
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Environ. Archaeol., 27 January 2025
Sec. Zooarchaeology
Volume 4 - 2025 | https://doi.org/10.3389/fearc.2025.1517380
This article is part of the Research TopicAquatic Transformations: Archaeozoology and Applied Historical Ecology in Wetland and Intertidal EcosystemsView all articles
Oysters are an almost ubiquitous presence in coastal archaeological sites globally. Southeast Queensland is no exception, with oysters frequently the dominant taxon in midden deposits. It has been estimated the total number of oysters at Booral Shell Mound in the Great Sandy Strait to be more than 5.9 million individuals. This paper moves beyond just the number of oysters to examine the structure of populations within the deposits at two Southeast Queensland sites, Booral Shell Mound and White Patch 3, from an Applied Historical Ecology approach. In doing so, the nature and sustainability of First Nations marine resource exploitation may be determined. Additionally, environmental factors influencing molluscan population dynamics can be elucidated. Historical accounts provide insights into observed collection practices in the early colonial period, as well as the persistence of First Nations oystering and other marine resource exploitation in the mid-late 19th century in response to participation in the wider economy of early Brisbane. Reasons for the late nineteenth-early Twentieth century collapse of Southeast Queensland oyster populations are examined and attempts to revive the oyster industry reviewed.
Coastal Southeast Queensland stretches from K'gari in the north, to the border of northern New South Wales in the south and possesses one of the best documented and intensively scrutinized coastal archaeological records in Australia (McNiven, 2006, p. 120; Ulm, 2002, p. 79; Figure 1), with approximately 2000 midden sites and artifact scatters recorded, although relatively few have been excavated and estimates of dated sites vary (e.g., Robins et al., 2015; Ulm, 2006; Ulm and Hall, 1996). Following a hiatus of some 20 years in new archaeological investigations in the region, there has in recent years been a rise in consultancy-based heritage studies and the numbers of dated sites have no doubt increased, but the information is confined to the gray literature and remain unreported publicly.
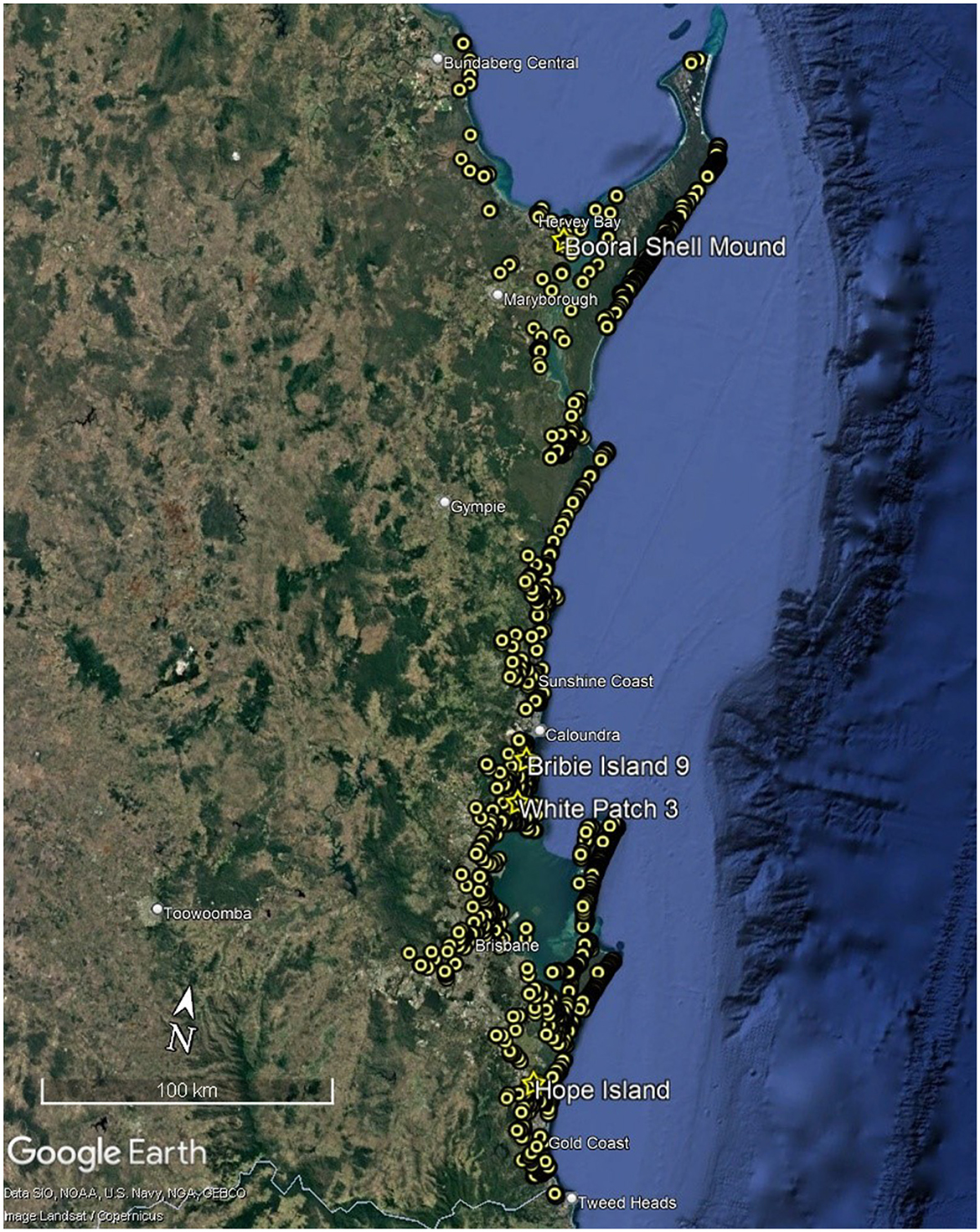
Figure 1. Recorded sites in Southeast Queensland including the sites mentioned in the text (Source Google Earth Pro 2024 and DATSIP database).
The earliest evidence for oystering in Southeast Queensland comes from Hope Island, a site near the mouth of the Coomera River (Figure 2) with a median age of 4,906 cal BP (Walters et al., 1987). Bribie Island 9 in the north of Bribie Island (Figure 2), has evidence of oystering from 3,465 cal BP (Smith, 1992). Excavated in 1987, the Hope Island assemblage was unavailable for further analysis, as the Kombumerri People of the Gold Coast region considered the most appropriate use of the material was as an interpretive feature at the Yugambeh Museum. Oyster was recovered from three stratigraphic units at Bribie Island 9 but was highly fragmented in two of them. The analyses presented here are of oyster assemblages from Booral Shell Mound in the Great Sandy Strait (Figure 2) which demonstrates occupation of a period of ~2,200 years, and White Patch 3 fronting Pumicestone Passage on Bribie Island (Figure 2) which was in use for ~170 years.
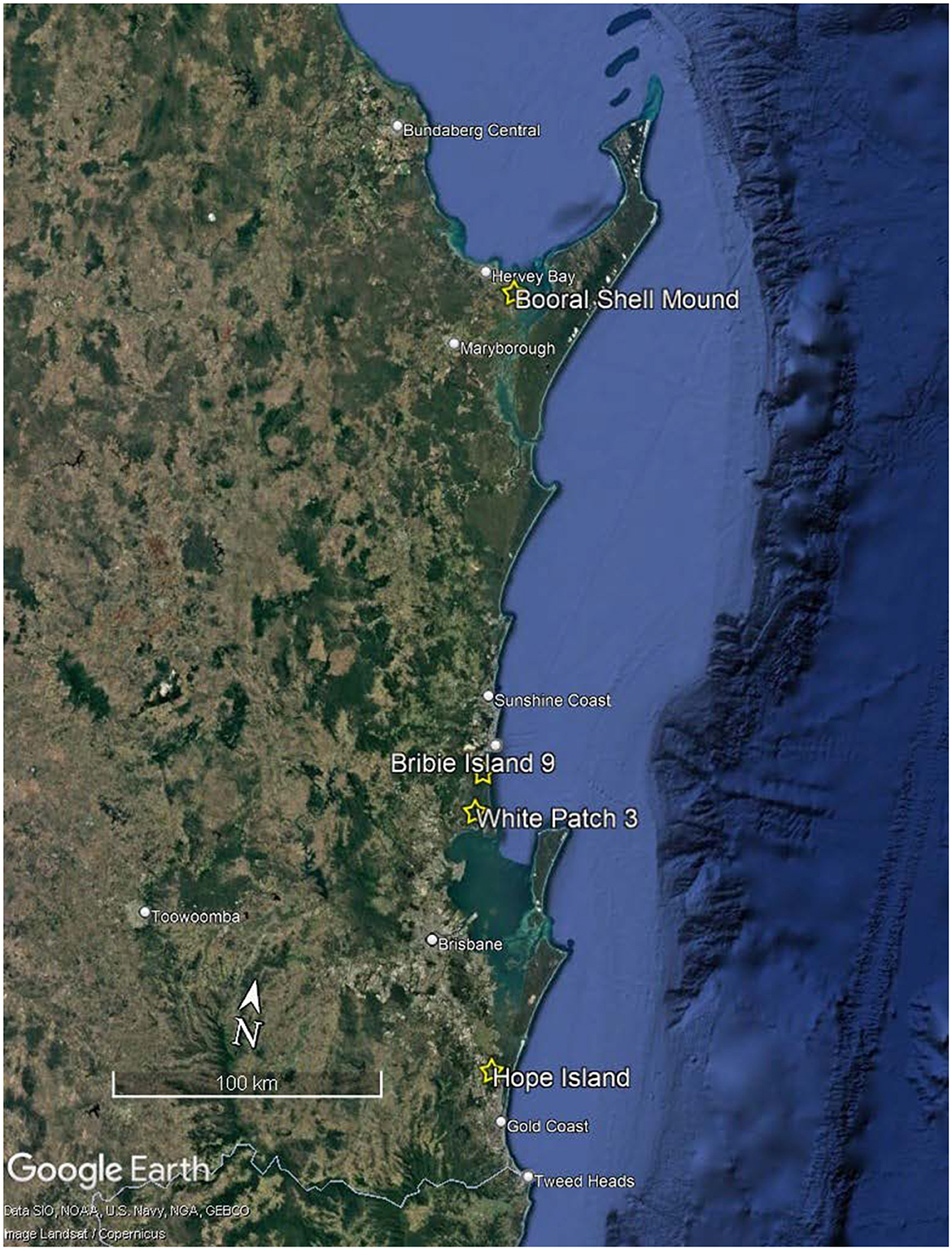
Figure 2. Location of Booral Shell Mound, BI9, White Patch 3 and Hope Island (Source Google Earth Pro 2024).
First Nations traditional lifeways were disrupted by the establishment of the Moreton Bay Penal Settlement, firstly at Redcliffe in 1824 and then at Brisbane/Meanjin in 1825, as well as the establishment of a pilot station and farm at Amity Point on Stradbroke Island/Minjerribah. The situation was exacerbated when Brisbane was opened to free settlers in 1842, and many First Nations peoples were displaced in the latter years of the 19th century. Nevertheless, they provided aid to the colonists in the form of labor in exchange for food, as well trading resources, particularly marine resources, which the early colonists had little means of otherwise obtaining. To an extent First Nations peoples were active participants in an economy transitioning from subsistence to commercialization, including the nascent oyster industry.
The oyster industry, based primarily in Southeast Queensland, flourished from the 1870s; it peaked in the late 19th-early 20th centuries before a decline from which it has never completely recovered. Similarly, there was a decline in the wild oyster populations although quantitative evidence for the extent of the areas lost is lacking: only commercial oystering was quantified by the number of bags produced each season. However, the naturally occurring subtidal oyster beds in Moreton Bay are considered functionally extinct.
