- 1Entomology Lab, Department of Zoology, Government College University, Faisalabad, Pakistan
- 2Centre of Excellence in Molecular Biology, University of the Punjab, Lahore, Pakistan
- 3Centre of Department of Biochemistry/US-Pakistan Center for Advance Studies in Agriculture and Food Security (USPCAS-AFS), University of Agriculture Faisalabad, Faisalabad, Pakistan
- 4Department of Biochemistry, Government College Women University, Faisalabad, Pakistan
- 5Department of Zoology, Government College University, Faisalabad, Pakistan
Housefly, Musca domestica, is considered responsible for transmitting a wide variety of human and veterinary diseases. Mostly, insecticides are being used for their control and more commonly, pyrethroid insecticides worldwide. However, resistance has been reported against various pyrethroid insecticides. Houseflies become resistant by two major mechanisms, i.e., target site insensitivity through knockdown resistance gene mutation (kdr) and enzyme detoxification. Thus, the current study was designed to monitor the frequency of pyrethroid resistance gene kdr in housefly populations of District Jhang. The flies were collected from seven sampling sites and then reared in the lab for molecular and biochemical assays. The amplification of template DNA was performed for knockdown resistance gene through the outer primers kdr1 and kdr4, and the inner primers kdr1 and kdr2 using PASA (PCR Amplification of Specific Alleles) method which specifically amplify the domain-II of kdr gene. Three populations were found homozygous susceptible (+/+; 42.85%), whereas two populations were found genetically homozygous resistant (−/−; 28.57%) which are insensitive to pyrethroid insecticides. Similarly, two populations were found heterozygous (+/−; 28.57%) for kdr suggesting thereby that at least 1/4th homozygous-resistant (−/−) housefly populations with insensitivity to pyrethroids would be produced in the future keeping in view the Mendelian ratio. Biochemical assay showed that homozygous-resistant populations had increased activity of Acetylcholinesterase (AChE), α-Carboxylesterases (α-Carboxyl), β-Carboxylesterase (β-Carboxyl), Alkaline Phosphatase (AkP), and Acidic Phosphatase (AcP) enzymes. In addition, heterozygous populations also showed increased activities of these enzymes. The current results would not only help avoid the indiscriminate load of insecticides onto the environment but also serve as a hallmark for the management of housefly populations in target areas in the future.
Introduction
Housefly is considered a major insect pest of animals as well as humans mainly due to its high rate of fecundity (Brown et al., 1995). It reduces the level of livestock activities through annoyance, upsetting animals during their times of feeding and resting, and also induces potential transmission of various pathogens (Cheeke, 2005; Forster et al., 2009). Manure and organic waste produced by overpopulated human beings in urban and rural areas as well provides feeding and breeding sites to these houseflies, thus causing dramatic increase in housefly population at these sites (Khamesipour et al., 2018).
Because of high fecundity, it poses serious concern of control. Although flies do not bite, they act as vectors of various pathogens i.e., virus, bacteria, fungi, protozoa, and nematodes. More than hundred pathogens are reported wherein houseflies serve as vectors (Khamesipour et al., 2018). Flies pick up pathogens from detritus, waste, and the other resources of sludge and then transfer vomits through their body parts, faeces, and contaminated mouth parts to humans, poultry, and various animals. The pathogens usually transferred by houseflies are Pseudomonas, Shigela, Salmonella, Staphylococcus, Campylobacter, Escherichia coli, Enterococcu, Klebsiella, Campylobacter, Acinetobacter, Trichuris, Chlamydia, and Strongyloides larvae, Entrobious vermicularis causing typhoid fever, food poisoning, tuberculosis, dysentery, opthalmic, anthrax, and infestation by parasitic worms (Khamesipour et al., 2018; Iqbal et al., 2014; Lord and Boston, 1904).
Increase in housefly population results in increase in nuisance in poultry farms, ultimately affecting the egg production of chickens. In addition, quality and appearance of eggs are compromised due to fly faeces (Howard and Wall, 1996). Different control measures are being used worldwide viz. avoidance to build-up of fly population through cleanliness, screening, waste management, etc. But chemical control remains the last and ultimate option. It has been established that chemical control of disease-causing vectors has become difficult because of their resistance toward chemicals. Since the instant reaction by pest control practitioners is to increase in dosage, which results in increased resistance level and contamination of the environment (Khamesipour et al., 2018; Abbas et al., 2014; Shono et al., 2004; Feyereisen, 1995; Kjaersgaard, et al., 2015). Physiologically, through resistance insects show certain characteristics such as decrease in uptake of insecticides, increase in detoxification, and alternation in target sites (Casida, 2016). Subsequently, metabolic resistance against insecticides also exists which increases mixed-function oxidase (MFO), glutathione S-transferase (GST) activity, alters esterases, and DDT dehydrochlorinase activities (Panini et al., 2016). Change in esterase level has been known as the mechanism of pyrethroid resistance in household insects such as mosquitoes and houseflies Musca domestica L. (Ahn et al., 1992).
Non-metabolic resistance factor was first documented conferring as rapid paralytic knock down and severe activity of Pyrethroids and DDT in housefly, Musca domestica in 1951 (Busvine, 1951). The mechanism is currently named as kdr (knockdown resistance) (Martins and Valle, 2012). Voltage Gated Sodium Channel (VGSC) is reported as the main target site of pyrethroid insecticides and is vital for electrical signaling within the nervous system. But insensitivity at this site is conferred by means of mutation in VGSC which serves as a primary mechanism of resistance against pyrethroids. The kdr mutation in sodium channel gene is caused due to a substitution of amino acids residue of Leucine to phenylalanine (L1014F) and has been associated with resistance in houseflies. Subsequent studies showed that resistant flies had alleles such as CYP6D1 and Vssc1 that contribute to resistance against permethrin and pyrethroids (Liu and Pridgeon, 2002; Rinkevich et al., 2006; Scott et al., 2013). It has been reported in United Arab Emirates that housefly populations causing detoxification as well as L1014F replacement of sodium channels suggest that there should be implication of management program for resistance against pyrethroids (Al-Deeb, 2014).
