- 1Department of Geology, Kansas State University, Manhattan, KS, United States
- 2Department of Civil Engineering, Kansas State University, Manhattan, KS, United States
- 3Department of Civil, Construction, and Environmental Engineering, San Diego State University, San Diego, CA, United States
- 4Department of Earth and Environmental Sciences, Tulane University, New Orleans, LA, United States
- 5Department of Agronomy, Kansas State University, Manhattan, KS, United States
- 6KTH-International Groundwater Arsenic Research Group, Department of Sustainable Development, Environmental Science and Engineering, KTH Royal Institute of Technology, Stockholm, Sweden
- 7International Center for Applied Climate Science, The University of Southern Queensland, Toowoomba, QLD, Australia
- 8Department of Geological Sciences, Stanford University, Stanford, CA, United States
HIGHLIGHTS
• Manganese and arsenic concentrations are elevated in Murshidabad groundwater.
• Manganese release appears to be independent of dissolved organic matter quality.
• Mineral precipitation and dissolution reactions impact fate of manganese.
• Arsenic concentrations are related to dissolved organic matter quantity and quality.
The prevalence of manganese (Mn) in Southeast Asian drinking water has recently become a topic of discussion, particularly when concurrent with elevated arsenic (As). Although Mn groundwater geochemistry has been studied, the link between dissolved organic matter (DOM) quality and Mn release is less understood. This work evaluates characteristics of DOM, redox chemistry, and the distribution of Mn within Murshidabad, West Bengal, India. Shallow aquifer samples were analyzed for cations, anions, dissolved organic carbon, and DOM properties using 3-dimensional fluorescence excitation emission matrices followed by parallel factor modeling analyses. Two biogeochemical regimes are apparent, separated geographically by the river Bhagirathi. East of the river, where Eh and nitrate () values are low, humic-like DOM coexists with high dissolved Mn, As, and Fe. West of the river, lower dissolved As and Fe concentrations are coupled with more protein-like DOM and higher and Eh values. Dissolved Mn concentrations are elevated in both regions. Based on the distribution of available electron acceptors, it is hypothesized that groundwater east of the Bhagirathi, which is more reducing and enriched in dissolved Fe and Mn but depleted in , is chemically dominated by Mn(IV)/Fe(III) reduction processes. West of the river where is abundant yet dissolved Fe is absent, and Mn(IV) likely buffer redox conditions such that Eh values are not sufficiently reducing to release Fe into the dissolved phase. The co-occurrence of humic-like DOM with dissolved As, Fe, and Mn in the more reducing aquifers may reflect complex formation between humic DOM and metals, as well as electron shuttling processes involving humic DOM, which may enhance metal(loid) release. Saturation indices of rhodochrosite (MnCO3) suggest that precipitation is thermodynamically favorable in a greater proportion of the more reducing sites, however humic DOM–Mn complexes may be inhibiting MnCO3 precipitation. Where dissolved arsenic concentrations are low, it is postulated that Mn(IV) reduction is oxidizing As(III) to As(V), increasing the potential for re-adsorption of As(V) onto relatively stable, un-reduced or newly precipitated Fe-oxides. Manganese release appears to be independent of DOM quality, as it persists in both humic and protein-like DOM environments.
Introduction
Throughout the Bengal Basin, elevated levels of manganese (Mn) and arsenic (As) have adversely impacted groundwater quality, prompting serious concerns to human health (Bhattacharya et al., 1997; Nickson et al., 1998; BGS and DPHE, 2001; Buschmann et al., 2008; Datta et al., 2009, 2011; Frisbie et al., 2009; Farooq et al., 2011; Sankar et al., 2014; Datta, 2015; Shrivastava et al., 2015; Kshetrimayum and Hegeu, 2016). Further, groundwater is the primary source of drinking water in many of these regions due to surface waters being contaminated by anthropogenic waste (McArthur et al., 2012a).
Although Mn is an essential trace nutrient, it has been reported to cause negative health effects when consumed in excess, including adverse impacts on maternity and birth outcomes (Yazbeck et al., 2006; Barrett, 2007; Hafeman et al., 2007; Grazuleviciene et al., 2009; Ljung et al., 2009; Spangler and Spangler, 2009; Wood, 2009; Zota et al., 2009), inhibiting the intellectual development of children (Woolf et al., 2002; Wasserman et al., 2006, 2008, 2011; Bouchard et al., 2011; Khan et al., 2012) and neurological problems associated with Parkinson's-like symptoms (Barceloux, 1999; Ono et al., 2002; Bouchard et al., 2007; Avelino et al., 2014). Many of these ailments have been documented in the Bengal Basin (Wasserman et al., 2006, 2008, 2011; Barrett, 2007; Hafeman et al., 2007; Ljung et al., 2009; Khan et al., 2012).
The Bureau of Indian Standards (BIS) has enforced an Acceptable Limit for Mn in drinking water of 0.1 mg L−1, and 0.3 mg L−1in the absence of an alternative drinking water source (BIS, 2012); however, it has been suggested that the Mn limit may be practically difficult to achieve, given the naturally occurring concentrations of Mn in groundwater. Further, the World Health Organization (WHO, 2011) revoked the guideline for acceptable Mn in drinking water of 0.4 mg L−1 because it was well above concentrations of Mn normally found in drinking water. Ljung and Vahter (2007) argued that 0.4 mg L−1 was originally too high, and numerous studies have suggested a re-evaluation of the guideline for Mn is required (Biswas et al., 2012a,b; Frisbie et al., 2012; McArthur et al., 2012b).
Manganese (II) is the most common Mn species in acidic to circumneutral pH groundwater, as it is more soluble than Mn(III) or Mn(IV) (Tebo et al., 2007). Whereas the precipitation of insoluble Mn(III)—Mn(IV)—oxy (hydroxides) at higher pH (Hem, 1985) is thermodynamically favorable, the activation energy is high and hence the reaction is slow in nature (Gounot, 1994). The biological oxidation of Mn(II) (Tebo et al., 1997), however, is kinetically favorable and has been shown to produce stable Mn(IV) bio-oxides (Tebo et al., 2004) that predominate in natural systems. Such Mn oxides are known to strongly adsorb As species in a similar fashion to Fe oxides (Morgan and Stumm, 1964; Young and Harvey, 1992; Manning et al., 2002; Deschamps et al., 2003; Foster et al., 2003; Toner et al., 2006; Wu et al., 2015). Furthermore, Mn(IV)—oxides can oxidize As(III) to As(V), limiting As mobility and toxicity (Golden et al., 1986). Arsenic—Mn precipitates have also been shown to form under such biogeochemical conditions (Tournassat et al., 2002).
Thermodynamically, Mn(IV) is a more favored terminal electron acceptor than Fe(III) by anaerobic microorganisms (Stumm and Morgan, 1981; McGuire et al., 2002; Bethke et al., 2011), and Mn(II) is mobilized as a result of reductive dissolution of Mn(IV)—oxides (Appelo and Postma, 2005; Buschmann et al., 2007). Further, the mobility of Mn(II) can be influenced by the precipitation of carbonate, sulfide, or phosphate phases (Nickson et al., 2000; McArthur et al., 2001; Buschmann et al., 2007; Nath et al., 2009; Sankar et al., 2014), sorption of Mn(II) onto sediment surfaces (Wersin et al., 1989), as well as complexation with dissolved organic matter (DOM) (Marshall, 1979; Gavin et al., 2001; Graham et al., 2002).
The influence of DOM on the geochemistry of trace metals has been documented extensively (Lovley and Phillips, 1988; Nealson and Saffarini, 1994; Lovley et al., 1996; Nickson et al., 2000; Smedley and Kinniburgh, 2002). Microbially mediated reductive dissolution of Mn(IV)/Fe(III) oxides in the presence of labile organic carbon has been shown to cause elevated levels of dissolved Mn(II), As(III), and Fe(II) in the groundwater of the Bengal Basin (Dowling et al., 2002; Smedley and Kinniburgh, 2002; Lovley et al., 2004; McArthur et al., 2004). Recently, multiple roles of biologically refractive (humic-like) DOM such as aqueous complexation (Sharma et al., 2010; Liu et al., 2011) and electron shuttling (Lovley et al., 1996, 1998, 1999; Scott et al., 1998; Kappler et al., 2004; Jiang and Kappler, 2008; Wolf et al., 2009; Mladenov et al., 2010, 2015) have been implicated in the mobilization of redox sensitive elements such as Fe and As. Formation of Mn—humic complexes have been shown to inhibit the adsorption of Mn in near surface environments (Gavin et al., 2001; Graham et al., 2002). Lovley et al. (1996) showed that the model humic substance (AQDS) was capable of shuttling electrons from anaerobic microorganisms to Fe(III)—oxides, and it was suggested that these findings were similar for Mn(IV)—oxides, yet no study to date has directly confirmed this. In addition, Mn(IV)—oxides have been shown to oxidize phenols to produce humic-like substances (Vodyanitskii, 2009), and lyse high molecular weight (HMW) biologically refractive humic and fulvic acids into low molecular weight (LMW) biologically labile organic compounds such as pyruvate, which can serve as an electron donor for microorganisms (Sunda and Kieber, 1994).
Absorbance and fluorescence spectroscopic techniques have been widely used to characterize chromophoric DOM sources and transformations. Three-dimensional excitation emission matrices (EEMs) contain fluorophores associated with DOM derived from both microbial and higher plant sources. In addition to evaluation of fluorescence peaks (Coble, 1996) and indices (Parlanti et al., 2000; Ohno, 2002; Zsolnay, 2003; Cory and McKnight, 2005; Hansen et al., 2016) found in the EEMs, fluorescence data can be further analyzed using a parallel factor (PARAFAC) multivariate modeling analysis. PARAFAC modeling of large EEM datasets identifies the underlying fluorescence components that comprise an EEM. PARAFAC models also provide concentrations and relative distributions of each modeled component in each sample. These techniques have been used to discriminate unique fluorescence components and investigate potential sources of DOM (Stedmon et al., 2003; Stedmon and Bro, 2008; Williams et al., 2013).
The quality of DOM and its potential roles in As mobilization have been investigated using a range of fluorescence and absorbance spectroscopic techniques in Bangladesh (Mladenov et al., 2010, 2015). Further, a PARAFAC model developed using samples from the same sites as in this study (West Bengal, India) identified four unique components, namely terrestrial humic-like, humic-like impacted by agriculture, protein-like, and microbial humic-like (Kulkarni et al., 2016). The association of DOM with Mn mobilization from aquifer sediments has not been examined to date. The objective of this study was therefore to highlight associations between Mn and As biogeochemistry in conjunction with DOM quality in the shallow groundwater of West Bengal, India.
Materials and Methods
Study Site
A total of 51 groundwater and 16 sediment samples were collected from 2 sites located on the west side (Nabagram and Kandi) and 4 sites located on the east side (Hariharpara, Beldanga, Naoda, and Khidirpur) of the North-South flowing river Bhagirathi in Murshidabad to investigate the biogeochemistry of Mn (Figure 1). Murshidabad is a district (~5,500 km2) in north-central West Bengal with a population of ~7.1 million (Census of India, 2011).
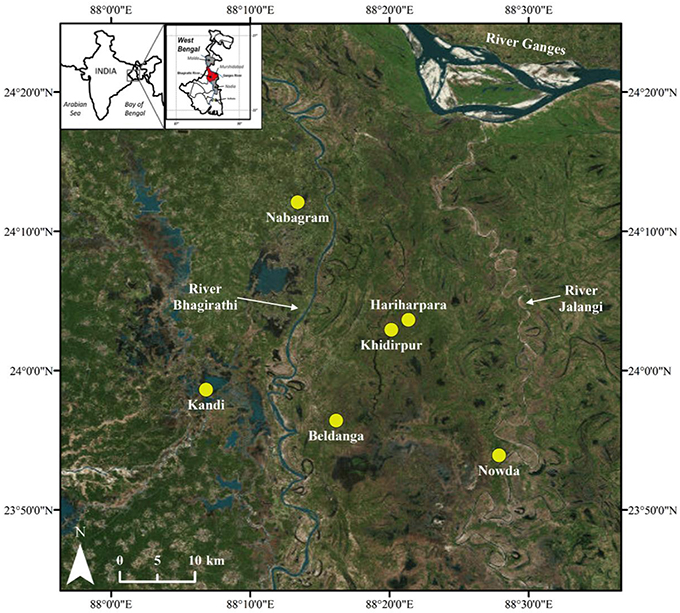
Figure 1. Map of the study area, including sampling sites. Nabagram and Kandi are west of the river Bhagirathi (HMLA); Hariharpara, Beldanga, and Naoda are east of the river Bhagirathi (HMHA); Khidirpur is east of the river Bhagirathi, but an HMLA site.
