- Department of Building Science and Materials, Technische Universität Berlin, Berlin, Germany
Introduction: A novel way to upcycle fine concrete or brick demolition waste (<2 mm) to akaliactivated lightweight aggregates (ALA) was described recently. As pollutant burdens in these precursors are closely controlled by federal law in Germany, the aggregates produced in this study could be used in direct contact with the environment.
Methods: Therefore, different parameters of ALA, lightweight expanded clay aggregates (LECA), and plant granulate were measured and compared, such as pH buffering, heavy metal leaching, pH, and conductivity in solution, pore size distribution, available water content (AWC), and dissolvable macronutrients. Additional plant growth experiments assessed the aggregate’s feasibility as a substrate compared to or as an improvement for lightly acidic soil, with different mixing ratios between LUFA reference soil and ALA.
Results: These investigations showed high phytotoxicity, which might be explained by salinization (∼3.6 or 4.6 times higher conductivities than plant granulate) and by ALA’s elevated pH (>12). The latter may be used for soil improvements like liming, but a neutralization capacity of only ∼1.7% compared to pure CaO was reached. Similar to this, ALA’s AWC stayed below 1/6 of LECA’s AWC. Both brick or concrete powder aggregates (BPA or CPA) provided comparable or higher amounts of Ca, K, and P relative to plant granulate and exhibited heavy metal loads below the German federal limit values.
Discussion: While these findings hinted that ALA could act as soil improvements in the future, this use case is not feasible without significant improvements to either ALA’s production process or post-treatment.
1 Introduction
Approximately 74% of all mineral construction and demolition waste is recycled in the European Economic Area (Williams et al., 2020). This number includes unquantified material streams used in lower-quality applications like filler materials in construction or backfilling. Caro et al. (2024) have shown that extensive environmental savings are accompanied by shifting these downcycle pathways to high-quality recycling (meaning replacing primary materials with higher environmental impacts). Moreover, the fine fraction, which is crushed <2 mm, has no standardized recycling path according to the standards DIN 4226-101 (2017a), DIN 4226-102 (2017b), and DIN 1045-2 (2023) and is therefore landfilled or downcycled. Against this background, an article by Wichmann et al. (2023) showed a new usage path for the fine fraction of recycled concrete or brick that produces granular brick or concrete powder aggregates (BPA or CPA) with high water absorption (up to 19%) (Wichmann et al., 2023). While the production of lightweight granulates through pelletization is a known technique, these experiments are usually done with various types of fly ashes (Gesoğlu et al., 2007; Jo et al., 2007; Bui et al., 2012; Yliniemi et al., 2016; Zafar et al., 2021), employ classic binders such as Portland cement [e.g., (Narattha and Chaipanich, 2018; Tajra et al., 2018)] or are produced using sintering at high temperatures (Kwek and Awang, 2021). A study by Tataranni et al. (2018) produced lightweight aggregates from digested spent bentonite clay and basalt powder (Tataranni et al., 2018).
Wichmann et al. suggested using their alkali-activated lightweight aggregates (ALA) as substitutes for lightweight aggregates in construction, similar to applications known for lightweight expanded clay aggregates (LECA). These are used widely in construction (Rashad, 2018) and also in water-filtering and plant cultivation [e.g., (Mlih et al., 2020; Hamid et al., 2022)]. The latter use cases are normally outside the applicability of ALAs because fly ash, as its most common precursor, tends to contain leachable pollutants, such as heavy metals [see (Meer and Nazir, 2018; Zhao et al., 2022), for example]. Yet, with the pollutant contamination of precursors for BPA and CPA being controlled according to DIN 4226-101 (2017a) and DIN 4226-102 (2017b), such usage might be possible. This study aimed to project plant cultivation on LECA to ALAs. With their high mineral content and elevated water absorption capacity, applications as plant substrates are imaginable. If feasible, ALA would potentially present an eco-friendly substitute for LECA as its production does not require burning at > 1,000°C (Rashad, 2018) and consumes fewer primary resources.
The idea of using alkali-activated materials to support plant growth is not self-explanatory, as the activators needed are strongly alkaline solutions (Song et al., 2024). A broadly similar approach could be seen in “planting concrete,” where coarse aggregates are bound by a cementitious matrix to allow plant growth to be mechanically stabilized underground. Jin et al. (2024) reported on the possibility of reducing pH levels by using sulfoaluminate cement in such applications (Jin et al., 2024). Utilization of ALAs for plant growth will have to address alkalinity as well. Therefore, a short examination of general parameters like pH, conductivity, nutrients, heavy metal leaching, and acid buffering potential was conducted and is presented in this article, alongside a simplified plant assessment, to investigate if plant survival on ALAs is at all possible.
2 Material and methods
Two fine construction demolition waste materials, concrete powder (CP) and brick powder (BP), were used for the following investigations. Detailed chemical and physical information on the powders can be found in Wichmann et al. (2023). The powders were alkali-activated with a sodium silicate solution consisting of 13.5 wt% SiO2, 12.7 wt% Na2O, and 73.8 wt% H2O, which equals molar ratios of 1.1 for SiO2/Na2O (mol mol−1) and 20 for H2O/Na2O (mol mol−1). More information regarding the composition of the alkaline solution may also be found in Wichmann et al. (2023). Through a pelletizer disk and a pelletization process, artificial aggregates, named concrete powder aggregates (CPA) and brick powder aggregates (BPA), were produced, as shown in Figure 1. Wichmann et al. (2023) further investigated compressive strength, loose bulk density, particle density, and water absorption of the aggregates (Wichmann et al., 2023). Additionally, investigations of the behavior of the aggregates within the composite material concrete were made. The mechanical and physical properties of these composites were reported by Wichmann et al. (2023) and Wichmann and Stephan (2023).

Figure 1. Alkali-activated lightweight aggregates. Brick powder aggregates are displayed on the left side, and cement powder aggregates are displayed on the right.
To enhance clarity and ease of referencing in the following matter, all experiments conducted for this article have been sorted into different experiment groups. While group A represents chemical experiments with subgroups A.1–A.3, and group B refers to the physical experiment and has no subgroups. Group C includes both biological experiments, C.1 and C.2.
