- 1School of Biological Science, Water Research Centre, Environment Institute, The University of Adelaide, Adelaide, SA, Australia
- 2Aquatic Ecodynamics, UWA School of Agriculture and Environment, The University of Western Australia, Crawley, WA, Australia
- 3School of Civil, Environmental and Mining Engineering, The University of Adelaide, Adelaide, SA, Australia
- 4Inland Waters and Catchment Ecology Program, South Australian Research and Development Institute, Aquatic Sciences, Henley Beach, West Beach, WA, Australia
- 5Goyder Institute for Water Research, Adelaide, SA, South Australia
Freshwater flows to estuaries shape habitat, transport nutrients to drive productivity, and generate a salinity gradient that impacts water quality and provides spawning cues for fish. The aim of this study was to quantify how environmental flows improved outcomes for a coastal lagoon system (the Coorong, South Australia), considering the export, and prevention of ingress, of salt from the system, and the increased available habitat for key fish biota. A hydrodynamic model was used to simulate salinity and water temperature, and to determine the salt exchange between the Coorong and ocean for the observed conditions with environmental water release included. Scenario simulations showed that maintaining river flow is shown to arrest salt intrusion from the ocean into the Coorong. Without environmental water, the net import of salt into the Coorong would have been considerably greater, ranging between 1.86 million tonnes in 2018–19 to approximately 2.33 million tonnes in 2019–20. The fresher conditions created by environmental water provision supported a considerable expansion of suitable fish habitat area, derived from a simple habitat index based on salinity and water temperature. Without environmental water the habitat suitable for mulloway would have contracted by 38% over the 3 year investigation period. A similar trend is evident for black bream, Tamar goby, greenback flounder, yelloweye mullet, congolli and smallmouth hardyhead. The results highlighted the importance of cumulative benefits from delivering environmental water over multiple years, with different results obtained if the environmental water provided regularly or just focused over a single year. The approach used in this work to relate hydrological changes from water management to indicators of habitat suitability through changes to physical attributes provides information to inform the evaluation of environmental watering, as well as a tool to support future decision making to maximise the benefits from this precious resource.
Introduction
Estuaries are some of the most productive ecosystems in the world yet they are under threat from reduced freshwater flows, excessive nutrient loads, rising sea levels, modified salinity regimes and changing geomorphology. Consequently estuaries are considered some of the most degraded ecosystems on earth (Vermeiren and Sheaves, 2014).
Freshwater flows to estuaries shape habitat (Loneragan and Bunn, 1999), transport nutrients to drive productivity (Mallin et al., 1993; Mallin and Paerl, 1994), generate a zone over which salinity grades from fresh to marine and provide spawning cues for fish (Reinfelds et al., 2013). Further, more freshwater flows scour sand to maintain an open river mouth, potentially controlling connectivity between marine and riverine ecosystems and facilitating fish movement (Milner et al., 2012).
Over-extraction and a drying climate has led to significantly reduced flows to many estuaries globally with potential consequences for estuarine biota (Gillanders et al., 2011). Notable examples of significantly reduced flows to estuaries include the Nile (Sarif El Din, 1977), Colorado (Rowell et al., 2005), Yellow (Liu and Zhang, 2002), Ebro (Ibáñez et al., 2020), Peel-Harvey (Huang et al., 2020) and Indus (Salik et al., 2016). Rivers of the Murray Darling Basin (MDB), Australia, are exposed to a multitude of stressors including high rates of water extraction for irrigated agriculture and decreasing precipitation (Leblanc et al., 2012). Flows in the Murray Darling Basin are highly variable but the changing climate is evident with low rainfall in recent decades and flow to South Australia exceeding the median flow in only three of the 20 years to 2020. A reduction in Autumn rainfall leading to low soil moisture and increase in evapotranspiration have been implicated in the low flows observed since 1997 in the Murrumbidgee River, a major tributary of the River Murray (Speer et al., 2021). Low flows in the last 2 decades has meant that there has been reduced connectivity between the main river channel and the adjacent floodplain, and abnormally low flows reaching the terminus of the system: the River Murray estuary and the Coorong (Brookes et al., 2021).
Agreements that take environmental water requirements into consideration in the allocation and management of river systems are being created globally, with notable examples of the Colorado River Minute 323 (IBWC, 2017) and Murray-Darling Basin Plan (Water Act 2007 (Commonwealth) (Australia)). The Water Act and Basin Plan established of the Commonwealth Environmental Water Holder who holds water entitlements to be delivered for environmental benefits, with the water purchased water from willing sellers or recovered through water efficiency or other projects. Evaluating the environmental response to these flows, and other environmental water held in the system, is necessary to determine how well the managed environmental flow is meeting the ecological objectives, and to inform future decision making on environmental flow releases.
Although it is widely recognised that estuaries rely on freshwater flows to maintain critical processes, the science to determine freshwater environmental flows for estuaries lags behind that of rivers and floodplains (Chilton et al., 2022). Characterising benefits of flow and setting environmental flow targets is difficult, however, it is critical for properly informed flow allocation and delivery in order to support multiple environmental objectives. These outcomes may include maintenance of habitat complexity which supports biodiversity, lateral and longitudinal connectivity to connect a river with the floodplain and aid dispersal of propagules, and flows to stimulate spawning and facilitate recruitment (Bunn and Arthington, 2002). In order to set an environmental flow target it is necessary to understand life history responses to altered flow regimes (Bunn and Arthington, 2002), set an ecological objective, and then determine the flow timing and duration to achieve the desired ecological outcome across a range of scales and ecological functions (Stein et al., 2021). For estuaries this includes the relationship between freshwater inflow, coastal dynamics and geomorphological processes (Adams and Van Niekerk, 2020). There remain challenges, however, in quantifying the links between flow and estuarine response in a way that can support decision-making. New tools are needed to help compare environmental outcomes that could be achieved from alternate scenarios of water delivery.
At the terminus of the Murray River lies the Coorong. The name Coorong is derived from the word kurangk which is the name given by the Ngarrindjeri, the traditional people of the region, meaning narrow neck (Bourman et al., 2019). The Coorong is an estuarine lagoonal system with a natural salinity gradient ranging from freshwater to hyper-saline at the extremity. Freshwater flows to the Murray Mouth and Coorong help maintain an open Murray Mouth, prevent ingress of seawater, maintain connectivity for diadromous fish and modify water level and salinity in the Coorong, which shapes the habitat for biota (Webster, 2010). Freshwater flows through the barrages (that control water flow from the river) are important to prevent extreme hyper-salinity while maintaining estuarine habitat and ecosystem health (Brookes et al., 2009). Whilst acknowledging that many factors shape the fish community, salinity has been identified as the primary driver of fish distribution and assemblage structure by influencing the extent of estuarine fish habitat in the Coorong (Ye et al., 2011; McNeil et al., 2013; Bice et al., 2018; Brookes et al., 2021). Early summer flows are likely to be particularly beneficial as they provide low salinity inflow to offset evaporation, where without these flows water level in the South Lagoon drop through evaporation, and the resulting evapo-concentration and extreme salinities degrade habitat conditions for key fish species.
The aim of this study was to determine how effective environmental flows are at exporting salt from the Murray Darling Basin, what impact they have on arresting seawater ingress which shapes the salinity gradient, and ultimately the availability of habitat for key fish biota in the Coorong, an estuarine lagoonal system at the terminus of the Murray River. This is undertaken using a finite volume hydrodynamic model for assessing the contribution of environmental water to the spatio-temporal distribution of lagoon salinity, and a habitat model based on laboratory experiment-derived salinity thresholds for fish mortality. Ultimately the aim is to assess and quantify the benefits of environmental flows for the improvement of estuarine fish habitat for several key species with different levels of salinity tolerance.
Methods
Site Description
The Lower Lakes and Coorong are the terminal waterbodies of the Murray-Darling River system (Figure 1). Typically, there is a regular seasonality to the flow pattern, with higher flows occurring over winter and spring. The highest flows generally occur in September to November and the lowest in February to April, but higher-flow events can occur at almost any time of year due to the catchment spanning from sub-tropical to Mediterranean climates Gibbs et al., 2019). The site represents a diverse collection of aquatic environments created where sharp hydrologic gradients intersect with a complex coastal geomorphology. The region is a place of natural beauty, high ecological value and is the spiritual home of the local indigenous people, the Ngarrindjeri.