Distinct geological settings on the east and west sides of the river Bhagirathi have been previously discussed in Datta et al. (2011), Sankar (2013), Sankar et al. (2014), and Kulkarni et al. (2016). Briefly, the geology of the sites located east of the river Bhagirathi consists of young Holocene sediments (~7,000 years before present), whereas Pleistocene sediments (12,300—48,600 years before present) occur to the west of the river (Acharya et al., 2000; Mukherjee et al., 2007; Neidhardt et al., 2013). The permeability of Holocene sands east of the river Bhagirathi is reported to be 40–60 m d−1, whereas the permeability of the Pleistocene sediments to the west are reported to be slightly lower (i.e., 20–30 m d−1; Mukherjee et al., 2007). The lower permeability of the Pleistocene sediments is attributed to the presence of secondary clays and iron oxides, which are thought to clog pore spaces within the sands (Ravenscroft et al., 2005).
Several studies have reported higher Fe and As concentrations in groundwater from the Holocene aquifers (Mukherjee and Bhattacharya, 2001; Bhattacharya et al., 2002; Datta et al., 2011; Sankar, 2013; Sankar et al., 2014), whereas lower Fe and As concentrations have been observed in groundwater from the Pleistocene aquifers (Datta et al., 2011; Hoque et al., 2011; Sankar et al., 2014; Kulkarni et al., 2016). The contrasting DOM quality at Hariharpara, Beldanga, Nabagram, and Kandi sites has been discussed in Kulkarni et al. (2016), who demonstrated the presence of more humic-like DOM in Hariharpara and Beldanga compared to Nabagram and Kandi.
Sample Collection and Storage
Groundwater samples were collected at various depths from drinking water tube wells at Nabagram (n = 8, 27–43 m depth), Kandi (n = 6, 21–37 m depth), Hariharpara (n = 12, 12–25 m depth), Beldanga (n = 13, 18–40 m depth), Naoda (n = 10, 18–40 m depth) and Khidirpur (n = 2, 8 m depth) in January of 2010 (Sankar et al., 2014) and later in January of 2015. After pumping and purging each well for ~15 min, groundwater samples were collected in acid washed HDPE bottles pre-rinsed three times with the collected sample.
Field parameters such as pH, Eh (Ag/AgCl adjusted to standard hydrogen electrode (SHE)), temperature, electrical conductivity, salinity, and total dissolved solids (TDS) were measured immediately after the sample was collected in a clean 5-gallon HDPE bucket using portable probes (HACH HQ11D and Mettler Toledo SG3). The samples were filtered using a 0.45 μm nitrocellulose membrane filter and acidified in the field using Optima Grade HNO3 (0.2 % v/v) for the analysis of cations (MnT, FeT, AsT, Ca2+, Mg2+) by high resolution inductively coupled plasma mass spectrometry (HR-ICP-MS). Filtered and unacidified samples were used for the analysis of anions (Cl−, Br−, F−, , , and ) by ion chromatography (IC). All collected samples were placed immediately on ice and preserved throughout transport to Kansas State University.
Dissolved organic carbon (DOC) and total dissolved nitrogen (TDN) concentrations were determined using a thermic oxidation method on a Shimadzu TOC/TN 5050A analyzer. The samples were filtered in the field using pre-combusted (4 h, 450°C) 0.7 μm glass fiber filters (GFF) to avoid the risk of leaching organics from filters (such as nitrocellulose filters). Filtered samples were acidified in the field using Optima Grade HCl (0.2 % v/v) to inhibit microbial activity (Burdige and Homstead, 1994; Tupas et al., 1994; Burdige and Gardner, 1998). Spectral characterization (absorbance and fluorescence) of DOM was conducted on a Jobin Yvon Horiba Aqualog benchtop fluorometer, using 0.7 μm GFF filtered but unacidified samples to avoid fluorescence quenching effects at pH lower than the natural pH of the sample (Spencer et al., 2007).
Sediment cores were collected at the time of sampling by locally hired drillers using the percussion hand drilling method. Samples were collected in PVC tubes in incremental depths ranging between ~10 and 40 m, followed by preservation in O2-impermeable Remel® bags (Mitsubishi Gas Company, Remel®, Cat no. 2019-11-02), along with O2 absorber pouches (Mitsubishi Gas Company, AnaeroPouch® Anaero; Cat no. 23-246-379), flushed with high-purity N2 gas, sealed, and shipped on ice to Kansas State University. Bulk Mn, As, and Fe concentrations in sediments were acquired via a bulk digestion method modified from Premarathna et al. (2010). Briefly, ~0.5 g of finely homogenized (<2 mm) sediments were treated in glass digestion tubes with 2.5 mL of 30 % H2O2 for 10 min, followed by an additional 0.5 mL of 30 % H2O2, and allowed to react for 12 h. The samples were then digested at 90°C until the volume reduced to ~1 mL. To this, 2.5 mL of freshly prepared aqua regia (1:3, HNO3: HCl) was added and left to react for 12 h. Samples were heated to 75°C (30 min), 90°C (30 min), 110°C (30 min) and then to 140°C until the total volumes were reduced to ~1 mL. Samples were diluted to 10 mL using Optima Grade 0.1 % HNO3, filtered through 2.5 μm (Whatman 42) filter paper and analyzed for FeT and MnT by a Varian 720-ES Inductively Coupled Plasma-Optical Emission Spectrometer (ICP-OES) and for AsT by Varian GTA 120 Graphite Tube Atomizer w/AA 240Z Zeeman Atomic Absorption Spectrometer (GTA-AAS). Three NIST standards (Montana II) were digested and yields of 101 % (As), 84 % (Fe), and 88 % (Mn) obtained. To minimize potential losses, 30 cm digestion tubes with constricted necks were utilized. Lower Fe and Mn yields were probably attributed to Fe and Mn binding to siliceous materials, which are not readily dissociated by aqua regia (Loeppert and Inskeep, 1996).
Fluorescence Analyses of DOM
Spectral acquisition for fluorescence analyses was performed using an excitation range of 240–450 nm in 3 nm increments and an emission range of 300–600 nm in 3.28 nm increments (instrument default) with an integration time of 0.25 seconds. Absorbance was simultaneously measured on the same instrument from 300 to 600 nm in 3 nm increments. Filtered samples were brought to room temperature prior to fluorescence and absorbance measurements. Ultrapure water (18.3 MΩ·cm Milli-Q) was used for Raman normalization (emission intensity at 350 nm) and for blank subtraction. Inner filter effect corrections were done using the absorbance of each sample. Detailed information on instrumental parameters and data processing is described elsewhere (Kulkarni et al., 2016). Corrected EEMs of 51 samples were fitted to a four component PARAFAC model which was validated by split half analysis and random initialization technique using the DOMFluor toolbox (Stedmon and Bro, 2008).
Indices based on fluorescence and absorbance properties are useful in deciphering the quality of DOM in aqueous environments. The absorbance at 254 nm (Abs254) provides insight regarding the degree of aromaticity of DOM, particularly when normalized to DOC concentration and the path length of incident light as specific UV absorbance (SUVA254) (Weishaar et al., 2003). The ratio of the absorption spectral slopes between 275 and 295 nm (S275−295) and 350 and 400 nm (S350−400), or SR, was calculated to determine whether DOM was marine-like (SR < 1) or terrestrially dominated with high chromophoric dissolved organic matter (CDOM) (SR > 1) (Helms et al., 2008). Freshness index (β: α) was calculated as the ratio of emission intensity at 380 nm to the maximum intensity between 420 and 435 nm at an excitation wavelength of 310 nm (Parlanti et al., 2000); lower values signify a greater extent of DOM decomposition (Wilson and Xenopoulos, 2008; Fellman et al., 2010). Fluorescence index (FI), the ratio of fluorescence intensities at 470 and 520 nm emission and at an excitation wavelength of 370 nm, was calculated to understand the source of DOM. Higher FI's (~1.7–1.9) represent microbial derivation pathways and lower FI's (~1.3–1.4) imply a terrestrial origin (McKnight et al., 2001; Cory and McKnight, 2005). To measure the extent to which DOM has undergone humification, the humification index (HIX) was computed as the ratio of peak area under the emission spectra at 435 to 480 nm to the peak area from 300 to 345 nm at an excitation wavelength of 254 nm (Zsolnay, 2003).
Equilibrium Chemical Speciation and Modeling
Aqueous geochemical modeling was performed using Visual MINTEQ ver. 3.1 to calculate the equilibrium distribution and relevant saturation indices for Mn and Fe species. Arsenic speciation from groundwater within the same sites as this study was assessed previously by passing acidified samples through anion exchange columns, analyzing the eluent (H3AsO30) via HR-ICP-MS as As(III), then back-calculating from AsT to obtain As(V) (Sankar et al., 2014).
Statistical Analyses
Correlations were assessed by computing two-tailed Pearson correlation coefficients and the Mann-Whitney-Wilcoxon (MWW) U test was used to delineate statistically significant variability among groups due to a non-parametric distribution of parameters and a relatively small sample size. All statistical analyses were performed using SPSS Statistics Software. Parenthetic values represent the mean ± 95 % confidence interval, and only correlations of statistical significance (p < 0.05) are presented.
Results
Hydrochemistry of Dissolved Mn, As, and Fe in Groundwater
Of the 51 tube wells sampled, 73 % contained total dissolved Mn (MnT) > 0.4 mg L−1 (revoked (WHO, 2011) guideline), 78 % contained total dissolved As (AsT) > 10 μg L−1 (WHO, 2011 guideline), and 57 % exceeded both of these guidelines (Figure 2, Figure S1). Only 6 % of wells had MnT < 0.4 mg L−1 and AsT < 10 μg L−1. Typically, high MnT (0.83 ± 0.14 mg L−1) and high AsT (330 ± 97 μg L−1) concentrations were observed in the tube wells located to the east of the river Bhagirathi and these sites are termed as HMHA sites for further discussion (Table 1). Conversely, high MnT (1.1 ± 0.29 mg L−1) and low AsT (9.0 ± 2.1 μg L−1) concentrations were observed (Table 1) in the tube wells located to the west of the river (HMLA). The total Fe concentrations (FeT) at HMHA sites (3.6 ± 0.93 mg L−1) were significantly higher than at HMLA sites (0.31 ± 0.10 mg L−1) (Table 1). The difference between AsT and FeT concentrations at the HMHA and HMLA sites was found to be statistically significant, but insignificant for MnT concentrations (Table 1). Nitrate () and sulfate () values were significantly higher in HMLA (2.8 ± 3.0 mg L−1; 16 ± 9.7 mg L−1) groundwater than in HMHA (0.21 ± 0.30 mg L−1; 5.0 ± 2.8 mg L−1) groundwater (Table 1). Khidirpur, despite being east of the river Bhagirathi, showed exceptionally low AsT concentrations (3.4 ± 0.26 μg L−1) and is therefore discussed as an HMLA site.
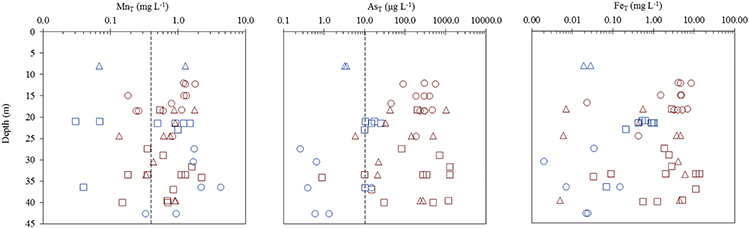
Figure 2. Vertical distribution of dissolved MnT, AsT, and FeTfor Beldanga ( ), Hariharpara (
), Hariharpara ( ), Naoda (
), Naoda ( ), Kandi (
), Kandi ( ), Nabagram (
), Nabagram ( ), and Khidirpur (
), and Khidirpur ( ). Dotted lines represent WHO limits for Mn (0.4 mg L−1—Revoked in 2011) and As (10 μg L−1).
). Dotted lines represent WHO limits for Mn (0.4 mg L−1—Revoked in 2011) and As (10 μg L−1).
