- 1State Key Laboratory of Freshwater Ecology and Biotechnology, Institute of Hydrobiology, Chinese Academy of Sciences, Wuhan, China
- 2University of Chinese Academy of Sciences, Beijing, China
- 3Key Laboratory of Aquatic Botany and Watershed Ecology, Wuhan Botanical Garden, Chinese Academy of Sciences, Wuhan, China
Periphyton is an ecological essential in freshwater lakes and rivers. Its abundance and biomass are very dynamic in various habitats and subject to various factors, for example, nutrient and light. Following flooding events, the transitional area adjacent to a river inlet and the shallow lake generates diverse habitats for periphyton with gradients in current velocity, suspended matters, nutrients, and light, which would strongly shape the growth and community of periphyton. In this study, three sampling sites were established around a river inlet in Erhai Lake, China, and a field survey was conducted in the sites from April to August (flooding seasons) in 2019 to investigate the abundance and biomass of periphyton and explore influential factors. The results showed that three study areas have different gradients of current velocity depending on the distance to the river inlet, thereby regulating the concentrations of nutrients and suspended matters, which strongly affected the periphyton community; to be specific, the biomass of periphyton was inhibited by the concentration of suspended matters and high concentrations of silicate mainly reduced the diversity of periphyton. Our results imply that the study on the driving factors of periphyton could help to understand its community assembly mechanism and biomass and species composition of periphyton can provide some reference for trophic state of the lake.
Introduction
Periphyton (attached on the surface of plants) is a major primary producer and an important component in clearwater and freshwater ecosystems. Periphyton is usually prevalent with the development of macrophyte and becomes a crucial factor affecting the ecological function of freshwater and biogeochemical processes (Roman and Sabater, 1999; Woodruff et al., 1999). For instance, periphyton can act as basal resources for both invertebrates and vertebrate consumers in benthic food webs (Hansson, 1992; Lamberti, 1996; Pusch et al., 1998; Muñoz et al., 2001). As the base of benthic food webs, periphyton community structure and diversity can be widely used to assess the trophic state and thus can be used to support proposed best management practices (Matlock et al., 1999; Smucker and Vis, 2009; Grimmett and Lebkuecher, 2017). Generally, the development of periphyton in shallow lakes and rivers has been attributed to the changes of physical, chemical, and biological factors, including light availability (Warren et al., 2017), water velocity (Townsend et al., 2012), nutrient concentrations (Myrstener et al., 2018), grazing by consumers (Jones and Sayer, 2003; Garcia et al., 2015), etc.
Light limitation is one of the main ecological factors influencing the development of periphyton (Vadeboncoeur et al., 2014; Warren et al., 2017). For example, more sufficient light obtained in a shallow littoral than a deeper littoral zone promoted periphyton growth and controlled the species composition and dynamics (Laura Sanchez et al., 2017; Hofmann et al., 2020). Increased current velocity limited periphyton development through shear stresses generated by high-speed flow that directly destroyed algae mat and prevented colonization, thus resulting in a large shift in periphyton biomass and communities (Saravia et al., 1998; Wilby et al., 1998; Shevchenko et al., 2019; Burrows et al., 2020; Spoljar et al., 2017). Furthermore, current velocity could increase suspended particulate transport efficiency (Jowett and Biggs, 1997; Mori et al., 2018) and consequently might result in the decrease of periphyton biomass due to abrasion (Francoeur and Biggs, 2006; Hoyle et al., 2017). Besides, nutrients are key factors for periphyton development. According to previous studies, the increase of nitrogen and phosphorus can lower the biomass of periphyton and alter its community composition (Myrstener et al., 2018; Lambrecht et al., 2019). In addition, adequate silicate in the water column contributes to the settlement of periphyton and proliferation (Admiraal et al., 1993; Nayar et al., 2005). Changes in the biomass and species composition of periphyton are regulated by biological groups, for example, invertebrates and vertebrate (Jones and Sayer, 2003; Cebrian et al., 2013). For example, benthic fish disturbance will potentially facilitate the release of nutrients from the sediment, thereby promoting periphyton growth (Meerhoff et al., 2007; Jeppesen et al., 2010), while invertebrates can remove periphyton biomass and alter community composition through direct grazing (Garcia et al., 2015; Mormul et al., 2017; Beck et al., 2019; Wolters et al., 2019).
The area around the river inlet is a hot spot zone-occurring complex environmental process. Processes occurring in the river inlet area have been poorly studied. Previous studies found that periphyton biomass, taxonomic diversity, and function are heavily dependent on hydrological regime (Biggs et al., 1999; Bondar-Kunze et al., 2015; Neverman et al., 2018). Especially, when a flooding event happens in the rainy season, the scouring effect of flooding and consequently high current velocity and suspended matter concentrations can act as a major reset mechanism to initiate new cycle metabolism and structural succession for the periphyton community and even the whole river ecosystem (Tett et al., 1978; Fisher et al., 1982; Lindström and Traaen, 1984; Reiter and Carlson, 1986; Townsend et al., 2012). Based on this, the structure of periphyton assemblages often reflects the impacts of trophic and hydrological state thus can be used to monitor water environment changes in the lake ecosystem.
Water flow also carries nutrients, which makes it difficult to establish a simple and causal relationship between characteristics of periphyton community and nutrients or water velocity in lakes. Therefore, this study aimed to determine how water velocity and nutrients might affect periphyton growth during the rainy season. Our study areas around the river inlet could be simplified as three types: estuary zone (E zone) in Miju River and Lake Erhai junction, estuary–lake mixing zone (E–L zone) from the river inlet to the range under the strong influence of the inlet, and lake littoral zone (L zone) that is the nearby littoral zone without the strong influence of the estuary inlet. We hypothesized that due to the inflow of rivers with the advent of the rainy season, the E zone and the E–L zone near the estuary of Erhai Lake may have increased the water velocity and a large amount of suspended matter compared with the L zone, both of which will be detrimental to the growth of periphyton. In addition, silicate from inflow of river may be expected to promote the growth of diatoms and change the community structure of the periphyton.
Materials and Methods
Study Area
Field investigations were carried out at the area around the river inlet area in Erhai Lake, China (25°55′N,100°78′E), and the area could be simplified as three types: estuary zone (E zone) in Miju River and Lake Erhai junction, estuary–lake mixing zone (E–L zone), and lake littoral zone (L zone) (Figure 1). Erhai Lake is the second largest freshwater lake in Yunnan Province. The Miju River with a length of 71.1 km and a basin area of 1310.8 km2 accounts for 54% of the total basin area of Erhai Lake (Yu et al., 2011). In addition, it is the largest water supply source to the Erhai Lake, accounting for more than 50% of the total inflow water (Liu et al., 2019).
