- 1Fishery Machinery and Instrument Research Institute of Chinese Academy of Fishery Sciences, Shanghai, China
- 2Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration, East China Normal University, Shanghai, China
- 3Shanghai Chenshan Plant Science Research Centre, Chinese Academy of Sciences, Shanghai Chenshan Botanical Garden, Shanghai, China
- 4College of Environmental Science and Engineering, Donghua University, Shanghai, China
This study investigated the synergetic effect of the combined calcium peroxide (CaO2) and microwave (MW) treatment on waste active sludge dewatering properties and organic contaminants’ removal. The optimal sludge dewaterability was obtained at CaO2 (20 mg/gVSS)/MW (70°C), and the capillary suction time decreased by 52% compared with raw sludge. Further investigation indicated that total extracellular polymeric substances (EPS), tightly bound EPS, total protein, and protein present in tightly bound EPS were closely correlated with sludge dewaterability. Tryptophan, aromatic protein–like substances and humic acid–like substances were the key compounds that affect sludge dewaterability. The charge neutralization and bridge effect of cation ions were strengthened when combined with MW irradiation. In addition, it was revealed that MW facilitated CaO2 to produce more hydroxyl and superoxide anion radicals. This study confirmed CaO2/MW to be an effective way to improve sludge dewatering and remove organic pollutants from sludge.
Introduction
The growing population and rising requirement for human activities have caused an increased yield of waste-activated sludge with high moisture content in wastewater treatment plants (WWTPs) in recent years (Liu et al., 2021). Treatment and disposal of sludge accounts for about 60% of the operating cost of WWTPs, which has become a critical issue (Kim et al., 2016; Liu et al., 2017). Dewatering is an important step in sludge treatment because proper dewatering can reduce the volume of sludge, thus reducing the cost of sludge transportation and disposal (Cao et al., 2021; Anjum et al., 2016). Therefore, it makes a strong economic incentive itself to improve the sludge dewaterability. In order to enhance sludge dewaterability, various treatment methods (e.g., chemical, physical, and biological) have been commonly applied, which can be used either separately or in combination (Cao et al., 2021; Kim et al., 2016; Liu et al., 2017; Akgul et al., 2017; Zheng et al., 2017).
Besides, a large number of organic pollutants in the sewage will be transferred to the sludge in the process of sewage treatment, which would result in the discharge of secondary pollutants during sludge disposal (e.g., incineration, composting, landfill, and agricultural utilization) (Li et al., 2015; Dubey et al., 2021; Lü et al., 2021). Common organic pollutants in sludge include not only traditional persistent organic pollutants (e.g., insecticide, polycyclic aromatic hydrocarbons, dioxin, and polychlorinated biphenyls) but also emerging contaminants (e.g., pharmaceuticals), endocrine-disrupting chemicals (e.g., poly brominated diphenyl ethers) (Zhang A. et al., 2015; Li et al., 2015; Dubey et al., 2021). Several studies have reported the possible threats for the environment derived from the agricultural use of sludge that contains toxic organic pollutants (Guo et al., 2020; Lü et al., 2021). For the purpose of fulfilling the requirements of the Environmental Protection Agency, sludge must be strictly disinfected prior to agricultural use or other final disposal (Lü et al., 2021). Recently, advanced oxidation processes (AOPs) have attracted much attention due to their excellent removal effect of organic pollutants in sludge (Qian et al., 2015; Wang and Li, 2016; Dubey et al., 2021). Some research has been done on the effect of AOPs on sludge treatment, whose result suggests that AOPs can be used as a highly efficient and environmentally friendly method for sludge processing (Zhang A. et al., 2015; Li et al., 2015; Dubey et al., 2021).
Calcium peroxide (CaO2) is a kind of solid inorganic peroxide compound with wide application, environmental friendliness, and safety, which can be considered as the “solid form” of hydrogen (H2O2) (Northup and Cassidy, 2008; Xu et al., 2020). CaO2 can gradually release H2O2 in humid environment, so as to maintain stable and continuous oxidation ability (Eq. 1) (Lu et al., 2017). H2O2 will produce hydroxyl radical (•OH) after obtaining a single electron (Eq. 2); hydroperoxyl (HO2•) and superoxide (•O2−) may also form in the system (Eqs. 3, 4) (Northup and Cassidy, 2008; Lu et al., 2017).
Nowadays, the use of CaO2-based AOPs in sludge treatment is a new and promising technique and has attracted increasing amount of attention (Li et al., 2015; Qian et al., 2015; Chen et al., 2016; Ping et al., 2018). CaO2 can be used as an effective method to oxidize organic pollutants and to facilitate sludge reuse (Xu et al., 2020). Researches have also demonstrated that CaO2 has superior performance in improving sludge dewatering (Chen et al., 2016; Wu and Chai, 2016). The rupture of the hydrophilic functional group caused by radical oxidation and the high bind affinity of calcium ions (Ca2+) with extracellular polymeric substances (EPS) have been confirmed to be a contributing factor to the enhancement of sludge dewaterability (Chen et al., 2016; Wu and Chai, 2016; Wu et al., 2018). However, the enhancement of sludge dewatering properties is limited with single CaO2 treatment (Chen et al., 2016). Research has shown that both ferrous iron and montmorillonite can catalyze the formation of •OH from CaO2, thus exhibiting synergistic effects on enhancing sludge dewaterability (Amina et al., 2018; Wu et al., 2018). Chen et al. (2016) opined that the combination of CaO2 and chemical flocculation exhibits superior performance in sludge dewatering performance. This may be due to the fact that the existence of CaO2 promotes the transformation of bound water to free water. Therefore, the combined technology provides a valuable research direction for the research of sludge treatment based on CaO2 in the future (Zheng et al., 2019).
In recent years, microwave (MW) irradiation has been widely used as an efficient and environmentally friendly sludge treatment method. Its main advantages are high efficiency, good selectivity, low cost, and strong controllability (Yu et al., 2009; Liu et al., 2016; Jiang et al., 2021). Our preliminary studies showed that the application of the combined treatment of CaO2 and MW is an efficient technique for sludge reuse (Wang and Li, 2016). MW irradiation promoted the production of •OH from CaO2, thus improving the oxidation effect of CaO2. This combination can reduce the dosage of chemicals and improve the resource utilization effect of sludge (Wang and Li, 2016). However, the performance of CaO2/MW treatment on sludge dewaterability and organic pollutants’ removal remain less studied, and it is meaningful to study whether there is synergy in these processes.
This study focused on exploring the practicality of combined CaO2 and MW treatments on improving WAS dewatering performance and the organic pollutants’ degradation. Capillary suction time (CST) was characterized to evaluate sludge dewatering performance. The relationship between sludge disintegration degree and EPS fractions of CST was established. The influence of Ca2+ on surface charge of sludge flocs was assessed. Three-dimensional excitation–emission matrix (EEM) fluorescence spectroscopy, Fourier-transformed infrared (FTIR) spectroscopy, was also performed to allow a better understanding of the possible mechanisms of sludge dewatering. Electron paramagnetic resonance (EPR) spectroscopy combined with radical scavenging was conducted for radical identification and evaluation of the main functional reactive oxygen species (ROS). Increasing knowledge on sludge dewaterability and toxic pollutants’ removal will provide theoretical basis for the development of innovative technologies in sludge treatment field.
Materials and Methods
Chemicals
CaO2 (80%) and spin trap 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) were purchased from Fisher in the United States. Isopropanol (IPA, C3H8O, 99%) and chloroform (CF, CHCl3, 99%) were bought from Aladdin Reagent Co. Ltd (Shanghai, China). Other chemicals involved in this research were of analytical grade and purchased from Sinopharm Chemical Reagent (Shanghai, China).
Physical and Chemical Properties of Sludge
The raw sludge sample was collected from the secondary sedimentation tank of a large sewage treatment plant in Shanghai, China. The plant adopts the anoxic–oxic process to treat wastewater of 75,000 m3/d, with service population of 200,000. The sludge is filtered by a 1-mm × 1-mm screen and precipitated at 4°C for 24 h before use. The physical and chemical properties of the concentrated sludge were tested (Table 1).
