
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci. , 04 January 2023
Sec. Interdisciplinary Climate Studies
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.1093513
The decay of litter in the air (that is, standing litter) and on the ground is an essential process of litter decomposition for many plant species. However, the contribution of standing litter to litter decomposition (e.g., CO2 emission) is still ambiguous, especially for non-leaf litter. In this study, we examined the CO2 emission from reed litter (Phragmites communis) in coastal wetlands in the Yellow River Delta (YRD), China. The results showed that the soil litter released more CO2 than the standing litter due to its rapid loss of labile organic carbon and high enzyme activities (that is, invertase and β-glucosidase). In contrast, cumulative CO2 emissions from standing litter were equivalent to 56%–70% of those on the soil surface, indicating that CO2 emissions from standing litter cannot be ignored. The sheath litter had the highest cumulative CO2 emission per unit of dry biomass among the three types of litter. Taking into account the biomass per unit area, the non-leaf litter (that is, culm and sheath) emitted more CO2 than leaf litter. On the daily scale, the litter released more CO2 at night than in the daytime, because low air temperature and high relative air humidity at night can help dew formation, accelerating CO2 emission at night. On the seasonal scale, air temperature and relative air humidity were positively related to CO2 emission, leading to rapid CO2 emission in summer and fall. The Q10 value of CO2 emission from standing litter (an average of 1.44) was lower than that of litter on the ground (an average of 2.16) due to a low residual rate of recalcitrant organic carbon in standing litter. Our findings highlight that standing litter decomposition should not be overlooked and suggest that more attention should be paid to the decay of non-leaf litter in the coastal wetland of the YRD.
Wetlands only account for 2%–6% of the global land area, but their carbon storage accounts for 12%–20% of the carbon storage of the terrestrial ecosystem, which is an essential global carbon pool (Kayranli et al., 2010). Coastal wetland is an important type of wetland due to its huge carbon sink and its crucial role in mitigating climate change (Wang et al., 2021). Litter decomposition is a vital component in the carbon cycle of the ecosystem, regulating carbon storage of terrestrial ecosystems and atmospheric CO2 concentration (Wang et al., 2015a; b; Wang et al., 2017; Liu et al., 2021). In the wetland ecosystem, many plants do not fall off the ground immediately after senescence, but stand in the air for a long time, that is, standing litter (Kuehn et al., 2004; Zhang et al., 2014a). Microorganisms, such as fungi, began to invade and decompose litter in the air (Kuehn et al., 2011). Until now, most studies concentrated on the process of decomposition of the litter on the surface of soil or sediment (e.g., Rejmankova and Sirova, 2007; Zhang et al., 2022), while research on the decomposition of standing litter is still insufficient.
The decay of litter in the air is a crucial stage of litter decomposition, which contributes significantly to the complete litter decomposition process (Zhang et al., 2014a). CO2 emission is a component of standing litter decomposition (Wang et al., 2017; Gong et al., 2019). In the wetland ecosystem, CO2 emission from standing litter will potentially contribute to ecosystem CO2 emission (Kuehn and Suberkropp, 1998). Until now, the contribution of CO2 release from standing litter is still uncertain. Previous studies found that standing litter released similar CO2 as litter on the ground (Kuehn and Suberkropp, 1998), even more CO2 than litter on the ground (Gliksman et al., 2018). However, the CO2 emission from standing litter was 12% of that on the soil surface in a subtropical forest ecosystem (Mao et al., 2021), or the contribution of CO2 emission from standing litter to ecosystem respiration can be negligible in a freshwater marsh due to a low proportion of 1.12% (Zhang et al., 2014b). Due to the difference in the quality of the litter of different organs, the CO2 emissions from various types of litter (e.g., culm and sheath) are also different, and the CO2 emission rate of standing leaf and sheath litter was higher than that of culm litter (Kuehn et al., 1999 and 2004; Evans et al., 2020). If differences in the biomass of different plant organs are taken into account, their impacts on CO2 emissions from litter, especially standing litter, will be more diversified. Therefore, CO2 emissions from standing litter are of great significance to further elucidate the clarity of gas emissions from ecosystems.
The quality of organic carbon (OC) in the litter was a vital factor influencing the temperature sensitivity (Q10) of CO2 emissions. So far, the relationships between the quality of the OC and the Q10 value of CO2 emissions are still uncertain. Fierer et al. (2005) found that a higher quality of OC resulted in a lower temperature sensitivity of OC decomposition. Some studies found that recalcitrant OC fractions had higher Q10 values than labile fractions (Davidson and Janssens, 2006; Moinet et al., 2020). Even studies showed that the decomposition of recalcitrant OC was not sensitive to increasing temperature (Giardina and Ryan, 2000). The difference in litter decomposition in the air and on the ground may change the proportion of recalcitrant and labile OC in the litter, thus resulting in the various responses of CO2 emission to temperature at different decomposition interfaces. However, it is still unknown whether the temperature sensitivity (Q10) of CO2 emissions from standing litter is similar to that on the ground.
The Yellow River Delta (YRD) is one of the youngest wetlands in the warm temperate zone of China (Qin et al., 2010). Reed (Phragmites communis) is one of the major plant species in coastal wetlands of the YRD. Reed litter, especially sheath and culm litter, can remain in the air for several months or even longer after senescence. During this time, the litter has started to decompose (that is, the decomposition of standing litter). However, it is still undetermined whether the standing litter of reeds released CO2 emissions similar to those on the ground in the YRD, especially for non-leaf litter (i.e., sheath and culm). The objectives of this study are 1) to investigate the difference in CO2 emission and its temperature sensitivity (Q10) between standing litter and litter on the ground, and 2) to examine the difference in litter decomposition between leaf, sheath, and culm litter. This study is expected to better understand the characteristics of standing litter decomposition in coastal wetlands and provide scientific evidence for the management of carbon pools in coastal wetlands.
The Yellow River Delta is located in Shandong province, China, with an area of 12,038 km2. This area belongs to a warm temperate continental monsoon climate with a mean air temperature of 11.7°C–12.8°C, an annual evaporation of 1,900–2,400 mm and an annual precipitation of 530–630 mm. About 70% of the rain occurs between July and September. The soil types are Calcaric Fluvisols, Gleyic Solochaks, and Salic Fluvisols (FAO; Guan et al., 2019; Lu et al., 2021). The research site was located in the southern part of the YRD Nature Reserve, a nontidal wetland. The main vegetation species were Tamarix Chinensis Lour., Phragmites communis (Cav.) Trin. ex Stued. and Suaeda salsa (Linn.) Pall. At this site, the soil does not flood for most of the year, but it is easy to temporarily flood after heavy rainfall in summer and fall. According to the field investigation, dead reeds do not fall directly to the soil surface and their aboveground part can stand until the end of the next growing season, especially for the culm and sheath litter, resulting in a standing litter decomposition process.
