
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 31 January 2022
Sec. Toxicology, Pollution and the Environment
Volume 9 - 2021 | https://doi.org/10.3389/fenvs.2021.811466
This article is part of the Research TopicChallenges in Characterizing Nano- to Macro-Plastics and Adhered Substances in the Aquatic EnvironmentView all 7 articles
This study was conducted to explore the protective potential of three different antioxidant supplements, lycopene, citric acid, and Chlorella, against reproductive injuries induced by microplastics (MPs) in freshwater mature male catfish. A total of 150 mature male African catfish (Clarias gariepinus) were assigned to five treatment groups as follows: control group fish were fed with control diet, the second group fish were fed with 500 mg/kg MP diet, and the remaining three groups of fish were fed with 500 mg/kg MP diet plus lycopene (500 mg/kg diet), citric acid (30 g/kg diet), and Chlorella (50 g/kg diet), respectively, for 15 days. Ingestion of MPs significantly decreased serum luteinizing hormone, follicle-stimulating hormone, sex steroid (testosterone and estradiol) levels and sperm count, spermatocrit, motility, and viability. It also induced histological alterations and degenerative changes in testicular tissues. Administration of lycopene and Chlorella with MP diets maintained hormone levels comparable to those in the control group, enhanced sperm quality, and decreased testicular histological damage. Chlorella was more effective in enhancing sperm quality, and lycopene was more efficient in alleviating testicular tissue damage. Citric acid supplementation was irrelevant in mitigating MP-induced injury. This study indicated that both lycopene and Chlorella ameliorated the MP-induced reproductive dysfunction by improving reproductive hormonal levels, sperm parameters, and histological configuration, whereas the citric acid dose used in this study was not effective in ameliorating the MP-induced reproductive stress. Additional research and monitoring of MP-induced pollution in freshwater ecosystems are required to avoid the severity of reproductive toxicity in freshwater fish.
Plastic is a global environmental pollutant whose levels have grown rapidly due to developments in plastic manufacturing (Vijver et al., 2020). Plastic production advancements have increased in the past 6 decades by almost 560 times (Plastics Europe, 2020). The expected annual amount of plastics that enter the marine environment is more than 9.5 million tons, and these plastics disintegrate into microplastics (MPs) (particles measuring <5 mm in length) through mechanical and biological degradation (Law and Thompson, 2014; Boucher and Friot, 2017; Law, 2017). MPs pollution has a large geographical magnitude as it is dispersed by surface water currents (Wang et al., 2017). Freshwater can act as a source, a transfer medium, and a sink for MPs (Klein et al., 2018). Freshwater systems receive MPs directly from several primary sources and hence they accumulate various MPs (Law, 2017; Luo et al., 2019). Recent research has shown that freshwater ecosystems have become increasingly loaded with MPs pollution (Koelmans et al., 2019; Li et al., 2020). Although the variability of plastic polymers, polyethylene plastic (PE), with the chemical formula (C2H4)n, represents one of the supreme world extensively used (de Sà et al., 2018). PE exist in numerous products, for instance plastic bags, camera films, fibers, storage containers and wrapping, toys, etc. (Lusher et al., 2015). Due to the widespread dissemination of MPs in the environment, they are easily gulped by fish, bivalves, and other aquatic organisms (Lusher et al., 2015; Nadal et al., 2016). Fish may ingest MPs directly and/or by feeding on other organisms (Carlos de Sá et al., 2015). Ingested MPs can induce gut impasse and altered gut function, which can lead to reduced nutrition (Jovanović, 2017). Recent researches have described that MPs prompt oxidative stress and apply adverse effects on the antioxidant defense systems of some studied invertebrates (Jeong et al., 2017; Yu et al., 2018). Moreover, in some studied fish MPs can amend antioxidant biomarkers and induce lipid peroxidation as described for; zebrafish (Danio rerio) (Qiao et al., 2019; Wan et al., 2019), red tilapia (Oreochromis sp.) (Ding et al., 2018), sheepshead minnows (Cyprinodon variegatus) (Choi et al., 2018), and for Nile tilapia (Oreochromis niloticus) as well (Hamed et al., 2019; Ismail et al., 2021). Mps prompting oxidative stress and lipid destruction in brains of European sea bass (Dicentrarchus labrax) (Barboza et al., 2018).
Exposure to MPs has been reported as a reason of reproductive stress and gonadal impairment (Ismail et al., 2021, Karami et al., 2016; Rochman et al., 2014; Wang et al., 2019). There are studies reporting that MPs induced fecundity reduction in mature female medaka (Oryzias latipes) (Zhu et al., 2020). Moreover, MPs exposure by brood fish delayed hatching rate, heart rate and growth in marine medaka (Oryzias melastigma) offspring (Wang et al., 2019). MPs are also known to induce oxidative stress levels, elevate apoptosis levels and testicular histological impairments (Qiang and Cheng, 2021), and significantly decrease sperm velocity in oysters (Sussarellu et al., 2016). Moreover, MPs exposure induced testicular deteriorating changes and prompted testis-ova with drop in both LH and T serum levels in tilapia male (Ismail et al., 2021).
Lycopene has been described as a potent natural antioxidant that mitigates oxidative stress responses in some fish species subjected to several toxicants or any other stress circumstances (Amarowicz, 2011; Yonar, 2012; Dawood et al., 2020). Addition of lycopene to fish feed was found to increase growth parameters (Rashidian et al., 2020) and maintain fish wellbeing by improving both antioxidative and immune responses (Dawood et al., 2020). Lycopene is known to exert several positive effects on human health as it can be incorporated into the treatment for male infertility and other syndromes (Grabowska et al., 2019).
Citric acid and its salts are increasingly being examined as dietary supplements in fish feed due to their acidifying characteristics that can improve nutrient utilization, gastrointestinal condition, and digestive enzyme activity (Vielma et al., 1999; Lim et al., 2015). Citric acid supplementation to fish diet was found to chelate calcium and phosphorus and increase their solubility in the rainbow trout (Pandey and Satoh, 2008), red seabream (Pagrus major) (Hossain et al., 2007), yellowtail (Seriola quinqueradiata) (Sarker et al., 2012), and turbot (Scophthalmus maximus) (Dai et al., 2018). Citric acid supplements were found to reduce oxidative damage and alleviate inflammatory responses in the turbot (S. maximus) (Chen et al., 2018; Zhao et al., 2020).
Applications of microalgae supplementation have been developed recently; for instance, Chlorella vulgaris has established to develop, immunity, aquatic remedy, stress enhancement, and disease resistance in fish (Nicula et al., 2018; Mekkawy et al., 2020; Sahin et al., 2014). C. vulgaris used as feed supplementation alleviated the negative effect of chlorpyrifos (CPF) exposure and maintained the growth performance and biochemical parameters (Abu-Srea et al., 2018) ,also, C. vulgaris modulated the expression of genes encoding antioxidant enzymes and stress immune-related genes (Zahran et al., 2020) in the Nile tilapia.
This study was conducted to determine the harmful effects of MP consumption on the reproductive performance of the freshwater mature male African catfish (C. gariepinus). Diets containing MPs were applied for 15 days. Moreover, to evaluate the ability to ameliorate the MPs-induced reproductive impairment, three different antioxidants (lycopene, citric acid, and Chlorella) were applied in combination with MPs-supplemented diets. Hormonal profiles, testicular histology, and sperm quality parameters were used as indicators for male reproductive status between the different experimental groups.