Sludge Treatment
This batch test was performed in a batch of 1 L glass bottles, and the volume of sludge added was 500 ml. Microwave processing was conducted with a microwave apparatus (Galanz, P70D20 TL-D4, China). For CaO2/MW treatment, before MW irradiation, appropriate amount of CaO2 (10, 20, 30, 40, and 50 mg/gVSS) was added, after which sludge samples were heated to the target temperatures (40, 50, 60, 70, 80, and 90°C) and placed in an air-bath shaker (200 rpm). Since CaO2 was dissolved slowly in water and could keep oxidation state for a long time, the sampling time was selected at 12 h according to the instructions provided in Li et al.’s article (Li et al., 2015). The sludge samples without any treatment were used as control. All the tests were conducted in parallel.
Analytical Methods
Analysis of Sludge Properties
Capillary suction time (CST) was detected by a 304M (Triton, England) to determine the dewatering ability of the sludge; zetal potential was measured by Zetasizer Nano ZS instrument (Malvern Instruments Ltd. Co., United Kingdom); chemical oxygen demand (COD), total solids, volatile suspended solids, and total suspended solids were measured referring to the standard method (APHA, 2005). DDSCOD is the ratio of the increment of soluble COD (SCOD) in the CaO2/MW treatment group to the biggest increment of SCOD, which can be calculated with the following formula:
where TCOD represents the total COD in raw sludge, SCOD0 represents the soluble COD in raw sludge, and SCOD represents the soluble COD in the CaO2/MW treatment group.
Analysis of Extracellular Polymeric Substances
EPS was extracted from raw sludge and digested sludge by the process adopted by Niu et al. (2013). Dissolved organic carbon was determined by using the total organic carbon analyzer (TOC-L, SHIMADZU Inc., Japan). Proteins were measured with the modified Lowry method (Frølund et al., 1996), and polysaccharides were determined with the phenol–sulfuric acid method (Gerhardt et al., 1994). The fluorescence spectra were measured using a fluorescence spectrophotometer (F-4500, Hitachi, Japan). The excitation range was 200–400 nm, and the emission was 220–550 nm. The scanning rate is 12, 000 nm/min, and the excitation and emission slit bandwidths are 5 nm. The data were processed with Origin 8.0. The extracted EPS was freeze-dried by a lyophilizer (FD-1A-50, Beijing Boyikang Laboratory Instruments Co., Ltd., China) and was then analyzed by FT-IR (Nicolet 5,700, Thermo Nicolet Co., United States) in the wavelength range of 400–4,000 cm−1, with the resolution of 2 cm−1 and the scanning times of 64 scans/min.
Analysis of Organic Contaminants
The sludge samples were freeze-dried and then dissolved in a mixture of methanol and acetate buffer solution (pH 5.0), treated with ultrasound for 30 min, and separated by centrifugation. This operation is repeated three times. The supernatant was filtered through 0.45 μm filter membranes and was then extracted with dichloromethane under neutral (pH = 7), acidic (pH = 2), and alkaline (pH = 12) conditions. The next step was to dry the supernatant with anhydrous sodium sulfate and concentrate the supernatant in a water bath at 38°C. The samples were analyzed by Agilent 7890A-5975C GC/MS (Agilent, United States) to identify organic species. The carrier gas was helium, and the products were separated by a fused-silica capillary column (30.0 m × 0.25 mm × 0.25 m, HP-5MS). The column temperature was controlled at 4°C for 3 min, which was then heated to 300°C in 30°C/min increments and lasted for 5 min.
Analysis of Reactive Oxygen Species
Free radicals were detected by electron paramagnetic resonance (EPR) using an ESP300 (Bruker, Germany). Spin trap 5, 5-dimethyl-1-pyrroline-N-oxide (DMPO) was selected as a spin trap. FeSO4(NH4)2SO4 (1.0 mmol/L, 5 μl), phosphate-buffered saline (50 mmol/L, pH = 7.4, 5 μl), DMPO (0.9 mol/L, 5 μl), and 5 μl sludge samples were mixed together, and then measured immediately. IPA and CF were used as scavenger for •OH and •O2−, respectively (Amina et al., 2018).
Correlation Analysis
SPSS software (SPSS version 19.0, SPSS Inc.) was employed to analyze the sludge dewaterability and its influencing factors. Pearson’s correlation coefficient (R) was used to evaluate the linear correlation between two parameters. R ranges between −1 and +1, with −1 denoting completely negative correlation, +1 means completely positive correlation, and 0 means no correlation. Within the 95% confidence interval, the correlation was considered statistically significant (p < 0.5).
Results and Discussion
Effect of CaO2/MW Treatment on Sludge Dewatering Performance
Effect of CaO2 Dosages and MW Temperature on Sludge Dewatering
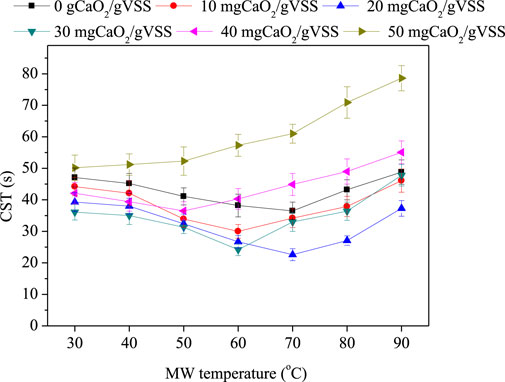
The change of sludge dewatering performance under different CaO2 dosages and MW temperature was investigated (Figure 1). Both CaO2 doses and MW radiation impact the CST values. The combination of proper CaO2 dosages and MW temperature presents synergetic effect on improving sludge dewatering behaviors. The optimal dehydration performance was at a CaO2 dosage of 20 mg/gVSS with MW temperature of 70°C. The CST value reduced by 52% compared with the raw sludge.
The test with single CaO2 at room temperature shows that the CST decreased from 47.1 to 44.2, 39.3, 36.1, and 42.1 s at the CaO2 dosages of 10, 20, 30, and 40 mg/gVSS, respectively, whereas at the CaO2 dosage of 50 mg/gVSS, the CST again increased to 50.2 s. The results were in line with the results of the research done by Chen et al. (2016), in no enhancement of filtration performance at lower CaO2 dosages due to the weak oxidation, and in which the dewaterability destroyed at higher CaO2 dosages due to the release of excess EPS. Tests with a single MW treatment show that when the temperature was under 60°C, the CST values decreased as the temperature increased. However, when the temperature increased above 60°C, the CST value increased gradually and even exceeded that of the raw sludge. This result is corroborated by Yu et al. (2009), who found that there is appropriate microwave energy that could enhance the sludge dewatering performance. One of the possible explanations for this phenomenon is that the sludge flocs, which are reservoir of water, are decomposed into smaller fragments and turn bound water into free water. The damage of dewaterability under MW treatment over 60°C could be ascribed to the disruption of the sludge structure and the formation of fine particles (Yu et al., 2009; Liu et al., 2016). The hybrid treatment witnessed that at the CaO2 dosages of 10, 20, 30, and 40 mg/gVSS, CST values slightly decreased until the temperature elevated to 60, 70, 60, and 50°C, respectively, after which the CST values gradually increased. At the CaO2 dosage of 50 mg/gVSS, the CST values increased gradually from 49 to 79 s with the elevation of MW temperature. It suggests that when CaO2 dosage is between 0 and 40 mg/gVSS, there is a suitable MW temperature which would lead to optimal dewatering performance; however, when CaO2 dosage exceeded 40 mg/gVSS, the dewatering performance gradually worsened with the increase of MW temperature.
