- 1Laboratory of Hydrogeoscience and Biological Engineering, L.G. Rich Environmental Laboratory, Department of Environmental Engineering and Earth Sciences, Clemson University, Anderson, SC, United States
- 2State Key Laboratory of Hydroscience and Engineering, Department of Hydraulic Engineering, Tsinghua University, Beijing, China
- 3Department of Agricultural Engineering, Gyeongsang National University, Jinju, South Korea
- 4Department of Plants, Soils and Climate, Utah State University, Logan, UT, United States
- 5Laboratory of Soil and Water Engineering, Department of Civil and Environmental Engineering, Rensselaer Polytechnic Institute, Troy, NY, United States
- 6Unité ECOSYS, AgroParisTech, Université Paris-Saclay, Thiverval-Grignon, France
Groundwater contamination by oocysts of the waterborne pathogen Cryptosporidium parvum is a significant cause of animal and human disease worldwide. Although research has been undertaken in the past to determine how specific physical and chemical properties of soils affect the risk of groundwater contamination by C. parvum, there is as yet no clear conclusion concerning the range of mobility of C. parvum that one should expect in field soils. In this context, the key objective of this research was to determine the magnitude of C. parvum transport in a number of soils, under conditions in which fast and preferential transport has been successfully prevented. C. parvum oocysts were applied at the surface of different soils and subjected to artificial rainfall. Apparently for the first time, quantitative PCR was used to detect and enumerate oocysts in the soil columns and in the leachates. The transport of oocysts by infiltrating water, and the considerable retention of oocysts in soil was demonstrated for all soils, although differences in the degree of transport were observed with soils of different types. More oocysts were found in leachates from sandy loam soils than in leachates from loamy sand soils and the retention of oocysts in different soils did not significantly differ. The interaction of various processes of the hydrologic system and biogeochemical mechanisms contributed to the transport of oocysts through the soil matrix. Results suggest that the interplay of clay, organic matter, and Ca2+ facilitates and mediates the transfer of organic matter from mineral surfaces to oocysts surface, resulting in the enhanced breakthrough of oocysts through matrices of sandy loam soils compared to those of loamy sand soils. Although the number of occysts that penetrate the soil matrix account for only a small percentage of initial inputs, they still pose a significant threat to human health, especially in groundwater systems with a water table not too distant from the soil surface. The results of the research demonstrate a critical need for the simultaneous study of the interaction of various processes affecting oocysts transport in the subsurface, and for its expansion into complex systems, in order to obtain a coherent picture of the behavior of C. parvum oocysts in soils.
Introduction
The presence of pathogenic bacteria, viruses, and protozoa in drinking water is a significant cause of animal and human disease in many parts of the world. One of these pathogens, the zoonitic protozoan Cryptosporidium parvum, causes cryptosporidiosis, a common gastrointestinal disease associated with severe gastroenteritis and diarrhea (Smith, 1992). Outside of the lower intestines of humans and domestic or wild animals, where C. parvum carries out the active part of its cycle, it is present in the environment in the form of 4- to 6-μm-long ovoid-shaped oocysts, with a double wall that is resistant to most oxidation processes (e.g., ozonation and chlorination) typically used for water treatment (Korich et al., 1990). Although the median infective dose of C. parvum oocysts for humans is reported at 30 oocysts (Dupont et al., 1995), evidence suggests that as few as ten may be enough to cause infection in humans (Smith, 1992).
During the past three decades, the presence of C. parvum in surface waters and groundwaters in the United States and Great Britain (Galbraith et al., 1987; LeChevallier et al., 1991; Rose et al., 1991) has been connected with several major outbreaks of Cryptosporidiosis (Hayes et al., 1989; Mackenzie et al., 1994; Smith and Rose, 1998). The presence of C. parvum oocysts in drinking water supplies results from a number of processes. For example, infected hosts, such as cows or deer may defecate in streams and shallow ponds, and their feces may end up on the soil via direct release or land spreading of manure. The land application of municipal or industrial wastewater sludge may also contribute significant numbers of oocysts at the soil surface. Here, runoff may carry oocysts to nearby waterbodies, or rain infiltration may transport oocysts in the subsoil to groundwater. Among the different pathways for transporting oocysts, rain infiltration was generally considered until a few years ago to be of little significance, in line with the common assumption that soils are effective at filtering a wide range of pathogens (Tim et al., 1988). Studies of packed columns with saturated flow by Brush et al. (1999) and Harter et al. (2000) and undisturbed columns with unsaturated flow (Mawdsley et al., 1996a), however, suggested that C. parvum oocysts could be transported rapidly downward through the soil. Detailed experimental observations have since confirmed this to be the case, especially through soil macropores and in karstic geological terrain through fractured bedrocks (Boyer and Kuczynska, 2003; Darnault et al., 2003, 2004; Kuczynska et al., 2003; Boyer et al., 2009; Ramirez et al., 2009; Petersen et al., 2012; McLaughlin et al., 2013). In particular, Darnault et al. (2003, 2004) demonstrated that preferential flow in the form of fingered flow and macropores flow in unsaturated soils allowed the fast transport of C. parvum oocysts, leading to breakthrough concentrations above infection doses.
Based on experimental evidence, it seems well established that C. parvum oocysts can move in soils through preferential pathways in relatively large amounts. It is also possible however that in many situations, typical rainfall events are of insufficient intensity for macropores to conduct much water or for fingers to develop to transport C. parvum. Such migration then occurs through the soil matrix, where oocysts are subjected closely to a number of physico-chemical processes, many of them similar to those that have been documented extensively for solutes. Some of the key mechanisms involved have been studied in detail. Changes in the solution chemistry induce the modification of van der Waals interactions, steric repulsions, and cation bridging that impact the processes controlling the attachment of C. parvum oocysts to collector in porous media (LeChevallier et al., 1991; Byrd and Walz, 2005, 2007; Liu et al., 2009; Janjaroen et al., 2010; Park et al., 2012; Balthazard-Accou et al., 2014). Steric interactions have been shown to hinder the impact of ionic strength on the oocysts' Debye length (Liu et al., 2009; Janjaroen et al., 2010). The presence of Ca2+ also enhances the deposition of C. parvum on silica surface compared to Na2+ solution (Janjaroen et al., 2010). Studies of the influence of ionic strength, natural organic matter (NOM), and surface charge on C. parvum oocyst transport demonstrate that electrostatic effects dominate hydrophobic effect (Hsu et al., 2001; Dai and Hozalski, 2002; Bradford and Bettahar, 2005; Cortis et al., 2006; Kim et al., 2010; Metge et al., 2010). Straining and release of C. parvum oocysts in porous media have been demonstrated to be a key mechanism controlling the transport and retention of C. parvum oocysts (Harter et al., 2000; Logan et al., 2001).
Whereas several of the processes that can, theoretically, affect the transport of C. parvum oocysts in soils have been studied separately, a coherent picture has yet to emerge of how these various processes interact in any given soil, and of the extent to which they determine the transport of oocysts. A complete analysis of these questions would clearly require very extensive experiments, with different types of soils and a full factorial design in each case. Unfortunately, such a program is extremely onerous, in part because each measurement of oocyst concentration in water and especially in soil samples costs a very significant amount of money. Therefore, preliminary experiments are recommended to elucidate the range of behaviors expected among soils with contrasting properties prior to any intensive and expensive research effort. If the transport of oocysts among selected soils with varied physico-chemical conditions does not vary appreciably, and in no case is cause for serious concern, there may be little justification for conducting the type of detailed factorial experiment needed to assess which parameters or combinations of parameters are most influential, and under what conditions.
In this context, the primary objective of the research detailed here involves examining the difference in the transport and retention of C. parvum oocysts in soils among a set of soil columns with contrasting properties, subjected to simulated rainfall under laboratory conditions. Comparisons are made in terms of traditional breakthrough curves (BTCs) of C. parvum oocysts, but also relative to distribution profiles of C. parvum oocysts within the soil columns. Based on the outcome of these comparisons, a secondary objective is to determine the need for further research to improve our ability to predict C. parvum oocysts in field soils.
Materials and Methods
Cryptosporidium parvum Oocysts
Original stock suspensions of purified and viable C. parvum oocysts (Iowa isolate–Cat# P102C@1 × 10/9) were obtained from Waterborne Inc. (New Orleans, LA). The concentration of the stock oocyst suspension (109 oocysts in 50 mL) was determined using a Neubauer hemocytometer at a magnification of 200×. The stock of C. parvum oocysts was suspended in 50 mL de-ionized (DI) water with penicillin, streptomycin, gentamicin and amphotericin B. The C. parvum oocysts were stored in the dark at 4°C for 18 months. Prior to each experiment, the stock oocyst suspension was mixed in a vortexer at 3,000 rpm for 15 min to disperse the C. parvum oocysts that had settled to the bottom of the 50 mL centrifuge tube. This mixing process was designed to optimize the uniformity and consistency of the inoculum used to prepare the experimental oocysts concentration for use in the soil column experiments.
