Glob. Change 2, 7. doi:10.3389/ffgc.2019.00007
Edmonds, J. W., Weston, N. B., Joye, S. B., Mou, X., and Moran, M. A. (2009). Microbial community response to seawater amendment in low-salinity tidal sediments. Microb. Ecol. 58 (3), 558–568. doi:10.1007/s00248-009-9556-2
Elliott, M., Mander, L., Mazik, K., Simenstad, C., Valesini, F., Whitfield, A., et al. (2016). Ecoengineering with ecohydrology: successes and failures in estuarine restoration. Estuar. Coast. Shelf Sci. 176, 12–35. doi:10.1016/j.ecss.2016.04.003
Empadinhas, N., and Da Costa, M. S. (2008). Osmoadaptation mechanisms in prokaryotes: distribution of compatible solutes. Int. Microbiol. 11 (3), 151–161. doi:10.2436/20.1501.01.55
Etesami, H., and Beattie, G. A. (2018). Mining halophytes for plant growth-promoting halotolerant bacteria to enhance the salinity tolerance of non-halophytic crops. Front. Microbiol. 9, 148. doi:10.3389/FMICB.2018.00148
Ferla, M., Cordella, M., Michielli, L., and Rusconi, A. (2007). Long-term variations on sea level and tidal regime in the lagoon of Venice. Estuar. Coast. Shelf Sci. 75, 214–222. doi:10.1016/j.ecss.2007.03.037
Ferronato, C., Speranza, M., Ferroni, L., Buscaroli, A., Vianello, G., and Vittori Antisari, L. (2018). Vegetation response to soil salinity and waterlogging in three saltmarsh hydrosequences through macronutrients distribution. Estuar. Coast. Shelf Sci. 200, 131–140. doi:10.1016/J.ECSS.2017.10.019
Findlay, S. E. G., and Kelly, V. R. (2011). Emerging indirect and long-term road salt effects on ecosystems. Ann. N. Y. Acad. Sci. 1223 (1), 58–68. doi:10.1111/j.1749-6632.2010.05942.x
Fiola, J. C., Rabenhorst, M. C., Scaduto, E., Seitz, C. R., and Rankin, K. M. S. (2020). Soil biogeochemistry of the capillary fringe in laboratory mesocosms with contrasting soil textures. Soil Sci. Soc. Am. J. 84 (3), 1011–1021. doi:10.1002/saj2.20076
Flowers, T. J., Munns, R., and Colmer, T. D. (2015). Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 115 (3), 419–431. doi:10.1093/aob/mcu217
Fontolan, G., Pillon, S., Bezzi, A., Villalta, R., Lipizer, M., Triches, A., et al. (2012). Human impact and the historical transformation of saltmarshes in the marano and grado lagoon, northern adriatic sea. Estuar. Coast. Shelf Sci. 113, 41–56. doi:10.1016/J.ECSS.2012.02.007
Frankenberger, W. T., and Bingham, F. T. (1982). Influence of salinity on soil enzyme activities. Soil Sci. Soc. Am. J. 46 (6), 1173–1177. doi:10.2136/SSSAJ1982.03615995004600060011X
Frihy, O. E., and El-Sayed, M. K. (2013). Vulnerability risk assessment and adaptation to climate change induced sea level rise along the Mediterranean coast of Egypt. Mitig. Adapt. Strateg. Glob. Change 18 (8), 1215–1237. doi:10.1007/s11027-012-9418-y
Fu, G., Han, J., Yu, T., Huangshen, L., and Zhao, L. (2019). The structure of denitrifying microbial communities in constructed mangrove wetlands in response to fluctuating salinities. J. Environ. Manage. 238, 1–9. doi:10.1016/j.jenvman.2019.02.029
Galliari, J., Santucci, L., Misseri, L., Carol, E., Alvarez, M., and del, P. (2021). Processes controlling groundwater salinity in coastal wetlands of the southern edge of South America. Sci. Total Environ. 754, 141951. doi:10.1016/j.scitotenv.2020.141951
Gardner, L. R. (2005). Role of geomorphic and hydraulic parameters in governing pore water seepage from salt marsh sediments. Water Resour. Res. 41 (7), 1–11. doi:10.1029/2004wr003671
Geilfus, C. M. (2018). Chloride: from nutrient to toxicant. Plant Cell Physiol. 59 (5), 877–886. doi:10.1093/pcp/pcy071
German, D. P., Weintraub, M. N., Grandy, A. S., Lauber, C. L., Rinkes, Z. L., and Allison, S. D. (2011). Optimization of hydrolytic and oxidative enzyme methods for ecosystem studies. Soil Biol. Biochem. 43 (7), 1387–1397. doi:10.1016/j.soilbio.2011.03.017
German, D. P., Marcelo, K. R. B., Stone, M. M., and Allison, S. D. (2012). The michaelis-menten kinetics of soil extracellular enzymes in response to temperature: a cross-latitudinal study. Glob. Change Biol. 18 (4), 1468–1479. doi:10.1111/j.1365-2486.2011.02615.x
Ghollarata, M., and Raiesi, F. (2007). The adverse effects of soil salinization on the growth of Trifolium alexandrinum L. and associated microbial and biochemical properties in a soil from Iran. Soil Biol. Biochem. 39 (7), 1699–1702. doi:10.1016/j.soilbio.2007.01.024
Giambastiani, B. M. S., Macciocca, V. R., Molducci, M., and Antonellini, M. (2020). Factors affecting water drainage long-time series in the salinized low-lying coastal area of Ravenna (Italy). Water 12 (1), 256. doi:10.3390/w12010256