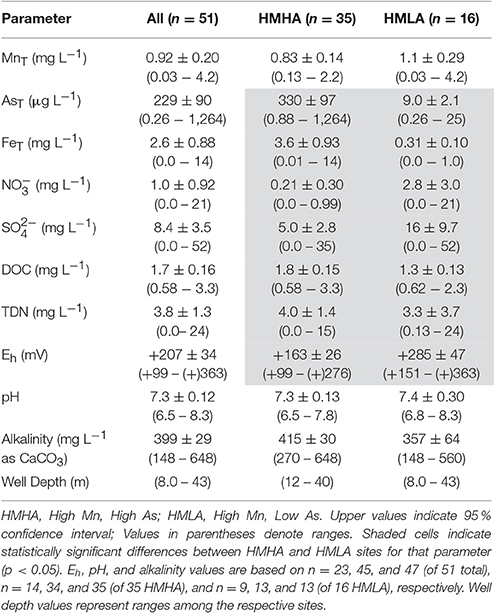
Table 1. Distribution of groundwater hydrogeochemical parameters within all sites, HMHA sites, and HMLA sites.
Based on Visual MINTEQ speciation modeling, the dominant species of Mn in all studied sites was Mn2+ (~73–89 % of MnT), with and MnCO3(aq)comprising the remaining ~4–17 % and ~7–9 % of MnT, respectively (Figure S4A). There was no significant difference in the percentages of Mn2+, , and MnCO3(aq) between the HMHA (Mn2+ = 81.6 ± 2.78 %; = 7.52 ± 0.60 %; MnCO3(aq) = 10.7 ± 2.66 %) and HMLA (Mn2+ = 83.3 ± 4.90 %; = 7.42 ± 1.05 %; MnCO3(aq) = 9.03 ± 4.04 %) sites (Table S4A), and all dissolved Mn species were of the oxidation state Mn(II). Iron speciation was more variable, with the dominant species being Fe2+ (~30–95 % of FeT), (~0–67 % of FeT), (~2–7 % of FeT), and FeOH+ (~0–1 % of FeT) (Figure S4B). Thus, Fe(II) and Fe(III) were present in proportions of ~32–99 % and 0–67 % of FeT, respectively. There was significantly more Fe2+ in HMHA (87.4 ± 6.31 % of FeT) than in HMLA (49.9 ± 19.9 % of FeT) sites, and there was significantly less (the only dominant Fe(III) species) in HMHA (6.87 ± 6.48 % of FeT) relative to HMLA (46.2 ± 21.7 % of FeT) sites (Table S4B). Percentages of were not significantly different between HMHA (5.16 ± 0.48 % of FeT) and HMLA sites (3.23 ± 1.79 % of FeT) (Table S4B). Analytically determined As speciation by a previous study of these same sites demonstrated that 55–98 % of AsT was As(III) in HMHA sites, whereas the only sample analyzed from HMLA sites was 36 % As(III) (Sankar et al., 2014).
Physical parameters (e.g., pH, Eh, alkalinity, depth) were not significantly different between HMHA and HMLA sites, excluding Eh, which was significantly lower in HMHA (+163 ± 26 mV) relative to HMLA (+285 ± 47 mV) sites (Table 1). Correlations of physical parameters with MnT, AsT, and FeT are presented in Tables S1–S3. A weak positive correlation was observed between MnT and AsTin HMHA groundwater, however, a significant positive correlation between MnT and AsT in Naoda was observed (Figure S2, Tables S1, S2). At Hariharpara, a positive correlation between FeT and AsT was apparent (Figure S2, Tables S2, S3). In all HMLA sites, AsT and FeTwere positively correlated (Figure S2, Tables S2, S3). Positive correlations between MnT and AsT and between AsT and FeT were observed in groundwater from the Nabagram location (Tables S1–S3).
Dissolved Organic Matter Quality
Dissolved organic carbon (DOC) concentrations at the HMHA sites (1.8 ± 0.15 mg L−1) were significantly higher than at HMLA sites (1.3 ± 0.13 mg L−1). Total dissolved nitrogen concentrations (TDN) at HMHA sites (4.0 ± 1.4 mg L−1) and HMLA sites (3.3 ± 3.7 mg L−1) were not significantly different (Table 1). At the HMHA sites, groundwater DOC was positively correlated with MnT (Figure S2, Table S1) and also with AsT (Figure S2, Table S2), but not with FeT (Figure S2, Table S3). By contrast, in HMLA groundwater, no significant correlation of DOC with MnT, AsT or FeT (Figure S2, Tables S1–S3) was found. Isolating just Kandi samples revealed a significant positive correlation between MnT and DOC concentrations, however (Table S1).
Absorbance at 254 nm (Abs254) intensities for groundwater from the HMHA sites (0.047 ± 0.006) were significantly higher than for groundwater from the HMLA sites (0.029 ± 0.007) (Table 2). Specific ultraviolet absorbance at 254 nm (SUVA254) values at HMHA sites (2.60 ± 0.28 L mg−1 m−1) and HMLA sites (2.43 ± 0.39 L mg−1 m−1) did not vary significantly (Table 2). It should be noted that ferric iron concentrations can artificially enhance Abs254 and SUVA254 values due to similar absorbance spectra (Weishaar et al., 2003), and that the variability of FeT concentrations between HMHA and HMLA sites (Table 1) may influence the observed variability in Abs254 (Table 2); therefore, SUVA results should be interpreted with caution. Spectral slope ratios (SR) at HMHA sites (1.16 ± 0.11) and HMLA sites (1.44 ± 0.29) were not significantly different (Table 2). Within HMLA groundwater, significant positive correlations were observed between SUVA254 and AsT (Table S2) and SUVA254 and FeT (Table S3). SUVA254 was also positively correlated with AsT (Table S2) and MnT (Table S1) in groundwater from Nabagram. In Beldanga, only AsT exhibited a positive correlation with SUVA254 (Table S2).
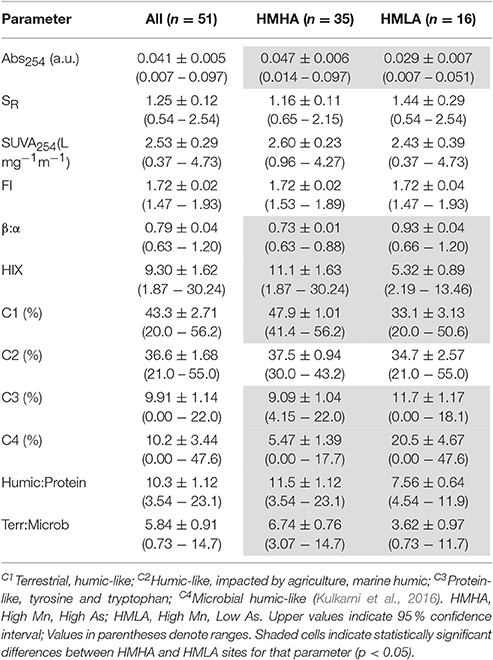
Table 2. Distribution of groundwater dissolved organic matter parameters within all sites, HMHA sites, and HMLA sites.
Fluorescence index (FI), freshness index (β:α) and humification index (HIX) at HMHA sites were 1.72 ± 0.02, 0.73 ± 0.01, and 11.1 ± 1.63, respectively, whereas they were 1.72 ± 0.04, 0.93 ± 0.04, and 5.32 ± 0.89, respectively, at the HMLA sites (Table 2). Fluorescence index (FI) did not vary significantly between HMHA and HMLA groundwater, whereas β:α was significantly lower in HMHA groundwater than in HMLA groundwater, and HIX was significantly higher in HMHA groundwater than in HMLA (Table 2).
A negative correlation was observed in all sites between AsT and β:α (Table S2) and between FeT and β:α (Table S3), whereas a positive correlation was observed between AsT and HIX (Table S2). At the HMLA sites, a negative correlation was found between MnT and β:α (Table S1), whereas AsT and FeT were positively correlated with β:α (Tables S2, S3). In Nabagram, AsT was negatively correlated with β:α (Table S2). A positive correlation was observed between FI and FeT in Naoda (Table S3), and in Beldanga FI was negatively correlated with AsT, and AsT with β:α (Table S2).
The PARAFAC model identified 4 fluorescence components (Table 2, Figure 3, Table S5): (1) a terrestrial humic-like (C1) component; (2) a humic-like component influenced by agricultural and wastewater activities (C2); (3) a protein-like component similar to tryptophan and tyrosine (C3); and (4) a microbial humic-like (C4) component. The four components identified were similar to components C1–C4 found in an earlier study in this region (Kulkarni et al., 2016). On average, the % C1 in groundwater from the HMHA sites (47.9 % ± 1.01) was significantly higher than % C1 in groundwater from the HMLA sites (33.1 % ± 3.13) (Table 2). In all sites and within HMLA sites, % C1 was positively correlated with AsT concentrations (Table S2), and also with FeT concentrations at HMLA sites (Table S3). The component % C2 in groundwater from the HMHA sites (37.5 % ± 0.94) and at the HMLA sites (34.7 % ± 2.57) was not significantly different (Table 2). Groundwater from HMLA sites shows positive correlations between % C2 and AsT (Table S2) and with FeT (Table S3). Component C3 (%) was significantly higher in groundwater from the HMLA sites (11.7 % ± 1.17) than in groundwater from the HMHA sites (9.09 % ± 1.04) (Table 2). Positive correlations between AsT and % C3 at all sampled locations (Table S2) and between AsT and % C3 at Beldanga (Table S2) were observed. The proportion of the DOM pool attributed to Component C4 (% C4) was significantly higher in groundwater from the HMLA sites (20.5 % ± 4.67) than in groundwater from the HMHA sites (5.47 % ± 1.39) (Table 2). A positive correlation between AsT and % C4 in groundwater from the HMLA sites was observed (Table S2). No significant correlations were observed between MnT and any of the proportions of the total DOM pool (Table S1).
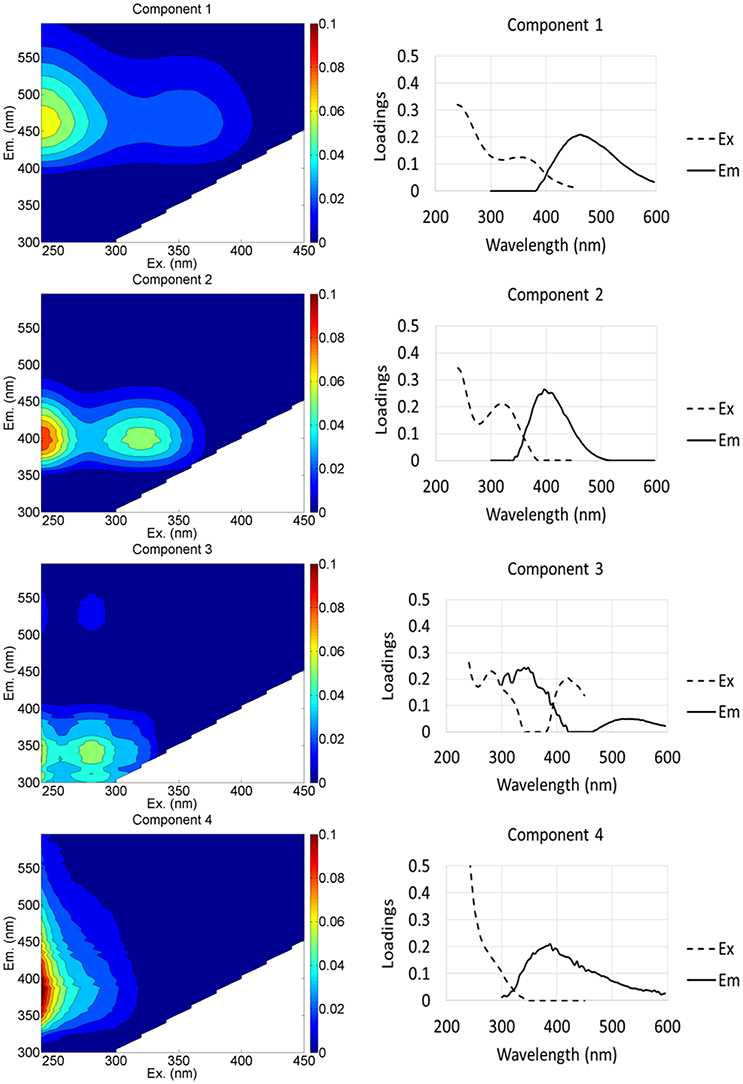
Figure 3. EEM spectra (left) and emission-excitation curves (right) showing loadings of four PARAFAC components identified in the model.