At the end of October 2020, three sampling points were established in the reed growing area to collect the aboveground litter. In the laboratory, the reed sample was divided into leaf, sheath, and culm litter. The litter sample was cut to a length of approximately 5 cm and oven-dried to constant weight at 70°C after cleaning with a soft brush. Ten grams of the sample were placed in a nylon litter bag (20 cm × 20 cm) with a mesh size of 1 mm. In this experiment, 96 litterbags were prepared (three types of litter × four repetitions × two decomposition interfaces × four sample dates). In November 2020, litterbags were placed at the original sampling point. For each type of litterbag, sixteen litterbags were placed on the soil surface and fixed with nails, and the others were suspended in the air. According to Zhang et al. (2014a), the litterbags in the air were fixed on a horizontal nylon net at a height of 1 m, equivalent to 3/4 of the mean height of the reed.
Litter CO2 emission was measured on the 90th (that is, in winter), 180th (that is, in spring), 270th (that is, in summer), and 360th (that is, in autumn) days. On each sampling date, the CO2 emission rate was tested for a whole day at 14:00, 18:00, 24:00, next at 6:00, 10:00 and 14:00. Before sampling, four litterbags of each litter type were collected. The dust on the surface of the litter was removed with a soft brush and then the sample was placed in a new and clean litterbag (20 cm × 20 cm). A PVC pipe wrapped with thermal insulation was used to measure CO2 emissions. This pipe has a diameter of 25 cm and a height of 30 cm, with one end closed and the other covered. A three-way valve and a temperature probe were installed on the cover of the PVC pipe. The new litterbag with the sample was placed in the PVC pipe and the pipe was sealed using a lid for 30 min. Gas samples with a volume of 50 ml were collected at the beginning and end of sealing, respectively. Each gas sample was stored in a vacuum bag. The CO2 concentration was measured by gas chromatography (Agilent 7890A, United States). The difference in CO2 concentration at the beginning and end of sealing is the CO2 emitted by the litter sample. When CO2 emissions were tested, air temperature and relative air humidity were measured in situ on the surface of the soil and in the air (that is, at a height of 1 m).
In the laboratory, the fresh litter was weighed and divided into two parts. A part of the fresh litter was oven-dried at 70°C to test the moisture content. The dry weight of each fresh sample was used to calculate the CO2 emission rate. The other part of the fresh litter sample was cut to <2 mm and was used to test the β-glucosidase and invertase activities using the method of Guan (1986). The concentrations of labile (LOC) and recalcitrant (ROC) OC in the litter sample were measured using the sample at the beginning (i.e., day 0) and end of experiment (i.e., day 360) and an acid hydrolysis approach (Rovira and Vallejo, 2002). The initial content of OC was measured by the dry combustion method using a Multi N/C 2100 analyzer (Analytik Jena, Germany). The initial content of total phosphorus (TP) was measured by the ammonium molybdate method after H2SO4-H2O2 oxidation (Kuo, 1996). The initial content of total nitrogen (TN) was determined by Kjeldahl digestion using a Kjeltec Auto Analyzer (Foss 8,400, Denmark).
The CO2 emission rate and the cumulative CO2 emission were calculated using the method of Tao et al. (2022). Cumulative CO2 emissions from 18:00 to 6:00 the next day were defined as CO2 emissions at night, and cumulative CO2 emissions from 6:00 to 18:00 were defined as CO2 emissions in the daytime.
Where R represents the CO2 emission rate, mg kg−1 h−1. P represents the standard atmospheric pressure, Pa. V represents the volume of the PVC pipe, cm3. c represents the difference in CO2 concentration at the beginning and end of sealing, ppm. t represents the sealing time, 0.5 h. r represents the universal gas constant. T represents the absolute air temperature, K. M is the molecular mass of CO2, g mol−1 m represents the dry weight of the litter sample, kg.
Where Rn and Rn+1 represent the CO2 emission rate of any two adjacent sampling times, mg kg−1 h−1. (Tn+1 − Tn) represents the time intervals between any two adjacent samples, h.
The temperature sensitivity (Q10) of CO2 emission was calculated following the method of Luo et al. (2001).
Where R represents the CO2 emission rate, mg kg−1 h−1. T represents the air temperature, °C. A and k represent constants.
The LOC loss ratio (%) and the residual ROC ratio (%) were calculated as follows.
Where M0 and M360 represent the litter mass on days 0 and 360, g. L0 and L360 represent the LOC concentration on days 0 and 360, mg g−1. R0 and R360 represent the ROC concentration on days 0 and 360, mg g−1.
The Shapiro-Wilk test was used to test the normality of data, and nonnormal data were logarithmically transformed before analysis. The difference in cumulative CO2 emission, enzyme activities, Q10 value, LOC loss ratio, and residual ROC ratio were compared separately using a one-way analysis of ANOVA with Tukey’s HSD test (Tamhane’s test when equal variances were not assumed) at a 95% confidence level. Data on CO2 production were also analyzed using a three-way analysis of variance (ANOVA) with sample date, decomposition interface and litter types as independent factors. Data on air temperature and relative air humidity were analyzed using a two-way analysis of variance (ANOVA) with sample date and decomposition interface as independent factors. Pearson’s correlation coefficients between CO2 emission and factors (that is, air temperature, relative air humidity, enzyme activities, and LOC loss ratio) and the relationship coefficients between the Q10 value and residual ROC ratio were also calculated. All statistical analyzes were conducted using SPSS 25.0 software (SPSS Inc. United States) and the figures were drawn using Origin 9.0 (OriginLab, Northampton, MA, United States).
Litter types, decomposition interfaces, and sample date significantly affected cumulative CO2 emission (Supplementary Table S1; p <0.001). Cumulative CO2 emissions from standing litter (that is, leaf, culm, and sheath) were less than those on the surface of the soil (Supplementary Figure S1; Figure 1A; p <0.001). The cumulative CO2 emissions of the standing leaf, culm, and sheath were 56.76%, 66.67%, and 69.19% of that on the soil surface. For standing litter, the cumulative CO2 emission from sheath litter is 1.33 and 1.36 times that of leaf and culm litter (p <0.05). On the surface of the soil, the cumulative CO2 emission of the sheath litter was 1.30 times the culm litter (p <0.05) and was similar to the leaf litter (Supplementary Figure S1; Figure 1A).
