- 1Fishery Machinery and Instrument Research Institute, Chinese Academy of Fisheries Sciences, Shanghai, China
- 2Key Laboratory of Aquaculture Facilities Engineering, Ministry of Agriculture and Rural Affairs, Shanghai, China
- 3Shanghai Ocean University, Shanghai, China
Denitrification and anaerobic ammonium oxidation (anammox) are the key processes of nitrogen removal in aquaculture pond sediment. However, the reaction characteristics remain unclear. In this study, considering the sediment of conventional freshwater fishponds as the object, we set the optimal conditions of organic carbon, temperature, and total nitrates for denitrification and anammox. We found that the abundance and diversity of denitrifying bacteria and anammox bacteria in the two groups were significantly different. Candidatus brocadia is the most important bacteria in aquaculture pond sediments. The removal efficiencies of nitrite (NO2−-N), nitrate (NO3−-N), ammonia nitrogen (NH4+-N), and total organic carbon (TOC) in the anammox optimal conditions group were 97.99%, 93.05%, 54.92%, and 58.82%, respectively; however, those in the denitrification optimal conditions group were 99.82%, 86.10%, 45.74%, and 70.76%, respectively. Comparing each optimal condition, the removal efficiency of NO2−-N and TOC in the denitrification optimal condition groups was higher, whereas those of NO3−-N and NH4+-N were higher in the anammox optimal condition groups. We provide a reference for resolving nitrogen pollution in aquaculture pond sediments.
1 Highlights
1) Denitrification and anammox in pond aquaculture are mainly regulated by organic carbon content, temperature, and nitrite.
2) Denitrification has higher removal efficiency of NO2--N and TOC, and anammox has a higher removal efficiency of NO3--N and NH4+-N.
3) In the optimal conditions for denitrification and anammox, denitrification has higher nitrogen removal efficiency, but the role of anammox cannot be ignored.
4) Candidatus brocadia is the most important bacteria for nitrogen regulation in aquaculture ponds.
2 Introduction
Nitrogen pollution is the main cause of water quality deterioration in pond aquaculture. The sediment includes pollutants collected from the area of pond aquaculture. Nitrogen cycling in polluted water sediments includes ammoniation, nitrification, denitrification (Table, 1984; Sauthier et al., 1998), and anaerobic ammonium oxidation (anammox) (Van de Graaf et al., 1996). Denitrification and anammox are two transformation pathways which permanently remove fixed nitrogen from the system by converting it to gaseous nitrogen (N) (Castine 2013). Since the nitrogen conversion processes require various microorganisms, the conditions for denitrification and anammox are highly specific. Biological nitrogen removal processes that have been identified to date include denitrification, anammox, and denitrification-dependent anaerobic methane oxidation (Damo) (Hinrichs et al., 1999). Except for a few denitrifying bacteria that can denitrify the sediment in an aerobic environment, almost all denitrification processes are carried out in low oxygen or anaerobic environments; anaerobic ammonia oxidation and denitrifying anaerobic methane oxidation especially can be performed only at oxygen concentrations of <2 μM. The three denitrification processes are collectively referred to as biological anaerobic denitrification (Thamdrup 2012). Denitrifying microorganisms widely exist in marine, river, soil, wetland, and other ecosystems and have a high taxonomic diversity (Tiedje 1988). Ten different families of bacteria, fungi, and archaea exhibit denitrifying properties (Verbaendert et al., 2011). Many studies of denitrification have been reported to date, but they mainly focus on environments such as oceans and wetlands. In recent years, with the increased aquaculture environmental pollution, research on denitrification and anammox in aquaculture environments has gradually increased and become a research hotspot.
Pond aquaculture undeniably offers coniderable potential for food production worldwide. In China, 48.79% aquatical production is from pond aquaculture (FAMA, 2022). However, with the continuous pursuit of high yield, a large number of feeds and fertilizers are used in aquaculture, which leads to the accumulation of organic matter in pond aquaculture sediment and the production of a large amounts of nitrites, ammonia, and other substances, causing a deterioration of water quality and endangering aquaculture animals (Sun et al., 2017; Wei 2017). Sediment is the main sink of eutrophication-related nutrients in pond aquaculture. It results from the sedimentation of particulate matter. In the soil layer below 15 cm, with the increase in the depth of the soil layer, the total phosphorus (TP) content increases, whereas the total nitrogen (TN) shows a downward trend. Organic matter is mainly concentrated at 5–10 cm, and nitrogen-containing organic matter is the main pollutant in the pond sediment (Liu 2011). In ponds with intensive production of staple carp, sludge deposition is ∼10–12 cm per year (Zhang 1989). In grass carp aquaculture ponds, the mud (air-dried sample) generally contains 3% organic matter, ∼0.01–0.1% available nitrogen, 0.2% of TN, 0.2% of TP, and ∼0.7–1% of potassium (Yao 2010). A study showed that the sediment comprises ∼14–53% of N and ∼39–67% of P from feed (Christopher et al., 2003). The organic matter produced by aquaculture is deposited on the pond bottom, and during denitrification, NO3−-N is reduced to nitrogen (N2) and NH4+-N. Generally, the lack of oxygen in the sediment is favorable for denitrification and inhibits nitrification, resulting in the accumulation of more NH4+-N in the sediment (Yue & Huang 2003). Under the activity of bacteria, excessive NH4+-N, NO2--N, methane (CH4), H2S, and other toxic substances, which are harmful to fish, are produced, affecting the safety of aquaculture (Boyd 1995). Nitrogen cycling in pond sediments is a complex process, and the reaction efficiency greatly varies. Guo et al. (2011) found that the denitrification rate of Grasscarp culture sediment ranges from 0 to 734.15 μmol/(m2. d), with an ammoniation rate 0–41.25 mmol/(m2. d) (Guo et al., 2011). The anammox process contributed 1.2%–15.3% to sediment dinitrogen gas production (Shen et al., 2016).
