- 1Center for Ecological Research, Northeast Forestry University, Harbin, China
- 2Key Laboratory of Sustainable Forest Ecosystem Management-Ministry of Education, School of Forestry, Northeast Forestry University, Harbin, China
- 3Heilongjiang Sanjiang Plain Wetland Ecosystem Research Station, Fuyuan, China
Restoration of reclaimed marshes has great effects on soil biological processes. However, the responses of soil microbial properties (microbial biomass and enzyme activities) to natural restoration of reclaimed marshes is poorly studied, especially in a long restoration chronosequence. This study assessed the responses of soil microbial properties to natural restoration and investigated the relationships between soil microbial properties and soil physico-chemical and plant properties. We selected a restoration chronosequence (1, 4, 8, 13, 17, 27 years) after farmland abandonment, a soybean field, and a natural marsh in Sanjiang Plain, northeast China. For each site, we analyzed the soil microbial biomass carbon and nitrogen (MBC and MBN), four enzymes (β-glucosidase, invertase, catalase, urease) activities, soil physico-chemical properties at 0–50 cm depths, and plant properties (biomass, height, and coverage). The MBC and MBN contents increased with restoration time, but MBN content slowed down after 8 years of restoration. After 27 years of restoration, the soil MBC and MBN contents were 15.7 and 3.2 times of those in the soybean field, but the largest contents of MBC and MBN in the restored sites were 7.78%, 27.76% lower than those in natural marshes, respectively. Moreover, soil enzyme activities and the geometric mean of enzymatic activities (GME) also increased with restoration but slowed down after 13 years of restoration. After 27 years of restoration, the GME was 2.9 times than that in the soybean field, but the largest GME in the restored sites was 31.15% lower than that in the natural marsh. MBC and MBN contents, soil enzyme activities, and GME had significant relationships with soil C:N ratio, organic carbon, nutrients (total nitrogen, available nitrogen, total phosphorus), bulk density, moisture content, pH, plant properties, (i.e. biomass, height, and coverage) (p < 0.01). Redundancy analysis revealed that soil C:N ratio, pH, moisture content, total nitrogen and phosphorus were main factors affecting MBC and MBN contents and enzyme activities. In conclusion, soil microbial properties can respond positively to the natural restoration process of the reclaimed marshes and were significantly correlated with specific parameters of soil physico-chemical and plant properties.
Introduction
Wetland restoration has become more important in the past 2 decades (Euliss et al., 2006; Marton et al., 2014a), as disturbed/degraded wetland have been found to reduce the functions of water storage, flood control, carbon (C) and nitrogen (N) sequestration, biodiversity conservation, etc. (Jiang et al., 2015; Yu et al., 2017; Qi et al., 2021). The reclaimed wetlands can increase carbon emission (CO2), decrease soil moisture and C and N storage, and change microbiological properties (MBC and enzyme activities) (Song et al., 2012; Bai et al., 2013). Natural restoration is an effective way to restore the degraded ecosystem because it can reduce anthropogenic disturbance (Walker et al., 2007) and cost less compared to artificial restoration (Zahawi et al., 2014).
Soil microorganisms play a great role in the biogeochemical process of wetlands (Sousa et al., 2015) and can provide nutrients for the development and function of soil and plants (Li et al., 2015; Xu et al., 2020). Soil microbial properties such as microbial biomass and enzyme activities are essential components of wetlands (Xiao et al., 2015). They are considered to be more sensitive parameters than physico-chemical properties important indicators and thus could reflect the changes in soil properties after ecosystem restoration (Araujo et al., 2013; Zhang et al., 2015; Kabiri et al., 2016). Soil microbial biomass is indexed to measure the active components of soil organic matter (SOM). It is closely related to nutrient cycling and thus is extensively considered as an indicator of soil fertility and ecosystem productivity (Singh and Gupta, 2018). Soil enzymes are derived from the exudates of plant roots and microorganisms and the decomposition products of residues in the soil (Sinsabaugh et al., 2009; Joniec, 2018). In particular, β-glucosidase (GLU), invertase (INV), catalase (CAT) are the main enzymes in the cycling of soil C, while urease (URE) is a key enzyme in the cycling of soil N (Baddam et al., 2016; Zhao et al., 2018). Soil microbial properties can be affected by soil properties such as soil C and N content, pH, moisture content, bulk density, and nutrients (Kotroczó et al., 2014; Baddam et al., 2016; Wang et al., 2019). They can also be affected by plant properties such as plant biomass, species composition, and age (Yuan and Yue, 2012; Xu et al., 2020). Moreover, soil microbial properties can also be influenced by land use/cover change (Raiesi and Beheshti, 2014; Feng et al., 2019). For example, Babujia et al. (2010) and Zhang et al. (2018) found that soil microbial biomass and enzyme activities can be influenced by the ecosystem restoration after farmland abandonment.
Farmland abandonment accelerates plant recovery and increases the input of organic matter through above- and below-ground biomass (Novara et al., 2017; Romero-Díaz et al., 2017), which can increase the soil microbial biomass and enzyme activity (Jiang et al., 2009; Wang B. et al., 2011). Raiesi and Salek-Gilani (2018) showed that soil enzyme activities increased after 4–45 years of farmland abandonment. Feng et al. (2019) found that MBC and MBN contents and enzyme activities increased with restoration time in degraded forests, but some enzyme activities decreased after 11 year restoration. However, the effects of a long natural restoration time on soil microbial biomass and enzyme activities in abandoned reclaimed marshes after farmland abandonment are rarely reported. It is necessary to study the effects of marsh ecosystem on soil microbial properties after restoration, and help clarify the changes in SOM and soil function.
Sanjiang Plain is one of the most typical temperate marsh distribution areas in the world (Brinson and Malvárez, 2002). It is the region most severely affected by tillage and also the largest wetlands restoration area in China (Mao et al., 2018). We chose a restoration chronosequence (1, 4, 8, 13, 17, 27 years after soybean field abandonment), a soybean field (SF), and a natural marsh (NM) in Sanjiang Plain to investigate MBC and MBN contents and activities of four enzymes including GLU, INV, CAT, and URE. The objectives of this study were to: (1) assess how these microbial properties respond to restoration of reclaimed marshes after farmland abandonment; (2) investigate the relationships between soil microbial properties with soil physico-chemical and plant properties. We hypothesized that (1) soil MBC and MBN contents and the activities of GLU, INV, CAT, and URE will increase with restoration time and that (2) soil MBC and MBN contents and the activities of GLU, INV, CAT, and URE will have significant relationships with soil physico-chemical and plant properties.
Materials and Methods
Study Area Description
The study was conducted in July 2019 at the Sanjiang National Nature Reserve of Fuyuan City. GPS was used to locate the study area (48°3′37.97″–48°9′05.82″N, 134°31′46.66″–134°36′05.51″E), which is located in the Sanjiang Plain of Northeast China (Figure 1). The study area belongs to a temperate climate, with an annual average temperature of 2.52°C and precipitation of 558 mm (falling mainly from June to September) (Song et al., 2009). In the past 50 years, the natural wetland has decreased from 3.53 million hm2 to only 0.81 million hm2 in Sanjiang Plain, becoming one of the fastest reduction areas of natural wetland in China, with 91% of the reduced wetland being transformed into farmland (Song K. et al., 2014). The cropping pattern in this area is one crop a year, sowing in mid-May, harvesting in mid-October, and plowing in November. However, the area of restored wetlands has gradually increased since the 1990s owing to the establishment of wetland nature reserves in the Sanjiang Plain.
