- 1CAS Key Laboratory of Crust-Mantle Materials and Environment, School of Earth and Space Sciences, University of Science and Technology of China, Hefei, Anhui, China
- 2The Second Institute of Hydrological and Engineering Geological Prospecting of Anhui Geological Prospecting Bureau, Wuhu, Anhui, China
Groundwater provides drinking water to city and rural residents; which is also one of the chief water sources for commercial and agricultural activities in Jieshou City. We collected and analyzed the samples of 18 underground water source wells in Jieshou. We investigated whether the water was of acceptable quality and had characteristics that exceeded the standard. This study was conducted to determine the chemical characteristics of groundwater and abnormally high super-standard ions found in groundwater. The hydrogeological conditions of the study area were analyzed through data collection; through sample collection and sample testing, the characteristics and types of water chemistry were analyzed by means of mathematical statistics analysis and the Piper chart. The genesis of water chemistry was discussed using the Gibbs chart and correlation analysis; the proportional coefficient of ion molar concentration was used to judge the source, origin, and forming process of groundwater chemical composition. The results show that the groundwater is classified as marginally alkaline water, with a composition of Na-HCO3. The cations are mainly Na+, and the anions are mainly HCO3−. According to the Ⅲ water standard of groundwater quality standard and comparing the content of each ion, Na+ and F− are the primary abnormal super-standard ions, and ions and compounds are the main occurrence states. The concentrations of Na+ and F− exceed the standard for class Ⅲ water. There was a positive correlation between the abnormal Na+ and F−, and the concentration of F− increased with the increase in monitoring depth. The causes of abnormal ions were mainly determined by the lithology of the aquifer in the study area, and most of them are fluorine-containing rocks, which are transferred into groundwater through leaching or hydration. The enrichment of Na+ and F− is influenced by the local primary geological setting, hydrochemical type, hydrogeological conditions, pH and artificial activities, and the primary geological setting is the main influencing factor.
1 Introduction
Groundwater resources characterized by wide distribution and stable water yield are vital water sources for agricultural irrigation, industries, and city life. In the northern part of China, groundwater as a crucial drinking water source for cities has been widely developed and utilized (Zhang et al., 2021). Epidemiological studies have shown that long-term high-fluoride intake from drinking water may lead to dental or skeletal fluorosis, which is suspected to be the leading cause of endemic fluorosis in some areas.
The water quality of the centralized underground water source directly affects the drinking water and the health of the residents. Hence, it is of great significance to study the water quality and its chemical characteristics for the rational development and utilization of underground water resources and the healthy drinking water of residents (Li et al., 2021; Xiao et al., 2021).
Several studies investigated the geochemical characteristics of groundwater under different climate conditions and geological background conditions and discovered that the groundwater in some areas shows high levels of salinity, nitrogen, fluoride, arsenic, etc., which can affect the groundwater quality (Lapworth et al., 2017; Adimalla, 2019), China (Wu and Sun, 2016; Yin et al., 2019; Li et al., 2021; Xiao et al., 2021). The geochemical characteristics of groundwater are closely related to factors such as the hydrogeological condition, the water-bearing media’s lithology, the aquifer’s hydrodynamic characteristics, and water–rock (soil) interactions (Liu et al., 2015; Kumar and Kuriachan, 2020). Some solutes in groundwater are gradually enriched under specific geological circumstances, reaching quantities that impair irrigation and drinking; this process is generally irreversible (Zhu et al., 2017).
Fuyang City is located in the northwestern part of Anhui province, with the most population in this province, and its industrial and agricultural water use is mainly sourced from groundwater. In recent years, despite a severe water shortage caused by water quality deterioration, serious groundwater resource pollution, and a lack of regulation and storage, the urban areas of Fuyang City, Taihe County, Linquan County, and Jieshou City have continued to exploit large amounts of groundwater to maintain average production and life, so groundwater quality and drinking water safety issues are significant concerns (Liu et al., 2012).
Jieshou is a county under Fuyang. The city has 23 centralized groundwater-type water sources, including one county-level source and 22 for villages, towns, and rural regions, supplying 729,500 people with 71,420 m3 daily. Using the Groundwater quality standard GB/T14848-2017 class III standard (GB, 2017), samples of water that exceed the standard (1.0 mg/L) are called “over-standard.”
According to the results of water quality testing conducted by local water source administrative departments, 13 of the 23 groundwater sources exceeded the standard for sodium content, and all 23 groundwater sources exceeded the class III groundwater standard of 1.0 mg/L for fluoride content, with some exceeding the standard by 1.12 times.
Fluoride is a crucial non-metallic halogen in the human body. Fluoride may fortify teeth and bones and minimize the occurrence of dental caries when administered in sufficient amounts. Nonetheless, if the human body consumes an excessive amount of fluoride, it will have negative effects on the bones and lead to conditions such as bone pain, rickets, and dental fluorosis (Zhang, 2014). High fluoride content in groundwater affects the exploitation and utilization of groundwater resources and the safety of drinking water for residents. Therefore, it has been studied worldwide (Karunanidhi et al., 2019; Yousefi et al., 2019; Haji et al., 2021).
This study evaluated county-level groundwater-type water sources from 18 drinking water source wells to analyze the hydrogeochemical characteristics and abnormal over-standard ions and also explored the cause of abnormal ions. Moreover, this study aims to 1) understand the geochemical characteristics of the groundwater in county-level water sources in Jieshou City, 2) determine abnormal over-standard ions, and 3) analyze the cause of abnormal ions.
2 Materials and methods
2.1 Study area
Jieshou is situated in the northwestern region of Anhui province, along the border between Anhui and Henan. It is at 115°15′ and 115°32′ east and 33°00′ and 33°30′ north. It borders Taihe County to the east, Fuyang City to the southeast, Linquan County to the south, Shenqiu County of Henan province to the west, and Dancheng County of Henan province to the north. Due to historic Yellow River floods, the administrative territory of Jieshou City shows the geomorphic characteristic of a deposition plain, linking the Yellow River flood plain and the Hejian Plain. The terrain along the Quan River is flat, high in the northwest and low in the southeast, and low-lying. On the majority of the planet’s surface, a Cenozoic Quaternary sedimentary layer is covered by sandy soil. The city has a maximum width of 25 km from east to west and a maximum length of 58 km from south to north.
There is a semi-humid monsoon climate in a warm temperature zone in Jieshou City, with an annual average temperature of 15.4°C and an annual average precipitation of 886.5 mm. There are two major tributaries of the Huai River that flow from east to west: the Quan River and the Ying River.
2.2 Description of an aquifer
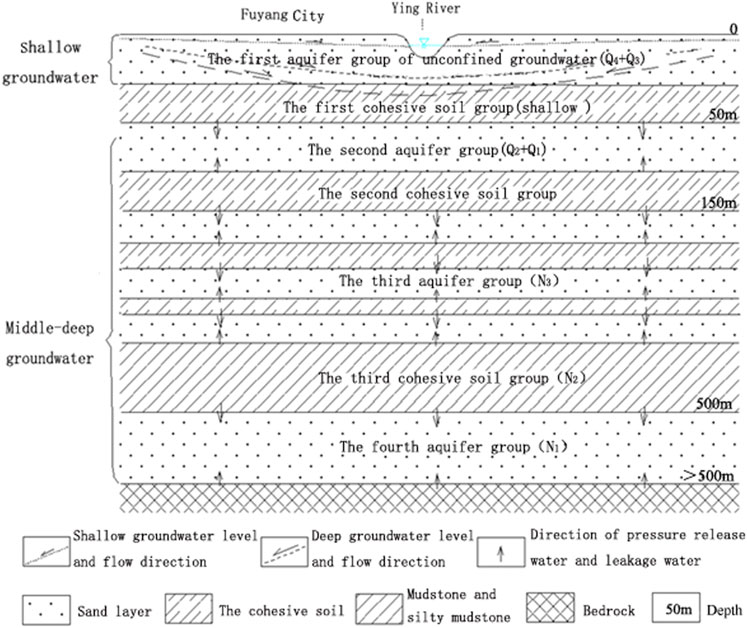
The groundwater in the region of study consists mainly of unconfined rock pore water. According to aquifer characteristics, groundwater’s buried state, hydrodynamic properties, and the link between atmospheric precipitation and surface water, the unrestrained rock porous aquifer group is classified into the shallow porous aquifer group and the deep porous aquifer group.
The shallow groundwater exists in the 50 m shallow Holocene and Late Pleistocene layers and is directly tied to atmospheric precipitation and surface water. It may be referred to as the first aquifer group (shallow); the middle-deep groundwater exists in the stratum 50 m below the surface and is unrelated to precipitation or surface water. According to the hydrogeological structure and exploitation situation, the middle-deep groundwater of unconfined rock is divided into three aquifer groups, namely, the second aquifer group (buried depth of 50–150 m), the third aquifer group (buried depth of 150–500 m), and the fourth aquifer group (buried depth of 500–1,000 m) (buried depth greater than 500 m). The details of Fuyang’s aquifer structure are given in Figure 1.