Giblin, A. E., Weston, N. B., Banta, G. T., Tucker, J., and Hopkinson, C. S. (2010). The effects of salinity on nitrogen losses from an oligohaline estuarine sediment. Estuaries Coast. 33 (5), 1054–1068. doi:10.1007/s12237-010-9280-7
Gil, R., Boscaiu, M., Lull, C., Bautista, I., Lidón, A., Vicente, O., et al. (2013). Are soluble carbohydrates ecologically relevant for salt tolerance in halophytes? Funct. Plant Biol. 40 (9), 805. doi:10.1071/FP12359
Glass, C., and Silverstein, J. (1999). Denitrification of high-nitrate, high-salinity wastewater. Water Res. 33 (1), 223–229. doi:10.1016/S0043-1354(98)00177-8
Glenn, E. P., Pitelka, L. F., and Olsen, M. W. (1992). “The use of halophytes to sequester carbon,” in Natural sinks of CO2 (Dordrecht: Springer), 251–263.
Gonneea, M. E., Mulligan, A. E., and Charette, M. A. (2013). Climate-driven sea level anomalies modulate coastal groundwater dynamics and discharge. Geophys. Res. Lett. 40 (11), 2701–2706. doi:10.1002/grl.50192
Gopalakrishnan, T., Hasan, M. K., Haque, A. T. M. S., Jayasinghe, S. L., and Kumar, L. (2019). Sustainability of coastal agriculture under climate change. Sustainability 11 (24), 7200. doi:10.3390/su11247200
Grimaldi, C., Thomas, Z., and Merot, P. (2009). “Chloride accumulation in the soil and groundwater under a downslope oak hedge reveals the impressive evapotranspiration of wooded linear structures below a temperate climate,” in EGU general assembly conference abstracts, Vienna, April 19–24, 2009, 4246.
Gryndler, M., Rohlenová, J., Kopecký, J., and Matucha, M. (2008). Chloride concentration affects soil microbial community. Chemosphere 71 (7), 1401–1408. doi:10.1016/j.chemosphere.2007.11.003
Guimond, J. A., and Michael, H. A. (2021). Effects of marsh migration on flooding, saltwater intrusion, and crop yield in coastal agricultural land subject to storm surge inundation. Water Resour. Res. 57 (2), e2020WR028326. doi:10.1029/2020WR028326
Guimond, J., and Tamborski, J. (2021). Salt marsh hydrogeology: a review. Water 13 (4), 543. doi:10.3390/w13040543
Gunde-Cimerman, N., Plemenitaš, A., and Oren, A. (2018). Strategies of adaptation of microorganisms of the three domains of life to high salt concentrations. FEMS Microbiol. Rev. 42, 353–375. doi:10.1093/femsre/fuy009
Hale, R. L., and Groffman, P. M. (2006). Chloride effects on nitrogen dynamics in forested and suburban stream debris dams. J. Environ. Qual. 35 (6), 2425–2432. doi:10.2134/JEQ2006.0164
Haywood, B. J., Hayes, M. P., White, J. R., and Cook, R. L. (2020). Potential fate of wetland soil carbon in a deltaic coastal wetland subjected to high relative sea level rise. Sci. Total Environ. 711, 135185. doi:10.1016/j.scitotenv.2019.135185
Henman, J., and Poulter, B. (2008). Inundation of freshwater peatlands by sea level rise: uncertainty and potential carbon cycle feedbacks. J. Geophys. Res. 113 (G1), 1011. doi:10.1029/2006JG000395
Herbert, E. R., Boon, P., Burgin, A. J., Neubauer, S. C., Franklin, R. B., Ardon, M., et al. (2015). A global perspective on wetland salinization: ecological consequences of a growing threat to freshwater wetlands. Ecosphere 6 (10), art206. doi:10.1890/ES14-00534.1
Hines, J., Megonigal, J. P., and Denno, R. F. (2006). Nutrient subsidies to belowground microbes impact aboveground food web interactions. Ecology 87 (6), 1542–1555. doi:10.1890/0012-9658(2006)87[1542:nstbmi]2.0.co;2
Hobbie, J. E., and Hobbie, E. A. (2013). Microbes in nature are limited by carbon and energy: the starving-survival lifestyle in soil and consequences for estimating microbial rates. Front. Microbiol. 4, 324. doi:10.3389/fmicb.2013.00324
Hobbs, R. J., Cramer, V. A., and Kristjanson, L. J. (2003). What happens if we cannot fix it? Triage, palliative care and setting priorities in salinising landscapes. Aust. J. Bot. 51 (6), 647. doi:10.1071/BT02109
Humphries, M. S., Kindness, A., Ellery, W. N., Hughes, J. C., Bond, J. K., Barnes, K. B., et al. (2011). Vegetation influences on groundwater salinity and chemical heterogeneity in a freshwater, recharge floodplain wetland, South Africa. J. Hydrology 411 (1-2), 130–139. doi:10.1016/j.jhydrol.2011.09.041
IPCC (2013). Climate change 2013: the physical science basis. Contribution of working group I to the fifth assessment report of the intergovernmental panel on Climate Change. Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press.
