
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 29 January 2021
Sec. Soil Processes
Volume 8 - 2020 | https://doi.org/10.3389/fenvs.2020.585464
This article is part of the Research TopicDesertification and RehabilitationView all 16 articles
Salinization is a major threat to the sustainability of land and water resources, especially in arid and semiarid regions. Understanding the water uptake from different soil depths for desert plants is useful for exploring salinity-tolerance mechanism in desert plants in extremely-arid and salinity-affected area. To understand water uptake from different soil depths for desert plants in Dunhuang, NW China, we used oxygen isotope composition in plant xylem water and soil water to determine the water sources in three different saline sites differing in their degree of soil electrical conductance (site 2 < site 1 < site 3). The co-existing desert plants in each saline site extracted different depth of soil water respectively: K. foliatum mainly used shallow soil water (0–20 cm); H. caspica and N. tangutorum mainly used deep soil water (40–200 cm); A. sparsifolia used water from the 120–200 cm soil layers, while T. ramosissima and E. angustifolia mainly extracted deeper soil water (>200 cm). Compared to that in saline site 2, Tamarix ramosissima and Alhagi sparsifolia can switch their water sources to deeper soil water when enduring more salt stress. Also, a significant and positive correlation between soil EC and soil water δ18O values was observed, indicating the evaporation would cause increase in salt concentration and isotopic enrichment in the upper soil profile. Overall, our results suggest that plants may explore deeper soil water to adapt to salt stress under severe salinity. This work may contribute to selecting salt-tolerant plants species which is vital to saline soil rehabilitation and utilization.
Saline soil, as an important soil resource, amounts to more than 800 million hectares, which comprises over 6% of the world’s total land area (Munns and Tester, 2008). Presently, the total area of saline soil resources is about 1.0×108 hm2 in China (Wang, 1993), and the saline lands account for 7.74% of the total land area in Dunhuang, NW China (Sang, 2006). It is clear that abundant ecological, economic and social benefits could be attained if these saline lands are remedied and developed sufficiently. In recent decades, climate changes have profoundly affected natural and human systems (Field and Barros, 2014). Thereinto, climatic warming has intensified soil moisture evaporation and drives the soil salt to move upward, which exacerbate soil salinization (Xiao et al., 2010). There has been a temporal increase in magnitude and intensity of salt-affected soils (Qadir et al., 2000) because of irrational human practices, such as excessive fertilizer use, irrational irrigation and deforestation. To date, soil salinization has been a worldwide problem and has attached much significance by governments and scientists (Wang, 2009). Salt accumulation in the soil generally change the soil texture and decrease the soil porosity, and consequently reduce the soil aeration and water conductance, causing differences in water use among plant species.
Soil water is a key restricting factor influencing the survival of desert plants of saline lands in arid regions. Soil depths of water uptake for plants could be determined by comparing the stable isotope hydrogen (D) and oxygen compositions (18O) of soil water and plant xylem water, as the isotope composition of xylem water remains unchanged during water transport from roots to stems (Ehrlinger and Dawson, 1992; Dawson et al., 2002; Šantrůček et al., 2007). In desert ecosystems, coexisting plant species may absorb water from different sources, such as soil water at different depths, groundwater, rain, etc. The differences in root distribution have been considered to be the mechanisms for the coexistence of diversified plant species in desert ecosystems (Zhou et al., 2013; Tiemuerbieke et al., 2018). For example, most shallow-rooted grasses take advantage of water in the shallow soil layers, while shrubs utilize a deeper soil water (Soriano and Sala, 1984). Moreover, some plant species shift soil depth of water uptake under altered environmental conditions. Chen et al. (2017) reported that the main water sources for Caragana microphylla shifted from topsoil to deeper soil in the dry season. Zhu et al. (2014) found that the 3-year-old Tamarix ramosissima Ledeb. and Lycium barbarum L. accessed more water in the deep profile after applying irrigation. Zhai et al. (2016) made a prediction of plant vulnerability to salinity increase in a coastal ecosystem, and found that hammock trees that took up water with higher salinities, had higher δ18O values of plant stem water. In addition, high soil salinity may make plant water uptake increasingly difficult owing to the changes of soil texture (Mahajan and Tuteja, 2005; Yang et al., 2007). Therefore, plants may switch to more stable water sources under some environmental stress. Thus, plants may access more deeper soil water when suffering from increasingly salinity stress in desert systems.
To date, little is known about the water sources of desert plant species in saline lands in extremely-arid regions. Little rainfall, strong evaporation and rapid groundwater table lowering (Bai, 2009) undoubtedly affects the water utilization of desert plant in saline lands in Dunhuang. Thus, the objectives of the present study are 1) to determine the soil depth of water uptake for coexsiting desert plant species in each saline site; 2) to compare the soil depth of water uptake for common plants (Tamarix ramosissima and Alhagi sparsifolia) across different saline sites. This study may provide insight into plant-soil water relation of desert plants of saline lands in extremely arid regions and will help us to fully understand the responses of species to ongoing climate changes.
Our study sites were located in the westernmost point of Hexi Corridor of Gansu province, within Dunhuang City (39°40′–41°35′N, 92°13′–95°30′E; average altitude of 1,138 m), northwestern China. The study area is characterized by a typical warm temperate continental arid climate with low rainfall and intense evaporation. The average annual precipitation (1938–2003) was 39.8 mm, with more than 67% occurring from June to August, while the mean annual potential evapotranspiration (PET) was 2,486 mm (Zhang, 2008). The mean annual temperature was 9.8°C, and the minimum and maximum mean monthly temperatures are −15.6°C and 32.8°C, respectively (Zhang, 2008). In the sampling year of 2011, the annual precipitation was 38.3 mm, with 27.3 mm occurring in June-August (Data from the National Meteorological Information Centre, China Meteorological Administration). The predominant soils in study area are salinized silts and sands containing clay interlayers. Vegetation are representative of those occurring throughout Dunhuang area, and are dominated by T. ramosissima and A. sparsifolia. Water table in this study area is about 60 m below the surface (Cui, 2014). Three saline sites differing in their degree of soil electrical conductance (EC, site 2 < site 1 < site 3) were selected in this study. Detailed site information including locations, dominant species and their life forms, is shown in Table 1.
Plant and soil sampling took place in three sites in July 2011. At each site, three plant communities (ca. 5 × 5 m2) were randomly selected, where all sampling and measurements were conducted. 2-4 dominant plant species present in each community were studied for water uptake. Two common species, T. ramosissima and A. sparsifolia, were sampled in the three saline sites (Table 1).
