- 1Department of Environment, Land and Infrastructure Engineering (DIATI), Polytechnic of Turin, Turin, Italy
- 2Department of Life Sciences and Systems Biology (DBios), University of Turin, Turin, Italy
- 3European Commission Joint Research Centre, Ispra, Italy
The MesoHABitat SImulation Model (MesoHABSIM) is the preferred method to calculate spatio-temporal variation in the fish habitat availability in Italian rivers. With the aim of improving the applicability of the MesoHABSIM approach in the Italian territory, we carried out a systematic review of physical habitat preferences for 31 freshwater fish species and three freshwater lampreys, representing 75% of the total indigenous freshwater fish community of Italy. Information related to suitable ranges of depth, flow velocity, biotic/abiotic substrates, covers/shelters was collected and summarized for two critical life stages (adult and juvenile) and two bioperiods (rearing/growth and spawning). Overall, 250 publications were reviewed, classified as 206 peer-reviewed papers, 20 books, 7 PhD thesis, and 17 grey literature sources. Our analysis revealed substantial deficits of information about habitat requirements for more than 30% of Italian freshwater fish species. This information is particularly scarce for the most threatened endemic species, especially for their most critical bioperiod (i.e., spawning). With the aim of preserving freshwater fish biodiversity as required in the EU Biodiversity Strategy for 2030 (European Commission, 2020), accurate information on physical habitat requirements for spawning is crucial. As an example application of MesoHABSIM, the collected habitat preference information was used to define and apply mesohabitat suitability criteria for one fish species (Telestes muticellus) in a regulated river reach of Argentina Creek (Province of Imperia, Italy). This analysis demonstrates the potential for applying information from the current review to other fish species.
Introduction
Preserving freshwater ecosystems is one of the most difficult challenges humans face globally. Although these ecosystems provide crucial services to society, they are one of the world’s most threatened environments (Dudgeon et al., 2006). Hydrological and morphological alterations related to water abstractions and hydropower generation are identified as high impact pressures causing degradation of river ecosystems and biodiversity loss (Renöfält et al., 2010). Since the 1980s, Habitat Suitability Models (HSMs), such as PHABSIM (Bovee, 1982), have been promoted and applied to enhance water resources management and preserve aquatic ecosystems. Among these models, the recent MesoHABitat SImulation Model (MesoHABSIM, Parasiewicz, 2001), has proven to be adequate for assessing habitat availability for riverine fish (Yi et al., 2017). The MesoHABSIM model, commonly, uses habitat suitability criteria, or species distribution models, to predict the amount of available habitat for a certain species or life stage in regulated rivers (Parasiewicz et al., 2013). The habitat suitability criteria can be built using empirical data collected in environmental reference sites with natural conditions of river morphology, flow regime, fish community composition (Vezza et al., 2014a; Muñoz-Mas et al., 2016). Alternatively, these criteria can be derived by collecting available information from the literature or using expert knowledge (Parasiewicz et al., 2013; Koutrakis et al., 2019). Despite its critical relevance, the availability of such biological information is still scattered and limited to the most common species or those of particular economic value (e.g., Alcaraz-Hernández et al., 2016). This results in a reduced capacity to widely implementing HSMs in regulated rivers, to ensure adequate habitat for the entire fish community, especially for the most critical life stages.
Starting from 2017, HSMs have been increasingly used in Italy. The Decrees n. 29/STA-13.02.2017 and n. 30/STA-13.02.2017 of the Ministry for Environment, Land and Sea Protection established the MesoHABSIM as a reference method for environmental flows design and impact assessment of water abstractions (Vezza et al., 2017). MesoHABSIM is a methodological approach that uses geomorphic units (GUs or mesohabitats, Belletti et al., 2017) as the spatial unit of analysis. After segmenting the river into homogeneous hydro-morphological reaches, a multiple, stage-dependent description of GUs provides basic maps, which are used to calculate the spatio-temporal variation in aquatic habitat availability. Standardized data collection is required to describe GUs in terms of wetted area, frequency distribution of water depth, flow velocity and substrate, presence of cover, water surface gradient, Froude number and velocity standard deviation.
With the aim of improving the applicability of the MesoHABSIM approach in the Italian territory, we carried out a systematic review focused on physical habitat preferences for 31 freshwater fish species and three freshwater lampreys of Italian rivers. Collected information was organized into a nationwide database and summarized using the MesoHABSIM protocol and standards. As an example of application, two literature-based biological models for adult and juvenile life stages of Italian vairone (Telestes muticellus) were built and used to assess fish habitat availability in a regulated reach of the Argentina Creek (Province of Imperia, NW Italy). This analysis demonstrates the potential for applying information from the current review to other fish species.
