
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Health , 04 October 2023
Sec. Environmental Epidemiology
Volume 2 - 2023 | https://doi.org/10.3389/fenvh.2023.1268828
Background: Exposure to outdoor artificial light at night (LAN) disrupts circadian rhythms and is suspected of increasing the risk of breast cancer. To date, this is an understudied aspect of environmental pollution. In this study, we sought to assess the specific role of exposure to outdoor artificial light at night in breast cancer, independently of air pollution-related effects.
Methods: Data from a French population-based case-control study, including 1,185 incident breast cancer cases and 1,282 controls enrolled in 2005–2007, were used. Outdoor LAN exposure data were obtained using radiance-calibrated images from the Defense Meteorological Satellite Program (DMSP) for 1995–2006 by cross-referencing the DMSP images and the geocoded locations of residences in ArcGIS. The odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were obtained using logistic regression adjusting for multiple potential confounders, including air pollution.
Results: The OR for overall breast cancer unadjusted for air pollution per interquartile range increase in LAN exposure was 1.05 (95% CI: 0.92–1.20). The OR decreased to 0.98 (95% CI: 0.81–1.17) after adjustment for ambient NO2 levels. Subgroup analyses showed slightly higher ORs in postmenopausal women (OR per IQR increase: 1.07; 95% CI: 0.85–1.35) and a positive association for HER2-positive breast tumors (OR: 1.55; 95% CI: 1.03–2.31).
Conclusion: Our results do not provide evidence that outdoor LAN exposure is associated with increased risk of breast cancer. However, an association was suggested for the HER2-positive subtype of breast cancer. Further large-scale studies with more precise exposure assessment methods, including blue light and indoor exposure measurements, and considering environmental exposures correlated with LAN exposure such as air pollution, are needed.
In 2020, 2.3 million new breast cancer cases were observed, making it the most frequently diagnosed cancer and a primary cause of death in women (1). Breast cancer is associated with an extensive range of risk factors, including hereditary and genetic factors, reproductive and hormonal factors (2–5), overweight after menopause, or lifestyle-related and environmental factors (6, 7). Emerging evidence points toward a link between light pollution and breast cancer. Over the past century, extensive development and use of electric light have made exposure to artificial light at night (LAN) ubiquitous in modern societies. The Atlas of night sky brightness shows that more than 80% of the world and more than 99% of the United States and European population live under night-light-polluted skies (8, 9), with a continuous increase in light emissions worldwide at a rate of 2.2% per year (10).
Recent experimental and epidemiologic evidence supports the hypothesis that LAN exposure is a carcinogen for breast cancer. Exposure to artificial LAN decreases or delays the production and secretion of melatonin, a hormone the pineal gland produces in the dark phase of the 24-h cycle. Disruptions in circadian rhythm associated with changes in the sleep-wake and melatonin cycles have been implicated to be carcinogenic, particularly hormone-dependent cancers such as breast cancer, due to their deleterious effects on the functioning of biological pathways such as hormone signaling, cell proliferation, DNA repair or inflammation pathways (11, 12).
The International Agency for Research on Cancer (IARC) categorized “shift work involving circadian disruption” as probably carcinogenic (Group 2A) in 2007 (13). In 2019, the IARC evaluation of “night work” based on additional studies resulted in the same classification, with consistent evidence of an association with breast cancer (14). Exposure to indoor LAN during night shifts has been hypothesized to be responsible for the development of cancer (15) through disruption of circadian rhythms, such as the suppression of the nocturnal secretion of melatonin and its oncostatic effects (11). While the IARC evaluation primarily focused on occupational exposures to LAN associated with night-shift work, the environmental exposure to LAN, subsequent circadian disruption, and its potential carcinogenic effects in the general population are poorly understood.
Ecological studies have shown that the incidence of breast cancer was higher in geographic areas with higher levels of light pollution assessed from nighttime satellite photometry data (16–21). A few case‒control and cohort studies (22–29) using satellite-based imagery to measure exposure to outdoor LAN have examined the association between LAN exposure in the visible range (350–600 nm) and breast cancer risk, with inconclusive results. Some studies reported that breast cancer was increased in women with high exposure to outdoor LAN (22, 23, 25, 26, 28), while others did not (24, 29–31). Of note, breast cancer was positively associated with the Melatonin Supression Index an indicator of blue light exposure (−480 nm) developed by Aubé et al. (32) and used in the MCC-Spain case‒control study (24). Exposure to outdoor LAN is often accompanied by exposure to other environmental factors that have been associated with breast cancer risk, either positively, such as air pollution (33) and noise pollution (34, 35), or negatively, such as exposure to green spaces (36). Only two cohort studies that accounted for potential confounding by aforementioned environmental factors (29, 30) reported no association between LAN exposure and breast cancer. To examine the independent effects of LAN exposure on breast cancer incidence, it seems necessary to account for factors that correlate with outdoor LAN, notably air pollution. Altogether, the potential health effects of outdoor LAN exposure deserve to be explored thoroughly due to its potentially important public health impact. Here, using data from the CECILE study, we aimed to examine the association between outdoor LAN exposure and breast cancer risk after adjusting for potential confounders such as air pollution. We also aimed to assess possible modifications of this association.
