
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 22 July 2022
Sec. Freshwater Science
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.903984
This article is part of the Research Topic Biodiversity in a changing world: How do species traits reflect anthropogenic changes in aquatic ecosystems? View all 8 articles
 Yasmine Costa Moreira1,2
Yasmine Costa Moreira1,2 Simone Jaqueline Cardoso2,3
Simone Jaqueline Cardoso2,3 Isabel Cristina Vidal Siqueira-Castro4
Isabel Cristina Vidal Siqueira-Castro4 Juliane Araújo Greinert-Goulart5
Juliane Araújo Greinert-Goulart5 Regina Maura Bueno Franco4
Regina Maura Bueno Franco4 Caio Graco-Roza6*
Caio Graco-Roza6* Roberto Júnio Pedroso Dias1,2*
Roberto Júnio Pedroso Dias1,2*Assessing functional diversity of communities is an efficient method to link community composition to ecosystem quality. Still, studies using functional traits of microeukaryote ciliate communities in biological wastewater treatment plants are lacking. The present work explores the functional diversity of the ciliate protist community in a wastewater treatment plant (WWTP) operating with a combined UASB-activated sludge system, and specifically to: 1) investigate the taxonomic and functional composition of the ciliate communities over time; 2) compare taxonomic and functional diversity indices with regard to its applicability in WWPS; 3) assess the relationship between the ciliate community’s functional composition and the WWTPs temporal conditions; and 4) investigate the potential use of functional diversity as an indicator of WWTP efficiency. Totally, we recorded 21 ciliate species throughout 37 samplings. The number of species was low compared to other plants. Bacterivorous and flake-forming species were the main functional strategies found in the samples. The correlation between taxonomic and functional richness was significant, indicating a functionally redundant community. There was a correlation between the Simpson and Rao’s quadratic entropy indexes suggesting that loss of taxonomic diversity leads to a loss of functional diversity. The homogeneity of the measured physical and chemical data led to functional homogenization and redundancy (homogenous CWM) of the ciliate community. The functional diversity is positively correlated with parameters of removal efficiency, indicating a promising application in WWTPs. Future studies will broaden knowledge on functional diversity in biological wastewater treatment systems, this being a first step with the unprecedented application of this methodology in artificial ecosystems.
Sanitation is essential for human life quality, preventing diseases and assuring dignity of life to people. Among all scopes of sanitation, sanitary wastewater is considered one of the most critical for still presenting great scarcity of collection and treatment (WHO WORLD HEALTH ORGANIZATION, 2018). Nowadays, the biological treatment is the most widely used method worldwide with the activated sludge being one of the most known (Metcalf and Eddy Inc, 2003). However, recent studies indicate the benefits of sequential anaerobic-aerobic systems, such as lower energy requirements (even generating energy trough the anaerobic reactor using the methane produced), as well as lower excess sludge production and stabilization, and greater operational simplicity (Sikosana et al., 2019; Vinardell et al., 2020).
Due to the wide application of the activated sludge system, more studies are needed on its biological composition (e.g., microbial composition) and its spatial and temporal dynamics. For instance, heterotrophic single-celled microeukaryotes (Protozoa) have been recorded within biological systems since the creation of the term “activated sludge” (Ardern and Lockett 1914), but the importance of these microorganisms to the purification process was not initially understood (Curds, 1963). Currently, various functions of protozoans in these systems are known, e.g., predation on disperse bacteria and purification of final effluent. Besides evaluating effluent quality and its correlation to decrease in density of Escherichia coli in the system (Madoni, 2003, 2011; Dubber and Gray, 2011; Foissner, 2016).
Among the single-celled microeukaryotes, the ciliates stand out (Alveolata, Ciliophora), as perhaps the most observed group in activated sludges. They are commonly found in aquatic and terrestrial environments, mostly as free-living organisms (Fenchel, 1987; Lynn, 2008), being a diverse and relatively well-characterized group in activated sludge. Ciliates, with over 8,000 described species, play an essential role in the functioning of microbial food webs, being a mediator of matter and energy transfer across trophic levels (Lynn, 2008; Xu et al., 2014). One of the possible ways to use Ciliates to monitor the quality of the activated sludge is through the Sludge Biotic Index (SBI) (Madoni, 1994). This index is bounded to zero and 10, and uses information about the ciliate density, species richness and dominant groups to categorize the system’s performance in four classes: I (8–10, very good performance), II (6–7, good performance), III (4–5, bad performance) and IV (0–3, terrible performance).
Historically, it is possible to separate the use of protists as indicators in wastewater treatment systems in three Ages (Foissner, 2016): During the Age of Discovery and Exploitation (1914–1950), the importance of the presence of these microorganisms to the wastewater purification process in activated sludges was recognized; in the Age of Bloom (1950–2000), there were advances in the understanding of the role of protists in these systems, broadening practical applications as bioindicators; and in the Age of Decline (2000–2016), there was a decrease of papers and taxonomists dedicated to studying these artificial ecosystems. Although there may be a saturation in the field regarding the of description of species and the study of species in these systems, we still lack ecological and molecular data for these artificial ecosystems to better understand the role and applied use of ciliates.
Recently, the Era of Integration (Clamp and Lynn, 2017) emerged based on collaboration between hierarchical levels or different disciplines, aiming at solving complex and large-scale problems mostly. Within this Era, arises the need to include new tools aiming the facilitation of data integration from different systems, which enable studying spatial and temporal variations of communities. Particularly, the integration of functional traits has been highlighted as an excellent tool for knowledge integration due to its ability to compare responses from communities with different taxonomic compositions.
The integrative approach, including integrative ecology and, more specifically, functional ecology, allows for a better understanding of the multidimensional aspects of ecosystems (Clamp and Lynn, 2017), including artificial systems. According to Tilman (2001), functional diversity is “the value and variation of species and their characteristics which influence the functioning of communities”. Functional diversity is based on morphological, physiological or behavioral characteristics related to performance and the relationship of organisms with the biotic and abiotic conditions of their environment (Mcgill et al., 2006; Violle et al., 2007). Functional diversity connects the species properties to the ecosystem processes through functional traits (Weisse, 2017). Studies on functional diversity using ciliates as model focus on the following approaches: spatial/temporal changes in the community structure (Xu et al., 2016; Bai and Xu, 2019; Liu et al., 2019; Sikder et al., 2019; Xu and Soininen, 2019; Zhong et al., 2019a,b; Xu et al., 2020), correlation between functional traits of ciliates and environmental changes (Zhong et al., 2017; Xu et al., 2018d; Bai and Xu, 2019; Sikder et al., 2019; Gui et al., 2020; Guo et al., 2020; Xu et al., 2020), vertical trophic-functional patterns in the community (Al et al., 2018b), identification of indicators to assess environmental quality (Xu et al., 2018a,b), and use of new methodological approaches (Weisse, 2017; Zhong et al., 2017; Bai et al., 2019).
Previous studies about functional diversity of ciliates in aquatic ecosystems highlighted 1feeding type (i.e., bacterivore, algivore, carnivore), 2body size classes (e.g.,< 50 μm; 50–150 μm; > 150 µm), 3form of locomotion (i.e., sessile, crawling and free-swimming), 4respiration type (aerobic or anaerobic), 5body shape (flattened or cylindrical), 6body flexibility (flexible or non-flexible), 7cyst formation, 8defense mechanisms (i.e., toxicysts, cell projections), and 9sensibility to abiotic parameters (i.e., temperature, salinity, pH) (Weisse, 2017; Xu et al., 2018b,c,d; Zhong et al., 2019a,b; Xu and Soninen, 2019; Xu et al., 2020). To our knowledge, there is no study about functional diversity of ciliates in wastewater treatment plants.
Although there is a large number of papers in the literature about checklist and ecology of ciliates in activated sludges, few of them evaluate the microfauna of the activated sludge of combined anaerobic-aerobic systems (UASB-activated sludge) (Liu et al., 2008; Siqueira-Castro et al., 2016b). Siqueira-Castro et al. (2016b) recently evaluated in Brazil the ciliates in the activated sludge of a combined anaerobic-aerobic system, as well as related that data to the physical and chemical variables of the plant. In that study, 24 samples of the plant were analyzed, focusing on the composition/abundance of ciliates and their correlation to environmental data. In the current study, we have used the data from that previous one (Siqueira-Castro et al., 2016b), as well as new data collected in the same period, to perform an unprecedented study on functional diversity of ciliates in a wastewater treatment plant. Thus, our aims were to: 1investigate taxonomic and functional composition and diversity of the ciliate community, as well as provide data on its temporal dynamics; 2compare and correlate the indices used in the taxonomic and functional diversities; 3assess the relationship between composition and functional traits of the ciliate community and the environmental conditions; and 4investigate the potential application of a functional approach as an indicator metric of efficiency in WWTPs.
The data used in this study was collected in the Piçarrão Wastewater Treatment Plant, in the city of Campinas (São Paulo, Brazil) (22°53′58″S and 47°08′57″W), which is a combined anaerobic-aerobic system comprised of two distinct technologies, a high-rate reactor (UASB) and activated sludge. The Piçarrão WWTP is capable of treating 0.56 m³/s of domestic wastewater and has a mixed liquor volume of 9,385.2 m³ in tank, with a 22-h time of hydraulic retention and 4-days sludge retention (for greater details, see Siqueira-Castro et al., 2016b).
The sampling period lasted 24 months, between April 2010 and March 2012, and 37 samplings were made. To carry out the physical and chemical analyses of the water, the samples were collected at the entrance of the WWTP (raw sewage), before passing through the bar screens, and at the exit of the plant (treated sewage), after passing through the secondary clarifier. The samples for the assessment of ciliate diversity were collected in the aeration tank.
The sanitation company of the city of Campinas (SANASA, 2012) was responsible for carrying out the physical and chemical analyses of the entrance (raw sewage) and exit (treated sewage) samples. The measured parameters were: biochemical oxygen demand (BOD5), chemical oxygen demand (COD), dissolved oxygen (DO), total kjeldahl nitrogen (TKN), nitrite (NO2), nitrate (NO3), total phosphate (TP), total alkalinity (TA), turbidity, pH, temperature, oils and greases, surfactants, sulfate, sulfide, total solids (TS) total fixed solids (FS), total volatile solids (VS.), total dissolved solids (TDS), dissolved fixed solids (DFS), dissolved volatile solids (DVS), total suspended solids (TSS), suspended fixed solids (SFS), suspense volatile solids (SVS), and settleable solids (SS) (Siqueira-Castro et al., 2016b).
The estimation of the abundance values of ciliates in vivo was carried out via optical microscopy (DIC), in a Sedgwick-Rafter chamber (CETESB, 2009), and the observation of the microorganisms was done in a 200x magnification under 3 h following the sampling from the aeration tank. The samples were diluted with mineral water in a 1:2–1:3 ratio (making sure the flakes would not hinder visualization). The counting followed the standard of 100 fields of a 1 mm2 area, randomly distributed, until the entire chamber was viewed (CETESB, 1985). The results were expressed in number of individuals per milliliter of mixed liquor (CETESB, 2009).
The identification of the ciliates at species level was carried out based on in vivo observations, silver impregnation, and images from scanning electron microscopy (Foissner, 2014). The main guides and papers used for the identification were Foissner et al. (1991, 1992, 1994, 1995), Foissner and Berger (1996), Berger (1999, 2006, 2011), Lynn and Small (2002), Berger and Foissner (2004), and Serrano et al. (2008). The checklist complete data and images of the ciliate species found are available in Siqueira-Castro et al. (2016b).
Eight morphological, behavioral, and physiological characteristics were selected as functional traits and subdivided into 21 categories. All traits affect aptitude via effects on growth, reproduction, survival and impacting or being impacted by environmental factors. The traits received equal weighting, being: feeding preference (bacterivore, carnivore, omnivore), body size (<50 µ—small, 50 < e < 150 µ—intermediate, > 150 µ—big), form of locomotion (free-swimming, sessile, crawling), body flexibility (flexible or non-flexible), body geometry (cylindrical or dorsoventrally flattened), respiration type (aerobic or anaerobic), flake formation (forming or non-forming), body shape (elongated - length two times the width, oval/rounded, pedunculated with tentacle, pedunculated without tentacle) (Supplementary Figure S1).