Materials and Methods
Literature Review
In an effort to develop quantitative relationships between physical habitat conditions and the distribution of autochthonous Italian fish species, we reviewed 250 bibliography sources published over the last 8 decades, describing habitat preferences of 34 autochthonous freshwater species (31 Osteichthyes and 3 Petromyzontiformes). These species (Figure 1) represent the 75% of the total indigenous freshwater fish community of Italy (Bianco, 1995). Due to the scope of the paper, we focused exclusively on riverine species. As habitat use by freshwater fish shifts during their life cycle (Gaudin, 2001), we explored information on habitat requirements for two main life stages (adult and juvenile) and two bioperiods (rearing/growth and spawning). For catadromous and anadromous species (e.g., Anguilla anguilla and Alosa fallax), we considered only the freshwater phase of corresponding life cycles. Furthermore, to simplify the data analysis, the juvenile stage of freshwater lampreys was associated to the ammocoete phase.
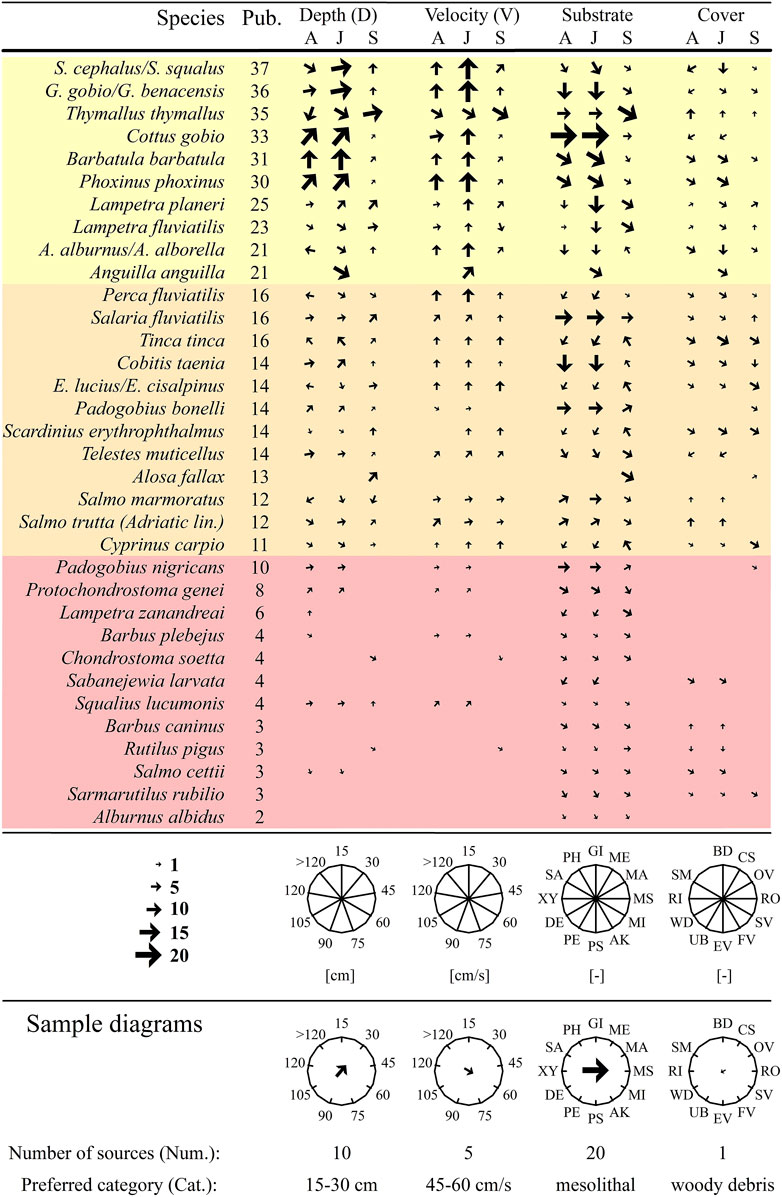
FIGURE 1. List of autochthonous Italian freshwater species included in the study. The orientation of the arrows suggests the preferred category (Cat.) of habitat parameters for each species, life stage (A = adult, J = juvenile) and bioperiod (S = spawning), and the size expresses the corresponding number of sources (Num.) reporting the same information. For a clearer depiction of the result, species are sorted according to the corresponding number of bibliography sources found (Pub.). Background colors are used to highlight the three groups of species: species with quite copious information (yellow), species with limited available data (orange), and species with very poor information (red). Water depth (D) and current velocity (V) are represented by nine categories of 15 cm (from 0 up to >120 cm) and 15 cm/s (from 0 up to >120 cm/s) respectively. Substrate categories codes: GI, gigalithal; ME, megalithal; MA, macrolithal; MS, mesolithal; MI, microlithal; AK, akal; PS, psammal; PE, pelal; DE, detritus; XY, xylal; SA, sapropel; PH, phytal. Cover categories codes: BD, boulder; CS, canopy shading; OV, overhanging vegetation; RO, roots; SV, submerged vegetation; FV, floating vegetation; EV, emerging vegetation; UB, undercut bank; WD, woody debris; RI, riprap; SM, shallow margins. For a better understanding, sample diagrams are given at the bottom of the Figure. The numerical values depicted as oriented arrows are reported in Supplementary Table S3 in the Supplementary Material.
