- Ministry of Ecology and Environment, Nanjing Institute of Environmental Sciences, Nanjing, China
In recent years, biochar has been considered as an effective adsorbent and soil conditioner due to its abundant carbon and high porosity. This study applied a kind of biochar from wheat straw pyrolysis to remediate phenanthrene-contaminated water and soil. The performance of the biochar in the removal of phenanthrene was discussed by liquid phase adsorption and soil incubation experiments. Furthermore, this work explored the enhancement effect of wheat straw biochar on soil microbial numbers and soil properties. The result of liquid phase adsorption indicated, 92.2% of phenanthrene was removed after incubating 0.6 g/L of wheat straw biochar for 4 h. Pseudo-second-order kinetic model (R2 = 0.99823) and Langmuir isotherm model (R2 = 0.99577) described the removal of phenanthrene by wheat straw biochar well. In soil incubation experiment with an initial phenanthrene content of 11.2 mg/kg, 89.1% of phenanthrene was removed at biochar dosage of 12% (w/w, wheat straw biochar/soil) after 30 days of incubation. In addition, the number of soil microorganisms, soil pH and organic matter (SOM) content increased after wheat straw biochar treatment. At the dosage of 12%, soil microbial count increased to 9.8 × 108 CFU/g-soil, soil pH increased by 1.8 units and SOM increased by 8.5 folds. The addition of wheat straw biochar not only improved soil quality, but also reduced the proportion of phenanthrene components, which could provide theoretical support for the resource utilization of agricultural waste.
1 Introduction
With the acceleration of industrialization and social and economic development, environmental pollution has become increasingly serious. In particular, a massive amount of polycyclic aromatic hydrocarbons (PAHs) generated from vehicle exhaust emissions, gas and coal tar production, wood processing, and oil spills were released into soil and water bodies, posing a major threat to the ecological environment and human health (Zhang et al., 2021; Guo et al., 2022). Today, PAHs are highly persistent in water and soil bodies and are difficult to handle due to their inherent properties such as low volatility and poor water solubility (Piscitelli et al., 2019). From this, the treatment of PAHs-contaminated water and soil remained a serious challenge.
PAHs have been detected in many wastewater and treated effluents, including the effluent from sewage treatment plants. These effluents have been identified as major sources of PAHs in aquatic environments, leading to increasing concerns about water safety (Qi et al., 2013; Qiao et al., 2018). Chemical oxidation and biological degradation have been applied to reduce the pollution and harm of water containing PAHs. However, the potential secondary pollution of chemical oxidation and the low survival rate and long treatment cycle of microorganisms limited their wide application in practical engineering. Due to the advantages of simple operation, short treatment time, high efficiency and recyclable adsorbent, the adsorption method has attracted academic research and engineering application in the treatment of wastewater containing PAHs (Qiao et al., 2018).
In order to reduce the adverse effects of PAHs on soil, a lot of researches have been carried out, in which microbial remediation was considered as one of the key solutions. For example, Li et al. (2021) designed a chemical oxidation + biological treatment strategy for phenanthrene-contaminated soil, and the removal efficiency increased by 33% after 63 days of remediation; Feng et al. (2012) analyzed the degradation of phenanthrene by halophilic strain and showed that at a salinity of 3% and a pH value of 9, the phenanthrene was almost completely depleted within 6 days. However, these microbial degraders would always be limited in natural ecosystems. That is, the microbial activity that determined the efficiency of microbial remediation was largely influenced by the hydrophobicity of PAHs (Li et al., 2020). In order to improve the bioremediation efficiency, two schemes were constructed: 1) inoculate exogenous microorganisms (isolated from a relatively stable PAH-contaminated soil environment) to replace the local microorganisms with weak degradation ability; 2) introduce specific biomass to stimulate the degradation ability of native microorganisms. Among them, since the introduced microorganisms were easily inactivated after inoculation in PAHs-contaminated soil, it could be easily failed to adopt bioaugmentation strategies, which has been demonstrated in many practical projects (Wang et al., 2018). Thus, the introduction of biomass in soil to stimulate the soil healing by indigenous microorganisms has become a better option.
Biochar was produced from the pyrolysis of biomass (forestry waste, crop straw, municipal sludge, etc.) and was considered to be an important adsorbent and soil conditioner in agricultural production (Palansooriya et al., 2022). Its large specific surface area and high porosity have attracted great attention in solving environmental problems, such as the removal of refractory organics and heavy metals from wastewater and soil. Qiao et al. (2018) used Enteromorpha prolifera biochar to treat water contaminated by pyrene and benzo[a]pyrene, and the adsorption efficiencies reached 92.5% and 85.2%. Rao et al. (2017) used poplar biochar to remediate phenanthrene-contaminated soil, and the experimental results showed that only 7.9% of phenanthrene remained in the soil after 21 days of treatment. Kong et al. (2018) remediated PAHs-contaminated soil using sawdust biochar, approximately 46% of PAHs were removed when the sawdust biochar content in soil was 5%. Due to the large specific surface area and high porosity, the biochar had strong adsorption ability to pollutants in water and soil bodies, and could improve the water holding capacity of soil (Jeffery et al., 2015). Due to the rich carbon, the biochar could provide better shelters and nutrients to soil microbes (Guo et al., 2021). These advantages of biochar were the main reasons for its good application potential in engineering.