A total of 150 mature male African catfish (weight 300–500 g, length 25–32 cm) were obtained from the Aquaponic Unit and transported to the Fish Biology and Pollution Laboratory in the Faculty of Science, Assiut University. The physicochemical parameters of rearing water were as follows: conductivity 261 mM/cm; pH 7.4; dissolved oxygen 6.9 mg/L; temperature 20.5°C, while light- dark hours were kept as 12/12. During the acclimatization period, fish were fed commercial feed about 3% of total body weight each day divided into two portions. The feed contained 30% protein and consisted of soybean meal, wheat bran, maize, crude protein, fats, crude fiber, fish meal, calcium, sodium chloride, vitamins, and mineral salt. Fish were distributed into five groups (30 fish/group), and each treatment group was separated and placed in aquaria in triplicates for an experimental duration of 15 days. During the experimental period fish groups were feed the same commercial feed for the control or commercial diet combined with the different tested supplement and/or MPs dose for experimental groups as following:
The first group was the control (ctr) group that was fed with the control diet , the second group (MPs) was fed with a diet containing MPs (500 mg/kg diet), the third group (MPs + lyco) was exposed to MPs (500 mg/kg diet) + lycopene (500 mg/kg diet), the fourth group (MPs + citr) was fed with a diet containing MPs (500 mg/kg diet) + citric acid (30 g/kg diet), and the fifth group (MPs + chl) was fed with a diet containing MPs (500 mg/kg diet) + Chlorella (50 g/kg diet).
After 15 experimental days, six fish from each experimental group were haphazardly selected and anesthetized by ice to lessen the handling stress. Blood samples were collected from the caudal vein, and testicular tissues were collected for histological analyses. The experimental design and fish treatments were agreed by the Faculty of Science Committee, Assiut University.
The MPs used in the experiment was powder of uneven polyethylene particles, more than 90% of the MPs particles were above 100 nm in size. The MPs raw powder was obtained from Toxemerge Pty Ltd. (Melbourne, Australia). To prepare the stock solution (2.5 g MP/L) purified water (Milli-Q) was used according to the manufacturer’s directions and kept at 4°C in the dark. The stock solution was sonicated before every use. Further dilutions were preformed from stock solution straightaway for each time of changing rearing water. The description of MP particles was done using light and transmission electron microscopy at TEMU, Assiut University (JEOL JEM-1200 EX II) FUTURE (Hamed et al., 2019). The selection of MPs treatment dose was done according to earlier study of Espinosa et al., 2019.
Lycopene and citric acid were bought from Sigma-Aldrich (Cairo, Egypt), and C. vulgaris extract was obtained from the National Research Center, Cairo, Egypt. The added doses of lyco, citr and chl were selected according to recommended doses of previous studies (Mahmoud et al., 2013; Carneiro et al., 2020).
Serum follicle-stimulating hormone (FSH) levels were measured as described by Knobil (1980) using a test kit (Cat. No. CANFSH-4060, Diagnostics Biochem Canada Inc.; Ontario, Canada), luteinizing hormone (LH) levels were assessed according to (Cumming et al., 1985) using a test kit (Cat. No. CAN-LH-4040, Diagnostics Biochem Canada Inc.; Ontario, Canada), and serum testosterone (T) levels were evaluated using an ELISA kit (CAN-TE-250, Diagnostics Biochem Canada Inc.; Ontario, Canada). Estradiol level was measured using an ELISA kit (CAN-E-430, Diagnostics Biochem Canada Inc.; Ontario, Canada), and hormone levels were measured at 450 nm using an automatic immunodiagnostic analyzer (Sorin Biomedica, Model: 0-2730; S/N = 0,654, Chemila SP.A., Italy).
After dissection, the testes were removed. A longitudinal incision was made on the testes, and the milt was collected into calibrated glass tubes. Sperm analysis was conducted in the laboratory using the removed sperm from the testicular specimens of each treatment group. Spermatocrit (%), sperm motility (min), and number of spermatozoa (×109) were calculated. Spermatocrit was calculated using the microhematocrit method described formerly (Ciereszko and Dabrowski, 1993). Sperm motility (period elapsed between activation and cessation of any propulsive movement) was calculated using a drop of sperm placed on a slide, which was later covered with a coverslip to avoid sperm cell movement under the wave action and sample dehydration. The slide was detected under a microscope (OMAX) at 5-min intervals at a amplification of ×400 to assess sperm motility period. To determine the sperm count, the sperm sample was diluted with Billard solution at a dilution proportion of 1:200 (Billard, 1977). Next, the Neubauer hemocytometer was prepared and made grease-free with its coverslip before filling the counting chamber with the sperm solution. The solution was left undisturbed in the chamber for 1 minute to settle down the spermatozoa. The concentration of spermatozoa was detected by getting the sperm number in the diluted sample in the Neubauer hemocytometer under ×400 magnification (Rainis et al., 2003).
Sperm viability was analyzed by calculating the proportion of live and dead sperm cells in the ejaculate using the detection kit of Molecular Probes LIVE/DEAD Sperm Viability Kit (L-7011, Eugene, Oregon, United States) on a total of 100 sperm cells each time. After semen collection, the samples were diluted in HEPES-buffered saline solution with bovine serum albumin (10 mM HEPES, 150 mM NaCl, 10% BSA with pH 7.4) to attain acceptable cell densities. It was then stained with diluted SYBR 14 dye for 5–10 min at 36°C, followed by propidium iodide addition and incubation for 5–10 min at 36°C. Dead and live spermatozoa were observed and counted under a Zeiss Axioplan2 fluorescence microscope (×400) equipped with a digital 3 CCD color video camera (Sony, AVT-Horn).
After fixation, testicular tissues were dehydrated in arising of ethanol concentrations, flowed by clearing step in methyl benzoate, and then fixed in paraffin wax. Sections of 5-µm thickness were cut and stained with hematoxylin–eosin and then inspected under a microscope.
All data were expressed as mean ± standard error and verified for significance using one-way analysis of variance (ANOVA) then tested by Tukey’s test for multiple comparisons to indicate the significance of differences amongst experimental groups. Data representing spermatocrit, sperm motility, and viability were subjected to arcsine square root transformation before conducting ANOVA. Probability of significant differences was set at p < 0.05. Analyses were conducted using the SPSS® version 23.0 package (SPSS, 1998).
Both serum LH and FSH levels in different treatment groups indicated a significant decline (p < 0.05) for MPs and citr fish groups, whereas the levels in lyco and chl groups were similar to those of the ctr group (Figures 1A,B).
