
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Health, 25 February 2025
Sec. Environmental Epidemiology
Volume 4 - 2025 | https://doi.org/10.3389/fenvh.2025.1468404
Objective: Heavy metals are present in many environmental pollutants, and have cumulative effects on the human body, which can lead to several diseases, including osteoporosis (OP). However, limited information was known about the correlation between OP and heavy metals, especially in postmenopausal women. The current research was aimed to explore the association heavy metals and bone mineral density (BMD) with OP risk among postmenopausal women in the US.
Methods: This cross-sectional study enrolled participants in NHANES 2013–2014 and 2017–2020. ICP–MS was applied to detect five metals, namely, Pb, Cd, Hg, Se and Mn, in blood. BMD was measured through DXA and then converted to T-scores. At the same time, the impacts of exposure to single and mixed metals on OP were assessed using multivariable logistic regression, WQS, and BKMR models. The relationship was examined based on age and BMI.
Results: Totally 905 postmenopausal women were enrolled for final analysis. Among them, 161 (17.80%) participants had OP. Logistic regression indicated that, Cd [OR (95% CI): 1.815 (1.076, 3.061) and 2.180 (1.309, 3.631), separately, P for trend = 0.006] and Se [OR (95% CI): 0.570 (0.356, 0.914), 0.454 (0.276, 0.747) and 0.689 (0.433, 1.097), separately, P for trend = 0.071] were related to OP in the adjusted model 1. Similar results to model 1 were obtained by the rest models. Multivariate linear regression model analysis suggested that subjects who had the greatest quartile of Cd level (Q4) exhibited lower BMD within the entire femur (β = −0.112, P = 0.007; P for trend = 0.003) compared to those in Q1. The WQS analysis suggested that Cd was correlated positively with increased OP risk, whereas Se inversely associated. In BKMR analysis, exposure to mixed metals was significantly positively related to OP. In subgroup analysis, Cd's impact on OP risk was most pronounced in the 50–60 year age and 25–30 kg/m2 BMI subgroups, and Se offered protection in older age and higher BMI groups.
Conclusion: This is the first study to determine the correlation between OP and heavy metals among postmenopausal women in the US based on large data. The results showed that the increased mixed metal concentration may lead to an increased OP risk among postmenopausal women. Blood Cd level was associated with an increased OP risk, and blood Se level served as the predicting factor for OP. More investigations are warranted to demonstrate our findings and elucidate the underlying biological mechanism.
Osteoporosis (OP), the systemic metabolic disease, exhibits the representative characteristics of decreased bone mass, decreased bone mineral density (BMD), and deteriorating bone microstructure (1, 2). As of 2010, there were 10.2 million US adults aged over 50 years diagnosed with OP (2). By 2030, the OP population is estimated to be 13.5 million (3, 4). Osteoporosis increases the financial and medical burden of patients and is a health issue globally that needs to be addressed. There are different risk factors related to OP development and decreased BMD, including genetic, dietary, or environmental factors (5, 6).
Rapid advancements in industrialization and urbanization have resulted in increased exposure to heavy metals which can be found in air, food, soil and water worldwide (7, 8). These metals can infiltrate the body through multiple pathways, including absorption through the skin, inhalation, or consumption of heavy metals in food and/or drinking water. Continuous exposure to heavy metals can result in a disruption of the body's internal equilibrium, with these metals beginning to build up in the body and being utilized as replacements for crucial elements (9). For instance, lead can displace calcium, cadmium can take the place of zinc, and aluminum can substitute for many trace elements (9). Additionally, the accumulation of heavy metals can impair the body's key metabolic functions and upset the balance of antioxidants. Similarly, the operation of various hormones and the activity of vital enzymes can also be affected (10). In a word, the general mechanism involved in heavy metal-induced toxicity is recognized to be the production of reactive oxygen species resulting oxidative damage and health related adverse effects (9, 11–13). Traditional heavy metals include lead (Pb), cadmium (Cd), mercury (Hg), selenium (Se) and manganese (Mn). It has been discovered that exposure to heavy metals can lead to gene variations and induce an increased risk of disease occurrence, including degenerative diseases and fractures (13–18). Blood metal levels can be a good indicator of the extent of exposure and body heavy metal accumulation (19). Meanwhile, many studies have found that blood heavy metal accumulation within bones is suggested to promote bone resorption while changing bone mineral level, finally causing OP or even bone fracture (20). For example, animal studies have shown cadmium to stimulate the formation and activity of osteoclasts, breaking down the collagen matrix in bone (21); one observational study showed that daily or long-term Cd, Pb, and Hg exposure is negatively correlated with BMD (22). Cd is found to be independently related to the increased OP risk, Se is independently related to the decreased OP risk, while Pb, Mn and Hg do not significantly influence OP occurrence (23). However, the relation of blood heavy metals with OP risk is only analyzed in observational studies with small sample sizes at present (24). In addition, many populations are found with a heavy metal exposure risk, like post-menopausal women with particular vulnerability to OP (16). It was essential to explore the relation between heavy metals and OP, because people might experience heavy metal accumulation under certain working conditions and OP threatened human health, particularly for postmenopausal women. Considering the lack of existing data on postmenopausal women, more studies are required to better comprehend the association of variation trends of blood heavy metals (such as Pb, Hg, Cd, Se and Mn) with the OP risk of postmenopausal women, aiming to shed more lights on clinical practice.