There were and are many First Nations peoples in Southeast Queensland, forming complex socio-cultural networks. Rather than naming any individual group or groups, the term First Nations people/s is used throughout to avoid inadvertently incorrectly ascribing actions to a particular group.
Sixty-two molluscan taxa were identified from Booral Shell Mound and 14 marine molluscan taxa identified from White Patch 3, however the analyses reported here are confined to the oyster component from each site. Saccostrea cucullata (Hooded Oyster) and S. glomerata (Sydney Rock Oyster) are both recorded as occurring in Southeast Queensland (Healy and Potter, 2010; Lamprell and Healy, 1998). However, Healy et al. (2011, p. 176) note that Sydney Rock Oyster is sometimes considered to be a subspecies of Hooded Oyster, and other authors have also noted that the species can be difficult to differentiate morphologically (e.g., Buroker et al., 1979; Healy and Potter, 2010; Lam and Morton, 2006; Saville-Kent, 1891; Thomson, 1954). Oysters live attached to hard objects and are usually gregarious and often densely packed, leading to frequent morphological variability among individuals—what are described as separate species may in fact simply be ecophenotypical variations (Carpenter and Niem, 1998, p. 224). A conservative approach is taken here, and the oysters are referred to simply as Saccostrea spp.
The quantification methods employed were NISP (number of identified specimens) and MNI (minimum number of individuals). The method of calculating MNI was straightforward and involved sorting the bivalves into upper and lower valves using the non-repetitive elements (NRE) of hinges with >50% of features preserved. Counts were taken for each excavation unit (XU), tallied, and the higher number of left or right valves for the stratigraphic unit (SU) was used as the MNI (Grayson, 1984, 2001). This method avoids the aggregation effects associated with using counts from XUs when the SU is the main analytical unit (Grayson, 1984). The use of NISP and MNI also allows for calculation of the fragmentation ratio (NISP: MNI) for individual taxa, which may reflect the level of intensity of site use.
The height of the valves was measured along the dorsal-ventral axis, and length left to right laterally. The morphometric data are important in determining the composition of samples in terms of population structure, determining ratios of juveniles to adults, levels of exploitation and potential evidence of resource depletion, and biological, ecological, and environmental conditions. Breakage patterns were also noted, as was the condition of the shell (e.g., chalky, weathered, degraded, burned, or damaged by worms or borers) (e.g., Zuschin and Stanton, 2001).
Accumulation rates are frequently employed in behavioral interpretations of sites, particularly in assessing the intensity of occupation. Stein et al. (2003, p. 298) presented a quantitative method of calculating the rate of accumulation of deposits within archaeological sites to measure changing landscape use over time and to assist in the interpretation of stratigraphically complex sites. The method relies on the determination of 14C age and depth below surface for at least two points in a deposit, with accumulation rates calculated by dividing the thickness of the accumulation in centimeters by the duration of the accumulation in years:
Stein et al. (2003, p. 313) defined three categories of accumulation: slow, intermediate, and rapid. Slow accumulation rates are <2 cm/100 years, with material being mixed in the process of deposition due to factors such as treadage. Intermediate rates of accumulation are >2 cm/100 years and <50 cm/100 years, with material being buried before mixing. Rapid accumulation rates are >50 cm/100 years, with rapid burial preserving the contextual relationships between sediments and cultural material. Accumulation rates can be calculated for stratigraphic units, and also for the excavation units within them to provide a finer-grained profile. This method of calculating accumulation rates does not allow for extrapolation of basal dates but it is a reasonably accurate reflection of occupation intensity, and is the method employed in the present study.
Conventional radiocarbon determinations were calibrated using CALIB 8.2 (Stuiver and Reimer, 1993) and the SHCal20 dataset (Hogg et al., 2020). Statistical analyses were undertaken using IBM© SPSS© Statistics Version 22 with the appropriate tests determined by reference to Field (2014) and Pallant (2013).
Booral Shell Mound (BSM) lies on the Great Sandy Strait (GSS) opposite the southern end of K'gari (Fraser Island) (Figure 2) in the Great Sandy Region, an area comprising K'gari, southern Hervey Bay, the Cooloola sandmass, and the GSS. The GSS was Ramsar-listed as a wetland area of international significance in 1999. It is a double-ended estuary, with K'gari acting as a barrier blocking the outflow of the Mary River which is diverted north through the estuary. The Mary River is the largest source of freshwater in the GSS with an average annual discharge of 2300GL, with large inputs of sediment and freshwater occurring during flood episodes contributing to siltation and turbidity, and seagrass mortality (Campbell and McKenzie, 2004; McKenzie and Campbell, 2003; McKenzie et al., 2014). Other major freshwater sources are creek and drainage basin runoffs from K'gari and the Cooloola sandmass. The GSS comprises the largest tidal swamp within the Southeast Queensland bioregion and is made up of intertidal sand/mud flats, extended seagrass beds, mangrove forests, salt flats and salt marshes, freshwater Melaleuca wetlands and coastal wallum swamps.
BSM is part of a complex of sites on the historic property “Booral” originally recorded by the Queensland State Archaeology Branch between 1979 and 1982, and subsequently re-surveyed by McNiven (1994) for National Estate listing, and by McNiven and Frankland in 1989 to identify appropriate areas for excavation (Frankland, 1990). McNiven also mapped five fish traps on the rocky foreshore adjacent to the property. In 1989 BSM comprised a discrete undisturbed U-shaped mound approximately 14 m long and 1.4 m high near the top of a steep embankment approximately 10 m asl fronting the shore in an area of dense grass, shrubs and trees (Frankland, 1990; McNiven, 1994). The excavation was placed over the highest area of the mound and consisted of a 1 m × 0.5 m trench divided into two 50 cm × 50 cm squares designated A and B. There were 28 spits (excavation units, XUs) to a depth of 160 cm, although no cultural material was found below c.137 cm. Five stratigraphic units (SU) were identified and described; SUs 1–3 contained dense shell deposits and lay directly on top of each other with no sterile sediments in between, while SUs 4 and 5 represent the original ground surface (Frankland, 1990; McNiven, 1994). SU1 comprised XUs 1–7; SU2 XUs 8–16; SU3 XUs 17–25, and SU4 and SU5 the final three XUs 26–28. A series of six radiocarbon age determinations was obtained from charcoal in Square A (Table 1).
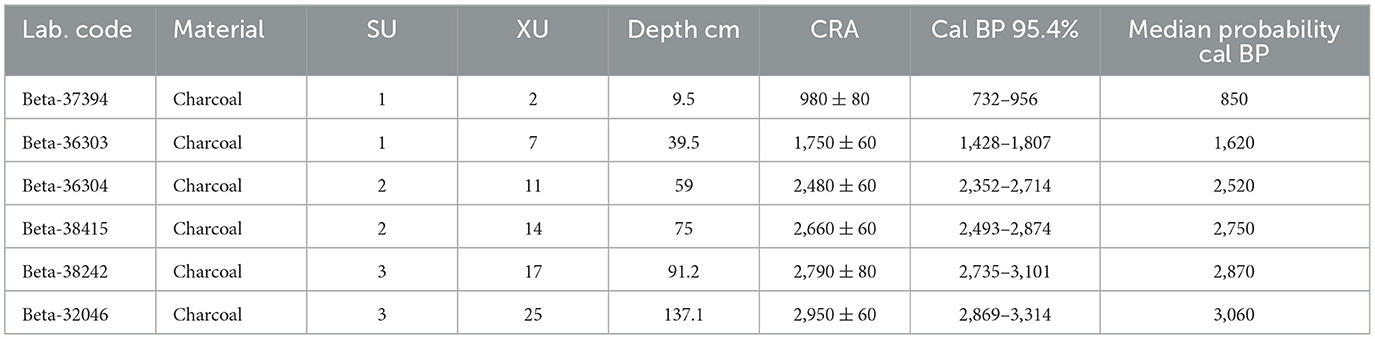
Table 1. Booral Shell Mound radiocarbon determinations (after Frankland, 1990).
Frankland (1990) analyzed the contents of Square A. The previously unsorted contents of Square B were analyzed for the present study. Due to the volume of material the decision was taken to sort and analyze every second excavation unit in addition to those units from which the radiocarbon age determinations were obtained, totaling 17 XUs, five from each SU containing cultural material, and one each from SU4 and SU5. The unsorted material was mechanically sieved through nested Endicott sieves, with the <2 mm fraction excluded from the analysis. NISP and MNI were calculated for all taxa as outlined in Materials and Methods. The total NISP for the 62 molluscan taxa identified to family, genus and species was 184,435, with a total MNI of 9,425. Only the Saccostrea component of the assemblage is discussed in detail here.
The formula developed by Stein et al. (2003, p. 300) outlined above was employed to determine potential variations in site use (Figure 3). For SU3 (3,059–2,868 cal BP), the rate of accumulation was 0.163 cm/yr or 16.3 cm per 100 years. Excavation units 16–14 (2,868–2,746 cal BP) at the base of SU2 returned a similar rate of accumulation of 0.162 cm/yr or 16.2 cm per 100 years, while the middle XUs 13–11 (2,746–2,521 cal BP) demonstrate a slowing of accumulation at 0.06 cm/yr, 6 cm per 100 years. The upper parts of SU2, 10–8 (2521–1620 cal BP), demonstrated a further slowing to 0.02 cm/yr or 2 cm per 100 years. SU1 (1620–845 cal BP) demonstrated a slight increase at 0.03 cm/yr, 3 cm per 100 years. The upper levels of SU2 fall into the category described by Stein et al. (2003, p. 313) as slow, while the remainder fall into the intermediate category (between 2 cm and 50 cm per 100 years). The authors consider that material deposited at this rate would be buried before being mixed. Although most of the site falls into the intermediate category, differences in the rates of accumulation are demonstrated throughout, reflecting potentially differing levels of site use. No rapid depositional events occur, instead suggesting continuous but perhaps not necessarily intensive use of the site.
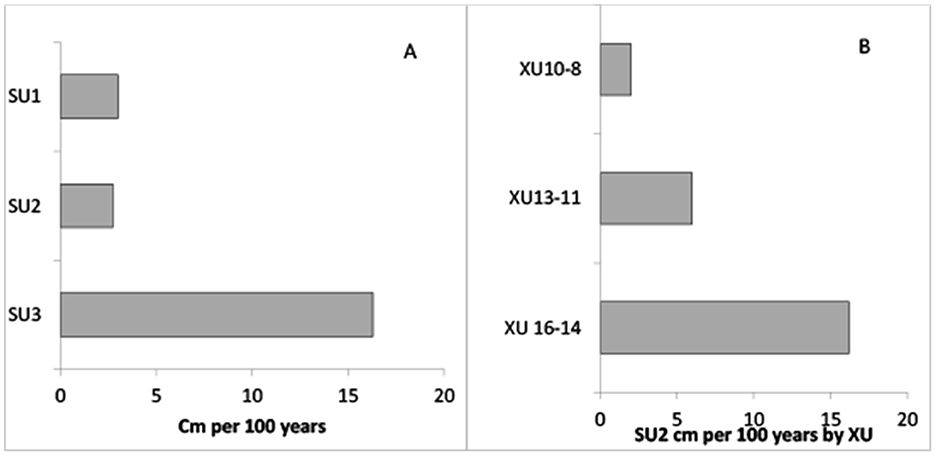
Figure 3. Sedimentation accumulation rates for BSM. (A) Accumulation rate per 100 years with average values for SU2 (B) SU2 accumulation rates by XU.