We exclusively considered researches performed in European rivers within the distribution area of the species as defined in Kottelat and Freyhof (2007). For Salmo trutta, we tried to purely focus to the Mediterranean/Adriatic lineage (Adriatic lin., Bernatchez, 2001) by exploring only researches performed within the circum-Mediterranean basins of Europe. To collect all available information, pubblications concerning 1) fish habitat preferences/use, 2) species distribution models, and 3) autoecological studies at different spatial scales (micro-, meso-, and macro-scale) were considered as suitable for the present study and reliable sources of information. Four environmental parameters, largely recognized as mainly influencing the patterns of habitat use by freshwater fish in rivers (Parasiewicz, 2007), were considered as target physical habitat descriptors: 1) water depth, 2) current velocity, 3) biotic/abiotic substrates, 4) covers/shelters. For water depth and velocity values, a source was included in the database only if quantitative information was present and, for every species, the entire range of reported values was considered. Concerning substrates and covers, the review revealed a significant difference in the classification and taxonomy adopted in each source. Therefore, prior to the database compilation, a standardizing process was implemented to ensure data consistency. It is important to highlight that a source could provide information for more than one species, life stages, bioperiods or parameters at once. The sources search was mainly carried out by leveraging the database of Web of Knowledge, Scopus and Google Scholar, as well as exploring the grey literature (Supplementary Table S4). Literature screening was achieved by using as search strings a combination of species’ scientific/common names, both in English and Italian language, and several habitat-related statements (see Supplementary Table S1 for details).
Data Analysis
The collected information were stored in a nationwide, easily accessible database, keeping track of the corresponding bibliography source, year of publication, river or study area, species, life stage, bioperiod. These data were then summarized into species-specific boards and organized according to life stages and bioperiods. During this step, habitat parameters were systematically stored and noted according to the MesoHABSIM protocol and standards (see Supplementary Table S2 for details). Specifically, water depth and velocity values were processed according to their frequency distribution and split in nine categories, respectively in 15 and 15 cm/s increments (range 0–120 cm, or cm/s, and above). Data on biotic/abiotic substrates were regrouped into 12 categories, following the classification proposed by Hauer et al. (2006), as reported in the MesoHABSIM standards. Finally, information concerning covers/shelters were summarized into 11 categories (Vezza et al., 2017).
For each species, life stages, bioperiods, and for every single category of the habitat parameters, it was therefore possible to calculate the number of sources reporting the same information. In this way, we could quantify the current available knowledge on habitat preferences of Italian freshwater fish and to estimate the preferred categories of habitat parameters for each species. Species or life stages for which information on habitat preferences were rare or not available were clearly derived and highlighted (Figure 1).