IPCC (2014). Climate change 2014: mitigation of climate change Cambridge. United Kingdom and New York, NY, USA: Cambridge University Press.
IPCC (2007). “Summary for policymakers,” in Climate change 2007: the physical science basis. contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change (Cambridge, United Kingdom and New York, NY, USA: Cambridge University Press).
Jackson, C. R., and Vallaire, S. C. (2009). Effects of salinity and nutrients on microbial assemblages in Louisiana wetland sediments. Wetlands 29 (1), 277–287. doi:10.1672/08-86.1
Jacoby, R. P., Taylor, N. L., and Millar, A. H. (2011). The role of mitochondrial respiration in salinity tolerance. Trends Plant Sci. 16 (11), 614–623. doi:10.1016/j.tplants.2011.08.002
James, K. R., Cant, B., and Ryan, T. (2003). Responses of freshwater biota to rising salinity levels and implications for saline water management: a review. Aust. J. Bot. 51 (6), 703. doi:10.1071/BT02110
Johnston, S. G., Slavich, P. G., Sullivan, L. A., and Hirst, P. (2003). Artificial drainage of floodwaters from sulfidic backswamps: effects on deoxygenation in an Australian estuary. Mar. Freshw. Res. 54 (6), 781. doi:10.1071/MF02016
Joye, S. B., and Hollibaugh, J. T. (1995). Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270 (5236), 623–625. doi:10.1126/science.270.5236.623
Kaniewski, D., Marriner, N., Morhange, C., Faivre, S., Otto, T., Van Campo, E., et al. (2016). Solar pacing of storm surges, coastal flooding and agricultural losses in the Central Mediterranean. Sci. Rep. 6, 25197. doi:10.1038/srep25197
Kirwan, M. L., and Guntenspergen, G. R. (2010). Influence of tidal range on the stability of coastal marshland. J. Geophys. Res. 115 (F2). doi:10.1029/2009jf001400
Kume, T., Umetsu, C., and Palanisami, K. (2009). Impact of the December 2004 tsunami on soil, groundwater and vegetation in the Nagapattinam district, India. J. Environ. Manage. 90 (10), 3147–3154. doi:10.1016/j.jenvman.2009.05.027
Kundzewicz, Z. W., and Döll, P. (2009). Will groundwater ease freshwater stress under climate change? Hydrol. Sci. J. 54 (4), 665–675. doi:10.1623/hysj.54.4.665
Ladeiro, B. (2012). Saline agriculture in the 21st century: using salt contaminated resources to cope food requirements. J. Bot. 2012, 1–7. doi:10.1155/2012/310705
Lamers, L. P. M., Govers, L. L., Janssen, I. C. J. M., Geurts, J. J. M., Van der Welle, M. E. W., Van Katwijk, M. M., et al. (2013). Sulfide as a soil phytotoxin-a review. Front. Plant Sci. 4, 268. doi:10.3389/fpls.2013.00268/
Lamers, L. P. M., Sarah-, J., F., Samborska, E. M., Van Dulken, I. A. R., Van Hengstum, G., and Roelofs, J. G. M. (2002). Factors controlling the extent of eutrophication and toxicity in sulfate-polluted freshwater wetlands. Limnol. Oceanogr. 47 (2), 585–593. doi:10.4319/LO.2002.47.2.0585
Lamers, L. P. M., Ten Dolle, G. E., Van Den Berg, S. T. G., Van Delft, S. P. J., and Roelofs, J. G. M. (2001). Differential responses of freshwater wetland soils to sulphate pollution. Biogeochemistry 55 (1), 87–101. doi:10.1023/A:1010629319168
Lemanowicz, J., Siwik-Ziomek, A., and Koper, J. (2019). Enzymatic variation of soils exposed to the impact of the soda plant in terms of biochemical parameters. Int. J. Environ. Sci. Technol. 16 (7), 3309–3316. doi:10.1007/S13762-018-1959-5
Leprince, F., and Quiquampoix, H. (1996). Extracellular enzyme activity in soil: effect of pH and ionic strength on the interaction with montmorillonite of two acid phosphatases secreted by the ectomycorrhizal fungus hebeloma cylindrosporum. Eur. J. Soil Sci. 47 (4), 511–522. doi:10.1111/j.1365-2389.1996.tb01851.x
Li, X., Li, F., Singh, B. P., Cui, Z., and Rengel, Z. (2006). Decomposition of maize straw in saline soil. Biol. Fertil. Soils 42 (4), 366–370. doi:10.1007/S00374-005-0042-9
Liu, B., Zhao, W., Wen, Z., Yang, Y., Chang, X., Yang, Q., et al. (2019). Mechanisms and feedbacks for evapotranspiration-induced salt accumulation and precipitation in an arid wetland of China. J. Hydrol. 568, 403–415. doi:10.1016/j.jhydrol.2018.11.004