In Pakistan, chemical pesticides were consistently used for the control of housefly and vast research work has been done for its chemical control. However, to our knowledge, there is no molecular work or any reports available on the frequency of pyrethroid insecticide resistance for kdr alleles. Therefore, the current study was designed to investigate the frequency of pyrethroid insecticide resistance kdr allele in housefly population from different locations of District Jhang, Punjab, Pakistan.
Materials and Methods
Collection of Houseflies
The collection of adult houseflies was undertaken from seven different sampling sites from District Jhang viz. Civil lines, 18-hazari, Head trimu, Nawaz chowk, Malhumorr, Sattelite town, and Haveli bahadur shah during 2017–18 (Figure 2).
Bioassays for Susceptibility/Resistance Against Pyrethroid Insecticides in Housefly Populations
The collected flies were reared in Entomology Lab of Department of Zoology, Government College University Faisalabad, on artificial diet under optimum conditions in 40 cm × 25 cm × 30 cm cages covered with mesh screen having cloth sleeves at their opening described by Keiding and Arevad (1964). Houseflies were maintained in cages with sugar-soaked cotton wool in small-sized beakers. Full fat fresh milk was also provided, soaked in cotton wool and wheat bran with yeast-moistened water and dry milk after emergence of flies to increase the production of eggs. Flies take about 10 days to complete their life cycle; food was changed after an interval of 2–3 days depending on the number of larvae. Bioassays were conducted at temperature 25 ± 2°C, 60 ± 5% relative humidity, and 12:12 (L/D) photoperiod. The collected fly samples from each respective site were pooled and used for mortality bioassays and molecular as well as biochemical assays. The lab strain considered as reference strain was used as control.
The insecticides used were of analytical standards and purified chromatographically above 90% purity level. Stock solution of 1 mM was prepared and kept in acetone. Insecticides used in bioassays included Lambda-cyhalothrin, Deltamethrin, Cypermethrin, Chlorpyrifos, and Tetramethrin. All of these chemicals were applied with respect to technical material diluted to the required concentration in Acetone.
A completely randomized experimental design was used. Five concentrations (2.5, 5, 10, 20, and 40 ppm) of each insecticide were prepared and replicated for three times. Approximately 60 flies were used in each concentration causing >0% and <100% mortality. In case of each treatment fresh dilution was used from the already-prepared stock solution. Mortality levels were assessed at 24, 48, and 72 h of exposure to insecticides. Mortality in control groups was also noted to obtain the corrected mortality according to Abbot’s formula (Abbot, 1925). Ataxic individuals considered died (Khan et al., 2014).
Here P is the % corrected mortality, C is the % mortality in the nontreated group, and T is the % mortality in the treated group.
Evaluation of Resistance of Pyrethroid Insecticides in Musca domestica Populations
For the evaluation of resistance, three generations of M. domestica pooled sample from District Jhang were reared. The adult mortality had been recorded after 72 h; the flies that remained alive were allowed to complete their three generations. Twenty adult flies were used to evaluate the resistance after 48 h feeding on the corresponding pyrethroid insecticides. Mortality data were recorded after 48 h of insecticide treatment on the emerged flies of the F1 and F2 (parental) generations (Sultana et al., 2016).
The number of emerging flies was recorded after 2 weeks and, meanwhile, the mortality was calculated. The resistance level was measured in each succeeding generation to evaluate the increase in the level of resistance following the protocol of Singh and Prakash (2013). The resistance ratios (Resistant/Susceptible) were estimated by dividing the LD50 for resistant strain with the LD50 for the Lab/reference strain.
PCR Amplification of Specific Alleles for Kdr Alleles
The TNE buffer method was used for the extraction of DNA (Ashraf et al., 2016; Zahoor et al., 2017). PASA was performed as described by Huang et al. (2004) using two outer allele-specific primers kdr1, 5′-AAGGATCGCTTCAAGG-3′and kdr4, 5′-TTCACCCAGTTCTTAAAACGAG-3′of 10 pmol and two inner primers kdr2, 5′-TCGTGATCGGCAATT-3′ kdr3, 5′-GTCAACTTACCACAAG-3′ of 40 pmol. The PCR reaction included 2 µL of genomic DNA, 10 pmol of each outer primer and 40 pmol of each inner primer, 12.5 µL Taq PCR Master Mixture, and 8.5 µL filtered nuclease-free water. Initial denaturation for 2 min followed by 40 cycles was performed at 95°C, 45 s at 94°C, 30 and 90 s and a final step extension for 10 min at 72°C. Each PCR reaction also included a negative control to avoid any contamination in reaction. The PCR amplified fragments were analyzed through electrophoresis on 1.5% agarose gel stained with Ethidium bromide and visualized under UV light documentation system. kdr1 and kdr4 primers amplified control fragment of 480 bp. kdr1 and kdr3 primers amplified susceptible allele fragment of 200 bp, whereas kdr2 and kdr4 primers amplified the fragment of 280 bp for kdr type (resistant) allele. The kdr mutation was identified by using direct DNA sequencing of voltage-gated sodium channels gene (kdr gene) (Eurofins scientific INC).
Enzyme Assay
Preparation of Whole-Body Homogenate
For enzyme assay adult houseflies were washed properly with distilled water and then dried with bloating paper. Flies were homogenized with ice-cold 20 mM sodium phosphate buffer (pH 7.0) with the help of Teflon hand homogenizer and then homogenate was centrifuged at 8,000 rpm at 4°C for 20 min. The solutions prepared for homogenization were kept at 4°C before use. The homogenates were then stored on ice for further use (Younes et al., 2011).