We referred to specialized literature to choose the functional traits (Madoni et al., 1993; Madoni, 1994; Madoni and Bassanini, 1999; Foissner, 2016; Weisse, 2017; Zhong et al., 2017; Xu et al., 2018b,c,d; Zhong et al., 2019a,b; Xu and Soninen, 2019; Xu et al., 2020). These characteristics were chosen for representing the behavior (feeding preference, form of locomotion, flake formation), morphology (body size, flexibility, geometry, and shape) and metabolism (respiration type) of the ciliates. After determining the traits, the classification of the ciliates according to their respective traits was carried out based on an identification/morphology atlas and specialized literature (Foissner et al., 1991, 1992, 1994, 1995; Foissner and Berger, 1996; Lynn and Small, 2002; Lynn, 2008; Xu et al., 2018d).
The SBI calculation was performed according to the proposed by Madoni (1994). The samples collected from the aeration tank and quantified via Sedgewick Rafter chamber resulted in the abundance of the microfauna and small flagellates. To obtain the dominant group, ciliates and testate amoebae were identified at the genus and/or species level, while flagellates, rotifers and nematodes were classified in big groups.
The taxonomic diversity of ciliate protozoans was calculated using species richness (Magurran, 2005), Shannon index (Shannon, 1948; Magurran, 2005), Simpson index (Simpson, 1949; Magurran, 2005) and species evenness (Pielou, 1975; Magurran, 2005).
For the characterization of functional diversity of the communities, the following functional diversity indices were used: functional richness (FRic), functional evenness (FEve) and functional divergence (FDiv) (Petchey and Gaston, 2002; Villéger et al., 2008; Laliberté and Legendre 2010), based on Gower’s dissimilarity method modified by Pavoine et al. (2009). These indices are based on the traits of the species in the community and express the functional differences between the species in the multidimensional space (Villéger et al., 2008; Mouchet et al., 2010), the dispersion or trait divergence Index being important as it contributes for the understanding of the consequences of species loss with information on their abundance and the distance between traits (Leps et al., 2006; Petchey and Gaston, 2006; Ricotta and Moretti, 2011; Wong and Dowd, 2015). Additionally, Rao’s quadratic entropy (1982) was also used.
The functional composition was assessed through the CWM (Community Weighted Mean value) (Pla et al., 2011) for the functional traits of the communities. CWM is calculated as a mean of the trait values, weighted by the relative abundance of the species (Ricotta and Moretti, 2011). The sum of CWM values (total CWM) was used for each trait throughout the 37 sampling instances, in order to assess the importance of these traits for the community.
The Pearson and Spearman correlations were used to test the correlations between species richness and functional richness (FRic), between CWM and Shannon index, between the taxonomic and functional evenness (FEve), between RaoQ index and Simpson index, and between SBI and functional divergence (FDiv). Additionally, they were also used to generate a correlation table between the environmental parameters and the CWM of the traits.
A principal component analysis (PCA) was carried out to extract indicators from the physical and chemical variables, capable of synthesizing a great portion of the fluctuation of the variables. Moreover, with the intention of quantifying what proportion of the variation in species composition and traits between samplings may be explained by environmental data, we used a redundancy analysis (RDA) followed by a variance analysis (ANOVA) of the RDA results to check if the model was significant.
All the analyses were carried out in the R software version 3.2.2 (R Core Team R 2015) using the ade4, (Chessel et al., 2004), vegan (Oksanen et al., 2012) and FD (Laliberté and Legendre, 2010; Laliberté et al., 2014) packages, with the exception of the taxonomic diversity indices, which were generated in PAST (Hammer et al., 2001).
A total of 21 active ciliate species were found in the samples throughout the analysis of the 37 collected samples in the combined anaerobic-aerobic system of Piçarrão WWTP. The abundance and diversity of the species fluctuated temporally (Figure 1). Between the first and the eighth sample, the total abundance values were slightly greater than in the forthcoming samples, however, with a lower diversity of species (Figure 1). Contrastingly, species richness had their lowest values in the initial samples, suggesting a niche partition a higher degree of niche complementarity (Figure 4A).
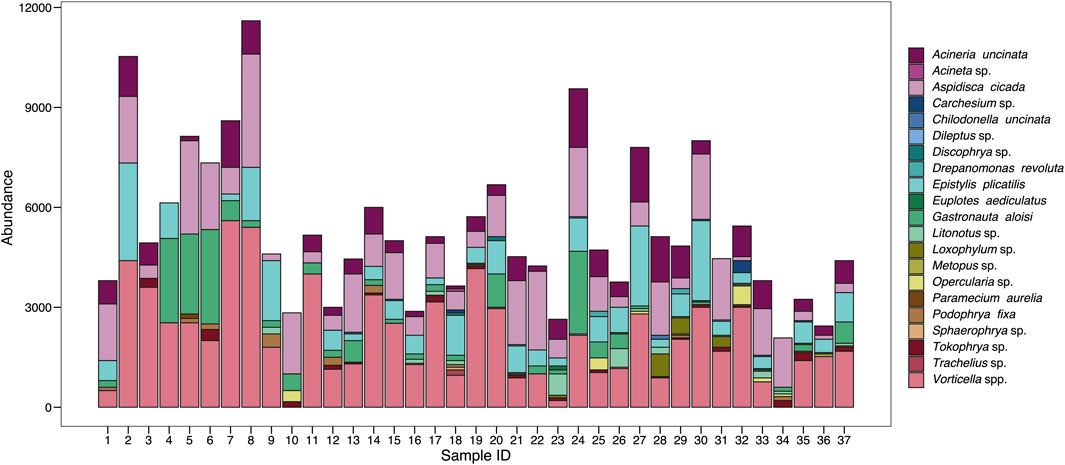
FIGURE 1. Abundance of the different ciliate species recorded throughout 37 samplings in a wastewater treatment plant in Brazil.
The most abundant and representative species in the ciliate community were Vorticella spp. (40%) and Aspidisca cicada (21%), which altogether comprised more than half of the entire community. These are included in the sessile and crawling groups, respectively, which were the most abundance functional groups observed in the system throughout the sampling period, with 54% and 32% of representativeness (Table 1).
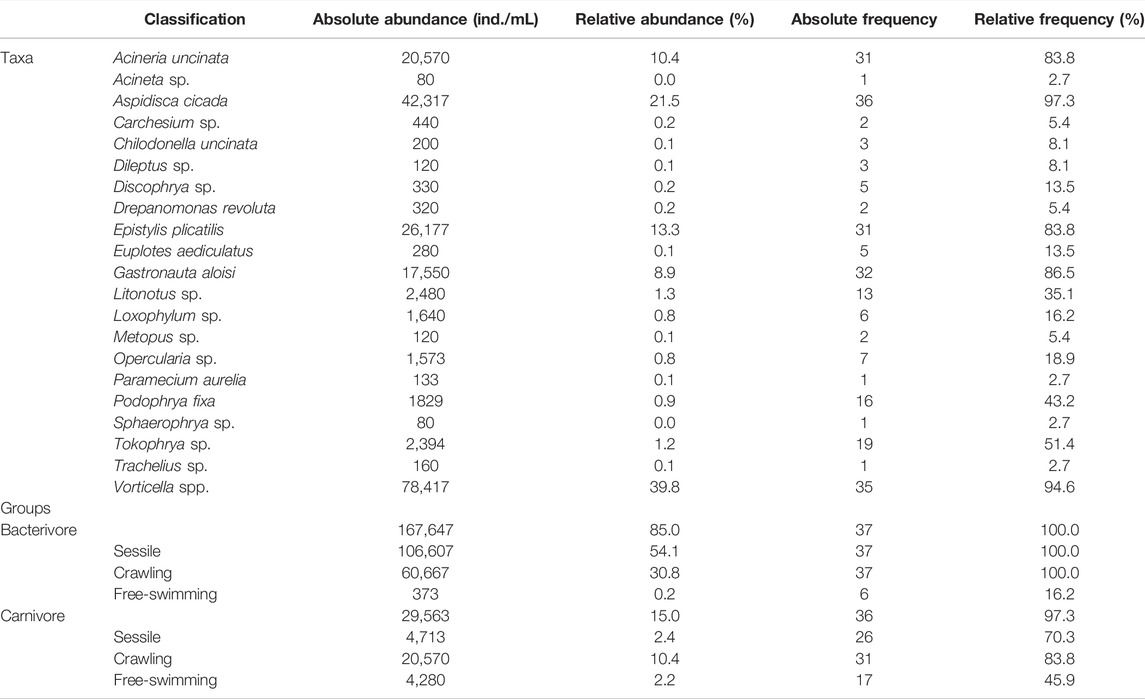
TABLE 1. Absolute and relative abundance and frequency of active ciliates found in the Piçarrão WWTP throughout the 37-sampling period.
With high heterogeneity, only 29% of the ciliatofauna was present in at least 50% of the samples, species such as Acineria uncinata (84%), Aspidisca cicada (97%), Epistylis plicatilis (84%), Gastronauta aloisi, Tokophrya sp. (51%) and Vorticella spp. (95%) were the most frequent (Table 1). These species are part of the sessile and crawling functional groups present in all samples, and carnivore ciliates were observed in 97% of samples (Table 1).
The proportion and representativeness of the traits recorded for the ciliate community presented a temporal homogeneity. Most traits occurred throughout the sampling period (Figure 2). Noteworthy, samples 10 and 34 were the ones with the lowest CWM values, mainly due to presenting little representativeness of individuals with flexible body type, which may be connected to a greater susceptibility to predation (Figure 3). Although there has been a change in taxonomic and functional composition (Figures 1, 2A–H), the greater homogeneity seen in the functional approach suggests that several species may perform the same function and occupy the same niche in this system, playing similar roles in this artificial ecosystem (Figure 3).
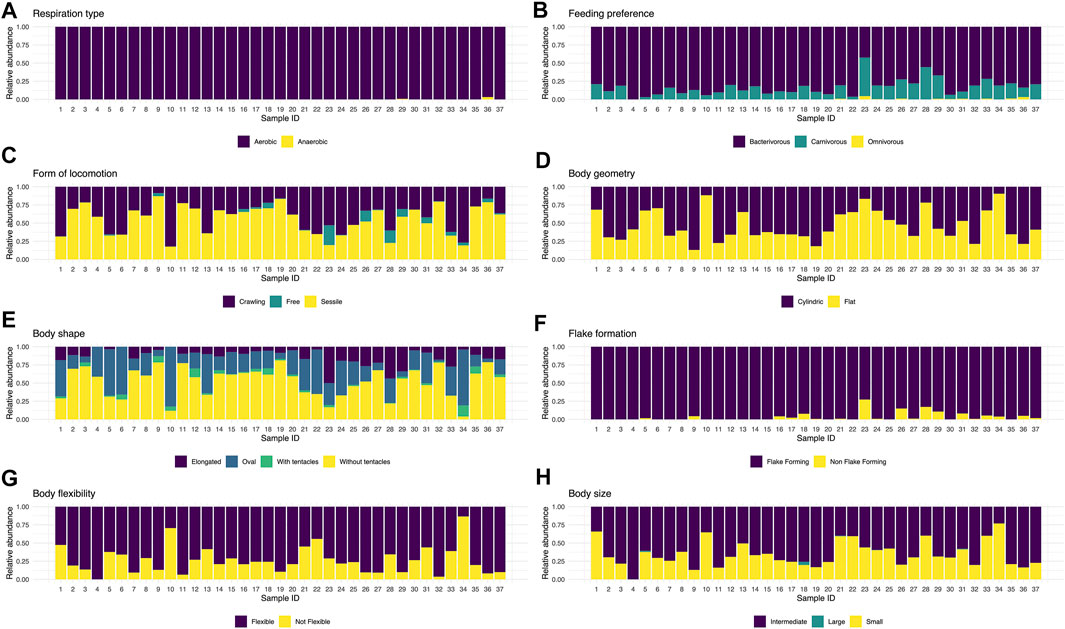
FIGURE 2. CWM value for the different functional traits chosen for the ciliates recorded throughout 37 samplings in a wastewater treatment plant in Brazil, Piçarrão WWTP.