We investigated the pH, conductivity, and macronutrients of ALA in a solution in experiment A.1. We chose to use plant granulate (PG) over LECA for this experiment, as it was burned at a lower temperature than commercial LECA (according to and available by Liapor GmbH and Co. KG). The reason for this was that sintering generally reduces the mobility of metals (Ren et al., 2021) and, therefore, probably nutrients as well. In using PG, the comparison to the ALAs was conducted with the more favorable reference for plant growth regarding nutrient release. This was only done here; all other experiments used LECA for comparison. The metered macronutrients included N, S, P, Mg, K, and Ca, which are vital for plant growth and, in contrast to micronutrients, are needed in large quantities by plants (Kirkby et al., 2024; Riaz et al., 2020). More information about the biochemistry of each macronutrient is given in extensive detail by Hawkesford et al. (2024). We also measured concentrations of Al, Fe, Na, and Si. Nitrogen (N) was not analyzed, as acidification for transport and storage was done with HNO3.
Experiment A.2 investigated titration curves to pH 7 for ALAs and LECA. Geopolymers are considered more acid-resistant than ordinary Portland concrete because they primarily comprise aluminosilicates (Gluth et al., 2022; Castillo et al., 2022). Therefore, this test was intended to show how much of the aggregates’ inner surface had been covered by these Al-O-Si structures. Given the ALA’s production method, it is imaginable that surfaces were in contact with inconsistent amounts of activator solution and thus been left with varying reactivity. Regarding this, commercially available LECA with a size distribution similar to BPA/CPA was used as reference material as they possess more inert surfaces than PG. Titration was meant to roughly simulate the acid attack expected in nature in a speed-up manner as rain is mildly acidic with a pH of approximately 5.6 (Prakash et al., 2023).
As all aggregates were manufactured from possibly contaminated materials, experiment A.3 was conducted to assess such burdens in BPA and CPA. Heavy metals and polycyclic aromatic hydrocarbons (PAH) are common pollutants in demolished concrete or brick (Müller, 2018). As their mobility in the environment is relevant for a hazard assessment, a sequential extraction was performed, following pedological methods (Gleyzes et al., 2002). These aim for a stepwise dissolution of different chemical fractions in soil and the release of therein-bound pollutants. Accordingly, it becomes possible to predict their mobility in the environment if the dissolution rate of the carrying fraction is known. Following the terminology used by Gleyzes et al. (2002), these fractions are exchangeable, acid-soluble (carbonate-bound), oxide-bound, oxidizable (organically bound), and residual. The oxidizable fraction was disregarded as no relevant quantities of organic material were expected in BPA or CPA. Concentrations of As, Cd, Cr, Cu, Ni, Pb, and Zn were measured as they often appear in cements and their raw materials (Gleis et al., 2003) and can be highly toxic to humans and plants in elevated concentrations (Tchounwou et al., 2012; Nagajyoti et al., 2010). The highly toxic Hg was not metered as it seldom occurs concentrated in clinker and is mainly a problem in the burning process, where it volatilizes and only partly remains in the produced cement (Zheng et al., 2012).
Pore space and size distribution play viable roles in interacting with the environment. For one thing, variably charged surface complexes can influence the proton or cation balance of the free solution (Amelung et al., 2018b); the larger the surface area, the more significant the effect. On the other hand, pores can take in and hold water against gravity due to capillary action (Amelung et al., 2018a), which can prevent soil desiccation and is utilized when using LECA as a plant growth substrate. Plants can access this water if it is held in pores sized between 0.2 μm and 50 μm; the total volume of these pores is called available water capacity (AWC) (Minasny and McBratney, 2018). Water in pores outside these dimensions is either drained as capillary action is too weak to hold it against gravity or inaccessible as capillary action is too strong for plant roots to overcome (>15,000 hPa) (Amelung et al., 2018a). Regarding the importance of the AWC, experiment B determined this value for ALA and LECA.
In experiment group C, tests were performed with standard reference soils offered by LUFA Speyer over various aggregate–soil mass ratios. LUFA 2.1 and 2.2 were chosen as lightly acidic soils (CaCl2, pH: 4.6 and 5.5) to accommodate the high pH values of the aggregates shown in preliminary measurements. Regarding this, Eruca sativa was selected as the test organism, which grows best at pH 6 to pH 7, according to Kniepenkerl by Bruno Nebelung GmbH. These experiments did not follow standard procedures stated in ISO 11269-2 (2012) and were meant only to gain information on the plant toxicity of ALAs in the soil in the most general way. Therefore, the results should be viewed with caution as some experiment conditions have not been sufficiently controlled. It was not possible nor advisable to derive any quantitative information from these tests by statistical analysis.
2.1 Chemical analysis (experiment group A)
2.1.1 pH, conductivity, and macronutrients (experiment A.1)
Initially, 25 g CPA, BPA, and a plant granulate (PG) available from Seramis (Westland Deutschland GmbH) based on clay were shaken for 96 h in 75 mL de-ionized water, while pH and conductivity were determined using handheld electrodes (accuracy: 0.01 (pH) and 0.5% (conductivity) at 10 min, 60 min, and 180 min as well as after the experiment. This experiment was loosely related to DIN EN 12457-4 (2003) but used a smaller liquid-to-solid (L/S) ratio than 10 L kg−1 and ignored the 0.45-µm filtration step as measurements were only meant to be comparative. For the same reason and to obtain information over various contact times, shaking time differed from DIN EN 12457-4 (2003). The test was run with two replicates of each sample.
After the experiment, samples were taken from the liquid supernatant of all flasks to obtain further data about any dissolved plant macronutrients. Again, this experiment was only comparative. Samples were centrifuged at 3,000 G, passed through 0.45-µm filters, and finally acidified with 1 mL 0.1 mol L−1 HNO3 (on a 14-mL sample). Concentrations were determined using inductively coupled plasma optical emission spectrometry (ICP-OES) with an iCAP 6300 Duo by Thermo Fischer Scientific.