In this study, three statistical approaches, namely, multivariable logistic regression, Bayesian kernel machine regression (BKMR), and weighted quantile sum regression (WQS), were used for assessing single and mixed effects of OP with whole blood Pb, Cd, total Hg, Mn, and Se levels in the NHANES. Additionally, we also conducted stratified analyses to reveal the health impacts of these metals across different demographic subgroups. Association studies and prediction of OP might assist postmenopausal women to prevent OP and reduce exposure to risk factors.
The National Health and Nutritional Examination Surveys (NHANES), first started in 1959 as the continuous program, conducts cross-sectional examinations of population health and nutrient in the US every year. Demographic, dietary, laboratory test and questionnaire data are released at 2-year intervals. This study recruited women aged ≥50 years who had complete information on blood heavy metals and BMD from NHANES in 2013–2014 and 2017–2020 (Figure 1). The subjects enrolled into this study provided informed consents. Our study protocols were approved by the ethics review board of National Center for Health Statistics (NCHS) and written consent was obtained. All studies were conducted in accordance with relevant guidelines/regulations.
After diluting the prepared whole blood samples, mass spectrometry was performed to directly determine blood heavy metal contents, including Pb, Cd, Hg, Se, and Mn. To uniformly distribute cellular components, during the dilution stage, a low portion of whole blood was obtained from a large whole blood sample. To dilute the blood samples, one portion of sample was mixed with one portion of water and 48 portions of diluent. Later, the sample was added onto the mass spectrometer via the inductively-coupled plasma ionization ion source (23, 25).
BMD measurements were carried out in different body parts (lumbar spine, total femur, and femoral neck) in NHANES. Scanning was carried out with Hologic QDR-4500A fan-beam densitometers (Hologic, Inc., Bedford, Massachusetts) based on dual-energy x-ray absorptiometry (DXA). In general, examination was performed on left hip, while examination on right hip was only conducted in the case of the self-reported pin, fracture, or replacement of left hip. Lumbar spine BMD was calculated as the mean of 1st–4th lumbar vertebrae. Pregnant women, patients with a history of bilateral hip pins/fractures/replacement or radiographic contrast material use, and patients with >450 lbs were excluded from DXA. OP was defined as the average peak BMD of lumbar spine, total hip and femoral neck based on the US NHANES III database and the associated standard deviation (SD) among white women with the age of 20–29 years. Subsequently, BMD levels of lumbar spine, total hip and femoral neck were converted to T-scores (26–28) with the constructed approach. T-score ≤−2.5, −2.5 < T-score ≤−1, and >−1 represented OP, osteopenia, and normal, respectively. We deemed normal subjects and those with osteopenia as non-OP.
Consistent with statements at the NHANES website, data were obtained at all study sites by the trained personnel following the specific procedures. Covariates included age, race, education, marital status, body mass index [BMI, calculated through the division of body weight (kg) by body height squared (m2)], smoking, diabetes, hypertension, parental OP history, and previous fractures. The digital weight scale was used to take body weight of patients (kg). The stadiometer equipped with the adjustable head piece and the fix vertical backboard was utilized to measure the standing height of patients.
