
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci. , 01 April 2015
Sec. Environmental Toxicology
Volume 3 - 2015 | https://doi.org/10.3389/fenvs.2015.00021
This article is part of the Research Topic Redox homeostasis managers in plants under environmental stresses View all 18 articles
The balance between accumulation of stress-induced polyamines and reactive oxygen species (ROS) is arguably a critical factor in plant tolerance to salt stress. Polyamines are compounds, which accumulate in plants under salt stress and help maintain cellular ROS homeostasis. In this review we first outline the role of polyamines in mediating salt stress responses through their modulation of redox homeostasis. The two proposed roles of polyamines in regulating ROS—as antioxidative molecules and source of ROS synthesis—are discussed and exemplified with recent studies. Second, the proposed function of polyamines as modulators of ion transport is discussed in the context of plant salt stress. Finally, we highlight the apparent connection between polyamine accumulation and programmed cell death induction during stress. Thus, polyamines have a complex functional role in regulating cellular signaling and metabolism during stress. By focusing future efforts on how polyamine accumulation and turnover is regulated, research in this area may provide novel targets for developing stress tolerance.
Global climate change and agronomic practices have contributed to increased soil salinity, which currently affects an estimated 45 million hectares of irrigated land (Rengasamy, 2010). Salt stress limits crop productivity and is imposed by an accumulation of cations (Na+, K+, Mg2+, Ca2+) and anions (Cl−, SO2−4, HCO−3) originating from water-soluble salts such as Na2SO4, NaHCO3, NaCl, and MgCl2 as well as less water-soluble salts including CaSO4, MgSO4, and CaCO3.These salts accumulate due to factors such as mineral erosion and crop irrigation with mineralized water or ocean water (Todorova et al., 2013).
High salt concentrations in soil cause both hyperionic and hyperosmotic stress in the intracellular environment. During the initial stages of salt stress, the high external solute concentration decreases the cellular water potential, which eventually imposes turgor loss and pleiotropic physiological responses including stomatal closure, growth inhibition, reduced pollen viability, inhibition of photosynthetic enzyme activity, sucrose accumulation, and inactivation of photosynthetic electron transport (Munns and Tester, 2008; Chaves et al., 2009; Biswal et al., 2011; Silva et al., 2011; Mittal et al., 2012; Shu et al., 2012; Jajoo, 2013). Long-term salt stress results in hyperaccumulation of Na+ leading to suppression of enzymatic activity, increased H2O2 and lipid peroxidation that ultimately causes leaf senescence (Sairam et al., 2002; Chinnusamy and Zhu, 2003; Allu et al., 2014).
Under normal conditions, the cytosol contains 100–200 mM of K+ and 1–10 mM of Na+ (Taiz and Zeiger, 2002). Excess NaCl is the most common cause of salt stress in plants and induces overaccumulation of Na+ and Cl− and depletion of K+ ions in the cell. This imbalance in the Na+:K+ ratio is a result of the competition between the ions for transport into the cell and is thought to produce detrimental effects due to changes in osmotic potential, nutrient limitation and ionic toxicity. Plants counteract these effects using multiple strategies including: (i) producing osmolytes like soluble sugars, organic acids, free amino acids, and accumulating potassium ions (Ahmad and Sharma, 2008; Ahmad et al., 2012), (ii) activating transporters that export sodium from the cell, (iii) limiting Na+ uptake into roots and leaves, (iv) sequestering Na+ ions into subcellular compartments, (v) altering photosynthetic rates, (vi) changing membrane structure, (vii) inducing antioxidative enzymes, and (viii) decreasing stomatal conductance (Jithesh et al., 2006; Ozgur et al., 2013). In addition, plant cells rapidly accumulate reactive oxygen species (ROS) in response to salt and other stresses, a response widely known as the “oxidative burst” (Mittler, 2002; Miller et al., 2008). The oxidative burst has an important role in inducing signaling events and is dependent on enzymes located in several subcellular compartments (Foyer and Noctor, 2005; Baxter et al., 2014). However, it is essential that ROS production be regulated, as excess ROS accumulation results in membrane lipid peroxidation, DNA damage, protein denaturation, carbohydrate oxidation, pigment breakdown, and ultimately leads to cell death (Scandalios, 1993; Noctor and Foyer, 1998). To counteract the potentially damaging effects of the oxidative burst, plants produce a diverse set of antioxidants whose regulation is not yet fully understood. While the interplay between ROS turnover and antioxidant accumulation during stress is quite complex, it is essential to understand how this system works for its potential in enhancing plant stress tolerance (Noctor and Foyer, 1998).
ROS are highly reactive forms of molecular oxygen and include the hydroxyl radical (HO·), superoxide (O2·−), hydrogen peroxide (H2O2), and singlet oxygen (1O2) (Dowling and Simmons, 2009; Shapiguzov et al., 2012). The reactivity and half-life of different ROS species are correlated to their mobility and diffusion distance in the cellular space. Among the ROS species present in plants, hydrogen peroxide is the most stable having a half-life of 1 ms, whereas singlet oxygen (1O2), superoxide (O·−2) and hydroxyl radicals (OH•) are short-lived species with half-lives of 1–4 μs to 1 nanosecond (Gechev et al., 2006; Moller et al., 2007). Although numerous subcellular compartments contribute to ROS production, the major sites of ROS generation include the chloroplast, mitochondria, and peroxisome (Figure 1) (Foyer et al., 2003; Mittler et al., 2004; Asada, 2006; Rhoads et al., 2006).
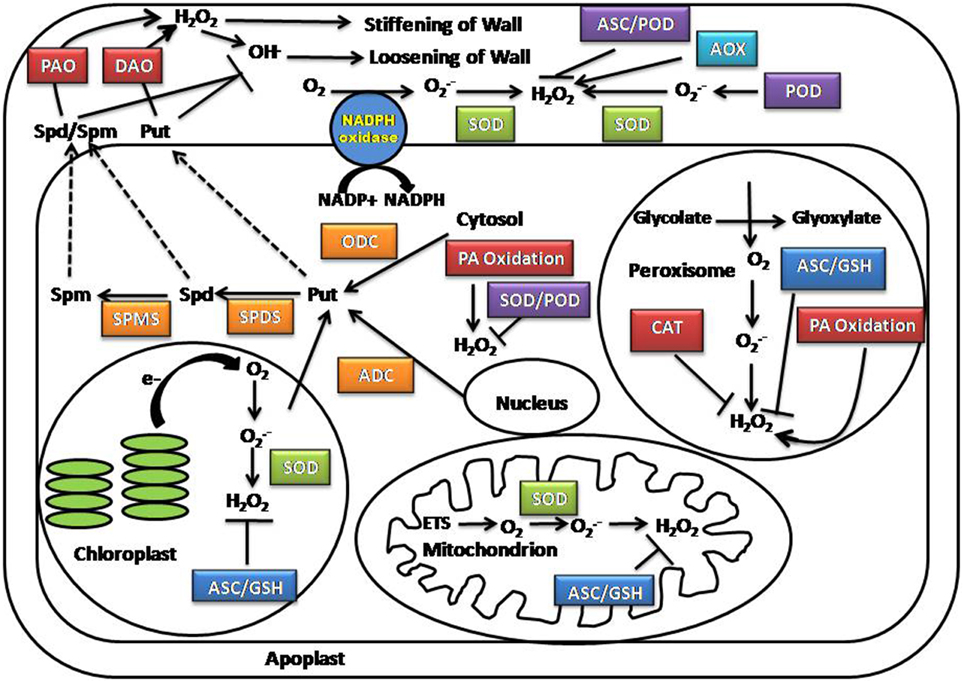
Figure 1. Location of ROS and polyamine production in the cell. AOX, Alternative oxidase; ADC, Arginine decarboxylase; ASC, Ascorbate; APX, Ascorbate peroxidase; CAT, Catalase; DAO, Diamine oxidases; ETS, Electron transport chain; GSH, Glutathione; ODC, Ornithine decarboxylase; PA, Polyamine; PAO, Polyamine oxidases; SPDS, Spermidine synthase; SPMS, Spermine synthase; SOD, Superoxide dismutase.
The chloroplast produces the highest levels of ROS under both normal conditions and salt stress. ROS generation occurs within both Photosystem I (PSI) and Photosystem II (PSII) reaction centers in the thylakoid membrane. During salt stress, ROS production is enhanced due to changes in membrane fluidity and protein complex formation, blocking the electron transfer from water to PSII (Chaves et al., 2009; Biswal et al., 2011; Silva et al., 2011; Jajoo, 2013). Another important site for ROS production is the mitochondria. During salt stress, mitochondrial respiration is disrupted; over-reduction of the ubiquinone pool facilitate the leakage of electrons from complexes I and III of the mitochondrial electron transport chain to molecular oxygen, resulting in O·−2 production (Noctor et al., 2007; Miller et al., 2010). Excess O2in the cell also increases the photorespiration rate, which produces O·−2 and 1O2 as by products (Allakhverdiev et al., 2002; Foyer and Noctor, 2003). Peroxisomes, which cater as a site for numerous metabolic processes such as photorespiration, β-oxidation of fatty acid, flavin oxidase pathway, dismutation of superoxide radicals and polyamine catabolism, also contribute significantly to ROS accumulation in plants subjected to salinity stress (Moschou et al., 2008a,b; Mohapatra et al., 2009). The effects of salt stress on peroxisomes and chloroplasts are interconnected. Reduced water availability and stomatal closure during salt stress causes reduction in the CO2 to O2 ratio in mesophyll cells. This facilitates the affinity of Rubisco to O2, thus increasing photorespiration and production of glycolate in chloroplasts. The end product of chloroplasts (glycolate) is oxidized by glycolate-oxidase in peroxisomes—a major pathway of H2O2 production (Noctor et al., 2002; Karpinski et al., 2003). In addition to organelles, enzymes localized in other cellular compartments, including the cytosolic polyamine oxidase (PAO) and diamine oxidase (DAO), plasma membrane NADPH oxidases, cell wall-associated peroxidases (POXs) and oxalate oxidases participate in ROS synthesis and may play a minor role in ROS production during salt stress (Kawano, 2003; Parida and Das, 2005; Ahmad and Sharma, 2008).