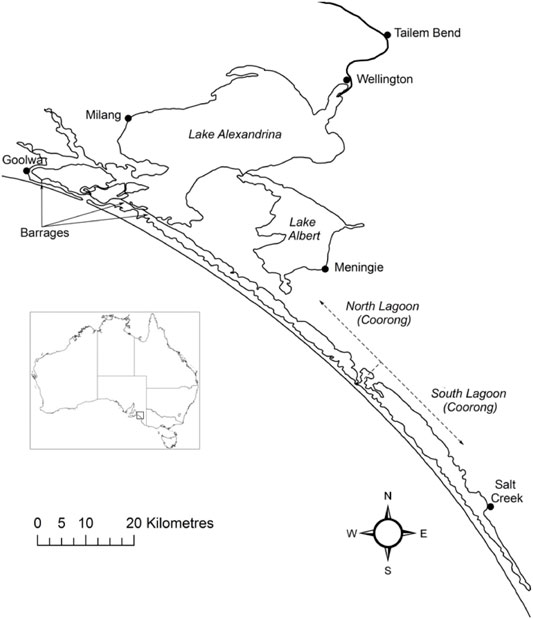
FIGURE 1. The Lower Lakes and Coorong. The freshwater in the lakes is isolated from the marine water by five barrages. Exchange with the ocean occurs through the Murray Mouth, which is located near Goolwa. The Coorong is comprised of the North and South lagoons.
Lake Alexandrina and Lake Albert are joined by a narrow waterway and have a combined area of 800 km2 (Bourman and Barnett, 1995). The River Murray enters Lake Alexandrina, which is separated from the Coorong and sea by five barrages that were constructed between 1935 and 1940, although less permanent sandbag and wooden barrages were constructed as early as 1914 (Fluin et al., 2007). Lake Alexandrina was estuarine prior to construction of the barrages, although paleolimnological evidence suggests the main body of the lake was predominantly fresh (Fluin et al., 2007), consistent with the higher flows leaving the Murray-Darling system prior to extensive river regulation.
The Coorong is a large coastal lagoon that is separated from the ocean by a Holocene dune system, the Younghusband Peninsula. The North Lagoon (area 95 km2) is historically estuarine and connected to the Southern Ocean by the Murray Mouth. The South Lagoon is typically hyper-saline under post-European conditions, has a similar, but slightly larger, area to the North Lagoon (Geddes and Butler, 1984) and is connected to the North Lagoon by a narrow channel near Parnka Point. The Coorong is shallow, with an average water depth of 1 m (Geddes and Butler, 1984), and consists of seven basins with maximum depths of 3–4 m when water levels peak in winter (Noye, 1974). The area north of Pelican Point is tidally influenced and mudflats are inundated and exposed daily depending on tide and wind.
Hydrodynamic Modelling
The interaction between freshwater flow over the barrages and from the Salt Creek, and seawater ingress through the Murray Mouth and transport of salt into the Coorong determine the salinity gradient within the Coorong. The salinity gradient plays a fundamental role in determining the available habitat for key biota in the Coorong. A hydrodynamic model has been used to represent the spatio-temporal variation in salinity, and the extent and persistence of hyper-salinity is essential for using the model to assess environmental flows.
The model platform used to assess the effects of environmental water delivery on the salt balance was the finite volume hydrodynamic model TUFLOW-FV, developed by BMT Global Pty Ltd. (BMTWBM, 2019). TUFLOW-FV is a flexible-mesh hydrodynamic model that accounts for variations in water level, salinity, temperature, and density in response to tides, inflows and surface thermodynamics. The TUFLOW-FV has been used extensively in the region for hydrological assessments (Ye et al., 2016; Ye et al., 2020). In this study, the model domain was configured to cover the Coorong lagoon and the mesh consists of triangular and quadrilateral elements of different size that are suited to simulating areas of complex estuarine morphometry (Figure 2). The model mesh contains 26,250 cells in total and the mean cell size is 10,338 m2. The model dynamically adjusts the time step to maintain a Courant–Friedrichs–Lewy value below 0.9 based on the current speeds and cell lengths, with a minimum time step of 0.1 s and a maximum of 15 s.
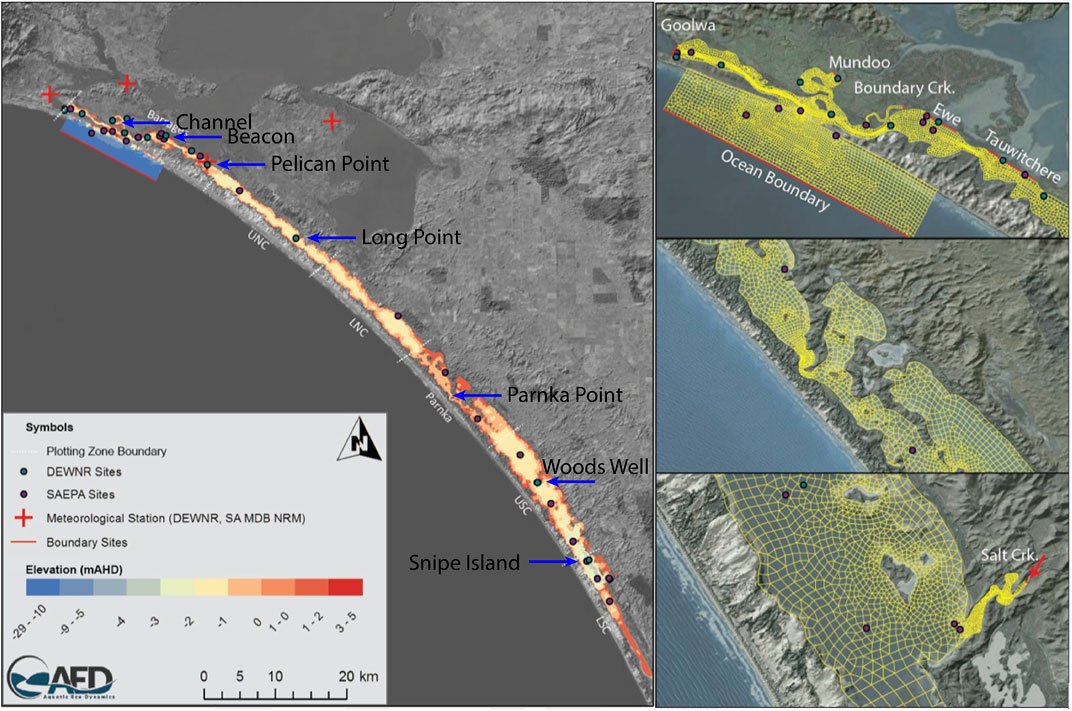
FIGURE 2. Overview of model domain applied in the Coorong using TUFLOW-FV. Coloured grids in maps on the left-hand side represent depths, i.e. increasing depth from deep (blue) to shallow (red). The map on the top-right corner shows the locations of five barrages: Goowa, Mundoo, Boundary Ck, Ewe, and Tauwitchere.
The model was configured as a 2D model as the shallow nature of the system means the water column is typically well mixed. The finite volume numerical scheme solves the conservative integral form of the nonlinear shallow water equations, as well as the advection and transport of scalar constituents such as salinity and temperature. Outside the Murray Mouth an open water level boundary was specified based on Barker Knoll tidal data, which were available at 10 min resolution (Figure 2). Inflow to the South Lagoon from the local catchment via Salt Creek was set based on available flow data from water. data.sa.gov.au (both curated by the South Australian Department of Environment and Water). Meteorological conditions were based on data from Narrung weather station nearby to the Coorong (Figure 2). Further details on model configuration are provided in Supplementary Material S1A. The performance of the hydrodynamic model in reproducing the estuary states had been assessed with a total of five monitoring sites spatially distributed from north to south of the lagoon (Figure 2 and Table 2).
Flow Scenarios
Modelling simulations were run from 1 July 2017 to 30 June 2020, i.e., a period of 3 years; this window was chosen as it began after the high flow event in 2016 which “reset” salinity levels in the Coorong (Figure 3). Because of the importance of salinity movement in this system, a detailed salt flux analysis was undertaken to understand the rate of salt accumulation in both the North and South of the Coorong. The salt load exported from the barrages and Salt Creek (imported into the Coorong) is a function of the flow volume and the salt concentrations in these inflows. Salinity in the Coorong is more complicated and needs to consider salt transportation (fluxes) in Coorong which varies with distance from the Murray Mouth, and subtle changes in water level gradients due to the interaction of the wind and tide. Additionally salt input to the Coorong includes input of water from the ocean which varies with the fluctuation in sea-level, and the seasonal change in the volume of water exiting the Murray Mouth, which acts to prevent seawater ingress.
