
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 04 January 2023
Sec. Soil Processes
Volume 10 - 2022 | https://doi.org/10.3389/fenvs.2022.1060277
The effects of different contents of biochar and vermicompost on the microbial and enzymatic activities of greenhouse soil were determined to provide a theoretical basis for improving the quality of greenhouse soil. The experiment was conducted in a greenhouse using potted tomatoes. Five treatments consisted of different amount ratios of organic amendments: 1% biochar (BC1), 3% biochar (BC3), 5% biochar (BC5), 3% vermicompost (VC3), and 5% vermicompost (VC5), with no addition of organic amendments as the control (CK). Compared with CK, the pH, organic matter content, and DOC concentration increased in treatment groups. The organic matter content of BC3 and BC5 significantly increased by 54.6% and 72.8%, respectively, and DOC concentration of BC3 significantly increased by 43.9%. Biochar and vermicompost significantly increased the diversity of bacterial and fungal communities in soil, as well as the abundance of Actinomycetes, Acidobacteria, Ascomycetes, and Aspergillus, and reduced the abundance of Aspergillus. The activities of urease and alkaline phosphatase were significantly increased, and the activity of nitrate reductase was inhibited in all treatment groups compared with CK. In addition, a highly significant positive correlation was observed among pH, Acidobacteria phylum abundance, and alkaline phosphatase activity in all treatments. DOC concentration was positively correlated with pH, organic matter content, Acidobacteria phylum abundance and alkaline phosphatase activity. Biochar and vermicompost were effective in improving the physicochemical properties of greenhouse soil, enhancing microbial diversity, and affecting enzymatic activities. Therefore, BC3 (3% biochar) had the most significant effect on community diversity and alkaline phosphatase and nitrate reductase activities. VC5 (5% vermicompost) had the best promotion effect on urease activity. This study highlights that biochar and vermicompost as organic amendments are recommended to improve the quality of greenhouse soils.
As an important natural resource in human production and life, soil is an important hub for material exchange and energy transfer in the ecosystem. In recent years, the contradiction between food demand and soil resources has become increasingly prominent with population growth, and the scientific and rational use of soil resources for the development of greenhouse cultivation has received considerable attention (Cai, 2019). In China’s greenhouse cultivation, conventional soil cultivation remains the main method used; thus, the quality of the soil in the greenhouse has become a limiting factor affecting the sustainable development of greenhouse cultivation (Van Groenigen et al., 2019). As the same crop is usually grown for several years in greenhouse cultivation, soil porosity and permeability are decreasing and soil crop succession barriers are frequent, limiting the quality of greenhouse cultivation products (Tang et al., 2021). Fertilizer application has become a common field management practice to improve soil fertility in greenhouse and increase crop yield and quality. However, unreasonable fertilizer application rates have also led to secondary salinization, soil caking and other problems (Mu et al., 2021; Ji et al., 2022). Selecting organic amendments as an alternative to chemical fertilizers not only ensures a regular supply of nutrients, but also alleviates soil secondary salinization or acidification arising from the long-term excessive application of chemical fertilizers (Chang et al., 2018; Shen et al., 2021; Zhang H. et al., 2022).
Given the unique ecological environment created by greenhouse cultivation, the effect of organic amendments addition on the microbial and enzymatic activity of greenhouse soils is becoming a hot topic of interest and research (Zhang Y. et al., 2022). As excellent soil conditioners, biochar and vermicompost can increase soil organic matter content, improve acidic soil, and promote soil water and fertilizer retention (Yang et al., 2015; Ronix et al., 2021; Zheng D. et al., 2021). Biochar is widely available, loose, and porous, and it is rich in functional groups. It can mitigate heavy metal contamination in soils, significantly improving crop yields and enhancing soil water storage and retention capacity (Alfadil et al., 2021). As a product of the degradation of organic waste by earthworms, vermicompost contains not only a large specific surface area, good agglomeration structure, good ion exchange and adsorption capacity, but also rich nutrients and beneficial microorganisms, which can effectively improve soil and its physicochemical properties and promote plant growth (Wang et al., 2021a). Studies have shown that long-term addition of organic amendments to soil has a positive impact on microbial biomass and enzymatic activity (Torres et al., 2015; Innangi et al., 2017; Luo et al., 2020). The applied organic materials can be decomposed by soil microorganisms or partly transformed and subsequently stabilized and accumulated as soil organic matter, improving soil fertility and promoting nutrient cycling (Wei et al., 2022). Microorganisms can be affected by the physicochemical properties of the soil. For example, the large specific surface area of soil aggregates provides protection for microorganisms (Liu Y. et al., 2022). In addition, the pH of the vermicompost is close to neutral, which is suitable for the reproduction of microorganisms, thereby causing a positive effect on the microorganisms (Van Groenigen et al., 2019). The increase of enzymatic activity may be caused by microbial changes or the higher stability of humic substances in organic amendments (Luo et al., 2018).
Soil, plants, and microorganisms form a complex soil microcosm system, and they coordinate with one another to maintain the balance of the soil ecosystem (He et al., 2022). Therefore, soil microorganisms not only play a key role in plant growth, but also contribute to the stability of soil ecosystems. Tomatoes are widely grown in China and in our greenhouses (Wu et al., 2022). At present, there are many related studies on tomato rhizosphere microorganisms and enzymatic activities (Nassal et al., 2018; Becagli et al., 2022). Therefore, this paper focuses on the study of organic amendments on tomato rhizosphere soil microorganisms and enzymatic activities, we selected potted tomato as the test crop, and applied different contents of biochar and vermicompost. This study aimed to investigate 1) how biochar and vermicompost affect soil physicochemical properties, microorganisms and enzymatic activity and 2) the possible correlation among soil physicochemical properties, soil microorganisms, and enzymatic activities. Furthermore, this experiment aimed to provide a theoretical basis for future scientific based application of organic amendments to improve soil quality in greenhouse.
The experiment was conducted in a plastic greenhouse in the water-saving garden of the Jiangning campus of Hohai University (31°57′N, 118°50′E) from July 2020 to January 2021. The planting soil used is yellow-brown loam, which is air-dried, sieved and placed into pots (specification is 28 cm in diameter and 38.5 cm in depth) (Figure 1) containing 10 kg of soil/pot. Tomato variety “Co-op 903” was planted when the tomato seedlings were five leaves and one shoot. Twenty grams of a nitrogen, phosphorus, and potassium compound fertilizer (15:15:15) was applied to each pot as a base fertilizer, and the plants were managed normally during the reproductive period.
The experiment was repeated three times for each treatment group. Six treatments were established: 1) soil untreated (CK), 2) soil amended with 1% biochar (BC1), 3) soil amended with 3% biochar (BC3), 4) soil amended with 5% biochar (BC5), 5) soil amended with 3% vermicompost (VC3) and 6) soil amended with 5% vermicompost (VC5).