High levels of ROS can damage the cell by inactivating enzymes, initiating lipid oxidation of membranes, and breaking DNA strands (Van Breusegem et al., 2001; Halliwell, 2006). Plants modulate ROS accumulation during salinity stress via enzymatic and non-enzymatic pathways. The cytosolic enzymatic antioxidants include superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), and enzymes that participate in the ascorbate-glutathione cycle (Figure 2). Non-enzymatic antioxidants include the lipid-soluble membrane-associated α-tocopherol, and β-carotene, which are products of lipid peroxidation. Polyamines belong to the category of water-soluble compounds with antioxidative properties alongside glutathione (GSH), ascorbate (ASC), polyphenols (flavonoids, tannins, and anthocyanins), proteinaceous thiols, proline, and glycine-betaine (Mittler, 2002; Ozgur et al., 2013; Todorova et al., 2013). Glycine-betaine is a key regulator in ROS homeostasis, which stabilizes PSII by preventing high salt (Na+ and Cl−)-induced dissociation of the regulatory extrinsic proteins (Papageorgiou and Murata, 1995). Some plants also use the alternative oxidase enzyme (AOX) to remove electrons from the ubiquinone pool and transfer them to oxygen to form water, thus preventing the over-reduction of ubiquinones and resulting in decrease of salt-induced ROS production in mitochondria (Smith et al., 2009; Miller et al., 2010). Unlike metazoans, plant cells do not have a mechanism to detoxify OH· enzymatically and to regulate the accumulation of OH·, rely on non-enzymatic antioxidants, and various mechanisms to prevent OH· formation (Bose et al., 2014).
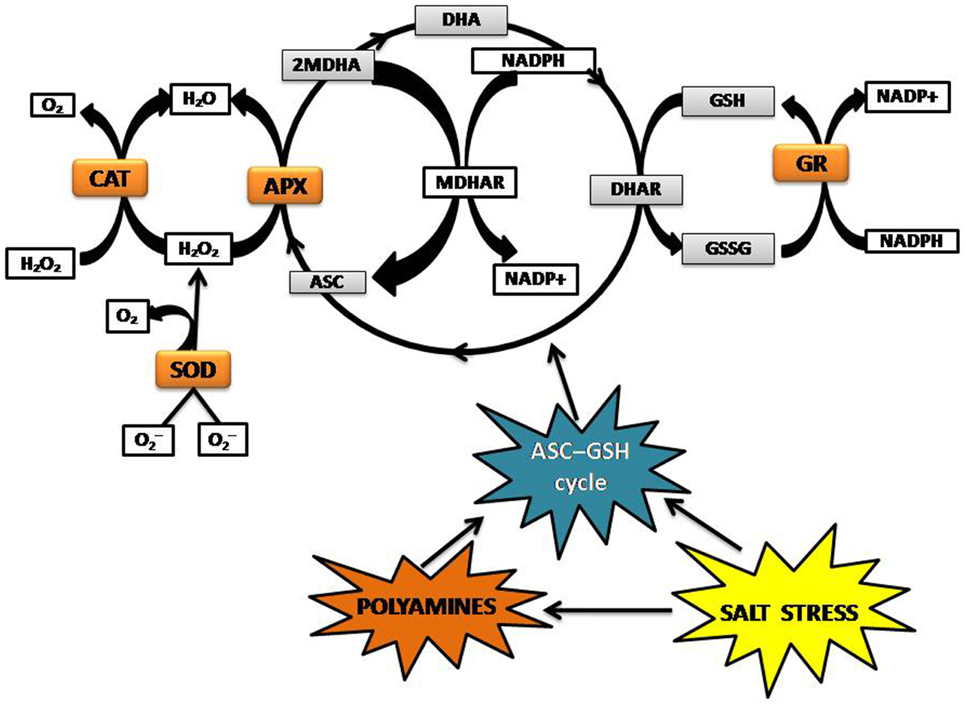
Figure 2. ROS detoxification mediated by ASC-GSHcycle. ASC, Ascorbate; APX, Ascorbate peroxidase; CAT, Catalase; DHA, Dehydroascorbate; GSH, Glutathione; GR, Glutathione reductase; GSSG, glutathione disulphide; MDHA, Monodehydroascorbate; MDHAR, NAD(P)H-dependent oxido-reductase; SOD, Superoxide dismutase.
Numerous studies have shown a correlation between antioxidant accumulation and plant salt stress tolerance; however recent evidence hints that this relationship is more complex than previously thought. Several polyols accumulating during salt stress (sorbitol, mannitol, myo-inositol, pinitol, and others) may be involved in scavenging hydroxyl radicals (Williamson et al., 2002). In particular, the osmolyte proline seems to be associated with the activation of ROS-scavenging enzymes during salt stress (Saradhi and Mohanty, 1997; Szabados and Savouré, 2010; Gupta and Huang, 2014). For example, exogenous application of proline improves salt tolerance in melon, and was associated with increased chlorophyll content, photosynthetic rate, reduced O·−2, and H2O2 accumulation, and increased levels of antioxidants (SOD, POD, CAT, APX, DHAR, and GR) (Yan et al., 2011). In addition, heightened levels of proline were observed in salt-tolerant transgenic rice overexpressing the DEAD-box helicase PDH45 which correlated with increased activation of antioxidant enzymes including SOD, APX, GPX, and GR under salt stress (Gill et al., 2013). It has also been shown that exogenous application of compatible solutes like glycine betaine, proline, mannitol, trehalose or myo-inositol, considerably reduced OH·− generated K+ efflux during salt stress through an unknown mechanism (Cuin and Shabala, 2007).
Thus, ROS production and detoxification during salt stress appears to involve multiple cellular locations and molecular mechanisms. While polyamines are only one of several compounds with antioxidative properties that accumulate in stressed plants, they seem to play a significant role in regulating stress tolerance as outlined below.
Polyamines, small aliphatic amines with proposed antioxidant effect, are ubiquitous across all living organisms (Hussain et al., 2011; Gupta et al., 2013). Endogenous levels of polyamines increase during exposure to abiotic stresses such as drought, salinity, chilling, heat, hypoxia, ozone, UV, and heavy metal exposure and are ubiquitously produced in all cells and tissues (Alcázar et al., 2010; Gill and Tuteja, 2010). The most abundant plant polyamines include putrescine (Put, 1, 4- diaminobutane), spermidine (Spd, N -3-aminopropyl-1, 4-diaminobutane) and spermine (Spm, bis (N -3-aminopropyl)-1,4-diaminobutane). Beside these, cadaverine (Cad, 1, 5-diaminopentane) has also been detected in several plant species, in particular in Gramineae, Leguminoseae and Solanaceae (Lutts et al., 2013). Another polyamine, thermospermine—a structural isomer of spermine—is synthesized by the action of thermospermine synthase (Takano et al., 2012). Putrescine (Put) is primarily synthesized by ornithine decarboxylase using ornithine as a substrate (Figure 1). Another alternative pathway for Put synthesis occurs through the action of arginine decarboxylase (ADC) followed by two successive steps catalyzed by agmatine iminohydrolase (AIH) and N-carbamoyl-Put amidohydrolase (CPA) (Fuell et al., 2010). Put can be used as a substrate to generate Spd by spermidine synthase (SPDS) and Spd can then converted to Spm by spermine synthase (SPMS). Other polyamine oxidation products include hydrogen peroxide and γ-aminobutyric acid, which are involved in plant development and stress responses (Tiburcio et al., 2014). The unique polycationic structure of polyamines suggest that they may be free radical scavengers, in line with some observations that their accumulation correlates with plant tolerance to biotic and abiotic stresses (Mehta et al., 2002; Walters, 2003; Groppa and Benavides, 2008; Gill and Tuteja, 2010; Gupta et al., 2013).
The interactions between polyamines, ROS and antioxidants are complex and induce diverse and apparently contradictory physiological effects during stress (Bhattacharjee, 2005; Gill and Tuteja, 2010; Pottosin et al., 2012, 2014; Velarde-Buendia et al., 2012). In particular, increased levels of cellular polyamines during abiotic stress (e.g., salinity) have shown dual effects. On one hand, exogenous polyamine application was correlated with higher plant tolerance to abiotic stress, partly due to the increased ability to inactivate oxidative radicals. On the other hand, polyamines were reported to decrease plant's capacity to withstand stress, possibly due to the increased levels of H2O2 resulted from polyamines' catabolism (Minocha et al., 2014). Indeed, both the anabolism and catabolism of the polyamine species were reported to increase during abiotic stress, with the net effect of raised cellular levels of ROS as well as antioxidant enzymes and metabolites (Pottosin et al., 2012, 2014; Minocha et al., 2014). In this review we have attempted to clarify the complex relationship between polyamines and ROS, focusing on the potential role of polyamine as a redox homeostasis manager during plant abiotic stress response.
Plant polyamines are thought to contribute to cellular responses during salt stress through modulation of ROS homeostasis via two distinct mechanisms (Takahashi and Kakehi, 2010). First, polyamines promote ROS degradation by scavenging free radicals and activating antioxidant enzymes during stress conditions (Gupta et al., 2013). Free polyamines are responsible for the detoxification of superoxide anions and hydrogen peroxide, while the conjugated polyamines are involved in scavenging other ROS (Langebartels et al., 1991; Kubis, 2005). Kuznetsov and Shevyakova (2007) have reported that conjugated polyamines show more antioxidant ability than free polyamines. Second, polyamines promote ROS production through polyamine catabolism in the apoplast (Yoda et al., 2006; Marina et al., 2008; Mohapatra et al., 2009; Campestre et al., 2011). While it is difficult to determine which of these mechanisms is most important during salt stress, manipulation of the polyamine biosynthetic pathways is correlated to abiotic stress resistance in several studies. For example, impaired expression of ADC1 or ADC2 significantly decreased Put levels and increased susceptibility to salt stress (Urano et al., 2004). When mouse ornithine decarboxylase (ODC) was introduced in Nicotiana tabacum, free polyamine content increased by 2-4 fold and germination increased by 33–45% on high salt medium (Kumria and Rajam, 2002). Transgenic Nicotiana tabacum plants overexpressing a S-adenosylmethionine decarboxylase (SAMDC) gene also demonstrated enhanced of soluble polyamines as well as increased seed weight, photosynthetic rate and expression of antioxidant enzymes (APX, MnSOD, and glutathione S-transferase) relative to untransformed lines (Wi et al., 2006). Increased polyamine accumulation (4–7%) was also observed in tobacco plants expressing the S-adenosylmethionine synthetase (SsSAMS2) gene, which supported up to 20% higher photosynthetic rates and biomass accumulation compared to the control (Qi et al., 2010). Similarly, introduction of SAMDC cDNA from Tritordeum into Oryza sativa produced higher free polyamine content (Put, Spd, Spm), and a reduction in salt-induced shoot growth repression compared to non-transgenic rice plants (Roy and Wu, 2002). Ectopic expression of SPDS orthologs from different source plants also improved growth and survival of young plants in Arabidopsis, European pear (Pyrus communis L.) and tomato suggesting the importance of this enzyme to cope up with saline environmental condition across diverse plant species (Kasukabe et al., 2004; Wen et al., 2008; Neily et al., 2011). Exogenous application of polyamines has also been shown to have a significant effect on the plant, and has been suggested to be a potential strategy to increase plant survival during salt stress. For example, Spm application promoted osmotic and salt stress tolerance in Arabidopsis and rice, which was thought to be due to enhanced polyphenol accumulation, CAT, and SOD enzyme activities (Sreenivasulu et al., 2000; Cheruiyot et al., 2007; Roychoudhury et al., 2011; Zrig et al., 2011; Radhakrishnan and Lee, 2013). In cucumber, Spm treatment enhanced salt tolerance (growth, photosynthetic rates) in a salt-sensitive cultivar, which was correlated to higher antioxidative enzyme activity and proline accumulation (Duan et al., 2008). Put application also increased the activity of antioxidant enzymes and carotenoids in leaf tissues of salt stressed Brassica juncea seedlings and enhanced seedling growth relative to the untreated controls (Verma and Mishra, 2005). Together, these studies indicate that altering polyamine accumulation through manipulation of biosynthetic pathways or direct application could have an effect on physiological responses to salt stress. Table 1 summarizes the effect of endogenously formed and exogenously applied polyamines in alleviating salt resistance via the modulation of cellular antioxidative components (enzymatic or non-enzymatic).
