- 1Department of Chemistry, Life Sciences, and Environmental Sustainability, University of Parma, Parma, Italy
- 2Environmental Protection, Monitoring and Biodiversity Conservation Department, National Institute for Environmental Protection and Research-National System for Environmental Protection (ISPRA/SNPA), Rome, Italy
Fungi are a significant food resource for soil fauna, whose grazing behavior can have a significant impact on their development. This relationship is an important aspect in soil functioning, with soil fungi acting as primary agents in decomposition processes. Being one of the most abundant groups among soil fauna, springtails can play a leading role in this context. Despite several previous studies on their epigeous fungal grazing behavior, data regarding the relationship between springtails and truffles are scarce. This study aimed to investigate food preferences of the springtail Folsomia candida for grazing on 12 different species of truffles, 11 belonging to Tuber genus, and 1 to Balsamia genus. We also evaluated how strongly this diet influences survival and reproduction of F. candida. In the first experiment, F. candida were allowed to choose freely between a cereal mixture (choice test) and 12 different species of truffle. In the second experiment, they were fed on the truffles only (no-choice test) for 28 days. Twelve truffle species were analyzed for survival and reproduction of F. candida. F. candida's feeding preference evolved over 72 h, beginning with a strong preference for the control and finally a general preference for truffles. Moreover, Collembola that fed on some Tuber species had a lower survival rate and fewer juveniles per adult compared to the control. Compared to other species, Tuber aestivum and Tuber melanosporum, which are well-known for their ability to produce brûlés, had a positive impact on collembolan fitness, whereas their palatability was not particularly prominent. Hence there was a relationship between diet and fitness in F. candida, whilst hardly any relationship was observed between fitness and feeding preference.
Introduction
The interaction between fungi and soil fauna is a key aspect in soil functioning, since both groups play an important role in the soil food web. Soil fauna has a significant impact on fungi, being the latter an important food resource for it (Hanlon, 1981; Jørgensen et al., 2003; Harold et al., 2005; Rotheray et al., 2011). Interactions between fungi and fungivores influence terrestrial biogeochemical cycles and can also induce fundamental changes in the performance of the plant–fungus association, through which fungal grazers affect mineralization, decomposition rates, and energy transport in soils (Ruess and Lussenhop, 2005; Fernandez et al., 2016).
The grazing of many groups of microarthropods can have either positive or negative effects on fungal communities, depending on the taxon and the abundance of animals (Bengtsson and Rundgren, 1983; Bretherton et al., 2006; Tordoff et al., 2008; Crowther et al., 2012). To prevent detrimental grazing some fungi have evolved defensive strategies, including the presence of crystal structures and other deposits on their hyphal surface, and the production of toxic or distasteful secondary metabolites (Böllmann et al., 2010).
Fungi are an important target in the feeding of springtails, one of the most abundant groups among soil fauna, and grazing by Collembola has produced evident effects on fungal development (Hopkin, 1997; Böllmann et al., 2010), either enhancing or decreasing mycorrhizal spore number (Bakonyi et al., 2002). Thus, their food choice could play a key role in this context. Despite springtails being generalists feeding on a wide range of foods, fungi are one of the main sources for most of them (Parkinson et al., 1979; Jørgensen et al., 2003; Chahartaghi et al., 2005). This group displays marked feeding preference behavior. Moreover, collembolan grazing seems to be species-specific, with selectivity depending on many fungal parameters, such as growing substrate, life stage, mycelium vitality and metabolic activity (Sabatini and Innocenti, 2000; Kaneda and Kaneko, 2004; Heděnec et al., 2013).
Whether the springtails' feeding preference is related to their development and fitness is not clearly known. Some authors (Pfeffer et al., 2010) suggest that Folsomia candida (Collembola, Isotomidae) is able to follow an optimal diet for its growth and reproduction rate by choosing to feed on what seems to be its favorite type of fungi. Furthermore, Heděnec et al. (2013) found that the fitness of F. candida strongly depended on the litter type rather than on fungal species. Truffles could play a crucial and interesting role here, since their presence influences the biochemical and physical composition of soil, especially in the rhizosphere, where they interact with soil fauna (Callot, 1999; Ricard, 2003; Granetti et al., 2005; García-Montero et al., 2012; Mello et al., 2013; Menta and Pinto, 2016). The genus Tuber, representing ectomycorrhizal fungi, produces hypogeous fruiting bodies that release secondary aromatic metabolites as adaptive strategy to attract feeders. This phenomenon is typical of subterranean organisms that strictly depend on animal activity for spore dispersion (Reyna Domenech, 2007). Tuber fungi also modify soil biogeochemistry (García-Montero et al., 2009; Trappe and Claridge, 2010) to such an extent that volatile compounds can inhibit the germination and growth of other plants around the host plant (Splivallo, 2008; Menta and Pinto, 2016), generating a burnt area called “brûlé,” which could affect soil fauna in many ways. Menta et al. (2014) tried to highlight the differences between soil fauna inside and outside the brûlé resulting from the peculiar environment created by Tuber aestivum (Vittadini). The authors showed that some collembolan families were more present in terms of abundance and frequency outside the brûlé, while a species Folsomia was abundant inside the brûlé. Some authors (Menta et al., 2014; Pinto et al., 2017) suggested that the conditions created by T. aestivum do not have a negative impact on Folsomia, which is known to graze on fungi and hyphae (Moore et al., 1985; Thimm and Larink, 1995; Fountain and Hopkin, 2005). Despite previous studies on the epigeous fungal grazing behavior of Collembola, data about the feeding interaction between springtails and truffles are still scarce (Parkinson et al., 1979; Chen et al., 1995; A'Bear et al., 2012; Heděnec et al., 2013).