This CECILE study, conducted in two French departments, Côte d'Or in the eastern part and Ille-et-Vilaine in the western part of the country, is a population-based case‒control study. All women aged 25–75 years residing in two departments with in situ or invasive breast tumors newly diagnosed during the study period (April 2005–March 2007) were eligible for inclusion. The cases were identified from the medical wards of the main cancer hospitals (Centre Eugène Marquis in Ille-et-Vilaine and Centre Georges-François Leclerc in Côte d'Or) and smaller public and private hospitals treating breast cancer patients in the two departments. Of the 1,556 eligible cases identified, 163 declined to participate, 151 could not be contacted, 7 died, and 2 had incomplete occupational history, resulting in 1,233 (79.3%) cases for inclusion in the study. The controls consisted of women from the general population residing in the same two departments when cases were diagnosed, without a previous history of breast cancer and frequency-matched by 10-year age group and department. The controls were recruited from random samples of private homes listed in the telephone directory. Women were first contacted by phone and invited to participate in the study within predefined quotas of socioeconomic status (SES) categories to reflect the distribution by SES in the general population of women in each department. Among the 1,731 eligible controls identified, 260 declined to participate, 154 could not be contacted for in-person interviews, and 2 had incomplete occupational history, resulting in 1,315 (76%) controls for inclusion in the study.
The local ethical committee approved the study protocol, and all subjects signed informed consent before enrolling in the study.
Women were interviewed in 60–90-min face-to-face interviews using standardized questionnaires. Information was obtained on sociodemographic characteristics, hormonal and reproductive factors [age at menarche and menopause, oral contraceptive use, menopausal hormonal therapy use (MHT), history of gynecological diseases, and outcomes of each pregnancy, breastfeeding], anthropometric factors (weight, height), personal medical history, family history of cancer, lifestyle-related factors (alcohol consumption, smoking, physical activities, dietary habits), and occupational and residential history. Only data obtained before or at the reference date (i.e., date of diagnosis for cases and date of consent for controls) were considered in the analysis.
Breast cancer cases were subclassified into 3 subtypes based on the information available from the pathology report: (i) hormone-receptor positive [i.e., estrogen receptor positive or progesterone receptor positive and human epidermal growth factor receptor 2 negative (ER-positive or PR-positive and HER2-negative), equivalent to the luminal A molecular subtype]; (ii) HER2-positive regardless of ER and PR status, equivalent to the luminal B and HER2-negative enriched molecular subtypes; and (iii) triple-negative tumors (ER-negative, PR-negative and HER2-negative). Tumors with more than 10% positive hormonal receptor cells were characterized as receptor-positive.
Outdoor exposure to LAN was assessed at each address occupied by women during the 10 years before the reference date (i.e., 1995–2007) by using the satellite images of the Operational Linescan System (OLS) available in the Defense Meteorological Satellite Program (DMSP) of the National Oceanic and Atmospheric US Administration (NOAA) (37). All the residential addresses occupied by women for 10 years before inclusion were geocoded. In this study, we used the Radiance Calibrated Nighttime Lights Products, high-dynamic range images with a spatial resolution of a 30-arc second grid −650 × 650 m) (38). The illuminance was measured in nanowatts per square centimeter per steradian (nW/cm2/sr). The radiance-calibrated images were available for the years 1996 (March 16, 1996–February 12, 1997), 1999 (January 19–December 11, 1999), 2000 (January 3–December 29, 2000), 2003 (December 30, 2002–November 27, 2003), 2004 (January 18–December 16, 2004) and 2006 (November 28, 2005–December 24, 2006). To estimate annual exposure over the 10 years before the reference date, the 1996 DMSP images were applied to 1995 and 1997, the 1999 images were applied to 1998, and the 2006 images were applied to 2005 and 2007. These images were projected in geographic information system software (GIS)—ArcGisPro 3.0 and cross-referenced with the geocoded locations of each address, which provided the luminosity value at each location. Then, the cumulative exposure to outdoor LAN over the 10 years was calculated as an average of annual exposures weighted on the length of stay at each address.
We considered the following covariates: age at reference, department of residence at reference, age at first full-term pregnancy, parity, menopausal status, oral contraceptive use, MHT use, family history of breast cancer in first-degree relatives, alcohol consumption, smoking, body mass index (BMI), night shift work, educational level as a proxy for SES, urbanization of the residential area at reference, and average annual exposure to air pollutants: nitrogen dioxide (NO2) and particulate matter (PM2.5 and PM10).
Unconditional logistic regression was used to calculate the estimates for the association between breast cancer and exposure to outdoor LAN, expressed as the mean annual exposure over the last 10 years in nW/cm2/sr. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were calculated for the 2nd and 3rd tertiles of outdoor LAN (T2 and T3) with reference to the lowest tertile (T1) and for one interquartile range (IQR = 159.9 nW/cm2/sr) increase in outdoor LAN exposure, all based on exposure distribution among controls. Adjustment sets of the association were identified from a directed acyclic graph (DAG) (see Supplementary Figure S1 in Supplemental Material) (30, 39). Model 1 was adjusted for the matching variables (age at recruitment as a continuous variable and department of residence at recruitment), as well as for urbanization of the area of residence at recruitment (main city center, suburbs, isolated cities, and rural areas, according to the INSEE classification) (32). Furthermore, Model 2 was adjusted for other potential confounders identified in the minimal adjustment set, including education (no school/primary education, basic secondary school, secondary school, university degree), age at first full-term pregnancy (<21 years, 22–24 years, 25–27 years, ≥28 years), parity (nulliparous, 1, 2 and ≥3), menopausal status and MHT use (premenopausal, postmenopausal with MHT use, postmenopausal without MHT use), history of breast cancer among 1st-degree relatives (yes, no), BMI (<18.5; 18.5–25; 25–30; ≥30 kg/m2, defined according to WHO classification), alcohol consumption (measured by the number of glasses per week: 0–3, 4–7, and ≥7 glasses per week), tobacco smoking (never, former and current smokers) and night shift work (never, ever: defined as having worked for at least 3 h between midnight and 5 a.m. in at least one job of minimum 6 months throughout the career). In Model 3, we further adjusted for air pollution using exposure to NO2, PM2.5, and PM10 (continuous variables µg/m3, measured as average annual exposure to each pollutant for 10 years before inclusion in the study, exposure assessment methods explained elsewhere) (40). In Model 3, we also assessed for possible collinearity between exposure to outdoor LAN and air pollution using the variance inflation factor (VIF), such that a VIF >5 indicated collinearity (41).