At present, there were two research views on the application of biochar in microbial remediation of organically contaminated soil. On the one hand, biochar prepared from high temperature pyrolysis of biomass reduced the bio-availability of organics due to its strong adsorption ability, thus reducing the biodegradation (Kołtowski et al., 2017). On the other hand, biochar obtained from low temperature pyrolysis promoted microorganisms reproduction due to its specific composition and structural characteristics, thus promoting the biodegradation (Bianco et al., 2021). Anyway, the addition of biochar in soil could reduce the toxicity to living microorganisms by reducing pollutants content and promoting microbial growth, which was beneficial to further remove pollutants in soil and improve soil fertility (Cabrera et al., 2014; Guo et al., 2021). Therefore, low-cost preparation and implantation of biochar in PAHs-contaminated water and soil had important scientific and practical significance. As one of the main foods for Chinese, wheat was grown in most parts of China, especially in the north. The widespread cultivation of wheat led to producing untold amounts of wheat straw each year. Wheat straw was regarded as agricultural solid waste in many countries, including China, and its disposal has caused serious environmental problems. From the perspective of renewable resource utilization, wheat straw could be used as a cheap and abundant material to produce biochar for environmental pollution control (Guo et al., 2019).
In this study, phenanthrene was taken as a representative of PAHs, and phenanthrene-contaminated waster and soil were selected as the remediation objects. The main purpose was to 1) evaluate the performance of wheat straw biochar in the removal of phenanthrene from liquid phase and soil; 2) discuss the possible kinetics and isotherms in liquid phase; and 3) investigate the amendment of soil characteristics. In summary, the interesting topics involved, the remediation of PAHs-contaminated soil and the investigation of the physicochemical properties in the remediation process by wheat straw biochar, were precisely the innovation of this research.
2 Materials and methods
2.1 Chemicals
The used chemicals including reagent-grade phenanthrene and HPLC-grade solvents such as methanol, acetone and hexane were acquired from Nanjing Chemical Reagent Co., Ltd., (Jiangsu, China).
2.2 Wheat straw and corresponding biochar
First, wheat straw collected in autumn from a farm in Langfang, China, was dried at 105°C for 4 h. Next, the dried wheat straw was ground using a ball mill at 3,000 r/min for 10 min, then sieved (0.5 mm) and the fraction under the sieve was collected. Third, the collected fractions were washed with distilled water and dried again. Then, the product obtained in the above step was pyrolyzed in a muffle furnace at 500°C in the absence of oxygen for 2 h. At last, the wheat straw biochar was successfully prepared.
The contents of C, H, and N in wheat straw biochar were determined by an elemental analyzer. The specific surface area, functional groups and micro-structure of wheat straw biochar were determined using Brunauer-Emmett-Teller (BET) analyzer, Fourier Transform Infrared Spectrometer (FTIR) and Scanning Electron Microscopy (SEM), respectively.
2.3 Wastewater treatment by wheat straw biochar
The phenanthrene containing water used in this study for the adsorption tests was a synthetic wastewater. Influences of wheat straw biochar dosage and initial phenanthrene concentration on the removal of phenanthrene were investigated at room temperature. After transferring a series of 100 ml solutions with a designed phenanthrene concentration of 0.5 mg/L (pH value not adjusted) to 300 ml glass tubes, different dosages of wheat straw biochar (0.2–1.2 g/L) were added separately and stirred at 200 rpm. After the wheat straw biochar was incubated for a period of time (1–7 h), the mixtures in the glass tubes were centrifuged (3,000 r/min, 15 min) to separate the supernatant and the wheat straw biochar. The supernatants were analyzed to determine residual phenanthrene. The amount of adsorbed phenanthrene at adsorption equilibrium (qe, mg/g) and the phenanthrene removal efficiency (η) was calculated by:
where, C0 and Ce (mg/L) were the initial and equilibrium phenanthrene concentrations of contaminated water, respectively; V (L) was the volume of contaminated water; m (g) was the weight of wheat straw biochar.
The effect of initial phenanthrene concentrations on phenanthrene removal by wheat straw biochar were investigated in a similar procedure. After transferring a series of 100 ml phenanthrene solutions at concentrations of 0.25–1.5 mg/L (pH value not adjusted) into 300 ml glass tubes, the wheat straw biochar with an optimum dosage from the biochar dosage-dependence experiments was respectively added and stirred at 200 rpm. Next, the wheat straw biochar was incubated for a period of time. This incubation time was obtained from the biochar dosage-dependence experiments. Then, the mixtures were separated and the collected supernatants were analyzed according to Eqs 1, 2.
2.4 Kinetic and isotherm models
Pseudo-first-order, second-order kinetics and intraparticle diffusion models were applied to characterize the kinetics of phenanthrene removal by wheat straw biochar, as shown in Eqs 3–5 (McKay et al., 1987).
where, t (min) was the adsorption time; qt (mg/g) was the amount of adsorbed phenanthrene at time t; k1 (min−1) and k2 [g/(mg·min)] were the rate constants of pseudo-first and second-order kinetic models, respectively; kd [g/(mg·min0.5)] and C were the rate constant and the intraparticle diffusion (intercept) constant of the intraparticle diffusion model.