In this study, we focused on the preference of F. candida for grazing on 12 different species of hypogeous truffles, 11 of which belonged to Tuber, and 1 to Balsamia. We aimed to improve our understanding of the strict relationship between soil fauna and hypogeous fungi by investigating whether the feeding preference influences survival and reproductive performance of F. candida. We expect that the fungi that show the greatest palatability influence F. candida fitness positively.
Materials and Methods
Hypogeous Fungi
We tested 12 species of hypogeous fungi, 11 belonging to Tuber and 1 belonging to Balsamia genus (Table 1). Soil fungi were collected in several municipalities located in Northern and Central Italy, in a period comprised between February 2015 and April 2016. All fungi were provided by ISPRA, the National System for Environmental Protection in Rome and classified and photographed according to their “Mycological biodiversity information system.” Table 1 reports the characteristics of the sites where the fungi were collected, the number of fungi used in the experiments for each species, and the vegetation cover of the areas. The number of truffles for each species varied between three and seven, depending on the availability of truffles during the study period. After collection, the truffle samples were gently brushed, washed with running water to remove soil residues, successively dried at room temperature for 2 h, and classified at species level using a microscope. They were then sliced (3 mm thickness) and dried at 27°C in airflow for 24 h (this treatment may have removed some VOCs but prevented proliferation of molds). The dried samples were placed into separate vacuum bags and sent to Parma University, where they were pulverized using a small grinder and immediately used for the test.
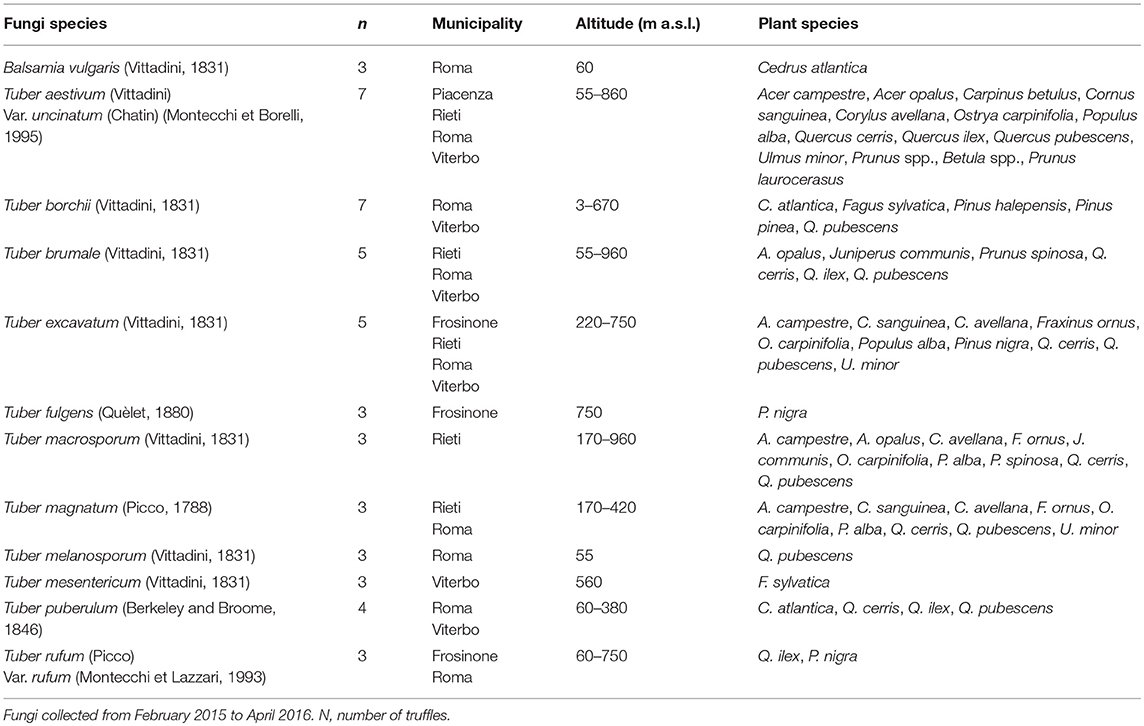
Table 1. Species of hypogeous fungi used in the study, number of fungi per species, municipality, altitude of the area, and plant species present in the area.
F. candida Cultures
The springtail F. candida Willem (Collembola: Isotomidae) is among the most intensively studied of all species of Collembola (Hopkin, 1997). This parthenogenetic species is widely distributed in many environments (Fountain and Hopkin, 2005). Cultures of this species are very easy to maintain and they are excellent for laboratory experiments due to their short reproductive cycle.
