
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Energy Res., 27 January 2025
Sec. Energy Storage
Volume 12 - 2024 | https://doi.org/10.3389/fenrg.2024.1465349
The global energy landscape is currently facing an unprecedented crisis. To address these difficulties, it is vital to create efficient and reliable energy storage and converting technologies. This review discusses the two important technologies; Water Splitting and Li-ion batteries for energy storage. Lithium-ion battery revolutionised convenient devices and electric motors with their higher energy-density, prolonged efficiency, and decreasing costs. Concurrently, Water splitting offers a pathway for hydrogen generation a clean fuel with high energy density, through electrolysis process. In this analysis, we will explore at the most recent breakthroughs, as well as the latest materials and catalysts, boosting the productivity and economic viability of water splitting. Electrode materials, electrolytes, and battery architectures that enhance performance and safety for Li-ion batteries are discussed. The integration of these technologies within renewable energy systems, highlighting their complementary roles in achieving carbon neutrality are also addressed in this review. We underscore the critical importance of water splitting and lithium-ion batteries in the sustainable energy landscape, through a comprehensive analysis of current research and future directions.
In order to rationalize the impending energy problem and ecological suffering, energy storage devices are essential (Kim et al., 2020). Lithium-ion batteries and water splitting both are essential innovations for storing energy and renewable energy. Water is split using electricity to produce hydrogen and oxygen gas through procedures including electrolysis and photo-electrochemical splitting (Bashir et al., 2021; Theerthagiri et al., 2020). This process can lead to sustainable energy solutions because the hydrogen produced is a source of renewable energy which might be utilized to power fuel cells (Zhang B. et al., 2021; Chen et al., 2022).
It’s a process of dividing water molecules using electrical energy. Also referred to as water decomposition. PEM and alkaline electrolysis both remain fundamental techniques for achieving this procedure. Whereas alkaline electrolysis uses a liquid alkaline electrolyte, PEM electrolysis uses a solid polymer electrolyte membrane solution. Water splitting happens at the anode and cathode in that order, which is driven by electric current in both processes.
Water splitting produces hydrogen, which is a storable, adaptable, and clean energy source. It may be utilised straight into fuel cells to produce power with minimal emissions and excellent efficiency. Hydrogen may also be transported and stored for use in various industries, power plants, and transportation-related uses. Water-splitting has a potential way for maintaining intermittent sustainable energy sources such as wind-solar power; it also enables grid stabilisation and decarbonisation programmes.
Lithium-ion battery (LIB) technology is developing quickly, which encourages its widespread use in mobile electronics, electric vehicles (EVs), and aircraft (Yang et al., 2024).
During charge and discharge cycle, Li-ions move interchangeably across the electrode interface of theses rechargeable batteries. These batteries utilize graphite or other carbon containing substances as anodes, and Li-containing compounds such as LCO or LFP as cathodes. Typically, a nonpolar solvent is utilized to dissolve the electrochemical intermediate, which is Li-salt. The batteries are well-suited for multiple functions because of the excellent energy-density, prolonged life-span, and minimal self-discharge percentage. In a scenario of sustainable energy integration, LIBs are critical to store surplus energy from renewables during low consumption intervals so that it is likely to be used over the periods of high consumption. This stabilises the system, lowers greenhouse gas emissions, and reduces reliance on fossil fuels. Lithium ions are transferred between electrodes during charging and discharging cycles, as opposed to lithium-ion batteries, which have been widely employed in electronic devices, E-cars, and grid storage (Shchurov et al., 2021; Wen et al., 2020). Because of its high energy density, extended cycle life, and low self-discharge rates, lithium-ion batteries have played an important role in facilitating the transition to electric vehicles and the use of renewable energy sources (Shchurov et al., 2021).
Water splitting and lithium-ion batteries are two significant technologies driving the transition to sustainable energy. LIBs are practical and scalable energy storage alternatives for diverse uses, whereas water splitting provides a technique for storing sustainable energy in the form of hydrogen. Combining these technologies with sustainable power sources, such as solar-wind energy, can provide a more robust, sustainable, and decentralised energy infrastructure that meets the growing global demand for clean, predictable energy.
A sustainable method for producing hydrogen, a clean energy source with a wide range of uses, is water splitting. Hydrogen production becomes emission-free by using renewable sources of electricity for electrolysis, like solar or wind energy. It reduces the dependence on nonrenewable sources and helps to mitigate the emission of greenhouse gases (Aravindan et al., 2023). Moreover, hydrogen has high storage and transfer efficiency, offering more options for energy transmission and storage. Water splitting thereby contributes to the decarbonization of industries including transportation, manufacturing, and power production, making the energy system more sustainable.
Together water splitting and lithium-ion batteries offer significant parts in solving energy sustainability and environmental concerns (Fu et al., 2022; Hassan et al., 2023; Budama et al., 2023). Both lithium-ion batteries and water splitting are essential for tackling environmental issues and energy sustainability, each providing a special contribution to the change towards a clean and sustainable energy landscape (Aravindan et al., 2023; Bashir et al., 2021).
Water splitting is significant in so many ways some are discussed here.
Employing renewable resources such as sunlight and wind-energy, water splitting offers sustainable method of creating hydrogen fuel (Yu et al., 2021; Acar et al., 2016; Rafique et al., 2020).
Water splitting produces hydrogen, which may be stored and utilised as a carrier of energy for multiple uses, such as transportation, industrial, and power generation. In order to combat grid instability and energy intermittency, it provides a way to store renewable energy produced from sporadic sources (Tee et al., 2017; Mlilo et al., 2021).
Water splitting produces hydrogen, which may be used in fuel cells to create energy with no emissions. This helps with the endeavor to decarbonize industries and sectors including power production, transportation, and industry. It provides a practical substitute for remnant fuels in addition of decreasing the special effects of climate alteration (Yu et al., 2021; Mehrpooya et al., 2021).
Lithium-ion batteries are critical for storing energy in Electric vehicles (EVs), portable gadgets, and grid energy systems due to the fact that their energy density is higher, long cycle life, and rapid charging capabilities. By reducing infrastructure investments, enhancing grid dependability, meeting demand with supply, and storing renewable energy throughout low demand, using it in high demand, they stabilise the system. Electric vehicles EVs run on lithium-ion batteries, which makes it possible to electrify transportation and lessen reliance on fossil fuels. When we compare EVs to cars having internal combustion engines, former are greener and more environment friendly, lowering Greenhouse Gas (GHG) release and pollution in the atmosphere.
Electric vehicles and widespread use of renewable energy are made possible by lithium-ion batteries. Through energy storage produced by sporadic re-newable resources like solar and wind power, LIBs contribute to improved grid stability and dependability and assist balance supply and demand. This capacity promotes the energy sector’s utilization of renewable sources, which lowers fossil fuel reliance and carbon emissions. Li-ion battery-powered vehicle electrification also improves air quality and public health by minimizing GHG emissions and air pollution. Lithium-ion batteries are therefore crucial to the shift towards a greener, more sustainable future.
In conclusion, Li-ion batteries and water splitting are essential technologies for boosting energy sustainability and addressing environmental issues. They make it possible to use renewable resources efficiently.
Through the process of water splitting, hydrogen can be produced directly from the breakdown of water into its constituent parts as can be seen in Equation 1. Breaking two O-H bonds requires the energy needed to divide a single H—O—H (water) molecule (Figure 1). Bond’s dissociation energy of each O—H bond is between 460 and 500 kJ/mol. The total energy required would be between 920 and 1,000 kJ/mol to split one water molecule and break both bonds. Please be aware that these are approximations as the real energy may vary depending on the particular circumstances surrounding the bond dissociation (Sorensen et al., 2020).
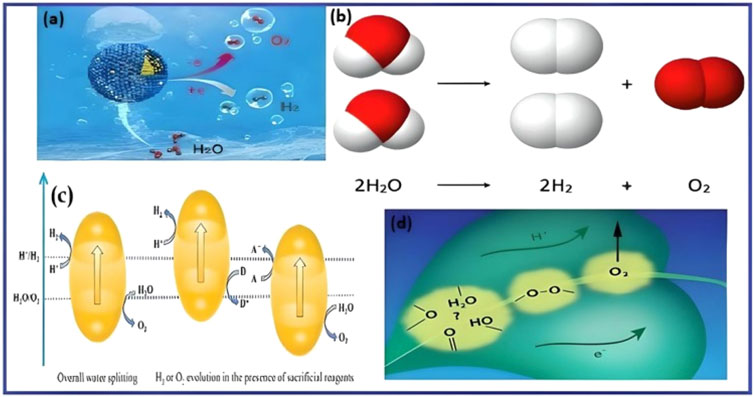
Figure 1. (A) A core-shell alloy nanocatalyst made of Au cores and AuIr2 alloy shell (Au@AuIr2) with partly oxidised surfaces improved water splitting efficiency in acidic conditions. Used with permission from Wang H. et al. (2021). (B) Diagram of the chemical equation of the electrolysis of water. Republished with permission from Ampah et al. (2024). (C) Schematic diagram of photocatalytic water splitting. Reproduced with permission from Junaid et al. (2023). (D) Photosynthetic water splitting (Davis et al., 2018).
Through different power sources, such as electrical current, thermal heat, or electromagnetic radiation, can provide H bonds. Water splitting procedures generally varies depending on whether an energy source—such as electrolysis, thermolysis, or photolysis—is used to carry out the reaction (Gopinath and Marimuthu, 2022).
Reduction occurs at the cathode i,e negative electrode, here hydrogen gas (H2) and hydroxide ions are formed from water molecules which had gained electrons. This reaction is shown in Equation 2 as,
The oxidation (Equation 3) process occurs, at anode i,e positive electrode, here to form oxygen gas (O2), protons (H+), and electrons. Water molecules lose electrons
Reaction involved in water-splitting is given by the equation,
Equation 4 illustrates the electrolysis of 1 mole of water produces 1 mole of hydrogen and half a mole of oxygen gas. The thermodynamic potentials and the first law of thermodynamics are employed in the process. Thermodynamic properties’ table, offers the relevant parameters, assuming 298K temperature and pressure of 1 atm (Petrovic, 2021). The two half-cell reactions that are utilised to accomplish water splitting are water reduction and water oxidation (Figure 2). The process HER is the name given to the reduction of protons on cathode;
Figure 2. (A) Schematic showing the anode and cathode locations for a two-electrode arrangement with NF/T (Ni3S2/MnS–O) for overall water splitting. Used by permission from Zhang et al. (2019). (B) The NiCo-nitrides/NiCo2O4/GF couple electrolyzer is depicted in the photos. Used by permission from Liu et al. (2019).
OER, the oxidation of water,
Water is hence very stable and very slightly conductive; electrolytes such as K3PO4, H₂SO₄, and KOH buffer increase the conductivity of water, resulting in an acidic, basic, and neutral medium.
In low pH medium;
In high pH medium;
In neutral media;
Water electrolysis, conducted under optimal pH conditions, has both advantages and disadvantages. In mediums which are acidic Equations 7, 8, the effectiveness of hydrogen reversible electrolysis (HER) is exceptional due to the availability of protons for immediate charge and discharge. However, OER can only be catalyzed by oxides of pricey and valuable ruthenium (Ru) or iridium (Ir) alloys at low over potential. Few electrocatalytically active oxides, such IrO2 and RuO2, have metallic-type conductivity. This is because the modest electronegativity difference between metals and oxygen prevents a band gap from forming (Anantharaj et al., 2021; Yu et al., 2023; Devadas et al., 2020; Qin et al., 2024). The use of less costly 3d transition metal oxides or hydroxides significantly increases OER efficiency in alkaline media, but minimal H2 generation occurs due to insufficient H+ supply (Liu et al., 2021) (Equations 9, 10).