Mancuso, M., Santucci, L., and Carol, E. (2020). Effects of intensive aquifers exploitation on groundwater salinity in coastal wetlands. Hydrol. Process. 34 (11), 2313–2323. doi:10.1002/hyp.13727
Martínez-Antonio, A., and Collado-Vides, J. (2003). Identifying global regulators in transcriptional regulatory networks in bacteria. Curr. Opin. Microbiol. 6 (5), 482–489. doi:10.1016/j.mib.2003.09.002
Marton, J. M., Herbert, E. R., and Craft, C. B. (2012). Effects of salinity on denitrification and greenhouse gas production from laboratory-incubated tidal forest soils. Wetlands 32 (2), 347–357. doi:10.1007/s13157-012-0270-3
Mazi, K., Koussis, A. D., and Destouni, G. (2014). Intensively exploited mediterranean aquifers: resilience to seawater intrusion and proximity to critical thresholds. Hydrol. Earth Syst. Sci. 18 (5), 1663–1677. doi:10.5194/HESS-18-1663-2014
McGinnis, D. F., Greinert, J., Artemov, Y., Beaubien, S. E., and Wüest, A. (2006). Fate of rising methane bubbles in stratified waters: how much methane reaches the atmosphere? J. Geophys. Res. 111 (9), C09007. doi:10.1029/2005JC003183
McLeod, E., Chmura, G. L., Bouillon, S., Salm, R., Björk, M., Duarte, C. M., et al. (2011). A blueprint for blue carbon: toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 9, 552–560. doi:10.1890/110004
Meiggs, D., and Taillefert, M. (2011). The effect of riverine discharge on biogeochemical processes in estuarine sediments. Limnol. Oceanogr. 56 (5), 1797–1810. doi:10.4319/LO.2011.56.5.1797
Meira, G. R., Andrade, M. C., Padaratz, I. J., Alonso, M. C., and Borba, J. C. (2006). Measurements and modelling of marine salt transportation and deposition in a tropical region in Brazil. Atmos. Environ. 40 (29), 5596–5607. doi:10.1016/j.atmosenv.2006.04.053
Millero, F. J., Feistel, R., Wright, D. G., and McDougall, T. J. (2008). The composition of standard seawater and the definition of the reference-composition salinity scale. Deep Sea Res. Part I Oceanogr. Res. Pap. 55 (1), 50–72. doi:10.1016/j.dsr.2007.10.001
Mishra, A., Sharma, S. D., and Khan, G. H. (2003). Improvement in physical and chemical properties of sodic soil by 3, 6 and 9 years old plantation of Eucalyptus tereticornis: biorejuvenation of sodic soil. For. Ecol. Manage. 184, 115–124. doi:10.1016/S0378-1127(03)00213-5
Mitra, S., Wassmann, R., and Vlek, P. L. G. (2005). An appraisal of global wetland area and its organic carbon stock. Curr. Sci. 88 (1), 25–35.
Moorhead, D. L., Rinkes, Z. L., Sinsabaugh, R. L., and Weintraub, M. N. (2013). Dynamic relationships between microbial biomass, respiration, inorganic nutrients and enzyme activities: informing enzyme-based decomposition models. Front. Microbiol. 4, 223. doi:10.3389/fmicb.2013.00223
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi:10.1146/annurev.arplant.59.032607.092911
Navrotskaya, S. E., and Chubarenko, B. V. (2013). Trends in the variation of the sea level in the lagoons of the Southeastern Baltic. Oceanology 53 (1), 13–23. doi:10.1134/S0001437012050128
Neubauer, S. C., Franklin, R. B., and Berrier, D. J. (2013). Saltwater intrusion into tidal freshwater marshes alters the biogeochemical processing of organic carbon. Biogeosciences 10 (12), 8171–8183. doi:10.5194/bg-10-8171-2013
Neukom, R., Gergis, J., Karoly, D. J., Wanner, H., Curran, M., Elbert, J., et al. (2014). Inter-hemispheric temperature variability over the past millennium. Nat. Clim. Chang. 4 (5), 362–367. doi:10.1038/nclimate2174
Nielsen, D. L., Brock, M. A., Rees, G. N., and Baldwin, D. S. (2003). Effects of increasing salinity on freshwater ecosystems in Australia. Aust. J. Bot. 51 (6), 655. doi:10.1071/BT02115
Noe, G. B., Krauss, K. W., Lockaby, B. G., Conner, W. H., and Hupp, C. R. (2013). The effect of increasing salinity and forest mortality on soil nitrogen and phosphorus mineralization in tidal freshwater forested wetlands. Biogeochemistry 114, 225–244. doi:10.1007/S10533-012-9805-1