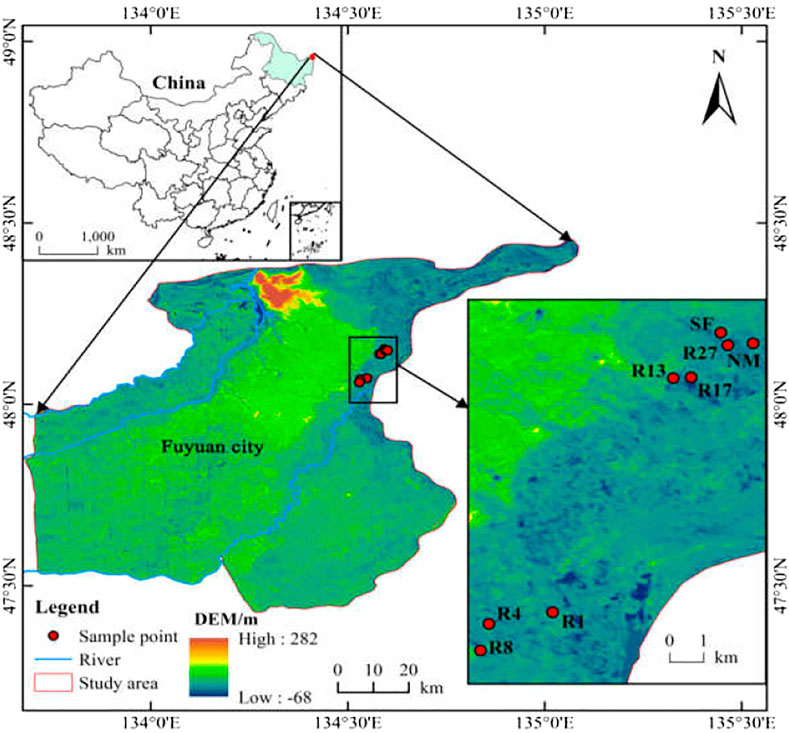
FIGURE 1. Location of the sampling sites in Sanjiang Plain, Northeast China. SF, soybean field; R1, R4, R8, R13, R17, and R27, restored sites after 1, 4, 8, 13, 17, and 27 years of farmland abandonment; NM, natural marsh.
Sampling Method
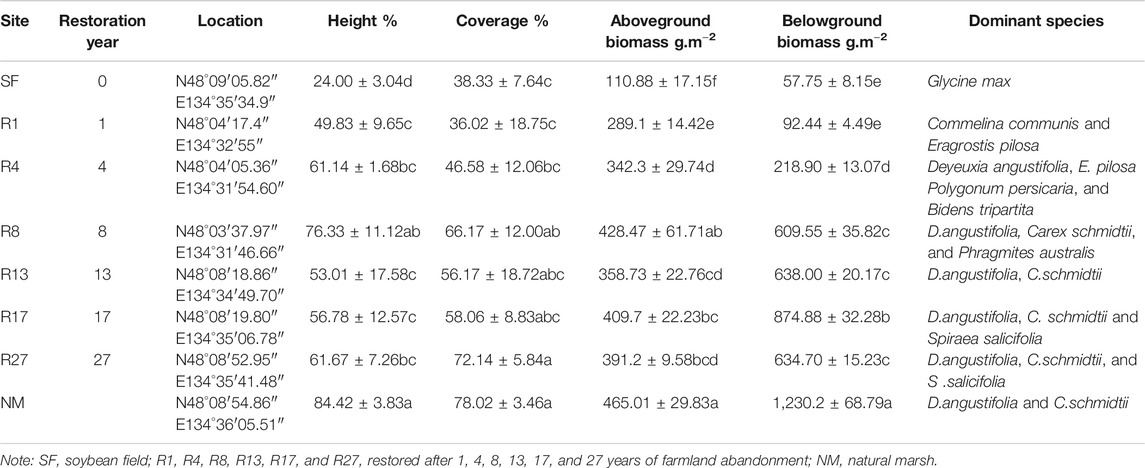
We selected eight sampling sites: one reclaimed marshland which has grown soybean field for more than ten years, six sites that have been abandoned for 1, 4, 8, 13, 17, and 27 years after growing soybeans for about 10 years, and one natural marsh. All the sites we selected are adjacent to rivers with similar hydrological conditions and topographies, which were formed due to alluviation. The plants in restored marshes showed an obvious transition trend from weed meadow (Commelina communis, Polygonum persicaria, Bidens tripartite, Echinochloa caudate) to Deyeuxia angustifolia and Carex schmidtii (Jin et al., 2020). The vegetation information of the study site is shown in Table 1. The soybean field and restored sites had been planted with soybeans for about 10 years before they were abandoned. Restored sites mainly rely on both the remaining seed bank of the restoration land and the hydrological conditions and vegetation of the natural marsh to achieve natural restoration. The natural marsh is dominated by two local typical wetland species of D.angustifolia-C.schmidtii. In each site, three 20 × 20 m plots were randomly set, and fifteen soil cores were collected by stainless steel sampler (5 cm diameter) after litter on the soil surface was removed. Each collected soil core with five layers (0–10 cm, 10–20 cm, 20–30 cm, 30–40 cm, 40–50 cm). The soil samples were stored in ziplock bags and brought back to the laboratory. Each soil sample was divided into two portions with one portion being stored at 4°C for the measurement of soil microbial biomass carbon and nitrogen (MBC and MBN) and available nitrogen (AN, NH4+-N and NO3−-N), and the other portion being air-dried for enzyme activity analyses and soil physico-chemical analyses (Ma et al., 2020).
In each sampling site, three 1 × 1 m quadrats were placed for the vegetation survey. Aboveground biomass was measured by harvesting all the aboveground plants. For belowground biomass in three quadrats, we obtained three complete cores of 0–50 cm depth at 10 cm intervals. The soil cores were put into 0.5 mm mesh sieve bags and cleaned. All plant samples were dried at 60°C to constant weight and weighed.
Laboratory Analysis
Soil bulk density (BD) was determined using the ring cutting method (5 cm inner diameter and 5 cm height). Soil moisture content (MC) was determined by drying the soil samples at 105°C for 24 h. Soil pH was determined using a potentiometric pH meter (SevenCompact S210, Swiss) (soil:water, 1:5). The total phosphorus (TP) was determined by the tcolorimetrical method with H2SO4-HClO4 as the digester. The available phosphorus (AP) was determinded by the colorimetrical method with HCl-H2SO4 as the digester. The total potassium (TK) was determined by acid fusion-flame spectrophotometry. Soil organic carbon (SOC) was determined by the dry combustion method and analyzed with a Multi N/C 2100 TOC analyzer (Analytik Jena, Germany). The available nitrogen (AN, NH4+-N and NO3−-N) was extracted with 1 mol/L KCl and then filtered. The total nitrogen (TN) was extracted by adding concentrated sulfuric acid and mixed catalyst to the soil sample, heating at high temperature, and then filtering. The filtrate of TN and AN was analyzed with an automatic continuous segmented flow analyzer (AA3, Seal Analytical, Germany). Soil MBC and MBN contents were determined by the fumigation-extraction method (Brookes et al., 1985; Vance et al., 1987). Soil information of the sampling site is shown in Table 2.
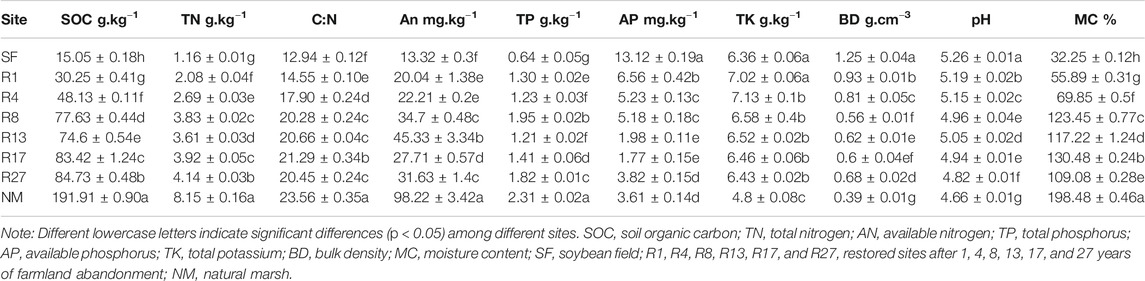
TABLE 2. Soil physico-chemical properties at the 0–50 cm depth in the eight sites (mean ± SD, n = 3).