Before starting the experiment, different doses of biochar and vermicompost were added, on a mass basis, to each treatment and mixed thoroughly with the soil. Soil, biochar and vermicompost were weighed in a mass ratio, where BC1, BC3, and BC5 contained 100, 300, and 500 g of biochar per pot. Similarly, VC3 and VC5 contained 300 g and 500 g vermicompost per pot. No organic amendments were applied to the CK. The biochar was purchased from Henan Lize Environmental Protection Technology Co., Ltd, which was corn stalk charcoal; the vermicompost was produced by fermenting pure, moist cow dung. Table 1 shows the specific physicochemical properties of biochar and vermicompost.
At the end of the experiment, soil samples were collected from each treatment group in replicate. Three tomatoes were randomly selected for each treatment, and they were uprooted to shake off and remove debris (Chaudhary et al., 2015; Edwards et al., 2015). We collected the soil sample attached to the root system, and divided it into two samples after mixing. One was put into the reagent tube and quickly stored in the refrigerator for the extraction of soil microbial genomic DNA, and the other was dried naturally for the determination of soil physicochemical properties and enzymatic activities.
Soil pH was measured by using a magnetic pH meter at a soil–water ratio of 1:5. Soil organic matter was determined by external heating with potassium dichromate (Li et al., 2012). The abovementioned indicators were based on air-dried soil samples. DOC was determined by taking deionized water as the extracting agent, weighing 10.000 g fresh soil sample, shaking for 1 h (250°r/min), centrifuging for 10 min (15,000°r/min), filtering (0.45 µm filter membrane), use the Shimadzu TOC-LCPH total organic carbon analyzer to determine the DOC mass fraction in the filtrate (Li et al., 2017). The soil samples used to determine soil microbial diversity were stored at 4°C. The microbial sequences were PCR amplified and sequenced using microbial diversity amplicon sequencing. After extraction of genomic DNA from samples for fungal diversity analysis, the ITS2 region of the internal transcribed spacer (ITS) was amplified using specific primers such as ITS3_KYO2 (GATGAAGAACGYAGYRAA) and ITS4 (TCCTCCGCTTATTGATATGC) with a barcode. The V3-V4 regions of the 16 S rDNA gene were amplified with primers 341 F (CCTACGGGNGGCWGCAG) and 806R (GGACTACHVGGGTATCTAAT). Fresh samples were collected for the determination of soil enzymatic activity after the experiment. Urease activity was determined by sodium phenol–sodium hypochlorite colorimetry (Khan et al., 2020), whereas the activity of alkaline phosphatase was determined by phenyldisodium phosphate colorimetry (Wang Z. et al., 2022). The activity of nitrate reductase was determined by using a kit from Beijing Solarbio Science & Technology Co., Ltd., based on sodium nitrite as standard solution. The soil samples were naturally air dried and sieved through 30–50 mesh. The principle is that nitrate reductase catalyzes the reduction of nitrate to nitrite, the nitrite produced can react with p-Aminobenzene Sulfonic Acid and α-naphthylamine quantitatively generates red azo compound; The generated red azo compound has a maximum absorption peak at 520 nm, which can be determined by ultraviolet spectrophotometry.
The alpha diversity index is based on the richness and evenness of species, reflecting the species diversity in a specific region or ecosystem. It estimates the species abundance and diversity of environmental communities through a series of statistical analysis indexes. Abundance-based coverage estimator (ACE) is used to estimate the total number of species in the sample. It uses low abundance species to estimate how many undiscovered species remain. Chao1 index speculates the expected number of species in the sample. The larger the value, the higher the species richness. Shannon–Wiener (Shannon) index not only cares about species richness, but also about species evenness. The essence of Simpson index is to comprehensively consider the richness and evenness of species in the sample (Chernov et al., 2015).
Data were statistically analyzed using Microsoft Excel 2010 and SPSS 26.0 Statistics (IBM, United States), and figures were processed using Origin 2021 and Auto CAD 2016. Significant difference among treatments was determined with Duncan’s multiple range test using analysis of variance (p < 0.05). The relationship among measured variables was analyzed using Pearson’s correlation coefficient analysis.
Different types and application contents of organic materials have different effects on soil physicochemical properties. As shown in Table 2, compared with CK, the addition of different organic materials increased the pH of the soil and the organic matter content. Treatment with additional biochar increased the pH of the soil more significantly, with BC1, BC3, and BC5 significantly increasing the pH by 1.55 units, 1.66 units, and 1.81 units respectively. Compared with the control, VC3 and VC5 increased the pH by 1.16 units and 1.29 units, respectively (Table 2). Compared with the control, the organic matter content of all treatments showed an increasing trend, except for VC3, which showed a slight decrease in organic matter content, with BC3 and BC5 showing a significant increase of 54.6% and 72.8%, respectively (Table 2). Therefore, biochar is more effective in enhancing the organic matter content of the soil compared with vermicompost. The dissolved organic carbon (DOC) content of the treatment added with an organic material was higher compared with the control, with BC3 being significantly higher by 43.9% (Table 2).
The diversity of the microbial community is positively correlated with plant’s ability to adapt to adversity. In this study, the V3-V4 region of 16S rRNA in 18 sample bacteria and the ITS (ITS2) region of 18 sample fungi were sequenced separately. After the application of organic materials, the alpha diversity index of tomato bacterial and fungal communities was improved compared with CK (Figure 2 and Figure 3). Except for the abundance-based coverage estimator (ACE), significant differences in Chao1, Simpson, and Shannon–Wiener (Shannon) indices were found between the treatment groups and the control group (p < 0.05), whereas the inter-group differences between the biochar-treated and vermicompost-treated groups was not significant (p > 0.05). In the biochar-treated group, all indices of BC3 reached the highest value, that is, the 3% biochar content had the most significant effect on enhancing the homogeneity and abundance of tomato bacterial community (Figure 2). The difference between the treatment group and control group was significant (Figure 3), and significant differences were observed in ACE and Chao1 indices of BC1 and BC5 (p < 0.05). The application of additional organic materials significantly increased the diversity of tomato bacterial and fungal communities.