2.3 Sample collection and statistical analysis
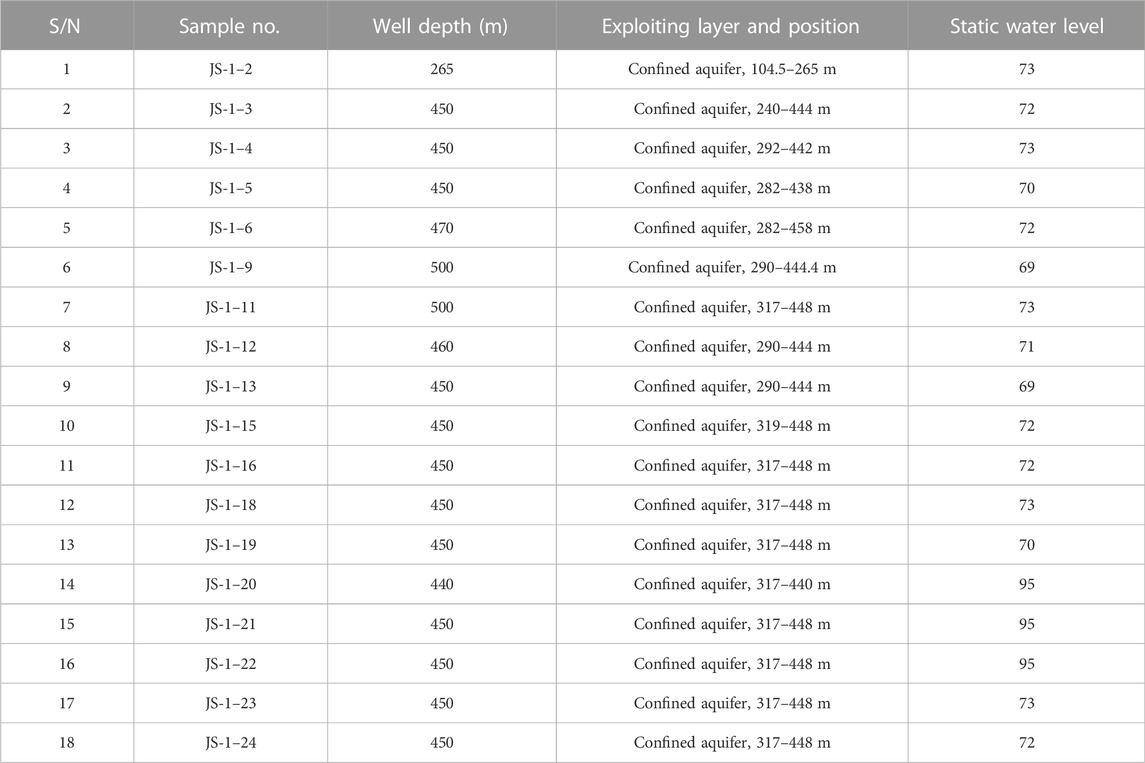
In this study, 18 groundwater samples were collected from the confined aquifer through pumping wells during July 2021. The information on water source wells is shown in Table 1. Sampling locations were recorded using the RTK (real-time kinematic) device (Figure 2). All the wells were the primary sources of drinking and domestic water use for residents. The sampling processes followed national technical regulations. Before sampling, the wells were pumped for 10 min to avoid stagnant water in the pipeline. The containers were brown polyethylene plastic bottles, rinsed with deionized water in the laboratory, and washed again using the groundwater to be sampled before sampling. Two replicates of each sample were collected to ensure the reliability and validity of the data.
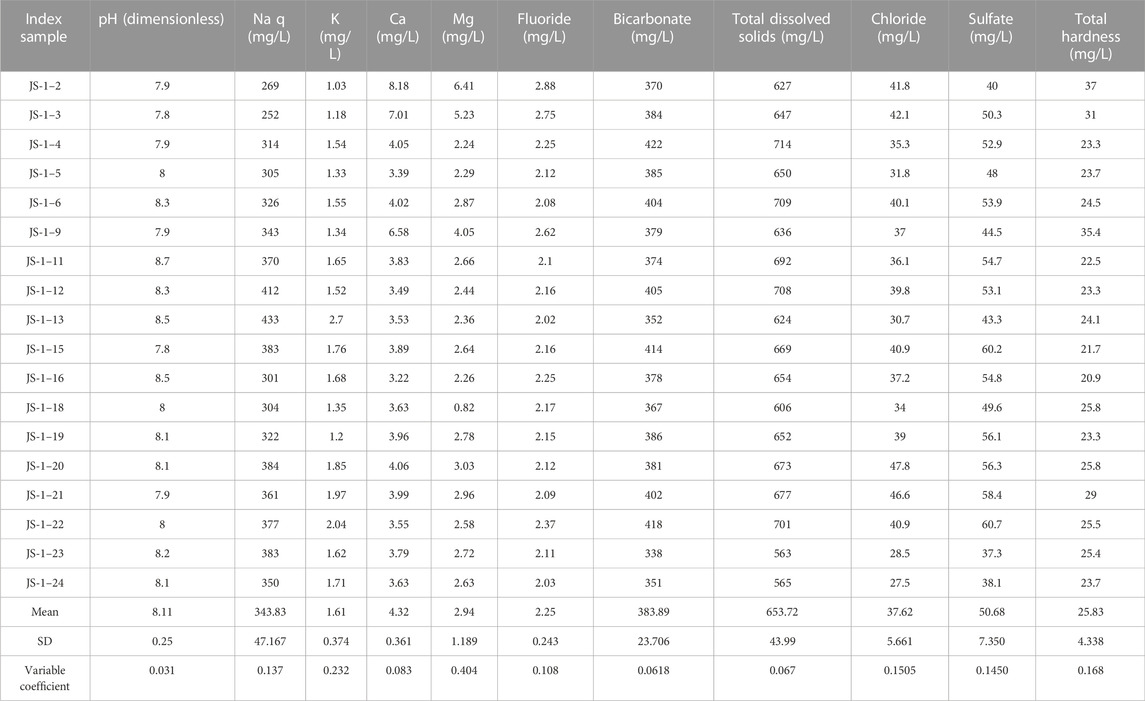
All samples were stored in a refrigerator at 4°C. All monitoring indicators for groundwater samples are shown in Table 2. The samples were mainly analyzed for pH, EC, total dissolved solids (TDS), total hardness (TH), main cations (K+, Ca2+, Na+, and Mg2+), main anions (HCO3−, CO32-, Cl−, and SO42-), nitrogen pollutants (NH4+, NO3−, and NO2−), and F−. Main cations were determined by ICP-OES (Avio 200, United States); Cl−, SO42-, and NO3− were determined by ion chromatographs (Eco IC and CIC-D100); F− was determined by a thundermagnetic ion activity meter (PXSJ-216); NH4+ and NO2− were determined by a visible light spectrophotometer (L2); and HCO3− and CO32- were determined by titration. All indexes were measured strictly according to the Quality Standard for Groundwater of the People’s Republic of China (GB/T 14,848–2017).
In this study, statistical andalyses were performed using Excel 2019. Correlation, factor analyses, and a graph of correlation were performed using SPSS 21.0. The diagram of the aquifer, the Piper diagram, and the Gibbs diagram were completed with the help of AutoCAD 2010 and Origin 2020. The proportional coefficient of ion molar concentration was used to assess the evolution process of groundwater chemical composition.
2.4 Groundwater quality assessment
In this study, the single-factor evaluation method was used to evaluate groundwater quality. Single-index evaluation refers to the analysis and evaluation of a single index to reflect the situation of a single pollutant in groundwater. In the evaluation of a single index, the groundwater quality category is determined by referring to the level limits of each participating index. When the limit values of indicators in different categories are the same, the quality standard is preferred instead of the inferior one, that is, when a factor can be identified as both the quality standard and the inferior standard, the quality standard is the first choice. Finally, the worst factor category was selected as the water quality category for the group.
The evaluation formula is Ii = Ci/C0; Ii is the water quality index of single factor i; Ci is the measured water quality value of a component; and C0 is the standard value of class Ⅲ in the Groundwater Quality Standard (GB/T 14,848–2017). Ii ≤ 1 indicates that the water quality factor meets the corresponding water quality standards; Ii ≥ 1 indicates that the water quality exceeds the corresponding water quality standard. The single-factor evaluation method can clearly identify the main pollution factors (abnormal superscalar factor) by comparing the representative values of each pollution factor concentration with the evaluation criteria (Hu, 2015).
