
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Environ. Chem., 18 March 2025
Sec. Sorption Technologies
Volume 6 - 2025 | https://doi.org/10.3389/fenvc.2025.1452837
This article is part of the Research TopicAdvances in the synthesis and utilization of waste-derived materials for water purificationView all 4 articles
Chromated Copper Arsenate (CCA) is a water-based mixture of heavy metals widely used as a timber preservative. Despite its efficacy in prolonging the lifespan of treated wood, CCA has become a subject of environmental scrutiny due to the leaching of toxic components into surrounding soil and water. CCA components in soil have been reported with levels as high as 3,300, 2,800 and 2,100 mg/kg for As, Cr and Cu, respectively; way above the recommended levels of 12, 64, 63 mg/kg for agricultural soils. Therefore, the use of CCA as a wood preservative has been restricted in most developed countries. Developing countries, however, continue to utilize CCA treated wood as utility poles. The elements of CCA have potential health risks upon dermal contact with CCA residues from treated structures as well as exposure from contaminated soil and water. There are also concerns about the disposal of CCA treated wood after use, with the current technology of landfilling being unsustainable because of the possibility of CCA leaching into underground water as well as the challenge of limited space for future disposal. Incineration and open burning as a way of disposal produce ash that is highly contaminated and the fumes contribute to air pollution with metals. There is therefore need for sustainable approached for disposal of wood waste. Since the leached elements end up in the environment, several remediation strategies such as chemical methods, bioremediation, phytoremediation and bioadsorption have been reported, as discussed in this review paper, towards sustainable solutions to CCA contamination with some strategies reporting 100% efficiency.
Chromated Copper Arsenate (CCA) (As2CrCuO9) is a water-based mixture of heavy metals/metalloid used as a wood preservative to increase its durability and protect it from fungal and bacterial degradation as well as attack by insects (Morais et al., 2021). Other names that refer to the same preservative are copper chrome arsenate or copper chromated arsenate. It is composed of 47.5% chromium oxide (CrO3), 18.5% copper oxide (CuO) and 34% arsenic pentoxide (As2O5), which constitutes CCA type C that is commonly applied in many countries since it provides a suitable combination of resistance to leaching as well as high efficacy (Moghaddam and Mulligan, 2008). Chromium enhances the fixation of copper and arsenic in the wood, copper provides resistance to fungi and bacteria while arsenic provides dual protection by preventing destruction of wood by insects and harsh weather conditions (Matos et al., 2010; Mohajerani et al., 2018). CCA has been in use since 1930s being the preservative of choice for treatment of wood intended for industrial use compared to other non-arsenic and non-chromium preservatives that include alkaline/ammoniacal copper quaternary (ACQ), copper azole, micronized copper azole, creosote, pentachlorophenol (Penta) (Bolin and Smith, 2013; Jones et al., 2019).
Treatment of wood with CCA involves high-pressure impregnation process at the treatment plants to provide a minimum retention of at least 21 kg/m3. During the fixation, Cr (VI) is reduced to Cr (III), which strongly binds to lignin by forming a complex (Kartal, 2003). The three metals once bound to wood exist in the forms represented in Equation 1 (Abd El-Fatah et al., 2004)
Wood species commonly used due to their durability include eucalyptus, pine, cedar and fir (Arriaga et al., 2023; KPLC, 2014). Wood treated with CCA has a variety of references that include “CCA treated timber,” “tanalised timber,” “pressure treated timber” and “permapine timber.” The treated wood is used in framings, outdoor playground equipment, fence posts, backyard decks, marine structures, garden edging, landscaping, picnic tables, electricity poles and in building (Coles et al., 2014; Hall and Beder, 2005; Morais et al., 2021). Through there are other options for electricity distribution and transmission such as concrete poles and steel pylons, wood treated poles are the ones commonly used due to their relatively low cost and lighter weight making them affordable (Muthike and Ali, 2021).
The use of CCA treated wood in residential areas in countries such as Australia, Canada and United States has been restricted due to the risk of environmental contamination and associated health effects (Hall and Beder, 2005). However, wood preservation by use of CCA continues in developing countries; an activity that takes place at the wood treatment plants. Effluents from these plants find their way into the environment where Cr(III) ions undergo oxidation reactions to form Cr(VI) ions. Cr(VI) compounds are of great concern owing to their high water solubility, permeability through biological membranes and subsequent interaction with proteins (Ambi et al., 2020; Okello et al., 2012; Stern, 2010). Cr(VI) is known to be highly toxic, mutagenic and carcinogenic to humans and animals (Sharma et al., 2022; Vignati et al., 2010). Arsenic is also a known carcinogen (Palma-Lara et al., 2020). Moreover, the toxic effects of CCA have been reported to be more severe than those of its individual constituents. Thus, the presence of these metals in the environment may result in acute and chronic effects in humans, aquatic organisms and the entire terrestrial ecosystem (Shanker et al., 2005; Stern, 2010). Animals and humans are therefore at risk when exposed to arsenic, chromium and copper from the CCA treated wood, dust or contaminated soil/water (Hall and Beder, 2005; Morais et al., 2021).
Because of the environmental impact of CCA, a clear understanding of its toxicity, fate and potential removal strategies is necessary. Therefore, this review focuses on CCA elements in the environment, their toxicity, removal strategies and sustainable alternatives to mitigate adverse effects and safeguard human health and ecosystems for future generations. It comprehensively delves into removal and immobilization of CCA components from a wide range of matrices, including soil, water and wood waste building on earlier reviews that focused on a single matrix such as wood waste (Lopes et al., 2019; Mohammed et al., 2022), or examined other heavy metals rather than specifically addressing the distinct components of CCA (Qasem et al., 2021; Wang et al., 2022; Yeo et al., 2021; Zeng et al., 2024).
To identify the relevant literature for inclusion in this article, Google scholar was used as the primary database for thorough search. Some of the keywords employed during the search included; “chromium” “copper” “arsenic” “CCA” “bioremediation” “decontamination” and “biosorption”. The choice of articles for inclusion was dependent on the titles and abstracts, which were found to be relevant to the subject matter that is, removal of chromium, copper and arsenic from contaminated matrices. The publications cited in this review encompasses journal articles, reports and theses. Data presented in graphical and tabular form in this work was extracted from the abstracts, methodologies, results and discussions of the studies covered. The years of publication considered in this review range between 2000 and 2023. The early years form the basis of our discussion whereas the recent years describe advances, which runs from 2018 up to 2023.
CCA treated wood has a lifetime of 40 years after which it is disposed of as wood waste since it no longer offers the much needed service (Xing et al., 2020). Besides polluting the environment with CCA elements through leaching during its use, disposing of the wood after use can lead to further environmental contamination (Townsend et al., 2004). Figure 1 shows the various stages that wood undergoes from planting to treatment and final disposal at the end of its service, with most of the stages contributing to environmental pollution.
The continued use of CCA treated wood places, humans and animals at great risk of exposure due to the possible leaching of CCA components into the environment. A study by Mercer and Frostick, (2012) showed that all the three elements of CCA leach from wood. The two leaching experiments employed (batch leaching and lysimeter) showed that freshly treated wood leach more than weathered wood losing 24% arsenic, 6% chromium and 18% copper compared to 0.28%, 0.08% and 1.14% for weathered wood. The concentration of the metal (loids) in leachate from the two types of wood exceeded the environmental quality standards (EQS) of the European Commission Water Framework Directive set at 50, 32 and 28 μg/L for arsenic, chromium and copper, respectively. The World Health Organization (WHO) guideline for Cr, Cu and As in drinking water is 0.05, 2 and 0.01 mg/L, respectively (WHO, 2011). In another study, soil samples near utility poles in Canada reported concentrations of up to 37.5, 65.5, and 38.9 mmol/kg for Cu, Cr, and As, respectively. Levels of Cu, Cr, and As from rainwater runoff of freshly treated poles exposed to natural rain recorded concentrations of 14.0, 77.7 and 55.8 μmol/L, with chromium and arsenic levels being higher than the recommended levels in drinking water of 15.7, 0.961 and 0.13 μmol/L by Canadian Council of Ministers of the Environment (CCME) (Coles et al., 2014). Leached CCA elements in rainwater runoff eventually drain into water bodies as illustrated in Figure 2.
Soil samples collected 20 cm from utility poles treated with CCA showed high contamination of 5,857, 3,815, and 3,797 mg/kg for As, Cr, and Cu, respectively, which were higher than the acceptable criteria for both industrial and residential soils (Villegas and Zagury, 2023). Another study on soil samples collected near utility poles reported concentrations of As, Cr and Cu at 265, 165 and 360 mg/kg, respectively (Gosselin and Zagury, 2020). Table 1 shows the levels of CCA components in contaminated soils from different parts of the world, with levels in most places exceeding the available guidelines by CCME. The presence of CCA components in soil and water bodies due to runoff paves the way for their uptake by plants and other organisms, eventually finding their way into the food chain.
Disposal of CCA treated wood waste is commonly done through landfilling of the wood itself or through incineration or a waste-to-energy process from which the ash is also landfilled (Choi et al., 2012). Unfortunately, the components can leach out of the treated wood contaminating the surrounding groundwater and soils, thus leading to human and animal exposure. Levels of arsenic and chromium in leachate from landfilled ash were reported to be 1.76 mg/L and 4.8 mg/L, which was 3 and 24 times greater compared to leachate from municipal waste landfills (Jambeck et al., 2007). Combustion and disposal of ash in landfills is of major concern because the process has been reported to convert Cr(III) to Cr(VI), which is more toxic and mobile (Song et al., 2006). A more worrying scenario is how to handle and reclaim abandoned wood treatment plants due to contamination of such locations and their surrounding environment with heavy metals. Soil collected from such a plant that had been in operation for more than 40 years reported very high concentrations of 2,800, 2,100 and 3,300 mg/kg for Cr, Cu and As, respectively (Beiyuan et al., 2018). Given the environmental risks associated with CCA, there is a great need for sustainable alternatives and strategies for the restoration of these spaces to make them useful again.