Bulk Sediment Geochemistry
Bulk sediment concentrations of Mn (278 ± 67.5 mg kg−1), As (6.61 ± 1.84 mg kg−1) and Fe (21.9 ± 4.82 g kg−1) at the HMHA sites were not significantly different than Mn (420 ± 122 mg kg−1), As (4.72 ± 0.36 mg kg−1) and Fe (19.2 ± 3.24 g kg−1) contents of the sediments from the HMLA sites (Table 3, Figure 4). Bulk As and Fe contents of sediments were positively correlated within all sites, within HMHA sites, and at Hariharpara (Table S4, Figure S3). Sediment Mn and Fe concentrations were positively correlated within the HMHA sites, at Hariharpara, and at Beldanga (Table S4, Figure S3). Similarly, sedimentary Mn and As were positively correlated at Hariharpara (Table S4, Figure S3).
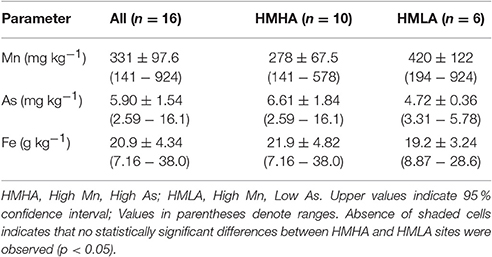
Table 3. Distribution of bulk sediment concentrations of Mn, As, and Fe within all sites, HMHA sites, and HMLA sites.
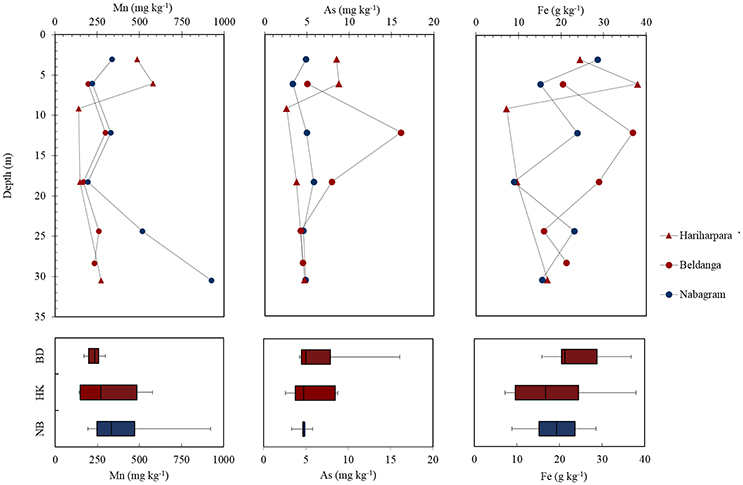
Figure 4. Depth plots of bulk sediment concentrations of Mn, As, and Fe in Hariharpara ( ), Beldanga (
), Beldanga ( ), and Nabagram (
), and Nabagram ( ). Box-and-whisker plots depict distribution of the elements in Nabagram (NB), Hariharpara (HK), and Beldanga (BD), respectively.
). Box-and-whisker plots depict distribution of the elements in Nabagram (NB), Hariharpara (HK), and Beldanga (BD), respectively.
Discussion
The distribution of dissolved MnT and AsT in groundwater of this study area appears to be controlled by redox processes as has been noted in previous studies in other regions of the Bengal Basin (Bhattacharya et al., 2002; McArthur et al., 2004, 2008; Buschmann et al., 2007; von Brömssen et al., 2007; Sankar et al., 2014). Elevated MnT was prevalent throughout all surveyed sites, yet AsT and FeT were constrained to groundwater with lower Eh and dissolved concentrations. Greater quantities of DOC and humic-like, terrestrial DOM coincided with low Eh, high AsT and FeT groundwater. By contrast, lower DOC concentrations and protein-like DOM were present within high Eh and low AsT and FeT groundwater. The distribution of MnT was seemingly unaffected by both DOC quantity and DOM quality, as MnT concentrations were consistently high. It should be noted here that Eh is employed as a qualitative measure of the general redox state of groundwater, as it is a difficult parameter to quantify due to the inherent redox disequilibrium in natural waters (Lindberg and Runnells, 1984; Stefansson et al., 2005). The discussions herein will attempt to correlate the release and accumulation of Mn, As, and Fe species with redox chemistry, carbonate chemistry and DOM properties.
Mobilization of Mn and As from Sediments to Groundwater
The solid phase concentrations of Mn, Fe, and As (Table 3) suggest that there are abundant metal(loid) concentrations in the shallow aquifers of Murshidabad. Hering and Kneebone (2002) experimentally showed that even 1.8 mg kg−1 of sedimentary As was enough to cause dissolved concentrations >10 μg L−1 under reducing conditions in the presence of sufficient labile organic carbon. Previously, we showed that both the HMHA and HMLA sites have substantial sedimentary organic matter (~10–20 % by weight) (Datta et al., 2011; Sankar, 2013; Mohajerin et al., 2014; Kulkarni et al., 2016), which could drive microbial reductive dissolution of both Mn and Fe minerals. Elevated dissolved concentrations of FeT and AsT in groundwater from other regions of the Bengal Basin have been described previously, and are thought to be the result of microbial reductive dissolution of Fe minerals under anoxic conditions (McArthur et al., 2001, 2004; Bhattacharya et al., 2002; Dowling et al., 2002; Roychowdhury et al., 2002; Horneman et al., 2004; Ravenscroft et al., 2005; Sankar et al., 2014). By contrast, relatively higher Eh values in HMLA sites may explain lower FeT (~0.31 mg L−1) and AsT (~9.0 μg L−1) concentrations. The average MnT and FeT concentrations in groundwater from the HMHA sites were 0.83 and 3.6 mg L−1 (i.e., MnT < FeT), whereas HMLA groundwater had average MnT and FeT concentrations of 1.1 and 0.31 mg L−1, respectively (i.e., MnT > FeT). These observations were consistent with the stoichiometry that 1 mole of acetate (simplest electron donor) would produce 8 moles of Fe(II) but only 4 moles of Mn(II) (e.g., Lovley and Phillips, 1988). Assuming that the same organic matter was used as an electron donor for both Fe(III) and Mn(IV) reduction, groundwater from the HMHA sites was apparently reducing enough to be dominated by Fe(III) reduction, releasing greater Fe(II) than Mn(II). In contrast, groundwater from the HMLA sites was less reducing. This may have led to Mn(IV) reduction remaining dominant and thus greater amounts of Mn(II) being released to solution relative to Fe(II).
Based on thermodynamics, it is suggested that after O2 and are reduced, Mn(IV) is commonly the next electron acceptor utilized by respiratory bacteria (Champ et al., 1979; Stumm and Morgan, 1981; Rittman and McCarty, 2001; McGuire et al., 2002; Bethke et al., 2011). Microbial Fe(III) reduction typically occurs after Mn(IV) is reduced. However, in natural systems, these zones are not sharply defined but rather overlap substantially [e.g., simultaneous Fe(III)— reduction or simultaneous reduction—CH4 generation] (Chapelle and Lovley, 1992; Postma and Jakobsen, 1996; Jakobsen and Postma, 1999; Kirk et al., 2004; Bethke et al., 2011). The abundance of electron acceptors also determines the prevalence of specific redox reactions by competitive exclusion of microbial communities (Lovley and Goodwin, 1988). For example, in aquifers with abundant Fe(III) but limited , iron-reducing bacteria would outcompete reducing bacteria by limiting the concentration of electron donor such that reduction cannot proceed (Chapelle and Lovley, 1992; Kirk et al., 2004).
Despite consistent TDN values between HMHA and HMLA sites, our analyses show that concentrations were predominantly below detection (i.e., < 0.1 mg L−1) in groundwater from the HMHA sites (~0.21 mg L−1; n = 28), yet significantly higher (~2.8 mg L−1; n = 13) in groundwater from the HMLA sites (also observed at Nabagram site, Sankar et al., 2014). This supports the notion that under sufficiently reducing conditions with an abundance of Fe(III) and Mn(IV) minerals and adequate supply of labile carbon, Mn(IV) and Fe(III) reduction can occur simultaneously, releasing Mn(II), Fe(II), and adsorbed AsT into HMHA groundwater. In contrast, higher concentrations of were coupled with relatively higher Eh, abundant Mn(IV) or Fe(III) bearing minerals and sufficient sedimentary labile carbon in HMLA sites. This may suggest that simultaneous and Mn(IV) reduction maintained the electron donor capacity to such a low level that Fe(III) reduction could not proceed, resulting in high dissolved Mn(II) but low Fe(II) and AsT concentrations. Contrasting regimes of high and low AsT groundwater (i.e., as in HMHA or HMLA in this study) are widespread throughout the Bengal Basin and other fluviodeltaic plains (Buschmann et al., 2007; von Brömssen et al., 2008; Bhattacharya et al., 2009; Bundschuh et al., 2010; Hug et al., 2011).
In the HMLA sites, it is expected that Mn(IV)—oxide bound AsT would accumulate in the aqueous phase upon reductive dissolution of Mn(IV)—oxides; however, this is not the case in the current study. Lower AsT concentrations may be attributed to the re-adsorption of AsT onto clay minerals, carbonate minerals, or incompletely reduced Fe(III)—oxides (Manning and Goldberg, 1997; McArthur et al., 2004; Guo et al., 2007; Bhattacharya et al., 2009).
The possibility that effectively competes with AsT for adsorption sites is considered negligible for groundwater from the HMLA sites because concentrations were below detection (i.e., < 0.1 mg L−1; Jain and Loeppert, 2000; Dixit and Hering, 2003; Stollenwerk et al., 2007). Mn(IV)—oxides have been shown to oxidize Fe(II) even in the presence of Fe(III) reducing microorganisms (Lovley and Phillips, 1988), which could precipitate Fe(III)—oxides. This provides additional sorption sites for AsT and maintains low Fe(II) and AsT concentrations in groundwater, as was shown experimentally by Wu et al. (2015). Several studies have reported the oxidation of As(III) by Mn(IV) and subsequent adsorption of As(V) onto Mn and Fe bearing minerals, specifically oxides (Oscarson et al., 1981; Sun et al., 1999; Manning et al., 2002; Amirbahman et al., 2006; Stollenwerk et al., 2007; Ehlert et al., 2014, 2016; Bai et al., 2016). Hence, the higher Mn(II) and lower Fe(II) and AsT concentrations in groundwater from the HMLA sites may be a product of Mn(IV) reduction and the associated oxidation of Fe(II) and As(III).
The above discussion mostly conforms to typical HMHA and HMLA sites, which are underlain by Holocene and Pleistocene sediments, respectively. A possible outlier may be the site of Khidirpur (Figure 1), a low AsT yet high MnT site, which is ~2 km southwest of Hariharpara (HMHA site). Due to the patchy distribution of AsT observed in the Bengal Basin and other fluviodeltaic plains (van Geen et al., 2003; Fendorf et al., 2010), this is not entirely surprising. In this context, one possible control could be paleointerfluvial Pleistocene deposition beneath Khidirpur (McArthur et al., 2011), which may have led to groundwater chemistry similar to other HMLA sites, overlying Pleistocene sediments. Another possible explanation could be that shallower sampling depths at Khidirpur (~8 m) relative to nearby HMHA sites (~12–25 m sampling depth) were related to higher Eh values observed in Khidirpur groundwater. It is possible that at ~8 m depth, a —Mn(IV) redox zone exists due to aeration induced by vertical mixing during the post-monsoon period. This is supported by the fact that the highest concentrations (average ~12 mg L−1) of all sampled wells (n = 51) were observed in Khidirpur, and MnT concentrations were also high (average ~0.67 mg L−1). The effects of vertical mixing on the geochemistry of shallow groundwater (<40 m) from the Nadia district (~160 km south of the current study site) have been observed during the post-monsoon period (Majumder et al., 2016). However, further investigations with detailed sediment analyses at Khidirpur would be necessary to understand the mechanism of AsT immobilization.
Influence of Dissolved Organic Matter Quality on Mn and As Mobilization
Analyses of spectral properties of fluorescent DOM suggest that the DOM in groundwater from the HMHA sites contains more humic-like (higher Humic: Protein ratio), terrestrial (higher Terrestrial: Microbial), and decomposed (lower β:α) organic compounds compared to the DOM in groundwater from the HMLA sites. These results are in agreement with previous investigations of DOM quality in West Bengal groundwater (Kulkarni et al., 2016).