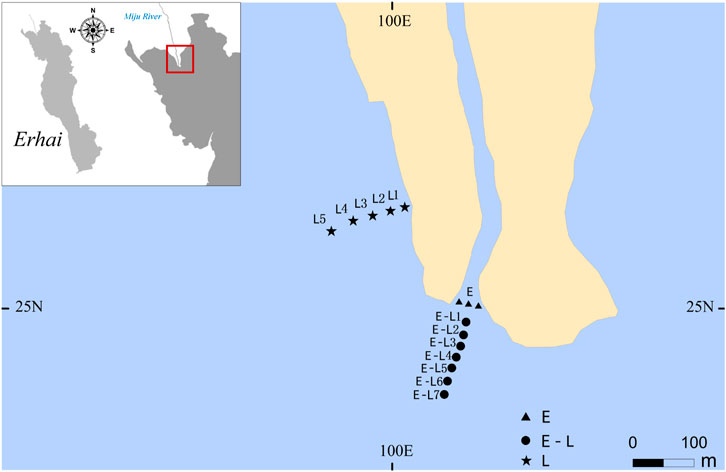
FIGURE 1. Sampling sites in Lake Erhai. E, estuary zone; E–L, estuary–lake mixing zone; L, lake littoral zone.
Field Samplings
In order to survey the impact of rainstorm scouring on periphyton biomass, and community structure in E zone, E–L zone, and L zone, three sampling sites at the E zone, seven sampling sites at the E–L zone and five sampling sites in the L zone were chosen, along a transection from the lakeshore to the pelagic zone, as shown in Figure 1. The mean of all samples at one sampling site was calculated.
The field investigation was performed from April to August 2019 (rainy season) with monthly sampling of periphyton (on the surface of plant leaves) and the water of trace elements (e.g., Fe, Mn, B, and SiO3), total nitrogen (TN) and total phosphorus (TP), suspended matter, and water velocity. Water depth (WD) at each site, representing the water depth of collecting macrophyte (P. wrightii), was measured at the same time.
Measurement of Water Samples
Water samples were collected with the Patalas Schindler sampler from the surface (0.5 m below the water surface), middle (middle of the water depth in that site),and the bottom (0.5 m above the sediment surface) mixed to obtain representative and comprehensive water samples at the 15 sampling sites and then sent to the laboratory immediately for measurement of total nitrogen (TN) (mg L−1), total phosphorus (TP) (mg L−1), iron (Fe) (mg L−1), manganese (Mn) (mg L−1), silicate (SiO3) (mg L−1), and boron (B) (mg L−1) following standard methods (APHA, 1999). In the meantime, water velocity at the 15 sampling sites was measured with Stalker II SVR and recorded with the unit of centimeters per second (cm s-1). The concentration of suspended matter was measured according to standard methods (GB T11901 1989) and calculated as mg L−1.
The Measurement of Periphyton Samples
A rotatable reaping hook covering a bottom area of 0.2 m2 was used to collect Potamogeton wrightii, and the collected macrophytes were carefully harvested. A macrophyte (P. wrightii) was randomly selected and placed into a sealed plastic bag with tap water, and then periphyton attached on the leaves of P. wrightii was removed by shaking the plant thoroughly. The obtained solution of periphyton was preserved with Lugol’s solution and concentrated to a final volume of 40 ml after sedimentation for 48 h. All taxa of algal abundances were counted with a counting chamber (0.1 ml) at 400 times magnification, and then calculated the mass of periphyton. The leaf area of P. wrightii was measured by a leaf area scanner (CI-202, CID Company, United States). The biomass of periphyton was calculated by the mass of periphyton attached on the leaf divided by the leaf area and expressed as μg cm−2.
The collection of attached matter (excluding algae but including bacteria, cell exudates, detritus, and all other organic matter residing) and determination of the leaf area was the same as that of periphyton. Furthermore, the determination of the quality of attachment matter was the same as the suspended matter. The biomass of attached matter was determined as quality per unit area of the leaf (μg cm−2).
Dominance Index and Simpson’s Diversity Index of Periphyton
Dominance index (Y = Ni/N × fi, where Y represents the dominance index, Ni represents the abundance of species i, N represents the total abundance, and fi represents the frequency of species i occurring at sampling sites) of dominant species in each river is listed in Table 1.
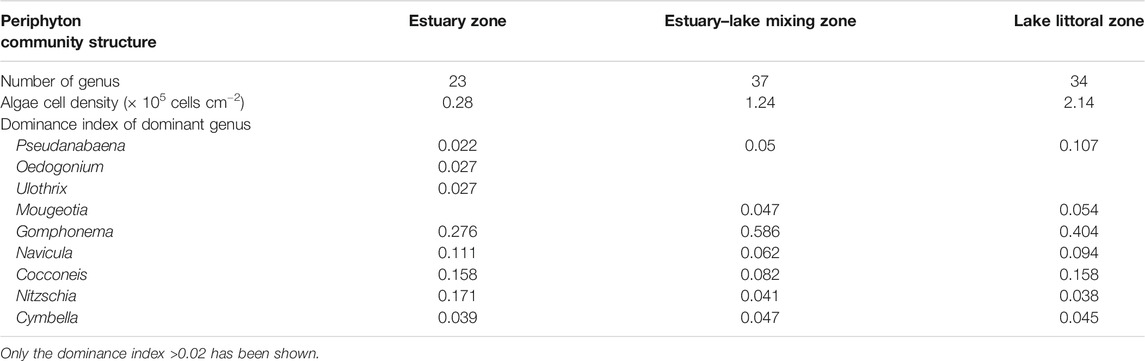
TABLE 1. Number of genus, algae cell density, and dominance index of dominant genus of periphyton of the three sampling zones.
Simpson’s diversity index is represented by the following formula:
where D represents the diversity index, s represents the numbers of total species, and
Statistical Analysis
Canonical analysis of principal coordinates (CAP) was used to test difference in community structure and environmental conditions among months and sampling location (Anderson and Willis, 2003). CAP is a variant of principal coordinate analysis (PCoA), aiming to find axis through a cloud of multivariate points that are best at discriminating among a priori defined groups (Clarke and Warwick, 2001). In this study, we used Bray–Curtis coefficients for periphyton biomass data and Euclidean distances for standardized environmental data of sampling location. The significant dissimilarities of communities and environmental conditions among months and sampling location were tested by applying one-way analysis of similarity (ANOSIM) (Clarke, 1993) based on permutation procedures with 999 runs. CAP and ANOSIM were carried out with the software Primer 6.0.