Soil β-glucosidase (GLU) activity was assayed using the substrate analogue para-nitrophenyl-β-d-glucopyranoside and expressed as μg p-nitrophenol (PNP) g−1.h−1 (Eivazi and Tabatabai, 1988). Soil invertase (INV) and urease (URE) activities were measured using conventional colorimetric methods (Song Y. et al., 2014). Before INV activity determination, soil samples were incubated with 15 ml of 8% sucrose solution and 5 ml of phosphate buffer (pH 5.5) at 37°C for 24 h, and INV activity expressed as mg glucose g−1.24 h−1. Before URE activity determination, soil samples were incubated with 10 ml of 10% urea solution and 20 ml of citric acid buffer (pH 6.7) at 37°C for 24 h, and URE activity expressed as mg NH4+-N g−1.24 h−1. Soil catalase (CAT) activity was determined by shaking soil samples with H2O2 as substrate for 20 min, then back-titration with a standard solution of 0.1 N KMnO4 and expressed as a mL.g−1 dry sample at 20 min (Wang et al., 2012).
To better illustrate the influence of restoration of reclaimed marshes on soil enzyme activities, we calculated the geometric mean of enzymatic activities (GME), because it can reflect the overall enzyme activity levels (Hinojosa et al., 2004). The GME was calculated as follows:
where GLU, INV, CAT, and URE represent β-glucosidase, invertase, catalase, and urease, respectively.
Statistical Analysis
One-way ANOVA was performed by the least significant difference (LSD) test (p < 0.05) to analyze the differences in soil microbial biomass carbon and nitrogen, enzyme activities, soil physico-chemical properties, and plant properties across different sites. The statistical analyses were performed by SPSS ver. 20.0 (SPSS Inc. United States). We applied the single sample K-S test in SPSS and the variance homogeneity test in one-way ANOVA to test the normal distribution and variance homogeneity of the data, respectively. Spearman correlation matrix was used to examine the relationships of soil microbial biomass, enzyme activities, the GME with the properties of soil physico-chemical and plant via the package “corrplot” in R 3.5.0 software. The influences of soil physico-chemical and plant properties on soil microbial carbon and nitrogen, and soil enzyme activities were evaluated by redundancy analysis (RDA). RDA was conducted using the Canoco 5.0 software (Microcomputer Power Inc. Ithaca, NY).
Results
Soil MBC and MBN Contents
The restoration time had significant effects on MBC and MBN contents (p < 0.05, Figure 2). The average MBC and MBN contents had significant differences in all sites (p < 0.05, Figure 2C), except for the MBN content between R13 and R17 sites (p = 0.70). The average MBC content of restored sites increased with restoration time, except for soil MBC content in the R13 site which was lower than in the restored R8 site. MBN content increased before 8 years of restoration and then fluctuated. The MBC and MBN contents in the R1, R4, R8, R13, R17, and R27 sites were significantly higher than those in the soybean field (p < 0.05). After 27 years of restoration, the MBC and MBN contents were 15.7 and 3.2 times of those in the soybean field, respectively. The largest contents of MBC and MBN in the restored sites were 7.78%, 27.76% lower than those in natural marshes, respectively. Soil MBC and MBN contents decreased with soil depth at all sites (p < 0.05, Figures 2A,B). The highest contents of MBC (4,834.29 mg.kg−1) and MBN (373.27 mg.kg−1) appeared at 0–10 cm in the natural marsh, while the lowest contents of MBC (27.54 mg.kg−1) and MBN (10.71 mg.kg−1) appeared at 40–50 cm in the soybean field. The differences in MBC and MBN contents of different sites in the surface layers (0–30 cm) were more obvious than those in the bottom layers (30–50 cm). For example, the MBC and MBN contents showed no significant difference at 30–40 cm and 40–50 cm in the R4, R8, and R17 sites with the R1 site (p > 0.05).
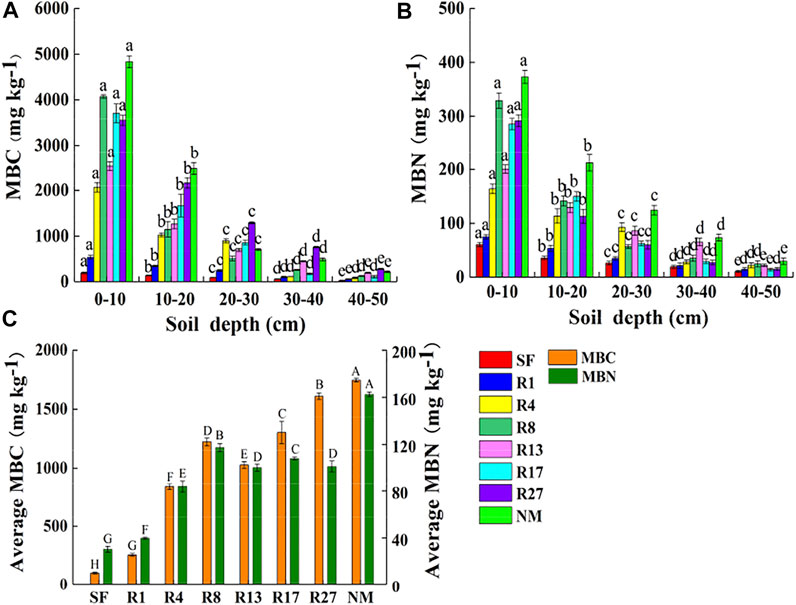
FIGURE 2. Distribution of MBC (A) and MBN (B) at 0–50 cm depth of soil, and the average MBC and MBN contents in different sites (C). SF, soybean field; R1, R4, R8, R13, R17, and R27, restored sites after 1, 4, 8, 13, 17, and 27 years of farmland abandonment; NM, natural marsh. Different lowercase letters in (A) and (B) indicate significant differences between different layers in the same site (p < 0.05). Different uppercase letters in (C) indicate significant differences of the average value of the five layers between different sites (p < 0.05).
Soil Enzyme Activities
The average activities of GLU, INV, CAT, and URE in the restored sites were significantly higher than in the soybean field but lower than in the natural marsh (p < 0.01, Figures 3E,F). After 27 years of restoration, the activities of GLU, INV, CAT, and URE were 3.5, 4.7, 2.7, 1.5 times of those in the soybean field, respectively. The largest activities of GLU, INV, CAT, and URE in the restored sites were 34.3, 26.74, 36.07, and 7.38% lower than those in natural marshes, respectively. A similar trend was observed in that the fluctuation of the average GLU, INV, and URE activities increased with restoration time (Figures 3E,F). However, the restoration rate of GLU and URE activities was fast in the first 8 years of restoration and then slowed down. The CAT activity increased with restoration time except for the R27 site, in which it declined compared to R17 (Figure 3F).
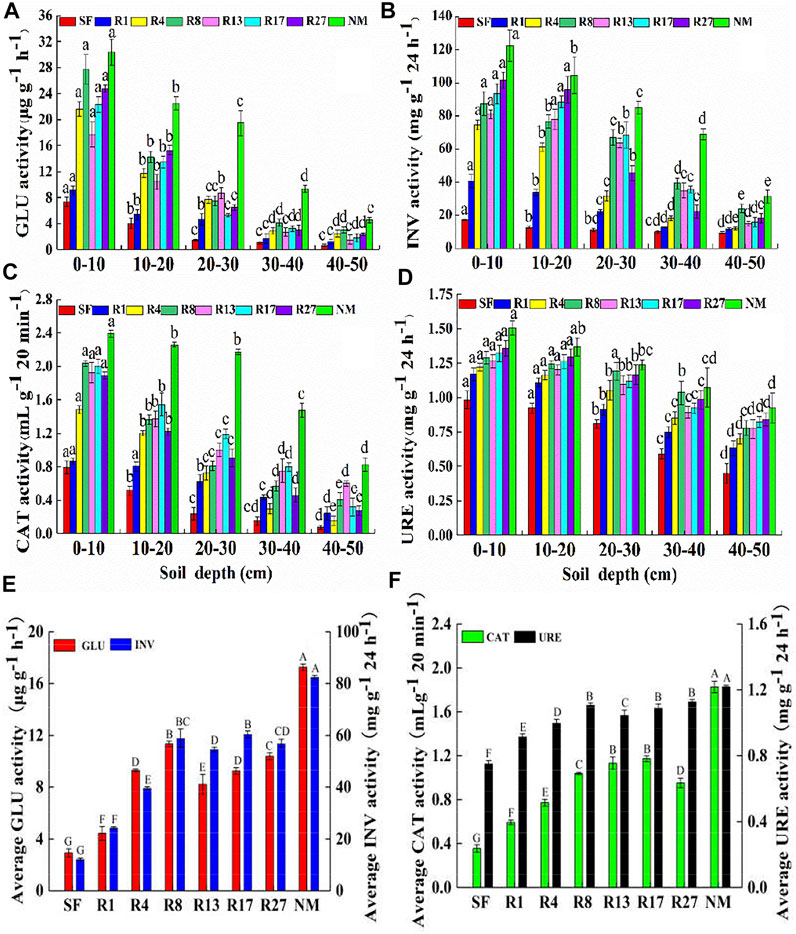
FIGURE 3. Distribution of soil enzyme activity at the soil 0–50 cm depth (A–D) and average soil enzyme activity of different sites (E–F). GLU, β-glucosidase; INV, invertase; CAT, catalase; URE, urease. SF, soybean field; R1, R4, R8, R13, R17, and R27, restored sites after 1, 4, 8, 13, 17, and 27 years of farmland abandonment; NM, natural marsh. Different lowercase letters in (A–D) indicate significant differences between different layers in the same site (p < 0.05). Different uppercase letters in (E–F) indicate significant differences of the average value of the five layers between different sites (p < 0.05).