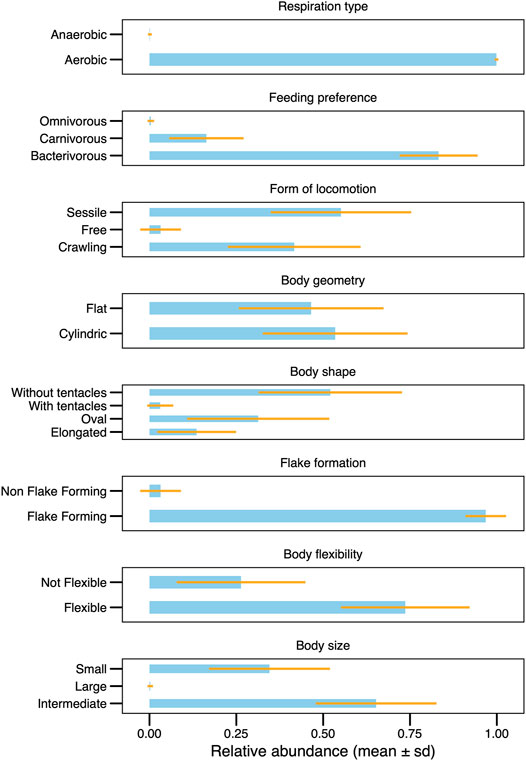
FIGURE 3. Mean values of CWM, showing and summarizing the functional characteristics in the ciliate community.
Aerobic respiration type was the predominant trait among the observed ones (Figure 2A), due to 95% of the community being comprised of aerobic species. Nonetheless, their use was important in the studied system because the treatment is initially composed by an anaerobic phase, followed by the aerobic one. The most consistent traits throughout the 37 sampling units were: aerobic respiration type, flake formation and bacterivore feeding type (Supplementary Figure S1). The latter two and others, such as body size between 50 and 150 μm, sessile and crawling forms of locomotion, body flexibility, cylindrical geometry, and the shapes oval and pedunculate without tentacle, were the most frequent, being present in all samples.
The profile of the community in this system had a predominance of sessile species without tentacle of intermediate size and a preference to bacterivore feeding, flexible, cylindrical and flake-forming (Figure 3). The functional traits of the recorded species in this study are listed in Supplementary Table S1 (in bold), as well as an ample list of ciliate species (∼190 species) which occur in distinct wastewater treatment systems around the world, with the intention of stimulating future applications of functional investigation in biological treatment systems.
Species richness varied between 3 and 12 species throughout the sampling period. There was a positive correlation between the taxonomic and functional richness values throughout the 37 samplings (0.66 correlation; p < 0.001; Figure 4A).
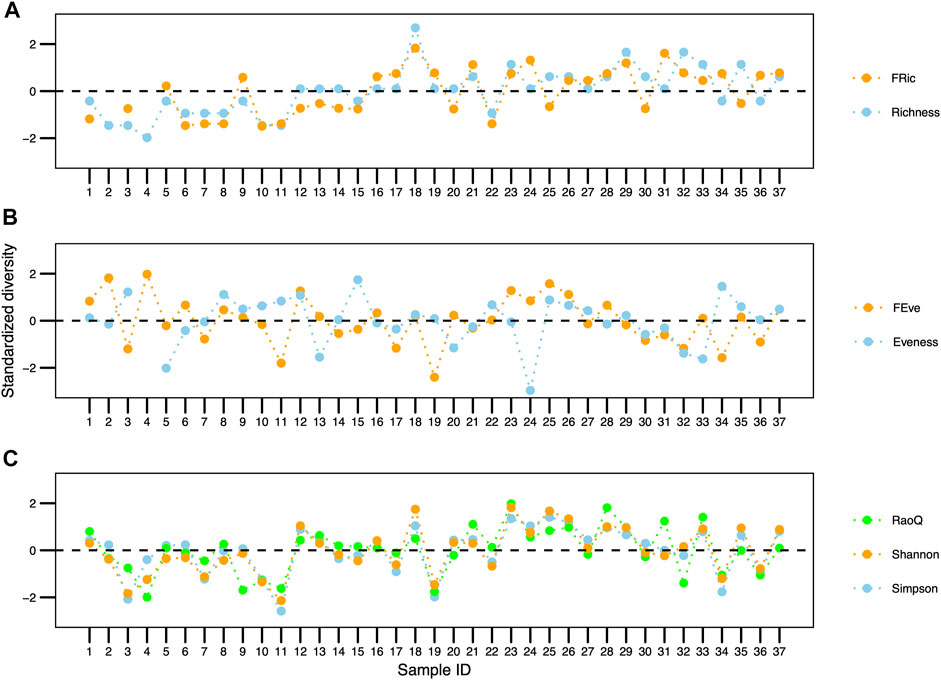
FIGURE 4. Comparison between taxonomic and functional traits of the ciliate community in a wastewater treatment plant in Brazil. (A) Richness, (B) Evenness, (C) Simpson, RaoQ indices and Shannon diversity.
There was a negative correlation, although non-significant (-0.07 correlation; p > 0.05), between the taxonomic and functional evenness data throughout the 37 samplings (Figure 4B). Regarding the Simpson, Shannon and Rao indices data, there was a positive and significant correlation (0.73 correlation; p < 0.001) (Figure 4C).
Functional divergence, with an average value of 0.87 ± 0.07, fluctuated between 0.7 and 0.98 (Figure 5). There was no significant correlation between SBI values and functional divergence (0.01 correlation; p > 0.05) (Figure 5).
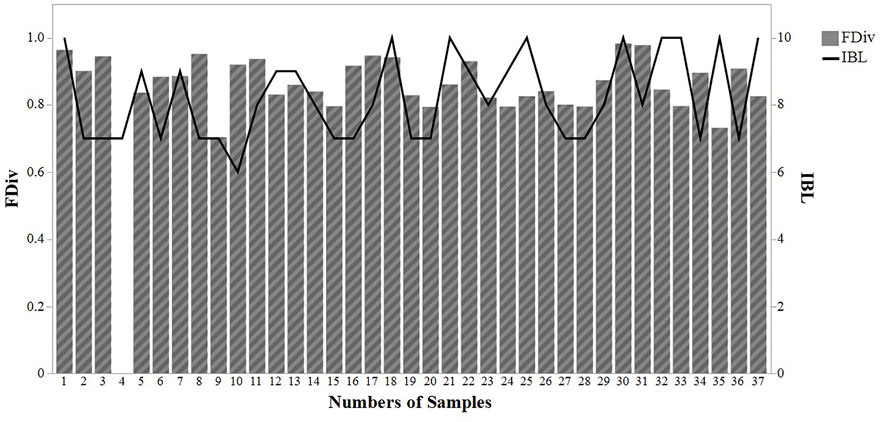
FIGURE 5. Comparison between functional divergence (FDVi) and sludge biotic index (IBL) values throughout 37 samplings in a wastewater treatment plant in Brazil.
The first two components of the PCA carried out with the abiotic data responded to 43.2% of total variance (PC1 = 24.4% and PC2 = 18.8%, Figure 6). The variables which most contributed to the first component were TKN, COD and N-NO3. However, no pattern regarding the sampling units was observed. In regard to the second component, the variables which most contributed were TS, Surf and BOD5, and there was a pattern in which a gradient of total solids was observed (Figure 6).
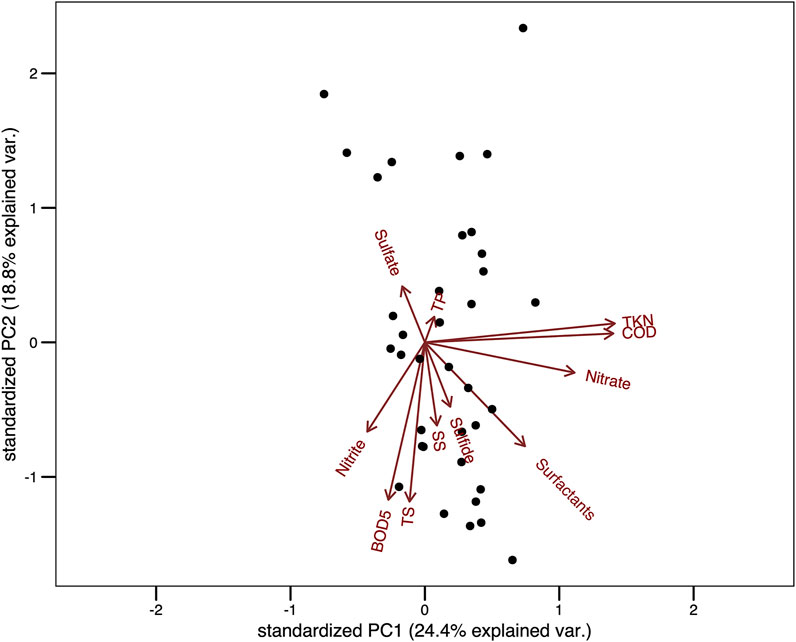
FIGURE 6. Principal Component Analysis (PCA) about environmental variables recorded throughout 37 samplings in a wastewater treatment plant in Brazil. Legend: TP, total phosphate; TS, total solids ; SS, settleable solids; TKN, total kjeldahl nitrogen; COD, chemical oxygen demand; BOD5, biochemical oxygen demand.
Regarding the efficiency parameters, we highlight the average removal efficiency of BOD5 being 88%, while COD was 86%, remaining constant throughout the sampling units (Figure 7). Nonetheless, the total solids value oscillated with a 47% amplitude. In general, the removal data presented in Figure 7 reinforce the homogeneity of the system throughout the 37 samplings.
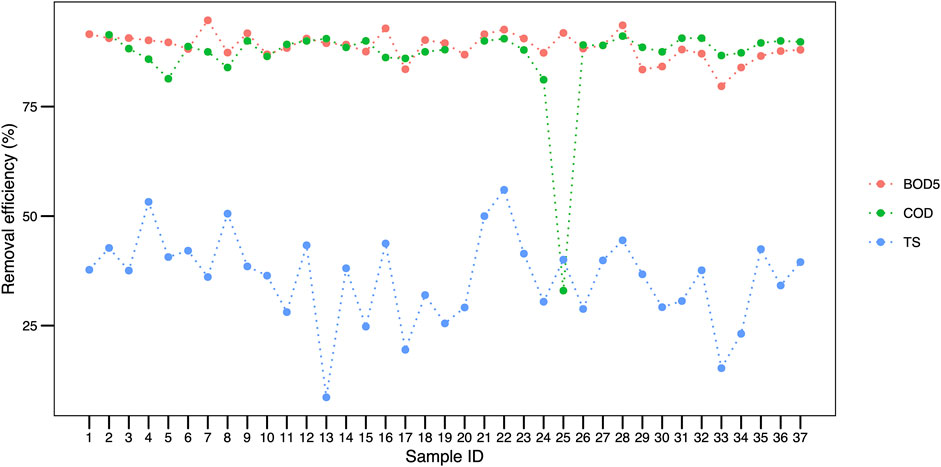
FIGURE 7. BOD5, COD and TS removal efficiency recorded throughout 37 samplings in a wastewater treatment plant in Brazil. Legend: BOD5, biochemical oxygen demand; COD, chemical oxygen demand; TS, total solids.
The relationship between the WWTP environmental conditions with the ciliate taxonomic composition was first presented by Siqueira-Castro et al. (2016b). However, we have observed that the species presented a greater value of correlation with environmental variables when compared to the ciliate community functional data (Table 2).