CCA has been reported to be toxic to humans and animals upon exposure, with CCA being more severe than the individual elements. Workers in wood treatment plants are majorly exposed to CCA through inhalation, while the normal population is exposed through dermal exposure once in contact with treated wood or ingestion of contaminated soil, food or water. Arsenic exist in three forms, i.e., trivalent arsenite (As (III)), pentavalent arsenate (As(V)) and elemental arsenic (As), with both arsenite and arsenate being toxic (Yeo et al., 2021). According to the United States Environmental Protection Agency (United States EPA), inorganic arsenic is a Group A carcinogen (Morais et al., 2021). Arsenic also affects the central nervous system, the immune system and can lead to fetal mortality. Chromium exists of two forms, i.e., Cr(III) and Cr(VI), with the latter being more toxic and a potential carcinogen to humans. Its high toxicity stems from its oxidative nature corroding the respiratory system and ultimately leading to lung cancer. Though copper is an essential trace element, high levels are detrimental to humans and animals, with diseases related to copper metabolism being reported (Morais et al., 2021).
Since CCA is able to leach into the environment, contaminating soil and exposing humans, especially children, a recent study by Villegas and Zagury (2023) carried out risk characterization of CCA contaminated soil. The study assessed different exposure pathways, including dermal, inhalation and oral ingestion for industrial and residential settings. High hazard index (HI) greater than one was reported for oral and dermal pathways from the residential and industrial scenarios while the inhalation pathway resulted in a lower HI of less than one. The oral pathway contributed the highest carcinogenic risk for As and Cr(VI), with risk values greater than the acceptable value of less than 10−4. These findings point to the need for strategies of preventing environmental pollution to protect humans from possible exposure.
There are concerns on contamination of the environment by CCA elements through leaching from treated wood. Incineration of CCA treated wood produces ash contaminated with metals, which need to be removed before the ash is placed in landfills. A study by Solo-gabriele et al. (2002) indicated that the contribution of these metals in ash could be as high as 36% by weight for wood with high CCA retention of 40 kg/m3. Open burning has been reported to emit up to 14% of arsenic to the environment (Wasson et al., 2005). On the other hand, landfills take up much space and metals are able to leach to underground water with time. Thus, there is an urgent need for environmentally sustainable strategies for handling wood waste to reduce environmental contamination. The use of wood waste fiber to produce reinforced polypropylene composites of high tensile strength for use in the construction industry has been reported (Nelson et al., 2023). Pyrolysis has also been explored as a potential alternative for disposal of wood that is out of service (Junk, 2022). Several methods have been proposed for the remediation of CCA-contaminated soils, water and wood waste. These include chemical remediation (Gezer et al., 2006; Janin et al., 2012) bioremediation (Xing et al., 2023), phytoremediation (Li et al., 2022), and physical methods such as soil washing and adsorption (Frighetto et al., 2016) among others as discussed in this section.
Chemical remediation involves the application of chemical amendments, such as iron oxide or calcium hydroxide or any other viable chemical, to immobilize and precipitate heavy metals, reducing their bioavailability and mobility in the environment. Organic acids such as citric, acetic, formic, oxalic, fumaric, tartaric, gluconic, and maleic, as well as mineral acids, such as sulphuric, hydrochloric, nitric, and phosphoric have been utilized (Frighetto et al., 2016). The use of NaOH and citric acid buffer at a pH of 3.5 achieved 100% extraction of copper from wood after 40 days. An optimized method using oxalic acid and metal tolerant bacteria (Bacillus Licheniformis CC01) afforded 78% 97% and 93% removal of copper, chromium and arsenic, respectively (Clausen, 2000). Another study which used the same bacteria and oxalic acid achieved very high removal efficiency of 90% copper (CuO), 80% chromium (CrO3), and 100% arsenic (As2O5). Acids convert the CCA elements into their water-soluble form.
Ethylenediaminetetracetic acid (EDTA) as a chelating agent has been widely used to form soluble complexes with metals. A 1% EDTA solution removed 60% copper, 13% chromium, and 25% arsenic from treated chips after 24 h (Kartal, 2003). Oleic acid was able to remove 96% copper, 78% chromium and 96% arsenic at a pH or 2.0 in 2 days. A high pH of 5.0 was found to be ineffective, especially in removal of chromium (Gezer et al., 2006). Leaching of CCA metals by use of sulphuric acid followed by precipitation with calcium hydroxide reported 98% removal of arsenic, 93% chromium and 96% copper from wood (Janin et al., 2012). Electro-removal method based on application of an electric field led to a reduction of 79.5, 87.4, and 81.3% in the mean concentrations of Cu, Cr, and As from treated waste wood. This method has been found to be effective since the electrolytic fluid containing the metals can be reused in production of CCA to provide further protection of wood (José de Castro et al., 2021). The application of super critical fluid extraction (SFE) technology using CO2 and organophosphorus chelating agents achieved extraction efficiencies of 63.5, 28.6, and 31.3% for Cu, Cr, and As, respectively, from wood waste (Abd El-Fatah et al., 2004). Though chemical methods, have showed a level of efficiency in remediation of wood waste, their disadvantage stems from the fact that chemicals are harmful and they lead to secondary environmental pollution (Xing et al., 2023). Traces of chemicals in decontaminated wood also limits its use in making blended products when mixed with other substances like geopolymer cement (Can and Sivrikaya, 2022; Elmira et al., 2023). As an alternative, bioremediation is hereby discussed.
Bioremediation involves the use of microorganisms to eliminate or reduce the concentrations of pollutants. Microorganisms such as fungi generate organic acids that enhance the acidity of the substrate, promoting the solubility of chromium and arsenic, thus facilitating their removal. Oxalic acid, among other organic acids displays a number of properties including chelating ability, reducing agent and ability to create a lower pH for removal of CCA elements. A study using oxalic acid producing fungi Fomitopsis palustris, Coniophora puteana, and Laetiporus sulphureus reported arsenic removal rates of 100% and 85% for F. palustris and L. sulphureus (Kartal et al., 2004). Xing et al. (2023) demonstrated Yarrowia lipolytica ability to remove 82.8% copper, 43.1% chromium and 63.8% arsenic through the production of oxalic, malic, acetic and citric acids.
Besides bioremediation, bioprocessing methods that take advantage of bioremediation and biodegradation by use of fungi have been explored as alternatives to disposal of CCA wood waste in landfills. Four fungal isolates, Meruliporia incrassata (TFFH-294), Antrodia radiculosa (MJL-630), M. incrassata (Mad-563) and A. radiculosa (FP-90848-T) degraded CCA-treated wood by more than 20% of the original dry weight of the wood (Illman and Yang, 2004). Similarly, another study used five isolates of brown-rot fungi to decay treated wood indicating mass losses of up to 60% and 53% by Crustoderma sp. KUC8611 and F. palustris, respectively. The removal of CrO3 (79%), As2O5 (87%), CuO (50%) by F. palustris was more efficient in CrO3 and As2O5 due to the high production of oxalic acid by the fungi (Choi et al., 2012). Fungi also remove these metals via bio-absorption into their cell structure as well as complexation with low molecular weight proteins (Kartal, 2003). While fungi have been reported to be efficient in removal of metals, they require several days to achieve this efficiency and may also result in transformation of wood constituents (Chang et al., 2012). Additionally, they may not assimilate all the components of CCA (Lopes et al., 2019).
Metal tolerant bacteria have been utilized to remove chromium; a highly stubborn metal in CCA. Three isolates, Acinetobacter calcoaceticus FN02, Aureobacterium esteroaromaticum VV03, and Klebsiella oxytoca CC08 were reported to release 98% of chromium, while Bacillus licheniformis CC01 released 93% of copper (Clausen, 2000). A mixed culture of lactic acid bacteria (Lactobacillus bulgaricus and Streptococcus thermophiles) was able to extract all three metal ions from the CCA treated wood within 4 days at 93% for copper, 86.5% for chromium, and 97.8% for of arsenic. These bacteria were also reported to produce pyruvic acid that aided in extraction of metals (Chang et al., 2012). Earthworms are other microorganisms that have shown potential to remove arsenic from CCA wood waste. Eisenia fetida, type of earthworms, were able to bioaccumulate arsenic in their tissues by feeding on CCA wood waste mixed with cow dung leading to their removal (Mohammed et al., 2022). Notwithstanding the promising results associated with bioremediation, long treatment time is a major drawback that should be probed to accelerate the process of acquiring results during research (Costa et al., 2022).
Pyrolysis is a thermochemical (physico-chemical) decomposition of biomass in limited supply or absence of oxygen to yield biochar, bio-oil and gases consisting of CO, CO2, and H2 that do not condense easily. Gmar et al. (2022) investigated a combination of pyrolysis and plastic encapsulation as a way of safely disposing contaminated wood. This study reported the highest biochar yield at 300°C with heavy metal retentions of 76, 91% and 83% for As, Cr and Cu, respectively. However, there was notable leaching of heavy metals from the biochars. To prevent leaching, encapsulation of the biochars with high-density polyethylene reduced leaching by 96, 95% and 91% for As, Cr and Cu, respectively. In another study, pyrolysis of CCA-treated wood released 41% of arsenic at 800°C, whereas gasification led to release of 90% arsenic. The release of arsenic was suppressed during pyrolysis by mixing organic sludge rich in iron and calcium with wood treated with CCA (Kato et al., 2021). Maximum recovery of arsenic from the solid state occurred at 475 C during pyrolysis as reported by Junk, (2022). At this temperature, the bio-oil yield was 29 wt% with a 606 ppm arsenic concentration representing a 6.7 wt% of the original arsenic. Pyrolysis reduces As(V) to As(III) as evidenced by the presence of (As2O3) in the bio-oil. Volatilization of arsenic was observed at temperatures above 475 C. It was noted that copper in CCA affects the production of bio-oil during pyrolysis, led to increased char yield and reduced arsenic recovery. Pyrolysis is an already established method for decontamination of CCA-treated wood waste as it involves fairly low capital. Compared to other similar technologies like gasification and incineration, less gaseous emissions are produced during pyrolysis. However, the energy requirements are high and further investigations are necessary to lower the cost of this process. Table 2 summarizes some of the strategies for removal of CCA elements from wood waste.