Saccostrea spp. dominated both the NISP (117,328, 63.62%) and MNI (5,323, 56.48%) determinations. Heights were measured for a total of 1,412 complete lower valves from the three SUs (for descriptive statistics see Table 2). The results of a Shapiro-Wilk test of normality (SU1 W = 0.992, df = 419, p = 0.033; SU2 W = 0.987, df = 352, p = 0.004; SU3 W = 0.975, df = 641, p = 0.000) indicated that valve heights did not follow a normal distribution. A one-way analysis of variance (ANOVA) was used to compare mean valve height by SU, with the results (F = 67.121, df = 2, p = 0.000) indicating a significant difference across the SUs. Levene's test of homogeneity of variance indicated that the variances for each group were significantly different (L = 5.082, df1 = 2, df2 = 1,409, p = 0.006). As the assumption of homogeneity of variance was violated, additional robust nonparametric tests of equality of means were conducted, with both Welch (W = 71.259, df1 = 2, df2 = 850.331, p = 0.000) and Brown-Forsythe (B = 70.496, df1 = 2, df2 = 1305.420, p = 0.000) test results also being significant. Post hoc testing using Tukey HSD indicated a significant difference at the 0.05 level for valve heights between SU2 and SUs 1 and 3 (Table 3). Although not dramatically different to SU1, SU2 has the smallest sample size, and this may have influenced the result.
Saccostrea individuals are sexually mature as males at a height of 20 mm and as females at 50–60 mm and can grow to heights of 60–80 mm, although commercially grown species can reach 100 mm (Catterall and Poiner, 1987; Lamprell and Healy, 1998). All SUs contained juvenile oysters, although these were relatively few (2.13%), as well as mature oysters at the upper ends of the height range (Figure 4). Overall, the picture is one of the harvesting of a relatively mature population not subject to over-exploitation.
Valve depth and morphology are environmentally influenced and there was little evidence of growth in tightly packed clusters or reefs. In SU1, 43 lower valves (3.7%) were cemented to other Saccostrea whole or partial valves (with one cemented to a Bembicium sp. shell); in SU2 45 lower valves (4.95%) were cemented to other whole or partial valves, and in SU3 only 31 valves (1.35%) were cemented to other whole or partial valves. All SUs contained lower valves with flat bases (SU1 n = 25, 2.25%; SU2 n = 204, 22.44%; SU3 n = 242, 10.6%), implying that they grew directly either on rock or a firmly packed surface. Kent (1992, p. 25; see also Saville-Kent, 1891) noted that “sand oysters” from bars of coarse firmly packed sand in the intertidal zone or in very shallow water have well-developed radial ribs and strongly colored valves caused by exposure to sunlight. In SU3, 223 lower valves (9.77%) exhibited fluted edges with well-developed radial ribs, and both upper and lower valves were observed to be relatively more deeply colored, being quite purple, than the upper SUs. The coloration may of course also be due to taphonomic factors, reflecting the relatively more rapid accumulation of sediments in the lowest section of the midden, which implies that material would be buried before extended surface exposure, abrasion or treadage.
The Saccostrea fragmentation ratios (NISP: MNI) varied across the SUs (SU1 31.23, SU2 21.06, and SU3 16.86). The ratios do not correspond with the estimated rates of accumulation of the deposit, although the high fragmentation rate in SU1 may indicate post-depositional and post-abandonment trampling. There was little worm or drill/borer damage noted on the complete and partial upper and lower valves in all SUs (1.24% of individuals), although drill and borer damage has been observed to be a contributory factor to shell breakage (Zuschin and Stanton, 2001) and may be more prevalent in the fragmented specimens. None of the remains exhibited signs of burning or exposure to high temperatures.
Bribie Island is the northernmost island of Moreton Bay (Figure 2). It is separated from the mainland on the north and west by Pumicestone Passage and bounded on the south and east by Moreton Bay and the Pacific Ocean. Pumicestone Passage is quite narrow, approximately 1.5 km at its widest point, with an average depth of <2 m. In the north near Bell's Creek, it can be waded at very low tides. The Passage is a “mesotidal, elongate back-barrier lagoon estuary with a tidal inlet at either end” (Lang et al., 1998, p. 89), divided into three parts: northern and southern tidal deltas, a microtidal central muddy estuarine basin fed on the west by three small tidal creeks, and bay-head deltas at Elimbah and Coochin Creeks, also on the western side.
The island is 32 km long and up to 8 km wide, and mostly between 5 m to 10 m in elevation, although some areas rise to 15 m. It is largely composed of north-south trending remnant Pleistocene aeolian dunes, with east-west trending Holocene dunes over the southern quarter of the Island, with the soil matrix composed entirely of podzols and siliceous sands (Hekel et al., 1979, p. 8–9; Willmott and Stevens, 1988). In east-west cross section the terrain exhibits low peaks and swales. There is no stone other than outcrops of coffee rock. A central barrier swamp extends north-south through the island fed from the northwest by Westaway Creek. There are also numerous small standing areas of water, and lagoons on the eastern side some of which are now breached and open to the sea. Bribie Island is typical of the coastal lowlands or wallum country characterized by Coaldrake (1961, p. 5). Petherick et al. (2008, p. 7) suggest that the wallum floristic communities, dominated by Banksia species, developed in the area throughout the Holocene. Bribie Island National Park covers approximately two-thirds of the island and includes a 4,000 ha commercial plantation which covers most of the Pleistocene dune ridges. More than 120 sites have been recorded on the Island with most of these identified as part of the Moreton Region Archaeological Project (MRAP)1 and the Bribie Island Forest Archaeological Project (BIFAP) (Figure 5).
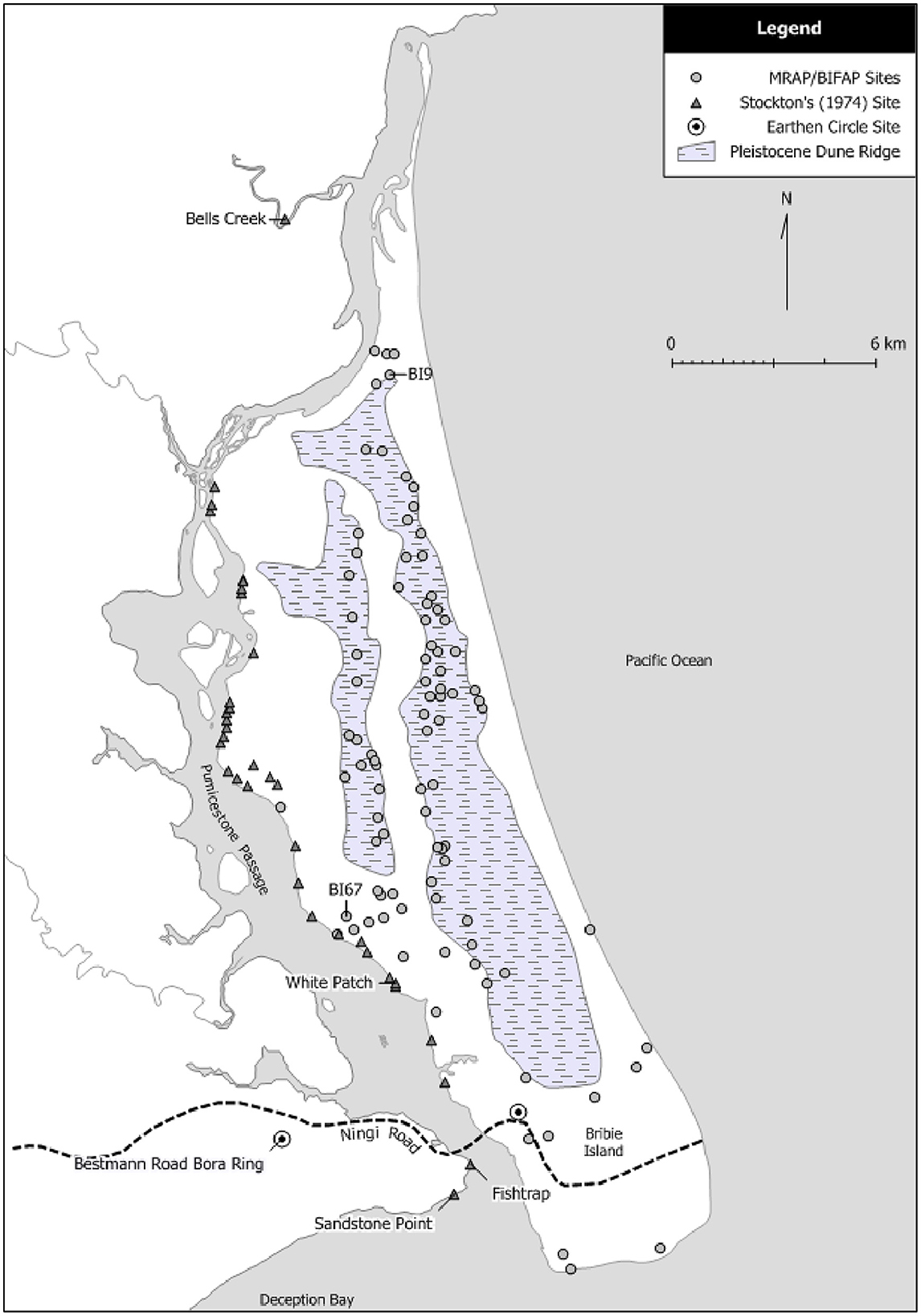
Figure 5. Recorded sites on Bribie Island (after Smith, 2016).
In 1974 Haglund excavated five midden sites (White Patch 1–5) recorded by Stockton (1974) on a sand ridge eroding landward on the southwest coast of Bribie Island facing Pumicestone Passage near White Patch (Figure 5), in the central microtidal estuarine basin (Lang et al., 1998). The sites are approximately 300 m southeast of site BI67, a large (c. 3 km2) undated midden scatter with cultural material to a depth of 50 cm (MRAP files). The notes and some material from the excavations were lodged with the Queensland Museum, but otherwise the work remained largely unreported until Crooks' (1982) BA Honors thesis. The general condition and content of four of the middens was considered poor, with White Patch 3 (WP3) considered to be in the best condition because of a relatively more sheltered position and was also the “richest” in “archaeological debris” (Crooks, 1982, p. 49). The maximum extent of the WP3 midden is unrecorded, but the original plan indicates 35 m2, on a ridge with contours from 5.5 m to 6.5 m in height. There are no details available regarding excavation methods or techniques for recovery of cultural material. Six squares totaling 4.5 m2 (Ulm, 2002, p. 81) were excavated; the analysis here is based on the WP3 material from square C50 held by the Queensland Museum.
The stratigraphic profile for squares C50 and C51 is reproduced in Figure 6 with the original unmatched scale bars retained. There is no accompanying description of the strata labeled 1.1 to 1.5, other than that there was an overlying humic layer, and that “The midden deposit forms a single unit composed of many distinct strata” (Haglund in Crooks, 1982, p. 60). Similarly, the two charcoal samples obtained for dating purposes came “from Level ‘b' ” and “Level ‘d' ” (Haglund in Crooks, 1982, p. 64–65), although the levels are not otherwise mentioned or described, and no depths are provided. It is presumed that Level “b” equates to the level labeled 1.2, and Level “d” to the level labeled 1.4. Gillespie and Temple (1979, p. 104) in their report on Sydney University Natural Radiocarbon Measurements state that the upper sample (SUA-480) came from the top of the midden deposit, and the lower (SUA-481) from the base of the midden 25 cm below although the exact location within the stratigraphic profile of the dating samples remains unknown. The upper sample returned a date of 450 ± 95 BP, and the lower sample a date of 670 ± 95 BP (Gillespie and Temple, 1979, p. 104; Haglund in Crooks, 1982, p. 64–65; Table 4). In order to attempt meaningful analysis of the shellfish and other cultural remains relative to the layers depicted in the stratigraphic profile rather than the arbitrary spits, stratigraphic units have been ascribed (Table 5). Some overlap between the stratigraphic units is inevitable because of the excavation within arbitrary spits.