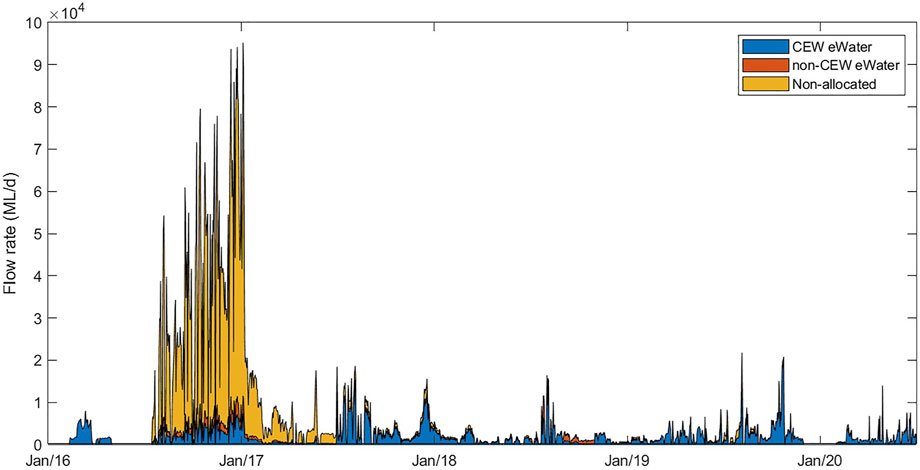
FIGURE 3. Overview of the flow rates through all barrages into the Coorong from three water sources assessed by the model simulations. The area plots show the proportion of flow that was considered non-allocated (“no eWater” scenario), the proportion of Commonwealth environmental water (CEWO) and the proportion of non-CEW environmental water (eWater). The cumulative of all these flows represents the “all water” (Base-case) scenario.
The contribution of environmental water to the transport of salt and habitat maintenance was assessed for three different flow scenarios:
1. Base-case scenario: a scenario with all environmental water (i.e. the observed flow);
2. Scenario 1: a scenario without any environmental water for the study period from 01/07/2017 to 30/06/2020 (i.e. counter-factual simulations assessing what would have happened if flows were not augmented with environmental water for 3 years) to study the cumulative effect of environmental water on the Coorong system; and
3. Scenario 2: a hybrid scenario with observed flow from 01/07/2017 to 30/06/2019, then no environmental water from 01/07/2019 to 30/06/2020 to provide insights into the specific benefit of the 2019–2020 water year provision, and the cumulative benefits of multi-year environmental watering.
The Criteria for Suitable Habitat
Salinity, water temperature and water levels from scenarios with and without environmental water were used to estimate habitat extent of fish. The fish model calculates spatio-temporal probabilities of habitat suitability for juveniles of seven key species, mulloway (Argyrosomus japonicus), black bream (Acanthopagrus butcheri), greenback flounder (Rhombosolea tapirina), yelloweye mullet (Aldrichetta forsteri), congolli (Pseudaphritis urvillii), Tamar goby (Afurcagobius tamarensis) and smallmouth hardyhead (Atherinosoma microstoma), based on laboratory experiment-derived lethal concentration salinity thresholds. The experiment provided salinity thresholds under two temperature settings, 23 and 14°C, representing the average summer and winter condition in the lagoon, which indicated most fish species had higher salinity tolerance in cooler months (McNeil et al., 2013). The model adopts a seasonal effect by account for temperature sensitivity to the salinity thresholds (higher salinity tolerance at lower temperatures) to calculate the habitat suitability index, HSI, according to:
where the salinity is temperature dependent based on:
where:
The parameters for the focus species of interest are described Table 1. Figure 4 shows an example plot of the HSI functions for mulloway. In addition, habitat is deemed unsuitable if water is less than 0.1 m deep. The computed HSI ranges between 0 and 1, where 0 represents least suitable and one represents most suitable.
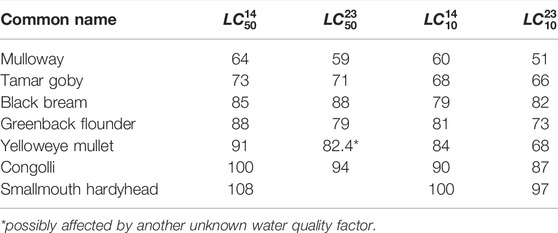
TABLE 1. Summary of lethal concentration for 50% of the population,
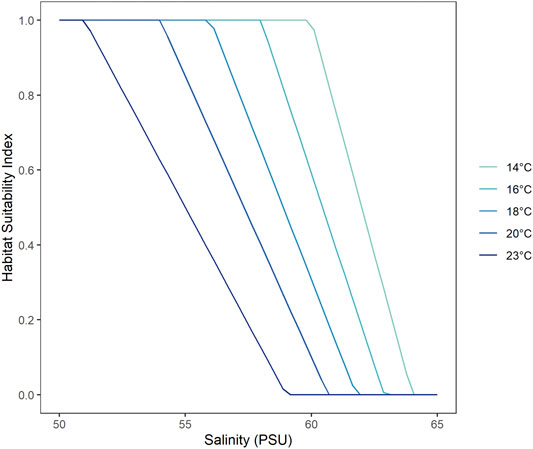
FIGURE 4. Habitat Suitability Index (HSI) as a function of salinity tolerance for mulloway, where a HSI value of one represents the most suitable conditions and 0 the least suitable. The salinity thresholds are a function of water temperature where fish is able to tolerate higher salinities at lower temperatures.
Results
Spatio-Temporal Salinity Distribution and Model Performance
Overall, the model was able to accurately reproduce salinity in the lagoon at the five assessment sites (Figure 5), and captured well the variations in time and space. The goodness of the fit statistics for the model performance in salinity (from the base case scenario with the actual environmental flow) against the observation showed generally high regression coefficient (R ≥ 0.5535) and low mean absolute errors and root-mean square errors (MAE≤11.89 PSU, RMS≤16.54 PSU, comparing to the observed salinity range of 0.14–122.13 PSU) (Table 2). The assessment results suggest that in its present form, the model is suitable for assessing management scenarios associated with different flow conditions.
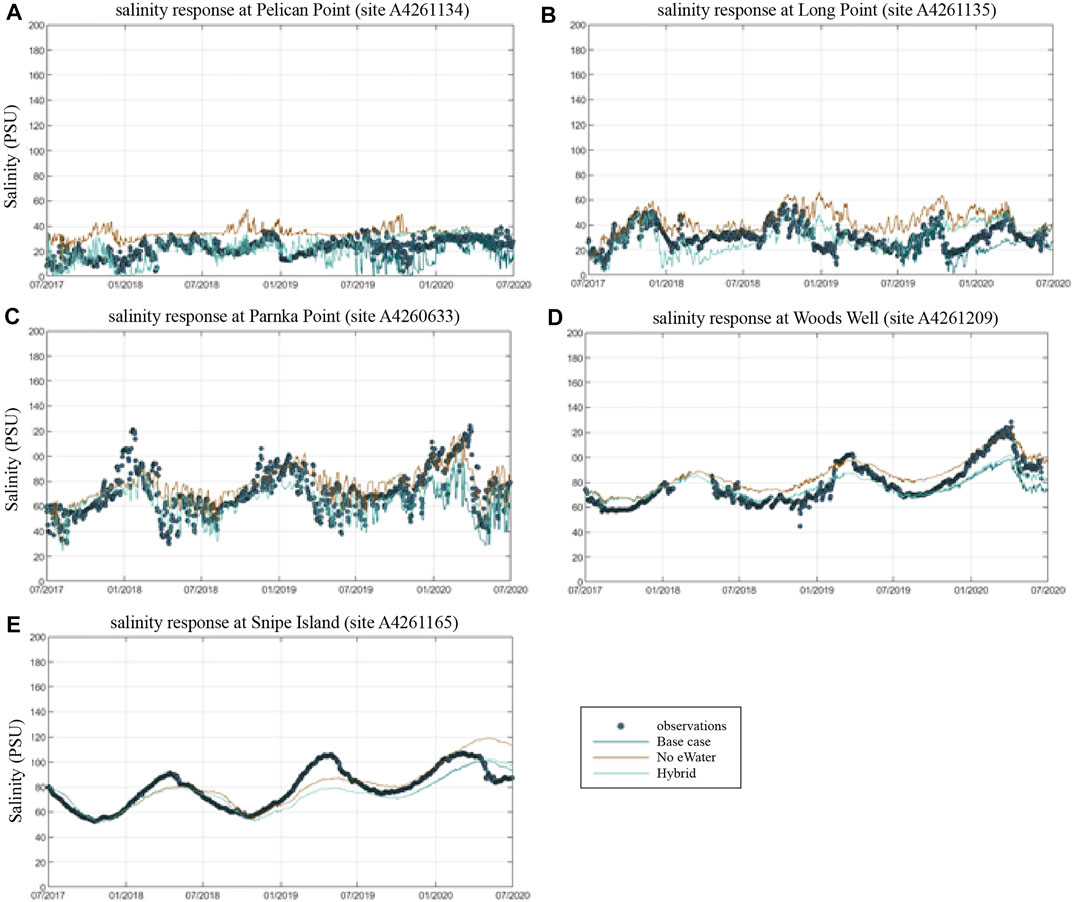
FIGURE 5. Comparison of measured and simulated salinity at key monitoring points within the Coorong lagoon, moving from the Murray Mouth into the South Lagoon. Model simulations for the Base-case (observed conditions), and Scenario 1 (no eWater) and Scenario 2 (mixed) are shown. A4261134 = Pelican Point (A), A4261135 = Long Point (B), A2460633 = Parnka Point (C). A4261209 = Woods Well, A4261165 = Snipe Island.