2.1.2 Titration (experiment A.2)
BPA and CPA were added to water (de-ionized, 20 g per 200 mL) and then neutralized to pH 7 using an automated titrator. The experiment used a TITRONIC® 300 piston burette to add dynamic quantities of 0.1 mol L−1 nitric acid (HNO3) every 2 min. The volume to be added was calculated from the delta between the current pH value and the target value but was set to at least 200 µL (or 0 µL below a ΔpH of 0.1) to avoid an asymptotic approach as well as a larger relative error by accumulation. This volume was protocolled alongside temperature and pH with every addition.
The acid volume needed to theoretically neutralize the solution (Va) was calculated using Equation 1, which assumes neutrality if the number of protons added (ca,H × Va) is equal to the number of OH− ions in the current solution (cc,OH × Vc). In praxis, dissolution and buffer reactions must be expected, and the deviation between calculated and measured values was used to estimate said reactions. Used here were the current basin volume (Vc), proton concentration in the HNO3 stock solution (ca,H), and the current proton concentration (cc,OH), calculated from pH readings according to Equation 2. Two replicate specimens were examined for CPA, BPA, and LECA, respectively.
with
As the added amount of acid was unlikely to converge before the experiments ended, limit values were extrapolated for each aggregate from arithmetic means of both replicates using a model for limited growth (Equation 3).
Furthermore, the pH buffering effect of the aggregates in solution was assessed by determining the logarithmic deviation between expected (ce,H) and actual H+ concentration after every HNO3 addition over the quantity of acid added. The expected concentration was calculated using Equation 4, where cc,H is the proton concentration before the addition step.
with
2.1.3 Heavy metal leaching (experiment A.3)
Ammonium nitrate was used to solute exchangeable cations following ISO 19730 (2008), while carbonate-bound heavy metals were obtained using buffered acetic acid (1 mol L−1 H3COONa, acidified to pH 5 with CH3COOH). The oxide-bound fraction was dissolved with 0.2 mol L−1 ammonium oxalate, acidified to pH 5 with HNO3. Finally, aqua regia was employed to dissolve any residual minerals following the specifications of ISO 11466 (1995). Except for aqua regia, all the above experiments used liquid–solid ratios of 25 mL to 10 g. In addition, samples were centrifuged at 1,000 G and then passed through 0.45-µm filters, after which the residuum was used for subsequent extraction, while the filtrates were analyzed for As, Cd, Cr, Cu, Ni, Pb, and Zn via ICP-OES.
2.2 Porosity (experiment group B)
The pore space was measured with a Microtrac BELPORE mercury porosimeter for two replicates of aggregates and LECA.
2.3 Plant growth experiments with Eruca sativa (experiment group C)
Further experiments were conducted to assess the environmental toxicity of using ALA to support plant growth. These were oriented toward ISO 11269-2 (2012) but simplified as only comparative data were to be obtained. Simplifications included no sieving of the samples (already-defined granulate sizes), no determination of cation exchange capacity or organic content for ALA, and no calculation of no observed effect concentration (NOEC), median lethal doses (LD50), or other coefficients. It should be noted that the standard stipulates the application of fertilizer. To reduce its effect on the soil pH, fertilizer was only given once with the first watering. All experiments assessed seed germination and early growth using three replicate plant pots per specimen with 10 seeds each in a climate chamber (see Figure 2) at 20°C and artificial lighting (16/8 h). Humidity was maintained between 50% and 70% (fluctuations caused by chamber size). The soil CaCl2-pH was determined after all experiments for every aggregate–soil specimen following ISO 10390 (2022).

Figure 2. Germination and early growth of E. sativa. Germination was observable for 5% CPA (A), yet not for 15% (B). It was normal in the reference soil (C). After 25 days, shoots from (A) were shriveled (D). (E) shows efflorescence in a 100% BPA sample from the pilot experiment, while (F) displays the climate chamber used in both tests. All displayed buckets had diameters of 108 mm.
2.3.1 Pilot experiment (experiment C.1)
The pilot experiment was conducted in order to get a first impression of the effect of BPA and CPA on plant growth and therefore used lightly acidic LUFA 2.2. The ALAs were mixed with soil in ratios of 1:3 (25%), 1:1 (50%), and 3:1 (75%) and tested alongside samples containing 100% LUFA 2.2 and, respectively, 100% BPA or CPA. A paper tissue was inlaid in the latter samples to provide an initial hold for the seeds.
2.3.2 Subsequent experiment (experiment C.2)
Regarding the distinct results of C.1, the experiment aimed to lessen the ALA’s phytotoxic effect. It was thus repeated with the more acidic LUFA 2.1 instead of LUFA 2.2 to partly counterbalance the ALA’s pH levels. Furthermore, aggregate–LUFA ratios were adjusted to 1:19 (5%), 1:9 (10%), and 3:17 (15%).
3 Results
Solutions detailed in A.1 showed strongly elevated pH values for both aggregates, measured at pH 12.3 (BPA) and pH 12.6 (CPA) compared to pH 7.0 for PG (arithmetic mean of two replicates, respectively). Shaking time seemed to have little impact on this, as pH levels after 3 h exhibited a pH difference of only 0.01 (BPA) or −0.1 (CPA) compared to the measurements taken after 96 h. Measurements of conductivity resulted in 1,092 μS cm−1 or 1.382 μS cm−1 for BPA and CPA, which differed by approximately 72 μS cm−1 or 291 μS cm−1 over the same time window. In contrast, PG solution conductivity was determined at 300 μS cm−1. All measured data may be accessed in the supplementary materials. Measurements of dissolvable macronutrients in these solutions indicate that the higher conductivities for BPA and CPA were mainly due to Na+ ions, with average concentrations of ∼2 g kg−1, while PG merely lies at ∼3% of these values (∼62 mg kg−1). Similar observations were made for Si (PG at ∼11%) and S (PG at ∼3%), which exist in oxidized speciation. Interestingly, aggregates and PG mainly showed comparable amounts of macronutrients in the case of Ca, K, Mg, and P, where BPA or CPA contained approximately 393%, 88%, 75%, and 117% or 113%, 79%, 2.8%, and 478% of the PG levels. The main difference when comparing both aggregates is that much higher amounts of Ca are available from BPA, while CPA makes more P and considerably less Mg available. Significantly lower levels of Al were found in ALA, suggesting that most aluminum was bound in aluminosilicates, while Fe levels were similar for all granulates.