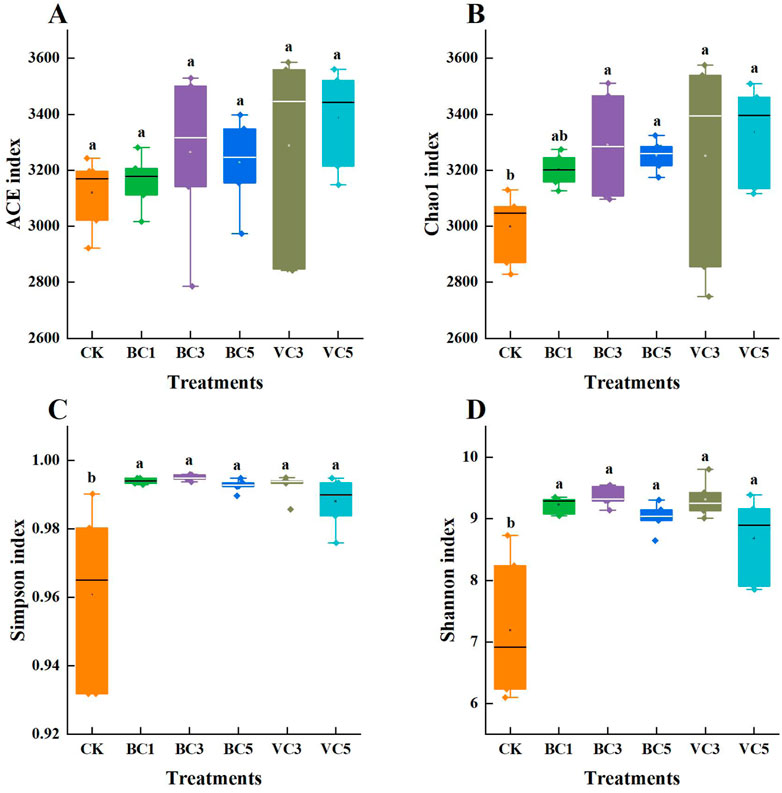
FIGURE 2. Alpha diversity index of soil bacterial communities. (A) ACE index; (B) Chao1 index; (C) Simpson index; (D) Shannon index. CK, soil untreated; BC1, soil amended with 1% biochar; BC3, soil amended with 3% biochar; BC5, soil amended with 5% biochar; VC3, soil amended with 3% vermicompost; VC5, soil amended with 5% vermicompost.
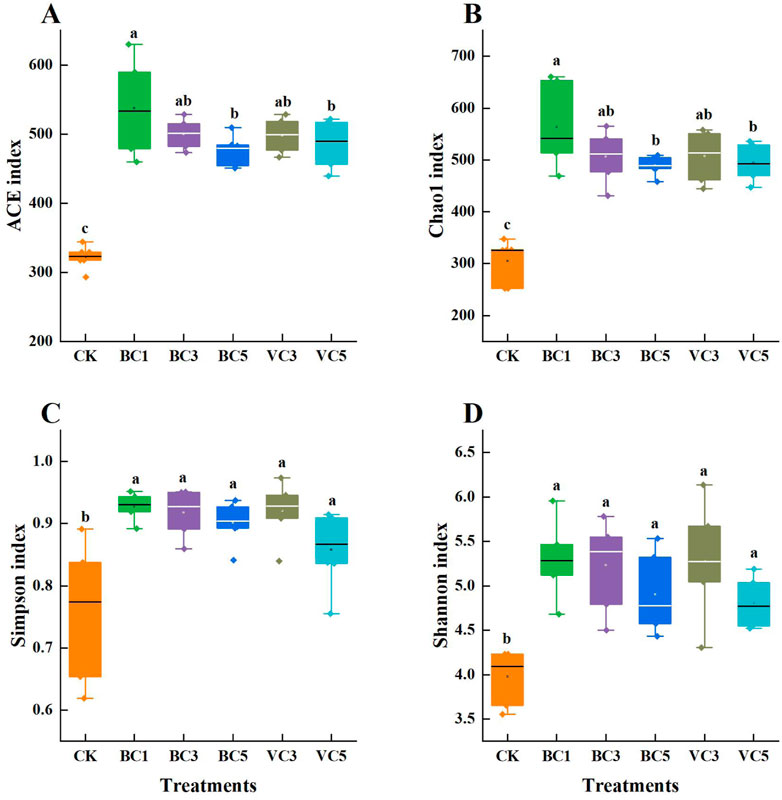
FIGURE 3. Alpha diversity index of soil fungi communities. (A) ACE index; (B) Chao1 index; (C) Simpson index; (D) Shannon index. CK, soil untreated; BC1, soil amended with 1% biochar; BC3, soil amended with 3% biochar; BC5, soil amended with 5% biochar; VC3, soil amended with 3% vermicompost; VC5, soil amended with 5% vermicompost.
Amplicon sequences of each group of samples were analyzed and species annotated (Figure 4). The dominant populations of the tomato bacterial community at the phylum level included Proteobacteria, Acidobacteria and Actinobacteria, which accounted for more than 90% of the overall relative abundance; the dominant species in the fungal community included Ascomycota and Anthophyta, with the relative abundance of these two phyla accounting for more than 90% of the total.
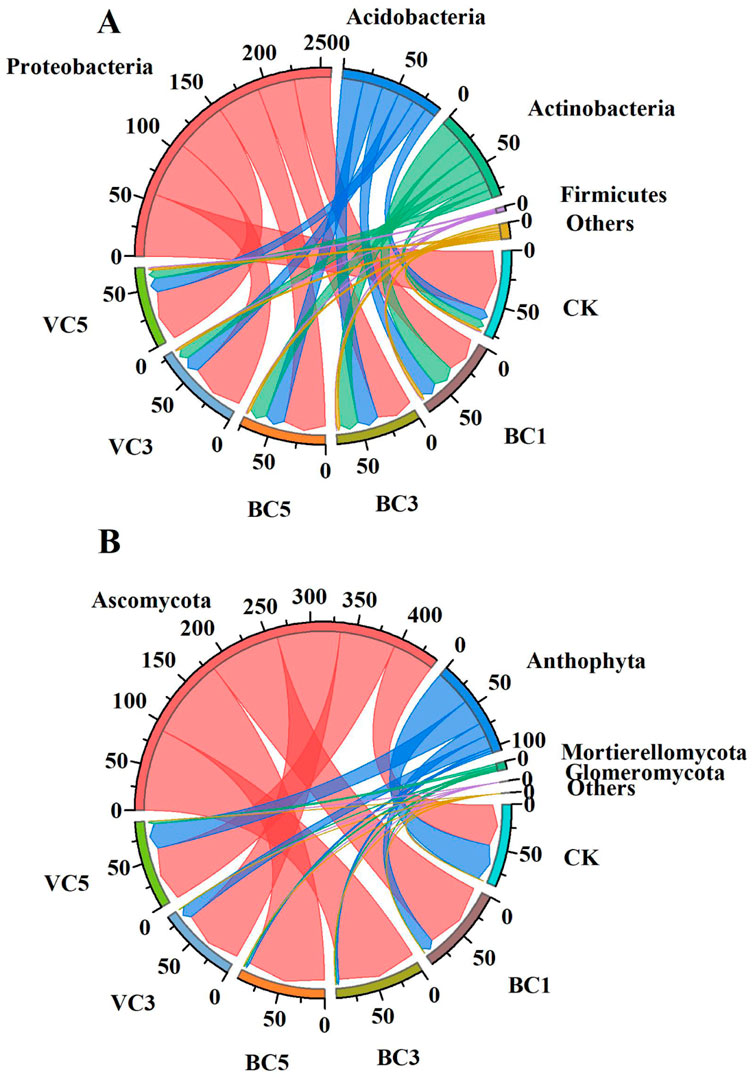
FIGURE 4. Chord diagram of tomato microbial community distribution. (A) Bacterial community. (B) Fungal community. CK, soil untreated; BC1, soil amended with 1% biochar; BC3, soil amended with 3% biochar; BC5, soil amended with 5% biochar; VC3, soil amended with 3% vermicompost; VC5, soil amended with 5% vermicompost.