Redundancy analysis (RDA) was used to select the significant driving factors of the environmental variables related to periphyton biomass, abundance, and diversity index. The variables of multicollinearity were evaluated by variance inflation factors (VIF, excluded variables with VIF > 5). Then, significant driving factors were selected by the forward selection with Monte Carlo permutations for further analysis. All biological abundance data and environmental data were log(x + 1) transformed to satisfy normality. RDA was run using the “vegan” package in the R environment (R version 3.6.2). The contributions of the explanatory variables were parted using the function “varpart” in the R package “vegan”.
Periphyton data obtained after initiation of the experiment at three sampling locations were analyzed by RM-ANOVA (shown in Table 2), and multiple means comparisons were performed by Student–Newman–Keuls (SNK). RM-ANOVA analyses were conducted with IBM SPSS Statistics 22.
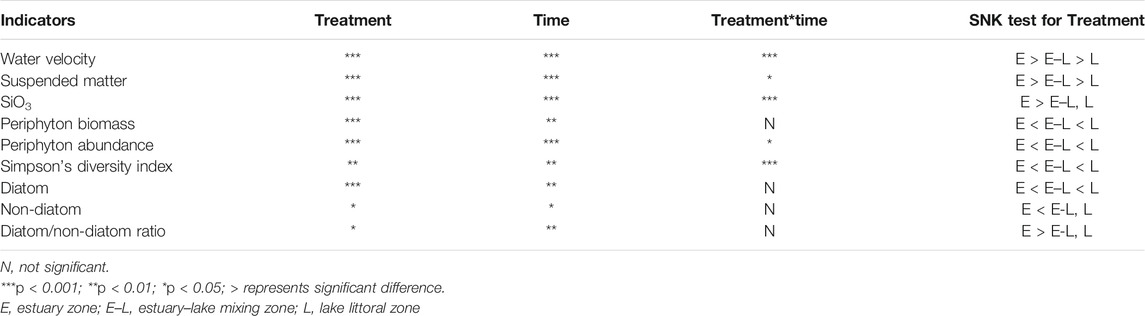
TABLE 2. Statistical results of the RM-ANOVA analysis about the water velocity, suspended matter, SiO3, periphyton biomass, and abundance, diversity index.
The linear regression was conducted with the Pearson correlation analysis to assess the relationship among water velocity, suspended matter, and periphyton biomass, and the relationship between silicate and the periphyton biomass of non-diatom and the periphyton biomass ratio of diatoms to non-diatoms.
Results
The Variation of Periphyton Community and Environmental Indicators
A total of 41 taxa were identified for periphyton during the studied period. Bacillariophyceae (18) was the dominant class, followed by Chlorophyceae (12), and other classes including Cyanophyceae, Dinophyceae, Euglenophyceae, and Cryptophyceae. The numbers of genus, cell density, and dominant species in each sampling zone are listed in Table 1.
According to CAP and ANOSIM, both of periphyton community structure and environmental indicators differed significantly among three sampling zones (R = 0.219, p = 0.001, Figure 2A; R = 0.513, p = 0.001, Figure 2C) and among months (R = 0.345, p = 0.001, Figure 2B; R = 0.237, p = 0.001, Figure 2D).
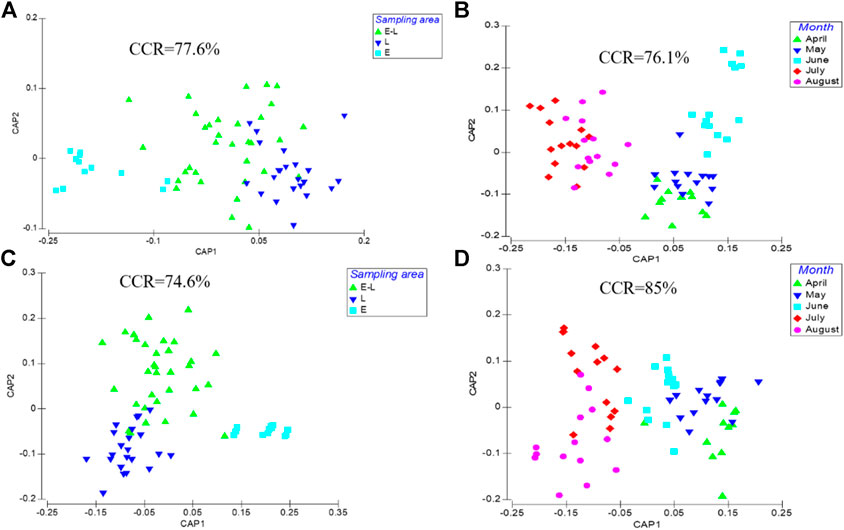
FIGURE 2. Canonical analysis of principal coordinate ordination plot (CAP) for periphyton biomass data in sampling location (A) and month (B) using the Bray-Curtis coefficient and environmental data in sampling location (C) and month (D) using standardized Euclidean distance. CCR represents the correct classification rate. E, estuary zone; E–L, estuary–lake mixing zone; L, lake littoral zone.
The Significant Driving Factors Selected by Redundancy Analysis
All variables can explain 50.2% of the variation in the periphyton community, and the concentration of suspended matters and silicate can significantly explain 37.5% of the variation in the periphyton community (Figure 3). The suspended matter was the most important variable with an explanatory power of 31.2%, followed by silicate with an explanation of 8.8%. The suspended matter interacts weakly with silicate, explaining the 2.4% periphyton variance (Figure 3B).