From Figure 3 the oxygen evolution reaction can be seen. This reaction has slow kinetics, so it is frequently seen as the primary barrier in splitting of water, limiting the effectiveness of the energy change. There’s been much research done on the OER mechanism in acidic environments. On the other hand, alkaline media rather than acidic media are used for the majority of experimental investigations. In both acidic and alkaline conditions, the generally recognised OER process involves four electron/proton transfer stages.
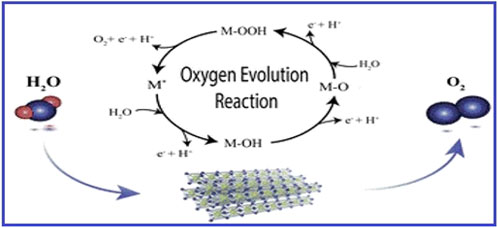
Figure 3. A diagram of Oxygen Evolution Reaction. Reproduced with consent from (Schuler et al., 2020).
In an environment which is acidic, the OER exhibits considerable sensitivity to pH; water molecule undergo oxidation, leading to the release of H+ + e− pairs and oxygen molecules. However, in an alkaline condition, hydroxyl groups (OH−) oxidised to H2O and O2, releasing e− in the process. By utilising oxides or hydroxides of less costly 3d transition metals, OER efficiency rises considerably in alkaline media; nevertheless, H2 generation is minimal because at high pH, there isn’t a rapid H+ supply to the cathode. Water splitting happens in marketable electrolyzers at 1.8–2.0 V, which is a significant cell potential that is 0.55–0.77 V greater than 1.23 V theoretical value. To break reaction system’s energy-related barrier, this extra potential—also referred to as over potential is required.
The catalyst used to catalyse the reaction has a complete influence on its kinetics like Ir-Ru catalysts work efficiently in low pH media as in comparison to alkaline ones. In alkaline conditions, transition metal-based catalysts such as those consisting of Fe, nickel Ni, and Co speed up OER more effectively (Jamesh and Harb, 2021; Liang et al., 2021; Xie et al., 2022).
In the process H2(g) is produced at the cathode during electrolysis, and in water splitting it’s a basic electrochemical mechanism. For the creation of hydrogen (that holds considerable potential as a sustainable and clean energy source). Development of effective water splitting methods require an understanding of the HER.
Hydrogen gas (H2) and hydroxide ions (OH−) are formed when water molecules (H2O) undergo reduction at the cathode (Equation 13), where they pick up electrons (e−). The general response is shown as:
At the electrode-electrolyte interface, electrochemical reduction of water molecules takes place. Whereas cathode offers a surface for this process. The electrode material, surface shape, electrolyte composition, pH, temperature, and applied voltage are among of the variables that affect the HER’s kinetics.
To increase the HER’s kinetics and reduce the overpotential required for hydrogen evolution, efficient catalysts are frequently used. Catalysts encourage the transmission of electrons, facilitate the release of hydrogen gas, and aid in the water molecules’ excitation and adsorption on electrode’s surface (Liu et al., 2022; Peng and Wei, 2020). Because of the strong catalytic activities and sturdiness, transition metals like platinum are frequently utilised as HER catalysts (Bhatt and Lee, 2020). However, research into substitute catalysts based on elements found on Earth, like transition metal oxides, sulphides, phosphides, and carbon-based compounds, has increased because of the expensive rate in addition with restricted availability of noble metals (Noor et al., 2021; Zhang M. et al., 2020). Significant advancements have been made in the last several years in the creation of economical and effective catalysts for the HER. The development of hybrid designs, surface engineering, heteroatom doping, and nanostructured materials has shown promise in boosting the durability and catalytic performance of HER catalysts (Yu et al., 2021; Chen Z. et al., 2021).
Therefore, understanding the principles and dynamics of HER is essential for advancement of water-splitting technologies and realisation for hydrogen’s potential as a clean and sustainable power source. Research in catalyst design, electrochemical engineering, and materials science could contribute to additional advancements in productivity and scalability in water electrolysis for the creation of hydrogen and it can be seen in Figure 4 (Peng and Wei, 2020; Wang S. et al., 2021; Tao et al., 2022). A potential greater than the thermodynamic potential is needed to perform water splitting practically. By utilising highly effective, long-lasting, and environmentally friendly HER and OER electrocatalysts and improving the architecture of the electrolyzer to reduce other resistances, electrochemical water splitting can be made more economical and environmentally benign (Govind Rajan et al., 2020; Bie et al., 2022).

Figure 4. (A) Electrode process of gas electrode. (B): PPT-POP and its composite materials’ electrochemical behaviour in relation to HER. (A) LSV, (B) Tafel plot, (C) impedance and (D) stability studies. Copied with approval from Rajagopal et al. (2020).
Using electrical energy, the procedure of electrolysis converts water into hydrogen and oxygen gases. This takes place at two electrodes: anode and cathode. Former undergoes oxidation, releasing protons and oxygen gas, while the later reduces protons in the water to generate hydrogen gas. While electrolysis compatible with diverse energy systems, including renewable ones like solar or wind power, its scalability and efficiency are limited by the large amount of energy and infrastructure it requires (Aravindan and Kumar, 2023; Ikuerowo et al., 2024; Fragiacomo et al., 2022).
The large sum of energy needed to fuel this reaction is one of the main obstacles for electrochemical reaction. When the bonds between molecules of water are broken, hydrogen and oxygen gases are released. This process requires electrical energy (Yu et al., 2021; Du et al., 2021). The electrolysis system’s efficiency, the water feedstock’s cleanliness, and the operating environment are some of the variables that affect how much energy is needed. Infrastructure for power production, delivery, and conversion is needed for electrolysis systems, in addition to specialised equipment for managing the electrolyte and separating gases. Large-scale electrolysis facilities in particular may find it expensive and resource-intensive to establish and maintain this infrastructure. Furthermore, the infrastructure needs of electrolysis are further increased by the demand for a dependable source of power and grid connection (Zhang C. et al., 2020; Seifert, 2022).
Technological constraints, cost-effectiveness, and energy availability are some of the issues that restrict the scalability of electrolysis (Arsad A. et al., 2023). While electrolysis may be used at many different sizes, ranging from major industrial facilities to small-scale electrolyzers for on-site hydrogen generation, scaling up electrolysis to satisfy significant hydrogen demand necessitates significant expenditures in energy supply and infrastructure. Even with technological advancements in electrolysis, there are still issues with the process’s overall efficiency, especially when taking energy losses in the production, conversion, and transmission of electricity into account (Grigoriev et al., 2020). Enhancing the economic feasibility and environmental sustainability of electrolysis-based hydrogen generation requires improving the efficiency of electrolysis systems through developments in electrolyzer design, electrode materials, and system integration.
One promising method for producing hydrogen from solar energy is photoelectrochemical water splitting. It provides a viable route for environmental friendly formation of hydrogen via the water-splitting process with solar energy. Photoelectrodes in PEC cells are made of semiconductor materials. Semiconductor are used to collect solar radiation and produce electron-hole pairs, or excitons. At the cathode, the produced electrons are utilised to decrease water, while at the anode, the holes take part in oxidation processes.
Principle: Photoelectrodes submerged in an electrolyte solution make up PEC cells. Redox processes begin at the electrode-electrolyte interface when photons with enough energy are absorbed by the photoelectrodes and form electron-hole pairs.
Anode Reaction: Water oxidation occurs at the anode, producing oxygen gas and protons, as can be seen from Equation 14;
Cathode Reaction: Proton reduction proceeds at the cathode, yielding hydrogen gas as depicted in Equation 15;
PEC techniques provide a number of benefits, such as the ability to integrate with current photovoltaic technology, high theoretical efficiency, and direct solar-driven water splitting. But obstacles including scalability, poor efficiency, and material stability prevent them from being widely used (Dai et al., 2020; Fernandez-Ibanez et al., 2021; Grape et al., 2020). Figures 5, 6 give the visual presentation of the different PEC and PSC tandem cells by using different materials.
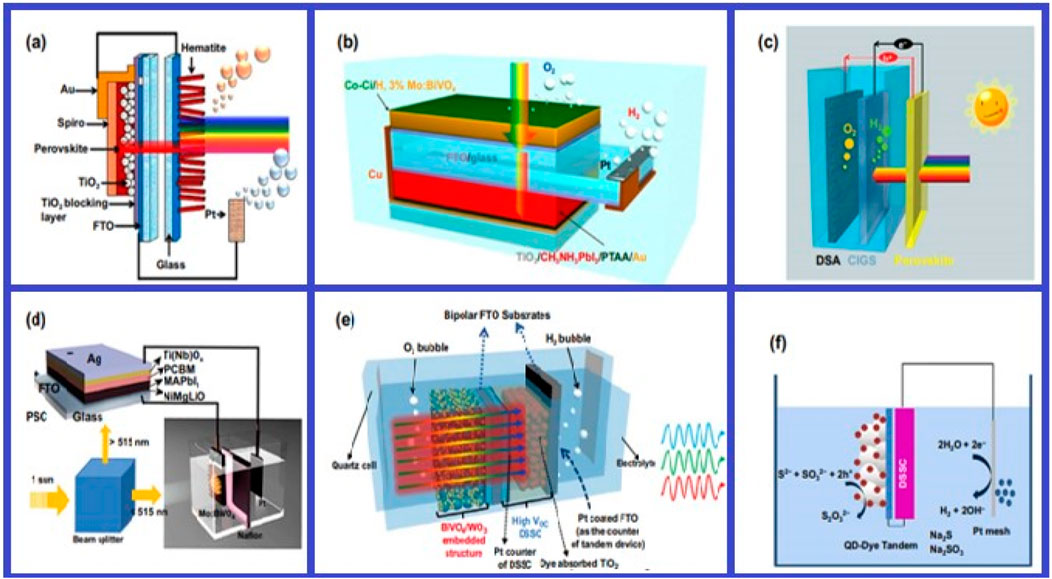
Figure 5. (A) Diagram of the PSC/hematite photoanode tandem cell with dual junctions. Printed with authorization from Dimitriou and Tsujimura (2017). (B) Setup included a tandem cell TiO2/CH3NH3PbI3 and a Co–Ci/H with 3% Mo:BiVO4. Reproduced with authorization (Kim et al., 2015). (C) Diagram of the tandem water-splitting perovskite and CIGS used. Republished with authorization of Luo et al. (2015). (D) The PEC-PSC tandem device’s setup. Republished with authorization of Qiu et al. (2016). (E) Diagram illustrating the photoanode tandem structure of WO3/BiVO4. Republished with authorization of Shi et al. (2015). (F) Plan for the tandem cell PEC-DSSC. Republished with authorization of Zhou et al. (2018).