In further analyses, we assessed the modification of the association between outdoor LAN exposure and breast cancer by using an interaction term between LANs and effect modifiers such as department, menopausal status, night shift work, urbanization, education, and BMI. We also assessed the association of LAN exposure with different tumor subtypes.
Exposure to outdoor LAN initially ranged from 0 to 1,128.61 nW/cm2/sr with a negatively skewed distribution. The values of LAN beyond “upper quartile (Q3) + 1.5*IQR” were flagged as outliers (42, 43) and excluded from the final analysis, as they highly distort the distribution. Out of 2,549 women, 56 had missing geocoded addresses and missing values for LAN, while 26 cases and 10 controls had outlying values for LAN, leaving 2,467 women for the main analysis.
Descriptive characteristics of the study participants by case and control status are shown in Table 1. The distribution by age and department, the matching variables, was similar in cases and controls. In our data, cases lived more often than controls in urban areas. Compared to controls, cases were more educated, had an earlier age at menarche, lower parity, later age at 1st full-term pregnancy, and more frequently had a family history of breast cancer. Premenopausal cases were, on average, thinner than controls, whereas BMI did not differ significantly among postmenopausal women. In these women, cases were more frequently current users of MHT than controls. No difference was observed between the two groups in oral contraceptive use, alcohol consumption, smoking status, or night shift work. The mean annual exposure to NO2, PM2.5, and PM10 was slightly higher in cases than in controls.
Table 2 shows the distribution of the exposure among cases and controls. Exposure to outdoor LAN was found to be significantly higher among the controls who resided in central cities (median, IQR: 232.4, 72.2–358.0 nW/cm2/sr) and suburbs (median, IQR: 110.7, 63.3–203.6 nW/cm2/sr) (p < 10−4) (Supplementary Figure S2 in Supplemental Material). Levels of exposure were relatively higher among women with university degrees and with higher exposure to NO2 and PM2.5 (p < 10−4). There was no significant difference in the exposure level by night shift work (p > 0.05).
The odds ratios for breast cancer associated with outdoor LAN exposure are shown in Table 3. In Model 1, with basic adjustment for age, department, and urbanization, the odds ratios in T2 and T3 compared to T1 were 1.11 (95% CI: 0.86–1.41) and 1.25 (95% CI: 0.95–1.63), respectively. The OR for a one interquartile range (IQR) increase in LAN exposure was 1.09 (95% CI: 0.96–1.24). Further adjustment for reproductive and lifestyle-related factors in Model 2 reduced the ORs at T2 and T3 and per IQR increase in LAN. Additional adjustment for NO2 used as a marker of air pollution resulted in further reduction of the ORs in T2 (1.05; 95% CI: 0.81–1.37) and T3 (1.10; 95% CI: 0.78–1.56) and for one IQR increase in LAN to 0.98 (95% CI: 0.81–1.52). Alternative adjustment for PM2.5 or PM10 in Model 3 also reduced the ORs, although only a minor reduction was observed for PM10.
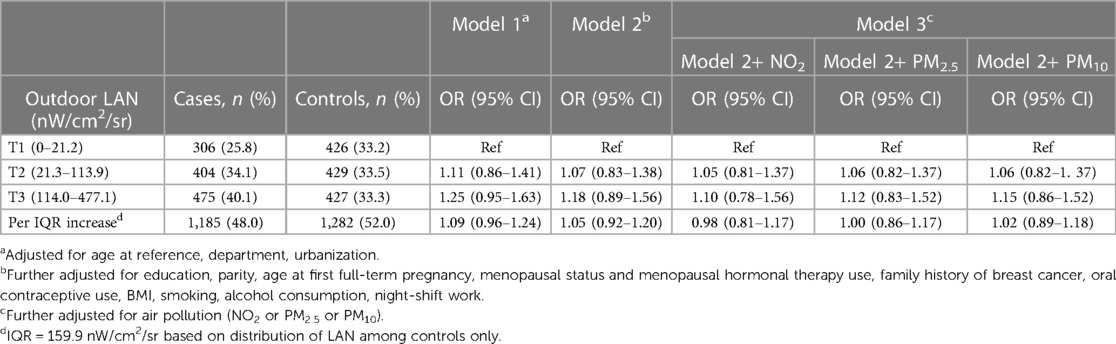
Table 3. Association of outdoor LAN and risk of breast cancer after adjusting for different covariates.
In Table 4, we explored the effect modification by department, urbanization, education, menopausal status, night shift work, and BMI, also comparing the effect before and after adjusting for exposure to NO2. These factors had no statistically significant effect modification (p-values for interaction >0.05). Further adjustment for air pollution (NO2) decreased the ORs in most of the strata.