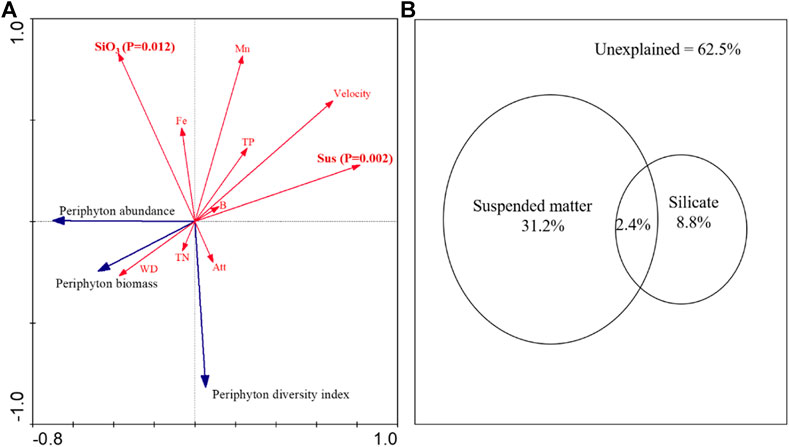
FIGURE 3. Ordination biplot of the redundancy analysis (RDA) for dominant periphyton biomass, abundance, diversity index and environmental variables (A); variance partitioning of periphyton community structure explained by suspended matter and silicate (B). In a: Monte Carlo permutation tests identified that suspended matter (p = 0.002) and silicate (p = 0.012) explained significant proportions. Blue vectors: periphyton biomass, abundance, and Simpson’s diversity index. Red vectors, environmental variables; Att, attached matter; Sus, suspended matter; Velocity, water velocity; WD, water depth; SiO3, silicate; TN, total nitrogen; TP, total phosphorus; Fe, iron; Mn, manganese; B, boron.
The Concentration Variation of Total Nitrogen and Total Phosphorus
The concentration of TN and TP were the highest in the E zone, followed by the E-L zone, and the lowest in L zone from June to August 2019 (rainy season). Meanwhile, the change trend of TN and TP was similar in the three sampling zones during the experiment (Figures 4A,B).
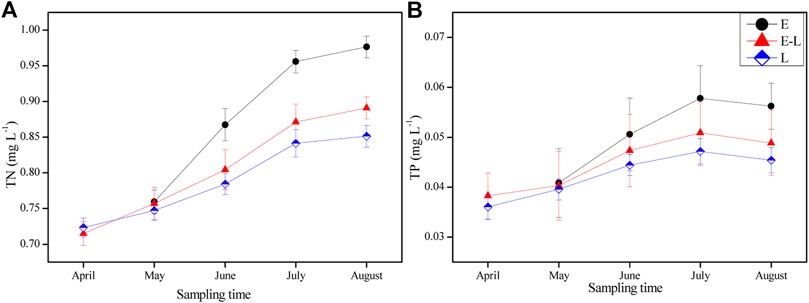
FIGURE 4. Variation of total nitrogen (TN) and total phosphorus (TP) in different sampling zones from April to August. E, estuary zone; E–L, estuary–lake mixing zone; L, lake littoral zone.
Water Velocity, Suspended Particulate, and Silicate
Water velocity, the concentration of suspended matter, and silicate were the highest in E zone, which was significantly higher than that in E–L and L zones, and the lowest in L zone. The above three indicators fluctuated over time in the three sampling zones (Table 2; Figures 5A,C,E), but the silicate concentration had been increasing in E zone from April to August 2019 (Figure 5E).
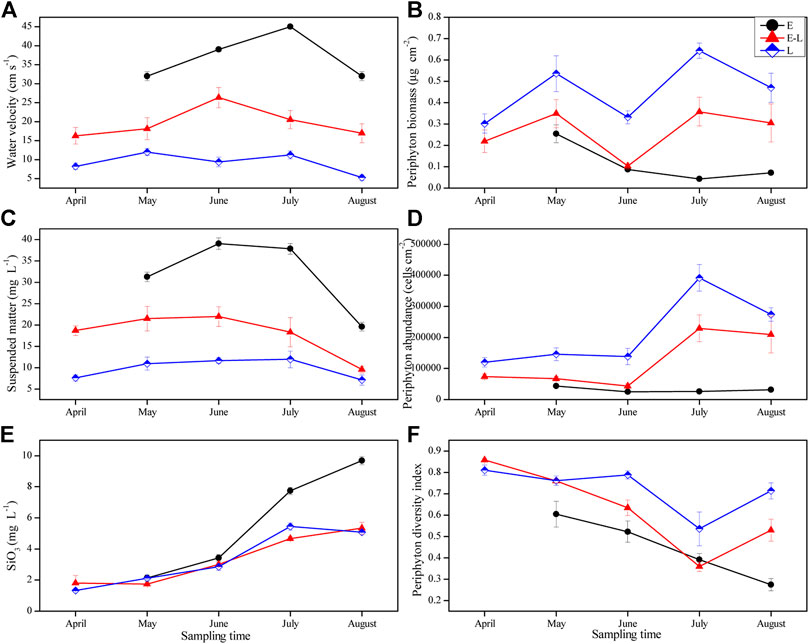
FIGURE 5. Variation of water velocity, suspended matter, silicate, periphyton biomass, abundance and diversity index in different sampling zones from April to August. E, estuary zone; E–L, estuary–lake mixing zone; L, lake littoral zone.
Periphyton Biomass, Abundance, and Simpson’s Diversity Index
In the three sampling zones, there were significant differences in periphyton biomass, abundance, and diversity index, which had changed with time (Table 2). During the experiments, periphyton biomass and abundance were the highest in L zone and the lowest in E zone (Figures 5B,D). As for the diversity index, that in the L zone is the highest, followed by that in the E–L zone from May to July, and a continuously decreasing trend from May to August was observed in E zone (Figure 5F).
The Correlation Among Water Velocity, Suspended Matter, and Periphyton Biomass
Our results also showed that suspended matter concentration was positively correlated with flow velocity (p < 0.001) (Figure 6A), while negatively correlated with the biomass of periphyton (p < 0.001) (Figure 6C). In addition, water flow velocity had a significantly negative correlation with the biomass of periphyton (p < 0.05) (Figure 6B).
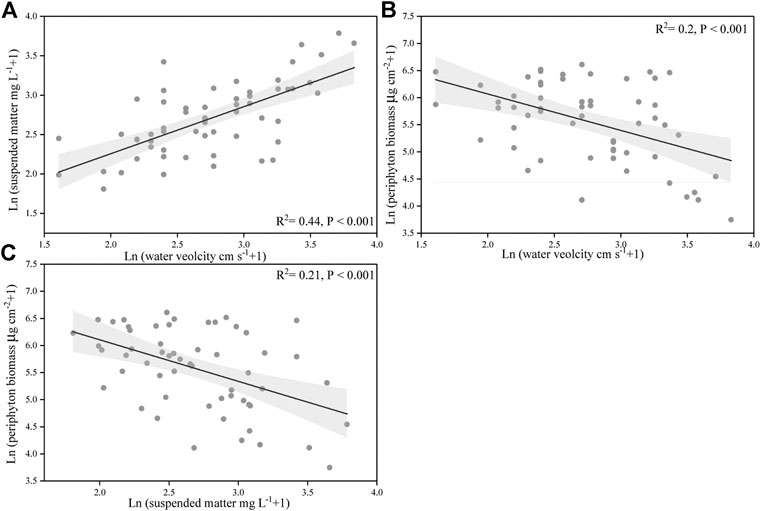
FIGURE 6. Relationships between the suspended particulate concentration, water velocity, and periphyton biomass. The data were ln(X + 1)-transformed. The line indicates linear regression for all the data, and its correlation coefficients and significance level were labeled. Gray shade are 95% point-wise confidence bands.