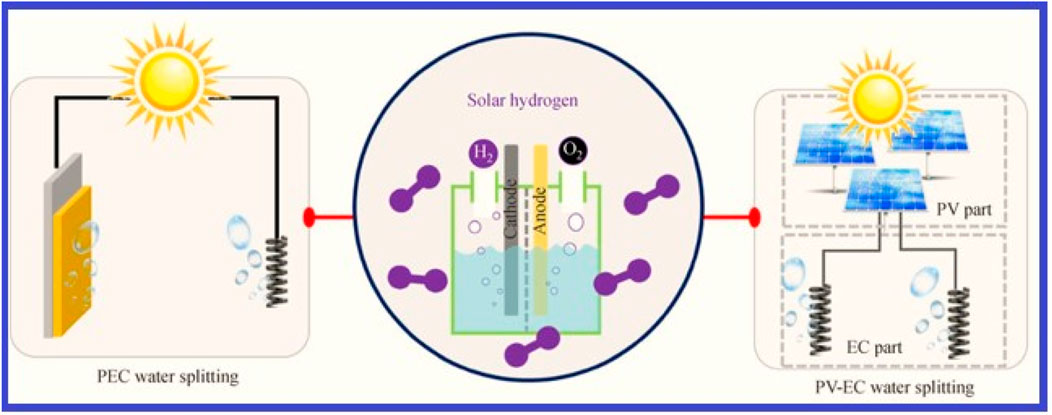
Figure 6. Two methods for producing solar hydrogen using PV-EC and PEC water splitting (Wang Z. et al., 2021).
Rechargeable batteries of the lithium-ion variety are frequently found in energy storage systems, electric cars, and portable devices. These batteries are made up of a separator, an electrolyte, and positive and negative electrodes (Francis et al., 2020). Electrodes, are usually made of materials like LiCoO2 (positive electrode) and graphite (negative electrode), serve as the sites for electrochemical reactions during charging and discharging. After the battery reaches full charge, lithium ions transferred from the LiCoO2 to the graphite electrode. Inversely, following discharge, lithium ions return to positive electrode (Quilty et al., 2023; Rouhi et al., 2021). Lithium ions can travel through the separator, a permeable membrane sandwiched between anode and cathode that prevents short-circuit and direct contact. During charging and discharging, the electrolyte—a conductive solution or gel—helps move lithium ions between the two electrodes (Quilty et al., 2023). It is made up of lithium salts that have been dissolved in a solvent, like a solution of ethylene and dimethyl carbonates. In addition to offering a stable environment for electrochemical processes to take place, the electrolyte is essential for facilitating ion movement (Quilty et al., 2023; Chen M. et al., 2021).
Charge transfer is accomplished by applying a voltage between the two electrodes using an external power source. Both cathode and anode materials oxidise and reduce throughout this process. When a battery is discharging, the lithium ions that have been stored move back through the electrolyte to the positive electrode, producing electrical current that may power electronics (Rouhi et al., 2021; Jiang et al., 2022).When comparing lithium-ion batteries to other rechargeable battery chemistries, they provide an energy density that is unmatched. Because of their high energy storage capacity per-unit volume or weight, they are the best choice for applications where weight and space are crucial considerations. Because of this, Li-ion battery is now a go-to opportunity for supplying power to portable electronic devices including wearable technology, computers, tablets, and smartphones (Wen et al., 2020; Galos et al., 2021). Electronic gadgets can function longer between charges because to lithium-ion batteries’ high energy density. Devices utilised in distant or mobile environments, where access to power sources may be restricted, should pay special attention to this. Longer runtime for users means less frequent recharging, which improves productivity and ease.
This batteries is now an adaptable power source for various uses beyond consumer devices due to their small size and lightweight design (Deng and Aifantis, 2023). Electric cars (EVs), drones, power tools, medical equipment, and renewable energy storage systems are among the many applications for them. Their high energy density makes it possible to store and deliver energy efficiently in a variety of settings, which promotes innovation and technological advancements in a wide range of sectors (Wu et al., 2020). These batteries can maintain charge for extended periods, and are known for their low self-discharge rate (Roth et al., 2023). This is beneficial for infrequently used devices or backup power applications. They also have an extended cycle life, allowing them to withstand hundreds or thousands of battery cycles without noteworthy capacity drop (Gao et al., 2022). The durability contributes to the longevity and reliability of lithium-ion battery-powered devices, hence reducing the overall ownership cost and the frequency of battery replacements. They are sensitive to deep discharging as in Figure 7C we can see the high discharge capacity of SC and PC. These are also sensitive to overcharging, which can cause irreversible damage to electrodes and electrolyte (Ouyang et al., 2022). Overcharging, on the other hand, can cause excessive heat buildup and thermal runaway. To mitigate these risks, lithium-ion batteries have built-in protection circuits and management systems that automatically shut down charging or discharging processes (Mollaei, 2020).
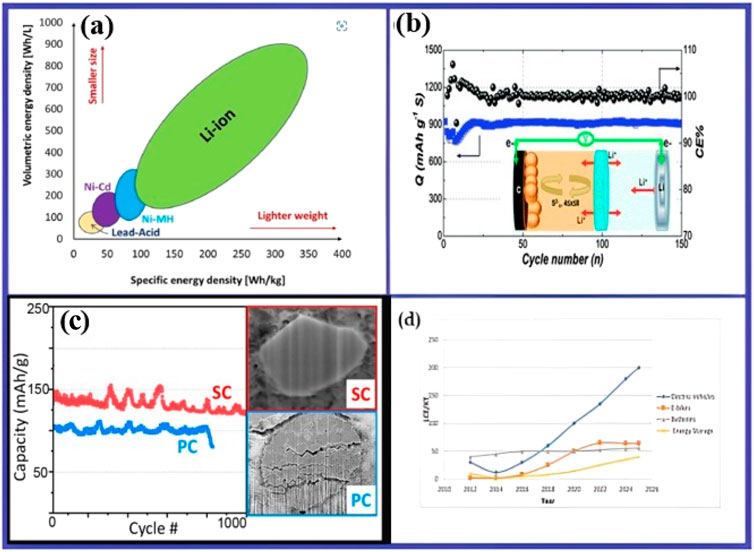
Figure 7. (A) Volumetric and specific energy densities of different battery types. Republished by permission of Nkembi et al. (2024). (B) Schematic diagram showing the Li2S usage in a II-phase electrolyte divided by a lithium super ionic conductor. Reprinted with permission from Wang et al. (2015). (C) Schematic diagram showing high discharge capacity of SC and PC. Reprinted with permission from Kim et al. (2022). (D) Various markets of LIBs. Reprinted from Eftekhari (2019), Khan et al. (2023a).
Recent research is focusing on the advances in the energy-density of LIBs that are essential for extending device runtime, increasing electric vehicle driving range, and enhancing renewable energy storage efficiency. Figures 7A, D give us an understanding of the research done so far in this area and the future of the LIBs. Strategies include optimizing electrode materials, enhancing electrolyte formulations, and exploring advanced battery designs. Safety is a top priority, with recent developments focusing on robust-battery management systems, thermal management techniques, and safer chemistries to minimize thermal runaway and battery fires. Despite their numerous benefits, lithium-ion batteries have faced challenges in cost and affordability (Durmus et al., 2020). Advancements in battery manufacturing processes, electrode fabrication techniques, and materials synthesis aim to reduce production costs and improve the economic viability of lithium-ion batteries (Zhang et al., 2023). This includes developing scalable manufacturing methods, recycling initiatives, and optimizing supply chains to minimize costs associated with raw materials and components. Research is underway to improve new materials for lithium-ion batteries, aiming to enhance sustainability, performance, and environmental impact. Alternative electrodes like silicon-based anodes as depicted in Figure 7B, high-nickel cathodes, and SSEs (Solid-state electrolytes) offer high energy density and enhanced cycling performance.
These batteries are powered via a series of electrochemical processes involving electrode materials, electrolytes, and charging/discharging mechanisms.
Graphite and lithium metal oxide such as lithium cobalt oxide are used as negative and positive electrodes, these are essential to the battery’s functioning. Lithium ions are released from cathode and intercalated to the anode during charging, while reverse happens during discharge (Quilty et al., 2023).
The negative electrode in a Li-ion battery releases Li+ ions to positive electrode. Graphite or other carbon-based materials are commonly used for this purpose. During charging, these ions are subsequently intercalated into the graphite lattice, releasing electrons that power the electronics that are connected. The layered structure of graphite makes reversible lithium ion intercalation possible. Lithium ions return to the positive electrode as the battery is depleted, producing electrical current.
The positive electrode, or cathode, in a lithium-ion battery is typically composed of lithium metal oxides, such as LiCoO2, LiFePO4, or LiMn2O4. During battery discharge, Li+ ions migrate out from anode and return to cathode material, completing an electrochemical process and producing electrical energy. This process occurs during charging and goes to the negative electrode via electrolyte, where they intercalate into the graphite anode. The energy-density, voltage, and life-span of a lithium-ion battery are all influenced by the specific cathode material that is used in it.
Sony Corporation in 1991, first introduced the battery into the market. These are presently the most popular and extensively used rechargeable batteries—have helped PEDs (portable electronic devices) enter a new era. Due to a variety of benefits, including 3.6 V higher voltage, lower self-discharge rate, high energy content, light-weight, excellent safety, and remarkable cycle performance, maintenance-free design, their introduction has been very competitive with other battery kinds. Due to these benefits, Li-ion batteries—which were formerly mostly supplied by Ni-MH and Ni-Cd batteries—are the ideal choice for energy storage in small-sized PEDs, including smart-phones, laptops, cameras, and other electronic devices. Meanwhile, such batteries are growing more and more common for use in aircraft, electric vehicles, and the military (Liang et al., 2019; Costa et al., 2021). Because they are lightweight and small, Figure 8 shows they may be used for lengthy period of time without requiring recharging, which makes them a useful and trustworthy power source for portable gadgets like laptops, tablets, and smartphones. It is impossible to undervalue the contribution lithium-ion batteries provide to smooth communication, productivity, and entertainment—especially in the fast-paced digital world of today (Riaz et al., 2021; Khan et al., 2020; Maiyalagan and Elumalai, 2021). Lithium-ion batteries’ energy storage capacity is essential in order to extend the driving range and improving general performance of ECs. The broad adoption of electric cars is being fueled by the automotive industry’s continued acceptance of sustainable mobility, which results in lowering the emission of GHG and a cleaner environment (Wen et al., 2020; Khan et al., 2023b; Hasan et al., 2021; Ahsan et al., 2022). Furthermore, the stability and effectiveness of renewable energy sources are significantly impacted by the incorporation of Li-ion battery into grid systems for storing energy. These batteries are essential for maintaining grid balance and a steady supply of electricity during times of high demand because they collect and store surplus energy produced by solar power and wind power throughout times of peak output. Supporting the shift to a stronger and sustainable energy infrastructure requires such competence (Kebede et al., 2022; Rana et al., 2023; Datta et al., 2021). To further improve lithium-ion battery performance, safety, and cost, research and development activities must be sustained. Meeting the changing demands of these vital applications and advancing energy storage technology will need research into novel electrode and electrochemical materials in addition to improvements in battery management systems.