Estimation of Acetyl Cholinesterase Activity
For 50 µL of homogenate, addition of 50 µL of 2.6 mM acetylcholine chloride and 1 ml of 20 Mm sodium phosphate buffer (pH7.0) was made. Incubation was then performed at 25°C for 5 min. To stop the reaction, 400 µL of 0.3% fast blue B salt was added. The OD value was recorded at 405 nm (Younes et al., 2011).
Estimation of Carboxyl Esterase Activity
For 50 µL homogenate, 1 ml of 20 Mm PBS (pH 7.0) and 50 µL of each α-naphthyl acetate and
Estimation of Acid and Alkaline Phosphatases Activity
For 50 µL of homogenate, 50 µL of 50 mM PBS (pH 7.0) and 100 µL of 20 mM p-nitro phenyl phosphate were added to estimate the activity of acid phosphatase activity. For alkaline phosphatases activity 50 µL homogenate was mixed with 50 µL of 50 mM Tris HCl buffer (pH9.0) and 100 µL of 20 mM p-nitrophenyl phosphate. Both the solutions were incubated at 37°C for 15 min and the reaction was stopped by adding 0.5M NaOH solution. The OD values were then recorded at 440 nm (Younes et al., 2011).
Statistical Analysis
The recorded data were corrected by using Abbott’s formula (Abbot, 1925) and subjected to the evaluation of variance (ANOVA) using Statistica 13.0 for Windows. Post hoc testing was also carried out using the Tukey HSD test. A significant level of 5% was taken into consideration for all statistical tests. A value of p < 0.05 was considered statistically significant. The fingerprints were observed in a UV high-clarity fluorescent machine and their images were saved using the SynGene Gel credentials method.
Results
Bioassays for Susceptibility/Resistance in Housefly Populations
To evaluate the resistance against insecticides lab strain of Musca domestica was treated with five insecticides Lambda cyhalothrin, deltamethrin, chlorpyrifos, cypermethrin, and tetramethrin, having used different concentrations viz. 2.5, 5, 10, 20, and 40% at exposure times of 24, 48, and 72 h, respectively. The maximum percentage mortality percentage of Lambda cyhalothrin was found at a concentration of 40 ppm (51.6%) after 48 h and 20 ppm (46.15%) after 72 h followed by 20 ppm concentration. It was found that the mortality was increased with increase in concentrations but not decreased with exposure time; moreover, low mortality was found at lower concentrations. Similar results were found with Deltamethrin; maximum mortality percentage was found at 40 ppm (51.6%) after 48 h and 49.01% after 24 h, respectively. Highest mortality rate was observed in case of Chlorpyrifos. Flies showed lowest resistance against Chlorpyrifos. At 20 ppm concentration mortality rate was found 57.1, 42.8, and 50% after 24, 48, and 72 h, respectively. Highest mortality percent (57.1%) was observed at 40 ppm concentration after 24 h. With Cypermethrin, mortality rate was found decreased with increase in exposure time (30, 28, and 9.8%) at 40 ppm after 24, 48, and 72 h, respectively. Tetramethrin showed least mortality among all tested insecticides. Overall, highest mortality rate (57.1429%) was observed at 40% concentration of Chlorpyrifos, and least mortality (14.7368%) was observed in case of Tetramethrin at same concentrations (Table 1). The observed mortality rate was Chlorpyrifos > Lambda Cyhalothrin > Deltamethrin > Cypermethrin > Tetramethrin.
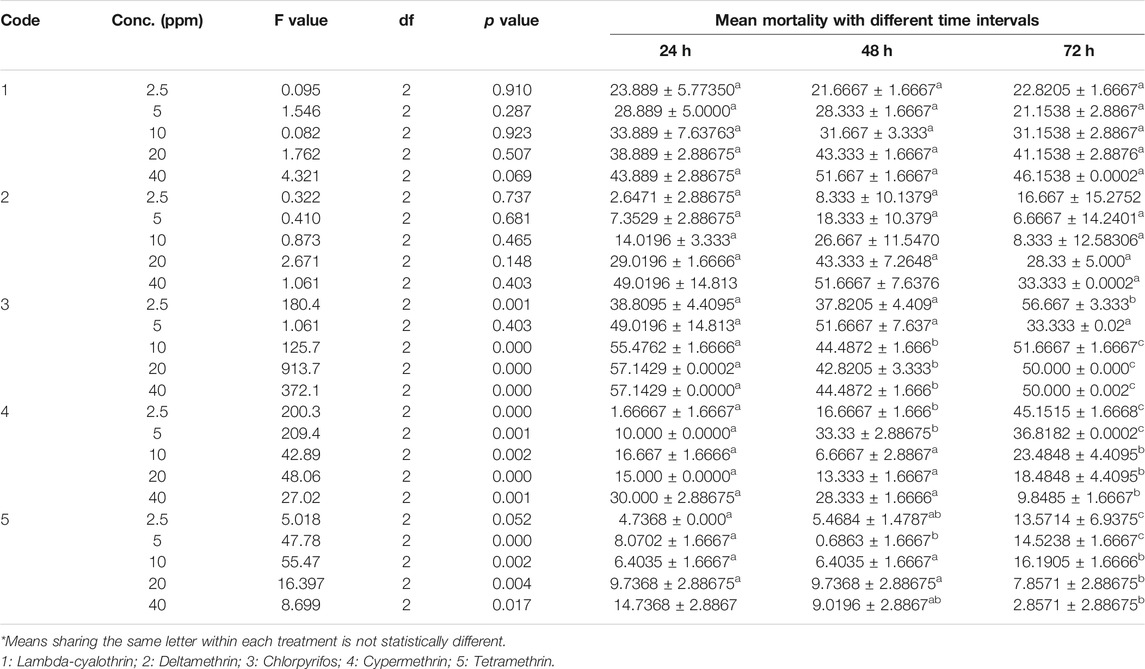
TABLE 1. Mean mortality of housefly (Musca domestica L.) larvae after 72 h of exposure to 30% concentration of insecticides.