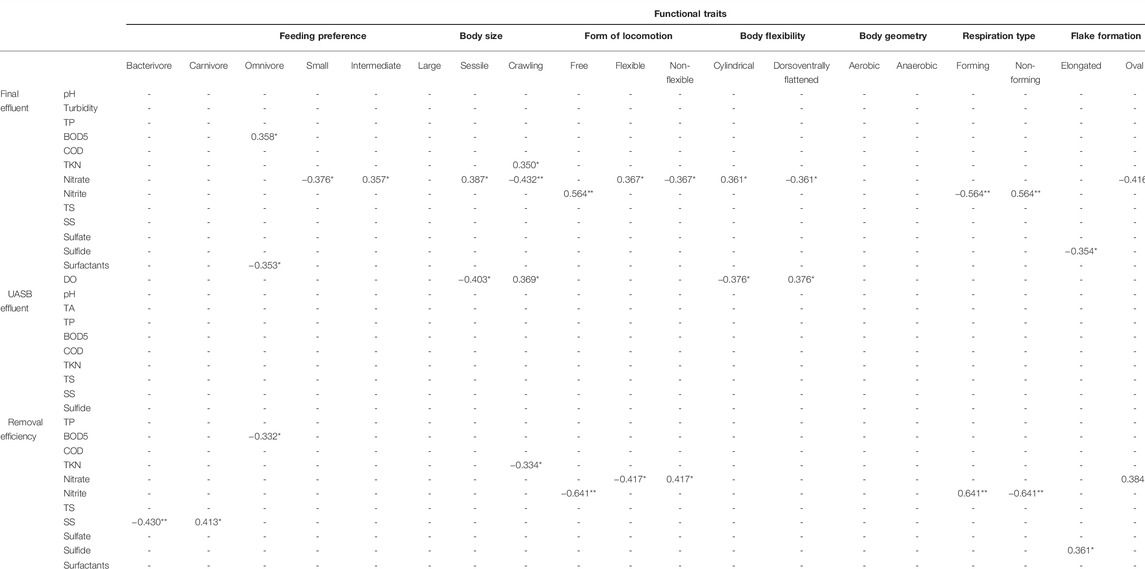
TABLE 2. Correlation between the functional traits attributed to the ciliate community in a WWTP in Brazil and environmental data. **p < 0.01 (significant correlation), *p < 0.05 (significant correlation).
Bacterivorous ciliates, an important trait in the community, were negatively correlated to the removal efficiency of settleable solids, whereas the 50–150 µm size trait was positively correlated to nitrate in the final effluent. Sessile and crawling traits had correlation to more than one environmental factor, namely: TKN, nitrate and OD in the final effluent and TKN removal efficiency (Table 2).
In the RDA of the taxonomic composition explained and environmental conditions, there was no significant correlation between distribution and abundance of species and environmental variables (p > 0.05) (Figure 8A). In this case, 30% of the variation in species abundance in the samplings is explained by environmental variables. The first RDA axis explained 20% and was positively correlated to total phosphorus removal efficiency (47%) and settleable solids removal efficiency (38%). It was also negatively correlated to nitrite (−31%) and TKN (−19%) removal efficiency. The second axis, with a 10% explanation, was strongly correlated to nitrate removal efficiency (41%).
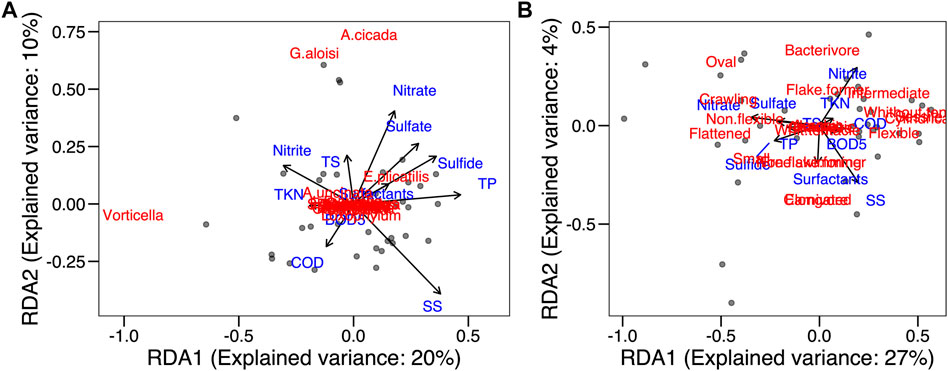
FIGURE 8. Redundancy analysis. (A) species and environmental variables; (B) traits and environmental variables.
In the RDA of functional composition (CWM values) and environmental conditions, no significance was found (Figure 8B), reflecting a functional redundancy the community. The functional characteristics of the species were very similar, and densities of traits were redundant. In this analysis, 31% of the variation in the CWM value in the samplings was explained by environmental variables. The first RDA axis explained 27% and was correlated positively to settleable solids removal efficiency (28%) and negatively to nitrate removal efficiency (−48%). The second axis explained 4% and was correlated negatively to settleable solids removal efficiency (−68%) and positively to nitrite removal efficiency (68%).
In both the taxonomic and functional compositions, the WWTP environmental conditions explained little of the diversity variability, which reflects the homogeneity of the physical-chemical conditions of the WWTP, once it is a controlled experimental operational system.
The number of ciliate species (21 species) recorded in this study was lower than those from other studies in continental waters. It can be explained by the WWTP systems conditions which were very homogenous along the whole sampling period. The restricted and particular environment of activated sludges, especially in WWTPs where there is a combined anaerobic (UASB) and aerobic (activated sludge) system, may act as an environmental filter, selecting species with similar traits, influencing the survival and reproduction of species and a consequent change in their presence or absence and in their abundance (Cadotte and Tucker, 2017). Literature shows that ciliates diversity varies depending on the method used by wastewater treatment plants. There is usually a smaller diversity in combined (anaerobic and aerobic) systems compared to conventional activated sludge systems (Liu et al., 2008).
In a previous study at the same system, Siqueira-Castro et al. (2016b) recorded 36 species, as they included the species cultivated in the lab days after sampling, since there was a focus on the morphological characterization of the ciliates to create a more detailed checklist. In the present study, we only took into account the active specimen analyzed on the day of sampling, as the formation of cysts could mask the function of these organisms in the system.
Although species richness of ciliates in continental waters and natural systems (rivers, streams, lakes) is high (>60 taxa) (Madoni, 1993, 2005; Sola et al., 1996; Foissner, 1997; Madoni and Zangrossi, 2005; Yang et al., 2012; Basuri et al., 2020), in WWTPs, especially those which operate with activated sludges, species richness is generally lower (<40 species) (Madoni et al., 1993; Martín-Cereceda et al., 1996; Dubber and Gray, 2011). Lately in Brazil there have been some studies about richness and species description of ciliates in WWTPs operating with activated sludges (Silva and Silva-Neto, 2001; Paiva and Silva-Neto, 2004; Bento et al., 2005; Ginoris et al., 2007; Oliveira et al., 2009; Siqueira-Castro et al., 2009; Fernandes and Silva-Neto, 2013). In our study, a lower number of taxa was to be expected as it is a combined anaerobic followed by aerobic system (UASB + activated sludge) that also did not take into account species that left cyst form while cultivated at the lab.
The 21 active ciliate species in this study, compared to the 36 species recorded by Siqueira-Castro et al. (2016b) for the same WWTP, was due to the exclusion of taxa kept at the lab days after sampling, likely in cyst form in the analyzed samples on the day of collection. According to Priya et al. (2008), anaerobic ciliates have lower growth efficiency than aerobic organisms, and the composition and availability of substrate constitute important factors influencing ciliate species richness in WWTPs. For instance, small alterations in pH range result in the formation of cysts in ciliates from the Metopus genus (Narayanan et al., 2007).
The temporal dynamics of species diversity, abundance and frequency found in the present study was relatively heterogenous, similarly to previous studies. Although there have been studies analyzing the ciliate community for months or even years (Madoni et al., 1993; Salvadó et al., 1995; Martín-Cereceda et al., 1996; Bento et al., 2005; Arévalo et al., 2009), very few studies have analyzed the temporal dynamics of the community in detail. The temporal heterogeneity of the ciliate community in WWTPs is mainly due to the fluctuation of environmental conditions and ecological and trophic relationships in this system. For instance, Vorticella spp. and Gastronauta aloisi both presented high abundances when the BOD5 in the UASB effluent was increased, such as occurred to the bacterivore crawling ciliates.
The biotic data obtained via SBI varied between classes I and II in this study, very good performance and good performance of the system, respectively, and the removal efficiencies of the plant remained high throughout the study for the parameters required by Brazilian law, indicating, as mentioned by Siqueira-Castro et al. (2016b), a well-functioning WWTP. Additionally, the density of ciliates in this study, with an average greater than 106 ind./L, also indicates a well-functioning WWTP, according to Madoni (1994).
Knowledge about functional traits allows for the investigation and understanding of different levels of biological organization, from individual (morphology and physiology) to ecosystem dynamics (Violle et al., 2007), such as we applied it in the UASB-activated sludge artificial ecosystem. The composition observed through the functional traits was more homogenous than the taxonomic one, as the species found had the similar functional characteristic, and play a similar role in the functioning of the ecosystem, occupying similar niches. The most significant functional traits found in this study was bacterivore feeding habit, indicating a bacterial control performed by ciliates with a consequent clearer final effluent; flake-forming indicating great frequency of ciliates aiding flaking in the WWTP; and the aerobic respiration type, indicating continuous and adequate aeration of the system throughout the 37 samplings. Their relationship to the dynamics of the combined UASB-activated sludge system helped to explain the high efficiency of the WWTP. Among the attributed traits in the present study, flake formation stands out as an innovative trait specific to these artificial ecosystems and may prove to be an important tool for the understanding of system efficiency.
The choice of traits was an important phase of the study, directly influencing applied interpretation and extrapolation (Petchey and Gaston, 2006), making it important to determine traits/characteristics which guide the response of species or the community to environmental change and, also, the effect of the species or the community on ecosystem functions (Suding et al., 2008; Weisse, 2017). Many of the traits used in this study were based on research involving limnic and marine ciliates, however, since they are influenced by the ecosystem, there was an adaptation for the WWTP using orientation provided by Suding et al. (2008) and Weisse (2017).
The feeding habit trait (Curds and Cockburn, 1970a,b) is related to feed resources, nutrient cycling, interactions in the food web such as top-down and bottom-up control, and energy flow (Pratt and Cairns, 1985; Litchman et al., 2013). This trait had a correlation to BOD5 and settleable solids removal (Table 2). Additional to the direct application of the feeding trait, due to the importance of ciliates in the microbial loop, another important application for this trait is its direct relation to the removal of oocysts and cysts of parasitic organisms in the WWTP. Giardia spp. cysts and Cryptosporidium spp. oocysts are parasitic protozoans commonly found in WWTPs and, recently, Siqueira-Castro et al. (2016b) recorded eight ciliate species preying on these cysts and oocysts in the Piçarrão WWTP (Campinas, Brazil). These pathogenic protozoans are a significant concern for human health, as they are estimated to be the main cause of the four billion diarrhea cases that happen globally each year (Baldursson and Karanis 2011; Kotloff et al., 2012; Swaffer et al., 2014). The removal and inactivation of these resistant forms of pathogenic protozoans in residuary waters is becoming more and more important due to the contamination of bodies of water by sewage effluents (Monis et al., 2014), which widens our understanding about the role of ciliates in the removal efficiency of WWTPs operating with activated sludge.
Body size, a trait with implications regarding food chain (Azam et al., 1983; Weisse, 2017), is proportionally correlated to the size of prey, growth rate, basal metabolism, mortality, excretion, biomass, all affecting the ecosystem flows (Litchman et al., 2013; Hébert et al., 2017). In Chinese coastal waters (Yellow Sea), Zhao et al. (2016) used body size and feeding habit and obtained as a result seasonal variation in ciliate body size in four sampling seasons with a difference in temperature, pH, nutrients, and other environmental variables. In the same environment, Xu et al. (2016) claimed that the body-size spectrum may be used as an important metric in the biomonitoring of water quality.
Form of locomotion is a trait that has been used in ciliate studies in wastewater treatment plants (Curds, 1973; Madoni et al., 1993) and reflects in distinct metabolic rates between ciliate species (Jackson and Berger, 1984; Fenchel, 1987). In our study, form of locomotion had a correlation with several environmental variables (Table 2). Although feeding habit and form of locomotion have already been used in studies on the efficiency of wastewater treatment systems (Neville, 1946; Curds and Vandyke, 1966; Curds and Cockburn, 1970a,b; Curds, 1973; Pratt and Cairns, 1985; Madoni et al., 1993; Madoni, 1994; Martín-Cereceda et al., 1996; Liu et al., 2008; Arévalo et al., 2009; Dubber and Gray, 2011), we have not found data in the literature with functional diversity using functional indices and analyses.
Shape, geometry, and flexibility of the body were correlated to environmental variables connected to nutrients, such as nitrite and nitrate (Table 2). Most of the species (95%) found active in this study are aerobic. In aerobic systems (activated sludges) this might seem a redundant trait, however, in a combined anaerobic-aerobic system its use was of interest.