Plant xylem sample were collected from the non-photosynthetic tissues during the morning period, and were immediately placed in 8 ml glass vials (National Scientific Company, United States) after removing the bark and phloem. The glass vials were sealed by parafilm (Alcan Packaging, WI, United States) and stored in a cool ice chest for delivery to the laboratory. In the laboratory, xylem samples were stored at −20°C before water extraction.
Concurrent with plant xylem sampling, three soil pits per site near studied plants were augured using a hand auger. Soil samples were collected at depths of 0–20 cm, 20–40 cm, 40–60 cm, 60–80 cm, 80–100 cm, 100–120 cm, 120–160 cm and 160–200 cm. Then each soil sample was separated into three parts for measurements for soil water content (SWC), soil EC, pH, ion content and stable isotope composition, respectively. Fresh soil for determination of SWC were sealed in soil tin. The soil samples for measurements of soil EC, pH, ion content were stored in plastic bags, then sieved and air-dried later. The soil samples for stable isotope analysis were immediately put into 10-ml screw-cap glass vials, then sealed with Parafilm and placed in a portable cooler for transporting back to the laboratory. To prevent evaporative isotopic fractionation, the soil samples for stable isotope analysis were stored in a refrigerator (at −20°C) in the laboratory until water extraction.
Water was extracted from plant stems and soil samples by cryogenic vacuum distillation (Dawson et al., 1993; Ehleringer et al., 2000; Horton et al., 2003). Water samples were measured for isotopic ratios of hydrogen (δD) and oxygen (δ18O) in an isotope ratio mass spectrometer (DELTA V Advantage, Thermo Fisher Scientific, Inc., Waltham, MA, United States) interfaced with an elemental analyzer (Flash EA1112 HT, Thermo Fisher Scientific, Inc., Waltham, MA, United States). The stable hydrogen and oxygen isotope were expressed as delta (δ) values per mil (%) relative to Vienna standard mean ocean water (V-SMOW) (Einbond et al., 1996), as shown in the following equation:
where Rsample and Rstandard are the ratio of the heavy to the light isotope in a sample and the standard, respectively. The analytical error for δD and δ18O were ±1% and ±0.2%, respectively.
Based on the similarities in δ18O values for the soil water in each layer, and δ18O values of xylem water, we divided the soil profile into five major sections (0–20 cm, 20–40 cm, 40–60 cm, 60–80 cm and 80–200 cm) in the three saline lands. The isotopic composition for each depth was according to the average value of samples within each interval. The isotope values of each interval and the xylem water were analyzed by the IsoSource software (the multi-source mass balance approach) to evaluate the contribution of each soil depth to xylem water. The IsoSource mixing model (http://www.epa.gov/wed/pages/models/stableIsotopes/isosource/isosource.htm) (Phillips and Gregg, 2003) used stable isotope values to determine the relative contributions of soil water to xylem water. The source increment was defined as 1% and mass balance tolerance was defined as 0.1%.
SWC was determined by a conventional loss-on-drying method and expressed in percentage of gravimetric water content [(g water/g dry soil) × 100%] (Wang and Chen, 2010). Electrical conductivity of 1:5 water extracts, made by adding 25 g of deionized water to 5 g of each sample (Dehaan and Taylor, 2002), were used to reflect soil EC, using a DDS-307 Conductivity Meter (LeiCi Co. Ltd., Shanghai, China). Soil pH was determined by a pH-3D pH meter (ZhiGuang Co. Ltd., Shanghai, China). Ca2+ and Mg2+ were determined by EDTA complexing titration, and Na+ and K+ contents were measured with flame spectrometry using a flame photometer (FP640; Shanghai Precision Science Instrument, China). Cl- was determined with AgNO3 titration, HCO3− and CO32− were determined using titration with hydrochloric acid, and SO42+ was indirectly determined through titration with EDTA (Qadir et al. 2007).
All statistical analyses were performed with SPSS software (version 17.0, SPSS Inc., Chicago, IL, United States). Multiple comparisons of isotope values for the soil water from all individual layers used a one-way analysis of variance (ANOVA) with Fisher’s least significant difference method. Significance was determined at the 95% confidence level (α = 0.05). Pearson’s correlation was calculated to determine the relationship between δ18O values, SWC, soil EC, and pH in soil profile. Charting was processed using the software Origin 9.0 (OriginLab Corp., Northampton, MA, United States).
Soil water content profiles varied significantly in the three saline sites (Figure 1). Overall, SWC was higher in the saline site three than that in the saline site one and saline site 2 (p < 0.05). In the saline site 1, SWC in 0–80 cm soil profile was significantly higher than that in 80–200 soil profile (p < 0.05): SWC varied little in the 0–80 cm soil layers, and showed a little decrease in 80–100 cm soil layers, and then increased in 100–200 cm soil layers. Significant soil profile differences were detected in the saline site 2 (p < 0.01): a peak of 11.3% in SWC occurred in 20–40 cm soil layer, and SWC showed little variation in 60–120 cm soil layers, and then increased steadily with depth to 11.3% again in 160–200 cm soil layers. In the saline site 3, SWC showed a decreasing trend from surface soil to deeper soil depths.
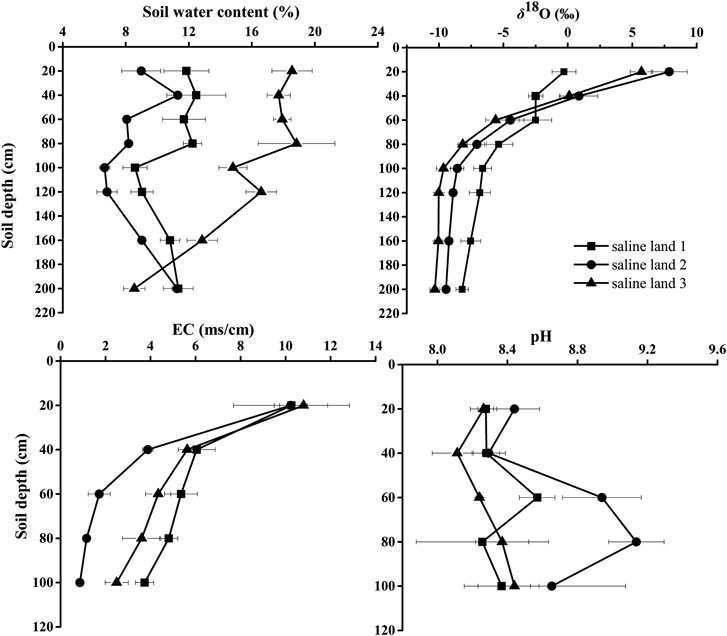
FIGURE 1. Variation in soil water content, soil water δ18O values, soil electrical conductivity (EC) and pH along soil profiles in the three saline sites. Data are presented as mean ± 1 SE.