Soils
The four different soils used in this study were collected in bulk form from fallow and cultivated pasture fields in the U.S. states of Illinois and Utah. The difference in the textural characteristics of these soils resulted in their classification as loamy sand and sandy loam based on their particle size analyses. One of the loamy sand soils collected in Kankakee County, IL is classified as a coarse-loamy, mixed, superactive, mesic Typic Endoaquoll belonging to the Gilford series which is a very deep, poorly drained or very poorly drained soil, and that formed in loamy over sandy sediments on outwash plains, near-shore zones (relict), and flood-plain steps. The Gilford soil series has a negligible potential for surface runoff, with a saturated hydraulic conductivity that is high in the upper part and very rapid in the lower part. Its soil permeability is moderately rapid in the upper part and rapid in the lower part. The other loamy sand soil, from Kankakee County, belongs to the Sparta series. The Sparta series is classified as a coarse-loamy, mixed, superactive, mesic Typic Endoaquoll, which is a very deep, and excessively drained soil. Formed in sandy outwash that has been reworked by wind these soils have a saturated hydraulic conductivity ranging from 10.00 to 100.00 micrometers per second. The Greenson sandy loam soils collected in Cache County (Utah) are classified as fine-silty, mixed, superactive, mesic Oxyaquic Calcixerolls; they are very deep, somewhat poorly drained, or moderately well-drained soils that formed from lacustrine deposits. Found on low lake terraces, the Greenson soil series is either somewhat poorly drained or moderately well-drained with a low-to-medium surface runoff, a slow-to-moderate permeability (moderately low to high saturated hydraulic conductivity), and a generally high organic matter content, from 3 to 9%. Collected in Cache County, UT, the Lewiston sandy loam soil is classified as coarse-loamy, mixed, superactive, mesic Aquic Calcixeroll that is a very deep, somewhat poorly drained soil formed in lacustrine sediments. Also found on lake terraces, the soil is somewhat poorly drained, has slow or very slow runoff and moderate permeability. The soil organic matter content ranges generally from 1 to 3%.
The physicochemical characteristics of these soils, presented in Table 1, were determined by the Utah State University Analytical Laboratories (USUAL). Soil texture and particle size analyses were performed using the hydrometer method (Gee and Bauder, 1986). Total carbon analyses were performed using a PrimacSLC Analyzer (Skalar Inc., Buford, GA, USA). Saturated soil paste extracts were prepared for the electrical conductivity and pH measurements (Rhoades, 1996). The cation exchange capacities of the soils were measured using the sodium acetate/ammonium acetate replacement method. Soil elements and ions were determined by ion chromatography using a Dionex ICS-3000 Ion Chromatography (IC) system. The soils were air-dried at 37°C, and sieved (2 mm), which were then stored in lidded buckets at room temperature until used.
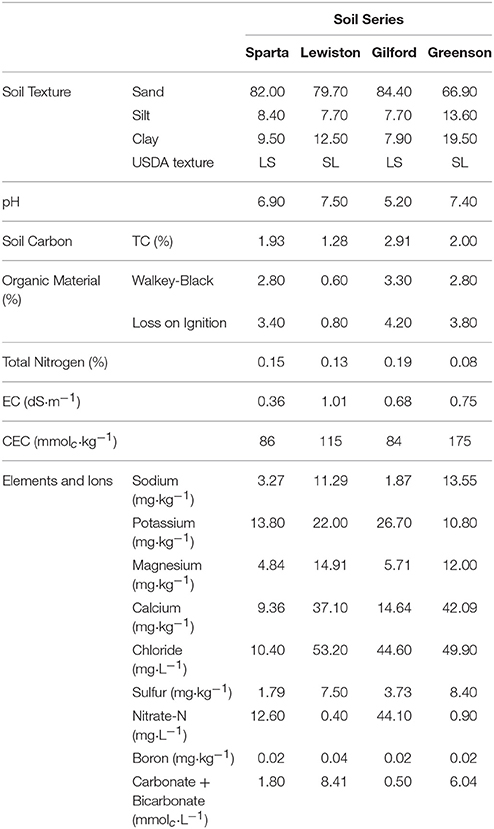
Table 1. Physicochemical properties of loamy sand and sandy loam soils from the four series of soils examined–Sparta, Lewiston, Gilford, and Greenson.
Artificial Rainfall Solutions and C. parvum Oocysts Inoculum Preparation
All solutions were prepared with deionized water (18 MΩ·cm−1 resistivity; Milli-Q, Millipore Corp, Bedford, MA). For all flow-through column experiments, the artificial rainfall solutions contained 1 mM KCl as a background electrolyte. Potassium bromide (KBr) was used as a conservative tracer and was measured using an Orion™ Bromide Electrode (ThermoFisher Scientific, Waltham, Massachusetts, USA). The preparation of the C. parvum oocysts inoculum consisted of adding about 1.5 × 106 C. parvum oocysts (quantified and enumerated using the qPCR method) from the stock oocysts suspension to 53 mL of artificial rainfall/background solution (1 mM KCl) that was spiked with 10 mM KBr tracer. Fifty of the 55 mL that constituted the inoculum were used in the flow-through column experiments and 5 mL were kept for further quantification of the initial input concentration (C0) of C. parvum oocysts applied to each column. The inoculum applied to the surface of each column contained a measured concentration of C. parvum oocysts of about 2.85 × 104 mL−1, corresponding to a total of 1.56 × 106 ± 575,000 oocysts.
Column Experimental and Rainfall Simulator Set-Up
The columns used in these experiments were composed of plastic rings with dimensions of 20 cm in length and 9.5 cm in internal diameter. A ring 5 cm in height was placed at the bottom of the column, followed by the placement of 2-cm rings to achieve a total column height of 30 cm, of which 20 cm were used for the experiments. Rings were compressed together with top and bottom column holders, which were held with four rods positioned parallel to the length of the column on the outside perimeter of the rings, and bolts to prevent sliding. The rods were bolted through a screen section of a funnel that also had an additional mesh to prevent soil particles from moving through. The column was then clamped to an individual stand. A separate clamp allowed connection of the bottom part of the funnel below the column and an additional rod built in the stand allowed connection of a nozzle above the column. The soil was packed into each column. To achieve a uniform and homogeneous soil packing and a soil bulk density of 1.5 g·cm−3 of soil, the total amount of soil (2,125 g) used to form the soil column was poured in three equal amounts and compacted with a rod. Four experimental columns were run simultaneously. One pump equipped with four cartridges dispensed the artificial rainfall solutions to the four nozzles, and a single air tube with brass couplings was used to pump air into these four nozzles. Rainfall was applied to the surface of each column using an individual nozzle (XA nozzle system ¼, 303 from BETE Fog Nozzle Inc., Greenfield, MA). The nozzle was connected to the water on one side through a peristaltic pump (Cole-Parmer, Vernon Hills, Illinois, USA) and to the air on the other side. The flow rate and the air pressure at the nozzle were adjusted at the pump and through a Parker Watts miniature precision regulator gauge (1/4 in; 60 psi; Parker Hannifin Corp., Cleveland, OH) to ensure that the nozzle spray covered the entire soil surface. The height of the nozzle above the soil surface was 10 cm.
Experimental Procedures/Rainfall Treatments and Leachate Collection
Each soil column experiment was performed in duplicate. Once the experimental columns were loaded with soils and placed in their stands, rainfall simulation was initiated with the artificial rainfall. Subsequent to achieving steady state and equilibrium conditions and a determination of constant outflow, C. parvum oocysts were released at the soil surface by pouring the 50 mL inoculum into the column. No rainfall was applied during the duration of the inoculum release at the soil surface until the inoculum had infiltrated. Rainfall was then resumed. In soil column experiments characterized by an occurrence of water ponding at the soil surface, the rainfall was discontinued until the ponded water had infiltrated and was then resumed. The soil flow velocities ranged from 0.09 to 0.16 cm·h−1 with all of the column outflows collected in 50 ml centrifuge tubes. After about 6 pore volumes (PV) had passed through the columns, the rainfall was stopped, the column set-up was dismantled, and the columns were sliced with a thin metal sheet. A total of 10 column layers were sliced: 1 cm for the top layer, 2 cm for each of the following seven rings, with the remaining 5 cm ring divided in two, to obtain two layers of approximately 2.5 cm each.
Soil Water Content
Each soil layer was sampled to determine the water content and the concentration of C. parvum oocysts in the soil profile. To establish the water content in the soil layers, three wet soil samples of 5 g each were collected randomly in each soil layer. These wet soil samples were then placed in aluminum foil cup holders, weighed, and oven dried at 105°C for 24 h. The weight of the dried samples was then recorded and the water content was established gravimetrically.
Isolation of C. parvum Oocysts from Soil
All of the soil remaining in each layer after sampling for water content was collected for purposes of measuring the concentration of C. parvum oocysts in the soil. 25 g of wet soil was placed in each of between 9 and 11 50 mL plastic centrifuge tubes, depending on the layer. The method developed by Koken et al. (2013) was used to isolate C. parvum oocysts from the soil. The C. parvum oocysts attached to soil particles were released by the addition of 20 mL of Tween 80 at two critical micelle concentrations (CMC) to each of the 50 mL centrifuge tubes, which were then fixed in a in a rotational shaker, perpendicular to the axis of rotation, for 24 h. Two tubes from each soil layer were selected to undergo the C. parvum oocyst isolation procedure, while the remaining tubes were stored. In each of the selected tubes, the soil was underlaid by 10 mL of cold sucrose solution with a density of 1.18 g·mL−1. These tubes were then centrifuged at 2,500 × g for 15 min to construct a sucrose concentration gradient and retain the C. parvum oocysts. The resulting supernatant was then transferred into a new 50 mL conical-bottomed centrifuge tube and subjected to centrifugation at 2,500 × g for 15 min. Thereafter, the supernatant was removed, leaving 4 mL in the tube. A first wash was performed by vortexing the tube, re-suspending the pellet by adding 35 mL of deionized water, followed by centrifugation at 2,500 × g for 15 min. A second wash was conducted following the procedure described in the first wash. For the third wash, supernatants were removed and the remaining 4 mL of the two tubes from each layer were vortexed and transferred into one 15 mL centrifuge tube. Deionized water was added to reduce the final volume to 12 mL. These 15 mL tubes were centrifuged at 2,500 × g for 15 min. In each case, the supernatant was removed to leave 3 mL of pellet in the 15 mL tube, and the pellet was re-suspended by vortex. All centrifugations involving 50 and 15 mL tubes were performed using a refrigerated centrifuge (Model 5810R, Eppendorf AG, Hamburg, Germany). A 300 μL aliquot was taken from the 3 mL, and placed in a microcentrifuge tube to which 900 μl of TE buffer was added. After vortexing briefly, the microcentrifuge tube was centrifuged at 14,000 × g in a microcentrifuge (Model 5424, Eppendorf AG) for 5 min. The supernatant was then removed, leaving a 0.5 mL sample for DNA extraction and PCR analyses.