Through data weighting, estimates for the US population were obtained and examined through layering and clustering. Continuous data with normal distribution were indicated by mean ± standard deviation (SD) during descriptive analysis and explored by Student's t-test, while those with non-normal distribution were indicated by median and quartile range and explored through non-parametric tests. Meanwhile, categorical data were expressed as frequency and frequency percentage and compared by Chi-squared test. Blood heavy metal levels (Pb, Cd, Hg, Se, and Mn) were determined based on quartiles (quartiles 1–4 indicating <25th, 25th–50th, 50th–75th, and >75th percentile). Logistic regression was carried out to study odds ratios (ORs) and 95% confidence intervals (CIs), aiming to examine the relation of blood heavy metals with OP risk. Quartile 1 was used for reference when evaluating blood heavy metals. Model 1 was adjusted for hypertension, diabetes, parental OP history, previous fractures, and marital status. Model 2 was adjusted for race, education level, and age based on Model 1. While Model 3 was adjusted for all associated covariates. All the exposures were ln-transformed before analysis. In addition, mixed effects were analyzed by adopting weighted quantile sum regression (WQS) models in both positive and negative directionality modes. Bayesian kernel machine regression (BKMR) provides a versatile and succinct estimation of the multivariate exposure-response relationship. The combined effects of each metal (“BKMR” packages) was assessed, while the dose-response relation of one individual metal with OP risk was also evaluated with the other metal levels being constant. This study established multivariate linear regression models to determine the relations of blood heavy metals with total spinal, total femoral, and femoral neck BMD. Subgroup analysis stratified by sex was also conducted to examine the relation of blood heavy metals with OP risk. Moreover, trend test was performed among elevating exposure groups using the multivariable models. Statistical analysis was completed with SPSS (version: 24.0; SPSS, Chicago, IL) and R (version 3.5.3). The significance level was set at p < 0.05.
After excluding non-qualified patients (Figure 1), there were totally 905 subjects enrolled into this study, including 161 cases with OP and 744 with no OP. Table 1 displays patient features with and with no OP. Compared with non-OP patients, OP patients were older and thinner, exhibiting low education levels, a parental OP history, and a lower probability as non-Hispanic Black. On the contrary, non-OP patients showed the lower blood Cd levels (P = 0.022). Total spinal, total femoral, and femoral neck BMD of non-OP patients were significantly higher than OP patients.
Multivariate logistic regression was performed to examine the relation of blood heavy metals with the OP risk, with effect value being indicated by OR and 95% CI (Table 2). The data from Model 1 was adjusted for hypertension, diabetes, history of osteoporosis in parents, previous fractures and marital status; in Model 2, race, education level, and age were adjusted based on Model 1; while Model 3 was adjusted for all covariates. In Model 1, a higher quartile of Cd [1.815 (95% CI: 1.076, 3.061); 2.180 (95% CI: 1.309, 3.631); P for trend = 0.006] was correlated with an increased OP risk. Cd level was also associated with the higher OP risk, and the significance was marginal [1.804 (95% CI: 1.037, 3.137); P for trend = 0.059] in Model 2. Blood Se level was significantly negatively related to the OP risk in Models 1, 2, and 3, i.e., (0.570 (95% CI: 0.356, 0.914); 0.454 (95% CI: 0.276, 0.747) (P for trend = 0.071) in Model 1; (0.519 (95% CI: 0.314, 0.857); 0.415 (95% CI: 0.245, 0.704); 0.558 (95% CI: 0.339, 0.917); (P for trend = 0.015) in Model 2; (0.507 (95% CI: 0.299, 0.862); 0.401 (95% CI: 0.231, 0.696); 0.515 (95% CI: 0.306, 0.866); (P for trend = 0.009) in Model 3. However, blood Pb, Hg, and Mn levels were not associated with DKD in three models (p > 0.05).
Our study utilized multivariate linear regression analysis for evaluating the correlation of blood heavy metals with BMD in two distinct models (Table 3). For Model 1, compared with lowest quartile (Q1) of Cd level, patients showing the highest quartile (Q4) exhibited lower total femoral BMD (β = −0.112, P = 0.007, P for trend = 0.003); compared with the lowest quartile (Q1) of Hg level, patients having highest quartile (Q2) showed the increased femoral neck BMD (β = 0.082, P = 0.040, P for trend = 0.310). Model 1 also revealed that Mn level was negatively related to total spinal and femoral neck BMD (β = −0.098, P = 0.015, P for trend = 0.005; β = −0.106, P = 0.009, P for trend = 0.007). After adjusting for all covariates, Mn level was also significantly positively related to total femoral and femoral neck BMD (β = 0.072, P = 0.033, P for trend = 0.753; β = 0.082, P = 0.020, P for trend = 0.978), but not significantly associated with total spinal BMD.
WQS models were utilized to analyze blood heavy metals that significantly contributed to the combined effect of mixed heavy metals (Pb, Cd, Hg, Se and Mn). Blood heavy metals were ranked according to their possible maximal weight in the mixture. Based on WQS under the positive directional mode, Cd was positively associated with the increased OP risk, whereas Se displayed the negative relation with an increased OP risk (Figure 2). Concerning BKMR results, Figure 3 displays the exposure-response results of individual metals and the combined effect on OP after being adjusted for confounders. After fixing additional metals at the 50th percentile, Se gradually had the decreased enhancing effect on OP, which was later weakened to near zero (Figure 3A). OP risk was significantly related to metal chemical mixture exposure (Figure 3B). The combined effect of 5 metals higher than 50th percentile significantly affected the OP risk ratio. The OP risk significantly increased with the heavy metals being higher than the set level (Figure 3B).