The three main genera at the bacterial level (Proteobacteria, Acidobacteria, and Actinobacteria) and the three main genera at the fungal level (Ascomycota, Anthophyta, and Mortierellomycota) were analyzed for variability.
Compared with CK, the addition of vermicompost had no significant effect on the three genera at the bacterial phylum level, and the abundance of each genus in VC5 was lower than that in the VC3 treatment group. The addition of 1% and 3% biochar significantly increased the abundance of Actinobacteria and significantly decreased the abundance of Proteobacteria in the soil. In addition, no evident regularity in the effect of changes in biochar content on the abundance of bacterial genera was observed. The addition of 3% and 5% of biochar significantly increased the relative abundance of the fungal Ascomycota, and the BC3 and BC5 treatment groups increased by 92.7% and 91.0%, respectively, compared with CK. Moreover, the addition of 3% and 5% of biochar significantly reduced the relative abundance of Anthophyta, and the BC3 and BC5 treatment groups decreased by 92.9% and 93.2%, respectively, compared with CK. The VC3 treatment group significantly increased the relative abundance of Mortierellomycota. The change in vermicompost content had no significant effect on the relative abundance of Ascomycota and Anthophyta. The results showed that the application of organic materials increased the abundance of Actinobacteria, Acidobacteria, Ascomycota and Mortierellomycota, and decreased the abundance of Proteobacteria and Anthophyta in the soil in Table 3.
Organic materials could affect the activity of soil enzymes. Biochar and vermicompost significantly increased urease activity compared with CK, which increased with the increase of organic material content. VC5 had the most significant effect on urease activity, with an increase of 222.76% (Figure 5). Biochar and vermicompost had a significantly positive effect on the activity of alkaline phosphatase. With the increase of biochar content, the activity of alkaline phosphatase initially increased and then decreased; the change in vermicompost content had no significant effect on the activity of alkaline phosphatase. The increased application of organic material inhibited the activity of nitrate reductase, with BC3 being the most effective treatment, with a 39.23% reduction. The activity of nitrate reductase initially decreased and then increased with the increase of biochar content. In the treatment group added with vermicompost, the activity of nitrate reductase increased with the increase of organic material content. The results showed that biochar and vermicompost significantly enhanced the activities of urease and alkaline phosphatase in soil and inhibited the activity of nitrate reductase.
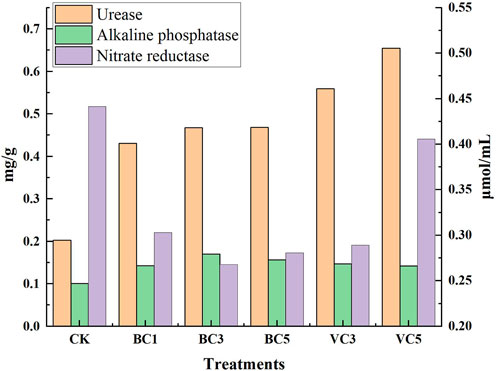
FIGURE 5. Effects of biochar and vermicompost on enzymatic activity in greenhouse soil. CK, soil untreated; BC1, soil amended with 1% biochar; BC3, soil amended with 3% biochar; BC5, soil amended with 5% biochar; VC3, soil amended with 3% vermicompost; VC5, soil amended with 5% vermicompost.
As shown in Table 4, the three major genera of bacteria were closely related to one another. The abundance of Proteobacteria was significantly and negatively correlated with the abundance of Acidobacteria and Actinobacteria (p < 0.05). The correlation among enzymes was significant, with a significantly negative correlation between nitrate reductase activity and alkaline phosphatase activity (p < 0.05). In addition, a certain relationship was observed between soil microorganisms and enzymatic activity, with a highly and significantly positive correlation between Acidobacteria phylum abundance and alkaline phosphatase activity (p < 0.01) and a significantly negative correlation with nitrate reductase activity (p < 0.05). Organic materials affect microbial and enzymatic activities by regulating soil physicochemical properties. A significantly positive correlation was observed among pH, dissolved organic carbon, and Acidobacteria phylum abundance (p < 0.05). In addition, a highly and significantly positive correlation with alkaline phosphatase activity (p < 0.01) and a significantly negative correlation with Aspergillus phylum abundance (p < 0.05) were observed. DOC was significantly and positively correlated with organic matter content, alkaline phosphatase activity, and Acidobacteria phylum abundance (p < 0.05).

TABLE 4. Correlation analysis among soil physicochemical properties, microbial abundance and enzyme.
In this study, biochar and vermicompost significantly altered soil physicochemical properties, microbial diversity and enzymatic activity. As good organic amendments, biochar and vermicompost can significantly change the physical and chemical properties of soil, such as soil bulk density and pH, and improve soil quality (Zhang et al., 2020; Guo et al., 2021; Li et al., 2022; Šimanský et al., 2022). Previous studies have suggested that the application of biochar can significantly increase the pH of the soil, particularly acidic soils (Lu et al., 2022). A similar effect was reported for vermicompost, which can increase the pH value of soil through the association of negatively charged functional groups with hydrogen ions (Wang et al., 2021b). Biochar and vermicompost are mostly alkaline in nature, with biochar having a strong ion exchange capacity and vermicompost having a certain acid-base buffering capacity (Zhao et al., 2015; Li J. et al., 2018). Thus, both organic amendments can increase the pH of the soil. Our studies have shown that after adding organic materials, the pH of the soil was significantly increased, and the acidic soil can be improved to weakly alkaline soil, which is consistent with the previous research results. Applying fertilizer, exogenous elements can be imported into the soil, thereby altering the nutrient content of the greenhouse soil (Hernández et al., 2014). As the core of soil fertility, soil organic carbon is inextricably linked to the material cycle and energy flow in the soil. DOC is an active part of organic carbon, which is fast, less stable, and easily oxidized (Kaiser and Kalbitz, 2012; Guillaume et al., 2022; Zhang Y. J. et al., 2022). Biochar and vermicompost can effectively increase the organic matter content of the soil (Liu M. et al., 2020; Bi et al., 2021), which accounts for less than 10% of soil’s solid phase composition but plays a critical role in driving microbial activity and promoting mineral transformation. Their content also characterizes the nutrient status and quality of the soil (Wang Q. et al., 2022). Here, our findings revealed that the application of organic materials could enhance the content of organic matter and DOC concentration in the soil, both of which increase with the proportion of vermicompost. A significantly positive correlation was observed between pH and DOC concentration, as well as soil organic matter and DOC. Therefore, under the experimental conditions, the improvement effect of biochar and vermicompost is more pronounced as the organic matter content increased. Furthermore, the improvement effect of biochar was more evident than that of vermicompost.
