
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Sci., 07 December 2020
Sec. Freshwater Science
Volume 8 - 2020 | https://doi.org/10.3389/fenvs.2020.502042
This article is part of the Research TopicTowards the Sustainable Use of African WetlandsView all 14 articles
Freshwater systems and their associated biodiversity are among the most threatened ecosystems globally. The greatest threats to freshwater fishes are the introduction and spread of non-native species, pollution, habitat degradation and loss, and overexploitation. While many regions across the world contain extensive networks of protected areas, these are largely ineffective for protecting riverine systems and their biodiversity. This is because they were designed with the aim of prioritising conservation of terrestrial biodiversity, with limited or no consideration for aquatic systems. The Cape Fold Ecoregion, located within the Western and Eastern Cape Provinces of South Africa, is home to the highest percentage of threatened freshwater fishes in the country. The region has an extensive protected area network that protects a wide array of ecosystems, but limited information exists on the role of protected areas in conserving the endemic freshwater fish fauna of this region. This study evaluated the value of protected areas for protection of freshwater fishes in the Western Cape Province by setting species conservation targets and then intersecting species distribution data with protected area polygons. Conservation targets were set to protect the minimum viable population required for long-term persistence, with a minimum of 10 subpopulations as a target. This, along with other factors such as population viability and protected area effectiveness was used to determine whether a species was effectively protected by the current protected area network. Species were classified into one of four categories; (1) “well protected,” (2) “moderately protected,” (3) “poorly protected,” and (4) “not protected.” Our results indicate that the majority of native fishes are inadequately protected within the current protected area network in the province. This is mainly a result of the linear nature of riverine ecosystems that exposes them to impacts and threats that emanate from outside of the protected area. These limitations are not unique to the CFE, and our findings have broader implications as they highlight the need for integrating both the riverine and terrestrial ecosystems in the design, expansion and management of protected areas. This will enhance and maximise conservation and protection of riverine systems and their unique biodiversity.
Freshwater fishes are one of the most imperilled vertebrate groups globally (Reid et al., 2013). The introduction and spread of non-native species, pollution, habitat degradation and loss, hydrological modifications, construction of instream barriers, excessive water abstraction, overexploitation and intensification of agricultural activities have been identified as the key threats to freshwater ecosystems and their biodiversity (Ricciardi and Rasmussen, 1999; Collares-Pereira and Cowx, 2004; Dudgeon et al., 2006; Darwall et al., 2011; Laurance et al., 2014). Because of these multiple impacts and threats, freshwater ecosystems continue to receive increasing global attention in a quest to determine effective ways to mitigate against the projected mass extinction of freshwater fishes (Ricciardi and Rasmussen, 1999; Azevedo-Santos et al., 2019). Although many regions across the world contain extensive networks of protected areas, their effectiveness in protecting riverine systems and their biodiversity has been increasingly questioned over recent years. This is because historically the designation of protected areas was largely informed by the need to protect terrestrial biodiversity (Thieme et al., 2012; Juffe-Bignoli et al., 2016; Azevedo-Santos et al., 2019). Despite being ranked as the most highly threatened ecosystems globally, freshwater systems have been overlooked in the designation of protected areas, and often, their inclusion in current protected areas has mainly been coincidental rather than intentional (Abell et al., 2011). The lack of integration of freshwater systems in protected area designation and establishment has been identified as the main reason for their limited efficacy in protecting freshwater fishes, for example large migratory fishes that require large areas to complete their life cycles are considered to be poorly protected in Brazil (Azevedo-Santos et al., 2019). Similarly, Chessman (2013) reported that protected areas offered no real benefit to the native fish in the Murray–Darling Basin of Australia, because they did not effectively mitigate threats related to non-native fish and alteration of water regimes. Lawrence et al. (2011) reported that while nearly two-thirds of native fishes occur in national parks in the United States, only 18% of highly imperilled fish species are represented within protected areas.
In a systematic evidence review of case studies of protected areas and freshwater biodiversity, Acreman et al. (2019) reported that just over half of these areas had positive outcomes for freshwater biodiversity. Reasons for reduced effectiveness include inadequate connectivity within freshwater ecosystems, lack of protection for migratory species beyond designated areas, limited control over threats from outside the protected area and the absence of a whole catchment approach (Acreman et al., 2019). From this, it is evident that the degree of protection for riverine ecosystems is largely dependent on the location of the protected area within the landscape. This is because the linear nature of riverine ecosystems exposes them to impacts and disturbances that occur outside the protected area and can be transmitted downstream from the point of impact for distances proportional to the scale and nature of the disturbance (Davies et al., 1993; Skelton et al., 1995). Protected areas located in headwater sections of catchments are also only likely to be effective in protecting a limited number of fish species that are associated with mountain streams, but will provide no protection to taxa in the lower sections of rivers with gentle gradients where there is increased intensity of human activities. Rivers are also inextricably linked to their catchments, and as such, some disturbances that may occur outside the protected area (for example introduction of non-native species) could spread and impact the whole catchment, including sections in the protected area (Wilkinson et al., 2018). The effectiveness of a protected area is therefore strongly determined by the extent of the catchment included within the protected area, as well as location and configuration of the reserve with respect to the catchment area (Skelton et al., 1995; Saunders et al., 2002; Collares-Pereira and Cowx, 2004; Juffe-Bignoli et al., 2016). For reserves located in headwater sections of rivers, instream barriers located in the lower sections can prevent upstream invasion and establishment of non-native fish species (Fausch et al., 2009; Ellender et al., 2011).
In southern Africa, the highest concentration of threatened freshwater fish is found in the Cape Fold Ecoregion (CFE) located at the southern tip of the continent, where more than 50% of the endemic fish fauna are listed in highly threatened categories of the IUCN as Critically Endangered (11%), Endangered (33%), and Vulnerable (11%). Indeed, evidence from previous and ongoing studies indicate that the number of threatened species in the CFE remains severely underestimated. Some of the species that are currently listed as Data Deficient or Least Concern, for example Cape kurper Sandelia capensis, Cape galaxias Galaxias zebratus, and chubbyhead barb Enteromius anoplus are complexes of several narrow range endemic lineages, some of which are likely to represent distinct species (Chakona et al., 2013; Bronaugh et al., 2020). Non-native piscivores and habitat degradation are ranked as the greatest threats to freshwater fishes of the CFE (Tweddle et al., 2009; Ellender and Weyl, 2014). A total of 15 introduced species have become established in the CFE, with some, such as common carp Cyprinus carpio, rainbow trout Oncorhynchus mykiss, basses (Micropterus spp.), and bluegill sunfish Lepomis macrochirus having widespread distributions that extend into rivers in formally protected areas (Jordaan et al., 2012). These species affect native species through predation, habitat alteration, competition for resources, the introduction of diseases and the disruption of ecological processes (De Moor and Bruton, 1988; Ellender and Weyl, 2014). The primary impact is predation and this has resulted in the extirpation of most native species from mainstream rivers and many tributaries within the CFE (Weyl et al., 2014; Van der Walt et al., 2016). Remnant populations of native species are now limited to upper reaches of tributaries above waterfalls and other barriers that prevent range expansion of non-native species (Skelton, 2001; Chakona et al., 2013, 2020).
While multiple anthropogenic impacts have transformed much of the landscape and riverscapes in the CFE, the region also has a comprehensive network of formally protected areas declared under the National Environmental Management: Protected Areas Act (Act 57 of 2003). These protected areas are essential tools for the conservation of biodiversity and prevent land-use practices that could impact ecosystem integrity (Gray et al., 2016). As with many other parts of the world, the current protected area network in the region is mostly a result of opportunistic reservations over time. In the past, there was limited formal conservation planning to ensure representation of both the patterns (taxa and land classes) and processes that underpin the persistence of biodiversity (DEA, 2016). Within the CFE, for example, establishment of protected areas almost exclusively focused on the protection of terrestrial ecosystems, especially endemic vegetation types (Wicht, 1945; Rebelo, 1997; van Wilgen et al., 2016). In recent years (since 2008), South Africa has invested in a National Protected Area Expansion Strategy (NPAES) to address this shortfall (DEA, 2016). Other areas that were also afforded protection included economically marginal regions that were less suitable for agricultural development or human habitation and where reservation costs were low (Rebelo, 1997). In the CFE, this resulted in a protected area network that predominantly comprises rugged, high altitude mountain areas, whereas the economically productive lowland areas have been heavily transformed through various land-use activities (Rouget et al., 2003b).
There is growing realisation that the existing protected area network is inadequate for conserving both aquatic and terrestrial biodiversity in South Africa, as for example, more than 30% of river types and 25% of vegetation types are not represented within conservation areas in the country (Rebelo, 1997; Nel et al., 2009). This prompted the need for expansion of protected areas, but there are concerns that these actions may not achieve the desired conservation outcomes as expansions are likely to be biased towards focusing on areas adjacent to existing protected (mainly high altitude) areas to facilitate management (Rouget et al., 2003a). This is a significant cause for concern given the ongoing and increasing levels of land transformation and loss of biodiversity in lowland areas (Rouget et al., 2003a; van Wilgen et al., 2016).