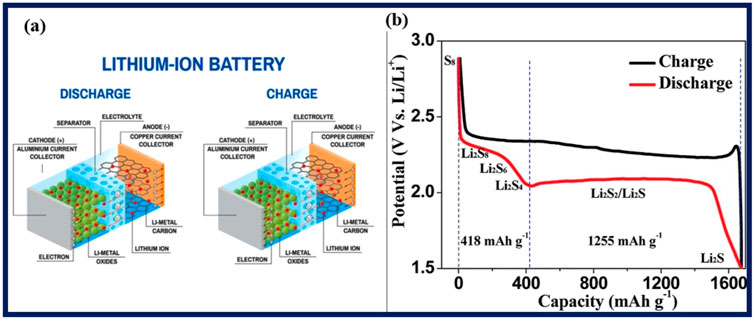
Figure 8. (A) Self-charge and discharge mechanism of LIB. (B) The usual lithium-sulfur battery charge-discharge curve. Copied with authorization by Guo and Liu (2019).
The vital advancement in energy-storing technology, notably for portable electronics, EVs, and grid energy storage systems, has been creation of the LIB (Chen et al., 2020). As the need for dependable and sustainable energy solutions continues to rise, investigation for the potential synergies between water splitting and lithium-ion batteries partakes as a viable path for resolving the issues related to energy generation and storage. When it comes to energy generation and storage, integrating water splitting technologies with lithium-ion batteries can provide a number of benefits (Xu et al., 2023). Water splitting may be used to make hydrogen, which can then be stored as energy. This is particularly helpful in scenarios where hydrogen may be electrolyzed using excess electricity from renewables. Once that’s done, hydrogen will be able to be stored and used as a clean energy source for a variety of applications, such as industrial usage or in fuel cell for electric vehicles.
Moreover, in situations where high energy density and long-term storage are critical, the addition of hydrogen as an energy carrier can improve the performance of lithium-ion batteries. The unpredictable character of renewable energy sources maybe addressed through combining the usage of lithium-ion batteries with hydrogen generated by water splitting, providing a more all-encompassing and environmentally friendly method of energy storage and consumption (Arsalis et al., 2022). Comprehensive investigations into the amalgamation of water splitting and lithium-ion batteries comprise the examination of cutting-edge substances as electrocatalysts, highly effective electrolysis procedures, and inventive techniques for storing and using the hydrogen generated. Furthermore, a cutting-edge field of study with great potential to completely transform the energy landscape is the development of smart energy management systems that seamlessly link water-splitting technology with lithium-ion batteries.
Water splitting technology is still developing, and its confluence with lithium-ion batteries has enormous potential to propel energy generating and storage systems towards sustainability and resilience (Zhong et al., 2024). The search for these synergies highlights how combining different energy technologies may be revolutionary in addressing the growing need for stable and sustainable energy in various sectors.
Both technologies may work well together to enhance energy storage and incorporating sustainable energy resources. Combining the beneficial characteristics of these two technologies can help to overcome the problems associated with energy generation and storage, especially when considering renewable energy sources. An important benefit is combining water splitting with lithium-ion batteries can be the possibility of improved energy utilization and storage (Zhang B. et al., 2021). One potential application for water splitting is the storage of surplus electricity formed by renewables. The process produces hydrogen. Discontinuous nature of renewable resources of energy maybe addressed by using this stored hydrogen as a clean energy source for variety of uses. A more thorough and sustainable method of energy storage and utilization may be accomplished by combining the extended-term storing capacity of lithium-ion batteries with the energy carrier qualities of hydrogen (Khakimov et al., 2024). Furthermore, there are important ramifications for electric cars from the confluence of lithium-ion batteries and water splitting (Sarker et al., 2024). A feasible alternative intended for increasing driving range and in general EC’s performance is the use of hydrogen produced by water splitting as a fuel source for fuel cells (Bethoux, 2020). This integration offers a workable option for the growth of sustainable transportation by addressing the shortfalls of traditional LIBs by regard to long term storage and energy density.
A thorough investigation into the incorporation of water splitting with lithium-ion batteries entails the search for cutting-edge electrocatalyst materials and highly effective electrolysis techniques. Another important area of research opportunity is the creation of creative ways to store and use the hydrogen that is created. To fully realise the benefits of this synergy, smart energy management system development that smoothly combines the two technologies is also essential. Beyond specific applications, the investigation of potential synergies between water splitting and lithium-ion batteries has wider ramifications for the grid-stability and renewable source’s integration (Zhu et al., 2022). Hydrogen produced by water splitting allows for capturing plus storing extra energy of renewable resources and be employed for a number of tasks, including industrial processes and the production of electricity. This technique increases the overall stability and efficacy of renewable resources by supporting the conversion to a new resilient and sustainable energy infrastructure.
An inventive strategy for longer span energy storing solutions is the investigation of hydrogen generated from water splitting as a green energy source for lithium-ion battery charging. This procedure includes electrolyzing water to create hydrogen, which theoretically may be used in fuel cells to generate energy for diverse uses, such as LIB charging (Khakimov et al., 2024).
Because it burns solely to produce water and has a high energy content per weight, hydrogen is becoming more and more popular as an alternative fuel. This might make hydrogen an ecologically benign choice. Hydrogen has the potential to store excess energy and supply electricity when required, which could facilitate the integration of intermittent renewable energy sources such as wind and solar power into the electrical grid. Fuel cells and other energy-conversion and storage systems benefit greatly from hydrogen (Stančin et al., 2020; Rashidi et al., 2022).
The foundation of the concept of using it as a clean energy source to charge lithium-ion batteries is due to hydrogen’s potential as a flexible energy carrier. The hydrogen generated as a result of electrolysis maybe stored and employed in fuel cells to create power again when needed. As hydrogen may be created during times of excess renewable energy and used during shortages, this technology may be very helpful for balancing energy needs. One of its main advantages is the capacity to produce hydrogen from renewable energy sources, which lessens reliance on traditional energy sources and makes the process sustainable. This is in line with international initiatives to move to a carbon-constrained economy. Additionally, this innovation provides a clean substitute for carbon-emitting fossil fuel-based energy sources, as the sole result of hydrogen fuel cells is water. To be practical, the hydrogen cycle—from generation to storage to electricity—must be made sufficiently efficient. The energy efficiency of converting water to hydrogen and subsequently electricity is currently less than that of charging batteries directly with electricity. A portion of this can be attributed to losses that occur during the electrolysis of water, fuel cell conversion, and hydrogen storage and transit (Tashie-Lewis and Nnabuife, 2021). This application does not, however, come without difficulties. Large and reliable storage systems are required because of the low-volumetric energy density of hydrogen (Hassan et al., 2021). Furthermore, the energy efficiency of electrolysis must be taken into account, as the process currently consumes significant amounts of electrical energy, which could offset some environmental benefits if the electricity is not derived from renewable sources (Lamy and Millet, 2020). In addition, safety issues arising from the flammability of hydrogen must be addressed, and the building of set-up for hydrogen making, storing, and transportation is a capital-intensive undertaking. When compared to other energy storage techniques, water electrolysis has a poorer energy efficiency. This is mostly because of energy losses that transpire during the electrolysis process and during the following fuel cell conversion of hydrogen back into electricity. As an energy storage medium, the total round-trip efficiency of hydrogen is frequently less than 50% (Adams et al., 2024). Electrolyzer efficiency normally varies from 60% to 80%, while fuel cell efficiency might vary from 40% to 60% (Escobar-Yonoff et al., 2021).
Other energy storage techniques, on the other hand, can achieve round-trip energy efficiency of more than 90% (Ameen et al., 2023). Because of this, direct electrical storage is currently more effective for uses requiring quick energy charging and discharging, such as grid energy storage or electric cars. The storage of hydrogen is a particularly difficult issue because of its low energy density by volume. Metal hydrides are one type of storage solution that must be carefully constructed to be safe, compact, and able to store enough hydrogen for practical application (Nguyen and Shabani, 2021). Finding effective and stable metal hydrides that can securely store hydrogen at acceptable pressures and temperatures is the subject of research.
Investigating distributed hydrogen production, in which hydrogen is produced at or close to the point of consumption, may help address the infrastructure problem by reducing the need for costly and large transportation networks. Research on safety practices is also continuing because of hydrogen’s flammability and requirement for handling at high pressures or low temperatures. To guarantee that hydrogen systems are inherently safe to operate, cutting-edge materials and technologies must be developed.
In conclusion, although using hydrogen to charge lithium-ion batteries is a promising idea, the subject is still in its infancy and will need to continue advancing due to technological, materials science, and system integration developments in order to get over the current challenges.
To increase overall sustainability, the following developments are being done in hydrogen production.
Scientists are experimenting with cutting-edge designs for electrolyzers, which can provide better efficiencies and can function well with sporadic energy supply notably are solid oxides and proton exchange membrane electrolyzers from renewable sources (Liu et al., 2023). Since electrolyzers are what induce water splitting, developing more efficient electrolyzer technologies is essential to increasing the practicality of hydrogen as a medium which can store energy. The two cutting-edge electrolyzer technologies are broken down as follows.
These electrolyzers shown in Figure 9 work at comparatively low temperatures, about 80°C (176°F), and they use a solid polymer electrolyte. Proton exchange membrane (PEM) electrolyzers are ideally perfect for linking with alternating renewable energy sources because of their short start-up periods and dynamic operation (Lopez et al., 2023). Renewable energy sources are highly effective due to their quick ramp-up/down capability, compact size, and high current densities, enabling them qualify for a range of hydrogen generation capacities.
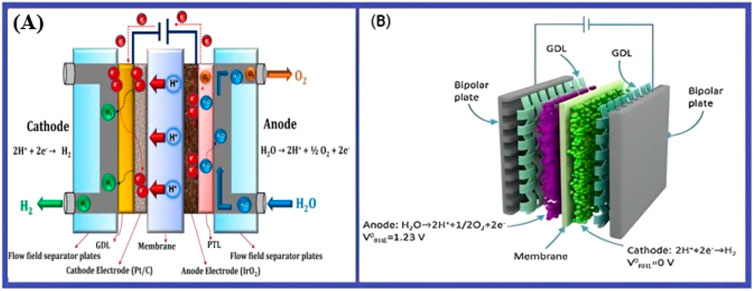
Figure 9. (A) Diagram illustrating the basic principles of PEM water electrolysis. Reprinted with the permission from Kumar and Lim (2022). (B) Schematic diagram of bipolar plates for hydrogen production through PEM (Feng et al., 2017).
These operate at temperatures that are significantly higher, usually between 1292°F and 1472°F (1292°C and 800°C). Since heat may be used for supplying a portion of the energy required for electrolysis, the high temperature functioning of SOEs (Solid Oxide Electrolyzers) has benefits for thermodynamics that allow for improved overall efficiency (Wang F. et al., 2021). This is especially helpful in environments like industrial ones where waste heat is accessible. Furthermore, direct splitting of carbon dioxide and water by SOEs has the potential to yield a combination of hydrogen and carbon monoxide, syngas, which may be utilised in a variety of chemical processes. Materials science advancements are enhancing the production of electrolyzers, lowering costs, increasing efficiency, and extending system lifetimes. Alternative catalyst materials and 3D printing are being explored for cost reduction and creative design. The overall goal of the drive for more efficient electrolyzer technologies is to guarantee that these devices are compatible and work in concert with the larger ecosystem of sustainable energy resources, in addition to optimising devices themselves (Arsad A. et al., 2023).