Generally, the dynamic adsorption of solute in liquid by an adsorbent depended on the equilibrium separation between the adsorbent and liquid phases (Widiastuti et al., 2011). In this study, the Freundlich and Langmuir isotherms showed as Eqs 6, 7 (Frendlich, 1906; Langmuir, 1918) were used to discuss the isotherms characteristics of phenanthrene removal by wheat straw biochar.
where, qm (mg/g) was the maximum amount of phenanthrene adsorbed on wheat straw biochar; kL was the Langmuir constant (L/mg); kf (mg/g) and 1/n were Freundlich empirical constants.
2.5 Soil remediation by wheat straw biochar
The fresh soil used in this work was collected from a university campus in Nanjing, China, and has no history of phenanthrene contamination. The content of soil organic matters (SOM) and pH value of the fresh soil were measured to be 8.7 g/kg and 6.8, respectively. After discharging gravel, plant roots and other residues, the fresh soil was air-dried and passed through a 2-mm screen. Subsequently, a phenanthrene-acetone solution was prepared and poured into the soil with a mass ratio of phenanthrene to soil of 12: 1 (mg: kg) and a soil moisture content of about 60%, and then, the mixture was mixed using a rotating oscillation in a fume hood to evaporate the acetone. Thereafter, the contaminated soil was homogenized to equilibrium in dark for 60 d, and finally an aged soil was obtained for the remediation experiments. Notably, phenanthrene content in the contaminated soil was 11.2 mg/kg.
To evaluate the performance of wheat straw biochar in remediation of phenanthrene-contaminated soil, 200 g soil samples were placed in a series of 800-ml plastic bowls and mixed with wheat straw biochar using wheat straw biochar/soil mass ratio of 4%, 8%, 12%, 16%, and 20%, respectively. Following, the mixtures were incubated at room temperature with a water-holding capacity of approximately 60% for 60 d. On day 10, 20, 30, 40, 50, and 60, the soil samples were selected and pre-treated to extract phenanthrene according to the method of Scelza et al. (2007) and the reduction of phenanthrene was analyzed.
2.6 Testing methods
The concentration of phenanthrene was detected using high performance liquid chromatography (HLPC) method according to Rao et al. (2017). A HPLC (Agilent 1200, America) equipped with a 150 mm × 4.6 mm × 5 μm chromatographic column (Eclipse XDB-C18) and a UV detector was applied. The quantity of soil microorganisms was counted by the plate counting method. The CO2 emission were measured using a procedure reported by Bao et al. (2020). The pH value of soil was tested in a 1:2.5 (w/v) suspension of distilled water by a pH meter. The SOM content was detected by the K2Cr2O7 volumetric method.
3 Results and discussion
3.1 Wheat straw biochar characteristics
Supplementary Table S1 showed that the pH value of wheat straw biochar was alkaline, which was ascribed to the removal of acidic functional groups when the wheat straw was pyrolyzed under high temperature conditions (500°C). The H/C ratio was an index always adopted to assess the carbonation degree and aromaticity of biochar, and generally speaking, the lower the H/C ratio, the higher the carbonation degree and aromaticity. This was ascribed to the changes of organic components in the biochar during pyrolysis, in this case, the long chains were broken and the dense rings were formed, which enhanced the carbonization and aromaticity. According to Xu et al. (2014), the high carbonization and aromaticity of biochar facilitated π-π reactions between its aromatic components and aromatic matters, thus contributing to the removal of aromatic contaminants. The decline of the O/C ratio after the pyrolysis implied a decrease in wheat straw biochar polarity and an increase in hydrophobicity. According to Guo et al. (2021), the low polarity of biochar was beneficial to its adsorption for non-polar organic matter, and the high hydrophobicity was beneficial to the growth of microorganisms. The C/N molar ratio of wheat straw biochar and soil was similar, indicating that the implantation of wheat straw biochar in soil remediation process hardly changed the soil C/N ratio. The results of BET test demonstrated that the wheat straw biochar had a relatively larger specific surface area and a higher porosity than the wheat straw. These characteristics could promote the phenanthrene adsorption by biochar in the treatment of phenanthrene containing water, and could also supply more habitat/unit volume for microorganisms in soil remediation so as to promote their growth and reproduction.
The microstructure of the wheat straw biochar in Supplementary Figure S1 showed that it had a multi-channel surface structure with micro-pores less than 10 μm in diameter. The pyrolysis process at 500°C not only decomposed some organic substances in wheat straw, but also volatilized some volatile substances. As a result, the wheat straw biochar structure contained multiple channels and micro-pores, which could provide large numbers of possible binding sites for phenanthrene adsorption and/or provide a better habitat for microorganisms.
Abundant functional groups were observed from the FTIR spectra of wheat straw biochar surface in Supplementary Figure S2. The peaks at 3,420, 2,930, 1,640, and 1,510 cm−1 suggested the existence of O–H of the water molecule, the aliphatic C–H, ethers C=O and aromatic C=C. A peak at 1,060 cm−1 was detected and assigned to the phenols C–O and aliphatic C–O–C. Meanwhile, the peak at 618 cm−1 indicated the presence of the aromatic C–H. Furthermore, an additional peak at 476 cm−1 manifested the existence of the Si–O–Si. According to Guo et al. (2021) and Zhang et al. (2018), when biochar was added to soil, its hydrophilic groups were conducive to better survival of microorganisms. Furthermore, some functional groups formed hydrogen bonds with phenanthrene on the surface of biochar, which could promote the adsorption of phenanthrene and the utilization by microorganisms.