Several studies have now demonstrated the important role of humic and biologically refractory DOM in mobilizing FeT and AsT via aqueous complex formation (Sharma et al., 2010; Liu et al., 2011). Formation of complexes between metals and DOM acts to keep those constituents in solution (Gavin et al., 2001). Another important role for humic substances in Bengal Basin groundwater is the ability of quinone moieties in humic DOM to shuttle electrons between Fe-reducing bacteria and Fe minerals (Lovley et al., 1996, 1998; Scott et al., 1998; Jiang and Kappler, 2008; Mladenov et al., 2010, 2015). By serving as electron shuttles, humic substances have the capability to accelerate reductive dissolution of Fe minerals, and the electron shuttling capacity has been shown to be very high in groundwater fulvic acids isolated from the Bengal Basin. This potential electron shuttling role by humic DOM is supported by higher concentrations of dissolved FeT and AsT in groundwater from the HMHA sites compared to groundwater from the HMLA sites. By contrast, the less aromatic and humic DOM that characterizes groundwater from the HMLA sites is expected to contribute far less to electron shuttling or complexation reactions.
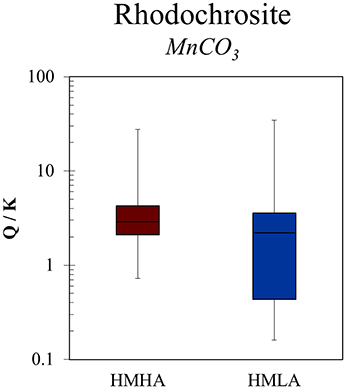
Graham et al. (2002) showed that humic substances in topsoil (0–15 cm) formed aqueous complexes with dissolved Mn(II) under reducing conditions and therefore maintained higher Mn(II) concentrations in solution. This was partially based on humic—Mn(II) complexation inhibiting the ability for Mn precipitation reactions, as evidenced by a previous investigation (Gavin et al., 2001). In our study, because more humic-like DOM is present in groundwater from the HMHA sites relative to the HMLA sites, substantially higher MnT concentrations could be expected in groundwater from the HMHA sites as a result of humic—Mn(II) complexation. However, saturation indices for the mineral rhodochrosite (MnCO3) indicated supersaturation in ~93 % of samples from the HMHA sites (Figure 5), suggesting precipitation (Lovley and Phillips, 1988) of Mn(II) with excess that is present due to oxidation of organic matter (Ying et al., 2011). For HMLA groundwater, supersaturated conditions for rhodochrosite were observed in 63 % of samples (Figure 5). Despite differences in rhodochrosite saturation calculations and DOM quality, MnT concentrations were similar in groundwater from the HMHA and HMLA sites. It is possible that humic DOM—Mn(II) complexation inhibits the precipitation of rhodochrosite in HMHA groundwater, resulting in comparable concentrations of dissolved Mn between the two regions. This is contrary to the expectation that greater MnT would be dissolved in HMHA groundwater based on: (i) humics acting as electron shuttles to catalyze Mn(IV) reduction, (ii) inherently lower Eh values, and (iii) a greater abundance of DOC for heterotrophic microbial metabolisms to catalyze Mn(IV) reduction.
Spatial Distribution of Dissolved Mn and As: Implications for Human Health
This study shows concentrations of groundwater MnT and AsT far exceed their recommended health limits in drinking water. The fact that 73 % of the tube wells sampled exceed the revoked WHO limit of MnT in drinking waters of 0.4 mg L−1 substantiates the notion that the reimplementation of a guideline value is prudent. Geogenic MnT contamination of groundwater is widespread throughout West Bengal, as well as in groundwater from Bangladesh, the Mekong Delta, and some parts of Europe, and recent advances in understanding its neurotoxicity are receiving attention worldwide (Wasserman et al., 2006, 2008, 2011; Barrett, 2007; Bouchard et al., 2007, 2011; Hafeman et al., 2007; Grazuleviciene et al., 2009; Ljung et al., 2009; Spangler and Spangler, 2009; Wood, 2009; Zota et al., 2009; Khan et al., 2012). Furthermore, the co-occurrence of MnT with AsT in groundwater is of particular concern, where the release of both may be linked to the dissolution of metal-oxides under reducing conditions. Although regional studies such as Buschmann et al. (2008) and McArthur et al. (2004) have documented an inverse relationship between dissolved Mn and As in groundwater, it is important to note that such deductions are most often applicable to basin-wide investigations. Probing these relations within a single sampling site, or a constrained series of sites as in the current study, may reveal unique trends that are heterogeneous and difficult to generalize. Effectively assessing an area for MnT and AsT contamination requires both of these perspectives—knowledge of regional and local scale relationships—in order to predict or evaluate the quality of water in a given well.
This study affirms that elevated AsT does not exclude the possibility of elevated MnT, especially in reducing, geogenically derived systems rich in labile DOM. Likewise, elevated MnT does not imply the absence of AsT. Knowledge of redox conditions, most easily attained via sediment color (Biswas et al., 2012b), can be a useful tool in estimating whether high Mn waters may be afflicted with high As concentrations. Nonetheless, it is apparent that obtaining “safe” drinking water from subsurface aquifers in Murshidabad is a serious challenge. Oxidized sediments similar to those in HMLA regions, i.e., brown sand aquifers and paleointerfluves, have been posed as alternative drinking water sources in SE Asia, however, the prevalence of dissolved MnT in these systems has raised concerns (von Brömssen et al., 2007; McArthur et al., 2008, 2012b; Biswas et al., 2012b, 2014 and references therein). Other strategies such as filtration and rainwater have been implemented, but due to cost and maintenance their application is not practical at this time (Hossain et al., 2015). It is important that remediation strategies and alternative water sources continue to be developed so that the inhabitants of regions such as SE Asia may eventually have access to safe drinking water supplies.
Conclusion
Geogenic MnT contamination in West Bengal groundwater significantly exceeds the revoked WHO guideline of 0.4 mg L−1, and only 6 % of the surveyed tube wells met the guidelines for both MnT and AsT. The release and accumulation of these metal(loid)s is strongly related to their redox chemistry, DOM characteristics, and the availability of electron acceptors and carbonate ligands. Relationships between MnT and DOM quality suggest that MnT release persists in conjunction with both protein-like and humic-like DOM, whereas dissolved AsT is strongly associated with humic-like, terrestrial DOM. Where Eh values are lower (e.g., HMHA), concentrations are negligible and Mn(IV) and Fe(III) are likely the dominant electron acceptors for microorganisms, leading to elevated Mn(II), Fe(II), and in the groundwater. Saturation indices of rhodochrosite imply a net sink for aqueous Mn(II) in HMHA sites, yet MnT concentrations are not significantly higher than in the samples with higher Eh values (e.g., HMLA), detectable , and low AsT and FeT. It is postulated that humic—Mn(II) complexation was inhibiting rhodochrosite precipitation, yet further work is required to understand these mechanisms.
Author Contributions
MV and SD are the principal executors of the field work, research and preparing this manuscript; MV is the principle researcher in this project; HK and NM contributed to the DOM modeling and interpretation and editing the manuscript; KJ, NK, PB contributed in data interpretation and shaping up of the manuscript; GH, JW, and MG contributed in data interpretation and editing sediment analyses, GH also contributed in data interpretation of the sediment geochemistry.
Funding
This work has been funded by National Science Foundation Grant Proposal Numbers (1) NSF-EAR1014947 (Datta-KState) and (2) NSF EAR-1014946 (Johannesson-Tulane), and (3) Sigma Xi Grants in Aid G20141015720343 (Vega-KState).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors would like to thank the National Science Foundation (NSF), Sigma Xi, and Kansas State University for support for field trips and project management to West Bengal. The authors would also like to thank the Department of Geology at K-State for funding, as well as the Departments of Agronomy and Biology for analytical assistances. Finally, the authors are greatly indebted to the inhabitants of Murshidabad who willingly helped in the logistical aspects of field sampling throughout the field excursions, and for their understanding and continuous support for these research efforts.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fenvs.2017.00029/full#supplementary-material
References
Acharya, S. K., Lahiri, S., Raymahashay, B. C., and Bhowmik, A. (2000). Arsenic toxicity of groundwater in parts of the Bengal basin in India and Bangladesh: the role of Quaternary stratigraphy and Holocene sea-level fluctuation. Environ. Geol. 39, 1127–1137. doi: 10.1007/s002540000107
Amirbahman, A., Kent, D. B., Curtis, G. P., and Davis, J. A. (2006). Kinetics of sorption and abiotic oxidation of arsenic(III) by aquifer materials. Geochim. Cosmochim. Acta 70, 533–547. doi: 10.1016/j.gca.2005.10.036
Appelo, C. A. J., and Postma, D. (2005). Geochemistry, Groundwater and Pollution, 2nd Edn. Amsterdam: A.A. Balkema Publishers. doi: 10.1201/9781439833544
Avelino, M. A., Fusao, E. F., Pedroso, J. L., Arita, J. H., Ribeiro, R. T., Pinho, R. S., et al. (2014). Inherited manganism: the “cock-walk” gait and typical neuroimaging features. J. Neuro. Sci. 341, 150–152. doi: 10.1016/j.jns.2014.03.057
Bai, Y., Yang, T., Liang, J., and Qu, J. (2016). The role of biogenic Fe-Mn oxides formed in situ for arsenic adsorption and oxidation in aquatic ecosystems. Water Res. 98, 119–127. doi: 10.1016/j.watres.2016.03.068
Barrett, J. R. (2007). Manganese and infant mortality: well water may raise death rates in Bangladesh. Environ. Health. Perspect. 115, A363. doi: 10.1289/ehp.115-a363a
Bethke, C. M., Sanford, R. A., Kirk, M. F., Jin, Q., and Flynn, T. M. (2011). The thermodynamic ladder in geomicrobiology. Am. J. Sci. 311, 183–210. doi: 10.2475/03.2011.01
BGS and DPHE (2001). Arsenic Contamination of Groundwater in Bangladesh, eds D. G. Kinniburgh and P. L. Smedley, British Geological Survey Technical Report WC/00/19. Keyworth: British Geological Survey.
Bhattacharya, P., Chatterjee, D., and Jacks, G. (1997). Occurrence of Arsenic-contaminated groundwater in alluvial aquifers from delta plains, eastern India: options for safe drinking water supply. Int. J. Water Resour. D. 13, 79–92. doi: 10.1080/07900629749944
Bhattacharya, P., Hasan, M. A., Sracek, O., Smith, E., Ahmed, K. M., von Brömssen, M., et al. (2009). Groundwater chemistry and arsenic mobilization in the Holocene flood plains in south-central Bangladesh. Environ. Geochem. Health 31, 23–43. doi: 10.1007/s10653-008-9230-5
Bhattacharya, P., Jacks, G., Ahmed, K. M., Routh, J., and Khan, A. A. (2002). Arsenic in groundwater of the Bengal Delta Plain Aquifers in Bangladesh. Bull. Environ. Contam. Toxicol. 69, 538–545. doi: 10.1007/s00128-002-0095-5
Biswas, A., Bhattacharya, P., Mukherjee, A., Nath, B., Alexanderson, H., Kundu, A. K., et al. (2014). Shallow hydrostratigraphy in an arsenic affected region of Bengal Basin: implication for targeting safe aquifers for drinking water supply. Sci. Total. Environ. 485–486, 12–22. doi: 10.1016/j.scitotenv.2014.03.045
Biswas, A., Nath, B., Bhattacharya, P., Halder, D., Kundu, A. K., Mandal, U., et al. (2012a). Testing tubewell platform color as a rapid screening tool for arsenic and manganese in drinking water wells. Environ. Sci. Technol. 46, 434–440. doi: 10.1021/es203058a
Biswas, A., Nath, B., Bhattacharya, P., Halder, D., Kundu, A. K., Mandal, U., et al. (2012b). Hydrogeochemical contrast between brown and grey sand aquifers in shallow depth of Bengal Basin: consequences for sustainable drinking water supply. Sci. Tot. Environ. 431, 402–412. doi: 10.1016/j.scitotenv.2012.05.031
Bouchard, M. F., Sauve, S., Barbeau, B., Legrand, M., Brodeur, M., Bouffard, T., et al. (2011). Intellectual impairment in school-age children exposed to manganese from drinking water. Environ. Health Perspect. 119, 138–143. doi: 10.1289/ehp.1002321
Bouchard, M., Mergler, D., Baldwin, M., Panisset, M., Bowler, R., and Roels, H. A. (2007). Neurobehavioral functioning after cessation of manganese exposure: a follow-up after 14 years. Am. J. Ind. Med. 50, 831–840. doi: 10.1002/ajim.20407
Bundschuh, J., Litter, M. I., and Bhattacharya, P. (2010). Targeting arsenic-safe aquifers for drinking water supplies. Environ. Geochem. Health 32, 307–315. doi: 10.1007/s10653-010-9308-8
Burdige, D. J., and Gardner, K. G. (1998). Molecular weight distribution of dissolved organic carbon in marine sediment pore waters. Mar. Chem. 62, 45–64. doi: 10.1016/S0304-4203(98)00035-8
Burdige, D. J., and Homstead, J. (1994). Fluxes of dissolved organic carbon from Chesapeake Bay sediments. Geochim. Cosmochim. Acta 58, 3407–3424. doi: 10.1016/0016-7037(94)90095-7
Bureau of Indian Standards (2012). IS 10500: 2012 Drinking Water – Specification, 2nd Revision. New Delhi: BIS.