Evaluation of Resistance of Pyrethroid Insecticides in Musca domestica Populations
The toxicity of four pyrethroid insecticides and resistance ratios of three generations of houseflies was recorded based on their LD50values. It is to mention here that Chlorpyrifos was found with high mortality, hereby conferring lowest resistance. Hence, it was not further studied for the evaluation of resistance. The flies with higher LD50 values were considered resistant in successive generations. Low level of resistance was found against Lambda Cyhalothrin when compared with Cypermethrin, Deltamethrin, and Permethrin (Table 2). Resistance ratios (RR) ranged between 0.947 and 0.976 in case of Lambda Cyhalothrin. With Deltamethrin and Cypermethrin, moderate level of resistance (RR) was found with a range between 1.033–1.183 and 1.1058–1.224, respectively. Maximum resistance was found in Tetramethrin with RR ranging between 1.257 and 1.462. Nevertheless, a reduction in % age mortality was also observed in successive generations for Permethrin, Deltamethrin, and Cypermethrin (Table 2).
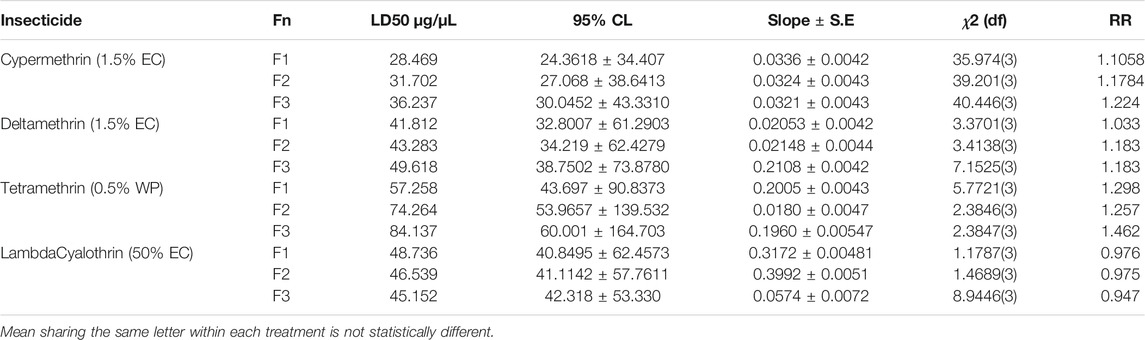
TABLE 2. Evaluation of resistance of pyrethroid insecticides in Musca domestica from district Jhang.
Molecular Assay
The four kdr primers designed by macrogen company were used for PASA (PCR Amplification of Specific Alleles) following the protocol of Huang et al. (2004). Kdr1 and kdr4 amplified fragment of 480 bp, kdr1 and kdr3 amplified 200 bp susceptible allelic fragments while kdr2 and kdr4 amplified 280 bp kdr type allelic fragments in the domain-II of kdr gene. Kdr5, kdr7, and kdr9 were also used as control during optimization to evaluate whether gene was actually amplified or not. Three populations were found homozygous susceptible (+/+; 42.85%), whereas two populations were found genetically homozygous resistant (−/−; 28.57%) which are insensitive to pyrethroid insecticides. Similarly, two populations were found heterozygous (+/−; 28.57%) for kdr. Hence, following the Mendelian ratio in future generation, at least 1/4th homozygous resistant (−/−) housefly populations would be produced which would increase the insensitivity to pyrethroid insecticides (Figure 1). The mutation was identified at position 1014 on domain II of transmembrane six of sodium channels.
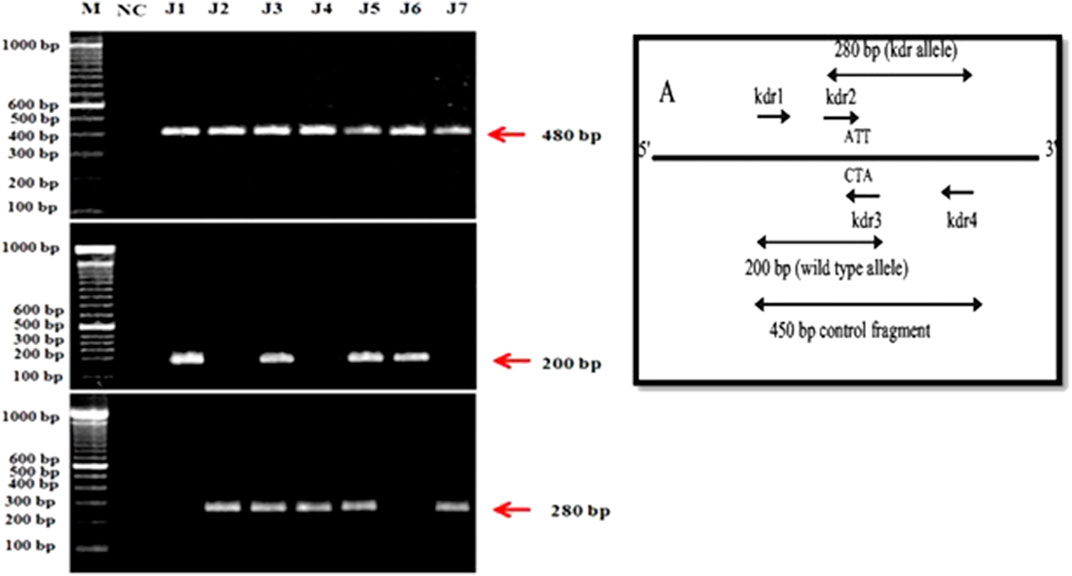
FIGURE 1. PCR amplification of housefly, Musca domestica L. Samples from District Jhang. (A): kdr alleles. (B): A control fragment amplified using kdr1 and kdr4 primers. (C): 200-bp susceptible allele fragment amplified kdr1 and kdr3. (D): 280-bp kdr allele fragment amplified by kdr2 and kdr4.