Significant site differences were detected in soil EC, especially in 20–100 cm soil layers, with lower soil EC values in the saline site two than that in the saline site one and saline site 3 (p < 0.05). Significant variations of soil EC among soil depths were observed in each saline site (p < 0.05). Soil EC decreased with soil depth and the highest soil EC was recorded in 0–20 cm soil layer in each saline site (Figure 1). Based on the equivalent-ratio of Cl−/SO42−, saline soil can be divided into four types of saline soil, which are chlorinate (≥4) sulfate-chlorinate (4−1), chlorinate-sulfate (1−0.5) and sulfate (<0.5) solonchak respectively (Wen, 2014). In this research, the soil salinization type of the three saline sites were the same type of saline soil which was sulfate solonchak (Figure 2).
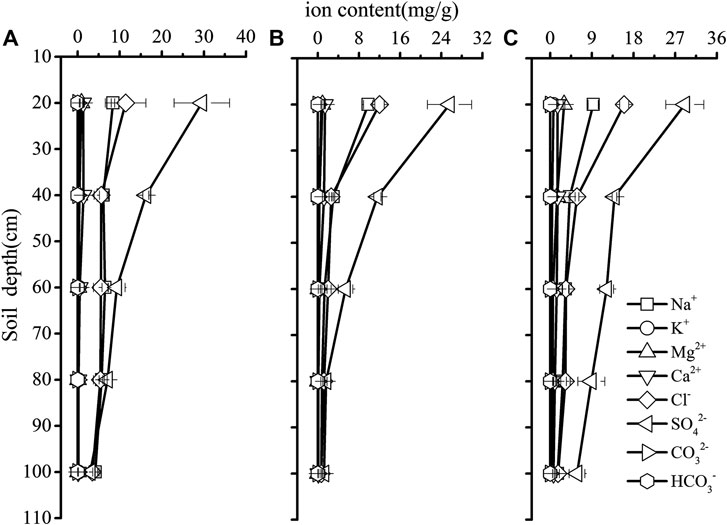
FIGURE 2. Soil salinity distribution in the saline site 1 (A), saline site 2 (B) and saline site 3 (C). Data are presented as mean ± 1 SE.
No significant difference in soil pH value was observed across the three saline sites (p > 0.05), except that the soil pH in 40–60 cm soil layer was higher significantly in the saline site two than that in the saline site 3 (p < 0.05). In each saline site, no apparent variation was found in soil pH value among the soil layers (saline site 1: p = 0.80; saline site 2: p = 0.15; saline site 3: p = 0.31).
There were linear relationships between δD and δ18O for soil water and xylem water in the study sites (Figure 3). δD and δ18O for Dunhuang were estimated by the Online Isotopes in Precipitation Calculator (OIPC; http://www.waterisotopes.org/) and the predicted Local Meteoric Water Line (pLMWL) was created. All the samples of soil water plotted below the global meteoric water line (GMWL) and pLMWL, indicating the strong evaporation enrichment and the extremely dry conditions. It can be noted that lower δD and δ18O values were found for xylem water, indicating that the studied plants took up water predominantly from soil water with lower δD and δ18O values.
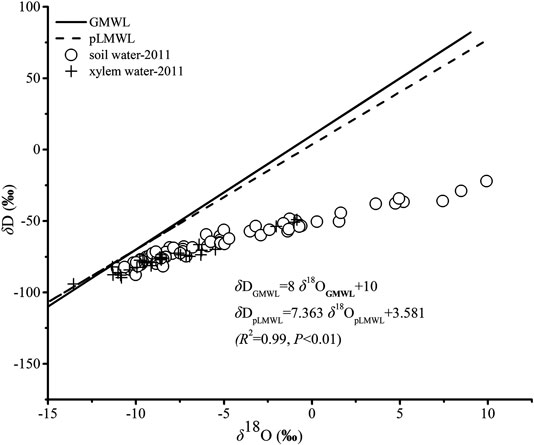
FIGURE 3. Relationship between δD and δ18O in soil water and xylem water. The Global Meteoric Water Line (GMWL) and the predicted Local Meteoric Water Line (pLMWL) were also presented.
The δ18O values of soil water across the three saline sites differed significantly (p < 0.05) in some soil layers (0–20 cm and 80–200 cm) (Figure 1). The δ18O values of soil water in each saline site showed a clear decreasing trend along the soil profile. The δ18O values of soil water decreased rapidly 6.31, 16.45 and 15.36% from 0 to 100 cm in the saline land 1, saline land two and saline land three, respectively. In contrast, from 100 to 200 cm, no trend in δ18O values of soil water was observed. The soil water in 0–20 cm soil layer had the highest mean δ18O value (−0.30, 7.87, and 5.71% for saline site 1, saline site 2 and saline site 3, respectively). The δ18O values of soil water varied significantly with soil depth, with variation in the upper layers (0–100 cm) greater than in the deep soil layers (>100 cm).
Table 2 summarizes the xylem water δ18O values collected in the three saline sites. Variations in the δ18O values of xylem water among plant species were observed in each saline site. In the saline site 1, K. foliatum had significantly higher δ18O values than A. sparsifolia, E. angustifolia and T. ramosissima (p < 0.01). In the saline site 2, no apparent variation in the xylem water δ18O values was observed between T. ramosissima and A. sparsifolia. In the saline site 3, T. ramosissima had significantly lower xylem water δ18O values than A. sparsifolia, H. capsica and N. tangutorum (p < 0.01). For the same plant species, no significant difference in mean xylem water δ18O values was observed across the different saline sites (T. ramosissima: p = 0.07; A. sparsifolia: p = 0.66), whereas multiple comparison tests found that the xylem water of T. ramosissima in the saline site two was significantly higher in 18O by −1.41% than that in the saline site 3.