The F. candida came from 15 laboratory cultures at Parma University. They were reared according to ISO guidelines (ISO 11267, 1999), maintained at 20 (±2°C with 50–55% RH, and fed weekly on a pulverized mixture of dried organic cereals (20% wheat, 20% oats, 20% rye, 20% spelt, and 20% rice). The animals used for egg deposition (aimed to obtain the age-synchronized juveniles used in the test) were collected from all 15 breeding containers and mixed to prevent them originating from a single breeding line.
All animals used in the tests were age-synchronized to 10 days by removing eggs from the deposition cultures and, once hatched, inserting juveniles into Petri dishes, with moistened breeding substrate 8:1 (w/w) plaster of Paris: activated carbon powder.
Feeding Preference Test
In this experiment F. candida's feeding preference was tested for each fungi species separately with a binary option method consisting in allowing them to choose between a fungus species and the cereal mixture. Cereal mixture was the food used for cultures and during the synchronization phase.
Petri dishes filled with a 0.5 cm plaster layer of Paris mixed with charcoal (8:1) were used for the experiments. Two small hollows (5 × 5 × 3 mm and distant 5 cm one to each other) were made at opposite sides of the Petri dish; one hollow contained 1 gr of a pulverized fungi sample and the other one contained the same quantity of pulverized cereal mixture. There were five replicates for each truffle-control. Ten same age individuals of F. candida were transferred from the breeding substrate to the test substrate via an exhauster. No mortality was observed during the process. All the experiments were conducted at 20°C in dark/light 12:12 h conditions. Using a stereomicroscope, the number of F. candida feeding on either the truffle or the cereal mixture was checked after 24, 48, and 72 h. The count was made without removing the lid from Petri dishes to avoid Collembola displacement as result of the disturbance.
Survival and Reproduction Test
In this second experiment, survival and reproduction of F. candida were tested following the ISO guidelines 11267 (1999). Petri dishes were filled with a 0.5 cm plaster layer of Paris mixed with charcoal (8:1), and one hollow (5 × 5 × 3 mm) in the center was filled with one pulverized fungi sample. Five replicates were prepared for each fungi sample and for the cereal mixture. Ten F. candida individuals aged 10–12 days were added to each Petri dish using an exhauster. No mortality events occurred during the process. All experiments were as in Feeding Preference Test, and all fungi were tested at the same time (July, 2016). The Petri dishes were incubated for 28 days and aerated once a week. At the end of this period, the number of surviving adults and the juveniles were recorded using a stereomicroscope with floatation technique (ISO 11267/99).
Statistical Analysis
The Friedman test, followed by the Wilcoxon test for post-hoc comparisons, is the non-parametric alternative to the one-way ANOVA with repeated measures which was applied here to evaluate differences in collembolan feeding preference behavior among checks at 24, 48, and 72 h. Interspecific fungi differences on the number of F. candida were analyzed using the Kruskal-Wallis test, a non-parametric approach used to compare multiple independent samples, and the Mann-Whitney test with the Bonferroni correction for post-hoc analysis. The Wilcoxon test was used to highlight differences between the F. candida grazing on cereal mixture vs. truffle to analyze feeding preference. To evaluate the differences on survival and reproduction between truffles and with cereal mixture, the Dunn test and Bonferroni correction were applied. The Kendall's tau was calculated to investigate the correlation between diet and fitness, in order to test the relationship between n. of F. candida grazing on truffles and: (i) n. of juveniles, (ii) n. of juveniles per adults introduced at the beginning, (iii) n. of juveniles per adults survived at the end of the experiment. A p-value ≤ 0.05 was considered significant. All statistical analyses were performed using R 3.6.0 software (R Core Team, 2017).
Results
Feeding Preference
The number of F. candida feeding on truffles differed between checking hours (p < 0.001), with an increasing at 48 h (p < 0.001) and 72 h (p < 0.001) compared to 24 h (Figure 1). F. candida showed different responses with different truffle species (p < 0.001), and when compared to the cereal mixture. In general, F. candida showed a preference for most truffle species except for T. excavatum and T. fulgens compared to the cereal mixture (Figure 1). T. macrosporum, T. magnatum, and T. mesentericum were preferred significantly to the cereal mixture at the end of the experiment (Figure 1). In particular, the preference percentage of grazing F. candida obtained by T. macrosporum was higher than 85%.
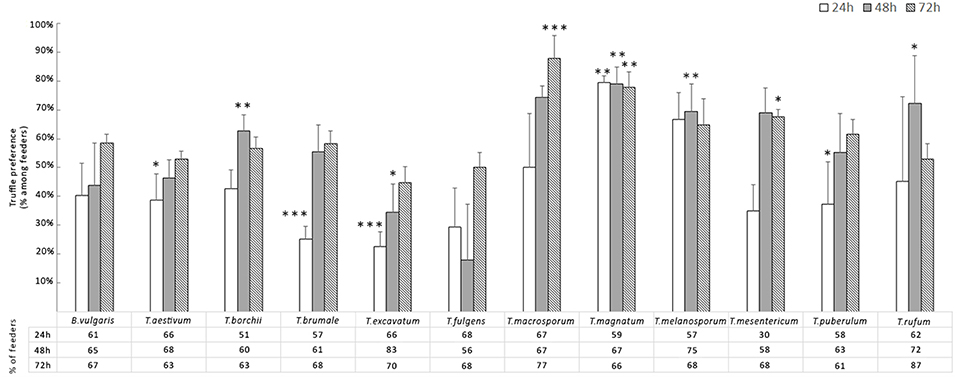
Figure 1. Average percentage ± Standard Error of F. candida that expressed truffle preference 24, 48, and 72 h after being introduced to feeding arenas. Asterisks correspond to significant differences between truffle and cereal mixture (control): *p < 0.05, **0.05> p > 0.01, ***p < 0.01.