In this study, urease activity was significantly higher in all treatments compared with CK, and the promotion effect increased with the increase of organic material applied. The increase in urease activity may be due to the amount of organic material applied, improving the physicochemical properties of the soil and providing a suitable environment for microbial colonization (El-Bassi et al., 2021; Lopes et al., 2021; Wang G. et al., 2022). Urease and alkaline phosphatase are hydrolases; the former can hydrolyze urea, and the latter can catalyze the mineralization of soil organic phosphorus compounds. Nitrate reductase plays a role in denitrification, and its activity, together with that of urease, can reflect the nitrogen use and transformation capacity of the soil (Li C. et al., 2018; Fu et al., 2020). Studies have revealed that vermicompost has a significant effect on plant nitrogen metabolism (Raza et al., 2022). Contrary to the results of previous studies, nitrate reductase activities in this study showed a decreasing trend compared with CK (Faccin and Di Piero, 2022), probably because that the application of organic amendments increases the organic matter in the soil, which increases the substrate available to microorganisms, thus enhancing the activity of denitrification enzymes. (Yang et al., 2020). The decrease in the relative abundance of Proteobacteria may also affect the carbon and nitrogen cycle in the soil. Contrary to previous research results, the results of this study showed that the alkaline phosphatase activity was inhibited under the action of vermicompost (Song et al., 2022). Combined with the results of correlation analysis, alkaline phosphatase activity was significantly and positively correlated with pH, DOC concentration, and Acidobacteria abundance, which may be due to the fact that vermicompost increased soil pH, thereby inhibiting enzymatic activity. The soluble salt content in vermicompost is high, and salt affects the abundance of Acidobacteria, thereby inhibiting the activity of phosphatase (Wang et al., 2016). DOC concentration and the activity of alkaline phosphatase showed a trend of first increasing and then decreasing with the increase of biochar dosage. On the one hand, it may be because that 1% biochar served as a habitat, which improved microbial abundance and enzyme activity. However, biochar at higher dosage may adsorb substrate needed by the phosphatase. On the other hand, we speculated that biochar changed the DOC concentration, thereby affecting the activity of alkaline phosphatase. Therefore, further studies are necessary to investigate the specific mechanism. Vermicompost can promote the increase in the number of bacteria and fungi and microbial biomass in soil, significantly enhancing microbial diversity and changing the microbial community structure (Maji et al., 2017). Biochar addition will affect the community structure of bacteria and fungi in the soil, significantly increasing the relative abundance of potentially beneficial bacteria and significantly decreasing the relative abundance of potentially pathogenic bacteria in the soil, which is beneficial to plant growth (Hou et al., 2022; Yang et al., 2022). The Actinomycetes phylum contains many bacteria that produce antimicrobial substances that are effective in suppressing soil-borne diseases, promoting nutrient cycling, and maintaining plant health (Liu H. et al., 2022). This finding indicated that the addition of biochar and vermicompost can significantly increase the diversity of bacterial and fungal communities, as well as the relative abundance of beneficial microorganisms. Organic amendments enhance the diversity and activity of microorganisms by adding nutrients to the soil and using its porous nature to provide a breeding ground for microorganisms (An and Tang, 2017). The diversity of the bacterial community increases with the increase of vermicompost levels, and the increase in bacterial community diversity in BC3 is the highest among the biochar treatment groups, indicating that the effect of biochar on bacterial community diversity does not increase indefinitely with the increase of biochar levels.
The physicochemical properties of the soil can also affect the microbial community. As biochar contains a large number of soluble nutrient elements, it can directly influence the physicochemical properties of the soil, and it has a positive effect on the activity of soil microorganisms. A study has demonstrated that Proteobacteria, Actinobacteria, and Acidobacteria are significantly correlated with the organic carbon content in aggregates (Zhao et al., 2018). Proteobacteria and Acidobacteria belong to eutrophic and oligotrophic groups, respectively. The former is suitable for growth under high carbon conditions, whereas the latter is suitable for growth under low carbon conditions (Liu P. et al., 2020; Su et al., 2022). In this study, correlation analyses showed that pH was negatively correlated with Proteobacteria but positively correlated with Acidobacteria. Proteobacteria are related to nitrogen fixation and carbon and nitrogen cycle (Li et al., 2021), and Actinobacteria associated with anabolic functions (Li et al., 2020; Zheng J. et al., 2021), the relative abundance of Proteobacteria decreased and the relative abundance of Actinobacteria increased, which may affect some metabolic functions. In addition, there was a significant positive correlation between Acidobacteria and DOC, which was consistent with previous studies. Proteobacteria is negatively correlated with DOC, contrary to previous studies (Zhao et al., 2018). Recent studies have borne out claims that Proteobacteria was key group which changes SOC mineralization and DOC structure (Cong et al., 2022). Under the conditions of this experiment, we speculate it may be that the increase of DOC was not significant, so the effect on the utilization of carbon by Proteobacteria was limited, and it may also be affected by pH.
In this study, correlation analysis and high-throughput sequencing methods were used to explore the effects of different contents of biochar and vermicompost on microbial and enzymatic activities of greenhouse soil. Based on the results, the following conclusions can be drawn:
1) Biochar and vermicompost have positive effects on soil physicochemical properties, and they showed a trend that the improvement effect increased with the increase of organic material content. The improvement effect of biochar was better than vermicompost.
2) The application of 3% biochar can effectively improve the homogeneity and richness of bacterial community.
3) 5% vermicompost had the best promotion effect on urease activity (with an increase of 222.76%). 3% biochar had the most obvious inhibition effect on the activity of nitrate reductase (with a decrease of 39.23%). It also has the most significant effect on alkaline phosphatase activity (with an increase of 68.6%).