Concentration of C. parvum Oocysts in Effluents
Soil effluent samples were collected in 50 mL centrifuge tubes and the method developed by Koken et al. (2013) was again used to concentrate C. parvum oocysts from the soil. Selected tubes were centrifuged at 2,500 × g for 15 min to concentrate the C. parvum oocysts in the pellet. The supernatants were discarded and the remaining 5 mL containing the pellets were transferred into 15 mL centrifuge tubes. Deionized water was added to each 15 mL tube to bring the final volume to 8 mL. The tubes were then spun at 2,500 × g for 15 min. The supernatant was removed and the remaining 1.7 mL were transferred to microcentrifuge tubes. These tubes were centrifuged at 14,000 × g for 5 min. The supernatants were removed, leaving 0.5 mL, to which 900 μL of TE buffer was added. After being vortexed, the microcentrifuge tubes were centrifuged at 14,000 × g for 5 min, and the supernatant was removed to leave 0.25 mL of pellet to which 0.25 mL of TE buffer was added, yielding a 0.5 mL of sample for DNA extraction and PCR analyses. All centrifugation processes involving 50 mL and 15 mL tubes were performed using an Eppendorf 5810 R refrigerated centrifuge. All centrifugations involving microcentrifuge tubes were performed in an Eppendorf 5424 microfuge.
DNA Extraction and qPCR Analyses of C. parvum Oocysts
The molecular biological methods used for the detection and enumeration of C. parvum oocysts, which include DNA extraction and qPCR analyses, using the procedures described in detail by Koken et al. (2013). Briefly, 7-point standard curves were prepared with DNA from 10,000; 1,000; 100; 10; 1; 0.1; and 0.01 oocysts (per 5 μL volume), which were obtained by pooling two samples from the C. parvum oocyst stock suspension. Each standard curve was used during the analysis of six microtiter plates containing samples obtained from soil and leachates. Six plates were run, with each plate containing its own set of standards for the standard curve. After DNA isolation, each sample was resuspended in 30 μL of DNA elution buffer (Cat# D3004-4; Zymo Research, Irvine, CA). A 5 μL sample DNA was used for each qPCR reaction, and each sample was tested in triplicate. Samples were analyzed in a 20 μL reaction volume with 50 cycles per run using a QuantStudio 12K Flex Real Time PCR system (Life Technologies, Carlsbad, CA, USA). Taqman® chemistry was used, and the primers and probe indicated in Koken et al. (2013) were synthesized as PrimeTime® qPCR assays by Integrated DNA Technlogies (Coralville, Iowa, USA) on the basis of GenBank accession number AF190627 by Jothikumar et al. (2008) as follows: 5′-ACTTTTTGTTTGTTTTACGCCG-3′ (forward primer), 5′-AATGTGGTAGTT GCGGTTGAA-3′ (reverse primer) and 5′-FAM-ATTTATCTCTTCGTAGCGGCG-BHQ-3′ (probe). The results were compiled and analyzed using the QuantStudio software (Life Technologies). After analysis, the data from each plate were exported to an Excel spreadsheet. Mean data for each sample (averaging of replicates) were compiled as aliquot data. These values reflected the number of oocyst genomes in 5 μL of each sample. These data were used to calculate the number of oocysts in the entire 30 μL sample. The standard deviation for each sample was also computed.
Statistical Analysis
Results of the transport and retention of C. parvum oocysts in soils were expressed as the percentage of C. parvum oocysts retrieved in the soil leachates and matrices, which reflect the behavior of C. parvum oocysts in soils of different physicochemical properties. All statistical analyses were performed using SAS®Studio software. For the statistical analysis of the transport and retention results of C. parvum in soils, a t-test was applied to determine the least significant difference (LSD) for the percentage of C. parvum oocysts retrieved in the soil leachates and matrices of Sparta, Lewiston, Gilford, and Greenson series soils. Statistical significance was accepted at p < 0.05. Results are expressed in graphs as mean ± standard deviation.
Results
Soil Physicochemical Characteristics
Physicochemical analyses of the soils used in this study indicate that soils in the Greenson series and Lewiston series are sandy loam soils, while the Sparta and Gilford series were characterized as loamy sand soils (Table 1). The clay contents were highest in the Greenson series soil (19.5%), with Lewiston (12.5%) and Sparta (9.5%) series soils having less clay and the Gilford series soil (7.9%) having the least clay. The organic matter content ranged from 1.28% in Lewiston series to 2.91% in Gilford series soils. The lowest pH values were observed in the Gilford (5.2) and Sparta (6.9) series soils, while the highest pH values were reported in Greenson (7.4) and Lewiston (7.5) series soils. The total calcium concentrations ranged from a low of 9.36 mg·kg−1 for the Sparta series, to a high of 42.09 mg·kg−1 for the Greenson series soil. With the addition of artificial rainfall, the highest flow velocity (0.16 cm·h−1) was observed in the Lewiston series soil, while the lowest flow velocity (0.10 cm·h−1) was reported in the Greenson series soil (Table 2).
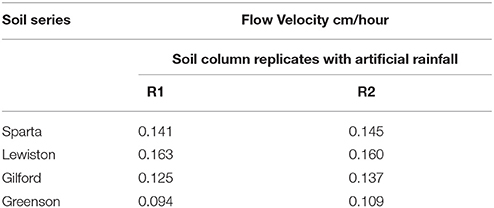
Table 2. Flow velocities from soil columns packed with the four series of soils examined–Sparta, Lewiston, Gilford, and Greenson–resulting from application of rainfall treatments.
Tracer tests were used to evaluate the hydraulic properties of the soils. The concentration of bromide ions entering the column, C0, and in the effluent, C, were used to calculate BTCs as C/C0 as a function of PV (Figure 1). The non-reactive chemical tracer added to the C. parvum oocysts mixture was measured in every leachate sample as mg·L−1 of leachate. No correlation was observed between the number of C. parvum oocysts in the leachate and the tracer concentration. Additionally, the BTCs of the tracer and the C. parvum oocysts did not display the same behavior. The tracer BTCs in the Sparta, Lewiston, and Gilford experiments presented a monotonic increase, with a C/C0 value reaching a peak ranging from about 0.13 to 0.20 that occurred at 0.56 to 1 PV, and then a monotonic decrease. Regarding the Greenson series, the tracer BTCs contained two peaks, a first peak at the 0.12 PV and a second peak at 1.5–3.5 PV, possibly indicating a fast and slow convective transport; however this possible dual flow had no effect on the mobility and transport of C. parvum oocysts.
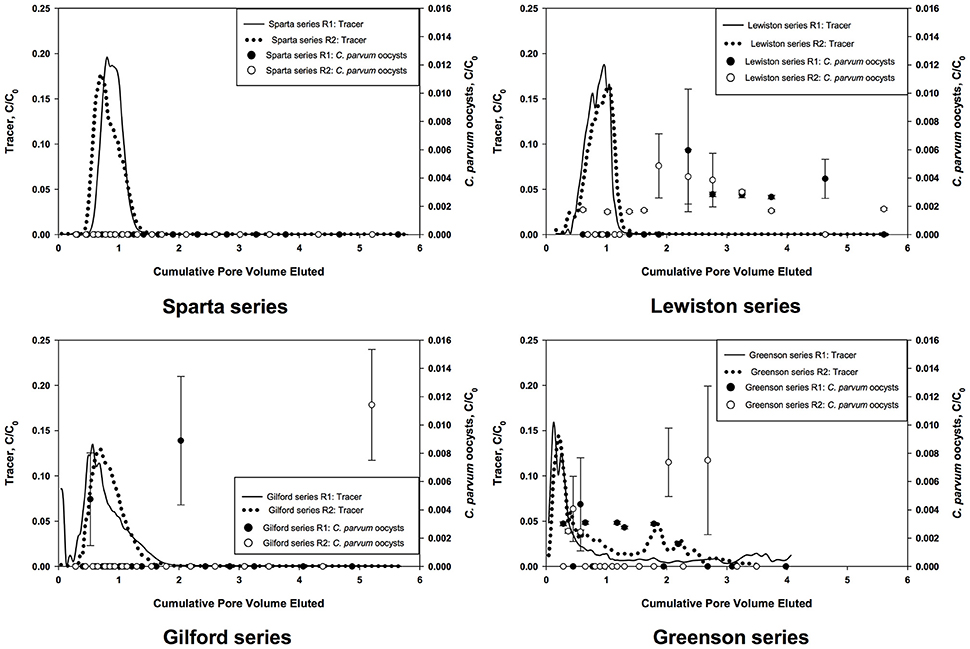
Figure 1. Breakthrough curves of C. parvum oocysts and bromide tracer in loamy sand and sandy loam soils from the four series of soils examined–Sparta, Lewiston, Gilford, and Greenson–during simulated rainfall.
Even though the experiments were conducted under unsaturated conditions, with free drainage at the bottom of the columns, the degree of saturation for the replicates of soil columns of all four soil series is high, always above 70% (Figure 2). The effect of such high saturations on C. parvum oocysts was not determined, but given the size of these oocysts, it is more than likely that their transport in the soils would have been even slower, and their retention more intense had the degree of saturation been significantly lower.