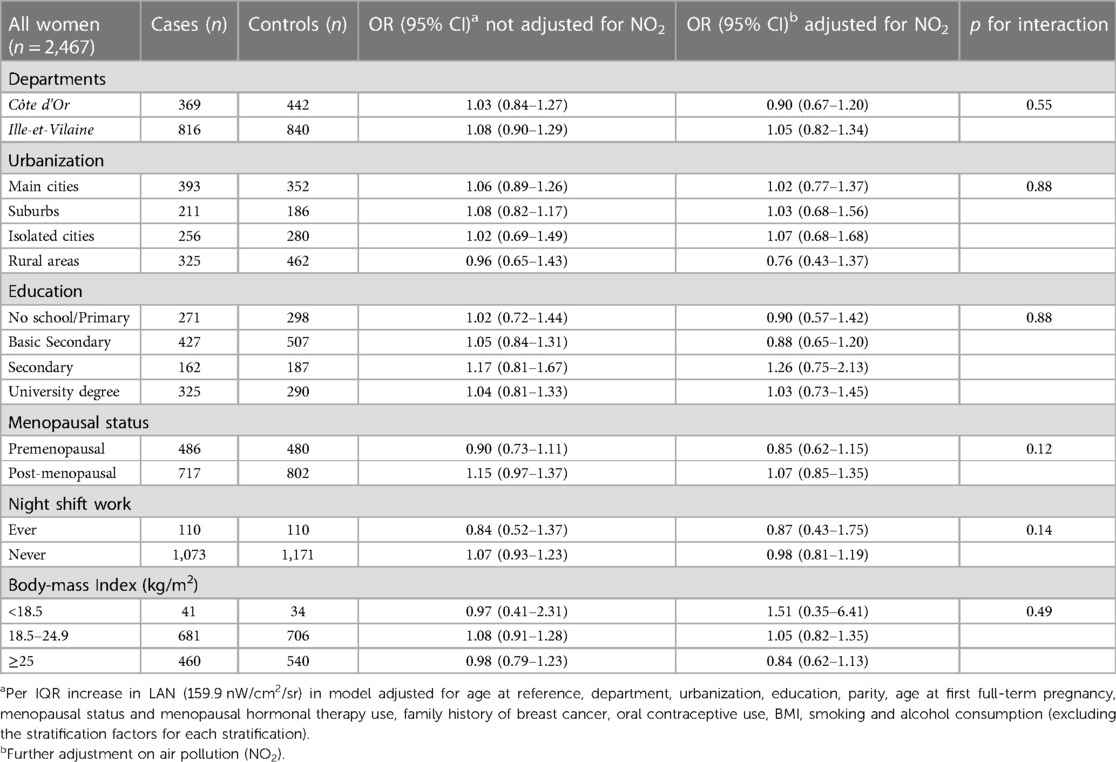
Table 4. Effect modification of the association of outdoor LAN and breast cancer risk by variables of interest.
When looking at breast cancer subtypes (Table 5), a positive association with LAN exposure was observed for HER2-positive breast cancer that persisted after adjustment for either air pollutant NO2, PM10 or PM2.5 (e.g., OR adjusted for NO2 1.55; 95% CI: 1.03–2.31). This association was driven by HER2-positive breast cancers in postmenopausal women (e.g., OR adjusted for NO2 2.15; 95% CI: 1.27–3.63), but was not observed in premenopausal women.
In this study, we did not find conclusive evidence of an association between exposure to outdoor LAN and breast cancer risk. The odds ratios for the association between LAN exposure and breast cancer were further reduced towards unity after adjustment for air pollution, an environmental exposure that is correlated with outdoor LAN. Stratification by menopausal status, urbanization, education, night shift work, or BMI showed no association between outdoor LAN exposure and breast cancer in any subgroup. Analyses by breast cancer subtype found no association with hormone receptor-positive and HER2-negative tumors (ER-positive or PR-positive/HER2-negative), but an association with HER2-positive tumors was indicated based on a small number of cases.
Previously conducted case‒control (23, 24, 31) or cohort studies (22, 25, 26, 28–30) have examined breast cancer risk as a function of environmental exposure to LAN assessed at the study subjects' home addresses, with inconsistent results. These studies measured exposure across the full spectrum of visible light from DMSP-OLS data, except the Spanish MCC-Spain Study (24) which assessed light intensity from nighttime photographs taken by astronauts aboard the International Space Station (ISS). Five studies reported that women with the highest exposure to LAN had a minor but significantly augmented risk of breast cancer compared to the group with the lowest exposure (22, 23, 25, 26, 28), while four other studies showed no increase in risk related to LAN exposure assessed in the full range of visible light (24, 29–31).
One of the main issues that emerges from these discordant results is the consideration of potential confounders, particularly environmental exposures. The two cohort studies that reported no association with breast cancer risk (29, 30) were also the only studies to consider other environmental exposures which correlate with outdoor LAN such as air pollution, green spaces and noise. These environmental covariates have also been suspected as breast cancer risk factors (33–36), and may therefore confound the association of breast cancer with outdoor LAN. The Nurses Cohort Study in Denmark reported a decreased hazard ratio after adjustment for air pollution and road traffic noise (30); the US Sister Study cohort showed no association between LAN and breast cancer after adjustment for air pollution (NO2, PM2.5), noise pollution, and proximity to green spaces (29). Our study also found that adjusting for NO2 exposure, a proxy for road-traffic-related air pollution associated with breast cancer risk by a previous study (33), further reduced the ORs associated with outdoor LAN exposure. This finding is consistent with the two recent cohorts and suggests that the confounding by NO2 or other environmental exposures that correlates with outdoor LAN may be responsible for the non-null associations between outdoor LAN and breast cancer observed in previous studies that did not consider these environmental covariates (23, 25, 26, 28). Therefore, environmental exposures in urban settings that are likely to correlate with outdoor LAN, need to be considered carefully to identify a possible independent effect of outdoor LAN on breast cancer risk. It is also essential to exercise caution while considering highly correlated factors such as outdoor LAN and air pollution. In our study, air pollution was correlated with outdoor LAN, which increased the risk of variation inflation and bias in our statistical models. To address this issue, we assessed multicollinearity using VIF and found no evidence of collinearity between air pollution and exposure to outdoor LAN in full models. Future studies on large datasets should attempt to disentangle and investigate the independent effects of outdoor LAN and air pollution exposures on breast cancer risk and their potential interactions.