Comparing the 12 truffles at 24 h, T. magnatum showed a higher number of F. candida feeders than T. aestivum (p < 0.01), T borchii (p < 0.01), T. brumale (p < 0.001), T. puberulum (p < 0.05), and T. excavatum (p < 0.001); this last truffle also differed from T. melanosporum (p < 0.05). At 48 h only T. aestivum differed significantly from T. magnatum (p < 0.05), and T. excavatum from T. borchii (p < 0.05). At the end of the experiment (72 h), only T. macrosporum showed higher F. candida feeders than T. aestivum, T. borchii, T. excavatum (p < 0.01 for all), and T. rufum (p < 0.05).
Survival and Reproduction
Results showed differences between truffles as regards collembolan survival (p < 0.001, Figure 2). B. vulgaris, T. aestivum, T. borchii, T. melanosporum, T. mesenterivum, and T. puberulum highlighted a survival percentage close to the cereal mixture (Figure 2). T. excavatum, T. macrosporum and T. magnatum did not differ from control. Differently, T. rufum determined significant lower survival when compared with the cereal mixture, but still higher than 50%. On the other hand, T. brumale and T. fulgens determined survival percentages lower than 50%, and significantly lower than the cereal mixture.
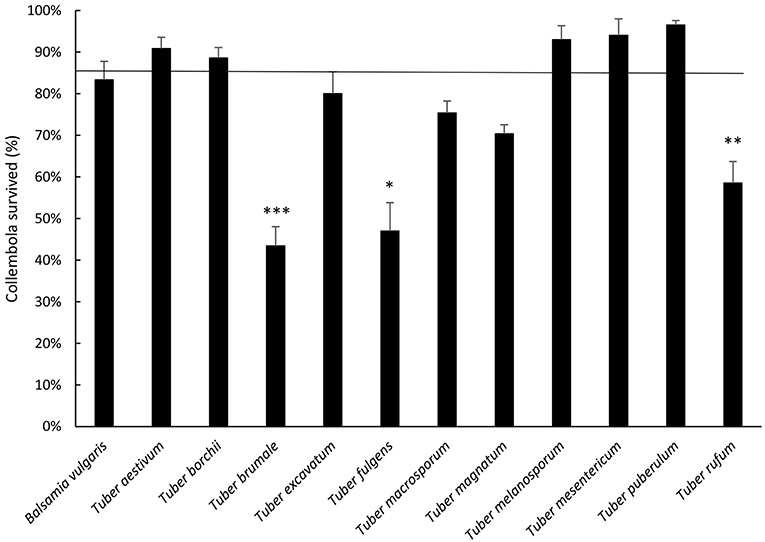
Figure 2. Average percentage ± Standard Error of F. candida surviving adults after 28 days' feeding on truffles. The horizontal line represents cereal mixture (control). Asterisks correspond to significant differences between truffle and control: *p < 0.05, **0.05> p > 0.01, ***p < 0.01. For the differences between truffles, see Supplementary Table 1.
When comparing the truffles, T. aestivum, T. borchii, T. melanosporum, T. mesentericum, and T. puberulum, led to higher survival when compared with T. brumale, T. fulgens, T. magnatum, and T. rufum. T. aestivum and T. puberulum showed higher survival when compared to T. macrosporum (Figure 2; see Supplementary Table 1 for statistical significances).
Results on reproduction of F. candida highlighted differences in the number of juveniles per adult depending on the species of truffle (p < 0.001, Figure 3). All truffle species, except T. borchii, T. melanosporum, and T. mesentericum, led to a lower number of juveniles when compared to the cereal mixture, but the differences resulted significant for T. fulgens, T. brumale, and T. magnatum only (p ≤ 0.001 for all).
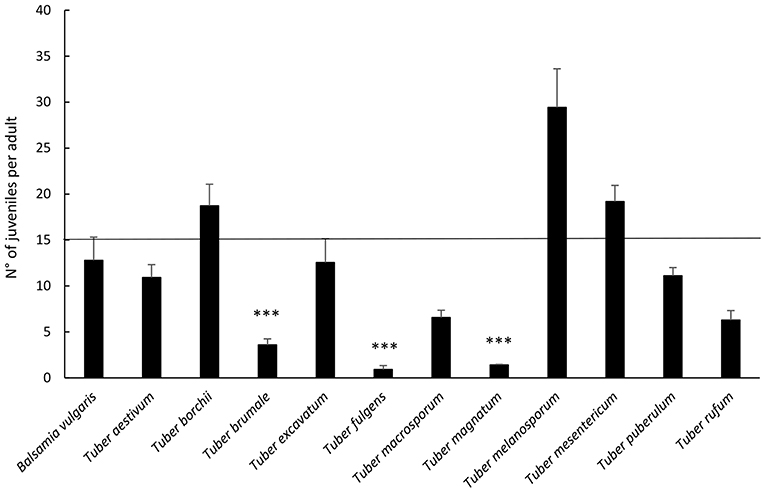
Figure 3. Average percentage ± Standard Error of F. candida juveniles (numbers of juveniles/surviving adults) after 28 days' feeding on truffles. The horizontal line represents cereal mixture (control). Asterisks correspond to significant differences between truffle and control: *p < 0.05, **0.05 > p > 0.01, ***p < 0.01. For the differences between truffles, see Supplementary Table 1.