Datasets are available on request: The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This work was financially supported by the National Key Research and Development Program of China (Grant No. 2019YFD1001900). We are grateful to GENEDENOVO (Guangzhou, China) for assisting in bioinformatics in this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1060277/full#supplementary-material
Alfadil, A. A., Xia, J., Shaghaleh, H., Alhaj Hamoud, Y., Ibrahim, J. N., Hamad, A. A. A., et al. (2021). Wheat straw biochar application improves the morphological, physiological, and yield attributes of maize and the physicochemical properties of soil under deficit irrigation and salinity stress. J. Plant Nutr. 44, 2399–2420. doi:10.1080/01904167.2021.1918156
An, Q., and Tang, W. (2017). The influences of straw biochar on growth and absorption of soil functional microorganisms. J. Shenyang Agric. Univ. 48, 411–417. doi:10.3969/j.issn.1000-1700.2017.04.004
Becagli, M., Arduini, I., and Cardelli, R. (2022). Using biochar and vermiwash to improve biological activities of soil. Agriculture 12, 178. doi:10.3390/agriculture12020178
Bi, Y., Kuzyakov, Y., Cai, S., and Zhao, X. (2021). Accumulation of organic compounds in paddy soils after biochar application is controlled by iron hydroxides. Sci. Total Environ. 764, 144300. doi:10.1016/j.scitotenv.2020.144300
Cai, Z. (2019). Scientific and technological issues of nutrient management under greenhouse cultivation in China. Acta Pedol. Sin. 56, 36–43. doi:10.11766/trxb201805310
Chang, T. T., Zhang, Y. J., Xu, H. L., Shao, X. H., Xu, Q. C., Li, F. L., et al. (2018). Osmotic adjustment and up-regulation expression of stress-responsive genes in tomato induced by soil salinity resulted from nitrate fertilization. Int. J. Agric. Biol. Eng. 11, 126–136. doi:10.25165/j.ijabe.20181103.2952
Chaudhary, D. R., Gautam, R. K., Yousuf, B., Mishra, A., and Jha, B. (2015). Nutrients, microbial community structure and functional gene abundance of rhizosphere and bulk soils of halophytes. Appl. Soil Ecol. 91, 16–26. doi:10.1016/j.apsoil.2015.02.003
Chernov, T. I., Tkhakakhova, A. K., and Kutovaya, O. V. (2015). Assessment of diversity indices for the characterization of the soil prokaryotic community by metagenomic analysis. Eurasian Soil Sc. 48, 410–415. doi:10.1134/s1064229315040031
Cong, P., Zheng, X., Han, L., Chen, L., Zhang, J., Song, W., et al. (2022). Biogas residue biochar still had ecological risks to the ultisol: Evidence from soil bacterial communities, organic carbon structures, and mineralization. J. Soils Sediments 22, 03269. doi:10.1007/s11368-022-03269-x
Edwards, J., Johnson, C., Santos-Medellín, C., Lurie, E., Podishetty, N. K., Bhatnagar, S., et al. (2015). Structure, variation, and assembly of the root-associated microbiomes of rice. Proc. Natl. Acad. Sci. U. S. A. 112, E911–E920. doi:10.1073/pnas.1414592112
El-Bassi, L., Azzaz, A. A., Jellali, S., Akrout, H., Marks, E. A. N., Ghimbeu, C. M., et al. (2021). Application of olive mill waste-based biochars in agriculture: Impact on soil properties, enzymatic activities and tomato growth. Sci. Total Environ. 755, 142531. doi:10.1016/j.scitotenv.2020.142531
Faccin, D., and Di Piero, R. M. (2022). Extracts and fractions of humic substances reduce bacterial spot severity in tomato plants, improve primary metabolism and activate the plant defense system. Physiol. Mol. Plant Pathol. 121, 101877. doi:10.1016/j.pmpp.2022.101877
Fu, Y., Zhang, Z., Yang, X., Wang, C., Lan, T., Tang, X., et al. (2020). Nitrate reductase is a key enzyme responsible for nitrogen-regulated auxin accumulation in Arabidopsis roots. Biochem. Biophys. Res. Commun. 532, 633–639. doi:10.1016/j.bbrc.2020.08.057
Guillaume, T., Makowski, D., Libohova, Z., Bragazza, L., Sallaku, F., and Sinaj, S. (2022). Soil organic carbon saturation in cropland-grassland systems: Storage potential and soil quality. Geoderma 406, 115529. doi:10.1016/j.geoderma.2021.115529
Guo, L., Bornø, M. L., Niu, W., and Liu, F. (2021). Biochar amendment improves shoot biomass of tomato seedlings and sustains water relations and leaf gas exchange rates under different irrigation and nitrogen regimes. Agric. Water Manag. 245, 106580. doi:10.1016/j.agwat.2020.106580
He, Y., Lan, Y., Zhang, H., and Ye, S. (2022). Research characteristics and hotspots of the relationship between soil microorganisms and vegetation: A bibliometric analysis. Ecol. Indic. 141, 109145. doi:10.1016/j.ecolind.2022.109145
Hernández, T., Chocano, C., Moreno, J., and García, C. (2014). Towards a more sustainable fertilization: Combined use of compost and inorganic fertilization for tomato cultivation. Agric. Ecosyst. Environ. 196, 178–184. doi:10.1016/j.agee.2014.07.006
Hou, J., Pugazhendhi, A., Phuong, T. N., Thanh, N. C., Brindhadevi, K., Velu, G., et al. (2022). Plant resistance to disease: Using biochar to inhibit harmful microbes and absorb nutrients. Environ. Res. 214, 113883. doi:10.1016/j.envres.2022.113883
Innangi, M., Niro, E., D Ascoli, R., Danise, T., Proietti, P., Nasini, L., et al. (2017). Effects of olive pomace amendment on soil enzyme activities. Appl. Soil Ecol. 119, 242–249. doi:10.1016/j.apsoil.2017.06.015
Ji, C., Ye, R., Yin, Y., Sun, X., Ma, H., and Gao, R. (2022). Reductive soil disinfestation with biochar amendment modified microbial community composition in soils under plastic greenhouse vegetable production. Soil Tillage Res. 218, 105323. doi:10.1016/j.still.2022.105323
Kaiser, K., and Kalbitz, K. (2012). Cycling downwards – dissolved organic matter in soils. Soil Biol. Biochem. 52, 29–32. doi:10.1016/j.soilbio.2012.04.002
Khan, M. Q., Rahman, K., Ghani, U., Basharat, A., Qamar, S. A., and Bilal, M. (2020). Synergistic effect of inhibitors (allylthiourea and 1, 2, 4-triazole) on the activity of wheat soil urease to reduce nitrogen loss. Case Stud. Chem. Environ. Eng. 2, 100059. doi:10.1016/j.cscee.2020.100059
Li, C., Ahmed, W., Li, D., Yu, L., Xu, L., Xu, T., et al. (2022). Biochar suppresses bacterial wilt disease of flue-cured tobacco by improving soil health and functional diversity of rhizosphere microorganisms. Appl. Soil Ecol. 171, 104314. doi:10.1016/j.apsoil.2021.104314
Li, C., Ma, S., Shao, Y., Ma, S., and Zhang, L. (2018). Effects of long-term organic fertilization on soil microbiologic characteristics, yield and sustainable production of winter wheat. J. Integr. Agric. 17, 210–219. doi:10.1016/s2095-3119(17)61740-4
Li, D., Chen, X., Peng, Z., Chen, S., Chen, W., Han, L., et al. (2012). Prediction of soil organic matter content in a litchi orchard of South China using spectral indices. Soil Tillage Res. 123, 78–86. doi:10.1016/j.still.2012.03.013
Li, J., Gu, K., Tang, C., Wang, H., and Shi, B. (2018). Advances in effects of biochar on physical and chemical properties of soils. J. Zhejiang Univ. Sci. 52, 192–206. doi:10.3785/j.issn.1008-973X.2018.01.025
Li, J., Zhang, X., Chen, Y., Jin, X., Ma, Z., Ji, D., et al. (2020). Potential functions of actinobacteria diversity in cyanobacteria and moss crusts in the southeastern Tengger Desert. Acta Ecol. Sin. 40, 1590–1601. doi:10.5846/stxb201901070055
Li, T., Guo, Z., Kou, C., Lv, J., Zhang, X., and Yang, X. (2017). Effects of extraction conditions on the test results of soil dissolved organic carbon. Ecol. Environ. Sci. 26, 1878–1883. doi:10.5846/stxb201901070055
Li, X., Dong, W., Song, A., Li, Y., Lu, Y., Wang, E., et al. (2021). Effects of straw addition on soil biological N2-fixation rate and diazotroph community properties. Sci. Agric. Sin. 54, 980–991. doi:10.3864/j.issn.0578-1752.2021.05.010
Liu, H., Dong, Y., Shen, M., Sun, F., Wang, X., Liu, J., et al. (2022). Characteristics of rhizosphere microbial communities in a disease- suppressive soil of tomato bacterial wilt and its disease-suppressive transmission mechanism. Acta Pedol. Sin. 59, 1125–1135. doi:10.3864/j.issn.0578-1752.2021.05.010
Liu, M., Wang, C., Liu, X., Lu, Y., and Wang, Y. (2020). Saline-alkali soil applied with vermicompost and humic acid fertilizer improved macroaggregate microstructure to enhance salt leaching and inhibit nitrogen losses. Appl. Soil Ecol. 156, 103705. doi:10.1016/j.apsoil.2020.103705
Liu, P., Xiao, J., Sun, B., Gao, M., Zhang, S., Yang, X., et al. (2020). Variation of bacterial community structure and the main influencing factors in Eum-orthic Anthrosols under different fertilization regimes. Plant Nutr. Fertilizer Sci. 2, 307–315. doi:10.11674/zwyf.19102
Liu, Y., Wang, P., and Wang, J. (2022). Formation and stability mechanism of soil aggregates: Progress and prospect. Acta Pedol. Sin., 5, 1–18.