The activities of GLU, INV, CAT, and URE decreased significantly with soil depths in all sites (Figures 3A–D). The highest activities of GLU, INV, CAT, and URE appeared at 0–10 cm in the natural marsh, which were 6.6, 3.9, 2.9, and 1.6 times than those in 40–50 cm of the same site, respectively. The highest GLU and CAT activities of restored sites appeared at 0–10 cm of the R8 site, which were 9.2 and 5.0 times than those in 40–50 cm of the same site, respectively (Figures 3A,C). The highest INV and URE activities of restored sites appeared at 0–10 cm in the R27 sites, which were 5.6 and 1.6 times than those in 40–50 cm of the same site, respectively (Figures 3B,D).
The GME was obtained based on the calculation of four soil enzyme activities in this study. The variation of GME was affected by restoration time and soil depth. The average GME had significant differences among all sites (p < 0.05, Figure 4B) except for the GME between R8 and R17 sites and between R17 and R27 sites. The growth rate was fast in the first 8 years of restoration and then slower down. After 27 years of restoration, the GME was 2.9 times than that in the soybean field, but was 31.15% lower than the natural marsh. The GME declined with soil depths (Figure 4A). The largest GME appeared at 0–10 in the natural marsh, which was 3.2 times than that in 40–50 cm of the same site. The highest GME of restored sites appeared at 0–10 cm in the R27 sites, which were 5.0 times than that in 40–50 cm of the same site.
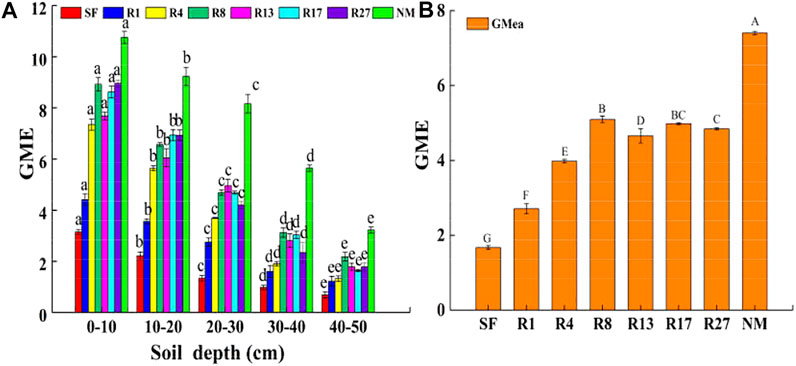
FIGURE 4. Distribution of the geometric mean of enzyme activity (GME) at the soil 0–50 cm depth (A) and the average GME in different sites (B). SF, soybean field; R1, R4, R8, R13, R17, and R27, restored sites after 1, 4, 8, 13,17, and 27 years of farmland abandonment; NM, natural marsh. Different lowercase letters in (A) indicate significant differences between different layers in the same site (p < 0.05). Different uppercase letters in (B) indicate significant differences of the average value of the five layers between different sites (p < 0.05).
Relationships of Soil Microbial Properties With Environmental Factors
The relationships of soil microbial biomass, enzyme activities with environmental factors (soil physico-chemical and plant properties) were shown by the Spearman rank correlation matrix (Figure 5). Soil microbial biomass, enzyme activities, and GME were negatively correlated with BD, pH, AP (p < 0.001), and TK (p < 0.05). Among enzyme activities, GLU activity was negatively related to AP (p < 0.05), and its relationship with TK was negative but not significantly.
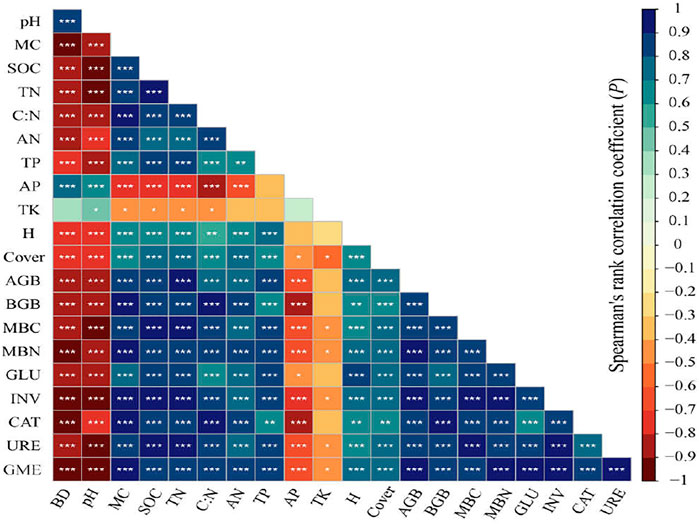
FIGURE 5. Correlation matrix of soil microbial biomass, enzyme activities, soil physico-chemical properties, and plant properties. The correlation coefficient (r-value) is represented by different colors. ***p < 0.001; **p < 0.01; *p < 0.05. BD, bulk density; MC, moisture content; SOC, soil organic carbon; TN, total nitrogen; AN, available nitrogen; TP, total phosphorus; AP, available phosphorus; TK, total potassium; H, plant height, Cover, plant coverage; AGB, aboveground biomass; BGB, belowground biomass; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; GLU, β-glucosidase; INV, invertase; CAT, catalase; URE, urease; GME, geometric mean of enzyme activity.
Soil MBC and MBN contents, and enzyme activities were positively correlated with MC, SOC, TN, C:N ratio, AN, TP, AGB, BGB, H, and Cover (p < 0.001). The first two axes of the RDA (Figure 6) accounted for 99.6% of the MBC and MBN variance, with the first axis accounting for 99.54% of the variance (Figure 6A). Soil C:N ratio, pH, MC were the most important factors affecting soil MBC and MBN contents, explained 98.6% of the total variance. The first two axes of the RDA accounted for 99.68% of the enzyme activities variance, with the first axis accounting for 99.24% of the variance (Figure 6B). Soil C:N ratio, TN, and MC were the most important factors affecting soil enzyme activities, explained 98.9% of the total variance.