Effect of Disintegration Degree on Sludge Dewatering Performance
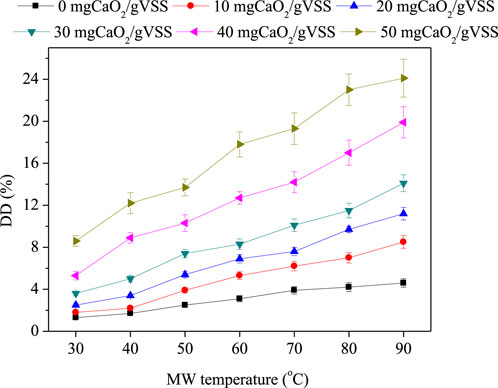
DDSCOD reflects the dissolved amount of organic substance of sludge. Hence, DDSCOD is closely related to sludge dewatering performance. In general, the DDSCOD values increase with CaO2 dosages and MW temperatures (Figure 2). The study shows that when the MW temperature increased from room temperature to 90°C, DDSCOD increased from 1.3 to 4.6%, 1.8 to 8.5%, 2.5 to 11.2%, 3.6 to 14.1%, 5.3 to 19.9%, and 8.6% to 24.1 at CaO2 dosages of 0, 10, 20, 30, 40, and 50 mg/gVSS, respectively. According to previous studies, both MW and CaO2 treatments were capable of disintegrating the sludge structure and microbial structure, and the combination of MW and CaO2 treatment synergistically improved the disruption of sludge flocs (Wang and Li, 2016).
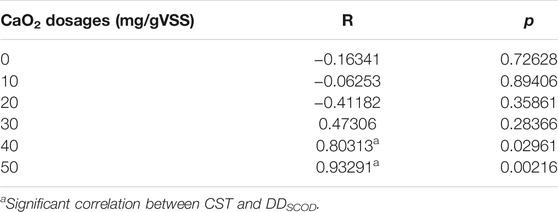
The optimal sludge dewatering performance was observed at DDSCOD of 4.8, 5.3, 7.6, and 8.3% at the treatment conditions of CaO2 (10 mg/gVSS)/MW(60°C), CaO2 (20 mg/gVSS)/MW(70°C), and CaO2 (30 mg/gVSS)/MW(60°C), with the corresponding CST values being 30, 22.6, and 24.2 s, respectively. Similar results were observed in other studies that an appropriate degree of sludge decomposition was crucial to sludge dewaterability (Yu et al., 2009; Chen et al., 2016). The correlation of disintegration degree and sludge dewaterability was also reflected by Pearson’s correlation between DDSCOD and CST (Table 2). It indicates that the DDSCOD was closely related to the dewatering performance at the CaO2 dosages of 40 and 50 mg/gVSS. Yet when CaO2 dosages were lower than 40 mg/gVSS, there appeared no correlation with DDSCOD and CST, which suggests that it is some other factors that are influencing sludge dewaterability. Because of its superior performance in improving sludge dewaterability, a CaO2 dosage of 20 mg/gVSS was determined to be the standard dosage that would be used for further mechanism investigation.
Effects of CaO2/MW Treatment on Extracellular Polymeric Substances
Effects of CaO2/MW Treatment Extracellular Polymeric Substance Distribution and Composition
Many studies have shown that the composition and concentration of EPS have an effect on sludge dewaterability (Zhen et al., 2012; Zhang et al., 2016); therefore, this study explored the distribution and composition of EPS in sludge. The changes of EPS fractions, protein, and polysaccharide concentration in different EPS fractions with the addition of 20 mg CaO2/gVSS at different MW temperatures were recorded in Figure 3. The reason why LB-EPS and SEPS concentration increased gradually with MW temperature might be the conversion of TB-EPS (Zhang et al., 2016). However, the total EPS (sum up of TB-EPS, LB-EPS, and SEPS) and TB-EPS content decreased first until the MW temperature elevated to 70°C. The variation of extracted total EPS is a synchronized process of generation and degradation (Li et al., 2016). The generation was mainly obtained by the intracellular substances in microbial cells. The visual results of the BacLight test (Figure 4) showed that the proportion of living cells to total cells was 81.8% in the raw sludge, with the addition of 20 mg CaO2/gVSS; the ratio then decreased to 80.0, 78.8, 76.0, 74.7, 73.0, 71.6, and 70.1% at room temperature, 40, 50, 60, 70, 80, and 90°C, respectively. It can be implied from the results that CaO2/MW treatment not only accelerates the disruption of EPS but also facilitates microbial cell lysis. The organic matter in cells could be released into the aqueous phase, leading to the enhancement of EPS monitoring quantity. The degradation is achieved by the chemical oxidation of CaO2 (Chen et al., 2016), but according to previous investigation, the mineralize effect of CaO2 can be neglected compared with an abundant amount of soluble organics (Li et al., 2015; Wang and Li, 2016). Therefore, it can be speculated that the lower EPS content was related to the compact floc structure since Ca2+ can compress the EPS structure through bridging and electrical neutralization. Therefore, Ca2+ plays an important role in the reconstruction of the sludge structure. The reconstruct sludge flocs were more compact and difficult to be extracted by heating and centrifuging (Wu and Chai, 2016). The optimal sludge dewatering performance was observed at the lowest total EPS and TB-EPS content. The finding is according with other reports, where it has been observed that the lower extractable EPS is always associated with better sludge dewatering properties (Zhang et al., 2016).

FIGURE 3. Variations of EPS concentration treated with 20 mgCaO2/gVSS and different MW temperatures: (A) TOC concentration of the SEPS, LB-EPS, and TB-EPS; (B) protein content in SEPS, LB-EPS, and TB-EPS; (C) polysaccharide content in SEPS, LB-EPS, and TB-EPS.
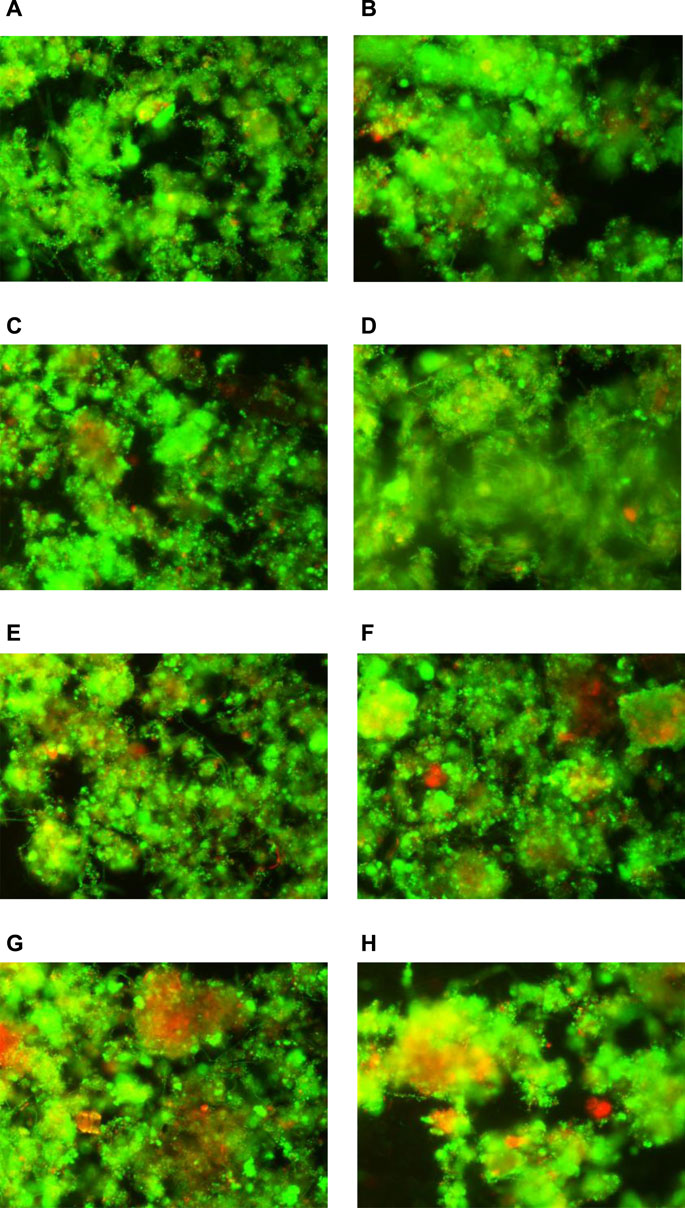
FIGURE 4. Photos of live–dead: (A) raw sludge; (B) 20 mgCaO2/gVSS; (C) CaO2 (20 mg/gVSS)/MW (40°C); (D) CaO2 (20 mg/gVSS)/MW (50°C); (E) CaO2 (20 mg/gVSS)/MW (60°C); (F) CaO2 (20 mg/gVSS)/MW (70°C); (G) CaO2 (20 mg/gVSS)/MW (80°C); (H) CaO2 (20 mg/gVSS)/MW (90°C).