3.2 Phenanthrene removal from contaminated water
3.2.1 Performances of wheat straw biochar in phenanthrene removal
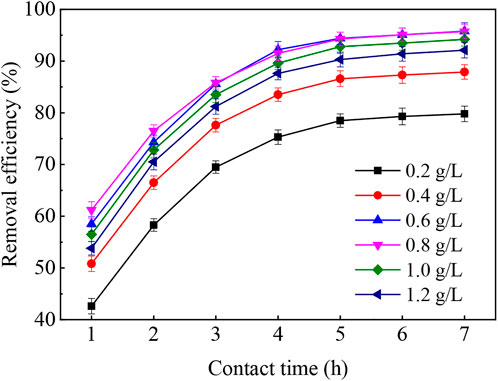
It could be seen from Figure 1 that under different dosages of the wheat straw biochar, phenanthrene was rapidly adsorbed, and more than 80% of phenanthrene was removed. In particular, after incubation with 0.6 g wheat straw biochar in 1 L contaminated water for 4 h, the phenanthrene removal efficiency reached 92.2%, and the corresponding amount of phenanthrene adsorbed on per unit mass of wheat straw biochar (qe) calculated from Eq. 1 was 0.77 mg/g. The adsorption capacity of wheat straw biochar was similar to that reported by Guo et al. (2021).
The increase in the dosage of wheat straw biochar contributed to the removal of phenanthrene, which was related to the adsorption sites and specific surface area provided by the wheat straw biochar. This reason was well agreed with that observed by Zielińska and Oleszczuk (2015). However, excess wheat straw biochar was not conducive to the removal of phenanthrene, because the surface active groups and adsorption sites interfered with each other, resulting in the aggregation effect of adsorbents, which further reduced the effective adsorption area and the number of active sites per unit mass of wheat straw biochar (Bhattacharyya and Gupta, 2009). Previous literature reported that physical interaction was not the only mechanism for phenanthrene removal by biochar, and a fact that could not be ignored was that the aromatic groups of wheat straw biochar could promote hydrophobic and π–π interactions with phenanthrene (Sun et al., 2011).
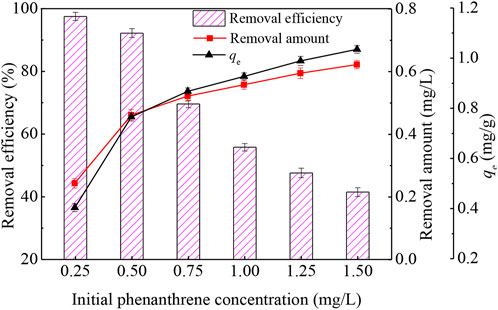
The initial concentration of phenanthrene in contaminated water was another important factor affecting the absorptive performance of the wheat straw biochar. Figure 2 showed that when the amount of wheat straw biochar was adjusted to 0.6 g/L, with the increase of the initial concentration of phenanthrene, the removal efficiency decreased and the removal amount increased. The reason was that the increase of the initial concentration of phenanthrene enhanced the driving force generated by the concentration gradient, which promoted phenanthrene adsorption by biochar. The adsorption capacity of phenanthrene increased from 0.41 to 1.04 mg/g until the adsorption equilibrium was reached. At this moment, the adsorption sites of wheat straw biochar were almost saturated (Cheng et al., 2016).
3.2.2 Kinetic and isotherm models
From the parameter values of the three kinetics equations in Supplementary Table S2, the pseudo-second-order kinetic model had the highest R2 value of 0.99823, while the intraparticle diffusion model had the lowest R2 value of 0.87172. Although the R2 value (0.94059) of pseudo-first-order kinetic model also higher than 0.90, the predicted qe (0.43 mg/g) did not match the experimentally measured value (0.77 mg/g). In comparison, the predicted qe by the pseudo-second-order kinetic model (0.90 mg/g) was the closest to the measured value from experiments. The adsorption mechanism of biochar reported in the literature was mainly surface adsorption, and the kinetic constant k2 was used to evaluate the adsorption rate (Koodyńska et al., 2012). The higher k2 value (1.34) for the removal of phenanthrene by wheat straw biochar indicated that the adsorption reached its equilibrium rapidly (Rao et al., 2017). Thus, the kinetic characteristics of phenanthrene removal were in line with the assumption of the pseudo-second-order model, which meant that the removal of phenanthrene by wheat straw biochar was more consistent with chemical adsorption (Rao et al., 2017).
From the parameter values of the two isotherms equations in Supplementary Table S3, it could be seen that the Langmuir isotherm model had a higher R2 value of 0.99577, indicating that the Langmuir isotherm was better than the Freundlich isotherm to describe the isotherm characteristics of the removal of phenanthrene by wheat straw biochar. However, Cheng et al. (2016) reported an opposite conclusion that the Freundlich isotherm model well described Cd2+ adsorption by peanut shell biochar because the adsorption process was heterogeneous adsorption. The Freundlich isotherm model was the best empirical equation to describe the heterogeneous adsorption process (Murugesan et al., 2011). In the present study, the removal of phenanthrene by wheat straw biochar did not fit well with the Freundlich isotherm, suggesting that the adsorption of phenanthrene was homogeneous.