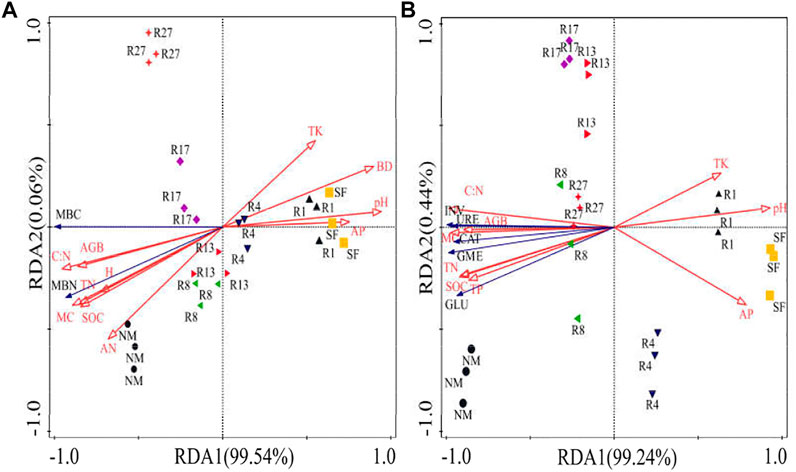
FIGURE 6. Redundancy analysis (RDA) of the effect of soil physico-chemical properties and plant properties on soil microbial biomass (A) and enzyme activities (B). BD, bulk density; MC, moisture content; SOC, soil organic carbon; TN, total nitrogen; AN, available nitrogen; TP, total phosphorus; AP, available phosphorus; TK, total potassium; AGB, aboveground biomass; H, plant height; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; GLU, β-glucosidase; INV, invertase; CAT, catalase; URE, urease; GME, geometric mean of enzyme activity.
Discussion
Response of Soil Microbial Biomass to Restoration of Reclaimed Marshes
Our results showed an increasing trend of MBC and MBN with restoration time (Figure 2). The results support part of our first hypothesis that soil microbial biomass will increase over restoration time. This result is consistent with previous studies (Song et al., 2012; Zhang et al., 2018; Feng et al., 2019; Morales-Londoño et al., 2019) on the changes of soil microbial biomass in the process of ecosystem restoration after abandonment. Song et al. (2012) reported that the soil MBC content increased in the restored sites with restoration time after 12 years of farmland abandonment. Several recent studies also reported the increase of soil MBC and MBN contents after the ecosystem restoration (Zhang et al., 2018; Feng et al., 2019; Morales-Londoño et al., 2019). Our study also showed an increasing trend of soil MBC and MBN during the 27 years of restoration. However, after 27 years of restoration, the MBC and MBN contents were still lower than those of the natural marsh. Soil MBC and MBN contents increased with restoration time may be attributed to the following reasons. Firstly, the restoration of vegetation increased plant coverage and above and below ground biomass (Table 1), which caused the increase of SOM and nutrient elements availability (Table 2), thus improving the soil microbial environment and the soil microbial process (Allison and Jastrow, 2006; Wang et al., 2017; Li et al., 2020). Secondly, the increasing MC of restored sites creates an anaerobic condition of the soil, resulting in lower decomposition of SOM after the farmland abandonment (Yang et al., 2019), which is conducive to carbon and nitrogen accumulation. MBC and MBN were basic fractions of soil active carbon and nitrogen pools and were positively related to SOM (Schnürer et al., 1985). Therefore, the increase of carbon and nitrogen is beneficial to the increase of microbial biomass. The variation of MBC and MBN contents after farmland abandonment indicates that they can be used as sensitive indicators of ecosystem response to the natural restoration of reclaimed marshes.
The contents of MBC and MBN decreased with soil depth in all sites, which is consistent with findings of the recent studies (Feng et al., 2019; Mgelwa et al., 2019). This result may be related to the decrease of the substrate input of plant residues (such as roots and secretions) reduced with soil depth, which directly caused the MBC and MBN contents with soil depth (Wichern et al., 2003).
Response of Soil Enzyme Activities to Restoration of Reclaimed Marshes
Our results of soil enzyme activities partly supported our first hypothesis that soil enzyme activities of the restored sites would increase with restoration time though there were fluctuations in the later stage of restoration. Our results are similar to previous studies that soil enzyme activities increased during natural restoration after farmland abandonment in the restored rangeland ecosystems (Raiesi and Salek-Gilani, 2018). Plant restoration after farmland abandonment had a positive effect on the enzyme activities which was due to increased organic matter input and improved soil physico-chemical and microbial properties (Cao et al., 2008; Shang et al., 2014). Moreover, continuous and abundant input of organic matter can provide sufficient nutrients for the growth of microorganisms and also increase the surface adsorption of organic matter by enzymes and their substrates (Raiesi and Salek-Gilani, 2018; Yu et al., 2019). Our results indicate that even after 27 years of farmland abandonment, the four enzyme activities in the restored sites still could not reach the level of the natural marsh. The results are similar to a recent study showed that soil enzyme activities after 45 years of farmland abandonment were lower than those in the natural sites (Raiesi and Salek-Gilani, 2018). It may take a longer time (or hundreds of years) for the soil enzyme activities of abandoned farmland to recover to the level of natural marshes, because the hydrology, soil, and vegetation of these restored sites have not recovered to the same level as those of natural marshes.
Our results also showed that there were significant differences of soil enzyme activities across the five soil depths in all sites (p < 0.05, Figures 3A–D). The soil enzyme activities decreased with soil depth in all sites, which were consistent with the previous reports (Zhang et al., 2015; Bai et al., 2018). This result was related to the fact that there are more organic matter and plant roots in the surface soil depth than in the deeper soil, which leads to the decline of the enzyme activities with soil depth (Xiao et al., 2015; Ma et al., 2020).
Similar to the four enzyme activities, the GME was also higher in the restored sites than that in the soybean field but lower than in the natural marsh. This result also responses to the finding by Raiesi and Salek-Gilani (2018) that the GME increased with farmland abandonment time. Besides, the growth rate of GME was faster in the early stage than in the later stage of restoration. The result may be due to fluctuation of the plant biomass, SOC and TN contents in the later stage of restoration (Table 1 and 2) because they are the main sources of nutrients and energy for the survival of soil microorganisms. Compared with a single enzyme, the GME has a more stable temporal variability (Paz-Ferreiro and Fu, 2016), which can better reflect the relationship between soil enzyme activities and environmental factors in the process of restoration.
Relationships of Soil Microbial Properties With Soil Physico-Chemical and Plant Properties
Natural restoration after farmland abandonment had great influences on the soil physico-chemical properties, plant properties, and microbial properties (Li et al., 2020; Zhang et al., 2016). In our restoration chronosequence, plant biomass, height, coverage, and nutrients content significantly increased compared to the soybean field (Table 1 and 2), which is consistent with the previous studies (Li et al., 2018; Marton et al., 2014b; Wang H. et al., 2011). In this study, soil MBC, MBN, the four soil enzyme activities, and the GME were positively correlated with MC, SOC, TN, C:N ratio, AN, TP, AGB, BGB, H, and Cover (p < 0.001), and were negatively correlated with BD, pH (p < 0.001) (Figure 5). These results support our second hypothesis that soil microbial biomass and enzyme activities have significant relationships with soil physico-chemical characteristics and plant properties. Wang et al. (2020) also found that soil GLU, URE, CAT were positively related to SOC, TN, TP, MC (p < 0.01), but INV was only positively related to SOC (p < 0.01) and TN (p < 0.01). Li et al. (2018) found that soil microbial biomass and enzyme activities had significant positive relationships with SOC, TN, and negative relationships with BD, and pH. Plant characteristics (biomass, height, coverage) also had significant effects on soil enzyme activities (Araujo et al., 2013; Qiang et al., 2020). RDA showed that among these affecting factors of soil and plant properties, the C:N ratio, pH, and MC were crucial explanatory factors affecting soil MBC and MBN contents (Figure 6A). The C:N ratio, TN, and TP were crucial explanatory factors affecting the soil enzyme activities and the GME (Figure 6B).
Soil C, N, and P can regulate the available nutrients for soil microbes, thus affecting the microbial properties and the changes in the soil C: N stoichiometry during the process of restoration of reclaimed marshes. The C:N ratio can reflect the degree of decomposition of SOM, and a high soil C:N ratio can slow down the decomposition rate of SOM, which is beneficial to the accumulation of carbon and nitrogen (Baisden et al., 2002; Marty et al., 2017). Soil MC as the main property of marsh plays an important role in restoration of reclaimed marshes. With the increase of marsh MC, the permeability of soil becomes weaker, which can depress soil respiration, inhibiting organic carbon decomposition (Pan et al., 2015), thus gradually minimizing the difference in hydrology conditions between restored marshes and natural marshes (Yang et al., 2019). Soil MC could also affect the production and turnover of enzymes by mediating the microbial biomass content (Steinweg et al., 2013). Soil pH can not only regulate the decomposition and mineralization of SOM but also influences the species and activities of microorganisms and the rate of soil enzymes participating in biochemical reactions (Dick et al., 2000). The decrease of soil pH will reduce the decomposition rate of soil organic matter (Mazurczyk and Brooks, 2018). Therefore, high soil C:N ratio, MC, and low pH contribute to the increase of soil microbial biomass content and enzyme activities in this study.