The Correlation Between Silicate and Periphyton
We also found that silicate concentration was strongly negatively correlated with Simpson’s diversity index of periphyton and was negatively related with non-diatoms biomass (p < 0.001 for both) (Figures 7A,B). Meanwhile, the concentration of silicate has a significant positive correlation with the periphyton biomass ratio of diatoms to non-diatoms (p < 0.001) (Figure 7C).
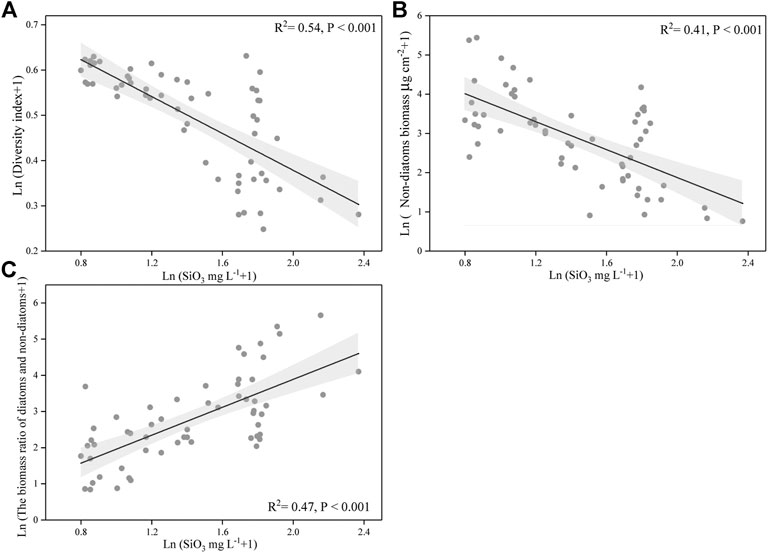
FIGURE 7. Relationships between the silicate concentration and diversity index of periphyton, and the silicate concentration and non-diatoms biomass, and the silicate concentration and the biomass ratio of diatoms and non-diatoms. The data were log-transformed. The line indicates linear regression for all the data, and its correlation coefficients and significance level were labeled. Gray shade are 95% point-wise confidence bands.
Discussion
This study revealed a significant temporal and spatial difference of periphyton community structure and environmental indicators in Erhai Lake. E zone boasted the highest water velocity, suspended matters, and silicate concentration, significantly higher than that in E–L and L zones, while has the lowest biomass, abundance, and diversity of periphyton during the rainy season. Moreover, there was indeed a significant positive correlation between water velocity and suspended matter, and both of them have an adverse effect on the periphyton. Meanwhile, silicate generated a particularly strong negative influence on non-diatoms and diversity of periphyton, but it has a significantly positive relationship with the ratio of diatoms to non-diatoms. Consequently, altering periphyton community and biomass were ultimately regulated by water velocity, suspended matters, and silicates during the rainy season.
Current velocity and suspended matters might play an important role in regulating periphyton biomass, including shear stress generated by high-speed flow (Horner and Welch, 1981; Lindström and Traaen, 1984; Biggs, 1996; Shevchenko et al., 2019), and light attenuation and abrasion effect due to mobilized suspended matter by enhanced current velocity (Francoeur and Biggs, 2006; Mori et al., 2018; Izagirre et al., 2009), which are not conducive to the growth and proliferation of periphyton. In the present study, water velocity (> 20 cm s−1) and suspended matters concentrations (> 18 mg L−1) significantly reduced periphyton biomass. The same period of flood season and the rainy season, the precipitation accounts for 84.9–93.2% of annual precipitation from May to October in the Erhai Lake basin (Li et al., 2017) and water velocity, nutrient, and suspended matter load increase during the flood season (Yang et al., 2017). Generally, flow velocity varied from 2 to 70 cm s−1 in the freshwater ecosystem (Rusanov and Khromov, 2016), and velocity can reach 90 cm s−1 during flood period (Jowett and Biggs, 1997). A decrease in periphyton biomass with increasing current velocity was observed by Ryder et al. (2006) and periphyton biomass depended inversely on current velocity within the range from 2 to 60 cm s−1 (Rusanov and Khromov, 2016). In addition, current velocity can positively facilitate the transport efficiency of suspended particulates (Jowett and Biggs, 1997; Mori et al., 2018), and thereby is detrimental to the accumulation of periphyton biomass by abrasion and shading effect (Daviescolley et al., 1992; Parkhill and Gulliver, 2002; Izagirre et al., 2009; Hoyle et al., 2017). In the present study, suspended matters were enhanced by 69% in E zone compared with that in L zone, and periphyton biomass reduced by 297%. Izagirre et al. (2009) reported that suspended matter loads (> 6 g L−1) initially reduced algal growth and algal photosynthetic efficiency showed a quick decrease, thus leading to the loss of periphyton biomass.
Silicon (Si) is an essential element of diatoms, and it plays a key role in forming valves and completing cell cycle (Koester et al., 2016). Diatoms account for the majority of periphyton; therefore, silicate may play an important role in the development and community structure of periphyton (Lee et al., 2019). In this study, there was indeed a significant negative correlation between silicate concentrations (1.2–9.7 mg L−1) and Simpson’s diversity index of periphyton, and a positive correlation between silicate and the ratio of diatoms and non-diatoms, altering the periphyton community. Hillebrand and Sommer (2000) reported that enhanced Si supply decreased periphyton diversity. Besides, Hafner et al. (2018) observed that changes in silicate concentration affected periphyton community structure due to exploitation and competition among taxa. In addition, silicate concentrations and ratio of diatoms to non-diatoms were higher in E zone than in L and E-L zones, which may be attributed to the expansion and reproduction of diatoms through the absorption and utilization of silicate, and to the competition advantage of diatoms to non-diatoms. Therefore, taking these reasons into account could offer an explanation for the varied periphyton community.