We caculated the contributions of water used by plant species from five ranges of soil depths (0–40 cm, 20–40 cm, 40–60 cm, 60–80 cm and 80–200 cm) by the IsoSource model. The IsoSource outputs showed that, in the saline site 1, K. foliatum used average 68.3% soil water from 0 to 20 cm soil layers, and there was no IsoSource solution for A. sparsifolia, E. angustifolia and T. ramosissima, because the mean xylem water δ18O values were beyond the confine of those of potential water sources (Table 3). Then we inferred the soil depth of water extraction by A. sparsifolia, E. angustifolia and T. ramosissima by the overlapping δ18O values for xylem water and soil water (Figure 4). In the saline site 1, A. sparsifolia extracted water from the 120–200 cm soil layers, while the δ18O values of T. ramosissima and E. angustifolia were more negative than those of 0–200 cm soil water, which indicated that T. ramosissima and E. angustifolia mainly extracted deeper soil water (>200 cm).
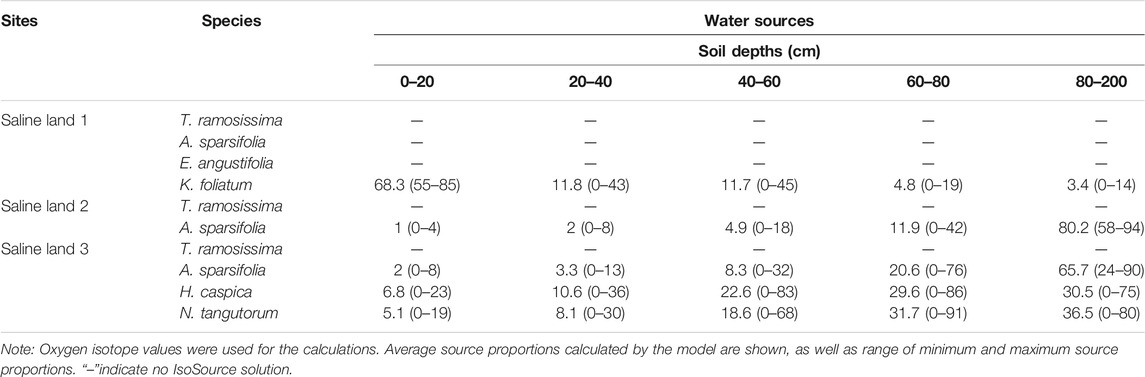
TABLE 3. Properties of feasible water sources (%) for desert plants in the three saline lands [mean (min-max)].
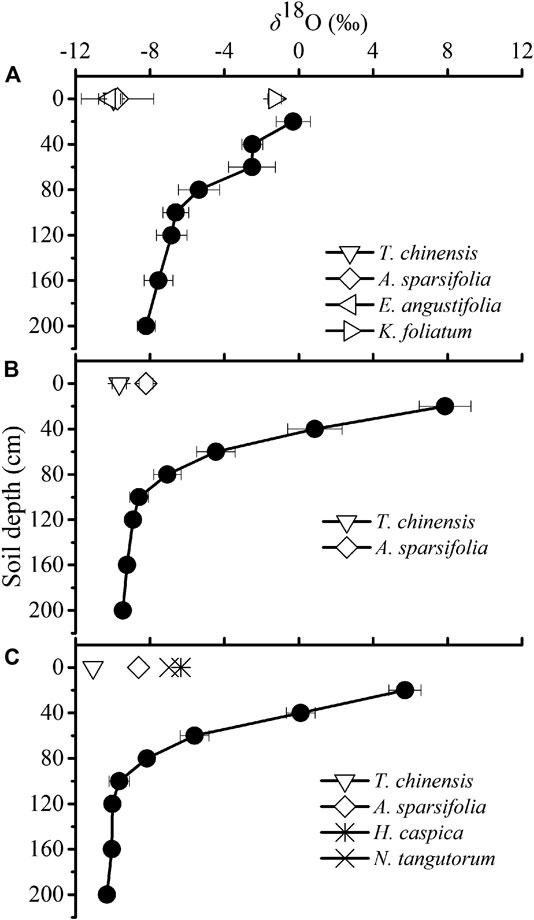
FIGURE 4. Variation in the δ18O value of soil water in the saline site 1(A), saline site 2 (B) and saline site 3 (C). Xylem water δ18O values of the dominant species are also presented in each saline site. Data are presented as mean ± 1 SE.
The IsoSource outputs showed that A. sparsifolia primarily used soil water from 80 to 200 cm (average 80.2%) and there was no IsoSource solution for T. ramosissima in the saline site 2 (Table 3). Overlapping δ18O values for xylem water of T. ramosissima and soil water were detected in the saline site 2. We inferred T. ramosissima utilized water from 120-200 cm soil layers (Figure 4).
The IsoSource outputs showed that average 65.7% of water source of A. sparsifolia came from 80 to 200 cm soil layers, H. caspica and N. tangutorum used little water from depths of 0–20 cm (average 6.8% and 5.1%, respectively), instead derived most of its water from deeper soil layers (40–200 cm) in the saline site 3 (Table 3). There was no IsoSource solution for T. ramosissima, and the δ18O values of T. ramosissima were more negative than those of 0–200 cm soil water. So, we inferred that T. ramosissima mainly extracted soil water at depths of >200 cm in the saline site 3 (Figure 4).
The relationship between SWC and soil water δ18O values in 0–200 cm soil profile was analyzed for each saline site, and the result showed that SWC correlated positively with soil water δ18O values for 0–200 cm soil profile in the saline site 1 (R = 0.42, p > 0.05) and saline site 3 (R = 0.49, p < 0.05), while no obvious correlation was observed in the saline site 2 (R = 0.20, p = 0.35). Whereas, it was found that there was no significant correlation between SWC and soil water δ18O values in 0–200 cm soil profiles of the whole study area either (R = 0.18, p = 0.13). The correlations among SWC, soil water δ18O, soil EC, and soil pH values in the 0–100 cm soil profile were also analyzed for each saline site. For the 0–100 cm soil profile, soil EC correlated positively with soil water δ18O values in each saline site, but no significant correlation was observed between SWC and soil water δ18O values (Table 4).
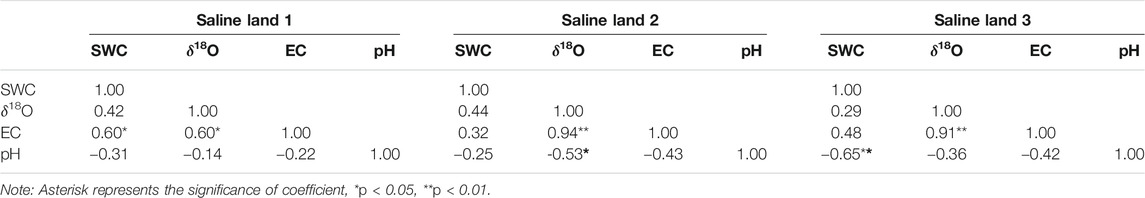
TABLE 4. Relationship among soil water content (SWC), soil water oxygen composition, soil electrical conductivity (EC) and pH (0–100 cm).