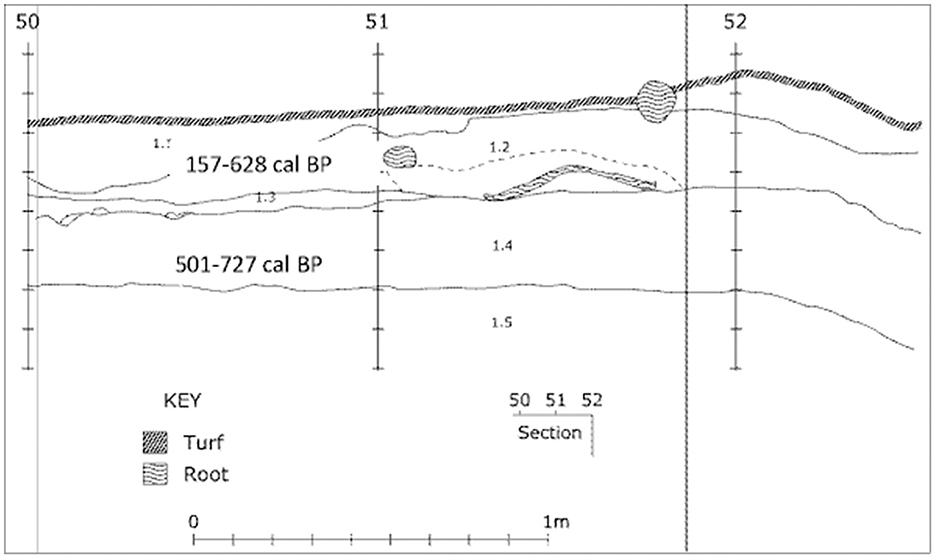
Figure 6. Stratigraphic profile of WP3 Square C50 showing the approximate location of calibrated age ranges (Smith, 2016, after Crooks, 1982). Descriptions of strata not available.
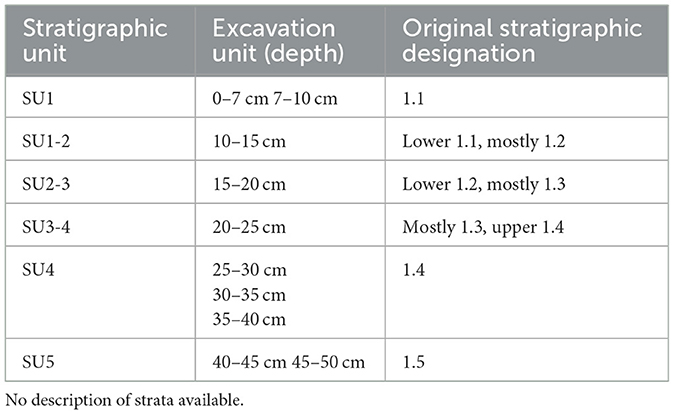
Table 5. Ascribed stratigraphic units for WP3 square C50 (after Haglund, in Crooks, 1982).
The molluscan assemblage consisted of 16 marine and two terrestrial taxa, with a total NISP of 6,508 and a total MNI of 771. Anadara trapezia (cockle) was the most abundant taxon, with a NISP of 686 accounting for 10.54% of the total NISP while the MNI of 227 accounted for 29.44% of the total MNI. The next most abundant taxon Saccostrea spp. NISP of 615 accounted for 9.65% of the total NISP, while the MNI of 169 accounted for 21.92% of the total MNI. Heights were obtained from 46 complete lower valves or cups from SUs 1–2 to 5 (no complete valves were recovered from SU1); the descriptive statistics are detailed in Table 6. Although a smaller sample than that of complete upper valves or lids (n = 111), the lower valves of Saccostrea spp. exhibit morphological responses to the substrates to which they attach, and to environmental changes. They are also better indicators of the overall size of individuals due to the variability in hinge height which is not reflected in the upper valves. It is acknowledged that the individual SU samples are small and do not lend themselves to meaningful statistical analyses. Despite these sample size issues, the information provides an approximate indication of valve height variation through the midden deposit. The mean values for all SUs indicate sexually mature males (≥20 mm), while the maximum values for all SUs indicate the presence of sexually mature females (≥50 mm) (Catterall and Poiner, 1987, p. 120). This represents the harvesting of a relatively mature population not subject to over-exploitation.
Nine of the 19 complete lower valves (47.36%) from SU2.3 exhibited very thin shell structure, and were flattened along one lateral margin, indicating growth directly either on rock or a firmly packed surface. Another lower valve was cemented to at least three other fragmented lower valves. Two of the complete lower valves in SU3.4 were cemented to two other valves, while in SU5 four whole lower valves (57.14%) were cemented together. SU4 also had two complete lower valves and two partial lower valves that exhibited very thin shell structure. SU2.3 and SU4, coincident with the thin-walled valves, also had the lowest minimum valve heights, with SU4 also having the lowest mean value. It is again acknowledged that the sample sizes are small, however the co-occurrence of thin walled and smaller valves is unlikely to be unrelated. Post-depositional taphonomic factors including physical degradation and particularly chemical alteration must be a consideration, as roasting over high heat can cause the conchiolin which forms the periostracum of valves to degrade and become friable; these types of valves usually also have a covering of small re-crystallized calcium carbonate fragments. Slow degradation of the conchiolin by bacterial decomposition or oxidation (e.g., oxygen dissolved in water) leaves valves with exposed calcium carbonate prisms (Kent, 1992, p. 15). The WP3 Saccostrea spp. remains did not otherwise exhibit evidence of exposure to high temperatures (e.g., change in color), nor did they exhibit calcium carbonate prisms. Ante-mortem factors such as those described by Gosling (2003, p. 7) and which result in thinning of the conchiolin include mechanical abrasion, fouling organisms (e.g., algal blooms), parasites and diseases. The condition of the whole valves suggests that they were not tightly packed in clusters or on oyster reefs and this may have rendered them more susceptible to abrasion by suspended sediments during freshwater flood events (from the tributary creeks flowing into Pumicestone Passage and the Brisbane River) or extreme tidal ranges influenced by storms. Equally there may have been two episodes during the formation of the midden when the oysters were exposed to diseases that affected both their growth rates and shell structure.
Although the physical evidence of the gathering of oysters by First Nations people in Southeast Queensland is abundant, direct ethnohistorical or ethnographical descriptions of the methods employed are few, but nonetheless useful.
Tom Petrie, whose family arrived in Brisbane in 1837, commented that “[First Nations people] … would eat oysters raw, but were very fond of them roasted, too, probably because they opened so easily then” (Petrie, 1904, p. 74–75) but offered no information on gathering practices. The 1841 account by Christopher Eipper, a German Presbyterian missionary interested in the local First Nations people with a view to converting them, is more useful. In August Eipper and some companions had traveled to the Toorbul area opposite Bribie Island and described women oyster gathering in Pumicestone Passage:
There was a canoe, in which they rowed to one of the small Islands … where they gathered the oysters out of the mud into the boat. When they had thus gathered a great quantity, they went back to the shore, and made a fire, into which all the oysters were put, to cleanse them from the mud, and being thus stewed at the same time, they are eaten, and taste very well (The Colonial Observer, 14 October 1841 p. 2).
An alternative method of gathering was recorded by George Watkins, pharmacist and dispenser at the Dunwich Benevolent Asylum on North Stradbroke Island/Minjerribah from 1868 to 1888:
The beach yield crustacea and shell-fish [sic]; among the latter oysters, pearl oysters, cockles and mussels of which the three last were always roasted. Low water was a working time for … [First Nations women] especially. They went out with dilly-bags and short spears, returning with the former filled with shellfish, coral, eels, crabs &c. (Watkins, 1891: p. 44).
G. K. Jackson of the Queensland Museum recorded the observations of Mr. Potts, an early European settler on the Mooloolah River:
…the [First Nations people] used to dive for oysters, either from the bank or from their canoes …The oysters, of course, grew on the bed of the river. One can easily pick the parts where they are most abundant, as nearby are numerous shells (Jackson, 1939, p. 291).
The observations of Eipper (1841) and Potts (Jackson, 1939) are particularly interesting in view of the nature of the assemblages from BSM and WP3 and are discussed further below.
Hall (1982) estimated the population of First Nations peoples in the Moreton Region at the time the Moreton Bay settlement was established to be around 4,000 individuals. During the convict period the European population comprised convicts, soldiers and their families, and civil servants and their families, and reached a peak of about 1,200 in 1831 (Steele, 1975). In the 1841 census, prior to the arrival of free settlers, the European population of Moreton Bay numbered 230 (Sydney Gazette and New South Wales Advertiser, 4 September 1841, p. 2). The first free census of 1846 recorded 839 people in Brisbane and its suburbs; the population grew to 2,543 by the 1851 census (Moreton Bay Courier, 6 December 1851, p. 2). Relations between the colonists and the First Nations peoples were not always harmonious, and records of hostility and brutality, sometimes on the part of the First Nations peoples but more frequently on the part of the colonists and colonial administration, occur all too commonly. However, in 1840 following an incident where the German missionaries who lived about 10 km east of Brisbane town fired on First Nations people they believed were intending to steal some of their potato crop, the Commandant, Lieutenant Gorman, wrote to the Colonial Secretary “I regret very much that the Missionaries fired on the [First Nations people] as we are on excellent terms with them for forty miles round.” (in Steele, 1975, p. 268).
The necessity for good relations between First Nations people and the incursive Europeans was not the disparity in numbers in the early days (and which could, and on occasions were, countered by the Europeans' superior firepower) but the fact that the Europeans were in what was in many respects an alien land. Some of the colonists came from Sydney and surrounding areas, a landscape and temperate climate quite different from sub-tropical Southeast Queensland, but most were immigrants who came directly from Britain. The local First Nations people knew the when, where, and how of obtaining resources.
In the 1850s there were six or seven First Nations camps at Breakfast Creek, some 7 km east of Brisbane town, where men fished with tow-row nets, particularly targeting mullet coming up the river, which the women then took into town to sell (Phillips, 1929). McMahon (1924) recorded the memories of an octogenarian Brisbane resident H. H. Ensor who recalled seeing up to 100 First Nations people in Brisbane town while on his way to school, all of whom had fish for sale. They came mainly from the camps at Breakfast Creek, “in which big bough fences formed traps, into which the fish got at high tide, and were easily caught when the water ebbed.” Norman Creek which flowed into the Brisbane River through its southern bank was also an area of First Nations camps. Mullet and bream were caught in nets in their hundreds and were taken by the women to be sold in the markets of South Brisbane (Lack, 1950; Melton, 1919). In 1861 one of the colonists, Walker, began seine-netting in Moreton Bay, with the report of his venture in the Courier of 17 August stating: “Until very recently the inhabitants of Brisbane depended mostly for a supply of fish upon the [First Nation people] of this locality, very much, no doubt, to the profit, in a pecuniary sense, of [them],” This was no mean feat given that the population in the 1861 census was 6,041 (Moreton Bay Courier, 7 May 1861, p. 2). Walker's venture was reported to have largely driven First Nations fishers from the field (Courier, 17 August 1861, p. 2). However, not all settlers lived close to the markets at North and South Brisbane. The Carmichael family who had a 50-acre block at Tingalpa on the southside approximately 13 km southeast of the town in the 1870s traded cakes of tobacco with First Nations people who would “spear us plenty of fish” (Carmichael, 1930). On Stradbroke Island/Minjerribah, First Nations people camped near Dunwich at different times of the year and supplied the residents of the Benevolent Asylum with fish (Kennedy, 1872). During the 1880s at Redcliffe on the coast northwest of Brisbane were First Nations people working as fishermen with a government-supplied boat (Parry-Okeden, 1930), and in the 1890s at Wynnum (Lovekin, 1896).