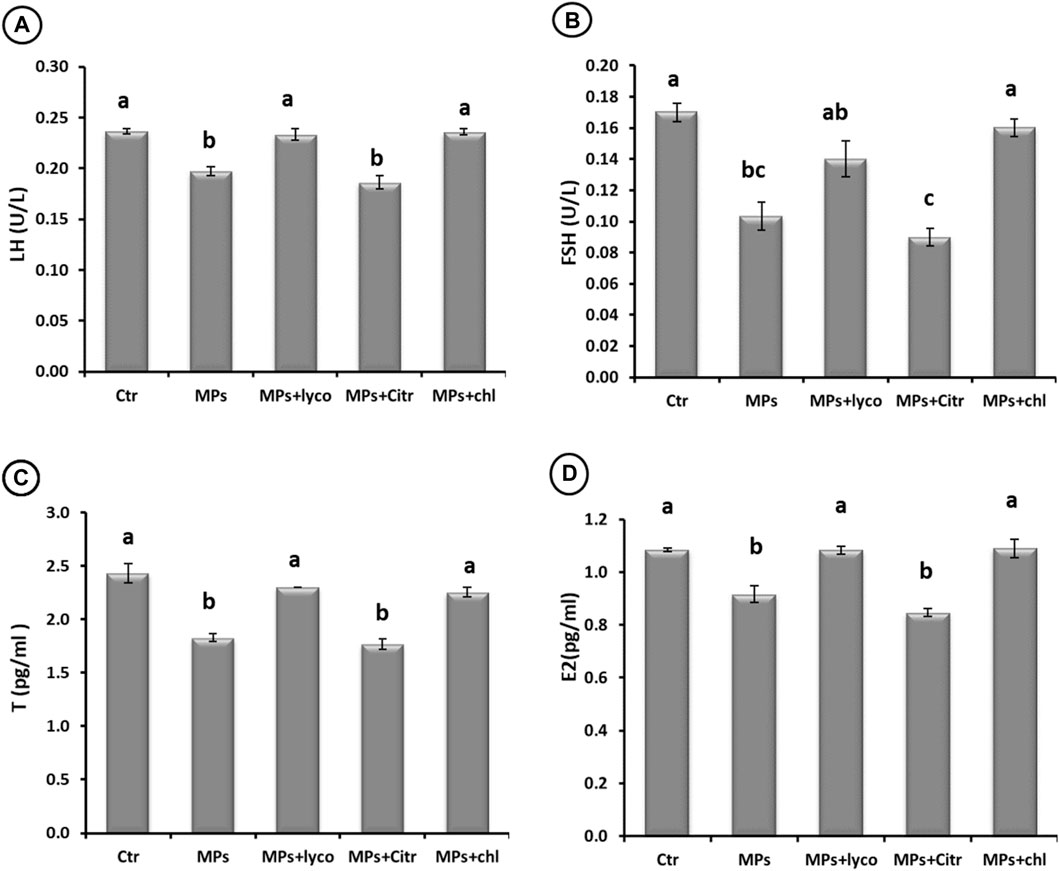
FIGURE 1. Effect of ingestion of MPs and ameliorative effect of lycopene (lyco), citric acid (Citr), and Chlorella (chl) or 15 days on (A) luteinizing hormone (LH), (B) follicle-stimulating hormone (FSH), (C) testosterone (T), and (D) estradiol (E2) levels of mature male African catfish (C. gariepinus). Data are demonstrated as mean ± SE, n = 6. Bars carrying different superscripts (A–C) are significantly different at (p < 0.05).
Regarding steroid profiles, both T and E2 levels showed a comparable pattern of a significant decline (p < 0.05) for MPs and citr groups comparing to the ctr group. For the fish groups lyco and chl, the serum T and E2 levels were comparable to those in the ctr group (Figures 1C,D).
Semen analysis revealed that the MPs prompted a decrease in spermatocrit, number of sperms, motility, and number of live sperms, as shown in Table 1. The MP group displayed significant decreases (p < 0.05) in all of the analyzed factors. Both lyco and chl groups displayed comparable high levels of spermatocrit and sperm viability percentage but less than those in the ctr group. The fifth group (chl) displayed significant increases (p < 0.05) in the number of sperms and motility percentage compared with the other treatment groups but similar to those of the ctr group. The citr group exhibited significant decreases (p < 0.05) in all the evaluated quality parameters compared to those of ctr, lyco, and chl groups, as shown in Table 1. Comparison of results showed that the citr and MP groups had comparable data and recorded less sperm quality compared with other groups.
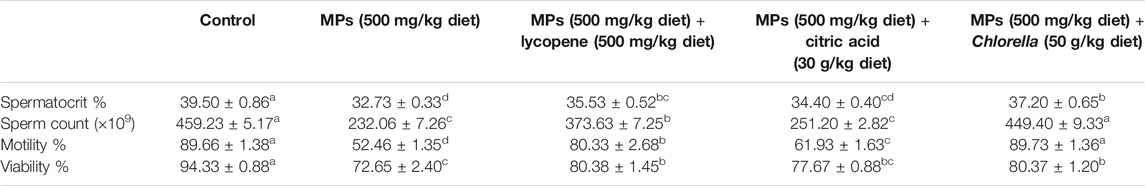
TABLE 1. Effect of ingestion of MPs and ameliorative effect of lycopene, citric acid, and Chlorella for 15 days on sperm quality of mature male African catfish (C. gariepinus). Data are demonstrated as mean ± SE. Different superscript letters within the same row denoted a significant difference (p < 0.05).
Light microscopic investigation revealed that C. gariepinus testicular sections obtained from the control group contained of lobules with diverse germ cells (spermatogonia, spermatocyte cyst, and spermatid cyst) that were detached from each other by interstitial tissue. Mature spermatozoa were accumulated in the lumen of testicular lobules (Figure 2A).
