
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 28 November 2022
Sec. Water and Wastewater Management
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.1065770
This article is part of the Research TopicWastewater Treatment: Removal of Recalcitrant CompoundsView all 7 articles
Fluoroquinolone antibiotics attract increasing attention in the water treatment field because of the potential adverse effects on aquatic ecosystems and human health. The graphitic carbon nitride (g-C3N4) based photocatalysis has been demonstrated as an economically feasible and environmentally benign process to control these persistent contaminants. In this study, a new visible-light-driven of reduced graphene oxide (rGO) and nanoscale zero-valent iron (nZVI) co-modified g-C3N4-based photocatalyst was synthesized via ultrasonication-assisted chemisorption method. The optimized nZVI-loaded rGO/g-C3N4 (10% IGCN) showed a reaction rate enhancement of 2.12∼3.69-fold and 1.20∼1.68-fold for the degradation of ofloxacin (OFL), norfloxacin (NOR), and ciprofloxacin (CIP) compared to that of carbon-doped g-C3N4 (MCB0.07) and rGO-supported g-C3N4 (7.5% GCN) under the irradiation of simulated visible light, respectively. The enhanced photocatalytic activity can be ascribed to the synergistic effect of nZVI and rGO to improve the separation of charge carriers and boost the harvest of visible light. The degradation mechanisms were explored by scavenger tests and X-ray photoelectron spectroscopy (XPS), indicating that holes (h+) played a dominant role in the decomposition of OFL, NOR, and CIP. The piperazine ring and C–N between the piperazine ring and benzene were the primary attack sites of h+. In addition, the ring-opening oxidation of benzene (C=C bond) connected by the C–F bond may also be an essential step. This study shed light on the degradation mechanism of OFL, NOR, and CIP under visible light irradiation of the 10% IGCN and provided theoretical support for the practical application of photocatalysis in treating antibiotics-containing water.
Fluoroquinolones (FQs), one class of broad-spectrum antibiotics widely used in human and veterinary medicine (Zheng et al., 2021; Adeleye et al., 2022), are the most frequently detected antibiotics in aquatic environment considered as a crucial class of emerging pollutants with concentrations ranging from ng/L to μg/L (Lu et al., 2021; Zhou et al., 2021), which not only poses a risk to human health, but also promotes the emergence of antibiotic-resistant bacteria and their resistances (Wallmann et al., 2021; Mangla et al., 2022). In virtue of the stability of quinolone backbone (Zhu et al., 2016), low biodegradability, and high adsorption affinity (Oberoi et al., 2019; Lu et al., 2021; Saya et al., 2022), FQs generally cannot been completely removed by conventional water and wastewater treatment technologies. Therefore, discharge from wastewater treatment plants have become the major pathway for the ubiquity of FQs residues entering in aqueous system. Thus, an innovative, feasible, and environmentally benign process urgently needs to be developed and utilized, and the fate of the FQs requires intensive and further investigations.
Solar-energy-enabled photocatalysis is considered a sustainable process to degrade persistent environmental pollutants via the attack of photogenerated reactive species such as holes (h+) and reactive oxygen species (ROS) (•OH, 1O2, •O2−/•HO2, and H2O2) (Zheng et al., 2019; Schnabel et al., 2021; Zewdie et al., 2021). Graphitic carbon nitride (g-C3N4) has attained significant attention for the degradation of organic pollutants in water because of its appealing electronic band structure, excellent physicochemical properties, and abundance (Ong et al., 2016; Balakrishnan and Chinthala, 2022). Also, the g-C3N4 photocatalysts can harvest and potentially utilize more sunlight and can be reactive under artificial light, which takes apparent advantages in water purification over conventional photocatalysts (Cui et al., 2021; Lin et al., 2022). However, the photocatalytic activity of original g-C3N4 (e.g., synthesized from melamine) was limited due to the small specific surface area, weak response of visible light, and strong recombination rate of photogenerated electron-hole (e−-h+) pairs (Zheng et al., 2017; Nemiwal et al., 2021). Recently, different innovative approaches (e.g., doping, heterojunctions, crystal phase, and facet control) were explored to minimize the drawbacks and enhance the photocatalytic efficiency. Our previous research proved that the C-doped structure could produce ROS under thermodynamically favorable conditions, localize h+ to improve charge separation, and enhance the photocatalytic degradation of persistent organic micropollutants (Zheng et al., 2017; Chang et al., 2019).
Reduced graphene oxide (rGO), which presents a similar carbon network and sp2 conjugated π structure with g-C3N4, has attracted considerable attention as an efficient supporter of g-C3N4 to increase the conductivity and accelerate the separation of photo-generated charge carriers due to π-conjugated built-in electric field in the interface between g-C3N4 and rGO (Saha et al., 2020). Yuan et al. (2019) reported that the photocatalytic degradation efficiency of tetracycline on rGO/g-C3N4 was more than 80% higher than that of original g-C3N4. Xie et al. (2022) found that a hybrid catalyst of 7.5% rGO/g-C3N4 presented higher inactivation efficiency on ofloxacin-resistant bacteria under light irradiation. In general, the excellent electron transfer ability of rGO was impregnated to realize the practical separation of photo-generated carriers of g-C3N4. The hybrid catalyst of rGO/g-C3N4 by related research does show good photocatalytic activity. Consequently, constructing three-phase, composite-based rGO/g-C3N4 by combining with metals (Au, Ag, Pd, etc.), non-metals (N, B, S, P, F, I, etc.), and other semiconductors (TiO2, Bi2WO6, etc.) were explored by many researchers in order to improve further its photocatalytic performance (Kasinathan et al., 2020).
Nanoscale zero-valent iron (nZVI) is also of increasing interest to researchers because of its environmentally harmless, good conductivity, strong reducibility, and high reactivity to organic pollutants (Wang et al., 2020; Wu et al., 2022a). Ma et al. (2021) has synthesized nZVI@Ti3C2-based MXene nanosheets. The vast majority of ranitidine is found to be degraded with 91.1% of removal efficiency, indicating that nZVI@Ti3C2-based MXene has a synergistic effect enhancing the catalyst chemical reactivity and stability. Wang et al. (2017) has reported a photocatalyst named Fe0/C3N4/MoS2 with improved carrier separation efficiency through capturing the photo-generated e− by nZVI. In addition, although nZVI is easy to be oxidized to ≡FeII and ≡FeIII, it can be reduced to Fe0 by utilization in photo-generated e− (Wang et al., 2016; Wang et al., 2019), and further promoting the regeneration and reuse of nZVI. However, several disadvantages of nZVI (easily oxidation, severe aggregation, etc.) has not been effectively resolved. The previous studies have shown that carbon materials can be used as the carrier of nZVI dispersed in the liquid phase, and their application limitations can be remedied through synergistic effects (Fan et al., 2022; Wang et al., 2022).
Therefore, a new efficient strategy to prepare nZVI composites is urgently required. The combination of g-C3N4, rGO, and nZVI will be a potential visible-light-driven photocatalytic technology in accelerating the migration of photo-generated e− and h+ and enhancing photocatalytic efficiency. However, there are few reports on the combination of g-C3N4, rGO, and nZVI in the photocatalytic degradation of antibiotic contaminants. Also, because of the selectivity in contaminant degradation of g-C3N4-based photocatalysts and the resistance to the decomposition of most antibiotics for their robust molecular structures (Zheng et al., 2019; He et al., 2022), designing and fabricating g-C3N4-based photocatalysts with high activities requires further research.
Herein, in the present work, the modification of g-C3N4 by rGO and nZVI was realized via ultrasonication-assisted chemisorption. The nZVI-loaded rGO/g-C3N4 was characterized and the photodegradation of ofloxacin (OFL), norfloxacin (NOR), and ciprofloxacin (CIP) by nZVI-loaded rGO/g-C3N4 were evaluated by scavenger-quenching experiment. The main photo-generated reactive species involved in the degradation of OFL, NOR, and CIP were explored by scavenger-quenching experiments. And the X-ray photoelectron spectroscopy (XPS) was used to study the changes of the surface characteristic groups of nZVI-loaded rGO/g-C3N4 before and after photocatalysis, and speculated the degradation mechanism and possible pathway. Meanwhile, the stability of nZVI-loaded rGO/g-C3N4 was evaluated through cycle experiment. This study will open a new horizon in the modification of g-C3N4 and provide a feasible treatment technology to remove FQs pollutants from water.
To improve the separation efficiency of photo-generated charge carriers, we first introduce the rGO onto substrate g-C3N4 to form n% GCN composite. After the optimization of rGO introduction on the substrate g-C3N4, the electron mediator nZVI was then introduced to form n% IGCN composite.
The substrate g-C3N4, which is named MCB0.07, was prepared by melamine (2 g), cyanuric acid (1.93 g), and barbituric acid (0.07 g) according to our previous study (Zheng et al., 2016). Then, the rGO was introduced on the basis of MCB0.07 (n% GCN, n stood for the mass ratio of graphene oxide (GO) to melamine). Melamine (2 g), cyanuric acid (1.93 g), barbituric acid (0.07 g) and a certain volume of GO dispersion liquid were added into the anhydrous ethanol of 40 ml. The powder was first stirred for 3 h (500 r/min) at 25°C, then ultrasonic treatment for 3 h, and finally dried in 70°C oven to obtain gray-white powder without liquid. The gray-white powder was then transferred into a covered alumina crucible and calcined in a muffle furnace at 550°C for 4 h with a heating rate of 2.3°°C/min. After natural cooling, the obtained solid is grounded into powder, which was denoted as n% GCN. In order to prove that GO was thermally reduced to rGO at high temperatures, the GO dispersion was dried in an oven at 70°C, and heated in a muffle furnace at 550°C for 4 h with a heating rate of 2.3°°C/min.