Nitrogen regulation is an important method to maintain a good water environment for pond aquaculture. For example, Wang et al. (2013) found that the aquaculture environment and season have a significant impact on the structure of anammox bacteria in the sediment of ponds (Wang et al., 2013). Gao et al. (2018) found that organic carbon can inhibit anammox bacteria in the sediment of ponds and promote denitrifying bacteria. A temperature of 25–35°C is conducive for denitrification and anammox (Gao et al., 2018). Liao and Wu found that there was a synergistic association between ammonia-oxidizing bacteria and anammox bacteria in the surface sediments of ponds (Liao and Wu, 2013). Nowadays, denitrification has has become a key research topic in the field of water treatment (Shi Y et al., 2022; Shitu A et al., 2022). Numerous studies have shown that organic carbon, temperature, and nitrite are the basic conditions for controlling denitrification and anammox. However, denitrification and anammox have different requirements for organic carbon, temperature, and nitrite. The same organic carbon, temperature, and nitrate conditions cannot meet the needs of denitrification as well as those of anammox (Yu et al., 2016). Under low concentrations of organic carbon, anammox bacteria and denitrifying bacteria can cooperate to remove nitrogen, and excess organic carbon inhibits the activity of anammox bacteria (Liu et al., 2014). Because nitrite controls denitrification and anammox, a higher denitrification effect in the water environment is observed only within a specific concentration range of nitrate. Beyond this concentration range, denitrifying bacteria are inhibited, thereby reducing the denitrification effect (Li et al., 2013). The bacteria in the anammox process are autotrophic, absorbing and fixing CO2 as a carbon source (Mulder et al., 1995). All studies show that it is difficult to synchronize denitrification and anammox in aquatic environments. Thus, controlling the synchronous reaction of denitrification and anammox is a research challenge.
In this study, we used gene cloning libraries, real-time fluorescence quantitative PCR, and diversity analysis to reveal the characteristics of denitrification and anammox in pond sediments. We hope that our study will provide a theoretical basis for the regulation of nitrogen in sediment and suggest an important way to alleviate nitrogen pollution in aquaculture ponds and improve aquaculture efficiency.
3 Materials and methods
3.1 Sediment collection and processing
The pond sediment was taken from Maogang aquaculture farms (N30°57′1.89″, E 121°08′52.21″), Shanghai, China. The pond aquaculture species were Ctenopharyngodon Idella (Grass carp), Malobrama amblycephala (Bream), Hypophthalmichthys molitrix (Silver carp), and Aristichys Nobilis (Bighead carp). The maximum culturing density was 0.82 kgm−3. We collected 0–10 cm surface sediment using a Peterson mud collector, immediately placed the samples in sterile plastic bags after collection, removed air, and transported the samples to the laboratory to be stored in cold storage.
3.2 Experimental equipment and methods
According to the effects of organic carbon, temperature, and nitrite on denitrification and anammox in ponds (Gao et al., 2018), we set up two experimental groups: a denitrification group and an anammox group. Each group consists of three 500-ml triangular flasks. Before the experiment, 100 g of sediment was dried under natural conditions, and 500 ml of purified water was inoculated in each triangular flask of the two groups; in this process, only water was added not inoculated (Van de Graaf et al., 1996). The optimal reaction conditions of the denitrification group were set as 28°C, 150 mg/L NaNO2, and 150 mg/L starch. In the anammox group, the optimal conditions were set as 34°C and 300 mg/L NaNO2. Thereafter, each bottle was purged with N2 for 10 min to eliminate the influence of O2 on denitrification and anammox reactions. To control the temperature and other conditions, we put the two groups of experimental triangular flasks in different intelligent artificial climate incubators (RHQ-1000).
According to Chen et al. (2009) and Yang et al. (2012), we set the experimental period as 20 days. After the start of the experiment, three water samples were repeatedly collected in the triangular flasks once a day to measure the physical and chemical indicators. When the nitrite (NO2−-N) in the denitrification and anammox groups was less than 0.1 mg/L, we added 150 mg/L NaNO2 to the denitrification groups and 300 mg/L NaNO2 to the anammox groups. Two grams of the sediment were collected every 5 days and used to detect physical and chemical indicators and flora. For each experimental system, we collected four types of sediment.
3.3 Physical and chemical indices of water determination
NO3−-N levels were determined using ultraviolet spectrophotometry, NO2−-N levels were determined using N-(1-Naphthyl) ethylenediamine spectrophotometry, and NH4+-N levels were determined using Nessler’s reagent (SEPA and AQSIQ, 2002). TOC was analyzed using the Multi N/C2100 system, with the measurement range being 0–30000 ppm and the maximum carbon content of solids being 150 mg. The spectrophotometer was a Model 721 spectrophotometer produced by Shanghai Precision Scientific Instruments Co., Ltd.
3.4 DNA extraction and PCR amplification
Total DNA was extracted using the FastDNA spin kit for soil (Mpbio, United States) from matrix samples (collected sediments). DNA quality was detected by 1% agarose gel electrophoresis. A total of 8 samples were obtained and divided into 2 parts, one for the analysis of flora structure and diversity, and the other for quantitative analysis.
The nirS gene of denitrifying bacteria and the 16S rRNA gene of anammox bacteria were amplified by nested PCR. The primer pair Cd3aF-R3cdR used in the nested denitrification method is referred to in the reported literature (Lipsewers et al., 2016). The primer pair Pla46f-630r was used in the first round of anaerobic ammonia oxidation, and the primer pair Amx368f-Amx820r was used in the second round. The PCR reaction system includes Premix Ex Taq 12.5 μl, forward primer 1 μl, reverse primer 1 μl, formwork 1 μl, and ddH2O 9.5 μl (Zhu et al., 2011).