Application of the MesoHABSIM Model
With the aim of highlighting the capabilities of the current review, two biological models were developed for adult and juvenile life stages of T. muticellus for their application in the MesoHABSIM modelling approach (Figures 2A,D). The information collected from literature was used to define conditional habitat suitability criteria, according to the indications reported in Parasiewicz et al. (2013). Two binary presence/absence models were designed to distinguish between suitable and not suitable mesohabitats and depicted, for clarity, using decision trees (see e.g., Koutrakis et al., 2019). The conditional models were built using the highest frequencies of habitat parameters reported in the literature. In addition, the mesohabitat was considered as suitable by imposing that at least 25% of the mesohabitat area should be characterized by a certain range of water depth, velocity, substrates, and a certain category of cover. The developed models were applied to a regulated reach of the Argentina Creek (Province of Imperia, NW of Italy), where geo-spatial information on GUs composition and empirical data on fish community were available. In 2015 a complete application of the MesoHABSIM methodology (Vezza et al., 2014b; Vezza et al., 2017) was carried out in the Argentina Creek to assess the environmental impact of a hydropower plant. From this data collection campaign, we extracted two hydro-morphological surveys carried out at 0.25 m3/s and 2.2 m3/s. Fish data were collected with backpack electrofishing, by sampling 20 GUs at the lowest flow condition (0.25 m3/s), assessing the presence/absence of adult and juvenile T. muticellus within each GUs (see Supplementary Material for details). This information was then used to validate the prediction performances of the developed biological models for adult and juvenile T. muticellus. The validation was evaluated using four performance metrics, i.e., accuracy, sensitivity, specificity, and true skill statistic (TSS), which are commonly used in species distribution models (Vezza et al., 2015b). The validated presence/absence models were finally applied and the habitat availability for adult and juvenile T. muticellus was calculated at the two considered discharges (Figures 2B,C,E,F).
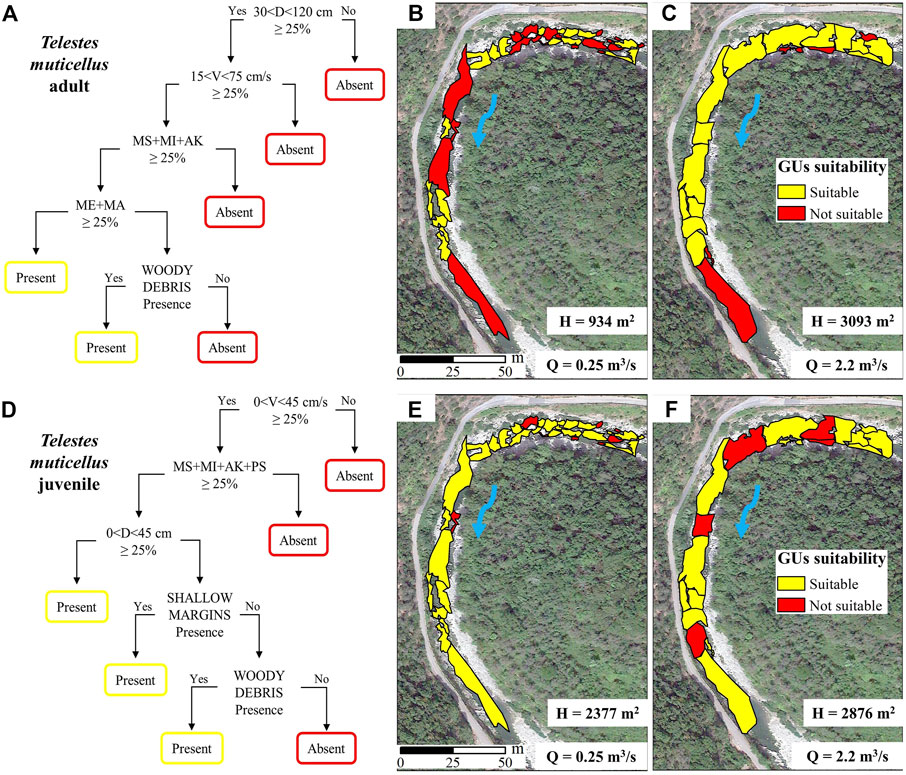
FIGURE 2. Presence/absence biological models for (A) adult and (D) juvenile life stages of Telestes muticellus, depicted by using a decision tree representation. Habitat suitability maps expressing the available habitat (H) assessed for adults T. muticellus at (B) 0.25 m3/s and (C) 2.2 m3/s, and for juveniles T. muticellus at (E) 0.25 m3/s and (F) 2.2 m3/s, in the Argentina Creek.
Results
Overall, we considered 250 bibliography sources, most of which (206) were peer-reviewed researches and the remaining were represented by books or book sections (20), PhD thesis (7) and grey literature sources (i.e., governmental or NGOs reports; 17). These bibliographic sources are listed in Supplementary Table S4, provided in the Supplementary Material.