3.3 Phenanthrene removal from contaminated soil
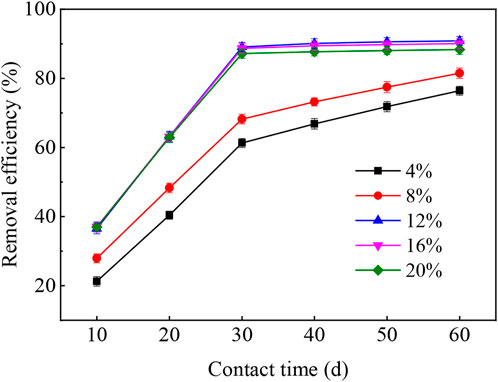
Figure 3 described the performance of wheat straw biochar in remediation of phenanthrene-contaminated soil with an initial phenanthrene content of 11.2 mg/kg. The efficiency of phenanthrene removal was positively dependent on the dosage of wheat straw biochar. The experimental results showed that phenanthrene was quickly removed within 30 days after adding different amounts of wheat straw biochar in the microbial remediation process of phenanthrene-contaminated soil, and phenanthrene was slowly removed after 30 days of remediation. Meanwhile, as the amount of wheat straw biochar increased from 4% to 12%, active adsorption sites increased and more surface was available for the adsorption of phenanthrene (Karthikeyan et al., 2007). At day 30, the removal efficiency of phenanthrene reached 89.1% when the wheat straw biochar dosage was adjusted to 12%. The phenanthrene removal efficiencies were almost balanced between 12% and 20% wheat straw biochar. Therefore, wheat straw biochar with optimum dosage of 12% was selected to remediate the phenanthrene-contaminated soil. The wheat straw biochar had a relatively larger specific surface area and higher porosity, which were considered by Peng et al. (2016) to be beneficial to the retention of phenanthrene in the pores of biochar. In addition, the benefits of wheat straw biochar on microbial numbers were also the main reasons for promoting phenanthrene removal from soils (as shown in Section 3.4).
3.4 Microbial quantity and CO2 emission
3.4.1 Microbial quantity
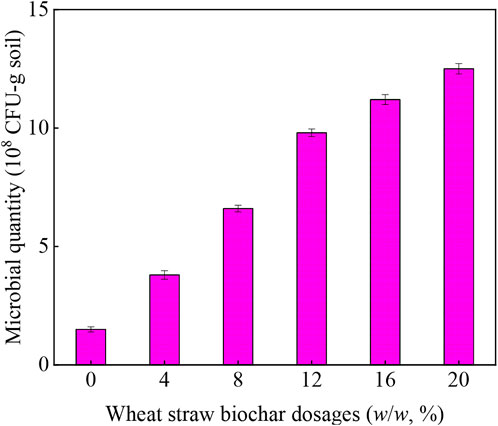
The changes in soil microbial numbers expressed in colony forming units (CFU) during remediation with wheat straw biochar was shown in Figure 4. It was clear that the microorganisms quantity increased significantly because the microorganisms were stimulated by wheat straw biochar. In the soil without wheat straw biochar addition, the microbial count was 1.5 × 108 CFU/g-soil, but at the end of soil remediation with different dosages of biochar, the microbial count increased to 3.8× 108–1.25 × 109 CFU/g-soil (9.8 × 108 CFU/g-soil at biochar dosage of 12%). The stimulating effect of wheat straw biochar led to a significant increase in the number of microorganisms. These results implied that wheat straw biochar promoted microbial reproduction, thereby actively promoting the decomposition of phenanthrene in soil. Some studies claimed that biochar could reduce the effective utilization (or called “bio-availability”) of PAHs by soil microorganisms due to its strong adsorption, which was not conducive to the removal of PAHs (Kołtowski et al., 2017), but this phenomenon was not found in this study.
Due to its large specific surface area and high porosity, wheat straw biochar provided a better habitat for microorganisms than the phenanthrene-contaminated soil, which was favorable for their attachment and growth. It is generally believed that these two factors dominated the growth of microorganisms during the remediation of phenanthrene-contaminated soil: 1) Wheat straw biochar could act as a slow-release nutrient source to promote sustainable growth and reproduction of microorganisms because it contained carbon and nitrogen, and could enrich nutrients and water from the soil by adsorption (Zhang et al., 2018); 2) Wheat straw biochar could also make soil physical properties better by cutting down the soil bulk density and ameliorating the aeration of soil, thereby increasing microbial growth (Guo et al., 2021). These were the reasons why wheat straw biochar could promote the growth of microorganisms during the remediation of phenanthrene-contaminated soil.
3.4.2 CO2 emission
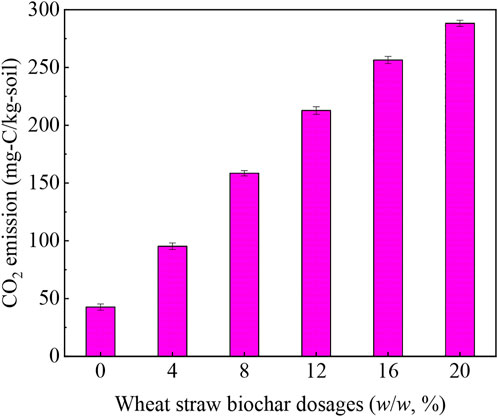
In addition to the number of microorganisms, this study also measured the CO2 emission levels under different dosages of wheat straw biochar during soil remediation to evaluate the effect of wheat straw biochar on microbial reproduction. Figure 5 showed that during the process of soil remediation, the CO2 emission level was positively correlated with the usage of wheat straw biochar, again implying the enhancement of the reproduction and metabolism of soil microorganisms. Bao et al. (2020) and Guo et al. (2021) also revealed similar information. In the presence of 4%–20% of wheat straw biochar, CO2 emission reached 95.3–288.2 mg-C/kg-soil. According to previous studies, since the raw material of biochar was biomass, during the soil remediation process, biochar itself would be degraded by microorganisms (which was also considered to be one of the reasons for providing nutrients for microorganisms and improving soil fertility), leading to a higher level of CO2 emissions (Guo et al., 2021). Therefore, although the presence of wheat straw biochar increased CO2 emission in soil, the contribution by the decomposition of wheat straw biochar itself was unclear. In the soil control group without wheat straw biochar, the CO2 emission level (42.7 mg-C/kg-soil) was much lower than that with wheat straw biochar, indicating that the CO2 release was mainly caused by microbial activity. This result confirmed that wheat straw biochar promoted microbial activity and phenanthrene degradation during soil remediation, which was consistent with the findings showed in Section 3.3 and Section 3.4.1.