3.5 Clone library construction and sequencing
After separating the nested PCR sample by agarose gel electrophoresis, a specific fragment size was cut from the gel, purified using the GeneJET Gel Extraction Kit (Thermo), ligated using a pEASY-T1 cloning kit, and then transformed into Trans1-T1 competent cells. Transformed cells carrying the target gene were cultured for 1 h in LB medium without ampicillin and then inoculated on solid LB medium containing ampicillin and the blue spot detection reagent. After overnight culture of each sample, 20 single colonies were obtained. Positive clones were detected by colony PCR, from which 18 were selected, inoculated in 1 ml LB liquid medium with ampicillin, cultured at 37°C for 10 h, and then, sent to Shanghai Invitrogen Co., Ltd. for sequencing.
3.6 Real-time PCR and data analysis
The nirS copy numbers in denitrifying bacteria were quantified in eight samples using the nirS-specific primers, Cd3aF and R3cdR levels and the 16SrRNA copy numbers in anammox bacteria were quantified using the specific primers, AMX-808-F and AMX-1040-R. The PCR conditions have been described previously (Noredal et al., 2007; Dang et al., 2009). The PCR reaction volume was 20 μl and included the following: 1 μl template DNA, 0.8 μl forward and reverse primers each, 2 × Master mix (Roche) 10 μl, and 7.4 μl ddH2O.
3.7 Colony structure and diversity analysis
The sequence was edited using DNAStar software; the carrier sequence was removed, and the operational taxonomic units (OTUs) were determined using Mothur software. Each representative OTU sequence was selected as an OTU, and similar sequences were searched and downloaded using the NCBI-BLAST alignment tool. MEGA5.05 was used to align similar sequences with representative sequences through multiple sequence alignments, and a phylogenetic tree was constructed using the neighbor-joining method. The Shannon index (Xu et al., 2011), Simpson index, species richness (Chao) (Cui 2011), and coverage ratio were calculated to analyze microbial diversity.
3.8 Data processing
The diversity level and water index data were analyzed using Statistical Product Service Solutions (SPSS 20.0) software.
4 Results
4.1 Abundance of denitrifying bacteria and anammox bacteria
Through molecular biology experiments, we found that the total abundances of denitrifying bacteria in denitrification and anammox groups under optimal conditions for organic carbon content, temperature, and nitrite were 2.43 × 1011 copies g−1 and 2.04 × 1011 copies g−1, respectively, while the abundances of anammox bacteria were 6.16 × 108 copies g−1 and 9.49 × 108 copies g−1, respectively.
In the denitrification group, the abundances of denitrifying bacteria in the four samples were 2.60 × 1010 copies g−1, 5.86 × 1010 copies g−1, 8.06 × 1010 copies g−1, and 7.78 × 1010 copies g−1, respectively. In the anammox group, the abundances of anammox bacteria in the four samples were 1.35 × 108 copies g−1, 2.10 × 108 copies g−1, 4.16 × 108 copies g−1, and 1.88 × 108 copies g−1, respectively. The results showed that the total abundance of denitrifying bacteria in the denitrification group was significantly higher than that in the anammox group (Figure 1A). Similarly, the total abundance of anammox bacteria in the anammox group was significantly higher than that in the denitrification group (Figure 1B). Thus, the denitrification group was positive for denitrifying bacteria while the anammox group was suitable for anaerobic ammonia-oxidizing bacteria.
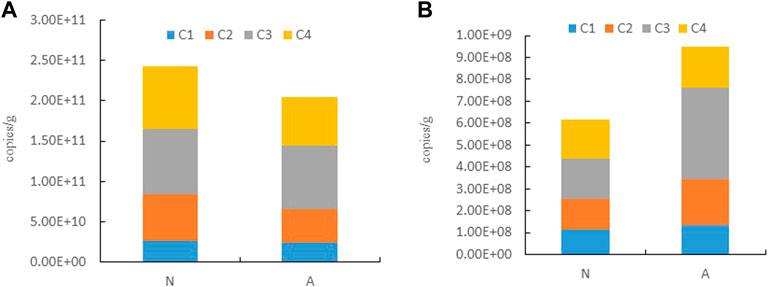
FIGURE 1. Abundance of denitrifying and anammox bacteria in the two conditions (A) denitrifying bacteria. (B) anammox bacteria. N: denitrification group. A: anammox group. C1, C2, C3, and C4 indicate the four sediments collected.
4.2 Diversity of denitrifying bacteria
4.2.1 Diversity level of the nirS gene
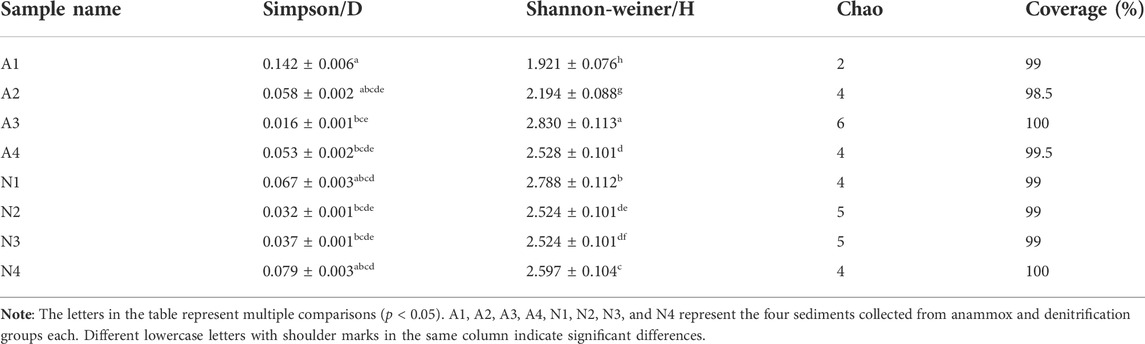
Using Mothur to analyze the sequence data of denitrifying bacteria in the denitrification and anammox groups, we found the clone coverage rate of the nirS gene in the two groups to be over 95%, indicating that the results of this clone library indeed represent the diversity level of denitrifying bacteria. The Shannon index of the denitrifying bacteria in the denitrification group was higher than that of the denitrifying bacteria in the anammox group, whereas the Simpson index showed the opposite trend, indicating that the diversity level of denitrifying bacteria in the denitrification group was relatively high. The diversity of denitrifying bacteria in the four sediments of denitrification and anammox groups did not show an increasing trend with time (Table 1).