Flake formation is an important characteristic in biological systems of sewage treatment and acts as an indicator of the system quality, as flake formation is an essential principle in this treatment system (Arregui et al., 2010). That is the main motivation to use this trait, which is new in studies on ciliate functional diversity for presenting as an exclusive characteristic of wastewater treatment systems that require flocculation, as is the case of activated sludge. It is interesting to highlight its correlation to nitrite in the final effluent (Table 2).
The search for adequate functional traits has been occurring for different taxonomic groups, as more representative traits with a clearer correlation to environmental variables are investigated. Functional diversity has proven to be an important branch of biodiversity research, being the most efficient in the detection of effect and response of biodiversity in the functioning of the ecosystem (Díaz et al., 2007; Suding et al., 2008; Laureto et al., 2015; Hébert et al., 2017). Our study makes clear the greater homogeneity of functional traits throughout the sampling units when compared to taxonomic data, in line with the homogenous efficiency environmental data reported for the WWTP. With the intention of promoting future applications of this functional approach in studies of biological wastewater treatment plants, we have compiled a list of ciliate species in these ecosystems, as well as their functional traits (Supplementary Table S1).
The functional approach allows for a better understanding of the functioning of several environments, using distinct groups of organisms, such as bacteria (Fuhrman, 2009), phytoplankton (Cardoso et al., 2017), plants (Cohen et al., 2014) and zooplankton (Setubal and Riccardi, 2020; Setubal et al., 2020), while data with ciliates are scarce (Weisse, 2017). Studies with a functional approach involving ciliates have been carried out in different ecosystems, such as the soil (Coûteaux and Darbyshire, 1998), coastal waters (Xu et al., 2016; Zhao et al., 2016; Zhong et al., 2017; Al et al., 2018a; Xu et al., 2018d; Bai and Xu, 2019; Bai et al., 2019; Guo and Xu, 2019; Xu and Soininen, 2019; Zhong et al., 2019a,b; Gui et al., 2020; Guo et al., 2020), and wetlands (Xu et al., 2018c,d; Liu et al., 2019; Xu et al., 2020), while it is rare to find one in a limnic environment.
Studies on the functional approach of ciliate communities in wastewater treatment plants are old and used mainly form of locomotion and feeding habit as important traits to understand efficiency and functioning of WWTPs operating with activated sludges (Neville, 1946; Curds and Vandyke, 1966; Curds and Cockburn, 1970a,b; Curds, 1973; Pratt and Cairns, 1985; Madoni et al., 1993; Madoni, 1994; Martín-Cereceda et al., 1996; Liu et al., 2008; Arévalo et al., 2009; Dubber and Gray, 2011, Madoni, 2011; Foissner, 2016). A clear comprehension of the functional traits related to the functioning and efficiency of removal in sewage treatment systems is a valuable tool for the optimization of the treatment, reflecting directly on the field of sanitation.
The taxonomic and functional composition and structure differed throughout the sampling period, the functional approach remaining more homogenous. Among the indices and parameters analyzed, species richness and functional richness were correlated positive and significantly, as there was a low number of ciliate species and a great number of attributed traits. In studies in WWTPs operating with activated sludges with a greater number of active ciliate species (Curds and Cockburn, 1970a,b; Madoni et al., 1993; Salvadó et al., 1995; Martín-Cereceda et al., 1996; Zhou et al., 2006, 2008; Dubber and Gray, 2011), there will potentially be new results, such as demonstrated in studies on ciliate communities in aquatic environments (Xu et al., 2016; Zhong et al., 2017; Al et al., 2018a,b; Xu et al., 2018c,d; Bai and Xu, 2019; Liu et al., 2019; Xu and Soninen, 2019; Zhong et al., 2019a,b; Gui et al., 2020; Xu et al., 2020). The low and little variable values of functional diversity presented in our study highlight a greater redundancy and a stable community (Weisse, 2017), in line with the BOD5 and COD removal data, thus occupying a smaller volume in the functional multidimensional space (Villéger et al., 2008).
The taxonomic and functional evenness and the diversity, using Shannon and CWM indices, were negatively correlated, demonstrating a new viewpoint and possible future interpretation for the structure and composition of the ciliatofauna. Meanwhile, the linear relationship between the Simpson and Rao indices suggests that taxonomic diversity loss also resulted in a functional diversity loss. The low values obtained for Rao’s index reinforce the idea of species selection (Leps et al., 2006). The low richness of active ciliate species recorded in this study indicates a potential functional redundancy of this community, however, these species play an essential role in the functioning of the ecosystem.
The functional divergence or functional complexity values did not have a correlation to the SBI data. As the SBI uses other biotic components of the ecosystem, it is necessary to widen the functional metrics for the entire biota of the activated sludge to better compare these metrics. Biodiversity and the ecosystem processes are related not only through number of species, but also through their interactions. The classic diversity measures are based on the assumption that all individuals have similar ecological roles, however, this does not occur in a community. The species richness, and Shannon and Simpson diversity indices are based on suppositions that do not occur in practice: all species are equal; all individuals are equal, independently of size (Magurran, 2005). The indices based on taxonomy (taxa identification) present unimodal or idiosyncratic relationships, whereas the ones based on characteristics present monotonic relationships under a perturbation regime (Smeti et al., 2019). For these reasons, biodiversity must be studied in the context of classic measures and also of structure and functioning of the community (Frainer et al., 2014), and being the diversity component which influences dynamics, productivity, stability and energy and nutrient balance, functional diversity has great ecological importance (Tilman, 2001). When we look at function, we take into consideration morphological, behavioral and ecological characteristics of individuals, providing good parameters to evaluate processes and the influence of disturbances (Villéger et al., 2008).
Studies on the functional approach of ciliate communities in wastewater treatment plants are old and used mainly form of locomotion and feeding habit as important traits to understand efficiency and functioning of WWTPs operating with activated sludges (Neville, 1946; Curds and Vandyke, 1966; Curds and Cockburn, 1970a, Curds and Cockburn, 1970b; Curds, 1973; Pratt and Cairns, 1985; Madoni et al., 1993; Madoni, 1994; Martín-Cereceda, 1996; Liu et al., 2008; Arévalo, 2009; Dubber and Grey, 2011, Madoni, 2011; Foissner, 2016). A clear comprehension of the functional traits related to the functioning and efficiency of removal in sewage treatment systems is a valuable tool for the optimization of the treatment, reflecting directly on the field of sanitation.
According to Xu et al. (2018b), the relationship between taxonomic and functional diversity should be analyzed in an integrative manner. The combination of these approaches is more informative and provides a greater understanding of the relationships between the structure of the ciliate community and the ecosystem functions. The present work corroborates the idea that both approaches could be evaluated together and supply a wider viewpoint on the functioning and efficiency of WWTPs.
The principal component analysis (PCA) demonstrated a high contribution of nitrogen inthe growth and development of the microorganisms. In our study, nitrogen removal efficiency was correlated positively to the number of pedunculated ciliates with tentacles (suctorian ciliates) and negatively to crawling ciliates (Table 1), these two traits being important metrics for the future monitoring of WWTPs.
The principal component (PCA) and redundancy (RDA) analyses demonstrated that the physical and chemical characteristics of the water in the Piçarrão WWTP presented little variability throughout the 2 years of sampling, with high removal efficiency during the whole study, as reported by other studies on the use of anaerobic reactors with post-treatment (Souza and Foresti, 1996; Coletti et al., 1997; Von Sperling and Chernicharo, 1998). Most ciliate species from our samples were bacterivorous (Figure 2B). Species from this functional group are expected to thrive in rich ecosystems, because of the high abundance of bacteria. Therefore, the high correlation between ciliate abundance and nutrients highlights and indirect effect organically rich environments in the abundance of bacteria which will serve as resource for the extant ciliate community (Figure 8).
The secondary treatment, beginning in UASB, continues into the aerobic reactor with recirculation of activated sludge (Siqueira-Castro et al., 2016a). In this process, the system acts as an environmental filter, selecting species and functional traits in this environment. Since the approach through functions is more homogenous, due to several species being able to have the same functional traits, the system is particularly redundant. The few correlation reports between functional traits and abiotic components (Table 2) show the need for new studies using a greater number of plants being sampled, and also plants with more heterogenous characteristics, as presented in the work of Madoni (1994), who investigated 44 wastewater treatment plants of the activated sludge type in Italy, heterogenous in efficiency and removal profile. Due to the clear correlation between the homogeneity of composition and structure in the functional approach with the homogeneity of abiotic data throughout the 37 samplings, we highlight the great potential of this approach in future studies in WWTPs and also its facilitated application by professionals not specialized in ciliate taxonomy and identification, as the identification of most functional traits do not require ample knowledge about the biology, ecology and morphology of ciliates.
The main conclusions of this study are: 1) the composition and structure of the ciliate community in the Piçarrão WWTP fluctuated throughout the sampling period, the taxonomic composition being more heterogenous than the functional composition; 2) the ciliate community in the Piçarrão WWTP (UASB-activated sludge) is functionally redundant, which may provide stability to the system and to the wastewater treatment; 3) the taxonomic and functional indices and metrics showed correlation, however, further research is necessary, as there was a low number of active species and high number of functional traits listed for this community; 4) the homogeneity of the measured physical and chemical data was followed by functional homogeneity and redundancy (homogenous CWM) of the ciliate community; 5) the functional approach has proven promising for future application in WWTPs operating with the combined UASB-activated sludge system and even more with only activated sludges, as there was a correlation of functional traits with clear removal efficiency parameters in the WWTP. The profile of the community in this system had a predominance of sessile species without tentacle of intermediate size and preference for bacterivorous, flexible, cylindrical and flake-forming food. In addition to presenting promising traits such as flake formation and body size.
An important bottleneck of ciliate analysis in monitoring wastewater plants in the need for taxonomic knowledge for identifying species correctly. Here, we used several morphological functional traits which can be estimated independently of taxonomic identification and provide a cost-effective measure of community variation in space and time. Our results showed that both taxonomic and functional composition varied similarly throughout the study, and therefore, using functional traits as a surrogate for studying wastewater treatment plants becomes a sound option. Finally, our study paves the way for using integrative metrics of functional diversity to investigate patterns of ciliate community composition and also to tackle applied questions such as the water treatment in wastewater treatment plants with combined UASB.
As future perspectives we suggest the investigation of new WWTPs with a wider range of variation in abiotic data and removal heterogeneity, as well as the functional composition and structure of the community, to attest this hypothesis of great synchrony between the microbiota function and the environmental data. Another perspective is the development of a future multimetric index involving the history of microbiota traits related to environmental data, as well as including the functional traits which were first described and presented in this study.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
YM: Conceptualization, Methodology, Formal analysis, Data curation, Writing—original draft. SC: Conceptualization, Formal analysis, Validation, Writing—review and editing, Supervision. IS-C: Conceptualization, Methodology, Writing—review and editing. JG-G: Conceptualization, Methodology, Writing—review and editing. RB: Conceptualization, Validation, Writing—review and editing, Funding. CG-R: Conceptualization, Validation, Writing—review and editing, Supervision. RD: Conceptualization, Methodology, Validation, Writing—review and editing, Funding, Supervision.