3.5 Amelioration of soil properties
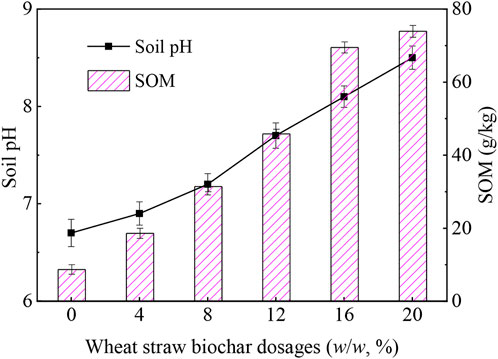
Compared with the untreated contaminated soil, pH value of wheat straw biochar-treated soil increased significantly after 60 days (Figure 6). As the wheat straw biochar usage increased from 4% to 20%, soil pH increased by 0.2–1.8 units. It is well known that soil pH was controlled by alkaline cations in soil. The addition of wheat straw biochar to soil increased soil pH value because wheat straw biochar itself contained these base cations (Liu, 2019). As a result, the contents of some exchangeable cations (such as H+ and Al3+) decreased, which increased soil pH value.
Figure 6 also depicted that SOM, one of the soil quality indicators, increased by 2.1–8.5 folds as the amount of wheat straw biochar increased from 4% to 20%. Cao et al. (2011) reported that due to the large stable carbon content of wheat straw biochar, small molecular organics were easily adsorbed to soil surface, and the subsequent catalytic activity on the surface of wheat straw biochar promoted the aggregation of adsorbed organics, resulting in the formation of SOM. Similar conclusions were revealed by Ahmed et al. (2016). Researchers had proposed that the implantation of biochar into soil was one of the effective means to increase SOM, which directly affected soil quality (Roldan, 2003). Therefore, the application of wheat straw biochar could make soil quality better.
4 Conclusion
This work provided a kind of wheat straw biochar that could effectively remediate phenanthrene-contaminated water and soil. The results of liquid phase adsorption experiments showed that the wheat straw biochar could remove more than 80% of phenanthrene at different dosages. Moreover, 89.1% of the phenanthrene was removed by 12% of wheat straw biochar for incubating 30 days in the contaminated soil with initial phenanthrene contents of 11.2 mg/kg. Furthermore, the increase of soil microorganisms, CO2 emission, soil pH value and SOM content under different wheat straw biochar dosage conditions indicated that wheat straw biochar improved the soil quality. Specifically, when the amount of wheat straw biochar in soil was adjusted to 4%–20%, the number of microorganisms increased to 3.8× 108–1.25 × 109 CFU/g-soil with a CO2 emission of 95.3–288.2 mg-C/kg-soil, in addition, soil pH increased by 0.2–1.8 units and SOM content increased by 2.1–8.5 folds. Therefore, agricultural biochar could be applied to effectively remove phenanthrene from water and soil, which would be a feasible way to recycle agricultural waste.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CD, YG, JL, YC employed in Ministry of Ecology and Environment, Nanjing Institute of Environmental Sciences.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.1039603/full#supplementary-material
References
Ahmad, M., Ok, Y. S., Rajapaksha, A. U., Lim, J. E., Kim, B. Y., Ahn, J. H., et al. (2016). Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: Chemical, microbial and spectroscopic assessments. J. Hazard. Mat. 301, 179–186. doi:10.1016/j.jhazmat.2015.08.029
Bao, H. Y., Wang, J. F., Zhang, H., Li, J., Li, H., and Wu, F. Y. (2020). Effects of biochar and organic substrates on biodegradation of polycyclic aromatic hydrocarbons and microbial community structure in PAHs-contaminated soils. J. Hazard. Mat. 385, 121595. doi:10.1016/j.jhazmat.2019.121595
Bhattacharyya, K. G., and Gupta, S. S. (2009). Kaolinite and montmorillonite as adsorbents for Fe(III), Co(II) and Ni(II) in aqueous medium. Appl. Clay Sci. 41, 1–9. doi:10.1016/j.clay.2007.09.005
Bianco, F., Race, M., Papirio, S., Oleszczuk, P., and Esposito, G. (2021). The addition of biochar as a sustainable strategy for the remediation of PAH-contaminated sediments. Chemosphere 263, 128274. doi:10.1016/j.chemosphere.2020.128274
Cabrera, A., Cox, L., Spokas, K., Hermosin, M. C., Cornejo, J., and Koskinen, W. C. (2014). Influence of biochar amendments on the sorption-desorption of amino-cyclopyrachlor, bentazone and pyraclostrobin pesticides to an agricultural soil. Sci. Total Environ. 470, 438–443. doi:10.1016/j.scitotenv.2013.09.080
