
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Sci. , 19 March 2015
Sec. Environmental Toxicology
Volume 3 - 2015 | https://doi.org/10.3389/fenvs.2015.00020
This article is part of the Research Topic Redox homeostasis managers in plants under environmental stresses View all 18 articles
Plants can maintain growth and reproductive success by sensing changes in the environment and reacting through mechanisms at molecular, cellular, physiological, and developmental levels. Each stress condition prompts a unique response although some overlap between the reactions to abiotic stress (drought, heat, cold, salt or high light) and to biotic stress (pathogens) does occur. A common feature in the response to all stresses is the onset of oxidative stress, through the production of reactive oxygen species (ROS). As hydrogen peroxide and superoxide are involved in stress signaling, a tight control in ROS homeostasis requires a delicate balance of systems involved in their generation and degradation. If the plant lacks the capacity to generate scavenging potential, this can ultimately lead to death. In grapevine, antioxidant homeostasis can be considered at whole plant levels and during the development cycle. The most striking example lies in berries and their derivatives, such as wine, with nutraceutical properties associated with their antioxidant capacity. Antioxidant homeostasis is tightly regulated in leaves, assuring a positive balance between photosynthesis and respiration, explaining the tolerance of many grapevine varieties to extreme environments. In this review we will focus on antioxidant metabolites, antioxidant enzymes, transcriptional regulation and cross-talk with hormones prompted by abiotic stress conditions. We will also discuss three situations that require specific homeostasis balance: biotic stress, the oxidative burst in berries at veraison and in vitro systems. The genetic plasticity of the antioxidant homeostasis response put in evidence by the different levels of tolerance to stress presented by grapevine varieties will be addressed. The gathered information is relevant to foster varietal adaptation to impending climate changes, to assist breeders in choosing the more adapted varieties and suitable viticulture practices.
Plants are able to maintain growth and reproductive success by sensing changes in the surrounding environment and reacting through mechanisms at the molecular, cellular, physiological, and developmental levels. These response mechanisms enable plants to react rapidly, within hours or days, to extreme environmental conditions that could otherwise be injuring or lethal. Understanding stress responses is one of the most important issues in plant research nowadays. Both biotic and abiotic stresses can promote the onset of oxidative stress through the accumulation of reactive oxygen species (ROS). Worldwide, extensive agricultural losses are attributed to drought, often in combination with heat (Mittler, 2006). The available scenarios for climate change suggest increases in aridity in Mediterranean climate regions (Jones et al., 2005), where grapevine traditionally grows. This species is an extremely important crop worldwide, at the economic and cultural levels. In Southern Europe, post flowering phases of the growth cycle usually occur under high temperatures, excessive light and drought conditions at soil and/or atmospheric level. In such situations plants are affected by a combination of abiotic and biotic stresses, triggering synergistic or antagonistic physiological, metabolic or transcriptomic responses unique to each stress combination. Oxidative stress also arises in in vitro propagation commonly applied to ornamental species, also used to rapidly propagate grapevine scions for grafting (Carvalho and Amâncio, 2002).
The ultimate “price” to pay for photosynthetic O2 release and plant aerobic metabolism is the production of ROS. ROS production can also be increased by stress conditions (Apel and Hirt, 2004). When photosynthesis is inhibited, absorption of light energy can be in excess to what can be used by the photosynthetic processes, resulting in ROS production and accumulation. The same is true when stress induced slowdown of other ROS processing metabolic mechanisms results in ROS accumulation. Climate change forecasts indicate a high probability of extreme temperature episodes, both high and low, a decrease in water availability as well as increases in carbon oxide and ozone in the atmosphere. All these factors impact plant growth and development by negatively affecting antioxidant homeostasis, hampering the adaptation to environmental stressors (Munné-Bosch et al., 2013).
A molecule is classified as “antioxidant” when it is able to quench ROS without itself undergoing conversion into a destructive radical, thus interrupting the cascades of uncontrolled oxidation. In that category are included, among others, the metabolites ascorbic acid (AsA, also termed vitamin C), glutathione (GSH), and carotenoid pigments. The ROS signaling or degradation pathways depend on antioxidant redox buffering enzymes such as superoxide dismutase (SOD), catalase (CAT), peroxiredoxins (Prx), ascorbate peroxidase (APX), and glutathione reductase (GOR) (Carvalho et al., 2006; Vidigal et al., 2013).
Upon abiotic stress gene expression profiles are altered and usually genes assigned to the functional categories “protein metabolism and modification,” “signaling” and “antioxidative response” undergo significant changes (Carvalho et al., 2011; Rocheta et al., 2014), thus enhancing the common attributes of abiotic stress defense pathways.
The nutraceutical properties of the grape berry and its derivatives, namely wine, are commonly associated with the antioxidant properties of the phenolic compounds they contain (Tenore et al., 2011; De Nisco et al., 2013), from simple flavonoids like anthocyanins to condensed proanthocyanidins (PAs, tannins), which can be solubilized into the vacuole or linked to cell wall polysaccharides, so, it is of great interest to understand their antioxidant mechanisms in planta.
The different levels of tolerance to stress presented by grapevine varieties relates directly to the genetic plasticity of the antioxidant homeostasis. Some varieties keep low basal levels of antioxidant metabolites thus having to synthesize them at the onset of stress. Such varieties have a slower response than those with higher basal levels of antioxidant metabolites (Carvalho et al., 2014). This is put in evidence by the different pattern of antioxidant metabolites in normal growth conditions, as shown in Table 1.
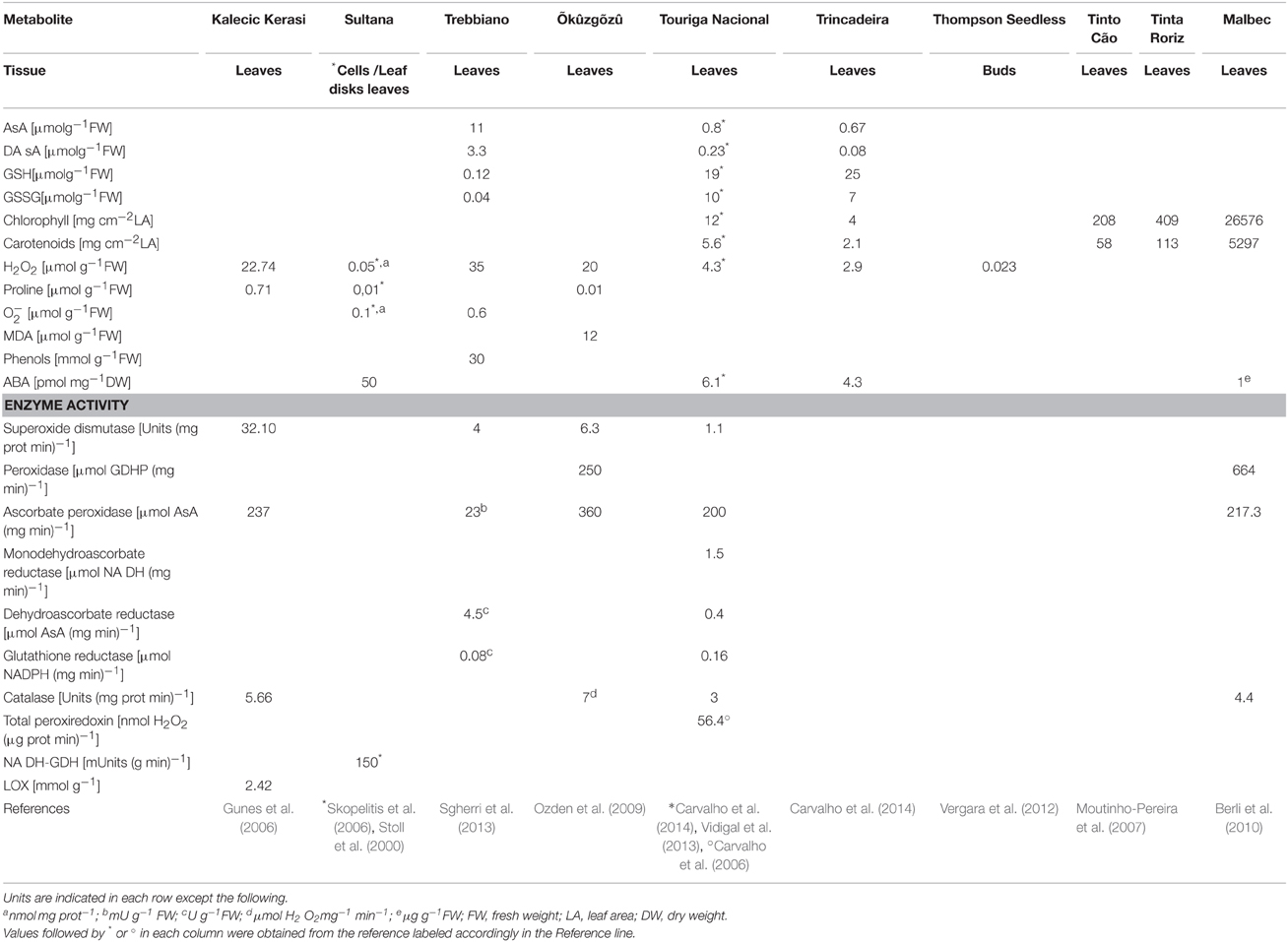
Table 1. Concentration of antioxidative metabolites and activities of antioxidative enzymes quantified in several grapevine varieties and in different tissues.
The first players in antioxidant homeostasis, which in normal conditions induce the need for detoxification/scavenging, are ROS themselves. In chloroplasts O−2 produced in the Mehler reaction occurs in normal conditions, commonly increasing upon stress. It is now believed that O−2 formation is the first step in a chain reaction leading to the control and regulation of several cellular processes (Apel and Hirt, 2004), with ROS integrated into signaling pathways (Mullineaux et al., 2006), often in crosstalk with hormonal regulation (Fujita et al., 2006). A mechanism of acclimation of Nicotiana benthamiana to high light, driven by the hormone abscisic acid (ABA) and by the accumulation of H2O2, also involving Prxs was recently described in Vidigal et al. (2014).
When the energy from triplet excited chlorophylls is transferred to molecular oxygen, 1O2 is formed. This ROS is a strong electrophile agent that can react with lipids, proteins, and DNA (Triantaphylidès and Havaux, 2009). However, 1O2 also reacts with target mediator molecules which trigger signaling cascades that lead either to programmed cell death or to acclimation (Ramel et al., 2012). In grapevine leaves, 1O2 and also H2O2 are generated in trace-element stress, such as that caused by boron in excess (Gunes et al., 2006).
Peroxisomes are probably the major sites of intracellular H2O2 production, although O−2 and nitric oxide radicals (NO·) are also produced in peroxisomes. The photoinhibition that is verified in grapevine leaves upon drought and salinity stress is accompanied of an increase in transcription of genes coding for peroxisome glycolate oxidase, catalase and several photorespiratory enzymes (Cramer et al., 2007).