A clear salinity gradient from the ocean mouth (Murray Mouth) to the southern lagoon is observed from both the field observations and model results, with the salinity varied from seawater level (∼36 PSU) to up to 122 PSU in the southern lagoon (Figure 6). The salinity change also presents a clear seasonal signal, with higher salinity in summer and lower salinity in winter (Figure 6) due to the seasonal patterns in precipitation and evaporation. At the site close to the Murray Mouth (site A4261134, Figure 5A), strong fluctuations in the salinity in daily to weekly scales can be observed, indicating the interactions between the barrage flows and the ocean intrusion in this region. The daily to weekly fluctuation signals became weaker with sites further from the Murray Mouth, whilst the seasonal signal became stronger (site A4261209, Figure 5D and site A4261165, Figure 5E).
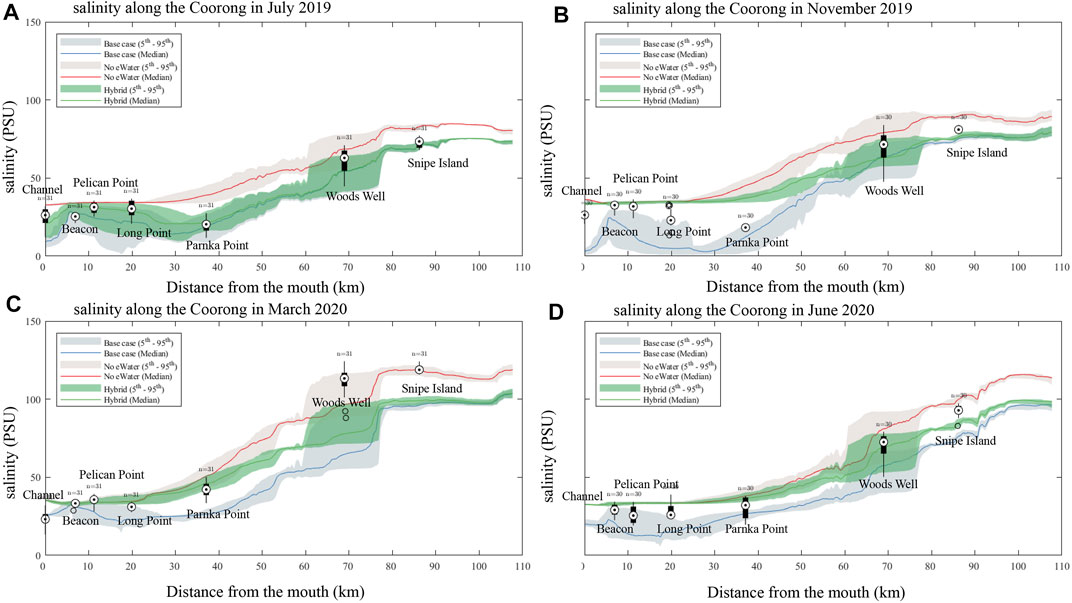
FIGURE 6. Comparison of measured and simulated salinity along the length of the Coorong lagoon (box-whisker), moving from the Murray Mouth into the South Lagoon. Model simulations for the “All water” (base-case observed conditions), and the no environmental water scenarios (“no eWater” and “hybrid”) are shown for July (A), March 2020 (C) and June 2020 (D). November 2019 (B). The shaded areas represent the 5 - 95% confidence interval.
Salt Flux Analysis
The environmental water is shown to significantly reduce the salinity levels. The impact was mostly obvious in the northern lagoon around the barrage inputs (0–40 km from the Murray mouth), but can be also observed along the Coorong to the southern lagoon. The effect of the environmental water in reducing the salt level is more significant in summer time (Figure 6B) where a difference of up to 30 PSU can be observed between the “base case” scenario and “no eWater” scenario around the barrage input region of north lagoon.
The comparison of scenario 1 (3-years no-eWater simulation) and scenario 2 (no eWater just in the last year) provides insights into the multi-year cumulative effects of salt transport in the lagoon. For example, in the time of November 2019, the median salinity at the north lagoon from scenario two is basically the same to scenario 1, however, in the south lagoon the salinity difference is still up to ∼20 PSU higher, indicating reduced salinity transport within the lagoon between north and south.
In 2018–19 and 2019–20 water years the only water that exited the barrages was Commonwealth environmental water and so all salt export over the barrages is attributable entirely to this (Table 3). Salt export from the Murray Darling Basin to the Coorong through the barrages was 496,936 tonnes in 2017–18, 532,333 tonnes 2018–19 and 623,999 tonnes in 2019–20. If there had not been environmental water in 2018–19 and 2019–20 then no salt would have been exported from the basin.
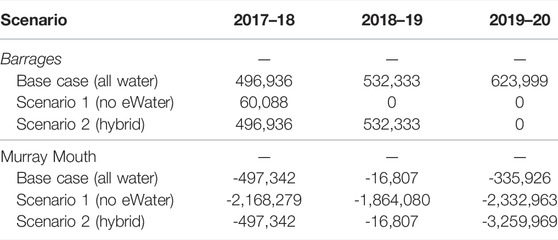
TABLE 3. Three year record of modelled salt export (tonnes) over the barrages to the Coorong estuary and through the Murray Mouth into the Southern Ocean.
Additionally, the salt concentration in the barrage flows is below the seawater concentration, so the flows from the barrages also serve to reduce salt ingress from seawater into the Murray Mouth and Coorong. In all 3 years, 2017–18 to 2019–20, there was a net import of salt into the Coorong (through the Murray Mouth), which is expected for years with low flow over the barrages that is not sufficient to replace the evaporation volume from the Coorong lagoon. Without environmental water, the net import of salt into the Coorong would have been considerably greater, ranging between 1.86 million tonnes in 2018–19 to approximately 2.33 million tonnes in 2019–20. Environmental water decreased salt import by approximately two million tonnes (Table 3), all of which is attributable to water held and delivered by the Commonwealth Environmental Water Holder. For the “hybrid” scenario (Scenario 2), the salt import from ocean into the Coorong was small in the first 2 years with the environmental water, then increased to 3.26 million tonnes in the last year without the environmental flow (Table 3), due to the environmental flow in the first 2 years creating a fresher environment inside the lagoon that enhanced the salt exchange in the last year.
Freshwater flowing from the barrages limits salt influx from the ocean and maintains lower salinity conditions in the Coorong. Salt from the Murray Mouth can travel southward as ocean water replenishes water that is evaporating in the Coorong. The dominant direction of salt flux is southward although it can move northward (negative flux in Supplementary Figure SB1, Supplementary B) when river flows over the barrages cease, the head of water decreased and the net flow of water is northwards. Further to the South at Parnka Point there are much higher flux rates as the salt concentrations are higher and so the rate of salt movement is higher (Supplementary Figure SB1, Supplementary B). This is evident in the cumulative flux of salt at Parnka Point which is considerably higher in both the northward and southward vectors (Figure 7) than salt flux at Long Point.
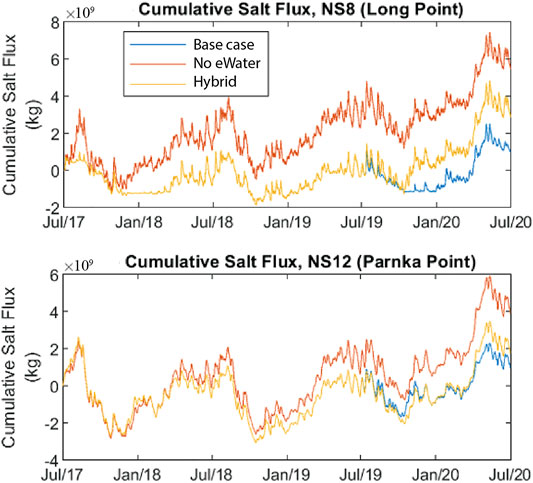
FIGURE 7. Cumulative net southward amount of salt mass into the North Lagoon (through Long Point) and South Lagoon (through Parnka Point) in the Coorong from July 2017–June 2020. Scenarios include with “All water”, without environmental water (“no eWater 3 years”) and without any environmental water in 2019–20 (“no eWater”). eWater delivery maintained this flux to be close to zero over the period of interest; even 1 year of no environmental water over the barrages contributes to salt accumulation in the North and South Lagoon. Note that only CEW water contributed to the eWater entering the Coorong (see Supplementary A for barrage flow amounts).