Cao, X., Ma, L., Liang, Y., Gao, B., and Harris, W. (2011). Simultaneous immobilization of lead and atrazine in contaminated soils using dairy manure biochar. Environ. Sci. Technol. 45, 4884–4889. doi:10.1021/es103752u
Cheng, Q., Huang, Q., Khan, S., Liu, Y., Liao, Z., Li, G., et al. (2016). Adsorption of Cd by peanut husks and peanut husk biochar from aqueous solutions. Ecol. Eng. 87, 240–245. doi:10.1016/j.ecoleng.2015.11.045
Feng, T. C., Cui, C. Z., Dong, F., Feng, Y. Y., Liu, Y. D., and Yang, X. M. (2012). Phenanthrene biodegradation by halophilic Martelella, sp. AD-3. J. Appl. Microbiol. 113, 779–789. doi:10.1111/j.1365-2672.2012.05386.x
Guo, J. Y., Jiang, J. Y., Chen, Y. H., Wen, X. Y., Chen, W. J., Wang, Y. F., et al. (2022). Synthesis of nZVI-BC composite for persulfate activation to degrade pyrene: Performance, correlative mechanisms and degradation pathways. Process Saf. Environ. Prot. 162, 733–745. doi:10.1016/j.psep.2022.04.051
Guo, J. Y., Jiang, S. L., and Pang, Y. J. (2019). Rice straw biochar modified by aluminum chloride enhances the dewatering of the sludge from municipal sewage treatment plant. Sci. Total Environ. 654, 338–344. doi:10.1016/j.scitotenv.2018.10.429
Guo, J. Y., Yang, S. Q., He, Q. L., Chen, Y. H., Zheng, F., Zhou, H. B., et al. (2021). Improving benzo(a)pyrene biodegradation in soil with wheat straw-derived biochar amendment: Performance, microbial quantity, CO2 emission, and soil properties. J. Anal. Appl. Pyrolysis 156, 105132. doi:10.1016/j.jaap.2021.105132
Jeffery, S., Bezemer, T. M., Cornelissen, G., Kuyper, T. W., Lehmann, J., Mommer, L., et al. (2015). The way forward in biochar research: Targeting trade-offs between the potential wins. GCB Bioenergy 7, 1–13. doi:10.1111/gcbb.12132
Karthikeyan, S., Balasubramanian, R., and Iyer, C. S. (2007). Evaluation of the marine algae Ulva fasciata and Sargassum sp. for the biosorption of Cu (II) from aqueous solutions. Bioresour. Technol. 98, 452–455. doi:10.1016/j.biortech.2006.01.010
Kołtowski, M., Hilber, I., Bucheli, T. D., Charmas, B., Skubiszewska-Zięba, J., and Oleszczuk, P. (2017). Activated biochars reduce the exposure of polycyclic aromatic hydrocarbons in industrially contaminated soils. Chem. Eng. J. 310, 33–40. doi:10.1016/j.cej.2016.10.065
Kong, L. L., Gao, Y. Y., Zhou, Q. X., Zhao, X, Y., and Su, Z. W. (2018). Biochar accelerates PAHs biodegradation in petroleum-polluted soil by biostimulation strategy. J. Hazard. Mat. 343, 276–284. doi:10.1016/j.jhazmat.2017.09.040
Koodyńska, D., Wntrzak, R., Leahy, J. J., Hayes, M. H. B., Kwapiński, W., and Hubicki, Z. (2012). Kinetic and adsorptive characterization of biochar in metal ions removal. Chem. Eng. J. 197, 295–305. doi:10.1016/j.cej.2012.05.025
Langmuir, I. (1918). The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 143, 1361–1403. doi:10.1021/ja02242a004
Li, D., Zhao, Y. Q., Wang, L. P., Wei, S. H., and Huang, S. M. (2021). Remediation of phenanthrene contaminated soil through persulfate oxidation coupled microbial fortification. J. Environ. Chem. Eng. 9, 106098. doi:10.1016/j.jece.2021.106098
Li, Y. B., He, J. Z., Qi, H. N., Li, H., Boyd, S. A., and Zhang, W. (2020). Impact of biochar amendment on the uptake, fate and bioavailability of pharmaceuticals in soil-radish systems. J. Hazard. Mat. 398, 122852. doi:10.1016/j.jhazmat.2020.122852
Liu, C. G. (2019). Enhancement of dewaterability and heavy metals solubilization of waste activated sludge conditioned by natural vanadium-titanium magnetite-activated peroxymonosulfate oxidation with rice husk. Chem. Eng. J. 359, 217–224. doi:10.1016/j.cej.2018.11.139
McKay, G., Otterburn, M. S., and Aga, J. A. (1987). Intraparticle diffusion process occurring during adsorption of dyestuffs. Water Air Soil Pollut. 36, 381–390. doi:10.1007/bf00229680
Murugesan, A., Ravikumar, L., SathyaSelvaBala, V., SenthilKumar, P., Vidhyadevi, T., Kirupha, S. D., et al. (2011). Removal of Pb (II), Cu (II) and Cd (II) ions from aqueous solution using polyazomethineamides: Equilibrium and kinetic approach. Desalination 271, 199–208. doi:10.1016/j.desal.2010.12.029
Palansooriya, K. N., Li, J., Dissanayake, P. D., Suvarna, M., Li, L., Yuan, X., et al. (2022). Prediction of soil heavy metal immobilization by biochar using machine learning. Environ. Sci. Technol. 56, 4187–4198. doi:10.1021/acs.est.1c08302