L-ascorbic acid (AsA) is an abundant metabolite playing important roles in plant stress physiology as well as in growth and development. AsA is a key antioxidant (Conklin and Loewus, 2001), able to directly eliminate several different ROS (Potters et al., 2002). Both the chloroplastic lipophilic antioxidant α-tocopherol (vitamin E) and carotenoid pigments (carotenes and xanthophylls) depend on AsA for regeneration from oxidized radicals (Potters et al., 2002). AsA is also the most important H2O2 reducing substrate, acting together with glutathione (GSH, γ-L-Glu-L-Cys-Gly) in the ascorbate-glutathione cycle (see Section The Ascorbate-Glutathione Cycle) (Noctor and Foyer, 1998). GSH is a multifunctional metabolite in plants, being a major reservoir of non-protein reduced sulfur, and a crucial element in cellular defense and protection, preventing the denaturation of proteins caused by oxidation of thiol groups during stress, reacting chemically with a wide range of ROS (Noctor et al., 2002). In grapevine, AsA and GSH pools and their adjustments upon stress seem to be variety dependent and under tight control (Carvalho et al., 2014). In cv. “Touriga Nacional” facing oxidative stress the existing AsA and GSH pools assure the cell buffering capacity while in cv. “Trincadeira” AsA and GSH need to be synthesized de novo, leading to a slower response that may be insufficient to maintain the redox pool at working levels (Table 1).
Carotenoids protect the photosynthetic apparatus against photo-oxidative damage not only by quenching the triplet states of chlorophyll molecules (Koyama, 1991) but also by scavenging ROS, protecting pigments and unsaturated fatty acids from oxidative damage (Edge et al., 1997). In the grapevine variety “Trincadeira” subjected to heat stress, carotenoids play an important role in leaf ROS scavenging, in tandem with ascorbate and glutathione, the usual first line of antioxidative defense in plants (Carvalho et al., 2014, Table 1).
The metabolism of proline, including proline oxidation, is extremely important in the response to stress, as it is one of the most widespread osmoprotectants (Kiyosue et al., 1996), increasing in conditions of water deficit, as shown in the grapevine cv. Riesling (Bertamini et al., 2006). Also in grapevine, ROS generated by salinity stress signal the expression of GDH α-subunit, GDH acting as an anti-stress enzyme not only by detoxifying ammonia but also by producing glutamate which is channeled to proline synthesis (Skopelitis et al., 2006). Artificially increased proline levels also lead to the decrease of H2O2 and malondialdehyde. It was thus suggested that the crosstalk between proline and H2O2 could play an important role in the response to oxidative stress in grapevine leaves (Ozden et al., 2009, Table 1). Proline accumulation increased by two-fold upon salinity stress and by three-fold after water stress, and was accompanied by an increase in transcript abundance of plasma membrane proline transporters and of pyrroline-5-carboxylate synthetase (P5CS), the enzyme that catalyzes the first two steps in proline biosynthesis (Cramer et al., 2007). In parallel, there was an increased transcript abundance of proline dehydrogenase, presumably to enable the removal of excess proline, which can be toxic if allowed to over accumulate (Cramer et al., 2007). Different types of stress can reduce proline levels as it happens in excess boron, leading to an increased lipid peroxidation and APX depletion (Gunes et al., 2006).
Redox homeostasis comprises the interaction of ROS with antioxidant molecules forming an interface for metabolic and environmental signals, thus modulating the induction of appropriate acclimation processes or cell death programs. In the chloroplasts, a decrease of CO2 fixation together with an over-reduction of the ETC is the foremost source of ROS production during stress; in mitochondria over-reduction of the respective ETC is also a chief mechanism of ROS generation (Yoshida et al., 2007) and in peroxisomes, H2O2 is produced when glycolate is oxidized to glyoxylic acid during photorespiration (Mittler et al., 2004).
ROS signaling pathways depend upon a strict homeostatic regulation accomplished through antioxidant redox buffering. Antioxidants determine the lifetime and the specificity of the ROS signal or processing products. In this process, enzymes such as SOD, CAT, Prx, APX, and GOR are the key players in antioxidant homeostasis (Carvalho et al., 2006; Vidigal et al., 2013). See Table 1 for reference values of enzyme activity in different grapevine varieties. A thorough search of the genes coding for these enzymes in grapevine was performed in NCBI (http://www.ncbi.nlm.nih.gov/) and 297 sequences were retrieved, including 109 peroxidases (Supplementary Table 1). Functional annotation is still incomplete and many of those sequences are redundant.
Phylogenetic dendrograms of grapevine non-redundant sequences of APX, SOD, CAT, GOR, and Prx gene families were generated based on the members annotated so far of Vitis vinifera, Arabidopsis thaliana, Populus trichocarpa, and Oryza sativa (var. japonica) retrieved from NCBI (Supplementary Figure 1). From the analysis of those dendrograms it was observed that V. vinifera has as many isoforms as A. thaliana and also has the highest sequence homology with this species. However, further annotation is still necessary such as in the case of GOR, for which no records of peroxisome and/or mitochondria isoforms are available (Figure 1).
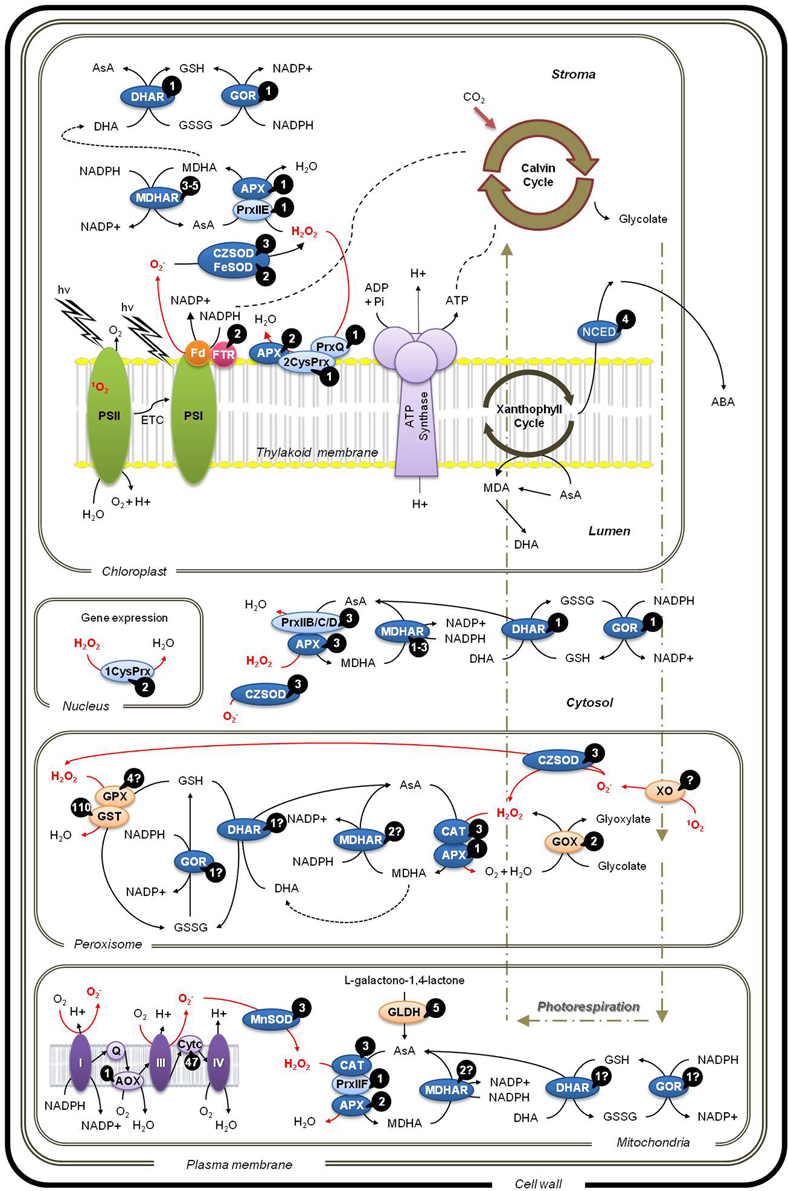
Figure 1. Localization of reactive oxygen species (ROS) scavenging pathways in different cellular compartments. ROS: 1O2, hydroxyl radical; H2O2, hydrogen peroxide; O−2, superoxide. Water-water cycle and ascorbate-glutathione cycle: APX, ascorbate peroxide; GOR, glutathione reductase; SOD, superoxide dismutase; CAT, catalase; DHAR, dehydroascorbate reductase; MDHAR, monodehydroascorbate reductase. Peroxiredoxin-mediated alternative water–water cycle: 1CysPrx, 1-cysteine peroxiredoxin; 2CysPrx, 2-cysteine peroxiredoxin; PrxII; Type II-peroxiredoxin; PrxQ, peroxiredoxin Q. ABA, abscisic acid; AOX, alternative oxidase; AsA, ascorbate; Cytc, cytochrome c; DHA, dehydroascorbate; ETC; electron transport chain; Fd, ferredoxin; FTR, ferredoxin-thioredoxin reductase; GLDH, L-galactono-1,4-lactone dehydrogenase; GOX, glycolate oxidase; GPX, glutathione peroxidase; GSH, reduced glutathione; GSSG, oxidized glutathione; GST, glutathione S-transferase; MDA, monodehydroascorbate; NCED, 9-cis-epoxycarotenoid dioxygenase; PSI and II, photosysthem I and II; Q, Coenzyme Q; XO, xanthine oxidase. Numbers in black circles indicate known isoforms in V. vinifera. The question marks indicate uncertainty on the actual number of isoforms, based in NCBI records; the given number is the most likely value.
Under normal conditions, electrons obtained from the splitting of water molecules at PS II are channeled through the photosynthetic apparatus and transferred to molecular oxygen by PS I. Under stress conditions that decrease CO2 availability due to stomata closure or increase exposure to continuous excessive light there is an excess of electron transfer toward molecular oxygen, generating O−2 ions in PS I, through the Mehler reaction (Asada, 2006). In this situation, redox homeostasis can be guaranteed in two consecutive steps, the membrane attached copper/zinc superoxide dismutase (CZSOD) which converts O−2 into H2O2 that is redirected to the ascorbate-glutathione cycle, where it is converted to water. This whole process is referred to as the water–water cycle (Rizhsky et al., 2003) as depicted in Figure 1. In “Trincadeira” grapevines submitted to heat stress both CZSOD and FeSOD are induced to scavenge plastidial O−2 and the resulting H2O2 is scavenged by the MDHAR-GOR branch of the ascorbate-glutathione cycle (Carvalho et al., 2014).