Assessment of outdoor LAN exposure through the DMSP data has several limitations, including low resolution, saturation effects in urban areas, and no information on spectral components of the light. Compared with the DMSP images, the ISS images used by Garcia-Saenz et al. (24) allowed a more elaborate evaluation of exposure to outdoor LAN. In addition to a higher resolution (i.e., 30 m in urban areas) compared to −650 m for the calibrated DMSP data in the present study, ISS images provide information on three spectral bands of visible light (red, green, blue). Although Garcia-Saenz et al. (24) reported no association of breast cancer with outdoor visual LAN used as an indicator of total luminance, they found a positive association of breast cancer with the Melatonin Suppression Index (MSI), a proxy measure of exposure to the blue light spectrum (32). This finding is in accordance with the observation that blue light is the most efficient spectral component of light to suppress nocturnal melatonin production (44) which in turn, could be linked with an elevated risk of breast cancer (45). In our study, we could not use ISS images to assess exposure to blue light because of their unavailability during the study period, i.e., 2005–2007. Further studies using ISS images could be of great interest to further examine the association of breast cancer with blue light.
Our study did not explore the effect of indoor exposure or the use of electronic devices, even though exposure from electronic devices, indoor lighting, and sleep settings plays an important role. Some case‒control studies (16, 27, 46–48) assessed exposure to indoor LAN using interviews on sleep habits (such as using lights, curtains/blinds/shutters or electronic devices, or visibility at night) but provided conflicting results. Only a few studies have measured both indoor and outdoor LAN (24, 25, 29, 49). Garcia-Saenz et al. mutually adjusted for indoor and outdoor exposure along with other confounders (24) and reported a significant association between breast cancer and outdoor LAN for the blue light spectrum of light. Conversely, Sweeney et al. (29) reported no association with outdoor LAN exposure, even among those who reported indoor LAN exposure from outdoor sources. Further studies could benefit from precise measurements of outdoor and indoor exposure using sensor-based measurements of indoor LAN and considering the sleep habits of using curtains/blinders/sleep masks which can cancel out outdoor exposure or the use of electronic devices at night. Such precise measurements could help to estimate the intensity and amount of outdoor LAN that penetrates the sleeping area and assess the risk attributable to each type of exposure.
Similar to our findings, some studies provided no evidence for effect modification by menopausal status (24, 30, 31), while some contradictorily suggested a higher risk for premenopausal women (25, 26).
Although many studies have proven that night shift work is associated with increased breast cancer risk (48, 50–52), our study showed an insignificant association with night-shift workers, based on small numbers of night-shift workers, thus the need to interpret the results cautiously. Nevertheless, this result is comparable to the results from the Danish Nurses Cohort with a larger sample size (n = 27,713) (30).
A higher OR for association of HER2-positive breast tumors compared to other subtypes in our study is a new finding. Unlike our study, the MCC Spain study (24), reported no association between LAN exposure (assessed using MSI) and HER2-positive tumors, and a positive association with HER2-negative tumors. While the possible mechanisms behind a differential association by HER2 subtype are not known, these contradictory results warrant further investigation. On the other hand, a few studies have examined the association of LAN exposure according to the hormone receptor positive (ER/PR-positive) or hormone receptor negative (ER/PR-negative) tumors and provided conflicting results for these subtypes (22, 25, 26, 28, 30).
One strength of this study is the outdoor LAN assessment for 10 years before recruitment, taking into account the residential history and corresponding changes in the level of exposure. Only a few studies have taken the residential history (23–26, 30) while others have considered a single assessment of exposure (25) or assessment at a single address (24).
We also used a large dataset providing adequate information on multiple potential risk factors for breast cancer and adjusted for many possible confounders for this association of breast cancer and LAN exposure. As mentioned earlier, the DMSP images used in our study have several limitations, including low resolution and no differentiation between the spectral components of the light. LAN assessment derived from DMSP data has also been criticized for the problem of saturation and inability to capture individual-level exposure, which leads to a risk of collinearity with other urban factors such as air pollution (53, 54), traffic-related noise (34, 35) or green spaces (36). We used the radiance-calibrated DMSP images, which improved the resolution and provided sufficient variation of the luminosity values in urban areas, thus reducing the problems associated with luminosity saturation (38). We attempted to account for confounding by air pollutants such as NO2, PM2.5, and PM10 but not for other environmental factors, such as exposure to green spaces, which possibly correlates negatively with outdoor LAN exposure or traffic-related noise which needs to be considered in future studies on outdoor LAN exposure.
Despite careful design and execution, some errors due to selection and recall biases inherent to the study design could not be ruled out. Selection bias was minimized by integrating in the models, the degree of urbanization at recruitment, accounitng at least partially, for the probability of selection of cases from urban areas than rural areas. Residual confounding arising from unassessed variables such as indoor exposure and residential greenness remains.