Peng, P., Lang, Y. H., and Wang, X. M. (2016). Adsorption behavior and mechanism of pentachlorophenol on reed biochars: pH effect, pyrolysis temperature, hydrochloric acid treatment and isotherms. Ecol. Eng. 90, 225–233. doi:10.1016/j.ecoleng.2016.01.039
Piscitelli, L., Malerba, A. D., Mezzapesa, G. N., Dumontet, S., Mondelli, D., Miano, T., et al. (2019). Potential microbial remediation of pyrene polluted soil: The role of biochar. Soil Res. 57, 807–813. doi:10.1071/sr19075
Qi, W. X., Liu, H. J., Pernet, C., and Qu, J. H. (2013). Polycyclic aromatic hydrocarbons in wastewater, WWTPs effluents and in the recipient waters of Beijing, China. Environ. Sci. Pollut. Res. 20, 4254–4260. doi:10.1007/s11356-012-1435-6
Qiao, K. L., Tian, W. J., Bai, J., Dong, J., Zhao, J., Gong, X. X., et al. (2018). Preparation of biochar from Enteromorpha prolifera and its use for the removal of polycyclic aromatic hydrocarbons (PAHs) from aqueous solution. Ecotoxicol. Environ. Saf. 149, 80–87. doi:10.1016/j.ecoenv.2017.11.027
Rao, M. A., Giuseppe, D. R. S., Scelza, R., and Conte, P. (2017). Biochar based remediation of water and soil contaminated by phenanthrene and pentachlorophenol. Chemosphere 186, 193–201. doi:10.1016/j.chemosphere.2017.07.125
Roldan, A. (2003). No-tillage, crop residue additions, and legume cover cropping effects on soil quality characteristics under maize in Patzcuaro watershed (Mexico). Soil Tillage Res. 72, 65–73. doi:10.1016/s0167-1987(03)00051-5
Scelza, R., Rao, M. A., and Gianfreda, L. (2007). Effects of compost and bacterial cells on decontamination and chemical and biological properties of an agricultural soil artificially contaminated with phenanthrene. Soil Biol. biochem. 39, 1303–1317. doi:10.1016/j.soilbio.2006.12.006
Sun, K., Ro, K., Guo, M., Novak, J., Mashayekhi, H., and Xing, B. (2011). Sorption of bisphenol A, 17α-ethinyl estradiol and phenanthrene on thermally and hydrothermally produced biochars. Bioresour. Technol. 102, 5757–5763. doi:10.1016/j.biortech.2011.03.038
Wang, B., Teng, Y., Xu, Y., Chen, W., Ren, W., Li, Y., et al. (2018). Effect of mixed soil microbiomes on pyrene removal and the response of the soil microorganisms. Sci. Total Environ. 640, 9–17. doi:10.1016/j.scitotenv.2018.05.290
Widiastuti, N., Wu, H. W., Ang, H. M., and Zhang, D. K. (2011). Removal of ammonium from greywater using natural zeolite. Desalination 277, 15–23. doi:10.1016/j.desal.2011.03.030
Xu, D., Zhao, Y., Sun, K., Gao, B., Wang, Z., Jin, J., et al. (2014). Cadmium adsorption on plant- and manure-derived biochar and biochar-amended sandy soils: Impact of bulk and surface properties. Chemosphere 111, 320–326. doi:10.1016/j.chemosphere.2014.04.043
Zhang, G. X., Guo, X. F., Zhu, Y. E., Liu, X. T., Han, Z. W., Sun, K., et al. (2018). The effects of different biochars on microbial quantity, microbial community shift, enzyme activity, and biodegradation of polycyclic aromatic hydrocarbons in soil. Geoderma 328, 100–108. doi:10.1016/j.geoderma.2018.05.009
Zhang, L., Qiu, X., Huang, L., Xu, J., Tang, H., Li, Z., et al. (2021). Microbial degradation of multiple PAHs by a microbial consortium and its application on contaminated wastewater. J. Hazard. Mat. 419, 126524. doi:10.1016/j.jhazmat.2021.126524
Keywords: phenanthrene containing water treatment, soil remediation, wheat straw biochar, soil characteristics, soil microorganism
Citation: Ding C, Gan Y, Luo J and Cui Y (2022) Wheat straw biochar and its performance in treatment of phenanthrene containing water and microbial remediation of phenanthrene contaminated soil. Front. Environ. Sci. 10:1039603. doi: 10.3389/fenvs.2022.1039603
Received: 08 September 2022; Accepted: 14 October 2022;
Published: 25 October 2022.
Edited by:
Junyuan Guo, Chengdu University of Information Technology, ChinaReviewed by:
Xin Qian, Nanjing University, ChinaXi Zhu, Zhongnan University of Economics and Law, China
Copyright © 2022 Ding, Gan, Luo and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yibin Cui, Y2lueWIyM0BzaW5hLmNvbQ==
 Chengcheng Ding
Chengcheng Ding Yonghai Gan
Yonghai Gan Jun Luo
Jun Luo