The nZVI modification was introduced on the basis of 7.5% GCN (n% IGCN, n was the mass ratio of nZVI and n% GCN) by a facile ultrasonication-assisted chemisorption method. All n% IGCN samples were freshly prepared to prevent the oxidation of nZVI. Specifically, a certain amount of 7.5% GCN and nZVI were dispersed in 10 ml of ultrapure water (ultrapure water was under N2 gas flow in advance) to prepare n% IGCN stock solution. Before the photocatalytic experiment, the n% IGCN stock solution was ultrasonicated for 1 min (KQ-300DE, 60 kHz) to achieve a uniform suspension. The synthesized schematic of the n% IGCN photocatalyst is shown in Figure 1.
The surface micro-morphology of samples was detected by field emission scanning electron microscopy (FE-SEM, Sigma 300, Germany) equipped with energy dispersive X-ray spectroscopy (EDS), and transmission electron microscopy (TEM, JEM-2100, Japan). The specific surface area of the samples was determined at 77 K using a gas adsorption instrument (NOVA-2000e, United States) after vacuum degassing under 300°C for 12 h, then pore structure was calculated. The crystal structures of samples were investigated using an X-ray diffractometer (XRD, Rigaku Dmax-2500, Japan) with Cu-Kα radiation (λ = 1.5406 Å) in the scanning range of 2θ = 5–90°. Fourier transform infrared spectroscopy (FTIR, Nicolet iS5, United States) measurement was carried out to characterize the functional groups in the 400–4,000 cm−1 range, and prepare KBr for use as a blank control group.
The valences and existing forms of the elements on the samples were analyzed using X-ray photoelectron spectroscopy (XPS, EscaLab Xi+, United States) with monochromated Al-Kα radiation (hυ = 1,486.6 eV) as the excitation source, and the binding energy values were calibrated concerning C1s = 284.80 eV. All the binding energies of functional groups were determined according to the NIST XPS Database. All materials were vacuum freeze-drying for 24 h to remove the interference of residual H2O and prevent the oxidation of some components. The diffuse reflectance spectroscopy (DRS, UV-3600, Japan) was used to further evaluate the optical properties of the catalysts with BaSO4 as the background level. The detailed calculation method of band gap (Eg) was given in Supplementary Text S1.
Photoluminescence (PL, FLS-980, GBR) spectra were obtained with an excitation wavelength of 345 nm and an emission range between 400 and 650 nm to determine the transport characteristics of charge carriers. The transient photocurrent responses and electrochemical impedance spectroscopy (EIS) were measured with an electrochemical analyzer (CS310H, China). The measurement method was given in Supplementary Text S2.
Photocatalytic degradation experiments were conducted in a photochemical reaction apparatus (66924-1000HX-R1, Newport, United States) with direct research grade arc lamp source equipped with an ozone-free xenon lamp (1000 W) (6295NS, Newport, United States), liquid filter (6213NS, Newport, United States), and a 400 nm filter to filter infrared and UV light out. The reaction temperature was maintained at 25 ± 0.2°C by a circulating water system. The distance between the light source and the liquid surface was 13.5 cm. The intensity of light with a wavelength greater than 400 nm (λ > 400 nm) was 220.3 mW/cm2. The photocatalytic reaction was carried out in a 300 ml jacket beaker. The initial concentration of phenol or different antibiotics (OFL, NOR, and CIP) was 10 mg/L, and the pH was maintained at 7.0 by adding phosphate buffer solution (PBS). The photocatalyst loading was 1 g/L, and the solution was magnetically stirred at 500 r/min. The solution and photocatalyst were mixed at dark for 20 min to reach adsorption-desorption equilibrium before the photocatalytic reaction. During the photocatalytic reaction under light irradiation, a certain amount of liquid sample was collected at regular time intervals and stored in a 4°C refrigerator after passing through a 0.22 μm filter membrane for further analysis.
Scavenger tests were employed by quenching selected reactive species generated in the photocatalytic system to study the contribution of a specific reactive species to the degradation of pollutants. The isopropyl alcohol (IPA), L-histidine (L-His), p-Benzoquinone (BQ), catalase, and ethylenediaminetetraacetic acid disodium salt dihydrate (EDTA-2Na) were used to quench the •OH, 1O2, •O2−, H2O2, and h+, respectively. The concentration of IPA, L-His, and catalase was 0.1 mol/L, 10 mmol/L, and 1,000 U/mL, respectively. The concentrations of BQ and EDTA-2Na were 4 mmol/L, respectively. The inhibition rate of the scavenger to the reaction is shown in Eq. 1:
Where N0, Na is the degradation percentage of pollutants with and without adding scavenger, respectively.
The concentration of phenol was determined by 4-aminoantipyrine spectrophotometry (HJ503-2009). The dissolved organic carbon (DOC) of the solution was determined by total organic carbon analyzer (Vario TOC, Germany). The temperature, pressure, and flow rate of the combustion tube were set at 850 ± 1°C, 1,000 ± 30 Pa, and 180 ± 5 ml/min, respectively. The water sample was tested after passing through a 0.22 μm membrane.
The concentrations of OFL, NOR, and CIP were analyzed using high performance liquid chromatography (HPLC, 1,260 Infinity II, United States) equipped with Venusil MP C18 reversed-phase chromatographic column (4.6 mm × 250 mm × 5 μm). The specific HPLC analysis method of OFL, NOR, and CIP were summarized in Supplementary Table S1.
The fluoride ion concentration was detected by ion chromatography (IC, Dionex ICS-1100, United States) equipped with AS23 (4 nm × 250 nm) column.
The regenerated photocatalysts were collected by using a 0.22 μm membrane, washed with ultrapure water for several times with ultrasonication, and dried at 105°C for 24 h. The regenerated photocatalysts were then used to degrade OFL, NOR, and CIP for multiple cycles as described in Section 2.3.
The loading of rGO and nZVI was optimized using phenol degradation kinetics as a performance indicator. 0%–10% rGO was introduced for the synthesis of n% GCN, and then 0%–15% nZVI was applied for the synthesis of n% IGCN. The degradation of phenol on various composites is shown in Supplementary Figures S1, S2 and Supplementary Tables S2, S3. It showed that 7.5% GCN and 10% IGCN had the highest degradation effects on phenol under the visible light (λ > 400 nm), which were 79.7% and 86%. Compared to the 72.7% degradation of phenol by MCB0.07, the removal efficiency of n% GCN and n% IGCN has been effectively improved.
The photocatalytic degradation of OFL, NOR, and CIP on MCB0.07, 7.5% GCN, and 10% IGCN are shown in Figure 2. Under visible light (λ > 400 nm) irradiation, the degradation of the antibiotic conformed well to the first-order kinetics. The reaction rate constants of MCB0.07, 7.5% GCN, and 10% IGCN were 0.0150 min−1, 0.0264 min−1, and 0.0318 min−1 for the degradation of OFL, 0.0122 min−1, 0.0274 min−1, and 0.0450 min−1 for the degradation of NOR, and 0.0158 min−1, 0.0242 min−1, and 0.0406 min−1 for the degradation of CIP, respectively. The degradation rate of 10% IGCN for the three antibiotics was the highest, indicating that the loading of rGO and nZVI can effectively improve the photocatalytic activity of g-C3N4. As shown in Supplementary Figure S3, the oxidation of OFL, NOR, and CIP is not complete, which indicates the existence of oxidation intermediates. The photolysis of OFL, NOR, and CIP under visible light irradiation, shown in Supplementary Table S4, are 8.28%, 8.10%, and 9.64%, respectively, which show the little effect on the photocatalytic degradation process.
The adsorption of OFL, NOR, and CIP on synthesized photocatalysts was evaluated under dark conditions. As shown in Supplementary Figure S4, it was found that the adsorption capacity of three quinolone antibiotics for the same photocatalytic material is not much different, but the adsorption capacity of different photocatalytic materials for the three quinolone antibiotics is obviously different. The removal efficiencies of OFL, NOR, and CIP were 14.63%, 14.24%, and 15.90% using MCB0.07, respectively. Moreover, the removal efficiencies of OFL, NOR, and CIP were 5.88%, 6.91%, and 6.60% using 10% IGCN, respectively. The decreasing adsorption capacity of MCB0.07, 7.5% GCN and 10% IGCN for fluoroquinolone antibiotics is related to the reduction of active sites due to the loading of rGO and nZVI as shown by the decrease of BET surface area (Supplementary Figure S5) and pore volume (Supplementary Figure S6). The adsorption of OFL, NOR, and CIP on each photocatalyst was comparable and the photocatalytic experiments were conducted after the adsorption reaching the equilibrium. Therefore, the adsorption effect was neglectable to photocatalytic degradation. Secondly, MCB0.07 has the highest static adsorption rate, but its ability that produces reactive oxygen species is relatively poor. While 10% IGCN has a weak adsorption capacity, it has a solid ability to produce reactive oxygen species (the removal effect of 10% IGCN for three antibiotics was seen in Section 3.3). Therefore, the strong adsorption capacity is not necessarily related to the high organic matter removal capacity.