Comparing truffles, the highest reproduction rate was supported by T. melanosporum, significantly different from T. aestivum, T. brumale, T. fulgens, T. macrosporum, T. magnatum, T. puberulum, and T. rufum (Figure 2; see Supplementary Table 1 for statistical significances).
Instead, T. fulgens determined the lowest number of juveniles, differing from all the other species of truffles except for T. brumale and T. magnatum. Furthermore, although T. brumale proved better compared to T. magnatum, it caused a significantly lower reproduction rate than the other species of truffle, except for T. excavatum, T. fulgens, T. macrosporum, and T. rufum (Figure 2; see Supplementary Table 1 for statistical significances).
No correlation was highlighted between diet, in term of n. of F. candida grazing on truffles, and fitness (n. of juveniles: tau: −0.039, p: not significant; n. of juvenile per adults introduced at the beginning, tau: −0.039, p: not significant; n. of juveniles per adults survived at the end of the experiment tau: −0.045, p: not significant).
Discussion
The aim of this study was to observe the grazing behavior of F. candida fed on twelve species of hypogeous fungi, and to evaluate if different fungi could affect survival and reproduction of this species. Consequently the aim was then to understand how much feeding preference was related to fitness of this collembolan species. It is a well-known fact that F. candida can exhibit distinct feeding preferences depending on the fungal species (Tordoff et al., 2008), and this study confirmed both this aspect and the important role of this species in spore dispersion and regulation of fungal community. The interesting addition is that not only do different fungal species have various palatability for this collembolan, but also that fungi were preferred as food resource when the springtails could choose between fungi and the usual food (cereal mixture in this study) that these animals had been used to feeding on for numerous generations. The feeding preference trend observed in this study showed an increment of truffle palatability already 48 h after the beginning of the experiment. This suggests that springtails need a short time to modify their feeding habits. Truffles exhibit their maximum sensorial properties when fresh. With a shelf-life of 7–10 days, truffles quickly lose their flavor intensity and start to spoil (Campo et al., 2017). Recent studies by Splivallo et al. (2015) and Splivallo and Ebeler (2015) show that bacteria associated with truffle-ascocarps and sulfur-containing volatiles, such as thiophene derivates, contribute to truffle aroma. Nevertheless, classical preservation method, like hot air drying (HAD) or dehydration of truffles, reducing water content and microbial growth, slow down enzymatic and chemical activities. The resulting microbial inhibition could partially explain the variations in truffle species preferences often observed throughout the first experiment. Moreover, the rehydration of the dried and pulverized truffle samples could have reactivated the microbiome, which could have served as food resource, being F. candida a fungivorous species.
We must however consider that F. candida showed variability between fungi. T. macrosporum and T. magnatum were the two more palatable species, while T. excavatum was the least favorite. Truffles attract arthropods with volatile compounds to facilitate spore dispersion through the grazers' digestive tract and, in this way, compensate their hypogeum condition (Reyna Domenech, 2007). This strategy could be particularly effective on those blind or reduced-eyesight soil-dwelling species that use odors as clues like F. candida. The VOCs profiles of Tuber spp. are highly complex and are far from being fully described (Vita et al., 2015). Moreover, the geographical origin contributes to the specific variation in VOC profiles, as reported by Üstün et al. (2018) for the white truffle T. magnatum. Besides, bacteria associated with truffle-fruiting bodies contribute to truffle aroma, making the system even more complex. In this study, the food source was not characterized in terms of biochemical composition because the focus is on the capacity shown by F. candida to discriminate among different truffle species and on the effects of these species on survival and reproduction of F. candida. Our results show that there is a substantial variation between species of truffle not only in their palatability but also in the effects on survival and reproduction rate of F. candida, in agreement with previous studies (Heděnec et al., 2013). Hubert et al. (2004) suggested the existence of four fungal groups in terms of attractiveness and suitability for grazers' development: (i) preferred and suitable for growth; (ii) preferred, but unsuitable; (iii) avoided, but suitable; and (iv) avoided and unsuitable. Our results fall into the first two categories, since we found that compared with the food provided during breeding, truffle was preferred in many cases but not always suitable for collembolan development. Several authors concluded that collembolans are able to select an optimal diet in order to maximize their fitness (Sabatini and Innocenti, 2000; Jørgensen et al., 2008). However, the current study suggests an inconsistent link, if not a discrepancy, between F. candida grazing preference and reproduction, in accord with other experiments, such as Heděnec et al. (2013), where discrepancies between food choice and food suitability emerged. Böllmann et al. (2010) suggested that this repellent characteristic has