Oysters were sold on the streets in Brisbane town, and along with crabs by traveling groups to households that were remote from the settlement (Kerkhove, 2013). Sandgate, in the late 1800s a resort town overlooking Moreton Bay, became a popular center with First Nations groups for oyster and crab sales, with customers coming from a wide radius (Blake and Osborne, 2008; Craig, 1908 in Kerkhove, 2013).
In October 1847 the Moreton Bay Courier reported that First Nations people had been netting “immense quantities” of prawns which were abundant in the river during the season, and superior to those obtained from southern coasts. “The [First Nations people] dispose of them to the inhabitants for a mere trifle; a loaf of bread or a fig of tobacco being the only remuneration they expect to receive for five or six quarts of prawns,” This report is particularly interesting, as it is the only historical reference to First Nations people prawning. Petrie (1904) devoted four chapters to First Nations foodstuffs and the methods by which they were obtained but does not mention prawns. It seems unlikely that First Nations people did not regularly take prawns when they were in season; they certainly possessed the technology to do so, although here are no recorded instances of recovery of prawn shells from midden deposits. It is tempting to speculate that the First Nations people were responding to market demand in an entrepreneurial spirit.
A common theme in records of transactions of sales and trade or bartering between First Nations peoples and Europeans is the cheapness of the goods supplied. Quantities of seafood and other foodstuffs such as honey could be obtained by householders for a cake of tobacco, a loaf of bread, a cooked meal, tea and sugar. That First Nations peoples did sell fish for cash in a larger market is evidenced by McMahon (1924), and also by Monks (2006, in Kerkhove, 2013) who quotes prices as three pence for a codfish, a shilling for five or six mullet, and a penny for other fish. Craig (1908, in Kerkhove, 2013) recorded crabs being sold at Sandgate for sixpence each. Accumulation of material wealth was not a focus for First Nations peoples, who had cultural obligations of reciprocity and exchange: when there was an abundance or surplus it was shared. To an extent the seafood trade reflects those cultural obligations, but equally it is difficult not to view the Europeans' approach as exploitation of people many of them considered inferior.
The collapse of oyster populations in Southeast Queensland in the late 19th-early 20th century, and by extension, the loss of viability of the commercial oyster industry, is mirrored by similar events along the eastern and south-eastern coast of Australia where subtidal populations have become functionally extinct. Causes of the collapse have been variously ascribed to historical overfishing, increased sedimentation, and the introduction of diseases (e.g., Beck et al., 2011; Kirby, 2004; Ogburn et al., 2007; Diggles, 2013; Saville-Kent, 1891). A further contributing factor is economics. These are considered here in the context of Moreton Bay and the Great Sandy Strait. Taken together, it becomes clear while reasons for the collapse of oyster populations themselves may be fairly apparent, the reasons for the collapse of the oyster industry are more complex.
Early historical descriptions of the oyster populations in Moreton Bay speak to their abundance and extent. Backhouse, a Quaker missionary and naturalist, visited the Moreton Bay settlement in 1836 and described a beach on Stradbroke Island/Minjerribah:
We took a walk upon a part of the beach, where the variety of shell-fish was great. The Rock Oysters were attached to the portions of the various Mangroves, within the influx of the sea. Drift Oysters were in large masses, below the high-water mark; among them were various species of Cypraea, Cowrie, Conus, &c. Common and Pearl Oysters were thinly scattered, lower down on the shore (Backhouse, 1843, p. 375).
Archer (1862, in Smith, 1985, p. 12) noted of Pumicestone Passage:
The water teems with fish, great and small and as for the oysters, I never saw anything like it. This day we saw something like a reef of rock about three feet out of the water and three hundred yards long … we found it to be a huge and apparently solid bed of oysters, big enough to load several large ships.
Saville-Kent, the Queensland Commissioner of Fisheries, ranked the oysters in the region based on the habitat they occupied and conditions of growth: bank oysters, oyster reefs, mangrove oysters, dredge oysters, and drift oysters (Saville-Kent, 1891, p. 5–6). Bank oysters grow in the intertidal zone on level banks, attached to stones or dead oyster shell cultch, or more often to the shells of the Hercules Club whelk, Pyrazus ebeninus. Some of the best oysters to be had were those in clusters of four or five attached to a whelk. This had unfortunate consequences for the whelk which was so burdened by the load it could no longer move to feed, and eventually starved to death. In Pumicestone Passage bank oysters were almost exclusively attached to ironstone pebbles on a substratum of gravel and mud; these are the kind of oysters Eipper observed being collected in 1841. Oyster banks occurred throughout Moreton Bay and further north in Wide Bay at the northern end of the Great Sandy Strait. Saville-Kent considered bank oysters to be the most valuable commercially, not only as the largest numbers of oysters sent to market were bank oysters, but because of the employment provided (Saville-Kent, 1891, p. 5).
Oyster reefs had an outer crust of live oysters up to 12 inches (30 cm) thick over a base of dead oysters, with the original substratum usually being gravel and coarse sand or large pebbles. The oysters tended to be smaller than bank oysters but grew well if carefully detached from the reef and placed on banks. This use of reef oysters to seed banks meant that “few if any reefs are to be found in their pristine massive condition throughout the oyster grounds of the Southern district.” (Saville-Kent, 1891, p. 5). Mangrove oysters grow on the exposed roots and respiratory shoots of mangroves, principally white mangrove (Avicennia marina), and could form extensive beds rivaling those of typical oyster reefs and were thus useful for separation and cultivation on banks. Dredge oysters were those occurring below the ebb tide while drift oysters were those lying loose and separately on the bed of the water, supposed to have been washed from off the banks or beds. Although dredge oysters had previously been harvested in large numbers, at the time of Saville-Kent's report the contribution was about 20% (Saville-Kent, 1891, p. 6). Fison (1889), the Inspector of Fisheries, wrote in 1889 that seeding dredge sections was a waste of time and money, and they should be left to recover naturally. Diggles (2013, p. 569) has interpreted this to mean that smothering of subtidal oyster beds after a flood in 1887 signaled the beginning of recruitment failure for oyster reefs in subtidal areas.
Oysters are available year-round in Southeast Queensland, but their condition is poorer during the winter months, and they tend to be in peak condition during spawning. This occurs during spring and summer when water temperatures are higher, usually between September and February (White and Beumer, 1997). Commercial oystering in Moreton Bay had begun in the 1840s when local First Nations people, paid by the bag, were employed to collect bank oysters in Pumicestone Passage and other areas for transport to the markets in Brisbane (Smith, 1985; Welsby in Thomson, 1967). The trade was largely unregulated, with individuals helping themselves “where, when, and how they chose” (Smith, 1981, p. 46). Until the 1863 Act for the Protection of Oyster Fisheries was passed live oysters as well as oyster shells mined from First Nations middens were burned to produce lime for mortar used in the construction of buildings in the Moreton Bay settlement. The Act prohibited the burning of live oysters, but over-exploitation of beds in some areas had already occurred (Smith, 1981, 1985). Dredging of oysters from boats in subtidal regions of the Bay began around 1865, but again were unlicensed and poorly regulated. Concerns were raised about the continued viability of the oyster industry, with most of the catch being exported to Sydney and Melbourne, with fears that without a closed season the beds would be dredged out. The passage of the 1874 Act for the Protection of Oysters and Encouragement of Oyster Fisheries licensed oyster banks and allowed for the auction of dredge sections. It was followed shortly after by the registration of the Moreton Bay Oyster Company which remained the largest company of its kind before closing in the 1960s (Smith, 1981, 1985).
Production peaked in 1891 when the beds produced a bumper crop following recovery from a flood in 1887; 21,000 sacks worth £29,000 were exported (Smith, 1981). Given that the standard sack or bag was equal to 100 dozen (Ogburn et al., 2007) this represents a staggering 25.2 million oysters. Of these, 1.26 million were dredge oysters (based on Saville-Kent (1891) observation that dredge oysters comprised 20% of the crop). Fison (1894) reported exports of 18,795 packages worth £22,063 in 1892, falling to 14,923 packages worth £17,832 in 1893, with no indication of improvements in the southern markets. Fisheries accounts also showed a decline in revenue and rents in 1893 and 1894.
During the 1870s the Moreton Bay Oyster Company, James Clark, and R W Leftwich and Sons acquired bank oyster leases in the Great Sandy Strait and Tin Can Inlet at its southern end (Brown, 2000). Apart from sales of the oysters themselves, the oyster fisheries became an important source of cultch for the Moreton Bay oyster fisheries, particularly after 1895. In the mid-1890s Jules Tardent, a K'gari forester, inspected the GSS oyster banks and commented on “the astronomical quantity of seed-oysters, stretching for miles” (Brown, 2000, p. 174). Dredging began in 1902 and continued until the last dredging section was forfeited in 1919 (Smith, 1985, p. 3–4). After the Moreton Bay oyster decline began and competition from producers from New Zealand and New South Wales in the markets became greater, some oystermen over-exploited their resources, stripping beds and thieving stock from other oystermen. The oyster leases were relatively isolated, making adequate policing difficult, and the costs of acquiring, developing and protecting them deterred investors. After 50 years of working in the area, Leftwich and Sons sold their leases to the Moreton Bay Oyster Company, and left the business (Brown, 2000, p. 175).
In 1887, a flood killed all the oysters in the southern part of the Bay, the rivers bringing down immense deposits of mud which simply smothered the bivalves. Then in 1892 and 1893, floods in the northern end of the Bay smothered the Bribie beds. About the same time, the dreaded mudworm disease made its appearance at the mouth of the Coomera River (The Queenslander, 8 September 1906).
Flooding in the creeks and rivers of Southeast Queensland is a fact of life of which the early European explorers were aware. In 1824 Oxley and Cunningham noted extensive bank erosion and flood debris high above the banks in the Brisbane River catchment, leading Oxley to comment: “… an inundation: a flood would be too weak an expression to use for a collection of water rising to the height (full 50 feet)” (in Cook, 2019, p. 6). The sedimentation transported by the floods discharges into Moreton Bay and has done so for millennia. In a study of the corals of northern Moreton Bay, Hekel et al. (1979) found the clear water coral genus Acropora was replaced by self-cleaning mud-resistant Favia and demonstrated that this change had taken place in the last 3,000 years, most likely between 1,000 and 2,000 years ago. They posited that it may have been due to a change in the direction of discharge from the Brisbane River from a northerly to a more easterly direction, climate change with either decreased humidity or a change from an equable to a seasonal climate, or a fall in sea level of about 1 m (Hekel et al., 1979). Walters (1992) considered that the change in the coral facies reflected an increase in the human population of the catchment area in the mid-late Holocene, associated with land management regimes such as firing the country to clear it. This resulted in episodes of erosion with sediments washed down the creeks into the river, and ultimately into the Bay where they formed large areas of mud and sand flats (Walters, 1992, p. 176). Certainly, increased sedimentation in Moreton Bay long pre-dates European settlement. However, with the advent of European settlement and the associated clearing of land for agricultural and pastoral purposes as well as urbanization, the run-off and associated sediment load in the Brisbane River increased exponentially. Increased siltation reduced the chances of successful recruitment, as oyster spat prefer to settle on clean surfaces.