To observe the morphological and microstructural features of as-prepared samples, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) images were investigated. As shown in Figure 3A, the MCB0.07 showed an abundant porous structure distributing evenly and densely on the surface, which was consistent with previous studies (Zheng et al., 2016; Chang et al., 2019). The original GO exhibits an irregular layered flaky structure with rough edges and smooth surfaces, shown in Figure 3D (Liu et al., 2017). According to the previous work, after 550°C treatment, GO was reduced to rGO (Shanavas et al., 2019; Hu et al., 2021), while the wrinkles and curls could be observed from rGO (Agarwal and Zetterlund, 2021). However, although the porous structure of 7.5% GCN and the interface between rGO and MCB0.07 can be observed (Figure 3B), the pore of 7.5% GCN was inapparent and messy than MCB0.07. This may be due to the introduction of rGO layers, which upset the pore structure of MCB. This was consistent with the Brunauer-Emmett-Teller (BET) results. The pristine nZVI particles exhibit uncontrollable agglomeration, shown in Figure 3E. After loading nZVI on 7.5% GCN to synthesize 10% IGCN, it was observed that nZVI spherical particles were successfully attached to the sheet layer of 7.5% GCN with slight agglomeration (Figure 3C).
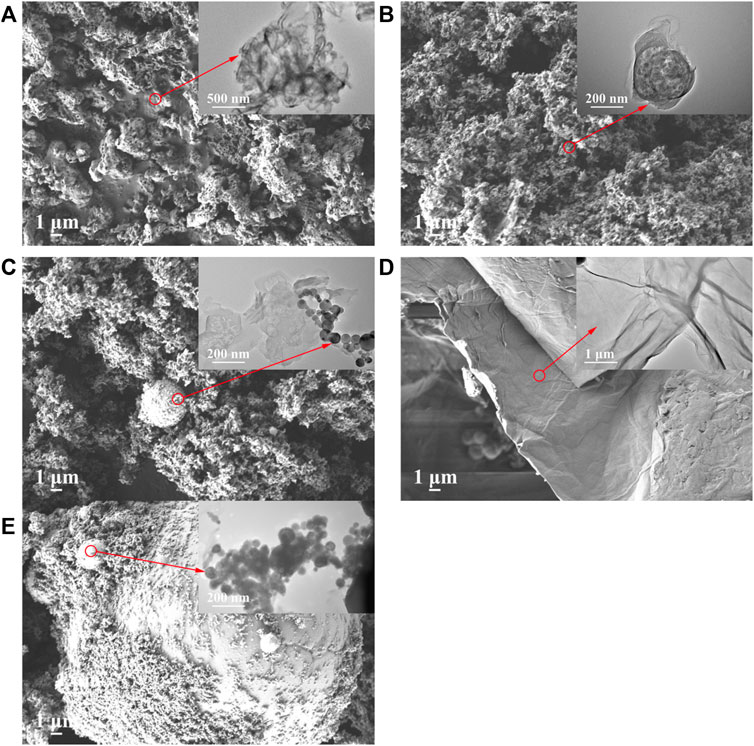
FIGURE 3. SEM and TEM of g-C3N4 series modified samples (A) MCB0.07, (B) 7.5% GCN, (C) 10% IGCN, (D) GO and (E) nZVI (the corresponding TEM image in the upper right corner).
The energy dispersive X-ray spectroscopy (EDS) of MCB0.07, 7.5% GCN, and 10% IGCN composites showed that C and N were uniformly distributed, and O was also presented due to incomplete polymerization of cyanuric acid or barbituric acid on g-C3N4 and the residual O-containing functional groups of rGO (Supplementary Figures S7, S8). As shown in Supplementary Table S5, the weight ratio of C and O in 7.5% GCN and 10% IGCN increased, while the weight ratio of N decreased compared with MCB0.07. This can indicate that rGO was successfully introduced to the sample since rGO has residual O-containing groups (Hu et al., 2021). The presence of about 2.52% Fe on 10% IGCN indicated the successful loading of nZVI, and Fe was also uniformly distributed on the samples.
The pore structures and BET specific surface area of the as-synthesized samples were further characterized by the N2 adsorption-desorption isotherm method. As shown in Supplementary Figure S5, MCB0.07, 7.5% GCN, and 10% IGCN all presented a representative type-IV curve with a high adsorption capacity at high relative pressures, indicating that they all have the highly porous structure (Li et al., 2015). In addition, mesopores were the main pore structure of three photocatalysts (Supplementary Figure S6; Table 1). However, the BET surface area and total pore volume of MCB0.07, 7.5% GCN, and 10% IGCN showed a decreasing trend. MCB0.07 showed the highest BET surface area (104.139 m2/g) and pore volume (0.270 cm3/g). Although the BET surface area of GO was determined to be 181.991 m2/g, the BET surface area and total pore volume of 7.5% GCN dropped to 79.558 m2/g and 0.225 cm3/g, respectively. This may be explained by the fact that the pores of MCB0.07 were destroyed by the rGO lamellar texture (Shao et al., 2016). With further loading of nZVI, nZVI may block the part of the pores on 10% IGCN as a result of the smaller size of nZVI particles (Rao et al., 2021), the BET surface area and total pore volume of 10% IGCN further decreased to 53.739 m2/g and 0.163 cm3/g, respectively. However, the removal efficiency of OFL, NOR, and CIP on 10% IGCN was higher than the other two photocatalysts (Figure 2), which indicated that the enhanced photocatalytic activity of the samples has no direct relationship with the BET surface areas, even though the parts of reactive sites of g-C3N4 were sacrificed due to the modification of nZVI and rGO.
The crystal structures of the as-prepared samples are determined by X-ray diffraction (XRD), shown in Figure 4. Two distinct and typical diffraction peaks at 13.0° and 27.34° (ICDD No. 87–1,526) were observed for MCB0.07, 7.5% GCN, and 10% IGCN, which are indexed to g-C3N4. The stronger peak at 27.34° was assigned to (002) crystal plane originating from the interlayer-stacking structure of a conjugated aromatic system (Liu et al., 2019), while the weaker peak at 13.0° corresponded to the (001) crystal plane originated from in-plane structural packing motif of tri-s-triazine units (Chang et al., 2019), respectively. However, the characteristic peaks of 7.5% GCN and 10% IGCN exhibited a slight shift compared with MCB0.07, which was attributed to the lattice strain generated in the hybridization process of MCB0.07 and rGO (Chen et al., 2020b). The GO sample presented a strong diffraction peak at 10.8° corresponding to the (001) crystal plane (Feng et al., 2022). Nevertheless, a new diffraction peak at 25.2° is detected after the GO treated under 550°C, shown in Supplementary Figure S9, being in accord with (002) crystal plane (Jaiswal et al., 2020), which provided the evidence for the conclusion that the GO was thermally reduced to rGO. However, the diffraction peaks of rGO were not detected in 7.5% GCN and 10% IGCN. It may be due to the content of rGO being too low to be detected (Tang et al., 2022). After nZVI loaded on 7.5% GCN, three typical diffraction peaks at 44.94°, 65.26°, and 82.46° were observed in the image of 10% IGCN, corresponding to (110), (200), and (211) crystal planes (ICDD No. 87–0722) (Huang et al., 2020), respectively. This indicated that the nZVI was successfully loaded without apparent oxidation, which may be credited with the protective effect of graphene materials on nZVI (Wang et al., 2019).
The molecular structures of the as-prepared samples were further examined by Fourier-transform infrared (FTIR) spectroscopy. As shown in Figure 5, the MCB0.07, 7.5% GCN, and 10% IGCN showed the typical absorption peaks with g-C3N4. The broad peaks between 2,850 and 3,600 cm−1 were from the stretching vibration of O–H bands and N–H components (Wang et al., 2019; Xiao et al., 2021). This indicated that the presence of unpolymerized–NH2/=NH groups at the defect sites of the aromatic ring in the cyclic g-C3N4 structure was identified (Shanavas et al., 2019). Besides, according to the study of (Huang et al., 2021), the incomplete polymerized fragments of g-C3N4 play a leading role in the separation of charge carriers. In addition, the peaks at 1,200–1,650 cm−1 can be attributed to the skeletal stretching vibration modes of a heptazine heterocyclic ring (Li et al., 2015) containing the N–(C)3 or bridging C–NH–C units (Kim et al., 2020). The sharp peak at 812 cm−1 was the breathing mode of the tri-s-triazine ring (Xiao et al., 2021). Compared with the GO sample, the characteristic peaks of GO were not detected in the 7.5% GCN and 10% IGCN samples. This may be explained by the carboxyl, carbonyl, and ester bonds on the surface of GO were reduced at 550°C to form rGO. In addition, as a result of the small amount of rGO in 7.5% GCN and 10% IGCN, the typical peaks were not obvious or merged with the peaks of g-C3N4. That was mutually supportive of the XRD results.