Conclusion
This research provided evidence for the responses of soil microbial biomass and enzyme activities to national restoration of reclaimed marshes. Compared with the soybean field, restoration of reclaimed marshes significantly increased the soil MBC and MBN contents, soil enzyme activities, and the GME of the restored sites. The MBC content increased with restoration time and the MBN content increased in the first 8 years of restoration and then slowed down in the studied sites. Generally, the GLU, INV, CAT activities, and the GME increased in the first 8 years of restoration and then fluctuated. The CAT activity increased in the first 17 years of restoration and then decreased in the R27 site. However, the MBC and MBN contents, soil enzyme activities of all these restored sites were lower than the natural marsh. Our results indicate that soil microbial properties can be gradually restored through natural restoration, but it may take a long time. We found that in the observed environmental factors, soil C:N ratio, pH, MC, TN, and TP were the key factors affecting soil microbial biomass and enzyme activities.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
CW: Methodology, Software, Formal analysis, Investigation, Writing-Original draft preparation and Writing-Reviewing and Editing. HL: Investigation. XS: Resources, Supervision, Project administration, Funding acquisition and Reviewing. TC: Supervision.
Funding
This research was funded by the National Natural Science Foundation of China (No. 31870443), The Natural Science Foundation of Heilongjiang Province of China (No. LH 2020C033), and Central Universities Basic Fund of China (No. 2572020BA06).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Qingbo Wang, Di Wu, Chenglin Liu for their support in field sampling. We also thank Sen Lu, Mei Gao for their assistance in laboratory analysis.
References
Allison, S. D., and Jastrow, J. D. (2006). Activities of Extracellular Enzymes in Physically Isolated Fractions of Restored Grassland Soils. Soil Biol. Biochem. 38, 3245–3256. doi:10.1016/j.soilbio.2006.04.011
Araújo, A. S. F., Cesarz, S., Leite, L. F. C., Borges, C. D., Tsai, S. M., and Eisenhauer, N. (2013). Soil Microbial Properties and Temporal Stability in Degraded and Restored Lands of Northeast Brazil. Soil Biol. Biochem. 66, 175–181. doi:10.1016/j.soilbio.2013.07.013
Babujia, L. C., Hungria, M., Franchini, J. C., and Brookes, P. C. (2010). Microbial Biomass and Activity at Various Soil Depths in a Brazilian Oxisol after Two Decades of No-Tillage and Conventional Tillage. Soil Biol. Biochem. 42, 2174–2181. doi:10.1016/j.soilbio.2010.08.013
Baddam, R., Reddy, G. B., Raczkowski, C., and Cyrus, J. S. (2016). Activity of Soil Enzymes in Constructed Wetlands Treated with Swine Wastewater. Ecol. Eng. 91, 24–30. doi:10.1016/j.ecoleng.2016.01.021
Bai, J., Xiao, R., Zhang, K., Gao, H., Cui, B., and Liu, X. (2013). Soil Organic Carbon as Affected by Land Use in Young and Old Reclaimed Regions of a Coastal Estuary Wetland, China. Soil Use Manag. 29, 57–64. doi:10.1111/sum.12021
Bai, X., Zeng, Q., Fakher, A., Dong, Y., and An, S. (2018). Characteristics of Soil Enzyme Activities and Microbial Biomass Carbon and Nitrogen under Different Vegetation Zones on the Loess Plateau, China. Arid Land Res. Manage. 32, 438–454. doi:10.1080/15324982.2018.1501621
Baisden, W. T., Amundson, R., Cook, A. C., and Brenner, D. L. (2002). Turnover and Storage of C and N in Five Density Fractions from California Annual Grassland Surface Soils. Glob. Biogeochem. Cycles 16, 64–71. doi:10.1029/2001GB001822
Brinson, M. M., and Malvárez, A. I. (2002). Temperate Freshwater Wetlands: Types, Status, and Threats. Envir. Conserv. 29, 115–133. doi:10.1017/S0376892902000085
Brookes, P. C., Landman, A., Pruden, G., and Jenkinson, D. S. (1985). Chloroform Fumigation and the Release of Soil Nitrogen: A Rapid Direct Extraction Method to Measure Microbial Biomass Nitrogen in Soil. Soil Biol. Biochem. 17, 837–842. doi:10.1016/0038-0717(85)90144-0
Cao, C., Jiang, D., Teng, X., Jiang, Y., Liang, W., and Cui, Z. (2008). Soil Chemical and Microbiological Properties along a Chronosequence of Caragana Microphylla Lam. Plantations in the Horqin sandy Land of Northeast China. Appl. Soil Ecol. 40, 78–85. doi:10.1016/j.apsoil.2008.03.008
Dick, W. A., Cheng, L., and Wang, P. (2000). Soil Acid and Alkaline Phosphatase Activity as pH Adjustment Indicators. Soil Biol. Biochem. 32, 1915–1919. doi:10.1016/S0038-0717(00)00166-8
Eivazi, F., and Tabatabai, M. A. (1988). Glucosidases and Galactosidases in Soils. Soil Biol. Biochem. 20, 601–606. doi:10.1016/0038-0717(88)90141-1
Euliss, N. H., Gleason, R. A., Olness, A., McDougal, R. L., Murkin, H. R., Robarts, R. D., et al. (2006). North American Prairie Wetlands Are Important Nonforested Land-Based Carbon Storage Sites. Sci. Total Environ. 361, 179–188. doi:10.1016/j.scitotenv.2005.06.007
Feng, C., Ma, Y., Jin, X., Wang, Z., Ma, Y., Fu, S., et al. (2019). Soil Enzyme Activities Increase Following Restoration of Degraded Subtropical Forests. Geoderma 351, 180–187. doi:10.1016/j.geoderma.2019.05.006
Hinojosa, M. B., García-Ruíz, R., Viñegla, B., and Carreira, J. A. (2004). Microbiological Rates and Enzyme Activities as Indicators of Functionality in Soils Affected by the Aznalcóllar Toxic Spill. Soil Biol. Biochem. 36, 1637–1644. doi:10.1016/j.soilbio.2004.07.006
Jiang, J.-P., Xiong, Y.-C., Jiang, H.-M., Ye, D.-Y., Song, Y.-J., and Li, F.-M. (2009). Soil Microbial Activity during Secondary Vegetation Succession in Semiarid Abandoned Lands of Loess Plateau. Pedosphere 19, 735–747. doi:10.1016/S1002-0160(09)60169-7
Jiang, T.-T., Pan, J.-F., Pu, X.-M., Wang, B., and Pan, J.-J. (2015). Current Status of Coastal Wetlands in China: Degradation, Restoration, and Future Management. Estuarine, Coastal Shelf Sci. 164, 265–275. doi:10.1016/j.ecss.2015.07.046
Jin, X., Sun, X., Li, H., Zhao, D., Li, D., Wang, L., et al. (2020). Changes of Plant Species Diversity and Biomass with Reclaimed Marshes Restoration. J. For. Res. 32, 133–142. doi:10.1007/s11676-020-01104-y
Joniec, J. (2018). Enzymatic Activity as an Indicator of Regeneration Processes in Degraded Soil Reclaimed with Various Types of Waste. Int. J. Environ. Sci. Technol. 15, 2241–2252. doi:10.1007/s13762-017-1602-x
Kabiri, V., Raiesi, F., and Ghazavi, M. A. (2016). Tillage Effects on Soil Microbial Biomass, SOM Mineralization and Enzyme Activity in a Semi-arid Calcixerepts. Agric. Ecosyst. Environ. 232, 73–84. doi:10.1016/j.agee.2016.07.022
Kotroczó, Z., Veres, Z., Fekete, I., Krakomperger, Z., Tóth, J. A., Lajtha, K., et al. (2014). Soil Enzyme Activity in Response to Long-Term Organic Matter Manipulation. Soil Biol. Biochem. 70, 237–243. doi:10.1016/j.soilbio.2013.12.028
Li, J., Shangguan, Z., and Deng, L. (2020). Dynamics of Soil Microbial Metabolic Activity during Grassland Succession after farmland Abandonment. Geoderma 363, 114167. doi:10.1016/j.geoderma.2019.114167