Immobilization techniques prevent the free movement of contaminants in the matrix and surrounding media through solidification or stabilization. Solidification involves changes in the physical properties of a waste that include compressive strength, permeability and encapsulation, while stabilization involves chemical changes of the hazardous constituents in a matrix to a less soluble, mobile or toxic form. Zeolite modified with iron has been shown reduce the amount of arsenic in contaminated soil as evidenced by reduced uptake of arsenic by plants. The arsenic concentration in leaves was reported to have reduced from 36 mg/kg to 15 mg/kg (Alessandro, 2019). A novel electrokinetics method for removal of Cr(VI) in contaminated soils reported 85.50% efficiency (Xu et al., 2017). By incorporating permeable reactive barrier (PRB) consisting of hydrocalumite (CaAl-LDH), the removal efficiency increased to 96.49%. A similar approach for removal of arsenic in contaminated soils involved coupling electro kinetics with PRB consisting of iron-manganese-carbon layered double hydroxide (Fe/Mn/C-LDH). Bamboo was used in the synthesis of the Fe/Mn/C-LDH fillers as the template due to its porous nature. The study established that the rate of removal of arsenic from contaminated soils was influenced by electric field strength, PRB position, moisture content as well as type of PRB filler. Maximum removal rates were 95.71% when the PRB filler was positioned in the middle, where the soil pH ranged between 5 and 8 providing suitable conditions for arsenic adsorption onto the PRB filler Fe/Mn/C-LDH. Moisture content of 35% gave the best removal rates whereas a voltage gradient of 2 V/cm gave the optimal arsenic immobilization (Zhu et al., 2022).
Stabilization of soil contaminated with arsenic by use of acid mine drainage-treated sludge (AMDS), which is a problematic waste in coal mining has been reported. The sludge is composed of iron and calcium minerals with high adsorption capacity for heavy metals (Amanda and Moersidik, 2019). The stabilization efficiency of acid mine drainage sludge was higher than 85% by the use of 3% of AMDS, which was attributed to ligand exchange between the Fe- (oxide) hydroxide and the arsenate to form a new complex, the cation bridge effect between the AMDS surface and the arsenate as well as co-precipitation (Tak et al., 2023). Starch stabilized Fe/Cu nanoparticles (0.04 wt% of starch) are capable of in situ stabilization of arsenic and chromium in soil contaminated with CCA. Treatment of contaminated soil with 0.4 g/L nanoparticles at a soil: liquid ratio of 0.1 reduced leachable arsenic in water from 55 to 4.2 μg/L through immobilization to nanoparticles (Babaee et al., 2017).
Phytoremediation has gained widespread application in the remediation of soils contaminated with heavy metals due to its environmentally friendly nature and associated low cost. This method involves processes such as phytoextraction (involving uptake of heavy metals), phytoaccumulation (involving accumulation and translocation of heavy metals), phytovolatilization (emission of heavy metals to the atmosphere in volatile form) and phytostablization (stabilization of heavy metals in the root zone of plants) (Li et al., 2022; Shah and Daverey, 2020). Phytoaccumulation of arsenic from contaminated soils was conducted using fern (Pteris vittata) as a trap plant. Soil and cow dung mixture (ratio of 1:1) was spiked with arsenic in the form of arsenic trioxide at different concentrations of 0 ppm (A0), 500 ppm (A1), 1,000 ppm (A2) and 2,000 ppm (A3). After 60 days, the fern had accumulated 0.9 ppm, 1769.9 ppm, 15,332.6 ppm and 23,837.2 ppm for A0, A1, A2 and A3, respectively. From the results, the soil seems to have been contaminated with arsenic prior to the experiment since the reported levels were higher than the spiked amount (Uddin et al., 2015). Fern is reported to be a hyperaccumulator, thus an excellent plant for phytoremediation.
The use of maize (Zea mays L.) in phytostabilization of contaminated soils from a CCA wood treatment plant amended with sewage sludge biosolid as an additional stabilizer has been reported. The results showed that phytostabilization using maize was greatly improved by the application of the sewage sludge biosolid through reduced mobility (Nakiguli et al., 2020). In another study, Fomitopsis arundinacea, M. sativa, and S. purpurea were investigated for their potential in phytoaccumulation of pentachlorophenol (PCP) and CCA. The experiment involved mechanical scarification and amendment of the soil with commercial soil to enhance germination of the plants by reducing the severe soil mineralization and compaction. Thereafter, the herbaceous species were sown manually followed by irrigation and were monitored for 4 years. At the end of the experiment, aerial tissues of F. arundinacea, M. sativa, and S. purpurea had accumulated 6.5, 10, and 8.75 mg/kg of copper, respectively, with chromium and arsenic being below detection limit. This was attributed to their interactions with soil particles, which reduces their bioavailability, and competition with other ions like phosphates present in the soil. Also the elements might have been translocated in the roots only and not the other parts of the plant (Yanitch et al., 2020). The use of plants in phytoremediation of contaminated soils is therefore an effective method owing to their ability to accumulate multiple contaminants from soils and also contribute to phytostabilization (Priya et al., 2023). However, this phytoaccumulation has limitations regarding safe disposal of harvested plants after phytoremediation process as well as recovery of the heavy metals. More research is therefore needed on sustainable ways of disposing contaminated plants (Shah and Daverey, 2020).
Some of the sustainable ways that can be considered to avoid re-entry of heavy metals into the environment may include; pyrolysis and stabilization with agents like cement to immobilize the contaminants. Another way that can be considered is treatment of the plant biomass with microorganisms, which convert biomass into soluble organic compounds that dissolve the heavy metals, thus reducing the volume occupied by biomass and consequently aiding the management of the waste. In addition, the plants can be used in the production of bio-energy. Moreover, when the harvested plants are mixed with other organic materials and left to decompose, an amendment that is rich in nutrients is produced which can be applied in agriculture (Priya et al., 2023; Singh et al., 2022). For the recovery of metals, suitable chemicals like acids can be used to recover metals when in high concentrations. Table 3 gives a comparison of the performance of the different remediation approaches in contaminated soils from recent studies.
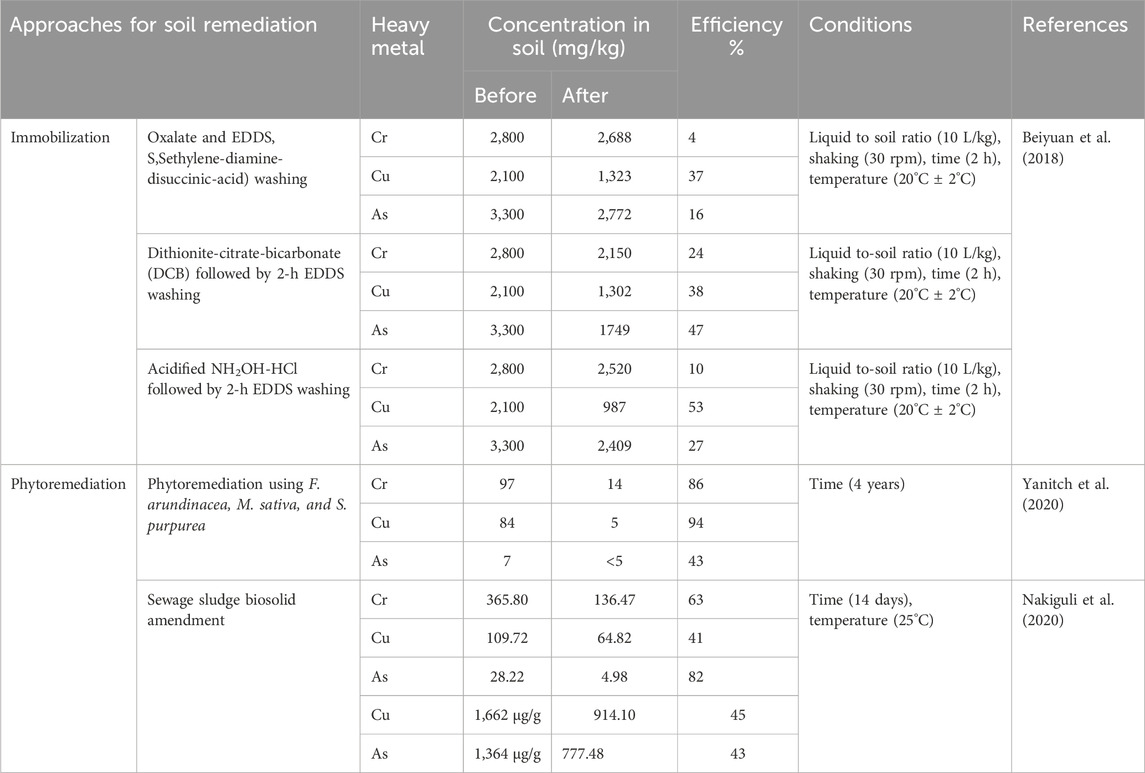
Table 3. Comparative studies for remediation of soils contaminated with chromium, copper and arsenic.
Adsorption, in the context of remediation, refers to the process by which contaminants in water, air, or soil adhere to the surface of a solid material (adsorbent), effectively removing them from the environment. This occurs due to physical or chemical interactions between the contaminant molecules and the adsorbent surface. Hexavalent chromium usually exists in water/wastewater as oxyanions such as chromate (CrO42−), hydrogen chromate (HCrO4−), and dichromate (Cr2O72−), which are mostly toxic, carcinogenic and mutagenic and do not precipitate easily using conventional precipitation methods. Similarly, the predominant forms of arsenate are dihydrogen arsenate (H2AsO4−), hydrogen arsenate (HAsO42−) and arsenate ion (AsO43−) (Saikia et al., 2011). The removal of heavy metals from contaminated water by use of adsorbents is explained by different adsorption mechanisms such as complexation, electrostatic interactions, ion exchange, redox reactions, physical adsorption and precipitation (Wu et al., 2016). Figure 3 shows some of the adsorption mechanisms leading to removal of chromium. Several adsorbents have been utilized for removal of CCA elements as outlined in the sections that follow.