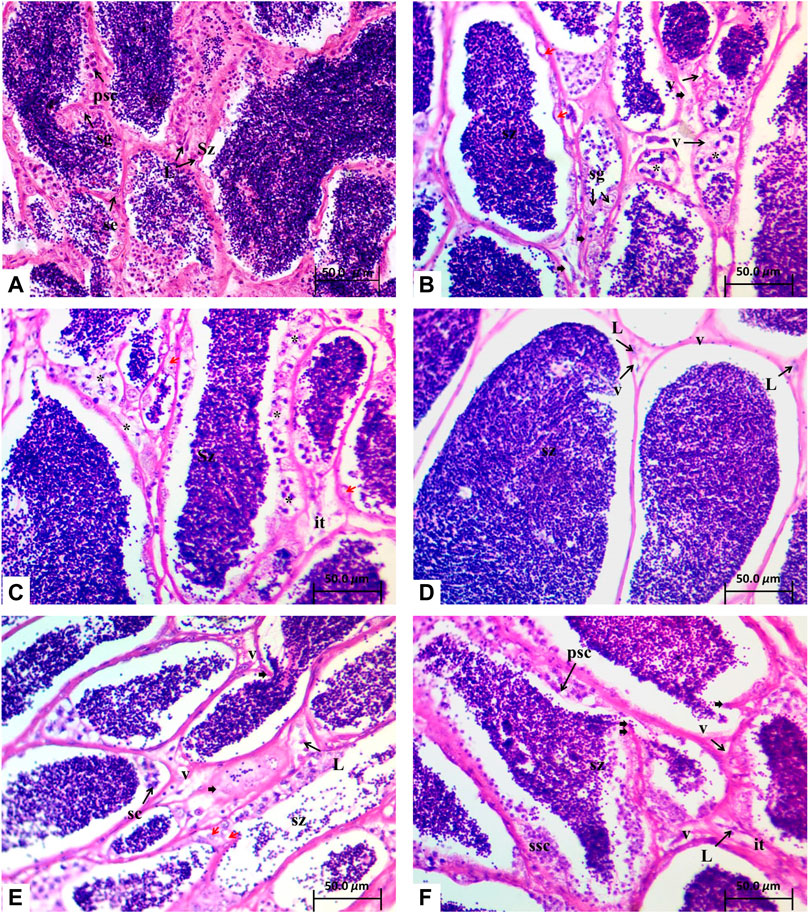
FIGURE 2. Testes tissues of C. gariepinus after exposure to microplastics for 15 days: (A) control (ctr) fish group with fully developed mature testis with nests of spermatogonia (sg) along the lobule wall, primary spermatocytes (psc), Leydig cells (L), and Sertoli cells (Se) and filled with mature spermatozoa (Sz). (B–C) microplastics-exposed group (MPs) with disorganized lobule structure (black arrow heads), vacuolation of interstitial tissue (v), hypertrophied spermatogonia (red arrow) and less amount of spermatozoa (Sz), degeneration of germ cells (*) and diminished interstitial cells (it), and degenerated Leydig cells (L). (D) Lycopene-exposed group (lyco) with normal amounts of spermatozoa (Sz), few vacuoles (v), and Leydig cells (L). (E) Citric acid-exposed group (citr) with less amount of spermatozoa (Sz), disorganized lobule structure (black arrow heads), vacuolated interstitial tissue (v), hypertrophied spermatogonia (red arrow), and degenerated Leydig cells (L). (F) Chlorella-exposed group (chl) with lobules occupied with spermatozoa (Sz) and interstitial tissue (it) with few vacuoles (v) and Leydig cells (L), disorganized lobule structure (black arrow heads), secondary spermatocytes (ssc), hematoxylin–eosin staining.
The testes tissues of C. gariepinus fed with diet containing MPs only (MP group) demonstrated a disorganized lobule structure accompanied by a reduced number of germinal cells. The interstitial tissue displayed high vacuolation with reduced number of interstitial cells and reduced number or absence of Leydig cells. Furthermore, there was degeneration of different germ cells with vacuolation along with hypertrophied spermatogonia and less amount of spermatozoa (Figures 2B,C).
In the third group of fish fed with diet containing MPs and supplemented with lyco, the testes tissues showed seminiferous tubules supported by a normal thin connective tissue accompanied with high amounts of spermatozoa, and the interstitial tissue displayed less vacuolization and few Leydig cells (Figure 2D).
The testicular sections of the fourth fish group (citr) exhibited less amount of spermatozoa, disorganized lobule structure, vacuolated interstitial tissue, hypertrophied spermatogonia, and degenerated Leydig cells (Figure 2E).
The testicular sections of the fifth fish group (chl) showed lobules with accumulated mature spermatozoa and spermatocyte cysts, and the interstitial tissue displayed few vacuoles and clusters of Leydig cells (Figure 2F).
Our findings provide key insights into the effects of dietary exposure of MPs on the reproductive ability and sperm quality of freshwater fish and the effects of three different antioxidants on the MP-induced toxicity.
Exposure to MPs affected the gonadal axis by inhibiting the synthesis of T and E2, decreasing the quality and quantity of sperms, inducing testicular tissue alterations, and finally leading to reproductive impairment. Similarly, previous studies have reported decreased sperm velocity in oyster after exposure to MPs (Sussarellu et al., 2016) and decreases in the number and motility of sperm and upsurge in sperm deformity rate in male mice treated with different doses of MPs (Xie et al., 2020). However, in contrast to our findings, MP exposure was found to significantly increase the level of T in male O. melastigma (Wang et al., 2019).
Other previous studies have conclusively demonstrated that MPs exposure induced testicular alterations in some fish species such as the Japanese medaka (Rochman et al., 2014; Wang et al., 2019) and zebrafish (Qiang and Cheng, 2021). Histological alterations consisting of vacuolation of interstitial tissue, disordered seminiferous lobule organization, dissolution of basal membrane (Rochman et al., 2014), loose arrangement of germ cells, and reduction of thickness of the testicular basement membrane have also been reported (Qiang and Cheng, 2021). Wang et al. (2019) explained different histological alterations in testicular tissues after MPs exposure, including an expansion in the interstitial tissue, seminiferous arrangement changes, the dissolution of the basal membrane, and a loose arrangement of spermatocytes.
The histological alterations observed in our study also included degeneration of germ cells in addition to vacuolized interstitial tissue and reduced number of Leydig cells. The decline and degeneration of both Leydig and Sertoli cells are always a sign of androgen suppression and degeneration of germ cells, because Leydig cells are the major site of androgen synthesis and release, and Sertoli cells provide structural support and nutrition to the developing germ cells (Duan et al., 2017).
Accumulating evidence confirms that the reproductive damage caused by MPs is due to oxidative damage (Xie et al., 2020). Oxidative stress has been reported to be accompanying with declining reproductive performance in oysters (Sussarellu et al., 2016) and marine medaka (Wang et al., 2019) exposed to MPs. Moreover, Xie et al. (2020) proposed that MPs induced reproductive toxicity in male mice through an oxidative stress. Oxidative stress can also target and detrimentally affect reproduction (Prokić et al., 2019). Qiang and Cheng (2021) proposed that overall stress in detoxification and the induction of activity of the antioxidant enzyme are contributing factors in reproductive impairments. Gonads are most susceptible to the oxidative stress, as gonads go through successive cell division with more mitochondrial oxygen intake and unsaturated fatty acids (Asadi et al., 2017). In addition, ingestion of MPs by fish and aquatic organisms was found to block the digestive system, decline the growth rate, and suppress the production of different enzymes (Wright et al., 2013; Jovanović, 2017).
Regarding the possible pathway by which MPs induce reproductive impairment, the decline in both LH and FSH levels after MP treatment in our study could be due to the indirect effect of MPs on the hypothalamus–pituitary- gonadal (HPG) axis and their suppressive effects on the synthesis and secretion of gonadotropins, which results in the disruption of production of sex steroid and degeneration of testicular tissue. These findings are consistent with those described by Wang et al. (2019) for the male marine medaka and by Karami et al. (2016) who described that MPs also declined the production of the hypothalamus gonadotropin-releasing hormone (GnRH) in the African catfish (C. gariepinus).
In this study, we detected an increasing number of dead sperms as a consequence of MPs ingestion in all of the experimental groups compared with the control and lycopene- and Chlorella-fed groups, which showed significantly reduced (p < 0.05) number of dead sperms and enhanced viability. According to some authors, MPs enter cells and produce oxidative stress through reactive oxygen species (ROS) generation, which then activates several biological responses consisting of inflammation, oxidative stress-induced signaling, and apoptosis pathways (Asharani et al., 2009; Bhabra et al., 2009; Tripathi et al., 2011). In a previous study on the marine copepod (Paracyclopina nana), it was observed that ingestion of MPs induced ROS production and activated the signaling pathways involved in the propagation of oxidative stress and those related to cell death process (Jeong et al., 2017). Studies have also confirmed that stressors such as exposure to pollutants can generate testicular oxidative stress and prompt apoptosis of germ cells, consequently disturbing the process of spermatogenesis (Asadi et al., 2017; Calivarathan et al., 2019).