Li, J., Tong, X., Awasthi, M. K., Wu, F., Ha, S., Ma, J., et al. (2018). Dynamics of Soil Microbial Biomass and Enzyme Activities along a Chronosequence of Desertified Land Revegetation. Ecol. Eng. 111, 22–30. doi:10.1016/j.ecoleng.2017.11.006
Li, J., Zhou, X., Yan, J., Li, H., and He, J. (2015). Effects of Regenerating Vegetation on Soil Enzyme Activity and Microbial Structure in Reclaimed Soils on a Surface Coal Mine Site. Appl. Soil Ecol. 87, 56–62. doi:10.1016/j.apsoil.2014.11.010
Ma, W., Li, G., Wu, J., Xu, G., and Wu, J. (2020). Response of Soil Labile Organic Carbon Fractions and Carbon-Cycle Enzyme Activities to Vegetation Degradation in a Wet Meadow on the Qinghai-Tibet Plateau. Geoderma 377, 114565. doi:10.1016/j.geoderma.2020.114565
Mao, D., Luo, L., Wang, Z., Wilson, M. C., Zeng, Y., Wu, B., et al. (2018). Conversions between Natural Wetlands and farmland in China: a Multiscale Geospatial Analysis. Sci. Total Environ. 634, 550–560. doi:10.1016/j.scitotenv.2018.04.009
Marton, J. M., Fennessy, M. S., and Craft, C. B. (2014a). Functional Differences between Natural and Restored Wetlands in the Glaciated Interior Plains. J. Environ. Qual. 43, 409–417. doi:10.2134/jeq2013.04.0118
Marton, J. M., Fennessy, M. S., and Craft, C. B. (2014b). USDA Conservation Practices Increase Carbon Storage and Water Quality Improvement Functions: an Example from Ohio. Restor. Ecol. 22, 117–124. doi:10.1111/rec.12033
Marty, C., Houle, D., Gagnon, C., and Courchesne, F. (2017). The Relationships of Soil Total Nitrogen Concentrations, Pools and C:N Ratios with Climate, Vegetation Types and Nitrate Deposition in Temperate and Boreal Forests of Eastern Canada. Catena 152, 163–172. doi:10.2134/jeq2013.04.011810.1016/j.catena.2017.01.014
Mazurczyk, T., and Brooks, R. P. (2018). Carbon Storage Dynamics of Temperate Freshwater Wetlands in Pennsylvania. Wetlands Ecol. Manage. 26, 893–914. doi:10.1007/s11273-018-9619-6
Mgelwa, A. S., Hu, Y.-L., Xu, W.-B., Ge, Z.-Q., and Yu, T.-W. (2019). Soil Carbon and Nitrogen Availability Are Key Determinants of Soil Microbial Biomass and Respiration in Forests along Urbanized Rivers of Southern China. Urban For. Urban Green. 43, 126351. doi:10.1016/j.ufug.2019.05.013
Morales-Londoño, D. M., Meyer, E., Kunze, A., Gonzalez, D., Prieto-Benavides, O. O., Armas, R. D., et al. (2019). Are Microbial Activity and Arbuscular Mycorrhizal Fungal Community Influenced by Regeneration Stages? A Case Study in Southern Brazil Coastal Atlantic Rain Forest. Appl. Soil Ecol. 138, 94–98. doi:10.1016/j.apsoil.2019.02.028
Novara, A., Gristina, L., Sala, G., Galati, A., Crescimanno, M., Cerdà, A., et al. (2017). Agricultural Land Abandonment in Mediterranean Environment Provides Ecosystem Services via Soil Carbon Sequestration. Sci. Total Environ. 576, 420–429. doi:10.1016/j.scitotenv.2016.10.123
Pan, T., Zeng, L., Zeng, C., and Wang, W. (2015). Effects of Spartina Alterniflora Invasion on Soil Organic Carbon in the Bare Tidal Flat Wetland of Minjiang River Estuary. Sci. Soil Water Conserv. 13, 84–90. (in Chinese). doi:10.16843/j.sswc.2015.01.013
Paz‐Ferreiro, J., and Fu, S. (2016). Biological Indices for Soil Quality Evaluation: Perspectives and Limitations. Land Degrad. Dev. 27, 14–25. doi:10.1002/ldr.2262
Qi, Q., Zhang, D., Zhang, M., Tong, S., Wang, W., and An, Y. (2021). Spatial Distribution of Soil Organic Carbon and Total Nitrogen in Disturbed Carex Tussock Wetland. Ecol. Indicators 120, 106930. doi:10.1016/j.ecolind.2020.106930
Qiang, W., Yang, B., Liu, Y., Qi, K., Yang, T., and Pang, X. (2020). Effects of Reclamation Age on Soil Microbial Communities and Enzymatic Activities in the Sloping Citrus Orchards of Southwestern China. Appl. Soil Ecol. 152, 103566. doi:10.1016/j.apsoil.2020.103566
Raiesi, F., and Beheshti, A. (2014). Soil Specific Enzyme Activity Shows More Clearly Soil Responses to Paddy rice Cultivation Than Absolute Enzyme Activity in Primary Forests of Northwest Iran. Appl. Soil Ecol. 75, 63–70. doi:10.1016/j.apsoil.2013.10.012
Raiesi, F., and Salek-Gilani, S. (2018). The Potential Activity of Soil Extracellular Enzymes as an Indicator for Ecological Restoration of Rangeland Soils after Agricultural Abandonment. Appl. Soil Ecol. 126, 140–147. doi:10.1016/j.apsoil.2018.02.022
Romero-Díaz, A., Ruiz-Sinoga, J. D., Robledano-Aymerich, F., Brevik, E. C., and Cerdà, A. (2017). Ecosystem Responses to Land Abandonment in Western Mediterranean Mountains. Catena 149, 824–835. doi:10.1016/j.catena.2016.08.013
Schnürer, J., Clarholm, M., and Rosswall, T. (1985). Microbial Biomass and Activity in an Agricultural Soil with Different Organic Matter Contents. Soil Biol. Biochem. 17, 611–618. doi:10.1016/0038-0717(85)90036-7
Shang, Z.-H., Cao, J.-J., Guo, R.-Y., Long, R.-J., and Deng, B. (2014). The Response of Soil Organic Carbon and Nitrogen 10years after Returning Cultivated alpine Steppe to Grassland by Abandonment or Reseeding. Catena 119, 28–35. doi:10.1016/j.catena.2014.03.006
Singh, J. S., and Gupta, V. K. (2018). Soil Microbial Biomass: a Key Soil Driver in Management of Ecosystem Functioning. Sci. Total Environ. 634, 497–500. doi:10.1016/j.scitotenv.2018.03.373
Sinsabaugh, R. L., Hill, B. H., and Follstad Shah, J. J. (2009). Ecoenzymatic Stoichiometry of Microbial Organic Nutrient Acquisition in Soil and Sediment. Nature 462, 795–798. doi:10.1038/nature08632
Song, C., Xu, X., Tian, H., and Wang, Y. (2009). Ecosystem-Atmosphere Exchange of CH4 and N2O and Ecosystem Respiration in Wetlands in the Sanjiang Plain, Northeastern China. Glob. Change Biol. 15, 692–705. doi:10.1111/j.1365-2486.2008.01821.x
Song, K., Wang, Z., Du, J., Liu, L., Zeng, L., and Ren, C. (2014). Wetland Degradation: its Driving Forces and Environmental Impacts in the Sanjiang Plain, China. Environ. Manage. 54, 255–271. doi:10.1007/s00267-014-0278-y
Song, Y., Song, C., Tao, B., Wang, J., Zhu, X., and Wang, X. (2014). Short-term Responses of Soil Enzyme Activities and Carbon Mineralization to Added Nitrogen and Litter in a Freshwater Marsh of Northeast China. Eur. J. Soil Biol. 61, 72–79. doi:10.1007/10.1016/j.ejsobi.2014.02.001
Song, Y., Song, C., Yang, G., Miao, Y., Wang, J., and Guo, Y. (2012). Changes in Labile Organic Carbon Fractions and Soil Enzyme Activities after Marshland Reclamation and Restoration in the Sanjiang Plain in Northeast China. Environ. Manage. 50, 418–426. doi:10.1007/s00267-012-9890-x
Sousa, R. F. d., Fernandes Brasil, E. P., de Figueiredo, C. C., and Leandro, W. M. (2015). Soil Microbial Biomass and Activity in Wetlands Located in Preserved and Disturbed Environments in the Cerrado Biome. Biosci. J. 31, 1049–1061. doi:10.14393/BJ-v31n4a2015-26176
Steinweg, J. M., Dukes, J. S., Paul, E. A., and Wallenstein, W. D. (2013). Microbial Responses to Multi-Factor Climate Change: Effects on Soil Enzymes. Front. Microbiol. 4, 146. doi:10.3389/fmicb.2013.00146
Vance, E. D., Brookes, P. C., and Jenkinson, D. S. (1987). An Extraction Method for Measuring Soil Microbial Biomass C. Soil Biol. Biochem. 19, 703–707. doi:10.1016/0038-0717(87)90052-6
Walker, L. R., Walker, J., and Hobbs, R. J. (2007). Linking Restoration and Ecological Succession. Netherlands: Springer, 5–21.