Furthermore, biological factors in lakes also play an important role in regulating the development of periphyton communities through bottom-up and top-down effects, and this may partly explain the change of its community (Garcia et al., 2015; Mormul et al., 2017; Beck et al., 2019). However, with the lack of snail and fish data in our study, it was not possible to evaluate their impacts on the periphyton, which may help to explain why all the measured environment indicators did not have a higher explanation (only 50.2%) for the changes in the community structure of periphyton.
Conclusion
In conclusion, we found that current velocities and suspended matters had an impact on the development of periphyton biomass. Both of them are not conducive to the development of periphyton through shear stresses generated by high-speed flow that directly destroys algae mat and mobilization of the suspended particulates to prevent colonization. In addition, altering periphyton community was regulated primarily by silicate. The increase in silicate concentration significantly reduced Simpson’s diversity index of periphyton and enhanced the ratio of diatoms to non-diatoms, due to exploitation and competition among taxa.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding authors.
Author Contributions
WR performed this experiment, analyzed data, and wrote the manuscript; YY, ZZ, HW, CY, and QCC contributed to field investigations; LN, YC, and XZ revised the manuscript and gave important and critical input; TC designed the research.
Funding
This study was supported by the National Science Foundation of China (Grant No. 31930074).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Admiraal, W., Mylius, S. D., de Ruyter van Steveninck, E. D., and Tubbing, D. M. J. (1993). A Model of Phytoplankton Production in the Lower River Rhine Verified by Observed Changes in Silicate Concentration. J. Plankton Res. 15, 659–682. doi:10.1093/plankt/15.6.659
Anderson, M. J., and Willis, T. J. (2003). Canonical Analysis of Principal Coordinates: A Useful Method of Constrained Ordination for Ecology. Ecology. 84, 511–525. doi:10.1890/0012-9658(2003)084[0511:caopca]2.0.co;2
APHA (1999). Standard Methods for the Examination of Water and Wastewater. 20th ed. Washington DC: American Public Health Association.
Beck, W. S., Markman, D. W., Oleksy, I. A., Lafferty, M. H., and Poff, N. L. (2019). Seasonal Shifts in the Importance of Bottom-Up and Top-Down Factors on Stream Periphyton Community Structure. Oikos. 128, 680–691. doi:10.1111/oik.05844
Biggs, B. J. F. (1996). Hydraulic Habitat of Plants in Streams. Regul. Rivers: Res. Mgmt. 12, 131–144. doi:10.1002/(sici)1099-1646(199603)12:2/3<131::aid-rrr385>3.0.co;2-x
Biggs, B. J. F., Tuchman, N. C., Lowe, R. L., and Stevenson, R. J. (1999). Resource Stress Alters Hydrological Disturbance Effects in a Stream Periphyton Community. Oikos. 85, 95–108. doi:10.2307/3546795
Bondar-Kunze, E., Tritthart, M., and Hein, T. (2015). The Influence of Short Term Water Level Fluctuations and Desiccation Stress on Periphyton Development at a Riparian Zone of a Large Regulated River. fal. 186, 283–296. doi:10.1127/fal/2015/0654
Burrows, R. M., Beesley, L., Douglas, M. M., Pusey, B. J., and Kennard, M. J. (2020). Water Velocity and Groundwater Upwelling Influence Benthic Algal Biomass in a sandy Tropical River: Implications for Water-Resource Development. Hydrobiologia. 847, 1207–1219. doi:10.1007/s10750-020-04176-3
Cebrian, J., Stutes, J., and Christiaen, B. (2013). Effects of Grazing and Fertilization on Epiphyte Growth Dynamics under Moderately Eutrophic Conditions: Implications for Grazing Rate Estimates. Mar. Ecol. Prog. Ser. 474, 121–133. doi:10.3354/meps10092
Clarke, K. R., and Warwick, R. M. (2001). Change in the marine Communities: An Approach to Statistical Analysis and Interpretation, New York: Mount Sinai Journal of Medicine New York.
Clarke, K. R. (1993). Non-parametric Multivariate Analyses of Changes in Community Structure. Austral Ecol. 18, 117–143. doi:10.1111/j.1442-9993.1993.tb00438.x
Davies-Colley, R. J., Hickey, C. W., Quinn, J. M., and Ryan, P. A. (1992). Effects of clay Discharges on Streams. Hydrobiologia. 248, 215–234. doi:10.1007/bf00006149
Fisher, S. G., Gray, L. J., Grimm, N. B., and Busch, D. E. (1982). Temporal Succession in a Desert Stream Ecosystem Following Flash Flooding. Ecol. Monogr. 52, 93–110. doi:10.2307/2937346
Francoeur, S. N., and Biggs, B. J. F. (2006). Short-term Effects of Elevated Velocity and Sediment Abrasion on Benthic Algal Communities. Hydrobiologia. 561, 59–69. doi:10.1007/s10750-005-1604-4
Garcia, E. A., Townsend, S. A., and Douglas, M. M. (2015). Context Dependency of Top-Down and Bottom-Up Effects in a Northern Australian Tropical River. Freshw. Sci. 34, 679–690. doi:10.1086/681106
Grimmett, M. R., and Lebkuecher, J. G. (2017). Composition of Algae Assemblages in Middle Tennessee Streams and Correlations of Composition to Trophic State. J. Freshw. Ecol. 32 (1), 363–389. doi:10.1080/02705060.2017.1314228
Hafner, D., Car, A., Jasprica, N., Kapetanović, T., and Dupčić Radić, I. (2018). Relationship between marine Epilithic Diatoms and Environmental Variables in Oligotrophic bay, NE Mediterranean. Medit. Mar. Sci. 19, 223–239. doi:10.12681/mms.14151
Hansson, L. -A. (1992). The Role of Food Chain Composition and Nutrient Availability in Shaping Algal Biomass Development. Ecology. 73, 241–247. doi:10.2307/1938735
Hillebrand, H., and Sommer, U. (2000). Diversity of Benthic Microalgae in Response to Colonization Time and Eutrophication. Aquat. Bot. 67, 221–236. doi:10.1016/s0304-3770(00)00088-7
Hofmann, A. M., Geist, J., Nowotny, L., and Raeder, U. (2020). Depth-distribution of lake Benthic Diatom Assemblages in Relation to Light Availability and Substrate: Implications for Paleolimnological Studies. J. Paleolimnol. 64, 315–334. doi:10.1007/s10933-020-00139-9
Horner, R. R., and Welch, E. B. (1981). Stream Periphyton Development in Relation to Current Velocity and Nutrients. Can. J. Fish. Aquat. Sci. 38, 449–457. doi:10.1139/f81-062
Hoyle, J. T., Kilroy, C., Hicks, D. M., and Brown, L. (2017). The Influence of Sediment Mobility and Channel Geomorphology on Periphyton Abundance. Freshw. Biol. 62, 258–273. doi:10.1111/fwb.12865
Izagirre, O., Serra, A., Guasch, H., and Elosegi, A. (2009). Effects of Sediment Deposition on Periphytic Biomass, Photosynthetic Activity and Algal Community Structure. Sci. Total Environ. 407, 5694–5700. doi:10.1016/j.scitotenv.2009.06.049
Jeppesen, E., Meerhoff, M., Holmgren, K., González-Bergonzoni, I., Teixeira-de Mello, F., Declerck, S. A. J., et al. (2010). Impacts of Climate Warming on lake Fish Community Structure and Potential Effects on Ecosystem Function. Hydrobiologia. 646 (1), 73–90. doi:10.1007/s10750-010-0171-5
Jones, J. I., and Sayer, C. D. (2003). Does the Fish-Invertebrate-Periphyton Cascade Precipitate Plant Loss in Shallow Lakes? Ecology. 84, 2155–2167. doi:10.1890/02-0422
Jowett, I. G., and Biggs, B. J. F. (1997). Flood and Velocity Effects on Periphyton and silt Accumulation in Two New Zealand Rivers. New Zealand J. Mar. Freshw. Res. 31, 287–300. doi:10.1080/00288330.1997.9516767
Koester, J., Brownlee, C., and Taylor, A. R. (2016). Algal Calcification and Silicification, in Encyclopedia of Life Sciences, Chichester: John Wiley & Sons.