more influence on feeding preference than fungi palatability. Indeed, T. magnatum showed high palatability but a low reproduction rate for F. candida, hence easily unsuitable in terms of quality. Furthermore, a link between attractiveness and unsuitability could constitute a mechanism of counterbalance to prevent the damages of overgrazing, since by reducing the reproduction the number of collembola that graze on truffle will be smaller. Considering this hypothesis, truffles, acting on F. candida fitness and collembolan population, can indirectly modify soil biochemical and physical composition and, consequently, change soil microbial community in terms of bacteria and other microorganisms that this species uses as food source. Therefore, the effects of some truffle species are direct, modifying soil biogeochemical properties, and indirect, acting on soil living community and, consequently, on soil food web, organic matter decomposition rate and biogeochemical processes that take place in the soil. The two species of truffles T. aestivum and T. melanosporum are known for their ability to create brûlés, affecting soil biogeochemistry (García-Montero et al., 2009) and soil fauna community (Menta et al., 2014; Pinto et al., 2017). Our results showed high collembolan survival and reproduction rate for both these species, even if their palatability did not differ significantly from other food resources. In particular, feeding on T. melanosporum resulted in the highest reproduction rate observed in this study, supporting Scheu and Simmerling hypothesis 2004 that compounds judged tasteful by Collembola may differ from those useful to enhance their fitness. These results suggest that truffle species able to create brûlés could be potentially valuable resources in terms of fitness for F. candida. Menta et al. (2014) proposed that T. aestivum metabolites could attract Folsomia genus unlike other soil microarthropod taxa that were more abundant outside the brûlés.
In conclusion, our data show that truffle species differed not only in their palatability but also in their effects on fitness of F. candida, highlighting an inconsistency between preference and suitability. Other studies should be conducted to understand if the difference in response of F. candida was caused by the identity of the metabolites produced by truffle species, and if similar effects are induced on other fungal grazers. This study aims to stimulate studies for better understand the extremely complex relationship between truffles, microbiome, and soil fauna, not only for extending the scientific knowledges but also for increasing the success in consistent truffle yield. Considering latter aspect, F. candida can play an important role in the truffle cultivation, at least for the truffles species that showed both high palatability and fitness for this Collembola.
Ethics Statement
This study was carried out in accordance with the recommendations of the ISO 11267:1999.
Author Contributions
CM formulated the idea, directed the study, conducted the experiments, and wrote the manuscript. BB helped in the statistical analysis and in the writing of the manuscript. SR conducted the experiments and the statistical analysis, and contributed to writing the manuscript. CS provided and classified all the truffles used in the experiments and participated in the writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank all the people that participated in the collection of truffles, in particular the staff of the Fungi Special Project, National System for Environmental Protection, Rome, Italy(http://www.isprambiente.gov.it/it/temi/biodiversita/lispra-e-la-biodiversita/attivita-e-progetti/progetto-speciale-funghi-1).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2019.00114/full#supplementary-material
References
A'Bear, A. D., Boddy, L., and Hefin, J. T. (2012). Impacts of elevated temperature on the growth and functioning of decomposer fungi are influenced by grazing Collembola. Glob. Change Biol. 18, 1823–1832. doi: 10.1111/j.1365-2486.2012.02637.x
Bakonyi, G., Posta, K., Kiss, I., Fábián, M., Nagy, I., and Nosek, J. N. (2002). Density-dependent regulation of arbuscular mycorrhiza by Collembola. Soil Biol. Biochem. 34, 661–664. doi: 10.1016/S0038-0717(01)00228-0
Bengtsson, G., and Rundgren, S. (1983). Respiration and growth of a fungus, Mortierella isabellina, in response to grazing by Onychiurus armatus (Collembola). Soil Biol. Biochem. 15, 469–473. doi: 10.1016/0038-0717(83)90013-5
Böllmann, J., Elmer, M., Wöllecke, J., Raidl, S., and Hüttl, R. F. (2010). Defensive strategies of soil fungi to prevent grazing by Folsomia candida (Collembola). Pedobiologia 53, 107–114. doi: 10.1016/j.pedobi.2009.06.003
Bretherton, S., Tordoff, G. M., Jones, T. H., and Boddy, L. (2006). Compensatory growth of Phanerochaete velutina mycelial systems grazed by Folsomia candida (Collembola). Microbial. Ecol. 58, 33–40. doi: 10.1111/j.1574-6941.2006.00149.x
Campo, E., Marco, P., Oria, R., Blanco, D., and Venturini, M. E. (2017). What is the best method for preserving the genuine black truffle (Tuber melanosporum) aroma? An olfactometric and sensory approach. Food Sci. Technol. 80, 84–91. doi: 10.1016/j.lwt.2017.02.009
Chahartaghi, M., Langel, R., Scheu, S., and Ruess, L. (2005). Feeding guilds in Collembola based on nitrogen stable isotope ratios. Soil Biol. Biochem. 37, 1718–1725. doi: 10.1016/j.soilbio.2005.02.006
Chen, B., Snider, R. J., and Snider, R. M. (1995). Food preference and effects of food type on the life history of some soil Collembola. Pedobiologia 39, 496–505.