This work was partially supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico). CNPq provided research grant to YM (Master’s fellowship) and RD (Bolsa de Produtividade PQ). CG-R was supported by Sakari Alhopuro Foundation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.903984/full#supplementary-material
Supplementary Figure S1 | Supplementary Figure S1: In vivo photomicrographs of the microfauna present in activated sludge of combined UASB-activated sludge system in the Piçarrão WWTP, Campinas. a—Acineria uncinata (1b; 2d; 3h; 4j; 5m; 6n; 7p; 8s); b—Acineta sp. (1b; 2e; 3g 4k; 5m; 6n; 7q; 8t); c—Aspidisca cicada (1a; 2d; 3h; 4k; 5m; 6n; 7q; 8t); d—Carchesium polypinum colony (1a; 2e; 3g; 4j; 5l; 6n; 7q; 8v); e—Discoprhya sp. (1b; 2e; 3g 4k; 5l; 6n; 7q; 8u); f—Drepanomonas revoluta (1a; 2d; 3h; 4j; 5m; 6n; 7q; 8s); g—Epistylis plicatilis (1a; 2e; 3g; 4j; 5l; 6n; 7q; 8v); h—Euplotes aediculatus (1c; 2e; 3h; 4j; 5m; 6n; 7q; 8s); i—Gastronauta aloisi (1a; 2e; 3h; 4j; 5m; 6n; 7q; 8t); j—Litonotus sp. (1b; 2e; 3i; 4j; 5m; 6n; 7r; 8s); k—Metopus sp. (1c; 2e; 3i; 4k; 5m; 6p; 7r; 8s); l—Loxophyllum sp. (1b; 2e; 3i; 4j; 5m; 6n; 7r; 8s); m—Opercularia sp. (1a; 2e; 3g; 4j; 5l; 6n; 7q; 8v); n—Paramecium aurelia (1a; 2f; 3i; 4k; 5m; 6n; 7r; 8s); o—Podophrya fixa (1b; 2d; 3g 4k; 5l; 6n; 7q; 8u); p—Vorticella sp. (1a; 2e; 3g; 4j; 5l; 6n; 7q; 8v). Traits: 1: feeding type (a—bacterivorous, b—carnivorous, c—omnivorous); 2: body size (d - <50 μm, e—50 < and <150 μm, f—>150 µm); 3: locomotion (g–sessile, h—crawling, i—free-swimming); 4: flexibility (j–flexible, k—non-flexible); 5: body geometry (l—cylindrical, m–dorsoventrally flattened); 6: respiration type (n–aerobic, o–facultative, p–anaerobic); 7: flake formation (q–form flakes, r–do not form flakes); 8: body shape (s–elongated, t–oval/rounded, u–pedunculate with tentacle, v–pedunculate without tentacle).
Supplementary Table S1 | Composition of ciliated protist species present in wastewater treatment systems and their respective functional traits. 1—feeding type: a- bacterivorous, b- carnivorous, c- omnivorous; 2—body size: d- <50 µm, e- 50 µm < and < 150 µm, f- >150 µm; 3—locomotion: g- sessile, h- crawling, i- free-swimming; 4—body flexibility: j- flexible, k- non-flexible; 5—body volume/geometry: l- cylindrical, m- dorsoventrally flattened; 6—respiration type: n- aerobic, o- facultative, p- anaerobic; 7—flake formation: q- forming, r- non forming; 8—body shape: s- elongated, t- oval/rounded without tentacle, u- pedunculated with tentacle, v- pedunculated without tentacle.
Abdullah Al, M., Forruq, R. M., Akhtar, A., Alam, M. W., Sikder, M. N. A., Warren, A., et al. (2018b). Seasonal Shift in Community Structure of Periphytic Ciliates in Estuarine Waters in the Northern Bay of Bengal, Bangladesh. Ocean. Sci. J. 53 (4), 707–718. doi:10.1007/s12601-018-0048-5
Abdullah Al, M., Gao, Y., Xu, G., Wang, Z., Warren, A., and Xu, H. (2018a). Trophic-Functional Patterns of Biofilm-Dwelling Ciliates at Different Water Depths in Coastal Waters of the Yellow Sea, Northern China. Eur. J. Protistology 63, 34–43. doi:10.1016/j.ejop.2018.01.003
Ardern, E., and Lockett, W. T. (1914). Experiments on the Oxidation of Sewage without the Aid of Filters. J. Chem. Technol. Biotechnol. 33, 523–539. doi:10.1002/jctb.5000331005
Arévalo, J., Moreno, B., Pérez, J., and Gómez, M. A. (2009). Applicability of the Sludge Biotic Index (SBI) for MBR Activated Sludge Control. J. Hazard. Mater. 167 (1-3), 784–789. doi:10.1016/j.jhazmat.2009.01.057
Arregui, L., Pérez-Uz, B., Zornoza, A., and Serrano, S. (2010). A New Species of the Genus Metacystis (Ciliophora, Prostomatida, Metacystidae) from a Wastewater Treatment Plant. Eukaryot. Microbiol. 57 (4), 362–368. doi:10.1111/j.1550-7408.2010.00484.x
Azam, F., Fenchel, T., Field, J., Gray, J., Meyer-Reil, L., and Thingstad, F. (1983). The Ecological Role of Water-Column Microbes in the Sea. Mar. Ecol. Prog. Ser. 10, 257–263. doi:10.3354/meps010257
Bai, X., Guo, C., and Xu, H. (2019). An Approach to Identifying Homogeneity in Community Functioning of Periphytic Ciliates in Colonization Surveys for Marine Bioassessment. Ecol. Indic. 102, 394–400. doi:10.1016/j.ecolind.2019.02.063
Bai, X., and Xu, H. (2019). Indication of Spatial Variations in Annual Cycle of Functional Traits of Periphytic Ciliates to Environmental Heterogeneity in Coastal Waters. Ecol. Indic. 98, 193–199. doi:10.1016/j.ecolind.2018.10.043
Baldursson, S., and Karanis, P. (2011). Waterborne Transmission of Protozoan Parasites: Review of Worldwide Outbreaks - an Update 2004-2010. Water Res. 45 (20), 6603–6614. doi:10.1016/j.watres.2011.10.013
Basuri, C. K., Pazhaniyappan, E., Munnooru, K., Chandrasekaran, M., Vinjamuri, R. R., Karri, R., et al. (2020). Composition and Distribution of Planktonic Ciliates with Indications to Water Quality in a Shallow Hypersaline Lagoon (Pulicat Lake, India). Environ. Sci. Pollut. Res. 27, 18303–18316. doi:10.1007/s11356-020-08177-6
Bento, A. P., Sezerino, P. H., Philippi, L. S., Reginatto, V., and Lapolli, F. R. (2005). Caracterização da microfauna em estação de tratamento de esgotos Do tipo lodos ativados: um instrumento de avaliação e controle Do processo. Eng. Sanit. Ambient. 10 (4), 329–338. (in portuguese). doi:10.1590/S1413-41522005000400009
Berger, H., and Foissner, W. (2004). Illustrated Guide and Ecological Notes to Ciliate Indicator Species (Protozoa, Ciliophora) in Running Waters, Lakes, and Sewage Plants. Handb. Angew. Limnol. Grundlagen‐Gewässerbelastung‐Restaurierung‐Aquatische Ökotoxikologie‐Bewertung‐Gewässerschutz, 1–160. doi:10.1002/9783527678488.hbal2003005
Berger, H. (2006). Monograph of the Urostyloidea (Ciliophora, Hypotrichia). Monogr. Biol. 85, 1–1303.
Cadotte, M. W., and Tucker, C. M. (2017). Should Environmental Filtering Be Abandoned? Trends Ecol. Evol. 32 (6), 429–437. doi:10.1016/j.tree.2017.03.004
Cardoso, S. J., Nabout, J. C., Farjalla, V. F., Lopes, P. M., Bozelli, R. L., Huszar, V. L. M., et al. (2017). Environmental Factors Driving Phytoplankton Taxonomic and Functional Diversity in Amazonian Floodplain Lakes. Hydrobiologia 802 (1), 115–130. doi:10.1007/s10750-017-3244-x
CETESB (1985). Manual técnico da microbiologia para sistemas de lodos ativados operando com esgotos domésticos. São Paulo, SP: Companhia de Tecnologia de Saneamento Ambiental. (in Portuguese).
CETESB (2009). Microbiologia de Lodos Ativados. São Paulo, SP: Companhia de Tecnologia de Saneamento Ambiental. (in Portuguese).
Chessel, D., Dufour, A. B., and Thioulouse, J. (2004). The Ade4 Package - I: One-Table Methods. R. News 4 (1), 5–10. https://cran.r-project.org/doc/Rnews/.
Clamp, J. C., and Lynn, D. H. (2017). Investigating the Biodiversity of Ciliates in the ‘Age of Integration'. Eur. J. Protistology 61, 314–322. doi:10.1016/j.ejop.2017.01.004
Cohen, J. S., Rainford, S.-K. D., and Blossey, B. (2014). Community-Weighted Mean Functional Effect Traits Determine Larval Amphibian Responses to Litter Mixtures. Oecologia 174 (4), 1359–1366. doi:10.1007/s00442-013-2856-8
Coletti, F. J., Povinelli, J., and Daniel, L. A. (1997). “Pós-tratamento por lodos ativados de efluentes provenientes de processos anaeróbios de tratamento de esgoto sanitário; Determinação de constantes cinéticas,” in Anais: 19° Congresso Brasileiro de Engenharia Sanitária Ambiental (Foz do Iguaçu, 97. set/(in Portuguese).
Coûteaux, M.-M., and F. Darbyshire, J. (1998). Functional Diversity Amongst Soil Protozoa. Appl. Soil Ecol. 10 (3), 229–237. doi:10.1016/S0929-1393(98)00122-X
Curds, C. R., and Cockburn, A. (1970a). Protozoa in Biological Sewage-Treatment Processes-I. A Survey of the Protozoan Fauna of British Percolating Filters and Activated-Sludge Plants. Water Res. 4 (3), 225–236. doi:10.1016/0043-1354(70)90069-2
Curds, C. R., and Cockburn, A. (1970b). Protozoa in Biological Sewage-Treatment Processes-II. Protozoa as Indicators in the Activated-Sludge Process. Water Res. 4 (3), 237–249. doi:10.1016/0043-1354(70)90070-9
Curds, C. R. (1963). The Flocculation of Suspended Matter by Paramecium Caudatum. J. General Microbiol. 33 (3), 357–363. doi:10.1099/00221287-33-3-357
Curds, C. R. (1973). The Role of Protozoa in the Activated-Sludge Process. Am. Zool. 13 (1), 161–169. doi:10.1093/icb/13.1.161
Curds, C. R., and Vandyke, J. M. (1966). The Feeding Habits and Growth Rates of Some Fresh-Water Ciliates Found in Activated-Sludge Plants. J. Appl. Ecol. 3 (1), 127–137. doi:10.2307/2401669
Díaz, S., Lavorel, S., de Bello, F., Quétier, F., Grigulis, K., and Robson, T. M. (2007). Incorporating Plant Functional Diversity Effects in Ecosystem Service Assessments. Proc. Natl. Acad. Sci. U.S.A. 104 (52), 20684–20689. doi:10.1073/pnas.0704716104
Dubber, D., and Gray, N. F. (2011). The Influence of Fundamental Design Parameters on Ciliates Community Structure in Irish Activated Sludge Systems. Eur. J. Protistology 47 (4), 274–286. doi:10.1016/j.ejop.2011.05.001
Fenchel, T. (1987). Ecology of Protozoa: The Biology of Free-Living Phagotrophic Protists. Berlin & Tokyo: Springer.
Fernandes, N. M., and Silva Neto, I. D. d. (2013). Morphology and 18S rDNA gene sequence of Spirostomum minus and Spirostomum teres (Ciliophora: Heterotrichea) from Rio de Janeiro, Brazil. Zool. (Curitiba) 30 (1), 72–79. doi:10.1590/S1984-46702013000100009
Foissner, W. (2014). An Update of 'basic Light and Scanning Electron Microscopic Methods for Taxonomic Studies of Ciliated Protozoa'. Int. J. Syst. Evol. Microbiol. 64 (1), 271–292. doi:10.1099/ijs.0.057893-0
Foissner, W., and Berger, H. (1996). A User-Friendly Guide to the Ciliates (Protozoa, Ciliophora) Commonly Used by Hydrobiologists as Bioindicators in Rivers, Lakes, and Waste Waters, with Notes on Their Ecology. Freshw. Biol. 35, 375–482. doi:10.1111/j.1365-2427.1996.tb01775.x
Foissner, W., Berger, H., and Kohmann, F. (1992). Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems—Band II: Peritrichia, Heterotrichida, Odontostomatida. Informationsberichte des Bayer, 5/92. Heft: Landesamtes für Wasserwirtschaft.
Foissner, W., Berger, H., and Kohmann, F. (1994). Landesamtes für Wasserwirtschaft, 1/94 Landesamtes für Wasserwirtschaft. Heft 1/91.Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems—Band III: Hymenostomata, Prostomatida, Nassulida. Informations berichte des Bayer
Foissner, W., Berger, H., and Kohmann, F. (1995). Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems—Band IV: Gymenostomatea, Loxodes, Suctoria. Informationsberichte des Bayer. Heft: Landesamtes für Wasserwirtschaft. 1/95.