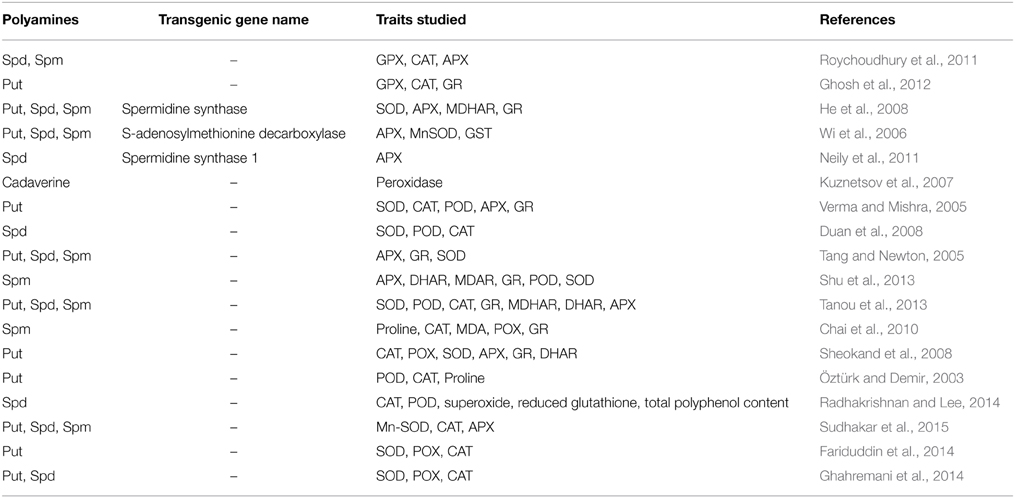
Table 1. Effect of polyamines in the regulation of various enzymatic and non-enzymatic antioxidant components in salt stressed plants.
Engineering consistent polyamine accumulation may not be so simple however, as plants also exhibit increased polyamine degradation during salt stress and thus polyamine turnover appears to be highly regulated. During salt stress, intracellular polyamines are exported from the cytosol to the apoplast, against the electrochemical gradient, and oxidized by DAO and/or PAO to generate hydrogen peroxide that is further converted to OH· via the Fenton reaction (Pottosin et al., 2014). For example, polyamine degradation occurs through oxidative deamination catalyzed by aminooxidases such as the copper-containing DAO and flavoprotein-containing PAO. DAO exhibits high affinity for diamines, while PAO oxidizes secondary amine groups from Spd and Spm (Alcazar et al., 2006). While dicotyledonous plants predominantly accumulate DAO, monocotyledonous plants usually accumulate more PAO than DAO (Šebela et al., 2001; Cona et al., 2006). The oxidative deamination of Put produces Δ 1-pyrroline, H2O2, and NH3 by DAO whereas activity of PAO resulted in the formation of Δ 1–pyrroline (from Spd oxidation) or 1-(3-aminopropyl)-pyrroline (from Spm oxidation), along with 1, 3-diaminopropane and H2O2 (Federico and Angelini, 1991). Both DAO and PAO are localized to the cytoplasm and cell wall and are involved in production of the hydrogen peroxide required for cell wall stiffening (Cona et al., 2003; Kuznetsov and Shevyakova, 2007) (Figure 1). These enzymes seem to contribute to changes in growth during salt stress since increased PAO accumulation in the expansion zone of maize leaves enhanced both ROS accumulation and elongation (Rodríguez et al., 2009; Shoresh et al., 2011). Moreover, high salt (400 mM NaCl) or ROS application induces DAO activity in the leaves and roots of the halophyte Mesembryanthemum crystallinum further implicating that these enzymes play a role in salt stress (Shevyakova et al., 2006).
Polyamines are also hypothesized to promote salt stress tolerance through their direct or indirect effects on ion transport (Figure 3) (Demidchik and Maathuis, 2007; Pandolfi et al., 2010; Bose et al., 2011). For instance, polyamines including Spd, Spm, and Put affect ion transport indirectly by interacting with plasma membrane phospholipids and enhancing membrane stability. Polyamine-enhanced membrane stability has been shown to have a significant effect on both H+/ATPase and Ca2+/ATPase transporters during salinity stress (Roy et al., 2005; Pottosin and Shabala, 2014). Ca2+ channel regulation mediated by polyamines and H2O2 in response to salt stress leads to the rapid rise in the intracellular concentration of Ca2+ that, subsequently, enforces a positive feedback on the ROS production via the membrane-localized NADPH-oxidase (Takeda et al., 2008; Bose et al., 2014). Sudden exposure to salt stress is reflected in the alterations of turgor that is sensed by rapid increase in cellular cGMP, produced by the action of receptor kinase cyclase. This in turn activates the root-localized cyclic nucleotide-gated channels allowing the inward flow of Ca2+, thus cGMP signal is converted to Ca2+ signal during salinity (Demidchik and Maathuis, 2007). On the other hand, a rise in cGMP can directly inactivate root voltage-independent non-selective cation channels (VI-NSCC) by reducing the influx of toxic Na+ (Rubio et al., 2003). Salt-stress elicited Ca2+ signals activate signaling molecules including the SOS3 calcium-binding protein and the serine/threonine protein kinase SOS2 which in turn activate the membrane Na+/H+ antiporter SOS1 leading to Na+ efflux (Zhu, 2003). If we consider the above mentioned reports, one can easily observe an indirect cumulative effect of polyamines and ROS in regulating the cellular Ca2+ that is important for salt response. In contrast, Spm may directly affect ion transport during salt stress by blocking inward-rectifying K+ channels (KIRC) and non-selective cation channels (NSCCs), limiting Na+ influx, and K+ efflux (Liu et al., 2000; Shabala et al., 2007; Zhao et al., 2007; Zepeda-Jazo et al., 2008). Put and Spm have shown strong potential in reducing the hydroxyl radical-induced K(+) efflux and the respective non-selective current. This synergistic effect between ROS and polyamines was much more pronounced in a salt-sensitive barley variety than salt-tolerant one (Velarde-Buendia et al., 2012). Subsequently, an increased external [Ca2+] activated depolarization-activated NSCCs (DA-NSCCs), inhibited Na+ -induced K+ efflux, thus ameliorating Na+ toxicity in plants (Shabala et al., 2006). During salinity, exogenous application of spermidine has been found to block VI-NSCC reducing the inward flow of Ca2+ and Na+ and the outward flow of K+ in barley seedlings (Zhao et al., 2007). It has been reported that polyamine accumulation under salt stress has a tendency to make the overall tonoplast cation conductance more K+ selective, thus considered to lead to higher vacuolar Na+ sequestration and an improved cytosolic K+/Na+ homeostasis (Zepeda-Jazo et al., 2008). Absence of Spm causes an imbalance in Ca2+ homeostasis in the Arabidopsis mutant plant and showed hypersensitivity to salinity, suggesting its involvement in modulating the activity of certain Ca2+- permeable channels and changes in Ca2+ allocation compared to unstressed state, which may prevent Na+ and K+ entry into the cytosol, enhance Na+ and K+ influx into the vacuole, or suppress Na+ and K+ release from the vacuole (Yamaguchi et al., 2006). Moreover, vacuolar Cation/H+ Exchangers (CAX) are found to be over-expressed and both FV and SV channels (FV, fact-activating vacuolar channel; SV, slow-activating vacuolar channel) suppressed during salinity, resulting into an overall increase in vacuolar Ca2+ (Cheng et al., 2004; Pottosin et al., 2004). Dobrovinskaya et al. (1999) reported that cellular polyamines strongly inhibited FV and SV channels whose reduced activity is essential for conferring salinity tolerance in the facultative halophyte Chenopodium quinoa (Bonales-Alatorre et al., 2013). However, more research is required to understand this interaction as well as the putative interactions between polyamines and vacuolar transport systems (Pottosin and Shabala, 2014).