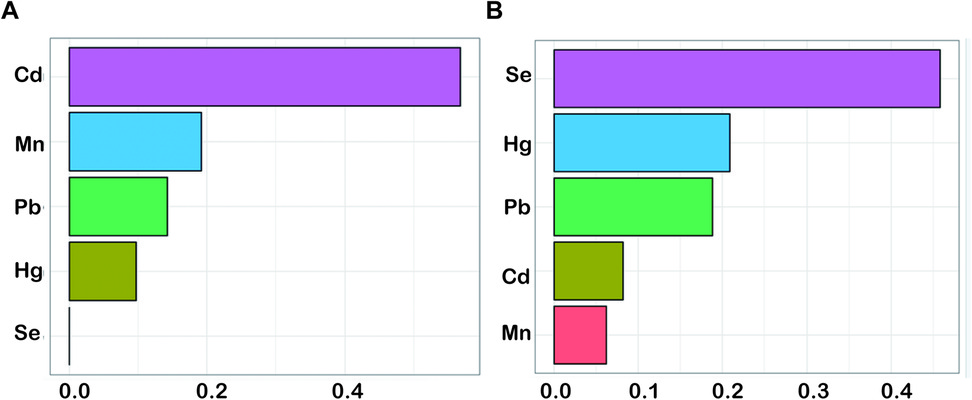
Figure 2. The WQS model regression index weights of ln-transformed blood concentrations of five metals for osteoporosis. (A) positive WQS model; (B) negative WQS model. The model was adjusted for history of hypertension, diabetes, osteoporosis in parents, previous fractures, marital status, race, education level, age, history of smoking and BMI. BMI, body mass index; WQS, weighted quantile sum; Cd, cadmium; Pb, lead; Hg, mercury; Mn, manganese; Se, selenium.
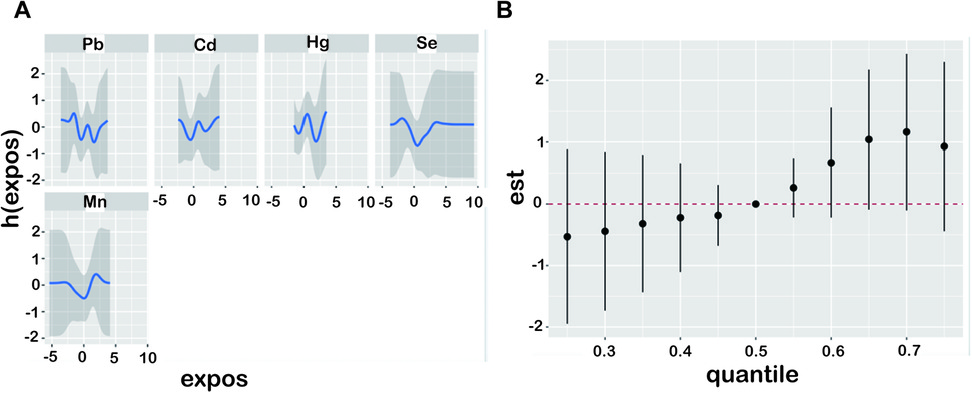
Figure 3. Exposure-response and joint effect of metals on osteoporosis were estimated by BKMR models. The model was adjusted for history of hypertension, diabetes, osteoporosis in parents, previous fractures, marital status, race, education level, age, history of smoking and BMI. (A) Presents univariate exposure-response functions and 95% confidence intervals for each metal exposure fixed at the median. (B) Joint effect of the mixture on z-scores of five metals (Cd, Pb, total Hg, Mn, and Se) on osteoporosis risk. BKMR, Bayesian kernel machine regression; BMI, body mass index; Cd, cadmium; Pb, lead; Hg, mercury; Mn, manganese; Se, selenium.