New catalyst development, increased efficiency, and improved scalability have been the main goals of recent developments in water splitting technology. Below is a summary of some significant developments.
The need for creating multifunctional, high-efficiency electrocatalysts with long-term stability is growing quickly in order to commercialize sustainable hydrogen production using affordable water electrolysis equipment (Janani et al., 2021). The creation of affordable catalysts for water electrolysis is crucial to the effort to increase the effectiveness of water-splitting technologies. By focusing on optimization of electrocatalysts, researchers aim to minimize the energy needed to initiate water-splitting process, thereby increasing its overall efficacy. The exploration of diverse catalytic materials, including transition metal oxides, carbides, and nitrides, presents an avenue for achieving cost-effective and highly efficient electrocatalysts (Das et al., 2022). Leveraging these advanced materials can lead to improved kinetics of water oxidation and hydrogen evolution, thus accelerating the entire process of water splitting. With respect to integrating water-splitting with lithium-ion batteries, the utilization of cost-effective electrocatalysts can further contribute to enhancing the overall conversion efficiency of renewable electricity into storable hydrogen (Liu et al., 2021). This underscores the pivotal role of cost-effective catalysts in advancing high-efficiency electrolysis processes and promoting the seamless integration of water-splitting technologies with energy storage systems.
Here are some of the most notable developments in this field.
Numerous anode materials based on transition metal phosphide (TMP) have been developed recently due of their excellent specific capacity and thermal stability (Park et al., 2023).
TMPs that exhibit good catalytic efficiency for the HER and the OER include nickel phosphide (Ni₂P) and cobalt phosphide (CoP). TMPs offer active sites for the later progression of hydrogen adsorption. They are reasonably priced, have good stability, and have strong catalytic activity.
Perovskite oxides like Ba₀.₅Sr₀.₅Co₀.₈Fe₀.₂O₃-δ are effective for OER because of their adjustable electronic structure and high electrical conductivity (Liu et al., 2024a). They facilitate oxygen evolution by providing multiple oxidation states for transition metals, enhancing oxygen adsorption and formation. The coupling oxygen OER in anode compartments in basic or acidic solutions is often determined by their slow kinetics. Energy barriers in reversible hydrogen electrode (RHE) reactions are measured as overpotential (η), in order to attain a suitable current density (j) of 10 mA cm−, must be overcome. IrO2 and RuO2, two examples of precious and rare metal oxides, are acknowledged as benchmark materials for accelerating OER; nonetheless, they’re costly, they have poor stability, and scarcity prevent their widespread commercial application. Transition metal oxides, sulphides, phosphides, carbon-based materials, and composites such cubic perovskite oxides have been thoroughly investigated for their desirable inherent catalytic activity in an effort to find high-performance, affordable alternatives. Figure 10 provides an overview of the different perovskite oxides.
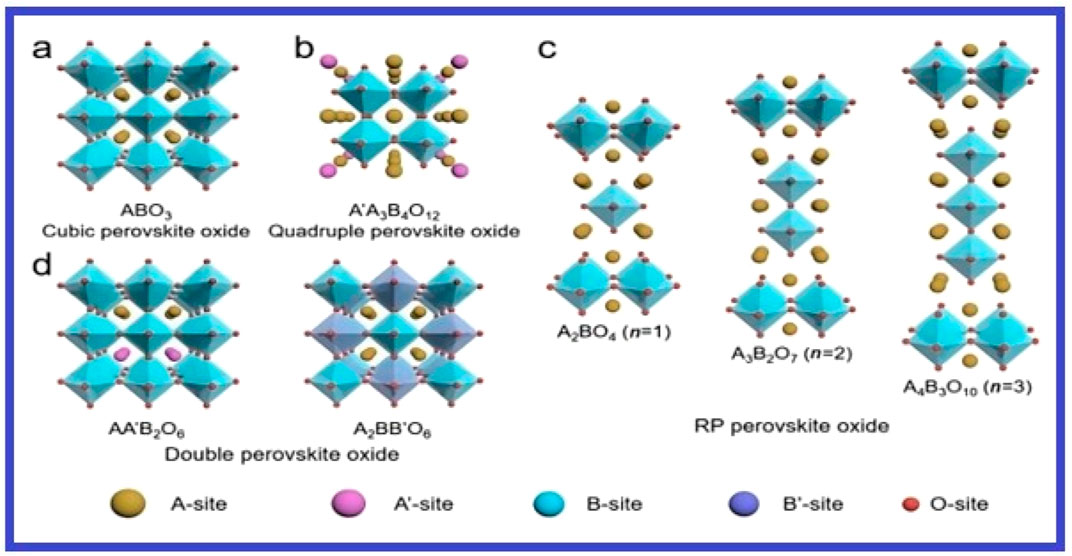
Figure 10. Crystal structures of various kinds of perovskite oxides: (A) Cubic (ABO3), (B) Quadruple (A′A3B4O12), (C) A-site ordered (AA′B2O6) and B-site ordered (A2BB′O6) as well as (D) RP (An+1BnO3n+1, n = 1, 2 and 3) perovskite oxides. Reproduced by approval from Liu et al. (2024b).
Single-metal atoms scattered on a supplementary material, provide excellent catalytic activity with low metal consumption in SACs. For water splitting processes, isolated metal atoms offer consistent, extremely active locations.
Energy conversion technologies heavily rely on electrocatalysts, and single-atom-catalysts is a novel class of catalysts. Due to their high atom utilization efficiency and inherent activity, single-atom catalysts (SACs), which are made up of atomically scattered metal atoms anchored to a support, have garnered a lot of interest like Pd as Figure 11 shows. By virtue of their special qualities and maximal atom-utilization efficiency, SACs enable atomic economy and the effective use of metal resources. It is difficult to create and retain metal centres as atomically distributed locations, nevertheless (Mitchell and Pérez-Ramírez, 2021). The goal of recent developments in wet-chemistry synthetic techniques for SACs is to stabilise individual metal atoms against aggregation and migration. SACs are reviewed along with their existing problems and future development opportunities. SACs have electrochemical applications in the CO2 reduction reaction (CO2RR), Hydrogen evolution reaction (HER), and oxygen reduction reaction (ORR) (Zhang and Guan, 2020).
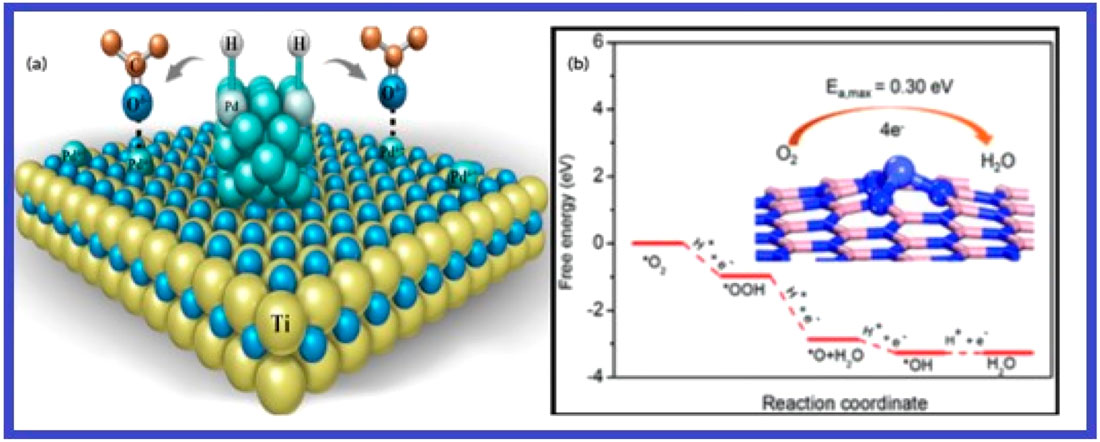
Figure 11. (A) Pd+: Pd single-atom sites for activation of C=O Pd: H2 dissociation sites in Pd nanoparticles. Reprinted from Kuai et al. (2020) with permission. (B) A potential electrocatalyst for the oxygen reduction reaction is a cobalt single-atom catalyst based on a two-dimensional boron nitride material. Published by permission of the Deng et al. (2019).
It’s a two-dimensional material with layered structure and large surface area, has been thoroughly investigated for HER (Ishag and Sun, 2021). Edges in MoS₂ layers provide active sites for hydrogen adsorption and evolution. Abundant, cost-effective, and can be engineered to improve catalytic performance.
With an indirect bandgap of 1.2 eV, bulk molybdenum diseminate is a vertical arrangement of layers linked by weak van der Waals contacts. Its electromagnetic spectrum absorption lies in the visible area, making it useful for a variety of processes like disinfection, energy storage, hydrogen production, and the breakdown of pollutants. Several research has provided an overview of recent developments in 2D MoS2-based material applications and photocatalytic methods (Yuan Y. et al., 2021). Figures 12, 13 illustrate the monolayer MoS2 and related 2D materials in a device for photoelectric applications.
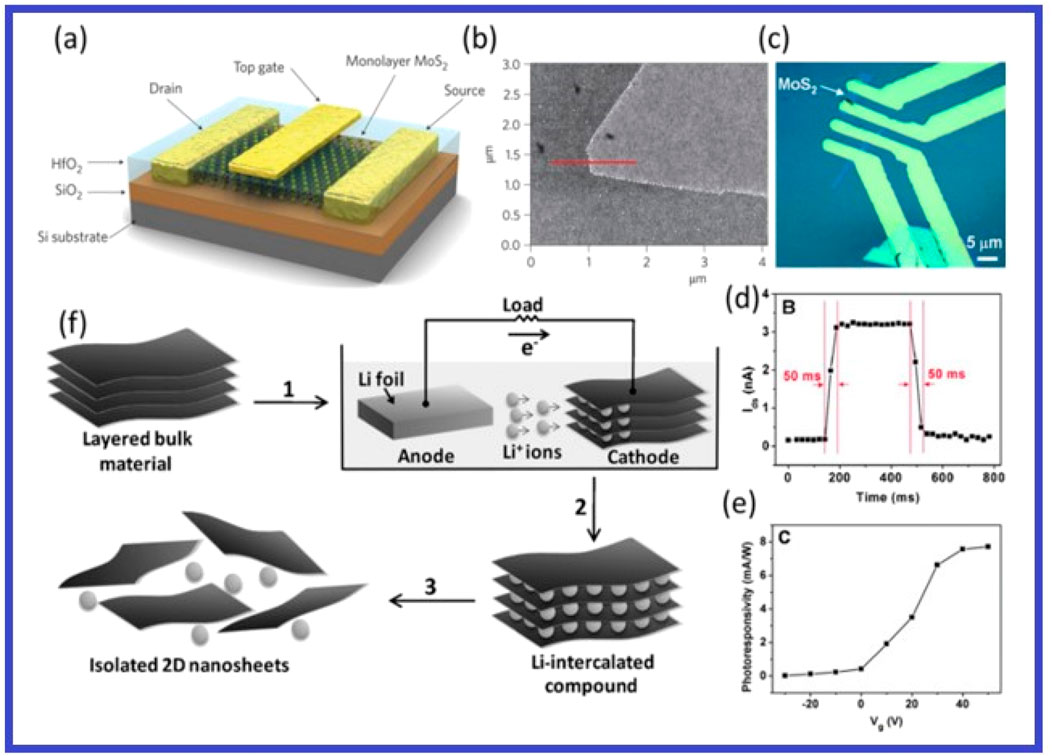
Figure 12. MoS2’s chemical exfoliation and electrical efficiency. (A) MoS2 monolayer transistor schematic view in three dimensions. (B) An AFM picture of single MoS2 coat. The red line crosses MoS2 and reaches to Si substrate, which has an oxide layer that is 270 nm thick. (C) FET device’s optical picture created using a single layer of MoS2. (D) The rate at which a single-layer MoS2 phototransistor switches on and off at Vds = 1 V and Plight = 80 μW. (E) How the gate voltage (Vds = 1 V, Plight = 80 μW) affects photoresponsivity. (F) The technique of electrochemical lithiation, which creates 2D nanosheets from layered bulk material. (A, B) republished with permission from Radisavljevic et al. (2011). Copyright 2011, Rights Managed by Nature Publishing Group. (C–E) reprinted with permission from Yin et al. (2012). Copyright 2012 American Chemical Society. (F) republished with permission by Zeng et al. (2011).