The data obtained from the titration experiment in A.2 are shown in Figure 3 which shows the pH level over time, using a moving average over 10 preceding values. LECA graphs exhibit oscillating behavior, most likely attributed to the minimal addition of 0.2 mL HNO3, which increases the H+ concentration strongly near pH 7. Afterward, the level rises slowly to pH 7 while no acid is added. This is noteworthy as LECA has inert surfaces (Rashad, 2018) and releases only small amounts of minerals into the surrounding solution. BPA and CPA graphs show a straighter progression, seemingly because proton-neutralizing processes had higher rate constants here. The varying increase in both curves before 35 h is initially due to the non-constant acid addition. Thus, in panel b), which shows the accumulated acid addition over time, changes in the addition rate are visible after ∼8 h (BPA and CPA) and ∼30 h (CPA) 35 h (BPA), which corresponds to the gradient changes in panel a) at roughly the same time. The slight time offset could be explained by the system diffusion-related inertia. Panel b) furthermore displays that about the same quantities of acid are needed to neutralize BPA and CPA, whereby the difference between replicates after 67 h is higher than between both aggregates. Limit points of the acid volume needed for neutralization were extrapolated to ∼0.62 mol kg−1 and ∼0.58 mol kg−1 for BPA and CPA (see Equation 3). For LECA, the experiment endpoint was assumed as the limit point at ∼0.054 mol kg−1. Panel c) displays the logarithmic deviation between the expected and actual pH level over the amount of added HNO3. The panel clearly shows that this deviation for both LECA and BPA/CPA aggregates mainly depends on the amount of acid added and appears to be independent of the initial pH value. For LECA, the expected and actual values approach each other more quickly, but the systematics of all curves appear to be similar.
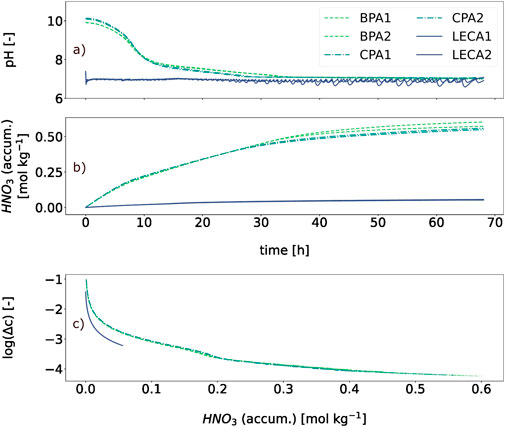
Figure 3. Titration experiment. Shown are the course of pH level (A) and the added amount of HNO3 (B) over the experiment duration; (C) shows the logarithmic deviation from the expected pH level. BPA and CPA show initial pH values approximately 10 but fall quickly with the addition of acid. A similar progression is shown for added amounts of HNO3 for both aggregates, where observable differences first appear after 30 h. In the last panel, Δc ranges from 0.1 to 10–4 and behaves similarly for all materials.
Figure 4 displays the heavy metal loads for all examined fractions. In general, all replicate findings show good accordance with each other and therefore seem valid. The recovery rate of CPA was lost due to a machine error. Otherwise, validity is supported by recovery rates of BPA and LECA, which differed by less than 20% from the fraction sums and are not shown here to increase clarity. Regarding BPA and CPA, contaminant profiles seemed similar for the residual fraction while showing more variance in the other dissolution steps. More As was exchangeable bound in BPA, whereas Cr and Cu loads were higher for CPA and comparable for the other metals. Carbonate-bound Cu was also found in greater concentrations in CPA, but more Cr and Pb could be detected in BPA with otherwise similar levels between both aggregates. Oxide-bound contaminants only differed notably for Zn and Cr (both higher in BPA). For LECA, Cu, Ni, and Zn levels were clearly elevated compared with the aggregates in the residual fraction, while almost no Pb was found. Regarding Ni and Pb, this was also true in every other fraction, but lighter loads of Cu and Zn were found here (except for exchangeable Zn). More As was measured in the dissolved oxides and carbonates in LECA than in BPA/CPA, yet not in the exchangeable fraction. Almost no Cr was measured in any fraction except the residual, and only marginal amounts of Cd were determined for all materials in all fractions. None of the observed pollutants exceeded the limited values stated in the German Federal Soil Protection Act (BBodSchV).
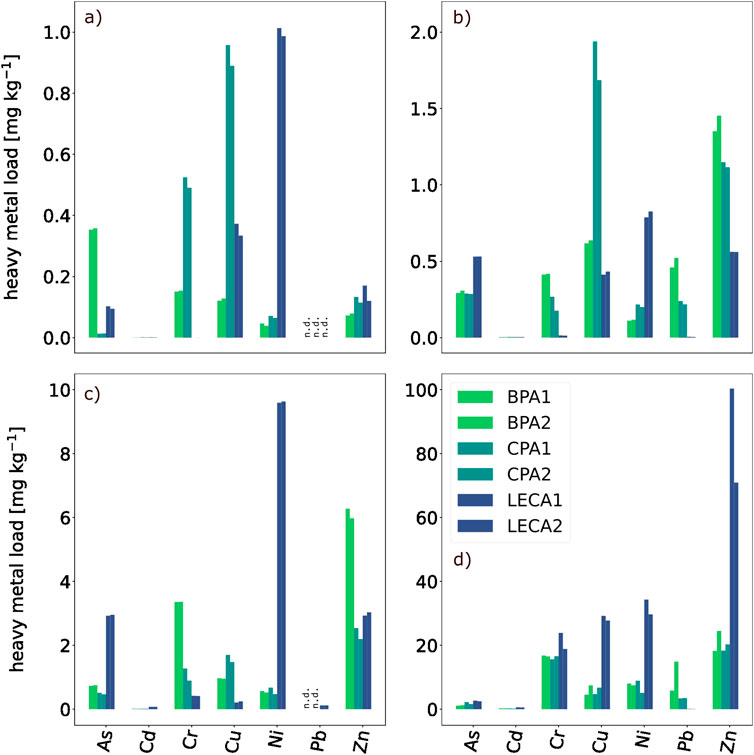
Figure 4. Heavy metal loads after sequential extraction. Shown are exchangeable (A), carbonate-bound (B) and oxide-bound (C) cations, and the residual fractions. Negative values were measured for Pb in (A, C), where Pb concentrations lay below the detection limit of the ICP-OES, and matrix differences between specimen and calibration probably caused negative readings. Therefore, negative concentrations were regarded as not detectable (n.d.). Furthermore, the error of this measurement was estimated by calculating the random measurement uncertainty using t × s/√n (t = Student t factor, s = standard deviation, n = sample size) and using the highest error for all measurements per panel. Therefore, the measurement errors were ∼0.01 mg kg−1 in (A), 0.37 mg kg−1 in (B), 0.55 mg kg−1 in (C), and 42.99 mg kg−1 in (D).