2.5 Analysis of available data
Based on the collected geological background and surface environmental data of the study area, the lithology and rock-forming minerals of groundwater aquifers were analyzed, and the source, precipitation, and migration mechanisms of abnormal ions were studied. Combined with the previous monitoring data, the causes of abnormal ions in the groundwater of the centralized drinking water source in Jieshou City were comprehensively analyzed.
3 Results and discussion
3.1 Hydrochemical characteristics
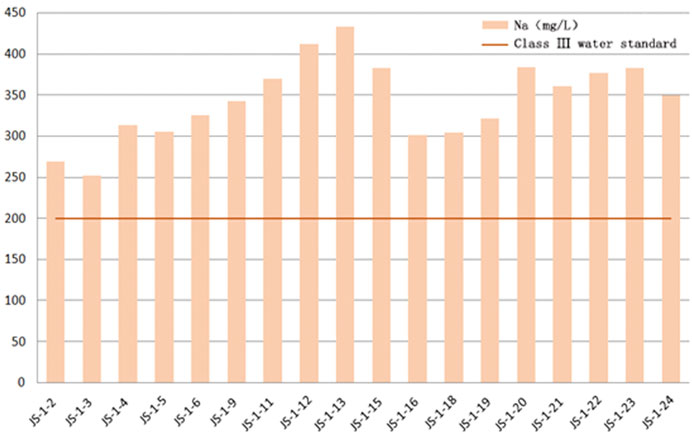
Using a mathematical statistic of the chemical indexes of county-level water sources in Jieshou City, the 18 groundwater samples collected this time have a pH value of 7.8–8.7, falling into the category of weak alkaline water. The major anion in the water is HCO3−, with an ionic concentration range of 338–422 mg/L, a mean value of 383.89 mg/L, a standard deviation of 23.706 mg/L, and a variable coefficient of 0.0618. The second major anion is SO42-, with an ionic concentration range of 37.3–60.2 mg/L, a mean value of 50.678 mg/L, a standard deviation of 7.350 mg/L, and a variable coefficient of 0.1450. The third anion is Cl−, with a content range of 38.1–47.8 mg/L, a mean value of 31.617 mg/L, a standard deviation of 5.661 mg/L, and a variable coefficient of 0.1505. The content variation trend of Cl− shows a certain positive correlation with SO42-. The major positive ion in the water is Na+, with an ionic concentration variation range of 252–433 mg/L and a mean value of 343.83 mg/L, followed by Ca2+ and Mg2+, with an average ionic concentration of 4.322 mg/L and 2.943 mg/L, respectively. Due to the similar physicochemical properties of magnesium and calcium, their contents in groundwater samples from water source wells show a positive correlation; the content of K+ is minimal, with a mean value of 1.612 mg/L (Table 3).
The TDS concentration range is 563–714 mg/L, falling into the category of highly mineralized water. All samples were tested without Cu, Mn, Zn, Be, Ni, Co, Ti, Cr, or Cr(VI).
3.2 Hydrochemical type
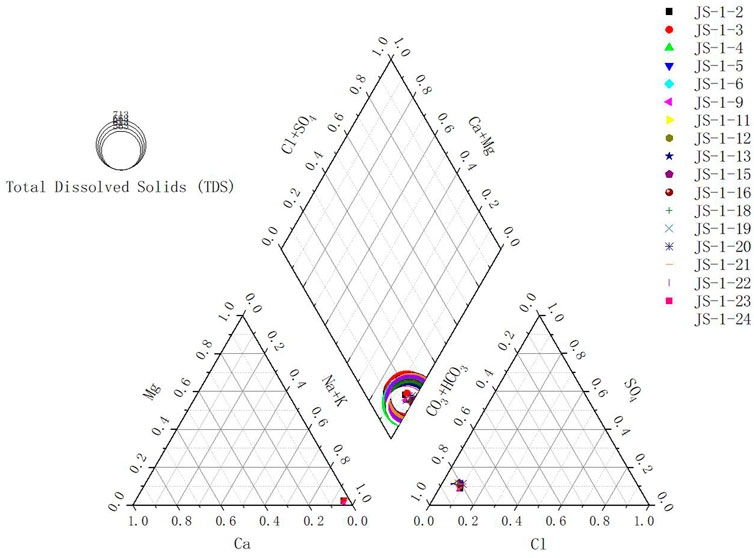
Piper diagrams are widely used to study groundwater chemical types and can effectively reflect hydrochemical types and characteristics (Liu et al., 2020). The hydrochemical type can reflect the content proportion of the major anions and positive ions in the water body, which is usually represented by a piper trilinear chart. According to the proportion of different ions, the hydrochemical type is divided into 16 categories. For the water source wells in Jieshou City, the plot results are shown in Figure 3.
The plot results indicate that since most water source wells are concentrated in an urban area and their depths are very close, the hydrochemical types of groundwater samples from water source wells in Jieshou City are very similar, showing that Na+ and HCO3− are dominant. Their hydrochemical type falls into the Na-HCO3-Cl-SO4 type, approximating the Na-HCO3 type. There could be multiple reasons for the formation of the Na-HCO3-Cl-SO4 type, including the following: 1) when the runoff condition of deep groundwater is not good, the concentrations of Cl−and Na+ lead to the hydrochemical types of groundwater being the Na-HCO3-Cl-SO4 type and 2) the dissolution of gypsum and calcite resulted in the enrichment of SO42−and Ca2+ (Gao et al., 2020).
3.3 Determination of abnormally enriched ions
With reference to the Quality Standard for Ground Water GB/T14848-2017, these research results are taken as the basis for determining whether the content of a hydrochemical constituent in the groundwater exceeds the standard. The groundwater whose indexes can meet the class III water standard can be taken as drinking water.
From the determination results of the samples and the previously monitored data, it is discovered that the ions and chemical constituents, in the groundwater samples of Jieshou City, that exceed national standards can be divided into two categories. Only few samples contain such ions over the standard in one category. An occasional contingency or an error may arise in these ions during the test and cause their level to exceed the standard by a small extent. Hence, they are not the focus of this study. In the other category, according to previous and current monitoring data, some ions are generally enriched to an extent far greater than the limit specified in the National Quality Standard for Ground Water GB/14848-2017. In particular, in the results of water source wells, some groundwater samples fall into the hydrochemical constituent of class IV or even class V groundwater. Such a class of hydrochemical constituent is considered abnormally enriched ions.
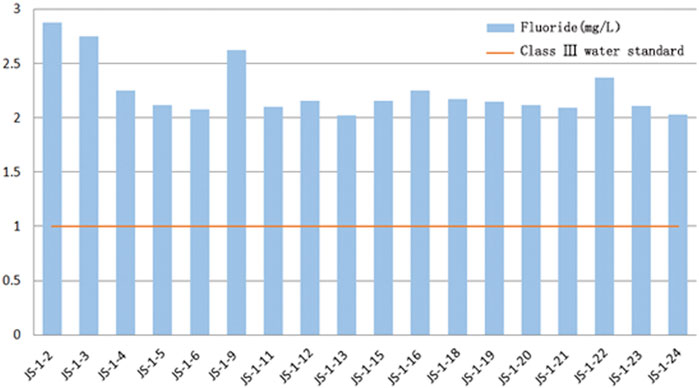
In the test results this time, the major abnormal ions are Na+ and F−. In accordance with the Quality Standard for Ground Water GB/T 14848–2017, class III groundwater is required to contain sodium less than 200 mg/L. Still, in the water source well samples of Jieshou City, the sodium content range is between 252 and 433 mg/L, significantly exceeding the class III water standard, and some samples even exceed the class IV water standard of 400 mg/L. The abnormally enriched F− is another significant feature. In the water source well samples collected this time, the fluoride content range was between 2.02 and 2.88 mg/L, which exceeds the admissible limit of 1 mg/L in the “Quality Standard for Ground Water GB/T 14,848–2017.” Most of the GW samples from the study area exceeded the standard. Therefore, Na+ and F− are determined as abnormally enriched ions (Kong et al., 2020).
3.4 Occurrence state of abnormal ions
3.4.1 Occurrence state of fluoride
Fluoride, a VII A group element in the periodic table of the elements with an atomic number of 9, belongs to the halogen element. Its simple substance has very strong reactivity, and it is easy to get an electron to form F−. Fluoride mainly exists in the form of an ion or combined state in the water–soil system, in which migration and transformation are closely related to the occurrence state of fluoride. When the environmental conditions change, the occurrence state of fluoride will also vary accordingly.