Similar results were obtained for protein in TB-EPS, LB-EPS, and SEPS, since proteins were the largest fractions in EPS matrix (Figure 3B). Polysaccharide content in LB-EPS and SEPS increased gradually from 9 to 14 mg/L and 15 to 21 mg/L, respectively, with the elevation of MW temperature from room temperature to 90°C. However, those in TB-EPS declined from 46 to 31 mg/L (Figure 3C). This phenomenon indicated that the higher MW temperature facilitated the transformation of polysaccharides in TB-EPS to the filtrate and LB-EPS. The changes of total polysaccharide concentration were not obvious (Figure 3C). The different variation tendency of protein and polysaccharide may be due to their distribution pattern in sludge, since proteins mainly exist in the pellet, while carbohydrates distributed uniformly in sludge component (Shao et al., 2009).
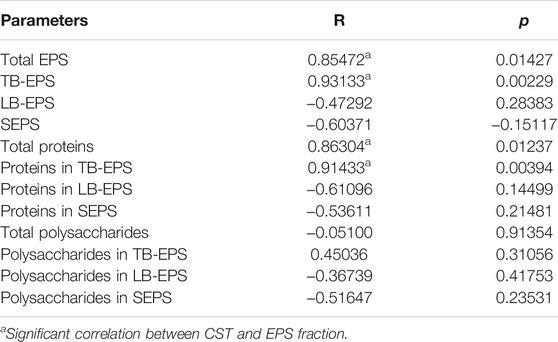
Correlation analysis was conducted to offer insights into the correlation between the EPS compound and sludge dewatering under CaO2 (20 mg/gVSS)/MW (70°C) conditioning (Table 3). The Pearson’s correlation result demonstrated that CST correlated with total EPS (R = 0.85472, p < 0.05), TB-EPS (R = 0.93133, p < 0.01), total protein (R = 0.86304, p < 0.05), and protein in TB-EPS (R = 0.91433, p < 0.01), while CST has no correlation with LB-EPS, SEPS, proteins in TB-EPS, proteins in SEPS, and polysaccharides. It implies that sludge dewaterability is to a large extent determined by total EPS, TB-EPS, total protein, and protein present in TB-EPS. It is due to the TB-EPS that covers the surface of the cell that provides protection for microbial cells insides the sludge flocs. Microbial cells and TB-EPS can bind a lot of water, so the presence of a large number of TB-EPS and cells will make dehydration more difficult. Therefore, TB-EPS and microbial cell lysis are conducive to release the water trapped in sludge into free water, thus increasing solid–liquid separation (Zhang et al., 2016). Protein is the main component of EPS, which is more important than polysaccharide in flocculation, sedimentation, and dehydration of sludge (Zhen et al., 2012; Yang et al., 2017).
Three-Dimensional Excitation–Emission Matrix Spectra of Sludge
Different EPS components (such as SEPS, LB-EPS, and tightly bound TB-EPS) have significant effects on bioflocculation, sludge settling, and dewatering (Chen et al., 2016). In order to identify the key organics related to sludge dewaterability, an EEM analysis was conducted. Each EEM fluorescence spectrum illustrated in Figure 5 gives some information about the compositions of EPS mixtures. Three main fluorescent peaks could be identified in SEPS, LB-EPS, and TB-EPS of raw sludge and treated sludge. The first peak which happened at the excitation/emission wavelengths (Ex/Em) of 250/320–380 (Peak A) was ascribed to aromatic protein–like substances; the second peak identified at the Ex/Em of 290/320–380 (Peak B) was identified to tryptophan protein–like substances; the third peak observed at the Ex/Em of 350/430–450 (Peak C) was referred to humic acid–like substances (Zhen et al., 2012; Chen et al., 2016; Yang et al., 2017).
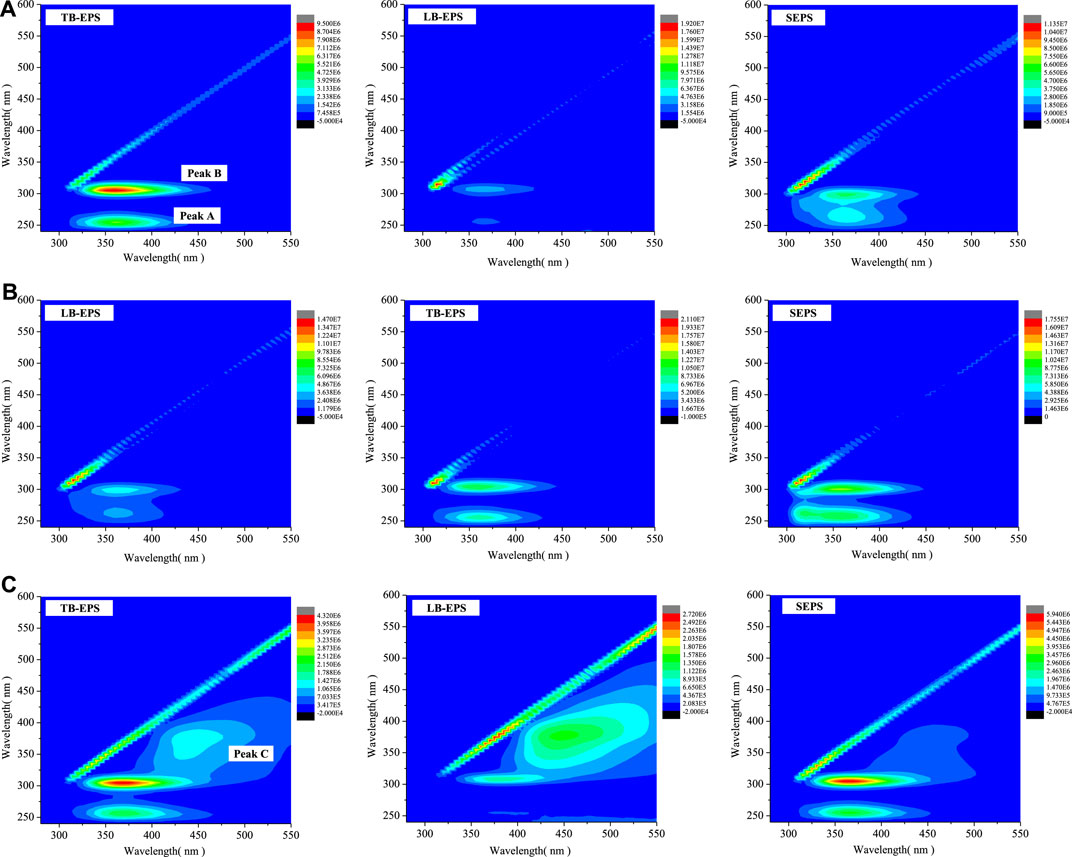
FIGURE 5. Excitation–emission matrix fluorescence spectra of the different extracellular polymeric substance (EPS) fractions: (A) raw sludge; (B) sludge treated by CaO2 (20 mg/gVSS)/MW(70°C); (C) sludge treated by CaO2 (50 mg/gVSS)/MW(80°C).