Due to the low flow over the barrages in 2017–18 for the no eWater scenario, and no flow in subsequent years, there was considerably more salt imported from the ocean into the Murray Mouth region, resulting in greater southward salt flux (Supplementary Figure SB2, Supplementary B). Without environmental water the cumulative southward salt flux in 2019–20 would be three times greater than with environmental water (Figure 7). Environmental water reduced the salt load to the South Lagoon, measured as salt flux southward at Parnka Point, by over 3.244 million tonnes over the 3-year period between July 2017 and July 2020. If there was a year without environmental water there would be excess salt accumulation in the Coorong. For the “hybrid” scenario when assuming environmental water was delivered in the first 2 years (2017–18 and 2018–19) but not in 2019–20, salt seems to have accumulated at a slower rate but at the end of the simulation period, the net accumulated flux is still more than twice what would have occurred had environmental flows been delivered in 2019–20 (Figure 7).
Monthly salt exports with and without environmental water delivery for July 2017–July 2020 show how seasonally dynamic salt export was in the Coorong (Supplementary Figure SB). Environmental water delivery maintained this flux to be close to zero over the period of interest.
Fish Habitat
In general, the environmental flows led to fresher conditions in the Coorong and a consistent expansion of suitable fish habitat area; an example of the new habitat area created by environmental water is shown as a map for mulloway habitat suitability (Figure 8). Distribution maps for other species have different ranges but similar overall patterns (Supplementary Figure SC). Note that this analysis shows the ∆HSI, that is the change between scenarios; in this case a value of one represents an area that was unsuitable under the “No eWater 3 years” scenario (Scenario 1) becoming fully suitable under the relevant scenario (Base case scenario).
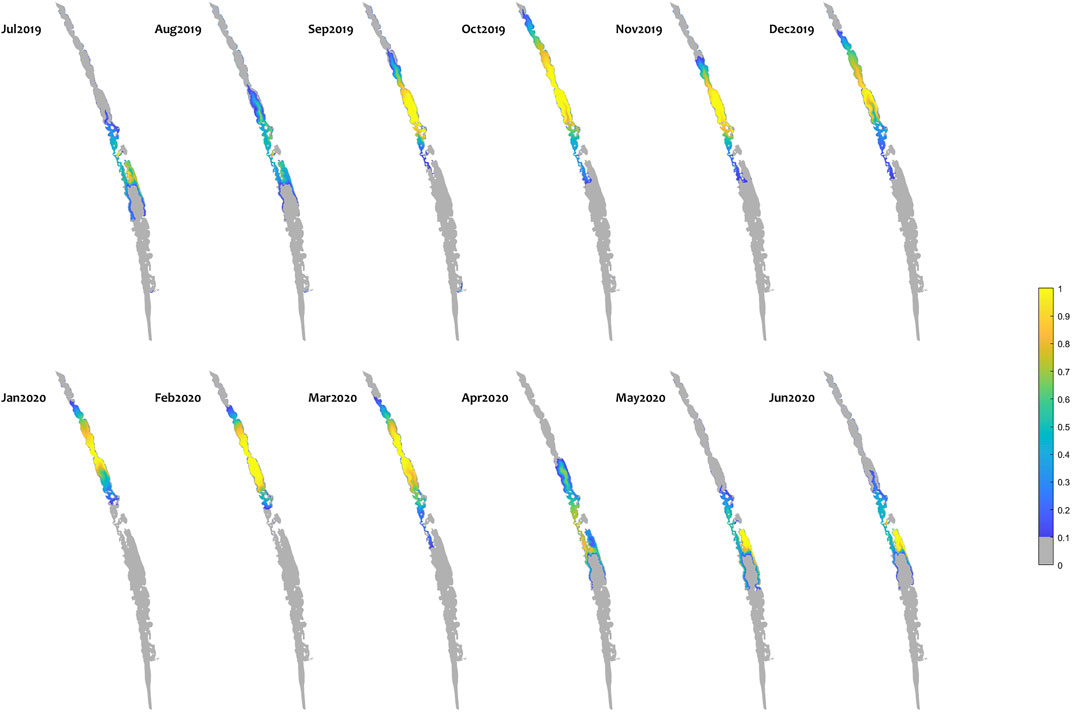
FIGURE 8. Monthly habitat area “gained” for mulloway due to environmental water delivery (calculated as the difference between habitat suitability index (HSI) in the “All water” (base-case) and “no eWater 3years” scenario. Large areas of the middle lagoon have an increase in habitat quality of 1, highlighting areas that would not be viable without environmental water, but became suitable due to the ongoing water delivery since 2017–18. The improvement in habitat score means salinity and temperature conditions are suitable for Mulloway. Other features of the environment such as food resources and appropriate sediment will also determine whether mulloway expand into a habitat. It may take some time for ecosystem restoration to reach a point where fish populations are supported in the expanded habitat.
To summarise the suitable habitat area for each species, the sum of HSI-weighted area (i.e. HSI×area) in each grid cell for each scenario was computed. Figure 9A shows the suitable fish habitat averaged over all months in each year. Without environmental water (No eWater 3 years) the habitat suitable for mulloway would have contracted by 16% in 2017–18, 33% by 2018–19 and 38% in 2019–20 (Figure 9B).
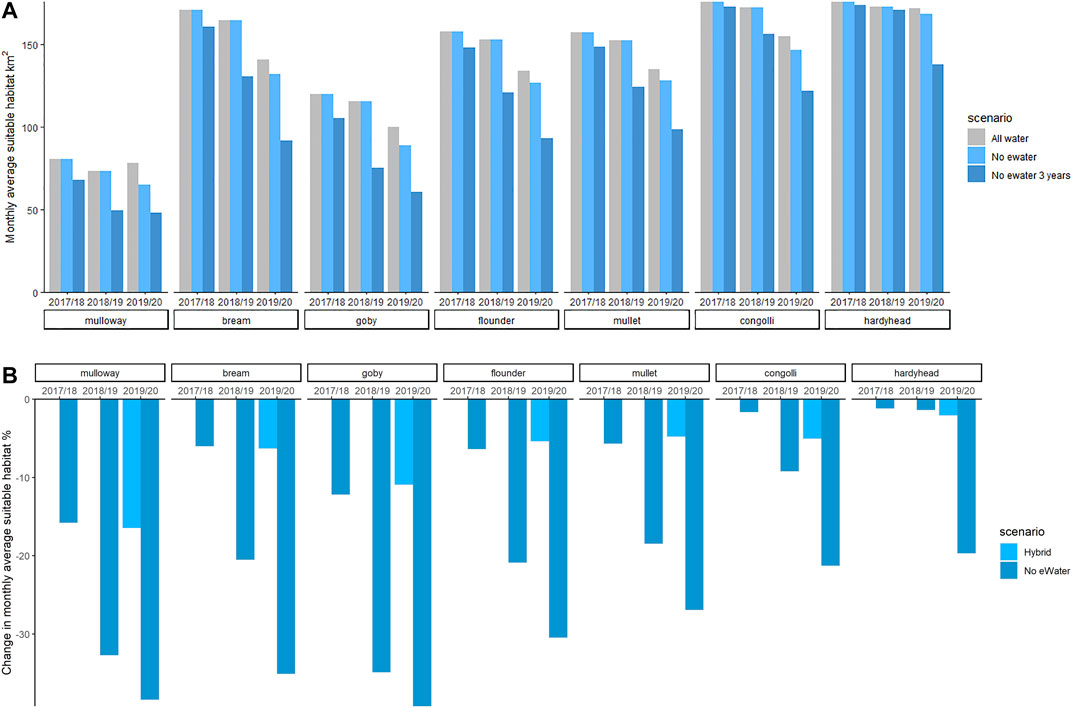
FIGURE 9. Habitat area (HSI-weighted) of juvenile stages of key fish species for the three scenarios (top). Change in area (%) that would have been in the case of no environmental water is shown in the bottom panel. Environmental water gives a large habitat expansion for all species and this increases year on year. The environmental water modifies the salinity conditions to be expanding the habitat that the fish can exploit. Other features such as food resources and appropriate sediment will also influence whether fish are able to exploit the available habitat.