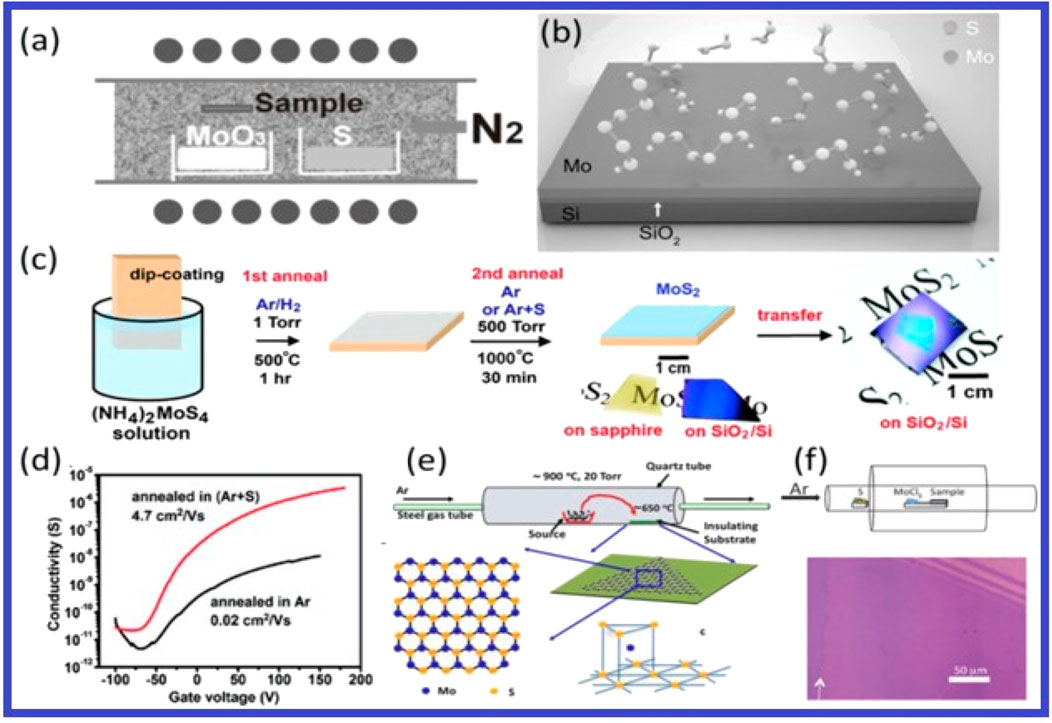
Figure 13. General CVD research of MoS2. (A) Sulfurization of powdered MoO3. Republished with approval by Lee et al. (2012). (B) Sulfurization films of Mo. (C) Schematic diagram of the two-step thermal disintegration of (NH4)2.MoS4. (D) The representative transfer curves for the devices invented using the MoS2 trilayers. Panel c, d reproduced with permission from Liu et al. (2012). (E) Vapor-solid growth from MoS2 powder. Reprinted with permission from Wu et al. (2013). (F) reprinted with permission from McCreary et al. (2014).
These are effective for OER in alkaline solutions due to their layered structure and synergetic effects between nickel and iron. The hydroxide layers facilitate efficient oxygen evolution through electron transfer processes. Low cost, higher activity, and good stability.
Huang with colleagues developed a flexible electrospinning strategy for creating porous nitrogen-doped carbon nanofibers containing NiFe alloy nanoparticles (Jiang et al., 2021). These nanoparticles in basic solution, showed an overpotential of 294 mV and excellent stability. Meng and colleagues used a rapid joule heating method to load the NiFe alloy onto MoO2 surfaces as represented in Figure 14, resulting in superior activity in alkaline seawater. The interfaces of NiFe alloy/MoO2 could improve OER performance (Zhang, 2024).
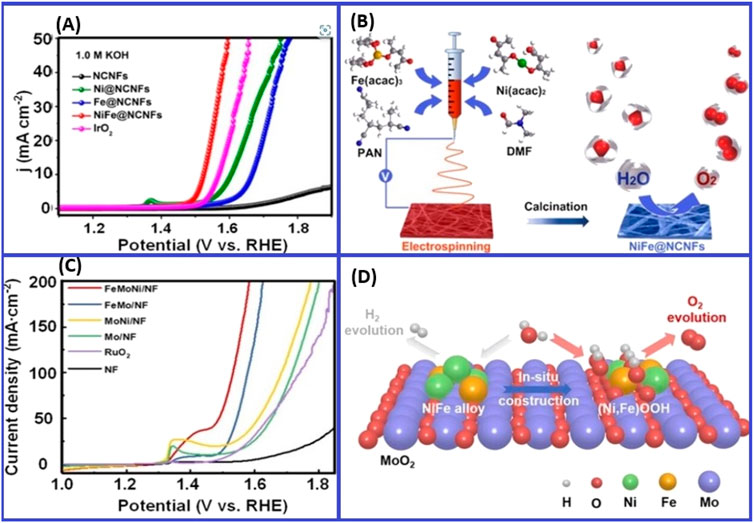
Figure 14. (A) Electrochemical efficiency for OER and Linear Sweep Voltammetry curves intended for diverse catalysts on a scanning rate of 5 mV s–1. Copied with approval from Wei et al. (2020). (B) The production procedure of NiFe@NCNFs (Wei et al., 2020). (C) LSV curves depicting the comparative performance of various catalysts. (D) Graphic representation, illustrating in-situ development of the NiFe alloy/MoO2 and hydrogen evolution reaction/OER for the catalyst. Reprinted with permission from Zhao Z. et al. (2024).
Research in water splitting technology aims to reduce energy input and production costs by developing more efficient catalysts, integrating renewable energy sources, optimizing reactor designs, and improving system efficiency (Yu et al., 2021; Li et al., 2020). Key research directions include developing non-precious metal catalysts like Transition Metal Dichalcogenides (TMDs) and Nickel-Iron Bimetallics, which are cheaper and show high activity for OER and HER. Single atom catalysts SACs optimize the utilization of metal atoms by dispersing them at the atomic level on a support material, resulting in high catalytic efficiency, lower metal usage, and reduced costs. The combination of sustainable energy resources, like photovoltaic integrated electrolyzers and wind-powered electrolyzers, can reduce reliance on external electricity sources and provide a sustainable and economical method of water-splitting. Flow cell designs improve mass transport and efficiency by allowing reactants to flow through the reaction chamber (Ma et al., 2021). 3D-printed or nanostructured electrodes increase reaction surface area, reducing energy input. Research focuses on minimizing overpotentials for HER and OER through electrocatalyst coatings and optimized electrolyte composition. Efficient thermal management systems are being designed to utilize excess heat produced throughout electrolysis procedure, enhancing total system efficacy. Examples of these improvements include porous nickel foam electrodes and nanostructured titanium dioxide.
For hydrogen to be widely used as a sustainable energy source, transportation and storage are essential. Transport and storage expenses can be minimised by using chemical hydrogen storage, which stores hydrogen in chemical compounds. Onsite generation of hydrogen, which involves developing small-scale units, can eliminate the need for transportation and reduce overall costs. Government and private sector funding, such as the U.S. Department HFTO (Energy’s Hydrogen and Fuel Cell Technologies Office) and the European Union’s Horizon 2020, is driving research and development in this field (Kopasz et al., 2022). International collaborations between countries and research institutions are accelerating advancements in hydrogen research. The combination of innovative materials, advanced engineering, and collaborative research is opening the door to additional economical and efficient hydrogen production (Arsad S. et al., 2023).
With notable improvements in energy density, charging speed, and safety, recent developments in lithium-ion battery technology have improved high-performance energy storage in grid storage, electric vehicles, and portable devices while also focusing on cost effectiveness, lifetime, and safety. A thorough depiction of some of the noteworthy developments in Li-ion battery technology can be found here.
Due to its exceptional qualities, including its plentiful supplies, environmental friendliness, exceptional reversible capacity, and a somewhat appropriate working potential, silicon (Si) has garnered a lot of interest as an anode (Zhao H. et al., 2024).
Silicon has a capacity much superior to traditional graphite anodes, it is a material that has the potential to increase energy density. Comparing it with graphite, silicon can store almost 10 times as much lithium. These anodes have energy capacity of approximately 11 times more than that of graphite-based anodes (Zhang C. et al., 2021). While, silicon is inexpensive, safe, and one the abundant elements on earth. Its discharge potential is also extremely low at 0.37 V vs. Li/Li+. But these problems need to be fixed if anodes are being used in military applications. Most silicon-based anodes exhibit low Li+ diffusion rate (≈10–13 cm2s−1), significant volume expansion (≈400%), and poor electronic conductivity (≈10–3 S cm−1). (Toki et al., 2024). The inclusion of carbon materials is a frequent technique for improving conductivity (Figure 15A). Many techniques have been proposed for buffering the volume increase, including pore creation, thin-film manufacturing, and the use of shape-preserving shell designs that can adjust to volumetric changes (Figure 15B). Carbon nanomaterials offer good ionic and electrical conductivity, desirable mechanical properties, and various structural designs for lithium-ion batteries. From Figure 16 it can be observed that a potential approach for Si-based anodes for LIBs is morphological tweaking using nanoparticles, nanowires, nanospheres, and/or nanotubes. Research and development of silicon-based anode materials for lithium-ion batteries that can be recharged are underway.