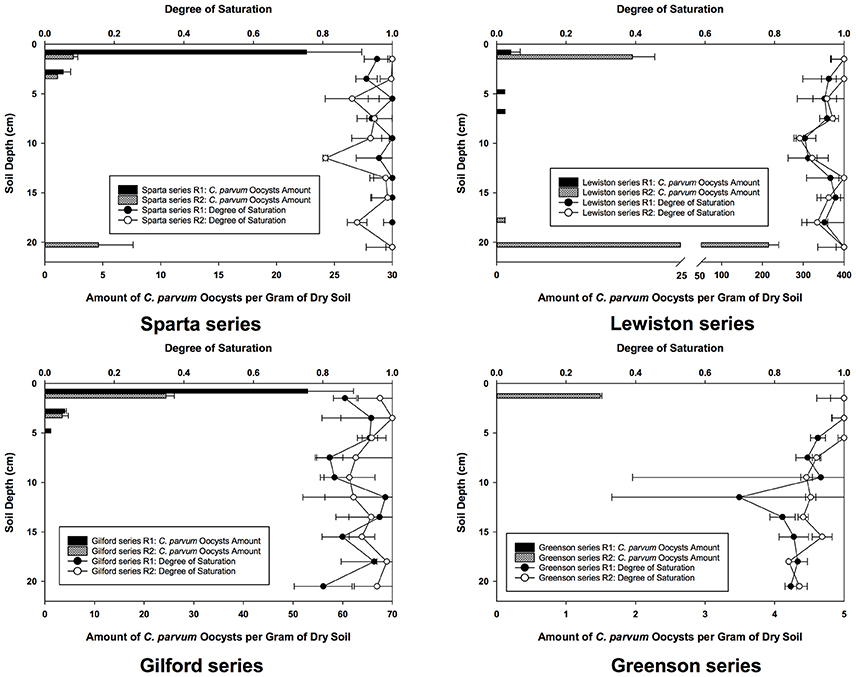
Figure 2. Spatial distribution of C. parvum oocysts recovered from soil columns and degree of saturation in soil profiles in loamy sand and sandy loam soils from the four series of soils examined–Sparta, Lewiston, Gilford and Greenson–following the application of rainfall treatments, and six pore volumes.
Prevalence and Concentration of C. parvum Oocysts in Soil Leachates
The leachates from all four soil series were analyzed to assess the prevalence and concentrations of C. parvum oocysts. Leachate samples were collected over about 6 PV of each soil column. The numbers of C. parvum oocysts in the soil leachates from two replicates for each of the four soils are represented in the breakthrough curves (BTCs) as C/C0, where C is concentration of C. parvum oocysts detected in leachates and C0 is the concentration of C. parvum oocysts detected in the input solution (Figure 1). A visual inspection of the soil columns did not reveal any cracks or holes.
In the Sparta series soil, C. parvum oocysts were not detected in the leachates of either of the replicates (Figure 1). The BTCs of the tracer, however, displayed a fast increase and a tailing decrease. The heights of the tracer BTCs were measured as C/C0 ranging from 0.17 to 0.20. The peaks of the BTC of the tracer occurred at 0.70 and 0.80 PV.
In the Lewiston series soil, C. parvum oocysts were detected in the soil leachates of both replicates (Figure 1). The peaks of the C. parvum oocysts BTC were observed at 2.35 PV for a C/C0 value of 0.006 and at 1.86 PV for a C/C0 value of 0.005, for the first and second replicates (R1 and R2), respectively. The peak of the tracer BTCs occurred at about 1.00 PV with C/C0 values of approximately 0.16 to 0.18. These results indicate the transport of C. parvum oocysts within the soil matrix and their prevalence in the soil leachates in considerable concentration.
In the Gilford series soil, C. parvum oocysts were present in only a few samples (Figure 1), with C/C0 values of 0.004 at 0.53 PV and 0.008 at 2.03 PV for R1, and 0.011 at 5.2 PV for R2. The peak of the tracer BTCs reached a C/C0 value of approximately 0.13 at 0.56 PV and 0.69 PV, respectively for replicates 1 and 2.
In the Greenson series soil, C. parvum oocysts were present in the leachates of both soil columns (Figure 1). Their peaks reached values of C/C0 of 0.004 at 0.56 PV and 0.007 at 2.68 PV, respectively, for R1 and R2. Two peaks were observed in each tracer BTC; an early and a late peak occurred in each of the replicates. The BTCs of the tracer displayed early peaks at approximately 0.12 for R1, and 0.20 PV for R2. Late and smaller peaks in the BTCs of the tracer were observed between 1.5 and 2 PV in R2, and at approximately 3.5 PV in R1. The occurrence of two peaks demonstrated the presence of fast and slow infiltration processes in this soil series for the tracer; however this dual-flow phenomenon did not induce two different transport behaviors for C. parvum oocysts.
Spatial Distribution and Concentration of C. parvum Oocysts in Soils
Following the flow and transport experiments in soil, the soil columns were sliced in layers and soil samples were analyzed to establish the spatial distribution and concentration of C. parvum oocysts in the soil profile. The numbers of C. parvum oocysts in the soil matrices from the two replicates for each of the four soils are presented as number of C. parvum oocysts per gram of dry soils (Figure 2).
In the Sparta series soil (Figure 2), C. parvum oocysts were detected in the top layers of the soil profiles of each of the replicates and in the deepest layer of the second replicate (R2). The highest concentration of C. parvum oocysts was detected in the top layer of the first replicate (R1). Although C. parvum oocysts were not detected in the soil leachates, their transport through the soil matrices of Sparta series was established, as they were detected and enumerated in the topmost soil layers of R1 and R2, and the deepest soil layer of R2.
In the Lewiston series soil (Figure 2), C. parvum oocysts were detected in the soil matrices of both of the R1 and R2 soil column replicates. The highest number of C. parvum oocysts in the soil column of replicate 1 was detected in the top layer, with about 2 C. parvum oocysts per gram of dry soil. In contrast, the highest number of C. parvum oocysts in the soil column of R2 was found in the deepest layer, with about 215 oocysts per gram of dry soil. Our results demonstrated the transport of C. parvum oocysts throughout the soil matrix of the Lewiston series soil.
In the Gilford series soil (Figure 2), C. parvum oocysts were found only in the top layers of the soil column in both replicates, whereas in the Greenson series soil (Figure 2), no C. parvum oocysts were detected in the soil column from R1, while only a few oocysts detected in the top layer the soil column from R2.
Cumulative Recovery of C. parvum Oocysts in Leachates and Soils
Throughout the transport experiments, higher numbers of C. parvum oocysts were detected in the soil matrices than in the soil leachates of all four soil series. Although the relative proportions of C. parvum oocysts in the soil matrices and soil leachates from the different soil series varied, the trends of total numbers of C. parvum oocysts recovered from each of the replicates of each soil series differed (Table 3). The highest recovery of the number of C. parvum oocysts in soil matrices and leachates was 2.718% in the soil column of replicate 2 of the Lewiston series soil. The majority of C. parvum oocysts recovered were from the soil matrices in all of our experiments, except in the first replicate of the Greenson series soil, where C. parvum oocysts were not detected in the soil matrices. The distribution of percentage recovery between soil matrices and leachates in this case (Greenson first replicate) was attributed to soil leachate only. Similarly, no C. parvum oocysts were detected in the leachates of either replicate of the Sparta series soil, which resulted in C. parvum oocysts being recovered only from the soil matrices.
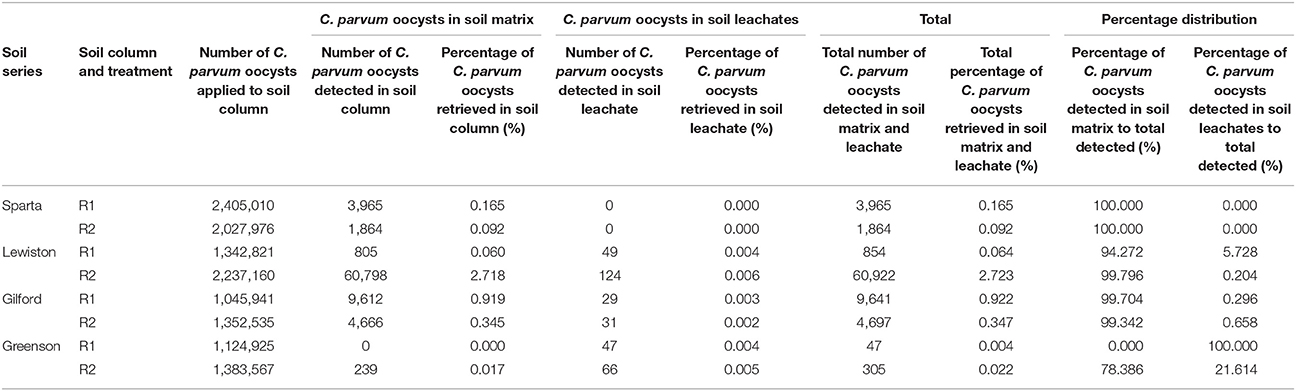
Table 3. Recovery of C. parvum oocysts in soil matrix and leachate of loamy sand and sandy loam soils from four different series–Sparta, Lewiston, Gilford, and Greenson–following the application of rainfall treatments, at an approximate six-pore volume.
The recovery of C. parvum oocysts in the leachates was very low, ranging from 0 to 0.006% (Table 3). C. parvum oocysts were detected in the leachates of three out of four soil types. Overall, the recovery of C. parvum oocysts in leachates was impacted by the soil type. Significance differences (p < 0.05) were detected among the percentages of C. parvum oocysts retrieved in the soil leachates of the Sparta, Lewiston, Gilford, and Greenson series (Figure 3). In sandy loam soils, the highest C. parvum oocyst recovery was observed in leachates from the Lewiston series soil (0.004–0.006%). In loamy sand soils, the highest recovery of C. parvum oocysts was detected in the leachates from the Gilford series soil (0.002–0.004%).
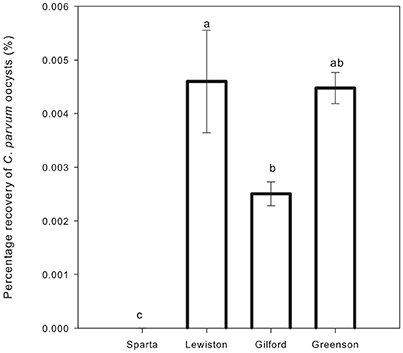
Figure 3. Percentage recovery of C. parvum oocysts in soil leachates from loamy sand and sandy loam soils from the four series of soils examined–Sparta, Lewiston, Gilford and Greenson–following the application of rainfall treatments. Significance levels are p < 0.05.