Foissner, W., Blatterer, H., Berger, H., and Kohmann, F. (1991). Taxonomische und ökologische Revision der Ciliaten des Saprobiensystems—Band I: Cyrtophorida, Oligotrichida, Hypotrichia, Colpodea. Informationsberichte des Bayer, 1/91. Heft: Landesamtes für Wasserwirtschaft.
Foissner, W. (1997). Faunistic and Taxonomic Studies on Ciliates (Protozoa, Ciliophora) from Clean Rivers in Bavaria (Germany), with Descriptions of New Species and Ecological Notes. Limnologica 27 (2), 179–238.
Foissner, W. (2016). Protists as Bioindicators in Activated Sludge: Identification, Ecology and Future Needs. Eur. J. Protistology 55, 75–94. doi:10.1016/j.ejop.2016.02.004
Frainer, A., McKie, B. G., and Malmqvist, B. (2014). When Does Diversity Matter? Species Functional Diversity and Ecosystem Functioning across Habitats and Seasons in a Field Experiment. J. Anim. Ecol. 83 (2), 460–469. doi:10.1111/1365-2656.12142
Fuhrman, J. A. (2009). Microbial Community Structure and its Functional Implications. Nature 459 (7244), 193–199. doi:10.1038/nature08058
Ginoris, Y. P., Amaral, A. L., Nicolau, A., Coelho, M. A. Z., and Ferreira, E. C. (2007). Development of an Image Analysis Procedure for Identifying Protozoa and Metazoa Typical of Activated Sludge System. Water Res. 41 (12), 2581–2589. doi:10.1016/j.watres.2007.02.006
Gui, Y., Bai, X., Zhong, X., Sikder, M. N. A., and Xu, H. (2020). Seasonal Variability in Biological Trait Pattern of Biofilm-Dwelling Protozoa in Colonization Surveys for Marine Bioassessment. Mar. Pollut. Bull. 160, 111604. doi:10.1016/j.marpolbul.2020.111604
Guo, C., Gui, Y., Bai, X., Sikder, M. N. A., and Xu, H. (2020). Seasonal Variation in Biological Trait Distribution of Periphytic Protozoa in Coastal Ecosystem: A Baseline Study for Marine Bioassessment. Mar. Pollut. Bull. 160, 111593. doi:10.1016/j.marpolbul.2020.111593
Guo, C., and Xu, H. (2019). Use of Functional Distinctness of Periphytic Ciliates for Monitoring Water Quality in Coastal Ecosystems. Ecol. Indic. 96, 213–218. doi:10.1016/j.ecolind.2018.09.008
Hammer, Ø., Harper, D. A. T., and Ryan, P. D. (2001). PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 4 (1), 1–9.
Hébert, M.-P., Beisner, B. E., and Maranger, R. (2017). Linking Zooplankton Communities to Ecosystem Functioning: toward an Effect-Trait Framework. J. Plankton Res. 39 (1), 3–12. doi:10.1093/plankt/fbw068
Jackson, K. M., and Berger, J. (1984). Survival of Ciliate Protozoa under Starvation Conditions and at Low Bacterial Levels. Microb. Ecol. 10 (1), 47–59. doi:10.1007/bf02011594
Kotloff, K. L., Blackwelder, W. C., Nasrin, D., Nataro, J. P., Farag, T. H., van Eijk, A., Adegbola, R. A., Alonso, P. L., Breiman, R. F., Golam Faruque, A. S., Saha, D., Sow, S. O., Sur, D., Zaidi, A. K. M., Biswas, K., Panchalingam, S., Clemens, J. D., Cohen, D., Glass, R. I., Mintz, E. D., Sommerfelt, H., and Levine, M. M. (2012). The Global Enteric Multicenter Study (GEMS) of Diarrheal Disease in Infants and Young Children in Developing Countries: Epidemiologic and Clinical Methods of the Case/control Study. Clin. Infect. Dis. 55 (Suppl. l_4), S232–S245. doi:10.1093/cid/cis753
Laliberté, E., and Legendre, P. (2010). A Distance-Based Framework for Measuring Functional Diversity from Multiple Traits. Ecology 91 (1), 299–305. doi:10.1890/08-2244.1
Laliberté, E., Zemunik, G., and Turner, B. L. (2014). Environmental Filtering Explains Variation in Plant Diversity along Resource Gradients. Science 345, 1602–1605. doi:10.1126/science.1256330
Laureto, L. M. O., Cianciaruso, M. V., and Samia, D. S. M. (2015). Functional Diversity: an Overview of its History and Applicability. Natureza Conservação 13 (2), 112–116. doi:10.1016/j.ncon.2015.11.001
Lepš, J., de Bello, F., Lavorel, S., and Berman, S. (2006). Quantifying and Interpreting Functional Diversity of Natural Communities: Practical Considerations Matter. Preslia 78 (4), 481–501. Available at: https://hal.archives-ouvertes.fr/halsde-00293183.
Litchman, E., Ohman, M. D., and Kiørboe, T. (2013). Trait-based Approaches to Zooplankton Communities. J. Plankton Res. 35 (3), 473–484. doi:10.1093/plankt/fbt019
Liu, H. C., Pu, X. J., Liu, J., and Du, W. H. (2019). Studies on the Diversity of Ciliate Species in Gahai Alpine Wetland of the Qinghai-Tibetan Plateau, China. Community Ecol. 20 (1), 83–92. doi:10.1556/168.2019.20.1.9
Liu, J., Yang, M., Qi, R., An, W., and Zhou, J. (2008). Comparative Study of Protozoan Communities in Full-Scale MWTPs in Beijing Related to Treatment Processes. Water Res. 42 (8-9), 1907–1918. doi:10.1016/j.watres.2007.11.020
Lynn, D., and Small, E. B. (2002). “Phylum Ciliophora,” in An Illustrated Guide to the Protozoa. Editors J. J. Lee, P. C. Bradbury, and G. F. Leedale (Society of Protozoologists, Lawrence).
Lynn, D. (2008). The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature. Springer Science & Business Media.
Madoni, P. (1994). A Sludge Biotic Index (SBI) for the Evaluation of the Biological Performance of Activated Sludge Plants Based on the Microfauna Analysis. Water Res. 28 (1), 67–75. doi:10.1016/0043-1354(94)90120-1
Madoni, P., and Bassanini, N. (1999). Longitudinal Changes in the Ciliated Protozoa Communities along a Fluvial System Polluted by Organic Matter. Eur. J. Protistology 35 (4), 391–402. doi:10.1016/S0932-4739(99)80048-0
Madoni, P. (1993). Ciliated Protozoa and Water Quality in the Parma River (Northern Italy): Long-Term Changes in the Community Structure. Hydrobiologia 264 (3), 129–135. doi:10.1007/BF00007283
Madoni, P. (2005). Ciliated Protozoan Communities and Saprobic Evaluation of Water Quality in the Hilly Zone of Some Tributaries of the Po River (Northern Italy). Hydrobiologia 541 (1), 55–69. doi:10.1007/s10750-004-4667-8
Madoni, P., Davoli, D., and Chierici, E. (1993). Comparative Analysis of the Activated Sludge Microfauna in Several Sewage Treatment Works. Water Res. 27 (9), 1485–1491. doi:10.1016/0043-1354(93)90029-H
Madoni, P. (2003). “Protozoa as Indicators of Wastewater Treatment Efficiency,” in The Handbook of Water and Wastewater Microbiology. Editors D. Mara, and N. Horan (London: Academic Press), 361–371. 361–371. doi:10.1016/B978-012470100-7/50023-6
Madoni, P. (2011). Protozoa in Wastewater Treatment Processes: A Minireview. Italian J. Zoology 78 (1), 3–11. doi:10.1080/11250000903373797
Madoni, P., and Zangrossi, S. (2005). Ciliated Protozoa and Saprobical Evaluation of Water Quality in the Taro River (Northern Italy). Italian J. Zoology 72 (1), 21–25. doi:10.1080/11250000509356648
Magurran, A. E. (2005). Measuring Biological Diversity. Oxford: Blackwell Science, 1–156. doi:10.1093/acprof:oso/9780198527855.001.0001
Martín-Cereceda, M., Serrano, S., and Guinea, A. (1996). A Comparative Study of Ciliated Protozoa Communities in Activated-Sludge Plants. FEMS Microbiol. Ecol. 21 (4), 267–276. doi:10.1016/S0168-6496(96)00062-1
Mcgill, B., Enquist, B., Weiher, E., and Westoby, M. (2006). Rebuilding Community Ecology from Functional Traits. Trends Ecol. Evol. 21 (4), 178–185. doi:10.1016/j.tree.2006.02.002
Metcalf and Eddy Inc (2003). Wastewater Engineering: Treatment and Reuse. New York: McGraw - Hill Book, 4
Monis, P., King, B., and Keegan, A. (2014). Removal and Inactivation of Cryptosporidium from Water. Cryptosporidium Parasite Dis., 515–552. doi:10.1007/978-3-7091-1562-6_13
Mouchet, M. A., Villéger, S., Mason, N. W. H., and Mouillot, D. (2010). Functional Diversity Measures: An Overview of Their Redundancy and Their Ability to Discriminate Community Assembly Rules. Funct. Ecol. 24 (4), 867–876. doi:10.1111/j.1365-2435.2010.01695.x
Narayanan, N., Priya, M., Haridas, A., and Manilal, V. B. (2007). Isolation and Culturing of a Most Common Anaerobic Ciliate, Metopus Sp. Anaerobe 13 (1), 14–20. doi:10.1016/j.anaerobe.2006.10.003
Neville, A. (1946). The Ecology and Function of Protozoa in Sewage Purification. Ann. Appl. Biol. 33 (3), 314–325. doi:10.1111/j.1744-7348.1946.tb06320.x
Nurul Azim Sikder, M., Bai, X., Warren, A., and Xu, H. (2019). An Approach to Determining Homogeneity in Taxonomic Breadth of Periphytic Ciliate Communities in Colonization Surveys for Bioassessment. Ecol. Indic. 107, 105671. doi:10.1016/j.ecolind.2019.105671
Oksanen, J., Kindt, R., Legendre, P., and O’Hara, R. B. (2012). Vegan: Community Ecology Package. Available at: http://cran.r-project.org
Oliveira, G. S. S. d., Araújo, C. V. d. M., and Fernandes, J. G. S. (2009). Microbiologia de Sistema de Lodos Ativados e Sua relação com o Tratamento de Efluentes Industriais: A Experiência da Cetrel. Eng. Sanit. Ambient. 14 (2), 183–191. doi:10.1590/S1413-41522009000200006
Paiva, T. D. S., and Silva–Neto, I. D. D. (2004). Description of Parentocirrus Brasiliensis Sp. N. (Ciliophora: Spirotrichea), a New Ciliate Protist Present in Activated Sludge. Zootaxa 504 (1), 1–10. doi:10.11646/zootaxa.504.1.1
Pavoine, S., Vallet, J., Dufour, A.-B., Gachet, S., and Daniel, H. (2009). On the Challenge of Treating Various Types of Variables: Application for Improving the Measurement of Functional Diversity. Oikos 118 (3), 391–402. doi:10.1111/j.1600-0706.2008.16668.x
Petchey, O. L., and Gaston, K. J. (2002). Functional Diversity (FD), Species Richness and Community Composition. Ecol. Lett. 5 (3), 402–411. doi:10.1046/j.1461-0248.2002.00339.x
Petchey, O. L., and Gaston, K. J. (2006). Functional Diversity: Back to Basics and Looking Forward. Ecol. Lett. 9 (6), 741–758. doi:10.1111/j.1461-0248.2006.00924.x
Pla, L., Casanoves, F., and Di Rienzo, J. (2011). Quantifying Functional Biodiversity. Springer, 1–8. doi:10.1007/978-94-007-2648-2_1
Pratt, J. R., and Cairns, J. (1985). Functional Groups in the Protozoa: Roles in Differing Ecosystems1,2. J. Protozool. 32 (3), 415–423. doi:10.1111/j.1550-7408.1985.tb04037.x
Priya, M., Haridas, A., and Manilal, V. B. (2008). Anaerobic Protozoa and Their Growth in Biomethanation Systems. Biodegradation 19 (2), 179–185. doi:10.1007/s10532-007-9124-8
R CORE TEAM. R (2015). A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing. Available at: http://www.R-project.org/.