Lopes, É. M. G., Reis, M. M., Frazão, L. A., Da Mata Terra, L. E., Lopes, E. F., Dos Santos, M. M., et al. (2021). Biochar increases enzyme activity and total microbial quality of soil grown with sugarcane. Environ. Technol. Innov. 21, 101270. doi:10.1016/j.eti.2020.101270
Lu, H., Li, K., Nkoh, J. N., Shi, Y., He, X., Hong, Z., et al. (2022). Effects of the increases in soil pH and pH buffering capacity induced by crop residue biochars on available Cd contents in acidic paddy soils. Chemosphere 301, 134674. doi:10.1016/j.chemosphere.2022.134674
Luo, A., Xia, D., Wang, X., Shi, D., Duan, J., Pi, Y., et al. (2020). Effects of organic materials on respiration and enzyme activity of upland yellow soil. Crop Res. 34, 568–573. doi:10.16848/j.cnki.issn.1001-5280.2020.06.11
Luo, G., Li, L., Friman, V., Guo, J., Guo, S., Shen, Q., et al. (2018). Organic amendments increase crop yields by improving microbe-mediated soil functioning of agroecosystems: A meta-analysis. Soil Biol. Biochem. 124, 105–115. doi:10.1016/j.soilbio.2018.06.002
Maji, D., Misra, P., Singh, S., and Kalra, A. (2017). Humic acid rich vermicompost promotes plant growth by improving microbial community structure of soil as well as root nodulation and mycorrhizal colonization in the roots of Pisum sativum. Appl. Soil Ecol. 110, 97–108. doi:10.1016/j.apsoil.2016.10.008
Mu, Y., Tang, D., Mao, L., Zhang, D., Zhou, P., Zhi, Y., et al. (2021). Phytoremediation of secondary saline soil by halophytes with the enhancement of γ-polyglutamic acid. Chemosphere 285, 131450. doi:10.1016/j.chemosphere.2021.131450
Nassal, D., Spohn, M., Eltlbany, N., Jacquiod, S., Smalla, K., Marhan, S., et al. (2018). Effects of phosphorus-mobilizing bacteria on tomato growth and soil microbial activity. Plant Soil 427, 17–37. doi:10.1007/s11104-017-3528-y
Raza, S. T., Wu, J., Rene, E. R., Ali, Z., and Chen, Z. (2022). Reuse of agricultural wastes, manure, and biochar as an organic amendment: A review on its implications for vermicomposting technology. J. Clean. Prod. 360, 132200. doi:10.1016/j.jclepro.2022.132200
Ronix, A., Cazetta, A. L., Ximenez, G. R., Spessato, L., Silva, M. C., Fonseca, J. M., et al. (2021). Biochar from the mixture of poultry litter and charcoal fines as soil conditioner: Optimization of preparation conditions via response surface methodology. Bioresour. Technol. Rep. 15, 100800. doi:10.1016/j.biteb.2021.100800
Shen, W., Hu, M., Qian, D., Xue, H., Gao, N., and Lin, X. (2021). Microbial deterioration and restoration in greenhouse-based intensive vegetable production systems. Plant Soil 463, 1–18. doi:10.1007/s11104-021-04933-w
Šimanský, V., Horák, J., and Bordoloi, S. (2022). Improving the soil physical properties and relationships between soil properties in arable soils of contrasting texture enhancement using biochar substrates: Case study in Slovakia. Geoderma Reg. 28, e00443. doi:10.1016/j.geodrs.2021.e00443
Song, X., Li, H., Song, J., Chen, W., and Shi, L. (2022). Biochar/vermicompost promotes Hybrid Pennisetum plant growth and soil enzyme activity in saline soils. Plant Physiol. biochem. 183, 96–110. doi:10.1016/j.plaphy.2022.05.008
Su, M., Ma, X., Hu, L., Zhao, L., Peng, J., Wang, H., et al. (2022). Effects of high-carbon basal fertilizers combined with nitrogen reduction on soil fertility and bacterial diversity. J. Agric. Biotechnol. 30, 1174–1185. doi:10.3969/j.issn.1674-7968.2022.06.013
Tang, L., Hamid, Y., Chen, Z., Lin, Q., Shohag, M. J. I., He, Z., et al. (2021). A phytoremediation coupled with agro-production mode suppresses Fusarium wilt disease and alleviates cadmium phytotoxicity of cucumber (Cucumis sativus L.) in continuous cropping greenhouse soil. Chemosphere 270, 128634. doi:10.1016/j.chemosphere.2020.128634
Torres, I. F., Bastida, F., Hernández, T., Albaladejo, J., and García, C. (2015). Enzyme activity, microbial biomass and community structure in a long-term restored soil under semi-arid conditions. Soil Res. 53, 553. doi:10.1071/sr14297
Van Groenigen, J. W., Van Groenigen, K. J., Koopmans, G. F., Stokkermans, L., Vos, H. M. J., and Lubbers, I. M. (2019). How fertile are earthworm casts? A meta-analysis. Geoderma 338, 525–535. doi:10.1016/j.geoderma.2018.11.001
Wang, F., Wang, X., and Song, N. (2021a). Biochar and vermicompost improve the soil properties and the yield and quality of cucumber (Cucumis sativus L.) grown in plastic shed soil continuously cropped for different years. Agric. Ecosyst. Environ. 315, 107425. doi:10.1016/j.agee.2021.107425
Wang, F., Zhang, W., Miao, L., Ji, T., Wang, Y., Zhang, H., et al. (2021b). The effects of vermicompost and shell powder addition on Cd bioavailability, enzyme activity and bacterial community in Cd-contaminated soil: A field study. Ecotoxicol. Environ. Saf. 215, 112163. doi:10.1016/j.ecoenv.2021.112163
Wang, G., Jin, Z., Wang, X., George, T. S., Feng, G., and Zhang, L. (2022). Simulated root exudates stimulate the abundance of Saccharimonadales to improve the alkaline phosphatase activity in maize rhizosphere. Appl. Soil Ecol. 170, 104274. doi:10.1016/j.apsoil.2021.104274