Available information on habitat preferences highly varied depending on the species (Figure 1). The highest number of publications was found for Squalius cephalus (37 Pub.), followed by Gobio gobio (36) and Thymallus thymallus (35). Conversely, very little information was found for several endemic Italian species (e.g., Sarmarutilus rubilio, Salmo cettii, Rutilus pigus, Barbus caninus: 3; Alburnus albidus: 2). From our analysis, Italian freshwater fish species can be classified into three groups, depending on the available knowledge and amount of publications. The first group can be composed by 10 species (from S. cephalus to A. anguilla, yellow group in Figure 1) with quite copious available information on habitat preferences (>20 Pub.) for almost all considered parameters, life stages and bioperiods. The second group (from Perca fluviatilis to Cyprinus carpio, orange group in Figure 1) comprises 12 species for which available data were found to be limited (10 < Pub. ≤20), and not covering all considered life stages and bioperiods. The third group (from Padogobius nigricans to Alburnus albidus, red group in Figure 1) is composed by the remaining 12 species for which information resulted very poor (≤10 Pub.) and several life stages or bioperiods exhibit absence of information. It is interesting to note that the third group is exclusively composed by purely Italian endemic species.
Considering life stages and bioperiods, the collected information on the rearing/growth bioperiod for juveniles (176 Pub.) and adults (135) was higher compared to those referring to spawning habitat preferences (98). Furthermore, focusing on the considered habitat parameters, we observed a greater amount of information regarding substrate preferences (208 Pub.), rather than depth (169), velocity (142) or cover (107). Preferred categories of water depth (D), varied significantly according to life stages and bioperiods (Figure 1). Moderate to high depth (from 30 to 120 cm) resulted suitable for the majority of adult species (24 species, 70% of the total). Conversely, for juvenile fish and spawning bioperiod, 26 species (77% of total) preferred shallow to medium water depth (from 0 to 75 cm). For velocity (V) no species exhibited a preference for a flow rate higher than 75 cm/s. Concerning abiotic substrates, for 22 species (65% of the total), the most preferred categories ranged from akal (AK) to macrolithal (MA), whereas for Cobitidae, Petromyzontidae and few species of Cyprinidae the preferred substrates were psammal (PS) and pelal (PE). For spawning, 26 species (76% of the total) preferred coarser grain sizes, mainly akal (AK), microlithal (MI) and mesolithal (MS). Phytal (PH) as spawning substrate was recorded for six species (18% of the total) of Cyprinidae, Cobitidae, and Esocidae. Finally, submerged vegetation (SV), boulder (BD), and woody debris (WD) were found to be the preferred covers for 20, four and two species, respectively.
Moderate to high water depth values (from 30 to 120 cm) and moderate velocities (from 15 to 75 cm/s) were found to be appropriate habitat parameters for the adult stage of T. muticellus. Whereas, its juvenile stage prefers shallow areas (water depth <45 cm) and low velocities (<45 cm/s). Considering substrate, coarser material (mainly AK, MI, and MS) resulted mostly preferred by both life stages, whereas WD was found to be the preferred cover (Figures 2A,D). The validation performance of conditional models for T. muticellus was quite good and considered acceptable: accuracy 67 and 81%, sensitivity 53 and 78%, specificity 100 and 100%, and TSS 53 and 78%, respectively for adult and juvenile life stages. Habitat availability (H) varied significantly with respect to the analyzed flow conditions, increasing from 934 m2 at 0.25 m3/s to 3,093 m2 at 2.2 m3/s (Figure 2B,C). Available habitat for juvenile T. muticellus was almost similar between the two surveyed discharges, showing a slight increase from 2,377 m2 at 0.25 m3/s to 2,876 m2 at 2.2 m3/s (Figure 2E,F). At 2.2 m3/s the GUs classified as not suitable (Figure 2F, red areas) for juvenile T. muticellus were mostly related to rapids, characterized by velocities much higher than 45 cm/s.
Discussion
The systematic review of habitat preferences for Italian freshwater fish revealed substantial deficits in knowledge and data availability for the considered species. In particular, for more than 30% of the Italian fish, such information resulted remarkably scarce (≤10 Pub.). Generally, a higher number of publications was found for species with a higher economic value or a larger distribution area. Indeed, the first group (from S. cephalus to A. anguilla in Figure 1) is composed by largely studied species that are distributed in more than 80% of European countries. Conversely, the third group (from P. nigricans to A. albidus in Figure 1) resulted exclusively consisting of the most threatened endemic species of the Italian territory, whose distribution ranges are restricted to certain Italian watercourses (Kottelat and Freyhof, 2007). These results are consistent with previous researches which pointed out that species with a higher socio-economic importance are generally better investigated (Smialek et al., 2019). In our opinion, future research and investments should be directed to study the habitat of endemic Italian species, to plan and better design habitat restoration actions.