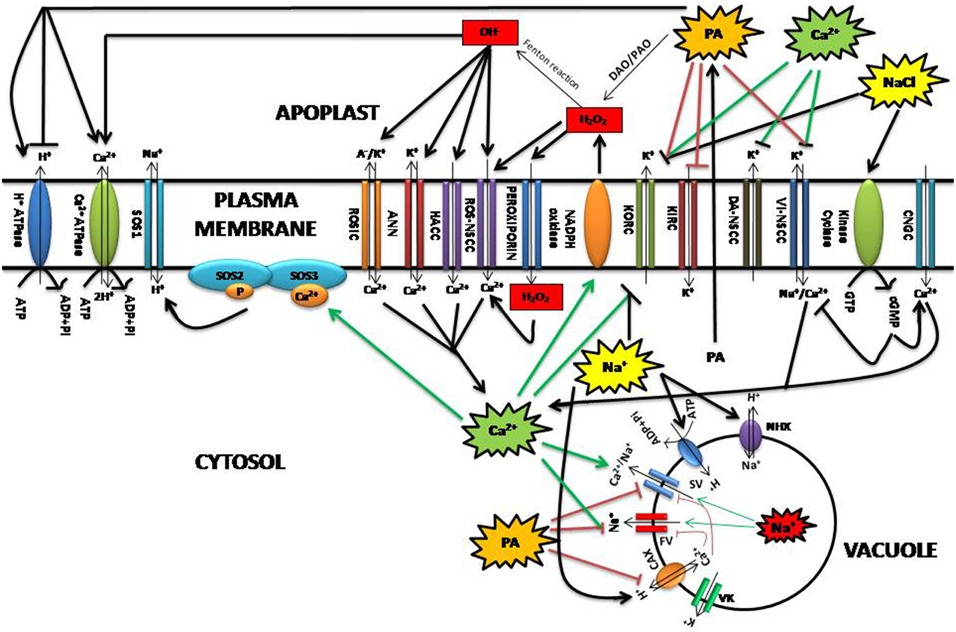
Figure 3. Relationship between polyamines and ROS during salinity in the context of ion transport regulation at plasma membrane and vacuole. ANN, Annexin-formed channel; CAX, Cation/H+ exchangers; CNGCs, Cyclic nucleotide-gated channels; cGMP, cyclic guanyl cyclase; DA-NSCCs, Depolarization-activated NSCCs; DAO, Diamine oxidases; FV, Fast vacuolar channel; HACC, Hyperpolarization-activated Ca2+ influx channel; KIRC, K+ inward-rectifying; KORC, K+ outward rectifying; VK, K+-selective channels; ROSIC, Non-selective voltage-independent conductance; NHX, Na+/H+ antiporters; PA, Polyamine; PAO, Polyamine oxidases; ROS-NSCC, ROS activated Non-selective cation channel; SOS1, SOS2, SOS3, respectively, Salt overly sensitive 1,2,3; SV, Slow vacuolar channel; VI-NSCCs, Voltage-independent nonselective cation channels.
Plants employ multi-level signal transduction to induce stress responses. The coordinated actions of hormones such as abscisic acid (ABA), ethylene, jasmonate, and auxin along with other signaling molecules like Ca2+, cyclic nucleotides, ROS and reactive nitrogen species such as NO form a complex signaling network (Neill et al., 2003; Tuteja and Sopory, 2008). Interestingly, ABA was found to be involved in regulating both biosynthetic and catabolic pathways for polyamines in Arabidopsis (Urano et al., 2004; Hussain et al., 2011). For example, exogenous application of ABA has been found to modulate the transcription and biosynthesis of polyamine metabolic enzymes such as ADC2, SPDS, and SPMS during stress (Alcazar et al., 2006; Hussain et al., 2011). On the other hand Put has been found to serve as a modulator of indispensable ABA increase under cold stress thus representing and reciprocal relationship between Put and ABA biosynthesis during the period of stress in order to increase plant adaptive potential (Cuevas et al., 2008, 2009; Urano et al., 2009). The transgenic tobacco plants overexpressing the ABA-biosynthetic enzyme 9-cis-epoxycarotenoid dioxygenase is associated with the ABA-induced production of H2O2, NO, and the subsequent induction of antioxidant enzymes conferring salt tolerance (Zhang et al., 2009). Recently, it has been shown that polyamines can induce the production of NO that serves as a signal-inducing salt resistance by increasing the K+ to Na+ ratio by stimulating the expression of the plasma membrane H+-ATPase and Na+/H+ antiport in the tonoplast (Zhao et al., 2004; Tun et al., 2006; Yamasaki and Cohen, 2006; Zhang et al., 2006). It was suggested that NO production induced by polyamines could be mediated either by H2O2, one reaction product of oxidation of polyamines by DAO and PAO, or by unknown mechanisms involving polyamines, DAO and PAO (Wimalasekera et al., 2011). Pre-treatment with H2O2 or sodium nitroprusside (NO donor) induced major antioxidant defense (SOD, catalase, APX, and GR), reduced protein carbonylation and accumulated leaf S-nitrosylated proteins, suggesting an overlap relation between NO and H2O2 signaling pathways in salinity acclimation (Tanou et al., 2009a,b).
In the light of these observations we have made an attempt to explore the interconnection(s) between polyamines, NO, ABA, and ROS as potential mediator(s) of stress responses. More research is needed to determine the exact nature of these intricate connections in the context of salt stress.
Plant cells employ dynamic activation of ROS production to regulate defense responses during stress. When ROS accumulation crosses a threshold value, cells enter into a genetically programmed necrotic process that leads to cellular suicide, which restricts the oxidative damage to a controlled number of cells and triggers pathways for nutrient recycling (de Pinto et al., 2006; Stowe and Camara, 2009). The key regulator of the switch between the cellular endurance and programmed cell death (PCD) under salt stress could be controlled by the interplay between polyamine and ROS homeostasis; specifically, the precise modulation of polyamine levels by the shift between polyamine anabolism and catabolism may result a lower polyamine concentration which, in turn may facilitate PCD (Moschou et al., 2008a; Toumi et al., 2010).
We have already discussed in our previous section that polyamines act as important regulators of ion homeostasis during salt stress. Modulation of the cellular K+ and Ca2+ concentrations regulate stress-related PCD pathways in plants (Moschou and Roubelakis-Angelakis, 2014). Plant polyamines are found to affect intracellular dynamics of both ions, thus suggesting their direct involvement in PCD (Wu et al., 2010; Zepeda-Jazo et al., 2011). Low cellular concentrations of K+ were shown to increase the activity of metacaspases and nucleases, thus promoting ROS- and salt-induced PCD (Demidchik et al., 2010). Salt stress led to high cytosolic [Ca2+] which promoted the opening of mitochondrial permeability transition pore (MPTP) and PCD induction in tobacco protoplasts (Lin et al., 2005). Mitochondrial depolarization and cytochrome-c release is a hallmark event during the PCD (Logan, 2008; Andronis and Roubelakis-Angelakis, 2010). Takahashi's group showed that 0.5 mM Spm pretreatment of tobacco leaf discs induced expression of the Salicylic acid (SA)-induced Protein Kinase (SIPK) and Wound-Induced Protein Kinase (WIPK) and caused mitochondrial dysfunction similar to the one observed during PCD in tobacco leaves (Takahashi et al., 2003).
Accumulation of metabolic derivatives of polyamines may also indirectly control PCD pathways (Moschou and Roubelakis-Angelakis, 2014). For example, tobacco plants with reduced or increased PAO expression demonstrated increased salt tolerance or PCD depending on the availability of intracellular polyamines (Moschou et al., 2008c). Expression of the Spm Oxidase (SMO) can also be linked to hydrogen peroxide production and PCD, providing additional support to the above presented view of PAO-induced PCD (Moschou and Roubelakis-Angelakis, 2014). It has also been reported that overexpression of PAO activates mitogen-activated protein kinases (MAPK)-mediated pathways during biotic stress (Moschou et al., 2009).
In sum, a connection between polyamine metabolism and PCD can be inferred, but more work is needed to determine the molecular mechanisms underlying this relationship.
This review outlines our current understanding of polyamines and their contributions to ROS homeostasis during salt stress, summarized in Figure 4. The figure depicts the possible cellular pathways by which polyamines modulate ROS homeostasis during salinity and the probable mode of action of endogenous and exogenous PAs into a single frame, so that one can easily view the current state of the field.
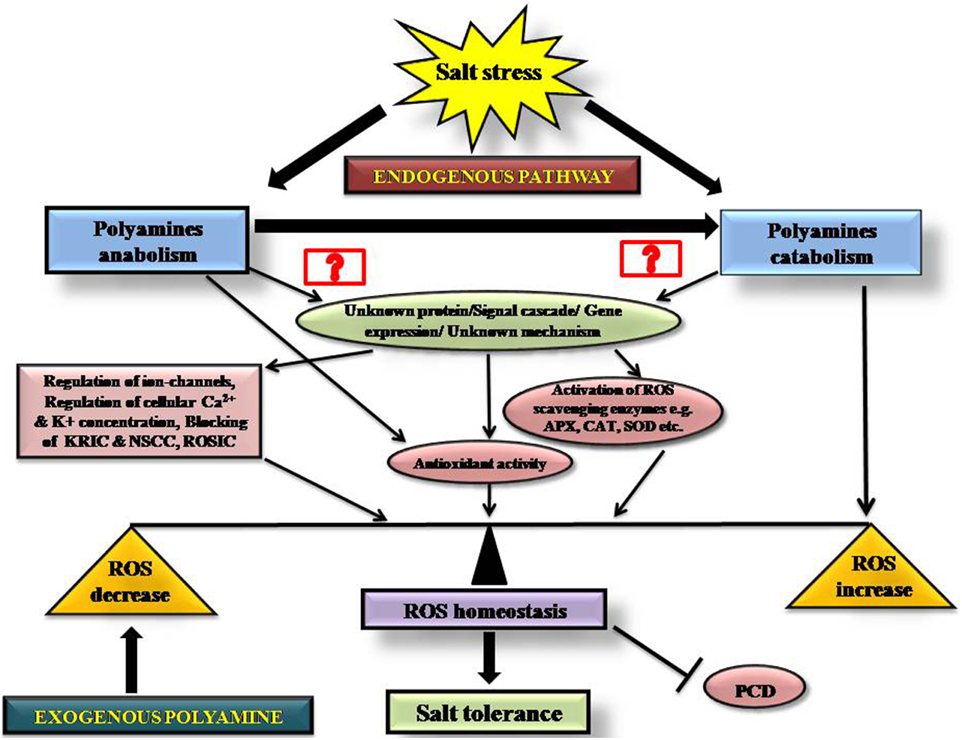
Figure 4. Schematic diagram showing the role of endogenous and exogenous polyamines in maintaining redox homeostasis during salinity stress.
Our literature review suggests that the regulation of polyamine metabolism is a complex process where the exact roles of polyamines in regulating ROS, ion transport and PCD are still to be discovered. For the field to progress there is a need to address several important aspects: (i) The identity of the cellular components that mediate the link between ROS synthesis, ROS signaling and polyamines; (ii) The mechanisms that these mediator components employ; and (iii) The potential organ- or tissue-specific differences in the composition and regulation of polyamine-ROS networks.
To solve these questions one should focus on several relevant processes including polyamine biosynthesis, transport and catabolism in parallel with the tissue-, species-, and salt stress dependent expression of various ion channels and transporters. Additionally, one should consider the nature of various ROS and polyamine species that accumulate in plants under stress and the sites of their subcellular synthesis, alongside changes in the polyamine and ROS scavenging systems.