Wang, Q., Yuan, J., Yang, X., Han, X., Lan, Y., Cao, D., et al. (2022). Responses of soil respiration and C sequestration efficiency to biochar amendment in maize field of Northeast China. Soil Tillage Res. 223, 105442. doi:10.1016/j.still.2022.105442
Wang, Z., Luo, G., Li, J., Chen, S., Li, Y., Li, W., et al. (2016). Response of performance and ammonia oxidizing bacteria community to high salinity stress in membrane bioreactor with elevated ammonia loading. Bioresour. Technol. 216, 714–721. doi:10.1016/j.biortech.2016.05.123
Wang, Z., Ma, S., Hu, Y., Chen, Y., Jiang, H., Duan, B., et al. (2022). Links between chemical composition of soil organic matter and soil enzyme activity in alpine grassland ecosystems of the Tibetan Plateau. Catena (Amst) 218, 106565. doi:10.1016/j.catena.2022.106565
Wei, G., Chen, F., Hu, Y., Wu, Y., Zhang, Q., and Li, X. (2022). Effect of reducing soil disinfestation with different organic materials on microbial community at tobacco planted soil. Chin. J. Soil Sci. 53, 1056–1066. doi:10.19336/j.cnki.trtb.2021090701
Wu, Z., Fan, Y., Qiu, Y., Hao, X., Li, S., and Kang, S. (2022). Response of yield and quality of greenhouse tomatoes to water and salt stresses and biochar addition in Northwest China. Agric. Water Manag. 270, 107736. doi:10.1016/j.agwat.2022.107736
Yang, H., Li, Z., Deng, M., Sheng, Z., Zhang, Y., Hu, F., et al. (2020). Effects of the combined application of different fertilizers and urea on nitrogen transformation enzyme activities in tea-garden soil from Chongqing. Chin. J. Appl. Environ. Biol. 26, 1107–1114. doi:10.19675/j.cnki.1006-687x.2019.09010
Yang, K., Jiang, Y., Wang, J., Cai, X., Wen, Z., Qiu, Z., et al. (2022). Tobacco straw biochar improved the growth of Chinese cherry (Prunus pseudocerasus) via altering plant physiology and shifting the rhizosphere bacterial community. Sci. Hortic. 303, 111244. doi:10.1016/j.scienta.2022.111244
Yang, L., Zhao, F., Chang, Q., Li, T., and Li, F. (2015). Effects of vermicomposts on tomato yield and quality and soil fertility in greenhouse under different soil water regimes. Agric. Water Manag. 160, 98–105. doi:10.1016/j.agwat.2015.07.002
Zhang, C., Li, X., Yan, H., Ullah, I., Zuo, Z., Li, L., et al. (2020). Effects of irrigation quantity and biochar on soil physical properties, growth characteristics, yield and quality of greenhouse tomato. Agric. Water Manag. 241, 106263. doi:10.1016/j.agwat.2020.106263
Zhang, H., Qian, W., Wu, L., Yu, S., Wei, R., Chen, W., et al. (2022). Spectral characteristics of dissolved organic carbon (DOC) derived from biomass pyrolysis: Biochar-derived DOC versus smoke-derived DOC, and their differences from natural DOC. Chemosphere 302, 134869. doi:10.1016/j.chemosphere.2022.134869
Zhang, Y., Gao, W., Luan, H., Tang, J., Li, R., Li, M., et al. (2022). Effects of a decade of organic fertilizer substitution on vegetable yield and soil phosphorus pools, phosphatase activities, and the microbial community in a greenhouse vegetable production system. J. Integr. Agric. 21, 2119–2133. doi:10.1016/s2095-3119(21)63715-2
Zhang, Y. J., Yu, S. G., Li, Z. J., Chang, T. T., Xu, Q. C., Xu, H. L., et al. (2022). Effects of excessive nitrogen fertilizer and soil moisture deficiency on antioxidant enzyme system and osmotic adjustment in tomato seedlings. Int. J. Agric. Biol. Eng. 15, 127–134. doi:10.25165/j.ijabe.20221502.5555
Zhao, F. Z., Fan, X. D., Ren, C. J., Zhang, L., Han, X. H., Yang, G. H., et al. (2018). Changes of the organic carbon content and stability of soil aggregates affected by soil bacterial community after afforestation. Catena (Amst) 171, 622–631. doi:10.1016/j.catena.2018.08.006
Zhao, H., Li, T., Zhao, Z., Liu, C., Wang, M., Li, D., et al. (2015). Cucumber seedling growth as influenced by worm cast medium added with vermiculite and nitrogen-phosphorus-potassium fertilizers. Acta Agric. Shanghai, 9, 13–20. doi:10.15955/j.issn1000-3924.2015.05.03
Zheng, D., Li, H., and Han, L. (2021). Soil Actinomycetes resource and their application. J. Green Sci. Technol. 2, 183–187. doi:10.16663/j.cnki.lskj.2021.02.064
Zheng, J., Wang, S., Wang, R., Chen, Y., Siddique, K. H. M., Xia, G., et al. (2021). Ameliorative roles of biochar-based fertilizer on morpho-physiological traits, nutrient uptake and yield in peanut (Arachis hypogaea L.) under water stress. Agric. Water Manag. 257, 107129. doi:10.1016/j.agwat.2021.107129
Keywords: biochar (BC), microbial community diversity, vermicompost (VC), soil quality (SQ), soil physicochemical properties
Citation: Wu Q, Zhang J, Liu X, Chang T, Wang Q, Shaghaleh H and Hamoud YA (2023) Effects of biochar and vermicompost on microorganisms and enzymatic activities in greenhouse soil. Front. Environ. Sci. 10:1060277. doi: 10.3389/fenvs.2022.1060277
Received: 03 October 2022; Accepted: 06 December 2022;
Published: 04 January 2023.
Edited by:
Yuncong Li, University of Florida, United StatesReviewed by:
Atif Muhmood, Ayub Agriculture Research Institute, PakistanCopyright © 2023 Wu, Zhang, Liu, Chang, Wang, Shaghaleh and Hamoud. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jie Zhang, emhhbmdqaWVqeGRAaGh1LmVkdS5jbg==; Yousef Alhaj Hamoud, eW91c2VmLWhhbW91ZDExQGhodS5lZHUuY24=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.