Peroxisomes are subcellular organelles with a single membrane that exist in almost all eukaryotic cells, containing as basic enzymatic constituents CAT and H2O2-producing flavin oxidases (Corpas et al., 2001; Figure 1). Despite their simplicity, they perform essential functions (Del Río et al., 2006); namely in the antioxidative metabolism. They have an essentially oxidative type of metabolism, and great metabolic plasticity, as their enzymatic content varies with the organism, cell/tissue-type and environmental conditions (Baker and Graham, 2002). Upon photo-oxidative stress the peroxisomal CAT is the most responsive of catalases in grapevine (Carvalho et al., 2011; Vidigal et al., 2013) and it was recently shown that peroxisomal CAT influences Prx activity in the cytosol of N. benthamiana (Vidigal et al., 2014).
Peroxidases are a large family of ubiquitous enzymes that have numerous roles in plant metabolism (For review, Passardi et al., 2005), including that of removing the H2O2 formed as a consequence of stress. In the grapevine genome 109 peroxidases were found (Supplementary Table 1). They use different electron donors, such as AsA, in the case of APX in the ascorbate-glutathione cycle; glutathione peroxidase (GPX) uses GSH as its reductant and the generically termed “peroxidases” use phenolic compounds (able to use guaiacol as substrate sometimes they are called “guaiacol-peroxidases”). In grapevine, GPX has been implicated in stress responses against Elsinoe ampelina and Rhizobium vitis and GPX is up-regulated in response to abiotic stresses such as drought and salinity (Cramer et al., 2007).
The main role of APX is the scavenging of H2O2 in the ascorbate-glutathione cycle, keeping its levels tightly controlled in order to maintain redox homeostasis, a similar role as that of GPX (Asada, 2006). Conversely, peroxidases oxidize a large variety of organic substrates and the resulting products are involved in important biosynthetic processes, such as lignification of the cell wall, degradation of IAA, biosynthesis of ethylene, wound healing, and defense against pathogens (Kvaratskhelia et al., 1997).
The reduction of H2O2 undertaken by AsA is only possible due to the activity of APX, an enzyme that uses two molecules of AsA to reduce H2O2 to water. AsA itself is oxidized to monodehydroascorbate (MDHA), an unstable compound that suffers spontaneous disproportionation to AsA and dehydroascorbate (DHA) (Potters et al., 2002). Monodehydroascorbate reductase (MDHAR) regenerates AsA from MDHA, using NADPH as a reducing agent and DHA is reduced to AsA through the action of dehydroascorbate reductase (DHAR), using GSH as the reducing agent. In this reaction GSH is oxidized to GSSG that, in turn is reduced back to GSH using NADPH as reducing potential. This is the ascorbate-glutathione cycle, also called Foyer-Halliwell-Asada cycle (Figure 1), where AsA and GSH act together to detoxify H2O2 in a cycle of oxidation-reduction, without being consumed and using electrons derived from NAD(P)H (Noctor and Foyer, 1998).
In grapevine, function of the ascorbate-glutathione cycle and its contribution to the detoxification process depend upon several factors, namely, the type, intensity and duration of the stress and the genotype under study. This became quite evident in a study comparing two greenhouse-grown genotypes (“Trincadeira” and “Touriga Nacional”) subjected to heat stress with previous acclimation to moderate heat stress (Carvalho et al., 2014). The levels of expression of the plastidial SOD genes (CZSOD and FeSOD) were induced, mostly in “Trincadeira,” suggesting the scavenge of chloroplast O−2 as a consequence of over-reduction of the ETC, while in “Touriga Nacional” only an increase of O−2 scavenging in the mitochondria (through MnSOD) was observed. H2O2 accumulation induced the expression of CAT, APX1, and APX3, together with the MDHAR-GOR branch of the ascorbate-glutathione cycle in both genotypes. This, together with similar results obtained for micropropagated grapevine subjected to high light (Carvalho et al., 2006) and of plants under viral infection (Sgherri et al., 2013) shows that, upon a severe stress, MDHAR alone is unable of maintaining the AsA pool in the reduced form, thus calling for the function of the whole ascorbate-glutathione cycle. After 3 days of acclimation to high light (Carvalho et al., 2006) or 24 h after the end of heat stress (Carvalho et al., 2014), the MDHAR branch of the cycle can keep the ascorbate pool reduced. However, the activation of the ascorbate-glutathione cycle is not, on its own, a trustworthy indicator of oxidative stress, as its activity can be triggered by differentiation of emerging structures in developing plants (Carvalho and Amâncio, 2002; Carvalho et al., 2006).
Glutathione S-transferases (GSTs) are enzymes that detoxify cytotoxic compounds by conjugation of GSH to several hydrophobic, electrophilic substrates (Marrs, 1996). Plant GSTs have been intensively studied because of their ability to detoxify herbicides, and several GSTs conferring herbicide tolerance have been characterized in many major crop species. Another plant GST subclass is implicated in stress responses, including those arising from pathogen attack, oxidative stress, and heavy-metal toxicity. In grapevine, the induction of GSTs upon pathogen attack occurs in parallel with that of phenylalanine ammonia-lyase (PAL) and stilbene synthase (STS) (Aziz et al., 2004). GSTs also play a role in the cellular response to auxins and during the normal metabolism of plant secondary products like anthocyanins and cinnamic acid. In grapevine, 107 GST isoforms were found (Supplementary Table 1) and seven genes belonging to the phi class and 57 belonging to the tau class, the two most important classes of plant-specific GSTs (Edwards et al., 2000), are implicated in anthocyanin metabolism during berry development (Zenoni et al., 2010), GST1 and GST4 being implicated in anthocyanin transport to the vacuole (Conn et al., 2008). Also in grapevine, the expression of several GSTs genes was affected by defoliation (Pastore et al., 2013).
Prxs catalyze the reduction of H2O2, alkylhydroperoxides, and peroxynitrite to water, alcohols, or nitrite, respectively (for review, Dietz, 2011; Figure 1). Prxs are redox sensitive proteins that can undergo reversible oxidation–reduction and as a result, switch “on” and “off” depending on the redox state of the cell. In V.vinifera, under conditions of light and heat stress, Prx activity decreased as a result of the decrease in H2O2 levels while, under water stress, Prx activity increased, mirroring the increase in H2O2 (Vidigal et al., 2013).
2CysPrx is the most abundant stromal protein, protecting the photosynthetic apparatus against oxidative stress (König et al., 2003). When in deficiency leads to inhibition of photosynthesis, decrease in chlorophyll and impaired grapevine development (Vidigal et al., 2013). In light of its function in photosynthesis, Vv2CysPrx01 (Vidigal et al., 2013), and Vv2CysPrxB were up-regulated in grapevine under light stress while Vv2CysPrxA was down-regulated under similar light stress conditions (Carvalho et al., 2011). These results were different from those obtained in A. thaliana, where an increase in light intensity resulted in little consequence to the expression of 2CysPrxA and 2CysPrxB (Horling et al., 2003). Transcription of 2CysPrxA is induced by H2O2 and repressed by ABA (Baier et al., 2004). In grapevine several ABA-responsive-genes were consistently up-regulated (Carvalho et al., 2011), which could be an explanation for the discrepancy in Prx expression between these studies. The other possibility could be connected to the dual function of Prx, both in antioxidant defense and in signaling (Dietz, 2003). Vv2CysPrx01 was up-regulated in grapevine under heat and water stress (Vidigal et al., 2013) a result that could be related with the chaperone function of 2CysPrx (Kim et al., 2010) and its role in drought tolerance (Rey et al., 2005).
PrxQ has a specific function in protecting photosynthesis, different from that of 2CysPrx (Lamkemeyer et al., 2006). In grapevine under abiotic stresses, VvPrxQ was either repressed or unresponsive (Vidigal et al., 2013), the same result as after 24 h of high light in in vitro propagated plants (Carvalho et al., 2011). However, in the same work, after 48 h, PrxQ transcripts increased significantly, pointing to a delayed transcription response. PrxQ is responsive to H2O2 and ABA (Guo et al., 2004), and in grapevine, neither H2O2 nor ABA increased upon light stress, another explanation for the verified discrepancies.
1CysPrx is a seed specific Prx that is targeted to the cytosol (Dietz, 2011). In grapevine leaves Vv1CysPrx03 is located in the cytosol and is connected to drought and heat tolerance (Vidigal et al., 2013).
PrxIIC was very responsive to light stress in grapevine, with the tendency to increase with time (Carvalho et al., 2011). In A. thaliana, PrxIIE expression was highly induced by light stress (Horling et al., 2003), while in grapevine it was down-regulated (Carvalho et al., 2011; Vidigal et al., 2013). However, the up-regulation of PrxIIE in grapevine under water stress, correlating well with the increase in H2O2, suggests a role in drought tolerance (Vidigal et al., 2013). The expression of the mitochondrial isoform, VvPrxIIF, was unaltered upon high light (Carvalho et al., 2011; Vidigal et al., 2013), in agreement with results obtained in poplar under photo-oxidative conditions or heavy-metal treatments (Gama et al., 2007). Conversely, it was up-regulated in grapevine under heat and water stress, with strong correlation with H2O2 and ABA (Vidigal et al., 2013) suggesting a role for VvPrxIIF under light independent stress conditions.
Two new possible chloroplast Prx genes were identified in grapevine, VvPrxII-1 and VvPrxII-2 (Vidigal et al., 2013). Transcript levels of VvPrxII-2 showed a strong response to heat stress and an analysis of its promoter region revealed the presence of the ABA-responsive element ABRE. Furthermore, the up-regulation of VvPrxII-2 was positively correlated with ABA concentration, suggesting that this Prx gene may play a role in ABA-mediated heat tolerance (Vidigal et al., 2013) while VvPrxII-1 transcripts were down-regulated under light stress and significantly up-regulated under water stress.
Phytohormones are essential for the ability of plants to adapt to stresses by mediating a wide range of adaptive responses often by the regulation of gene expression mediated by the ubiquitin–proteasome degradation of transcriptional regulators (Santner and Estelle, 2009). One of the key players in the response of plants to abiotic stress, especially when involving water shortage, is ABA. However, other hormones such as cytokinins (CK), salicylic acid (SA), ethylene (ET), and jasmonic acid (JA) play significant roles in keeping cell homeostasis under oxidative stress, and their interactions are schematized in Figure 2.
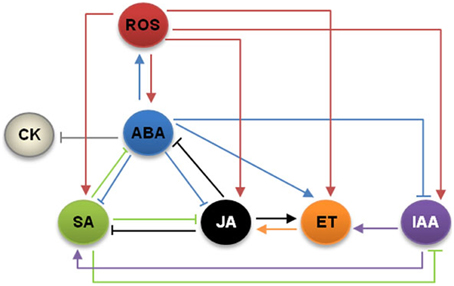
Figure 2. Crosstalk with hormone signals. ROS, reactive oxygen species; ABA, abscisic acid; CK, cytokinins; SA, salicylic acid; JA, jasmonic acid; ET, ethylene; IAA, auxins.