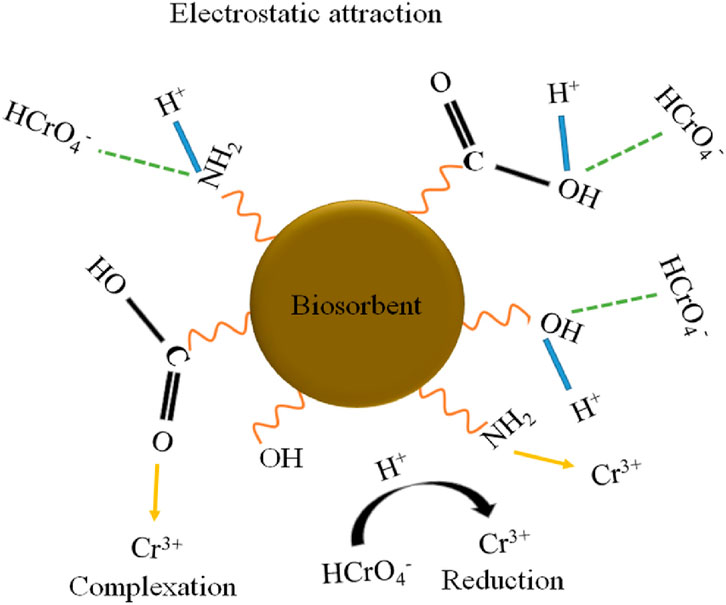
Figure 3. Adsorption mechanisms for removal of chromium from aqueous environment, reproduced with permission from Garg et al. (2023).
A study by Khaodhiar et al. (2000) reported on removal of copper, arsenate and chromate in a single and multi-solute electrolyte system of NaNO3 using iron-oxide coated sand. At pH above 6.0, 90% of copper was adsorbed. In a binary system of copper and arsenate, the amount of copper adsorbed was shown to increase due to formation of copper arsenate complex as well as the formation of inner-sphere complex that decreases the surface charge of iron oxide. Chromate or even a mixture of chromate and arsenate did not affect adsorption of copper. Highest adsorption of 35% for chromium was achieved at lower pH of 3.0. Copper was shown to influence the adsorption of chromate positively by increasing the surface charge of the iron oxide. Arsenate was also found to adsorb at low pH values with the presence of copper or chromate having no influence on the amount adsorbed. Manganese oxide sorbents have also been evaluated for removal of copper, chromium and arsenic from water reporting appreciable removal. Manganese coated sand was used for removal of chromium, copper and arsenic from a synthetic leachate solution using a sorbent dosage of 20 g/L. For a synthetic leachate solution containing 2, 1 and 0.2 mg/L total chromium, copper and arsenic, respectively, the sorbent reported 70%, 98%, 99% removal of the heavy metals (Wu et al., 2016).
Many other studies have reported on use of various sorbents to a wide range of heavy metals including the elements of CCA. Chitosan coated fly ash (SiO2 – based), showed adsorption capacity of 36.22 mg/g for Cr(III, VI), 28.65 mg/g for Cu(II), and 19.10 mg/g for As(V). The multiple functional groups in chitosan enhance its adsorption capacity, with Cu(II) achieving the highest removal efficiency of 89.5% using 0.3 g of adsorbent (Adamczuk and Kołodyńska, 2015). In another study, magnetic biochars modified with chitosan showed potential for heavy metal removal owing to chitosan having amine functional group on the surface of the sorbent as well as its large surface area for adsorption. Chitosan combined with magnetic loofah biochar (CMLB) was tested for Cr(VI) and Cu(II) ions removal from wastewater where it exhibited higher adsorption capacity in comparison to the pristine biochar. The magnetic property of this adsorbent enhanced the separation of CMLB from aqueous solutions. The highest adsorption capacity for Cr(III) and Cu(II) was reported as 30.14 mg/g and 54.68 mg/g, respectively using 40% CMLB where equilibrium was attained after 18 h. Also, it was observed that lower pH values (1.0–6.0) favored existence of Cr(VI) in the form HCrO4− and Cr2O72− which resulted to higher adsorption capacity of the sorbent. Adsorption capacity decreased with increase in pH which led to competition between hydroxide complexes and Cr(VI) ions for active sites (Xiao et al., 2019).
Activated alumina powder (AAP) has shown promising results for removal Cr(III) and Cu(II) from industrial waste water. At pH 5, precipitation of Cr and Cu occurred for high metal ion concentration. The percentage removal of Cr(III) and Cu(II) was maximum at pH 4.7 and pH 3, respectively. Results of the study indicated that equilibrium for Cr(III) and Cu(II) was attained at 50 min and 40 min, respectively. The highest removal of Cu(II) from a 0.1 M solution was reported as 99.8% using 10 g of sorbent whereas for Cr(III) it was 99.4% using 2 g of AAP from a 0.01 M solution. Additionally, the effect of particle size on the removal of heavy metal ions was studied where all the particle sizes (125, 250 and 420 mm) resulted to 99% removal of the ions. Cr(III) and Cu(II) adsorption increased as the temperature increased (Rajurkar et al., 2011).
Biomass-derived adsorbents are desirable in that they can be reused several times to remove metals without loss in their efficiency, thus reducing the cost of remediation. Some of the naturally available materials that have been utilized as adsorbents in removal of Cr(III), Cu(II) and As(V) in single and multi-solute system include; activated carbon, corncob, apple and orange peelings, oak and pine bark, biopolymers among many others. Naturally occurring biopolymers (chitin and chitosan) are rich in amine and hydroxyl groups that can form complexes with metal ions. Hassoune et al. (2018) reported 80.64 and 26.25 mg/g maximum uptake for the removal of Cr(VI) from water using gelatin-chestnut (GC) and gelatin-quebracho (GQ) biopolymer adsorbents, respectively. Chitosan, a biopolymer extracted from shrimp shell reported 98.5% and 97.4% adsorption efficiencies for arsenic and chromium, respectively after modification using NaOH and H2SO4 that enhanced functional groups increasing the adsorption capacity (Rahman et al., 2023). CCA treated sawdust exposed to a solution containing chitin achieved 74% copper, 62% chromium, and 63% arsenic removal, while chitosan removed 57% copper, 43% chromium, and 30% arsenic.
Azadirachta indica leaf was used to produce activated charcoal for Cu(II) adsorption. In batch experiments, 91.5% removal was achieved at 46°C with a 10 g/L dose. Column experiments optimized at 5 mL/min flow rate, 5 mg/L concentration, and 20 cm bed height showed a maximum adsorption capacity of 185.8 mg/g (Patel, 2020). Ambi et al. (2020) used Hibiscus sabdariffa calyces extract, rich in antioxidants, for Cr (VI) removal reporting promising results consistent with Clovis et al. (2020). Use of tea waste has gained ground in adsorption because it is an effective and inexpensive technique. Nagra et al. (2023) used tea waste in column sorption experiment, achieving 92% As(V) removal at pH 5. Tea waste showed competitive adsorption for Cu(II) and Pb(II) from aqueous solutions with a maximum Cu(II) adsorption capacity 37.17 mg/g in single element solutions and 28.41 mg/g in mixed solutions (Pasgar et al., 2022). Competition between Cr(VI), Cu(II) and As(V) ions during adsorption should therefore be investigated through research for efficiency in their removal. At a at pH 2, Jeyaseelan and Gupta (2016) reported 99% removal efficiency for Cr(VI) using green tea leaves.
Numerous studies have been conducted using water hyacinth, both in its raw form and activated form, to evaluate its capability in treatment of waste water containing heavy metals (Abbas et al., 2021; Ajibade et al., 2013; Gogoi et al., 2017; Lissy and Madhu, 2011; Panneerselvam and Priya, 2023). Water hyacinth (Eichhornia crassipes) shoot powder (WHSP) removed more than 98% of Cu(II) and Cr(VI) from both standard and tannery solutions as reported by (Sarkar et al., 2017). On the other hand, Huynh et al. (2021) reported lower removal efficiencies of 60.8% for As(V) and 60.7% for Cu(II) using water hyacinth grown in water contaminated with 0.5 mg/L arsenic and 5 mg/L after 30 days. Polyflavonoids tannins from the barks of Rhizophora apiculata mangrove, showed adsorption capacity of 8.78 mg/g for Cu (II) at pH 5 (Oo et al., 2009). Furthermore, flavonoids such as quercetin and their derivatives can reduce Cr(VI) to Cr(III) as reported by Okello et al. (2012), therefore their use in the removal of Cr(VI) has an added advantage. Though adsorption is also an alternative in removal of CCA elements, bioremediation stands out as more versatile than the use of adsorbents that require regeneration by use of chemicals (Kartal and Imamura, 2005). During regeneration, if the metals are not captured for recycling in CCA, the process may lead to further environmental degradation. Comparative studies on various biosorbents for removal of CCA components from aqueous solutions are summarized in Table 4.
Nanotechnology has been widely investigated for synthesis of highly efficient adsorbents for heavy metal removal. Iron oxide nanoparticles strongly adsorb arsenic and chromium due to their enhanced metal-binding capacity, improving the overall efficiency of adsorbents (Chowdhury and Yanful, 2010). Boruah et al. (2023) conducted a comprehensive literature review on iron-based nanomaterials for arsenic removal by looking at removal efficiency, factors influencing adsorption, and adsorbent regeneration capacity. The findings showed that magnetite and bimetallic nanomaterials are particularly effective for removing both Arsenic (III) and Arsenic (V). The findings corroborate the work of Parajuli et al. (2020) where magnetite nanoparticles derived from extracts of A. indica adsorbed As(V) successfully in the form of H2AsO4− through electrostatic attraction at low pH. Additionally, zero-valent iron nanoparticles have been shown to be efficient in removal of chromium (VI) with further benefits in reduction of chromium (VI) to Cr (III) in the presence of organic acids such as citric acid (Yang et al., 2017; Zhou et al., 2018).