In the three studied saline sites, a vertical isotopic gradient in soil water δ18O profiles was observed (Figure 4), with 18O-enrichment of surface soil water, indicating the occurrence of evaporation. The light isotope is easier to evaporate during evaporation, which makes the liquid water enrich in heavy isotopes, and evaporation mainly occurs in the soil surface layer (Li and Liu, 2008). Therefore, along the soil depths, the enrichment of heavy isotopes decreases (Figure 4). Zimmermann et al. (1967) reported the effect of soil water evaporation on hydrogen isotopes. They showed that evaporation at the surface of a saturated soil column leads to D-enrichment near the surface soil layer, which decreases exponentially with soil depths. The enrichment trend may occur as a result of several processes, including evaporation, change in isotopic composition of precipitation and mixing of new and old water. However, the average annual rainfall of Dunhuang is only 39.8 mm (Data from the National Meteorological Information Centre, China Meteorological Administration). Under such extremely arid climate conditions, the rain has evaporated before it infiltrates into the soil. Therefore, in this study, the impact of precipitation on soil water could be ignored. Similar soil water δ18O-profiles were also found in the Heihe Basin (Zhao et al., 2008; Zhou et al., 2011; Zeng and Ma, 2013). Zhao et al. (2008) evaluated water sources of riparian plants in the extremely arid region along the lower reaches of the Heihe River basin by stable isotope technique. They found that soil water isotope concentrations decreased approximately exponentially with depth to a relatively constant concentration. Zhou et al. (2011) used D and 18O to determine the water sources of sand dune plants in middle reaches of Heihe River, and showed δ D and δ18O values of soil water decreased as a typical exponential function of depth, except that some details of the exponential curve were altered by the infiltration of individual rainfall events. Zeng and Ma (2013) also found that δ D and δ18O values of soil water soil water decreased along soil depths in two different habitats (oasis-desert transitional zone and desert) in the Heihe River Basin.
In previous studies in arid regions, it was found that soil with less water content in the upper layers usually has more enriched δ18O values, and soil with more water content in the deeper soil layers has more depleted δ18O values (Wu et al., 2014; Liu et al., 2015; Zhou et al., 2015). However, no significant and negative correlations between SWC and soil water δ18O values were observed in each saline site. It is noted that significantly positive correlation or no obvious correlation were found between SWC and soil water δ18O values in this study, which is incongruous with previous result that soil water content is negatively correlated with soil water δ18O values (Zhu et al., 2014; Cui et al., 2017). Whereas, a significant and positive correlation between soil EC and soil water δ18O values in 0–100 cm soil profile was observed in each saline site (Table 4), indicating that intense evaporation exerted significant enrichment effects on soil water δ18O values in soil profiles and cause more soil salt assembled on soil surface. Zhu et al. (2014) investigated the water uptake for halophyte grown in Northern area of Ningxia plain (China) in contrasted water regimes, and found similar relations that significant and positive correlations between soil salt content and soil water δ18O values. The positive correlation between salinity and δ18O was also observed in many field studies on water table, such as Sternberg et al. (1991); Lin and Sternberg (1994), Ewe et al. (2007), which may result from mixing of freshwater with low δ18O values and saline seawater with high δ18O values. Zhai et al. (2016) found there was a curvilinear relationship between salinity and the δ18O values in the vadose zone. However, it was considered that high salinity will significantly restrain the evaporation fractionation of soil water, and thus δ D and δ18O values will be depleted (Li and Qiu. 2018; Din et al., 2020). In this study, heavy isotopes (18O) were enriched in the liquid phase as water evaporated due to intense soil evaporation (Li and Qiu. 2018). Meanwhile, stronger soil evaporation could cause more salt accumulation and even surface assembled.
Co-existing desert plant species in the same saline site exhibited obvious variations in depth of water uptake respectively. In the saline site 1, K. foliatum used average 68.3% soil water from 0 to 20 cm soil layers; A. sparsifolia extracted water from the 120–200 cm soil layers, while T. ramosissima and E. angustifolia mainly extracted deeper soil water (>200 cm). In the saline site 2, A. sparsifolia primarily used soil water from 80 to 200 cm (average 80.2%) and T. ramosissima utilized water from 120–200 cm soil layers. In the saline site 3, A. sparsifolia used average 65.7% soil water from 80 to 200 cm soil layers; H. caspica and N. tangutorum derived most of water from deeper soil layers (40–200 cm); T. ramosissima mainly extracted soil water at depths of >200 cm (Table 3). The xylem water δ18O values varied among the studied co-occurring plant species in the studied sites, which indicated that interspecific differences in water absorption. The differences in xylem water δ18O values is very small, but the water sources may vary among the co-occurring plant species (Min et al. 2019). The variations in soil depth of water uptake for co-occurring plant species would lead to niche segregation and complementary use of limited water resources, and facilitated plant species co-existence (Asbjornsen et al., 2008). In this study, K. foliatum depended mainly on shallow soil water, which is in accordance with its root distribution pattern (a relatively shallow root system with taproots penetrate to 20–30 cm deep and spindly adventitious roots) (Shi and Wang, 2003; Yang et al., 2013). A. sparsifolia utilized deep soil water in the three saline sites. Wang et al. (2017) got similar result that during the growing season, A. sparsifolia acquired soil water stored at 50–200 cm soil depth in the lower reaches of Tarim River. It has been reported that A. sparsifolia’s roots could penetrate up to 12–30 m deep (Shi, 2003). In this study E. angustifolia extracted soil water below the depth of 200 cm. E. angustifolia is a kind of deciduous tree, and it has been reported that their roots can extend into deeper soil layers (Shi and Qu, 2003). H. caspica and N. tangutorum mainly derived water from 40-200 cm soil depths in saline site 3. This is accordance with their root distribution that their absorbing roots mainly developed in 0–200 cm soil layers (Sun and Yu, 1992). In this study T. ramosissima attached soil water of middle or deep soil layers. There are many literatures on T. ramosissima’ water sources by stable isotope technique, which got the result that T. ramosissima was a facultative phreatophyte and mainly relied on deep soil water and groundwater (Zhou et al., 2013; Wang et al., 2017). For example, Studies in the southeastern Junggar Basin have shown that T. ramosissima obtained 90% of its water from deep soil water and groundwater (Zhou et al., 2013). The vertical root distribution seemed determine the soil depth from which plant species can potentially access water (Ehleringer et al., 1991; Xu and Li 2006; Zhou et al., 2015; Zhang et al., 2017). However, some study in the Gurbantonggut desert found that T. ramosissima mainly relied on middle to deep soil water (Tiemuerbieke et al., 2018). This inconsistency suggests that plant water uptake was determined by root activity rather than root presence (Dawson and Ehleringer, 1991; Prechsl et al., 2015). Moreover, Imada et al. (2013) reported that T. ramosissima’s fine root distribution was drastically changed by soil water and nutrient distribution. So, it is indicated that the distribution of active roots showed substantial ecological plasticity in response to soil water and nutrient. Overall, root is an important determinant of the availability of soil water and is closely related with plant–water relations (Nippert et al., 2010).