Drought and high salinity result in strong increases of plant ABA levels, accompanied by major changes in gene expression and in adaptive physiological responses (Seki et al., 2002; Rabbani et al., 2003). The expression of ABA-inducible genes that leads to stomatal closure, thus reducing water loss through transpiration and eventually restricting cellular growth, occurs promptly upon the sensing of stress (Peleg and Blumwald, 2011). Several loss of function mutants for genes related to de novo ABA synthesis, to ABA receptors and to downstream signaling are now available in several species (Fang et al., 2008; Cutler et al., 2010). In grapevine however, such tools are not available, thus ABA research has been taking place in a more classical approach.
To improve plant water status and decrease leaf temperature of grapevine plants different irrigation regimes are applied in Mediterranean vineyards (Costa et al., 2012). One method of applying regulated irrigation is by partial root-zone drying (PRD). The main rationale to use PRD in grapevine is the action of ABA in modeling stomatal conductance and the demonstration that, by keeping some root areas dry and others wet, the necessary hormonal signals to regulate stomatal conductance are provided by the dry root-zones and the water needed to prevent severe water deficit is delivered by the wet root-zones (Stoll et al., 2000). ABA accumulation depends both on an accelerated ABA biosynthesis under water deficit, and on the rate of ABA catabolism and conjugation, which is quite fast, and is the main factor controlling the disappearance of ABA signal (Jia and Zhang, 1997). Thus, the accumulation of this so-called stress ABA is controlled by a dynamic equilibrium between ABA biosynthesis at the level of NCED (9-cis-epoxycarotenoid dioxygenase) transcription and its catabolism and conjugation.
In berries, the onset of ripening (veraison) when anthocyanin accumulation begins in red varieties, is accompanied by a marked increase in ABA concentration (Deluc et al., 2009; Gambetta et al., 2010) and correlates well with sugar accumulation (Gambetta et al., 2010). ABA has been shown to activate anthocyanin biosynthetic genes and the anthocyanin-synthesis related VvmybA1 transcription factor (Jeong et al., 2004), and to induce the delay of expression of proanthocyanidin biosynthetic genes (VvANR and VvLAR2) (Lacampagne et al., 2009).
CK exert an opposite function as ABA, and CK levels decrease upon water shortage (Peleg and Blumwald, 2011). In grapevine the effect of ABA on root growth may be augmented by a reduction in CK concentration in the roots that leads to the enhanced ABA to CK ratios obtained in PRD irrigation cycles (Stoll et al., 2000), since it is known that root growth is inhibited by increased endogenous CK (Werner and Schmülling, 2009). In fact, it is possible to reverse the effects of PRD in stomatal conductance by exogenous application of benzyladenine, that also leads to lateral shoot development (Stoll et al., 2000). Also, when comparing fully irrigated and PRD vines a significant decrease in zeatin and zeatin riboside concentration in shoot tips and axillary buds is observed (Dry and Loveys, 1999).
SA is a phenolic plant growth regulator, with several physiological and biochemical functions (Raskin, 1992) under normal conditions and, especially, in the response to abiotic stresses, namely heat stress. SA is known to counteract the effects of heat stress by up-regulating the antioxidative system. The treatment of grapevine leaves with SA before, during and after heat stress maintained photosynthesis rates high, chiefly by keeping high levels of Rubisco activation state, and also accelerated the recovery of photosynthesis through effects on PS II function. These effects may be partially related to the presence of a heat shock protein, HSP21, during the recovery period in SA-treated leaves (Wang et al., 2010). Treatment with SA also protects mesophyll cells against cold and heat stress in leaves of young grape plants, affecting Ca2+ homeostasis, and enzymatic and non-enzymatic components of the ascorbate-glutathione cycle (Wang and Li, 2006). SA treatment also induced the expression of PAL and the synthesis of new PAL protein and increased its activity in grape berries (Wen et al., 2005). PAL is a crucial enzyme of the phenylpropanoid metabolism, catalyzing the formation of trans-cinnamic acid. Its induction results in the accumulation of phenolic acids and flavonoids, thus enhancing the quality of berries.
JA and their derivatives are known to play important roles in activating genes coding for proteins involved in the defense against abiotic (drought, salt, and ozone) and biotic (insects and microbial pathogens) stresses. The activity of JA responses can be regulated by antagonistic cross-talk with SA signaling (for review, Balbi and Devoto, 2008). In fact, SA can suppress the JA-dependent response to wounding and pathogen or insect attack (Leon-Reyes et al., 2010).
In grapevine, salt stress and biotic defense signaling share common pathways, e.g., the activity of a gadolinium-sensitive calcium influx channel and transient induction of JAZ/TIFY transcripts. Exogenous JA application can rescue growth in salt-sensitive Vitis riparia (Ismail et al., 2012). In line of these data, the authors proposed a model where the default pathway is salt stress signaling that is modulated by a parallel signal chain triggered by biotic factors downstream of JA signaling.
JAs are also described as promoting the synthesis and accumulation of the stilbene compound resveratrol in grapevine berries (Tassoni et al., 2005). A transcriptional study of the different berry tissues during development revealed that JA signaling genes are preferentially expressed in the pericarp while JA-biosynthesis genes have differential expressions, lipoxigenase-related genes in the pericarp while the conversion of linoleic acid to jasmonic acid appears to be seed-exclusive (Grimplet et al., 2007). Recently, high levels of expression of both JA and ethylene signaling related genes was reported in berries before veraison (Fasoli et al., 2012).
ET biosynthesis is induced in response to abiotic stresses and this hormone affects membrane permeability, osmotic potential (sugar and proline accumulation) and the control of cell water potential. Together with H2O2, ET acts as a signaling molecule in the response of grapevine buds to hypoxia, leading to the activation of antioxidative stress genes (Vergara et al., 2012). The oxidation of 1-aminocyclopropane-1-carboxylic acid (ACC) to ET is catalyzed by the membrane associated ACC oxidase. ACC oxidase has been reported to increase in response to cold in grapevine (Tattersall, 2006).
Auxins play an important role in fruit development, and the grape berry is no exception. Indole-3-acetic acid (IAA) content is high from anthesis to veraison, then declines to very low levels at maturation (Conde et al., 2007). Amidase (AMI1), responsible for the synthesis of IAA from indole-3-acetamide decreases its levels of gene expression during ripening (Pilati et al., 2007), in tandem with the decline of IAA levels.
Auxins have been implicated in the response to UV-acclimation in grapevine, genes belonging to auxin responsive SAUR and Aux/IAA family, auxin response factors and auxin transporter-like proteins are down-regulated in grapevine leaves exposed to low UV-B, supporting evidence for a role in the response to low UV-B fluence light (Pontin et al., 2010).
In vitro systems offer a practical and easily manipulated method for studying oxidative stress. In vitro cultures are usually grown in contained environments with low photon flux density and high relative humidity. Culture media contain high quantities of an organic carbon source and growth regulators, contributing to the development of characteristic features such as abnormal leaf anatomy, poor development of grana (Wetztein and Sommer, 1982) and low photosynthesis rates (Chaves, 1994). Oxidative stress due to photoinhibition is prone to occur upon transplantation to in vivo conditions. The extent in which photoinhibition affects the survival of the plant depends on its physiological status dictated by the prevailing environmental conditions, the efficiency of protective mechanisms against excess energy and the repair processes to restore normal photosynthesis (Krause and Weis, 1991). Nitro-oxidative abiotic stress can also cause damage to in vitro cultures and, in grapevine, procyanidins were shown to have a protective action against the damage caused by peroxisome peroxynitrite thus formed (Aldini et al., 2003).
Immediately after transplantation to ex vitro, in vitro propagated grapevine plants showed severely affected photosynthetic capacity, that recovered after 1 week (Carvalho et al., 2001) as clearly seen in the heat map of Figure 3. ROS concentration is maximal on the first 2 days after transfer while chlorophyll fluorescence indicators show symptoms of photoinhibition and the ROS scavenging machinery is activated. Photoinhibition symptoms are less severe when the first stages of in vivo growth are conducted at CO2 concentrations double the normal atmospheric values and light intensities six-fold higher than in vitro (Carvalho and Amâncio, 2002).
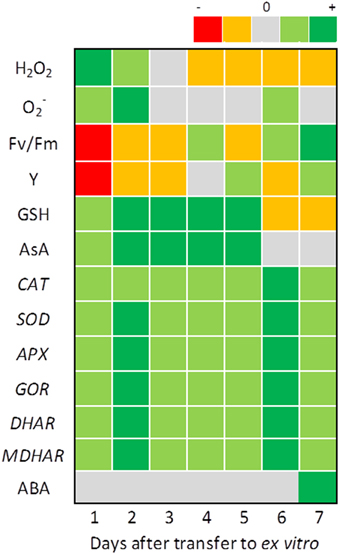
Figure 3. Monitoring changes in antioxidant homeostasis of in vitro propagated grapevine plants during the first 7 days of growth in ex vitro conditions. H2O2 was quantified in μmol g−1 FW; O−2 was visualized through nitroblue tetrazolium staining, Fv/Fm (maximum efficiency of PSII photochemistry in dark-adapted leaves), and Y (maximum quantum efficiency of PSII in light adapted leaves) were both measured using a Fluorimager chlorophyll fluorescence imaging system (Technologica Lda. Colchester, UK) and the Fluorchart software to isolate the individual leaves and calculate the values of the parameters, GSH and AsA were both quantified in μmol g−1 FW; CAT, SOD, APX, GOR, DHAR, and MDHAR expression was quantified through RT qPCR and ABA was quantified in nmol g−1 DW. The heat map represents the differences between the values measured at the moment of transfer to ex vitro (control) and those monitored for 7 days of ex vitro growth: dark green, very significant increase from the control; light green, significant increase from the control; gray, no significant differences to the control; orange, significant decrease from the control; red, very significant decrease from the control. Values were retrieved from Carvalho et al. (2006) and Vilela et al. (2007).
In the early stages of ex vitro growth a stabilization of Rubisco is observed (Carvalho et al., 2005), allowing photosynthesis to regain normal levels. In parallel, the activation of the ascorbate-glutathione cycle after 24 h of ex vitro growth helps to maintain cell redox homeostasis and regulates the antioxidative response (Carvalho et al., 2006). When the transcriptome of grapevine plants after transplantation was scanned an activation of signaling pathways up to 48 h was reported together with the up-regulation of the protein rescuing mechanism that involves the cooperation of HSP100 and HSP70, two ATP-dependent chaperone systems that remove non-functional and potentially harmful polypeptides deriving from misfolding, denaturation, or aggregation caused by stress (Carvalho et al., 2011). This was an unusually late and time-prolonged reaction when compared with “typical” abiotic stress responses (Mittler, 2006; Cramer, 2010). During this short period, H2O2 is accumulating and is used as a second messenger to trigger the pathways that are essential for plant survival at this delicate developmental phase (Vilela et al., 2008), as confirmed by the activation of genes related to stress defense pathways, hormones and protein metabolism in the same timeframe (Carvalho et al., 2011).