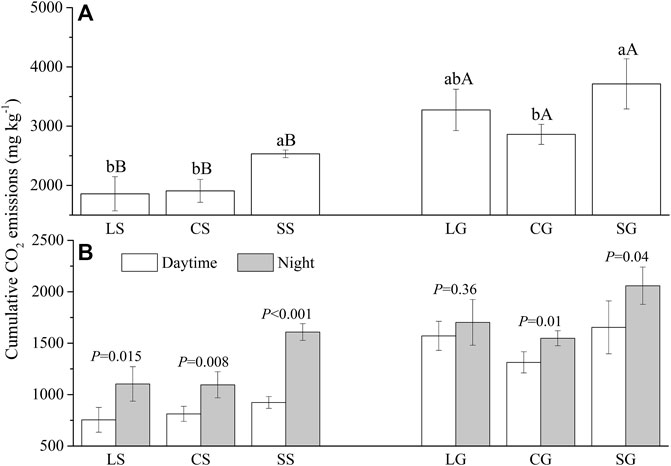
FIGURE 1. Cumulative CO2 emissions (A) from standing litter and litter on the ground and their distribution at night and in the day (B). LS, CS, and SS represent standing litter of leaf, culm, and sheath. LG, CG, and SG represent leaf, culm, and sheath litter on the ground. (A) Different lowercase letters represent a significant difference between three types of litter (p <0.05). Different capital letters represent the significant difference of the same litter type between standing litter and litter on the ground (p <0.05). (B) p values represent a significant difference between daytime and night (p <0.05).
On the daily scale, cumulative CO2 emission from the culm and sheath litter at night was greater than in the daytime at the two decomposition interfaces (p <0.05). Cumulative CO2 emissions from standing leaf litter at night were also higher than in the daytime (p <0.05). However, cumulative CO2 emission from leaf litter on the soil surface did not differ between daytime and night (Figure 1B). On the seasonal scale, the CO2 emission from standing litter on the 270th day was greater than on other sample dates (p <0.05). Similarly, CO2 emission on the 270th and 360th day was higher than on other sample dates for litter on the soil surface (Figure 1A; p <0.05).
The litter on the ground had a larger temperature sensitivity (Q10) of CO2 emission than the standing litter (p =0.004). For standing litter, the Q10 value of CO2 emission ranged from 1.34 to 1.60, with an average of 1.44. However, the Q10 value of CO2 emission from the litter on the ground ranged from 2.03 to 2.24, with an average of 2.16 (Figure 2).
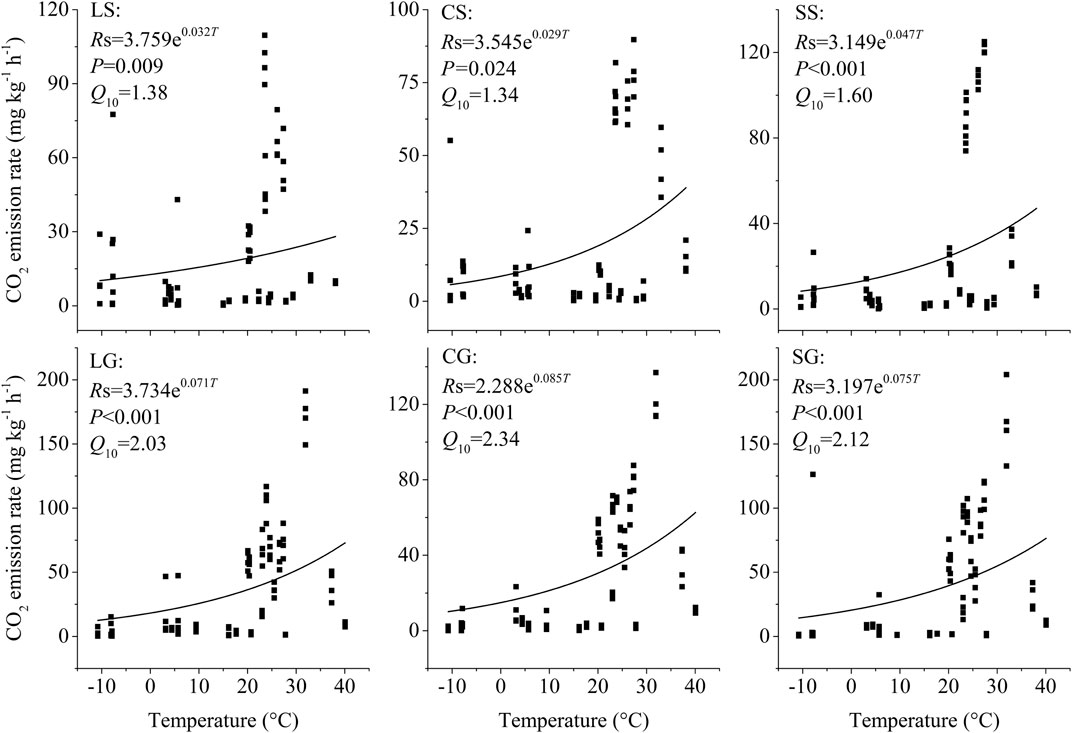
FIGURE 2. Temperature sensitivity (Q10) of CO2 emissoins from standing litter and litter on the ground. LS, CS, and SS represent standing litter of leaf, culm, and sheath. LG, CG, and SG represent leaf, culm, and sheath litter on the ground. Rs represents the CO2 emission rate. T represents the air temperature.
Leaf litter had larger β-glucosidase and invertase activities than non-leaf litter at both decomposition interfaces (p <0.05), and these enzyme activities of litter on the soil surface were greater than those in the air (Supplementary Figure S2; p <0.05). β-glucosidase and invertase activities were positively correlated with cumulative CO2 emission (Figure 3; p <0.05).
For standing litter, the LOC loss ratio of sheath litter was greater than that of leaf and culm litter (Figure 4A; p <0.05). Sheath litter had a higher LOC loss ratio than culm litter (Figure 4A; p <0.05), but had a similar LOC loss ratio to leaf litter on the soil surface. Overall, the litter on the soil surface had a higher LOC loss ratio than standing litter (Figure 4A; p <0.05). The LOC loss ratio was positively related to cumulative CO2 emission (Figure 4B; p <0.001). For leaf and culm litter, the residual ROC ratio on the soil surface was higher than in the air (p < 0.05). Sheath litter on the soil surface had a similar residual ROC ratio to standing litter (Figure 5A). Furthermore, the residual ROC ratio was positively related to the Q10 value (Figure 5B; p =0.02).