Salt stress constitutes a serious challenge to overcome in the quest of global increase in crop productivity. Understanding the underlying molecular mechanism of salt stress adaptation is the key to successful crop biotechnology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors acknowledge the support of technical facilities available at Presidency University, Kolkata. Financial assistance from DBT (RGYI) (Govt. of India) and W.B. State DST (Govt. of West Bengal) to BG and KG and DST-SERB (Govt. of India) to KG are also gratefully acknowledged.
Ahmad, P., Hakeem, K. R., Kumar, A., Ashraf, M., and Akram, N. A. (2012). Salt-induced changes in photosynthetic activity and oxidative defense system of three cultivars of mustard (Brassica juncea L.). Afr. J. Biotechnol. 11, 2694–2703. doi: 10.5897/AJB11.3203
Ahmad, P., and Sharma, S. (2008). Salt stress and phytobiochemical responses of plants–a review. Plant Soil Environ. 54, 89–99. doi: 10.1016/j.ecoenv.2004.06.010
Alcázar, R., Altabella, T., Marco, F., Bortolotti, C., Reymond, M., Koncz, C., et al. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231, 1237–1249. doi: 10.1007/s00425-010-1130-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Alcazar, R., Marco, F., Cuevas, J. C., Patron, M., Ferrando, A., Carrasco, P., et al. (2006). Involvement of polyamines in plant response to abiotic stress. Biotechnol. Lett. 28, 1867–1876. doi: 10.1007/s10529-006-9179-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Allakhverdiev, S. I., Nishiyama, Y., Miyairi, S., Yamamoto, H., Inagaki, N., Kanesaki, Y., et al. (2002). Salt stress inhibits the repair of photodamaged Photosystem II by suppressing the transcription and translation of psbA genes in Synechocystis. Plant Physiol. 130, 1443–1453. doi: 10.1104/pp.011114
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Allu, A. D., Soja, A. M., Wu, A., Szymanski, J., and Balazadeh, S. (2014). Salt stress and senescence: identification of cross-talk regulatory components. J. Exp. Bot. 65, 3993–4008. doi: 10.1093/jxb/eru173
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Andronis, E. A., and Roubelakis-Angelakis, K. A. (2010). Short-term salinity stress in tobacco plants leads to the onset of animal-like PCD hallmarks in planta in contrast to long-term stress. Planta 231, 437–448. doi: 10.1007/s00425-009-1060-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396. doi: 10.1104/pp.106.082040
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baxter, A., Mittler, R., and Suzuki, N. (2014). ROS as key players in plant stress signaling. J. Exp. Bot. 65, 1229–1240. doi: 10.1093/jxb/ert375
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bhattacharjee, S. (2005). Reactive oxygen species and oxidative burst: roles in stress, senescence and signal transduction in plants. Curr. Sci. 89, 1113–1121.
Biswal, B., Joshi, P. N., Raval, M. K., and Biswal, U. C. (2011). Photosynthesis, a global sensor of environmental stress in green plants: stress signalling and adaptation. Curr. Sci. 101, 47–56.
Bonales-Alatorre, E., Shabala, S., Chen, Z. H., and Pottosin, I. (2013). Reduced tonoplast fast-activating and slow-activating channel activity is essential for conferring salinity tolerance in a facultative halophyte, Quinoa. Plant Physiol. 162, 940–952. doi: 10.1104/pp.113.216572
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bose, J., Pottosin, I., Shabala, S. S., Palmgren, M. G., and Shabala, S. (2011). Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2:85. doi: 10.3389/fpls.2011.00085
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bose, J., Rodrigo-Moreno, A., and Shabala, S. (2014). ROS homeostasis in halophytes in the context of salinity stress tolerance. J. Exp. Bot. 65, 1241–1257. doi: 10.1093/jxb/ert430
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Campestre, M. P., Bordenave, C. D., Origone, A. C., Menéndez, A. B., Ruiz, O. A., Rodríguez, A. A., et al. (2011). Polyamine catabolism is involved in response to salt stress in soybean hypocotyls. J. Plant Physiol. 168, 1234–1240. doi: 10.1016/j.jplph.2011.01.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chai, Y. Y., Jiang, C. D., Shi, L., Shi, T. S., and Gu, W. B. (2010). Effects of exogenous spermine on sweet sorghum during germination under salinity. Biol. Plant. 54, 145–148. doi: 10.1007/s10535-010-0023-1
Chaves, M. M., Flaxes, J., and Pinheiro, C. (2009). Photosynthesis under drought and salt stress regulation mechanism from whole plant to cell. Ann. Bot. 103, 551–556. doi: 10.1093/aob/mcn125
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheng, N. H., Pittman, J. K., Zhu, J. K., and Hirschi, K. (2004). The protein kinase SOS2 activates the Arabidopsis H+/Ca2+ antiporters CAX1 to integrate calcium transport and salttolerance. J. Biol. Chem. 279, 2922–2926. doi: 10.1074/jbc.M309084200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cheruiyot, E. K., Mumera, L. M., Ngetich, W. K., Hassanali, A., and Wachira, F. (2007). Polyhenols as potential indicators for osmotic tolerance in tea (Camellia sinensis L.). Biosci. Biotechnol. Biochem. 71, 2190–2197. doi: 10.1271/bbb.70156
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Chinnusamy, V., and Zhu, J. K. (2003). “Plant salt tolerance. Topics in current genetics,” in Plant Responses to Abiotic Stress, Vol. 4, eds H. Hirt and K. Shinozaki (Berlin; Heidelberg: Springer), 241–270. doi: 10.1007/978-3-540-39402-0_10
Cona, A., Cenci, F., Cervelli, M., Federico, R., Mariottini, P., Moreno, S., et al. (2003). Polyamine oxidase, a hydrogen peroxide-producing enzyme, is up-regulated by light and down-regulated by auxin in the outer tissues of the maize mesocotyl. Plant Physiol. 131, 803–813. doi: 10.1104/pp.011379
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cona, A., Rea, G., Angelini, R., Federico, R., and Tavladoraki, P. (2006). Functions of amine oxidases in plant development and defence. Trends Plant Sci. 11, 80–88. doi: 10.1016/j.tplants.2005.12.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cuevas, J. C., López-Cobollo, R., Alcázar, R., Zarza, X., Koncz, C., Altabella, T., et al. (2008). Putrescine is involved in Arabidopsis freezing tolerance and cold acclimation by regulating abscisic acid levels in response to low temperature. Plant Physiol. 148, 1094–1105. doi: 10.1104/pp.108.122945
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cuevas, J. C., Lopez-Cobollo, R., Alcázar, R., Zarza, X., Koncz, C., Altabella, T., et al. (2009). Putrescine as a signal to modulate the indispensable ABA increase under cold stress. Plant Signal Behav. 4, 219–220. doi: 10.4161/psb.4.3.7861
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cuin, T. A., and Shabala, S. (2007). Compatible solutes reduce ROS-induced potassium efflux in Arabidopsis roots. Plant Cell Environ. 30, 875–885. doi: 10.1111/j.1365-3040.2007.01674.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Demidchik, V., Cuin, T. A., Svistunenko, D., Smith, S. J., Miller, A. J., Shabala, S., et al. (2010). Arabidopsis root K+- efflux conductance activated by hydroxyl radicals: single-channel properties, genetic basis and involvement in stress-induced cell death. J. Cell Sci. 123, 1468–1479. doi: 10.1242/jcs.064352
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Demidchik, V., and Maathuis, F. J. M. (2007). Physiological roles of nonselective cation channels in plants: from salt stress to signalling and development. New Phytol. 175, 387–404. doi: 10.1111/j.1469-8137.2007.02128.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
de Pinto, M. C., Paradiso, A., Leonetti, P., and De Gara, L. (2006). Hydrogen peroxide, nitric oxide and cytosolic ascorbate peroxidase at the crossroad between defence and cell death. Plant J. 48, 784–795. doi: 10.1111/j.1365-313X.2006.02919.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dobrovinskaya, O. R., Muñiz, J., and Pottosin, I. I. (1999). Inhibition of vacuolar ion channels bypolyamines. J. Membr. Biol. 167, 127–140. doi: 10.1007/s002329900477
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dowling, D. K., and Simmons, L. W. (2009). Reactive oxygen species as universal constraints in life-history evolution. Proc. Biol. Sci. 276, 1737–1745. doi: 10.1098/rspb.2008.1791
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Duan, J., Li, J., Guo, S., and Kang, Y. (2008). Exogenous spermidine affects polyamine metabolism in salinity-stressed Cucumis sativus roots and enhances short-term salinity tolerance. J. Plant Physiol. 165, 1620–1635. doi: 10.1016/j.jplph.2007.11.006
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fariduddin, Q., Mir, B. A., Yusuf, M., and Ahmad, A. (2014). 24-epibrassinolide and/or putrescine trigger physiological and biochemical responses for the salt stress mitigation in Cucumis sativus L. Photosynthetica 52, 464–474. doi: 10.1007/s11099-014-0052-7
Federico, R., and Angelini, R. (1991). “Polyamine catabolism in plants,” in Biochemistry and Physiology in Plants, eds R. D. Slocum and H. E. Flores (Boca Raton; Ann Arbor; London: CRC Press), 41–56.
Foyer, C. H., and Noctor, G. (2003). Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol. Plant. 119, 355–364. doi: 10.1034/j.1399-3054.2003.00223.x
Foyer, C. H., and Noctor, G. (2005). Oxidant and antioxidant signaling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Environ. 28, 1056–1071. doi: 10.1111/j.1365-3040.2005.01327.x
Foyer, C. H., Parry, M., and Noctor, G. (2003). Markers and signals associated with nitrogen assimilation in higher plants. J. Exp. Bot. 54, 585–593. doi: 10.1093/jxb/erg053
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fuell, C., Elliott, K. A., Hanfrey, C. C., Franceschetti, M., and Michael, A. J. (2010). Polyamine biosynthetic diversity in plants and algae. Plant Physiol. Biochem. 48, 513–520. doi: 10.1016/j.plaphy.2010.02.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gechev, T. S., Van Breusegem, F., Stone, J. M., Denev, I., and Laloi, C. (2006). Reactive oxygen species as signals that modulate plant stress responses and programmed cell death. Bioessays 28, 1091–1101. doi: 10.1002/bies.20493
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ghahremani, M., Ghanati, F., Bernard, F., Azad, T., Gholami, M., and Safari, M. (2014). Ornithine-induced increase of proline and polyamines contents in tobacco cells under salinity conditions. Aust. J. Crop Sci. 8, 91–96.