Buschmann, J., Berg, M., Stengel, C., and Sampson, M. L. (2007). Arsenic and manganese contamination of drinking water resources in Cambodia: coincidence of risk areas with low relief topography. Environ. Sci. Tech. 41, 2146–2152. doi: 10.1021/es062056k
Buschmann, J., Berg, M., Stengel, C., Winkel, L., Sampson, M. L., Trang, P. T. K., et al. (2008). Contamination of drinking water resources in the Mekong delta floodplains: arsenic and other trace metals pose serious health risks to population. Environ. Int. 34, 756–764. doi: 10.1016/j.envint.2007.12.025
Census of India (2011). Available online at: http://www.census2011.co.in/census/district/7-murshidabad.html
Champ, D. R., Gulens, J., and Jackson, R. E. (1979). Oxidation-reduction sequences in ground water flow systems. Can. J. Earth Sci. 16, 12–23. doi: 10.1139/e79-002
Chapelle, F. H., and Lovley, D. R. (1992). Competitive exclusion of sulfate reduction by Fe(III)-reducing bacteria: a mechanism for producing discrete zones of high-iron ground water. Ground Water 30, 29–36. doi: 10.1111/j.1745-6584.1992.tb00808.x
Coble, P. G. (1996). Characterization of marine and terrestrial DOM in seawater using excitation-emission matrix spectroscopy. Mar. Chem. 51, 325–346. doi: 10.1016/0304-4203(95)00062-3
Cory, R. M., and McKnight, D. M. (2005). Fluorescence spectroscopy reveals ubiquitous presence of oxidized and reduced quinones in dissolved organic matter. Environ. Sci. Technol. 39, 8142–8149. doi: 10.1021/es0506962
Datta, S. (2015). Hydrological aspects of arsenic contamination of groundwater in eastern India. Adv. Agron. 132, 75–137. doi: 10.1016/bs.agron.2015.02.001
Datta, S., Mailloux, B., Jung, H. B., Hoque, M. A., Stute, M., Ahmed, K. M., et al. (2009). Redox trapping of arsenic during groundwater discharge in sediments from the Meghna riverbank in Bangladesh. Proc. Natl. Acad. Sci. U.S.A. 106, 16930–16935. doi: 10.1073/pnas.0908168106
Datta, S., Neal, A. W., Mohajerin, T. J., Ocheltree, T., Rosenheim, B. E., White, C. D., et al. (2011). Perennial ponds are not an important source of water or dissolved organic matter to groundwaters with high arsenic concentrations in West Bengal, India. Geophys. Res. Lett. 38, 1–5. doi: 10.1029/2011GL049301
Deschamps, E., Ciminelli, V. S. T., Weidler, P. G., and Ramos, A. Y. (2003). Arsenic sorption onto soils enriched in Mn and Fe minerals. Clays Clay Minerals 51, 197–204. doi: 10.1346/CCMN.2003.0510210
Dixit, S., and Hering, J. G. (2003). Comparison of arsenic(V) and arsenic(III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ. Sci. Technol. 37, 4182–4189. doi: 10.1021/es030309t
Dowling, C. B., Poreda, R. J., Basu, A. R., and Peters, S. L. (2002). Geochemical study of arsenic release mechanisms in the Bengal Basin groundwater. Water. Resour. Res. 38, 12-1–12-18. doi: 10.1029/2001wr000968
Ehlert, K., Mikutta, C., and Kretzchmar, R. (2014). Impact of birnessite on arsenic and iron speciation during microbial reduction of arsenic-bearing ferrihydrite. Environ. Sci. Technol. 48, 11320–11329. doi: 10.1021/es5031323
Ehlert, K., Mikutta, C., and Kretzchmar, R. (2016). Effects of manganese oxide on arsenic reduction and leaching from contaminated floodplain soil. Environ. Sci. Technol. 50, 9251–9261. doi: 10.1021/acs.est.6b01767
Farooq, S. H., Chandrasekharam, D., Norra, S., Berner, Z., Eiche, E., Thambidurai, P., et al. (2011). Temporal variations in arsenic concentration in the groundwater of Murshidabad District, West Bengal, India. Environ. Earth Sci. 62, 223–232. doi: 10.1007/s12665-010-0516-4
Fellman, J. B., Hood, E., and Spencer, R. G. M. (2010). Fluorescence spectroscopy opens new windows into dissolved organic matter dynamics in freshwater ecosystems: a review. Limnol. Oceanogr. 55, 2452–2462. doi: 10.4319/lo.2010.55.6.2452
Fendorf, S., Michael, H., and van Geen, A. (2010). Spatial and temporal variations of groundwater arsenic in south and southeast Asia. Science 328, 1123–1127. doi: 10.1126/science.1172974
Foster, A. L., Brown, G. E., and Parks, G. A. (2003). X-ray adsorption fine structure study of As(V) and Se(IV) sorption complexes on hydrous Mn oxides. Geochim. Cosmochim. Acta 67, 1937–1953. doi: 10.1016/S0016-7037(02)01301-7
Frisbie, S. H., Mitchell, E. J., Dustin, H., Maynard, D. M., and Sarkar, B. (2012). World Health Organization discontinues its drinking-water guideline for manganese. Environ. Health. Perspect. 120, 775–778. doi: 10.1289/ehp.1104693
Frisbie, S. H., Mitchell, E. J., Mastera, L. J., Maynard, D. M., Yusuf, A. Z., Siddiq, M. Y., et al. (2009). Public health strategies for western Bangladesh that address arsenic, manganese, uranium, and other toxic elements in drinking water. Environ. Health Perspect. 117, 410–416. doi: 10.1289/ehp.11886
Gavin, K. G., Graham, M., Kirika, A., and Britton, A. (2001). “Manganese-humic interactions in the catchment, water and sediment of Loch Bradan, S.W. Scotland,” in Understanding and Managing Organic Matter in Soils, Sediments, and Waters, eds R. S. Swift and K. M. Spark (St. Paul, MN: International Humic Substances Society), 437–443.
Golden, D. C., Chen, C. C., and Dixon, J. B. (1986). Ion exchange, thermal transformations and oxidizing properties of birnessite. Clays Clay Minerals 34, 511–520. doi: 10.1346/CCMN.1986.0340503
Gounot, A. M. (1994). Microbial oxidation and reduction of manganese: consequences in groundwater and applications. FEMS Microbiol. Rev. 14, 339–349. doi: 10.1111/j.1574-6976.1994.tb00108.x
Graham, M. C., Gavin, K. G., Farmer, J. G., Kirika, A., and Britton, A. (2002). Processes controlling the retention and release of manganese in the organic rich catchment of Loch Bradan. Appl. Geochem. 17, 1061–1067. doi: 10.1016/S0883-2927(02)00012-4
Grazuleviciene, R., Nadisauskiene, R., Buinauskiene, J., and Grazulevicius, T. (2009). Effects of elevated levels of manganese and iron in drinking water on birth outcomes. Pol. J. Environ. Stud. 18, 819–825.
Guo, H., Stüben, D., and Berner, Z. (2007). Adsorption of arsenic(III) and arsenic(V) from groundwater using natural siderite as the adsorbent. J. Colloid Interface Sci. 315, 47–53. doi: 10.1016/j.jcis.2007.06.035
Hafeman, D., Factor-Litvak, P., Cheng, Z., van Geen, A., and Ahsan, H. (2007). Association between manganese exposure through drinking water and infant mortality in Bangladesh. Environ. Health Perspect. 115, 1107–1112. doi: 10.1289/ehp.10051
Hansen, A. M., Kraus, T., Pellerin, B., Fleck, J., Downing, B., and Bergamaschi, B. (2016). Optical properties of dissolved organic matter (DOM): effects of biological and photolytic degradation. Limnol. Oceanogr. 61, 1015–1032. doi: 10.1002/lno.10270
Helms, J. R., Stubbins, A., Ritchie, J. D., Minor, E. C., Kieber, D. J., and Mopper, K. (2008). Absorption spectral slopes and slope ratios as indicators of molecular weight, source, and photobleaching of chromophoric dissolved organic matter. Limnol. Oceanogr. 53, 955–969. doi: 10.4319/lo.2008.53.3.0955
Hem, J. D. (1985). “Study and interpretation of the chemical characteristics of natural water, 3rd Edn.,” in US Geological Survey Water-Supply Paper 2254.
Hering, J. G., and Kneebone, P. E. (2002). “Biogeochemical controls on arsenic occurrence and mobility in water supplies,” in Environmental Chemistry of Arsenic, ed W. T. Frankenberger (New York, NY: Marcel Dekker, Inc.), 183–215.
Hoque, M. A., Burgess, W. G., Shamsudduha, M., and Ahmed, K. M. (2011). Delineating low-arsenic groundwater environments in the Bengal Aquifer System, Bangladesh. Appl. Geochem. 26, 614–623. doi: 10.1016/j.apgeochem.2011.01.018
Horneman, A., van Geen, A., Kent, D. V., Mathe, P. E., Zheng, Y., Dhar, R. K., et al. (2004). Decoupling of As and Fe release to Bangladesh groundwater under reducing conditions. Part I: evidence from sediment profiles. Geochim. Cosmochim. Acta 68, 3459–3473. doi: 10.1016/j.gca.2004.01.026
Hossain, M., Rahman, S. N., Bhattacharya, P., Jacks, G., Saha, R., and Rahman, M. (2015). Sustainability of arsenic mitigation interventions–an evaluation of different alternative safe drinking water options provided in Matlab, an arsenic hot spot in Bangladesh. Front. Environ. Sci. 3:30. doi: 10.3389/fenvs.2015.00030
Hug, S. J., Gaertner, D., Roberts, L. C., Schirmer, M., Ruettimann, T., Rosenberg, T. M., et al. (2011). Avoiding high concentrations of arsenic, manganese, and salinity in deep tubewells in Munshiganj District, Bangladesh. Appl. Geochem. 26, 1077–1085. doi: 10.1016/j.apgeochem.2011.03.012
Jain, A., and Loeppert, R. H. (2000). Effect of competing anions on the adsorption of arsenate and arsenite by ferrihydrite. J. Environ. Qual. 29, 1422–1430. doi: 10.2134/jeq2000.00472425002900050008x
Jakobsen, R., and Postma, D. (1999). Redox zoning, rates of sulfate reduction and interactions with Fe-reduction and methanogenesis in a shallow sandy aquifer, Romo, Denmark. Geochim. Cosmochim. Acta 63, 137–151. doi: 10.1016/S0016-7037(98)00272-5
Jiang, J., and Kappler, A. (2008). Kinetics of microbial and chemical reduction of humic substances: implications for electron shuttling. Environ. Sci. Technol. 42, 3563–3569. doi: 10.1021/es7023803
Kappler, A., Benz, M., Schink, B., and Brune, A. (2004). Electron shuttling via humic acids in microbial iron(III) reduction in a freshwater sediment. FEMS Microbiol. Ecol. 47, 85–92. doi: 10.1016/S0168-6496(03)00245-9
Khan, K., Wasserman, G. A., Liu, X., Ahmed, E., Parvez, F., Slavkovich, V., et al. (2012). Manganese exposure from drinking water and children's academic achievement. J. Neurotoxicol. 33, 91–97. doi: 10.1016/j.neuro.2011.12.002
Kirk, M. F., Holm, T. R., Park, J., Jin, Q., Sanford, R. A., Fouke, B. W., et al. (2004). Bacterial sulfate reduction limits natural arsenic contamination in groundwater. Geology 32, 953–956. doi: 10.1130/G20842.1
Kshetrimayum, K. S., and Hegeu, H. (2016). The state of toxicity and cause of elevated iron and manganese concentrations in surface water and groundwater around Naga Thrust of Assam-Arakan basin, Northeastern India. Environ. Earth Sci. 75:604. doi: 10.1007/s12665-016-5372-4
Kulkarni, H. V., Mladenov, N., Johannesson, K. H., and Datta, S. (2016). Contrasting dissolved organic matter quality in groundwater in Holocene and Pleistocene aquifers and implications for influencing arsenic mobility. Appl. Geochem. 77, 194–205. doi: 10.1016/j.apgeochem.2016.06.002
Lindberg, R. D., and Runnells, D. D. (1984). Ground water redox reactions: an analysis of equilibrium state applied to Eh measurements and geochemical modeling. Science 225, 925–927. doi: 10.1126/science.225.4665.925
Liu, G., Fernandez, A., and Cai, Y. (2011). Complexation of arsenite with humic acid in the presence of ferric iron. Environ. Sci. Technol. 45, 3210–3216. doi: 10.1021/es102931p
Ljung, K. S., Kippler, M. J., Goessler, W., Grander, G. M., Nermell, B. M., and Vahter, M. E. (2009). Maternal and early life exposure to manganese in rural Bangladesh. Environ Sci. Technol. 43, 2595–2601. doi: 10.1021/es803143z
Ljung, K. S., and Vahter, M. (2007). Time to re-evaluate the guideline value for manganese in drinking water? Environ. Health Perspect. 115, 1533–1538. doi: 10.1289/ehp.10316
Loeppert, R. H., and W. P., Inskeep (1996). “Iron,” in Methods of Soil Analysis. Part 3. Chemical Methods, SSSA Book Series 5, eds D. L. Sparks, A. L. Page, P. A. Helmke and R. H. Loeppert (Madison, WI: SSSA), 639–664.