Results obtained from the mercury porosimeter, as described in B, are summarized in Figure 5E, where LECA exhibits a larger pore volume in every size subclass. Overall, the AWC amounted to >300 mm³ g−1 for LECA, while neither BPA nor CPA reached AWCs above 50 mm³ g−1. However, specific surface areas for BPA (∼5 m2 g−1) and CPA (∼5.4 m2 g−1) were comparable with LECA (∼11.9 m2 g−1), as their pore size distribution is more alike for pores <0.2 µm, which do not contribute to AWC.
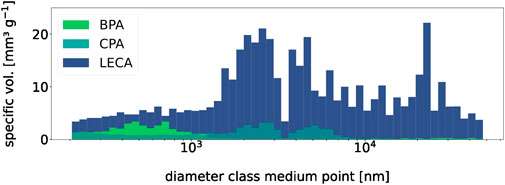
Figure 5. Pore size distribution. All bars are slightly transparent, and BPA readings remained near 0 mm3 g−1 for pore diameters > 103 nm.
The pilot experiment described in C was aborted after 18 days, as plant growth was solely observable in pure LUFA 2.2 mixtures after this time. Furthermore, only 3 of 30 E. sativa seeds germinated in the 25% CPA specimen, while all other aggregate–soil mixtures showed no germination at all. Every soil–aggregate pH measurement resulted in levels above 8.8; the data can be found in supplementary material. Pure BPA and CPA samples showed crystal growth on their surfaces (see Figure 2).
The subsequent E. sativa experiment showed higher germination rates for all mixtures, yet only those of 5% CPA (see Figure 2) were comparable with the reference sample (Figure 2C). During the early growth phase, however, no healthy development or growth comparable to the reference could be observed for any aggregate–soil mixtures (panels b and d). Measurements of the soil pH showed H+ concentrations that differed less than in the pilot experiment and lay within two magnitudes of each other. pH levels metered were ∼6.4 for LUFA 2.1 as well as ∼7.4, ∼8, and ∼7.8 for 5%, 10%, and 15% aggregate content (average between BPA and CPA). The former was noticeably elevated compared to the experiment’s beginning (pH 4.6), hinting at a buffering effect by the fertilizer.
4 Discussion
In experiment A.1, aggregates showed pH levels that clearly differ from the range of ordinary soil pH values, lying between pH 3 and pH 11 (Amelung et al., 2018b). The pH value influences practically all biogeochemical properties of soil (Neina, 2019), and alkaline levels can induce low P, Fe, Zn, and Mn availability in soils (Wilkinson et al., 2000), aside from being simply caustic at a certain point. In addition, conductivities are three- to four-fold elevated in comparison with PG. Higher osmotic pressures are thus to be expected in plant grow matrices or soils containing ALA, where high salinity can cause drought stress, dehydration, or salt/ion toxicity to plants (Wilkinson et al., 2000). While both aggregates provide dissolvable macronutrients in amounts broadly comparable to the reference material, Na+ ion concentrations dwarf their quantities and are more than 30 times higher in the PG samples. This probably stems from NaOH used in production, which can lead to leaf dehydration and is toxic to plants (Wilkinson et al., 2000). One possibility to address this could be additional washing steps after production, removing any quickly dissolvable ions, such as sodium, from the aggregate surfaces. However, macronutrients may become less available in this way if they are also residue from production rather than being more slowly dissolvable contents of the raw material. If they are, there would be no need to produce ALA from the feed grain to use these nutrients. Furthermore, such steps would increase the water consumption of producing aggregates and generate wastewater, raising questions about the utilization costs and meaningfulness of combining BPA/CPA production with these efforts. Another approach could be to modify the production process and lower the sodium content on the surface of the aggregates. However, due to the complexity of the pelletization process and the challenges in controlling the distribution of components, this may not be a feasible solution.
As for the increased pH levels, a washing step could decrease these values, the ultimate effect depending on the system’s buffering capacity. Using acid to neutralize the aggregates would increase their salt content further or produce even more wastewater. On the other hand, higher pH values may be helpful to soils with high acidity. To assess this possibility, the buffering capacity of BPA/CPA is again relevant. Experiment A.2 showed that ALA needed approximately ten times as much HNO3 in mol kg−1 as LECA to be neutralized, while in the LECA case, this was instead the amount of acid that could be neutralized by it as LECA initially had a neutral pH. Given the latter, the determined quantities for BPA and CPA do not suggest strong buffering characteristics (for a solid). This is illustrated in comparison with CaO, which is used alongside similar solids like CaOH or CaCO3 for soil liming. As 1 mol of CaO is capable of neutralizing 2 mol protons by providing OH− (Equation 5), only approximately 336 mg (molar mass: ∼56.08 g mol−1) of it would be needed to cancel out the ∼12 mmol HNO3 used on 20 g BPA/CPA in experiment A.2. Following Equation 1 however, only 0.02 mmol would have been enough to neutralize the free 200 mL solution.