The compositions and chemical states of 10% IGCN were analyzed by X-ray photoelectron spectroscopy (XPS). As shown in Supplementary Figure S10, 10% IGCN was mainly composed of C, N, O, and Fe. For a closer examination of C, N, O, and Fe peaks, high-resolution XPS scans over C 1s, N 1s, O 1s, and Fe 2p of 10% IGCN sample were obtained, respectively. The high-resolution XPS scans over C 1s of 10% IGCN contained three components located at 284.8, 286.4, and 288.4 eV. The peak at 284.8 eV is assigned to the alkyl carbon (C–C) (Zhou et al., 2018), which may be proof of the interaction between rGO and g-C3N4 (Palanivel et al., 2021). The peak of 286.4 and 288.4 eV are assigned to the C–O bonds (Wu et al., 2022b) and sp2 carbon (N–C=N) presented in the framework of g-C3N4 (Zhou et al., 2018), respectively, as shown in Supplementary Figure S11A. As shown in Supplementary Figure S11B, the high-resolution scans over N 1s peaks could be deconvoluted into three peaks with binding energies of 399, 400.3, and 401.4 eV, corresponding to the aromatic N with sp2-hybridization bonded with C (C–N=C) (Shanavas et al., 2019; Zeng et al., 2020), the N–(C)3 groups at the edges of heptazine units (Xiao et al., 2021), and the amino functions (C–NH) which are derived from the terminal amino groups on the surface (Zhou et al., 2018), respectively. As shown in Supplementary Figure S11C, the high-resolution XPS scans over O 1s of 10% IGCN showed that three typical peaks were found with binding energies located at 529.9, 531.5, and 532.9 eV, respectively. The peak at 529.9 eV attributes to the Fe–O bond (Wang et al., 2021b) because of the partial oxidation of nZVI. In addition, the peaks located at 531.5 and 532.9 eV are associated with the C=O bond and organic C–O bond (Chen et al., 2021) due to incomplete polymerization of cyanuric acid or barbituric acid. Most notably, according to the previous research (Xie et al., 2022), compared with 7.5% GCN, the binding energies of C 1s, N 1s, and O 1s of 10% IGCN exhibited a distinct increase, which revealed that an intense interaction could be found between nZVI and 7.5% GCN rather than a simple physical mixing. The Fe species that existed in 10% IGCN were composed of Fe0 and oxidation states (e.g., Fe2+ and Fe3+) (Supplementary Figures S11C,D). The peaks at 707.2 and 720.4 eV of Fe 2p high-resolution XPS scans corresponded to Fe0 2p3/2 and Fe 2p1/2 (Lv et al., 2021), respectively. It indicated that nZVI was loaded successfully. The presence of Fe3+ and Fe2+ was shown by the peaks of Fe 2p3/2 and Fe 2p1/2 at 711.9 and 725.9 eV and 710.6 and 724.3 eV (Ma et al., 2021), which represented that only a small amount of nZVI was inevitably oxidized on the particle surface in the preparation of 10% IGCN because of the high activity of nZVI (Liang et al., 2016; Kong et al., 2021; Rao et al., 2021).
The optical absorption properties of as-synthesized photocatalysts were characterized by measuring the UV-vis diffuse reflectance spectrum (UV-Vis DRS). The spectra (Supplementary Figure S12) indicate that the visible light absorption of MCB0.07 is greatly enhanced via rGO and nZVI modification. Notably, the absorption edges of MCB0.07, 7.5% GCN, and 10% IGCN were found to be 460 nm, 468 nm, and 599 nm, corresponding to the band gaps (Eg) of 2.70 eV, 2.65 eV, and 2.07 eV, respectively, which represented that the capability of harvesting and utilizing visible light photons were enhanced obviously. Compared to MCB0.07, a slightly red shift was observed in 7.5% GCN, which may attribute to the intrinsic absorption of black-colored rGO (Shao et al., 2016) and the enhancement of π-π*conjugation effect by rGO is indispensable for the change (Tang et al., 2022). With further loading of the nZVI, 10% IGCN showed an intensive absorption in visible light region. This is probably relevant to the presence of Fe energy level results in decreasing of the energy gap for electron transition (Kong et al., 2021) and the synergism of rGO and nZVI (Fan et al., 2022), indicating that the introduction of rGO and nZVI play a critical role in improving the absorption capacity of visible light. However, in general, the narrower Eg is, the faster recombination of photo-generated charge carriers is, while 10% IGCN was not affected during, which will be discussed below.
To further explore the photoelectric conversion capability and carrier separation efficiency, transient photocurrent response and electrochemical impedance spectroscopy (EIS) are performed, as shown in Figure 6A. The smaller the radius of the curve is, the lower the charge transfer resistance is. The radius of the curve for MCB0.07 was the largest and significantly larger than 7.5% GCN, indicating that the introduction of rGO accelerated the rate of charge transfer at the interface of the photocatalysts and reduced the charge transfer resistance of g-C3N4 (Jo et al., 2018), which could be attributed to the high charge-carrier mobility capability of the rGO sheets (Tong et al., 2015; Chen et al., 2017). With loading nZVI on 7.5% GCN, the curve radius of 10% IGCN reduced further due to the excellent conductivity of nZVI (Kong et al., 2021). Figure 6B shows that the transient photocurrent responses are recorded by switching the visible-light-on-off for five cycles. In general, the larger the photocurrent density is, the higher the photo-generated carrier mobility is. Obviously, the photocurrent density of 10% IGCN was significantly larger than that of MCB0.07 and 7.5% GCN, which meant that 10% IGCN had good separation efficiency of photo-induced e−- h+ pairs. Similarly, the photoluminescence (PL) spectrum result also confirms that 10% IGCN possesses a better photo-generated e−-h+ separation efficiency (Figure 6C) due to the gradually decreasing fluorescence intensity.
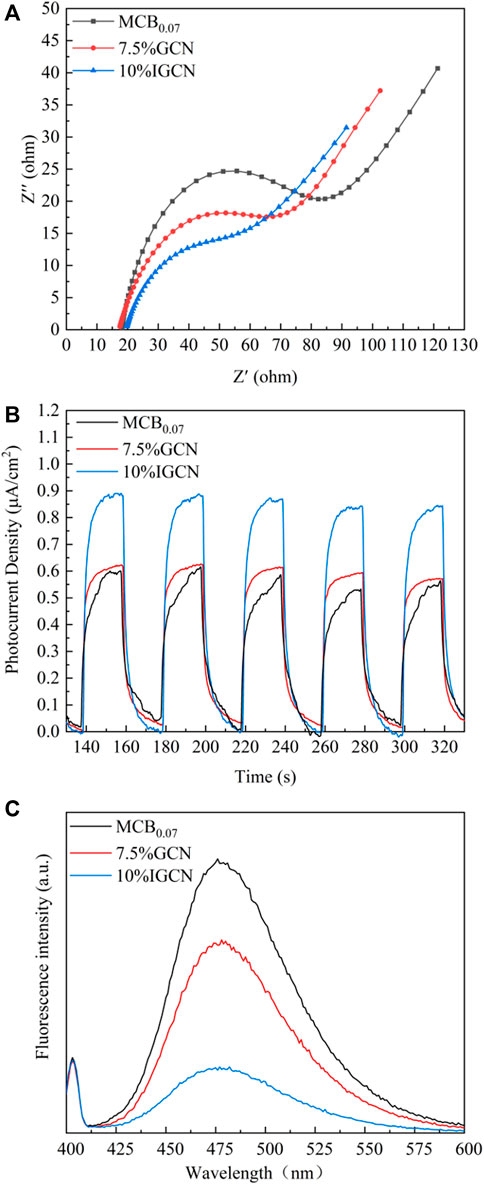
FIGURE 6. Photoluminescence spectrum (A), Electrochemical impedance spectroscopy (EIS) (B) and transient photocurrent responses (C) of as-prepared samples.
Notably, the narrower Eg of 10% IGCN was prone to induce photo-generated e−-h+ recombination, but the synergy between g-C3N4, rGO, and nZVI could effectively improve the transfer and separation efficiency of e−-h+ (Wang et al., 2019) and offset the negative effect of narrow Eg, combined with the EIS, transient photocurrent response and PL spectrum results. In addition, although Fe0 was oxidized due to inevitable oxidation, ≡FeII and ≡FeIII on the surface of material could capture e− and reduced to Fe0, which could improve the e−-h+ separation.
Under the visible light radiation (λ > 400 nm), the photocatalytic degradation of OFL, NOR, and CIP on 10% IGCN with time is displayed in Figure 7. The results showed that the degradation process of OFL, NOR, and CIP by 10% IGCN conforms to the first-order reaction rate equation (Table 2). The degradation rate of NOR is 0.045 min−1, which is higher than that of CIP (k = 0.0406 min−1) and OFL (k = 0.0318 min−1). Moreover, all three antibiotics had a more significant degradation rate. The difference in degradation rate may be related to the molecular structure and the molecular weight (Fang et al., 2021).

TABLE 2. Photocatalytic degradation rate constants and DOC removal rate constants of 10% IGCN during OFL, NOR, and CIP degradation.
To evaluate the mineralization of antibiotics on 10% IGCN, the change of DOC reaction rate constants in the solution was measured. As Figure 7B shows, the reaction rate constants of DOC for OFL, NOR, and CIP degradation by 10% IGCN were 0.0107 min−1, 0.011 min−1, and 0.0108 min−1, respectively. The reaction rate constants of DOC for the three antibiotics were not significantly different. Compared with the apparent degradation effects of OFL, NOR, and CIP in Figure 7A, the DOC results showed that all three antibiotics had degradation products. However, the oxazinyl in the OFL structure is not easy to break and open (Tian et al., 2020; Zhao et al., 2021), and the cyclopropyl in the CIP structure is not easy to fall off, either (Wang et al., 2018; Bai et al., 2020; Chen et al., 2020a). These molecular structures may have a specific impact on the mineralization of antibiotics.
To clarify the impacts of reactive species on the photocatalytic degradation of OFL, NOR, and CIP by 10% IGCN, isopropanol (IPA), L-histidine (L-His), 1,4-benzoquinone (BQ), EDTA-2Na, and catalase was introduced to the reaction system to trap •OH, 1O2, •O2−, h+, and H2O2.