Lovley, D. R., Coates, J. D., Blunt-Harris, E. L., Phillips, E. J. P., and Woodward, J. C. (1996). Humic substances as electron acceptors for microbial respiration. Nature 382, 445–448. doi: 10.1038/382445a0
Lovley, D. R., Fraga, J. L., Blunt-Harris, E. L., Hayes, L., Phillips, E. J. P., and Coates, J. D. (1998). Humic substances as a mediator for microbially catalyzed metal reduction. Acta. Hydrochim. Hydrobiol. 26, 152–157.
Lovley, D. R., Fraga, J. L., Coates, J. D., and Blunt-Harris, E. L. (1999). Humics as an electron donor for anaerobic respiration. Environ. Microbiol. 1, 89–98. doi: 10.1046/j.1462-2920.1999.00009.x
Lovley, D. R., and Goodwin, S. (1988). Hydrogen concentrations as an indicator of the predominant terminal electron-accepting reactions in aquatic sediments. Geochim. Cosmochim. Acta 52, 2993–3003. doi: 10.1016/0016-7037(88)90163-9
Lovley, D. R., Holmes, D. E., and Nevin, K. P. (2004). Dissimilatory Fe(III) and Mn(IV) reduction. Adv. Microb. Physiol. 49, 219–286. doi: 10.1016/S0065-2911(04)49005-5
Lovley, D. R., and Phillips, E. J. P. (1988). Novel mode of microbial energy metabolism-organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl. Environ. Microbiol. 54, 1472–1480.
Majumder, S., Datta, S., Nath, B., Neidhardt, H., Sarkar, S., Roman-Ross, G., et al. (2016). Monsoonal influence on variation of hydrochemistry and isotopic signatures: Implications for associated arsenic release in groundwater. J. Hydrol. 535, 407–417. doi: 10.1016/j.jhydrol.2016.01.052
Manning, B. A., Fendorf, S. E., Bostick, B., and Suarez, D. L. (2002). Arsenic (III) oxidation and arsenic (V) adsorption reactions on synthetic birnessite. Environ. Sci. Technol. 36, 976–981. doi: 10.1021/es0110170
Manning, B. A., and Goldberg, S. (1997). Adsorption and stability of arsenic (III) at the clay-mineral-water interface. Environ. Sci. Technol. 31, 2005–2011. doi: 10.1021/es9608104
Marshall, K. C. (1979). “Biogeochemistry of manganese minerals,” in Biogeochemical Cycling of Mineral-Forming Elements, eds P. A. Trudinger and D. J. Swaine (Amsterdam: Elsevier/North Holland Publishing Co.), 253–286.
McArthur, J. M., Banerjee, D. M., Hudson-Edwards, K. A., Mishra, R., Purohit, R., Ravenscroft, P., et al. (2004). Natural organic matter in sedimentary basins and its relation to arsenic in anoxic ground water: the example of West Bengal and its worldwide implications. Appl. Geochem. 19, 1255–1293. doi: 10.1016/j.apgeochem.2004.02.001
McArthur, J. M., Nath, B., Banerjee, D. M., Purohit, R., and Grassineau, N. (2011). Palaeosol control on groundwater flow and pollutant distribution: the example of arsenic. Environ. Sci. Technol. 45, 1376–1383. doi: 10.1021/es1032376
McArthur, J. M., Ravenscroft, P., Banerjee, D. M., Milsom, J., Hudson-Edwards, K. A., Sengupta, S., et al. (2008). How paleosols influence groundwater flow and arsenic pollution: a model from the Bengal Basin and its worldwide implication. Water Resour. Res. 44:W11411. doi: 10.1029/2007WR006552
McArthur, J. M., Ravenscroft, P., Safiullah, S., and Thirlwall, M. F. (2001). Arsenic in groundwater: testing pollution mechanisms for sedimentary aquifers in Bangladesh. Water Resour. Res. 37, 109–117. doi: 10.1029/2000WR900270
McArthur, J. M., Sikdar, P. K., Hoque, M. A., and Ghosal, U. (2012a). Waste-water impacts on groundwater: Cl/Br ratios and implications for arsenic pollution of groundwater in the Bengal Basin and Red River Basin, Vietnam. Sci. Total Environ. 437, 390–402. doi: 10.1016/j.scitotenv.2012.07.068
McArthur, J. M., Sikdar, P. K., Nath, B., Grassineau, N., Marshall, J., and Banerjee, D. M. (2012b). Sedimentological Control on Mn, and Other Trace Elements, In Groundwater of the Bengal Delta. Environ. Sci. Technol. 46, 669−676. doi: 10.1021/es202673n
McGuire, J. T., Long, D. T., Klug, M. J., Haack, S. K., and Hyndman, D. W. (2002). Evaluating behavior of oxygen, nitrate and sulfate during recharge and quantifying reduction rates in a contaminated aquifer. Environ. Sci. Technol. 36, 2693–2700. doi: 10.1021/es015615q
McKnight, D. M., Boyer, E. W., Westerhoff, P. K., Doran, P. T., Kulbe, T., and Andersen, D. T. (2001). Spectrofluorometric characterization of dissolved organic matter for indication of precursor organic material and aromaticity. Limnol. Oceanogr. 46, 38–48. doi: 10.4319/lo.2001.46.1.0038
Mladenov, N., Zheng, Y., Miller, M. P., Nemergut, D. R., Legg, T., Simone, B., et al. (2010). Dissolved organic matter sources and consequences for iron and arsenic mobilzation in Bangladesh aquifers. Environ. Sci. Technol. 44, 123–128. doi: 10.1021/es901472g
Mladenov, N., Zheng, Y., Simone, B., Bilinksi, T. M., McKnight, D. M., Nemergut, D., et al. (2015). Dissolved organic matter quality in a shallow aquifer of Bangladesh: implications for arsenic mobility. Environ. Sci. Technol. 49, 10815–10824. doi: 10.1021/acs.est.5b01962
Mohajerin, T., Neal, A. W., Telfeyan, K., Sankar, M. S., Ford, S., Yang, N., et al. (2014). Geochemistry of tungsten and arsenic in aquifer systems: a comparative study of groundwaters from West Bengal, India, and Nevada, USA. Wat. Air. Soil. Poll. 225, 1792. doi: 10.1007/s11270-013-1792-x
Morgan, J. J., and Stumm, W. (1964). The role of multivalent metal oxides in limnological transformations as exemplified by iron and manganese. J. Water Poll. Contr. Fed. 36, 276–277.
Mukherjee, A. B., and Bhattacharya, P. (2001). Arsenic in groundwater in the Bengal Delta Plain: slow poisoning in Bangladesh. Environ. Rev. 9, 189–220. doi: 10.1139/a01-007
Mukherjee, A., Fryar, A. E., and Howell, P. D. (2007). Regional hydrostratigraphy and groundwater flow modeling in the arsenic-affected areas of the western Bengal basin, West Bengal, India. Hydrogeology 15, 1397–1418. doi: 10.1007/s10040-007-0208-7
Nath, B., Chakraborty, S., Burnol, A., Stüben, D., Chatterjee, D., and Charlet, L. (2009). Mobility of arsenic in the sub-surface environment: an integrated hydrogeochemical study and sorption model of the sandy aquifer materials. J. Hydrol. 364, 236–248. doi: 10.1016/j.jhydrol.2008.10.025
Nealson, K. H., and Saffarini, D. (1994). Iron and manganese in anaerobic respiration: environmental significance, physiology, and regulation. Ann. Rev Microbiol. 48, 311–343. doi: 10.1146/annurev.mi.48.100194.001523
Neidhardt, H., Biswas, A., Freikowski, D., Majumder, S., Chetterjee, D., and Berner, Z. A. (2013). Reconstructing the sedimentation history of the Bengal Delta Plain by means of geochemical and stable isotopic data. Appl. Geochem. 36, 70–82. doi: 10.1016/j.apgeochem.2013.06.017
Nickson, R. T., McArthur, J. M., Burgess, W. G., Ahmed, K. M., Ravenscroft, P., and Rahman, M. (1998). Arsenic poisoning in Bangladesh groundwater. Nature 395:338. doi: 10.1038/26387
Nickson, R. T., McArthur, J. M., Ravenscroft, P., Burgess, W. G., and Ahmed, K. M. (2000). Mechanism of arsenic release to groundwater, Bangladesh and West Bengal. Appl. Geochem. 15, 403–413. doi: 10.1016/S0883-2927(99)00086-4
Ohno, T. (2002). Response to comment on “Fluorescence inner-filtering correction for determining the humification index of dissolved organic matter”. Environ. Sci. Technol. 36:4196. doi: 10.1021/es020113d
Ono, K., Komai, K., and Yamada, M. (2002). Myoclonic involuntary movement associated with chronic manganese poisoning. J. Neurol. Sci. 199, 93–96. doi: 10.1016/S0022-510X(02)00111-9
Oscarson, D. W., Huang, P. M., Defosse, C., and Herbillon, A. (1981). Oxidative power of Mn(IV) and Fe(III) oxides with respect to As(III) in terrestrial and aquatic environments. Nature 291, 50–51. doi: 10.1038/291050a0
Parlanti, E., Worz, K., Geoffroy, L., and Lamotte, M. (2000). Dissolved organic matter fluorescence spectroscopy as a tool to estimate biological activity in a coastal zone submitted to anthropogenic inputs. Org. Geochem. 31, 1765–1781. doi: 10.1016/S0146-6380(00)00124-8
Postma, D., and Jakobsen, R. (1996). Redox zonation: equilibrium constraints on the Fe(III)/ - reduction interface. Geochim. Cosmochim. Acta 60, 3169–3175. doi: 10.1016/0016-7037(96)00156-1
Premarathna, H. L., McLaughlin, M. J., Kirby, J. K., Hettiarachchi, G. M., Beak, D., Stacey, S., et al. (2010). Potential availability of fertilizer selenium in field capacity and submerged soils. Soil Sci. Soc. Am. J. 74, 1589–1596. doi: 10.2136/sssaj2009.0416
Ravenscroft, P., Burgess, W. G., Ahmed, K. M., Burren, M., and Perrin, J. (2005). Arsenic in groundwater of the Bengal Basin, Bangladesh: Distribution, field relations, and hydrogeological setting. Hydrogeol. J. 13, 727–751. doi: 10.1007/s10040-003-0314-0
Rittman, B. E., and McCarty, P. L. (2001). Environmental Biotechnology: Principles and Applications. Boston, MA: McGraw-Hill Higher Education.
Roychowdhury, T., Uchino, T., Tokunaga, H., and Ando, M. (2002). Arsenic and other heavy metals in soils from an arsenic-affected area of West Bengal, India. Chemosphere 49, 605–618. doi: 10.1016/S0045-6535(02)00309-0
Sankar, M. S. (2013). Geochemical Significance of Arsenic and Manganese Toxicity in Groundwaters from Murshidabad District, West Bengal, India, [master's thesis]. Manhattan, KS: Kansas State University.