To the relation of blood heavy metals with OP risk, subgroup analyses stratified by age and BMI were conducted (Table 4). As suggested by logistic regression, the fourth quartile of Cd level was related to an increased OP risk (4.538 (95% CI: 1.309, 15.734; P for trend = 0.009; P for interaction = 0.001) in the 50–60 years subgroup. Additionally, Cd level was not significantly correlated with OP risk in the 60–70 and >70 years subgroups. However, in the 60–70 years subgroup, Se level exhibited negative relation with an increased OP risk [0.404 (95% CI: 0.172, 0.949); 0.362 (95% CI: 0.155, 0.849); P for trend = 0.010; P for interaction = 0.431]. Significant differences were also found in the >70 years subgroup. Based on BMI-stratified subgroup analysis, blood Cd level was significantly positively related to OP risk for the 25–30 kg/m2 subgroups, while no significant relationship was found in the <25 and >30 kg/m2 subgroups. However, the association of blood Se level with OP was significant in BMI (P for interaction = 0.001). The higher blood Se (Q4) levels were related to the decreased OP risk in the BMI 25–30 and >30 kg/m2 subgroups. In the Pb, Hg and Mn sections, all subgroups exhibited no significance.
Based on our knowledge, this is the first and largest population-based study analyzing the relation of blood single or combined heavy metals with an increased OP risk among the US postmenopausal women. Multivariate logistic regression showed that blood Cd and Se exposure were related to DKD. WQS model analysis further underscored the hazardous impact of blood Cd levels and the positive impact of blood Se levels. Mixed heavy metals were significantly positively related to OP in BKMR model. According to stratified analyses, Se was negatively associated with OP among age 60–70 years, BMI 25–30 and >30 kg/m2. Cd was positively associated with OP among age 50–60 years, BMI 25–30 kg/m2.
There are certain studies investigating how blood heavy metals affect bone health or the OP risk (16, 23, 24). For example, a study have shown a significant association between older women with higher blood lead levels and an increased risk of osteoporosis with a consequent susceptibility to bone fractures (29); A correlation has been observed between the concentration of cadmium in the blood and the likelihood of developing osteopenia and osteoporosis among Korean women who have undergone menopause. Nonetheless, additional longitudinal research is needed to establish the presence of a dose-response gradient and to mitigate potential selection biases, particularly in individuals suffering from osteoporosis of the femoral neck (16); The study emphasized the association between Cd and Pb in the blood and the incidence of osteoporosis in Saudi population (24). However, our study is the first to explore the correlation of blood heavy metals with factors including BMD and OP risk among the US postmenopausal women on the basis of big data. Our study examined the relations of blood heavy metals with bone health based on 2 NHANES cycles (2013–2014 and 2017–2020) in the US postmenopausal women, overcoming the problem of insufficient data observed in previous articles. Age, sex, and BMI are the conventional risk factors related to OP. The bone mass is the highest among young adults, which subsequently decreases with the increasing age (30). In addition, women experience rapider bone loss with age due to estrogen deficiency, while men undergo slower bone loss (30). Therefore, we focused on investigating the association of blood heavy metals with OP and bone density among postmenopausal women. The results of this study were consistent with many previous research reports. For example, blood Cd levels are associated with the osteopenia and OP risk among the Korean postmenopausal women (16). Blood Cd level increased the risk of OP, while blood Se level decreased the OP risk in the US middle-aged and older adult populations in a previous NHANES 2013–2014 and 2017–2018 study (23), consistent with our findings.
Bone is a target organ for toxic metals. These metals are correlated with lower BMD and OP (31). Cd exposure may result in the decreased BMD through multiple potential mechanisms, including suppressing proliferation, viability, and osteoblast differentiation of bone marrow mesenchymal stem cells (BMMSCs) via P2X7-PI3K-AKT and NF-κB pathways (32, 33). Cd has been recently suggested to cause apoptosis of bone osteoblasts through ROS (34). Overexposure to cadmium can decrease the synthesis of calcitriol, degrade the collagen matrix within bones, disrupt the mineralization process of bone cells, suppress the function of osteoblasts, and enhance the activity of osteoclasts, thereby adversely affecting bone health (22). Furthermore, Cd is found to inhibit Wnt/β-catenin pathway to suppress osteogenesis (35). The lower blood Se level is related to skeletal disorder, in particular OP in women (36). Nevertheless, in some research, Se is not related to BMD among the healthy females. Se exposure can lead to an increased risk of OP via several mechanisms. At first, Se can promote BMMSCs differentiation into osteoblasts by decreasing mature osteoclast generation and differentiation (37). Second, Se influences osteoblastic differentiation and subsequent bone resorption through regulating oxidative stress (38–40). Third, Wnt/LRP8/ApoER2 pathway is the basic intracellular Se transport pathway to change bone metabolism (41). Based on animal studies, bone metabolism is altered after Se deprivation, which is associated with the decreased GPX1 activity, blood Ca content, pituitary growth hormone level, plasma insulin-like growth factor level, whereas the increased blood 1, 25-dihydroxyvitamin D3, urinary Ca, and parathyroid hormone contents (39, 42). Therefore, further studies are worth being conducted to determine the relationship between Cd and Se levels and OP and to explore the underlying mechanism.