Enzyme Assay
The effect of insecticides on the activity of Acetylcholine Esterase (AchE), Carboxylesterase (α- Carboxylesterases and β-Carboxylesterases), Acidic Phosphatase (AcP), Alkaline Phosphatases (AkP) is shown in Table 2. Maximum percent inhibition of AChE was observed in satellite town (29.434%) followed by Malhumor (23.256%). Low level of inhibition of AChE was shown by Haveli Bahadur Shah (13.598%). Maximum percent inhibition of AkP was observed in satellite town (149.08%) followed by civil lines (132.2664%), whereas very low percent inhibition was found in 18-Hazari (40.341%). Similarly, maximum percent inhibition of AcP was found in satellite town (125.250%) followed by civil lines (109.31%). Low level of inhibition of AcP was found in samples from Nawaz chowk (22.067%). Maximum percent inhibition of α- Carboxylesterases was shown by satellite town (32.4%) followed by Malhumor (25.14%), whereas low level of inhibition of α- Carboxyl was found in 18-Hazari (11.98%). Similarly, maximum percent inhibition of β-Carboxylesterases was observed in Satellite town (28.43%) followed by Malhumor (20.32%), whereas very low level of inhibition was found in 18-Hazari (5.95%). Overall, the percentage inhibition of Alkaline Phosphatases (AkP) and Acidic Phosphatase (AcP) was found high as compared with Acetylcholinesterase (AChE) and Carboxylesterase (α- Carboxyl and β-Carboxyl) activity (Table 3).
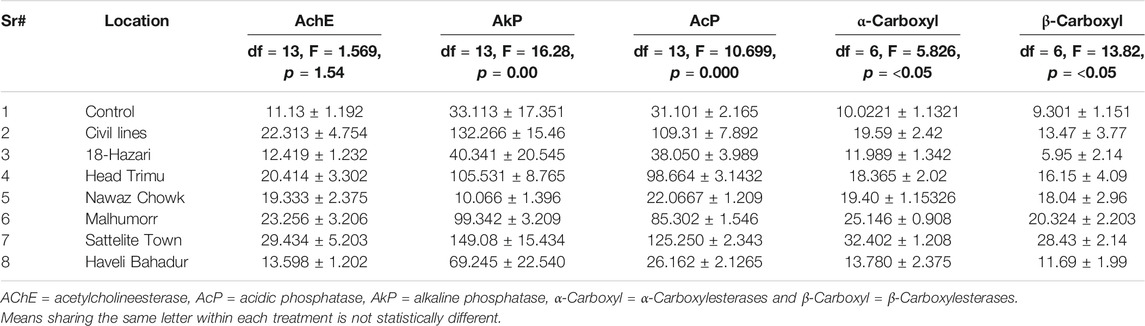
TABLE 3. Effect of insecticides on the percent enzyme inhibition in Musca domestica L. from District Jhang.
Discussion
During the present study, adult housefly (Musca domestica L.) was collected from seven different locations of Jhang, Punjab, Pakistan first to investigate the level of resistance against commercially used pyrethroid insecticides through bioassays and then to molecular genotyping of kdr mutation through PASA to reveal the frequency of kdr allele in field populations of housefly (kdr/kdr, kdr/sus, sus/sus). The flies were further used to find out the underlying modulation in the enzymatic activity due to pyrethroid resistance.
The percentage mortality of Musca domestica. L was recorded using five different concentrations of five insecticides with time exposure of 24 h, 48, and 72 h. It was recorded that with the increase in concentration of insecticides the mortality rate was also increased. Another factor that correlates with mortality is time exposure. It was already reported that prolonged exposure gave high mortality (Sultana et al., 2016 and 2019). Although all the other insecticides caused maximum mortality at exposure time of 72 h, Tetramethrin (2.85%) and Cypermethrin (9.84%) had decreased mortality when exposure time was increased. In addition, low level of resistance was found against Lambda Cyhalothrin when compared with Cypermethrin, Deltamethrin, and Permethrin consistent to our previous studies (Ranian et al., 2021). With Deltamethrin and Cypermethrin moderate level of resistance (RR) was found, whereas maximum resistance was found with Tetramethrin (Singh and Prakash, 2013).
In agreement with the molecular studies regarding kdr mutation by Huang et al. (2004), the kdr mutation was genotyped by allele-specific PCR (PASA) which revealed that this allele was present in the tested populations. The findings showed three types of genotypes with amplification of 280 bp homozygous-resistant allelic fragment (kdr/kdr). Some of the flies showed heterozygous genotype with amplification of two allelic fragments 280 bp for kdr and 200 bp or homozygous susceptible allelic fragment of 200 bp. The results were in agreement with the findings of Huang et al. (2004). It is to mention here that PASA did not work in true sense; rather the results were reproduced in separate PCR reactions. The confirmation of L1014F mutation in housefly provides us with evidence that Kdr-type resistance is present in flies (Figure 3). The changes in sequences reinforced the importance of conservation of Leucine residue in transmembrane six of domain II of sodium channels protein conferring the target site insensitivity against pyrethroid group of insecticides as discussed by Eleftherianos et al. (2008).
Two locations viz. Civil lines and Satellite town were found homozygous resistant (kdr/kdr) for housefly populations, whereas Head trimu and Malhumorr were found homozygous susceptible (sus/sus). Three sampling sites viz. 18-Hazari, Nawaz chowk, and Haveli bahadur shah were found heterozygous for kdr (kdr/sus). The percentage of heterozygous genotype (kdr/sus) was higher as compared with homozygous genotypes. Maybe, heterozygotes have fitness advantage through a pleiotropic effect as discussed by Foster et al. (2004). The other possibility is that susceptible strains could migrate infrequently into the population in that area. The percentage of homozygous-resistant genotype was 28% and that of homozygous-susceptible sus/sus genotype was 43%, whereas maximum percentage was found for heterozygous kdr/sus genotype as 29% (Figure 2).