In light of the ongoing and projected threats to the endemic freshwater fish fauna of the CFE (Ellender et al., 2017; Shelton et al., 2018), there is need for an assessment of the effectiveness of the current network of protected areas in mitigating these threats. Although previous studies have documented the distribution and conservation status of freshwater fishes in protected areas in the country and the region (Skelton et al., 1995; Impson et al., 2002; Russell, 2011), these studies did not evaluate long-term persistence. This requires some consideration for whether the population within the protected area is viable and what the minimum number of these populations are for persistence of the species into the future. The recent discovery of hidden diversity and description of new species within a number of fish genera in the CFE (e.g., Chakona et al., 2013) also necessitated the need to update information on species distribution ranges and evaluate the degree of protection afforded to them by formally protected areas. The aim of the present study was thus 2-fold: (i) to provide an updated inventory of species distributions in protected areas that reflect the latest taxonomic information and, (ii) to assess the effectiveness of the current protected area network in conserving native freshwater fishes of the CFE. Given that these protected areas were designated for protection of unique plant diversity, particularly in high altitude areas, it was predicted that headwater species would be better protected than lowland species.
Only native primary freshwater fishes were included in this assessment and both obligate and facultative catadromous species were excluded from the assessment. Other species excluded were those of marine origin that can complete their life cycle in freshwater such as the river goby Glossogobius calidus. Also excluded were estuarine and marine species that enter freshwater systems but do not maintain permanent breeding populations in these rivers such as moonies (Monodactylus spp.). Extra-limital populations of native fish species were not included in the assessment irrespective of the conservation status of that species and only populations within the native range of the species were considered. Data Deficient species were excluded from this assessment due to paucity of distribution data or as a result of taxonomic uncertainty.
For the taxa included in the assessment, specimen-linked point distribution data were obtained from the South African Institute for Aquatic Biodiversity (SAIAB, 2016) for each of the 31 taxa assessed. This dataset formed the basis of the 2016 Red List Assessment (RLA) of the freshwater fishes of South Africa (SANBI, 2016). Prior to the current study and the RLA, all distribution data were verified by taxon specialists and updated where relevant to ensure that all recently collected data were included and any data points based on possible misidentification of species were excluded. The cleaned distribution data were intersected with a polygon shapefile of South Africa’s protected area network (Government of South Africa, 2010) using ArcGIS software (Version 10). Protected areas recognised in terms of the South African National Environmental Management: Protected Areas Act (Act 57 of 2003) and considered secure into the future were included (Forest Wilderness Areas, Forest Nature Reserves, World Heritage Sites, Wilderness Areas, Provincial Nature Reserves, Mountain Catchment Areas and National Parks). This intersected data produced a list of protected areas for each species. To ensure that no species was excluded from a protected due to differences in point data precision, after intersecting the data, the inferred presence was determined by examining the points near the boundary of the protected area (≤1 km). Expert knowledge was used to infer the presence of these species in a protected area, based on their knowledge of the species ecology and whether suitable habitat was available inside the protected area. For future iterations of this analysis, these points should be ground-truthed. Further, when a protected area had multiple geographically isolated sections, these were assessed as individual protected areas and recorded individually (Supplementary Appendix 1).
As non-native invasive fishes are considered a primary threat to a large number of native freshwater fish taxa in Southern Africa (Tweddle et al., 2009), their presence on a protected area was one of the primary drivers affecting the scoring of the effectiveness of the protected area. This required additional input data on alien fish distributions and instream invasion barriers. The primary sources of this information was unpublished survey data and expert knowledge, supplemented with peer reviewed literature where available. While impacts associated with changes in water quality and quantity are also an important threat to freshwater fishes, available data was too limited for inclusion in the assessment.
Pfab et al. (2011) provided conservation targets for species persistence and the minimal number of subpopulations that are needed to ensure survival into the future. Ideally, conservation targets can be set in terms of a minimum viable population size of 10, 000 individuals or 10 viable subpopulations per species, supported by IUCN RLA criteria (IUCN 2001) and as proposed by Pfab et al. (2011), respectively. However, there is limited population density or abundance data available for the majority of freshwater fishes of the region. In this study, conservation targets could only be set in terms of the number of viable subpopulations and not actual population sizes. Subpopulation viability was determined based on information included in the latest RLAs. All subpopulations on protected areas were scored as either viable or not viable with default values of 1,000 or 100 individuals assigned, respectively (Supplementary Appendix 2). In the case of more than one population of a species present in the same protected area, the viability and protection level was assessed at the individual subpopulation level and not at the protected area level.
Furthermore, in the case of naturally range-restricted or rare species, allowances were made for adjustments in the conservation target to be less than 10 viable subpopulations (representing a target of 10,000 individuals). An example of this would be Barnard’s rock catfish Austroglanis barnardi which is naturally range-restricted and warranted a reduced conservation target of five viable subpopulations (representing a target of 5,000 individuals). When such adjustments were made, it was motivated and open to review from other freshwater fish experts. In future iterations of this analysis, a review process for such adjustments is recommended. In cases where species were lost from large sections of their natural distribution range due to threats, conservation targets were not adjusted.
In addition to determining species representation within protected areas, the effectiveness of the protected area in safeguarding the species was also assessed. A default value of one was assigned in the case of a protected area being highly effective in protecting the species against major threats and ensuring the long-term persistence of the population present within the protected area. Default values of 0.5 and 0.1 were assigned to “fair” and “poor” levels of protection afforded by the protected area, where the protected area was either moderately effective in mitigating some of the threat to the species (0.5) or completely ineffective in mitigating the major threats (0.1). Given that non-native invasive fishes, especially Micropterus spp. and O. mykiss are considered the primary threat to the majority of native Southern African freshwater fishes, any river within a protected area where these species have become established were given a poor protection score. This was irrespective of other variables such as habitat availability and water quality within the protected area. As many invasions occur from mainstream or downstream sources, only rivers where the headwaters are fully within the protected area and where known invasion barriers exist were rated as having a fully effective protection function for the species in question. Other threats were scored based on available data and expert knowledge of the protected area.
In summary, the protection category was assigned based on the number of individuals protected (a function of the number of populations on protected areas as a function of their viability and protection effectiveness), relative to the conservation target (set as 10,000 individuals unless stated otherwise). This methodology is presented in detail in Supplementary Appendix 2. Protection categories were: “not protected”: <5% of the conservation target met within protected areas; “poorly protected”: 5–49% of the conservation target met within protected areas; “moderately protected”: 50–99% of the conservation target met within protected areas; “well protected”: 100% + of the conservation target met within protected areas. These categories follow from well-established and accepted ecosystem categories for South African ecosystem protection level assessments (Driver et al., 2012). It must be noted that a constraint to effectively assessing all CFE fish species in terms of protection was variation in data quality and quantity and to address this, a confidence score (high, medium, or low) was awarded to each assessment.
Of the 45 known freshwater fish taxa in the CFE, 31 were assessed using the described methodology. Species native to the CFE but excluded from the current assessment due to either taxonomic uncertainty or limited distribution data were E. anoplus, Enteromius pallidus, G. zebratus, and S. capensis. The moggel Labeo umbratus was also excluded as it has a wide distribution range outside the CFE. For assessed taxa, a conservation target of 10 000 individuals were set with the exception of the Tradouw redfin Pseudobarbus sp. nov. “Tradouw,” Krom River redfin Pseudobarbus senticeps, Twee River redfin Sedercypris erubescens, Doring River redfin Pseudobarbus sp. nov. “Doring,” Galaxias sp. nov. “Goukou” and Barnard’s rock catfish Austroglanis barnardi (Table 1). These taxa are all highly range-restricted and a conservation target of 10,000 individuals were considered unrealistic. The conservation target was reduced to 5,000 individuals based on natural low abundance or restricted natural distribution ranges. It was found that 112 out of the 163 (67%) populations assessed was done with high confidence. Populations with low confidence scores were sites where recent survey data were not available and thus assessors relied on expert knowledge of the past conditions of those areas to score population viability and protected area effectiveness. Only eight (5%) populations received a low score, highlighting the need for surveys in these protected areas.