4.2.2 Phylogenetic analysis of nirS genes
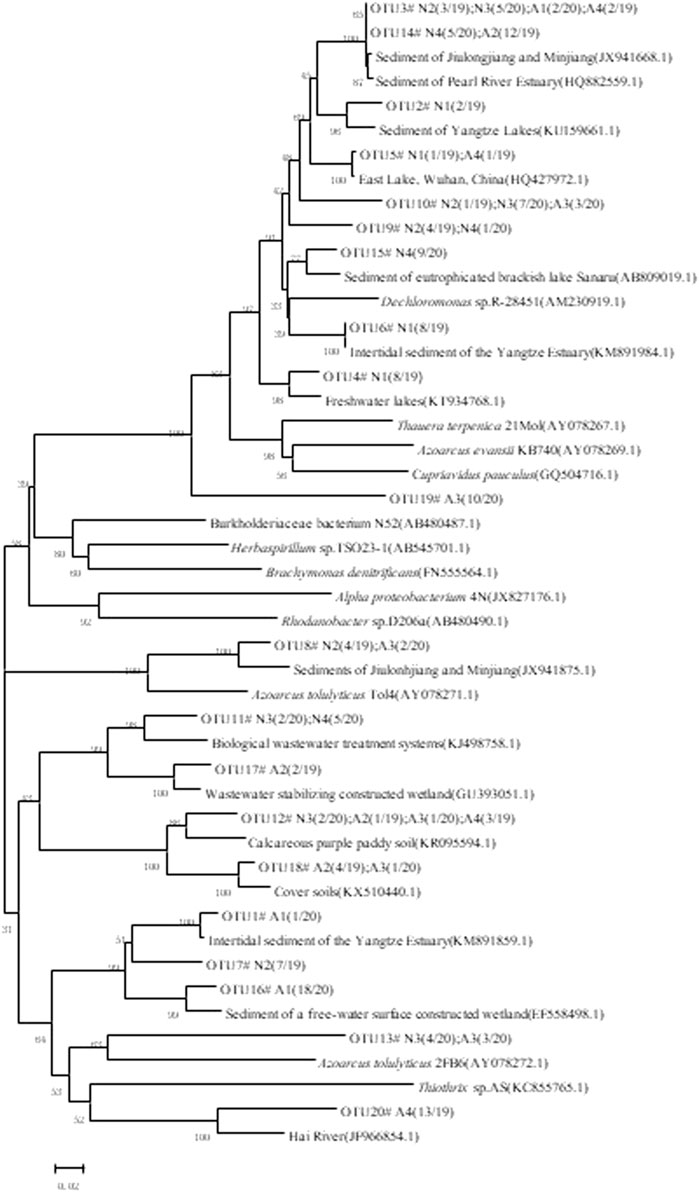
β-Proteobacteria was the most dominant group in the nirS gene library. The four sediments collected from denitrification and anammox groups (represented in the table as N1, N2, N3, and N4, and A1, A2, A3, and A4, respectively) were sequenced, and 156 valid sequences were obtained. The OTUs were divided based on Mothur’s 3% variance (97% sequence similarity for clustering OTUs), resulting in 20 OTUs. Among them, the number of OTUs from N1, N2, N3, N4, A1, A2, A3, and A4 samples was 4, 5, 5, 4, 2, 4, 6, and 4, respectively, indicating that denitrifying bacteria in the denitrification system showed little difference in OTU numbers at different times.
Phylogenetic analysis was performed to compare and analyze the denitrifying nirS genes obtained from denitrification and anammox systems (Figure 2). From the phylogenetic tree, overall, 156 valid sequences could be classified into the phylum pseudomonadota, including the classes Alphaproteobacteria, Betaproteobacteria, and Gammaproteobacteria and the genera Dechloromonas, Burkholderiaceae, Thiothrix, Thauera, Azoarcus, Brachymonas, and Azospirillum. In the nirS gene cloning library, two OTUs belonged to Alphaproteobacteria and were 98% identical to the most similar nirS gene sequence in GenBank. Most of the similar sequences were derived from similar environments, such as the Pearl River estuary in China, the San Francisco Bay estuary (Mosier and Francis, 2010), and the Yangtze River estuary in China (Zheng et al., 2015). Two OTUs belonged to Gammaproteobacteria; the OTU and GenBank strain of the denitrifying bacteria Thiothrix sp. AS (KC855765.1) showed 99% sequence similarity with other sequences in the group, the most similar sequence being derived from the estuaries of San Francisco Bay (Mosier and Francis, 2010) and Jiaozhou Bay, China (Dang et al., 2010), and the Haihe River, China.