Öberg, G., Holm, M., Sandén, P., Svensson, T., and Parikka, M. (2005). The role of organic-matter-bound chlorine in the chlorine cycle: a case study of the stubbetorp catchment, Sweden. Biogeochemistry 75 (2), 241–269. doi:10.1007/s10533-004-7259-9
Oren, A. (1999). Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63 (2), 334–348. doi:10.1128/MMBR.63.2.334-348.1999
Oren, A. (2008). Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst. 4 (1), 2. doi:10.1186/1746-1448-4-2
Otlewska, A., Migliore, M., Dybka-Stępień, K., Manfredini, A., Struszczyk-Świta, K., Napoli, R., et al. (2020). When salt meddles between plant, soil, and microorganisms. Front. Plant Sci. 11, 553087. doi:10.3389/fpls.2020.553087
Oude Essink, G. H. P. (2001). Salt water intrusion in a three-dimensional groundwater system in The Netherlands: a numerical study. Transp. Porous Media 43 (1), 137–158. doi:10.1023/A:1010625913251
Pathak, H., and Rao, D. L. N. (1998). Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biol. Biochem. 30 (6), 695–702. doi:10.1016/S0038-0717(97)00208-3
Pattnaik, P., Mishra, S. R., Bharati, K., Mohanty, S. R., Sethunathan, N., Adhya, T. K., et al. (2000). Influence of salinity on methanogenesis and associated microflora in tropical rice soils. Microbiol. Res. 155 (3), 215–220. doi:10.1016/S0944-5013(00)80035-x
Pellegrini, P., and Fernández, R. J. (2018). Crop intensification, land use, and on-farm energy-use efficiency during the worldwide spread of the green revolution. Proc. Natl. Acad. Sci. U. S. A. 115 (10), 2335–2340. doi:10.1073/pnas.1717072115
Pereira, C. S., Lopes, I., Abrantes, I., Sousa, J. P., and Chelinho, S. (2019). Salinization effects on coastal ecosystems: a terrestrial model ecosystem approach. Phil. Trans. R. Soc. B 374 (1764), 20180251. doi:10.1098/rstb.2018.0251
Pereira, E. C., Vazquez de Aldana, B. R., Arellano, J. B., and Zabalgogeazcoa, I. (2021). The role of fungal microbiome components on the adaptation to salinity of Festuca rubra subsp. pruinosa. Front. Plant Sci. 12, 695717. doi:10.3389/fpls.2021.695717
Pereira, S. I. A., Moreira, H., Argyras, K., Castro, P. M. L., and Marques, A. P. G. C. (2016). Promotion of sunflower growth under saline water irrigation by the inoculation of beneficial microorganisms. Appl. Soil Ecol. 105, 36–47. doi:10.1016/j.apsoil.2016.03.015
Pfeffer, W. T., Harper, J. T., and O’Neel, S. (2008). Kinematic constraints on glacier contributions to 21st-century sea-level rise. Science 321, 1340–1343. doi:10.1126/science.1159099
Pilkey, O. H., Cooper, J. A. G., and Lewis, D. A. (2009). Global distribution and geomorphology of fetch-limited barrier islands. J. Coast. Res. 25 (4), 819–837. doi:10.2112/08-1023.1
Poffenbarger, H. J., Needelman, B. A., and Megonigal, J. P. (2011). Salinity influence on methane emissions from tidal marshes. Wetlands 31 (5), 831–842. doi:10.1007/s13157-011-0197-0
Qu, W., Li, J., Han, G., Wu, H., Song, W., Zhang, X., et al. (2019). Effect of salinity on the decomposition of soil organic carbon in a tidal wetland. J. Soils Sediments 19, 609–617. doi:10.1007/s11368-018-2096-y
Rath, K. M., Fierer, N., Murphy, D. V., and Rousk, J. (2019). Linking bacterial community composition to soil salinity along environmental gradients. ISME J. 13 (3), 836–846. doi:10.1038/s41396-018-0313-8
Rath, K. M., Maheshwari, A., Bengtson, P., and Rousk, J. (2016). Comparative toxicities of salts on microbial processes in soil. Appl. Environ. Microbiol. 82 (7), 2012–2020. doi:10.1128/aem.04052-15
Rath, K. M., and Rousk, J. (2015). Salt effects on the soil microbial decomposer community and their role in organic carbon cycling: a review. Soil Biol. Biochem. 81, 108–123. doi:10.1016/j.soilbio.2014.11.001
Reddy, K. R., and DeLaune, R. D. (2020). “Coastal wetlands: mississippi river deltaic plain coastal marshes, Louisiana,” in Biogeochemistry of wetlands. Editors K. R. Reddy, and R. D. DeLaune (Boca Raton, FL: CRC Press), 693–726.