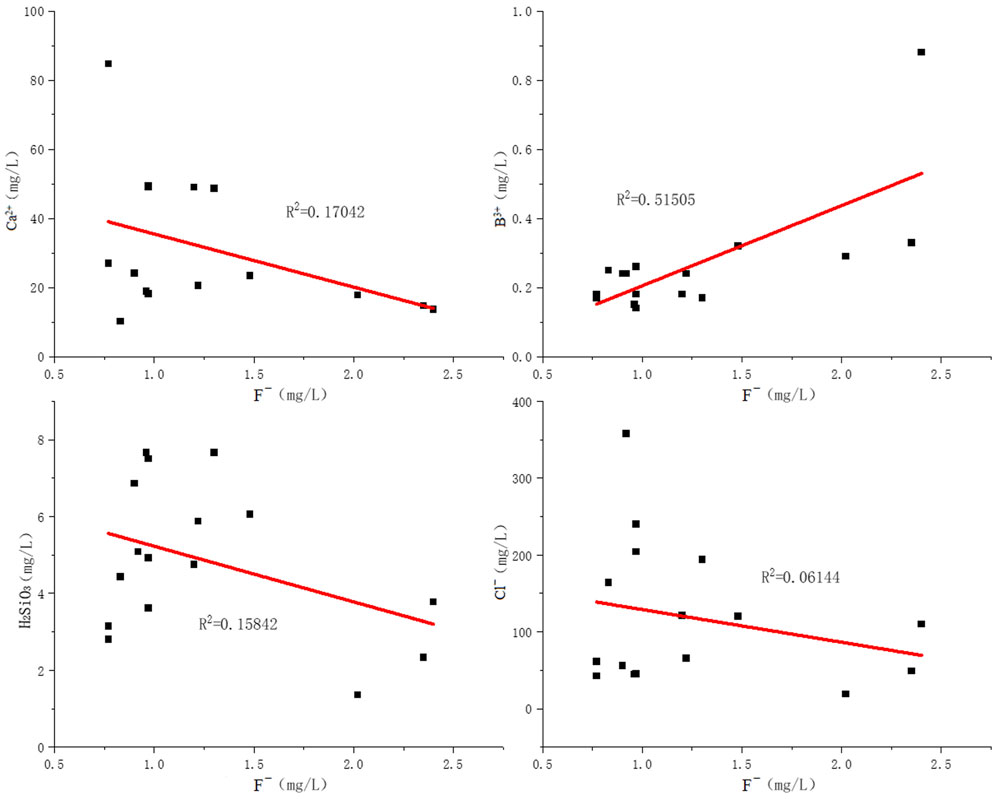
It is known from the test results that fluoride exists in groundwater mainly in the forms of F−, MgF+, CaF+, and NaF; the F− in groundwater is evidently positively correlated to the content of TDS, HCO3−, and Na+, while showing an unsharp negative correlation with Ca2+, Mg2+, Cl−, and SO42−.
Most water source wells in the county-level water sources of Jieshou City are more than 400 m deep. The groundwater at this depth is middle-deep groundwater, with weak water flow, slow horizontal circulation, and mainly vertical alternating. The salinity in the water is not easy to migrate, forming a superior storage condition for F−, and the hydrochemical action is dominated by evaporation and inspissation, facilitating the enrichment of fluoride and the formation of groundwater with high fluoride (Liu et al., 2015; Li et al., 2017).
3.4.2 Occurrence state of sodium
Sodium, an element of alkali metal with an atomic number of 11, can easily lose only one electron in the valence shell and form Na+. Due to its high reactivity, there is no simple sodium substance in nature. Most of sodium exists in minerals in the form of a compound. The content of sodium element in the earth’s crust is 2.27%, ranking it seventh in abundance in the earth’s crust and fifth among metal elements, and its abundance in the ocean is 1.08 × 104 mg/L. The majority of sodium salt minerals are highly soluble, but some sodium minerals have poor solubility or do not even dissolve. The common sodium minerals are montmorillonite [(Na,Ca)0.33(Al,Mg)2 [Si4O10](OH)2·nH2O], hornblende [(Ca,Na)2–3 [Mg, Fe(II),Fe(III), Al]5 [(Al,Si)8O22](OH)2], albite [Na2OAl2O3.6SiO2], etc (Rajmohan et al., 2021).
3.5 Distribution characteristics and change of abnormal ions
It can be known from the aforementioned test results that in the county-level water sources of Jieshou City, the Fe, Mn, and iodide levels conform to the class III water standard of groundwater, but the fluoride and sodium ions greatly exceed the standard by 1.88 times and 1.165 times, respectively. The over-standard fluoride and sodium ions show a positive correlation. The distribution of abnormal ions in the wells of water sources is shown in Figure 4, Figure 5.
1) Middle aquifer: JS-1–2 samples were collected from the aquifer (104.5–265 m) with a fluoride content of 2.88 mg/L at the maximum, which is 1.88 times the class III water standard of groundwater.
2) Deep aquifer: The rest of the samples were collected from the aquifer 280–448 m, with fluoride content between 2 and 2.4 mg/L, generally exceeding the standard by 1–1.4 times the class III water standard of groundwater.
3) The overall trend of vertical distribution shows that the fluoride concentration increases with the depth of monitoring wells, and the fluoride content is relatively higher in the deep aquifer. It is inferred that this relates to the calcium ion content in the groundwater to a certain extent.
The high fluoride content in groundwater has caused a great level of harm to human health (Liu et al., 2021). The analysis of fluoride distribution and its causes in groundwater can provide a basis for determining the source of fluoride and the safety of drinking water (Zhang et al., 2020; Liu et al., 2021).
3.6 Cause analysis of abnormal ions
3.6.1 Source of abnormal ions
1) Material source of fluoride
The main reason for high fluorion fluoride concentrations is the continuous dissolution of fluorinated minerals. The groundwater of the research area is unconfined pore water, and the middle-deep aquifer group is composed of Neogene–Early and Middle Pleistocene calcareous argillaceous glutenite and Quaternary system alluvial sand. According to the analysis of existing data, the fluoride released from the hydrolysis and corrosion of hornblende, pyroxene, gneiss, biotite, granite, evaporite, albite, fluorapatite, and fluorinated mineral bedrock makes a significant contribution to the high fluoride levels in groundwater (Yang et al., 2017). Different types of rocks always have different fluoride contents, determining the differentiation of fluoride background values in different regions. In natural conditions, rocks or fluorinated minerals may release the fluoride into the water body, atmosphere, and soil under the combined effects of water–soil action, volcanic movement, and bedrock weathering and then integrate with the regional fluoride geochemical abnormality to form the fluorosis area. High-fluoride rocks and minerals are the ultimate sources of regional fluoride pollution or fluorosis.
2) Regional background features of sodium
The county-level water sources of Jieshou City belong to middle-deep groundwater, and its aquifer is mainly composed of silty sand, fine sand, and medium sand. The sandstone contains many sodium-bearing minerals such as albite, which enters the groundwater via ion exchange action, so the aquifer lithology is the primary source of sodium. In addition, the research area is located in the Huaibei Plain, one of the regions with a potentially wide distribution of soda saline-alkali soil in China. The salty soda soil formed by various causes is rich in all kinds of sodium salts. This part of the sodium salt can probably enter the groundwater under the action of rain eluviation, which is an essential source of sodium in groundwater as well.