After overcoming the initial stress of transfer, plants undergo a new cycle of up-regulation of the antioxidative machinery, which reaches a maximum on day 6, and is not accompanied by photo-oxidative stress symptoms or increase in GSH/AsA pools (Carvalho et al., 2006). This coincides with the protrusion of new roots and the expansion of the first ex vitro leaf and culminates with a peak of ABA and H2O2 concentration on the seventh day after transfer, both produced in the newly-expanded and functional roots (Figure 3; Neves et al., 1998; Vilela et al., 2007). At this point, acclimatization to ex vitro is not yet complete but photo-oxidative stress is no longer a problem for the growing grapevine plants.
One of the first attempts at “cataloging” biotic stress response genes in grapevine was undertaken with the aid of the MapMan onthology, adjusted to encompass a few pathways in detail, such as phenylpropanoid, terpenoid, and carotenoid biosynthesis, very responsive upon biotic stress, with a marked effect on wine production and quality (Rotter et al., 2009). The authors describe an overview of transcriptional changes after the interaction of a susceptible grapevine with Eutypa lata, and show that the responsive genes belong to families known to take part in plant biotic stress defense, such as PR-proteins and enzymes of the phenylpropanoid pathway (Rotter et al., 2009).
Several stress response processes are common between biotic and abiotic stresses, exerting either synergistic or antagonistic actions, depending upon the specific stress combination that the plant is facing. One example is the role of dehydrins (DHNs) in the protection of plant cells from drought and also in host resistance to various pathogens. In the genus Vitis, the wild V. yeshanensis is tolerant to both drought and cold, and moderately resistant to powdery mildew due to the precocious induction of DHN1, occurring earlier in drought conditions than in V. vinifera and having more than one up-regulation peak during the infection with Erysiphe necator as compared to V. vinifera (Yang et al., 2012).
ROS signaling seems to have an important role in Plasmopara viticola resistance, as resistant varieties display a specific chronological set of events upon infection, that is not observed in susceptible genotypes, beginning with an increase in O−2, followed by a hypersensitive response, an increase in peroxidase activity in cells flanking the infection area and finally, an increased accumulation of phenolic compounds (Kortekamp and Zyprian, 2003). Specifically, the peroxidase activity after an infection with P. viticola is strongly correlated with resistance to P. viticola in field plants (Kortekamp and Zyprian, 2003).
In grapevine, the most ubiquitous reaction to fungal infection is the accumulation of phytoalexins. Since the 1970s that it is known that grapevine synthesizes resveratrol in response to fungal attacks (Peter and Pryce, 1977). Viniferins, products of resveratrol oxidation, are also produced in response to biotic and abiotic stresses, and also classified as phytoalexins. These compounds present biological activity against a wide range of pathogens and are considered as markers for plant disease resistance (Pezet et al., 2004b). Resveratrol is synthesized from coumaroyl CoA and malonyl CoA by STS (Figure 4). STS is closely related to chalcone synthase (CHS), the key enzyme in flavonoid-type compound biosynthesis leading to the production of chalcones while STS leads to the production of stilbenes (Figure 4). Indeed, under certain conditions, such as oxidative stress, the transcriptional response of VvSTS and VvCHS genes appears to be diametrically opposed suggesting that under those conditions, the plant refocuses its metabolism on stilbene biosynthesis, taking precedence over flavonol biosynthesis, as schematized in Figure 4, the pathway highlighted in red (Vannozzi et al., 2012).
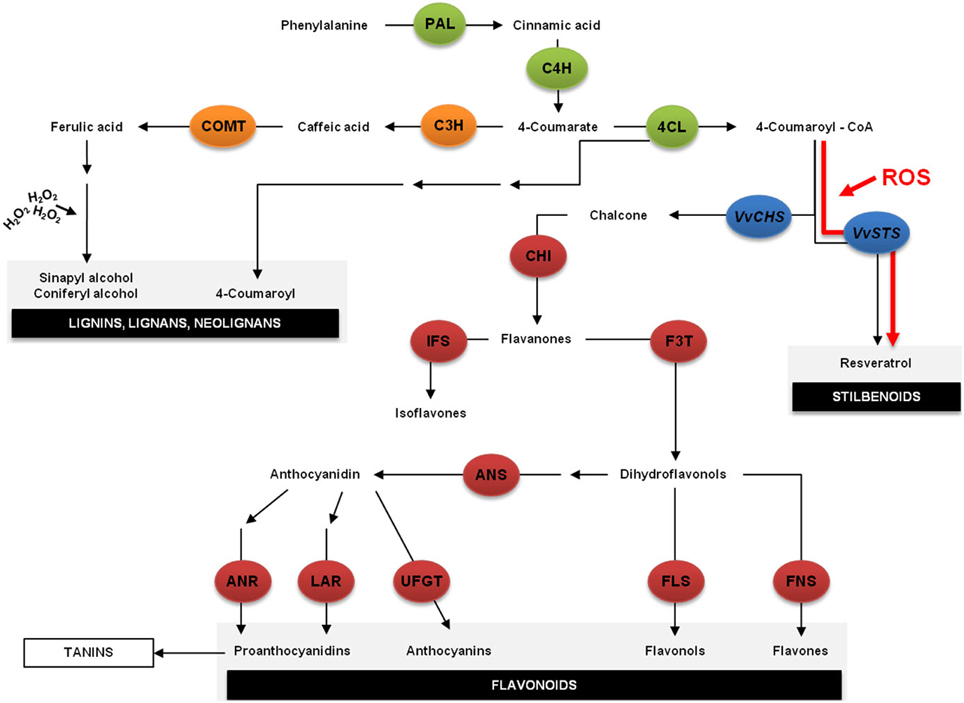
Figure 4. The phenylpropanoid pathway is part of the secondary metabolism and it is responsible for the synthesis of different classes of metabolites, such as lignins, flavonoids and stilbenoids. The first step is the deamination of phenylalanine to cinnamate by the action of phenylalanine ammonia lyase (PAL). Cinnamic acid is then hydroxylated by cinnamate-4-hydroxylase (C4H) to 4-coumarate, which is then activated to 4-coumaroyl-coenzyme A (CoA) by 4-coumaroyl—CoA ligase (4CL). After this reaction the main pathway is divided into two major branches: the flavonoid biosynthesis pathway and the lignin biosynthetic pathway. However, under stress the balance between the transcription rates of VvSTS and VvCHS appears to shift dramatically suggesting that the plant refocuses its metabolism on stilbene biosynthesis, taking precedence over flavonol biosynthesis. H2O2, hydrogen peroxide; ANR, anthocyanidin reductase; ANS, anthocyanidin synthase; C3H, coumaroyl 3-hydrolase; CHI, chalcone isomerase; COMT, caffeic acid/5-hydroxyferulic acid 3/5-O-methyltransferase; F3T, flavanone 3-hydroxylase; FLS, Flavonol synthase; FLS, flavonol synthase; FNS, flavone synthase; IFS, isoflavone synthase; LAR, leucoanthocyanidin reductase; ROS, reactive oxygen species; UFGT, UDP-glucose:flavonoid 3-O-glucosyl transferase; VvCHS, Vitis vinifera chalcone synthase; VvSTS, Vitis vinifera stilbene synthase.
Resistant grape genotypes artificially inoculated with P. viticola show very high amounts of stilbenes at the site of infection, that actively inhibit the motility of P. viticola zoospores and subsequent disease development (Pezet et al., 2004a). Interestingly, PAL seems to be constitutively expressed in resistant and susceptible genotypes, but was totally repressed in tissues after mock inoculation using the non-host pathogen Pseudoperonospora Cubensis. CHS and STS, however, had their expression increased after inoculation with P. viticola, indicating an activation of the resistance response, in accordance with the increase of stilbenes (Kortekamp, 2006).
The antioxidative response of the grapevine genotype “Trebbiano” when infected by the grapevine fanleaf virus was thoroughly scrutinized. At the early stages of infection, increases in H2O2 were observed and probably due to enhanced dismutation of O−2 by SOD, whereas, toward the late phase of infection, increases in AsA, GSH, and APX activity might be the reasons for H2O2 to regain control levels (Sgherri et al., 2013).
Upon infection by necrotrophic pathogens, which need to kill their host cells to gain access to nutrients, an activation of JA-dependent defense mechanisms takes place (Avanci et al., 2010). Many plant pathogens can either produce auxins themselves or manipulate host auxin biosynthesis to interfere with the host's normal developmental processes. In response, plants have evolved mechanisms to repress auxin signaling during infection as a defense strategy, mediated by the accumulation of SA. In grapevine, auxin responsive genes (including SAUR, Aux/IAA, auxin importer AUX1, auxin exporter PIN7) are also significantly repressed in pathogen resistance responses (Wang et al., 2007), supporting the hypothesis that down-regulation of auxin signaling contributes to induce immune responses in plants (Bari and Jonathan, 2009).
Plant species such as pear, tomato, strawberry, and pineapple show a specific oxidative stress response during fruit development, termed oxidative burst. The respective fruits are themselves named “climacteric.” As grapevine is not amongst them, it came somewhat as a surprise when Pilati et al. (2007) reported an oxidative burst in cv. “Pinot Noir” that began at veraison and was characterized by rapid accumulation of H2O2 and by the modulation of many ROS scavenging enzymes, previously thought not to be up-regulated in this species. This work comprised a thorough transcriptomic analysis of the grape berry in the stages close to veraison and the quantification of H2O2. The latter increased at the moment of veraison, reaching its maximum 1–2 weeks after, and then decreasing at a slower pace toward ripening. In tandem, transcripts coding APX, GPX, Prxs, Trxs, glutaredoxins, GSTs and metallothioneins were up-regulated, in accordance with the onset of a well-orchestrated antioxidative response. Shortly after, grape's oxidative burst was again reported and associated with high sugar content that impairs photosynthesis in the berries, possibly through ABA signaling (Lijavetzky et al., 2012). It must be referred that high levels of ACC oxidase transcript accumulation have been reported immediately preceding veraison, together with a peak in ACC accumulation and ET emission (Chervin et al., 2004). Proteomics studies also reported an increase in ROS scavenging enzymes toward ripening (Giribaldi et al., 2007; Negri et al., 2008). The subject remained wrapped in controversy because other authors did not obtain the same results (Terrier et al., 2005), until Rienth et al. (2014) shed some light onto what might be causing such disparity of results. The authors, in yet another transcriptomic assay of grape berries, found that the oxidative burst occurs markedly during the night, at ripening, following the same trend as sugar transport and phytoalexin synthesis. Together with H2O2, 1O2 was also found to increase in chloroplasts together with enzymatic peroxidation of membrane galactolipids (Pilati et al., 2014).