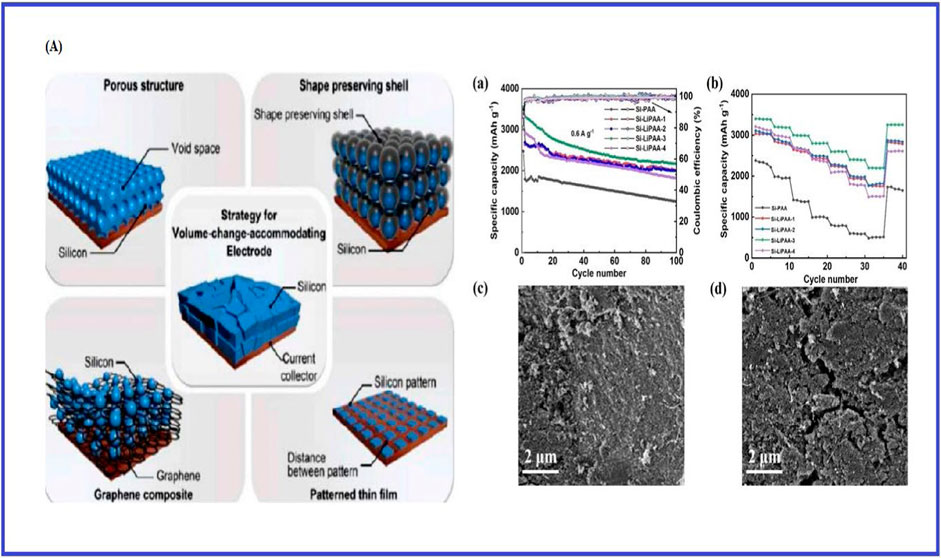
Figure 15. (A) recommended methodology and illustrative constructions of silicon/carbon composite anodes made using different techniques to boost conductivity [Republished from Ko et al. (2015)]. (B) (a) Diagrams showing the specific capacity and coulombic efficiency of several silicon anodes for 100 cycles at 0.6 a g⁻1 of current density. (b) A rate performance test chart with varying current densities for different silicon anodes. (c) After 100 cycles, the si−lipaa-3 electrode’s semi-images (d) under a high-magnification scanning electron microscope. Reprinted by permission of the Zhu et al. (2024).
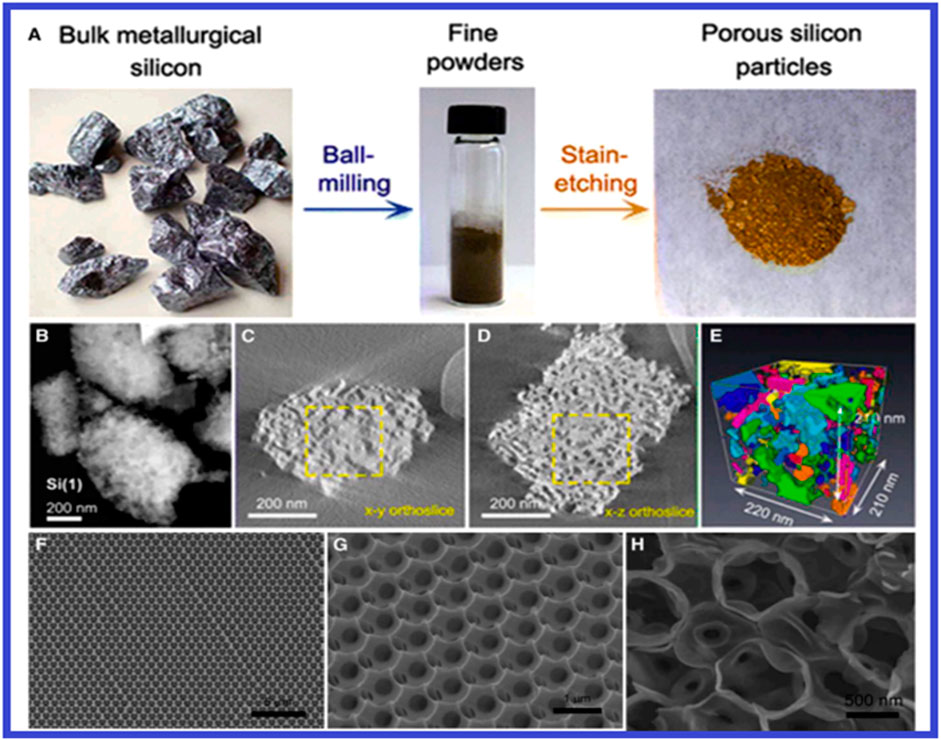
Figure 16. (A) The preparation method for the structural characterization of porous Si anodes (B–E) from the displayed HAADF-STEM tomography, porous Si is rebuilt. Porous Si templated from 890-nm pre-sintered silica microspheres before (F, G) and after (H) the CV test are shown in (F–H) SEM pictures. (Reproduced with permission from Ge et al. (2014). Copyright, 2007 Elsevier).
As shown in Figure 17, solid-state electrolytes can boost energy density and improve safety by substituting liquid ones because they don't catch fire. These electrolytes are suitable for use with lithium metal anodes and high-voltage cathodes. Ensuring stable interfaces and enough ionic conductivity (Song, 2020). Safe and effective electrolyte systems are necessary for high energy Li batteries. Although confined to cell voltage and with poor electrochemical stability, aqueous electrolytes are a promising class of electrolytes. Although they have limitations in kinetics, low mechanical stability, high electrode/electrolyte resistance, and poor interface stability, inorganic SSEs are nevertheless a promising material (Sun et al., 2022).
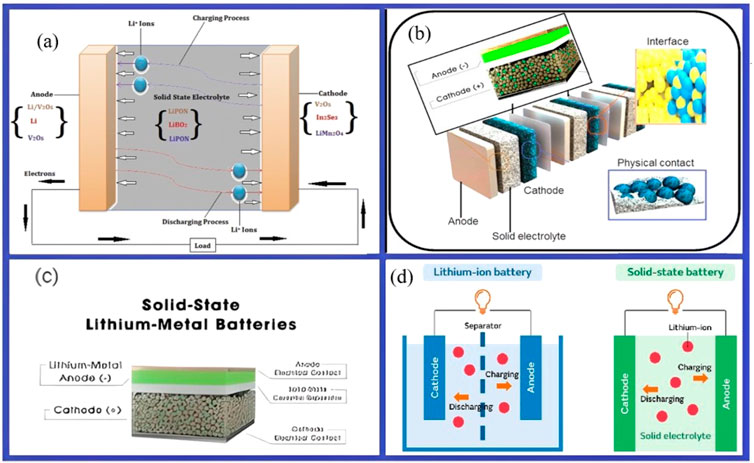
Figure 17. (A) Diagrammatic representation of a typical lithium-based solid-state battery, illustrating the direction of ion flow and a few potential choices for the anode, electrolyte, and cathode. Reproduced with permission from Sharma et al. (2024). (B) Diagram showing the bipolar stack solid-state battery cell (Famprikis et al., 2019). (C) The structure of a solid-state battery. (D) How lithium-ion batteries and solid-state batteries work (Qadir et al., 2024).
Small organic molecule ions are the building blocks of OIPCs (organic ionic plastic crystals), which are SSE. Because of their ionic composition, which produces non-volatility and very little vapor pressure even after melting, they are safe. Devices that use OIPCs include capacitors, dye-sensitized solar cells, proton exchange membrane fuel cells, and next-generation high-energy batteries. Important characteristics include their increased interfacial stability and capacity to generate low resistance, stable interphases at electrodes during charge-discharge cycling (Gutierrez-Pardo et al., 2021). Because of their favourable flexibility, OIPCs are interesting electrolyte materials that can enhance solid/solid contact for Solid state lithium batteries (SSLBs). It is essential to comprehend the connection between ion transport and phase behaviours while developing high-performing OIPC-based electrolytes Solid polymer electrolytes (SPEs) are used in higher energy density and all solid-state batteries due to their mechanical integrity and flexibility. They increase battery safety, especially for Li metal batteries. However, their poor coordination with Li+ ions restricts Li+ ion mobility. Ionic liquids and solvents are used as plasticizers or block copolymers to enhance alkali cation transport (Li et al., 2021; Durga et al., 2021).
The scientific community is focusing on LIB cathodes to increase voltage and capacity. These cathodes come in three varieties: polyanions, spinel, and layered. They have high theoretical capacity but have practical drawbacks like cost, surface deterioration, low conductivity, and lower energy density. To address these issues, the NMC (LiNixMnyCozO2) cathode is developed (shown in Figure 18), addressing issues like cost, surface deterioration, low conductivity, and lower energy density (Das et al., 2023). Each transition metal in the NMC multilayer cathode has a unique set of features.
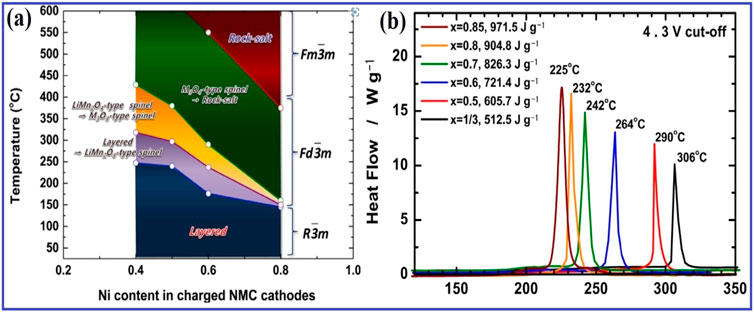
Figure 18. (A) Diagrammatic representation of the charged NMC cathode materials’ phase stability map during heating. (B) Li1-δ[NixCoyMnz]O2 materials’ DSC values (x = 1/3, 0.5, 0.6, 0.7, 0.8, and 0.85). Reproduced with permission from Tang et al. (2023).
For instance, cobalt provides stability and reduces cation mixing, manganese boosts the operating voltage, and nickel aids in increasing capacity—all of which help electric vehicles (EVs) function better over long distances. In the NMC multilayer cathode, every transition metal has a distinct set of properties. For instance, cobalt provides stability and reduces cation mixing, manganese boosts the operating voltage, and nickel aids in increasing capacity, all of which enhance the performance of electric vehicles (EVs) over long distances. Among the drawbacks of NMC include disordering, oxygen evolution, microcracking, and the irreversible rock-salt phase shift. These issues may lead to decreased capacity and cyclability. By doping NMC with transition and non-transition metals and coating it with metal oxides, phosphates, and fluorides, these can be reduced. Doping with aluminium, niobium, and zirconium improves NMC performance, inhibits unfavourable reactions, and provides better thermal stability. Phase transitions and corrosion are reduced by the coating layer, which acts as a barrier between the cathode and electrolyte. Li et al. were able to stabilise LiNi0.8Mn0.1Co0.1O2(NMC-811), maintain a high capacity, and halt H2O and CO2 processes during storage by doping the material with Al3+ ions (Park et al., 2022). The coating layer protects the electrode from HF produced by electrolytes and acts as an HF scavenger. Liu et al. were able to get 157.6 mAh g−1 at 10C and high capacity retention after 100 cycles after coating LiNi0.815Co0.15Al0.035O2 with LiTiO2 (Li L. et al., 2022). Zou et al. created a Li3PO4-coated NMC-811 cathode that produced 84.6% retention at 1C over 100 cycles following 7 days of air exposure. By reducing side reactions with the electrolyte and causing stability, combined doping and coating/bi-functional coating improved the cathode material. Bao et al. covered a LiNi0.6Mn0.2Co0.2O2 (NMC-622) cathode with ZrO2 and doped it with Zr4+ ions. This produced a twofold protective layer on top of the cathode material and stopped adverse evolutions.
With 49% of the market share, LIBs are the industry leader, followed by lead-acid (43%), NiMH (4%), and NiCd (3%) (Zhao et al., 2021). Due to its high specific power, high specific energy, and cost savings, low self-discharge rate LIBs are recommended. LIBs do have several drawbacks, too, such lengthy charge periods. For LIBs, 0.5°C–1°C is the optimal charging rate, with the CV phase consuming more than 60% of the charging time. The prolonged charging duration is a substantial obstacle to the general implementation of BEVs, as increasing pack capacity is necessary to alleviate “range anxiety,” which leads to even longer charging durations.