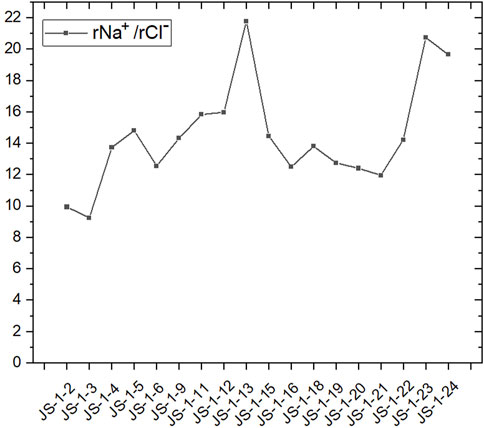
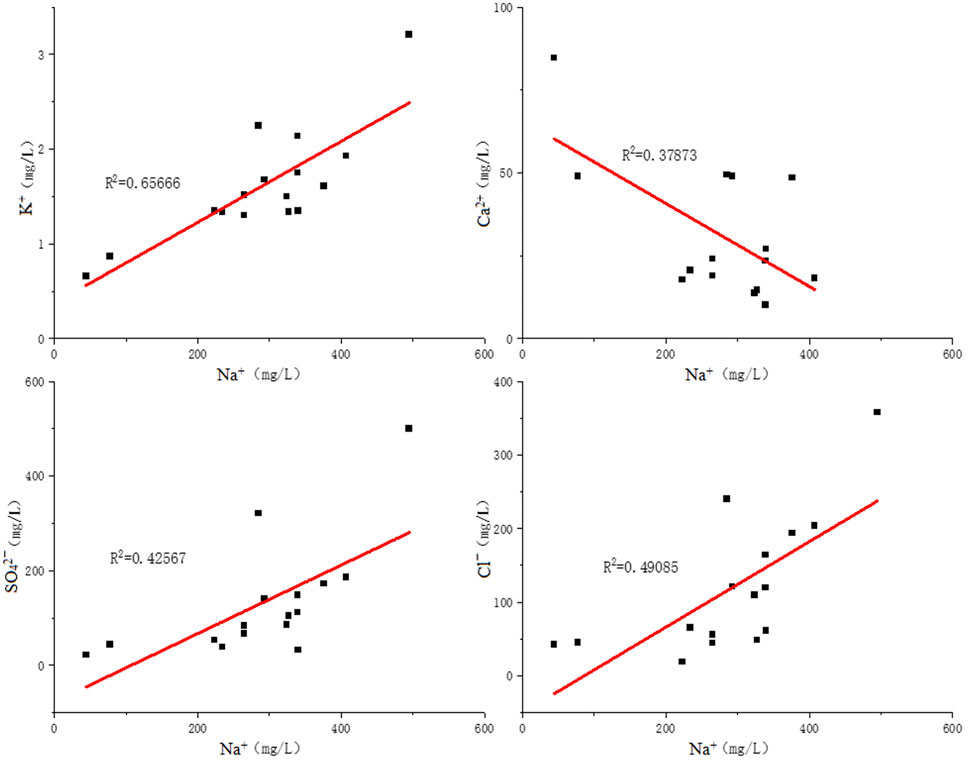
In studying the chemical composition of groundwater, some hydrogeochemical issues can often be studied by using the proportionality coefficient of ion molar concentration between various components. The proportionality coefficient of ion molar concentration can be applied to determine the source, cause, and forming process of groundwater’s chemical components; better describe the evolutionary process and characteristics of the hydrochemical component; and make a typical analysis of the hydrogeochemical evolution. rNa+/rCl− and rCa2+/rSO42- can reflect the source of main ions in groundwater. In the samples collected this time, the results of the rNa+/rCl− ratio in water source wells are far higher than 1 (Figure 6), indicating that the dissolving process of feldspar, niter, and other sodium-bearing minerals has happened; this further proves that feldspar and other sodium-bearing minerals are significant sources of sodium in the groundwater of Jieshou City (Zango et al., 2019).
3.6.2 Separation of abnormal ions
1) Groundwater formation mechanism
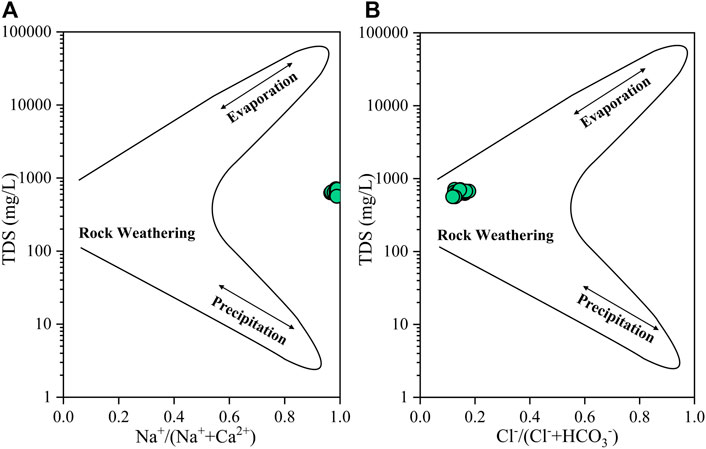
The soluble ions in the groundwater mainly originate from the weathering decomposition of rocks and soil and the atmospheric precipitation, so the Gibbs diagram (Gibbs, 1970) is often used to qualitatively assess the hydrochemical influence of regional rocks, atmospheric precipitation, and evaporation concentration on groundwater (Sunkari et al., 2020; Yu et al., 2020). The groundwater-tested hydrochemical data are projected into the Gibbs diagram. Rock weathering represents the rock weathering degree, precipitation represents the atmospheric precipitation degree, and evaporation represents the evaporation concentration degree. The results of this chart indicate that the main ion component of groundwater originates from the rock weathering process. In this weathering process, sodium-bearing minerals dissolve, allowing sodium to enter the groundwater (Hu, 2015).
The TDS content of the groundwater in Jieshou’s concentrated groundwater source is generally high, and the ratio of ρ(Na+)/ρ(Na++ Ca2+) is large (0.93–1.0), and the ratio of ρ(Cl−)/ρ(Cl− + HCO3−) is small (0.1–0.28) (Figure 7). The results show that the ionic characteristics of groundwater in Jieshou City are influenced by rock weathering and evaporation, of which the latter is more notable. In addition, the ρ (Na+)/ρ (Na++ Ca2+) ratio of water samples in most areas falls outside the Gibbs curve, indicating that the water may be affected by groundwater overextraction and other external factors, leading to the change of separation composition (Kong et al., 2020).
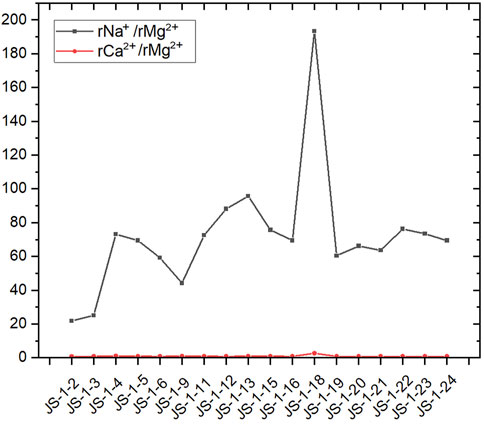
2) Mineralization degree
rNa+/rMg2+ and rCa2+/Mg2+ can reflect the degree of mineralization of groundwater. In general conditions, the calcium ion is a dominant cation in low-mineralized water. As the mineralization degree increases, sodium gradually becomes the dominant cation. In the water source well results of Jieshou City, rNa+/rMg2+ is far more abundant than rCa2+/rMg2+ (Figure 8), showing that the degree of mineralization of the deep well is significantly higher, leading to the enrichment of sodium. High salinity in groundwater is conducive to the dissolution of fluorite, allowing fluoride to enter the groundwater (Li et al., 2020). According to the molar ratios of rNa+/rMg2+ and rCa2+/rMg2+, the dominant interactions between groundwater and rocks can be distinguished, which are evaporation, dissolution, and silicate weathering, respectively (Xiao et al., 2015; Olea-Olea et al., 2020).
3) Ion exchange
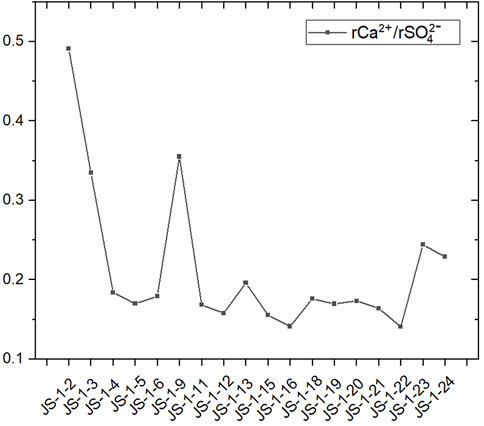
Ion exchange is one crucial approach for abnormally enriched ions to enter the groundwater. Affected by the ion radius and the electric charge of the ions, fluoride often enters the water body by exchanging hydroxide in water solution. At the same time, the sodium is more likely to exchange with the calcium ions in the water. In the research area, fluoride shows a significant correlation at a level of 0.059 (both sides), and the groundwater, with a pH value that is more alkaline, provides a good condition for the enrichment of fluoride. The adsorption capability and degree of the ions in groundwater relate to the ions’ concentration in the groundwater. Suppose the pH value of groundwater is higher (to be alkaline), then the degree of mineralization of groundwater increases. In that case, the alternate adsorption of cations in the water will also be enhanced, and the calcium ions existing in the groundwater will displace sodium from the sodium salt (Frommen and Groeschke, 2021). With the increase of sodium in the water, the amount of fluoride will also increase. Typically, the calcium ions in the groundwater come from the dissolution of gypsum, so its ratio to sulfate is basically maintained at 1:1, while most rCa2+/rSO42- ratios in the water source well samples of Jieshou City are less than 1 (Figure 9). This proves the loss of calcium, which displaces the sodium in minerals through ion exchange with sodium-bearing minerals, thus separating from the groundwater system.
4) Hydrodynamic condition of groundwater
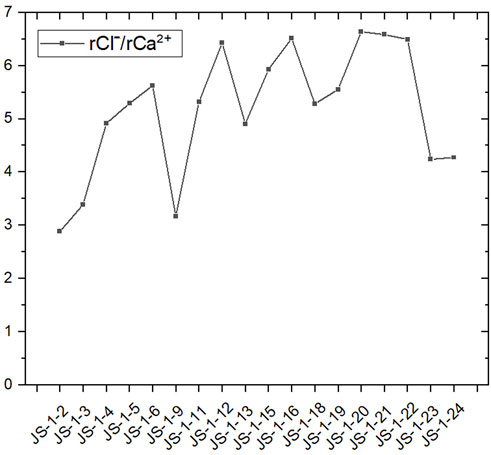
rCl−/rCa2+ can be used to reflect the hydrodynamic properties and lixiviation of groundwater. The hydrodynamic condition of shallow groundwater in Jieshou City changes considerably (Figure 10), but on average, the lixiviation of a water source well is evidently higher than the results of a monitor well, which is consistent with the higher enrichment of sodium and fluoride in water source wells. The results revealed the lixiviation, and the hydrodynamic condition is one of the causes of abnormally enriched ions in the groundwater of Jieshou City.