Rengasamy, P. (2006). World salinization with emphasis on Australia. J. Exp. Bot. 57 (5), 1017–1023. doi:10.1093/jxb/erj108
Revsbech, N. P., Jacobsen, J. P., and Nielsen, L. P. (2005). Nitrogen transformations in microenvironments of river beds and riparian zones. Ecol. Eng. 24 (5), 447–455. doi:10.1016/j.ecoleng.2005.02.002
Richardson, K., Steffen, W., Schellnhuber, H. J., Alcamo, J., Barker, T., Kammen, D. M., et al. (2009). “Climate change - global risks, challenges and decisions: synthesis report,” in Copenhagen climate conference (Copenhagen, Denmark: Museum Tusculanum).
Rickard, D., and Morse, J. W. (2005). Acid volatile sulfide (AVS). Mar. Chem. 97, 141–197. doi:10.1016/j.marchem.2005.08.004
Rietz, D. N., and Haynes, R. J. (2003). Effects of irrigation-induced salinity and sodicity on soil microbial activity. Soil Biol. Biochem. 35 (6), 845–854. doi:10.1016/S0038-0717(03)00125-1
Rignot, E., Velicogna, I., Van Den Broeke, M. R., Monaghan, A., and Lenaerts, J. (2011). Acceleration of the contribution of the Greenland and Antarctic ice sheets to sea level rise. Geophys. Res. Lett. 38 (5), L05503. doi:10.1029/2011GL046583
Rose, D. A., Konukcu, F., and Gowing, J. W. (2005). Effect of watertable depth on evaporation and salt accumulation from saline groundwater. Soil Res. 43 (5), 565. doi:10.1071/SR04051
Rysgaard, S., Thastu, P., Dalsgaard, T., Christensen, P. B., and Sloth, N. P. (1999). Effects of salinity on NH4+ adsorption capacity, denitrification in Danish estuarine sediments. Estuaries 22 (1), 21. doi:10.2307/1352923
Sambou, A., Theilade, I., Fensholt, R., and Ræbild, A. (2016). Decline of woody vegetation in a saline landscape in the Groundnut Basin, Senegal. Reg. Environ. Change 16 (6), 1765–1777. doi:10.1007/S10113-016-0929-z
Sardinha, M., Müller, T., Schmeisky, H., and Joergensen, R. G. (2003). Microbial performance in soils along a salinity gradient under acidic conditions. Appl. Soil Ecol. 23 (3), 237–244. doi:10.1016/S0929-1393(03)00027-1
Saviozzi, A., Cardelli, R., and Di Puccio, R. (2011). Impact of salinity on soil biological activities: a laboratory experiment. Commun. Soil Sci. Plant Analysis 42 (3), 358–367. doi:10.1080/00103624.2011.542226
Schoepfer, V. A., Bernhardt, E. S., and Burgin, A. J. (2014). Iron clad wetlands: soil iron-sulfur buffering determines coastal wetland response to salt water incursion. J. Geophys. Res. Biogeosci. 119 (12), 2209–2219. doi:10.1002/2014jg002739
Segarra, K. E. A., Comerford, C., Slaughter, J., and Joye, S. B. (2013). Impact of electron acceptor availability on the anaerobic oxidation of methane in coastal freshwater and brackish wetland sediments. Geochim. Cosmochim. Acta 115, 15–30. doi:10.1016/j.gca.2013.03.029
Segarra, K. E. A., Schubotz, F., Samarkin, V., Yoshinaga, M. Y., Hinrichs, K. U., Joye, S. B., et al. (2015). High rates of anaerobic methane oxidation in freshwater wetlands reduce potential atmospheric methane emissions. Nat. Commun. 6 (1), 7477. doi:10.1038/ncomms8477
Serrano, R. (1996). Salt tolerance in plants and microorganisms: toxicity targets and defense responses. Int. Rev. Cytol. 165, 1–52. doi:10.1016/s0074-7696(08)62219-6
Setia, R., Lewis, M., Marschner, P., Raja Segaran, R., Summers, D., Chittleborough, D., et al. (2013). Severity of salinity accurately detected and classified on a paddock scale with high resolution multispectral satellite imagery. Land Degrad. Dev. 24 (4), 375–384. doi:10.1002/ldr.1134
Setia, R., Marschner, P., Baldock, J., and Chittleborough, D. (2010). Is CO2 evolution in saline soils affected by an osmotic effect and calcium carbonate? Biol. Fertil. Soils 46 (8), 781–792. doi:10.1007/s00374-010-0479-3
Shao, P., Han, H., Sun, J., Yang, H., and Xie, H. (2022). Salinity effects on microbial derived-C of coastal wetland soils in the Yellow River delta. Front. Ecol. Evol. 10, 872816. doi:10.3389/fevo.2022.872816
Shvartsev, S. L. (2008). Geochemistry of fresh groundwater in the main landscape zones of the Earth. Geochem. Int. 46 (13), 1285–1398. doi:10.1134/s0016702908130016