As shown in Supplementary Figure S14 and Supplementary Table S6, after adding EDTA-2Na, the degradation kinetics of OFL, NOR, and CIP decreased to 0.0048, 0.0072, and 0.0086 min−1, respectively, indicating that h+ played a dominant role during the photocatalytic degradation of the three antibiotics. However, from the perspective of inhibition rate (Supplementary Figure S15), compared with h+, the contributions of individual reactive oxygen species (ROS) (•OH, 1O2, •O2−, and H2O2) do not show dramatic differences in the degradation of OFL, NOR, and CIP. In detail, the ROS inhibition rate of OFL, NOR, and CIP followed the orders: •OH (37.78%) > •O2− (32.05%) > H2O2 (30.79%) > 1O2 (23.84%), •OH (30.22%) > 1O2 (24.72%) > H2O2 (17.40%) > •O2− (16.79%), and ·•O2− (27.90%) > 1O2 (19.57%) > H2O2 (15.87%) > •OH (13.86%). It may be explained that the differences in the molecular structures of the antibiotics may cause the different interactions between them and the ROS (Zheng et al., 2016). Furthermore, although the scavengers quenched the target ROS, it affected the formation of other ROS through the free radical chain reaction.
Furthermore, the energy band structure of 10% IGCN was proposed to illustrate the possible photocatalytic degradation mechanism accounting for the enhancing in degradation efficiency and the complicated oxidation process. The Eg of g-C3N4 was obtained at 2.70 eV from the UV-Vis DRS results (Supplementary Figure S12B). The conduction band (CB) and valence band (VB) edge potentials of g-C3N4 can be estimated according to Supplementary Eqs S4, S6. Combined with XPS valence band spectra (Supplementary Figure S13), the calculated ECB and EVB of g-C3N4 are −1.12 eV and 1.58 eV, respectively, consistent with previous research (Chang et al., 2019).
Combined with Supplementary Table S7, the possible mechanism is proposed to explain the transformation of photo-induced charge carriers in the photocatalytic system of 10% IGCN. Under the visible light irradiation, the e− of g-C3N4 could be promoted to the CB, leaving h+ in the VB. However, it should be noted that the VB of g-C3N4 (+1.58 eV) was higher than the redox potential of •OH/H2O (+2.32 eV) as well as •OH/OH− (+1.99 eV), leading to the insufficient oxidation capacity of h+ to generate •OH, resulting in only being produced through the free radical chain reaction. Therefore, it provided evidence that the direct oxidation of pollutants induced by h+, existing on the surface of the photocatalyst, was the main step in the degradation of OFL, NOR, and CIP. Furthermore, the h+ was considered the starting point of the chain reaction, which was the source and necessary factor for producing the ROS (Zheng et al., 2019). As excellent electron mediators, e− could be transferred from CB of g-C3N4 to rGO and then to nZVI, which meant that more e− would be involved in the photocatalytic reaction. Moreover, owing to the interfacial interactions and synergistic effect between g-C3N4, rGO, and nZVI (Wang et al., 2019), the recombination of e− and h+ was largely inhibited, and the utilization of visible light was greatly improved, while the photocatalytic performance was enhanced by the following process. The CB of g-C3N4 (−1.12 eV) was lower than the redox potential of O2/•O2− (−0.33 eV), resulting in the capability to generate •O2−, which could be further oxidized into 1O2 by h+ (1O2/•O2− (+0.65 eV)), as well as reduced into H2O2 by e− (•O2−/H2O2 (+0.89 eV)). With the exception of producing the •O2−, the O2 could also capture the e− to generate H2O2 (O2/H2O2 (+0.28 eV)). As an electron trapper, H2O2 could be dissociated into the •OH (H2O2/•OH (+0.38 eV)) using e−, which may be an important source of •OH. Although ≡FeII or ≡FeIII could trap e− to some extent to inhibit the oxidation of nZVI (Raha and Ahmaruzzaman, 2020), the nZVI is inevitably oxidized into Fe2O3 after the reaction (Supplementary Figure S20), attributing to the strong oxidation of ROS and activity of nZVI (Kong et al., 2021).
XPS was used to study the residues of OFL, NOR, and CIP on the surface of 10% IGCN before and after photocatalytic reaction to deduce the possible degradation intermediates. As shown in Figure 8; Supplementary Figures S16, S17, there are indistinct differences in C 1s, N 1s, and O 1s spectra. In contrast, although the Fe 2p spectra show the disappearance of the characteristic peaks of nZVI, it is speculated that the oxidation on the surface of nZVI led to no detection. However, the F 1s spectra showed a considerable change before and after the reaction (from 688.8 eV to 686.6 eV), indicating that the benzene ring attached to the C–F bond occurred as the ring-opening oxidation.
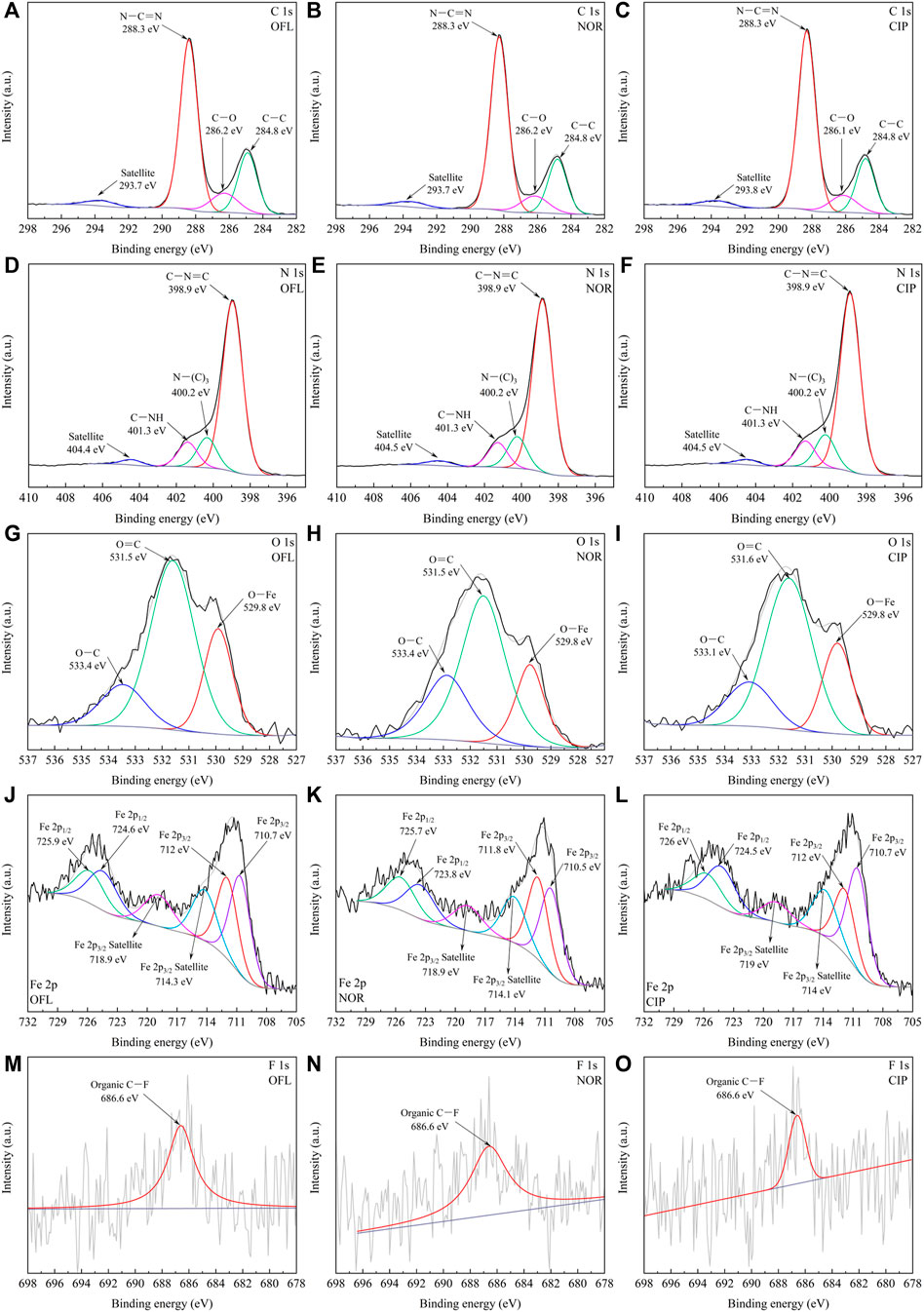
FIGURE 8. XPS spectra of 10% IGCN after the reaction, (A–C) C 1s, (D–F) N 1s, (G–I) O 1s, (J–L) Fe 2p, and (M–O) F 1s.
In summary, the possible photocatalytic degradation mechanisms of 10% IGCN in OFL, NOR, and CIP under visible light are proposed in Figure 9. As the main reactive species in the reaction, the h+ may attack the C–N bond between the piperazine ring and benzene binding at first (Fan et al., 2020; Hu et al., 2020; Hu et al., 2020; Su et al., 2022), according to the DFT calculation. Moreover, the cleavage on the piperazine ring induced by h+ was considered one of the degradation pathways because the two N atoms on the piperazine ring were also the most active sites (Hu et al., 2020; Zhang et al., 2020; Zhao et al., 2021). And then, the products could be further oxidized by •OH and •O2−, etc. Moreover, the ring-opening oxidation of the benzene ring (C=C bond) connected by the C–F bond may also be an important step. With the exception of the above conclusion, the defluorination is observed definitely because of the increasing concentration of F− in OFL, NOR, and CIP solution after the photocatalytic reactions (Table 3). •OH and •O2− were important ROS in the defluorination process, but their mechanisms differed. Combined with the contribution of ROS, it is speculated that the fluorine was substituted by •OH directly in the defluorination of OFL and NOR (Jin et al., 2019; Liu et al., 2020; Zhang et al., 2020; Wang et al., 2021a). However, the defluorination process in CIP solution may be triggered by •O2− (Wang et al., 2018), which subsequently underwent •OH substitution, leading to the formation of the defluorination product. It can be seen that the OFL, NOR, and CIP molecules were gradually decomposed by h+ and ROS and finally mineralized.