5) Hydrochemical condition
Regarding fluoride, it is known from the groundwater test data of Jieshou City that as far as the distribution area of calcareous water in groundwater is concerned, the fluoride contained in it is lower. In comparison, the fluoride contained in the sodium water distribution area is high, but as far as fluoride is concerned, its calcium salt and sodium salt have different solubilities. In general conditions, the solubilities of calcium fluoride and sodium fluoride in water are 16 mg/L and 42 × 103 mg/L, respectively. When the sodium fluoride is hydrolyzed, the fluoride will exist in the groundwater as ions. Due to its low solubility, the calcium fluoride will exist in water as sediment. Thus, the groundwater shows the phenomenon of “high calcium and low fluoride or high sodium and high fluoride” (Singh et al., 2020). In other words, if the cations in groundwater are mainly calcium ions, in view of the solubility of calcium fluoride, the groundwater will contain less fluoride; but when the cations in groundwater are mainly sodium, on account of the strong solubility of sodium fluoride, there will be significant fluoride enrichment. The relationship between fluoride and other ions or elements is shown in Figure 11. Furthermore, the bicarbonate and carbonate contained in groundwater could cause a chemical reaction with calcium fluoride to generate calcium carbonate or calcium bicarbonate, further accelerating its hydrolysis and thus increasing the fluoride content in groundwater (Yu et al., 2015; Zhang et al., 2020).
Regarding sodium, the hydrochemical component results of groundwater in Jieshou City also demonstrate some beneficial chemical conditions for the enrichment of sodium in groundwater or show other ions’ content level features coupled with the sodium enrichment process. The details are as follows:
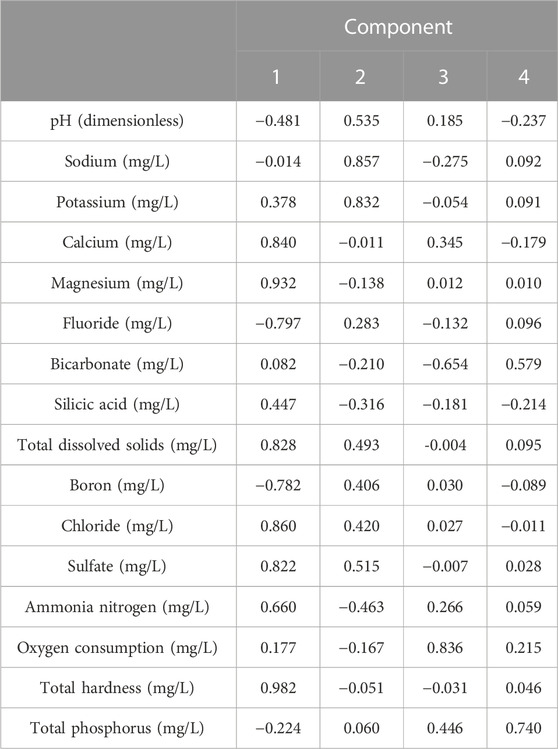
(1) Factor analysis was performed using IBSS 2021 statistical software, and it was discovered that the second major elements, sodium and potassium, contribute greatly to the extraction results, indicating that the alkali metals have a common source (natural mineral); meanwhile, other ions that show larger positive loads are boron, chloride, and sulfate; all these ions can be explained as the results of the lixiviation process of nature, and these provide a basis for the mineral source of sodium (Table 4).
(2) Correlation analysis shows that the sodion sodium content is strongly negatively correlated with calcium ions this time. This verifies that in the process of ion exchange, the increase of sodium content can restrain the calcium ion content; sodium has a positive correlation with boron, chloride, and sulfate ions; sodium has a weak positive correlation with pH value, indicating that the alkali environment is in favor of the dissolution of sodium. The groundwater of Jieshou City exactly shows weak alkali environment, providing a favorable condition for the enrichment of sodium in the water body. The correlation of sodion and other ions is shown in Figure 12.
3.6.3 Migration mechanism of abnormal ions
1.) Migration mechanism of fluoride
The formation lithology of the research area mainly includes the Quaternary silt, silty fine sands, and clay; the Neogene mudstone and siltite; the Paleogene sand shale and siltite; and the Cretaceous glutenite. The type of groundwater is unconfined rock pore water. The aquifer group’s Earth shell generally contains a high content of fluoride minerals; the clay minerals are rich in kaolinite, montmorillonite, and hydromica, and the sand layer contains mica, apatite, fluorite, hornblende, etc. During weathering, eluviation, and pollution production, they are transferred to groundwater under lixiviation or hydration action and become an important source of natural groundwater. The main reactions are as follows:
1) The decomposition equation of biotite under alkaline conditions (high pH value) is as follows:
2) The decomposition equation of hornblende under alkaline conditions is as follows:
3) The chemical reaction equation of CaF2 (fluorite) is as follows:
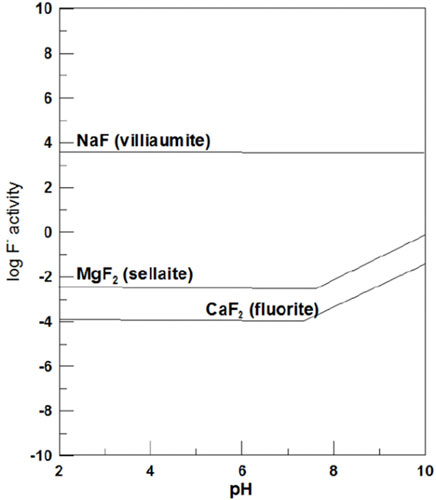
Equations (1) and (2) represent the replacement action, and Eq. (3) represents the hydrolysis reaction of fluorite. The relation between the solubility of fluoride-rich minerals (frequently observed in fluorite) and the pH value is shown in Figure 13 (Elrashidi and Lindsay, 1986), in which the solubility of fluorite and sellaite significantly increases with the pH value. According to the groundwater test results of Jieshou City, the pH value of deep water in county-level water sources is between 7.8 and 8.7, showing alkalinity. Therefore, the reaction described in Equations (2) and (3) is the main reaction of fluoride rise. Many domestic and international scholars have carried out a great deal of empirical research on this. Alkaline water is beneficial to the dissolution of F−-containing minerals (Lü et al., 2016). The Ca2+ concentration promotes further dissolution of F−-containing minerals such as fluorite, thus releasing F− into groundwater (Liu et al., 2015; Li et al., 2019).
2.) Migration mechanism of sodium
In general, sodium exists as sodium in the water body. However, in addition to existing as ions in minerals, they may also be adsorbed on the surface of negatively charged clay particles through electrostatic interaction. Therefore, the process of sodium entering the water body mainly includes the sodium-bearing minerals dissolving, a part of the indissolvable minerals exchanging ions with other cations in the water body, and the sodium adsorbing on the surface of mineral particles de-adsorbing.
Within the administrative area of Jieshou City, there are some thick, unconfined Quaternary fluvial and lacustrine sediments deposits on the surface layer, which is also the leading development site of groundwater in the city (Hu, 2015). The sediments are rich in various sodium-bearing minerals such as feldspar, niter, and clay minerals and are easy to enter the water body through the aforementioned process, which provides a solid foundation for the source of sodium in the groundwater of Jieshou City (Figure 14). Some sodium-containing minerals such as fluorite and montmorillonite can absorb a cation in GW and release an intrinsic cation. The higher the concentration of cations in GW, the stronger its adsorption capacity (Frommen and Groeschke, 2021).
3.6.4 Influential factors of abnormal ion enrichment
1) Climate and hydrogeological conditions
The research area is located in a semi-arid region with low rainfall, high evaporation, and intense evaporation inspissation action. The shallower the groundwater depth is, the stronger the evaporation inspissation will be. When water is evaporated, the salinity will accumulate in the residual water, causing the total dissolved solids to rise. When evaporation inspissation occurs, the salts precipitate in order of increasing solubility. Because of the rising concentration of a chemical component in water and the precipitation of salts, the proportion of chemical components in water also changes. The evaporation inspissation causes the region to form a sodium carbonate distribution area and increases the fluoride and sodium content in the water.