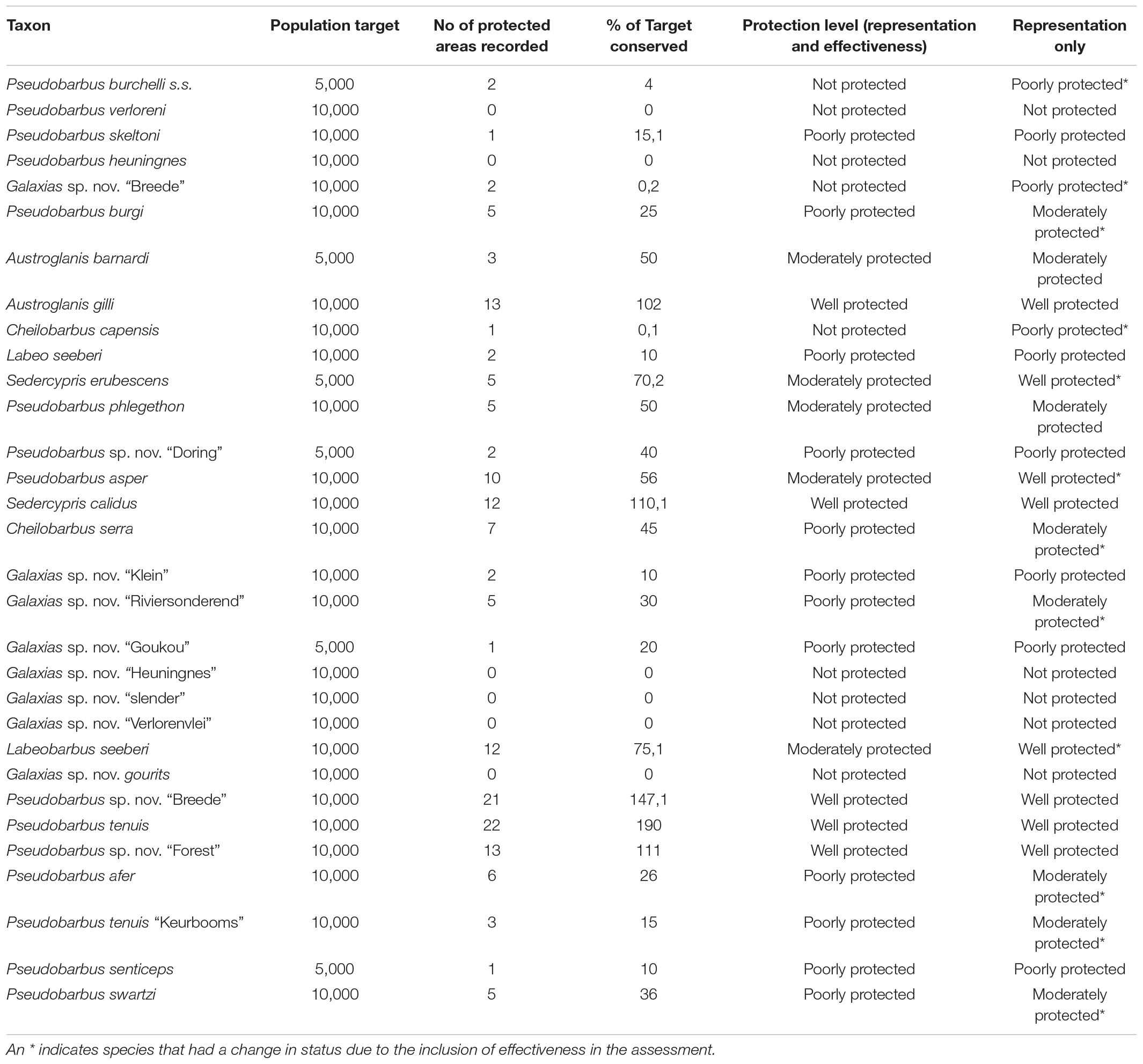
Table 1. Summary of protection level assessment results, with population target, number of protected areas taxa occur in, percentage target that is met, final protection level and protection level based on representation only.
Results indicated that 21 of the 31 taxa (68%) are either “poorly protected” (39%), “not protected” (10%) or are absent from any form of formally protected area (19%). These categories indicate that less than 49% of the conservation target for these taxa are being met within the current protected area network. For the remaining 10 taxa that were assessed, five (16%) were “moderately protected” with an additional five (16%) considered “well protected” (Table 1 and Figure 1). In terms of presence on formally protected areas, 11 taxa (35%) occurred on 1–3 protected areas with an additional seven taxa (23%) present on 4–8 protected areas. Three taxa (10%) was recorded from 9 to 12 protected areas with 2 species (6%) present on 13–18 protected areas and a further two being present on >18 formally protected areas (Figure 2).
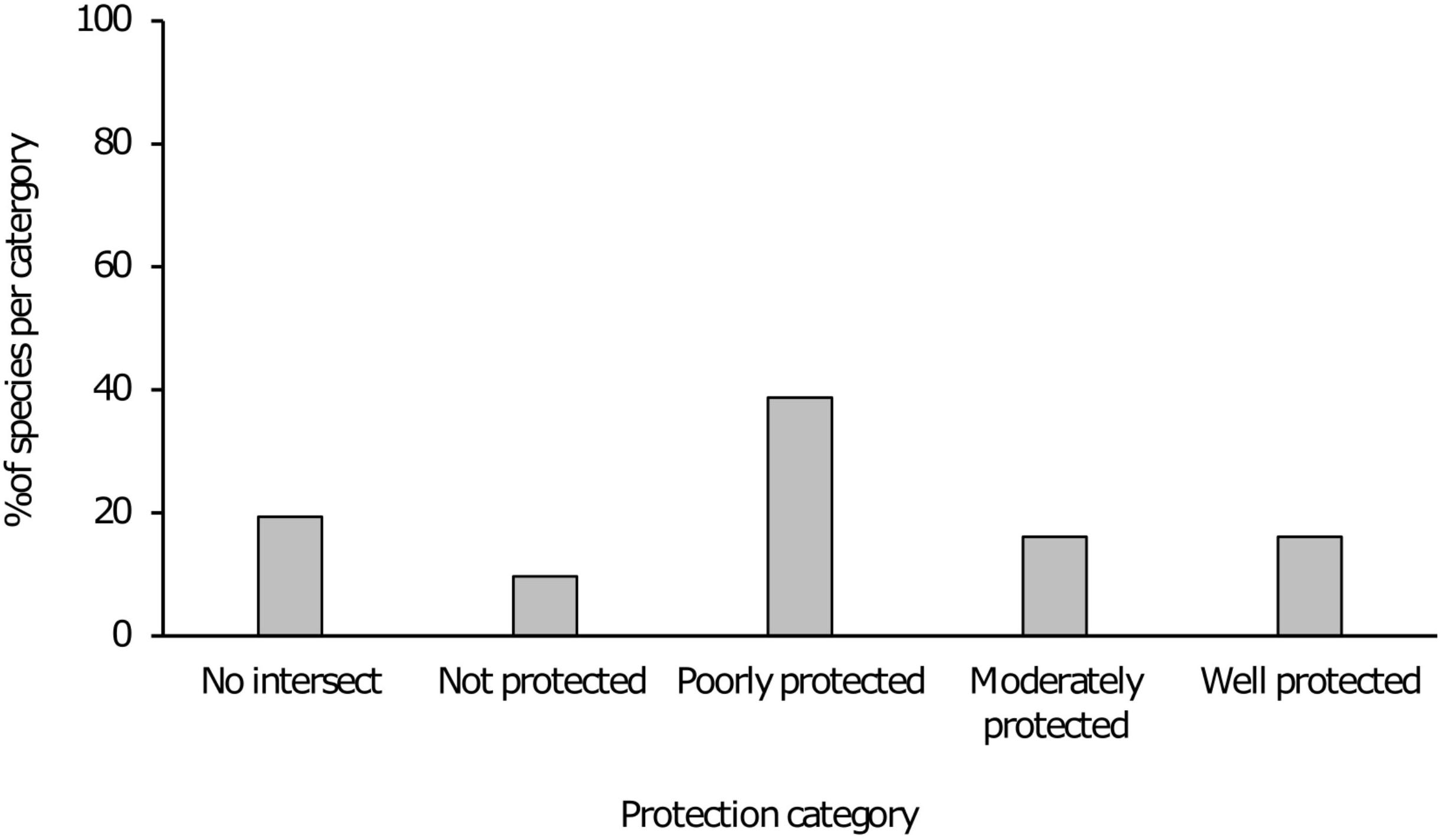
Figure 1. Bar chart indicating various protection level categories and the percentage of taxa in each category.