Wang, B., Liu, G. B., Xue, S., and Zhu, B. (2011). Changes in Soil Physico-Chemical and Microbiological Properties during Natural Succession on Abandoned farmland in the Loess Plateau. Environ. Earth Sci. 62, 915–925. doi:10.1007/s12665-010-0577-4
Wang, B., Xue, S., Liu, G. B., Zhang, G. H., Li, G., and Ren, Z. P. (2012). Changes in Soil Nutrient and Enzyme Activities under Different Vegetations in the Loess Plateau Area, Northwest China. Catena 92, 186–195. doi:10.1016/j.catena.2011.12.004
Wang, L., Pang, X., Li, N., Qi, K., Huang, J., and Yin, C. (2020). Effects of Vegetation Type, fine and Coarse Roots on Soil Microbial Communities and Enzyme Activities in Eastern Tibetan Plateau. Catena 194, 104694. doi:10.1016/j.catena.2020.104694
Wang, L., Yan, B., Prasher, S. O., Ou, Y., Bian, Y., and Cui, H. (2019). The Response of Microbial Composition and Enzyme Activities to Hydrological Gradients in a Riparian Wetland. J. Soils Sediments 19, 4031–4041. doi:10.1007/s11368-019-02373-9
Wang, Y., Ji, H., Wang, R., Guo, S., and Gao, C. (2017). Impact of Root Diversity upon Coupling between Soil C and N Accumulation and Bacterial Community Dynamics and Activity: Result of a 30 Year Rotation experiment. Geoderma 292, 87–95. doi:10.1016/j.geoderma.2017.01.014
Wang, H., Wang, R., Yu, Y., Mitchell, M. J., and Zhang, L. (2011). Soil Organic Carbon of Degraded Wetlands Treated with Freshwater in the Yellow River Delta, China. J. Environ. Manage. 92, 2628–2633. doi:10.1016/j.jenvman.2011.05.030
Wichern, F., Richter, C., and Joergensen, R. G. (2003). Soil Fertility Breakdown in a Subtropical South African Vertisol Site Used as a home Garden. Biol. Fertil. Soils. 37, 288–294. doi:10.1007/s00374-003-0596-3
Xiao, Y., Huang, Z., and Lu, X. (2015). Changes of Soil Labile Organic Carbon Fractions and Their Relation to Soil Microbial Characteristics in Four Typical Wetlands of Sanjiang Plain, Northeast China. Ecol. Eng. 82, 381–389. doi:10.1016/j.ecoleng.2015.05.015
Xu, J., Liu, B., Qu, Z.-L., Ma, Y., and Sun, H. (2020). Age and Species of Eucalyptus Plantations Affect Soil Microbial Biomass and Enzymatic Activities. Microorganisms 8, 811. doi:10.3390/microorganisms8060811
Yang, L., Jiang, M., Zhu, W., Han, L., and Qin, L. (2019). Soil Bacterial Communities with an Indicative Function Response to Nutrients in Wetlands of Northeastern China that Have Undergone Natural Restoration. Ecol. Indicators 101, 562–571. doi:10.1016/j.ecolind.2019.01.037
Yu, P., Tang, X., Zhang, A., Fan, G., and Liu, S. (2019). Responses of Soil Specific Enzyme Activities to Short-Term Land Use Conversions in a Salt-Affected Region, Northeastern China. Sci. Total Environ. 687, 939–945. doi:10.1016/j.scitotenv.2019.06.171
Yu, S., Cui, B., Gibbons, P., Yan, J., Ma, X., Xie, T., et al. (2017). Towards a Biodiversity Offsetting Approach for Coastal Land Reclamation: Coastal Management Implications. Biol. Conservation 214, 35–45. doi:10.1016/j.biocon.2017.07.016
Yuan, B.-C., and Yue, D.-X. (2012). Soil Microbial and Enzymatic Activities across a Chronosequence of Chinese pine Plantation Development on the Loess Plateau of China. Pedosphere 22, 1–12. doi:10.1016/S1002-0160(11)60186-0
Zahawi, R. A., Reid, J. L., and Holl, K. D. (2014). Hidden Costs of Passive Restoration. Restor. Ecol. 22, 284–287. doi:10.1111/rec.12098
Zhang, C., Liu, G., Xue, S., and Wang, G. (2016). Soil Bacterial Community Dynamics Reflect Changes in Plant Community and Soil Properties during the Secondary Succession of Abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 97, 40–49. doi:10.1016/j.soilbio.2016.02.013
Zhang, W., Qiao, W., Gao, D., Dai, Y., Deng, J., Yang, G., et al. (2018). Relationship between Soil Nutrient Properties and Biological Activities along a Restoration Chronosequence of Pinus Tabulaeformis Plantation Forests in the Ziwuling Mountains, China. Catena 161, 85–95. doi:10.1016/j.catena.2017.10.021
Zhang, Y. L., Chen, L. J., Chen, X. H., Tan, M. L., Duan, Z. H., Wu, Z. J., et al. (2015). Response of Soil Enzyme Activity to Long-Term Restoration of Desertified Land. Catena 133, 64–70. doi:10.1016/j.catena.2015.04.012
Zhao, J., Gong, L., An, S., Li, Y., and Chen, X. (2018). Correlation between Soil Organic and Inorganic Carbon and Environmental Factors in Cotton fields in Different Continuous Cropping Years in the Oasis of the Northern Tarim Basin. Environ. Sci. 7, 3374–3381. (in Chinese). doi:10.13227/j.hjkx.201711099
Keywords: geometric mean of enzymatic activities, plant properties, restoration time, soil C:N ratio, sanjiang plain
Citation: Wang C, Li H, Sun X and Cai T (2021) Responses of Soil Microbial Biomass and Enzyme Activities to Natural Restoration of Reclaimed Temperate Marshes After Abandonment. Front. Environ. Sci. 9:701610. doi: 10.3389/fenvs.2021.701610
Received: 28 April 2021; Accepted: 28 May 2021;
Published: 10 June 2021.
Edited by:
Rahul Mahadev Shelake, Gyeongsang National University, South KoreaReviewed by:
Rajesh Waghunde, Bharuch, IndiaYahya Kooch, Tarbiat Modares University, Iran
Jincai Ma, Jilin Unversity, China
Copyright © 2021 Wang, Li, Sun and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoxin Sun, c3VueGlhb3hpbkBuZWZ1LmVkdS5jbg==; Tijiu Cai, dGlqaXUuY2FpQG5lZnUuZWR1LmNu
 Chunguang Wang1,2
Chunguang Wang1,2 Xiaoxin Sun
Xiaoxin Sun