The stability performance of 10% IGCN photocatalyst is tested through the repeating experiment for OFL, NOR, and CIP degradation under the visible light irradiation, shown in Supplementary Figure S18. After four cycles, the removal efficiency of OFL, NOR, and CIP had a decrease from 82.71% to 71.92%, from 91.70% to 82.36%, and from 88.94% to 79.16%, respectively at 60 min, indicating the photo stability of the catalyst. The XRD patterns of the initial and used 10% IGCN composite for OFL, NOR, and CIP degradation were also conducted to further testify the stability of photocatalyst. As illustrated in Supplementary Figure S19, the diffraction peaks of nZVI are weakened, and the new peaks are consistent with Fe2O3 (ICDD. No. 39–0238), indicating that nZVI should be surface oxidation rather than complete oxidation, which is mutually explained with XPS results. However, the photocatalytic performance of 10% IGCN did not decrease significantly, which may be due to the existence of heterojunction between Fe2O3 and g-C3N4 (Shanavas et al., 2019).
In summary, a new visible-light-responsive 10% IGCN photocatalyst was prepared via co-modifying by nZVI and rGO on g-C3N4. It shows an improved photocatalytic performance on the degradation of antibiotics due to the enhanced visible light harvest and photogenerated charge separation. The 10% IGCN exhibited excellent photocatalytic performance for the degradation of OFL, NOR, and CIP under the irradiation of λ > 400 nm, with reaction rate constants, were 0.0318 min−1, 0.045 min−1, and 0.0406 min−1, respectively. According to the quenching experiments, h+ was identified as the main reactive species and played a crucial role in producing ROS. At the beginning of the reaction, h+ could directly attack the piperazine ring or C–N bond (between the piperazine ring and benzene). The ring-opening oxidation of the benzene ring (C=C bond) linked by the C–F bond was also a vital oxidation process of OFL, NOR, and CIP. In addition, the ROS-mediated defluorination reaction was also observed. After four cycles, the 10% IGCN maintained good photocatalytic performance and demonstrated promising potential for recycling. This work provides a new strategy to improve the photocatalytic performance of g-C3N4 by loading nZVI and rGO, and the mechanism study sheds light on the practical application of 10% IGCN on the degradation of fluoroquinolone antibiotics.
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
CL: Conceptualization, data curation, formal analysis, investigation, methodology, writing—original draft. YX: Data curation, investigation, methodology. YJ: Data curation, investigation. YD: Data curation. QZ: Writing—review and editing. YS: Project administration, supervision, writing—review and editing.
This research work was supported by the National Key R&D Program of China (2022YFE0104900); the National Natural Science Foundation of China (22278007).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1065770/full#supplementary-material
Adeleye, A. S., Xue, J., Zhao, Y., Taylor, A. A., Zenobio, J. E., Sun, Y., et al. (2022). Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mat. 424, 127284. doi:10.1016/j.jhazmat.2021.127284
Agarwal, V., and Zetterlund, P. B. (2021). Strategies for reduction of graphene oxide – a comprehensive review. Chem. Eng. J. 405, 127018. doi:10.1016/j.cej.2020.127018
Bai, F., Ni, S., Tang, Y., Pan, X., and Zhao, Z. (2020). Ciprofloxacin transformation in aqueous environments: Mechanism, kinetics, and toxicity assessment during •OH-mediated oxidation. Sci. Total Environ. 699, 134190. doi:10.1016/j.scitotenv.2019.134190
Balakrishnan, A., and Chinthala, M. (2022). Comprehensive review on advanced reusability of g-C3N4 based photocatalysts for the removal of organic pollutants. Chemosphere 297, 134190. doi:10.1016/j.chemosphere.2022.134190
Chang, X., Yao, X., Ding, N., Yin, X., Zheng, Q., Lu, S., et al. (2019). Photocatalytic degradation of trihalomethanes and haloacetonitriles on graphitic carbon nitride under visible light irradiation. Sci. Total Environ. 682, 200–207. doi:10.1016/j.scitotenv.2019.05.075
Chen, F., An, W., Liu, L., Liang, Y., and Cui, W. (2017). Highly efficient removal of bisphenol A by a three-dimensional graphene hydrogel-AgBr@rGO exhibiting adsorption/photocatalysis synergy. Appl. Catal. B Environ. 217, 65–80. doi:10.1016/j.apcatb.2017.05.078
Chen, F., Liu, L., Chen, J., Li, W., Chen, Y., Zhang, Y., et al. (2021). Efficient decontamination of organic pollutants under high salinity conditions by a nonradical peroxymonosulfate activation system. Water Res. 191, 116799. doi:10.1016/j.watres.2020.116799
Chen, L., Ni, R., Yuan, T., Gao, Y., Kong, W., Zhang, P., et al. (2020a). Effects of green synthesis, magnetization, and regeneration on ciprofloxacin removal by bimetallic nZVI/Cu composites and insights of degradation mechanism. J. Hazard. Mat. 382, 121008. doi:10.1016/j.jhazmat.2019.121008
Chen, T., Zhang, J., Ge, H., Li, M., Li, Y., Liu, B., et al. (2020b). Efficient extraction of uranium in organics-containing wastewater over g-C3N4/GO hybrid nanosheets with type-II band structure. J. Hazard. Mat. 384, 121383. doi:10.1016/j.jhazmat.2019.121383
Cui, Y., An, X., Zhang, S., Tang, Q., Lan, H., Liu, H., et al. (2021). Emerging graphitic carbon nitride-based membranes for water purification. Water Res. 200, 117207. doi:10.1016/j.watres.2021.117207
Fan, M., Zhang, P., Wang, C., Tang, J., and Sun, H. (2022). Tailored design of three-dimensional rGOA-nZVI catalyst as an activator of persulfate for degradation of organophosphorus pesticides. J. Hazard. Mat. 428, 128254. doi:10.1016/j.jhazmat.2022.128254
Fan, Y., Zhou, Z., Feng, Y., Zhou, Y., Wen, L., and Shih, K. (2020). Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites. Chem. Eng. J. 383, 123056. doi:10.1016/j.cej.2019.123056
Fang, L., Miao, Y., Wei, D., Zhang, Y., and Zhou, Y. (2021). Efficient removal of norfloxacin in water using magnetic molecularly imprinted polymer. Chemosphere 262, 128032. doi:10.1016/j.chemosphere.2020.128032
Feng, Y., Chen, G., Zhang, Y., Li, D., Ling, C., Wang, Q., et al. (2022). Superhigh co-adsorption of tetracycline and copper by the ultrathin g-C3N4 modified graphene oxide hydrogels. J. Hazard. Mat. 424, 127362. doi:10.1016/j.jhazmat.2021.127362
He, W., Liu, L., Ma, T., Han, H., Zhu, J., Liu, Y., et al. (2022). Controllable morphology CoFe2O4/g-C3N4 p-n heterojunction photocatalysts with built-in electric field enhance photocatalytic performance. Appl. Catal. B Environ. 306, 121107. doi:10.1016/j.apcatb.2022.121107
Hu, K., Li, R., Ye, C., Wang, A., Wei, W., Hu, D., et al. (2020). Facile synthesis of Z-scheme composite of TiO2 nanorod/g-C3N4 nanosheet efficient for photocatalytic degradation of ciprofloxacin. J. Clean. Prod. 253, 120055. doi:10.1016/j.jclepro.2020.120055
Hu, X., Hu, X., Peng, Q., Zhou, L., Tan, X., Jiang, L., et al. (2020). Mechanisms underlying the photocatalytic degradation pathway of ciprofloxacin with heterogeneous TiO2. Chem. Eng. J. 380, 122366. doi:10.1016/j.cej.2019.122366
Hu, Y., Zhou, C., Wang, H., Chen, M., Zeng, G., Liu, Z., et al. (2021). Recent advance of graphene/semiconductor composite nanocatalysts: Synthesis, mechanism, applications and perspectives. Chem. Eng. J. 414, 128795. doi:10.1016/j.cej.2021.128795
Huang, C., Wen, Y., Ma, J., Dong, D., Shen, Y., Liu, S., et al. (2021). Unraveling fundamental active units in carbon nitride for photocatalytic oxidation reactions. Nat. Commun. 12 (1), 320. doi:10.1038/s41467-020-20521-5
Huang, X., Zhang, F., Peng, K., Liu, J., Lu, L., and Li, S. (2020). Effect and mechanism of graphene structured palladized zero-valent iron nanocomposite (nZVI-Pd/NG) for water denitration. Sci. Rep. 10 (1), 9931. doi:10.1038/s41598-020-66725-z
Jaiswal, A., Pal, S., Kumar, A., and Prakash, R. (2020). Metal free triad from red phosphorous, reduced graphene oxide and graphitic carbon nitride (red P-rGO-g-C3N4) as robust electro-catalysts for hydrogen evolution reaction. Electrochimica Acta 338, 135851. doi:10.1016/j.electacta.2020.135851