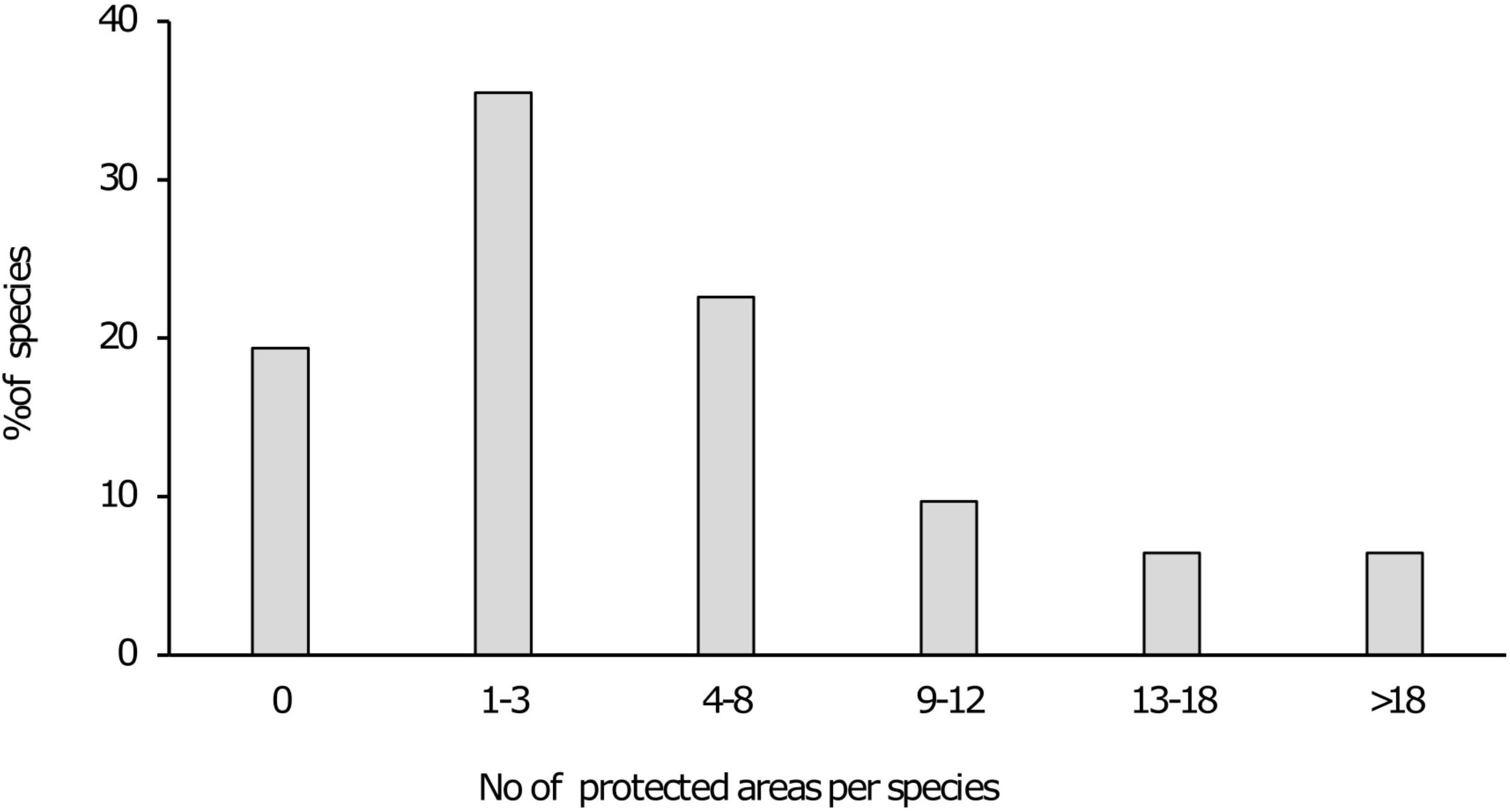
Figure 2. Bar chart indicating the percentage of species present within various numbers of protected areas.
When considering protection level relative to conservation status, it is evident that the majority of taxa in the “no intersect” to “poorly protected” categories comprise Critically Endangered (CR) and Endangered (EN) taxa (Figure 3). The only Vulnerable (VU) taxa in these categories are two Galaxias lineages Galaxias sp. nov. “Gouritz” and Galaxias sp. nov. “Goukou,” both of which are listed under category D2 for small and restricted populations that are at high risk from future threats. The only Near Threatened (NT) species listed in the “poorly protected” category is the Clanwilliam sawfin Cheilobarbus serra but it must be noted that this species is close to meeting the criteria for being “moderately protected” as 45% of the conservation target is being met within formally protected areas (Table 1). For the category of “moderately protected” one taxon is listed as CR, two as EN and one each as VU and NT respectively. Of the five taxa that were assessed as “well protected” four are listed as NT with one taxon listed as VU (Figure 3).
When excluding protected area effectiveness from the analysis and only considering the number of viable populations within a protected area, all species improved in terms of the percentage of the conservation target met within protected areas and 12 species changed protection category (Table 1 and Figure 4). Three species changed status from “not protected” to “poorly protected.” These species had one to two populations within a protected area, however these populations were severely impacted by non-native fish species as the protected areas did not provide any mitigation against this threat. Six species changed from “poorly protected” to “moderately protected” and three moved from “moderately” to “well protected.” When considering reduced protected areas effectiveness, the primary driver was the presence of non-native fish species within the protected area and the lack of control measures. Secondly, impacts associated with poor land-use practices occurring outside the boundaries of the protected area also served to reduce the effectiveness of the protected area.
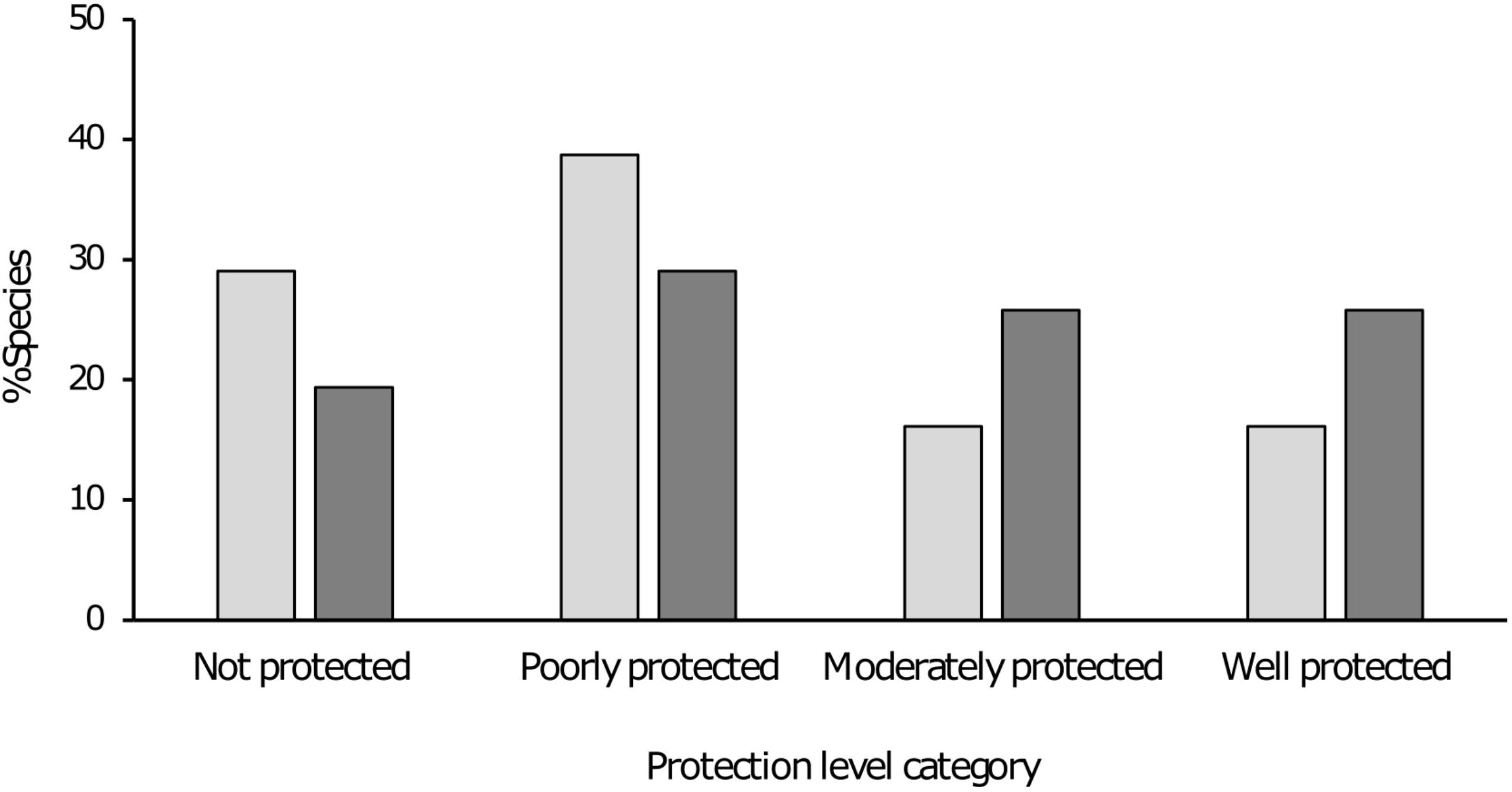
Figure 4. Bar chart indicating the percentage taxa within each protection level category depending on method of assessment, protection level including effectiveness (grey), and protection level excluding effectiveness (dark grey).