Green synthesis, devoid of chemical use, presents a futuristic approach and departure from chemical synthesis in the production of nanoparticles for heavy metal adsorption. Plants contain numerous reducing agents and are considered as the main factory for green synthesis (Shafey, 2020). Co-precipitation of FeCl3.6H2O and FeSO4.7H2O using A. indica leaves extract was applied in the green synthesis of magnetite (Fe3O4) nanoparticles (MNPs) in an inert environment, where the molar ratio of the iron salts was 2:1. The maximum adsorption capacity of the MNPs was 62.89 mg/g at pH 2 for As(V) (Parajuli et al., 2020). A comparative study on removal of arsenic from water using iron oxide nanoparticles synthesized through a green procedure utilizing five different leaf extracts is reported by Kamath et al. (2020). The researchers used the following leaves for green synthesis; black tea leaves (Camellia sinensis), oak tree leaves (Quercus viriniana), green tea leaves (C. sinensis), pomegranate leaves (Punica granatum) and eucalyptus leaves (Eucalyptus globulus) reporting adsorption capacities of 18.98, 32.05, 13.70, 11.65 and 39.84 mg/g, respectively. This study introduced unprecedented oak leaves as potential adsorbents in green synthesis with a very high adsorption capacity that can be useful in nanotechnology. Vaseghi et al. (2019) explored a simple method for the removal of chromium and copper from aqueous solutions through a simultaneous process of bio-reduction and adsorption using Eryngium campestre leaf extract. In the study, nanoparticles were produced by reduction of metal ions by the phytochemicals (mainly phenolic acids and flavonoids) found in the leaf extract achieving removal efficiencies of 98.92% and 98.16% for Cu and Cr at pH 7, respectively. Incorporation of green synthesis in nanotechnology therefore provides a synergistic approach in adsorption of Cr(VI), Cu(II) and As(V), presenting a promising avenue for future research.
Several arsenic free alternatives for CCA have been developed with copper as the primary active component. This is because copper provides the best fungicide protection and exhibits low mammalian toxicity. These alternatives include: acid copper chromate, alkaline copper quat, ammoniacal copper citrate, copper azole, copper dimethyldithio-carbamate, copper HDO, and borates. Since they lack arsenic in their formulation, these alternatives have an advantage of eliminating leaching of arsenic, which is of great concern due to its toxicity in the environment. However, wood treated with these alternatives is 10%–30% more costly than CCA treated wood due to the high cost of the chemicals. In addition, with the exception of acid copper chromate, these alternatives have a propensity of corrosiveness to metal fasteners compared to CCA Leaching of these CCA alternatives has also been reported, with the release of copper being higher or greater than in the CCA chemical. This is attributed to the higher proportion of copper in the CCA alternatives. Nevertheless, presence of copper and co-biocides into the environment is of less concern to environmentalists, since the associated mammalian toxicity is lower as opposed to arsenic in CCA (Lebow, 2004; Solo-gabriele et al., 2004). A study by Belizário et al. (2023) reported Micronized Copper Azol type C (MCA-C), which consists of tebuconazole, propiconazole, and micronized copper to be a potential alternative to CCA. The MCA-C alternative does not contain chromium or arsenic and therefore offers a suitable choice for wood preservative. Other possible CCA alternatives include; creosote, copper naphthenate, pentachlorophenol and ammoniacal copper zinc arsenate (Mankowski et al., 2023).
CCA elements in the environment is a major concern due to the associated risk of human and animal exposure. Therefore, the management of CCA waste is in tandem with the spirit of sustainable development goals (SDGs). Several remediation strategies have been highlighted in this review including chemical remediation, bioremediation, pyrolysis, immobilization, phytoremediation and adsorption. Despite the high efficiencies displayed by chemical remediation, it presents a major drawback since it is associated with secondary contaminants. On the other hand, pyrolysis as a way of disposal of wood waste could be a better alternative since it produces waste that is manageable but the energy requirements are too high. Recycling of CCA treated wood in what is referred to as waste-to-energy process as well as conversion of the wood waste to wood chips is a promising route of dealing with CCA treated wood for sustainable development. These processes lead to reduction of space occupied by waste that can be utilized for agriculture. Use of landfills is also greatly reduced thus abating underground water contamination as well production of useful energy that can be converted into different forms.
The use of microorganisms in bioremediation is a viable approach as they do not introduce secondary pollutants to the environment. Additionally, their ability to generate organic acids enhances the solubility of heavy metals, thereby facilitating the removal of the contaminants. However, the efficiency of microorganisms typically manifests after several days, making this method time-intensive. Furthermore, their application may result in the transformation of wood constituents. Bioadsorbents offer an effective and sustainable alternative for the removal of heavy metals from the environment due to their eco-friendly nature and low cost, presenting a promising approach to heavy metal remediation. Their natural abundance ensures a reliable and continuous supply, and their non-toxic properties prevent the introduction of harmful chemicals into the environment, making them a safer and more sustainable option. However, their disposal after use presents a major challenge. Given that each remediation technique has its own drawbacks and the increasing need for sustainable solutions, a synergistic approach that integrates multiple strategies for the removal of chromium, copper and arsenic should be the future focus in addressing CCA contamination.
SA: Writing–original draft. EN: Conceptualization, Funding acquisition, Supervision, Writing–review and editing. VO: Conceptualization, Funding acquisition, Supervision, Writing–review and editing.
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Machakos University internal research grant awarded to Veronica A. Okello and Elizabeth N. Ndunda.
The authors express their gratitude to Machakos University for the award of the internal research grant that supported this work. In addition, Sharolyne Atiang’ is grateful for Machakos University Chancellor’s scholarship that has supported her tuition fee.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbas, N., Butt, M. T., Ahmad, M. M., and Deeba, F. (2021). Phytoremediation potential of Typha latifolia and water hyacinth for removal of heavy metals from industrial wastewater. Chem. Int. 7 (2), 103–111. doi:10.5281/zenodo.4559406
Abd El-Fatah, S., Goto, M., Kodama, A., and Hirose, T. (2004). Supercritical fluid extraction of hazardous metals from CCA wood. J. Supercrit. Fluids 28 (1), 21–27. doi:10.1016/S0896-8446(03)00005-6
Adamczuk, A., and Kołodyńska, D. (2015). Equilibrium, thermodynamic and kinetic studies on removal of chromium, copper, zinc and arsenic from aqueous solutions onto fly ash coated by chitosan. Chem. Eng. J. 274, 200–212. doi:10.1016/j.cej.2015.03.088
Adedeji, G. A., David, I. O., Onakpoma, I., and Aiyeloja, A. A. (2023). Contamination: insights from an empirical evaluation of soil and grass around CCA-treated wooden electric poles in a Nigerian university. Afr. J. Agric. Technol. Environ. 12 (2), 73–81.
Ajibade, F. O., Aderian, K. A., and K, E. C. (2013). Phytoremediation efficiencies of water hyacinth in removing heavy metals phytoremediation efficiencies of water hyacinth in removing heavy metals in domestic sewage (A case study of university of ilorin, Nigeria). Int. J. Eng. Sci. (IJES) 2 (2), 16–27. doi:10.6084/m9.figshare.940965
Alessandro, V. (2019). The remediation of arsenic in chromated copper arsenate contaminated soil using natural and modified zeolite. Sacramento: California State University.
Amanda, N., and Moersidik, S. S. (2019). Characterization of sludge generated from acid mine drainage treatment plants. J. Phsyics Conf. Ser. 1351, 012113–012116. doi:10.1088/1742-6596/1351/1/012113
Ambi, A. A., Isa, M. T., Ibrahim, A. B., Bashir, M., Ekwuribe, S., and Sallau, A. B. (2020). Hexavalent chromium bioremediation using Hibiscus Sabdariffa calyces extract: process parameters, kinetics and thermodynamics. Sci. Afr. 10, e00642–e00649. doi:10.1016/j.sciaf.2020.e00642
Arriaga, F., Wang, X., Íñiguez-González, G., Llana, D. F., Esteban, M., and Niemz, P. (2023). Mechanical properties of wood: a review. Forests 14 (6), 1202. doi:10.3390/f14061202
Babaee, Y., Mulligan, C. N., and Rahaman, M. S. (2017). Arsenic immobilization in soil using starch-stabilized Fe/Cu nanoparticles: a case study in treatment of a chromated copper arsenate (CCA)-contaminated soil at lab scale. J. Soils Sediments 18 (4), 1610–1619. doi:10.1007/s11368-017-1882-2
Beiyuan, J., Lau, A. Y. T., Tsang, D. C. W., Zhang, W., Kao, C., Baek, K., et al. (2018). Chelant-enhanced washing of CCA-contaminated soil: coupled with selective dissolution or soil stabilization. Sci. Total Environ. 612, 1463–1472. doi:10.1016/j.scitotenv.2017.09.015
Belizário, A. C., Oliveira Lopes de, F., Icimoto, I. H., and Vairo, M. (2023). Performance comparison between preservative products concerning termites attack on pine timber. Rev. Árvore 47, e4724. doi:10.1590/1806-908820230000024
Bhat, P., Jain, N., Naik, N., Samrot, A. V., Pai, J. B., and Salmataj, S. A. (2023). Adsorptive removal of chromium from simulated industrial waste water using jungle geranium-derived biosorbents. ES Mater. Manuf. 22, 1–16. doi:10.30919/esmm1070
Bolin, C. A., and Smith, S. T. (2013). Life cycle assessment of CCA-treated wood highway guard rail posts in the US with comparisons to galvanized steel guard rail posts. J. Transp. Technol. 3, 58–67. doi:10.4236/jtts.2013.31007
Boruah, H., Tyagi, N., Gupta, S. K., Chabukdhara, M., and Malik, T. (2023). Understanding the adsorption of iron oxide nanomaterials in magnetite and bimetallic form for the removal of arsenic from water. Front. Environ. Chem. 11, 1104320. doi:10.3389/fenvs.2023.1104320
Can, A., and Sivrikaya, H. (2022). Removal of copper, chromium, and arsenic from CCA-treated wood using glycerol/choline chloride deep eutectic solvent. Environ. Sci. Proc. 22 (1), 1–7. doi:10.3390/iecf2022-13037
CCME (2007). “Canadian soil quality guidelines for the protection of environmental and human health,” in Canadian council of ministries of the environment. 100719 Contents.