Diggles (2013, p. the multiple ≥8-meter flood events in 1893 (commonly known as the “Black February” floods), may have introduced enough sediment and nutrients into the previously sand-dominated Pumicestone Passage system to begin to alter its structure. This view can be extrapolated to Moreton Bay more widely, with increased organic enrichment and sedimentation providing a habitat in which the mudworm Polydora spp. could thrive, particularly in the lower intertidal zone where dredge oysters occurred. At the height of the infestation in 1899, the number of banks being collected had reduced from 421 to 292, and dredge sections from 36 to 18 (Smith, 1981, p. 53).
Mudworms are spionid polychaetes, the most abundant group of segmented worms in the marine environment (Glasby, 2011, p. 103); Vohra (1965, p. 197) recorded 16 polychaete genera around Victoria Point and Dunwich in Moreton Bay. One group of genera, the polydorids, burrow into mollusk shells and other calcareous substrata. Shell damage occurs through the formation of U-shaped burrows where the shell matrix is etched and dissolved by an acidic secretion of the worm. Worms may also enter the shell through the gills, or while the valves are open during feeding (Rouse, 2000, p. 268). Huntley and Scarponi (2015, p. 151) found that spionids thrive in environments that are organically enriched and prone to disturbance, with high sedimentary input and dynamic levels of salinity. A higher prevalence of polychaete boring occurs in less predictable, highly fluctuating environments with low taxonomic diversity, and are typically found on shallow infaunal suspension-feeding bivalves.
Discussion remains as to the source of the mudworm disease, which was first reported from the Hunter River catchment in central New South Wales in 1880. Initially it was thought that the disease had spread from infected oysters imported from New Zealand. The Sydney markets imported New Zealand oysters in winter when Queensland oysters were unavailable; the oysters also had the advantage of being relatively cheap as they did not attract royalty payments. If there was a glut in the market or the oysters needed fattening, they were moved to estuaries such as the Hawkesbury and Hunter Rivers, as well as further north. Ogburn et al. (2007, p. 278–279) presented data demonstrating that the first reports of mass mortality events from mudworm disease usually occurred within a year either of the introduction of New Zealand oysters into the estuaries, or the movement of cultch and oysters from infected areas. The evidence seems compelling, however, there is nothing to suggest that mudworm was a problem in New Zealand at that time and in fact mudworm was not reported in New Zealand oysters until the early 1970s and then only in oysters from the north of the country (Read, 2010 in Diggles, 2013). An alternative hypothesis, that polydorid spionids were endemic in eastern Australian estuaries and increased in numbers in response to increased siltation as the result of colonial-era land clearing, is favored by Diggles (2013, p. 570) as “a more parsimonious explanation for the rise of mudworm and the demise of subtidal oyster reefs in Pumicestone Passage and Moreton Bay” based on the “historical epidemiological evidence, modern scientific understanding of settlement cues of spionid polychaete larvae and recent taxonomic work on Australian spionids.” Considering Vohra's (1965) observations on spionid polychaetes in Moreton Bay, Diggles' (2013) explanation seems the most likely. Smith (1985, p. 31) reports that not all oyster beds were equally affected, with those sections on soft, muddy bottoms most heavily infected and longest in recovery, while sections on clean firm substrate were frequently unaffected. Similarly, banks on higher ground escaped the infestation but were decimated in softer lower areas. By 1925 the Bay was largely free of mudworm disease, but the damage, much of it irreversible, was done. Mudworm did not reach the Great Sandy Strait, which had different problems including high levels of predation by Trachotus anak, a fish known as the giant oystercatcher and up to a meter long, which targets bank oysters (Brown, 2000; Smith, 1985; Department of Agriculture and Fisheries, 2022).
There were also several economic pressures on the commercial oyster industry. Concurrent with the worst of the mudworm epidemic was the 1898 breakthrough of the bar at Jumpinpin which separated what is now known as South Stradbroke Island from what became “North” Stradbroke Island/Minjerribah. The resultant tidal inflow scoured out the most productive banks and sections in southern Moreton Bay. In contrast to the New South Wales industry which responded quickly to the mudworm epidemic by enforcing requirements for timber and wire mesh trays to keep oysters above the bottom and away from potential mudworm infection, thus greatly increasing production the Queensland industry response was slow. Smith (1985, p. 42–43) outlines industry problems including the use of unsuitable materials, lack of experienced oystermen with available capital to invest, selection of unsuitable sites for tray and rack cultivation, increased labor costs and shortages of skilled labor, and attempts by some companies to monopolize the industry. The New South Wales industry had become self-reliant by the early 1920s and posed serious competition. By 1936 Queensland was importing New South Wales oysters and spat, depressing both prices and demand for the local product, resulting in many oystermen leaving the industry. More recently, oysters in southeast Queensland have also been affected by QX (“Queensland Unknown”) disease identified in 1960s as being caused by the single-celled parasite Marteilia sydneyi which infects oysters between January and April; the parasite essentially causes the oyster to starve to death over a period of some weeks and can result in >95% mortality within populations (Adlard and Nolan, 2015).
There is still a commercial oyster industry operating in Moreton Bay under the aegis of the Queensland Department of Agriculture and Fisheries (DAF), with the most recent Oyster Industry Management Plan for Moreton Bay Marine Park (MBMP) released in 2015. Non-commercial traditional oystering is not within its scope. There are four oyster growing areas, at Moreton Island, North Stradbroke Island, Pimpama River, and Pumicestone Passage. There is limited natural spatfall within the Bay, and most spat is imported from New South Wales with the oysters generally grown to maturity and fattened with tray cultivation and adjustable longline systems. Mature oysters are often moved to areas suited to fattening the oysters prior to sale and major harvesting takes place between August and April depending on the location (Department of Agriculture and Fisheries, 2015, p. 2). In stark contrast to the glory days of the late 19th century, the industry now accounts for 0.8% of the total Australian market (McDougall, 2020, p. 6). Although the 2015 Management Plan provides the tenure for long-term investment required for oyster farming, it reported that most authorized areas were producing few, if any, oysters, with annual returns by authority holders revealing around 50% of licensed oyster areas had nil production. In recent years, total edible oyster production decreased by 42.2%, from 87,407 dozen in 2020–21 to 50,547 dozen in 2021–22. Annually the industry is valued at ~$500, 000 (Department of Agriculture and Fisheries, 2015, p. 2, 2022:6). West et al. (2019) observed that the inefficiency of production in existing oyster-growing areas is likely due to the short harvest season, QX disease, poor water quality and the relatively high proportion of hobbyist farmers in the industry. According to the DAF 2015 Plan there are about a dozen full and part-time growers responsible for the majority of MBMP's oyster production.
In terms of non-commercial oysters, Gillies et al. (2018) reported a possible remnant oyster reef near Dunwich on North Stradbroke Island/Minjerribah. They note that little has been done to address the protection or restoration of shellfish ecosystems which in part, is due to “the shifting baseline syndrome, as a large proportion of the loss occurred in the late 1800s and early 1900s, outside the living memory of most coastal users” (Gillies et al., 2018, p. 16). Localized trials of “oyster gardening” on pontoons in canal estates on Bribie Island fronting Pumicestone Passage, as well as oyster reef balls (successful in Chesapeake Bay and Tampa Bay in the US), three-dimensional Besser® block walls, and natural oyster shell cultch have been successful in growing oysters in Pumicestone Passage if they are kept free of silt (Boström-Einarsson et al., 2023; Diggles, 2018). The gardens consisted of monocultural rock oysters, and polycultural gardens of rock oysters, leaf oysters (Isognomon ephippium) and hairy mussels (Trichomya hirsuta). Boström-Einarsson et al. (2023, p. 246–248) reported total of 56 invertebrate taxa were found in the polyculture gardens, and 36 in the monoculture gardens, comprising mostly amphipods, decapod crustaceans and polychaetes. Twelve fish taxa were identified across all oyster gardens, with 10 species present in polyculture cages, and five species present in monoculture and control cages, dominated by the Oyster Blenny and juvenile Butter Bream. Clearly the polycultural gardens are more successful in attracting a greater variety of species and creating an ecosystem. Diggles (2018, p. 30) considers the restoration of subtidal shellfish reefs in Pumicestone Passage using natural recruitment processes feasible, particularly “if appropriately designed clean settlement substrates (preferably natural shell cultch) are placed into the ecosystem during natural recruitment periods in late spring and throughout the summer months.”
In view of the concerns of Gillies et al. (2018) it is heartening that community-driven projects have recently begun in both Moreton Bay and the Great Sandy Strait with a view to reef restoration/re-establishment with the goal of forming ecosystems rather than a monocultural commercial resource. The Moreton Bay project is a partnership between OzFish, Healthy Land and Waterways, and the Australian Government's National Landcare Program. Over a 10-year period, the project will involve construction and deployment of 50,000 triangular-shaped “robust oyster baskets” (ROB) containing recycled oyster shells over an area of 100 hectares near the Port of Brisbane. Surveys on ROBs in place since 2019 showed the average number of shellfish on each ROB to be 626 in the subtidal zone, and 2,536 in the intertidal. “Other animals” numbered 1,120 in the subtidal and 748 in the intertidal (https://ozfish.org.au/projects/moreton-bay-shellfish-reef-restoration/). The Great Sandy Strait program is a partnership between The Nature Conservancy, Butchulla Native Title Aboriginal Corporation, The Queensland Government and the local community and aims to deliver shellfish reef restoration within the Great Sandy Strait to help improve biodiversity, water quality, and wetland function. It seems appropriate that the area to be restored/re-established is adjacent to where Booral Shell Mound is located (https://www.natureaustralia.org.au/newsroom/great-sandy-strait-new-project/).
The importance of the involvement of First Nations people in oyster reef and ecosystem restoration extends beyond ecological considerations. Gibbs et al. (2023) consider “Coastal and oyster reef restoration research is uniquely positioned to revive TEK [traditional ecological knowledge], bolster the cultural revitalization of First Nations peoples, and create sustained conservation outcomes.” The Great Sandy Strait restoration, co-managed from the outset by the Butchulla Native Title Aboriginal Corporation, is an example of this approach. The Butchulla view on the collaborative project is that “It is important we continue taking steps together in the journey of healing country. These shellfish reefs are an important part of Butchulla cultural heritage, and it is great to work in partnership on a project restoring our Sea Country,” (https://www.natureaustralia.org.au/newsroom/great-sandy-strait-new-project/, Aunty Joy Bonner). The acknowledgment of the shellfish reefs as a key element of First Nations cultural heritage rather than just a food resource reflects the deep connection to all aspects of the seascape, landscape, and sky scape of which people are a part. Elsewhere in Southeast Queensland, the Quandamooka people of Moreton Bay continue ancestral practices of oyster reef management to ensure sustainability of harvests (Ross and Quandamooka, 1995 in Reeder-Myers et al., 2022); the challenge of recognizing the characteristics of such practices in the archaeological record remains.
The archaeological evidence from BSM demonstrates that people were harvesting oysters in the GSS from at least 3,000 years ago, and that the oyster harvest was sustainable. During ~2,300 years of discard at the site, there were several phases of differing occupational intensity, but no evidence of large, rapid depositional events. Instead, there appears to have been long-term, perhaps seasonal, low-level occupation and discard, although the evidence suggests that the most intensive use of the site was in the period following its initial occupation. The morphological characteristics of the oysters from the assemblage indicate that they did not originate from densely packed reefs, instead resembling bank oysters. The differences in morphology and color of the valves from the lowest level of the deposit, SU3, resemble the characteristics of sand oysters (Kent, 1992; Saville-Kent, 1891) and while acknowledging that taphonomic factors may be at play, the slight increase (albeit in very small numbers) of clustered valves in the upper two SUs may reflect the development of the oyster population being exploited. The valve morphology also speaks to the antiquity and persistence of bank oysters in the Southeast Queensland region. The preference for bank (and in view of the information provided to Jackson in 1939, dredge oysters in some areas) may be the reason that the massive reefs described by Saville-Kent (1891) and Archer (1862 in Smith, 1985) developed—they were not regularly or heavily harvested, and any use was small-scale. As Rick et al. (2016, p. 6572) observed for Chesapeake Bay, hand collection of oysters in nearshore, shallow-water/fringing reefs may “have left significant oyster populations in deeper water free from human harvest. This would have limited the long-term impacts on regional oyster populations by preserving a source population to supply recruits and by leaving substantial portions of the hard substrate and/or overall 3D reef structure intact.” Once commercial fishing began, exploitation of the reefs was at a very much larger scale, changing the reef structures and leading to degradation and collapse. Although not explicit in the literature, no doubt some of the reefs were also dredged.