Based on available distribution data and using the methodology that considers both species presence and protected area effectiveness, there is evidence that the current protected area network within the CFE is inadequate for the protection of many freshwater fish taxa of the region. Alarmingly, 84% of taxa are under-protected (16% “moderately protected,” 39% “poorly protected,” 10% “not protected,” and 19% are completely absent from formally protected areas). Only 16% of taxa can be regarded as “well protected,” indicating that 100% or more of the conservation target is being met within formally protected areas. This is in contrast to Impson et al. (2002) who reported the freshwater fishes of the CFE, including threatened taxa, to be “well protected,” as only two of the 19 known taxa were not recorded in protected areas. These apparently conflicting results must however be interpreted with caution and can be explained with three arguments. Firstly, there has been major taxonomic revisions in the past decade, resulting in a different compliment of species included in the present assessment to those assessed by Impson et al. (2002). Secondly, while the former study considered national parks, provincial nature reserves and local authority nature reserves, the present study included all areas declared under the National Environmental Management: Protected Areas Act and thus assessed additional areas such as mountain catchment areas and stewardship areas. Finally, while Impson et al. (2002) only reported presence of native species in protected areas, the present study quantified the number of populations per area and evaluated the population viability as well as the effectiveness of the protected area in terms of mitigating major threats to the species, in order to give a better indication of protected area functioning.
The poor protection afforded to native freshwater fishes by protected areas, is not unique to the CFE, but is consistent with findings from other regions including the United States (e.g., Lawrence et al., 2011; Jenkins et al., 2015; Grantham et al., 2017), Europe (e.g., Keith, 2000; Hermoso et al., 2015; Miranda and Pino-del-Carpio, 2016), South America (Azevedo-Santos et al., 2019), and Australia (Januchowski-Hartley et al., 2011). The limited protection afforded by these areas are a result of either limited representation of species, inadequate management focused towards freshwater ecosystems or a combination of both. In order to meet a set conservation targets, a species should not only be present and viable in the protected area, but the protected area must function to protect the species against significant threats. In the case of freshwater fishes of the CFE, the primary threats are non-native piscivores and loss of critical habitats or deterioration in habitat quality (Tweddle et al., 2009; Ellender and Weyl, 2014). Protected areas by their very nature are designed to safeguard against land-use impacts and land transformation, and are thus automatically protected from direct threats to habitat diversity and quality (Keith, 2000). This is however only true when the headwaters of the system as well as the catchment area of the river is protected. Effective conservation of riverine ecosystems thus require protection of entire catchments, which is often not feasible due to scale and existing land-use practices (Skelton et al., 1995). Historical and ongoing land transformation have resulted in major impacts on both terrestrial and aquatic habitats in lowland areas (Rouget et al., 2003a,b). Consequently, the middle and lower reaches of most rivers are impacted by unsustainable water abstraction, modification of natural flow regimes, sedimentation and pollution (Russell, 2011; de Moor and Day, 2013; Shelton et al., 2018). These impacts, coupled with widespread and established populations of non-native piscivores in mainstem habitats have resulted in highly fragmented native fish populations that persist in headwater streams that act as sanctuary areas (Chakona and Swartz, 2012).
Given the degraded state of the majority of mainstem rivers in the region, coupled with reserve bias to high altitude areas, it is expected that species rated as being “well protected” are likely to be headwater specialists or widespread species that occur in headwater habitats as well as in downstream sections of larger tributaries. This is supported by our findings as species that were evaluated as being “well protected” are mainly small-bodied minnows. Some of these are headwater specialists, such as the slender redfin Pseudobarbus tenuis. Others are habitat generalists which can occur in high altitude mountain streams while also extending into lower reaches of larger tributaries such as the Breede River redfin Pseudobarbus sp. nov. “Breede” and the Clanwilliam redfin Sedercypris calidus. Endemic to the Gouritz system, P. tenuis is present in headwater streams on at least 22 formally protected areas and the majority of these populations are viable and can be considered “well protected” based on recent surveys of Swartberg Nature Reserve Complex and associated provincial reserves. well protected headwater streams and rivers will however only serve to protect native fish fauna in the absence of predatory non-native species such as O. mykiss and Micropterus spp. The typical cool and clear headwater streams of the Cape Fold Mountains provide ideal habitat for the invasion and establishment of O. mykiss. This can significantly reduce the protection value of a protected area even in the absence of other threats. Oncorhynchus mykiss is a global invader with deleterious impacts on native biota in many parts of its introduced range (Lintermans, 2000; Morgan et al., 2004; Arismendi et al., 2009). For example, Shelton et al. (2014b) reported that in the Breede River system, O. mykiss significantly reduced the densities of three native fish species and completely displaced native fishes at more than 50% of the study sites. While some Pseudobarbus sp. nov. “Breede” populations are impacted by the presence of O. mykiss and other invasive fishes, many populations occur in protected areas upstream of invasion barriers, or in tributaries where O. mykiss have not yet been introduced, such as the Langeberg and Riviersonderend provincial reserves and their associated mountain catchment areas.
In cases of species with more restricted current distribution ranges, the presence of O. mykiss on protected areas may have a more significant effect. In the case of the Berg River redfin Pseudobarbus burgi, five of the eight known subpopulations occur on formally protected areas where the headwaters are within the protected area, thereby safeguarding against upstream impacts (Jordaan et al., 2017). However, because O. mykiss has become established in these protected areas, the degree of protection for P. burgi was evaluated as fair, resulting in the species being listed as “poorly protected.” If the reserves where P. burgi and O. mykiss co-occur (Jonkershoek and Haweqwa provincial reserves and their associated mountain catchment areas) can change management objectives to remove the trout from these rivers, the protection value of these areas would increase and the species would be “moderately protected.” The feasibility of O. mykiss removal from these rivers however remains to be determined.
Two other species that were evaluated as being “well protected,” S. calidus and the Clanwilliam rock catfish Austroglanis gilli, are both endemic to the Olifants-Doring River system in the CFE. These two species have several well protected populations within the greater Cederberg Nature Reserve complex where they persist in headwater streams upstream of invasion barriers (Van der Walt et al., 2017a,b). Micropterus species are established invaders in the Olifants-Doring River system where especially smallmouth bass M. dolomieu have severely impacted native freshwater fishes in the system. Van der Walt et al. (2016) conducted a comprehensive study of Micropterus invasion in 41 tributaries of the Olifants-Doring River system and demonstrated the critical role that instream barriers play in restricting the movement of non-native species and thus preventing localised extinctions of native fishes in headwater streams situated in protected areas. Shelton et al. (2014a) also highlighted the important role of instream barriers in preventing upstream movement of M. dolomieu in the Witte River, a tributary of the Breede river system located in the Haweqwa Nature Reserve. Rahel (2013) proposed intentional fragmentation as an active management strategy in cases where non-native species pose a threat to remnant populations of native species. Typically, a barrier is constructed, followed by removal of non-native species, and reintroduction of native species into upstream segments in a strategy known as isolation management (Novinger and Rahel, 2003). This strategy was successfully implemented in the Rondegat River in the CFE where M. dolomieu was removed to allow a range expansion of the native fish fauna (Weyl et al., 2014). This strategy should however be evaluated within the context of potential long-term genetic impacts associated with fragmentation and isolation (Fausch et al., 2009; Chakona et al., 2020).
When considering the protection of larger cyprinids that are more reliant on mainstream habitat, the present study showed that only one species, the Clanwilliam yellowfish Labeobarbus seeberi can be considered to be “moderately protected.” This species is endemic to the Olifants-Doring River system and occurs in 12 protected areas where it has several viable populations upstream of invasion barriers. Removal of M. dolomieu from the Rondegat River allowed this species to expand its range by about 4 km (Weyl et al., 2014). The two other large cyprinids endemic to this system, the Clanwilliam sandfish Labeo seeberi and the Clanwilliam sawfin Cheilobarbus serra were evaluated as being “poorly protected.” However, C. serra is very close to meeting the criteria for “moderately protected.” Although this species is present on seven protected areas, the effectiveness for some of these areas was rated as fair, given that the headwaters are not protected and thus open to potential invasion by non-native species and impacts related to poor land-use. Examples are the Winterhoek mountain catchment area where the headwaters of the Olifants River originate and the Oorlogskloof Provincial Nature Reserve with the Oorlogskloof River, which has its source closer to the town of Calvinia in the Northern Cape Province.
In contrast to C. serra, Labeo seeberi is only present in two protected areas and narrowly meets the criteria for being “poorly protected.” This species was historically widespread in the Olifants-Doring system prior to the introduction of non-native fish and the construction of large instream dams such as Clanwilliam Dam (Van Rensburg, 1966; De Moor and Bruton, 1988). It now persists as a few fragmented and non-viable isolated subpopulations with the exception of the viable population in the Oorlogskloof Provincial Nature Reserve. The whitefish Cheilobarbus capensis (formerly Barbus andrewi), is currently restricted to the Breede River system and is also poorly represented within the current protected area network as it only intersects with a single protected area, Bontebok National Park. Here the mainstem Breede River is dominated by non-native fishes, and the presence and survival of C. capensis is uncertain. This species is listed as EN and it persists mainly within man-made impoundments outside of the formal protected area network (Impson et al., 2017).