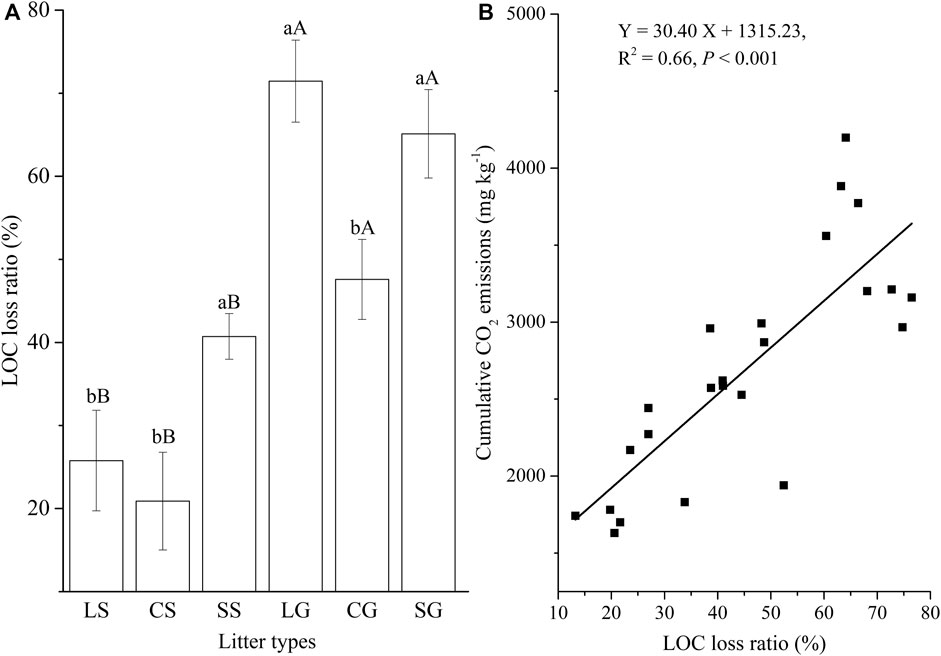
FIGURE 4. Loss ratio of labile organic carbon (LOC) (A) and its relationship with cumulative CO2 emissions (B). LS, CS, and SS represent standing litter of leaf, culm, and sheath. LG, CG, and SG represent leaf, culm, and sheath litter on the ground. Different lowercase letters represent significant difference between three types of litter (p <0.05). Different capital letters represent the significant difference of the same litter type between standing litter and litter on the ground (p <0.05).
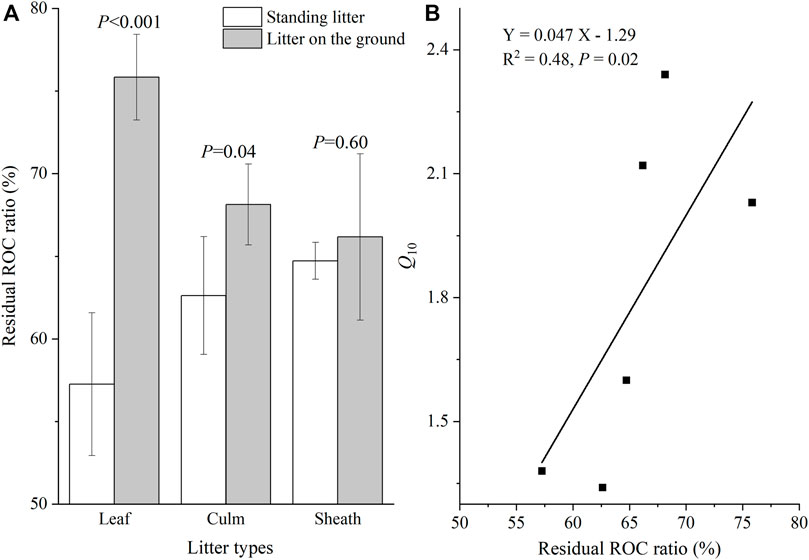
FIGURE 5. Recalcitrant organic carbon (ROC) residual ratio (A) and its relationship with the Q10 value (B).
The sample date significantly affected the air temperature and relative air humidity (Supplementary Table S2; p <0.001). Air temperature and relative air humidity on days 270 and 360 were higher than those on days 90 and 180. The decomposition interface did not influence the air temperature and relative air humidity. The air temperature in the daytime was higher than at night, but the relative air humidity was higher at night than in the daytime (Supplementary Figure S3; p < 0.05).
On the daily scale, the CO2 emission rate was negatively related to air temperature and positively associated with relative air humidity on the 90th, 180th and 270th day for standing litter, and similar relationships were observed on the 180th and 270th day for litter on the soil surface (Table 1). However, the CO2 emission rate had a positive relationship with the air temperature and a negative relationship with the relative air humidity on day 360 (Table 1). On the seasonal scale, air temperature and relative air humidity were positively related to cumulative CO2 emissions (Table 2; p <0.001).
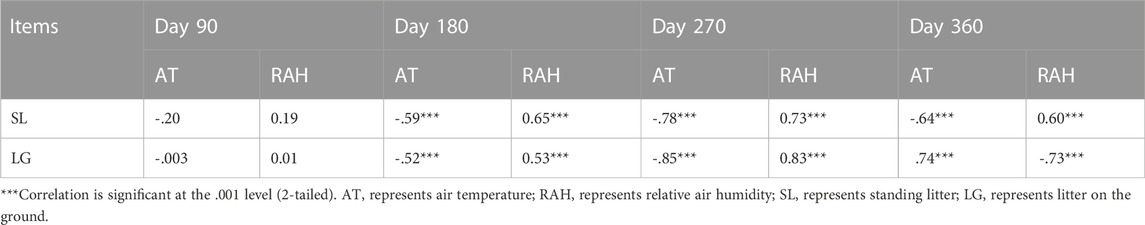
TABLE 1. Relationship between CO2 emission rate and environmental factors on the daily scale (n = 72).
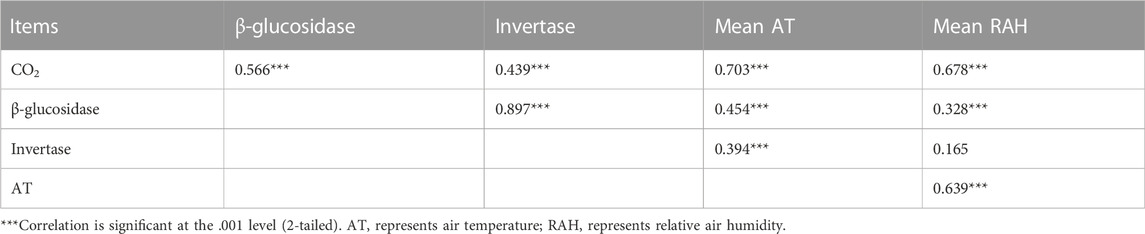
TABLE 2. Relationship between cumulative CO2 emissions, enzyme activities, and environmental factors on the seasonal scale (n = 96).