Extensive literature research shows that the fluoride contained in the shallow groundwater of the north Anhui area exceeds the standard (Ding et al., 2009; Wu et al., 2011); the shallow regional groundwater has a certain hydraulic connection to deep groundwater; and leaking recharge from shallow groundwater to deep groundwater will bring chemical constituents to the latter. Some studies have demonstrated (Xu et al., 2009) that the fluoride content of groundwater is negatively correlated with runoff conditions. If the groundwater runoff condition is good and the circulation alternation is quick, the fluoride content will be lower; in contrast, if the groundwater runoff condition is poor and the circulation alternation is slow, fluoride is likely to enrich.
2) Chemical type of groundwater
The fluoride concentration in groundwater can be affected by chemical constituents and the hydrochemical type of groundwater. It is known from the analysis that the hydrochemical type of research area is the Na-HCO3 type. According to the test results, the samples with high Ca2+ concentrations have low fluoride concentrations. In conformity with existing research results, the regions with high fluoride concentrations in groundwater often have high concentrations of Ca2+, HCO3−, and Na+ (Guo et al., 2007; Su et al., 2013). This phenomenon occurs because when groundwater’s water-bearing medium is calcium, Na+ exchanges cations with Ca2+, elevating the Ca2+ concentration in groundwater and increasing CaF2 solubility; thus, fluoride content increases in groundwater. High concentrations of HCO3− and Na+ is beneficial for the dissolution of CaF2 (Xu et al., 2009).
3) pH
A large number of studies have indicated that the pH value of groundwater is positively correlated with fluoride concentration (Han et al., 2022; Su et al., 2009; He et al., 2010; Lu et al., 2014). This is mainly because when groundwater pH is high, the OH− concentration rises in groundwater, and OH− has a radius close to F−. Also, equivalent electrovalence makes it easier to displace F− in fluorinated minerals. In addition, the meta-alkaline groundwater is beneficial to the dissolution of fluorinated minerals.
4) Human factors
In the research area, the buried depth of the deep aquifer is considerable, generally greater than 200 m, and the upper aquiclude is a thick clay stratum with good water-proof performance. In natural conditions, shallow groundwater is evidently affected by climatic conditions and human factors, but deep groundwater is less affected.
However, human exploitation of deep groundwater can damage the aquifer structure, change the original hydrodynamic condition of groundwater, cause groundwater from high-fluoride aquifers to flow into a low-fluoride aquifer, and elevate its fluoride concentration. In some regions, the overexploitation of deep groundwater leads to groundwater depression cone and leakage recharge from the upper high-fluoride aquifer to deep groundwater, thus increasing the fluoride concentration of deep groundwater.
4 Conclusion
According to the regional data of the past and this sampling analysis, the groundwater is classified as marginally alkaline water with a composition of Na-HCO3-Cl-SO4; the cations are mainly Na+, and the anions are mainly HCO3−. The fluoride and sodium in the groundwater from centralized drinking water sources in Jieshou City are abnormally enriched ions. Their contents have exceeded the thresholds specified in the class III water standard of the National Quality Standard for Ground Water: sodium, 200 mg/L and fluoride, 1.0 mg/L. Some samples even exceed the class IV water standard.
The abnormal enrichment of ions in the centralized drinking water sources of Jieshou City is caused by natural geological factors, hydrogeological conditions, and groundwater’s hydrochemical type. The primary geological setting is the main influencing factor, and human factors have little influence on it. The high content of F− and Na+ is caused by geological factors and hydrogeological conditions, while the aquifer group’s Earth shell generally contains a high content of fluoride minerals, and the sand layer contains mica, apatite, fluorite, and hornblende, etc. During weathering, eluviation, and pollution, they are transferred to groundwater under lixiviation or hydration action and become a significant source of fluoride in natural groundwater. In addition, the high sodium ion content is also related to the type of water chemistry and ion exchange.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GS: sample collecting, experimentation, methodology, software, and writing—original draft. GL: conceptualization and review and editing. YL: investigation and sample collecting. GW: software and experimentation.
Funding
The authors acknowledge the support from the National Key Research and Development Project of China (2020YFC1908601, 2020YFC1908602) and the National Natural Science Foundation of China (41972166).
Acknowledgments
We wish to thank the editor and the reviewers for their helpful suggestions and comments that significantly improved the research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adimalla, N. (2019). Groundwater quality for drinking and irrigation purposes and potential health risks assessment: A case study from semi-arid region of south India. Expo. Health 11, 109–123. doi:10.1007/s12403-018-0288-8
Ding, D., Xu, G. Q., and He, X. W. (2009). Analysis on hydrochemical characteristics and influential factors of the fluorine in shallow groundwater of Huaibei plain. Water Resour. Prot. 25 (2), 64–68.
Elrashidi, M. A., and Lindsay, W. L. (1986). Chemical-Equilibria of fluorine in soils-a theoretical development. Soil Sci. 141 (4), 274–280. doi:10.1097/00010694-198604000-00004
Frommen, T., Groeschke, M., Nolscher, M., Koeniger, P., and Schneider, M. (2021). Anthropogenic and geogenic influences on peri-urban aquifers in semi-arid regions: Insights from a case study in northeast jaipur, Rajasthan, India. India Hydrogeol. J. 29 (3), 1261–1278. doi:10.1007/s10040-021-02301-7
Gao, Y., Qian, H., Ren, W., Wang, H., Liu, F., and Yang, F. (2020). Hydrogeochemical characterization and quality assessment of groundwater based on integrated-weight water quality index in a concentrated urban area. J. Clean. Prod. 260, 121006. doi:10.1016/j.jclepro.2020.121006
GB (2017). General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. Bingjing: Standardization Administration of the People’s Republic of China, 1–14.
Gibbs, R. J. (1970). Mechanisms controlling world water chemistry. Science 170 (3962), 1088–1090. doi:10.1126/science.170.3962.1088
Guo, Q., Wang, Y., Ma, T., and Ma, R. (2007). Geochemical processes controlling the elevated fluoride concentrations in groundwaters of the Taiyuan Basin, Northern China. J. Geochem. Explor. 93 (1), 1–12. doi:10.1016/j.gexplo.2006.07.001
Haji, M., Karuppannan, S., Qin, D., Shube, H., and Kawo, N. S. (2021). Potential human health risks due to groundwater fluoride contamination: A case study using multi-techniques approaches (gwqi, FPI, gis, hhra) in bilate river basin of southern main Ethiopian rift, Ethiopia. Ethiop. Arch. Environ. Contam. Toxicol. 80 (1), 277–293. doi:10.1007/s00244-020-00802-2
Han, C., Liu, J., Gao, Z., Xu, Y., Zh, Y., Han, Z., et al. (2022). Chemical characteristics, evolution, and quality of groundwater and processes controlling its fluoride concentration features: Case study of a typical high-fluoride areas in the southwestern shandong plain, China. Environ. Sci.Poll. Res. 29, 19003–19018. doi:10.1007/s11356-021-16928-2
He, J., Zhang, F. C., and Han, S. B. (2010). Analysis on distribution features and cause of high-fluorine groundwater in Northern China [J]. Geol. China 37 (3), 621–626.
Hu, Y. H. (2015). “Study on geochemical characteristics of water environment in groundwater sources of northern Anhui [D],”Doctoral dissertation. (Huainan, China: Anhui University of Science and Technology).
Karunanidhi, D., Aravinthasamy, P., Subramani, T., Roy, P. D., and Srinivasamoorthy, K. (2019). Risk of fluoride-rich groundwater on human health: Remediation through managed aquifer recharge in a hard rock terrain, south India. South. India. Nat. Res. Res. 29 (4), 2369–2395. doi:10.1007/s11053-019-09592-4
Kong, L., Wang, Z., and Wang, B. (2020). An analysis of the hydro-chemical characteristics and causes of drinking water source of concentrated deep groundwater in Fuyang city [J]. China Rural Water Hydropower 21(3), 78–82.
Kumar, P. J. S., and Kuriachan, L. (2020). Chemometric appraisal of groundwater quality for domestic, irrigation and industrial purposes in lower Bhavani River basin. Tamil Nadu, India. Int. J. Environ. Anal. Chem. 2020, 1770241. doi:10.1080/03067319.2020.1770241
Lapworth, D. J., Krishan, G., Macdonald, A. M., and Rao, M. S. (2017). Groundwater quality in the alluvial aquifer system of northwest India: New evidence of the extent of anthropogenic and geogenic contamination. Sci. Total Environ. 599–600, 1433–1444. doi:10.1016/j.scitotenv.2017.04.223
Li, J., Shi, Z., Liu, M., Wang, G., Liu, F., and Wang, Y. (2021). Identifying anthropogenic sources of groundwater contamination by natural background levels and stable isotope application in Pinggu basin, China. J. Hydrol. 596, 126092. doi:10.1016/j.jhydrol.2021.126092
Li, J. X., Wang, Y. T., Zhu, C. J., Xue, X., Qian, K., Xie, X., et al. (2020). Hydrogeochemical processes controlling the mobilization and enrichment of fluoride in groundwater of the North China Plain. Sci. Total Environ. 730, 138877. doi:10.1016/j.scitotenv.2020.138877
Li, J., Zhou, H., Qian, K., Xie, X., Xue, X., Yang, Y., et al. (2017). Fluoride and iodine enrichment in groundwater of north China plain: Evidences from speciation analysis and geochemical modeling. Sci. Total Environ. 598, 239–248. doi:10.1016/j.scitotenv.2017.04.158
Li, P., He, X., Li, Y., and Xiang, G. (2019). Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese loess plateau: A case study of tongchuan, northwest China. Northwest China. Expo. Health 11, 95–107. doi:10.1007/s12403-018-0278-x
Liu, F., Wang, X., and Zhu, P. F. (2012). Analysis of antimony distribution and environment assessment in collapsed lake water body in Huaibei mining area [J]. JUSTC 42 (1), 242.