Crowther, T. W., Littleboy, A., Jones, T. H., and Boddy, L. (2012). Interactive effects of warming and invertebrate grazing determine the outcomes of competitive fungal interactions. FEMS Microbiol. Ecol. 18, 419–426. doi: 10.1111/j.1574-6941.2012.01364.x
Fernandez, C. W., Langley, J. A., Chapman, S., McCormack, M. L., and Koide, R. T. (2016). The decomposition of ectomycorrhizal fungal necromass. Soil Biol. Biochem. 93, 38–49. doi: 10.1016/j.soilbio.2015.10.017
Fountain, M. T., and Hopkin, S. P. (2005). Folsomia candida (Collembola): a “standard” soil arthropod. Annu. Rev. Entomol. 50, 201–222. doi: 10.1146/annurev.ento.50.071803.130331
García-Montero, L. G., Quintana, A., Valverde-Asenjo, I., and Díaz, P. (2009). Calcareous amendments in truffle culture: a soil nutrition hypothesis. Soil Biol. Biochem. 41, 1227–1232. doi: 10.1016/j.soilbio.2009.03.003
García-Montero, L. G., Valverde-Asenjo, I., Moreno, D., Díaz, P., Hernando, I., Menta, C., et al. (2012). “Influence of edaphic factors on edible ectomycorrhizal mushrooms: new hypotheses on soil nutrition and C sinks associated to ectomycorrhizae and soil fauna using the Tuber Brûlé model,” in Edible Ectomycorrhizal Mushrooms, Vol. 34, eds A. Zambonelli and G. M. Bonito (Berlin: Springer), 83–104. doi: 10.1007/978-3-642-33823-6_6
Granetti, B., De Angelis, A., and Materozzi, G. (2005). Umbria terra di tartufi. Terni: Regione Umbria–Gruppo Micologico Ternano, 303pp.
Hanlon, R. G. D. (1981). Influence of grazing by collembola on the activity of senescent fungal colonies grown on media of different nutrient concentration. Oikos 36, 362–367. doi: 10.2307/3544634
Harold, S., Tordoff, G. M., Jones, T. H., and Boddy, L. (2005). Mycelial responses of Hypholoma fasciculare to collembolan grazing: effect of inoculum age, nutrient status and resource quality. Mycol. Res. 109, 927–935. doi: 10.1017/S095375620500331X
Heděnec, P., Radochová, P., Nováková, A., Kaneda, S., and Frouz, J. (2013). Grazing preference and utilization of soil fungi by Folsomia candida (Isotomidae: Collembola). Eur. J. Soil Biol. 55, 66–70. doi: 10.1016/j.ejsobi.2012.12.005
Hopkin, S. P. (1997). Biology of the Springtails (Insecta: Collembola). New York, NY: Oxford University Press.
Hubert, J., Jarošík, V., Mourek, J., Kubátová, A., and Ždárková, E. (2004). Astigmatid mite growth and fungi preference (Acari:Acaritida): comparisons in laboratory experiments. Pedobiologia 48, 205–214. doi: 10.1016/j.pedobi.2003.12.005
ISO 11267 (1999). Soil Quality-Inhibition of Reproduction of Collembola (Folsomia candida) by Soil Pollutants. Rep. No. ISO 11267:1999(E). Geneva: Int. Stand. Organ, 16pp.
Jørgensen, H. B., Elmholt, S., and Petersen, H. (2003). Collembolan dietary specialisation on soil grown fungi. Biol. Fertil. Soils 39, 9–15. doi: 10.1007/s00374-003-0674-6
Jørgensen, H. B., Hedlund, K., and Axelsen, J. A. (2008). Life-history traits of soil collembolans in relation to food quality. Appl. Soil Ecol. 38, 146–151. doi: 10.1016/j.apsoil.2007.10.003
Kaneda, A., and Kaneko, N. (2004). The feeding preference of a collembolan (Folsomia candida Willem) on ectomycorrhiza [Pisolithus tinctorius (Pers.)] varies with mycelial growth condition and vitality. Appl. Soil Ecol. 27, 1–5. doi: 10.1016/j.apsoil.2004.04.001
Mello, I. N. K., Braga, F. R., Monteiro, T., Freitas, L. G., Araujo, J. M., Soares, F. E. F., et al. (2013). Biological control of infective larvae of Ancylostoma spp. in beach sand. Rev. Iberoam. Micol. 31, 114–118. doi: 10.1016/j.riam.2013.05.003
Menta, C., García-Montero, L. G., Pinto, S., Conti, F. D., Baroni, G., and Maresi, M. (2014). Does natural “microcosm” created by Tuber aestivum affect soil microarthropods? A new hypothesis based on Collembola in truffle culture. Appl. Soil Ecol. 84, 31–37. doi: 10.1016/j.apsoil.2014.06.012
Menta, C., and Pinto, S. (2016). “Biodiversity and ecology of soil fauna in relation to truffle,” in True Truffle (Tuber spp.) in the World. Soil Biology, Vol. 47, eds A. Zambonelli, M. Iotti, and C. Murat (Cham: Springer), 319–331. doi: 10.1007/978-3-319-31436-5_19
Moore, C., John, T. V. S., and Coleman, D. C. (1985). Ingestion of vesicular-arbuscular mycorrhizal hyphae and spores by soil microarthropods. Ecology 66, 1979–1981. doi: 10.2307/2937394
Parkinson, D., Visser, S., and Whittaker, J. B. (1979). Feeding preferences for certain litter fungi by Onychiurus subtenuis. Soil Biol. Biochem. 11, 529–535. doi: 10.1016/0038-0717(79)90013-0
Pfeffer, S. P., Khalili, H., and Filser, J. (2010). Food choice and reproductive success of Folsomia candida feeding on copper-contaminated mycelium of the soil fungus Alternaria alternate. Pedobiologia 54, 19–23. doi: 10.1016/j.pedobi.2010.08.003
Pinto, S., Gatti, F., Garcìa-Montero, L. G., and Menta, C. (2017). Does soil fauna like truffles just as humans do? One-year study of biodiversity in natural brûlés of Tuber aestivum Vittad. Sci. Total Environ. 584–585, 1175–1184. doi: 10.1016/j.scitotenv.2017.01.181
R Core Team (2017). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. Available online at: https://www.R-project.org/
Ricard, J. M. (2003). La truffe. Guide technique de trufficulture. Paris: Centre Technique Interprofessionnel des Fruits et L'egumes CTIFL.