Jin, X., Zhou, X., Sun, P., Lin, S., Cao, W., Li, Z., et al. (2019). Photocatalytic degradation of norfloxacin using N-doped TiO2: Optimization, mechanism, identification of intermediates and toxicity evaluation. Chemosphere 237, 124433. doi:10.1016/j.chemosphere.2019.124433
Jo, W., Kumar, S., Eslava, S., and Tonda, S. (2018). Construction of Bi2WO6/RGO/g-C3N4 2D/2D/2D hybrid Z-scheme heterojunctions with large interfacial contact area for efficient charge separation and high-performance photoreduction of CO2 and H2O into solar fuels. Appl. Catal. B Environ. 239, 586–598. doi:10.1016/j.apcatb.2018.08.056
Kasinathan, M., Thiripuranthagan, S., and Sivakumar, A. (2020). A facile fabrication of Br-modified g-C3N4/rGO composite catalyst for enhanced visible photocatalytic activity towards the degradation of harmful dyes. Mat. Res. Bull. 130, 110870. doi:10.1016/j.materresbull.2020.110870
Kim, J., Kim, H., Choi, J., and Baek, K. (2020). Bifunctional iron-modified graphitic carbon nitride (g-C3N4) for simultaneous oxidation and adsorption of arsenic. Environ. Res. 188, 109832. doi:10.1016/j.envres.2020.109832
Kong, W., Yue, Q., Gao, Y., Li, Q., Xu, X., Kong, Y., et al. (2021). Enhanced photodegradation of sulfadimidine via PAA/g-C3N4-Fe0 polymeric catalysts under visible light. Chem. Eng. J. 413, 127456. doi:10.1016/j.cej.2020.127456
Li, Y., Wang, J., Yang, Y., Zhang, Y., He, D., An, Q., et al. (2015). Seed-induced growing various TiO2 nanostructures on g-C3N4 nanosheets with much enhanced photocatalytic activity under visible light. J. Hazard. Mat. 292, 79–89. doi:10.1016/j.jhazmat.2015.03.006
Liang, Z., Wen, Q., Wang, X., Zhang, F., and Yu, Y. (2016). Chemically stable and reusable nano zero-valent iron/graphite-like carbon nitride nanohybrid for efficient photocatalytic treatment of Cr(VI) and rhodamine B under visible light. Appl. Surf. Sci. 386, 451–459. doi:10.1016/j.apsusc.2016.06.010
Lin, J., Tian, W., Zhang, H., Duan, X., Sun, H., Wang, H., et al. (2022). Carbon nitride-based Z-scheme heterojunctions for solar-driven advanced oxidation processes. J. Hazard. Mat. 434, 128866. doi:10.1016/j.jhazmat.2022.128866
Liu, W., Li, Y., Liu, F., Jiang, W., Zhang, D., and Liang, J. (2019). Visible-light-driven photocatalytic degradation of diclofenac by carbon quantum dots modified porous g-C3N4: Mechanisms, degradation pathway and DFT calculation. Water Res. 151, 8–19. doi:10.1016/j.watres.2018.11.084
Liu, W., Zhou, J., and Yao, J. (2020). Shuttle-like CeO2/g-C3N4 composite combined with persulfate for the enhanced photocatalytic degradation of norfloxacin under visible light. Ecotoxicol. Environ. Saf. 190, 110062. doi:10.1016/j.ecoenv.2019.110062
Liu, Y., Jin, W., Zhao, Y., Zhang, G., and Zhang, W. (2017). Enhanced catalytic degradation of methylene blue by α-Fe2O3/graphene oxide via heterogeneous photo-Fenton reactions. Appl. Catal. B Environ. 206, 642–652. doi:10.1016/j.apcatb.2017.01.075
Lu, J., Ji, Y., Chovelon, J., and Lu, J. (2021). Fluoroquinolone antibiotics sensitized photodegradation of isoproturon. Water Res. 198, 117136. doi:10.1016/j.watres.2021.117136
Lv, H., Niu, H., Zhao, X., Cai, Y., and Wu, F. (2021). Carbon zero-valent iron materials possessing high-content fine Fe0 nanoparticles with enhanced microelectrolysis-Fenton-like catalytic performance for water purification. Appl. Catal. B Environ. 286, 119940. doi:10.1016/j.apcatb.2021.119940
Ma, Y., Lv, X., Xiong, D., Zhao, X., and Zhang, Z. (2021). Catalytic degradation of ranitidine using novel magnetic Ti3C2-based MXene nanosheets modified with nanoscale zero-valent iron particles. Appl. Catal. B Environ. 284, 119720. doi:10.1016/j.apcatb.2020.119720
Mangla, D., Annu, , , Sharma, A., and Ikram, S. (2022). Critical review on adsorptive removal of antibiotics: Present situation, challenges and future perspective. J. Hazard. Mat. 425, 127946. doi:10.1016/j.jhazmat.2021.127946
Nemiwal, M., Zhang, T. C., and Kumar, D. (2021). Recent progress in g-C3N4, TiO2 and ZnO based photocatalysts for dye degradation: Strategies to improve photocatalytic activity. Sci. Total Environ. 767, 144896. doi:10.1016/j.scitotenv.2020.144896
Oberoi, A. S., Jia, Y., Zhang, H., Khanal, S. K., and Lu, H. (2019). Insights into the fate and removal of antibiotics in engineered biological treatment systems: A critical review. Environ. Sci. Technol. 53 (13), 7234–7264. doi:10.1021/acs.est.9b01131
Ong, W., Tan, L., Ng, Y. H., Yong, S., and Chai, S. (2016). Graphitic carbon nitride (g-C3N4)-based photocatalysts for artificial photosynthesis and environmental remediation: Are we a step closer to achieving sustainability? Chem. Rev. 116 (12), 7159–7329. doi:10.1021/acs.chemrev.6b00075
Palanivel, B., Lallimathi, M., Arjunkumar, B., Shkir, M., Alshahrani, T., Al-Namshah, K. S., et al. (2021). rGO supported g-C3N4/CoFe2O4 heterojunction: Visible-light-active photocatalyst for effective utilization of H2O2 to organic pollutant degradation and OH radicals production. J. Environ. Chem. Eng. 9 (1), 104698. doi:10.1016/j.jece.2020.104698
Raha, S., and Ahmaruzzaman, M. (2020). Enhanced performance of a novel superparamagnetic g-C3N4/NiO/ZnO/Fe3O4 nanohybrid photocatalyst for removal of esomeprazole: Effects of reaction parameters, co-existing substances and water matrices. Chem. Eng. J. 395, 124969. doi:10.1016/j.cej.2020.124969
Rao, Z., Zhu, N., Wei, X., Li, F., Wu, P., Dang, Z., et al. (2021). Efficient peroxydisulfate activation with nZVI/CuO@BC nanocomposite derived from wastes for degradation of tetrabromobisphenol A in alkaline environment. J. Hazard. Mat. 417, 126029. doi:10.1016/j.jhazmat.2021.126029
Saha, D., Visconti, M. C., Desipio, M. M., and Thorpe, R. (2020). Inactivation of antibiotic resistance gene by ternary nanocomposites of carbon nitride, reduced graphene oxide and iron oxide under visible light. Chem. Eng. J. 382, 122857. doi:10.1016/j.cej.2019.122857
Saya, L., Malik, V., Gautam, D., Gambhir, G., BalendraSingh, W. R., Hooda, S., et al. (2022). A comprehensive review on recent advances toward sequestration of levofloxacin antibiotic from wastewater. Sci. Total Environ. 813, 152529. doi:10.1016/j.scitotenv.2021.152529
Schnabel, T., Jautzus, N., Mehling, S., Springer, C., and Londong, J. (2021). Photocatalytic degradation of hydrocarbons and methylene blue using floatable titanium dioxide catalysts in contaminated water. Water Reuse 11 (2), 224–235. doi:10.2166/wrd.2021.118
Shanavas, S., Mohana Roopan, S., Priyadharsan, A., Devipriya, D., Jayapandi, S., Acevedo, R., et al. (2019). Computationally guided synthesis of (2D/3D/2D) rGO/Fe2O3/g-C3N4 nanostructure with improved charge separation and transportation efficiency for degradation of pharmaceutical molecules. Appl. Catal. B Environ. 255, 117758. doi:10.1016/j.apcatb.2019.117758
Shao, L., Jiang, D., Xiao, P., Zhu, L., Meng, S., and Chen, M. (2016). Enhancement of g-C3N4 nanosheets photocatalysis by synergistic interaction of ZnS microsphere and RGO inducing multistep charge transfer. Appl. Catal. B Environ. 198, 200–210. doi:10.1016/j.apcatb.2016.05.056
Su, Q., Li, J., Yuan, H., Wang, B., Wang, Y., Li, Y., et al. (2022). Visible-light-driven photocatalytic degradation of ofloxacin by g-C3N4/NH2-MIL-88B(Fe) heterostructure: Mechanisms, DFT calculation, degradation pathway and toxicity evolution. Chem. Eng. J. 427, 131594. doi:10.1016/j.cej.2021.131594
Tang, Z., He, W., Wang, Y., Wei, Y., Yu, X., Xiong, J., et al. (2022). Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. 311, 121371. doi:10.1016/j.apcatb.2022.121371
Tian, Y., He, X., Chen, W., Tian, X., Nie, Y., Han, B., et al. (2020). Significant enhancement of photo-Fenton degradation of ofloxacin over Fe-Dis@Sep due to highly dispersed FeC6 with electron deficiency. Sci. Total Environ. 723, 138144. doi:10.1016/j.scitotenv.2020.138144