The assemblage from WP3 suggests that occupation was episodic over a period of ~170 years although the inability to calculate sediment accumulation rates makes the level of use difficult to determine. Oysters are present throughout the midden with the valve heights indicating a mature population at the commencement of deposition. There is evidence of decreased valve heights in SU 2–3 and SU4 (although it is acknowledged that the sample sizes are very small) preceded and succeeded by larger valves; the cause of the decrease is unlikely to be resource depression. Disease or sub-optimal environmental conditions may be factors, demonstrating that even prior to the European incursion the waters of Moreton Bay could be less than pristine (cf Walters, 1992). There are no comparative sites from which information may derived on how widespread or localized these sub-optimal environments were, as there are no other excavated or dated sites on the western side of Bribie Island. There have been extensive excavations at Sandstone Point on the mainland which has ages that overlap WP3, but the analyses of the shellfish have been quantitative rather than qualitative (Nolan, 1986; Walters, 1986). There is no evidence of resource depression or over-exploitation at WP3. Given the richness of the estuarine environment, people just moved somewhere else. The midden scatters along Pumicestone Passage (Figure 4) attest to the availability of habitat patches suitable for exploitation.
Ethnographic analogy should be employed cautiously, but the accounts of Eipper (1841) and Jackson (1939) would seem to support the collection of bank and dredge oysters by First Nations groups observed archaeologically. Eipper (1841), Petrie (1904) and Watkins (1891) all describe oysters as being “roasted” with the implication of prolonged exposure to high temperatures, however no evidence of burning was found on any of the archaeological valves, suggesting that they had only briefly been exposed to low temperature fires, if indeed this was the method used to open them.
The participation of First Nations people in the economy of early Brisbane, in the provision of fish, oysters, and other seafoods was an extension of traditional cultural obligations of reciprocity and trade. It can be argued that these obligations were misunderstood and exploited by the colonists. It can also be argued that, despite the exploitation, First Nations people contributed to the establishment of the oyster and fishing industries that became commercially successful ventures in the role not only of skilled laborers but also because of their specialized local knowledge. First Nations people taught the early European settlers how to fish in the Great Sandy Strait and Tin Can Inlet and played a critical role in the commercial dugong fishery during the 1850s−1870s. First Nations people were also involved in the oyster industry during the 1870s and through into the 20th century. Many locals became professional fishermen, with Percy Wheeler being one of the first Butchulla to obtain a commercial fishing license in 1903 (Woolley, 2016).
The Southeast Queensland commercial oyster industry, based in Moreton Bay, is worth ~$500,000 per year, with a total crop in 2021–2022 of 50,547 dozen, or 606,564 oysters. This is in stark contrast to the value of recreational fin-fishing in Southeast Queensland, including Moreton Bay, which has been estimated at $156 million to $194 million and thus contributes significant economic benefits, especially locally, with boating, bait and fishing tackle industries heavily reliant on this activity (Thurstan et al., 2019). Attempts to re-invigorate the oyster industry include searching for another Queensland native species (non-endemic species are considered a biosecurity hazard), but there is still the need to manage diseases, particularly QX (McDougall, 2020). It begs the question, in view of the inefficiencies noted by West et al. (2019), of why? Is it really worth the effort? Perhaps it is time for the Southeast Queensland oyster fisheries to be considered “boutique” and marketed as such to emphasize their exclusivity and cachet.
The application of an applied historical ecology approach has allowed the identification of sustainable oyster harvesting over a period of some 2,200 years at Booral Shell Mound in the Great Sandy Strait, while also reflecting the development of the oyster population within the harvesting catchment. At White Patch 3, two episodes of sub-optimal environmental conditions have been identified. Exploration of the notion of overfishing leading to the collapse of Southeast Queensland oyster populations, and by association, the commercial oyster industry has instead revealed a complex set of causes. Problems with poor water quality and increased siltation within Moreton Bay and the Great Sandy Strait remain, but the prospects of restoration of oyster reefs in some areas are becoming more positive with the involvement of grass roots (seagrass roots?) groups who are on the ground and water and have a keen interest in the restoration of ecosystems rather than perpetuating a monoculture. They have moved beyond the “shifting baseline.” The involvement of First Nations people in restoration and rehabilitation projects fulfills the ethos of what is good for Country comes first.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
TS: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The analyses were partly funded through an Australian Postgraduate Award to TS.
Access to the assemblages was granted by the Queensland Museum Aboriginal and Torres Strait Islander Consultative Committee and facilitated by Brit Asmussen and Nick Hadnutt of the Queensland Museum. Research space in the University of Queensland Archaeological Science Laboratories was provided by the School of Social Science. Special thanks go to the editorial team of Patrick Faulkner, Iain McKechnie, and Katherine Woo for their invitation to contribute to this special issue.
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Gen AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^Moreton Region Archaeological Project (MRAP) Files. Department of Anthropology and Sociology, The University of Queensland.
Adlard, R. D., and Nolan, M. J. (2015). Elucidating the life cycle of Marteilia sydneyi, the aetiological agent of QX disease in the Sydney rock oyster (Saccostrea glomerata). International J. Parasitology 45, 419–426. doi: 10.1016/j.ijpara.2015.02.002
Backhouse, J. (1843). A Narrative of a Visit to the Australian Colonies. Hamilton, Adams and Co: London.
Beck, M. W., Brumbaugh, R. D., Airoldi, L., Carranza, A., Coen, L. D., Crawford, C., et al. (2011). Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience 61, 107–116. doi: 10.1525/bio.2011.61.2.5
Blake, T., and Osborne, P. (2008). Deception Bay: The History of a Seaside Community. Caboolture: Caboolture Shire Council.
Boström-Einarsson, L., Martinez-Baena, F., Diggles, B., Firby, L., and McLeod, I. M. (2023). An ecological assessment of Australia's first community oyster gardens. Ecol. Manag. Restor. 23, 244–251. doi: 10.1111/emr.12565
Buroker, N. E., Hershberger, W. K., and Chew, K. K. (1979). Population Genetics of the Family Ostreidae. I. Intraspecific Studies of Crassostrea gigas and Saccostrea commercialis. Mar. Biol. 54, 157–169. doi: 10.1007/BF00386595
Campbell, S. J., and McKenzie, L. J. (2004). Flood related loss and recovery of intertidal Seagrass meadows in southern Queensland, Australia. Estuarine Coast. Shelf Sci. 60, 477–490. doi: 10.1016/j.ecss.2004.02.007
Carpenter, C. E., and Niem, V. H. (1998). The Living Marine Resources of the Western Pacific, Volume 1. Virginia: Old Dominion University Press.
Catterall, C. P., and Poiner, I. R. (1987). The potential impact of human gathering on shellfish populations, with reference to some NE Australian intertidal flats. Oikos 50, 114–122. doi: 10.2307/3565407
Coaldrake, J. E. (1961). The Ecosystems of the Coastal Lowlands (“Wallum”) of Southern Queensland. Melbourne: CSIRO.
Cook, M. (2019). A River With a City Problem: A History of Brisbane Floods. St Lucia, Queensland: University of Queensland Press.
Crooks, J. (1982). Report on Three Excavations at Pumicestone Passage, Moreton Bay, Southeast Queensland. Unpublished BA (Hons) thesis, Department of Anthropology and Sociology, The University of Queensland, Brisbane.
Department of Agriculture and Fisheries (2015). Oyster Industry Plan for Moreton Bay Marine Park. Department of Agriculture and Fisheries, State of Queensland.
Department of Agriculture and Fisheries (2022). Aquaculture Production Summary for Queensland 2021–22. Department of Agriculture and Fisheries, State of Queensland.
Diggles, B. K. (2013). Historical epidemiology indicates water quality decline drives loss of oyster (Saccostrea glomerata) reefs in Moreton Bay, Australia. N. Z. J. Mar. Freshwater Res. 47, 561–581. doi: 10.1080/00288330.2013.781511
Diggles, B. K. (2018). “Annual pattern of settlement of sydney rock oyster (Saccostrea glomerata) spat in pumicestone passage, Moreton Bay,” in Proceedings of the Royal Society of Queensland. doi: 10.5962/p.357815
Eipper, C. (1841). Observations made on a journey to the natives at Toorbul, August 12, 1841. Colonial Obser. 1:10.
Field, A. (2014). Discovering Statistics Using IBM SPSS Statistics: and Sex and Drugs and Rock ‘n' roll. (4th edn.) Los Angeles: Sage.
Fison, C. S. (1889). Report on the Oyster Fisheries of Moreton Bay and the Great Sandy Strait. Brisbane: Government Printer. doi: 10.5962/bhl.title.12854
Frankland, K. (1990). Booral: A Preliminary Investigation of an Archaeological Site in the Great Sandy Strait Region, South-East Queensland. Unpublished BA (Hons) thesis, Department of Anthropology and Sociology, The University of Queensland, Brisbane.
Gibbs, M., Ross, P., Scanes, E., Gibbs, J., Rotolo-Ross, R., and Parker, L. (2023). Extending conservation of coastal and oyster reef restoration for First Nations cultural revitalization. Conserv. Biol. 37:e14158. doi: 10.1111/cobi.14158
Gillespie, R., and Temple, R. B. (1979). Sydney university natural radiocarbon measurements 5. Radiocarbon 21, 95–106. doi: 10.1017/S0033822200004227
Gillies, C. L., McLeod, I. M., Alleway, H. K., Cook, P., Crawford, C., Creighton, C., et al. (2018). Australian shellfish ecosystems: Past distribution, current status and future direction. PLoS ONE 13:e0190914. doi: 10.1371/journal.pone.0190914
Glasby, C. (2011). “Marine Bristle Worms (Class Polychaeta),” in Wild Guide to Moreton Bay and Adjacent Coasts 2nd edition, ed. P. Davie (Brisbane: Queensland Museum), 103–105.
Gosling, E. (2003). Bivalve Molluscs: Biology, Ecology and Culture. Oxford: Blackwell Publishing.re doi: 10.1002/9780470995532
Grayson, D. K. (1984). Quantitative Zooarchaeology: Topics in the Analysis of Archaeological Fauna. Orlando: Orlando Academic Press.
Grayson, D. K. (2001). The archaeological record of human impacts on animal populations. J. World Prehist. 15, 1–68. doi: 10.1023/A:1011165119141
Hall, J. (1982). “Sitting on the crop of the bay: an archaeological and historical sketch of aboriginal settlement and subsistence in Moreton Bay, southeast Queensland,” in Coastal Archaeology in Eastern Australia: Proceedings of the 1980 Valla Conference on Australian Prehistory, ed. S. Bowdler (Occasional Papers in Prehistory No. 11. Canberra: Department of Prehistory, Research School of Pacific Studies, The Australian National University), 79–95.