Grapevine can be considered a model for fruit species. Several transcriptomic studies are now complementing the existing information on abiotic stress responses of many grapevine varieties, previously described at the physiological level. Knowledge of gene expression patterns points to specific varietal responses and to different levels of stress tolerance, confirming the high phenotypic plasticity of this species. The results obtained so far suggest that some varieties keep redox homeostasis without an apparent boost in their antioxidant pool, just adjusting the activities of antioxidant enzymes and/or the accumulation of antioxidant molecules, while others need to synthesize those antioxidant molecules de novo. The former demonstrate a well-timed and efficient ROS removal and a broad plasticity in adapting to environmental shifts. At the genomic level, for instance, grapevine Prx isoforms are specifically targeted and highly responsive to major abiotic stresses. Examples are the gene expression of cytosol PrxIIE apparently with a role in grapevine drought tolerance, while in other species it was reported as only responding to light; and the identification of two new possible chloroplast Prx genes, VvPrxII-1, and VvPrxII-2, the former down-regulated by light stress and up-regulated by water stress, the latter induced by heat stress in tandem with increased ABA concentration and with an ABRE sequence in its promoter.
Specific development processes can shift redox homeostasis. An obvious example is the oxidative burst in berries, a singular feature occurring in this non-climacteric species during veraison, mostly during the night. Specifically, this metabolic event is accompanied by sugar transport and resveratrol synthesis. Reports from in vitro grapevine systems reveal stress and developmental-related signaling mediated by ROS in growing leaves and roots.
As a whole, the phenotypic plasticity of different grapevine varieties which behave as more tolerant to environmental aggressions that cause oxidative stress can improve crop yield and quality, and thus the species economic value.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by Fundação para a Ciência e Tecnologia (FCT) through CBAA Funding (PestOE/AGR/UI0240/2011) and the post-doc grants SFRH/BPD/85767/2012 and SFRH/BPD/43898/2008 to LC and PV, respectively. This work also benefited from European project KBBE InnoVine (ref. 311775) and the European COST Action FA1106 “QualityFruit.”
The Supplementary Material for this article can be found online at:: http://www.frontiersin.org/journal/10.3389/fenvs.2015.00020/abstract
Supplementary Table 1. Complete list of isoforms for the major antioxidative response genes in grapevine: superoxide dismutase, catalase, ascorbate peroxidase, dehydroascorbate reductase, monodehydroascorbate reductase, glutathione reductase, glutathione peroxidase, glutathione S-transferase, peroxidase, peroxiredoxin and thioredoxin. Accessions were retrieved from NCBI (http://www.ncbi.nlm.nih.gov/) and the gene name, symbol, grape gene accession numbers and gene ID are given.
Supplementary Figure 1. Dendrogram analysis of APX, SOD, CAR, GOR, and Prx of V. vinifera, Populus trichocarpa; Oryza sativa L. ssp. Japonica and Arabidopsis thaliana, with the respective intracellular locations and confidence that the sequences belong within the respective group.
Aldini, G., Carini, M., Piccoli, A., Rossoni, G., and Facino, R. M. (2003). Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sci. 73, 2883–2898. doi: 10.1016/S0024-3205(03)00697-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Apel, K., and Hirt, H. (2004). Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 55, 373–399. doi: 10.1146/annurev.arplant.55.031903.141701
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Asada, K. (2006). Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 141, 391–396. doi: 10.1104/pp.106.082040
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Avanci, N. C., Luche, D. D., Goldman, G. H., and Goldman, M. H. S. (2010). Jasmonates are phytohormones with multiple functions, including plant defense and reproduction. GMR 9, 484–505. doi: 10.4238/vol9-1gmr754
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Aziz, A., Heyraud, A., and Lambert, B. (2004). Oligogalacturonide signal transduction, induction of defense-related responses and protection of grapevine against Botrytis cinerea. Planta 218, 767–774. doi: 10.1007/s00425-003-1153-x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baier, M., Ströher, E., and Dietz, K. (2004). The acceptor availability at photosystem I and ABA control nuclear expression of 2-Cys Peroxiredoxin-A in Arabidopsis thaliana. Plant Cell Physiol. 45, 997–1006. doi: 10.1093/pcp/pch114
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Baker, A., and Graham, I. (2002). Plant Peroxisomes. Biochemistry, Cell Biology and Biotechnological Applications. Dordrecht: Kluwer Academic Publishers.
Balbi, V., and Devoto, A. (2008). Jasmonate signaling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol. 177, 301–318. doi: 10.1111/j.1469-8137.2007.02292.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Berli, F. J., Moreno, D., Piccoli, P., Hespanhol-Viana, L., Silva, M. F., Bressan-Smith, R., et al. (2010). Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 33, 1–10. doi: 10.1111/j.1365-3040.2009.02044.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bari, R., and Jonathan, J. (2009). Role of plant hormones in plant defence responses. Plant Mol. Biol. 69, 473–488. doi: 10.1007/s11103-008-9435-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Bertamini, M., Zulini, L., Muthuchelian, K., and Nedunchezhian, N. (2006). Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 44, 151–154. doi: 10.1007/s11099-005-0173-0
Carvalho, C., and Amâncio, S. (2002). Antioxidant defence system in plantlets transferred from in vitro to ex vitro: effects of increasing light intensity and CO2 concentration. Plant Sci. 162, 33–40. doi: 10.1016/S0168-9452(01)00524-6
Carvalho, L. C., Coito, J. L., Colaço, S., Sangiogo, M., and Amâncio, S. (2014). Heat stress in grapevine: the pros and cons of acclimation. Plant. Cell Environ. doi: 10.1111/pce.12445. [Epub ahead of print].
Carvalho, L. C., Esquível, M. G., Martins, I., Pinto Ricardo, C., and Amâncio, S. (2005). Monitoring the stability of Rubisco in micropropagated grapevine (Vitis vinifera L.) by two-dimensional electrophoresis. J. Plant Physiol. 162, 365–374. doi: 10.1016/j.jplph.2004.09.013
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Carvalho, L. C., Os, M. L., Chaves, M. M., and Amâncio, S. (2001). Chlorophyll fluorescence as an indicator of photosynthetic functioning of in vitro grapevine and chestnut plantlets under ex vitro acclimatization. Plant Cell. Tissue Organ Cult. 67, 271–280. doi: 10.1023/A:1012722112406
Carvalho, L. C., Vilela, B. J., Mullineaux, P. M., and Amâncio, S. (2011). Comparative transcriptomic profiling of Vitis vinifera under high light using a custom-made array and the affymetrix genechip. Mol. Plant 6, 1038–1051. doi: 10.1093/mp/ssr027
Carvalho, L. C., Vilela, B. J., Vidigal, P., Mullineaux, P. M., and Amâncio, S. (2006). Activation of the ascorbate−glutathione cycle is an early response of micropropagated Vitis vinifera L. Explants transferred to ex vitro. Int. J. Plant Sci. 167, 759–770. doi: 10.1086/503919
Chaves, M. M. (1994). “Environmental constrains to photosynthesis in ex vitro plants,” in Physiology, Growth and Development of Plants in Culture, eds P. J. Lumsden, J. R. Nicholas, and W. J. Davies (Dordrecht, Kluwer Academic Publishers), 1–18.
Chervin, C., El-Kereamy, A., Roustan, J.-P., Latché, A., Lamon, J., and Bouzayen, M. (2004). Ethylene seems required for the berry development and ripening in grape, a non-climacteric fruit. Plant Sci. 167, 1301–1305. doi: 10.1016/j.plantsci.2004.06.026
Conde, C., Silva, P., Fontes, N., Dias, A. C. P., Tavares, R. M., Sousa, M. J., et al. (2007). Biochemical changes throughout grape berry development and fruit and wine quality. Food 1, 1–22.
Conklin, P. L., and Loewus, F. A. (2001). Biosynthesis of ascorbic acid in plants: a renaiscance. Annu. Rev. Plant Biol. Plant Mol. Biol. 52, 437–467. doi: 10.1146/annurev.arplant.52.1.437
Conn, S., Curtin, C., Bézier, A., Franco, C., and Zhang, W. (2008). Purification, molecular cloning, and characterization of glutathione S-transferases (GSTs) from pigmented Vitis vinifera L. cell suspension cultures as putative anthocyanin transport proteins. J. Exp. Bot. 59, 3621–3634. doi: 10.1093/jxb/ern217
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Corpas, F. J., Barroso, J. B., and del Río, L. (2001). Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 6, 145–150. doi: 10.1016/S1360-1385(01)01898-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Costa, J. M., Ortuño, M. F., Lopes, C. M., and Chaves, M. M. (2012). Grapevine varieties exhibiting differences in stomatal response to water deficit. Func. Plant Biol. 39, 179–189. doi: 10.1071/FP11156
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cramer, G. R. (2010). Abiotic stress and plant responses from the whole vine to the genes. Aust. J. Grape Wine Res. 16, 86–93. doi: 10.1111/j.1755-0238.2009.00058.x
Cramer, G. R., Ergül, A., Grimplet, J., Tillett, R. L., Tattersall, E. A. R., Bohlman, M. C., et al. (2007). Water and salinity stress in grapevines: early and late changes in transcript and metabolite profiles. Funct. Integr. Genomics 7, 111–134. doi: 10.1007/s10142-006-0039-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Cutler, S. R., Rodriguez, P. L., Finkelstein, R. R., and Abrams, S. R. (2010). Abscisic acid: emergence of a core signaling network. Annu. Rev. Plant Biol. 61, 651–679. doi: 10.1146/annurev-arplant-042809-112122
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Del Río, L. A., Sandalio, L. M., Corpas, F. J., Palma, M., and Barroso, J. B. (2006). Reactive oxygen species and reactive nitrogen species in peroxisomes. Production, scavenging, and role in cell signalling. Plant Physiol. 141, 330–335. doi: 10.1104/pp.106.078204
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Deluc, L. G., Quilici, D. R., Decendit, A., Grimplet, J., Wheatley, M. D., Schlauch, K., et al. (2009). Water deficit alters differentially metabolic pathways affecting important flavor and quality traits in grape berries of Cabernet Sauvignon and Chardonnay. BMC Genomics 10:212. doi: 10.1186/1471-2164-10-212
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
De Nisco, M., Manfra, M., Bolognese, A., Sofo, A., Scopa, A., Tenore, G. C., et al. (2013). Nutraceutical properties and polyphenolic profile of berry skin and wine of Vitis vinifera L. (cv. Aglianico). Food Chem. 140, 623–629. doi: 10.1016/j.foodchem.2012.10.123
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dietz, K.-J. (2003). Plant peroxiredoxins. Annu. Rev. Plant Biol. 54, 93–107. doi: 10.1146/annurev.arplant.54.031902.134934
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dietz, K.-J. (2011). Peroxiredoxins in plants and cyanobacteria. Antioxid. Redox Signal. 15, 1129–1159. doi: 10.1089/ars.2010.3657
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Dry, P., and Loveys, B. R. (1999). Grapevine shoot growth and stomatal conductance are reduced when part of the root system is dried. Vitis 38, 151–156.