A similar trend is evident for black bream, Tamar goby, greenback flounder, yelloweye mullet, congolli and smallmouth hardyhead (Supplementary Figure SC). Three years without environmental water reduced suitable goby habitat by 39% (Figure 9B). This scenario commenced after high flow in 2016–17. If the starting conditions were more saline then we could expect a more rapid contraction in suitable habitat if no environmental water was available.
If we consider just 1 year (2019–20) of without environmental water (“No eWater”), the suitable habitat area contracted by between 2 and 17% for the seven fish species within that period. Mulloway habitat was the most sensitive to a single 1-year reduction in environmental water. Smallmouth hardyhead can tolerate very saline conditions and their habitat was least affected by the 1-year reduction in environmental water.
Discussion
Environmental water dilutes salt in Lake Alexandrina, the Murray Mouth estuary and along the length of the Coorong. Without environmental water delivered for the period in question, river flow over the barrages would only have occurred in 2017–18, and not in 2018–19 or 2019–20. Without environmental water from 2017–20, the river flow would have only transported ∼40% of salt from the river catchment that was exported when additional environmental water was included. This reduction in salt export from the river system implies that without the environmental water the salt would be accumulating upstream in wetlands and floodplains.
The median salinity in the Murray Mouth in 2019–20 was 21.3 PSU which was lower than 2018–19 (median salinity 30.9 PSU), similar to 2017–18 (26.2 PSU) but higher than in 2016–17 (11.3 PSU). The 2016–17 fresher conditions reflected generally high river flows throughout the basin, and flow into South Australia peaked at 94,600 ML/d. The period following this natural flow pulse notably benefited from the Commonwealth environmental water, which created fresher conditions at the Murray Mouth in 2019–20 compared to the without environmental water scenario.
Further away from the Murray Mouth, salinity throughout the Coorong is not only a function of riverine inflows, but also the nature of tidal exchange. When barrage flows are low, seawater enters into the Murray Mouth and more salt is then transported down the Coorong where it is subject to evapo-concentration. Dedicated environmental water made up 100% of flow over the barrages, thus preventing some salt load reaching the South Lagoon; this is measured as salt flux southward at Parnka point, which was estimated have been over 3.2 million tonnes from July 2017 to June 2020. It is also evident that environmental water flowing over the barrages is required in every year to reduce excessive salt accumulation within the Coorong. If environmental water had not been delivered in the last of the 3 year action period (2019–20) an additional 1.7 million tonnes of salt would have accumulated in the South Lagoon. This highlights the importance of continued delivery in periods of low flow, as the benefits amplified by the final year.
Prior to our study period during the Millennium Drought, from 2007–08 to 2009–10, flow over the barrages ceased and the import of salt into the Coorong resulted in salinity in the South Lagoon that was five times seawater salinity, and led to the demise of much of the aquatic life (Brookes et al., 2009). Environmental water provides freshening flows but also acts to inhibit seawater intrusion, thereby maintaining more appropriate salinity conditions, particularly in the South Lagoon. Given that barrage releases now almost entirely (up to 100%) depend on Commonwealth environmental water provision in dry years, it is a critical allocation for limiting salt flux into the South Lagoon and the subsequent cycle of salinity build up due to evapo-concentration. Even 1 year without barrage flow can result in a large flux of salt moving southwards (∼1.7 million tonnes net southwards flux).
MDBA (2021) report salt export over the barrages as 510,000, 360,000 and 430,000 tonnes/year in 2017/18, 2018/19 and 2019/20, respectively. The approach used in MDBA (2021) is slightly different to that used here, where a hydrological model is used (as opposed to hydrodynamic model used here) to undertake a similar water balance, based on river (Lock 1) inflows, diversions and losses to estimate flow over the barrages, with the salinity of that flow assumed to be the average salinity of Lake Alexandrina derived for multiple observation stations within the lake (MDBA, 2013). Given the differences in approaches the exact salt loads are different between that reported here and MDBA (2021), however the magnitudes are similar. As noted in MDBA (2013) the approach taken here (2D hydrodynamic model) is the only method capable of estimating salt export to the Southern Ocean from the River Murray System and Coorong. This work has challenged the assumption that salt export over the barrages is a necessary and appropriate surrogate for salt export to the Southern Ocean (MDBA, 2013), where it is export to the ocean that is required to be reported on by the Basin Plan.
The fish habitat suitability model presented here is intentionally simplified, noting that salinity is the primary driver for habitat condition in this system, and considering our goal was an indicator that could interface with the modelling scenario outputs and readily inform management decisions. The estuarine fish species that inhabit the Coorong vary slightly in their tolerance to salinity with yellow-eye mullet, congolli and smallmouth hardyhead able to tolerate more saline conditions. Without environmental water, fish habitat contracts quickly and significantly. Even after the high flow year in 2016–17, if there was no environmental water in 2017–18, significant habitat contraction would have occurred. As mulloway and Tamar goby have the smallest area of suitable habitat, this contraction would have the most profound impact on these species, followed by black bream, greenback flounder and yellow-eye mullet. Habitat for the more salinity tolerant congolli and smallmouth hardyhead would not have changed as significantly in 2017–18 as for the other species. Nonetheless, consecutive years of no environmental water is likely to reduce suitable habitat by up to 39% within 3 years and even the highly salt-tolerant smallmouth hardyhead experienced a 20% contraction of suitable habitat. This result highlights the importance of using multiple years of flow when undertaking habitat assessment, as systems with a large retention time have a “memory” and salinity is a function of antecedent conditions as well as annual inputs and outputs.
Fish can move in response to changing salinity and habitat suitability, however it is generally considered advantageous to have a greater area of habitat with suitable water quality (e.g., salinity) and abundant food resources to support the long-term maintenance of fish populations. Habitat assessment should also consider that different conditions may be needed at different life stages, considering that feeding, spawning, nursery and refuge grounds are different for many estuarine dependent fish species (Bice et al., 2018). For example, Barletta et al. (2005) observed that when estuarine salinity rose in the dry season the fish moved upstream for spawning and shelter for young-of-year juveniles. As rainy season flows increased they observed a decrease in salinity and movement of fish downstream to inshore areas. We acknowledge that there are also more complex drivers of habitat, and besides salinity, the distribution and abundance of estuarine fish is affected by water velocities, prey abundance (Bottom and Jones, 1990) and seagrass extent (Griffiths, 2001). Elevated flow can flush juveniles from the estuary to off-shore where they experience less productive and hospitable conditions. Low flow can lead to the formation of sandbars at the estuary mouth, which coupled with an increase in stratification, can lead to low oxygen and conditions unsuitable for some species (Gale et al., 2006; Cottingham et al., 2014, 2018). Excessive nutrient loading, turbidity and poor sediment quality can also degrade habitat and reduce abundance (Hallett et al., 2016; Valesini et al., 2017).
The predictions of habitat in these simulations can be considered as the potential (or “realisable”) niche for the fish communities since these results are based on fish tolerance to salinity. As a tool for planning environmental flow delivery this provides a relative assessment of total available habitat if we also consider that many other drivers of habitat mentioned above similarly correlate with salinity, making salinity a good primary indicator. Nonetheless, care should be taken in interpreting and communicating these reported habitat areas, bearing in mind other ecological constraints on population recovery are not captured in the index formulation. Others have attempted to build more complex empirical relationships between multiple drivers and presence/abundance (e.g., Zucchetta et al., 2010), though lack of regular monitoring data and difficulty interfacing with scenarios from these dynamic models remain challenging. Furthermore, for fisheries management the flow releases would also need to consider the flow necessary to induce spawning in the flow cued spawning species (e.g., Bream), the salinity tolerance of prey and a sustainable level of commercial fishing. More traditional food-web and population models could be used for this purpose rather than the habitat modelling presented here.
Environmental water planning in Australia currently considers both flow variability in rivers and water regimes in wetlands. Water regimes describe the extent, duration and depth of inundation and longer-term planning considers the frequency of years that environmental flows or inundation need to occur to achieve the desired environmental outcome. It is generally accepted that increasing the number of years that environmental water is available builds ecosystem resilience as there is a cumulative effect from e-water delivery. Ecosystem resilience strengthens the capacity to cope with drought or dry periods when they arise. In the case of the Coorong the environmental water from the River Murray freshens the lagoons and expands the area with suitable habitat to support fish communities. Just 1 year without environmental water would result in a contraction of suitable habitat and continued contraction if flow is not maintained. While Coorong salinity is an easily measured parameter to assess the benefits from cumulative years of environmental water delivery, there is evidence that other features of rivers show a cumulative response to e-watering. For example, Catelotti et al. (2015) determined that measuring inundation frequencies over a period of five to 10 years provided the best timeframe to explain observed variation in river red gum health. Given the evidence that multiple consecutive years with environmental water can build resilience in ecosystem habitat and populations this may prove to be an important objective in future planning for environmental flow.