In addition to non-native fishes, impacts associated with poor land-use practices and inadequate management of surface water resources can affect native fish populations both within and outside formally protected areas. Most of the CFE has a typical Mediterranean climate characterised by winter rainfall and hot and mostly dry summers. These climatic conditions, coupled with the water demand of a rapidly increasing population, result in severe utilisation pressure on water resources (Allsopp et al., 2014). This is not unique to the Western Cape Province, but is characteristic of many arid and semi-arid areas of the world (Collares-Pereira and Cowx, 2004). Unsustainable water abstraction can have deleterious consequences for freshwater fishes and their aquatic ecosystems, including reduced habitat, suboptimal flow and temperature conditions, restriction of migration and destruction of spawning areas (Maceda-Veiga, 2013). Species such as the smallscale redfin Pseudobarbus asper, which is adapted to mainstem river conditions, are especially vulnerable to excessive water abstraction, unsustainable land-use activities and anthropogenic pollution (Skelton, 2001). This species has a natural distribution range that includes both the Gouritz and Gamtoos systems and it currently occurs on 10 protected areas. Despite this, P. asper narrowly meets the criteria for “moderately protected” as many of the lowland protected areas where it occurs are at risk of non-native fish invasion from both upstream and downstream sources, with excessive water abstraction in upstream areas posing considerable alteration of hydrological regimes in downstream sections. Examples are the Swartberg and Anysberg Provincial Nature Reserves that both have viable P. asper populations, but these populations are being impacted by significant water abstraction upstream of the reserves.
Impacts related to water over-abstraction are likely to worsen in future given climate change models that predict an increasingly dry climate, with a reduced period of river flow and temperature increases, thereby reducing water quality and quantity for aquatic species such as freshwater fish (Dallas et al., 2019). Shelton et al. (2018) reported that native fish species are vulnerable to the impacts of climate change, specifically in the CFE. Climate change was however not included in the current assessment of protection level of species due to the high level of uncertainty of specific impacts on each species and how they interact within the protected areas. Future assessments need to include climate change as a threat requiring mitigation. This is possible as the National Protected Area Expansion Strategy (NPAES) includes as part of its criteria for protected area expansion the need for it to be resilient to climate change. With the recent drought (2015-present) affecting many parts of the CFE, boreholes have been installed in some protected areas in the region as precautionary measures to ensure water security in highly populated areas. However, the impact of these boreholes within protected areas are currently unknown.
Our results further illustrate that only considering species representation within protected areas and not protected area effectiveness as well, can lead to an overestimation of the value of protected areas for freshwater fishes of the CFE. This may be misleading for conservation prioritisation efforts and associated conservation interventions. The latter is highly relevant for the management of non-native fishes, which play a significant role in determining whether a protected area can function to protect fish species, as they are the main driver for reduced protection. Given the highly threatened status of the majority of freshwater fish species of the CFE, the ongoing discovery of new highly threatened lineages and the well-documented impacts of non-native fishes, there is a significant need for preventing new invasions and managing the impacts of invasions in formally protected areas. Weyl et al. (2015) highlighted the complexities associated with managing non-native fish invasions on protected areas once utilisation of a species is established. An example of conflicting management objectives is the management of introduced trout populations on Limietberg Nature Reserve for sustainability by a local angling group, which is incompatible with conservation objectives for the newly described Giant redfin P. skeltoni. Historically, this species was widely distributed across the Breede River system (Kadye et al., 2016), but currently it persists as three fragmented subpopulations as a result of the impacts of invasive fish. It was assessed as being “poorly protected” as well as Endangered, highlighting the need for active conservation intervention. It should also be noted, that the understanding of how multiple threats interact can assist in adaptive management decisions, and recent work using Bayesian Networks and Adverse Outcome Pathways research could be explored to improve understanding of multiple interacting threats (Mitchell et al., 2018) and provide important insights on effective management strategies.
In conclusion, the methodology used in this study can provide an indication of how well species are protected for persistence into the future, as the current protection level of a species relative to a minimum viable population is considered. Species identified as being “not protected” or “poorly protected” should, along with threatened species, be prioritised for identification and implementation of active conservation interventions and inclusion in protected area expansion processes. Acreman et al. (2019) proposed a number of actions to enhanced protected area effectiveness, most of which are applicable to the current scenario. Active conservation interventions that can be implemented on-reserve include the localised management of non-native fish to benefit highly threatened native fish fauna, coupled with the construction of barriers to prevent re-invasion (Weyl et al., 2014). These intervention measures should aim to include an active environmental awareness component to involve stakeholders, especially in the cases where so-called “conflict species” are involved. Other on-reserve management actions that will benefit freshwater fishes include the management of invasive alien vegetation to maintain intact riparian zones and to minimise the impact of fires. Forest fires are often overlooked as a threat to aquatic ecosystems but can cause significant impacts such as excessive sedimentation, bank destabilisation, changes in shading patterns and the release of toxic polycyclic aromatic hydrocarbons (Maceda-Veiga, 2013). Threats related to the alteration of flow regimes and surface water availability originate mostly outside protected areas and are therefore much more challenging to mitigate. In these cases, conservation managers are largely reliant on efficient implementation of national legislation, such as the National Water Act (Act 36 of 1998). Water use on protected areas, such as the utilisation of boreholes or diversion weirs, are mostly included in formal protected area management plans with clear goals and strategies towards sustainability and conservation of aquatic ecosystems, both on the protected area and downstream (Nel et al., 2009).
A limitation of the methodology is that some species would never be classified as “well protected” due to their small total population size and highly restricted natural distribution ranges. These species are thus not necessarily “under protected” due to the network not including them, there are merely not enough populations to meet the conservation target of 10 viable populations. Furthermore, the analysis was conducted with the best available distribution data for the species considered and for some species there are few recent records. More field surveys are required to confirm the status of populations within various protected areas and to identify pressures that may not be alleviated by the protected areas. Based on this assessment as well as the species Red List assessments, the primary threat to these species are non-native fishes. Hence improved distribution data for non-native fishes in protected areas, along with the geographical location of invasion barriers, will enhance biodiversity conservation and management in the CFE. Impacts unrelated to non-native fish such as pollution, changes in flow regimes and impacts on habitat quality may be more challenging to assess spatially, especially those located in lowland areas. Multiple stressors can also interact to produce antagonistic, additive or synergistic effects (Jackson et al., 2016). Modelling the risk of invasive species relative to other stressors should be explored to inform future assessments of protected area effectiveness. In order to test the robustness of the methodology and replicability of the results, it should be applied to other ecoregions of Southern Africa. It can also be expanded to other Mediterranean-type ecosystems such as the Iberian Peninsula that has a high number of threatened freshwater fishes and is faced with similar threats to the CFE (Maceda-Veiga, 2013). The outcome of this work should not only inform conservation planning and protected area expansion but should also form part of the development of protected area management plans and their associated monitoring and management goals. Protection level assessments of species should also be considered during Red List Assessments, as this would inform the conservation actions needed for the specific species being assessed.
All datasets generated for this study are included in the article/Supplementary Material.
DvdC collated all datasets, contributed to the conceptualisation and write-up of the manuscript and provided comprehensive GIS support. AC contributed to the write-up of the manuscript and provided unpublished distribution data to improve the accuracy of the assessments therein. MJ contributed to the conceptualisation and collation of datasets and lead the write-up of the manuscript. All authors contributed meaningfully to this manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ms. Lize von Staden of the South African National Biodiversity Institute (SANBI) initially developed the principles of the assessment methodology used in this manuscript. Ms. Tilla Raimondo (SANBI) and Mr. Riaan van der Walt (formerly CapeNature) provided assistance and scientific inputs during the assessment process. We wish to thank the reviewers for their useful contributions which has greatly improved the quality of this work. MJ wish to thank Ms. Vicky Hudson of CapeNature for useful inputs and discussions during the write-up of this work. The authors wish to acknowledge financial support from SANBI for publication fees.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2020.502042/full#supplementary-material
Abell, R., Thieme, M., Ricketts, T. H., Olwero, N., Ng, R., Petry, P., et al. (2011). Concordance of freshwater and terrestrial biodiversity. Conserv. Lett. 4:127–136. doi: 10.1111/j.1755-263X.2010.00153.x
Acreman, M., Hughes, K. A., Arthington, A. H., Tickner, D., and Duenas, M. A. (2019). Protected areas and freshwater biodiversity: a novel systematic review distils eight lessons for effective conservation. Conserv. Lett. 13:e12684. doi: 10.1111/conl.12684
Allsopp, N., Anderson, P., Holmes, P., Melin, A., and O’Farrell, P. (2014). “People, the Cape Floristic Region, and sustainability,” in Fynbos: Ecology, Evolution, and Conservation of a Megadiverse Region, eds G. A. Verboom, J. F. Colville, and N. Allsopp, (Oxford: Oxford University Press), 337–360.