Lamberti, G. A. (1996). The Role of Periphyton in Benthic Food Webs. Algal Ecol. 17, 533–572. doi:10.1016/b978-012668450-6/50046-1
Lambrecht, R. W., Tavares, D. A., Santos, T. R., and Ferragut, C. (2019). Responses of Periphyton Biomass and Nutrient Status to Experimental Enrichment and its Relationships with Changes in Seston Nutrient Content and Chlorophyll-A. Hydrobiologia. 836, 141–153. doi:10.1007/s10750-019-3947-2
Lee, S. S., Bishop, I. W., Spaulding, S. A., Mitchell, R. M., and Yuan, L. L. (2019). Taxonomic Harmonization May Reveal a Stronger Association between Diatom Assemblages and Total Phosphorus in Large Datasets. Ecol. Indicators. 102, 166–174. doi:10.1016/j.ecolind.2019.01.061
Li, Y., Li, B., Zhang, K., Zhu, J., and Yang, Q. (2017). Study on Spatiotemporal Distribution Characteristics of Annual Precipitation of Erhai Basin. J. China Inst. Water Resour. Hydropower Res. 15 (3), 234–240. doi:10.13244/j.cnki.jiwhr.2017.03.012
Lindström, E. -A., and Traaen, T. S. (1984). Influence of Current Velocity on Periphyton Distribution and Succession in a Norwegian Soft Water River. SIL Proc. 1922-2010. 22, 1965–1972. doi:10.1080/03680770.1983.11897602
Liu, J., Xu, J., Chen, J., Hong, X., and Zhou, M. (2019). Spatio-temporal Distribution Characteristics of Water Quality in Miju River and Erhai Lake. J. Coast. Res. 93, 31–38. doi:10.2112/si93-005.1
Matlock, M. D., Storm, D. E., Smolen, M. D., Matlock, M. E., McFarland, A. M. S., and Hauck, L. M. (1999). Development and Application of a Lotic Ecosystem Trophic Status index. Trans. ASAE. 42 (3), 651–656.
Meerhoff, M., Clemente, J. M., de Mello, F. T., Iglesias, C., Pedersen, A. R., and Jeppesen, E. (2007). Can Warm Climate-Related Structure of Littoral Predator Assemblies Weaken the clear Water State in Shallow Lakes? Glob. Change Biol. 13 (9), 1888–1897. doi:10.1111/j.1365-2486.2007.01408.x
Mori, T., Miyagawa, Y., Onoda, Y., and Kayaba, Y. (2018). Flow-velocity-dependent Effects of Turbid Water on Periphyton Structure and Function in Flowing Water. Aquat. Sci. 80, 12. doi:10.1007/s00027-017-0552-1
Mormul, R. P., Ahlgren, J., and Brönmark, C. (2017). Snails Have Stronger Indirect Positive Effects on Submerged Macrophyte Growth Attributes Than Zooplankton. Hydrobiologia. 807, 165–173. doi:10.1007/s10750-017-3391-0
Muñoz, I., Real, M., Guasch, H., Navarro, E., and Sabater, S. (2001). Effects of Atrazine on Periphyton under Grazing Pressure. Aquat. Toxicol. 55, 239–249. doi:10.1016/s0166-445x(01)00179-5
Myrstener, M., Rocher-Ros, G., Burrows, R. M., Bergstrom, A.-K., Giesler, R., Sponseller, R. A., et al. (2018). Persistent nitrogen limitation of stream biofilm communities along climate gradients in the Arctic. Glob. Change Biol. 24 (8), 3680–3691. doi:10.1111/gcb.14117
Nayar, S., Goh, B. P. L., and Chou, L. M. (2005). Settlement of marine Periphytic Algae in a Tropical Estuary. Estuarine, Coastal Shelf Sci. 64, 241–248. doi:10.1016/j.ecss.2005.01.016
Neverman, A. J., Death, R. G., Fuller, I. C., Singh, R., and Procter, J. N. (2018). Towards Mechanistic Hydrological Limits: A Literature Synthesis to Improve the Study of Direct Linkages between Sediment Transport and Periphyton Accrual in Gravel-Bed Rivers. Environ. Manage. 62, 740–755. doi:10.1007/s00267-018-1070-1
Parkhill, K. L., and Gulliver, J. S. (2002). Effect of Inorganic Sediment on Whole-Stream Productivity. Hydrobiologia. 472, 5–17. doi:10.1023/a:1016363228389
Pusch, M., Fiebig, D., Brettar, I., Eisenmann, H., Ellis, B. K., Kaplan, L. A., et al. (1998). The Role of Micro‐organisms in the Ecological Connectivity of Running Waters. Freshw. Biol. 40, 453–495. doi:10.1046/j.1365-2427.1998.00372.x
Reiter, M. A., and Carlson, R. E. (1986). Current Velocity in Streams and the Composition of Benthic Algal Mats. Can. J. Fish. Aquat. Sci. 43, 1156–1162. doi:10.1139/f86-144
Roman, A. M., and Sabater, S. (1999). Effect of Primary Producers on the Heterotrophic Metabolism of a Stream Biofilm. Freshw. Biol. 41, 729–736. doi:10.1046/j.1365-2427.1999.00413.x
Rusanov, A. G., and Khromov, V. M. (2016). Longitudinal Distribution of Periphyton Algae in the Moskva River under Eutrophication. Water Resour. 43, 513–521. doi:10.1134/s0097807816030131