In the case of the Coorong, a site of international significance, it has become evident that prolonged periods of low flow have not only led to increases in salinity that impact on fish communities, but the extreme salinities have also contributed to the loss of the seagrass Ruppia tuberosa (Kim et al., 2015; Brookes et al., 2009) and benthic macroinvertebrates (Dittmann et al., 2015). The continued commitment to deliver environmental water over periods of prolonged drought is needed to allow the long-term re-establishment of benthic communities in the Coorong in order to support fish populations over the long-term. To this end, we therefore further recommend extension of the habitat modelling approach to allow for prediction of Ruppia spp and benthic macroinvertebrate suitability, allowing a wider view of the benefits of e-water on the lagoon system. Whilst managing excessively high salinity in the South Lagoon through environmental water provision has been the focus here, we also highlight the need for consideration of other complementary actions that can be used to restore and re-establish high-quality habitat.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
Ethical review and approval was not required for the animal study because this is a modelling study.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research project has been funded via the Commonwealth Environmental Water Office’s Monitoring, Evaluation and Research Program (Flow-MER).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the traditional owners of Country in the Murray–Darling Basin, and their Elders past and present, and their cultural, social, environmental, spiritual and economic connection to their lands and waters. In particular, the Ngarrindjeri Nation and the First Peoples of the River Murray and Mallee as traditional owners of the land and water on which this publication is focused.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.796623/full#supplementary-material
References
Adams, J. B., and Van Niekerk, L. (2020). Ten Principles to Determine Environmental Flow Requirements for Temporarily Closed Estuaries. Water 12, 1944. doi:10.3390/w12071944
Barletta, M., Barletta-Bergan, A., Saint-Paul, U., and Hubold, G. (2005). The Role of Salinity in Structuring the Fish Assemblages in a Tropical Estuary. J. Fish Biol. 66, 45–72. doi:10.1111/j.0022-1112.2005.00582.x
Bice, C. M., Wedderburn, S. D., Hammer, M. P., Ye, Q., and Zampatti, B. P. (2018). "Fishes of the Lower Lakes and Coorong: A Summary of Life History, Population Dynamics and Management," in Natural History of the Coorong, Lower Lakes, and Murray Mouth Region (Yarluwar-Ruwe) Editor L. Mosley. (Australia: Royal Society of South Australia), 371–399. doi:10.20851/natural-history-cllmm-3.6
Bottom, D. L., and Jones, K. K. (1990). Species Composition, Distribution, and Invertebrate Prey of Fish Assemblages in the Columbia River Estuary. Prog. Oceanography 25, 243–270. doi:10.1016/0079-6611(90)90009-q
Bourman, R. P., and Barnett, E. J. (1995). Impacts of River Regulation on the Terminal Lakes and Mouth of the River Murray, South Australia. Aust. Geog Stud. 33, 101–115. doi:10.1111/j.1467-8470.1995.tb00688.x
Bourman, R. P., Murray-WallaceDeirdre, C. V. D. R., Ryan, D. D., Belperio, A. P., and Harvey, N. (20192019). “Geomorphological Evolution of the River Murray Estuary, South Australia,” in Natural History of the Coorong, Lower Lakes, and Murray Mouth Region (Yarluwar-Ruwe). Editor L. Mosley (Royal Society of South Australia). doi:10.20851/natural-history-cllmm-2.2
Brookes, J., Aldridge, K., Hipsey, M., Busch, B., Ye, Q., Gibbs, M., et al. (2021). “Ecological Condition of the Lower Lakes and Coorong,” in Murray Darling Basin Australia: Its Future Management (Elsevier). doi:10.1016/b978-0-12-818152-2.00005-x
Brookes, J. D., Aldridge, K. T., Bice, C. M., Deegan, B., Ferguson, G. J., Paton, D. C., et al. (2015). Fish Productivity in the Lower Lakes and Coorong, Australia, during Severe Drought. Trans. R. Soc. South Aust. 139, 189–215. doi:10.1080/03721426.2015.1074338
Brookes, J. D., Lamontagne, S., Aldridge, K. T., Benger, S., Bissett, A., Bucater, L., et al. (2009). An Ecosystem Assessment Framework to Guide Management of the Coorong. Final Report of the CLLAMMecology Research Cluster. CSIRO: Water for a Healthy Country National Research Flagship. Canberra: CSIRO Publishing. doi:10.4225/08/585ac4318b01f
Bunn, S. E., and Arthington, A. H. (2002). Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manage. 30, 492–507. doi:10.1007/s00267-002-2737-0
Catelotti, K., Kingsford, R. T., Bino, G., and Bacon, P. (2015). Inundation Requirements for Persistence and Recovery of River Red Gums ( Eucalyptus camaldulensis ) in Semi-arid Australia. Biol. Conservation 184, 346–356. doi:10.1016/j.biocon.2015.02.014
Chilton, D., Hamilton, D., Nagelkerken, I., Cook, P., Hipsey, M. R., Reid, R., et al. (2022). Environmental Flow Requirements of Estuaries: Providing Resilience to Current and Future Climate and Direct Anthropogenic Changes. Front. Environ. Sci. 9, 764218. doi:10.3389/fenvs.2021.764218
Cottingham, A., Hesp, S. A., Hall, N. G., Hipsey, M. R., and Potter, I. C. (2014). Marked Deleterious Changes in the Condition, Growth and Maturity Schedules of Acanthopagrus butcheri (Sparidae) in an Estuary Reflect Environmental Degradation. Estuarine, Coastal Shelf Sci. 149, 109–119. doi:10.1016/j.ecss.2014.07.021
Cottingham, A., Huang, P., Hipsey, M. R., Hall, N. G., Ashworth, E., Williams, J., et al. (2018). Growth, Condition, and Maturity Schedules of an Estuarine Fish Species Change in Estuaries Following Increased Hypoxia Due to Climate Change. Ecol. Evol. 8, 7111–7130. doi:10.1002/ece3.4236
Dittmann, S., Baring, R., Baggalley, S., Cantin, A., Earl, J., Gannon, R., et al. (2015). Drought and Flood Effects on Macrobenthic Communities in the Estuary of Australia's Largest River System. Estuarine, Coastal Shelf Sci. 165, 36–51. doi:10.1016/j.ecss.2015.08.023
Fluin, J., Gell, P., Haynes, D., Tibby, J., and Hancock, G. (2007). Palaeolimnological Evidence for the Independent Evolution of Neighbouring Terminal Lakes, the Murray Darling Basin, Australia. Hydrobiologia 591, 117–134. doi:10.1007/s10750-007-0799-y
Gale, E., Pattiaratchi, C., and Ranasinghe, R. (2006). Vertical Mixing Processes in Intermittently Closed and Open Lakes and Lagoons, and the Dissolved Oxygen Response. Estuarine, Coastal Shelf Sci. 69, 205–216. doi:10.1016/j.ecss.2006.04.013
Geddes, M. C., and Butler, A. I. (1984). Physicochemical and Biological Studies on the Coorong Lagoons, South Australia, and the Effect of Salinity on the Distribution of the Macrobenthos. Trans. R. Soc. South Aust. 108, 51–62.
Gibbs, M. S., Joehnk, K., Webster, I., and Heneker, T. (2019). “Hydrology and Hydrodynamics of the Lower Lakes, Coorong and Murray Mouth,” in Natural History of the Coorong, Lower Lakes, and Murray Mouth Region (Yarluwar-Ruwe). Editor L. Mosley (Royal Society of South Australia), 197–216.
Gillanders, B. M., Elsdon, T. S., Halliday, I. A., Jenkins, G. P., Robins, J. B., and Valesini, F. J. (2011). Potential Effects of Climate Change on Australian Estuaries and Fish Utilising Estuaries: a Review. Mar. Freshw. Res. 62, 1115–1131. doi:10.1071/mf11047
Griffiths, S. P. (2001). Factors Influencing Fish Composition in an Australian Intermittently Open Estuary. Is Stability Salinity-Dependent? Estuarine, Coastal Shelf Sci. 52, 739–751. doi:10.1006/ecss.2000.0756
Hallett, C. S., Valesini, F. J., Clarke, K. R., and Hoeksema, S. D. (2016). Effects of a Harmful Algal Bloom on the Community Ecology, Movements and Spatial Distributions of Fishes in a Microtidal Estuary. Hydrobiologia 763, 267–284. doi:10.1007/s10750-015-2383-1
Huang, P., Hennig, K., Kala, J., Andrys, J., and Hipsey, M. R. (2020). Climate Change Overtakes Coastal Engineering as the Dominant Driver of Hydrological Change in a Large Shallow Lagoon. Hydrol. Earth Syst. Sci. 24, 5673–5697. doi:10.5194/hess-24-5673-2020
Ibáñez, C., Caiola, N., and Belmar, O. (2020). Environmental Flows in the Lower Ebro River and delta: Current Status and Guidelines for a Holistic Approach. Water 12, 2670. doi:10.3390/w12102670
IBWC (2017). Extension of Cooperative Measures and Adoption of a Binational Water Scarcity Contingency Plan in the Colorado River Basin, Minute No 323. Available at: https://www.ibwc.gov/Files/Minutes/Min323.pdf.