Arismendi, I., Soto, D., Penaluna, B., Jara, C., Leal, C., and León−Muñoz, J. (2009). Aquaculture, non−native salmonid invasions and associated declines of native fishes in Northern Patagonian lakes. Freshw. Biol. 54, 1135–1147. doi: 10.1111/j.1365-2427.2008.02157.x
Azevedo−Santos, V. M., Frederico, R. G., Fagundes, C. K., Pompeu, P. S., Pelicice, F. M., Padial, A. A., et al. (2019). Protected areas: A focus on Brazilian freshwater biodiversity. Divers. Distrib. 25: 442–448. doi: 10.1111/ddi.12871
Bronaugh, W. M., Swartz, E. R., and Sidlauskas, B. L. (2020). Between an ocean and a high place: coastal drainage isolation generates endemic cryptic species in the Cape kurper Sandelia capensis (Anabantiformes: Anabantidae) Cape Region, South Africa. J. Fish Biol. 96:1087–1099. doi: 10.1111/jfb.14182
Chakona, A., and Swartz, E. R. (2012). Contrasting habitat associations of imperilled endemic stream fishes from a global biodiversity hot spot. BMC Ecol. 12:19. doi: 10.1186/1472-6785-12-19
Chakona, A., Gouws, G., Kadye, W. T., Jordaan, M. S., and Swartz, E. R. (2020). Reconstruction of the historical distribution ranges of imperilled stream fishes from a global endemic hotspot based on molecular data: Implications for conservation of threatened taxa. Aquat. Conserv. 30, 144–158. doi: 10.1002/aqc.3251
Chakona, A., Swartz, E., and Gouws, G. (2013). Evolutionary drivers of diversification and distribution of a southern temperate stream fish assemblage: Testing the role of historical isolation and spatial range expansion. PLoS One 8:e70953. doi: 10.1371/journal.pone.0070953
Chessman, B. C. (2013). Do protected areas benefit freshwater species? A broad−scale assessment for fish in Australia’s Murray–Darling Basin. J. Appl. Ecol. 50:12104. doi: 10.1111/1365-2664.12104
Collares-Pereira, M. J., and Cowx, I. G. (2004). The role of catchment scale environmental management in freshwater fish conservation. Fish Manag. Ecol. 11, 303–312. doi: 10.1111/j.1365-2400.2004.00392.x
Dallas, H., Shelton, J., Paxton, B., Weyl, O., Reizenberg, J., Bloy, L., et al. (2019). Assessing the effect of climate change on native and non-native freshwater fishes of the Cape Fold Ecoregion, South Africa. Water Research Commission Report No K5/2337. Pretoria: Water Research Commission.
Darwall, W. R., Holland, R. A., Smith, K. G., Allen, D., Brooks, E. G., Katarya, V., et al. (2011). Implications of bias in conservation research and investment for freshwater species. Conserv. Lett. 4, 474–482. doi: 10.1111/j.1755-263X.2011.00202.x
Davies, B. R., O’Keeffe, J. H., and Snaddon, C. D. (1993). A synthesis of the ecological functioning, conservation and management of South African river ecosystems. WRC Report no TT62/93. Pretoria: Water Research Commission.
de Moor, F. C., and Day, J. A. (2013). Aquatic biodiversity in the Mediterranean region of South Africa. Hydrobiologia 719, 237–268. doi: 10.1007/s10750-013-1488-7
De Moor, I. J., and Bruton, M. N. (1988). Atlas of alien and translocated indigenous aquatic animals of southern Africa. South African National Scientific Programmes Report 144. Pretoria: South African National Scientific Programmes.
DEA (2016). National Protected Areas Expansion Strategy for South Africa 2016. Pretoria: Department of Environmental Affairs.
Driver, A., Sink, K. J., Nel, J. N., Holness, S., Van Niekerk, L., Daniels, F., et al. (2012). National Biodiversity Assessment 2011: An assessment of South Africa’s biodiversity and ecosystems. Synthesis Report. Pretoria: South African National Biodiversity Institute and Department of Environmental Affairs.
Dudgeon, D., Arthington, A. H., Gessner, M. O., Kawabata, Z.-I., Knowler, D. J., Lévêque, C., et al. (2006). Freshwater biodiversity: importance, threats, status and conservation challenges. Biol. Rev. 81, 163–182. doi: 10.1017/S1464793105006950
Ellender, B. R., and Weyl, O. L. F. (2014). A review of current knowledge, risk and ecological impacts associated with non-native freshwater fish introductions in South Africa. Aquat. Invasions 9, 117–132. doi: 10.3391/ai.2014.9.2.01
Ellender, B. R., Wasserman, R. J., Chakona, A., Skelton, P. H., and Weyl, O. L. F. (2017). A review of the biology and status of Cape Fold Ecoregion freshwater fishes. Aquat. Conserv. 27, 867–879. doi: 10.1002/aqc.2730
Ellender, B. R., Weyl, O. L., and Swartz, E. R. (2011). Invasion of a headwater stream by non-native fishes in the Swartkops River system. South Africa. Afr. Zool. 46, 39–46. doi: 10.3377/004.046.0116
Fausch, K. D., Rieman, B. E., Dunham, J. B., Young, M. K., and Peterson, D. P. (2009). Invasion versus isolation: trade−offs in managing native salmonids with barriers to upstream movement. Conserv. Biol. 23, 859–870. doi: 10.1111/j.1523-1739.2008.01159.x
Government of South Africa (2010). National Protected Area Expansion Strategy for South Africa 2008, Priorities for expanding the protected area network for ecological sustainability and climate change adaptation. Pretoria: Department of Environmental Affairs.
Grantham, T. E., Fesenmyer, K. A., Peek, R., Holmes, E., Quiñones, R. M., Bell, A., et al. (2017). Missing the boat on freshwater fish conservation in California. Conserv. Lett. 10, 77–85. doi: 10.1111/conl.12249
Gray, C. L., Hill, S. L., Newbold, T., Hudson, L. N., Börger, L., Contu, S., et al. (2016). Local biodiversity is higher inside than outside terrestrial protected areas worldwide. Nat. Commun. 7:12306. doi: 10.1038/ncomms12306
Hermoso, V., Filipe, A. F., Segurado, P., and Beja, P. (2015). Effectiveness of a large reserve network in protecting freshwater biodiversity: a test for the Iberian Peninsula. Freshw. Biol. 60, 698–710. doi: 10.1111/fwb.12519
Impson, N. D., Bills, I. R., and Cambray, J. A. (2002). “A conservation plan for the unique and highly threatened freshwater fishes of the Cape Floral Kingdom” in Conservation of Freshwater Fishes: Options for the Future. Oxford: Blackwell Science, 432–440.
Impson, D., Jordaan, M., and Van der Walt, R. (2017). Pseudobarbus capensis,” in The IUCN Red List of Threatened Species 2017. (Switzerland: International Union for Conservation of Nature), doi: 10.2305/IUCN.UK.2017-3.RLTS.T2560A100114381.en
Jackson, M. C., Loewen, C. J. G., Vinebrooke, R. D., and Chimimba, C. T. (2016). Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob. Chang. Biol. 22, 180–189. doi: 10.1111/gcb.13028
Januchowski-Hartley, S. R., Pearson, R. G., Puschendorf, R., and Rayner, T. (2011). Fresh waters and fish diversity: distribution, protection and disturbance in tropical Australia. PLoS One 6:e25846. doi: 10.1371/journal.pone.0025846
Jenkins, C. N., Van Houtan, K. S., Pimm, S. L., and Sexton, J. O. (2015). US protected lands mismatch biodiversity priorities. Proc. Natl. Acad. Sci. U.S.A. 112, 16. doi: 10.1073/pnas.1418034112
Jordaan, M. S., Impson, D., and van der Walt, R. (2012). “Freshwater fish,” in Western Cape Province State of Biodiversity Report, ed. A. A. Turner, (Stellenbosch: CapeNature Scientific Services), 67–86.
Jordaan, M., Van der Walt, R., Swartz, E. R., and Impson, D. (2017). Pseudobarbus burgi. The IUCN Red List of Threatened Species 2017. Switzerland: International Union for Conservation of Nature, doi: 10.2305/IUCN.UK.2017-3.RLTS.T107660562A100170651.en
Juffe−Bignoli, D., Harrison, I., Butchart, S. H., Flitcroft, R., Hermoso, V., Jonas, H., et al. (2016). Achieving Aichi Biodiversity Target 11 to improve the performance of protected areas and conserve freshwater biodiversity. Aquat. Conserv. 26:aqc.2638. doi: 10.1002/aqc.2638
Kadye, W. T., Chakona, A., and Jordaan, M. S. (2016). Swimming with the giant: coexistence patterns of a new redfin minnow Pseudobarbus skeltoni from a global biodiversity hot spot. Ecol. Evol. 6:2328. doi: 10.1002/ece3.2328
Keith, P. (2000). The part played by protected areas in the conservation of threatened French freshwater fish. Biol. Conserv. 92, 265–273.