Under the optimal treatment conditions, the fluorescence intensities of tryptophan protein–like substances and aromatic protein–like substances in TB-EPS demonstrated a tendency to weaken, whereas their counterpart in LB-EPS and SEPS increased compared with raw sludge (Figure 5B). This is in agreement with the analysis of EPS variation in Effects of CaO2/MW treatment extracellular polymeric substances 12 distribution and composition. The result implies that tryptophan protein–like substances and aromatic protein–like substances play a major role in improving sludge dewatering performance. Furthermore, under CaO2 (50 mg/gVSS)/MW (80°C) treatment conditions (Figure 5C) which led to the poor dewatering behavior compared with the raw sludge, the intensities of tryptophan protein–like substances and aromatic protein–like substances in TB-EPS, LB-EPS, and S-EPS components were all enhanced significantly compared to the raw sludge. It is indicated that more EPS could be extracted from sludge flocs. In addition, humic acid–like substances were also released into EPS fractions, which was in accordance with the study of Ping et al. (2018) where it was observed that the humic acid–like substances were the only fluorescence products in the CaO2 group at high temperature. The presence of these substances in EPS may lead to the deterioration of sludge dewatering performance since they were more difficult to be chemically oxidized than protein (Ping et al., 2018). The result of this study corroborates the finding of Zhen et al. (2012), who found that except for proteins, sludge dewatering is also affected by humic acid substances.
Effect of Cation Ions on Sludge Dewaterability
Surface Charge of Sludge Flocs
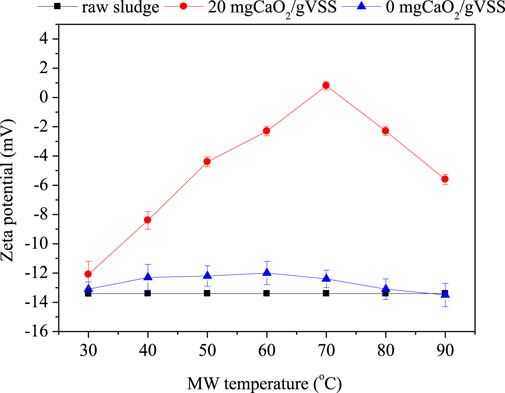
The variation of surface charge with zeta potential under different treatment conditions is shown in Figure 6. The surface of untreated raw sludge was negatively charged and the zeta potential was −13.4 mV. The contribution of several polymeric compounds, such as protein, polysaccharide, and humus, to the sludge-negative surface has been reported (Yu et al., 2014). The sludge particles repelled each other via electrostatic and maintaining a relatively stable state, which inhibited sludge aggregation and resulted in poor dewatering performance (Guan et al., 2012; Yu et al., 2014). Surface charge of sludge was significantly affected by CaO2 and MW temperature. Without the addition of CaO2, the variation of zeta potential was not obvious, whereas in the presence of the addition of 20 mgCaO2/gVSS, with the increase of temperature, the zeta potential increased gradually and then decreased at the turning point of 70°C. The less negative charge was mainly due to the electrostatic neutralization of calcium ions (Yu et al., 2014; Wu and Chai, 2016). As the charge inside the sludge is not reachable, the flocs’ disruption and cell lysis caused by MW and CaO2 treatment transfer the function groups from the particle components to the soluble phase, which provides more binding sites for Ca2+ (Guan et al., 2012). According to the DLVO theory, the decrease of zeta potential is usually accompanied by a compressed electric double layer (Li et al., 2012). The compression of the double electric layer leads to the decrease of the interface water. Hence, in addition to releasing interstitial water through sludge disintegration, CaO2 can also convert interface water into bulk water by neutralizing the zeta potential and diluting the electric double layer (Wu and Chai, 2016). The subsequent increase of surface charge was likely a result of the emergence of more negative charged groups by further cleavage of the sludge structure (Yu et al., 2014). This also well interprets the variation of EPS in Figure 3. The optimum dehydration occurred at the minimum zeta potential values of 0.8 mV. The result is also in line with the previous literatures (Li et al., 2016).
FTIR Spectra of Extracellular Polymeric Substances
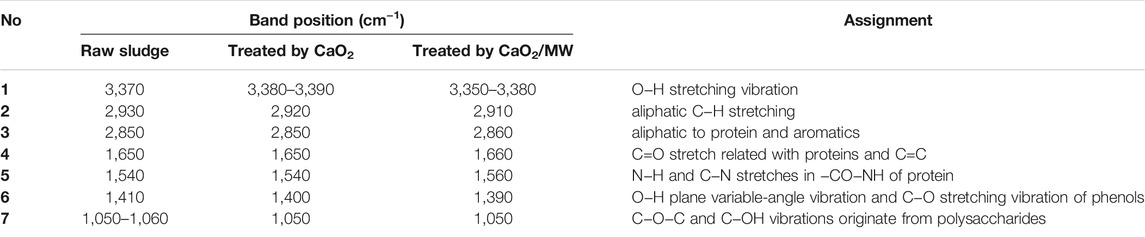
The interaction between Ca2+ and sludge flocs can be analyzed by FTIR. The FTIR spectrum of EPS extracted from raw sludge and treated sludge is illustrated in Figure 7. The FTIR spectrum can be divided into six main regions, which are connected with many functional groups: 3350−3390 cm-1 represents the O−H groups stretching vibrations present in polymeric substances; 2910-2930 and 2850-2860 cm-1 proved the appearance of aliphatic chains present in polysaccharides, proteins, and humus; 1,650–1,660 cm−1 can ascribed to the stretching vibrations of C=O and C=C of amidesⅠ; 1,540–1,560 cm−1 was due to the N−H stretches in −CO−NH of amidesⅡ; 1,390–1,410 cm−1 was ascribed to O−H plane variable angle vibration and C−O tensile vibration of phenols; 1,050–1,060 cm−1 (O−H groups) represents the characteristic absorption peak of polysaccharides (Guan et al., 2012; Chen et al., 2016; Yang et al., 2017).
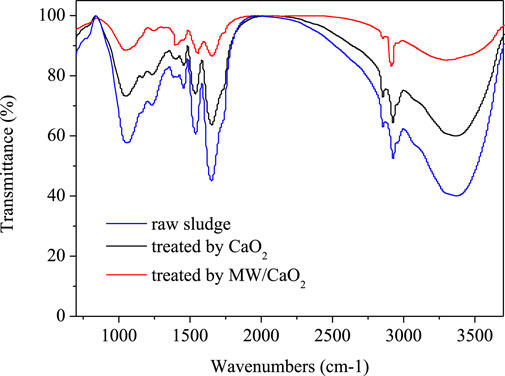
FIGURE 7. Fourier-transform infrared spectra of raw sludge, CaO2 (20 mg/gVSS) treated sludge, and CaO2 (20 mg/gVSS)/MW (70°C) treated sludge.
The vibration of characteristic bands is listed in Table 4. The transformation of O−H and O−H stretching vibration indicates that H+ is replaced by Ca2+ (Guan et al., 2012). In addition, Ca2+ could react with functional groups in protein, resulting in the shift of C−N and N−H vibration (Guan et al., 2012). This interaction can precisely reduce the charge of flocs, as described in Surface charge of sludge flocs. The transformation of bands in CaO2/MW-treated sample was more obvious than that of single CaO2 treatment sample, indicating that the MW treatment strengthened the interaction between Ca2+ and protein. The vibration of C−O−C stretches and C−OH vibrations that originated from polysaccharides were not obvious in the presence of CaO2, implying that the bridging between Ca2+ and protein plays a dominating role in the dehydration process.
Effect of CaO2/MW on Organic Pollutants
Removal Efficiency of Organic Pollutants
Since the presence of organic pollutants in sludge causes further concerns for sludge management, the removal of these substances in sludge has important environmental significance (Li et al., 2015; Dubey et al., 2021; Lü et al., 2021). The organic contaminants detected in different sludge samples are listed in Table 5. The total number of species detected in this study was 33, only 12 of which were the same as those that had been detected by the previous study (Li et al., 2015). The newly found organic components mainly originate from PPCPs (e.g., cimetidine, muskone, and estriol), which have attracted more attention from scholars due to their threats to environment and human health (Zhang X. et al., 2015). Other organic components mainly originate from interior decoration material (e.g., benzene and benzyl alcohol) and pesticides (e.g., methylbenzene). These organic substances have different levels of toxicity, limiting the sludge recycle and disposal.