4.3 Diversity of anammox bacteria
4.3.1 Diversity level of the 16S rRNA gene
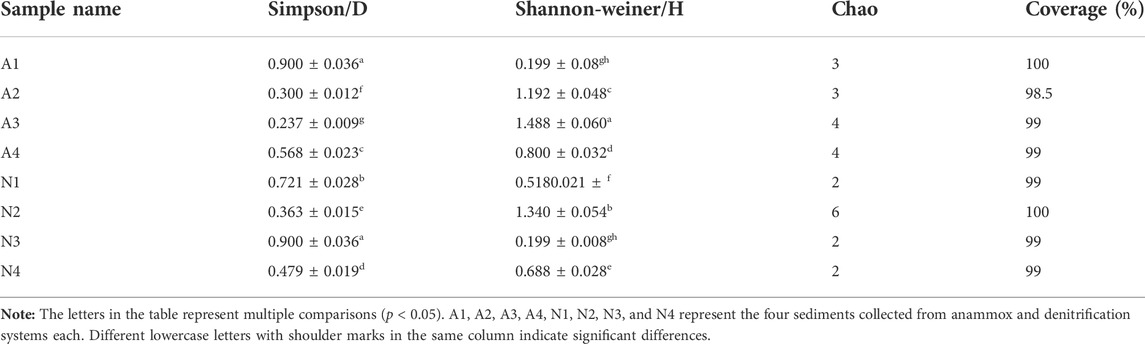
The diversity of anammox bacteria in the four sediments of denitrification and anammox groups was analyzed using the Mothur method. Results showed the cloning coverage ratio of the 16S rRNA gene in anammox bacteria to be above 95% in the two groups (Table 2), indicating that the clone library represents the diversity level of anammox bacteria quite well. The Shannon index of anammox bacteria in the anammox group was higher than that in the denitrification group, and the Simpson exponent of the denitrification group was larger, indicating that the diversity of anammox bacteria in the anammox group was relatively high. The diversity levels of anammox bacteria in all four sediments of the anammox group tended to increase with time.
4.3.2 Phylogenetic analysis of 16S rRNA gene
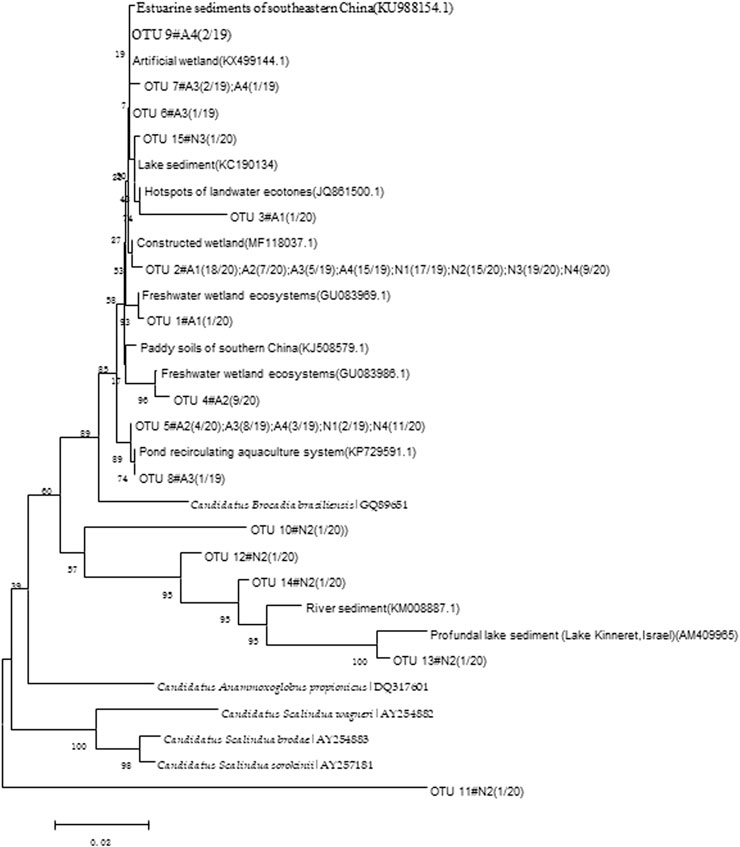
The four sediments collected from the denitrification and anammox groups (represented in the tables as N1, N2, N3, and N4, and A1, A2, A3, and A4, respectively) were sequenced, and 157 effective sequences were obtained. The OTUs were divided into 15 units based on a 3% variance in Mothur. Among them, the number of OTUs in N1, N2, N3, N4, A1, A2, A3, and A4 were 2, 6, 2, 2, 3, 3, 4, and 4, respectively. The results showed that anammox bacteria had differences in OTU numbers at different times in the anammox group and that there were considerable differences with time in the denitrification group. Phylogenetic analysis was used to compare and analyze the anammox bacterial 16S rRNA genes obtained from the denitrifying and anammox groups (Figure 3). The denitrifying and anammox groups mainly contained two types of anammox bacteria: Candidatus brocadia and Candidatus anamomoxoglobus. Ten OTUs belonged to Candidatus brocadia, and their similarity with the most similar 16S rRNA gene sequence in GenBank was 96%. Among them, OTU2 showed the highest abundance across all OTUs in the clone library, similar to that of anammox bacteria found in constructed wetlands, with a similarity of 99% (Lee et al., 2014). A higher abundance of OTU (OTU5) was similar to that of anammox bacteria found in recirculating aquaculture groups, with a similarity of 100% (Van et al., 2011). Most of the similar sequences in the remaining taxa were derived from Chinese estuaries, rivers, and lakes (Wang et al., 2013). Sequence similarity of anammox bacteria among OTU10, OTU12, OTU13, and OTU14 and those in deep-water sediments in the lake was 94–97% (Schwarz et al., 2007).