There are certain strengths in this study. Firstly, this work was carried out on the basis of the nationwide survey, in which BMD was measured by expert scientists with established methods. Secondly, this study is the first to focus on examining the relation of blood heavy metals with parameters including OP and BMD in the US postmenopausal women. Our findings showed that Se might prevent OP occurrence, while Cd promoted OP occurrence. After adjusting for confounders, subgroup analyses stratified by age and BMI were carried out to determine the association of Cd and Se with the changed OP risk. Nevertheless, some limitations must be pointed out in this work. Firstly, the present cross-sectional study might inevitably induce residual confounding due to other unmeasured factors, regardless of adjustment of some covariates. Secondly, we measured blood heavy metals once only, which might be unable to indicate the continuous exposure, causing measurement errors. Moreover, WQS and BKMR cannot be employed to analyze weighted data, probably leading to bias of the model results. However, it is necessary to perform a cohort study to more accurately understand the effects of metals on OP.
To conclude, NHANES 2013–2014 and 2017–2020 data from the United States suggest among postmenopausal women that single and mixed blood metal levels are significantly related to OP. Blood Cd level increases the OP risk, whereas blood Se level protects from OP occurrence, revealing that appropriately increasing Se intake can postpone OP occurrence and progression in the US postmenopausal women. Association studies and prediction of OP might assist postmenopausal women to prevent OP and reduce exposure to risk factors. Further experimental investigations and prospective cohort studies are required to validate the connections and to clarify fundamental mechanisms at play.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving humans were approved by the Institutional Review Board of the National Center for Health Statistics. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
SP: Methodology, Software, Writing – original draft. GZ: Supervision, Writing – review & editing. DW: Supervision, Writing – review & editing. ZH: Data curation, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors acknowledge the data from the National Health and Nutrition Examination Survey (NHANES).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Hendrickx G, Boudin E, Van Hul W. A look behind the scenes: the risk and pathogenesis of primary osteoporosis. Nat Rev Rheumatol. (2015) 11:462–74. doi: 10.1038/nrrheum.2015.48
2. Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. (2014) 29:2520–6. doi: 10.1002/jbmr.2269
3. Duan W, Meng X, Sun Y, Jia C. Association between polycyclic aromatic hydrocarbons and osteoporosis: data from NHANES, 2005–2014. Arch Osteoporos. (2018) 13:112. doi: 10.1007/s11657-018-0527-4
4. Carlson BC, Robinson WA, Wanderman NR, Sebastian AS, Nassr A, Freedman BA, et al. A review and clinical perspective of the impact of osteoporosis on the spine. Geriatr Orthop Surg Rehabil. (2019) 10:2151459319861591. doi: 10.1177/2151459319861591
5. Lunde A, Tell GS, Pedersen AB, Scheike TH, Apalset EM, Ehrenstein V, et al. The role of comorbidity in mortality after hip fracture: a nationwide Norwegian study of 38,126 women with hip fracture matched to a general-population comparison cohort. Am J Epidemiol. (2019) 188:398–407. doi: 10.1093/aje/kwy251
6. Rizzoli R, Biver E, Brennan-Speranza TC. Nutritional intake and bone health. Lancet Diabetes Endocrinol. (2021) 9:606–21. doi: 10.1016/S2213-8587(21)00119-4
7. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. Exp Suppl. (2012) 101:133–64. doi: 10.1007/978-3-7643-8340-4_6
8. Ahamad MI, Rehman A, Mehmood MS, Mahmood S, Zafar Z, Lu H, et al. Spatial distribution, ecological and human health risks of potentially toxic elements (PTEs) in river Ravi, Pakistan: a comprehensive study. Environ Res. (2024) 263:120205. doi: 10.1016/j.envres.2024.120205