Leaf litter had higher content of TN and TP than non-leaf litter, while non-leaf litter had higher content of total OC than leaf litter (Supplementary Table S3; p <0.05). Culm litter had the highest C/N, C/P ratios and the smallest N/P ratio among three types of litter (Supplementary Table S3; p <0.05). Cumulative CO2 emission was not related to the contents of OC, TN, TP and stoichiometric ratios of C/N, C/P, and N/P (Supplementary Table S4).
Standing litter plays a vital role in CO2 emission from litter. However, the contribution of standing litter to litter CO2 emissions is still uncertain. Standing litter can release more (Gliksman et al., 2018), or similar (Kuehn and Suberkropp, 1998), or less (Zhang et al., 2014b; Mao et al., 2021) CO2 than litter on the ground. In this study, although standing litter released less CO2 than litter on the soil surface (Supplementary Figure S1; Figure 1), it was equivalent to 56%–70% of that on the soil surface, indicating that CO2 emission from standing reed litter should not be ignored in the YRD.
The microorganism is a vital factor driving litter decomposition and CO2 emission (Evans et al., 2020; Logan et al., 2021), and fungal decomposers had colonized standing litter even at the beginning of plant senescence (Newell, 2002; Chimney and Pietro, 2006). Enzymes (e.g., β-glucosidase) were involved in the decomposition and transformation of OC (Sinsabaugh, 2010; Song et al., 2017; Miao et al., 2020; Chen et al., 2022). Additionally, labile organic substrates stimulated microbial activity and accelerated litter decomposition (Kuzyakov et al., 2000; de Graaff et al., 2010). Higher β-glucosidase activity (e.g., Song et al., 2017; Tao et al., 2022) and LOC concentrations (Don and Kalbitz, 2005; Wang L. et al., 2015) resulted in faster litter decomposition or CO2 emission. Compared to litter on the surface of soil or sediment, standing litter could not absorb water and immobilize nutrients from the soil by microbes (He et al., 2013; Zhang et al., 2021), reducing microbial growth and its activity. In this study, the litter on the surface of the soil had larger activities of β-glucosidase and invertase than the standing litter, especially on days 180 and 360 (Supplementary Figure S2). Such enzyme activities were positively related to CO2 emission (Figure 3), resulting in rapid CO2 emission. Moreover, the LOC loss ratio of the litter on the soil surface was higher than that in the air (Figure 4), which further explained the rapid release of CO2 from the litter on the soil surface.
The types of litter had a significant effect on CO2 emission rates. Standing sheath litter had the highest cumulative CO2 emission per unit mass of dry biomass among the three types of litter. The leaf and sheath litter had higher cumulative CO2 emissions per unit mass of dry biomass than the culm litter on the soil surface (Figure 1). Usually, LOC decomposed more quickly than ROC (see, e.g., Kuzyakov et al., 2000; de Graaff et al., 2010; Tao et al., 2013). In this experiment, the sheath had the most considerable LOC loss ratio among the three types of standing litter. Leaf and sheath litter had a greater LOC loss ratio than the culm litter on the soil surface. The LOC loss ratio was positively related to cumulative CO2 emission (Figure 4), indicating that rapid LOC decomposition of leaf and sheath litter accelerated CO2 emission. Previous studies found that litter stoichiometry did not constrain the litter decomposition (Aerts et al., 2012), and carbon quality rather than stoichiometry controlled the litter decomposition (Hättenschwiler and Jørgensen, 2010). In this experiment, non-significant relationships between cumulative CO2 emission and stoichiometric ratios were observed (Supplementary Table S4). Thus, we concluded that OC quality rather than stoichiometry was the major factor adjusting CO2 emission from litter in the YRD.
A study in the adjacent area showed that the biomass of the reed leaf, culm, and sheath was 1264.32, 3667.58, and 1123.78 g m−2 (Zan et al., 2011). Based on the results of Zan et al. (2011), we estimated that cumulative CO2 emissions of standing culm and sheath litters were 2.97 and 1.21 times that of leaf litter, while cumulative CO2 emission from culm and sheath litter was 2.54 and 1.01 times that of leaf litter on the soil surface in the YRD. In other words, the reed litter without leaves in the YRD contributed about 80% of total CO2 emissions from the litter regardless of the decomposition interface. Therefore, non-leaf litter (e.g., culm and sheath) of reeds may be the main contributor to CO2 emissions from litter in the YRD. This study highlights the importance of CO2 emission from non-leaf litter, and subsequent studies should focus on the decomposition of non-leaf litter.
Air temperature and relative air humidity were vital factors in adjusting litter CO2 emission (Zhang et al., 2014b; Wang et al., 2017), but their effects on litter CO2 emission varied at different time scales. On the daily scale, we found that the litter released more CO2 emissions at night than in the day, especially for the standing litter (Figure 1B). The previous study observed that, in Mediterranean grasslands, water vapor from the atmosphere stimulated microbial activity and litter decomposition at night (Dirks et al., 2010; Gliksman et al., 2017), and similar results were found in a semi-arid grassland ecosystem due to outstanding absorption of water from the atmosphere overnight for standing litter (Wang et al., 2017). Microbes are mainly r-strategy organisms with short lifespans, responding rapidly to changes in water supply (Jacobson et al., 2015). High air humidity at night was positively associated with microbial activity, especially for standing litter (Wang et al., 2017). Similarly, high relative air humidity at night and dew condensation were observed to adjust microbial activity (McHugh et al., 2015) and led to rapid CO2 emission (Wang et al., 2017).
Previous study found that non-rainfall moisture, such as humidity and dew, was a key driver of microbial respiration from standing litter (Evans et al., 2020). When there was no precipitation, the maximum rate of CO2 emission occurred in the evening and early morning when dew condensed (Kuehn et al., 2004). Moreover, the total PLFAs in litter were positively related to relative humidity at night, especially for the standing litter (Wang et al., 2017). In the coastal wetland of this research area, relative air humidity at night exceeded 70% and even approached 100%, higher than in the daytime. The air temperature at night was lower than in the daytime (Supplementary Figure S3). Higher air humidity and lower air temperature at night may help dew condensation, thereby promoting microbial respiration. Our investigation found that the maximum rate of CO2 emission occurred from 18:00 to 6:00 (Supplementary Figure S1). Our results also showed that, on the daily scale, the CO2 emission rate had a negative relationship with air temperature and a positive relationship with relative air humidity (Table 1). Therefore, we speculate that lower air temperature and higher relative air humidity at night may help dew formation, providing moisture for microorganisms, and thus accelerating CO2 emission.