Sankar, M. S., Vega, M. A., Defoe, P. P., Kibria, M. G., Ford, S., Telfeyan, K., et al. (2014). Elevated arsenic and manganese in groundwaters of Murshidabad, West Bengal, India. Sci. Tot. Environ. 488–489, 570–579. doi: 10.1016/j.scitotenv.2014.02.077
Scott, D. T., McKnight, D. M., Blunt-Harris, E. L., Kolesar, S. E., and Lovley, D. R. (1998). Quinone moieties act as electron acceptors in the reduction of humic substances by humics-reducing microorganisms. Environ. Sci. Technol. 32, 2984–2989. doi: 10.1021/es980272q
Sharma, P., Ofner, J., and Kappler, A. (2010). Formation of binary and ternary colloids and dissolved complexes of organic matter, Fe and As. Environ. Sci. Technol. 44, 4479–4485. doi: 10.1021/es100066s
Shrivastava, A., Barla, A., Yadav, H., and Bose, S. (2015). Arsenic contamination in shallow groundwater and agricultural soil of Chakdaha block, West Bengal, India. Front. Environ. Sci. 2:50. doi: 10.3389/fenvs.2014.00050
Smedley, P. L., and Kinniburgh, D. G. (2002). A review of the source, behaviour and distribution of arsenic in natural waters. Appl. Geochem. 17, 517–568. doi: 10.1016/S0883-2927(02)00018-5
Spangler, A. H., and Spangler, J. G. (2009). Groundwater manganese and infant mortality rate by county in North fCarolina: an ecological analysis. EcoHealth 6, 596–600. doi: 10.1007/s10393-010-0291-4
Spencer, R. G. M., Bolton, L., and Baker, A. (2007). Freeze/thaw and pH effects on freshwater dissolved organic matter fluorescence and absorbance properties from a number of UK locations. Water Res. 41, 2941–2950. doi: 10.1016/j.watres.2007.04.012
Stedmon, C. A., and Bro, R. (2008). Characterizing dissolved organic matter fluorescence with parallel factor analysis: a tutorial. Linmol. Oceanagr. Methods 6, 572–579. doi: 10.4319/lom.2008.6.572
Stedmon, C. A., Markager, S., and Bro, R. (2003). Tracing dissolved organic matter in aquatic environments using a new approach to fluorescence spectroscopy. Mar. Chem. 82, 239–254. doi: 10.1016/S0304-4203(03)00072-0
Stefansson, A., Arnorsson, S., and Sveinbjörnsdottir, A. E. (2005). Redox reaction and potentials in natural water at disequilibrium. Chem. Geol. 221, 289–311. doi: 10.1016/j.chemgeo.2005.06.003
Stollenwerk, K. G., Breit, G. N., Welch, A. H., Yount, J. C., Whitney, J. W., Foster, A. L., et al. (2007). Arsenic attenuation by oxidized aquifer sediments in Bangladesh. Sci. Tot. Environ. 379, 133–150. doi: 10.1016/j.scitotenv.2006.11.029
Stumm, W., and Morgan, J. J. (1981). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. New York, NY: Wiley.
Sun, X., Doner, H. E., and Zavarin, M. (1999). Spectroscopy study of arsenite oxidation on Mn-xubstituted goethite. Clays Clay Minerals 47, 474–480. doi: 10.1346/CCMN.1999.0470409
Sunda, W. G., and Kieber, D. J. (1994). Oxidation of humic substances by manganese oxides yields low-molecular-weight organic substrates. Nature 367, 62–64. doi: 10.1038/367062a0
Tebo, B. M., Bargar, J. R., Clement, B. G., Dick, G. J., Murray, K. J., Parker, D., et al. (2004). Biogenic manganese oxides: properties and mechanisms of formation. Annu. Rev. Earth Planet. Sci. 32, 287–328. doi: 10.1146/annurev.earth.32.101802.120213
Tebo, B. M., Clement, B. G., Dick, G. J., Hurst, C. J., Crawford, R. L., Garland, J. L., et al. (2007). “Biotransformations of manganese,” in Manual of Environmental Microbiology, 3rd Edn., eds C. J. Hurst, R. L. Crawford, J. L. Garland, D. A. Lipson, A. L. Mills, and L. D. Stetzenbach (Washington, DC: ASM Press) 1223–1238. doi: 10.1128/9781555815882.ch100
Tebo, B. M., Ghiorse, W. C., van Waasbergen, L. G., Siering, P. L., and Caspi, R. (1997). “Bacterially-mediated mineral formation: insights into manganese(II) oxidation from molecular genetic and biochemical studies,” in Geomicrobiology: Interactions Between Microbes and Minerals, eds J. F. Banfield and K. H. Nealson (Washington, DC: Mineralogical Society of America), 225–266.
Toner, B., Manceau, A., Webb, S. M., and Sposito, G. (2006). Zinc sorption to biogenic hexagonal-birnessite particles within a hydrated bacterial biofilm. Geochim. Cosmochim. Acta 70, 27–43. doi: 10.1016/j.gca.2005.08.029
Tournassat, C., Charlet, L., Bosbach, D., and Manceau, A. (2002). Arsenic(III) oxidation by birnessite and precipiation of manganese(II) arsenate. Environ. Sci. Technol. 36, 493–500. doi: 10.1021/es0109500
Tupas, L. M., Popp, B. N., and Karl, D. M. (1994). Dissolved organic carbon in oligotrophic waters: experiments on sample preservation, storage, and analysis. Mar. Chem. 45, 207–216. doi: 10.1016/0304-4203(94)90004-3
van Geen, A., Zheng, Y., Versteeg, R., Stute, M., Horneman, A., Dhar, R., et al. (2003). Spatial variability of arsenic in 6000 tube wells in a 25 km2 area of Bangladesh. Water Resour. Res. 39:1140. doi: 10.1029/2002WR001617
Vodyanitskii, Y. N. (2009). Mineralogy and geochemistry of manganese: a review of publications. Eurasian Soil Sci. 42, 1170–1178. doi: 10.1134/S1064229309100123
von Brömssen, M., Jakariya, M., Bhattacharya, P., Ahmed, K. M., Hasan, M. A., Sracek, O., et al. (2007). Targeting low-arsenic aquifers in groundwater of Matlab, Upazila, Southeastern Bangladesh. Sci. Tot. Environ. 379, 121–132. doi: 10.1016/j.scitotenv.2006.06.028
von Brömssen, M., Larsson, S. H., Bhattacharya, P., Hasan, M. A., Ahmed, K. M., Jakariya, M., et al. (2008). Geochemical characterization of shallow aquifer sediments of Matlab Upazila, Southeastern Bangladesh - implications for targeting low-As aquifers. J. Cont. Hydrol. 99, 137–149. doi: 10.1016/j.jconhyd.2008.05.005
Wasserman, G. A., Liu, X., Factor-Litvak, P., Gardner, J. M., and Graziano, J. H. (2008). Developmental impacts of heavy metals and undernutrition. Basic Clin. Pharmacol. Toxicol. 102, 212–217. doi: 10.1111/j.1742-7843.2007.00187.x
Wasserman, G. A., Liu, X., Parvez, F., Ahsan, H., Levy, D., Factor-Litvak, P., et al. (2006). Water manganese exposure and children's intellectual function in Araihazar, Bangladesh. Environ. Health Perspect. 114:124. doi: 10.1289/ehp.8030
Wasserman, G. A., Liu, X., Parvez, F., Factor-Litvak, P., Ahsan, H., Levy, D., et al. (2011). Arsenic and manganese exposure and children's intellectual function. Neurotoxicology 32, 450–457. doi: 10.1016/j.neuro.2011.03.009
Weishaar, J. L., Aiken, G. R., Bergamaschi, B., Fram, M. S., Fujii, R., and Mopper, K. (2003). Evaluation of specific ultraviolet absorbance as an indicator of the chemical composition and reactivity of dissolved organic carbon. Environ. Sci. Technol. 37, 4702–4708. doi: 10.1021/es030360x
Wersin, P., Charlet, L., Karthein, R., and Stumm, W. (1989). From adsorption to precipitation: sorption of Mn(II) on FeCO3(s). Geochim. Cosmochim. Acta 53, 2787–2796. doi: 10.1016/0016-7037(89)90156-7
Williams, C. J., Frost, P. C., and Xenopoulos, M. A. (2013). Beyond best management practices: pelagic biogeochemical dynamics in urban stormwater ponds. Ecol. App. 23, 1384–1395. doi: 10.1890/12-0825.1
Wilson, H. F., and Xenopoulos, M. (2008). Effects of agricultural land use on the composition of fluvial dissolved organic matter. Nat. Geosci. 2, 37–41. doi: 10.1038/ngeo391
Wolf, M., Kappler, A., Jiang, J., and Meckenstock, R. U. (2009). Effects of humic substances and quinones at low concentrations on ferrihydrite reduction by Geobacter metallireducens. Environ. Sci. Technol. 43, 5679–5685. doi: 10.1021/es803647r
Wood, R. J. (2009). Manganese and birth outcome. Nutr. Rev. 57, 415–420. doi: 10.1111/j.1753-4887.2009.00214.x
Woolf, A., Wright, R., Amarasiriwardena, C., and Bellinger, D. (2002). A child with chronic manganese exposure from drinking water. Environ. Health Perspect. 110:613. doi: 10.1289/ehp.02110613
Wu, Y., Li, W., and Sparks, D. (2015). The effects of iron(II) on the kinetics of arsenic oxidation and sorption on manganese oxides. J. Colloid Interface Sci. 457, 319–328. doi: 10.1016/j.jcis.2015.07.022
Yazbeck, C., Moreau, T., Sahuquillo, J., Takser, L., and Huel, G. (2006). Effect of maternal manganese blood levels on erythrocyte calcium-pump activity in newborns. Sci. Tot. Environ. 354, 28–34. doi: 10.1016/j.scitotenv.2004.12.012
Ying, S. C., Kocar, B. D., Griffis, S. D., and Fendorf, S. (2011). Competitive microbially and Mn oxide mediated redox processes controlling arsenic speciation and partitioning. Environ. Sci. Technol. 45, 5572–5579. doi: 10.1021/es200351m
Young, L. B., and Harvey, H. H. (1992). The relative importance of manganese and iron oxides and organic matter in the sorption of trace metals by surficial lake sediments. Geochim. Cosmochim. Acta 56, 1175–1186. doi: 10.1016/0016-7037(92)90055-N
Zota, A. R., Ettinger, A. S., Bouchard, M., Amarasiriwardena, C. J., Schwartz, J., Hu, H., et al. (2009). Maternal blood manganese levels and infant birth weight. Epidemiology 20, 367–373. doi: 10.1097/EDE.0b013e31819b93c0
Keywords: manganese, organic matter, West Bengal, arsenic, shallow aquifer
Citation: Vega MA, Kulkarni HV, Mladenov N, Johannesson K, Hettiarachchi GM, Bhattacharya P, Kumar N, Weeks J, Galkaduwa M and Datta S (2017) Biogeochemical Controls on the Release and Accumulation of Mn and As in Shallow Aquifers, West Bengal, India. Front. Environ. Sci. 5:29. doi: 10.3389/fenvs.2017.00029
Received: 22 March 2017; Accepted: 29 May 2017;
Published: 23 June 2017.
Edited by:
Ondra Sracek, Palacký University, Olomouc, CzechiaReviewed by:
Marco Petitta, Sapienza Università di Roma, ItalyTomas Navratil, Institute of Geology (ASCR), Czechia
Copyright © 2017 Vega, Kulkarni, Mladenov, Johannesson, Hettiarachchi, Bhattacharya, Kumar, Weeks, Galkaduwa and Datta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Saugata Datta, c2RhdHRhQGtzdS5lZHU=
 Michael A. Vega
Michael A. Vega Harshad V. Kulkarni
Harshad V. Kulkarni Natalie Mladenov
Natalie Mladenov Karen Johannesson
Karen Johannesson Ganga M. Hettiarachchi
Ganga M. Hettiarachchi Prosun Bhattacharya
Prosun Bhattacharya Naresh Kumar
Naresh Kumar Joseph Weeks
Joseph Weeks Madhubhashini Galkaduwa
Madhubhashini Galkaduwa Saugata Datta
Saugata Datta