Laurance, W. F., Sayer, J., and Cassman, K. G. (2014). Agricultural expansion and its impacts on tropical nature. Trends Ecol. Evol. 29, 107–116. doi: 10.1016/j.tree.2013.12.001
Lawrence, D. J., Larson, E. R., Liermann, C. A. R., Mims, M. C., Pool, T. K., and Olden, J. D. (2011). National parks as protected areas for US freshwater fish diversity. Conserv. Lett. 4, 364–371. doi: 10.1111/j.1755-263X.2011.00185.x
Lintermans, M. (2000). Recolonization by the mountain galaxias Galaxias olidus of a montane stream after the eradication of rainbow trout Oncorhynchus mykiss. Mar. Freshw. Res. 51, 799–804. doi: 10.1071/MF00019
Maceda-Veiga, A. (2013). Towards the conservation of freshwater fish: Iberian Rivers as an example of threats and management practices. Rev. Fish Biol. Fish. 23, 1–22. doi: 10.1007/s11160-012-9275-5
Miranda, R., and Pino-del-Carpio, A. (2016). Analysing freshwater fish biodiversity records and respective conservation areas in Spain. J. Appl. 32, 240–248. doi: 10.1111/jai.13027
Mitchell, C., Chu, V. R., Harris, M. J., Landis, W. G., von Stackelberg, K. E., and Stark, J. D. (2018). Using metapopulation models to estimate the effects of pesticides and environmental stressors to Spring Chinook salmon in the Yakima River Basin, WA. Seattle, WA: Salish Sea Ecosystem Conference.
Morgan, D. L., Gill, H. S., Maddern, M. G., and Beatty, S. J. (2004). Distribution and impacts of introduced freshwater fishes in Western Australia. N Z. J. Mar. Freshw. Res. 38, 511–523. doi: 10.1080/00288330.2004.9517257
Nel, J. L., Reyers, B., Roux, D. J., and Cowling, R. M. (2009). Expanding protected areas beyond their terrestrial comfort zone: identifying spatial options for river conservation. Biol. Conserv. 142, 1605–1616. doi: 10.1016/j.biocon.2009.02.031
Novinger, D. L., and Rahel, F. J. (2003). Isolation Management with Artificial Barriers as a Conservation Strategy for Cutthroat Trout in Headwater Streams. Conserv. Biol. 17, 772–781. doi: 10.1046/j.1523-1739.2003.00472.x
Pfab, M. F., Victor, J. E., and Armstrong, A. J. (2011). Application of the IUCN Red Listing system to setting species targets for conservation planning purposes. Biodivers. Conserv. 20, 1001–1012. doi: 10.1007/s10531-011-0009-0
Rahel, F. R. (2013). Intentional fragmentation as a management strategy in aquatic systems. Bioscience 63, 362–372. doi: 10.1525/bio.2013.63.5.9
Rebelo, A. G. (1997). “Conservation,” in Vegetation of Southern Africa, eds R. M. Cowling, D. M. Richardson, and S. M. Pierce, (Cambridge: Cambridge University Press), 571–590.
Reid, G. M., Contreras, MacBeath, T., and Csatádi, K. (2013). Global challenges in freshwater-fish conservation related to public aquariums and the aquarium industry. Int. Zoo Yearb. 47, 6–45. doi: 10.1111/izy.12020
Ricciardi, A., and Rasmussen, J. B. (1999). Extinction rates of North American freshwater fauna. Conserv. Biol. 13, 1220–1222. doi: 10.1046/j.1523-1739.1999.98380.x
Rouget, M., Cowling, R. M., Pressey, R. L., and Richardson, D. M. (2003a). Identifying spatial components of ecological and evolutionary processes for regional conservation planning in the Cape Floristic Region. South Africa. Divers. Distrib. 9, 191–210.
Rouget, M., Richardson, D. M., Cowling, R. M., Lloyd, J. W., and Lombard, A. T. (2003b). The current configuration of protected areas in the Cape Floristic Region, South Africa—reservation bias and representation of biodiversity patterns and processes. Biol. Conserv. 112, 129–145. doi: 10.1016/S0006-3207(02)00396-8
Russell, I. A. (2011). Conservation status and distribution of freshwater fishes in South African national parks. Afr. Zool. 46, 117–132. doi: 10.1080/15627020.2011.11407485
SAIAB (2016). Specimen-records of preserved specimens and observations in the database of the National Collection of Fishes of South Africa. Dataset / Occurrence. Grahamstown: South African Institute for Aquatic Biodiversity.
SANBI (2016). Red List of South African Species. South Africa: South African Biodiversity Institute.
Saunders, D. L., Meeuwig, J. J., and Vincent, A. C. J. (2002). Freshwater protected areas: Strategies for conservation. Conserv. Biol. 16, 30–41. doi: 10.1046/j.1523-1739.2002.99562.x
Shelton, J. M., Day, J. A., and Impson, N. D. (2014a). Preliminary evaluation of the impact of invasive smallmouth bass Micropterus dolomieu on native fish abundance in the Witte River. Cape Town Floristic Region, South Africa. Afr. Zool. 4, 277–282.
Shelton, J. M., Samways, M. J., and Day, J. A. (2014b). Predatory impact of non-native rainbow trout on endemic fish populations in headwater streams in the Cape Floristic Region of South Africa. Biol. Invasions 17, 365–379. doi: 10.1007/s10530-014-0735-9
Shelton, J. M., Weyl, O. L., Chakona, A., Ellender, B. R., Esler, K. J., Impson, N. D., et al. (2018). Vulnerability of Cape Fold Ecoregion freshwater fishes to climate change and other human impacts. Aquat. Conserv. 28, 68–77. doi: 10.1002/aqc.2849
Skelton, P. H., Cambray, J. A., Lombard, A., and Benn, G. A. (1995). Patterns of distribution and conservation status of freshwater fishes in South Africa. S. Afr. J. Zool. 30, 71–81.
Thieme, M. L., Rudulph, J., Higgins, J., and Takats, J. A. (2012). Protected areas and freshwater conservation: a survey of protected area managers in the Tennessee and Cumberland River Basins, USA. J. Environ. Manage. 109:21. doi: 10.1016/j.jenvman.2012.06.021
Tweddle, D., Bills, R., Swartz, E., Coetzer, W., Da Costa, L., Engelbrecht, J., et al. (2009). “The status and distribution of freshwater fishes,” in The status and distribution of freshwater biodiversity in Southern Africa, eds W. R. T. Darwall, K. G. Smith, D. Tweddle, and P. H. Skelton, (Gland: IUCN and South African Institute for Aquatic Biodiversity), 21–37.
Van der Walt, J. A., Weyl, O. L. F., Woodford, D. J., and Radloff, F. G. T. (2016). Spatial extent and consequences of black bass (Micropterus spp.) invasion in a Cape Floristic Region river basin. Aquat. Conserv. 26:aqc.2589. doi: 10.1002/aqc.2589
Van der Walt, R., Jordaan, M., and Impson, D. (2017b). Pseudobarbus calidus. The IUCN Red List of Threatened Species 2017: e.T2562A100139530. Switzerland: International Union for Conservation of Nature, doi: 10.2305/IUCN.UK.2017-3.RLTS.T2562A100139530.en
Van der Walt, R., Jordaan, M., Bills, R., and Impson, D. (2017a). Austroglanis gilli. The IUCN Red List of Threatened Species 2017: e.T2427A99448983. Switzerland: International Union for Conservation of Nature, doi: 10.2305/IUCN.UK.2017-3.RLTS.T2427A99448983.en
Van Rensburg, K. J. (1966). Die vis van die Olifantsrivier (weskus) met spesiale verwysing na die geelvis (Barbus capensis) en die saagvin (Barbus serra). Invest. Rep. Cape Depart. Nat. Conserv. 10, 1–14.
van Wilgen, B. W., Carruthers, J., Cowling, J. M., Esler, K. J., Forsyth, A. T., Gaertner, M., et al. (2016). Ecological research and conservation management in the Cape Floristic Region between 1945 and 2015: History, current understanding and future challenges. Trans. R. Soc. S. Afr. 71, 207–303. doi: 10.1080/0035919X.2016.1225607
Weyl, O. L. F., Ellender, B. R., Wasserman, R. J., and Woodford, D. J. (2015). Unintended consequences of using alien fish for human benefit in protected areas. Koedoe 57:1264. doi: 10.4102/koedoe.v57i1.1264
Weyl, O. L., Finlayson, B., Impson, N. D., Woodford, D. J., and Steinkjer, J. (2014). Threatened endemic fishes in South Africa’s Cape Floristic Region: a new beginning for the Rondegat River. Fisheries 39, 270–279. doi: 10.1080/03632415.2014.914924
Wicht, C. L. (1945). Report of the Committee on the Preservation of the Vegetation of the South Western Cape. Cape Town: Royal Society of South Africa.
Keywords: Cape Fold Ecoregion, freshwater fish, biodiversity, protected area effectiveness, invasive fish, conservation interventions
Citation: Jordaan MS, Chakona A and van der Colff D (2020) Protected Areas and Endemic Freshwater Fishes of the Cape Fold Ecoregion: Missing the Boat for Fish Conservation? Front. Environ. Sci. 8:502042. doi: 10.3389/fenvs.2020.502042
Received: 01 October 2019; Accepted: 13 November 2020;
Published: 07 December 2020.
Edited by:
Rebecca Elizabeth Tharme, Riverfutures Ltd., United KingdomReviewed by:
Stephen John Beatty, Murdoch University, AustraliaCopyright © 2020 Jordaan, Chakona and van der Colff. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martine S. Jordaan, bWpvcmRhYW5AY2FwZW5hdHVyZS5jby56YQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.