Cryptosporidium parvum oocysts were detected in the soil of all replicates of all four soils, except for the soil from the first replicate of the Greenson series soil. Overall, the C. parvum oocyst recoveries in the four different soils were not significantly different. No significant differences (p < 0.05) were observed among the percentages of C. parvum oocysts retrieved in the soil matrices for the Sparta, Lewiston, Gilford, and Greenson series (Figure 4). C. parvum oocysts recovery in soils ranged from 0 to 2.718%. The highest C. parvum oocyst recovery from sandy loam soils was reported for the Lewiston series soil (0.060–2.718%). For the loamy sand soils, the Sparta series and Gilford series, soils showed C. parvum oocyst recoveries ranging from 0.092 to 0.165% and 0.345 to 0.919%, respectively.
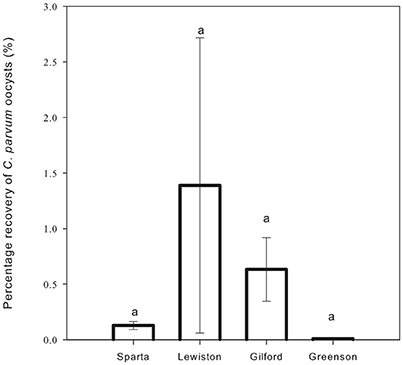
Figure 4. Percentage recovery of C. parvum oocysts in soil columns from loamy sand and sandy loam soils the four series of soils examined–Sparta, Lewiston, Gilford and Greenson–following application of rainfall treatments. Significance levels are p < 0.05.
Discussion
The fate and transport of C. parvum oocysts in soil were investigated in a series of laboratory soil column experiments subject to simulated artificial rainfall events in order to induce unsaturated flow conditions. The transport of C. parvum oocysts with infiltrating water through the vadose zone was demonstrated in all soils. However, differences in the degree of the transport of C. parvum oocysts were observed. While C. parvum oocysts were detected in the leachates of the Lewiston, Gilford, and Greenson series, they were not detected in the leachates of the Sparta series. More C. parvum oocyst transport and breakthrough occurred in sandy loam soils (Greenson and Lewiston series) than in loamy sand soils (Sparta and Gilford series).
Cryptosporidium parvum oocysts were detected in the soil matrices of all four soil types, except in the first replicate of the Greenson series soil, and were usually retained in the topmost layer in considerably larger quantity than that seen in the rest of the soil profile. The retention of microorganisms in upper soil layers has also been reported in a previous study (Gerba et al., 1975), and in particular with C. parvum oocysts (Mawdsley et al., 1996a). However, in several instances, additional C. parvum oocysts were detected in the deepest soil layer, as considerable transport had occurred. These findings indicate that the physicochemical properties of the soils have primary and direct control over the behavior of C. parvum oocysts.
The mobility behavior of C. parvum oocysts in soils was monitored in natural repacked and homogeneous soil columns that allowed investigation of the effects of soil physicochemical properties. Repacked soil columns provided a homogeneous environment to study the impacts of soil parameters and soil water chemistry on the mobility of C. parvum oocysts. A similar approach was used previously to study the fate and transport of other microorganisms (Trevors et al., 1990; Gannon et al., 1991a,b; van Elsas et al., 1991). Soil water chemistry, water content, and flow of water through soil have been reported as major factors affecting the extent of mobility of microorganisms in soils (Kuikman et al., 1990; Trevors et al., 1990; van Elsas et al., 1991; Balthazard-Accou et al., 2014). Mawdsley et al. (1996a) established the possible transport of C. parvum oocysts through three different and intact soil cores obtained from clay loam, silty loam, and loamy sand soils. Transport studies simulating the aquifer flow also examined the deposition processes and associated breakthrough of C. parvum oocysts in saturated porous media (Harter et al., 2000). Transport experiments in unsaturated flow porous media (Darnault et al., 2003, 2004) demonstrated that preferential flow in the form of fingered flow and macroporous flow in soils allowed the fast transport of C. parvum oocysts with breakthrough concentrations above infection doses. Therefore, the results presented here are consistent with previous research that also demonstrated the significant transport of C. parvum oocysts in soil.
The deposition processes of C. parvum oocysts have been investigated in model granular media—sand and glass beads—(Dai and Hozalski, 2002; Kim et al., 2010), and in natural media (Harter et al., 2000; Hijnen et al., 2005). In their analysis of the effect of mineral charges in the deposition of C. parvum oocysts in soils Harter et al. (2000) observed that the transport and retention of C. parvum oocysts in saturated porous media followed the first order attachment theory. Also experiments undertaken to investigate the fate and transport of C. parvum oocysts during infiltration processes demonstrated that they are subject to advection-dispersion and reversible adsorption processes (Brush et al., 1999). Additionally, Darnault et al. (2004) emphasized the effects of gas-water interfaces on the capture and retention of C. parvum oocysts during unsaturated flow.
Our discussion attempts to provide an understanding of the different environmental processes that govern the mobility of C. parvum oocysts in soils, as illustrated with the quantitative results from our different experimental systems. Our study clearly demonstrates that the transport and retention of C. parvum oocysts in natural soils varies by soil types. C. parvum oocysts have a negative surface charge under typical environmental conditions (Thomas et al., 2001; Hsu and Huang, 2002) due to carboxylate, carboxylic and phosphate groups on the surface of oocysts (Karaman et al., 1999). The glycocalyx that is located at the surface of the outer wall of C. parvum oocysts impacts the interactions between C. parvum oocysts and surfaces by inducing a steric repulsion (Nanduri et al., 1999; Jenkins et al., 2010; Liu et al., 2010; Dumėtre et al., 2012), which may hinder adsorption. In our experimental system, the presence of clay particles and organic matter, as well as dissolved ions in the soil water solution and soil matrices, are likely to have also impacted the surface properties of the C. parvum oocysts.
Effects of Soil Types on Transport and Retention of C. parvum Oocysts in Natural Soils
Although numerous studies have been undertaken to investigate the effects of soil type on the mobility and transport of microorganisms (i.e., bacteria and viruses) in soil, research on the fate and transport of protozoan cysts is scarce (Gerba et al., 1975; Bitton and Harvey, 1992). In soil, the mobility of bacteria is controlled by adsorption, straining, and sedimentation processes (Reddy et al., 1981; Gannon et al., 1991a,b; Tan et al., 1992), whereas in the case of viruses, mobility is governed mainly by adsorption as a results of their small size (Tan et al., 1992). For protozoa and their cysts, such as C. parvum oocysts, which have a diameter of 4–6 μm, adsorption, straining, and sedimentation are expected to impact their mobility in a manner similar to that of bacteria (Mawdsley et al., 1996b).
Soil type is a critical parameter that affects the mobility of microorganisms (Bitton et al., 1974; Bashan and Levanony, 1988; Tan et al., 1991; Huysman and Verstraete, 1993; Mawdsley et al., 1996a). The presence of clay and organic matter, in particular, affects the adsorption processes of bacteria and viruses as a result of their large surface area and negative charge (Reddy et al., 1981).
This study has demonstrated that leaching of C. parvum downward through the soil profile and the vadose zone does occur, although the extent of their transport is affected by the soil type. The soil type was a determinant in the transport of C. parvum oocysts through soil and into leachate, as larger quantities with concentrations of C. parvum oocysts found in sandy loam soils (i.e., Lewiston and Greenson series) as opposed to loamy sand soils (i.e., Sparta and Gilford series).
Interactions at Interfaces between C. parvum Oocysts and Soil Components: Interplay of Clay Particles, Organic Matter, and Calcium Ions
Potential interaction of the C. parvum oocysts with surfaces and interfaces they encounter, particularly the surfaces of soil particles, during the soil infiltration process is possible. Among the soil components, clay particles have the largest surface areas for potential interactions. Consequently, soil with high clay content provides the highest probability and potential for C. parvum oocysts to interact with clay surfaces, which may result in greater adsorption of C. parvum oocysts to soil clay particles (Balthazard-Accou et al., 2014). In soil, most of the organic matter (OM) is generally combined with minerals that consist of clay and silt particles (Cheshire et al., 2000). Therefore, since most of the OM in soil is bound to the clay and silt surfaces, we can postulate that clay-OM and silt-OM complexes may also facilitate the interplay between C. parvum oocysts and OM, as C. parvum oocysts collide with clay surfaces that are coated with OM. Soil that contains high clay and silt contents may enhance and promote a higher degree of interaction between OM present at the surface of minerals and C. parvum oocysts that encounter these surfaces during their transport through soil. Interactions of OM and surfaces of C. parvum oocysts is a complex phenomenon, and previous studies have suggested the influence of OM on the fate and transport of C. parvum oocysts in porous media. In particular, an increase in OM concentration has been found to induce an increase in the breakthrough of C. parvum oocysts in sand and a decrease in the collision efficiency (Abudalo et al., 2010). The adsorption of OM on the surface of C. parvum oocysts has been postulated to be mediated by the presence of the polyvalent cations Ca2+ (Dai and Hozalski, 2003).