Healy, J., Norman, M., Potter, D., and Willan, R. (2011). “Molluscs,” in P. Davie (ed.), Wild Guide to Moreton Bay and Adjacent Coasts 2nd edition (Brisbane: Queensland Museum), 119–196.
Healy, J. M., and Potter, D. G. (2010). A preliminary checklist of the marine bivalves (Mollusca: Bivalvia) of Moreton Bay, Queensland. Mem. Queensl. Museum-Nature 54, 235–252.
Hekel, H., Ward, W. T., Jones, M., and Searle, D. E. (1979). “Geological development of northern Moreton Bay,” in Northern Moreton Bay Symposium: The Proceedings of a Symposium held at the Abel Smith Lecture Theatre, University of Queensland, September 23–24, eds. A. Bailey, and N.C. Stevens (Brisbane: Royal Society of Queensland), 7–18.
Hogg, A. G., Heaton, T. J., Hua, Q., Palmer, J. G., Turney, C. S. M., Southon, J., et al. (2020). SHCal20 Southern Hemisphere calibration, 0–55,000 years cal BP. Radiocarbon 62, 759–778. doi: 10.1017/RDC.2020.59
Huntley, J. W., and Scarponi, D. (2015). Geographic variation of parasitic and predatory traces on mollusks in the northern Adriatic Sea, Italy: implications for the stratigraphic paleobiology of biotic interactions. Paleobiology 41, 134–153. doi: 10.1017/pab.2014.9
Jackson, G. K. (1939). Aboriginal middens of Point Cartwright district. Mem. Queensl. Mus. 11, 289–295.
Kennedy, E. B. (1872). “Dugong fishing in Queensland,” in South Australian Chronicle and Weekly Mail.
Kent, B. W. (1992). Making Dead Oysters Talk: Techniques for Analyzing Oysters from Archaeological Sites. Baltimore: Maryland Historical and Cultural Publications for Maryland Historical Trust, Historic St. Mary's City, Jefferson Patterson Park and Museum.
Kerkhove, R. (2013). Aboriginal trade in fish and Seafoods to settlers in nineteenth-century south-east Queensland: a vibrant industry? Queensl. Rev. 20, 144–156. doi: 10.1017/qre.2013.17
Kirby, M. X. (2004). Fishing down the coast: historical expansion and collapse of oyster fisheries along continental margins. Proc. Natl. Acad. Sci. USA. 101:13096. doi: 10.1073/pnas.0405150101
Lam, K., and Morton, B. (2006). Morphological and mitochondrial-DNA analysis of the Indo-West Pacific rock oysters (Ostreidae: Saccostrea species). J. Molluscan Stud. 70, 235–245. doi: 10.1093/mollus/eyl002
Lamprell, K. L., and Healy, J. M. (1998). Bivalves of Australia, Volume 2. Leiden: Backhuys Publishers.
Lang, S. C., McClure, S. T., Grosser, M., and Herdy, T. (1998). “Sedimentation and coastal evolution, northern Moreton Bay,” in Moreton Bay and Catchment, eds. I. R. Tibbetts, N. J. Hall, and W. C. Dennison (Brisbane: The University of Queensland), 81–92.
McDougall, C. (2020). Reinvigorating the Queensland Oyster Industry. Final Report to the Fisheries Research and Development Corporation, Griffith University, Brisbane, Queensland, November. CC BY 3.0.
McKenzie, L. J., and Campbell, S. J. (2003). Seagrass resources of the Booral Wetlands and the Great Sandy Strait: February/March 2002. Cairns: Queensland Department of Primary Industries.
McKenzie, L. J., Collier, C., and Waycott, M. (2014). Reef Rescue Marine Monitoring Program: Inshore Seagrass. Cairns: TROPwater, James Cook University.
McMahon, T. J. (1924). Brisbane 70 years ago: octogenarian's memories: an interesting story. Brisbane Courier.
McNiven, I. J. (1994). Booral: Cultural Heritage Management Plan. Unpublished report to the Queensland Department of Environment and Heritage, Maryborough.
McNiven, I. J. (2006). “Late moves on Donax: aboriginal marine specialisation in southeast Queensland over the last 6000 years,” in An Archaeological Life: Papers in Honour of Jay Hall, eds. S. Ulm, and I. Lilley (Brisbane: Aboriginal and Torres Strait Islander Studies Unit, The University of Queensland), 109–124.
Nolan, A. (1986). Sandstone Point: Temporal and Spatial Patterns of Aboriginal Site Use at a Midden Complex, South-East Queensland. Unpublished BA (Hons) thesis, Department of Anthropology and Sociology, The University of Queensland, Brisbane.
Ogburn, D. M., White, I., and McPhee, D. P. (2007). The disappearance of oyster reefs from Eastern Australian estuaries: Impact of colonial settlement or mudworm invasion? Coastal Manag. 35, 271–287. doi: 10.1080/08920750601169618
Pallant, J. (2013). SPSS Survival Manual: A Step by Step Guide to Data Analysis Using SPSS (5th edn). London: Routledge.
Parry-Okeden, U. E. (1930). Redcliffe in the early eighties: many memories of the early settlers. Sunday Mail 9:21.
Petherick, L. M., McGowan, H. A., Moss, P. T., and Kamber, B. S. (2008). Late quaternary aridity and dust transport pathways in eastern Australia. Quarter. Austral. 25, 2–11.
Petrie, C. C. (1904). Tom Petrie's Reminiscences of Early Queensland. Brisbane: Watson, Ferguson and Co.
Phillips, C. W. (1929). Old Brisbane: Blacks at the Hamilton: Memories of Mr C. W. Phillips. Brisbane Courier 29 March.
Reeder-Myers, L., Braje, T. J., Hofman, C. A., Elliott Smith, E. A., Garland, C. J., Grone, M., et al. (2022). Indigenous oyster fisheries persisted for millennia and should inform future management. Nat. Commun. 13:2383. doi: 10.1038/s41467-022-29818-z
Rick, T. C., Reeder-Myers, L. A., Hofman, C. A., Breitburg, D., Lockwood, R., Henkes, G., et al. (2016). Millennial-scale sustainability of the Chesapeake Bay Native American oyster fishery. Proc. Natl. Acad. Sci. U.S.A. 113, 6568–6573. doi: 10.1073/pnas.1600019113
Robins, R., Hall, J., and Stock, E. (2015). Geoarchaeology and the archaeological record in the coastal Moreton Region, Queensland, Australia. Quat. Int. 385, 191–205. doi: 10.1016/j.quaint.2015.02.060
Rouse, G. (2000). “The family spionidae,” in Polychaetes and Allies: The Southern Synthesis, eds. P. L. Beesley, G. B. Ross and C. J. Glasby (Fauna of Australia: Australian Biological Resources Study/CSIRO Publishing).
Smith, A. D. T. (1992). An Archaeology Site Location and Subsistence-Settlement Analysis of Bribie Island, Southeast Queensland. Unpublished B.A (Hons) thesis, The University of Queensland, Brisbane.
Smith, A. D. T. (2016). Archaeological Expressions of Holocene Cultural and Environmental Change in Coastal Southeast Queensland. Unpublished PhD thesis, School of Social Science, University of Queensland, Brisbane.
Smith, G. S. (1985). The Queensland Oyster Fishery: An Illustrated History. Brisbane: Queensland Department of Primary Industries.
Steele, J. G. (1975). Brisbane Town in Convict Days, 1824–1842. St Lucia: University of Queensland Press.
Stein, J. K., Deo, J. N., and Phillips, L. S. (2003). Big sites-short time: accumulation rates in archaeological sites. J. Archaeol. Sci. 30, 297–316. doi: 10.1006/jasc.2002.0838
Stockton, J. (1974). Report of an Archaeological Survey in the Vicinity of Bribie Island, Southeast Queensland. Unpublished B A (Hons) thesis, Department of Anthropology and Sociology, The University of Queensland, Brisbane
Stuiver, M., and Reimer, P. J. (1993). Extended 14C data base and revised CALIB 3.0 14C age calibration program. Radiocarbon 35, 215–230. doi: 10.1017/S0033822200013904
Thomson, A. K. (1967). The Collected Works of Thomas Welsby. Vols I and II. Brisbane: Jacaranda Press.
Thomson, J. M. (1954). The genera of oysters and the Australian species. Austral. J. Mar. Freshwater Res. 5, 132–168. doi: 10.1071/MF9540132
Thurstan, R., Fraser, K., Brewer, D., Buckley, S., Dinesen, Z., Skewes, T., et al. (2019). “Fishers and fisheries of Moreton Bay,” in Moreton Bay Quandamooka and Catchment: Past, present, and future, eds. I. R. Tibbetts, P. C. Rothlisberg, D. T. Neil, T. A. Homburg, D. T. Brewer, and A. H. Arthington (Brisbane, Australia: The Moreton Bay Foundation).
Ulm, S. (2002). Reassessing marine fishery intensification in southeast Queensland. Queensl. Archaeol. Res. 13, 79–96. doi: 10.25120/qar.13.2002.70
Ulm, S. (2006). Australian marine reservoir effects: a guide to ΔR values. Aust. Archaeol. 63, 57–60. doi: 10.1080/03122417.2006.11681838
Ulm, S., and Hall, J. (1996). Radiocarbon and cultural chronologies in southeast Queensland prehistory. Tempus 6, 45–62.
Vohra, F. C. (1965). Ecology of intertidal Zostera flats of Moreton Bay (Unpublished Ph.D thesis). Department of Zoology, The University of Queensland, Brisbane, QLD, Australia.
Walters, I. (1986). Another Kettle of Fish: The Prehistoric Moreton Bay Fishery. Unpublished Ph.thesis D, Department of Anthropology and Sociology, The University of Queensland, Brisbane.
Walters, I. (1992). Farmers and their fires, fishers and their fish: production and productivity in pre-European south-east Queensland. Dialect. Anthropol. 17, 167–182. doi: 10.1007/BF00258089
Walters, I., Lauer, P., Nolan, A., Dillon, G., and Aird, M. (1987). Hope Island: salvage excavation of a Kombumerri site. Queensl. Archaeol. Res. 4, 80–95. doi: 10.25120/qar.4.1987.173
Watkins, G. (1891). Notes on the aboriginals of stradbroke and Moreton Island. Proc. R. Soc. Queensl. 8, 40–50. doi: 10.5962/p.351167
West, E., Conacher, C., Dexter, J., Lee, P., Heidenreich, M., and Paterson, B. (2019). “Aquaculture in Moreton Bay,” in Moreton Bay Quandamooka and Catchment: Past, present, and future, eds. I. R. Tibbetts, P. C. Rothlisberg, D. T. Neil, T. A. Homburg, D. T. Brewer, and A. H. Arthington (Brisbane, Australia: The Moreton Bay Foundation).
White, P. M., and Beumer, J. P. (1997). Survey and Classification of Oyster Growing Areas 1993–1995. Unpublished Report to Queensland Department of Primary Industries, Brisbane.
Willmott, W. F., and Stevens, N. C. (1988). Rocks and Landscapes of the Sunshine Coast. Brisbane, QLD: Geological Society of Australia; Queensland Division.
Keywords: First Nations, coastal archaeology, Southeast Queensland, applied historical ecology, archaeomalacology
Citation: Smith T (2025) Three thousand years of oyster fisheries: a view from Southeast Queensland, Australia. Front. Environ. Archaeol. 4:1517380. doi: 10.3389/fearc.2025.1517380
Received: 26 October 2024; Accepted: 06 January 2025;
Published: 27 January 2025.
Edited by:
Iain McKechnie, University of Victoria, CanadaReviewed by:
Melanie Fillios, University of New England, AustraliaCopyright © 2025 Smith. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tam Smith, dGFtLnNtaXRoQHVxLmVkdS5hdQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.