Tong, Z., Yang, D., Shi, J., Nan, Y., Sun, Y., and Jiang, Z. (2015). Three-Dimensional porous aerogel constructed by g-C3N4 and graphene oxide nanosheets with excellent visible-light photocatalytic performance. ACS Appl. Mat. Interfaces 7 (46), 25693–25701. doi:10.1021/acsami.5b09503
Wallmann, L., Krampe, J., Lahnsteiner, J., Radu, E., van Rensburg, P., Slipko, K., et al. (2021). Fate and persistence of antibiotic-resistant bacteria and genes through a multi-barrier treatment facility for direct potable reuse. Water Reuse 11 (3), 373–390. doi:10.2166/wrd.2021.097
Wang, F., Feng, Y., Chen, P., Wang, Y., Su, Y., Zhang, Q., et al. (2018). Photocatalytic degradation of fluoroquinolone antibiotics using ordered mesoporous g-C3N4 under simulated sunlight irradiation: Kinetics, mechanism, and antibacterial activity elimination. Appl. Catal. B Environ. 227, 114–122. doi:10.1016/j.apcatb.2018.01.024
Wang, M., Yu, H., Wang, P., Chi, Z., Zhang, Z., Dong, B., et al. (2021a). Promoted photocatalytic degradation and detoxication performance for norfloxacin on Z-scheme phosphate-doped BiVO4/graphene quantum dots/P-doped g-C3N4. Sep. Purif. Technol. 274, 118692. doi:10.1016/j.seppur.2021.118692
Wang, X., Hong, M., Zhang, F., Zhuang, Z., and Yu, Y. (2016). Recyclable nanoscale zero valent iron doped g-C3N4/MoS2 for efficient photocatalysis of RhB and Cr(VI) driven by visible light. ACS Sustain. Chem. Eng. 4 (7), 4055–4063. doi:10.1021/acssuschemeng.6b01024
Wang, X., Lu, M., Ma, J., and Ning, P. (2019). Preparation of air-stable magnetic g-C3N4@Fe0-graphene composite by new reduction method for simultaneous and synergistic conversion of organic dyes and heavy metal ions in aqueous solution. Sep. Purif. Technol. 212, 586–596. doi:10.1016/j.seppur.2018.11.052
Wang, X., Pu, X., Yuan, Y., Xiang, Y., Zhang, Y., Xiong, Z., et al. (2020). An old story with new insight into the structural transformation and radical production of micron-scale zero-valent iron on successive reactivities. Chin. Chem. Lett. 31 (10), 2634–2640. doi:10.1016/j.cclet.2020.08.007
Wang, X., Wang, Y., Ma, J., and Ning, P. (2022). Band gap narrowing ternary phosphorus-doped g-C3N4/Fe0@expanded graphite carbon layer hybrid composite for effective degradation of tetracycline via multiply synergistic mechanisms. J. Environ. Chem. Eng. 10 (3), 107589. doi:10.1016/j.jece.2022.107589
Wang, X., Zhou, Z., Liang, Z., Zhuang, Z., and Yu, Y. (2017). Photochemical synthesis of the Fe0/C3N4/MoS2 heterostructure as a highly active and reusable photocatalyst. Appl. Surf. Sci. 423, 225–235. doi:10.1016/j.apsusc.2017.06.050
Wang, Y., Zhang, P., Li, T., Lyu, L., Gao, Y., and Hu, C. (2021b). Enhanced Fenton-like efficiency by the synergistic effect of oxygen vacancies and organics adsorption on FexOy-d-g-C3N4 with Fe‒N complexation. J. Hazard. Mat. 408, 124818. doi:10.1016/j.jhazmat.2020.124818
Wu, X., Wang, X., Xie, Y., Ren, N., Ma, J., and Ning, P. (2022a). Facile in-situ construction of highly dispersed nano zero-valent iron modified black TiO2 Z-scheme recyclable heterojunction with highly efficient visible-light-driven photocatalytic activity. Appl. Catal. B Environ. 310, 121325. doi:10.1016/j.apcatb.2022.121325
Wu, Z., Tong, Z., Xie, Y., Sun, H., Gong, X., Qin, P., et al. (2022b). Efficient degradation of tetracycline by persulfate activation with Fe, Co and O co−doped g−C3N4: Performance, mechanism and toxicity. Chem. Eng. J. 434, 134732. doi:10.1016/j.cej.2022.134732
Xiao, J., Liu, Q., Song, M., Li, X., Li, Q., and Shang, J. K. (2021). Directing photocatalytic pathway to exceedingly high antibacterial activity in water by functionalizing holey ultrathin nanosheets of graphitic carbon nitride. Water Res. 198, 117125. doi:10.1016/j.watres.2021.117125
Xie, Y., Yin, X., Jiao, Y., Sun, Y., and Wang, C. (2022). Visible-light-responsive photocatalytic inactivation of ofloxacin-resistant bacteria by rGO modified g-C3N4. Environ. Sci. Pollut. Res. 29, 63142–63154. doi:10.1007/s11356-022-20326-7
Yuan, D., Huang, W., Chen, X., Li, Z., Ding, J., Wang, L., et al. (2019). Introduction of in-plane π-conjugated heterojunction via rGO modulation: A promising approach to enhance photoexcited charge separation and transfer of g-C3N4. Appl. Surf. Sci. 489, 658–667. doi:10.1016/j.apsusc.2019.05.303
Zeng, Z., Fan, Y., Quan, X., Yu, H., Chen, S., and Zhang, S. (2020). Energy-transfer-mediated oxygen activation in carbonyl functionalized carbon nitride nanosheets for high-efficient photocatalytic water disinfection and organic pollutants degradation. Water Res. 177, 115798. doi:10.1016/j.watres.2020.115798
Zewdie, T. M., Habtu, N. G., Dutta, A., and Van der Bruggen, B. (2021). Solar-assisted membrane technology for water purification: A review. Water Reuse 11 (1), 1–32. doi:10.2166/wrd.2020.049
Zhang, D., Qi, J., Ji, H., Li, S., Chen, L., Huang, T., et al. (2020). Photocatalytic degradation of ofloxacin by perovskite-type NaNbO3 nanorods modified g-C3N4 heterojunction under simulated solar light: Theoretical calculation, ofloxacin degradation pathways and toxicity evolution. Chem. Eng. J. 400, 125918. doi:10.1016/j.cej.2020.125918
Zhao, G., Ding, J., Zhou, F., Chen, X., Wei, L., Gao, Q., et al. (2021). Construction of a visible-light-driven magnetic dual Z-scheme BiVO4/g-C3N4/NiFe2O4 photocatalyst for effective removal of ofloxacin: Mechanisms and degradation pathway. Chem. Eng. J. 405, 126704. doi:10.1016/j.cej.2020.126704
Zheng, D., Yin, G., Liu, M., Chen, C., Jiang, Y., Hou, L., et al. (2021). A systematic review of antibiotics and antibiotic resistance genes in estuarine and coastal environments. Sci. Total Environ. 777, 146009. doi:10.1016/j.scitotenv.2021.146009
Zheng, Q., Durkin, D. P., Elenewski, J. E., Sun, Y., Banek, N. A., Hua, L., et al. (2016). Visible-light-responsive graphitic carbon nitride: Rational design and photocatalytic applications for water treatment. Environ. Sci. Technol. 50 (23), 12938–12948. doi:10.1021/acs.est.6b02579
Zheng, Q., Shen, H., and Shuai, D. (2017). Emerging investigators series: Advances and challenges of graphitic carbon nitride as a visible-light-responsive photocatalyst for sustainable water purification. Environ. Sci. Water Res. Technol. 3 (6), 982–1001. doi:10.1039/C7EW00159B
Zheng, Q., Xu, E., Park, E., Chen, H., and Shuai, D. (2019). Looking at the overlooked hole oxidation: Photocatalytic transformation of organic contaminants on graphitic carbon nitride under visible light irradiation. Appl. Catal. B Environ. 240, 262–269. doi:10.1016/j.apcatb.2018.09.012
Zhou, C., Lai, C., Huang, D., Zeng, G., Zhang, C., Cheng, M., et al. (2018). Highly porous carbon nitride by supramolecular preassembly of monomers for photocatalytic removal of sulfamethazine under visible light driven. Appl. Catal. B Environ. 220, 202–210. doi:10.1016/j.apcatb.2017.08.055
Zhou, L., Han, P., Zhao, M., Yu, Y., Sun, D., Hou, L., et al. (2021). Biotransformation of lincomycin and fluoroquinolone antibiotics by the ammonia oxidizers aoa, aob and comammox: A comparison of removal, pathways, and mechanisms. Water Res. 196, 117003. doi:10.1016/j.watres.2021.117003
Keywords: visible light photocatalysis, modified graphitic carbon nitride, nanoscale zero-valent iron, fluoroquinolone antibiotics, degradation mechanism
Citation: Liu C, Xie Y, Jiao Y, Du Y, Zheng Q and Sun Y (2022) Visible-light-driven nanoscale zero-valent iron loaded rGO/g-C3N4 for fluoroquinolone antibiotics degradation in water. Front. Environ. Sci. 10:1065770. doi: 10.3389/fenvs.2022.1065770
Received: 10 October 2022; Accepted: 16 November 2022;
Published: 28 November 2022.
Edited by:
Dunia E. Santiago, University of Las Palmas de Gran Canaria, SpainReviewed by:
Sriram Mansingh, Siksha O Anusandhan University, IndiaCopyright © 2022 Liu, Xie, Jiao, Du, Zheng and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yingxue Sun, c3VueXhAdGguYnRidS5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.