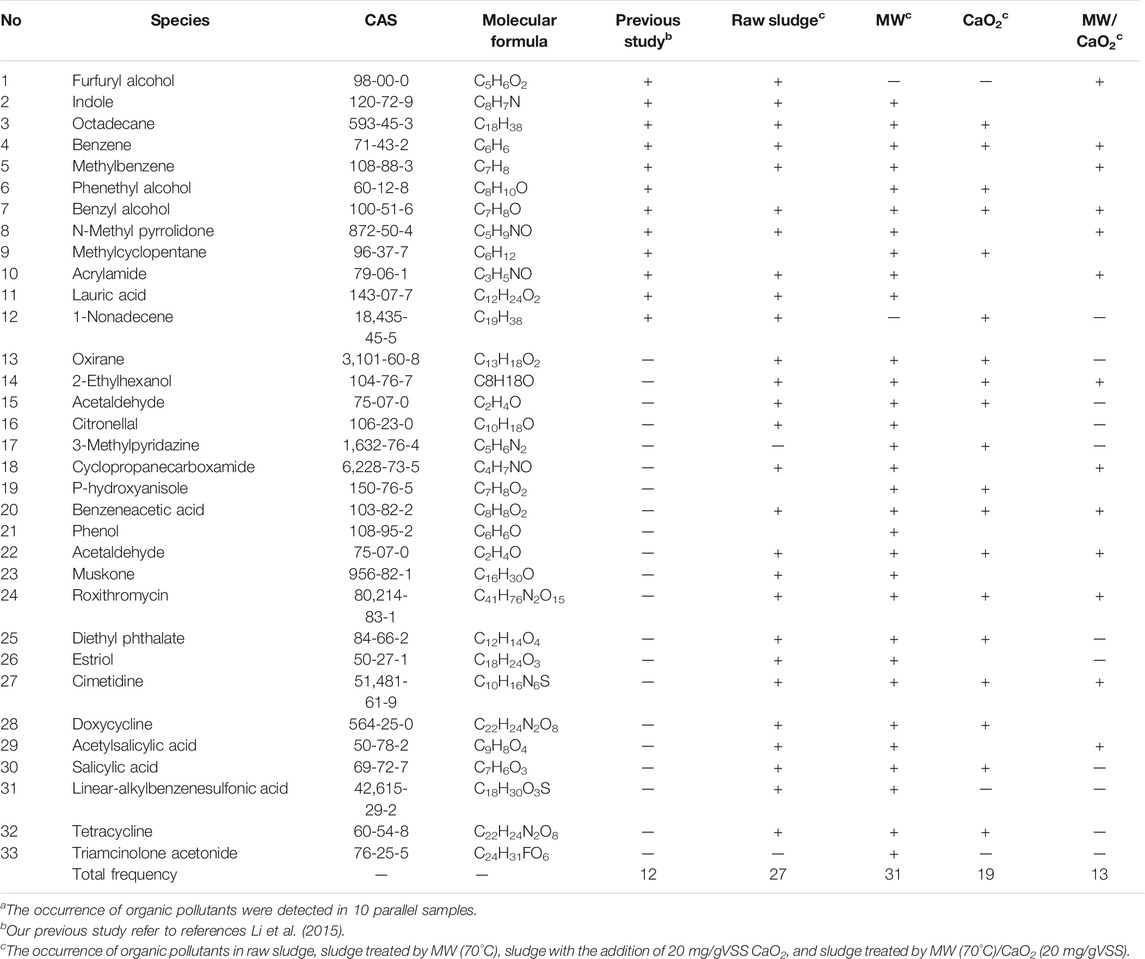
TABLE 5. Organic pollutants detected in different sludge samples.a
Twenty-seven kinds of organic substances were detected in raw sludge, which increased to 31 after MW irradiation. This might be due to the MW treatment that promoted disintegration of sludge and the collapse of sludge flocs that accelerated the release of organics adsorbed and entrapped in the sludge mixtures. However, the quantity of organic species decreased to 19 and 13 under the treatment of CaO2 and CaO2/MW, respectively. This means that CaO2 is efficient for the removal of organic pollutants, and the removal efficiency further improved when combined with MW irradiation. Therefore, CaO2/MW treatment makes a promising method for the removal of organic pollutants and facilitated the subsequent sludge treatment and disposal.
Analysis of Reactive Oxygen Species
The EPR technique was adopted to clarify the degradation mechanisms of organic contaminants. DMPO is a common spin probe that can trap •OH or superoxide anion radicals (•O2−) to produce DMPO-OH (quartet EPR signal) or DMPO-OOH (sextet EPR signal) spin adduct (Lipovsky et al., 2012; Zhou et al., 2019; Zheng et al., 2019). The ROS present in single CaO2 and the hybrid CaO2/MW-treated sludge samples are presented in Figure 8. Both DMPO-OH and DMPO-OOH spin adduct were observed in CaO2/MW-treated sludge samples (Figure 8B), while only DMPO-OH spin adduct was obtained in CaO2-treated sludge samples (Figure 8A). It is believe that MW can promote the generation of •OH radicals from CaO2, because the peak strength of DMPO-OH in the CaO2/MW group is higher than that of DMPO-OH in the CaO2 group. This is consistent with our previous study that MW irradiation can facilitate CaO2 to produce more •OH radicals (Wang and Li, 2016). Since DMPO-OOH spin adduct is unstable and can decompose to DMPO-OH spin adduct (Wang and Li, 2016), it is uncertain whether the spectrum presented in Figure 8A increased from the production of •O2− or/and •OH. Thus, IPA and CF were used to scavenge •OH and •O2−, respectively. As shown in Figure 8C, the existence of IPA abolished the quartet signal, while the influence of CF on the quartet signal can be neglected. This suggests that due to the scavenging ability of sludge and the expeditious changing of •O2−, the concentration of •O2− produced by CaO2 alone was not enough to be detected by DMPO. However, MW irradiation can improve the •O2− production by CaO2 and present an obvious DMPO-OOH spin adduct signal in Figure 8B.
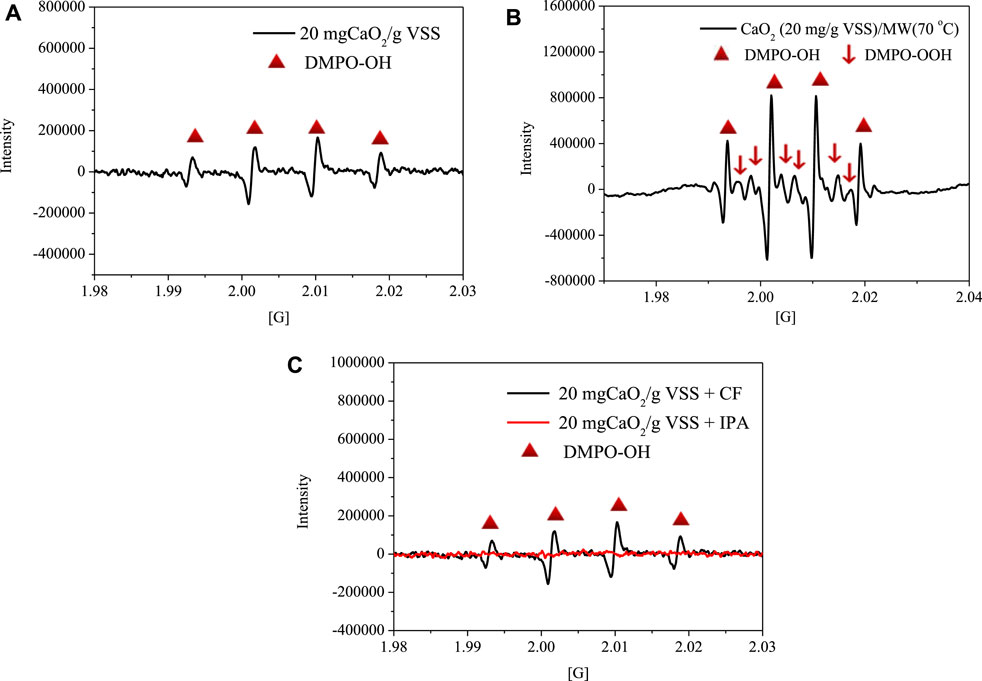
FIGURE 8. Electron paramagnetic resonance spectra of sludge samples under different treatment conditions.