Chang, Y. C., Choi, D. B., and Kikuchi, S. (2012). Enhanced extraction of heavy metals in the two-step process with the mixed culture of Lactobacillus bulgaricus and Streptococcus thermophilus. Bioresour. Technol. 103 (1), 477–480. doi:10.1016/j.biortech.2011.09.059
Choi, Y. S., Kim, J. J., Kim, M. J., Imamura, Y., Yoshimura, T., and Kim, G. H. (2012). Fungal biodegradation of CCA-treated wood and removal of its metal components. Chemosphere 88 (6), 725–729. doi:10.1016/j.chemosphere.2012.03.062
Chowdhury, S. R., and Yanful, E. K. (2010). Arsenic and chromium removal by mixed magnetite-maghemite nanoparticles and the effect of phosphate on removal. J. Environ. Manag. 91 (11), 2238–2247. doi:10.1016/j.jenvman.2010.06.003
Clausen, C. A. (2000). Isolating metal-tolerant bacteria capable of removing copper, chromium, and arsenic from treated wood. Waste Manag. Res. 18 (3), 264–268. doi:10.1034/j.1399-3070.2000.00128.x
Clovis, M., Machumi, F., and Innocent, E. (2020). Assessment of heavy metals in Hibiscus sabdariffa calyces and Moringa oleifera leaves collected from different areas in Tanzania. J. Ecobiotechnology 12, 17–21. doi:10.25081/jebt.2020.v12.6549
Coles, C. A., Arisi, J. A., Organ, M., and Veinott, G. I. (2014). Leaching of chromium, copper and arsenic from CCA-treated utility Poles. Appl. Environ. Soil Sci. 2014, 1–11. doi:10.1155/2014/167971
Costa, L. G. da, Brocco, V. F., Paes, J. B., Kirker, G. T., and Bishell, A. B. (2022). Biological and chemical remediation of CCA treated eucalypt poles after 30 years in service. Chemosphere 286 (1), 131629. doi:10.1016/j.chemosphere.2021.131629
Elmira, K. (2023). Kinetics study of oxalic acid remediation of chromated copper arsenate (CCA) -treated timber for potential re-use [University of New South Wales]. doi:10.26190/unsworks/24929
Frick, H., Tardif, S., Kandeler, E., Holm, P. E., and Brandt, K. K. (2019). Assessment of biochar and zero-valent iron for in-situ remediation of chromated copper arsenate contaminated soil. Sci. Total Environ. 655, 414–422. doi:10.1016/j.scitotenv.2018.11.193
Frighetto, S., Souza, H., Gampert, L., Maria, C., Azevedo, N., Maria, S., et al. (2016). Decontamination of CCA-treated eucalyptus wood waste by acid leaching. Waste Manag. 49, 253–262. doi:10.1016/j.wasman.2016.01.031
Gardner, D., Weindorf, D. C., and Flynn, M. (2013). Presence of chromium, copper, and arsenic in schoolyard soils. Soil Horizons 54 (2), 0–5. doi:10.2136/sh12-12-0032
Garg, R., Garg, R., Sillanpää, M., Alimuddin, Khan, M. A., Mubarak, N. M., Tan, Y. H., et al. (2023). Rapid adsorptive removal of chromium from wastewater using walnut-derived biosorbents. Sci. Rep. 13 (1), 6859. doi:10.1038/s41598-023-33843-3
Gezer, E. D., Yildiz, U., Yildiz, S., Dizman, E., and Temiz, A. (2006). Removal copper, chromium and arsenic from CCA-treated yellow pine by oleic acid. Build. Environ. 41 (3), 380–385. doi:10.1016/j.buildenv.2005.02.014
Giri, D. D., Jha, J. M., Srivastava, N., Hashem, A., Abd-Allah, E. F., Shah, M., et al. (2022). Sustainable removal of arsenic from simulated wastewater using solid waste seed pods biosorbents of Cassia fistula L. Chemosphere 287 (3), 132308. doi:10.1016/j.chemosphere.2021.132308
Gmar, M., Bouafif, H., Bouslimi, B., Braghiroli, F. L., and Koubaa, A. (2022). Pyrolysis of chromated copper arsenate-treated wood: investigation of temperature, granulometry, biochar yield, and metal pathways. Energies 15 (14), 5071. doi:10.3390/en15145071
Gogoi, P., Adhikari, P., and Maji, T. K. (2017). Bioremediation of arsenic from water with citric acid cross-linked water hyacinth (E. crassipes) root powder. Environ. Monit. Assess. 189 (8), 383. doi:10.1007/s10661-017-6068-2
Gosselin, M., and Zagury, G. J. (2020). Metal(loid)s inhalation bioaccessibility and oxidative potential of particulate matter from chromated copper arsenate (CCA)-contaminated soils. Chemosphere 238, 124557. doi:10.1016/j.chemosphere.2019.124557
Hall, N. L., and Beder, S. (2005). Treated timber, ticking time-bomb. The need for a precautionary approach to the use of copper chrome arsenate (CCA) as a timber preservative. New South Wales, Australia: University of Wollongong. Available online at: http://www.pc.gov.au/__data/assets/pdf_file/0013/21811/sub017.pdf.
Hassoune, J., Tahiri, S., El Krati, M., Luisa Cervera, M., and de la Guardia, M. (2018). Removal of hexavalent chromium from aqueous solutions using biopolymers. J. Environ. Eng. 144 (8), 04018060. doi:10.1061/(asce)ee.1943-7870.0001396
Huynh, A., Chen, Y., and Tran, B. N. T. (2021). A small-scale study on removal of heavy metals from contaminated water using water hyacinth. Processes 9, 1802. doi:10.3390/pr9101802
Illman, B. L., and Yang, V. W. (2004). Bioremediation and degradation of CCA-treated wood waste wood decay fungi isolation and culture conditions (issue september). Available online at: https://www.fs.usda.gov/research/treesearch/6360.
Jambeck, J., Weitz, K., Solo-gabriele, H., Townsend, T., and Thorneloe, S. (2007). CCA-Treated wood disposed in landfills and life-cycle trade-offs with waste-to-energy and MSW landfill disposal. Waste Manag. 27, 21–28. doi:10.1016/j.wasman.2007.02.011
Janin, A., Blais, J.-F., Mercier, G., Drogui, P., and Kervella, H. (2012). CCA-treated wood waste remediation process optimization with successive recirculation loops study. J. Environ. Eng. 138 (2), 200–207. doi:10.1061/(asce)ee.1943-7870.0000474
Jeyaseelan, C., and Gupta, A. (2016). Green tea leaves as a natural adsorbent for the removal of Cr(VI) from aqueous solutions. Air, Soil Water Res. 9, ASWR.S35227–19. doi:10.4137/ASWR.S35227
Jones, A. S., Marini, J., Solo-gabriele, H. M., Robey, N. M., and Townsend, T. G. (2019). Arsenic, copper, and chromium from treated wood products in the U.S. disposal sector. Waste Manag. 87, 731–740. doi:10.1016/j.wasman.2019.03.004
José de Castro, R., Kehl, G. D., Candaten, L., Dos Santos, G. D., da Silva, P. R. B., Trevisan, R., et al. (2021). Electro-removal of copper, chromium, and arsenic from chromated copper arsenate treated waste wood. Eng. Sanit. Ambient. 26 (2), 211–219. doi:10.1590/s1413-415220190319
Junk, G. S. (2022). Recovery of Arsenic from CCA-treated timber (issue march). Christchurch, New Zealand: University of Canterbury.
Kamath, V., Chandra, P., and Jeppu, G. P. (2020). Comparative study of using five different leaf extracts in the green synthesis of iron oxide nanoparticles for removal of arsenic from water. Int. J. Phytoremediation 22 (12), 1278–1294. doi:10.1080/15226514.2020.1765139
Kartal, S. N. (2003). Removal of copper, chromium, and arsenic from CCA-C treated wood by EDTA extraction. Waste Manag. 23 (6), 537–546. doi:10.1016/s0956-053x(02)00143-5Available online at: http://www.ncbi.nlm.nih.gov/pubmed/12909094.
Kartal, S. N., and Imamura, Y. (2005). Removal of copper, chromium, and arsenic from CCA-treated wood onto chitin and chitosan. Bioresour. Technol. 96 (3), 389–392. doi:10.1016/j.biortech.2004.03.004
Kartal, S. N., Munir, E., Kakitani, T., and Imamura, Y. (2004). Bioremediation of CCA-treated wood by brown-rot fungi Fomitopsis palustris, Coniophora puteana, and Laetiporus sulphureus. J. Wood Sci. 50 (2), 182–188. doi:10.1007/s10086-003-0544-8
Kato, T., Hatakeyama, T., and Sugawara, K. (2021). Release behavior of arsenic, chromium, and copper during heat treatments of CCA-treated wood. J. Material Cycles Waste Manag. 23 (4), 1636–1645. doi:10.1007/s10163-021-01246-z
Khaodhiar, S., Azizian, M. F., Osathaphan, K., and Nelson, P. O. (2000). Copper, chromium, and arsenic adsorption and equilibrium modeling in an iron-oxide-coated sand, background electrolyte system. Water, Air, Soil Pollut. 119 (1–4), 105–120. doi:10.1023/A:1005109325539
Kilpi-Koski, J., Penttinen, O. P., Väisänen, A. O., and van Gestel, C. A. M. (2019). An uptake and elimination kinetics approach to assess the bioavailability of chromium, copper, and arsenic to earthworms (Eisenia andrei) in contaminated field soils. Environ. Sci. Pollut. Res. 26 (15), 15095–15104. doi:10.1007/s11356-019-04908-6
KPLC (2014). Specif. Treat. Wood Poles. Part 1 Eucalyptus Poles, 1–13. Available online at: https://kplc.co.ke/img/full/P0rYKJZKGTVn_specificationfortreatedwoodpolesPart1Eucalyptuspoles.pdf.