Ryder, D. S., Watts, R. J., Nye, E., and Burns, A. (2006). Can Flow Velocity Regulate Epixylic Biofilm Structure in a Regulated Floodplain River? Mar. Freshw. Res. 57, 29–36. doi:10.1071/mf05099
Sánchez, M. L., Rodríguez, P., Torremorell, A. M., Izaguirre, I., and Pizarro, H. (2017). Phytoplankton and Periphyton Primary Production in Clear and Turbid Shallow Lakes: Influence of the Light Environment on the Interactions between These Communities. Wetlands. 37, 67–77. doi:10.1007/s13157-016-0840-x
Saravia, L. A., Momo, F., and Boffi Lissin, L. D. (1998). Modelling Periphyton Dynamics in Running Water. Ecol. Model. 114, 35–47. doi:10.1016/s0304-3800(98)00113-6
Shevchenko, T. F., Klochenko, P. D., Timchenko, V. M., and Dubnyak, S. S. (2019). Epiphyton of a cascade plain Reservoir under Different Hydrodynamic Conditions. Ecohydrol. Hydrobiol. 19, 407–416. doi:10.1016/j.ecohyd.2019.01.006
Smucker, N. J., and Vis, M. L. (2009). Use of Diatoms to Assess Agricultural and Coal Mining Impacts on Streams and a Multiassemblage Case Study. J. North Am. Benthological Soc. 28, 659–675. doi:10.1899/08-088.1
Spoljar, M., Zhang, C., Drazina, T., Zhao, G., Lajtner, J., and Radonic, G. (2017). Development of Submerged Macrophyte and Epiphyton in a Flow-Through System: Assessment and Modelling Predictions in Interconnected Reservoirs. Ecol. Indic. 75, 145–154.
Tett, P., Gallegos, C., Kelly, M. G., Hornberger, G. M., and Cosby, B. J. (1978). Relationships Among Substrate, Flow, and Benthic Microalgal Pigment Density in the Mechums River, Virginia 1. Limnol. Oceanogr. 23, 785–797. doi:10.4319/lo.1978.23.4.0785
Townsend, S. A., Garcia, E. A., and Douglas, M. M. (2012). The Response of Benthic Algal Biomass to Nutrient Addition over a Range of Current Speeds in an Oligotrophic River. Freshw. Sci. 31, 1233–1243. doi:10.1899/11-163.1
Vadeboncoeur, Y., Devlin, S. P., McIntyre, P. B., and Vander Zanden, M. J. (2014). Is There Light after Depth? Distribution of Periphyton Chlorophyll and Productivity in lake Littoral Zones. Freshw. Sci. 33, 524–536. doi:10.1086/676315
Warren, D. R., Collins, S. M., Purvis, E. M., Kaylor, M. J., and Bechtold, H. A. (2017). Spatial Variability in Light Yields Colimitation of Primary Production by Both Light and Nutrients in a Forested Stream Ecosystem. Ecosystems 20(1), 198–210. doi:10.1007/s10021-016-0024-9
Wilby, R. L., Cranston, L. E., and Darby, E. J. (1998). Factors Governing Macrophyte Status in Hampshire Chalk Streams: Implications for Catchment Management. Water Environ. J. 12, 179–187. doi:10.1111/j.1747-6593.1998.tb00170.x
Wolters, J. W., Reitsema, R. E., Verdonschot, R. C. M., Schoelynck, J., Verdonschot, P. F. M., and Meire, P. (2019). Macrophyte‐specific Effects on Epiphyton Quality and Quantity and Resulting Effects on Grazing Macroinvertebrates. Freshw. Biol. 64, 1131–1142. doi:10.1111/fwb.13290
Woodruff, S. L., House, W. A., Callow, M. E., and Leadbeater, B. S. C. (1999). The Effects of Biofilms on Chemical Processes in Surficial Sediments. Freshw. Biol. 41, 73–89. doi:10.1046/j.1365-2427.1999.00387.x
Yang, H., Li, H., and Li, L. (2017). Study on Temporal and Spatial Variation of Phosphorus Load in Erhai Inflow Riversand Eutrophication in Erhai Lake in 2015. PEARL RIVER. 38 (7), 77–79. doi:10.3969/j.issn.1001-9235
Keywords: flooding, current velocity, suspended matter, silicate, periphyton community structure
Citation: Ren W, Yao Y, Zhang Z, Cao Y, Yuan C, Wang H, Chou QC, Ni L, Zhang X and Cao T (2021) Changes of Periphyton Abundance and Biomass Driven by Factors Specific to Flooding Inflow in a River Inlet Area in Erhai Lake, China. Front. Environ. Sci. 9:680718. doi: 10.3389/fenvs.2021.680718
Received: 15 March 2021; Accepted: 10 May 2021;
Published: 28 May 2021.
Edited by:
Teresa Ferreira, University of Lisbon, PortugalReviewed by:
Haoping Wu, Guangdong University of Technology, ChinaJoanna Rosińska, Poznan University of Medical Sciences, Poland
Copyright © 2021 Ren, Yao, Zhang, Cao, Yuan, Wang, Chou, Ni, Zhang and Cao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolin Zhang, emhhbmd4bEBpaGIuYWMuY24=; Te Cao, Y2FvdGVAaWhiLmFjLmNu
 Wenjing Ren
Wenjing Ren Yiqian Yao1,2
Yiqian Yao1,2 Yu Cao
Yu Cao Hao Wang
Hao Wang Qing Chuan Chou
Qing Chuan Chou Leyi Ni
Leyi Ni Xiaolin Zhang
Xiaolin Zhang Te Cao
Te Cao