According to previous studies, •OH radical is the main functional ROS that is generated from CaO2 for organic substances degradation (Li et al., 2016; Zhang et al., 2018). There are many pathways for •OH to react with organic contaminants (e.g., mechanical oxidation–reduction reaction, radical chain reaction, and hydrogen atom transfer). Through these reaction ways, organic pollutants can be degraded or produce less toxic intermediates (Qian et al., 2015; Amina et al., 2018; Zhang et al., 2018). Furthermore, MW irradiation can facilitate CaO2 to generate more •OH and •O2− radicals, so the oxidation capacity of CaO2 is improved. The combined CaO2 and MW treatment can decompose a variety of organic pollutants in sludge and reduce the potential environmental risks in sludge reuse. In conclusion, CaO2/MW pretreatment improves sludge dewatering, reduces sludge volume, and reduces the cost of transporting sludge to the final disposal site. Therefore, CaO2/MW pretreatment can be used as an effective method in practical applications.
Conclusion
Combined CaO2 and MW treatment can synergistically enhance sludge dewaterability. The optimal dewaterability was obtained at CaO2 (20 mg/gVSS)/ MW (70°C). Under these conditions, the capillary suction time decreased by 52% compared with the raw sludge. Total EPS, LB-EPS, total protein, and protein in LB-EPS were closely related to the sludge dewaterability. When combined with MW, Ca2+ produced better performance in compressing sludge flocs via bridge and charge neutralization, contributing to the improvement of sludge filtration properties. MW irradiation facilitates the production of •OH and •O2− radicals by CaO2, thus improving the removal efficiency of organic contaminants.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material; further inquiries can be directed to the corresponding author.
Author Contributions
Conceptualization, JW and AZ; methodology, KS and LD; software, JW and YZ; writing—original draft, JW and XL; writing—review and editing, JW.
Funding
This work was supported by the National Key Research and Development Program of China (No. 2019YFD0900300), the Natural Science Foundation of Shanghai (No. 21ZR1479900), the Shanghai Sailing Program (No. 18YF1407500), China Agriculture Research System of MOF and MARA (CARS-48), and Shanghai Key Lab for Urban Ecological Processes and Eco-Restoration (SHUES 2021A14).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Akgul, D., Abbott, T., and Eskicioglu, C. (2017). Assessing Iron and Aluminum-Based Coagulants for Odour and Pathogen Reductions in Sludge Digesters and Enhanced Digestate Dewaterability. Sci. Total Environ. 598, 881–888. doi:10.1016/j.scitotenv.2017.04.141
Amina, X., Si, K., Si, Y., and Yousaf, B. (2018). Synergistic Effects and Mechanisms of Hydroxyl Radical-Mediated Oxidative Degradation of Sulfamethoxazole by Fe(II)-EDTA Catalyzed Calcium Peroxide: Implications for Remediation of Antibiotic-Contaminated Water. Chem. Eng. J. 353, 80–91. doi:10.1016/j.cej.2018.07.078Si
Anjum, M., Al-Makishah, N. H., and Barakat, M. A. (2016). Wastewater Sludge Stabilization Using Pre-treatment Methods. Process Saf. Environ. Prot. 102, 615–632. doi:10.1016/j.psep.2016.05.022
APHA (2005). Standard Methods for the Examination of Water and Wastewater. 21th ed. Washington, DC: American Public Health Association.
Cao, B., Zhang, T., Zhang, W., and Wang, D. (2021). Enhanced Technology Based for Sewage Sludge Deep Dewatering: A Critical Review. Water Res. 189, 116650. doi:10.1016/j.watres.2020.116650
Chen, Z., Zhang, W., Wang, D., Ma, T., Bai, R., and Yu, D. (2016). Enhancement of Waste Activated Sludge Dewaterability Using Calcium Peroxide Pre-oxidation and Chemical Re-flocculation. Water Res. 103, 170–181. doi:10.1016/j.watres.2016.07.018
Dubey, M., Mohapatra, S., Tyagi, V. K., Suthar, S., and Kazmi, A. A. (2021). Occurrence, Fate, and Persistence of Emerging Micropollutants in Sewage Sludge Treatment. Environ. Pollut. 273, 116515. doi:10.1016/j.envpol.2021.116515
Frølund, B., Palmgren, R., Keiding, K., and Nielsen, P. H. (1996). Extraction of Extracellular Polymers from Activated Sludge Using a Cation Exchange Resin. Water Res. 30, 1749–1758.
Gerhardt, P., Murray, R. G. E., Wood, W. A., and Krieg, N. R. (1994). Methods for General and Molecular Bacteriology. Washington, DC: American Society for Microbiology (ASM).
Guan, B., Yu, J., Fu, H., Guo, M., and Xu, X. (2012). Improvement of Activated Sludge Dewaterability by Mild thermal Treatment in CaCl2 Solution. Water Res. 46, 425–432. doi:10.1016/j.watres.2011.11.014
Guo, Y., Rene, E. R., Wang, J., and Ma, W. (2020). Biodegradation of Polyaromatic Hydrocarbons and the Influence of Environmental Factors during the Co-composting of Sewage Sludge and green forest Waste. Bioresour. Tech. 297, 122434. doi:10.1016/j.biortech.2019.122434
Jiang, M., Song, S., Liu, H., Wang, P., and Dai, X. (2021). Effect of Gentamicin Mycelial Residues Disintegration by Microwave-Alkaline Pretreatment on Methane Production and Gentamicin Degradation during Anaerobic Digestion. Chem. Eng. J. 414, 128790. doi:10.1016/j.cej.2021.128790
Kim, M. S., Lee, K.-M., Kim, H.-E., Lee, H.-J., Lee, C., and Lee, C. (2016). Disintegration of Waste Activated Sludge by Thermally-Activated Persulfates for Enhanced Dewaterability. Environ. Sci. Technol. 50, 7106–7115. doi:10.1021/acs.est.6b00019
Li, H., Wen, Y., Cao, A., Huang, J., Zhou, Q., and Somasundaran, P. (2012). The Influence of Additives (Ca2+, Al3+, and Fe3+) on the Interaction Energy and Loosely Bound Extracellular Polymeric Substances (EPS) of Activated Sludge and Their Flocculation Mechanisms. Bioresour. Tech. 114, 188–194. doi:10.1016/j.biortech.2012.03.043
Li, Y., Wang, J., Zhang, A., and Wang, L. (2015). Enhancing the Quantity and Quality of Short-Chain Fatty Acids Production from Waste Activated Sludge Using CaO2 as an Additive. Water Res. 83, 84–93. doi:10.1016/j.watres.2015.06.021
Li, Y., Yuan, X., Wu, Z., Wang, H., Xiao, Z., Wu, Y., et al. (2016). Enhancing the Sludge Dewaterability by Electrolysis/electrocoagulation Combined with Zero-Valent Iron Activated Persulfate Process. Chem. Eng. J. 303, 636–645. doi:10.1016/j.cej.2016.06.041
Lipovsky, A., Levitski, L., Tzitrinovich, Z., Gedanken, A., and Lubart, R. (2012). The Different Behavior of Rutile and Anatase Nanoparticles in Forming Oxy Radicals upon Illumination with Visible Light: An EPR Study. Photochem. Photobiol. 88, 14–20. doi:10.1111/j.1751-1097.2011.01015.x