9. Rehman K, Fatima F, Waheed I, Akash MSH. Prevalence of exposure of heavy metals and their impact on health consequences. J Cell Biochem. (2018) 119:157–84. doi: 10.1002/jcb.26234
10. Rusyniak DE, Arroyo A, Acciani J, Froberg B, Kao L, Furbee B. Heavy metal poisoning: management of intoxication and antidotes. EXS. (2010) 100:365–96. doi: 10.1007/978-3-7643-8338-1_11
11. Goutam Mukherjee A, Ramesh Wanjari U, Renu K, Vellingiri B, Valsala Gopalakrishnan A. Heavy metal and metalloid—induced reproductive toxicity. Environ Toxicol Pharmacol. (2022) 92:103859. doi: 10.1016/j.etap.2022.103859
12. Rzymski P, Tomczyk K, Rzymski P, Poniedzialek B, Opala T, Wilczak M. Impact of heavy metals on the female reproductive system. Ann Agric Environ Med. (2015) 22:259–64. doi: 10.5604/12321966.1152077
13. Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. (2021) 12:643972. doi: 10.3389/fphar.2021.643972
14. Parida L, Patel TN. Systemic impact of heavy metals and their role in cancer development: a review. Environ Monit Assess. (2023) 195:766. doi: 10.1007/s10661-023-11399-z
15. Lim HS, Lee HH, Kim TH, Lee BR. Relationship between heavy metal exposure and bone mineral density in Korean adult. J Bone Metab. (2016) 23:223–31. doi: 10.11005/jbm.2016.23.4.223
16. Kim ES, Shin S, Lee YJ, Ha IH. Association between blood cadmium levels and the risk of osteopenia and osteoporosis in Korean post-menopausal women. Arch Osteoporos. (2021) 16:22. doi: 10.1007/s11657-021-00887-9
17. Shetaia SA, Nasr RA, Lasheen ESR, Dar MA, Al-Mur BA, Zakaly HMH. Assessment of heavy metals contamination of sediments and surface waters of Bitter Lake, Suez Canal, Egypt: ecological risks and human health. Mar Pollut Bull. (2023) 192:115096. doi: 10.1016/j.marpolbul.2023.115096
18. Adimalla N, Chen J, Qian H. Spatial characteristics of heavy metal contamination and potential human health risk assessment of urban soils: a case study from an urban region of south India. Ecotoxicol Environ Saf. (2020) 194:110406. doi: 10.1016/j.ecoenv.2020.110406
19. Park S, Lee BK. Body fat percentage and hemoglobin levels are related to blood lead, cadmium, and mercury concentrations in a Korean adult population (KNHANES 2008–2010). Biol Trace Elem Res. (2013) 151:315–23. doi: 10.1007/s12011-012-9566-7
20. Bjorklund G, Pivina L, Dadar M, Semenova Y, Chirumbolo S, Aaseth J. Long-term accumulation of metals in the Skeleton as related to osteoporotic derangements. Curr Med Chem. (2020) 27:6837–48. doi: 10.2174/0929867326666190722153305
21. Kazantzis G. Cadmium, osteoporosis and calcium metabolism. Biometals. (2004) 17:493–8. doi: 10.1023/B:BIOM.0000045727.76054.f3
22. Jalili C, Kazemi M, Taheri E, Mohammadi H, Boozari B, Hadi A, et al. Exposure to heavy metals and the risk of osteopenia or osteoporosis: a systematic review and meta-analysis. Osteoporos Int. (2020) 31:1671–82. doi: 10.1007/s00198-020-05429-6
23. Huang Z, Wang X, Wang H, Zhang S, Du X, Wei H. Relationship of blood heavy metals and osteoporosis among the middle-aged and elderly adults: a secondary analysis from NHANES 2013 to 2014 and 2017 to 2018. Front Public Health. (2023) 11:1045020. doi: 10.3389/fpubh.2023.1045020
24. Banjabi AA, Kannan K, Kumosani TA, Yousef JM, Abulnaja KO, Moselhy SS. Association of blood heavy metal levels with osteocalcin abnormality and incidence of osteoporosis in Saudi subjects. Braz J Biol. (2021) 83:e248828. doi: 10.1590/1519-6984.248828
25. Xia F, Li Q, Luo X, Wu J. Identification for heavy metals exposure on osteoarthritis among aging people and machine learning for prediction: a study based on NHANES 2011–2020. Front Public Health. (2022) 10:906774. doi: 10.3389/fpubh.2022.906774
26. Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. (1998) 8:468–89. doi: 10.1007/s001980050093
27. Bass MA, Sharma A, Nahar VK, Chelf S, Zeller B, Pham L, et al. Bone mineral density among men and women aged 35–50 years. J Am Osteopath Assoc. (2019) 119:357–63. doi: 10.7556/jaoa.2019.064