Edge, R., McGarvey, D. J., and Truscott, T. G. (1997). The carotenoids as anti-oxidants—a review. J. Photochem. Photobiol. 41, 189–200. doi: 10.1016/S1011-1344(97)00092-4
Edwards, R., Dixon, D. P., and Walbot, V. (2000). Plant glutathione S-transferases: enzymes with multiple functions in sickness and in health. Trends Plant Sci. 5, 193–198. doi: 10.1016/S1360-1385(00)01601-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fang, J., Chai, C., Qian, Q., Li, C., Tang, J., Sun, L., et al. (2008). Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J. 54, 177–189. doi: 10.1111/j.1365-313X.2008.03411.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fasoli, M., Dal Santo, S., Zenoni, S., Tornielli, G. B., Farina, L., Zamboni, A., et al. (2012). The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 24, 3489–3505. doi: 10.1105/tpc.112.100230
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Fujita, M., Fujita, Y., Noutoshi, Y., Takahashi, F., Narusaka, Y., Yamaguchi-Shinozaki, K., et al. (2006). Crosstalk between abiotic and biotic stress responses: a current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 9, 436–442. doi: 10.1016/j.pbi.2006.05.014
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gama, F., Keech, O., Eymery, F., Finkemeier, I., Gelhaye, E., Gardeström, P., et al. (2007). The mitochondrial type II peroxiredoxin from poplar. Physiol. Plant 129, 196–206. doi: 10.1111/j.1399-3054.2006.00785.x
Gambetta, G. A., Matthews, M. A., Shaghasi, T. H., McElrone, A. J., and Castellarin, S. D. (2010). Sugar and abscisic acid signaling orthologs are activated at the onset of ripening in grape. Planta 232, 219–234. doi: 10.1007/s00425-010-1165-2
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Giribaldi, M., Perugini, I., Sauvage, F.-X., and Schubert, A. (2007). Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics 7, 3154–3170. doi: 10.1002/pmic.200600974
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Grimplet, J., Deluc, L. G., Tillett, R. L., Wheatley, M. D., Schlauch, K. A., Cramer, G. R., et al. (2007). Tissue-specific mRNA expression profiling in grape berry tissues. BMC Genomics 8:187. doi: 10.1186/1471-2164-8-187
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Gunes, A., Soylemezoglu, G., Inal, A., Bagci, E. G., Coban, S., and Sahin, O. (2006). Antioxidant and stomatal responses of grapevine (Vitis vinifera L.) to boron toxicity. Sci. Hortic. 110, 279–284. doi: 10.1016/j.scienta.2006.07.014
Guo, X.-L., Cao, Y.-R., Cao, Z.-Y., Zhao, Y.-X., and Zhang, H. (2004). Molecular cloning and characterization of a stress-induced peroxiredoxin Q gene in halophyte Suaeda salsa. Plant Sci. 167, 969–975. doi: 10.1016/j.plantsci.2004.05.004
Horling, F., Lamkemeyer, P., Ko, J., Finkemeier, I., Kandlbinder, A., Baier, M., et al. (2003). Dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis. Plant Physiol. 131, 317–325. doi: 10.1104/pp.010017
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ismail, A., Riemann, M., and Nick, P. (2012). The jasmonate pathway mediates salt tolerance in grapevines. J. Exp. Bot. 63, 2127–2139. doi: 10.1093/jxb/err426
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Jeong, S., Goto-Yamamoto, N., Kobayashi, S., and Esaka, M. (2004). Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 167, 247–252. doi: 10.1016/j.plantsci.2004.03.021
Jia, W., and Zhang, J. (1997). Comparison of exportation and metabolism of xylem-delivered ABA in maize leaves at different water status and xylem sap pH. Plant Growth Regul. 21, 43–49. doi: 10.1023/A:1005722121030
Jones, G. V., White, M. A., Cooper, O. R., and Storchmann, K. (2005). Climate change and global wine quality. Clim. Change 73, 319–343. doi: 10.1007/s10584-005-4704-2
Kim, K.-H., Alam, I., Lee, K.-W., Sharmin, S. A., Kwak, S.-S., Lee, S. Y., et al. (2010). Enhanced tolerance of transgenic tall fescue plants overexpressing 2-Cys peroxiredoxin against methyl viologen and heat stresses. Biotechnol. Lett. 32, 571–576. doi: 10.1007/s10529-009-0185-0
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kiyosue, T., Yoshiba, Y., Yamaguchi-Shinozaki, K., and Shinozaki, K. (1996). A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8, 1323–1335. doi: 10.1105/tpc.8.8.1323
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
König, J., Lotte, K., Plessow, R., Brockhinke, A., Baier, M., and Dietz, K.-J. (2003). Reaction mechanism of plant 2-Cys peroxiredoxin. Role of the C terminus and the quaternary structure. J. Biol. Chem. 278, 24409–24420. doi: 10.1074/jbc.M301145200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kortekamp, A. (2006). Expression analysis of defence-related genes in grapevine leaves after inoculation with a host and a non-host pathogen. Plant Physiol. Biochem. 44, 58–67. doi: 10.1016/j.plaphy.2006.01.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Kortekamp, A., and Zyprian, E. (2003). Characterization of Plasmopara-resistance in grapevine using in vitro plants. J. Plant Physiol. 160, 1393–1400. doi: 10.1078/0176-1617-01021
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Koyama, Y. (1991). Structures and functions of carotenoids in photosynthetic systems. J. Photochem. Photobiol. 9, 265–280.
Krause, G. H., and Weis, E. (1991). Chlorophyll fluorescence and photosynthesis: the basics. Annu. Rev. Plant Physiol. Plant Mol. Biol. 42, 313–349. doi: 10.1146/annurev.pp.42.060191.001525
Kvaratskhelia, M., Winkel, C., and Thorneley, R. N. F. (1997). Purification and characterization of a nove1 class 111 peroxidase isoenzyme from tea leaves. Plant Physiol. 1237–1245. doi: 10.1104/pp.114.4.1237
Lacampagne, S., Gagné, S., and Gény, L. (2009). Involvement of abscisic acid in controlling the proanthocyanidin biosynthesis pathway in grape skin: new elements regarding the regulation of tannin composition and leucoanthocyanidin reductase (LAR) and anthocyanidin reductase (ANR) activities and expression. J. Plant Growth Regul. 29, 81–90. doi: 10.1007/s00344-009-9115-6
Lamkemeyer, P., Laxa, M., Collin, V., Li, W., Finkemeier, I., Schöttler, M., et al. (2006). Peroxiredoxin Q of Arabidopsis thaliana is attached to the thylakoids and functions in context of photosynthesis. Plant J. 45, 968–981. doi: 10.1111/j.1365-313X.2006.02665.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Leon-Reyes, A., Van der Does, D., De Lange, E. S., Delker, C., Wasternack, C., Van Wees, S. C. M., et al. (2010). Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232, 1423–1432. doi: 10.1007/s00425-010-1265-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Lijavetzky, D., Carbonell-Bejerano, P., Grimplet, J., Bravo, G., Flores, P., Fenoll, J., et al. (2012). Berry flesh and skin ripening features in Vitis vinifera as assessed by transcriptional profiling. PLoS ONE 7:e39547. doi: 10.1371/annotation/fd93800a-3b3c-484d-97a9-190043309e4b
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Marrs, K. (1996). Functions and regulation of glutathione s-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 127–158. doi: 10.1146/annurev.arplant.47.1.127
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R. (2006). Abiotic stress, the field environment and stress combination. Trends Plant Sci. 11, 15–19. doi: 10.1016/j.tplants.2005.11.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Mittler, R., Vanderauwera, S., Gollery, M., and Van Breusegem, F. (2004). Reactive oxygen gene network of plants. Trends Plant Sci. 9, 490–498. doi: 10.1016/j.tplants.2004.08.009
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Moutinho-Pereira, J., Magalhães, N., Gonçalves, B., Bacelar, E., Brito, M., and Correia, C. (2007). Gas exchange and water relations of three Vitis vinifera L. cultivars growing under Mediterranean climate. Photosynthetica 45, 202–207. doi: 10.1007/s11099-007-0033-1
Mullineaux, P. M., Karpinski, S., and Baker, N. R. (2006). Spatial dependence for hydrogen peroxide-directed signaling in light-stressed plants. Plant Physiol. 141, 346–350. doi: 10.1104/pp.106.078162
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Munné-Bosch, S., Queval, G., and Foyer, C. H. (2013). The impact of global change factors on redox signaling underpinning stress tolerance. Plant Physiol. 161, 5–19. doi: 10.1104/pp.112.205690
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Negri, A. S., Prinsi, B., Rossoni, M., Failla, O., Scienza, A., Cocucci, M., et al. (2008). Proteome changes in the skin of the grape cultivar Barbera among different stages of ripening. BMC Genomics 9:378. doi: 10.1186/1471-2164-9-378
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Neves, C., Sá, C., and Amâncio, S. (1998). Histochemical detection of H2O2 by tissue printing as a precocious marker of rhizogenesis in grapevine. Plant Physiol. Biochem. 36, 817–824. doi: 10.1016/S0981-9428(99)80019-9
Noctor, G., and Foyer, C. H. (1998). Ascorbate and glutathione: keeping active oxygen under control. Annu. Rev. Plant Physiol. Plant Mol. Biol. 49, 249–279. doi: 10.1146/annurev.arplant.49.1.249
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Noctor, G., Gomez, L., Vanacker, H., and Foyer, C. H. (2002). Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signaling. J. Exp. Bot. 53, 1283–1304. doi: 10.1093/jexbot/53.372.1283
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ozden, M., Demirel, U., and Kahraman, A. (2009). Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 119, 163–168. doi: 10.1016/j.scienta.2008.07.031
Passardi, F., Cosio, C., Penel, C., and Dunand, C. (2005). Peroxidases have more functions than a Swiss army knife. Plant Cell Rep. 24, 255–265. doi: 10.1007/s00299-005-0972-6
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pastore, C., Zenoni, S., Fasoli, M., Pezzotti, M., Tornielli, G. B., and Filippetti, I. (2013). Selective defoliation affects plant growth, fruit transcriptional ripening program and flavonoid metabolism in grapevine. BMC Plant Biol. 13:30. doi: 10.1186/1471-2229-13-30
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peleg, Z., and Blumwald, E. (2011). Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 14, 290–295. doi: 10.1016/j.pbi.2011.02.001
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Peter, B., and Pryce, J. (1977). Oxidative dimerisation of 4-hydroxystilbenes in vitro: production of a grapevine phytoalexin mimic. J. Chem. Soc. Chem. Commun., 208–210.
Pezet, R., Gindro, K., Viret, O., and Richter, H. (2004a). Effects of resveratrol, viniferins and pterostilbene on Plasmopara viticola zoospore mobility and disease development. Vitis 43, 145–148.