Liu, H., Xiao, H., Fu, B., and Liu, H. (2017). Feasibility of Sludge Deep-Dewatering with Sawdust Conditioning for Incineration Disposal without Energy Input. Chem. Eng. J. 313, 655–662. doi:10.1016/j.cej.2016.09.107
Liu, J., Wei, Y., Li, K., Tong, J., Wang, Y., and Jia, R. (2016). Microwave-acid Pretreatment: A Potential Process for Enhancing Sludge Dewaterability. Water Res. 90, 225–234. doi:10.1016/j.watres.2015.12.012
Liu, X., Wu, Y., Xu, Q., Du, M., Wang, D., Yang, Q., et al. (2021). Mechanistic Insights into the Effect of Poly Ferric Sulfate on Anaerobic Digestion of Waste Activated Sludge. Water Res. 189, 116645. doi:10.1016/j.watres.2020.116645
Lü, H., Chen, X.-H., Mo, C.-H., Huang, Y.-H., He, M.-Y., Li, Y.-W., et al. (2021). Occurrence and Dissipation Mechanism of Organic Pollutants during the Composting of Sewage Sludge: A Critical Review. Bioresour. Tech. 328, 124847. doi:10.1016/j.biortech.2021.124847
Lu, S., Zhang, X., and Xue, Y. (2017). Application of Calcium Peroxide in Water and Soil Treatment: A Review. J. Hazard. Mater. 337, 163–177. doi:10.1016/j.jhazmat.2017.04.064
Niu, M., Zhang, W., Wang, D., Chen, Y., and Chen, R. (2013). Correlation of Physicochemical Properties and Sludge Dewaterability under Chemical Conditioning Using Inorganic Coagulants. Bioresour. Tech. 144, 337–343. doi:10.1016/j.biortech.2013.06.126
Northup, A., and Cassidy, D. (2008). Calcium Peroxide (CaO2) for Use in Modified Fenton Chemistry. J. Hazard. Mater. 152, 1164–1170. doi:10.1016/j.jhazmat.2007.07.096
Ping, Q., Lu, X., Zheng, M., and Li, Y. (2018). Effect of CaO2 Addition on Anaerobic Digestion of Waste Activated Sludge at Different Temperatures and the Promotion of Valuable Carbon Source Production under Ambient Condition. Bioresour. Tech. 265, 247–256. doi:10.1016/j.biortech.2018.06.007
Qian, Y., Zhou, X., Zhang, Y., Sun, P., Zhang, W., Chen, J., et al. (2015). Performance of α-methylnaphthalene Degradation by Dual Oxidant of Persulfate/calcium Peroxide: Implication for ISCO. Chem. Eng. J. 279, 538–546. doi:10.1016/j.cej.2015.05.053
Shao, L., He, P., Yu, G., and He, P. (2009). Effect of Proteins, Polysaccharides, and Particle Sizes on Sludge Dewaterability. J. Environ. Sci. 21, 83–88. doi:10.1016/s1001-0742(09)60015-2
Wang, J., and Li, Y. (2016). Synergistic Pretreatment of Waste Activated Sludge Using CaO2 in Combination with Microwave Irradiation to Enhance Methane Production during Anaerobic Digestion. Appl. Energ. 183, 1123–1132. doi:10.1016/j.apenergy.2016.09.042
Wu, B., and Chai, X. (2016). Novel Insights into Enhanced Dewatering of Waste Activated Sludge Based on the Durable and Efficacious Radical Generating. J. Air Waste Manag. Assoc. 66, 1151–1163. doi:10.1080/10962247.2016.1189858
Wu, B., Su, L., Dai, X., and Chai, X. (2018). Development of Sludge-Derived Mesoporous Material with Loaded Nano CaO2 and Doped Fe for Re-utilization of Dewatered Waste-Activated Sludge as Dewatering Aids. Chem. Eng. J. 335, 161–168. doi:10.1016/j.cej.2017.10.015
Xu, Q., Huang, Q.-S., Wei, W., Sun, J., Dai, X., and Ni, B.-J. (2020). Improving the Treatment of Waste Activated Sludge Using Calcium Peroxide. Water Res. 187, 116440. doi:10.1016/j.watres.2020.116440
Yang, Q., Sun, J., Wang, D., Wang, S., Chen, F., Yao, F., et al. (2017). Effect of Nickel on the Flocculability, Settleability, and Dewaterability of Activated Sludge. Bioresour. Tech. 224, 188–196. doi:10.1016/j.biortech.2016.11.018
Yu, J., Guo, M., Xu, X., and Guan, B. (2014). The Role of Temperature and CaCl2 in Activated Sludge Dewatering under Hydrothermal Treatment. Water Res. 50, 10–17. doi:10.1016/j.watres.2013.11.034
Yu, Q., Lei, H., Yu, G., Feng, X., Li, Z., and Wu, Z. (2009). Influence of Microwave Irradiation on Sludge Dewaterability. Chem. Eng. J. 155, 88–93. doi:10.1016/j.cej.2009.07.010
Zhang, A., Shen, X., Yin, X., Li, X., and Liu, Y. (2018). Application of Calcium Peroxide for Efficient Removal of Triamcinolone Acetonide from Aqueous Solutions: Mechanisms and Products. Chem. Eng. J. 345, 594–603. doi:10.1016/j.cej.2018.01.104
Zhang, A., Wang, J., and Li, Y. (2015a). Performance of Calcium Peroxide for Removal of Endocrine-Disrupting Compounds in Waste Activated Sludge and Promotion of Sludge Solubilization. Water Res. 71, 125–139. doi:10.1016/j.watres.2015.01.005
Zhang, W., Cao, B., Wang, D., Ma, T., and Yu, D. (2016). Variations in Distribution and Composition of Extracellular Polymeric Substances (EPS) of Biological Sludge under Potassium Ferrate Conditioning: Effects of pH and Ferrate Dosage. Biochem. Eng. J. 106, 37–47. doi:10.1016/j.bej.2015.11.004
Zhang, X., Gu, X., Lu, S., Miao, Z., Xu, M., Fu, X., et al. (2015b). Degradation of Trichloroethylene in Aqueous Solution by Calcium Peroxide Activated with Ferrous Ion. J. Hazard. Mater. 284, 253–260. doi:10.1016/j.jhazmat.2014.11.030
Zhen, G., Lu, X., Li, Y., Zhao, Y., Wang, B., Song, Y., et al. (2012). Novel Insights into Enhanced Dewaterability of Waste Activated Sludge by Fe(II)-activated Persulfate Oxidation. Bioresour. Tech. 119, 7–14. doi:10.1016/j.biortech.2012.05.115
Zheng, M., Daniels, K. D., Park, M., Nienhauser, A. B., Clevenger, E. C., Li, Y., et al. (2019). Attenuation of Pharmaceutically Active Compounds in Aqueous Solution by UV/CaO2 Process: Influencing Factors, Degradation Mechanism and Pathways. Water Res. 164, 114922. doi:10.1016/j.watres.2019.114922
Zheng, Y., Xing, M., Cai, L., Xiao, T., Lu, Y., and Jiang, J. (2017). Interaction of Earthworms-Microbe Facilitating Biofilm Dewaterability Performance during Wasted Activated Sludge Reduction and Stabilization. Sci. Total Environ. 581-582, 573–581. doi:10.1016/j.scitotenv.2016.12.166
Keywords: calcium peroxide/microwave (CaO2/MW), filterability, advanced oxidation technology, organic pollutants, reactive oxygen species (ROS)
Citation: Wang J, Shang K, Da L, Liu X, Zhao Y and Zhang A (2021) Synergetic Effect of Combined CaO2 and Microwave Treatment on Waste Active Sludge Dewaterability and Organic Contaminants’ Removal. Front. Environ. Sci. 9:734277. doi: 10.3389/fenvs.2021.734277
Received: 30 June 2021; Accepted: 09 August 2021;
Published: 30 September 2021.
Edited by:
Shuwen Yan, Fudan University, ChinaReviewed by:
Juan Lv, University of Shanghai for Science and Technology, ChinaMing Zheng, Shanghai University, China
Copyright © 2021 Wang, Shang, Da, Liu, Zhao and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ai Zhang, YWl6aGFuZ0BkaHUuZWR1LmNu
 Jie Wang
Jie Wang Kankan Shang2,3
Kankan Shang2,3 Yongjing Zhao
Yongjing Zhao Ai Zhang
Ai Zhang