28. Peng S, Zhang G, Wang D. Association of selenium intake with bone mineral density and osteoporosis: the national health and nutrition examination survey. Front Endocrinol (Lausanne). (2023) 14:1251838. doi: 10.3389/fendo.2023.1251838
29. Khalil N, Cauley JA, Wilson JW, Talbott EO, Morrow L, Hochberg MC, et al. Relationship of blood lead levels to incident nonspine fractures and falls in older women: the study of osteoporotic fractures. J Bone Miner Res. (2008) 23:1417–25. doi: 10.1359/jbmr.080404
30. Pouresmaeili F, Kamalidehghan B, Kamarehei M, Goh YM. A comprehensive overview on osteoporosis and its risk factors. Ther Clin Risk Manag. (2018) 14:2029–49. doi: 10.2147/TCRM.S138000
31. Berglund M, Akesson A, Bjellerup P, Vahter M. Metal-bone interactions. Toxicol Lett. (2000) 112-113:219–25. doi: 10.1016/S0378-4274(99)00272-6
32. Luo H, Gu R, Ouyang H, Wang L, Shi S, Y JI, et al. Cadmium exposure induces osteoporosis through cellular senescence, associated with activation of NF-kappaB pathway and mitochondrial dysfunction. Environ Pollut. (2021) 290:118043. doi: 10.1016/j.envpol.2021.118043
33. Ma Y, Ran D, Cao Y, Zhao H, Song R, Zou H, et al. The effect of P2X7 on cadmium-induced osteoporosis in mice. J Hazard Mater. (2021) 405:124251. doi: 10.1016/j.jhazmat.2020.124251
34. Al-Ghafari A, Elmorsy E, Fikry E, Alrowaili M, Carter WG. The heavy metals lead and cadmium are cytotoxic to human bone osteoblasts via induction of redox stress. PLoS One. (2019) 14:e0225341. doi: 10.1371/journal.pone.0225341
35. Wu L, Wei Q, Lv Y, Xue J, Zhang B, Sun Q, et al. Wnt/beta-catenin pathway is involved in cadmium-induced inhibition of osteoblast differentiation of bone marrow mesenchymal stem cells. Int J Mol Sci. (2019) 20:1519. doi: 10.3390/ijms20061519
36. Hoeg A, Gogakos A, Murphy E, Mueller S, Kohrle J, Reid DM, et al. Bone turnover and bone mineral density are independently related to selenium status in healthy euthyroid postmenopausal women. J Clin Endocrinol Metab. (2012) 97:4061–70. doi: 10.1210/jc.2012-2121
37. Li C, Wang Q, Gu X, Kang Y, Zhang Y, Hu Y, et al. Porous se@SiO(2) nanocomposite promotes migration and osteogenic differentiation of rat bone marrow mesenchymal stem cell to accelerate bone fracture healing in a rat model. Int J Nanomedicine. (2019) 14:3845–60. doi: 10.2147/IJN.S202741
38. Liu H, Bian W, Liu S, Huang K. Selenium protects bone marrow stromal cells against hydrogen peroxide-induced inhibition of osteoblastic differentiation by suppressing oxidative stress and ERK signaling pathway. Biol Trace Elem Res. (2012) 150:441–50. doi: 10.1007/s12011-012-9488-4
39. Cao JJ, Gregoire BR, Zeng H. Selenium deficiency decreases antioxidative capacity and is detrimental to bone microarchitecture in mice. J Nutr. (2012) 142:1526–31. doi: 10.3945/jn.111.157040
40. Vescini F, Chiodini I, Palermo A, Cesareo R, De Geronimo V, Scillitani A, et al. Selenium: a trace element for a healthy skeleton—a narrative review. Endocr Metab Immune Disord Drug Targets. (2021) 21:577–85. doi: 10.2174/1871530320666200628030913
41. Pietschmann N, Rijntjes E, Hoeg A, Stoedter M, Schweizer U, Seemann P, et al. Selenoprotein P is the essential selenium transporter for bones. Metallomics. (2014) 6:1043–9. doi: 10.1039/C4MT00003J
Keywords: heavy metals, bone mineral density (BMD), osteoporosis, NHANES, postmenopausal
Citation: Peng S, Zhang G, Wang D and He Z (2025) The impact of heavy metals on osteoporosis in postmenopausal women. Front. Environ. Health 4:1468404. doi: 10.3389/fenvh.2025.1468404
Received: 28 July 2024; Accepted: 11 February 2025;
Published: 25 February 2025.
Edited by:
Heba H. Abdel-Kader, National Institute of Oceanography and Fisheries (NIOF), EgyptReviewed by:
Muhammad Sajid, Northeast Normal University, ChinaCopyright: © 2025 Peng, Zhang, Wang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaoxiang Zhang, ejAxMDAyMDIwMjFAMTYzLmNvbQ==; Decheng Wang, d2RjMTM1MDExMzExMzVAMTYzLmNvbQ==; Zhiliang He, aHpsMTg4MTE1Mjc5NTNAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.