4.4 Nitrogen removal efficiency of denitrification and anammox groups
Before the experiment, we measured the air-dried sediment samples. The results showed that the contents of NO2−-N, NO3−-N, NH4+-N, and TOC were 45.87 mgL−1, 14.97 mgL−1, 66.1 mgL−1, and 108.67 mgL−1, respectively. Figure 4 shows the changes in NO2−-N, NO3−-N, NH4+-N, and TOC in denitrification and anammox groups, respectively. The removal rates ((initial concentration - last concentration)/initial concentration) for NO2−-N, NO3−-N, NH4+-N, and TOC in the denitrification group were 99.82%, 86.10%, 45.74%, and 70.76%, respectively (Figure 4A). The removal rates for NO2−-N, NO3−-N, NH4+-N, and TOC in the anammox group were 97.99%, 93.05%, 54.92%, and 58.82%, respectively (Figure 4B). The removal rates for NO2−-N and NO3−-N in the denitrification and anammox groups were over 97% and 85%, respectively. Theoretically, the removal times for NO2−-N, NO3−-N, and NH4+-N in the denitrification group were approximately 5, 7, and 14 days, respectively, whereas those in the anammox group were approximately 7, 8, and 12 days, respectively. The removal rate for NO2−-N and TOC were significantly higher in the denitrifying group than in the anammox group, whereas those for NO3−-N and NH4+-N in the anammox group were significantly higher than those in the denitrifying group. In conclusion, the denitrification efficiency was higher in the denitrification group, and ammonia oxidation was higher in the anammox group.
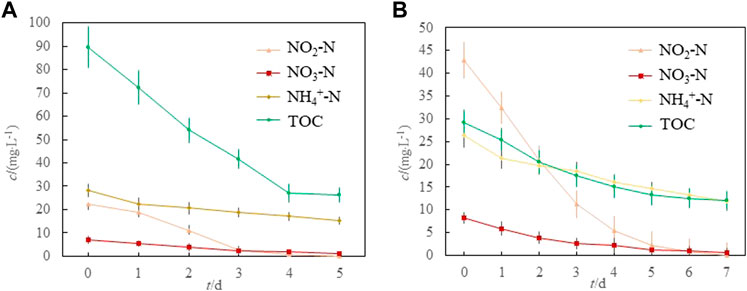
FIGURE 4. The NO2−-N, NO3−-N, NH4+-N, and TOC in denitrification and anammox groups (A) denitrifying group; (B). anammox group.
5 Discussion
Sediment is an important part of the aquaculture pond ecosystem. Sediments store nitrogen, phosphorus, and other substances in aquaculture ponds. Excessive accumulation of sediment causes deterioration of water bodies and increases the incidence of diseases in the farmed animals. Sediments are rich in microorganisms, and denitrifying bacteria and anammox bacteria can convert ammonia nitrogen into nitrogen. Thus, the study of the denitrification and anammox of pond sediment has attracted significant attention.
In this study, we found that the Shannon index for denitrifying bacteria in the denitrification group was larger than that in the anammox group, whereas the Simpson index showed the opposite trend. This further indicated the rates of denitrification and anammox to be regulated by organic carbon content, temperature, and nitrite.
In the experiment groups, we found that most sequences of the anammox bacterial 16S rRNA gene clone library belonged to Candidatus brocadia, and the most similar sequences were derived from constructed wetlands. This may be attributed to the abundance and diversity of anammox bacteria related to nutrient input from aquaculture ponds (Zeng et al., 2016). Fewer species have been found in these locations compared to those in nearby waters, and this may be attributed to the small scale, and single structure of the aquaculture pond on one hand, and a large number of nutrients added to the aquaculture pond on the other, resulting in the anammox oxidizing bacteria Candidatus brocadia becoming the dominant genus in the aquaculture pond. Some sequences in the library constructed in this study belonged to Dechloromonas, Burkholderia-ceae, Thiothrix, Thauera, Azoarcus, Brachymonas, and Azospirillum, which have been reported in other studies. In this study, we found two types of known anammox bacteria, Candidatus brocade,a and Candidatus kuenenia. It may also be that the aquaculture pond has more nutrients, which makes the Candidatus brocadia anammox bacteria become the dominant genus of the aquaculture pond.
In this study, we found that the removal efficiency of NO2−-N and TOC in the denitrification optimal conditions was higher, whereas that of NO3−-N and NH4+-N was higher in the anammox optimal conditions. This study provided a reference for resolving nitrogen pollution in the aquaculture pond sediment, and it further indicates that the organic carbon, temperature, and nitrate could regulate denitrification and anammox.
This study also suggests that, when regulating pond sediment, in addition to the collaborative application of denitrification and anaerobic ammonia oxidation, we should explore the role of denitrification and anammox, such as establish sequential batch reactions of denitrification and anammox to reduce nitrogen pollution. Overall, our study revealed the characteristics of the denitrification and anammox reactions in pond sediment and provided a reference for resolving nitrogen pollution in the culture environment, which will have great significance in green aquaculture in the future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XL: Conceptualization, Methodology, Formal analysis, Writing-original draft, Validation. MG: Formal analysis, Writing–original draft, Writing–review andamp; editing. JW: Methodology, Validation. ZG: Formal analysis, Investigation, Software. G-FC: Formal analysis, Investigation.
Acknowledgments
The authors would like to thank the “Modern agricultural industrial technology system in China” (grant NO. CARS-46) and the National Key R and D plan of China (grant NO. 2019YFD0900300) for financial support. The authors also thank the reviewer for their contribution in improving our manuscript. We thank www.wileyauthors.com/eeo/preparation for linguistic assistance during the preparation of this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Castine, S. (2013). Nitrogen removal and reuse in land-based aquaculture. Townsville, Australia: James Cook University.
Chen, T., Ping, Z., and Hu, B. L. (2009). Species diversity and ecological distribution of anaerobic ammonium-oxidizing bacteria. Chin. J. Appl. Ecol. 20 (5), 1229–1235.