In the present study, we evaluated the potential of three different antioxidant-supplemented diets (lyco, citr, and chl) combined with 500 mg of MPs to alleviate the reproductive damage induced by MPs. We detected that both lycopene- and Chlorella-supplemented diets were effective in this regard, but not the citric acid-supplemented diet. In both the lycopene- and Chlorella-supplemented groups, we observed significantly higher (p < 0.05) serum levels of LH, FSH, T, and E2 and sperm quality parameters than those in the MPs-treated group.
Lycopene is considered to be one of the most effective antioxidants, as lycopene supplementation has been reported to exert ameliorating characteristics against oxidative stress (Hedayati et al., 2019). Lycopene was found to strongly attenuate oxidative stress in C. gariepinus (Mahmoud et al., 2013) and Cyprinus carpio (Yonar, 2012) when provided as a dietary supplement. Moreover, lycopene represents one of the most promising antioxidants against reproductive toxicity (Zhao et al., 2020). Our results showed that the fish group fed with lycopene supplementation displayed few histological alterations. In agreement with our results, Zhao et al. (2020) described that lycopene could alleviate the damage to seminiferous tubules and spermatogenic cells in mice. Furthermore, lycopene could recover and protect against sperm and testicular damage in rats (Tripathy et al., 2017).
Regarding sperm quality, our results emphasized that lycopene supplementation ameliorated the damaging effect of MPs on sperm motility and number and spermatocrit percentage and enhanced the sperm viability. Several research have been conducted in human and animals testing lycopene supplementation, they have shown promising results in relieving male infertility, where the sperm number and viability were increased (Durairajanayagam et al., 2014). Moreover, lycopene could improve values of sperm motility, number, and density (Zhao et al., 2020). Similarly in agreement with our results, lycopene protects against acute zearalenone -induced decreased amount and sperm motility and testosterone content in male mice (Boeira et al., 2015), also lycopene can improve the decreased sperm number, and motility induced by Lipopolysaccharide (Aly et al., 2012).
In the present study, the Chlorella-supplemented diet was the most effective in maintaining sperm quality parameters comparable to those in the control. A previous study confirmed that Chlorella is a potent antioxidant, as the chlorophylls of C. vulgaris were found to inhibit lipid peroxidation by reducing ROS generation (Hsu et al., 2013). The antioxidant property of C. vulgaris can counteract and prevent the negative, disruptive effect of oxidative stress on spermatogenesis, male reproductive hormonal profiles, and (HPG) axis (Eissa et al., 2020).
Recently, Chlorella was used as an antioxidant to alleviate reproductive toxicity; the application of C. vulgaris against deltamethrin toxicity was found to restore spermatogenic activity, sperm viability, and sperm count in rats (Osama et al., 2019). Moreover, C. vulgaris extract ameliorated reproductive dysfunction by improving the FSH and T profiles, sperm parameters (motility, viability, and count), testicular alterations, and testicular antioxidant activities in albino rats (Eissa et al., 2020). C. vulgaris improves oxidative stress that affected the reproduction in New Zealand White rabbits (Sikiru et al., 2019). In recent study conducted on zebrafish, addition of C. vulgaris in meal diet up to 50 g/kg provided the high egg production, hatching rate and larval survival (Carneiro et al., 2020).
Our study demonstrated that citric acid supplementation was less effective in the detoxification of MPs. Studies have reported that citric acid exerted beneficial effects in terms of growth and nutrient utilization (Romano et al., 2016; Dai et al., 2018). Citric acid may play a vital role in heavy metal detoxification and decreasing oxidative damage in the worm Caenorhabditis elegans (Song et al., 2019). The less alleviating efficiency of citric acid supplementation in the present study can be associated to the tested dose, and fewer doses may be more effective. In fact, it has been reported that citric acid inclusion percentage is an important factor for its effectiveness (Dai et al., 2018). Romano et al. (2016) demonstrated that increasing the dietary citric acid supplement dose can induce liver damage and affect health status.
In conclusion, our study has validated that MP pollution in the aquatic environment can directly impact the reproductive function of freshwater fish such as the African catfish, wherein ingestion of diets containing 500 mg/kg MPs for 15 days induced testicular damage, diminished sperm quality and viability, and suppressed hormonal profiles. These results establish that intake of MPs is a potential source of reproductive stress. Furthermore, both lycopene and Chlorella supplements acted as potent antioxidants in detoxifying the reproductive damage induced by MPs, whereas citric acid was found to be an ineffective antioxidant in ameliorating the MPs-induced reproductive toxicity in male catfish. Other doses of citric acid could be considered in future research.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
This animal study was reviewed and approved by the Research Ethical Committee of the Faculty of Science, Assiut University.
Experimental design: AE-D. Experiment and analysis: AE-D, MH, RI. Data interpretation: AE-D, MH, RI. Writing and revision: AE-D, MH, RI. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, orclaim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abu-Srea, H., Risha, E., Zahran, E., and Abdalla, O. (2018). Protective Effects of Chlorella Vulgaris Dietary Supplementation on Growth Performance, Hematological and Biochemical Parameters in Nile tilapia (Oreochromis niloticus) Exposed to Chlorpyrifos. Ann. Vet. Anim. Sci. 5, 1–12.
Aly, H. A. A., El-Beshbishy, H. A., and Banjar, Z. M. (2012). Mitochondrial Dysfunction Induced Impairment of Spermatogenesis in LPS-Treated Rats: Modulatory Role of Lycopene. Eur. J. Pharmacol. 677, 31–38.
Amarowicz, R. (2011). Lycopene as a Natural Antioxidant. Eur. J. Lipid Sci. Technol. 113, 675–677. doi:10.1002/ejlt.201100157
Asadi, N., Bahmani, M., Kheradmand, A., and Rafieian-Kopaei, M. (2017). The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn. Res. 11, IE01. doi:10.7860/JCDR/2017/23927.9886
Asharani, P. V., Low Kah Mun, G., Hande, M. P., and Valiyaveettil, S. (2009). Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 3, 279–290. doi:10.1021/nn800596w
Barboza, L. G. A., Vieira, L. R., and Guilhermino, L. (2018). Single and Combined Effects of Microplastics and Mercury on Juveniles of the European Seabass (Dicentrarchus labrax): Changes in Behavioural Responses and Reduction of Swimming Velocity and Resistance Time. Environ. Pollut. 236, 1014–1019.
Bhabra, G., Sood, A., Fisher, B., Cartwright, L., Saunders, M., Evans, W. H., et al. (2009). Nanoparticles Can Cause DNA Damage across a Cellular Barrier. Nat. Nanotech 4, 876–883. doi:10.1038/nnano.2009.313
Billard, R. (1977). A New Technique of Artificial Insemination for Salmonids Using a Sperm Diluent. Fisheries 2, 24–25.
Boeira, S. P., Funck, V. R., Borges Filho, C., Del’Fabbro, L., Gomes, M. G. d., Donato, F., et al. (2015). Lycopene Protects against Acute Zearalenone-Induced Oxidative, Endocrine, Inflammatory and Reproductive Damages in Male Mice. Chemico-Biological Interactions 230, 50–57. doi:10.1016/j.cbi.2015.02.003
Boucher, J., and Friot, D. (2017). Primary Microplastics in the Oceans: A Global Evaluation of Sources. Switzerland: Iucn Gland.