Here, the behavior of LECA is noteworthy as it has inert surfaces (Rashad, 2018) and releases only small amounts of minerals into the surrounding solution. The surrounding solution remained neutral after the addition of ∼1.1 mmol HNO3, and LECA showed weaker but similar buffering behavior to the aggregates (Figure 3). One possible explanation for this could lie in the pore volume/surface of all examined granulates, where protons could react or be adsorbed (Amelung et al., 2018b). The H+ transport between pore water and free solution is a diffusion-controlled process, especially in smaller pores (Amelung et al., 2018b), which gives the system a certain inertia. However, the pore volume was small compared to the volume of the free solution, and, therefore, the proton loss by diffusion into the pore volume alone was probably negligible. A connection between the granulate inner surface and the proton loss, on the other hand, is imaginable. In the pore space, the surface-to-volume ratio increases sharply with decreasing pore diameter, leading to specific surface areas of >2,000 m2 g−1 for activated carbon (Hu et al., 2001). Aluminosilicates have variably charged complexes on their surfaces that interact with the proton balance of the pore solution (Walther, 1996), according to which the pH deviancy shown in Figure 3 could be explained by the occupation of the inner pore surfaces by protons. As both LECA and the aggregates have specific surfaces in the same value range (experiment B), this does not contradict the observed behavior. On the other hand, additional effects must have taken place, as LECA has a larger inner surface but buffers less. The most obvious of those may be basic ions on BPA/CPA’s inner surface, residuals from production. The aggregates might positively affect acidic soil, yet they are not nearly comparable to established techniques such as liming with CaO. As experiment B further highlighted, this light pH buffering is also not accompanied by a substantial increase in AWC, so a significantly promoting effect on plant growth seems unlikely. One possibility to increase the AWC could be either a thermal treatment of the aggregates to expand the aggregates and thereby increase the pore volume or to modify the grading curve of the fine wastes, leading to a lower packing density and producing more pore volume. However, expanding the aggregates is energy-intensive, and altering the grading curve requires significant labor. Therefore, these options are not economically advisable. Other studies successfully used foaming agents like hydrogen peroxide (H2O2) (Tataranni et al., 2018) or sodium metasilicate (Na2SiO3) (Zafar et al., 2021) to significantly increase the water absorption of ALAs, which a near doubling in the latter case. The detailed pore size distribution analysis provided by Tataranni et al. (2018) shows that the AWC of aggregates created in this way is even larger than the reference LECA samples used in their study (Tataranni et al., 2018). Thus, the use of such agents could be a feasible way to improve the ALA’s feasibility to support plant growth.
On the positive side, experiment A.3 did not indicate contamination with heavy metals, which would, however, fully depend on the raw material used in a specific case. In this study, the aggregate production process did not appear to have systematically or significantly influenced the mobility of heavy metals. Availability varied comparably between BPA, CPA, and LECA in all fractions and seemed mostly dependent on the initial raw material of each granulate. This differs from other studies reported by Song et al. (2024), where the alkali-activation of fly ash was able to reduce leachable heavy metal amounts significantly (Song et al., 2024), although these studies did not consider lightweight aggregates. In general, sintering was considered to be the most effective treatment to immobilize heavy metals in artificial lightweight aggregates, as reported by Ren et al. (2021).
Experiments C.1 and C.2 showed a prominent phytotoxic effect of ALAs. While the experiment design was not rigid enough to allow any quantitative analysis, viewing the fact that additions of 10% BPA/CPA or more resulted in the complete inhibition of growth in almost all cases allows this qualitative statement. A likely reason for the high phytotoxic effect could be the high salinity and pH of the aggregates, whereas the former might be the most prominent effect. This is illustrated by CaCl2-pH levels determined after C.2, where these values were near the optimum range for E. sativa, but toxic effects were still pronounced. In addition, the crystal growth observed in C.1 (Figure 2) underlines the salinity. As discussed above, washing the aggregates would reduce this problem but raises questions about the meaningfulness of this application. Further experiments in a more controlled environment are necessary to clarify the influence of salinity and pH beyond this first assessment.
In general, further research seems necessary at this point. Studies could include investigations similar to experiment C with washed ALAs, which should contain lesser amounts of salt but also provide fewer macronutrients or reduced acid-neutralizing capacity. If such an approach proves to be feasible as a plant substrate, additional examinations could concern themselves with a determination of the ecological or water footprint of ALA’s production and washing processes. These use concentrated chemicals and produce wastewater, which could outbalance the method’s ecological gains. Another research direction should be to increase ALA’s AWC with foaming agents like H2O2 because this might considerably improve ALA’s usability as soil improvement.
5 Conclusion
This study has shown that BPA and CPA exhibit high toxicity levels toward E. sativa, probably mainly due to their high salt contents. These might be reduced with additional effort, which would require further research and raise questions about the sustainability of such an application. Given their acid uptake capacity, the aggregates might be used to improve lightly acidic soils if salinity has been addressed, which, however, would most likely affect the aggregate’s proton uptake as well. In addition, ALA could perhaps be used to improve AWC in the soil if their own AWC could be increased via changes in production, for example, by the use of foaming agents. All things considered, alkali-activated powder aggregates proved to have interesting properties to support plant growth but would need extensive optimization before being viable candidates for such use cases.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MF: data curation, formal analysis, investigation, methodology, software, visualization, and writing–original draft. IW: funding acquisition, project administration, and writing–original draft. DS: conceptualization, funding acquisition, supervision, and writing–review and editing.
Funding
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. Financial support by the Federal Ministry of Education and Research in the framework of ReMin (grant number 033R259) in Germany is greatly appreciated.