A critical issue is increasing the knowledge related to spawning habitat requirements. Although reproduction is a crucial phase for freshwater fish life cycle, surprisingly we observed a general lack of information for this bioperiod. In particular, the information we found mainly derived from historical data and publications (e.g., Hardisty, 1944) or ecological reviews (e.g., Vriese et al., 1994; Mann, 1996) carried out in the Northern part of Europe. This indicates that spawning habitat requirements for Italian freshwater fish are not sufficiently investigated. Actually, we found only 11 studies (4% of the total) carried out in Italian rivers, which means, on average, less than one study per species. With the aim of preserving freshwater fish biodiversity as reported in the EU Biodiversity Strategy for 2030 (European Commission, 2020), accurate information on physical habitat requirements for spawning are crucially required. In this regard, this review can provide important insights to direct future researches on this significant topic.
Although we encountered several data regarding substrate preferences for almost all considered species and life stages, available information on the remaining environmental parameters was found to be more scattered. The data collection strategies, adopted in the considered publications for characterizing physical river habitat, seems to influence the amount of available information. For instance, for substrate classification, semi-quantitative procedures which use simple visual estimation were found to be extensively used (e.g., Copp, 1997; Klaar et al., 2004). This resulted in a larger amount of data regarding fish substrate preferences. Nevertheless, substrate classification was found to largely differ among the considered studies, making the comparison and aggregation of information difficult. For water depth and velocity, several publications reported only bulk estimation of mean values over an entire river reach, which can be considered a poor and low quality source of information in terms of physical habitat preferences (e.g., Giannetto et al., 2013). Finally, with respect to covers, the lower amount of data can be mainly related to the scale and spatial resolution used by the considered studies to analyze fish habitat requirements. This was specifically due to the wider application of micro-habitat suitability models (e.g., PHABSIM, Bovee, 1982), in which covers/shelters are not usually taken into account (e.g., Lamouroux et al., 1999).
Meso-scale HSMs derived from literature demonstrated high effectiveness in predicting fish distribution in the Argentina Creek. This result was in line with previous studies (Adamczyk et al., 2019; Koutrakis et al., 2019) that used literature information to infer habitat suitability for fish. However, it is important to state that biological models derived from literature may be currently built in Italy only for the first and second group of species (from S. cephalus to C. carpio in Figure 1). Due to the very limited amount of information we would exclude to build habitat suitability criteria for species of group three (from P. nigricans to A. albidus in Figure 1).
The MesoHABSIM approach has already proved high potential in assessing habitat availability for freshwater species in various hydro-morphological contexts of European rivers (Vezza et al., 2014b; Acuña et al., 2020). In particular, in Italy such model is currently used for impact assessment of hydropower and e-flows design in regulated rivers (Vezza et al., 2015a; Vezza et al., 2017). Nevertheless, one of the most important limitation for implementing MesoHABSIM to the entire Italian territory consists in a limited amount of available biological models, for which extensive field data collection and analysis are currently required. In this regard, the present systematic literature review represents a notable step forward. The analysis carried out for T. muticellus, can be repeated for other species and further literature-based models could be defined based on the collected information. However, we suggest that biological models derived from literature should be always locally validated before using them in impact assessment and e-flows design procedures.
Author Contributions
Conception and design of the study: PV. Data collection: GN, EQ, PV, CC, and IG. Fundamental support and supervision: SF. Drafting the manuscript: GN and PV. Revising the manuscript: SF, CC, EQ, and IG. All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Funding
This work was partially supported by the River Po Basin Authority and the Biodiv’ALP project.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank David Farò from the University of Trento for his support in finding papers written in German for grayling (Thymallus thymallus).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2021.634737/full#supplementary-material
References
Acuña, V., Jorda‐Capdevila, D., Vezza, P., De Girolamo, A. M., McClain, M. E., Stubbington, R., et al. (2020). Accounting for Flow Intermittency in Environmental Flows Design. J. Appl. Ecol. 57, 742–753. doi:10.1111/1365-2664.13590
Adamczyk, M., Parasiewicz, P., Vezza, P., Prus, P., and De Cesare, G. (2019). Empirical Validation of MesoHABSIM Models Developed with Different Habitat Suitability Criteria for Bullhead Cottus gobio L. As an Indicator Species. Water 11, 726. doi:10.3390/w11040726
Alcaraz-Hernández, J. D., Muñoz-Mas, R., Martínez-Capel, F., Garófano-Gómez, V., and Vezza, P. (2016). Generalized Additive Models to Predict Adult and Young Brown trout (Salmo trutta Linnaeus, 1758) Densities in Mediterranean Rivers. J. Appl. Ichthyol. 32, 217–228. doi:10.1111/jai.13025
Belletti, B., Rinaldi, M., Bussettini, M., Comiti, F., Gurnell, A. M., Mao, L., et al. (2017). Characterising Physical Habitats and Fluvial Hydromorphology: A New System for the Survey and Classification of River Geomorphic Units. Geomorphology 283, 143–157. doi:10.1016/j.geomorph.2017.01.032
Bernatchez, L. (2001). The Evolutionary History of Brown trout (Salmo trutta L.) Inferred from Phylogeographic, Nested Clade, and Mismatch Analyses of Mitochondrial DNA Variation. Evolution 55, 351–379. doi:10.1111/j.0014-3820.2001.tb01300.x
Bianco, P. G. (1995). Mediterranean Endemic Freshwater Fishes of Italy. Biol. Conservation 72, 159–170. doi:10.1016/0006-3207(94)00078-5
Bovee, K. D. (1982). A Guide to Stream Habitat Analysis Using the Instream Incremental Flow Methodology. Fort Collins, Colorado (USA).