Carlos de Sá, L., Luís, L. G., and Guilhermino, L. (2015). Effects of Microplastics on Juveniles of the Common Goby (Pomatoschistus Microps): Confusion with Prey, Reduction of the Predatory Performance and Efficiency, and Possible Influence of Developmental Conditions. Environ. Pollut. 196, 359–362.
Carneiro, W. F., Castro, T. F. D., Orlando, T. M., Meurer, F., de Jesus Paula, D. A., Virote, B. D. C. R., et al. (2020). Replacing Fish meal by Chlorella sp. Meal: Effects on Zebrafish Growth, Reproductive Performance, Biochemical Parameters and Digestive Enzymes. Aquaculture 528, 735612.
Chen, Z., Zhao, S., Liu, Y., Yang, P., Ai, Q., Zhang, W., et al. (2018). Dietary Citric Acid Supplementation Alleviates Soybean Meal-Induced Intestinal Oxidative Damage and Micro-ecological Imbalance in Juvenile Turbot, Scophthalmus maximus L. Aquac. Res. 49, 3804–3816. doi:10.1111/are.13847
Choi, J.S., Jung, Y.J., Hong, N.H., Hong, S.H., and Park, J.W. (2018). Toxicological Effects of Irregularly Shaped and Spherical Microplastics in a Marine Teleost, the Sheepshead Minnow (Cyprinodon variegatus). Mar. Pollut. Bull. 129, 231–240.
Ciereszko, A., and Dabrowski, K. (1993). Estimation of Sperm Concentration of Rainbow trout, whitefish and Yellow Perch Using a Spectrophotometric Technique. Aquaculture 109, 367–373. doi:10.1016/0044-8486(93)90175-x
Cumming, D. C., Vickovic, M. M., Wall, S. R., and Fluker, M. R. (1985). Defects in Pulsatile LH Release in Normally Menstruating Runners. J. Clin. Endocrinol. Metab. 60, 810–812. doi:10.1210/jcem-60-4-810
Dai, J., Li, Y., Yang, P., Liu, Y., Chen, Z., Ou, W., et al. (2018). Citric Acid as a Functional Supplement in Diets for Juvenile Turbot, Scophthalmus maximus L.: Effects on Phosphorus Discharge, Growth Performance, and Intestinal Health. Aquaculture 495, 643–653. doi:10.1016/j.aquaculture.2018.04.004
Dawood, M. A. O., Abdel‐Tawwab, M., and Abdel‐Latif, H. M. R. (2020). Lycopene Reduces the Impacts of Aquatic Environmental Pollutants and Physical Stressors in Fish. Rev. Aquacult. 12, 2511–2526. doi:10.1111/raq.12455
de Sà, L.C., Oliveira, M., Ribeiro, F., Rocha, T. L., and Futter, M. N (2018). Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future?. Sci. Total Environ 645, 1029e1039.
Ding, J., Zhang, S., Razanajatovo, R.M., Zou, H., and Zhu, W. (2018). Accumulation, Tissue Distribution, and Biochemical Effects of Polystyrene Microplastics in the Freshwater Fish Red Tilapia (Oreochromis niloticus). Environ. Pollut. 238, 1–9.
Duan, P., Hu, C., Quan, C., Yu, T., Huang, W., Chen, W., et al. (2017). 4-Nonylphenol Induces Autophagy and Attenuates mTOR-p70S6K/4EBP1 Signaling by Modulating AMPK Activation in Sertoli Cells. Toxicol. Lett. 267, 21–31. doi:10.1016/j.toxlet.2016.12.015
Durairajanayagam, D., Agarwal, A., Ong, C., and Prashast, P. (2014). Lycopene and Male Infertility. Asian J. Androl. 16, 420–425. doi:10.4103/1008-682X.126384
Eissa, M. M., Ahmed, M. M., Abd Eldaim, M. A., Orabi, S. H., Elbaz, H. T., Mohamed, M. A., et al. (2020). Methanolic Extract of Chlorella Vulgaris Protects against Sodium Nitrite-Induced Reproductive Toxicity in Male Rats. Andrologia 52, e13811. doi:10.1111/and.13811
Espinosa, C., Esteban, M., and Cuesta, A. (2019). Dietary Administration of PVC and PE Microplastics Produces Histological Damage, Oxidative Stress and Immunoregulation in European Sea Bass (Dicentrarchus labrax L.). Fish Shellfish Immunol.. doi:10.1016/j.fsi.2019.10.072
Grabowska, M., Wawrzyniak, D., Rolle, K., Chomczyński, P., Oziewicz, S., Jurga, S., et al. (2019). Let Food Be Your Medicine: Nutraceutical Properties of Lycopene. Food Funct. 10, 3090–3102. doi:10.1039/c9fo00580c
Hamed, M., Soliman, H. A. M., Osman, A. G. M., and Sayed, A. E.-D. H. (2019). Assessment the Effect of Exposure to Microplastics in Nile Tilapia (Oreochromis niloticus) Early Juvenile: I. Blood Biomarkers. Chemosphere 228, 345–350. doi:10.1016/j.chemosphere.2019.04.153
Hedayati, N., Naeini, M. B., Nezami, A., Hosseinzadeh, H., Wallace Hayes, A., Hosseini, S., et al. (2019). Protective Effect of Lycopene against Chemical and Natural Toxins: A Review. Biofactors 45, 5–23. doi:10.1002/biof.1458
Hossain, M. A., Pandey, A., and Satoh, S. (2007). Effects of Organic Acids on Growth and Phosphorus Utilization in Red Sea Bream Pagrus major. Fish. Sci. 73, 1309–1317.
Hsu, C.-Y., Chao, P.-Y., Hu, S.-P., and Yang, C.-M. (2013). The Antioxidant and Free Radical Scavenging Activities of Chlorophylls and Pheophytins. Fns 04, 1–8. doi:10.4236/fns.2013.48a001
Hussein, M. M. A., Elsadaawy, H. A., El-Murr, A., Ahmed, M. M., Bedawy, A. M., Tukur, H. A., et al. (2019). Endosulfan Toxicity in Nile tilapia (Oreochromis niloticus) and the Use of Lycopene as an Ameliorative Agent. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 224, 108573. doi:10.1016/j.cbpc.2019.108573
Ismail, R. F., Saleh, N. E., and Sayed, A. E. H. (2021). Impacts of Microplastics on Reproductive Performance of Male Tilapia (Oreochromis niloticus) Pre-fed on Amphora Coffeaeformis. Environ. Sci. Pollut. Res. Int. 28 (48), 68732–68744.