For the common species, despite no habitat effects on xylem water δ18O values, the depth of water uptake for common species differed across the three saline sites. According to Figure 4; Table 3, we compared the soil depth from which common plant species may access across the three saline sites, and observed that T. ramosissima and A. sparsifolia attached more shallow soil water in the saline site 2 than in the saline site 1 and 3. The soil EC in the saline site 2 was the lowest among the three saline sites. This accordance with changes in soil EC suggests that T. ramosissima and A. sparsifolia’s water use could be influenced, to some extent, by soil salinity. Thus, we attempted to propose that it was likely that the widely distributed desert plants exhibited certain plasticity in water use to access deeper water sources to cope with salt stress in the saline habitats. Similar results were reported that woody plants endured salt stress by spatial partitioning and temporal shift in water absorption in the Everglades ecotone and coastal ecosystems (Ewe et al., 1999, Ewe et al., 2007; Ewe and Sternberg 2002). It has been reported that desert plants may shifted to deeper soil water to suffer salt stress in Xinjiang, northwest China (Min et al., 2019). In this study, soil EC, SO42−, Cl− and Na+ decreased with soil depths (Figures 1, 2), and soil salinity declined with depths. Salt accumulation in the soil generally change the soil texture and decrease the soil porosity, and consequently reduce the soil aeration and water conductance (Min et al., 2019). Moreover, high soil salinity cause plants access water more difficultly (Mahajan and Tuteja 2005; Yang et al., 2007). Therefore, the ability to explore and utilized deeper water sources ensure these desert plants to acclimate to environments stresses.
Our research indicated that niche complementarity for water resources among coexisting desert species is the potential mechanism for water-limited and salinity-effected ecosystems, which could maintain a resilient community under drought stress and salt stress. In each saline site, contrasting soil depths of water use for each desert species were mainly determined by their distinct root distributions, which cause water source partitioning. The studied common plant species would access more deeper soil water in more saline habitat, implying T. ramosissima and A. sparsifolia had the ability to shift to deeper soil water to suffer salt stress. A better understanding of plants physiological responses to different soil salinities would facilitate to rehabilitate saline soil and provide a scientific basis for ecosystem protection and management in arid and semiarid environment.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication
The work was financially supported by the Opening Foundation of Key Laboratory of Desert and Desertification, Chinese Academy of Sciences (KLDD-2018–007), the Scientific and Technological Innovation Programs of Higher Education Institutions in Shanxi (2019L0483), National Natural Science Foundation of China (41671207).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
We would like to thank Qiao Zeng and Tianyang Fu for help during field and laboratory work.
Asbjornsen, H., Shepherd, G., Helmers, M. J., and Mora, G. (2008). Seasonal patterns in depth of water uptake under contrasting annual and perennial systems in the Corn Belt Region of the Midwestern. U.S. Plant and Soil. 308, 69–92. doi:10.1007/s11104-008-9607-3
Bai, Y. (2009). Assessment on agricultural ecological security in Dunhuang Oasis. MS dissertation. Lanzhou (China): Northwest Normal University.
Chen, D. S., Dong, Z. W., Gao, L., Chen, X. M., Peng, X. H., Si, B. C., et al. (2017). Water-use process of two desert shrubs along a precipitation gradient in Horqin Sandy Land. Chinese Journal of Plant Ecology 41 (12), 1262–1272. doi:10.17521/cjpe.2017.0219
Cui, Y.-Q., Ma, J.-Y., Feng, Q., Sun, J.-H., and Sun, W. (2017). Water sources and water-use efficiency of desert plants in different habitats in Dunhuang, NW China. Ecol. Res. 32 (2), 243–258. doi:10.1007/s11284-017-1433-8
Cui, Y.-Q. (2014). Water sources and water-use efficiency of desert plants in Dunhuang area, Gansu China. MS dissertation. Beijing (China): Chinese Acadamy of Sciencies.
Dawson, T., Ehleringer, J., Hall, A., and Farquhar, G. (1993). “Water sources of plants as determined from xylem-water isotopic composition: perspectives on plant competition, distribution, and water relations,” in Stable isotopes and plant carbon-water relations. Editors J. R. Ehleringer, and G. D. Farquhar (Cambridge, MA: Academic Press Inc.), 465–496.
Dawson, T., and Ehleringer, J. (1991). Streamside trees that do not use stream water. Nature 350 (6316), 335–337. doi:10.1038/350335a0
Dawson, T., Mambelli, S., Plamboeck, A., Templer, P., and Tu, K. (2002). Stable isotopes in plant ecology. Annu. Rev. Ecol. Systemat. 33, 507–559. doi:10.1146/annurev.ecolsys.33.020602.095451
Dehaan, R. L., and Taylor, G. R. (2002). Field-derived spectra of salinized soils and vegetation as indicators of irrigation-induced soil salinization. Remote Sensing of Environment 80 (3), 406–417. doi:10.1016/S0034-4257(01)00321-2
Din, J., Liu, Y. F., Liu, Q., and Wang, J. J. (2020). Dynamic variation characteristics of hydrogen and oxygen stable isotope composition in soil water after salt water irrigation in arid area. Saf. Environ. Eng. 27 (3), 32–39. doi:10.13578/j.cnki.issn.1671-1556.2020.03.005
Ehleringer, J. R., Phillips, S. L., Schuster, W. S. F., and Sandquist, D. R. (1991). Differential utilization of summer rains by desert plants. Oecologia 88 (3), 430–434.