Copp, G. H. (1997). Microhabitat Use of Fish Larvae and 0+ Juveniles in a Highly Regulated Section of the River Great Ouse. Regul. Rivers: Res. Mgmt. 13, 267–276. doi:10.1002/(SICI)1099-1646(199705)13:3<267::AID-RRR454>3.0.CO;2-B
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z.-I., Knowler, D. J., Lévêque, C., et al. (2006). Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. 81, 163–182. doi:10.1017/S1464793105006950
European Commission (2020). Biodiversity Strategy for 2030 - Bringing Nature Back into Our Lives. Brussels (BE): Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions.
Gaudin, P. (2001). Habitat Shifts in Juvenile Riverine Fishes. rs 12, 393–408. doi:10.1127/lr/12/2001/393
Giannetto, D., Carosi, A., Ghetti, L., Pedicillo, G., Pompei, L., and Lorenzoni, M. (2013). Ecological Traits ofSqualius Lucumonis (Actinopterygii, Cyprinidae) and Main Differences with Those of Squalius Squalusin the Tiber River Basin (Italy). Knowl. Managt. Aquat. Ecosyst. 409, 04. doi:10.1051/kmae/2013049
Habersack, H., Liedermann, M., and Hauer, C. (2006). The Application of Hydrodynamic Models as Integrative Tool between Flood protection and Ecological Demands Using a Morphologically Based Evaluation Method. Proc. Int. Conf. Fluv. Hydraul. - River Flow 2, 2001–2006. doi:10.1201/9781439833865.ch219
Hardisty, M. W. (1944). The Life History and Growth of the Brook Lamprey (Lampetra planeri). J. Anim. Ecol. 13, 110. doi:10.2307/1444
Klaar, M., Copp, G. H., and Horsfield, R. (2004). Autumnal Habitat Use of Non-native Pumpkinseed Lepomis gibbosus and Associations with Native Fish Species in Small English Streams. Folia Zool 53, 189–202.
Kottelat, M., and Freyhof, J. (2007). Handbook of European Freshwater Fishes. Cornol (CH): Publications Kottelat.