Ricotta, C., and Moretti, M. (2011). CWM and Rao's Quadratic Diversity: a Unified Framework for Functional Ecology. Oecologia 167, 181–188. doi:10.1007/s00442-011-1965-5
Salvadó, H., Gracia, M. P., and Amigó, J. M. (1995). Capability of Ciliated Protozoa as Indicators of Effluent Quality in Activated Sludge Plants. Water Res. 29 (4), 1041–1050. doi:10.1016/0043-1354%2894%2900258-9
SANASA (2012). Available at:www.sanasa.com.br (Accessed November 30, 2012).(In Portuguese).
Serrano, S., Arregui, L., Pérez-Uz, B., Calvo, P., and Guinea, A. (2008). Guidelines for Identification of Ciliates in Wastewater Treatments Plants, 120. London: IWA Publishing.
Setubal, R. B., and Riccardi, N. (2020). Long-Term Effects of Fish Biomanipulation and Macrophyte Management on Zooplankton Functional Diversity and Production in a Temperate Shallow Lake. Limnology 21 (3), 305–317. doi:10.1007/s10201-020-00617-z
Setubal, R. B., Sodré, E. d. O., Martins, T., and Bozelli, R. L. (2020). Effects of Functional Diversity and Salinization on Zooplankton Productivity: An Experimental Approach. Hydrobiologia 847 (13), 2845–2862. doi:10.1007/s10750-020-04276-0
Shannon, C. E. (1948). A Mathematical Theory of Communication. Bell Syst. Tech. J. 27 (3), 379623–423656. doi:10.1002/j.1538-7305.1948.tb01338.x
Sikosana, M. L., Sikhwivhilu, K., Moutloali, R., and Madyira, D. M. (2019). Municipal Wastewater Treatment Technologies: A Review. Procedia Manuf. 35, 1018–1024. doi:10.1016/j.promfg.2019.06.051
Silva, S. B., and da Silva-Neto, I. D. (2001). Morfologia dos protozoários ciliados presentes em um reator experimental de tratamento de esgoto por processo de lodos ativados. Rev. Bras. Zoociências 3 (2), 203
Siqueira-Castro, I. C. V., Greinert-Goulart, J. A., Bonatti, T. R., Yamashiro, S., and Franco, R. M. B. (2016a). First Report of Predation of Giardia Sp. Cysts by Ciliated Protozoa and Confirmation of Predation of Cryptosporidium Spp. Oocysts by Ciliate Species. Environ. Sci. Pollut. Res. 23 (11), 11357–11362. doi:10.1007/s11356-016-6689-y
Siqueira-Castro, I. C. V., Greinert-Goulart, J. A., Rossetto, R., Guimarães, J. R., and Franco, R. M. B. (2016b). Ciliated Protozoa Community of a Combined Uasb-Activated Sludge System in Southeastern Brazil. Environ. Sci. Pollut. Res. 23 (23), 23804–23814. doi:10.1007/s11356-016-7591-3
Siqueira-Castro, I. C. V., Paiva, T. d. S., and Silva-Neto, I. D. d. (2009). Morphology of Parastrongylidium estevesi nomb. nov. and Deviata brasiliensis sp. nov. (Ciliophora: Stichotrichia) from a Sewage Treatment Plant in Rio de Janeiro, Brazil. Zool. (Curitiba, Impr.) 26 (4), 774–786. doi:10.1590/S1984-46702009000400024
Smeti, E., von Schiller, D., Karaouzas, I., Laschou, S., Vardakas, L., Sabater, S., et al. (2019). Multiple Stressor Effects on Biodiversity and Ecosystem Functioning in a Mediterranean Temporary River. Sci. Total Environ. 647, 1179–1187. doi:10.1016/j.scitotenv.2018.08.105
Sola, A., Serrano, S., and Guinea, A. (1996). Influence of Environmental Characteristics on the Distribution of Ciliates in the River Henares (Central Spain). Hydrobiologia 324 (3), 237–252. doi:10.1007/BF00016396
Souza, J. T., and Foresti, E. (1996). Domestic Sewage Treatment in an Upflow Anaerobic Sludge Blanket – Sequencing Batch Reactor System. Water Sci. Technol. 33 (3), 73–84. doi:10.1016/0273-1223(96)00323-X
Suding, K. N., Lavorel, S., Chapin, F. S., Cornelissen, J. H. C., Díaz, S., Garnier, E., Goldberg, D., Hooper, D. U., Jackson, S. T., and Navas, M.-L. (2008). Scaling Environmental Change through the Community-Level: A Trait-Based Response-And-Effect Framework for Plants. Glob. Change Biol. 14 (5), 1125–1140. doi:10.1111/j.1365-2486.2008.01557.x
Swaffer, B. A., Vial, H. M., King, B. J., Daly, R., Frizenschaf, J., and Monis, P. T. (2014). Investigating Source Water Cryptosporidium Concentration, Species and Infectivity Rates During Rainfall-Runoff in a Multi-Use Catchment. Water Res. 67, 310–320. doi:10.1016/j.watres.2014.08.055
Tilman, D. (2001). Functional Diversity. Encycl. Biodivers. 3, 109–120. doi:10.1016/b0-12-226865-2/00132-2
Villéger, S., Mason, N. W. H., and Mouillot, D. (2008). New Multidimensional Functional Diversity Indices for a Multifaceted Framework in Functional Ecology. Ecology 89, 2290–2301. doi:10.1890/07-1206.1
Vinardell, S., Astals, S., Peces, M., Cardete, M. A., Fernández, I., Mata-Alvarez, J., et al. (2020). Advances in Anaerobic Membrane Bioreactor Technology for Municipal Wastewater Treatment: A 2020 Updated Review. Renew. Sustain. Energy Rev. 130, 109936. doi:10.1016/j.rser.2020.109936
Violle, C., Navas, M.-L., Vile, D., Kazakou, E., Fortunel, C., Hummel, I., et al. (2007). Let the Concept of Trait Be Functional! Oikos 116, 882–892. doi:10.1111/j.0030-1299.2007.15559.x
Von Sperling, M., and Chernicharo, C. A. L. (1998). “Selection of Wastewater Treatment Systems in Urban Areas. Comparison Between Conventional Aerobic Systems (Activated Sludge) and Anaerobic-Aerobic Systems (UASB Activated Sludge),” in Seminario Latinoamericano de Digestion Anaerobia (Viña del Mar. Anais, V Taller27–30.
Weisse, T. (2017). Functional Diversity of Aquatic Ciliates. Eur. J. Protistology 61, 331–358. doi:10.1016/j.ejop.2017.04.001
WHO UNICEF (2017). Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines. Geneva: World Health Organization, United Nations Children’s Fund.
WHO WORLD HEALTH ORGANIZATION (2018). Guidelines on Sanitation and Health. Geneva. CC BY-NC-SA 3.0 IGO.
Wong, M. C., and Dowd, M. (2015). Patterns in Taxonomic and Functional Diversity of Macrobenthic Invertebrates across Seagrass Habitats: A Case Study in Atlantic Canada. Estuaries Coasts 38 (6), 2323–2336. doi:10.1007/s12237-015-9967-x
Xu, G., Yang, E., Lee, Y., and Kang, S.-H. (2018a). Vertical Shift in Ciliate Body-Size Spectrum and its Environmental Drivers in Western Arctic Pelagic Ecosystems. Environ. Sci. Pollut. Res. 25 (19), 19082–19091. doi:10.1007/s11356-018-2094-z
Xu, G., Zhong, X., Al, M. A., Warren, A., and Xu, H. (2018b). Identifying Bioindicators Across Trait-Taxon Space for Assessing Water Quality in Marine Environments. Mar. Pollut. Bull. 131, 565–571. doi:10.1016/j.marpolbul.2018.04.044
Xu, H., Yong, J., and Xu, G. (2016). Bioassessment of Water Quality Status Using a Potential Bioindicator Based on Functional Groups of Planktonic Ciliates in Marine Ecosystems. Mar. Pollut. Bull. 110 (1), 409–414. doi:10.1016/j.marpolbul.2016.06.033
Xu, H., Zhang, W., and Jiang, Y. (2014). Do early Colonization Patterns of Periphytic Ciliate Fauna Reveal Environmental Quality Status in Coastal Waters? Environ. Sci. Pollut. Res. 21, 7097–7112. doi:10.1007/s11356-014-2615-3
Xu, Y., Fan, X., Warren, A., Zhang, L., and Xu, H. (2018d). Functional Diversity of Benthic Ciliate Communities in Response to Environmental Gradients in a Wetland of Yangtze Estuary, China. Mar. Pollut. Bull. 127, 726–732. doi:10.1016/j.marpolbul.2017.12.068
Xu, Y., and Soininen, J. (2019). Spatial Patterns of Functional Diversity and Composition in Marine Benthic Ciliates along the Coast of China. Mar. Ecol. Prog. Ser. 627, 49–60. doi:10.3354/meps13086
Xu, Y., Stoeck, T., Stoeck, T., Forster, D., Ma, Z., Zhang, L., et al. (2018c). Environmental Status Assessment Using Biological Traits Analyses and Functional Diversity Indices of Benthic Ciliate Communities. Mar. Pollut. Bull. 131, 646–654. doi:10.1016/j.marpolbul.2018.04.064
Xu, Y., Yao, S., Soetaert, K., and Fan, X. (2020). Effects of Salt Marsh Restoration on Eukaryotic Microbenthic Communities in the Yangtze Estuary. Mar. Ecol. Prog. Ser. 638, 39–50. doi:10.3354/meps13242
Yang, J., Jiang, Y., and Hu, X. (2012). The Relationship Between Protistan Community and Water Quality Along the Coast of Qingdao. Acta Eco Sin. 32 (6), 1703–1712. doi:10.5846/stxb201102250220
Zhao, L., Xu, G., Wang, Z., and Xu, H. (2016). Body-Size Spectra of Biofilm-Dwelling Protozoa and Their Seasonal Shift in Coastal Ecosystems. Eur. J. Protistology 56, 32–40. doi:10.1016/j.ejop.2016.07.003
Zhong, X., Xu, G., Min, G.-S., Kim, S., and Xu, H. (2019a). Can Tidal Events Influence Monitoring Surveys Using Periphytic Ciliates Based on Biological Trait Analysis in Marine Ecosystems? Mar. Pollut. Bull. 142, 452–456. doi:10.1016/j.marpolbul.2019.04.005
Zhong, X., Xu, G., Min, G.-S., Kim, S., and Xu, H. (2019b). Insight into Tidal Disturbance on Colonization Surveys for Marine Bioassessment Using Periphytic Ciliates Based on Biological Trait Analysis. Mar. Pollut. Bull. 149, 110584. doi:10.1016/j.marpolbul.2019.110584
Zhong, X., Xu, G., and Xu, H. (2017). Use of Multiple Functional Traits of Protozoa for Bioassessment of Marine Pollution. Mar. Pollut. Bull. 119 (2), 33–38. doi:10.1016/j.marpolbul.2017.03.043
Zhou, K., Xu, M., Dai, J., and Cao, H. (2006). The Microfauna Communities and Operational Monitoring of an Activated Sludge Plant in China. Eur. J. Protistology 42 (4), 291–295. doi:10.1016/j.ejop.2006.07.005
Keywords: functional diversity, Ciliophora, functional traits, wastewater treatment plant, taxonomic diversity
Citation: Moreira YC, Cardoso SJ, Siqueira-Castro ICV, Araújo Greinert-Goulart J, Bueno Franco RM, Graco-Roza C and Dias RJP (2022) Ciliate Communities Respond via Their Traits to a Wastewater Treatment Plant With a Combined UASB–Activated Sludge System. Front. Environ. Sci. 10:903984. doi: 10.3389/fenvs.2022.903984
Received: 25 March 2022; Accepted: 16 May 2022;
Published: 22 July 2022.
Edited by:
Songdong Shao, Dongguan University of Technology, ChinaReviewed by:
Qiongfang Zhuo, Dongguan University of Technology, ChinaCopyright © 2022 Moreira, Cardoso, Siqueira-Castro, Araújo Greinert-Goulart, Bueno Franco, Graco-Roza and Dias. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Caio Graco-Roza, Y2Fpby5yb3phQGhlbHNpbmtpLmZp; Roberto Júnio Pedroso Dias, cmp1bmlvZGlhc0Bob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.