As observed in transport experiments in the Greenson series soil, the highest combination of clay, organic matter, and Ca2+ resulted in the highest transport through the soil matrix and the highest release of C. parvum oocysts in leachates (Tables 1, 3 and Figure 1). These results demonstrate the role that clay particles, organic matter, and Ca2+ play in the transport of C. parvum oocysts. These results and proposed description of an enhanced transport process of C. parvum oocysts provided by adsorption of organic matter at the oocysts surface mediated by Ca2+ and promoted by the presence of clay are in agreement with similar proposed enhanced-transport phenomena in other contexts. In our study, sandy loam soils have a higher content of clay (12.5 and 19.5% for Lewiston and Greenson series, respectively) than loamy sand soils (9.5 and 7.5% for Sparta and Gilford series, respectively). Consequently, a soil that contains higher concentrations of clay and silt particles offers larger surface areas for C. parvum oocysts to potentially encounter and a higher probability of C. parvum oocysts to interact with these surfaces as they are transported through the soil. The largest CEC values were observed for sandy loam soils Lewiston (115 mmolc·kg−1) and Greenson (175 mmolc·kg−1) compared to loamy sand soils Sparta (86 mmolc·kg−1) and Gilford (840 mmolc·kg−1). In our experiments, a larger number of C. parvum oocysts were observed in leachates from soils with high clay content (0.004–0.006% oocysts recovered in water in Lewiston and Greenson series, with clay content of 12.5 and 19.5%, respectively) than in leachates from soils with low clay content (0–0.003% oocysts recovered in water in Sparta and Gilford series, with clay content of 9.5 and 7.5%, respectively). Indeed, direct and indirect effects of clay particles, as well as interplay effects of other soil parameters have been identified to significantly govern the fate and transport of C. parvum oocysts. Since clay and silt surfaces are the sites where most of the OM resides and the probability of interaction of C. parvum oocysts with OM increases as the percentage of clay and silt particles increases. As previously reported, when C. parvum oocysts are placed in the presence of elements from natural OM, OM may adsorb onto their surface and increase their negative charges and hydrophobicity which enhance the electrostatic repulsion between the OM-coated C. parvum oocysts and the negatively charged soil particle surfaces that they encountered, and therefore increase their mobility and impact their transport (Mawdsley et al., 1996a; Ongerth and Pecoraro, 1996; Considine et al., 2002; Dai and Hozalski, 2002, 2003; Mohanram et al., 2010, 2012). These phenomena may result in enhanced transport, as observed in sandy loam soils. Adsorption of organic matter on the surface of C. parvum oocysts induces an increase in zeta potential that counteracts the negative charge.
In addition, the presence of Ca2+ has been postulated as possible mediator in the coating of C. parvum oocysts by OM (Dai and Hozalski, 2003). Soils that had the highest concentration of Ca2+, Greenson series (42.09 mg·kg−1 and Lewiston series (37.10 mg·kg−1), and the highest clay content (12.5 and 19.5% for Lewiston and Greenson series, respectively) had the greatest breakthrough of C. parvum oocysts, as corroborated by the C. parvum oocysts BTC results and the analyses of the physicochemical properties of the soils. Our findings support and demonstrate the critical role of Ca2+ in mediating the transfer of OM from the surface of minerals to the surface of C. parvum oocysts. In particular, the Lewiston and Greenson soil series that had the highest soil Ca2+ concentration, the highest clay content, and the highest silt content resulted in the highest breakthrough of C. parvum oocysts among all soils, although the OM concentrations of all soils were similar (3.4, 0.8, 4.20, and 3.80% OM for Sparta, Lewiston, Gilford, and Greenson, respectively) (Tables 1, 3 and Figure 1). We can then postulate that the interplay of clay (and silt to some extent), OM, and Ca2+ facilitated and mediated the transfer of OM to C. parvum oocysts surface, which resulted in the breakthrough of C. parvum of oocysts through the soil. Based on our findings, this interplay between these biogeochemical compounds and processes—clay and silt content, presence of OM, Ca2+ content, and Ca2+ mediated transfer of OM from mineral surfaces to C. parvum oocysts—is critical in controlling the mobility and transport of C. parvum oocysts in soils. Our observations are also in agreement with previous research findings by Ferguson et al. (2003) and Peng et al. (2008) that also highlighted the role of soil texture and soil physico-chemical properties on the fate and transport of C. parvum oocysts in soil.
Recovery of C. parvum Oocysts in Natural Soil and Water Samples
Compared with the total number of C. parvum oocysts applied to the soil columns surface, a highest oocyst recovery rate reported for soil samples (2.718%) was considerably greater than the highest oocyst recovery rate reported for leachate samples (0.006%). These recovery results are in agreement with those from Petersen et al. (2012), with the highest recovery rate of oocysts at 9.8% for soil samples and 0.03% for leachate samples. The median of the efficiency of C. parvum oocysts recovery from the different soil matrices in our experiment was calculated as 0.128%. This is similar to the previously reported efficiency of C. parvum oocyst recovery from soils in other studies (Mawdsley et al., 1996a; Petersen et al., 2012). Mawdsley et al. (1996a) investigated the effect of incubation time on the number of C. parvum oocysts extracted from soil initially inoculated with C. parvum oocysts. Mawdsley et al. (1996a) found that the efficiency of C. parvum occyst recovery from soil decreases rapidly as time passes, from 61.6% for an incubation time of 15 min to 0.3% for an incubation time of 7 days. A substantially higher number of oocysts were recovered from the sandy loam soils (Lewiston and Greenson series) than the loamy sand soils (Sparta and Gilford series), as shown in Table 3. However, the distributions of C. parvum oocysts within the soil profiles were similar. Most of the C. parvum oocysts were encountered in the soil top layers (0–5 cm) and in the bottom layers (17.5–20 cm). Our C. parvum oocyst recoveries are in agreement with previously published results (Mawdsley et al., 1996a; Petersen et al., 2012). Also, our time of inoculation of C. parvum oocysts in soils and the volume of soil samples treated for C. parvum extraction are higher than in previously reported studies (Mawdsley et al., 1996a; Petersen et al., 2012).
Conclusion
Given the intrinsic difficulties involved in the completion of experiments relative to the transport of C. parvum oocysts in soil columns, and especially given the onerous nature of analytical measurements that are a requisite of these experiments, the research described here had from the outset a modest objective. Succinctly, this objective was the creation of a screening test to determine the expected range of transport patterns in the field under conditions that are the least favorable to oocyst transport (since the soils exhibit no preferential pathways) but are nevertheless likely to be found routinely in the field.
Experimental results we obtained demonstrate that C. parvum oocysts can be transported in the soil matrix. Although the number of C. parvum occysts that broke through the soil matrix accounted for only a small percentage of the initial inputs at the soil surface, they still pose a significant threat to human health, especially in groundwater systems where the water table of the aquifer is near the soil surface, as a small number of C. parvum oocysts can lead to the waterborne disease cryptosporidiosis. In the context of climate change and extreme hydrological events, the risk of transport of C. parvum oocysts and contamination of aquifers augment as matrix flow increases under wetter conditions. Under these wetter climate conditions, extreme precipitation events may result in a large amount of water infiltrating the soil, and higher than normal frequency of extreme precipitation events may result in more continuously wet soil conditions. Such wet soil conditions would lead to higher soil moisture and soil hydraulic conductivity that would favor the mobility and transport of C. parvum oocysts, particularly through the soil matrix.
The soils used in the experiments vary simultaneously according to a number of parameters, whose influence cannot be teased apart via simple screening tests. Nevertheless, experimental results suggest the interplay of clay particles, organic matter, and calcium ions on the mechanisms controlling the transport and retention of C. parvum oocysts in natural soils. In this context, our work makes clear the urgent need to conduct research so as to simultaneously analyze the interaction of various processes that can affect both the mobility and transport of C. parvum oocysts in the subsurface environment in natural complex soil systems. Although the interactions of these processes increase the difficulty of directly identifying and ranking the contribution of the hydrologic and biogeochemical processes that influence the transport of C. parvum oocysts, hypotheses can be developed from results of realistic experimental conditions identical, or similar, to conditions in either the field or in natural systems. Findings from such studies, resulting from experiments conducted in soils and enabling the interaction of various processes, may be coupled with findings from experiments designed to investigate the influence of these processes separately and to establish a fundamental understanding of how these individual processes influence C. parvum oocysts behavior in the environment. Therefore, a more coherent picture of the interaction and influence of these processes concerning the transport of C. parvum oocysts may emerge from individual process-based experiments that either do or do not support hypotheses formulated from experiments involving various interacting processes.
An extension of the current study is necessary to characterize the surface properties of C. parvum oocysts upon which depend the interactions of C. pavum oocyst with surfaces and therefore their mobility in the natural environment, in particular soil environment systems. An extension of this work must also include the study of the effects of climate change and extreme hydrologic events on the fate and transport of C. parvum occysts in soil. Among the many possible climate scenarios, the increase in soil wettability, an increase in the frequency of extreme precipitation events, and changes in biogeochemical cycles are likely to induce changes in the physical and biochemical properties of soil, as well as changes in the soil infiltration process, soil moisture, and hydraulic properties. Such climate change therefore will have direct and indirect effects on the risk of contamination of groundwater resources by C. parvum oocysts.
Author Contributions
The authors CD, AJ, and PB conceived and designed the experiment; CD, ZP, CY, and PB performed the experiment; CD, BL, ZP, AJ, and PB analyzed the data; and CD and PB wrote the paper.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the U.S. Department of Agriculture, National Institute of Food and Agriculture (NIFA), National Research Initiative (NRI) Competitive Grant Program under Grant numbers 2008-35102-19159 and 2008-35102-20653, and Clemson University. We also wish to acknowledge the technical assistance of David Powelson, Yirui Chen, and Linlin Mu in the completion of this study.
References
Abudalo, R. A., Ryan, J. N., Harvey, R. W., Metge, D. W. and Landkamer, L. (2010). Influence of organic matter on the transport of Cryptosporidium parvum oocysts in a ferric oxyhydroxide-coated quartz sand saturated porous medium. Water Res. 44, 1104–1113. doi: 10.1016/j.watres.2009.09.039
Balthazard-Accou, K., Fifi, U., Agnamey, P., Casimir, J. A., Brasseur, P., and Emmanuel, E. (2014). Influence of ionic strength and soil characteristics on the behavior of Cryptosporidium oocysts in saturated porous media. Chemosphere 103, 114–120. doi: 10.1016/j.chemosphere.2013.11.045
Bashan, Y., and Levanony, H. (1988). Adsorption of the rhizosphere Bacterium Azospirillum Brasilense Cd to soil, sand and peat particles. J. Gen. Microbiol. 134, 1811–1820. doi: 10.1099/00221287-134-7-1811
Bitton, G., and Harvey, R. W. (1992). “Transport of pathogens through soils and aquifers,” in Environmental Microbiology, ed R. Mitchell (New York, NY: Wiley-Liss), 103–124.