Ghosh, N., Das, S. P., Mandal, C., Gupta, S., Das, K., Dey, N., et al. (2012). Variations of antioxidative responses in two rice cultivars with polyamine treatment under salinity stress. Physiol. Mol. Biol. Plants 18, 301–313. doi: 10.1007/s12298-012-0124-8
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gill, S. S., Tajrishi, M., Madan, M., and Tuteja, N. (2013). A DESD-box helicase functions in salinity stress tolerance by improving photosynthesis and antioxidant machinery in rice (Oryza sativa L. cv. PB1). Plant Mol. Biol. 82, 1–22. doi: 10.1007/s11103-013-0031-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gill, S. S., and Tuteja, N. (2010). Polyamines and abiotic stress tolerance in plants. Plant Signal Behav. 5, 26–33. doi: 10.4161/psb.5.1.10291
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Groppa, M. D., and Benavides, M. P. (2008). Polyamines and abiotic stress: recent advances. Amino Acids 1, 35–45. doi: 10.1007/s00726-007-0501-8
Gupta, B., and Huang, B. (2014). Mechanism of salinity tolerance in plants: physiological, biochemical, and molecular characterization. Int. J. Genomics 2014:701596. doi: 10.1155/2014/701596
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gupta, K., Dey, A., and Gupta, B. (2013). Plant polyamines in abiotic stress responses. Acta Physiol. Plant. 35, 2015–2036. doi: 10.1007/s11738-013-1239-4
Halliwell, B. (2006). Reactive species and antioxidants. Redox biology is a fundamental theme of aerobic life. Plant Physiol. 141, 312–322. doi: 10.1104/pp.106.077073
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
He, L., Ban, Y., Inoue, H., Matsuda, N., Liu, J., and Moriguchi, T. (2008). Enhancement of spermidine content and antioxidant capacity in transgenic pear shoots overexpressing apple spermidine synthase in response to salinity and hyperosmosis. Phytochemistry 69, 2133–2141. doi: 10.1016/j.phytochem.2008.05.015
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Hussain, S. S., Ali, M., Ahmad, M., and Siddique, K. H. M. (2011). Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. Biotechnol. Adv. 29, 300–311. doi: 10.1016/j.biotechadv.2011.01.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jajoo, A. (2013). “Changes in photosystem II in response to salt stress,” in Ecophysiology and Responses of Plants under Salt Stress, Vol. 5, eds P. Ahmad, M. M. Azooz, and M. N. V. Prasad (New York, NY: Springer), 149–168. doi: 10.1007/978-1-4614-4747-4_5
Jithesh, M. N., Prashanth, S. R., Sivaprakash, K. R., and Parida, A. K. (2006). Antioxidative response mechanisms in halophytes: their role in stress defence. J. Gen. 85, 237–254. doi: 10.1007/BF02935340
Karpinski, S., Gabrys, H., Mateo, A., Karpinska, B., and Mullineaux, P. M. (2003). Light perception in plant disease defence signalling. Curr. Opin. Plant Biol. 6, 390–396. doi: 10.1016/S1369-5266(03)00061-X
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kasukabe, Y., He, L., Nada, K., Misawa, S., Ihara, I., and Tachibana, S. (2004). Overexpression of spermidine synthase enhances tolerance to multiple environmental stresses and up-regulates the expression of various stress-regulated genes intransgenic Arabidopsis thaliana. Plant Cell Physiol. 45, 712–722. doi: 10.1093/pcp/pch083
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kawano, T. (2003). Roles of the reactive oxygen species-generating peroxidase reactions in plant defense and growth induction. Plant Cell Rep. 21, 829–837. doi: 10.1007/s00299-003-0591-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kubis, J. (2005). The effect of exogenous spermidine on superoxide dismutase activity, H2O2 and superoxide radical level in barley leaves under water deficit conditions. Acta Physiol. Plant. 27, 289–295. doi: 10.1007/s11738-005-0005-7
Kumria, R., and Rajam, M. V. (2002). Ornithine decarboxylase transgene in tobacco affects polyamines, in vitro-morphogenesis and response to salt stress. J. Plant. Physiol. 159, 983–990. doi: 10.1078/0176-1617-00822
Kuznetsov, V., Shorina, M., Aronova, E., Stetsenko, L., Rakitin, V., and Shevyakova, N. (2007). NaCl- and ethylene-dependent cadaverine accumulation and its possible protective role in the adaptation of the common ice plant to salt stress. Plant sci. 172, 363–370. doi: 10.1016/j.plantsci.2006.09.012
Kuznetsov, V. V., and Shevyakova, N. I. (2007). Polyamines and stress tolerance of plants. Plant Stress 1, 50–71.
Langebartels, C., Kerner, K., Leonardi, S., Schraudner, M., Trost, M., Heller, W., et al. (1991). Biochemical-plant responses to ozone.1. differential induction of polyamine and ethylene biosynthesis in tobacco. Plant Physiol. 95, 882–889. doi: 10.1104/pp.95.3.882
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lin, J., Wang, Y., and Wang, G. (2005). Salt stress-induced programmed cell death via Ca2+-mediated mitochondrial permeability transition in tobacco protoplasts. Plant Growth regul. 45, 243–250. doi: 10.1007/s10725-005-5163-5
Liu, K., Fu, H. H., Bei, Q. X., and Luan, S. (2000). Inward potassium channel in guard cells as a target for polyamine regulation of stomatal movements. Plant Physiol. 124, 1315–1325. doi: 10.1104/pp.124.3.1315
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Logan, D. C. (2008). Having a swell time—mitochondrial morphology and plant cell death programmes. J. Micro. 231, 215–224. doi: 10.1111/j.1365-2818.2008.02037.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lutts, S., Hausman, J. F., Quinet, M., and Lefèvre, I. (2013). “Polyamines and their roles in the alleviation of ion toxicities in plants,” Ecophysiology and Responses of Plants under Salt Stress, Vol.12, eds P. Ahmad, M. M. Azooz, and M. N. V. Prasad (New York, NY: Springer), 315–353.
Marina, M., Maiale, S. J., Rossi, F. R., Romero, M. F., Rivas, E. I., Garriz, A., et al. (2008). Apoplastic polyamine oxidation plays different roles in local responses of tobacco to infection by the necrotrophic fungus Sclerotinia sclerotiorum and the biotrophic bacterium Pseudomonas viridiflava. Plant Physiol. 147, 2164–2178. doi: 10.1104/pp.108.122614
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mehta, R. A., Cassol, T., Li, N., Ali, N., Handa, A. K., and Mattoo, A. K. (2002). Engineered polyamine accumulation in tomato enhances phytonutrient content, juice quality, and vine. Nat. Biotechnol. 20, 613–618. doi: 10.1038/nbt0602-613
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miller, G. A. D., Suzuki, N., Ciftci-Yilmaz, S., and Mittler, R. O. N. (2010). Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 33, 453–467. doi: 10.1111/j.1365-3040.2009.02041.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Miller, G., Shulaev, V., and Mittler, R. (2008). Reactive oxygen signaling and abiotic stress. Physiol. Plant. 133, 481–489. doi: 10.1111/j.1399-3054.2008.01090.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Minocha, R., Majumdar, R., and Minocha, S. C. (2014). Polyamines and abiotic stress in plants: a complex relationship. Front. Plant Sci. 5, 1–17. doi: 10.3389/fpls.2014.00175
Mittal, S., Kumari, N., and Sharma, V. (2012). Differential response of salt stress on Brassica juncea: photosynthetic performance, pigment, proline, D1 and antioxidant enzymes. Plant Physiol. Biochem. 54, 17–26. doi: 10.1016/j.plaphy.2012.02.003
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R. (2002). Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 7, 405–410. doi: 10.1016/S1360-1385(02)02312-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R., Vanderauwera., S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mohapatra, S., Minocha, R., Long, S., and Minocha, S. C. (2009). Putrescine overproduction negatively impacts the oxidative state of poplar cells in culture. Plant Physiol. Biochem. 47, 262–271. doi: 10.1016/j.plaphy.2008.12.007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moller, I. M., Jensen, P. E., and Hansson, A. (2007). Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 58, 459–481. doi: 10.1146/annurev.arplant.58.032806.103946
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moschou, P. N., Delis, I. D., Paschalidis, K. A., and Roubelakis-Angelakis, K. A. (2008a). Transgenic tobacco plants overexpressing polyamine oxidase are not able to cope with oxidative burst generated by abiotic factors. Physiol. Plantarum. 133, 140–156. doi: 10.1111/j.1399-3054.2008.01049.x
Moschou, P. N., Paschalidis, K. A., Delis, I. D., Andriopoulou, A. H., Lagiotis, G. D., Yakoumakis, D. I., et al. (2008c). Spermidine exodus and oxidation in the apoplast induced by abiotic stress is responsible for H2O2 signatures that direct tolerance responses in tobacco. Plant Cell 20, 1708–1724. doi: 10.1105/tpc.108.059733
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moschou, P. N., Paschalidis, K. A., and Roubelakis-Angelakis, K. A. (2008b). Plant polyamine catabolism: the state of the art. Plant Signal Behav. 3, 1061–1066. doi: 10.4161/psb.3.12.7172
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moschou, P. N., and Roubelakis-Angelakis, K. A. (2014). Polyamines and programmed cell death. J. Exp. Bot. 65, 1285–1296. doi: 10.1093/jxb/ert373
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moschou, P. N., Sarris, P. F., Skandalis, N., Andriopoulou, A. H., Paschalidis, K. A., Panopoulos, N. J., et al. (2009). Engineered polyamine catabolism preinduces tolerance of tobacco to bacteria and oomycetes. Plant Physiol. 149, 1970–1981. doi: 10.1104/pp.108.134932
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neill, S. J., Desikan, R., and Hancock, J. T. (2003). Nitric oxide signalling in plants. New Phytol. 159, 11–35. doi: 10.1046/j.1469-8137.2003.00804.x
Neily, M. H., Baldet, P., Arfaoui, I., Saito, T., Qiu-li, L., Asamizu, E., et al. (2011). Overexpression of apple spermidine synthase 1 (MdSPDS1) leads to significant salt tolerance in tomato plants. Plant Biotechnol. 28, 33–42. doi: 10.5511/plantbiotechnology.10.1013a
Noctor, G., De Paepe, R., and Foyer, C. H. (2007). Mitochondrial redox biology and homeostasis in plants. Trends Plant Sci. 12, 125–134. doi: 10.1016/j.tplants.2007.01.005
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noctor, G., and Foyer, C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 249–279. doi: 10.1146/annurev.arplant.49.1.249
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noctor, G., Veljovic-Jovanovic, S., Driscoll, S., Novitskaya, L., and Foyer, C. H. (2002). Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann. Bot. 89, 841–850. doi: 10.1093/aob/mcf096
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ozgur, R., Uzilday, B., Sekmen, A. H., and Turkan, I. (2013). Reactive oxygen species regulation and antioxidant defence in halophytes. Funct. Plant Biol. 40, 832–847. doi: 10.1071/FP12389
Öztürk, L., and Demir, Y. (2003). Effects of putrescine and ethephon on some oxidative stress enzyme activities and proline content in salt stressed spinach leaves. Plant Growth Regul. 40, 89–95. doi: 10.1023/A:1023078819935
Pandolfi, C., Pottosin, I., Cuin, T., Mancuso, S., and Shabala, S. (2010). Specificity of polyamine effects on NaCl-induced ion flux kinetics and salt stress amelioration in plants. Plant Cell Physiol. 51, 422–434. doi: 10.1093/pcp/pcq007