Overall, this population-based case‒control study found no association between exposure to outdoor LAN and breast cancer risk. A positive association was found for HER2-positive type cancer when exposed to the highest level of outdoor LAN. There was no significant effect modification by menopausal status, night shift work, BMI, or urbanization. Further large-scale studies using more precise exposure assessments in indoor and outdoor settings and accounting for other environmental exposures, such as noise pollution and green spaces, are warranted to closely examine this association.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by CPPRB de Bicêtre—Hôpital de Bicêtre—Jan 18, 2005. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
NP: Formal Analysis, Writing – original draft. EC-D: Methodology, Writing – review & editing, Data curation. AB: Data curation, Writing – review & editing. EF: Conceptualization, Supervision, Writing – review & editing. PG: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
The CECILE study was supported by grants from the French National Institute of Cancer (INCa), the Fondation de France, the French Agency for Environmental and Occupational Health Safety (ANSES), and the League against Cancer. NP is funded by a doctoral allowance for her PhD from the Doctoral School of Public Health, Paris-Saclay University.
The authors kindly thank the volunteers and staff of the CECILE study, who contributed to study execution, data collection, and management. We also thank the Defense Meteorological Satellite Program (DMSP) of the National Oceanic and Atmospheric US Administration for making the satellite images publicly available.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvh.2023.1268828/full#supplementary-material
1. Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Breast cancer—epidemiology, risk factors, classification, prognostic markers, and current treatment strategies—an updated review. Cancers (Basel). (2021) 13(17):4287. doi: 10.3390/cancers13174287
2. Clavel-Chapelon F, E3N Group. Cumulative number of menstrual cycles and breast cancer risk: results from the E3N cohort study of French women. Cancer Causes Control. 2002. 13(9):831. doi: 10.1023/a:1020684821837
3. Xu YL, Sun Q, Shan GL, Zhang J, Liao HB, Li SY, et al. A case-control study on risk factors of breast cancer in China. Arch Med Sci. (2012) 8(2):303–9. doi: 10.5114/aoms.2012.28558
4. Hankinson SE, Eliassen AH. Endogenous estrogen, testosterone, and progesterone levels in relation to breast cancer. J Steroid Biochem Mol Biol. (2007) 106(1–5):24. doi: 10.1016/j.jsbmb.2007.05.012
5. Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer (Dove Med Press). (2019) 11:151. doi: 10.2147/BCTT.S176070
6. Rainey L, Eriksson M, Trinh T, Czene K, Broeders MJM, van der Waal D, et al. The impact of alcohol consumption and physical activity on breast cancer: the role of breast cancer risk. Int J Cancer. (2020) 147(4):931. doi: 10.1002/ijc.32846
7. Andersen ZJ, Stafoggia M, Weinmayr G, Pedersen M, Galassi C, Jørgensen JT, et al. Long-term exposure to ambient air pollution and incidence of postmenopausal breast cancer in 15 European cohorts within the ESCAPE project. Environ Health Perspect. (2017) 125(10):35. doi: 10.1289/EHP1742
8. Falchi F, Cinzano P, Duriscoe D, Kyba CCM, Elvidge CD, Baugh K, et al. The new world atlas of artificial night sky brightness. Sci Adv. (2016) 2(6):e1600377. doi: 10.1126/sciadv.1600377
9. Bará S. Anthropogenic disruption of the night sky darkness in urban and rural areas. R Soc Open Sci. (2016) 3(10):160541. doi: 10.1098/rsos.160541
10. Kyba CCM, Kuester T, De Miguel AS, Baugh K, Jechow A, Hölker F, et al. Artificially lit surface of earth at night increasing in radiance and extent. Sci Adv. (2017) 3(11):e1701528. doi: 10.1126/sciadv.1701528
11. Reiter RJ, Tan DX, Erren TC, Fuentes-Broto L, Paredes SD. Light-mediated perturbations of circadian timing and cancer risk: a mechanistic analysis. Integr Cancer Ther. (2009) 8(4):354–60. doi: 10.1177/1534735409352026
12. Menéndez-Menéndez J, Martínez-Campa C. Melatonin: an anti-tumor agent in hormone-dependent cancers. Int J Endocrinol. (2018) 2018:3271948. doi: 10.1155/2018/3271948
13. Straif K, Baan R, Grosse Y, Secretan B, El Ghissassi F, Bouvard V, et al. Carcinogenicity of shift-work, painting, and fire-fighting. Lancet Oncol. (2007) 8(12):1065–6. doi: 10.1016/S1470-2045(07)70373-X
14. Ward EM, Germolec D, Kogevinas M, McCormick D, Vermeulen R, Anisimov VN, et al. Carcinogenicity of night shift work. Lancet Oncol. (2019) 20(8):1058–9. doi: 10.1016/S1470-2045(19)30455-3
15. Lunn RM, Blask DE, Coogan AN, Figueiro MG, Gorman MR, Hall JE, et al. Health consequences of electric lighting practices in the modern world: a report on the national toxicology program’s workshop on shift work at night, artificial light at night, and circadian disruption. Sci Total Environ. (2017) 607–608:1073–84. doi: 10.1016/j.scitotenv.2017.07.056