Rotheray, T. D., Chancellor, M., Jones, T. H., and Boddy, L. (2011). Grazing by Collembola affects the outcome of interspecific mycelial interactions of cord-forming basidiomycetes. Fungal Ecol. 4, 1–14. doi: 10.1016/j.funeco.2010.09.001
Ruess, L., and Lussenhop, J. (2005). “Trophic interactions of fungi and animals,” in The Fungal Community Its Organization and Role in the Ecosystems, eds J. Dighton, J. F. White, and P. Oudemans (Boca Raton, FL: CRC Press), 581–598. doi: 10.1201/9781420027891.ch28
Sabatini, M. A., and Innocenti, G. (2000). Soil borne plant pathogenic fungi in relation to some collembolan species under laboratory conditions. Mycol. Res. 104, 1197–1201. doi: 10.1017/S0953756200003026
Scheu, S., and Simmerling, F. (2004). Growth and reproduction of fungal feeding Collembola as affected by fungal species, melanin and mixed diets. Oecologia 139, 347–353. doi: 10.1007/s00442-004-1513-7
Splivallo, R. (2008). “Biological significance of truffle secondary metabolites,” in Secondary Metabolites in Soil Ecology, Vol. 14, Soil Biology, ed P. Karlovsy (Berlin; Heidelberg: Springer-Verlag), 141–165. doi: 10.1007/978-3-540-74543-3_8
Splivallo, R., Deveau, A., Valdez, N., Kirchhoff, N., Frey-Klett, P., and Karlovsky, P. (2015). Bacteria associated with truffle-fruiting bodies contribute to truffle aroma. Env. Microb. 17, 2647–2660. doi: 10.1111/1462-2920.12521
Splivallo, R., and Ebeler, S. E. (2015). Sulfur volatiles of microbial origin are key contributors to human-sensed truffle aroma. Appl. Microb.Biotech. 99, 2583–2592. doi: 10.1007/s00253-014-6360-9
Thimm, T., and Larink, O. (1995). Grazing preferences of some Collembola for endomycorrhizal fungi. Biol. Fert. Soils 19, 266–268. doi: 10.1007/BF00336171
Tordoff, G. M., Boddy, L., and Jones, T. H. (2008). Species-specific impacts of Collembola grazing on fungal foraging ecology. Soil Biol. Biochem. 40, 434–442. doi: 10.1016/j.soilbio.2007.09.006
Trappe, J. M., and Claridge, A. W. (2010). The hidden life of truffles. Sci. Am. 302, 78–84. doi: 10.1038/scientificamerican0410-78
Üstün, N. S., Bulam, S., and Peksen, A. (2018). “Biochemical properties, biological activities and usage of truffles,” in Conference: International Congress on Engineering and Life Science (ICELIS 2018), 26–29 April 2018, Proceeding Book (Kastamonu), 772–778.
Keywords: springtail, Tuber, Balsamia, food quality, preference test, soil fauna, ectomycorrhizal fungi
Citation: Menta C, Siniscalco C, Bonati B and Remelli S (2019) Food Choice and Fitness of Folsomia candida (Collembola, Isotomidae) Fed on Twelve Species of Truffle. Front. Environ. Sci. 7:114. doi: 10.3389/fenvs.2019.00114
Received: 13 December 2018; Accepted: 01 July 2019;
Published: 16 July 2019.
Edited by:
Maria Luz Cayuela, Spanish National Research Council (CSIC), SpainReviewed by:
Matthieu Chauvat, Université de Rouen, FranceJuliane Filser, University of Bremen, Germany
Copyright © 2019 Menta, Siniscalco, Bonati and Remelli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Menta, Y3Jpc3RpbmEubWVudGFAdW5pcHIuaXQ=
 Cristina Menta
Cristina Menta Carmine Siniscalco
Carmine Siniscalco Beatrice Bonati
Beatrice Bonati Sara Remelli
Sara Remelli