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Papageorgiou, G., and Murata, N. (1995). The unusually strong stabilizing effects of glycine betaine on the structure and function of the oxygenevolving Photosystem II complex. Photosynth. Res. 44, 243–252. doi: 10.1007/BF00048597
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Parida, A. K., and Das, A. B. (2005). Salt tolerance and salinity effects on plants: a review. Ecotoxicol. Environ. Safe. 60, 324–349. doi: 10.1016/j.ecoenv.2004.06.010
Pottosin, I., and Shabala, S. (2014). Polyamines control of cation transport across plantmembranes: implications for ion homeostasis and abiotic stress signaling. Front. Plant Sci. 5, 1–16. doi: 10.3389/fpls.2014.00154
Pottosin, I., Velarde-Buendía, A. M., Bose, J., Zepeda-Jazo, I., Shabala, S., and Dobrovinskaya, O. (2014). Cross-talk between reactive oxygen species and polyamines in regulation of ion transport across the plasmamembrane: implications for plant adaptive responses. J. Exp. Bot. 65, 1271–1283. doi: 10.1093/jxb/ert423
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pottosin, I., Velarde-Buendía, A. M., Zepeda-Jazo, I., Dobrovinskaya, O., and Shabala, S. (2012). Synergism between polyamines and ROS intheinduction of Ca(2+) and K(+) fluxes in roots. Plant Signal. Behav. 7, 1084–1087. doi: 10.4161/psb.21185
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pottosin, I. I., Martínez-Estévez, M., Dobrovinskaya, O. R., Muñiz, J., and Schönknecht, G. (2004). Mechanism of luminal Ca2+and Mg2+ action on the vacuolar slowly activating channels. Planta 219, 1057–1070. doi: 10.1007/s00425-004-1293-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Qi, Y. C., Wang, F. F., Zhang, H., and Liu, W. Q. (2010). Overexpression of suadea salsa S –adenosylmethionine synthetase gene promotes salt tolerance in transgenic tobacco. Acta Physiol. Plant. 32, 263–269. doi: 10.1007/s11738-009-0403-3
Radhakrishnan, R., and Lee, I. J. (2013). Ameliorative effects of spermine against osmotic stress through antioxidants and abscisic acidchanges in soybean pods and seeds. Acta Physiol. Plant. 35, 263–269. doi: 10.1007/s11738-012-1072-1
Radhakrishnan, R., and Lee, I. J. (2014). Effect of low dose of spermidine on physiological changes in salt stressed cucumber plants. Russ. J. Plant Physiol. 61, 90–96. doi: 10.1134/S1021443714010129
Rengasamy, P. (2010). Soil processes affecting crop production in salt-affected soils. Funct. Plant Biol. 37, 613–620. doi: 10.1071/FP09249
Rhoads, D. M., Umbach, A. L., Subbaiah, C. C., and Siedow, J. N. (2006). Mitochondrial reactive oxygen species. Contribution to oxidative stress and interorganellar signaling. Plant Physiol. 141, 357–366. doi: 10.1104/pp.106.079129
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rodríguez, A. A., Maiale, S. J., Menéndez, A. B., and Ruiz, O. A. (2009). Polyamine oxidase activity contributes to sustain maize leaf elongation under saline stress. J. Exp. Bot. 60, 4249–4262. doi: 10.1093/jxb/erp256
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Roy, M., and Wu, R. (2002). Overexpression of S-adenosylmethionine decarboxylase gene in rice increases polyamine level and enhances sodium chloride-stress tolerance. Plant Sci. 163, 987–992. doi: 10.1016/S0168-9452(02)00272-8
Roy, P., Niyogi, K., Sengupta, D. N., and Ghosh, B. (2005). Spermidine treatment to rice seedlings recovers salinity stress induced damage of plasma membrane and PM-bound H+-ATPase in salt-tolerant and salt sensitive rice cultivars. Plant Sci. 168, 583–591. doi: 10.1016/j.plantsci.2004.08.014
Roychoudhury, A., Basu, S., and Sengupta, D. N. (2011). Amelioration of salinity stress by exogenously applied spermidine or spermine in three varieties of indica rice differing in their level of salt tolerance. J. Plant Physiol. 168, 317–328. doi: 10.1016/j.jplph.2010.07.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rubio, F., Flores, P., Navarro, J. M., and Martinez, V. (2003). Effects of Ca2+, K+ and cGMP on Na+ uptake in pepper plants. Plant Sci. 165, 1043–1049. doi: 10.1016/S0168-9452(03)00297-8
Sairam, R. K., Roa, K. V., and Srivastava, G. C. (2002). Differential response of wheat genotypes to long term salinity stress in relation to oxidative stress, antioxidant activity and osmolyte concentration. Plant Sci. 163, 1037–1046. doi: 10.1016/S0168-9452(02)00278-9
Saradhi, P., and Mohanty, P. (1997). Involvement of proline in protecting thylakoid membranes against free radical-induced photodamage. J. Photochem. Photobiol. B. Biol. 38, 253–257. doi: 10.1016/S1011-1344(96)07470-2
Šebela, M., Radová, A., Angelini, R., Tavladoraki, P., Frébort, I., and Pec, P. (2001). FAD-containing polyamine oxidases: a timely challenge for researchers in biochemistry and physiology of plants. Plant Sci. 160, 197–207. doi: 10.1016/S0168-9452(00)00380-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shabala, S., Cuin, T. A., and Pottosin, I. I. (2007). Polyamines prevent NaCl- induced K+ efflux from pea mesophyll by blocking non-selective cation channels. FEBS Lett. 581, 1993–1999. doi: 10.1016/j.febslet.2007.04.032
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shabala, S., Demidchik, V., Shabala, L., Cuin, T. A., Smith, S. J., Miller, A. J., et al. (2006). Extracellular Ca2+ ameliorates NaCl-induced K+ loss from Arabidopsis root and leaf cells by controlling plasmamembrane K+- permeable channels. Plant Physiol. 141, 1653–1665. doi: 10.1104/pp.106.082388
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shapiguzov, A., Vainonen, J. P., Wrzaczek, M., and Kangasjarvi, J. (2012). ROS-talk—how the apoplast, the chloroplast, and the nucleus get the message through. Front. Plant Sci. 3:292. doi: 10.3389/fpls.2012.00292
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sheokand, S., Kumari, A., and Sawhney, V. (2008). Effect of nitric oxide and putrescine on antioxidative responses under NaCl stress in chickpea plants. Physiol. Mol. Biol. Plants 14, 355–362. doi: 10.1007/s12298-008-0034-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shevyakova, N. I., Rakitin, V. Y., Stetsenko, L. A., Aronova, E. E., and Kuznetsov, V. V. (2006). Oxidative stress and fluctuations of free and conjugated polyamines in the halophyte Mesembryanthemum crystallinum L. under NaCl salinity. Plant Growth Regul. 50, 69–78. doi: 10.1007/s10725-006-9127-1
Shoresh, M., Spivak, M., and Bernstein, N. (2011). Involvement of calcium-mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Radical Biol. Med. 51, 1221–1234. doi: 10.1016/j.freeradbiomed.2011.03.036
Shu, S., Guo, S. R., Sun, J., and Yuan, L. Y. (2012). Effects of salt stress on the structure and function of the photosynthetic apparatus in Cucumis sativus and its protection by exogenous putrescine. Physiol Plant. 146, 285–296. doi: 10.1111/j.1399-3054.2012.01623.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Shu, S., Yuan, L. Y., Guo, S. R., Sun, J., and Yuan, Y. H. (2013). Effects of exogenous spermine on chlorophyll fluorescence, antioxidant system and ultrastructure of chloroplasts in Cucumis sativus L. under salt stress. Plant Physiol Biochem. 63, 209–216. doi: 10.1016/j.plaphy.2012.11.028
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Silva, E. N., Ribeiro, R. V., Ferreira-Silva, S. L., Viegas, R. A., and Silveira, J. A. G. (2011). Salt stress induced damages on the photosynthesis of physic nut young plants. Sci. Agric. (Piracicaba Braz). 68, 62–68. doi: 10.1590/S0103-90162011000100010
Smith, C. A., Melino, V. J., Sweetman, C., and Soole, K. L. (2009). Manipulation of alternative oxidase can influence salt tolerance in Arabidopsis thaliana. Physiol. Plant. 137, 459–472. doi: 10.1111/j.1399-3054.2009.01305.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sreenivasulu, N., Grimm, B., Wobus, U., and Weschke, W. (2000). Differential response of antioxidant compounds to salinity stress in salt tolerant and salt sensitive seedlings of foxtail millet (Setaria italica). Physiol. Plant. 109, 435–442. doi: 10.1034/j.1399-3054.2000.100410.x
Stowe, D. F., and Camara, A. K. (2009). Mitochondrial reactive oxygen species production in excitable cells: modulators of mitochondrial and cell function. Antioxid. Redoxn. Signal. 11, 1373–1414. doi: 10.1089/ars.2008.2331
Sudhakar, C., Veeranagamallaiah, G., Nareshkumar, A., Sudhakarbabu, O., Sivakumar, M., Pandurangaiah, M., et al. (2015). Polyamine metabolism influences antioxidant defense mechanism in foxtail millet (Setaria italica L.) cultivars with different salinity tolerance. Plant Cell Rep. 34, 141–156. doi: 10.1007/s00299-014-1695-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Szabados, L., and Savouré, A. (2010). Proline: a multifunctional amino acid. Trends Plant Sci. 15, 89–97. doi: 10.1016/j.tplants.2009.11.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takahashi, T., and Kakehi, J.-I. (2010). Polyamines: ubiquitous polycations with unique roles in growth and stress responses. Ann. Bot. 105, 1–6. doi: 10.1093/aob/mcp259
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takahashi, Y., Berberich, T., Miyazaki, A., Seo, S., Ohashi, Y., and Kusano, T. (2003). Spermine signalling in tobacco: activation of mitogen-activated protein kinases by spermine is mediated through mitochondrial dysfunction. Plant J. 36, 820–829. doi: 10.1046/j.1365-313X.2003.01923.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takano, A., Kakehi, J. I., and Takahashi, T. (2012). Thermospermine is not a minor polyamine in the plant kingdom. Plant Cell Physiol. 53, 606–616. doi: 10.1093/pcp/pcs019
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Takeda, S., Gapper, C., Kaya, H., Bell, E., Kuchitsu, K., and Dolan, L. (2008). Local positive feedback regulation determines cell shape in root hair cells. Science 319, 1241–1244. doi: 10.1126/science.1152505
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tang, W., and Newton, R. J. (2005). Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul. 46, 31–43. doi: 10.1007/s10725-005-6395-0
Tanou, G., Job, C., Rajjou, L., Arc, E., Belghazi, M., Diamantidis, G., et al. (2009b). Proteomics reveals the overlapping roles of hydrogen peroxide and nitric oxide in the acclimation of citrus plants to salinity. Plant J. 60, 795–804. doi: 10.1111/j.1365-313X.2009.04000.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tanou, G., Molassiotis, A., and Diamantidis, G. (2009a). Hydrogen peroxide- and nitric oxide-induced systemic antioxidant prime-like activity under NaCl-stress and stress-free conditions in citrus plants. J. Plant Physiol. 166, 1904–1913. doi: 10.1016/j.jplph.2009.06.012
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tanou, G., Ziogas, V., Belghazi, M., Christou, A., Filippou, P., Job, D., et al. (2013). Polyamines reprogram oxidative and nitrosative status and the proteome of citrus plants exposed to salinity stress. Cell Environ. Plant. 37, 864–885. doi: 10.1111/pce.12204
Tiburcio, A. F., Altabella, T., Bitrián, M., and Alcázar, R. (2014). The roles of polyamines during the lifespan of plants: from development to stress. Planta 240, 1–18. doi: 10.1007/s00425-014-2055-9
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Todorova, D., Katerova, Z., Sergiev, I., and Alexieva, V. (2013). “Role of polyamines in alleviating salt stress,” in Ecophysiology and Responses of Plants under Salt Stress, Vol. 13, eds P. Ahmad, M. M. Azooz, and M. N. V. Prasad (New York, NY: Springer), 355–379.