Liu, H., Guo, H., Yang, L., Wu, L., Li, F., Li, S., et al. (2015). Occurrence and formation of high fluoride groundwater in the hengshui area of the north China plain. Environ. Earth Sci. 74 (3), 2329–2340. doi:10.1007/s12665-015-4225-x
Liu, J., Gao, Z., Wang, Z., Xu, X., Su, Q., Wang, S., et al. (2020). Hydrogeochemical processes and suitability assessment of groundwater in the Jiaodong Peninsula, China. Environ. Monit. Assess. 192 (10), 384–417. doi:10.1007/s10661-020-08356-5
Liu, J., Peng, Y., Li, C., Gao, Z., and Chen, S. (2021). A characterization of groundwater fluoride, influencing factors and risk to human health in the southwest plain of Shandong Province, North China. North China. Ecotoxicol. Environ. Saf. 207, 111512. doi:10.1016/j.ecoenv.2020.111512
Lu, G. S., Han, B. P., and Wu, F. (2014). Discussion on high-fluorine groundwater features and cause in southwest Shandong area [J]. Geol. China 41 (1), 294–302.
Lü, J., Qiu, H., Lin, H., Yuan, Y., Chen, Z., and Zhao, R. (2016). Source apportionment of fluorine pollution in regional shallow groundwater at you’xi county southeast China. Chemosphere 158, 50–55. doi:10.1016/j.chemosphere.2016.05.057
Olea-Olea, S., Escolero, O., Mahlknecht, J., Ortega, L., Taran, Y., Moran-Zenteno, D. J., et al. (2020). Waterrock interaction and mixing processes of complex urban groundwater flow system subject to intensive exploitation: The case of Mexico city. J. South Am. Earth Sci. 103, 102719. doi:10.1016/j.jsames.2020.102719
Rajmohan, N., Masoud, M. H. Z., and Niyazi, B. A. M. (2021). Impact of evaporation on groundwater salinity in the arid coastal aquifer, Western Saudi Arabia. West. Saudi Arabia. Catena 196, 104864. doi:10.1016/j.catena.2020.104864
Singh, G., Rishi, M. S., Herojeet, R., Kaur, L., Priyanka, M., and Sharma, K. (2020). Multivariate analysis and geochemical signatures of groundwater in the agricultural dominated taluks of Jalandhar district, Punjab. India. J. Geochem Explor 208, 106395.
Su, C., Wang, Y., Xie, X., and Li, J. (2013). Aqueous geochemistry of high-fluoride groundwater in datong basin, northern China. J. Geochem. Explor. 135, 79–92. doi:10.1016/j.gexplo.2012.09.003
Su, K. Z., Xu, D. Q., and Tang, D. J. (2009). Study on adsorption performance of fluorine removal agent - natural plumbum soil[J]. Journal of Hefei University of Technology. Nat. Sci. Ed. 32 (7), 1042–1045.
Sunkari, E. D., Abu, M., Zango, M. S., and Wani, A. M. L. (2020). Hydrogeochemical characterization and assessment of groundwater quality in the kwahu-bombouaka group of the voltaian supergroup. Ghana J. Afr. Earth Sci. 169, 103899.
Wu, B. R., Wang, M. R., and Zhao, W. D. (2011). Analysis on distribution law and source of shallow high-fluorine groundwater in Huaibei plain in Anhui [J]. J. Hefei Univ. Technol. Nat. Sci. Ed. 33 (12), 1862–1865.
Wu, J., and Sun, Z. (2016). Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Mid-west China. Expo. Health 8, 311–329. doi:10.1007/s12403-015-0170-x
Xiao, J., Jin, Z. D., Wang, J., and Zhang, F. (2015). Hydrochemical characteristics, controlling factors and solute sources of groundwater within the Tarim River Basin in the extreme arid region, NW Tibetan Plateau. Quat. Int. 380–381, 237–246. doi:10.1016/j.quaint.2015.01.021
Xiao, Y., Liu, K., Hao, Q., Li, J., Zhang, Y., Cui, W., et al. (2021). Hydrogeochemical features and genesis of confined groundwater and health perspectives for sustainable development in urban hengshui, north China plain. North China plain. J. Chem. 2021, 1–15. doi:10.1155/2021/5578192
Xu, G. Q., Liu, J., and Zhu, Q. S. (2009). Analysis on distribution features and influential factors of fluorine in shallow groundwater of Huaibei plain in Anhui[J]. J. Water Res. Water Eng. 20 (5), 9–13.
Yang, N., Liu, J. F., and Liao, A. M. (2017). Distribution and influential factors of deep high-fluorine groundwater in typical area of northern Anhui[J]. Hydro. Eng. Geo., 20, 33–41.
Yin, S., Xiao, Y., Gu, X., Hao, Q., Liu, H., Hao, Z., et al. (2019). Geostatistical analysis of hydrochemical variations and nitrate pollution causes of groundwater in an alluvial fan plain. Acta geophys. 67, 1191–1203. doi:10.1007/s11600-019-00302-5
Yousefi, M., Ghalehaskar, S., Asghari, F. B., Ghaderpoury, A., Dehghani, M. H., Ghaderpoori, M., et al. (2019). Distribution of fluoride contamination in drinking water resources and health risk assessment using geographic information system, northwest Iran. Regul. Toxicol. Pharmacol. 107, 104408. doi:10.1016/j.yrtph.2019.104408
Yu, H., Gui, H. R., Zhao, H. H., Wang, M. C., Li, J., Fang, H. X., et al. (2020). Hydrochemical characteristics and water quality evaluation of shallow groundwater in Suxian mining area, Huaibei coalfield, China. Int. J. Coal Sci. Technol. 12, 825–835. doi:10.1007/s40789-020-00365-6
Yu, S., Sun, P. A., Du, W. Y., He, S. y., and Li, R. (2015). Effect of hydrochemistry characteristics under impact of human activity: A case study in the upper reaches of the xijiang river basin. Environ. Sci. 36 (1), 72–79.
Zango, M. S., Sunkari, E. D., Abu, M., and Lermi, A. (2019). Hydrogeochemical controls and human health risk assessment of groundwater fluoride and boron in the semi-arid North East region of Ghana. J. Geochem Explor 207, 106363. doi:10.1016/j.gexplo.2019.106363
Zhang, B. (2014). “Distribution law and cause analysis of high-fluorine groundwater in Northwest Shandong plain [D],”Master dissertation. (Beijing: China University Of Geosciences).
Zhang, Q., Qian, H., Xu, P., Li, W., Feng, W., and Liu, R. (2021). Effect of hydrogeological conditions on groundwater nitrate pollution and human health risk assessment of nitrate in Jiaokou Irrigation District. J. Clean. Prod. 298, 126783. doi:10.1016/j.jclepro.2021.126783
Zhang, Q., Xu, P., Qian, H., and Yang, F. (2020). Hydrogeochemistry and fluoride contamination in jiaokou irrigation district, central China: Assessment based on multivariate statistical approach and human health risk. Sci. Total Environ. 741, 140460. doi:10.1016/j.scitotenv.2020.140460
Keywords: centralized drinking water sources, abnormal ions, distribution characteristics, cause analysis, influential factors
Citation: Su G, Liu G, Li Y and Wang G (2023) Analysis of groundwater ion abnormality and its cause of centralized drinking water sources in Jieshou City, China. Front. Environ. Sci. 10:1055623. doi: 10.3389/fenvs.2022.1055623
Received: 28 September 2022; Accepted: 12 December 2022;
Published: 16 January 2023.
Edited by:
Zhanhong Wan, Zhejiang University, ChinaReviewed by:
Şehnaz Şener, Süleyman Demirel University, TurkeyQiang Xia, Chengdu University of Technology, China
Copyright © 2023 Su, Liu, Li and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guijian Liu, bGdqQHVzdGMuZWR1LmNu
 Guifen Su
Guifen Su Guijian Liu1*
Guijian Liu1*