Bitton, G., Lahav, N., and Henis, Y. (1974). Movement and retention of Klebsiella Aerogenes in soil columns. Plant Soil 40, 373–380. doi: 10.1007/BF00011519
Boyer, D. G., and Kuczynska, E. (2003). Storm and seasonal distributions of fecal coliforms and Cryptosporidium in a spring. J. Am. Water Resour. Assoc. 39, 1449–1456. doi: 10.1111/j.1752-1688.2003.tb04430.x
Boyer, D. G., Kuczynska, E., and Fayer, R. (2009). Transport, fate, and infectivity of Cryptosporidium parvum oocysts released from manure and leached through macroporous soil. Environ. Geol. 58, 1011–1019. doi: 10.1007/s00254-008-1580-x
Bradford, S. A., and Bettahar, M. (2005). Straining, attachment, and detachment of Cryptosporidium oocysts in saturated porous media. J. Environ. Qual. 34, 469–478. doi: 10.2134/jeq2005.0469
Brush, C. F., Ghiorse, W. C., Anguish, L. J., Parlange, J. Y., and Grimes, H. G. (1999). Transport of Cryptosporidium parvum oocysts through saturated columns. J. Environ. Qual. 28, 809–815. doi: 10.2134/jeq1999.00472425002800030011x
Byrd, T. L., and Walz, J. Y. (2005). Interaction force profiles between Cryptosporidium parvum oocysts and silica surfaces. Environ. Sci. Technol. 39, 9574–9582. doi: 10.1021/es051231e
Byrd, T. L., and Walz, J. Y. (2007). Investigation of the interaction force between Cryptosporidium parvum oocysts and solid surfaces. Langmuir 23, 7475–7483. doi: 10.1021/la0701576
Cheshire, M. V., Dumat, C., Fraser, A. R., Hillier, S., and Staunton, S. (2000). The interaction between soil organic matter and soil clay minerals by selective removal and controlled addition of organic matter. Eur. J. Soil Sci. 51, 497–509. doi: 10.1111/j.1365-2389.2000.00325.x
Considine, R. F., Dixon, D. R., and Drummond, C. J. (2002). Oocysts of Cryptosporidium parvum and model sand surfaces in aqueous solutions: an atomic force microscope (AFM) study. Water Res. 36, 3421–3428. doi: 10.1016/S0043-1354(02)00082-9
Cortis, A., Harter, T., Hou, L. L., Atwill, E. R., Packman, A. I., and Green, P. G. (2006). Transport of Cryptosporidium parvum in porous media: long-term elution experiments and continuous time random walk filtration modeling. Water Resour. Res. 42:W12S13. doi: 10.1029/2006WR004897
Dai, X., and Hozalski, R. M. (2003). Evaluation of microspheres as surrogates for Cryptosporidium parvum oocysts in filtration experiments. Environ. Sci. Technol. 37, 1037–1042. doi: 10.1021/es025521w
Dai, X. J., and Hozalski, R. M. (2002). Effect of NOM and biofilm on the removal of Cryptosporidium parvum oocysts in rapid filters. Water Res. 36, 3523–3532. doi: 10.1016/S0043-1354(02)00045-3
Darnault, C. J. G., Garnier, P., Kim, Y. J., Oveson, K. L., Steenhuis, T. S., Parlange, J. Y., et al. (2003). Preferential transport of Cryptosporidium parvum oocysts in variably saturated subsurface environments. Water Environ. Res. 75, 113–120. doi: 10.2175/106143003X140890
Darnault, C. J. G., Steenhuis, T. S., Garnier, P., Kim, Y. J., Jenkins, M. B., Ghiorse, W. C., et al. (2004). Preferential flow and transport of Cryptosporidium parvum oocysts through the vadose zone: experiments and modeling. Vadose Zone J. 3, 262–270. doi: 10.2113/3.2.736
Dumėtre, A., Aubert, D., Puech, P. H., Hohweyer, J., Azas, N., and Villena, I. (2012). Interaction forces drive the environmental transmission of pathogenic protozoa. Appl. Environ. Microbiol. 78, 905–912. doi: 10.1128/AEM.06488-11
Dupont, H. L., Chappell, C. L., Sterling, C. R., Okhuysen, P. C., Rose, J. B., and Jakubowski, W. (1995). The infectivity of Cryptosporidium parvum in healthy volunteers. N. Engl. J. Med. 332, 855–859. doi: 10.1056/NEJM199503303321304
Ferguson, C., Husman, A. M. R., Altavilla, N., Deere, D., and Ashbolt, N. (2003). Fate and transport of surface water pathogens in watersheds. Crit. Rev. Environ. Sci. Technol. 33, 299–361. doi: 10.1080/10643380390814497
Galbraith, N. S., Barrett, N. J., and Stanwellsmith, R. (1987). Water and disease after Croydon-A review of water-borne and water-associated disease in the Uk 1937-86. J. Inst. Water Environ. Manag. 1, 7–21. doi: 10.1111/j.1747-6593.1987.tb01184.x
Gannon, J. T., Manilal, V. B., and Alexander, M. (1991a). Relationship between cell surface properties and transport of bacteria through Soil. Appl. Environ. Microbiol. 57, 190–193.
Gannon, J. T., Mingelgrin, U., Alexander, M., and Wagenet, R. J. (1991b). Bacterial transport through homogeneous soil. Soil Biol. Biochem. 23, 1155–1160. doi: 10.1016/0038-0717(91)90028-I
Gee, G. W., and Bauder, J. W. (1986). “Particle-size analysis,” in Methods of Soil Analysis, Part 1. Physical and Mineralogical Methods, 2nd Edn., ed A. Klute (Madision, WI.: American Society of Agronomy and Soil Science Society of America), 383–411.
Gerba, C. P., Wallis, C., and Melnick, J. L. (1975). Fate of wastewater bacteria and viruses in soil. J. Irrig. Drain. Divis. ASCE 101, 157–174.
Harter, T., Wagner, S., and Atwill, E. R. (2000). Colloid transport and filtration of Cryptosporidium parvum in sandy soils and aquifer sediments. Environ. Sci. Technol. 34, 62–70. doi: 10.1021/es990132w
Hayes, E. B., Matte, T. D., Obrien, T. R., Mckinley, T. W., Logsdon, G. S., Rose, J. B., et al. (1989). Large community outbreak of Cryptosporidiosis due to contamination of a filtered public water supply. N. Engl. J. Med. 320, 1372–1376. doi: 10.1056/NEJM198905253202103
Hijnen, W. A. M., Brouwer-Hanzens, A. J., Charles, K. J., and Medema, G. J. (2005). Transport of MS2 phage, Escherichia coli, Clostridium perfringens, Cryptosporidium parvum and Giardia intestinalis in a gravel and a sandy soil. Environ. Sci. Technol. 39, 7860–7868. doi: 10.1021/es050427b
Hsu, B. M., and Huang, C. P. (2002). Influence of ionic strength and pH on hydrophobicity and zeta potential of Giardia and Cryptosporidium. Colloids Surf. A Physicochem. Eng. Aspects 201, 201–206. doi: 10.1016/s0927-7757(01)01009-3
Hsu, B. M., Huang, C. P., and Pan, J. R. (2001). Filtration behaviors of Giardia and Cryptosporidium-Ionic strength and pH effects. Water Res. 35, 3777–3782. doi: 10.1016/S0043-1354(01)00117-8
Huysman, F., and Verstraete, W. (1993). Effect of cell surface characteristics on the adhesion of bacteria to soil particles. Biol. Fertil. Soils 16, 21–26. doi: 10.1007/BF00336510
Janjaroen, D., Liu, Y. Y., Kuhlenschmidt, M. S., Kuhlenschmidt, T. B., and Nguyen, T. H. (2010). Role of divalent cations on deposition of Cryptosporidium parvum oocysts on natural organic matter surfaces. Environ. Sci. Technol. 44, 4519–4524. doi: 10.1021/es9038566
Jenkins, M. B., Eaglesham, B. S., Anthony, L. C., Kachlany, S. C., Bowman, D. D., and Ghiorse, W. C. (2010). Significance of wall structure, macromolecular composition, and surface polymers to the survival and transport of Cryptosporidium parvum oocysts. Appl. Environ. Microbiol. 76, 1926–1934. doi: 10.1128/AEM.02295-09
Jothikumar, N., da Silva, A. J., Moura, I., Qvarnstrom, Y., and Hill, V. R. (2008). Detection and differentiation of Cryptosporidium hominis and Cryptosporidium parvum by dual TaqMan assays. J. Med. Microbiol. 57, 1099–1105. doi: 10.1099/jmm.0.2008/001461-0
Karaman, M. E., Pashley, R. M., Bustamante, H., and Shanker, S. R. (1999). Microelectrophoresis of Cryptosporidium parvum oocysts in aqueous solutions of inorganic and surfactant cations. Colloids Surf. A Physicochem. Eng. Aspects 146, 217–225. doi: 10.1016/s0927-7757(98)00796-1
Kim, H. N., Walker, S. L., and Bradford, S. A. (2010). Coupled factors influencing the transport and retention of Cryptosporidium parvum oocysts in saturated porous media. Water Res. 44, 1213–1223. doi: 10.1016/j.watres.2009.09.041
Koken, E., Darnault, C. J. G., Jacobson, A., Powelson, D., and Hendrickson, W. (2013). Quantification of Cryptosporidium parvum in natural soil matrices and soil solutions using qPCR. J. Microbiol. Methods 92, 135–144. doi: 10.1016/j.mimet.2012.11.015
Korich, D. G., Mead, J. R., Madore, M. S., Sinclair, N. A., and Sterling, C. R. (1990). Effects of ozone, chlorine dioxide, chlorine, and monochloramine on Cryptosporidium parvum oocyst viability. Appl. Environ. Microbiol. 56, 1423–1428.