It should be noted that, on the daily scale, the rate of CO2 emission from soil litter on day 360 was positively related to the air temperature and negatively associated with relative air humidity, in conflict with the results on the 180th and 270th days (Table 1). When collecting samples on day 360, precipitation caused temporary flooding (about 0–3 cm depth) on the soil surface, which may alleviate the limitation of moisture on microbial respiration. Moreover, an incubation experiment found that increasing temperature accelerated CO2 emission from water-saturated litter (Zhang et al., 2014b). Thus, we reasoned that, under the condition of sufficient water, a higher air temperature in the daytime might stimulate microbial activity and microbial respiration compared to that at night on day 360.
Fungi made up the majority of the microorganisms in the standing litter (Findlay et al., 2002). Temperature was an important factor affecting seasonal dynamics of fungal biomass on standing litter (Verma et al., 2003). The biomass of fungi on litter increased exponentially with temperature (Suberkropp and Weyers, 1996). On the seasonal scale, cumulative CO2 emission and β-glucosidase activity were positively correlated with air temperature and relative air humidity (Table 2; p <0.001). Higher mean air temperature and relative air humidity (Supplementary Figure S3) in summer (i.e., the 270th day) and autumn (i.e., the 360th day) can be conducive to stimulating enzyme activity and CO2 emission. Overall, this study emphasized the importance of microenvironment fluctuations that influence litter CO2 emissions at different time scales.
The quality of OC can affect the Q10 of CO2 emissions, but their relationships are still uncertain. The earlier results showed that the ROC decomposition rate did not vary with temperature (Giardina and Ryan, 2000) or had a similar response to varied temperatures as the LOC decomposition (Fang et al., 2005). Since then, many studies have observed that ROC decomposition was more sensitive to temperature increase than LOC decomposition (see, e.g., Davidson and Janssens, 2006; Wang et al., 2018). We found that the soil litter had a higher Q10 value than the standing litter (Figure 2; p =0.004).
Compared to standing litter, the higher microbial activity and fungal biomass of litter on the soil surface triggered rapid decomposition (He et al., 2013; Zhang et al., 2015; Wang et al., 2017), which can result in an abundant accumulation of litter ROC fractions. A previous study in the YRD also found an increased proportion of ROC in reed litter after 2 years of in situ decomposition (Tao et al., 2019). In this study, the ground litter accumulated more ROC than standing litter (Figure 5A). According to the hypothesis of ‘carbon quality temperature’ (Davidson and Janssens, 2006), the abundant accumulation of ROC in soil litter may require considerable activation energy for the decomposition of OC, thus increasing the temperature sensitivity. In addition, the positive relationship between residual ROC ratio and the Q10 value (Figure 5B) further testified to the speculation mentioned above.
Our results suggest that, in the coastal wetlands of the YRD, although cumulative CO2 emission of standing reed litter was lower than that on the soil surface, it was equivalent to 56%–70% of that on the soil surface, indicating a non-negligible contribution of standing litter to litter CO2 emission. Taking into account the biomass of leaf and non-leaf organs, non-leaf litter (that is, sheath and culm) contributed about 80% of the total CO2 emission from the litter. CO2 emission at night was greater than in the daytime for three types of litter, because low air temperature and high relative humidity at night helped dew formation, thus stimulating microbial respiration. Litter on the soil surface had a higher Q10 value of CO2 emission than standing litter due to the high residual ratio of ROC. Our results emphasize the importance of CO2 emission from standing reed litter, especially for non-leaf litter.
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding author.
BT and JW designed the study, performed the research, analyzed data, and wrote the paper. QC performed the research. YJ and BZ wrote the paper.
This research was supported by the Shandong Province Natural Science Foundation, China (ZR2020MD004), the Liaocheng University Experimental Technology Foundation, China (26322170123), the Research Project on Teaching Reform in Universities in Shandong Province (M2018X052), and the Liaocheng ‘Water City Talents’ project: cooperation in the field of resources and environment (K19LC0301).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1093513/full#supplementary-material
Aerts, R., van Bodegom, P. M., and Cornelissen, H. C. (2012). Litter stoichiometric traits of plant species of high-latitude ecosystems show high responsiveness to global change without causing strong variation in litter decomposition. New Phytol. 196, 181–188. doi:10.1111/j.1469-8137.2012.04256.x
Chen, J., Zhang, Y., Kuzyakov, Y., Wang, D., and Olesen, J. E. (2022). Challenges in upscaling laboratory studies to ecosystems in soil microbiology research. Glob. change Biol. 2022, 16537. doi:10.1111/gcb.16537
Chimney, M. J., and Pietro, K. C. (2006). Decomposition of macrophyte litter in a subtropical constructed wetland in south Florida (USA). Ecol. Eng. 27, 301–321. doi:10.1016/j.ecoleng.2006.05.016
Davidson, E. A., and Janssens, I. A. (2006). Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440 (7081), 165–173. doi:10.1038/nature04514
De Graaff, M. A., Classen, A. T., Castro, H. F., and Schadt, C. W. (2010). Labile soil carbon inputs mediate the soil microbial community composition and plant residue decomposition rates. New Phytol. 188, 1055–1064. doi:10.1111/j.1469-8137.2010.03427.x
Dirks, I., Navon, Y., Kanas, D., Dumbur, R., and Grunzweig, J. M. (2010). Atmospheric water vapor as driver of litter decomposition in Mediterranean shrubland and grassland during rainless seasons. Glob. Change Biol. 16, 2799–2812. doi:10.1111/j.1365-2486.2010.02172.x