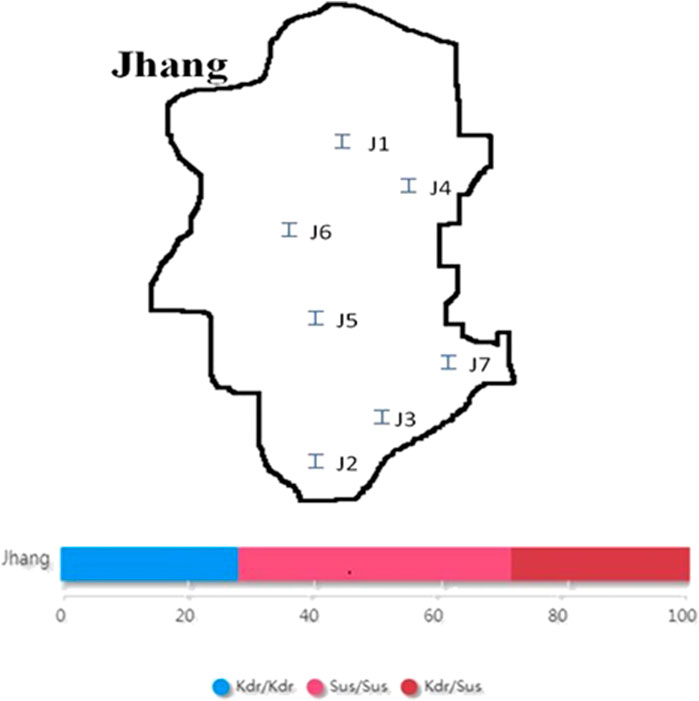
FIGURE 2. Map of District Jhang and frequency of homozygous sus/sus, heterozygous kdr/sus, and homozygous kdr/kdr loci. J1: Civil lines; J2: 18-Hazari; J3: Head trimu; J4: Nawaz chowk; J5: Malhumorr; J6: Sattelite town; J7: Haveli bahadur.
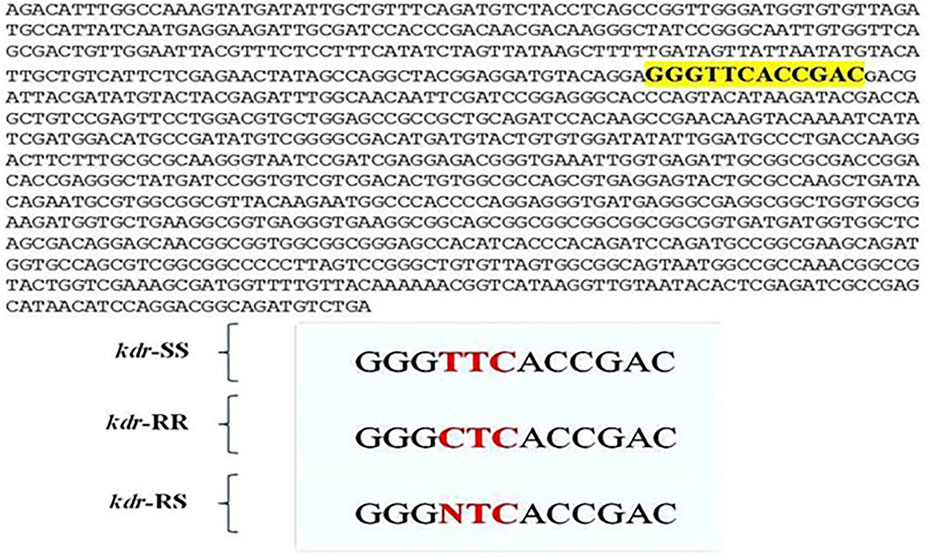
FIGURE 3. Kdr mutation in housefly Musca domestica L. population from district Jhang. Lower panel: The mutation was identified from collected samples of housefly, Musca domestica L. at position 1014 in domain II of transmembrane six of sodium channels. Three kinds of sequences were obtained viz. kdr homozygous resistant (kdr-RR), kdr homozygous susceptible (kdr-SS), and kdr resistant (kdr-RS).
The enzymatic activity of fly populations revealed modulation in activity level when compared with Lab strain. It has been described that enzymatic activity contributes to resistance against pyrethroids (Eleftherianos et al., 2008). In the present study, the metabolic enzyme activity of homozygous-resistant (kdr/kdr), homozygous-susceptible (sus/sus), and heterozygous (kdr/sus) samples varied among different populations. According to Qin et al. (2014) in the absence of mutation at the target sites the metabolic detoxification becomes the major resistance mechanism. Hence, the enzymatic activity changes to counter the effect of employed insecticides (Sawicki, 1978; Eleftherianos et al., 2008).
The main objective to relate biochemical assay of Lab strain with field populations was to notice the changes in detoxification enzyme levels when exposed to pyrethroid insecticides. The current findings of enzyme assay of field strains while comparing with the molecular assay revealed that percent inhibition of Acetylcholinesterase (AchE), Carboxylesterase (α- Carboxylesterases and β-Carboxylesterases), Alkaline phosphatase (AkP), and Acidic phosphatase (AcP) had increased with increase in resistance (kdr/kdr) and decreased with increase in susceptibility (sus/sus). In addition, the Alkaline phosphatases (AkP) and Acidic phosphatases (AcP) activity increased more as compared with Acetylcholine Esterase (AchE) and Carboxylesterase. The results of the present study could be helpful in the strategic development of proactive management plans of Musca domestica L. in Jhang, Pakistan and the analysis of resistance will be helpful in the future to devise a targeted control strategy against population of housefly.