Sloss, C. R., Jones, B. G., McClennen, C. E., de Carli, J., and Price, D. M. (2006). The geomorphological evolution of a wave-dominated barrier estuary: Burrill Lake, New South Wales, Australia. Sediment. Geol. 187, 229–249. doi:10.1016/j.sedgeo.2005.12.029
Smith, S. M., Roman, C. T., James-Pirri, M. J., Chapman, K., Portnoy, J., and Gwilliam, E. (2009). Responses of plant communities to incremental hydrologic restoration of a tide-restricted salt marsh in Southern New England (Massachusetts, U.S.A.). Restor. Ecol. 17 (5), 606–618. doi:10.1111/J.1526-100X.2008.00426.x
Smolders, A. J. P., Lamers, L. P. M., Lucassen, E. C. H. E. T., Van Der Velde, G., and Roelofs, J. G. M. (2007). Internal eutrophication: how it works and what to do about it—a review. Chem. Ecol. 22 (2), 93–111. doi:10.1080/02757540600579730
Sorensen, J., Tiedje, J. M., and Firestone, R. B. (1980). Inhibition by sulfide of nitric and nitrous oxide reduction by denitrifying Pseudomonas fluorescens. Appl. Environ. Microbiol. 39 (1), 105–108. doi:10.1128/aem.39.1.105-108.1980
Spencer, T., Schuerch, M., Nicholls, R. J., Hinkel, J., Lincke, D., Vafeidis, A. T., et al. (2016). Global coastal wetland change under sea-level rise and related stresses: the DIVA Wetland Change Model. Glob. Planet. Change 139, 15–30. doi:10.1016/j.gloplacha.2015.12.018
Stagg, C. L., Schoolmaster, D. R., Krauss, K. W., Cormier, N., and Conner, W. H. (2017). Causal mechanisms of soil organic matter decomposition: deconstructing salinity and flooding impacts in coastal wetlands. Ecology 98 (8), 2003–2018. doi:10.1002/ecy.1890
Stumm, W., and Morgan, J. J. (1996). Aquatic chemistry: chemical equilibria and rates in natural waters. New York: John Wiley.
Sun, R., Wang, X., Tian, Y., Guo, K., Feng, X., Sun, H., et al. (2022). Long-term amelioration practices reshape the soil microbiome in a coastal saline soil and alter the richness and vertical distribution differently among Bacterial, Archaeal, and Fungal communities. Front. Microbiol. 12, 768203. doi:10.3389/fmicb.2021.768203
Tobias, C., and Neubauer, S. C. (2019). “Salt marsh biogeochemistry - An overview,” in Coastal wetlands: an integrated ecosystem approach. Editors G. M. E. Perillo, E. Wolanski, D. R. Cahoon, and C. S. Hopkinson. 2nd edition (Amsterdam, Netherlands: Elsevier), 539–596. doi:10.1016/B978-0-444-63893-9.00016-2
Toldo, E. E., Dillenburg, S. R., Corrêa, I. C. S., and Almeida, L. E. S. B. (2000). Holocene Sedimentation in Lagoa dos Patos Lagoon, Rio Grande do Sul, Brazil. J. Coast. Res. 16, 816–822.
UNESCO (2016). Education for people and planet: creating sustainable futures for all. Global education monitoring report. UNESCO Publishing. Available at: http://uis.unesco.org/sites/default/files/documents/education-for-people-and-planet-creating-sustainable-futures-for-all-gemr-2016-en.pdf (Accessed May 1, 2021).
Valipour, M. (2014). Drainage, waterlogging, and salinity. Arch. Agron. Soil Sci. 60 (12), 1625–1640. doi:10.1080/03650340.2014.905676
Valjarević, A., Filipović, D., Milanović, M., and Valjarević, D. (2020). New updated world maps of sea-surface salinity. Pure Appl. Geophys. 177 (6), 2977–2992. doi:10.1007/S00024-019-02404-z
Vermeer, M., and Rahmstorf, S. (2009). Global sea level linked to global temperature. Proc. Natl. Acad. Sci. U. S. A. 106 (51), 21527–21532. doi:10.1073/PNAS.0907765106
Wang, K., Xu, J., Li, Y., Wang, H., Wei, Q., Liao, L., et al. (2020). Response of soil respiration and microbial biomass to soil salinity under different water content in the coastal areas of Eastern China. Eurasian Soil Sc. 53 (1), 82–89. doi:10.1134/S1064229320010159
Weissman, D. S., and Tully, K. L. (2020). Saltwater intrusion affects nutrient concentrations in soil porewater and surface waters of coastal habitats. Ecosphere 11 (2), e03041. doi:10.1002/ECS2.3041
Weston, N. B., Dixon, R. E., and Joye, S. B. (2006). Ramifications of increased salinity in tidal freshwater sediments: geochemistry and microbial pathways of organic matter mineralization. J. Geophys. Res. 111 (G1), G01009. doi:10.1029/2005JG000071