Don, A., and Kalbitz, K. (2005). Amounts and degradability of dissolved organic carbon from foliar litter at different decomposition stages. Soil Biol. Biochem. 37, 2171–2179. doi:10.1016/j.soilbio.2005.03.019
Evans, S., Todd-Brown, K. E. O., Jacobson, K., and Jacobson, P. (2020). Non-rainfall moisture: A key driver of microbial respiration from standing litter in arid, semiarid, and mesic grasslands. Ecosystems 23 (6), 1154–1169. doi:10.1007/s10021-019-00461-y
Fang, C. M., Smith, P., Moncrieff, J. B., and Smith, J. U. (2005). Similar response of labile and resistant soil organic matter pools to changes in temperature. Nature 433, 57–59. doi:10.1038/nature03138
Fierer, N., Craine, J. M., Mclauchlan, K., and Schimel, J. P. (2005). Litter quality and the temperature sensitivity of decomposition. Ecology 86 (2), 320–326. doi:10.1890/04-1254
Findlay, S. E. G., Dye, S., and Kuehn, K. A. (2002). Microbial growth and nitrogen retention in litter of Phragmites australis compared to Typha angustifolia. Wetlands 22, 616–625. doi:10.1672/0277-5212(2002)022[0616:mganri]2.0.co;2
Giardina, C. P., and Ryan, M. G. (2000). Evidence that decomposition rates of organic carbon in mineral soil do not vary with temperature. Nature 404 (6780), 858–861. doi:10.1038/35009076
Gliksman, D., Navon, Y., Dumbur, R., Haenel, S., and Grunzweig, J. M. (2018). Higher rates of decomposition in standing vs. surface litter in a Mediterranean ecosystem during the dry and the wet seasons. Plant Soil 428, 427–439. doi:10.1007/s11104-018-3696-4
Gliksman, D., Rey, A., Seligmann, R., Dumbur, R., Sperling, O., Navon, Y., et al. (2017). Biotic degradation at night, abiotic degradation at day: Positive feedbacks on litter decomposition in drylands. Glob. Change Biol. 23 (4), 1564–1574. doi:10.1111/gcb.13465
Gong, C., Song, C. C., Zhang, D., and Zhang, J. S. (2019). Litter manipulation strongly affects CO2 emissions and temperature sensitivity in a temperate freshwater marsh of northeastern China. Ecol. Indic. 97, 410–418. doi:10.1016/j.ecolind.2018.10.021
Guan, B., Xie, B. H., Yang, S., Hou, A. X., Chen, M., and Han, G. X. (2019). Effects of five years’ nitrogen deposition on soil properties and plant growth in a salinized reed wetland of the Yellow River Delta. Ecol. Eng. 136, 160–166. doi:10.1016/j.ecoleng.2019.06.016
Hättenschwiler, S., and Jørgensen, H. B. (2010). Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J. Ecol. 98, 754–763. doi:10.1111/j.1365-2745.2010.01671.x
He, X. B., Lin, Y. H., Han, G. M., and Ma, T. (2013). Litterfall interception by understorey vegetation delayed litter decomposition in Cinnamomum camphora plantation forest. Plant Soil 372, 207–219. doi:10.1007/s11104-013-1734-9
Jacobson, K., van Diepeningen, A., Evans, S., Fritts, R., Gemmel, P., Marsho, C., et al. (2015). Non-rainfall moisture activates fungal decomposition of surface litter in the Namib sand sea. PLOS ONE 10, e0126977. doi:10.1371/journal.pone.0126977
Kayranli, B., Scholz, M., Mustafa, A., and Hedmark, A. (2010). Carbon storage and fluxes within freshwater wetlands: A critical review. Wetlands 30 (1), 111–124. doi:10.1007/s13157-009-0003-4
Kuehn, K. A., and Suberkropp, K. (1998). Diel fluctuations in rates of CO2 evolution from standing dead leaf litter of the emergent macrophyte Juncus effuses. Aquat. Microb. Ecol. 14 (2), 171–182. doi:10.3354/ame014171
Kuehn, K. A., Gessner, M. O., Wetzel, R. G., and Suberkropp, K. (1999). Decomposition and CO2 evolution -from standing litter of the emergent macrophyte Erianthus giganteus. Microb. Ecol. 38 (1), 50–57. doi:10.1007/s002489900154
Kuehn, K. A., Ohsowski, B. M., Francoeur, S. N., and Neely, R. K. (2011). Contributions of fungi to carbon flow and nutrient cycling from standing dead Typha angustifolia leaf litter in a temperate freshwater marsh. Limnol. Oceanogr. 56 (2), 529–539. doi:10.4319/lo.2011.56.2.0529
Kuehn, K. A., Steiner, D., and Gessner, M. O. (2004). Diel mineralization patterns of standing-dead plant litter: Implications for CO2 flux from wetlands. Ecology 85 (9), 2504–2518. doi:10.1890/03-4082
Kuo, S. (1996). “Phosphorus,” in Methods of soil analysis. Part 3. Chemical methods. Editor D. L. Sparks (Madison: Soil Science Society of America and American Society of Agronomy), 869–919.
Kuzyakov, Y., Friedel, J. K., and Stahr, K. (2000). Review of mechanisms and quantification of priming effects. Soil Biol. Biochem. 32, 1485–1498. doi:10.1016/s0038-0717(00)00084-5
Liu, Y., Zhao, C., Guo, J., Zhang, L., Xuan, J., Chen, A., et al. (2021). Short-term phosphorus addition augments the effects of nitrogen addition on soil respiration in a typical steppe. Sci. Total Environ. 761, 143211. doi:10.1016/j.scitotenv.2020.143211
Logan, J. R., Jacobson, K. M., Jacobson, P. J., and Evans, S. E. (2021). Fungal communities on standing litter are structured by moisture type and constrain decomposition in a hyper-arid grassland. Front. Microbiol. 12, 596517. doi:10.3389/fmicb.2021.596517
Lu, G. R., Xie, B. H., Cagle, G. A., Wang, X. H., Han, G. X., Wang, X. J., et al. (2021). Effects of simulated nitrogen deposition on soil microbial community diversity in coastal wetland of the Yellow River Delta. Sci. Total Environ. 757, 143825. doi:10.1016/j.scitotenv.2020.143825
Luo, Y., Wan, S., Hui, D., and Wallace, L. L. (2001). Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413 (6856), 622–625. doi:10.1038/35098065
Mao, R., Wu, P. P., Xu, J. W., Wan, S. Z., and Zhang, Y. (2021). Leaf litter decomposition in the air should not be ignored in subtropical plantations of China. For. Ecol. Manag. 499, 119614. doi:10.1016/j.foreco.2021.119614
McHugh, T. A., Morrissey, E. M., Reed, S. C., Hungate, B. A., and Schwartz, E. (2015). Water from air: An overlooked source of moisture in arid and semi-arid regions. Sci. Rep. 5, 13767. doi:10.1038/srep13767
Miao, Y., Liu, M., Xuan, J., Xu, W., Wang, S., Miao, R., et al. (2020). Effects of warming on soil respiration during the non-growing seasons in a semiarid temperate steppe. J. Plant Ecol. 13, 288–294. doi:10.1093/jpe/rtaa013
Moinet, G. Y. K., Moinet, M., Hunt, J. E., Rumpel, C., Chabbi, A., and Millard, P. (2020). Temperature sensitivity of decomposition decreases with increasing soil organic matter stability. Sci. Total Environ. 704, 135460. doi:10.1016/j.scitotenv.2019.135460