Kumar, U., Singh, R. S., Mandal, J., Nayak, A. K., and Jha, A. K. (2022). Removal of As(III) and Cr(VI) from aqueous solutions by Bixa orellana leaf biosorbent and As(III) removal using bacterial isolates from heavy metal contaminated site. J. Indian Chem. Soc. 99 (5), 100334. doi:10.1016/j.jics.2021.100334
Kumpiene, J., Nordmark, D., Hamberg, R., Carabante, I., Simanavičienė, R., and Aksamitauskas, V. Č. (2016). Leaching of arsenic, copper and chromium from thermally treated soil. J. Environ. Manag. 183, 460–466. doi:10.1016/j.jenvman.2016.08.080
Lebow, S. (2004). Alternatives to chromated copper arsenate for residential construction. Available online at: https://www.fs.usda.gov/research/treesearch/6362.
Li, C., Yang, G., Liu, Z., and Cai, J. (2022). Overview of phytoremediation technology for heavy metal contaminated soil. Int. Conf. Environ. Renew. Energy Green Chem. Eng. 350, 01006–1015. doi:10.1051/e3sconf/202235001006
Lissy, A. M. P. N., and Madhu, G. (2011). Removal of heavy metals from waste water using water hyacinth. ACEEE Int. J. Transp. Urban Dev. 1 (1), 48–52.
Lopes, D. J. V., Stokes, C. E., and Bobadilha, G. dos S. (2019). The use of chemical and biological agents in the recovery of heavy metals from treated woods - a brief review. BioResources 14 (1), 2287–2299. doi:10.15376/biores.14.1.Lopes
Madhuranthakam, C. M. R., Thomas, A., Akhter, Z., Fernandes, S. Q., and Elkamel, A. (2021). Removal of chromium(VI) from contaminated water using untreated moringa leaves as biosorbent. Pollutants 1, 51–64. doi:10.3390/pollutants1010005
Mankowski, M., Kirker, G., and Shmulsky, R. (2023). Timber bridges. Mississippi, United States: Mississippi State University, SB923, 56. Available online at: https://www.fwrc.msstate.edu/pubs/TimberBridges_SB923.pdf
Matos, R. C., Vieira, C., Morais, S., Pereira, M. L., and Pedrosa, J. (2010). Toxicity of chromated copper arsenate: a study in mice. Environ. Res. 110 (5), 424–427. doi:10.1016/j.envres.2010.03.001
Mercer, T. G., and Frostick, L. E. (2012). Leaching characteristics of CCA-treated wood waste: a UK study. Sci. Total Environ. 427–428, 165–174. doi:10.1016/j.scitotenv.2012.04.008
Moghaddam, A. H., and Mulligan, C. N. (2008). Leaching of heavy metals from chromated copper arsenate (CCA) treated wood after disposal. Waste Manag. 28 (3), 628–637. doi:10.1016/j.wasman.2007.03.009
Mohajerani, A., Vajna, J., and Ellcock, R. (2018). Chromated copper arsenate timber: a review of products, leachate studies and recycling. J. Clean. Prod. 179, 292–307. doi:10.1016/j.jclepro.2018.01.111
Mohammed, A.-R., Süleyman, K., and Yüksek, T. (2022). Literature review: the role of earthworms in bioremediation of treated wood. Wood Industry Eng. 4 (1), 16–31.
Morais, S., Fonseca, H. M. A. C., Oliveira, S. M. R., Oliveira, H., Gupta, V. K., Sharma, B., et al. (2021). Environmental and health hazards of chromated copper arsenate-treated wood: a review. Int. J. Environ. Res. Public Health 18 (11), 5518. doi:10.3390/ijerph18115518
Muthike, G., and Ali, G. (2021). Concrete vs Wooden Poles - effects of the shift to concrete poles on tree growers (Issue 49). Available online at: https://www.kefri.org/assets/publications/articles/CONCRETEVSWOODENPOLES.pdf.
Nagra, M. A., Natasha, N., Bibi, I., Tariq, T., Naz, R., Ansar, S., et al. (2023). Biowaste-based sorbents for arsenic removal from aqueous medium and risk assessment. Environ. Geochem Health 45, 9017–9028. doi:10.1007/s10653-022-01402-w
Nakiguli, C. K., Ojok, W., Omara, T., Wasswa, J., and Ntambi, E. (2020). Mobility of chromium, copper and arsenic in amended chromated copper arsenate contaminated soils. Asian J. Appl. Chem. Res. 6 (4), 33–48. doi:10.9734/ajacr/2020/v6i430168
Nelson, J., Pickering, K. L., and Beg, M. D. H. (2023). Assessment of the potential of waste copper chromium and arsenic (CCA) -treated timber fibre reinforced polypropylene composites for construction. J. Compos. Sci. 7 (2), 48–68. doi:10.3390/jcs7020048
Okello, V. A., Mwilu, S., Noah, N., Zhou, A., Chong, J., Knipfing, M. T., et al. (2012). Reduction of hexavalent chromium using naturally-derived flavonoids. Environ. Sci. Technol. 46, 10743–10751. doi:10.1021/es301060q
Oo, C. W., Kassim, M. J., and Pizzi, A. (2009). Characterization and performance of Rhizophora apiculata mangrove polyflavonoid tannins in the adsorption of copper (II) and lead (II). Industrial Crops Prod. 30 (1), 152–161. doi:10.1016/j.indcrop.2009.03.002
Oppong, E., Nnuro, W. A., Ofori, I., and Amaniampong, A. (2021). Metal leaching from chromated copper arsenate (CCA)-Treated wood: implications on the environment. Int. Res. Material Environ. 1 (1), 14–52.
Opuru, F. E., Kibet, J. K., and Verdi, N. C. (2020). Biotoxic stratification of arsenic: chromated and ammoniacal arsenicals contamination of soil and ground water (river elbergon)-Kenya. IJAED J. Inorg. Chem. 1 (2), 1–10. Available online at: https://www.ijaed.com/journal/index.php/ijaed/article/view/15.
Palma-Lara, I., Martínez-Castillo, M., Quintana-Pérez, J. C., Arellano-Mendoza, M. G., Tamay-Cach, F., Valenzuela-Limón, O. L., et al. (2020). Arsenic exposure: a public health problem leading to several cancers. Regul. Toxicol. Pharmacol. 110, 104539–104638. doi:10.1016/j.yrtph.2019.104539
Panneerselvam, B., and Priya, S. K. (2023). Phytoremediation potential of water hyacinth in heavy metal removal in chromium and lead contaminated water. Int. J. Environ. Anal. Chem. 103 (13), 3081–3096. doi:10.1080/03067319.2021.1901896
Parajuli, K., Sah, A. K., and Paudyal, H. (2020). Green synthesis of magnetite nanoparticles using aqueous leaves extracts of Azadirachta indica and its application for the removal of as(V) from water. Green Sustain. Chem. 10 (04), 117–132. doi:10.4236/gsc.2020.104009
Pasgar, A., Nasiri, A., and Javid, N. (2022). Single and competitive adsorption of Cu2+ and Pb2+ by tea pulp from aqueous solutions. Environ. Health Eng. Manag. 9 (1), 65–74. doi:10.34172/EHEM.2022.08
Patel, H. (2020). Batch and continuous fixed bed adsorption of heavy metals removal using activated charcoal from neem (Azadirachta indica) leaf powder. Sci. Rep. 10 (1), 16895–16912. doi:10.1038/s41598-020-72583-6
Priya, A. K., Muruganandam, M., Ali, S. S., and Kornaros, M. (2023). Clean-up of heavy metals from contaminated soil by phytoremediation: a multidisciplinary and eco-friendly approach. Toxics 11 (5), 422. doi:10.3390/toxics11050422
Qasem, N. A. A., Mohammed, R. H., and Lawal, D. U. (2021). Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean. Water 4, 36. doi:10.1038/s41545-021-00127-0
Rahman, A., Haque, M. A., Ghosh, S., Shinu, P., Attimarad, M., and Kobayashi, G. (2023). Modified shrimp-based chitosan as an emerging adsorbent removing heavy metals (chromium, nickel, arsenic, and cobalt) from polluted water. Sustainability 15, 2431. doi:10.3390/su15032431
Rajurkar, N. S., Gokarn, A. N., and Dimya, K. (2011). Adsorption of Chromium(III), Nickel(II), and Copper(II) from aqueous solution by activated alumina. Clean. - Soil, Air, Water 39 (8), 767–773. doi:10.1002/clen.201000273
Saikia, J., Saha, B., and Das, G. (2011). Efficient removal of chromate and arsenate from individual and mixed system by malachite nanoparticles. J. Hazard. Mater. 186 (1), 575–582. doi:10.1016/j.jhazmat.2010.11.036
Sarkar, M., Rahman, A. K. M. L., and Bhoumik, N. C. (2017). Remediation of chromium and copper on water hyacinth (E. crassipes) shoot powder. Water Resour. Industry 17, 1–6. doi:10.1016/j.wri.2016.12.003
Shafey, A. M.El. (2020). Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: a review. Green Process. Synthesis 9 (1), 304–339. doi:10.1515/gps-2020-0031
Shah, V., and Daverey, A. (2020). Phytoremediation: a multidisciplinary approach to clean up heavy metal contaminated soil. Environ. Technol. and Innovation 18, 100774. doi:10.1016/j.eti.2020.100774
Shaibur, M. R., Tanzia, F. K. S., Nishi, S., Nahar, N., Parvin, S., and Adjadeh, T. A. (2022). Removal of Cr (VI) and Cu (II) from tannery effluent with water hyacinth and arum shoot powders: a study from Jashore, Bangladesh. J. Hazard. Mater. Adv. 7, 100102. doi:10.1016/j.hazadv.2022.100102
Shanker, A. K., Cervantes, C., Loza-Tavera, H., and Avudainayagam, S. (2005). Chromium toxicity in plants. Environ. Int. 31 (5), 739–753. doi:10.1016/j.envint.2005.02.003
Sharma, P., Singh, S. P., Parakh, S. K., and Tong, Y. W. (2022). Health hazards of hexavalent chromium (Cr (VI)) and its microbial reduction. Bioengineered 13 (3), 4923–4938. doi:10.1080/21655979.2022.2037273
Sheikh, Z., Amin, M., Khan, N., Khan, M. N., Sami, S. K., Khan, S. B., et al. (2021). Potential application of Allium Cepa seeds as a novel biosorbent for efficient biosorption of heavy metals ions from aqueous solution. Chemosphere 279, 130545. doi:10.1016/j.chemosphere.2021.130545
Singh, B. S. M., Singh, D., and Dhal, N. K. (2022). Enhanced phytoremediation strategy for sustainable management of heavy metals and radionuclides. Case Stud. Chem. Environ. Eng. 5, 100176. doi:10.1016/j.cscee.2021.100176
Solo-gabriele, H. M., Townsend, T. G., and Cai, Y. (2004). Environmental impacts of CCA-treated wood within Florida, USA environmental impacts of CCA-treated wood within Florida, USA. Available online at: https://www.irg-wp.com/irgdocs/details.php?ea5fde9b-3da5-403a-a7c5-006256cf9d5a.