Conclusion
It was concluded that Tetramethrin had maximum resistance and Lambda Cyhalothrin had low level of resistance, whereas Deltamethrin and Cypermethrin showed moderate level of resistance in housefly populations from Jhang. Based on the molecular data of kdr alleles, two sites Civil lines and Satellite town had pyrethroid-resistant flies. Overall, three populations were found homozygous susceptible (+/+) and two populations were found heterozygous (+/−) for kdr. Keeping in view the simple Mendelian genetics, heterozygous for mutation lead towards homozygous kdr mutants, thus, increasing thereby the frequency of resistant strains in a given area. The sodium channels containing the Leucine to Phenylalanine mutation are less sensitive to the toxic effect of pyrethroid group of insecticides. It was also concluded that Acetylcholinesterase (AChE), Acid Phosphatase (AcP), Alkaline Phosphatase (AkP) had been increased in resistant housefly populations. The study helps reveal that a combination of chemicals could be a better choice rather than using pyrethroid insecticide individually. Thus, despite having molecular approaches, being rather costly, the enzyme assays would simply be used as a marker to check the susceptibility level of housefly samples from a given area.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author Contributions
BR designed and performed the experiments, analyzed the data, and wrote the manuscript. MK supervised and helped in designing the experiments, statistical analysis of data and approved the final manuscript. MZ and KR helped in designing and performing the experiments and arrangement of data. AA, KM, HM, and FJ reviewed and finalized the manuscript and provided the guidance in preparing manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbas, N., Khan, H. A. A., and Shad, S. A. (2014). Cross-resistance, Genetics, and Realized Heritability of Resistance to Fipronil in the House Fly, Musca domestica (Diptera: Muscidae): a Potential Vector for Disease Transmission. Parasitol. Res. 113 (4), 1343–1352. doi:10.1007/s00436-014-3773-4
Abbott, W. S. (1925). A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 18 (2), 265–267. doi:10.1093/jee/18.2.265a
Ahn, Y. J., Funaki, E., and Motoyama, N. (1992). “Mechanisms of Resistance to Pyrethroids and DDT in a Japanese Strain of the Housefly,” in Neurotox’91. (Dordrecht: Springer), 257–269.
Al-Deeb, M. A. (2014). Pyrethroid Insecticide Resistance Kdr Gene in the House Fly, Musca domestica (Diptera: Muscidae), in the United Arab Emirates. As 05 (14), 1522–1526. doi:10.4236/as.2014.514163
Ashraf, H. M., Zahoor, M. K., Nasir, S., Majeed, H. N., and Zahoor, S. (2016). Genetic Analysis of Aedes aegypti Using Random Amplified Polymorphic DNA (RAPD) Markers from Dengue Outbreaks in Pakistan. J. Arthropod Borne Dis. 10 (4), 546–559.
Brown, J. K., Frohlich, D. R., and Rosell, R. C. (1995). The Sweetpotato or Silverleaf Whiteflies: Biotypes of Bemisia Tabaci or a Species Complex? Annu. Rev. Entomol. 40 (1), 511–534. doi:10.1146/annurev.en.40.010195.002455
Busvine, J. R. (1951). Mechanism of Resistance to Insecticide in Houseflies. Nature 168, 193–195. doi:10.1038/168193a0
Casida, J. E. (2016). Unexpected Metabolic Reactions and Secondary Targets of Pesticide Action. J. Agric. Food Chem. 64 (22), 4471–4477. doi:10.1021/acs.jafc.6b01564
Cheeke, P. R. (2005). Applied Animal Nutrition: Feeds and Feeding (No. 636.085 C414a Ej. 1 025345). Pearson Prentice Hall.
Eleftherianos, I., Foster, S. P., Williamson, M. S., and Denholm, I. (2008). Characterization of the M918T Sodium Channel Gene Mutation Associated with strong Resistance to Pyrethroid Insecticides in the Peach-Potato Aphid, Myzus persicae (Sulzer). Bull. Entomol. Res. 98 (2), 183–191. doi:10.1017/s0007485307005524
Feyereisen, R. (1995). Molecular Biology of Insecticide Resistance. Toxicol. Lett. 82-83, 83–90. doi:10.1016/0378-4274(95)03470-6
Förster, M., Klimpel, S., and Sievert, K. (2009). The House Fly (Musca domestica) as a Potential Vector of Metazoan Parasites Caught in a Pig-Pen in Germany. Vet. Parasitol. 160 (1), 163–167. doi:10.1016/j.vetpar.2008.10.087
Foster, K. R., Shaulsky, G., Strassmann, J. E., Queller, D. C., and Thompson, C. R. L. (2004). Pleiotropy as a Mechanism to Stabilize Cooperation. Nature 431 (7009), 693–696. doi:10.1038/nature02894
Howard, Z. R., O'Bryan, C. A., Crandall, P. G., and Ricke, S. C. (2012). Salmonella Enteritidis in Shell Eggs: Current Issues and Prospects for Control. Food Res. Int. 45 (2), 755–764. doi:10.1016/j.foodres.2011.04.030
Howard, J., and Wall, R. (1996). Autosterilization of the House Fly, Musca domestica (Diptera: Muscidae) in Poultry Houses in North-East India. Bull. Entomol. Res. 86 (4), 363–367.
Huang, J., Kristensen, M., Qiao, C.-l., and Jespersen, J. B. (2004). Frequency of Kdr Gene in House Fly Field Populations: Correlation of Pyrethroid Resistance and Kdr Frequency. J. Econ. Entomol. 97 (3), 1036–1041. doi:10.1093/jee/97.3.1036
Iqbal, W., Malik, M. F., Sarwar, M. K., Azam, I., Iram, N., and Rashda, A. (2014). Role of Housefly (Musca domestica, Diptera; Muscidae) as a Disease Vector; a Review. J. Entomol. Zoolog. Stud. 2 (2), 159–163.
Keiding, J., and Arevad, K. (1964). Procedure and Equipment for Rearing a Large Number of Housefly Strains. Bull. World Health Organ. 31 (4), 527–528.