Ehleringer, J. R., Roden, J., and Dawson, T. E. (2000). “Assessing ecosystem-level water relations through stable isotope ratio analyses,” in Methods in ecosystem science. Editors O. E. Sala, R. B. Jackson, H. A. Mooney, and R. W. Howarth (Heidelberg: Springer-Verlag), 181–198.
Ehrlinger, J., and Dawson, T. (1992). Water uptake by plants: perspectives from stable isotope composition. Plant Cell Environ. 15 (9), 1073–1082.
Einbond, A., Sudol, M., and Coplen, T. (1996). New guidelines for reporting stable hydrogen, carbon, and oxygen isotope-ratio data. Geochem. Cosmochim. Acta 60 (17), 3359–3360.
Ewe, S. M., and Sternberg, D. L. (2002). Seasonal water-use by the invasive exotic, Schinus terebinthifolius, in native and disturbed communities. Oecologia 133, 441–448. doi:10.1007/s00442-002-1047-9 |
Ewe, S. M., Sternberg, Lda. S., and Childers, D. L. (2007). Seasonal plant water uptake patterns in the saline southeast Everglades ecotone. Oecologia 152, 607–616. doi:10.1007/s00442-007-0699-x |
Ewe, S. M. L., Sternberg, L. D. S. L., and Busch, D. E. (1999). Water-use patterns of woody species in pineland and hammock communities of South Florida. For. Ecol. Manag. 118, 139–148.
Field, C. B., and Barros, V. R., and Intergovernmental Panel on Climate Change (2014). Climate Change 2014: Impacts,Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, United Kingdom: Cambridge University Press.
Horton, J., Hart, S., and Kolb, T. (2003). Physiological condition and water source use of Sonoran Desert riparian trees at the Bill Williams River, Arizona, United States. Isot. Environ. Health Stud. 39 (1), 69–82. doi:10.1080/1025601031000096772
Imada, S., Taniguchi, T., Acharya, K., and Yamanaka, N. (2013). Vertical distribution of fine roots of Tamarix ramosissima in an arid region of southern Nevada. J. Arid Environ. 92, 46–52. doi:10.1016/j.jaridenv.2013.01.006
Li, J. Z., and Liu, X. Z. (2008). Advances of stable hydrogen and oxygen isotope applied in spac water cycle. J. Desert Res. 28 (4), 787–794.
Li, T., and Qiu, G. Y. (2018). Hydrogen and oxygen stable isotope study on the difference of evaporation between salt and pure water. Trop. Geogr. 38 (6), 857–865. doi:10.13284/j.cnki.rddl.003084
Lin, G., and Sternberg, L. D. S. L. (1994). Utilization of surface water by red mangrove (Rhizophora mangle L.): an isotopic study. Bull. Mar. Sci. 54, 94–102.
Liu, S., Chen, Y., Chen, Y., Friedman, J., Hati, J., and Fang, G. (2015). Use of 2H and 18O stable isotopes to investigate water sources for different ages of Populus euphratica along the lower Heihe River. Ecol. Res. 30 (4), 581–587. doi:10.1007/s11284-015-1270-6
Mahajan, S., and Tuteja, N. (2005). Cold, salinity and drought stresses: an overview. Arch. Biochem. Biophys. 444, 139–158. doi:10.1016/j.abb.2005.10.018 |
Min, X. J., Zang, Y. X., Sun, W., and Ma, J. Y. (2019). Contrasting water sources and water-use efficiency in coexisting desert plants in two saline-sodic soils in northwest China. Plant Biol. (Stuttg) 21 (6), 1150. doi:10.1111/plb.13028 |
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi:10.1146/annurev.arplant.59.032607.092911 |
Nippert, J. B., Butler, J. J., Kluitenberg, G. J., Whittemore, D. O., Arnold, D., Spal, S. E., et al. (2010). Patterns of Tamarix water use during a record drought. Oecologia 162 (2), 283–292. doi:10.1007/s00442-009-1455-1 |
Phillips, D., and Gregg, J. (2003). Source partitioning using stable isotopes: coping with too many sources. Oecologia 136 (2), 261–269. doi:10.1007/s00442-003-1218-3 |
Prechsl, U. E., Burri, S., Gilgen, A. K., Kahmen, A., and Buchmann, N. (2015). No shift to a deeper water uptake depth in response to summer drought of two lowland and sub-alpine C₃-grasslands in Switzerland. Oecologia 177 (1), 97–111. doi:10.1007/s00442-014-3092-6 |
Qadir, M., Ghafoor, A., and Murtaza, G. (2000). Amelioration strategies for saline soils: a review. Land Degrad. Dev. 11 (6), 501–521. doi:10.1002/1099-145X(200011/12)11:63.0.CO;2-S
Qadir, M., Oster, J. D., Schubert, S., Noble, A. D., and Sahrawat, K. L. (2007). Phytoremediation of sodic and saline-sodic soils. Adv. Agron. 96, 197–247. doi:10.1016/S0065-2113(07)96006-X
Sang, X. F. (2006). Visual simulation and management of groundwater in Dunhuang Basin. MS dissertation. Lanzhou (China): Lanzhou University.
Šantrůček, J., Květoň, J., Šetlík, J., and Bulíčková, L. (2007). Spatial variation of deuterium enrichment in bulk water of snowgum leaves. Plant physiology 143 (1), 88–97. doi:10.1104/pp.106.089284 |
Shi, Y. J. (2003). Alhagi sparsifolia. J. Tradit. Chin. Vet. Med. 44–45. doi:10.13823/j.cnki.jtcvm.2003.s1.048
Shi, Y. J., and Qu, J. M. (2003). Elaeagnus angustifolia. J. Tradit. Chin. Vet. Med. 154–155. doi:10.13823/j.cnki.jtcvm.2003.s1.070
Shi, Y. J., and Wang, C. L. (2003). Kalidium foliatum. J. Tradit. Chin. Vet. Med. 142–144. doi:10.13823/j.cnki.jtcvm.2003.s1.047
Soriano, A., and Sala, O. (1984). Ecological strategies in Patagonian arid steppe. Vegetatio 56, 9–15.
Sternberg, L. D., Ish-Shalom-Gordon, N., Ross, M., and O'Brien, J. (1991). Water relations of coastal plant communities near the ocean/freshwater boundary. Oecologia 88 (3), 305–310. doi:10.1007/BF00317571
Sun, X., and Yu, Z. (1992). A study on root system of Nitraria Tangutorum. J. Desert Res. 12 (4), 50–54.