Solo-gabriele, H. M., Townsend, T. G., Messick, B., and Calitu, V. (2002). Characteristics of chromated copper arsenate-treated wood ash. J. Hazard. Mater. 28 (3), 213–232. doi:10.1016/s0304-3894(01)00311-9
Song, J., Dubey, B., Jang, Y., Townsend, T., and Solo-gabriele, H. (2006). Implication of chromium speciation on disposal of discarded CCA-treated wood. J. Hazard. Mater. 128, 280–288. doi:10.1016/j.jhazmat.2005.08.004
Stern, A. H. (2010). A quantitative assessment of the carcinogenicity of hexavalent chromium by the oral route and its relevance to human exposure. Environ. Res. 110 (8), 798–807. doi:10.1016/j.envres.2010.08.002
Tak, H., Kim, S., Kim, K., Wang, S., and Lee, M. (2023). Stabilization of as and heavy metal-contaminated soils by two mine drainage-treated sludges. Minerals 13 (2), 148. doi:10.3390/min13020148
Tokay, B., and Akpınar, I. (2021). A comparative study of heavy metals removal using agricultural waste biosorbents. Bioresour. Technol. Rep. 15, 100719. doi:10.1016/j.biteb.2021.100719
Townsend, T., Tolaymat, T., Solo-Gabriele, H., Dubey, B., Stook, K., and Wadanambi, L. (2004). Leaching of CCA-treated wood: implications for waste disposal. J. Hazard. Mater. 114, 75–91. doi:10.1016/j.jhazmat.2004.06.025
Uddin, A. F. M. J., Manirul, M. I., Mayeda, U., Roni, M. Z. K., and Mehraj, H. (2015). Evaluation of pteris vittata as trap plant to mitigate arsenic from arsenic contaminated soil. J. Sci. Technol. and Environement Inf. 1 (2), 75–80. doi:10.18801/jstei.010215.09
Usman, A. R. A., Lee, S. S., Awad, Y. M., Lim, K. J., Yang, J. E., and Ok, Y. S. (2012). Soil pollution assessment and identification of hyperaccumulating plants in chromated copper arsenate (CCA) contaminated sites, Korea. Chemosphere 87 (8), 872–878. doi:10.1016/j.chemosphere.2012.01.028
Uwumarongie-Ilori, E. G., and Okieimen, F. (2010). Extractive decontamination of heavy metals from CCA contaminated soil using organic acids. Afr. J. Environ. Sci. Technol. 4 (9), 567–576. doi:10.4314/ajest.v4i9.71313
Vaseghi, Z., Nematollahzadeh, A., and Tavakoli, O. (2019). Plant-mediated Cu/Cr/Ni nanoparticle formation strategy for simultaneously separation of the mixed ions from aqueous solution. J. Taiwan Inst. Chem. Eng. 96, 148–159. doi:10.1016/j.jtice.2018.10.020
Vignati, D. A. L., Dominik, J., Beye, M. L., Pettine, M., and Ferrari, B. J. D. (2010). Chromium(VI) is more toxic than chromium(III) to freshwater algae: a paradigm to revise? Ecotoxicol. Environ. Saf. 73 (5), 743–749. doi:10.1016/j.ecoenv.2010.01.011
Villegas, M. C. A., and Zagury, G. J. (2023). Incorporating oral, inhalation and dermal bioaccessibility into human health risk characterization following exposure to Chromated Copper Arsenate (CCA)-contaminated soils. Ecotoxicol. Environ. Saf. 249 (July 2022), 114446. doi:10.1016/j.ecoenv.2022.114446
Wang, Q., Zhu, S., Xi, C., and Zhang, F. (2022). A review: adsorption and removal of heavy metals based on polyamide-amines composites. Front. Chem. 10, 814643. doi:10.3389/fchem.2022.814643
Wasson, S. J., Linak, W. P., Gullett, B. K., King, C. J., Touati, A., Huggins, F. E., et al. (2005). Emissions of chromium, copper, arsenic, and PCDDs/Fs from open burning of CCA-treated wood. Environ. Sci. Technol. 39 (22), 8865–8876. doi:10.1021/es050891g
WHO (2011). Guidelines for drinking-water quality. Geneva, Switzerland: World Health Organization, 564. Available online at: https://www.who.int/publications/i/item/9789240045064.
Wu, L., Punia, S., and Khodadoust, A. P. (2016). Removal of chromium, copper, and arsenic from contaminated water using manganese oxide based adsorbents. Geo-Chicago 273, 62–69. doi:10.1061/9780784480168.007
Xiao, F., Cheng, J., Cao, W., Yang, C., Chen, J., and Luo, Z. (2019). Removal of heavy metals from aqueous solution using chitosan-combined magnetic biochars. J. Colloid Interface Sci. 540, 579–584. doi:10.1016/j.jcis.2019.01.068
Xing, D., Magdouli, S., Zhang, J., Bouafif, H., and Koubaa, A. (2023). A comparative study on heavy metal removal from CCA-treated wood waste by Yarrowia lipolytica: effects of metal stress. J. Fungi 9 (4), 469. doi:10.3390/jof9040469
Xing, D., Magdouli, S., Zhang, J., and Koubaa, A. (2020). Microbial remediation for the removal of inorganic contaminants from treated wood: recent trends and challenges. Chemosphere 258, 127429. doi:10.1016/j.chemosphere.2020.127429
Xu, Y., Xia, W., Hou, H., Zhang, J., and Qian, G. (2017). Remediation of chromium-contaminated soil by electrokinetics and electrokinetics coupled with CaAl-LDH permeable reaction barrier. Environ. Sci. Pollut. Res. 24, 20479–20486. doi:10.1007/s11356-017-9705-y
Yang, J., Zhong, L., and Liu, L. (2017). Chromium (VI) reduction in the nano- or micron-sized iron oxide - citric acid systems: kinetics and mechanisms. J. Environ. Chem. Eng. 5 (3), 2564–2569. doi:10.1016/j.jece.2017.05.011
Yanitch, A., Kadri, H., Frenette-Dussault, C., Joly, S., Pitre, F. E., and Labrecque, M. (2020). A four-year phytoremediation trial to decontaminate soil polluted by wood preservatives: phytoextraction of arsenic, chromium, copper, dioxins and furans. Int. J. Phytoremediation 22 (14), 1505–1514. doi:10.1080/15226514.2020.1785387
Yeo, K. F. H., Li, C., Zhang, H., Chen, J., Wang, W., and Dong, Y. (2021). Arsenic removal from contaminated water using natural adsorbents: a review. Coatings 11 (11), 1407–1418. doi:10.3390/COATINGS11111407
Zeng, X., Jin, Q., Wang, P., and Huang, C. (2023). Distribution and speciation of heavy metal(loid)s in soils under multiple preservative-treated wooden trestles. Toxics 11 (3), 249. doi:10.3390/toxics11030249
Zeng, Y., Lin, Y., Ma, M., and Chen, H. (2024). A review on the removal of heavy metals from water by phosphorus-enriched biochar. Minerals 14 (1), 61. doi:10.3390/min14010061
Zhou, L., Li, R., Zhang, G., Wang, D., Cai, D., and Wu, Z. (2018). Zero-valent iron nanoparticles supported by functionalized waste rock wool for efficient removal of hexavalent chromium. Chem. Eng. J. 339, 85–96. doi:10.1016/j.cej.2018.01.132
Keywords: copper, chromium, arsenic, CCA, immobilization, bioremediation, biosorption
Citation: Atiang’ S, Ndunda EN and Okello VA (2025) Advances in removal of chromated copper arsenate elements in wood waste, contaminated water and soils. Front. Environ. Chem. 6:1452837. doi: 10.3389/fenvc.2025.1452837
Received: 21 June 2024; Accepted: 25 February 2025;
Published: 18 March 2025.
Edited by:
Benton Otieno, Vaal University of Technology, South AfricaReviewed by:
Xiaoming Wan, Chinese Academy of Sciences (CAS), ChinaCopyright © 2025 Atiang’, Ndunda and Okello. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Veronica A. Okello, dm9rZWxsb0Bta3N1LmFjLmtl
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.