Christopher, J., Preston, N., Thompson, P. J., and Burford, M. (2003). Nitrogen budget and effluent nitrogen components at an intensive shrimp farm. Aquaculture 218 (1-4), 397–411. doi:10.1016/s0044-8486(03)00014-0
Cui, T. T. (2011). Intestinal flora diversity study of captive adult pandas in autumn based on 16S rDNA-RFLP technology. Ya'an, China: Sichuan Agricultural University.
Dang, H., Chen, R., Wang, L., Guo, L., Chen, P., Tang, Z., et al. (2010). Environmental factors shape sediment anammox bacterial communities in hypernutrified Jiaozhou Bay, China. Appl. Environ. Microbiol. 76 (21), 7036–7047. doi:10.1128/aem.01264-10
Dang, H., Wang, C., Jing, L., Li, , T., Fang, Wei, J., et al. (2009). Diversity and distribution of sediment nirS-encoding bacterial assemblages in response to environmental gradients in the eutrophied Jiaozhou Bay. Microb. Ecol. 58 (1), 161–169. doi:10.1007/s00248-008-9469-5
Gao, M. Y., Liu, X. G., Zeng, X. L., and Lu, S. M. (2018). Effects of organic carbon for denitrification and anaerobic ammonium oxidation in sediments of aquaculture pond. Chin. J. Environ. Eng. 1, 49–56.
Guo, Y. J., Wang, F., Dong, S. L., Gao, Q. F., Zhang, M. Z., and Tian, X. L. (2011). The rates of denitrification, nitrification and ammoniation in the enclosed sediment of Grasscarp under Different Polyculture Modes. Chin. Fish. Sci. 18 (4), 10.
Hinrichs, K., Uwe, H., John, M., Sylva, Sean, Brewer, P., Peter, G., et al. (1999). Methane-consuming archaebacteria in marine sediments. Nature 398, 802–805. doi:10.1038/19751
Kiani, S., Kujala, K., Pulkkinen, J. T., Aalto, S. L., Suurnakki, S., Kiuru, T., et al. (2020). Enhanced nitrogen removal of low carbon wastewater in denitrification bioreactors by utilizing industrial waste toward circular economy. J. Clean. Prod. 254 (1), 119973. doi:10.1016/j.jclepro.2020.119973
Lee, K. H., Wang, Y. F., Li, H., and Gu, J. D. (2014). Niche specificity of ammonia-oxidizing archaeal and bacterial communities in a freshwater wetland receiving municipal wastewater in Daqing, Northeast China. Ecotoxicology 23 (10), 2081–2091. doi:10.1007/s10646-014-1334-3
Li, W. L., Yang, B. Y., Chen, Y. Q., Yin, J., and Xu, S. L. (2015). Study on nitrogen removal characteristics of the denitrifying filter with different additional carbon sources. Technol. Water Treat. 41 (11), 82–85.
Li, Y. F., Wang, X., and Gao, Y. (2013). Study on effects of different organic matter, nitrite, e, and pH for denitrification nitrogen and phosphorus removal. J. Shenyang Archit. Univ. Nat. Sci. 29 (3), 531–537.
Liao, M. J., and Wu, G. (2013). “Investigation of ammonia-oxidizing microorganisms in aquaculture ponds,” in Academic annual meeting of Chinese Society of Environmental Sciences, Chengdu City, Sichuan Province, Oct. 22-23.
Lipsewers, Y. A., Hopmans, E. C., Meysman, F. J., Damsté, J. S. S., and Villanueva, L. (2016). Abundance and diversity of denitrifying and anammox bacteria in seasonally hypoxic and sulfidic sediment of the saline Lake Grevelingen. Front. Microbiol. 7, 1–15. doi:10.3389/fmicb.2016.01661
Liu, C., Li, Z., Zhang, Z., Wang, C., and Li, J. (2014). Nitrogen removal performance and sludge characteristics of anammox coupling heterotrophic denitrification. Chin. J. Environ. Eng. 8 (8), 3137–3142.
Liu, X. G. (2011). Study of pollution of pond culture and regulation technology of ecological engineering. Nanjing, China: Nanjing Agricultural University.
Liu, X. G., Wang, J., Wu, Z. F., Cheng, G. F., and Gu, Z. J. (2021). Anaerobic ammonium oxidation bacteria in a freshwater recirculating pond aquaculture system. Int. J. Environ. Res. Public Health 18 (9), 4941. doi:10.3390/ijerph18094941
Mosier, A. C., and Francis, C. A. (2010). Denitrifier abundance and activity across the San Fran cisco Bay estuary. Environ. Microbiol. Rep. 2 (5), 667–676. doi:10.1111/j.1758-2229.2010.00156.x
Mulder, A., Graaf, A. A., Robertson, L. A., and Kuenen, J. G. (1995). Anaerobic ammonium oxidation discovered in a denitrifying fluidized bed reactor. FEMS Microbiol. Ecol. 16 (3), 177–184. doi:10.1111/j.1574-6941.1995.tb00281.x
Noredal, T. I., Mats, J., Magnus, R., Pell, M., Mikael, H., and Sara, H. (2007). Silver (Ag+) reduces denitrification and induces enrichment of novel NirK genotypes in soil. FEMS Microbiol. Lett. 270 (2), 189–194. doi:10.1111/j.1574-6968.2007.00632.x
Sauthier, N., Grasmick, A., and Blancheton, J. P. (1998). Biological denitrification applied to a marine closed aquaculture system. Water Res. 32 (6), 1932–1938. doi:10.1016/s0043-1354(97)00406-5
Schwarz, J. I., Eckert, W., and Conrad, R. (2007). Community structure of archaea and bacteria in a profundal lake sediment Lake Kinneret (Israel). Syst. Appl. Microbiol. 30 (3), 239–254. doi:10.1016/j.syapm.2006.05.004
SEPA (State Environmental Protection Administration)AQSIQ (State general administration of the people’s republic of China for quality supervision and inspection and quarantine) (2002). “Environmental quality standards for surface water,” in National Standard of the People’s Republic of China GB3838.