Jeong, C.-B., Kang, H.-M., Lee, M.-C., Kim, D.-H., Han, J., Hwang, D.-S., et al. (2017). Adverse Effects of Microplastics and Oxidative Stress-Induced MAPK/Nrf2 Pathway-Mediated Defense Mechanisms in the marine Copepod Paracyclopina Nana. Sci. Rep. 7, 41323. doi:10.1038/srep41323
Jovanović, B. (2017). Ingestion of Microplastics by Fish and its Potential Consequences from a Physical Perspective. Integr. Environ. Assess. Manag. 13, 510–515. doi:10.1002/ieam.1913
Karami, A., Romano, N., Galloway, T., and Hamzah, H. (2016). Virgin Microplastics Cause Toxicity and Modulate the Impacts of Phenanthrene on Biomarker Responses in African Catfish ( Clarias gariepinus ). Environ. Res. 151, 58–70. doi:10.1016/j.envres.2016.07.024
Klein, E. Y., Van Boeckel, T. P., Martinez, E. M., Pant, S., Gandra, S., Levin, S. A., et al. (2018). Global Increase and Geographic Convergence in Antibiotic Consumption between 2000 and 2015. Proc. Natl. Acad. Sci. USA 115, E3463–E3470. doi:10.1073/pnas.1717295115
Knobil, E. (1980). The Neuroendocrine Control of the Menstrual Cycle. Recent Prog. Horm. Res. 36, 53–88. doi:10.1016/b978-0-12-571136-4.50008-5
Koelmans, A. A., Mohamed Nor, N. H., Hermsen, E., Kooi, M., Mintenig, S. M., and De France, J. (2019). Microplastics in Freshwaters and Drinking Water: Critical Review and Assessment of Data Quality. Water Res. 155, 410–422. doi:10.1016/j.watres.2019.02.054
Law, K. L. (2017). Plastics in the Marine Environment. Annu. Rev. Mar. Sci. 9, 205–229. doi:10.1146/annurev-marine-010816-060409
Law, K. L., and Thompson, R. C. (2014). Microplastics in the Seas. Science 345, 144–145. doi:10.1126/science.1254065
Li, C., Busquets, R., and Campos, L. C. (2020). Assessment of Microplastics in Freshwater Systems: A Review. Sci. Total Environ. 707, 135578. doi:10.1016/j.scitotenv.2019.135578
Lim, C., Lückstädt, C., Webster, C. D., and Kesius, P. (2015). “Organic Acids and Their Salts,” in Dietary Nutrients, Additives, and Fish Health (Hoboken, NJ, USA: Wiley), 305–319. doi:10.1002/9781119005568.ch15
Luo, W., Su, L., Craig, N. J., Du, F., Wu, C., and Shi, H. (2019). Comparison of Microplastic Pollution in Different Water Bodies from Urban Creeks to Coastal Waters. Environ. Pollut. 246, 174–182. doi:10.1016/j.envpol.2018.11.081
Lusher, A. L., Hernandez-Milian, G., O'Brien, J., Berrow, S., O'Connor, I., and Officer, R. (2015). Microplastic and Macroplastic Ingestion by a Deep Diving, Oceanic Cetacean: the True's Beaked Whale Mesoplodon Mirus. Environ. Pollut. 199, 185–191. doi:10.1016/j.envpol.2015.01.023
Mahmoud, U. M., Ebied, A. M., and Mohamed, S. M. (2013). Effect of lead on Some Haematological and Biochemical Characteristics of Clarias gariepinus Dietary Supplemented with Lycopene and Vitamin E. Egypt. Acad. J. Biol. Sci. C, Physiol. Mol. Biol. 5, 67–89. doi:10.21608/eajbsc.2013.16112
Mekkawy, I. A., Mahmoud, U. M., Moneeb, R. H., and Sayed, A. H. (2020). Significance Assessment of Amphora Coffeaeformis in Arsenic-Induced Hemato- Biochemical Alterations of African Catfish (Clarias gariepinus). Front. Mar. Sci. 7, 191. doi:10.3389/fmars.2020.00191
Nadal, M. A., Alomar, C., and Deudero, S. (2016). High Levels of Microplastic Ingestion by the Semipelagic Fish Bogue Boops boops (L.) Around the Balearic Islands. Environ. Pollut. 214, 517–523. doi:10.1016/j.envpol.2016.04.054
Nicula, M., Pacala, N., Stef, L., Pet, I., Dronca, D., Ahmadi, M., et al. (2018). Garlic and Chlorella Biomodulate Lead Toxicity on Manganese Homeostasis in Carassius gibelio Bloch. Rev. Chim. 69, 986–989.
Osama, E., Galal, A. A. A., Abdalla, H., and El-Sheikh, S. M. A. (2019). Chlorella Vulgaris Ameliorates Testicular Toxicity Induced by Deltamethrin in Male Rats via Modulating Oxidative Stress. Andrologia 51, e13214. doi:10.1111/and.13214
Pandey, A., and Satoh, S. (2008). Effects of Organic Acids on Growth and Phosphorus Utilization in Rainbow troutOncorhynchus Mykiss. Fish. Sci. 74, 867–874. doi:10.1111/j.1444-2906.2008.01601.x
Plastics Europe (2020). Plastics—the Facts 2019: An analysis of European plastics production, demand and waste data for 2011. Available at: https://www.plasticseurope.org/application/files/9715/7129/9584/FINAL_web_version_Pl astics_the_facts2019_14102019.pdf (2019) (Accessed October, 2020)
Prokić, M. D., Radovanović, T. B., Gavrić, J. P., and Faggio, C. (2019). Ecotoxicological Effects of Microplastics: Examination of Biomarkers, Current State and Future Perspectives. Trac Trends Anal. Chem. 111, 37–46.
Qiang, L., and Cheng, J. (2021). Exposure to Polystyrene Microplastics Impairs Gonads of Zebrafish (Danio rerio). Chemosphere 263, 128161. doi:10.1016/j.chemosphere.2020.128161
Qiao, R., Deng, Y., Zhang, S., Wolosker, M. B., Zhu, Q., Ren, H., et al. (2019). Accumulation of Different Shapes of Microplastics Initiates Intestinal Injury and Gut Microbiota Dysbiosis in the Gut of Zebrafish. Chemosphere 236, 124334–124334.
Rainis, S., Mylonas, C. C., Kyriakou, Y., and Divanach, P. (2003). Enhancement of Spermiation in European Sea Bass (Dicentrarchus labrax) at the End of the Reproductive Season Using GnRHa Implants. Aquaculture 219, 873–890. doi:10.1016/s0044-8486(03)00028-0
Rashidian, G., Rainis, S., Prokić, M. D., and Faggio, C. (2020). Effects of Different Levels of Carotenoids and Light Sources on Swordtail Fish (Xiphophorus Helleri) Growth, Survival Rate and Reproductive Parameters. Nat. Prod. Res. 3, 1–12. doi:10.1080/14786419.2020.1723091
Rochman, C. M., Kurobe, T., Flores, I., and Teh, S. J. (2014). Early Warning Signs of Endocrine Disruption in Adult Fish from the Ingestion of Polyethylene with and without Sorbed Chemical Pollutants from the marine Environment. Sci. Total Environ. 493, 656–661. doi:10.1016/j.scitotenv.2014.06.051
Romano, N., Simon, W., Ebrahimi, M., Fadel, A. H. I., Chong, C. M., and Kamarudin, M. S. (2016). Dietary Sodium Citrate Improved Oxidative Stability in Red Hybrid tilapia (Oreochromis sp.) but Reduced Growth, Health Status, Intestinal Short Chain Fatty Acids and Induced Liver Damage. Aquaculture 458, 170–176. doi:10.1016/j.aquaculture.2016.03.014
Sahin, K., Yazlak, H., Orhan, C., Tuzcu, M., Akdemir, F., and Sahin, N. (2014). The Effect of Lycopene on Antioxidant Status in Rainbow Trout (Oncorhynchus mykiss) Reared Under High Stocking Density. Aquaculture 418, 132–138.