16. Kloog I, Portnov BA, Rennert HS, Haim A. Does the modern urbanized sleeping habitat pose a breast cancer risk? Chronobiol Int. (2011) 28(1):76–80. doi: 10.3109/07420528.2010.531490
17. Kloog I, Stevens RG, Haim A, Portnov BA. Nighttime light level co-distributes with breast cancer incidence worldwide. Cancer Causes Control. (2010) 21(12):2059–68. doi: 10.1007/s10552-010-9624-4
18. Rybnikova N, Stevens RG, Gregorio DI, Samociuk H, Portnov BA. Kernel density analysis reveals a halo pattern of breast cancer incidence in Connecticut. Spat Spatiotemporal Epidemiol. (2018) 26:143–51. doi: 10.1016/j.sste.2018.06.003
19. Rybnikova N, Portnov BA. Population-level study links short-wavelength nighttime illumination with breast cancer incidence in a major metropolitan area. Chronobiol Int. (2018) 35(9):1198–208. doi: 10.1080/07420528.2018.1466802
20. Keshet-Sitton A, Or-Chen K, Huber E, Haim A. Illuminating a risk for breast cancer: a preliminary ecological study on the association between streetlight and breast cancer. Integr Cancer Ther. (2017) 16(4):451–63. doi: 10.1177/1534735416678983
21. Lamphar H, Kocifaj M, Limón-Romero J, Paredes-Tavares J, Chakameh SD, Mego M, et al. Light pollution as a factor in breast and prostate cancer. Sci Total Environ. (2022) 806(Pt 4):150918. doi: 10.1016/j.scitotenv.2021.150918
22. Xiao Q, James P, Breheny P, Jia P, Park Y, Zhang D, et al. Outdoor light at night and postmenopausal breast cancer risk in the NIH-AARP diet and health study. Int J Cancer. (2020) 147(9):2363–72. doi: 10.1002/ijc.33016
23. Bauer SE, Wagner SE, Burch J, Bayakly R, Vena JE. A case-referent study: light at night and breast cancer risk in Georgia. Int J Health Geogr. (2013) 12:23. doi: 10.1186/1476-072X-12-23
24. Garcia-Saenz A, Sánchez de Miguel A, Espinosa A, Valentin A, Aragonés N, Llorca J, et al. Evaluating the association between artificial light-at-night exposure and breast and prostate cancer risk in Spain (MCC-Spain study). Environ Health Perspect. (2018) 126(4):047011. doi: 10.1289/EHP1837
25. Hurley S, Goldberg D, Nelson D, Hertz A, Horn-Ross PL, Bernstein L, et al. Light at night and breast cancer risk among California teachers. Epidemiology. (2014) 25(5):697–706. doi: 10.1097/EDE.0000000000000137
26. James P, Bertrand KA, Hart JE, Schernhammer ES, Tamimi RM, Laden F. Outdoor light at night and breast cancer incidence in the Nurses’ health study II. Environ Health Perspect. (2017) 125(8):087010. doi: 10.1289/EHP935
27. Yang W, Shi Y, Ke X, Sun H, Guo J, Wang X. Long-term sleep habits and the risk of breast cancer among Chinese women: a case-control study. Eur J Cancer Prev. (2019) 28(4):323–9. doi: 10.1097/CEJ.0000000000000458
28. Xiao Q, Gierach GL, Bauer C, Blot WJ, James P, Jones RR. The association between outdoor artificial light at night and breast cancer risk in black and white women in the southern community cohort study. Environ Health Perspect. (2021) 129(8):87701. doi: 10.1289/EHP9381
29. Sweeney MR, Nichols HB, Jones RR, Olshan AF, Keil AP, Engel LS, et al. Light at night and the risk of breast cancer: findings from the sister study. Environ Int. (2022) 169:107495. doi: 10.1016/j.envint.2022.107495
30. Clarke RB. Outdoor light at night and breast cancer incidence in the danish nurse cohort. Environ Res. (2021) 194:110631. doi: 10.1016/j.envres.2020.110631
31. Ritonja J, McIsaac MA, Sanders E, Kyba CCM, Grundy A, Cordina-Duverger E, et al. Outdoor light at night at residences and breast cancer risk in Canada. Eur J Epidemiol. (2020) 35(6):579–89. doi: 10.1007/s10654-020-00610-x
32. Aubé M, Roby J, Kocifaj M. Evaluating potential spectral impacts of various artificial lights on melatonin suppression, photosynthesis, and star visibility. PLoS One. (2013) 8(7):e67798. doi: 10.1371/journal.pone.0067798
33. Gabet S, Lemarchand C, Guénel P, Slama R. Breast cancer risk in association with atmospheric pollution exposure: a meta-analysis of effect estimates followed by a health impact assessment. Environ Health Perspect. (2021) 129(5):57012. doi: 10.1289/EHP8419
34. Sørensen M, Poulsen AH, Kroman N, Hvidtfeldt UA, Thacher JD, Roswall N, et al. Road and railway noise and risk for breast cancer: a nationwide study covering Denmark. Environ Res. (2021) 195:110739. doi: 10.1016/j.envres.2021.110739
35. Abbasi M, Yazdanirad S, Dehdarirad H, Hughes D. Noise exposure and the risk of cancer: a comprehensive systematic review. Rev Environ Health. (2022). [online ahead of print]. doi: 10.1515/reveh-2022-0021
36. Zare Sakhvidi MJ, Yang J, Mehrparvar AH, Dzhambov AM, Ebrahimi AA, Dadvand P, et al. Exposure to greenspace and cancer incidence, prevalence, and mortality: a systematic review and meta-analyses. Sci Total Environ. (2022) 838(Pt 2):156180. doi: 10.1016/j.scitotenv.2022.156180
37. Dubach LL, Ng C. Compendium of meteorological space programs, satellites and experiments. Greenbelt, MD: National Space Science Data Center (1998).