Shen, L., Wu, H., Gao, Z., Ruan, Y. j., Xu, X. h., Li, J., et al. (2016). Evidence for anaerobic ammonium ox idation process in freshwater sediments of aquaculture ponds. Environ. Sci. Pollut. Res. 23 (2), 1344–1352. doi:10.1007/s11356-015-5356-z
Shi, Y., Liu, T., Yu, H., and Quan, X. (2022). Enhancing anoxic denitrification of low C/N ratio wastewater with novel ZVI composite carriers. J. Environ. Sci. (2), 12.
Shitu, A., Liu, G., Muhammad, A. I., Zhang, Y., Tadda, M. A., Qi, W., et al. (2022). Recent advances in application of moving bed bioreactors for wastewater treatment from recirculating aquaculture systems: A review. Aquac. Fish. 7 (3), 244–258. doi:10.1016/j.aaf.2021.04.006
Sun, Z. D., Wang, Y. Z., and Gao, K. Z. (2017). Biological ecological remediation technology of polluted water. Shandong Fish. 4, 52–55.
Table, T. S. (1984). Turnover of nitrogen compounds in the constructed wetland Oath freshen. Kassel, Germany: University Kassel Press.
Thamdrup, B. (2012). New pathways and processes in the global nitrogen cycle. Annu. Rev. Ecol. Evol. Syst. 43 (1), 407–428. doi:10.1146/annurev-ecolsys-102710-145048
Tiedje, J. M. (1988). Ecology of denitrification and dissimilatory nitrate reduction to ammonium. Environ. Microbiol. Anger, 179–244.
Van de Graaf, A. A., de Bruijn, P., Robertson, L. A., Jetten, M. S. M., and Kuenen, J. G. (1996). Autotrophic growth of anaerobic ammonium-oxidizing micro-organisms in a fluidized bed reactor. Microbiology 142 (8), 2187–2196. doi:10.1099/13500872-142-8-2187
Van, K., Harhangi, H. R., Flik, G., Jetten, M. S. M., Klaren, P. H. M., and dchjm, O. P. (2011). Anam mox bacteria in different compartments of recirculating aquaculture systems. Biochem. Soc. Trans. 39 (6), 1817–1821. doi:10.1042/bst20110743
Verbaendert, I., De Vos, P., Boon, N., and Heylen, K. (2011). Denitrification in gram-positive bacteria: An underexplored trait. Biochem. Soc. Trans. 39 (1), 254–258. doi:10.1042/bst0390254
Wang, Z. Y., Wang, C. H., Wang, Z. X., and Pei, Y. S. (2013). Enhancement of anaerobic ammonium oxidation in lake sediment by applying drinking water treatment residuals. Bioresour. Technol. 142 (8), 745–749. doi:10.1016/j.biortech.2013.06.016
Wei, L. (2017). Harmful treatment of nitrite in the late stage of prawn culture. Plant Dr. 1, 43–44.
Xu, Q., Zhang, F., Zhong-Qi, X. U., Jia, Y. L., and You, J. M. (2011). An analysis of the characteristics and the “dilution effect” of the Simpson index and Shannon-Wiener index. Pratacultural Sci. 28 (4), 527–531.
Yang, R. G., Zhang, L. H., and Liu, Q. (2012). Analysis of influence factors on wastewater biological treatment process of shortcut nitrification-denitrification. J. Northeast China Inst. Electr. Power Eng. 32 (1), 61–65.
Yao, H. L. (2010). Study on the ecology of integrated aquaculture pond in China. Beijing, China: Science Press.
Yu, D. S., Wei, S. J., Li, J., Qi, P. Q., and Guan, Y. J. (2016). Effect of temperature on simultaneous carbon and nitrogen removal by anaerobic ammonium oxidation and denitrification. China Environ. Sci. 36 (5), 1384–1391.
Yue, W. Z., and Huang, X. P. (2003). Advance in biogeochemistry studies on nitrogen and phosphorus in offshore sediment. J. Appl. Oceanogr. 22 (3), 407–414.
Zeng, X. L., Liu, X. G., Wu, Z. F., Shi, X., and Lu, S. M. (2016). [Community characteristics of ANAMMOX bacteria in subsurface flow constructed wetland (SSFCW) for processing of aquaculture waster water]. Environ. Sci. 37 (2), 615–621.
Zheng, Y. L., Hou, L. J., Liu, M., Gao, J., Yin, G. Y., Li, X. F., et al. (2015). Diversity, abundance, and distribution of nirS-harboring denitrifiers in intertidal sediments of the Yangtze estuary. Microb. Ecol. 70 (1), 30–40. doi:10.1007/s00248-015-0567-x
Keywords: Anammox, denitrification, sediment, pond aquaculture, regulation effect
Citation: Liu X, Gao M, Wang J, Gu Z and Cheng G-f (2022) Characteristics of denitrification and anammox in the sediment of an aquaculture pond. Front. Environ. Sci. 10:1023835. doi: 10.3389/fenvs.2022.1023835
Received: 20 August 2022; Accepted: 14 October 2022;
Published: 31 October 2022.
Edited by:
Zheng-Yang Huo, Renmin University of China, ChinaReviewed by:
Shiyang Zhang, Wuhan University of Technology, ChinaSamik Bagchi, Digested Organics, United States
Copyright © 2022 Liu, Gao, Wang, Gu and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingguo Liu, bGl1eGcxMjIzQDE2My5jb20=
 Xingguo Liu
Xingguo Liu Meiyun Gao3
Meiyun Gao3 Zhaojun Gu
Zhaojun Gu