Kuczynska, E., Boyer, D. G., and Shelton, D. R. (2003). Comparison of immunofluorescence assay and immunomagnetic electrochemiluminescence in detection of Cryptosporidium parvum oocysts in karst water samples. J. Microbiol. Methods 53, 17–26. doi: 10.1016/S0167-7012(02)00211-7
Kuikman, P. J., Vanelsas, J. D., Jansen, A. G., Burgers, S. L. G. E., and Vanveen, J. A. (1990). Population dynamics and activity of bacteria and protozoa in relation to their spatial distribution in soil. Soil Biol. Biochem. 22, 1063–1073. doi: 10.1016/0038-0717(90)90031-T
LeChevallier, M. W., Norton, W. D., and Lee, R. G. (1991). Occurrence of Giardia and Cryptosporidium Spp in surface water supplies. Appl. Environ. Microbiol. 57, 2610–2616.
Liu, Y., Janjaroen, D., Kuhlenschmidt, M. S., Kuhlenschmidt, T. B., and Nguyen, T. H. (2009). Deposition of Cryptosporidium parvum oocysts on natural organic matter surfaces: microscopic evidence for secondary minimum deposition in a radial stagnation point flow cell. Langmuir 25, 1594–1605. doi: 10.1021/la803202h
Liu, Y., Kuhlenschmidt, M. S., Kuhlenschmid, T. B., and Nguyen, T. H. (2010). Composition and conformation of Cryptosporidium parvum oocyst wall surface macromolecules and their effect on adhesion kinetics of oocysts on quartz surface. Biomacromolecules 11, 2109–2115. doi: 10.1021/bm100477j
Logan, A. J., Stevik, T. K., Siegrist, R. L., and Ronn, R. M. (2001). Transport and fate of Cryptosporidium parvum oocysts in intermittent sand filters. Water Res. 35, 4359–4369. doi: 10.1016/S0043-1354(01)00181-6
Mackenzie, W. R., Hoxie, N. J., Proctor, M. E., Gradus, M. S., Blair, K. A., Peterson, D. E., et al. (1994). A Massive outbreak in Milwaukee of Cryptosporidium infection transmitted through the public water supply. N. Engl. J. Med. 331, 161–167. doi: 10.1056/NEJM199407213310304
Mawdsley, J. L., Brooks, A. E., and Merry, R. J. (1996a). Movement of the protozoan pathogen Cryptosporidium parvum through three contrasting soil types. Biol. Fertil. Soils 21, 30–36. doi: 10.1007/BF00335990
Mawdsley, J. L., Brooks, A. E., Merry, R. J., and Pain, B. F. (1996b). Use of a novel soil tilting table apparatus to demonstrate the horizontal and vertical movement of the protozoan pathogen Cryptosporidium parvum in soil. Biol. Fertil. Soils 23, 215–220. doi: 10.1007/BF00336066
McLaughlin, S. J., Kalita, P. K., and Kuhlenschmidt, M. S. (2013). Fate of Cryptosporidium parvum oocysts within soil, water, and Plant environment. J. Environ. Manage. 131, 121–128. doi: 10.1016/j.jenvman.2013.09.017
Metge, D. W., Harvey, R. W., Aiken, G. R., Anders, R., Lincoln, G., and Jasperse, J. (2010). Influence of organic carbon loading, sediment associated metal oxide content and sediment grain size distributions upon Cryptosporidium parvum removal during riverbank filtration operations, Sonoma County, CA. Water Res. 44, 1126–1137. doi: 10.1016/j.watres.2009.11.033
Mohanram, A., Ray, C., Harvey, R. W., Metge, D. W., Ryan, J. N., Chorover, J., et al. (2010). Comparison of transport and attachment behaviors of Cryptosporidium parvum oocysts and oocyst-sized microspheres being advected through three minerologically different granular porous media. Water Res. 44, 5334–5344. doi: 10.1016/j.watres.2010.06.015
Mohanram, A., Ray, C., Metge, D. W., Barber, L. B., Ryan, J. N., and Harvey, R. W. (2012). Effect of dissolved organic carbon on the transport and attachment behaviors of Cryptosporidium parvum oocysts and carboxylate-modified microspheres advected through temperate humic and tropical volcanic agricultural soil. Environ. Sci. Technol. 46, 2088–2094. doi: 10.1021/es2003342
Nanduri, J., Williams, S., Aji, T., and Flanigan, T. P. (1999). Characterization of an immunogenic glycocalyx on the surfaces of Cryptosporidium parvum oocysts and sporozoites. Infect. Immun. 67, 2022–2024.
Ongerth, J. E., and Pecoraro, J. P. (1996). Electrophoretic mobility of Cryptosporidium oocysts and Giardia cysts. J. Environ. Eng. 122, 228–231. doi: 10.1061/(ASCE)0733-9372(1996)122:3(228)
Park, Y., Atwill, E. R., Hou, L., Packman, A. I., and Harter, T. (2012). Deposition of Cryptosporidium parvum oocysts in porous media: a synthesis of attachment efficiencies measured under varying environmental conditions. Environ. Sci. Technol. 46, 9491–9500. doi: 10.1021/es300564w
Peng, X., Murphy, T. M., and Holden, N. M. (2008). Evaluation of the effect of temperature on the die-off rate for Cryptosporidium parvum oocysts in water, soils, and feces. Appl. Environ. Microbiol. 74, 7101–7107. doi: 10.1128/AEM.01442-08
Petersen, H. H., Enemark, H. L., Olsen, A., Amin, M. G., and Dalsgaard, A. (2012). Transport of Cryptosporidium parvum oocysts in soil columns following applications of raw and separated liquid slurries. Appl. Environ. Microbiol. 78, 5994–6000. doi: 10.1128/AEM.07829-11
Ramirez, N. E., Wang, P., Lejeune, J., Shipitalo, M. J., Ward, L. A., Sreevatsan, S., et al. (2009). Effect of tillage and rainfall on transport of manure applied Cryptosporidium parvum oocysts through soil. J. Environ. Qual. 38, 2394–2401. doi: 10.2134/jeq2008.0432
Reddy, K. R., Khaleel, R., and Overcash, M. R. (1981). Behavior and transport of microbial pathogens and indicator organisms in soils treated with organic wastes. J. Environ. Qual. 10, 255–266. doi: 10.2134/jeq1981.00472425001000030001x
Rhoades, J. D. (1996). “Salinity: electrical conductivity and total dissolved solids,” in Methods of Soil Analysis. Part 3. Chemical Methods, ed D. L. Sparks (Madison, WI.: American Society of Agronomy and Soil Science Society of America), 417–435.
Rose, J. B., Gerba, C. P., and Jakubowski, W. (1991). Survey of potable water supplies for Cryptosporidium and Giardia. Environ. Sci. Technol. 25, 1393–1400. doi: 10.1021/es00020a005
Smith, H. V. (1992). Cryptosporidium and water-A review. J. Inst. Water Environ. Manag. 6, 443–451. doi: 10.1111/j.1747-6593.1992.tb00773.x
Smith, H. V., and Rose, J. B. (1998). Waterborne cryptosporidiosis: current status. Parasitol. Today 14, 14–22. doi: 10.1016/S0169-4758(97)01150-2
Tan, Y., Bond, W. J., and Griffin, D. M. (1992). Transport of bacteria during unsteady unsaturated soil-water flow. Soil Sci. Soc. Am. J. 56, 1331–1340. doi: 10.2136/sssaj1992.03615995005600050001x
Tan, Y., Bond, W. J., Rovira, A. D., Brisbane, P. G., and Griffin, D. M. (1991). Movement through soil of a biological control agent, Pseudomonas fluorescens. Soil Biol. Biochem. 23, 821–825. doi: 10.1016/0038-0717(91)90092-X
Thomas, F., Bard, E., Rouillier, M. C., Prelot, B., and Mathieu, L. (2001). Filtration-elution of Cryptosporidium oocysts assisted by electrostatic interactions. Colloids Surf. A Physicochem. Eng. Aspects 195, 135–142. doi: 10.1016/s0927-7757(01)00836-6
Tim, U. S., Mostaghimi, S., and Dillaha, T. A. (1988). Modeling the movement and persistence of bacteria and viruses in porous media. Kodak Professional, St. Joseph, Michigan, AZO Paper No. 88–2627.
Trevors, J. T., Vanelsas, J. D., Vanoverbeek, L. S., and Starodub, M. E. (1990). Transport of a genetically engineered Pseudomonas fluorescens strain through a soil microcosm. Appl. Environ. Microbiol. 56, 401–408.
Keywords: Cryptosporidium, microorganisms, groundwater, soil transport, qPCR
Citation: Darnault CJG, Peng Z, Yu C, Li B, Jacobson AR and Baveye PC (2017) Movement of Cryptosporidium parvum Oocysts through Soils without Preferential Pathways: Exploratory Test. Front. Environ. Sci. 5:39. doi: 10.3389/fenvs.2017.00039
Received: 10 December 2016; Accepted: 13 June 2017;
Published: 28 June 2017.
Edited by:
Maria Luz Cayuela, Consejo Superior de Investigaciones Científicas (CSIC), SpainReviewed by:
Siu Mui Tsai, University of São Paulo, BrazilPeter S. Hooda, Kingston University, United Kingdom
Copyright © 2017 Darnault, Peng, Yu, Li, Jacobson and Baveye. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christophe J. G. Darnault, Y2Rhcm5hdUBjbGVtc29uLmVkdQ==
 Christophe J. G. Darnault
Christophe J. G. Darnault Zhenyang Peng
Zhenyang Peng Chan Yu
Chan Yu Biting Li
Biting Li Astrid R. Jacobson
Astrid R. Jacobson Philippe C. Baveye
Philippe C. Baveye