38. Hsu FC, Baugh KE, Ghosh T, Zhizhin M, Elvidge CD. DMSP-OLS radiance calibrated nighttime lights time series with intercalibration. Remote Sens (Basel). (2015) 7(2):1855–76. doi: 10.3390/rs70201855
39. Amini H, Dehlendorff C, Lim YH, Mehta A, Jørgensen JT, Mortensen LH, et al. Long-term exposure to air pollution and stroke incidence: a danish nurse cohort study. Environ Int. (2020) 142:105891. doi: 10.1016/j.envint.2020.105891
40. Lemarchand C, Gabet S, Cénée S, Tvardik N, Slama R, Guénel P. Breast cancer risk in relation to ambient concentrations of nitrogen dioxide and particulate matter: results of a population-based case-control study corrected for potential selection bias (the CECILE study). Environ Int. (2021) 155:106604. doi: 10.1016/j.envint.2021.106604
41. Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol. (2019) 72(6):558–69. doi: 10.4097/kja.19087
42. Streiner DL. Statistics commentary series: commentary no. 26: dealing with outliers. J Clin Psychopharmacol. (2018) 38(3):170–1. doi: 10.1097/JCP.0000000000000865
43. El-Masri MM, Mowbray FI, Fox-Wasylyshyn SM, Kanters D. Multivariate outliers: a conceptual and practical overview for the nurse and health researcher. Can J Nurs Res. (2021) 53(3):316–21. doi: 10.1177/0844562120932054
44. Chang AM, Aeschbach D, Duffy JF, Czeisler CA. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc Natl Acad Sci U S A. (2015) 112(4):1232–7. doi: 10.1073/pnas.1418490112
45. Stevens RG, Zhu Y. Electric light, particularly at night, disrupts human circadian rhythmicity: is that a problem? Philos Trans R Soc B. (2025) 370(1667):20140120. doi: 10.1098/rstb.2014.0120
46. Li Q, Zheng T, Holford TR, Boyle P, Zhang Y, Dai M. Light at night and breast cancer risk: results from a population-based case–control study in Connecticut, USA. Cancer Causes Control. (2010) 21(12):2281–5. doi: 10.1007/s10552-010-9653-z
47. Keshet-Sitton A, Or-Chen K, Yitzhak S, Tzabary I, Haim A. Can avoiding light at night reduce the risk of breast cancer? Integr Cancer Ther. (2016) 15(2):145–52. doi: 10.1177/153473541561878
48. Davis S, Mirick DK, Stevens RG. Night shift work, light at night, and risk of breast cancer. J Natl Cancer Inst. (2001) 93(20):1557–62. doi: 10.1093/jnci/93.20.1557
49. Keshet-Sitton . Light and the city: breast cancer risk factors differ between urban and rural women in Israel. Available at: https://www-ncbi-nlm-nih-gov.proxy.insermbiblio.inist.fr/pmc/articles/PMC5739126/
50. Manouchehri E, Taghipour A, Ghavami V, Ebadi A, Homaei F, Latifnejad Roudsari R. Night-shift work duration and breast cancer risk: an updated systematic review and meta-analysis. BMC Womens Health. (2021) 21(1):89. doi: 10.1186/s12905-021-01233-4
51. O’Leary ES, Schoenfeld ER, Stevens RG, Kabat GC, Henderson K, Grimson R, et al. Shift work, light at night, and breast cancer on long island, New York. Am J Epidemiol. (2006) 164(4):358–66. doi: 10.1093/aje/kwj211
52. Cordina-Duverger E, Menegaux F, Popa A, Rabstein S, Harth V, Pesch B, et al. Night shift work and breast cancer: a pooled analysis of population-based case-control studies with complete work history. Eur J Epidemiol. (2018) 33(4):369–79. doi: 10.1007/s10654-018-0368-x
53. Kyba CCM, Aronson KJ. Assessing exposure to outdoor lighting and health risks. Epidemiology. (2015) 26(4):e50. doi: 10.1097/EDE.0000000000000307
Keywords: artificial light at night, circadian disruption, breast cancer, case-control study, hormone receptor, HER2 receptor
Citation: Prajapati N, Cordina-Duverger E, Boileau A, Faure E and Guénel P (2023) Exposure to outdoor artificial light at night and breast cancer risk: a population-based case-control study in two French departments (the CECILE study). Front. Environ. Health 2:1268828. doi: 10.3389/fenvh.2023.1268828
Received: 28 July 2023; Accepted: 19 September 2023;
Published: 4 October 2023.
Edited by:
Omar Hahad, Johannes Gutenberg University Mainz, GermanyReviewed by:
Marin Kuntic, Johannes Gutenberg University Mainz, Germany© 2023 Prajapati, Cordina-Duverger, Boileau, Faure and Guénel. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pascal Guénel cGFzY2FsLmd1ZW5lbEBpbnNlcm0uZnI=
Abbreviations BMI, body mass index; CI, confidence interval; DAG, directed acyclic graph; DMSP, defense meteorological satellite program; DNA, deoxyribonucleic acid; ER, estrogen receptor; GIS, geographic information system; HER, human epidermal growth receptor; IARC, International Agency for Cancer Research; IQR, interquartile range; ISS, International Space Station; LAN, light at night; MCC, multi-case‒control; MHT, menopausal hormone therapy; MSI, melatonin suppression index; NO2, nitrogen dioxide; NOAA, National Oceanic and Atmospheric US Administration; OLS, operational linescan system; OR, odds ratio; PM, particulate matter; PR, progesterone receptor; SES, socioeconomic status; VIF, variation inflation factor.
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.