Acknowledgments
We acknowledge support by the Open Access Publication Fund of TU Berlin.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenve.2024.1511300/full#supplementary-material
References
Amelung, W., Blume, H. P., Fleige, H., Horn, R., Kandeler, E., Kögel-Knabner, I., et al. (2018a). “Physikalische Eigenschaften und Prozesse,” in Scheffer/Schachtschabel Lehrbuch der Bodenkunde (Berlin, Heidelberg: Springer Spektrum). doi:10.1007/978-3-662-55871-3_6
Amelung, W., et al. (2018b). “Chemische Eigenschaften und Prozesse,” in Scheffer/Schachtschabel Lehrbuch der Bodenkunde (Berlin, Heidelberg: Springer Spektrum). doi:10.1007/978-3-662-55871-3_5
Bui, L. A., Hwang, C., Chen, C., Lin, K., and Hsieh, M. (2012). Manufacture and performance of cold bonded lightweight aggregate using alkaline activators for high performance concrete. Constr. Build. Mater. 35, 1056–1062. doi:10.1016/j.conbuildmat.2012.04.032
Caro, D., Lodato, C., Damgaard, A., Cristóbal, J., Foster, G., Flachenecker, F., et al. (2024). Environmental and socio-economic effects of construction and demolition waste recycling in the European Union waste recycling in the European Union. Sci. Total Environ. 908, 168295. doi:10.1016/j.scitotenv.2023.168295
Castillo, H., Collado, H., Droguett, T., Vesely, M., Garrido, P., and Palma, S. (2022). State of the art of geopolymers: a review. e-Polymers 22 (1), 108–124. doi:10.1515/epoly-2022-0015
DIN 4226-101 (2017a). Recycled aggregates for concrete in accordance with DIN EN 12620 — Part 101: types and regulated dangerous substances.
DIN 4226-102 (2017b). Recycled aggregates for concrete in accordance with DIN EN 12620 — Part 102: type testing and factory production control.
DIN EN 12457-4 (2003). Characterization of waste — leaching; Compliance test for leaching of granular waste materials and sludges — Part 4: one stage batch test at a liquid to solid ratio of 10 l/kg for materials with particle size below 10 mm (without or with limited size reduction); German version EN 12457-4:2002.
Gesoğlu, M., Özturan, T., and Güneyisi, E. (2007). Effects of fly ash properties on characteristics of cold-bonded fly ash lightweight aggregates. Constr. Build. Mater. 21 (Issue 9), 1869–1878. doi:10.1016/j.conbuildmat.2006.05.038
Gleis, M., Achternbosch, M., Bräutigam, K. R., Hartlieb, N., Kupsch, C., Richers, U., et al. (2003). Heavy metals in cement and concrete resulting from the Co-incineration of wastes in cement kilns with regard to the legitimacy of waste utilisation. (Berlin, Deutschland: Umweltbundesamt).
Gleyzes, C., Tellier, S., and Astruc, M. (2002). Fractionation studies of trace elements in contaminated soils and sediments: a review of sequential extraction procedures. TrAC Trends Anal. Chem. 21, 451–467. doi:10.1016/S0165-9936(02)00603-9
Gluth, G. J. G., Grengg, , Ukrainczyk, N., Mittermayr, F., and Dietzel, M. (2002). Acid resistance of alkali-activated materials: recent advances and research needs. RILEM Tech. Lett. 7, 58–67. doi:10.21809/rilemtechlett.2022.157
Hamid, S. H. A., Lananan, F., Noor, N. A. M., and Endut, A. (2022). Physical filtration of nutrients utilizing gravel-based and lightweight expanded clay aggregate (LECA) as growing media in aquaponic recirculation system (ARS). Aquac. Eng. 98, 102261. doi:10.1016/j.aquaeng.2022.102261
Hawkesford, M. J., Cakmak, I., Coskun, D., De Kok, L. J., Lambers, H., Schjoerring, J. K., et al. (2024). Chapter 6 - Functions of macronutrients. in Marschner's Mineral Nutrition of Plants. doi:10.1016/B978-0-12-384905-2.00006-6
Hu, Z., and Srinivasan, M. P. (2001). Mesoporous high-surface-area activated carbon. Microporous Mesoporous Mater. 43 (3), 267–275. doi:10.1016/S1387-1811(00)00355-3
ISO 10390 (2022). Soil, treated biowaste and sludge — determination of pH (ISO 10390:2021); German version EN ISO 10390:2022.
ISO 11269-2 (2012), Soil quality — determination of the effects of pollutants on soil flora — Part 2: effects of contaminated soil on the emergence and early growth of higher plants.
ISO 19730 (2008), Soil quality — extraction of trace elements from soil using ammonium nitrate solution.