Sarker, M. S. A., Satoh, S., Kamata, K., Haga, Y., and Yamamoto, Y. (2012). Supplementation effect(s) of organic acids and/or lipid to plant protein-based diets on juvenile yellowtail, Seriola quinqueradiata Temminck et Schlegel 1845, growth and, nitrogen and phosphorus excretion. Aquacult. Res. 43, 538–545. doi:10.1111/j.1365-2109.2011.02859.x
Sikiru, A. B., Arangasamy, A., Alemede, I. C., Guvvala, P. R., Egena, S. S. A., Ippala, J. R., et al. (2019). Chlorella vulgaris Supplementation Effects on Performances, Oxidative Stress and Antioxidant Genes Expression in Liver and Ovaries of New Zealand White Rabbits. Heliyon (9) 5, e02470. doi:10.1016/j.heliyon.2019.e02470
Song, S., Han, Y., Zhang, Y., Ma, H., Zhang, L., Huo, J., et al. (2019). Protective Role of Citric Acid against Oxidative Stress Induced by Heavy Metals in Caenorhabditis elegans. Environ. Sci. Pollut. Res. 26, 36820–36831. doi:10.1007/s11356-019-06853-w
SPSS (1998). SPSS for windows step by step: A simple study guide and reference, 17.0 update, 10/e. Chennai, TN, India: Pearson Education India.
Sussarellu, R., Suquet, M., Thomas, Y., Lambert, C., Fabioux, C., Pernet, M. E. J., et al. (2016). Oyster Reproduction Is Affected by Exposure to Polystyrene Microplastics. Proc. Natl. Acad. Sci. USA 113, 2430–2435. doi:10.1073/pnas.1519019113
Tripathi, A., PremKumar, K. V., Pandey, A. N., Khatun, S., Mishra, S. K., Shrivastav, T. G., et al. (2011). Melatonin Protects against Clomiphene Citrate-Induced Generation of Hydrogen Peroxide and Morphological Apoptotic Changes in Rat Eggs. Eur. J. Pharmacol. 667, 419–424. doi:10.1016/j.ejphar.2011.06.005
Tripathy, A., Ghosh, A., Dey, A., Pakhira, B. P., and Ghosh, D. (2017). Attenuation of the Cyproterone Acetate-Induced Testicular Hypofunction by a Novel Nutraceutical Lycopene: a Genomic Approach. Andrologia 49, 24. doi:10.1111/and.12709
Vielma, J., Ruohonen, K., and Lall, S. (1999). Supplemental Citric Acid and Particle Size of Fish Bone-Meal Influence the Availability of Minerals in Rainbow trout Oncorhynchus mykiss (Walbaum). Aquacult. Nutr. 5, 65. doi:10.1046/j.1365-2095.1999.00092.x
Vijver, M., Peijnenburg, W., and Abolahpur Monikh, F. (2020). Micro(nano)plastics in Aquatic Organisms, Transferability of Knowledge from Nanowires. Wetsus: European Centre for Sustainable Water Technology.
Wan, Z., Wang, C., Zhou, J., Shen, M., Wang, X., Fu, Z., et al. (2019). Effects of Polystyrene Microplastics on the Composition of the Microbiome and Metabolism in Larval Zebrafish. Chemosphere 217, 646–658.
Wang, J., Li, Y., Lu, L., Zheng, M., Zhang, X., Tian, H., et al. (2019). Polystyrene Microplastics Cause Tissue Damages, Sex-specific Reproductive Disruption and Transgenerational Effects in marine Medaka (Oryzias Melastigma). Environ. Pollut. 254, 113024. doi:10.1016/j.envpol.2019.113024
Wang, W., Ndungu, A. W., Li, Z., and Wang, J. (2017). Microplastics Pollution in Inland Freshwaters of China: a Case Study in Urban Surface Waters of Wuhan, China. Sci. Total Environ. 575, 1369–1374. doi:10.1016/j.scitotenv.2016.09.213
Wright, S. L., Thompson, R. C., and Galloway, T. S. (2013). The Physical Impacts of Microplastics on marine Organisms: a Review. Environ. Pollut. 178, 483–492. doi:10.1016/j.envpol.2013.02.031
Xie, X., Deng, T., Duan, J., Xie, J., Yuan, J., and Chen, M. (2020). Exposure to Polystyrene Microplastics Causes Reproductive Toxicity through Oxidative Stress and Activation of the P38 MAPK Signaling Pathway. Ecotoxicol Environ. Saf. 190, 110133. doi:10.1016/j.ecoenv.2019.110133
Yu, P., Liu, Z., Wu, D., Chen, M., Lv, W., and Zha, Y. (2018). Accumulation of Polystyrene Microplastics in Juvenile Eriocheir sinensis and Oxidative Stress Effects in the Liver. Aquat. Toxicol.. Amsterdam, Netherlands 200, 28–36.
Yonar, M. E. (2012). The Effect of Lycopene on Oxytetracycline-Induced Oxidative Stress and Immunosuppression in Rainbow trout (Oncorhynchus mykiss, W.). Fish Shellfish Immunol. 32, 994–1001. doi:10.1016/j.fsi.2012.02.012
Zahran, E., Elbahnaswy, S., Risha, E., and El-Matbouli, M. (2020). Antioxidative and Immunoprotective Potential of Chlorella Vulgaris Dietary Supplementation against Chlorpyrifos-Induced Toxicity in Nile tilapia. Fish. Physiol. Biochem. 46, 1549–1560. doi:10.1007/s10695-020-00814-8
Zhao, Y., Lin, J., Talukder, M., Zhu, S.-Y., Li, M.-Z., Wang, H.-R., et al. (2020). Aryl Hydrocarbon Receptor as a Target for Lycopene Preventing DEHP-Induced Spermatogenic Disorders. J. Agric. Food Chem. 68, 4355–4366. doi:10.1021/acs.jafc.9b07795
Keywords: microplastics, sex steroids, sperm quality, lycopene, testicular damage, citric acid, Chlorella
Citation: El-Din H. Sayed A, Hamed M and Ismail RF (2022) Natural Antioxidants can Improve Microplastics-Induced Male Reproductive Impairment in the African Catfish (Clarias Gariepinus). Front. Environ. Sci. 9:811466. doi: 10.3389/fenvs.2021.811466
Received: 08 November 2021; Accepted: 29 December 2021;
Published: 31 January 2022.
Edited by:
Sadasivam Anbumani, Indian Institute of Toxicology Research (CSIR), IndiaReviewed by:
Vladimir Mukhanov, A.O. Kovalevsky Institute of Biology of the Southern Seas, UkraineCopyright © 2022 El-Din H. Sayed, Hamed and Ismail. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alaa El-Din H. Sayed, YWxhYXNheWVkQGF1bi5lZHUuZWc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.