Koutrakis, E. T., Triantafillidis, S., Sapounidis, A. S., Vezza, P., Kamidis, N., Sylaios, G., et al. (2019). Evaluation of Ecological Flows in Highly Regulated Rivers Using the Mesohabitat Approach: A Case Study on the Nestos River, N. Greece. Ecohydrology & Hydrobiology 19, 598–609. doi:10.1016/j.ecohyd.2018.01.002
Lamouroux, N., Capra, H., Pouilly, M., and Souchon, Y. (1999). Fish Habitat Preferences in Large Streams of Southern France. Freshw. Biol. 42, 673–687. doi:10.1046/j.1365-2427.1999.00521.x
Mann, R. H. K. (1996). Environmental Requirements of European Non-salmonid Fish in Rivers. Hydrobiologia 323, 223–235. doi:10.1007/BF00007848
Muñoz-Mas, R., Fukuda, S., Vezza, P., and Martínez-Capel, F. (2016). Comparing Four Methods for Decision-Tree Induction: A Case Study on the Invasive Iberian Gudgeon (Gobio Lozanoi ; Doadrio and Madeira, 2004). Ecol. Inform. 34, 22–34. doi:10.1016/j.ecoinf.2016.04.011
Parasiewicz, P. (2001). MesoHABSIM: A Concept for Application of Instream Flow Models in River Restoration Planning. Fisheries 26, 6–13. doi:10.1577/1548-8446(2001)026<0006:m>2.0.co;2
Parasiewicz, P., Rogers, J. N., Vezza, P., Gortázar, J., Seager, T., Pegg, M., et al. (2013). “Applications of the MesoHABSIM Simulation Model,” in Ecohydraulics: An Integrated Approach (Chichester, UK: John Wiley & Sons, Ltd)), 109–124. doi:10.1002/9781118526576.ch6
Parasiewicz, P. (2007). The MesoHABSIM Model Revisited. River Res. Applic. 23, 893–903. doi:10.1002/rra.1045
Renöfält, B. M., Jansson, R., and Nilsson, C. (2010). Effects of Hydropower Generation and Opportunities for Environmental Flow Management in Swedish Riverine Ecosystems. Freshw. Biol. 55, 49–67. doi:10.1111/j.1365-2427.2009.02241.x
Smialek, N., Pander, J., Mueller, M., van Treeck, R., Wolter, C., and Geist, J. (2019). Do we Know Enough to Save European Riverine Fish?-A Systematic Review on Autecological Requirements during Critical Life Stages of 10 Rheophilic Species at Risk. Sustainability 11, 5011. doi:10.3390/su11185011
Vezza, P., Goltara, A., Spairani, M., Zolezzi, G., Siviglia, A., Carolli, M., et al. (2015a). “Habitat Indices for Rivers: Quantifying the Impact of Hydro-Morphological Alterations on the Fish Community,” in Engineering Geology For Society And Territory - Volume 3: River Basins, Reservoir Sedimentation and Water Resources. Editors G. Lollino, M. Arattano, M. Rinaldi, O. Giustolisi, J. C. Marechal, and G. Grant (Cham (CH): Springer International Publishing), 357–360. doi:10.1007/978-3-319-09054-2_75
Vezza, P., Muñoz-Mas, R., Martinez-Capel, F., and Mouton, A. (2015b). Random Forests to Evaluate Biotic Interactions in Fish Distribution Models. Environ. Model. Softw. 67, 173–183. doi:10.1016/j.envsoft.2015.01.005
Vezza, P., Parasiewicz, P., Calles, O., Spairani, M., and Comoglio, C. (2014a). Modelling Habitat Requirements of Bullhead (Cottus gobio) in Alpine Streams. Aquat. Sci. 76, 1–15. doi:10.1007/s00027-013-0306-7
Vezza, P., Parasiewicz, P., Spairani, M., and Comoglio, C. (2014b). Habitat Modeling in High-Gradient Streams: The Mesoscale Approach and Application. Ecol. Appl. 24, 844–861. doi:10.1890/11-2066.1
Vezza, P., Zanin, A., and Parasiewicz, P. (2017). Manuale tecnico-operativo per la modellazione e la valutazione dell’integrità dell’habitat fluviale. Roma (IT).
Vriese, F. T., Semmekrot, S., and Raat, A. J. P. (1994). Assessment of Spawning and nursery Areas in the River Meuse. Water Sci. Tech. 29, 297–299. (Publ by Pergamon Press Inc). doi:10.2166/wst.1994.0124
Keywords: freshwater fish, habitat preferences, habitat suitability models, MesoHABSIM, Italy, endemic species
Citation: Negro G, Fenoglio S, Quaranta E, Comoglio C, Garzia I and Vezza P (2021) Habitat Preferences of Italian Freshwater Fish: A Systematic Review of Data Availability for Applications of the MesoHABSIM Model. Front. Environ. Sci. 9:634737. doi: 10.3389/fenvs.2021.634737
Received: 28 November 2020; Accepted: 16 July 2021;
Published: 27 July 2021.
Edited by:
Angela Helen Arthington, Griffith University, AustraliaReviewed by:
Jeyaraj Antony Johnson, Wildlife Institute of India, IndiaFernando Mayer Pelicice, Federal University of Tocantins, Brazil
Copyright © 2021 Negro, Fenoglio, Quaranta, Comoglio, Garzia and Vezza. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Giovanni Negro, Z2lvdmFubmkubmVncm9AcG9saXRvLml0
 Giovanni Negro
Giovanni Negro Stefano Fenoglio2
Stefano Fenoglio2 Emanuele Quaranta
Emanuele Quaranta Claudio Comoglio
Claudio Comoglio Paolo Vezza
Paolo Vezza