Tiemuerbieke, B., Min, X. J., Zang, Y. X., Xing, P., Ma, J. Y., and Sun, W. (2018). Water use patterns of co-occurring C3 and C4 shrubs in the Gurbantonggut desert in northwestern China. Sci. Total Environ. 634, 341–354. doi:10.1016/j.scitotenv.2018.03.307 |
Wang, T. (2009). Review and prospect of research on oasification and desertification in arid regions. J. Desert Res. 29 (1), 1–9.
Wang, Y. S., and Chen, J. S. (2010). Study of stable isotope model for saturated soil water movement in the condition of evaporation. J. Sichuan Univ. (engineering science edition) 42 (1), 10–13. doi:10.3969/j.issn.1002-10.15961/j.jsuese.2010.01.012
Wang, Y. Y., Chen, Y. P., Li, W. H., Wang, R. Z., Zhou, Y. Y., and Zhang, J. P. (2017). Water sources of typial desert riparian plants in the lower reaches of Tarim River. J. Desert Res. 37 (6), 1150–1157. doi:10.7522/j.issn.1000-694x.2016.00103
Wen, J. (2014). “The present condition and an analysis of control measurements of the secondary salinization of soils in the Bachu county, Xinjiang,” in Proceedings of the annual conference of Chinese Society of Water Conservancy, Xuzhou, China, December 29, 2014 (Tianjin, China: Chinese Hydraulic Engineering Society). Editors W. Sui, Y. Sun, and C. Wang, 654–661.
Wu, Y., Zhou, H., Zheng, X.-J., Li, Y., and Tang, L.-S. (2014). Seasonal changes in the water use strategies of three co-occurring desert shrubs. Hydrol. Process. 28 (26), 6265–6275. doi:10.1002/hyp.10114
Xiao, G. J., Zhang, Q., Li, Y., Zhang, F. J., Wang, R. Y., and Luo, C. K. (2010). Impact of climatic warming on soil salinity and irrigation amount of Yellow River irrigation areas in Ningxia Hui Autonomous Region. Transactions of CSAE 26 (6), 7–13. doi:10.3969/j.issn.1002-6819.2010.06.002
Xu, H., and Li, Y. (2006). Water-use strategy of three central Asian desert shrubs and their responses to rain pulse events. Plant Soil 285 (1-2), 5–17. doi:10.1007/s11104-005-5108-9
Yang, C. W., Chong, J. N., Li, C. Y., Kim, C., Shi, D. C., and Wang, D. L. (2007). Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294, 263–276. doi:10.1007/s11104-007-9251-3
Yang, H. T., Li, X. R., Liu, L. C., Jia, R. L., Wang, Z. R., Li, X. J., et al. (2013). Biomass allocation patterns of four shrubs in desert grassland. J. Desert Res. 33 (5), 1340–1348. doi:10.7522/j.issn.1000-694x.2013.00197
Zeng, Q., and Ma, J. Y. (2013). Plant water sources of different habitats and its environmental indication in Heihe River basin. J. Glaciol. Geocryol. 35 (1), 148–155. doi:10.7522/j.issn.1000-0240.2013.0017
Zhai, L., Jiang, J., DeAngelis, D., and Sternberg, L. D. S. L. (2016). Prediction of plant vulnerability to salinity increase in a coastal ecosystem by stable isotope composition (δ18O) of plant stem water: a model study. Ecosystems 285 (19), 32–49. doi:10.1007/s10021-015-9916-3
Zhang, C., Li, X., Wu, H., Wang, P., Wang, Y., Wu, X., et al. (2017). Differences in water-use strategies along an aridity gradient between two coexisting desert shrubs (Reaumuria soongorica and Nitraria sphaerocarpa): isotopic approaches with physiological evidence. Plant Soil 419, 15–25. doi:10.1007/s11104-017-3332-8
Zhang, M. Y. (2008). Study on the carrying capacity of water resources in Dunhuang. MS dissertation. Lanzhou (China): Lanzhou University.
Zhao, L. J., Xiao, H. L., Cheng, G. D., Song, Y. X., Zhao, L., Li, C. Z., et al. (2008). A preliminary study of water sources of riparian plants in the lower reaches of the Heihe basin. Acta Geosci. Sin. 29 (6), 709–718. doi:10.1007/s40333-014-0037-1
Zhou, C. X., Sun, Z. Y., and Yu, S. W. (2011). Using D and 18O stable isotopes to determine the water sources of sand dune plants in Linze, middle reaches of the Heihe River. Geol. Sci. Technol. Inf. 30 (5), 103–109.
Zhou, H., Zhao, W., Zheng, X., and Li, S. (2015). Root distribution of Nitraria sibirica with seasonally varying water sources in a desert habitat. J. Plant Res. 128 (4), 613–622. doi:10.1007/s10265-015-0728-5 |
Zhou, H., Zheng, X. J., Tang, L. S., and Li, Y. (2013). Differences and similarities between water sources of Tamarix ramosissima, Nitraria sibirica and Reaumuria soongorica in the southeastern Junggar Basin. Chin. J. Plant Ecology 37 (7), 665–673. doi:10.3724/SP.J.1258.2013.00069
Zhu, L., Wang, Z. H., Mao, G. L., Zheng, S. X., and Xu, X. (2014). Water uptake from different soil depths for halophytic shrubs grown in Northern area of Ningxia plain (China) in contrasted water regimes. J. Plant Interact. 9 (1), 26–34. doi:10.1080/17429145.2012.751139
Keywords: stable oxygen isotope, desert plants, saline land, soil water utilization, Dunhuang
Citation: Cui Y-Q, Niu L-Q, Xiang J-L, Sun J-H, Xiao J-H and Ma J-Y (2021) Water Uptake from Different Soil Depths for Desert Plants in Saline Lands of Dunhuang, NW China. Front. Environ. Sci. 8:585464. doi: 10.3389/fenvs.2020.585464
Received: 21 July 2020; Accepted: 07 December 2020;
Published: 29 January 2021.
Edited by:
Atsushi Tsunekawa, Tottori University, JapanReviewed by:
Na Li, Chinese Academy of Science, ChinaCopyright © 2021 Cui, Niu, Xiang, Sun, Xiao and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian-Ying Ma, bWFqeTY1MkBuZW51LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.