Kim, D., Aldridge, K. T., Ganf, G. G., and Brookes, J. D. (2015). Physicochemical Influences on Ruppia Tuberosa Abundance and Distribution Mediated through Life Cycle Stages. Iw 5, 451–460. doi:10.5268/IW-5.4.709
Leblanc, M., Tweed, S., Van Dijk, A., and Timbal, B. (2012). A Review of Historic and Future Hydrological Changes in the Murray-Darling Basin. Glob. Planet. Change 80-81, 226–246. doi:10.1016/j.gloplacha.2011.10.012
Liu, C. M., and Zhang, S. F. (2002). Drying up of the Yellow River: its Impacts and Counter-measures. Mitigation Adaptation Strateg. Glob. Change 7, 203–214. doi:10.1007/s13753-016-0083-8
Loneragan, N. R., and Bunn, S. E. (1999). River Flows and Estuarine Ecosystems: Implications for Coastal Fisheries from a Review and a Case Study of the Logan River, Southeast Queensland. Aust. J. Ecol. 24, 431–440. doi:10.1046/j.1442-9993.1999.00975.x
Mallin, M. A., and Paerl, H. W. (1994). Planktonic Trophic Transfer in an Estuary: Seasonal, Diel, and Community Structure Effects. Ecology 75, 2168–2184. doi:10.2307/1940875
Mallin, M., Paerl, H., Rudek, J., and Bates, P. (1993). Regulation of Estuarine Primary Production by Watershed Rainfall and River Flow. Mar. Ecol. Prog. Ser. 93, 199–203. doi:10.3354/meps093199
McNeil, D. G., Westergaard, S., Cheshire, K. J. M., Noell, C. J., and Ye, Q. (2013). Effects of Hyper-saline Conditions upon Six Estuarine Fish Species from the Coorong and Murray Mouth. South Australian Research and Development Institute (Aquatic Sciences), Adelaide. Adelaide: SARDI. No. 700. 26pp.
MDBA (2013). Approach for Estimating Salt export from the River Murray System to the Southern Ocean, MDBA Publication No 15/13. Murray‒Darling Basin Authority Canberra.
MDBA (2021). Assessment of the Salt export Objective and Salinity Targets for Flow Management 2019–20, MDBA Publication No 04/21. Murray‒Darling Basin Authority Canberra.
Milner, N. J., Solomon, D. J., and Smith, G. W. (2012). The Role of River Flow in the Migration of Adult Atlantic salmon,Salmo salar, through Estuaries and Rivers. Fish. Manag. Ecol. 19, 537–547. doi:10.1111/fme.12011
Noye, J. (1974). The Coorong. Adelaide. Adelaide: Department of Adult Education, University of Adelaide.
Reinfelds, I. V., Walsh, C. T., van der Meulen, D. E., Growns, I. O., and Gray, C. A. (2013). Magnitude, Frequency and Duration of Instream Flows to Stimulate and Facilitate Catadromous Fish Migrations: Australian Bass (Macquaria Novemaculeataperciformes, Percichthyidae). River Res. Applic. 29, 512–527. doi:10.1002/rra.1611
Rowell, K., Flessa, K. W., Dettman, D. L., and Román, M. (2005). The Importance of Colorado River Flow to nursery Habitats of the Gulf Corvina (Cynoscion Othonopterus). Can. J. Fish. Aquat. Sci. 62, 2874–2885. doi:10.1139/f05-193
Salik, K. M., Hashmi, M. Z.-u. -R., Ishfaq, S., and Zahdi, W.-u. -Z. (2016). Environmental Flow Requirements and Impacts of Climate Change-Induced River Flow Changes on Ecology of the Indus Delta, Pakistan. Reg. Stud. Mar. Sci. 7, 185–195. doi:10.1016/j.rsma.2016.06.008
Sharaf El Din, S. H. (1977). Effect of the Aswan High Dam on the Nile Flood and on the Estuarine and Coastal Circulation Pattern along the Mediterranean Egyptian Coast. Limnol. Oceanogr. 22, 194–207. doi:10.4319/lo.1977.22.2.0194
Speer, M. S., Leslie, L. M., MacNamara, S., and Hartigan, J. (2021). From the 1990s Climate Change Has Decreased Cool Season Catchment Precipitation Reducing River Heights in Australia's Southern Murray-Darling Basin. Sci. Rep. 11, 16136. doi:10.1038/s41598-021-95531-4
Stein, E. D., Zimmerman, J., Yarnell, S. M., Stanford, B., Lane, B., Taniguchi-Quan, K. T., et al. (2021). The California Environmental Flows Framework: Meeting the Challenges of Developing a Large-Scale Environmental Flows Program. Front. Environ. Sci. 9, 769943. doi:10.3389/fenvs.2021.769943
Valesini, F. J., Cottingham, A., Hallett, C. S., and Clarke, K. R. (2017). Interdecadal Changes in the Community, Population and Individual Levels of the Fish Fauna of an Extensively Modified Estuary. J. Fish. Biol. 90, 1734–1767. doi:10.1111/jfb.13263
Vermeiren, P., and Sheaves, M. (2014). Predictable Habitat Associations of Four Crab Species Across the Low Intertidal Landscape of a Tropical Estuary over Time. Estuaries and Coasts 38, 285–295. doi:10.1007/s12237-014-9799-0
Webster, I. T. (2010). The Hydrodynamics and Salinity Regime of a Coastal Lagoon - the Coorong, Australia - Seasonal to Multi-Decadal Timescales. Estuarine, Coastal Shelf Sci. 90, 264–274. doi:10.1016/j.ecss.2010.09.007
Ye, Q., Bucater, L., and Short, D. (2011). Coorong Fish Intervention Monitoring during Barrage Releases in 2010/11. Adelaide: South Australian Research and Development Institute Aquatic Sciences. SARDI Publication No. F2011/000309-1. SARDI Research Report Series No. 559.
Ye, Q., Livore, J., Aldridge, K., Giatas, G., Hipsey, M., Joehnk, K., et al. (2016). Monitoring Ecological Response to Commonwealth Environmental Water Delivered to the Lower Murray River in 2013-14. SARDI Adelaide: South Australian Research and Development Institute. Final Report prepared for the Commonwealth Environmental Water Office.
Ye, Q., Giatas, G., Brookes, J., Furst, D., Gibbs, M., Oliver, R., et al. (2020). Commonwealth Environmental Water Office Long-Term Intervention Monitoring Project 2014‐2019: Lower Murray River Technical Report. SARDI Adelaide: Aquatic Sciences. A report prepared for the Commonwealth Environmental Water Office by the South Australian Research and Development Institute.
Keywords: environmental flows, salinity, mulloway (Argyrosomus japonicus), bream, estuary, freshwater flow requirements, salt export
Citation: Brookes JD, Huang P, Zhai SY, Gibbs MS, Ye Q, Aldridge KT, Busch B and Hipsey MR (2022) Environmental Flows to Estuaries and Coastal Lagoons Shape the Salinity Gradient and Generate Suitable Fish Habitat: Predictions From the Coorong, Australia. Front. Environ. Sci. 10:796623. doi: 10.3389/fenvs.2022.796623
Received: 17 October 2021; Accepted: 28 February 2022;
Published: 07 April 2022.
Edited by:
Avril C Horne, The University of Melbourne, AustraliaReviewed by:
Norman Mercado-Silva, Universidad Autónoma del Estado de Morelos, MexicoEleanor Gee, National Institute of Water and Atmospheric Research (NIWA), New Zealand
Copyright © 2022 Brookes, Huang, Zhai, Gibbs, Ye, Aldridge, Busch and Hipsey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Justin D. Brookes, SnVzdGluLmJyb29rZXNAYWRlbGFpZGUuZWR1LmF1
 Justin D. Brookes
Justin D. Brookes Peisheng Huang
Peisheng Huang Sherry Y. Zhai
Sherry Y. Zhai Matthew S. Gibbs
Matthew S. Gibbs Qifeng Ye4
Qifeng Ye4 Matthew R. Hipsey
Matthew R. Hipsey