Pezet, R., Gindro, K., Viret, O., and Spring, J.-L. (2004b). Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiol. Mol. Plant Pathol. 65, 297–303. doi: 10.1016/j.pmpp.2005.03.002
Pilati, S., Brazzale, D., Guella, G., Milli, A., Ruberti, C., Biasioli, F., et al. (2014). The onset of grapevine berry ripening is characterized by ROS accumulation and lipoxygenase-mediated membrane peroxidation in the skin. BMC Plant Biol. 14:87. doi: 10.1186/1471-2229-14-87
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pilati, S., Perazzolli, M., Malossini, A., Cestaro, A., Demattè, L., Fontana, P., et al. (2007). Genome-wide transcriptional analysis of grapevine berry ripening reveals a set of genes similarly modulated during three seasons and the occurrence of an oxidative burst at veraison. BMC Genomics 8:428. doi: 10.1186/1471-2164-8-428
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Pontin, M. A., Piccoli, P. N., Francisco, R., Bottini, R., Martinez-Zapater, J. M., and Lijavetzky, D. (2010). Transcriptome changes in grapevine (Vitis vinifera L.) cv. Malbec leaves induced by ultraviolet-B radiation. BMC Plant Biol. 10:224. doi: 10.1186/1471-2229-10-224
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Potters, G., De Gara, L., Asard, H., and Horemans, N. (2002). Ascorbate and glutathione: guardians of the cell cycle, partners in crime? Plant Physiol. Biochem. 40, 537–548. doi: 10.1016/S0981-9428(02)01414-6
Rabbani, M. A., Maruyama, K., Abe, H., Khan, M. A., Katsura, K., Ito, Y., et al. (2003). Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol. 133, 1755–1767. doi: 10.1104/pp.103.025742
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Ramel, F., Birtic, S., Ginies, C., Soubigou-Taconnat, L., Triantaphylidès, C., and Havaux, M. (2012). Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl. Acad. Sci. U.S.A. 109, 5535–5540. doi: 10.1073/pnas.1115982109
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Raskin, I. (1992). Role of salicylic acid in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 43, 439–463. doi: 10.1146/annurev.pp.43.060192.002255
Rey, P., Cuiné, S., Eymery, F., Garin, J., Court, M., Jacquot, J.-P., et al. (2005). Analysis of the proteins targeted by CDSP32, a plastidic thioredoxin participating in oxidative stress responses. Plant J. 41, 31–42. doi: 10.1111/j.1365-313X.2004.02271.x
Rienth, M., Torregrosa, L., Kelly, M. T., Luchaire, N., Pellegrino, A., Grimplet, J., et al. (2014). Is transcriptomic regulation of berry development more important at night than during the day? PLoS ONE 9:e88844. doi: 10.1371/journal.pone.0088844
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rizhsky, L., Liang, H., and Mittler, R. (2003). The water-water cycle is essential for chloroplast protection in the absence of stress. J. Biol. Chem. 278, 38921–38925. doi: 10.1074/jbc.M304987200
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rocheta, M., Becker, J. D., Coito, J. L., Carvalho, L., and Amâncio, S. (2014). Heat and water stress induce unique transcriptional signatures of heat-shock proteins and transcription factors in grapevine. Funct. Integr. Genomics 14, 135–148. doi: 10.1007/s10142-013-0338-z
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Rotter, A., Camps, C., Lohse, M., Kappel, C., Pilati, S., Hren, M., et al. (2009). Gene expression profiling in susceptible interaction of grapevine with its fungal pathogen Eutypa lata: extending MapMan ontology for grapevine. BMC Plant Biol. 9:104. doi: 10.1186/1471-2229-9-104
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Santner, A., and Estelle, M. (2009). Recent advances and emerging trends in plant hormone signaling. Nature 459:1071–1078. doi: 10.1038/nature08122
Seki, M., Narusaka, M., Ishida, J., Nanjo, T., Fujita, M., Oono, Y., et al. (2002). Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 31, 279–292. doi: 10.1046/j.1365-313X.2002.01359.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Sgherri, C., Ranieri, A., and Quartacci, M. F. (2013). Antioxidative responses in Vitis vinifera infected by grapevine fanleaf virus. J. Plant Physiol. 170, 121–128. doi: 10.1016/j.jplph.2012.09.016
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Skopelitis, D. S., Paranychianakis, N. V., Paschalidis, K., Pliakonis, E. D., Delis, I. D., Yakoumakis, D. I., et al. (2006). Abiotic stress generates ROS that signal expression of anionic glutamate dehydrogenases to form glutamate for proline synthesis in tobacco and grapevine. Plant Cell 18, 2767–2781. doi: 10.1105/tpc.105.038323
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Stoll, M., Loveys, B., and Dry, P. (2000). Hormonal changes induced by partial rootzone drying of irrigated grapevine. J. Exp. Bot. 51, 1627–1634. doi: 10.1093/jexbot/51.350.1627
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tassoni, A., Fornalè, S., Franceschetti, M., Musiani, F., Michael, A. J., Perry, B., et al. (2005). Jasmonates and Na-orthovanadate promote resveratrol production in Vitis vinifera cv. Barbera cell cultures. New Phytol. 166, 895–905. doi: 10.1111/j.1469-8137.2005.01383.x
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Tattersall, E. (2006). Changes in Gene Expression in Response to Abiotic Stress in Grapevine (Vitis Vinifera). Ph.D. thesis, University of Reno, Nevada: ProQuest.
Tenore, G. C., Troisi, J., Di Fiore, R., Manfra, M., and Novellino, E. (2011). Nutraceutical value and toxicological profile of selected red wines from Morocco. Food Chem. 129, 792–798. doi: 10.1016/j.foodchem.2011.05.022
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Terrier, N., Glissant, D., Grimplet, J., Barrieu, F., Abbal, P., Couture, C., et al. (2005). Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222, 832–847. doi: 10.1007/s00425-005-0017-y
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Triantaphylidès, C., and Havaux, M. (2009). Singlet oxygen in plants: production, detoxification and signaling. Trends Plant Sci. 14, 219–228. doi: 10.1016/j.tplants.2009.01.008
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vannozzi, A., Dry, I. B., Fasoli, M., Zenoni, S., and Lucchin, M. (2012). Genome-wide analysis of the grapevine stilbene synthase multigenic family: genomic organization and expression profiles upon biotic and abiotic stresses. BMC Plant Biol. 12:130. doi: 10.1186/1471-2229-12-130
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vergara, R., Parada, F., Rubio, S., and Pérez, F. J. (2012). Hypoxia induces H2O2 production and activates antioxidant defence system in grapevine buds through mediation of H2O2 and ethylene. J. Exp. Bot. 63, 4123–4131. doi: 10.1093/jxb/ers094
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vidigal, P., Carvalho, R., Amâncio, S., and Carvalho, L. (2013). Peroxiredoxins are involved in two independent signaling pathways in the abiotic stress protection in Vitis vinifera. Biol. Plant. 57, 675–683. doi: 10.1007/s10535-013-0346-9
Vidigal, P., Martin-Hernandez, A. M., Guiu-Aragonés, V., Amâncio, S., and Carvalho, L. C. (2014). Selective silencing of 2Cys and type-IIB Peroxiredoxins discloses their roles in cell redox state and stress signaling. J. Integr. Plant Biol. doi: 10.1111/jipb.12296. [Epub ahead of print].
Vilela, B. J., Carvalho, L. C., and Amâncio, S. (2008). Integration of stress produced reactive oxygen species in the stomatal regulation of micropropagated Vitis vinifera L. plantlets impaired in ABA signaling. Plant Signal. Behav. 3, 558–559. doi: 10.1007/s00299-007-0427-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Vilela, B. J., Carvalho, L. C., Ferreira, J., and Amâncio, S. (2007). Gain of function of stomatal movements in rooting Vitis vinifera L. plants: regulation by H2O2 is independent of ABA before the protruding of roots. Plant Cell Rep. 26, 2149–2157. doi: 10.1007/s00299-007-0427-3
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, D., Pajerowska-Mukhtar, K., Culler, A. H., and Dong, X. (2007). Salicylic acid inhibits pathogen growth in plants through repression of the auxin signaling pathway. Curr. Biol. 17, 1784–1790. doi: 10.1016/j.cub.2007.09.025
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, L.-J., Fan, L., Loescher, W., Duan, W., Liu, G.-J., Cheng, J.-S., et al. (2010). Salicylic acid alleviates decreases in photosynthesis under heat stress and accelerates recovery in grapevine leaves. BMC Plant Biol. 10:34. doi: 10.1186/1471-2229-10-34
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wang, L., and Li, S. (2006). Salicylic acid-induced heat or cold tolerance in relation to Ca2+ homeostasis and antioxidant systems in young grape plants. Plant Sci. 170, 685–694. doi: 10.1016/j.plantsci.2005.09.005
Wen, P.-F., Chen, J.-Y., Kong, W.-F., Pan, Q.-H., Wan, S.-B., and Huang, W.-D. (2005). Salicylic acid induced the expression of phenylalanine ammonia-lyase gene in grape berry. Plant Sci. 169, 928–934. doi: 10.1016/j.plantsci.2005.06.011
Werner, T., and Schmülling, T. (2009). Cytokinin action in plant development. Curr. Opin. Plant Biol. 12, 527–538. doi: 10.1016/j.pbi.2009.07.002
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Wetztein, H. I., and Sommer, H. E. (1982). Leaf anatomy of tissue cultured Liquidambar styraciflua (Hamamelidaceae) during acclimatization. Am. J. Bot. 69, 1579–1586. doi: 10.2307/2442913
Yang, Y., He, M., Zhu, Z., Li, S., Xu, Y., Zhang, C., et al. (2012). Identification of the dehydrin gene family from grapevine species and analysis of their responsiveness to various forms of abiotic and biotic stress. BMC Plant Biol. 12:140. doi: 10.1186/1471-2229-12-140
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Yoshida, K., Terashima, I., and Noguchi, K. (2007). Up-regulation of mitochondrial alternative oxidase concomitant with chloroplast over-reduction by excess light. Plant Cell Physiol. 48, 606–614. doi: 10.1093/pcp/pcm033
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Zenoni, S., Ferrarini, A., Giacomelli, E., Xumerle, L., Fasoli, M., Malerba, G., et al. (2010). Characterization of transcriptional complexity during berry development in Vitis vinifera using RNA-Seq. Plant Physiol. 152, 1787–1795. doi: 10.1104/pp.109.149716
PubMed Abstract | Full Text | CrossRef Full Text | Google Scholar
Keywords: ROS, ascorbate-glutathione cycle, oxidative burst, peroxiredoxins, hormone stress signals, in vitro stress, biotic stress
Citation: Carvalho LC, Vidigal P and Amâncio S (2015) Oxidative stress homeostasis in grapevine (Vitis vinifera L.). Front. Environ. Sci. 3:20. doi: 10.3389/fenvs.2015.00020
Received: 28 November 2014; Accepted: 02 March 2015;
Published: 19 March 2015.
Edited by:
Margarete Baier, Freie Universität Berlin, GermanyReviewed by:
Iris Finkemeier, Max Planck Institute for Plant Breeding Research, GermanyCopyright © 2015 Carvalho, Vidigal and Amâncio. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sara Amâncio, LEAF Research Centre, Departamento de Recursos Naturais, Ambiente e Território (DRAT), Instituto Superior de Agronomia, Universidade de Lisboa, Tapada da Ajuda, Calçada da Tapada, 1349-017 Lisboa, Portugalc2FtcG9ydEBpc2EudWxpc2JvYS5wdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.