Newell, S. Y. (2002). Fungal biomass and productivity in standing-decaying leaves of black needlerush (Juncus roemerianus). Mar. Freshw. Res. 52, 249–255. doi:10.1071/mf00068
Qin, Y., Yang, Z. F., and Yang, W. (2010). A novel index system for assessing ecological risk under water stress in the Yellow River Delta wetland. Procedia Environ. Sci. 2, 535–541. doi:10.1016/j.proenv.2010.10.058
Rejmankova, E., and Sirova, D. (2007). Wetland macrophyte decomposition under different nutrient conditions: Relationships between decomposition rate, enzyme activities and microbial biomass. Soil Biol. Biochem. 39, 526–538. doi:10.1016/j.soilbio.2006.08.022
Rovira, P., and Vallejo, V. R. (2002). Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: An acid hydrolysis approach. Geoderma 107 (1-2), 109–141. doi:10.1016/s0016-7061(01)00143-4
Sinsabauth, R. L. (2010). Phenol oxidase, peroxidase and organic matter dynamics of soil. Soil Biol. Biochem. 42, 391–404. doi:10.1016/j.soilbio.2009.10.014
Song, Y. Y., Song, C. C., Meng, H. N., Swarzenski, C. M., Wang, X. W., and Tan, W. W. (2017). Nitrogen additions affect litter quality and soil biochemical properties in a peatland of Northeast China. Ecol. Eng. 100, 175–185. doi:10.1016/j.ecoleng.2016.12.025
Suberkropp, K., and Weyers, H. (1996). Application of fungal and bacterial production methodologies to decomposing leaves in streams. Appl. Environ. Microbiol. 62, 1610–1615. doi:10.1128/aem.62.5.1610-1615.1996
Tao, B. X., Chen, Q. H., Wang, J. D., Zhang, B. H., Yuan, H. Y., and Chen, Y. J. (2022). Fertile island” of Tamarix Chinensis accelerated the carbon decomposition in the coastal wetlands of the Yellow River Delta, China. Catena 211, 106034. doi:10.1016/j.catena.2022.106034
Tao, B. X., Song, C. C., and Guo, Y. D. (2013). Short-term effects of nitrogen additions and increased temperature on wetland soil respiration, Sanjiang Plain, China. Wetlands 33, 727–736. doi:10.1007/s13157-013-0432-y
Tao, B. X., Zhang, B. H., Dong, J., and Liu, C. Y. (2019). Effect of organic carbon quality on the litter decomposition and temperature sensitivity of Phragmites australis in the Yellow River Delta, China. Acta Ecol. Sin. 39 (15), 5564–5572. (In Chinese with English abstract).
Verma, B., Robarts, R. D., and Headley, J. V. (2003). Seasonal changes in fungal production and biomass on standing dead Scirpus lacustris litter in a northern prairie wetland. Appl. Environ. Microbiol. 69 (2), 1043–1050. doi:10.1128/aem.69.2.1043-1050.2003
Wang, D., Liu, Y., Shang, Z. H., Tian, F. P., Wu, G. L., Warrington, D., et al. (2015a). Effects of grassland conversion from cropland on soil respiration on the semi-arid loess plateau, China. Air, Water 43 (7), 1052–1057. doi:10.1002/clen.201300971
Wang, D., Wu, G. L., Chang, X. F., Yang, Z., and Hao, H. M. (2015b). Effects of grazing exclusion on CO2 fluxes in a steppe grassland on the Loess Plateau (China). Ecol. Eng. 83, 169–175. doi:10.1016/j.ecoleng.2015.06.017
Wang, F. M., Sanders, C. J., Santos, I. R., Tang, J. W., Schuerch, M., Kirwan, M. L., et al. (2021). Global blue carbon accumulation in tidal wetlands increases with climate change. Natl. Sci. Rev. 8 (9), nwaa296–155. doi:10.1093/nsr/nwaa296
Wang, J., Liu, L. L., Wang, X., Yang, S., Zhang, B. B., Li, P., et al. (2017). High night-time humidity and dissolved organic carbon content support rapid decomposition of standing litter in a semi-arid landscape. Funct. Ecol. 31 (8), 1659–1668. doi:10.1111/1365-2435.12854
Wang, L., Throop, H. L., and Gill, T. (2015c). A novel method to continuously monitor litter moisture- A microcosm-based experiment. J. Arid Environ. 115, 10–13. doi:10.1016/j.jaridenv.2014.12.011
Wang, Q. K., Liu, S. G., and Tian, P. (2018). Carbon quality and soil microbial property control the latitudinal pattern in temperature sensitivity of soil microbial respiration across Chinese forest ecosystems. Glob. Change Biol. 24 (7), 2841–2849. doi:10.1111/gcb.14105
Zan, X. X., Xu, B. D., Ren, Y. P., Wang, X. L., and Cai, X. Y. (2011). The growth and dynamics of biomass of reed Phragmites australis in wetlands of Daguhe Estuary. Periodical Ocean Univ. China 41 (11), 27–33. (In Chinese with English abstract).
Zhang, X. H., Jiang, W., Jiang, S. S., Tan, W. W., and Mao, R. (2021). Differential responses of litter decomposition in the air and on the soil surface to shrub encroachment in a graminoid-dominated temperate wetland. Plant Soil 462, 477–488. doi:10.1007/s11104-021-04893-1
Zhang, X. H., Mao, R., Gong, C., Qiao, T. H., and Song, C. C. (2014b). CO2 evolution from standing litter of the emergent macrophyte Deyeuxia angustifolia in the Sanjiang Plain, Northeast China. Ecol. Eng. 63, 45–49. doi:10.1016/j.ecoleng.2013.12.002
Zhang, X. H., Song, C. C., Mao, R., Song, Y., and Meng, H. (2015). Comparing differences in early-stage decay of macrophyte shoots between in the air and on the sediment surface in a temperate freshwater marsh. Ecol. Eng. 81, 14–18. doi:10.1016/j.ecoleng.2015.04.020
Zhang, X. H., Song, C. C., Mao, R., Yang, G. S., Tao, B. X., Shi, F. X., et al. (2014a). Litter mass loss and nutrient dynamics of four emergent macrophytes during aerial decomposition in freshwater marshes of the Sanjiang plain, Northeast China. Plant Soil 385, 139–147. doi:10.1007/s11104-014-2217-3
Keywords: standing litter, CO2 emission, Q10, Phragmites communis, Yellow River Delta
Citation: Tao B, Wang J, Jiang Y, Chen Q and Zhang B (2023) CO2 emissions from reed litter in the air and on the soil surface in the Yellow River Delta, China. Front. Environ. Sci. 10:1093513. doi: 10.3389/fenvs.2022.1093513
Received: 10 November 2022; Accepted: 15 December 2022;
Published: 04 January 2023.
Edited by:
Donald Young, Virginia Commonwealth University, United StatesReviewed by:
Ning Zong, Institute of Geographic Sciences and Natural Resources Research (CAS), ChinaCopyright © 2023 Tao, Wang, Jiang, Chen and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baoxian Tao, dGFvYmFveGlhbkBzaW5hLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.