Weston, N. B., Vile, M. A., Neubauer, S. C., and Velinsky, D. J. (2011). Accelerated microbial organic matter mineralization following salt-water intrusion into tidal freshwater marsh soils. Biogeochemistry 102 (1), 135–151. doi:10.1007/s10533-010-9427-4
White, E., and Kaplan, D. (2017). Restore or retreat? Saltwater intrusion and water management in coastal wetlands. Ecosyst. Health Sustain. 3 (1), e01258. doi:10.1002/ehs2.1258
White, I., Melville, M. D., Wilson, B. P., and Sammut, J. (1997). Reducing acidic discharges from coastal wetlands in eastern Australia. Wetl. Ecol. Manag. 5 (1), 55–72. doi:10.1023/A:1008227421258
White, P. J., and Broadley, M. R. (2001). Chloride in soils and its uptake and movement within the plant: a review. Ann. Bot. 88 (6), 967–988. doi:10.1006/anbo.2001.1540
Wichern, J., Wichern, F., and Joergensen, R. G. (2006). Impact of salinity on soil microbial communities and the decomposition of maize in acidic soils. Geoderma 137, 100–108. doi:10.1016/j.geoderma.2006.08.001
Wicke, B., Smeets, E., Dornburg, V., Vashev, B., Gaiser, T., Turkenburg, W., et al. (2011). The global technical and economic potential of bioenergy from salt-affected soils. Energy Environ. Sci. 4 (8), 2669–2681. doi:10.1039/C1EE01029H
Wong, V. N. L., Dalal, R. C., and Greene, R. S. B. (2008). Salinity and sodicity effects on respiration and microbial biomass of soil. Biol. Fertil. Soils 44 (7), 943–953. doi:10.1007/s00374-008-0279-1
Wood, C., and Harrington, G. A. (2015). Influence of seasonal variations in sea level on the salinity regime of a coastal groundwater-fed wetland. Groundwater 53 (1), 90–98. doi:10.1111/gwat.12168
Wu, X., Wang, Y., Shen, C., and Zhao, Z. (2022). Variable-density flow and solute transport in stratified salt marshes. Front. Mar. Sci. 8, 804526. doi:10.3389/fmars.2021.804526
Xian, X., Pang, M., Zhang, J., Zhu, M., Kong, F., Xi, M., et al. (2019). Assessing the effect of potential water and salt intrusion on coastal wetland soil quality: simulation study. J. Soils Sediments 19, 2251–2264. doi:10.1007/s11368-018-02225-y
Yang, Z., Wang, T., Voisin, N., and Copping, A. (2015). Estuarine response to river flow and sea-level rise under future climate change and human development. Estuar. Coast. Shelf Sci. 156, 19–30. doi:10.1016/j.ecss.2014.08.015
Zhang, W. W., Wang, C., Xue, R., and Wang, L. J. (2019). Effects of salinity on the soil microbial community and soil fertility. J. Integr. Agric. 18, 1360–1368. doi:10.1016/S2095-3119(18)62077-5
Zhang, Y., Du, J., Zhao, X., Wu, W., Peng, B., Zhang, J., et al. (2014). A multi-proxy study of sedimentary humic substances in the salt marsh of the Changjiang Estuary, China. Estuar. Coast. Shelf Sci. 151, 295–301. doi:10.1016/j.ecss.2014.10.007
Zhao, Q., Bai, J., Gao, Y., Zhao, H., Zhang, G., Cui, B., et al. (2020). Shifts in the soil bacterial community along a salinity gradient in the yellow river delta. Land Degrad. Dev. 31, 2255–2267. doi:10.1002/ldr.3594
Zhao, Y., Li, T., Shao, P., Sun, J., Xu, W., and Zhang, Z. (2022). Variation in bacterial community structure in rhizosphere and bulk soils of different halophytes in the yellow river delta. Front. Ecol. Evol. 9, 816918. doi:10.3389/fevo.2021.816918
Keywords: salinization, see level rise, wetlands, GHG, coastal soils
Citation: Mazhar S, Pellegrini E, Contin M, Bravo C and De Nobili M (2022) Impacts of salinization caused by sea level rise on the biological processes of coastal soils - A review. Front. Environ. Sci. 10:909415. doi: 10.3389/fenvs.2022.909415
Received: 31 March 2022; Accepted: 06 July 2022;
Published: 04 August 2022.
Edited by:
Dionisios Gasparatos, Agricultural University of Athens, GreeceReviewed by:
Loredana Canfora, Council for Agricultural and Economics Research (CREA), ItalyMarcin Naliwajski, University of Łódź, Poland
Copyright © 2022 Mazhar, Pellegrini, Contin, Bravo and De Nobili. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisa Pellegrini, ZWxpc2EucGVsbGVncmluaUB1bml1ZC5pdA==
†ORCID: Elisa Pellegrini, https://orcid.org/0000-0002-6972-9540
 Sadat Mazhar
Sadat Mazhar Elisa Pellegrini
Elisa Pellegrini Marco Contin
Marco Contin Carlo Bravo
Carlo Bravo Maria De Nobili
Maria De Nobili