Jin, S., Zhang, Y., Yan, Y., Xu, Z., Li, A., Wang, J., et al. (2024). Influence of aggregate characteristics on the plant growing environment of the planting concrete with SAC. J. Clean. Prod. 445, 141179. doi:10.1016/j.jclepro.2024.141179
Jo, B., Park, S., and Park, J. (2007). Properties of concrete made with alkali-activated fly ash lightweight aggregate (AFLA). Cem. Concr. Compos. 29 (Issue 2), 128–135. doi:10.1016/j.cemconcomp.2006.09.004
Kirkby, E. A., Nikolic, M., White, P. J., and Xu, G. (2024). “Chapter 5 - mineral nutrition, yield, and source–sink relationships,” in Marschner's Mineral Nutrition of Higher Plants 85–133. doi:10.1016/B978-0-12-384905-2.00005-4
Kwek, S. Y., and Awang, H. (2021). Utilisation of recycled silt from water treatment and palm oil fuel ash as geopolymer artificial lightweight aggregate. Sustainability 13, 6091. doi:10.3390/su13116091
Meer, I., and Nazir, R. (2018). Removal techniques for heavy metals from fly ash. J. Mater Cycles Waste Manag. 20, 703–722. doi:10.1007/s10163-017-0651-z
Minasny, B., and McBratney, A. B. (2018). Limited effect of organic matter on soil available water capacity. Eur. J. Soil Sci. 69 (1), 39–47. doi:10.1111/ejss.12475
Mlih, R., Bydalek, F., Klumpp, E., Yaghi, N., Bol, R., and Wenk, J. (2020). Light-expanded clay aggregate (LECA) as a substrate in constructed wetlands – a review. Ecol. Eng. 148, 105783. doi:10.1016/j.ecoleng.2020.105783
Nagajyoti, P. C., Lee, K. D., and Sreekanth, T. V. M. (2010). Heavy metals, occurrence and toxicity for plants: a review. Environ. Chem. Lett. 8, 199–216. doi:10.1007/s10311-010-0297-8
Narattha, C., and Chaipanich, A. (2018). Phase characterizations, physical properties and strength of environment-friendly cold-bonded fly ash lightweight aggregates. J. Clean. Prod. 171, 1094–1100. doi:10.1016/j.jclepro.2017.09.259
Neina, D. (2019). The role of soil pH in plant nutrition and soil remediation. Appl. Environ. Soil Sci. (1), 1–9. doi:10.1155/2019/5794869
Prakash, J., Agrawal, S. B., and Agrawal, M. (2023). Global trends of acidity in rainfall and its impact on plants and soil. J. Soil Sci. Plant Nutr. 23, 398–419. doi:10.1007/s42729-022-01051-z
Rashad, A. M. (2018). Lightweight expanded clay aggregate as a building material – an overview. Constr. Build. Mater. 170, 757–775. doi:10.1016/j.conbuildmat.2018.03.009
Ren, P., Ling, T.-C., and Mo, K. H. (2021). Recent advances in artificial aggregate production. J. Clean. Prod. 291, 125215. doi:10.1016/j.jclepro.2020.125215
Riaz, M. U., Ayub, M. A., Khalid, H., Haq, M. A., Rasul, A., Rehman, M. Z., et al. (2020). “Fate of micronutrients in alkaline soils,” in Resources use efficiency in agriculture. Editors S. Kumar, R. S. Meena, and M. K. Jhariya (Singapore: Springer). doi:10.1007/978-981-15-6953-1_16
Song, Z., Zhang, Y., Xia, Y., Sun, C., and Wang, L. (2024). “Chapter 18 - recycling of municipal solid waste incineration fly ash into SCMs and aggregates’,” in Treatment and utilization of combustion and incineration residues. Editors L. Wang, D. Tsang, and J. Yan (Elsevier), 317–338. doi:10.1016/B978-0-443-21536-0.00030-7
Tajra, F., Elrahman, M. A., Chung, S.-Y., and Stephan, D. (2018). Performance assessment of core-shell structured lightweight aggregate produced by cold bonding pelletization process. Constr. Build. Mater. 179, 220–231. doi:10.1016/j.conbuildmat.2018.05.237
Tataranni, P., Besemer, G. M., Bortolotti, V., and Sangiorgi, C. (2018). Preliminary research on the physical and mechanical properties of alternative lightweight aggregates produced by alkali-activation of waste powders. Materials 11, 1255. doi:10.3390/ma11071255
Tchounwou, P. B., Yedjou, C. G., Patlolla, A. K., and Sutton, D. J. (2012). Heavy metal toxicity and the environment. Exp. Suppl. 101, 133–164. doi:10.1007/978-3-7643-8340-4_6
Walther, J. V. (1996). Relation between rates of aluminosilicate mineral dissolution, pH, temperature and surface charge. Am. J. Sci. 296, 693–728. doi:10.2475/ajs.296.7.693
Wichmann, I., Firdous, R., and Stephan, D. (2023). Upcycling of demolition material from concrete and brick for the production of cold-bound, alkali-activated lightweight aggregates. ’ Mater Struct. 56, 135. doi:10.1617/s11527-023-02216-7
Wichmann, I., and Stephan, D. (2023). “Mechanical and physical properties of concrete made of alkali-activated lightweight aggregates from construction demolition waste,” in Material today: proceedings. doi:10.1016/j.matpr.2023.05.533
Wilkinson, R. E., Clark, R. B., and Baligar, V. C. (2000). “Acidic and alkaline soil constraints on plant mineral nutrition,” in Plant-environment interactions (London: CRC Press), 146–190.
Williams, R., Artola, I., Beznea, A., and Nicholls, G. (2020). Limits of recycling: emerging challenges of waste management in europe. Final report. Trinomics B.V. Rotterdam, Netherlands. Available at: https://trinomics.eu/wp-content/uploads/2020/06/Trinomics-2020-Limits-of-Recycling.pdf September 30, 2024).
Yliniemi, J., Nugteren, H., Illikainen, M., Tiainen, M., Weststrate, R., and Niinimäki, J. (2016). ‚Lightweight aggregates produced by granulation of peat-wood fly ash with alkali activator. Int. J. Mineral Process. 149, 42–49. doi:10.1016/j.minpro.2016.02.006
Zafar, I., Rashid, K., and Ju, M. (2021). Synthesis and characterization of lightweight aggregates through geopolymerization and microwave irradiation curing. J. Build. Eng. 42, 102454. doi:10.1016/j.jobe.2021.102454
Zhao, X., Yang, J., Ning, N., and Yang, Z. (2022). Chemical stabilization of heavy metals in municipal solid waste incineration fly ash: a review. Environ. Sci. Pollut. Res. 29, 40384–40402. doi:10.1007/s11356-022-19649-2
Keywords: C&D waste, aluminosilicate, heavy metal mobility, plant substrate, liming, upcycling
Citation: Frohmüller MO, Wichmann I and Stephan D (2025) A short overview of the physicochemical properties of cold-bonded alkali-activated lightweight aggregates and preliminary examinations of their usability for plant growth. Front. Environ. Eng. 3:1511300. doi: 10.3389/fenve.2024.1511300
Received: 14 October 2024; Accepted: 27 December 2024;
Published: 17 February 2025.
Edited by:
Adeline Seak May Chua, University of Malaya, MalaysiaReviewed by:
Kim Hung Mo, Sunway University, MalaysiaChong Yang Chuah, University of Malaya, Malaysia
Copyright © 2025 Frohmüller, Wichmann and Stephan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Max O. Frohmüller, bS5mcm9obXVlbGxlckB0dS1iZXJsaW4uZGU=
 Max O. Frohmüller
Max O. Frohmüller Isabelle Wichmann
Isabelle Wichmann Dietmar Stephan
Dietmar Stephan