Toumi, I., Moschou, P. N., Paschalidis, K. A., Daldoul, S., Bouamama, B., Chenennaoui, S., et al. (2010). Abscisic acid signals reorientation of polyamine metabolism to orchestrate stress responses via the polyamine exodus pathway in grapevine. J. Plant Physiol. 167, 519–525. doi: 10.1016/j.jplph.2009.10.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tun, N. N., Santa-Catarina, C., Begum, T., Silveira, V., Handro, W., Floh, E. I. S., et al. (2006). Polyamines induce rapid biosynthesis of nitric oxide (NO) in Arabidopsis thaliana seedlings. Plant Cell Physiol. 47, 346–354. doi: 10.1093/pcp/pci252
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tuteja, N., and Sopory, S. K. (2008). Chemical signaling under abiotic stress environment in plants. Plant Signal. Behav. 3, 525–536. doi: 10.4161/psb.3.8.6186
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Urano, K., Maruyama, K., Ogata, Y., Morishita, Y., Takeda, M., Sakurai, N., et al. (2009). Characterization of the ABA regulated global responses to dehydration in Arabidopsis by metabolomics. Plant J. 57, 1065–1078. doi: 10.1111/j.1365-313X.2008.03748.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Urano, K., Yoshiba, Y., Nanjo, T., Ito, T., Yamaguchi-Shinozaki, K., and Shinozaki, K. (2004). Arabidopsis stress-inducible gene for arginine decarboxylase AtADC2 is required for accumulation of putrescine in salt tolerance. Biochem. Biophys. Res. Commun. 313, 369–375. doi: 10.1016/j.bbrc.2003.11.119
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Van Breusegem, F., Vranova, E., Dat, J. F., and Inzé, D. (2001). The role of active oxygen species in plant signal transduction. Plant Sci. 161, 405–414. doi: 10.1016/S0168-9452(01)00452-6
Velarde-Buendia, A. M., Shabala, S., Cvikrova, M., Dobrovinskaya, O., and Pottosin, I. (2012). Salt-sensitive and salt-tolerant barley varieties differ in the extent of potentiation of the ROS-induced K(+) efflux by polyamines. Plant Physiol. Biochem. 61, 18–23. doi: 10.1016/j.plaphy.2012.09.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Verma, S., and Mishra, S. N. (2005). Putrescine alleviation of growth in salt stressed Brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 162, 669–677. doi: 10.1016/j.jplph.2004.08.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Walters, D. R. (2003). Polyamines and plant diseases. Phytochemistry 64, 97–107. doi: 10.1016/S0031-9422(03)00329-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wen, X. P., Pang, X. M., Matsuda, N., Kita, M., Inoue, H., Hao, Y. J., et al. (2008). Overexpression of the apple spermidine synthase gene in pear confers multiple abiotic stress tolerance by altering polyamine titers. Transgenic Res. 17, 251–263. doi: 10.1007/s11248-007-9098-7
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wi, S., Kim, W. T., and Park, K. Y. (2006). Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 25, 1111–1121. doi: 10.1007/s00299-006-0160-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Williamson, J. D., Jennings, D. B., Guo, W. W., Pharr, D. M., and Ehrenshaft, M. (2002). Sugar alcohols, salt stress, and fungal resistance: polyols— multifunctional plant protection? J. Am. Soc. Horti. Sci. 127, 467–473.
Wimalasekera, R., Tebartz, F., and Scherer, G. F. (2011). Polyamines, polyamine oxidases and nitric oxide in development, abiotic and biotic stresses. Plant Sci. 181, 593–603. doi: 10.1016/j.plantsci.2011.04.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wu, J. Y., Shang, Z. L., Wu, J., Jiang, X. T., Moschou, P. N., Sun, W. D., et al. (2010). Spermidine oxidase-derived H2O2 regulates pollen plasma membrane hyperpolarization-activated Ca2+- permeable channels and pollen tube growth. Plant J. 63, 1042–1053. doi: 10.1111/j.1365-313X.2010.04301.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yamaguchi, K., Takahashi, Y., Berberich, T., Imai, A., Miyazaki, A., Takahashi, T., et al. (2006). The polyamine spermine protects against high salt stress in Arabidopsis thaliana. FEBS Lett. 580, 6783–6788. doi: 10.1016/j.febslet.2006.10.078
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yamasaki, H., and Cohen, M. F. (2006). NO signal at the crossroads: polyamine induced nitric oxide synthesis in plants. Trends Plant Sci. 11, 522–524. doi: 10.1016/j.tplants.2006.09.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yan, Z., Guo, S., Shu, S., Sun, J., and Tezuka, T. (2011). Effects of proline on photosynthesis, root reactive oxygen species (ROS) metabolism in two melon cultivars (Cucumis melo L.) under NaCl stress. Afr. J. Biotechnol. 10, 18381–18390. doi: 10.5897/AJB11.1073
Yoda, H., Hiroi, Y., and Sano, H. (2006). Polyamine oxidase is one of the key elements for oxidative burst to induce programmed cell death in tobacco cultured cells. Plant Physiol. 142, 193–206. doi: 10.1104/pp.106.080515
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zepeda-Jazo, I., Shabala, S., Chen, Z., and Pottosin, I. I. (2008). Na+-K+ transport in roots under salt stress. Plant Signal. Behav. 3, 401–403. doi: 10.4161/psb.3.6.5429
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zepeda-Jazo, I., Velarde-Buendía, A. M., Enríquez-Figueroa, R., Bose, J., Shabala, S., Muñiz-Murguía, J., et al. (2011). Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membranes. Plant Physiol. 157, 2167–2180. doi: 10.1104/pp.111.179671
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y., Tan, J., Guo, Z., Lu, S., He, S., Shu, W., et al. (2009). Increased abscisic acid levels in transgenic tobacco over-expressing 9 cis-epoxycarotenoid dioxygenase influence H2O2 and NO production and antioxidant defences. Plant Cell Environ. 32, 509–519. doi: 10.1111/j.1365-3040.2009.01945.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhang, Y., Wang, L., Liu, Y., Zhang, Q., Wei, Q., and Zhang, W. (2006). Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224, 545–555. doi: 10.1007/s00425-006-0242-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, F., Song, C. P., He, J., and Zhu, H. (2007). Polyamines improve K+/Na+ homeostasis in barley seedlings by regulating root ion channel activities. Plant Physiol. 145, 1061–1072. doi: 10.1104/pp.107.105882
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhao, L. Q., Zhang, F., Guo, J. K., Yang, Y. L., Li, B. B., and Zhang, L. X. (2004). Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol. 134, 849–857. doi: 10.1104/pp.103.030023
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zhu, J. K. (2003). Regulation of ion homeostasis under salt stress. Curr. Opin. Plant Biol. 6, 441–445. doi: 10.1016/S1369-5266(03)00085-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zrig, A., Tounekti, T., Vadel, A. M., Mohamed, H. B., Valero, D., Serrano, M., et al. (2011). Possible involvement of polyphenols and polyamines in salt tolerance of almond rootstocks. Plant Physiol. Biochem. 49, 1313–1322. doi: 10.1016/j.plaphy.2011.08.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: polyamine signaling, ROS, plant abiotic stress, salinity stress, redox homeostasis
Citation: Saha J, Brauer EK, Sengupta A, Popescu SC, Gupta K and Gupta B (2015) Polyamines as redox homeostasis regulators during salt stress in plants. Front. Environ. Sci. 3:21. doi: 10.3389/fenvs.2015.00021
Received: 11 December 2014; Accepted: 05 March 2015;
Published: 01 April 2015.
Edited by:
Naser A. Anjum, University of Aveiro, PortugalReviewed by:
Naser A. Anjum, University of Aveiro, PortugalCopyright © 2015 Saha, Brauer, Sengupta, Popescu, Gupta and Gupta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bhaskar Gupta, Department of Biological Sciences, Presidency University, 86/1 College Street, Kolkata 700073, IndiaYmhhc2thci5kYnNAcHJlc2l1bml2LmFjLmlu;Ymhhc2thcnpvb2xvZ3lAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.