Khamesipour, F., Lankarani, K. B., Honarvar, B., and Kwenti, T. E. (2018). A Systematic Review of Human Pathogens Carried by the Housefly (Musca domestica L.). BMC Public Health 18 (1), 1049. doi:10.1186/s12889-018-5934-3
Khan, H., Abbas, N., Shad, S. A., and Afzal, M. B. S. (2014). Genetics and Realized Heritability of Resistance to Imidacloprid in a Poultry Population of House Fly, Musca domestica L. (Diptera: Muscidae) from Pakistan. Pestic. Biochem. Physiol. 114, 38–43. doi:10.1016/j.pestbp.2014.07.005
Kjaersgaard, A., Blanckenhorn, W. U., Pertoldi, C., Loeschcke, V., Kaufmann, C., Hald, B., et al. (2015). Plasticity in Behavioural Responses and Resistance to Temperature Stress in. Musca domesticaAnimal Behav. 99, 123–130. doi:10.1016/j.anbehav.2014.11.003
Liu, N., and Pridgeon, J. W. (2002). Metabolic Detoxication and the Kdr Mutation in Pyrethroid Resistant House Flies, Musca domestica (L.). Pestic. Biochem. Physiol. 73 (3), 157–163. doi:10.1016/s0048-3575(02)00101-3
Martins, A. J., and Valle, D. (2012). The Pyrethroid Knockdown Resistance. Insecticides-Basic Other Appl. 17, 38.
Panini, M., Manicardi, G. C., Moores, G. D., and Mazzoni, E. (2016). An Overview of the Main Pathways of Metabolic Resistance in Insects. Invertebrate Survival J. 13 (1), 326–335. doi:10.25431/1824-307X/isj.v13i1.326-335
Qin, Q., Li, Y., Zhong, D., Zhou, N., Chang, X., Li, C., et al. (2014). Insecticide Resistance of Anopheles Sinensis and an. Vagus in Hainan Island, a Malaria-Endemic Area of China. Parasites Vectors 7 (1), 92. doi:10.1186/1756-3305-7-92
Ranian, K., Zahoor, M. K., Zahoor, M. A., Rizvi, H., Rasul, A., Majeed, H. N., et al. (2021). Evaluation of Resistance to Some Pyrethroid and Organophosphate Insecticides and Their Underlying Impact on the Activity of Esterases and Phosphatases in House Fly, Musca domestica (Diptera: Muscidae). Polish J. Environ. Stud. 30 (1), 327–336. doi:10.15244/pjoes/96240
Rinkevich, F. D., Zhang, L., Hamm, R. L., Brady, S. G., Lazzaro, B. P., and Scott, J. G. (2006). Frequencies of the Pyrethroid Resistance Alleles of Vssc1 and CYP6D1 in House Flies from the Eastern United States. Insect Mol. Biol. 15 (2), 157–167. doi:10.1111/j.1365-2583.2006.00620.x
Sawicki, R. M. (1978). Unusual Response of DDT-Resistant Houseflies to Carbinol Analogues of DDT. Nature 275 (5679), 443–444. doi:10.1038/275443a0
Scott, J. G., Leichter, C. A., Rinkevihc, F. D., Harris, S. A., Su, C., Aberegg, L. C., et al. (2013). Insecticide Resistance in House Flies from the United States: Resistance Levels and Frequency of Pyrethroid Resistance Alleles. Pestic. Biochem. Physiol. 107 (3), 377–384. doi:10.1016/j.pestbp.2013.10.006
Shono, T., Zhang, L., and Scott, J. G. (2004). Indoxacarb Resistance in the House Fly, Musca domestica. Pestic. Biochem. Physiol. 80 (2), 106–112. doi:10.1016/j.pestbp.2004.06.004
Singh, S., and Prakash, S. (2013). Development of Resistance in Tribolium castaneum, Herbst (Coleoptera: Tenebrionidae) towards Deltamethrin in Laboratory. Int. J. Scientific Res. Publications 3 (8), 1–4.
Sultana, K., Zahoor, M. K., Sagheer, M., Nasir, S., Zahoor, M. A., Jabeen, F., et al. (2016). Insecticidal Activity of weed Plants, Euphorbia Prostrata and Chenopodiastrum Murale against Stored Grain Insect Pest Trogoderma Granarium Everts, 1898 (Coleoptera: Dermestidae). Turkish J. Entomol. 40 (3), 291–301. doi:10.16970/ted.19938
Younes, M. W., Othman, S. E., Elkersh, M. A., Youssef, N. S., and Omar, G. A. (2011). Effect of Seven Plant Oils on Some Biochemicalparameters in Khapra Beetle Trogoderma Granarius Everts (Coleoptera: Dermestidae). Egypt. J. Exp. Biol. 7 (1), 53–61.
Keywords: pyrethroids, kdr, PASA, resistant, susceptible and enzyme inhibition
Citation: Riaz B, Kashif Zahoor M, Malik K, Ahmad A, Majeed HN, Jabeen F, Zulhussnain M and Ranian K (2022) Frequency of Pyrethroid Insecticide Resistance kdr Gene and Its Associated Enzyme Modulation in Housefly, Musca domestica L. Populations From Jhang, Pakistan. Front. Environ. Sci. 9:806456. doi: 10.3389/fenvs.2021.806456
Received: 31 October 2021; Accepted: 15 December 2021;
Published: 03 February 2022.
Edited by:
Mazhar Iqbal Zafar, Quaid-i-Azam University, PakistanReviewed by:
Renata Da Rosa, State University of Londrina, BrazilSofia Khalid, Fatima Jinnah Women University, Pakistan
Copyright © 2022 Riaz, Kashif Zahoor, Malik, Ahmad, Majeed, Jabeen, Zulhussnain and Ranian. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Muhammad Kashif Zahoor, a2FzaGlmLnphaG9vckBnY3VmLmVkdS5waw==
 Bushra Riaz1
Bushra Riaz1 Aftab Ahmad
Aftab Ahmad Humara Naz Majeed
Humara Naz Majeed Kanwal Ranian
Kanwal Ranian