The development of ChgOp systems often involves adhering to LIB’s operational mechanism in order to facilitate quick charging and/or restrict specific AMs. Akira Yoshino created the LIB, an electrochemical energy storage device that uses electrical energy, in 1985, and Sony introduced it to the market in 1991, during discharging and saved, in chemical bonds during charging. It is made up of a separator in lithium salt solution, an anode, and a cathode with two current collectors. Lithium transition-metal oxide, which contains Li-ions for intercalation and de-intercalation during discharging, is typically utilised as the cathode and graphite as the anode. A common cathode material is lithium nickel cobalt aluminium oxide (NCA), followed by lithium nickel manganese cobalt oxide (NMC), lithium iron phosphate (LFP), and lithium cobalt oxide (LCO). The separator acts as an insulator electrically. Figure 19 illustrates the LFP battery, which has 36% of the LIB market, is a rocking-chair battery because it uses Li-ions to move between electrodes and de-intercalate from LiFePO4.
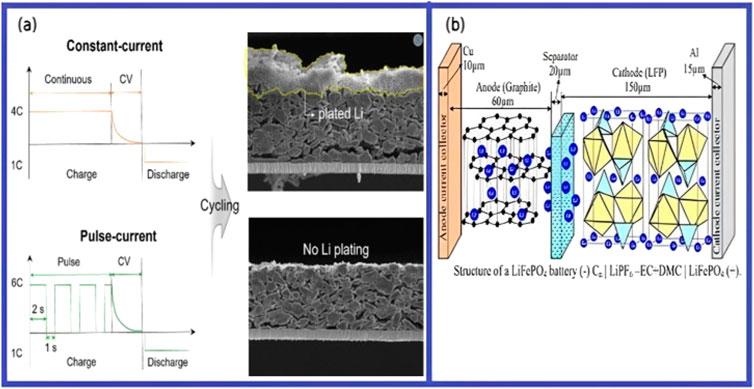
Figure 19. (A) Mechanistic understanding of the fast-charging based on pulse current to help suppress Li plating on the graphite anode. (B) LiFePO4 battery structure (−) C | LiPF6-EC + DMC | LiFePO4 (+). Reprinted by permission of the Jeong et al. (2023).
The creation of sustainable energy sources is one of the main issues facing humanity today. A workable solution to reduce our need on fossil fuels is green hydrogen. It is produced by electrolyzing water with renewable energy. Given these challenges, relying exclusively on fossil fuels is no longer practical, and it is imperative to investigate accessible and sustainable alternative energy sources. One such alternative that could be crucial in the transition to a more sustainable future is green hydrogen.
Sunlight is converted into electricity by photovoltaic panels, which powers an electrolyzer (Li J. et al., 2022). When sunlight is not available, the electrolyzer transforms water into hydrogen and oxygen, which are then stored and used by a fuel cell to produce energy. Sunlight is converted into electricity by solar panels, which is then either used within the system or sent to an electrolyzer for additional processing. An electrolyzer powered by surplus electricity splits water into hydrogen and oxygen, enabling continuous hydrogen generation. Hydrogen is stored in tanks or underground reservoirs for future use. When electricity demand rises or sunlight is unavailable, stored hydrogen is fed into a fuel cell. Unfortunately, large-scale solar hydrogen generation is not feasible due to the high cost of PV-powered electrolysis, or PV-electrolysis for short (Lee et al., 2022). This drives the development of photoelectrochemical (PEC) cells, which integrate the processes of electrolysis and light harvesting by directly converting solar energy into hydrogen fuel via water photoelectrolysis.
One of the maximum commonly available renewable-energy sources, is wind power. But it has two major drawbacks: it is unstable and variable. Put another way, although wind energy is one of the energy technologies that is developing the fastest, its generation fluctuation is known to be directly related to climatic variability and a growing number of primary technological and economic issues. The “Power-to-Gas” technology is one example of how wind energy may be stored and how hydrogen can be a viable choice. With this method, water is electrolyzed to make hydrogen and oxygen using extra power from wind turbines. After that, when the wind isn't blowing the hydrogen may be stored and utilised to power cars or produce energy. The idea of hydrogen being used as a fuel source is not new. Actually, Sir William Grove initially suggested it in 1839 after he had shown how the fuel cell worked. However, the high expense of manufacturing and storage has hampered the viability of using hydrogen as a common energy source. Recent technological developments have increased the appeal of hydrogen as a storage option for wind energy, including increases in the efficiency of electrolysis and the creation of more affordable storage options (Schrotenboer et al., 2022). Tanks or pipes already in place can be used to store or transport hydrogen, which can also be kept as a liquid or gas. There are still problems that must be set like the necessity of infrastructure to enable hydrogen production, storage, and distribution as well as the requirement to guarantee the security of hydrogen transit and storage (Rasul et al., 2022). On the other hand, hydrogen’s potential as a wind energy storage solution is encouraging and may lessen our reliance on fossil fuels while facilitating the switch to a more sustainable energy system.
Even though LIBs have come a long way since they were first introduced. Prior to LIBs meeting the needs of the automotive sector to the fullest, a number of technological challenges need to be addressed. High energy capacity, numerous charge-discharge cycles, and low self-discharge are lithium-ion batteries’ main advantages over other battery types. It functions as an energy storage device for power systems as well as a power source for electric vehicles. The non-aqueous electrolyte system known as LIBs, with its greater specific energy and energy density, has become a prominent technology in a number of sectors. Compared to aqueous rechargeable chemistries like NiMH and NiCd, they are able to sustain cell voltage levels three times higher (Yuan X. et al., 2021). However, only slight gains in nominal voltages result from the fundamental electrochemical coupling of high-energy devices being unchanged. Most particular energy gains are the result of engineering advances in cell/electrode optimisation and active material capacity. Future electrification projects may face difficulties as the rate of performance improvement has decreased. To direct research into the specific energy of batteries, the automobile industry has issued cathode- and anode-specific energy targets. While the EUCAR objectives reference earlier research on the materials requirements of the automobile industry, the USABC goals transform vehicle-level goals into targets at the materials level. In terms of useful value, petrol gives 5.3 kWh/kg, with an outstanding engine thermal productivity of 41% and an exceptional energy storing capacity of 12.9 kWh/kg (Masias et al., 2021). Although the USABC and EUCAR have different publication dates, vehicle-level aims, and goal-setting techniques, their gravimetric method and volumetric goals are similar. Comparing cathode targets also reveals similar, though not perfect, energy storage figures for gravimetric and volumetric methods. The materials chemistry community should be encouraged by the two consortia founded on automakers that have aligned their materials aims. One of the main obstacles to battery electric cars’ (BEVs’) acceptability in the market is their high cost in association to ICEVs (internal combustion engine vehicles).
This technology also has some drawbacks which make it somewhat challenging for the sustainability purpose. Li is an expensive metal. The cost of raw materials and refinement procedures rises due to the use of rare metals in lithium-ion batteries. As a result, lithium battery costs can differ between brands and be somewhat more expensive. It might be difficult to fix lithium-ion batteries because of their sophisticated technology and compliance with international safety regulations. Extreme temperatures have the potential to harm lithium batteries, which could pose a safety risk. Maintaining an incorrect temperature rate, performance degradation, temperature non-uniformity, and low-temperature performance are a few heat-related issues that Li-ion batteries encounter. Because of high cost of materials and the intricacy of the engineering involved, the battery pack is frequently the most costly part of an EV. However, over the past 10 years, significant investments in battery production capacity and a decline in the amount of cobalt in cathodes have allowed for an 80% cost reduction. If this trend continues, total cost of ownership parity between ICEVs and BEVs will be closer than it has ever been. Since purchase price parity is still elusive, further cost reduction is required to enhance market adoption. For long-distance users, particularly those without access to overnight or workplace charging, charging time is a major problem. Customers may charge up to 350 kW at speeds of up to 80% SOC with direct current fast charging, which can provide several hundred miles of range in around 40 min. However, faster charging rates are still the better option, especially for drivers without access to overnight or workplace charging. While the U.S. DOE’s long-term goal for ultra-rapid charging is 200 miles in 7.5 min, the USABC intends to attain 80% SOC in 15 min. LIBs have a high energy density due to their low electrochemical potential; however, this also raises the risk of accidental lithium plating, which can lead to irreversible capacity loss, reduced performance, and an elevated risk of thermal runaway and short circuiting. To improve rapid charging, new electrode chemistry, thermal management, and electrode engineering can be applied at the cell and battery levels. Electrode engineering can reduce voltage polarisation and facilitate lithium intercalation by adjusting thicknesses and porosities. Furthermore, electrode engineering can be used to modify the power-to-energy ratio of cells in a variety of ways.
As Figure 20 demonstrates, since its introduction, the LIB has undergone significant progress, enabling its specific energy content to almost triple. The more than threefold increase in LIB life satisfies most automotive calendar and cycle life requirements; nevertheless, complicated control methods and the preservation of cell energy reserves are still required. Furthermore, there has been a nearly two-fold reduction in LIB expenses. As practical pack sizes and battery system charge rates increase, fast charging has emerged as a new automotive goal. The initial energy content of LIBs, which is approximately 350 Wh/kg total cell energy, is not enough to meet automotive energy objectives when converted to useful, pack end-of-life values. Consequently, a number of Beyond Lithium Ion (BLI) research initiatives were started.
Figure 20. (A) a cross-sectional SEM picture of CNFs reveals sulfur deposition all over the cathode. (B) Diagram showing the carbonate-based electrolyte discharge from a lithium-sulfur battery. (C) CNFs in a SEM picture prior to deposition. (D) a cross-sectional SEM picture of CNFs reveals after sulfur deposition all over the cathode. Republished with authorization from Pai et al. (2022).
Analysing the difficulties related to photocatalytic overall water splitting is crucial because, although the process of producing hydrogen by photocatalytic overall water splitting is intriguing, the photocatalytic efficiency is not adequate. Photocatalytic total water splitting is very challenging due to several issues, including unfavourable thermodynamics, slow kinetics, dissolved oxygen, backward reaction, and side reaction. These challenges need to be solved for photocatalytic overall water splitting to be effective. An attractive and practical alternative to total water splitting is the combination of selective organic synthesis and selective organic synthesis to produce hydrogen (Navalón et al., 2022). Value-added oxidation products can be produced from the organic compounds by single-electron processes, which are far simpler to manage than four-electron oxygen. Our basic understanding of the activity trends in the two half-reactions of water splitting has rapidly advanced over the last few decades; however, valid and reliable reaction descriptors remain elusive, especially for OER and HER in the alkaline media, which are essential for controlling the structure design of promising catalysts and catalytic activity. Therefore, it is essential to develop advanced characterization techniques (such as in situ spectroscopic approaches) and theoretical simulation to clarify the underlying chemical process at the molecular level (Atkins et al., 2022).
The significant energy losses in a single cycle of hydrogen storage are a significant disadvantage. Regretfully, the water splitting process itself is an uphill reaction that is thermodynamically unfavorable and necessitates a certain external voltage (1.23 V in an ideal scenario) (YANG). Furthermore, in order to successfully create hydrogen and oxygen, a viable electrode surface must have a high activation energy. In order to counteract the energetically unfavorable aspect of water splitting, additional bias is introduced to the system. Only activation energy, which is discovered to be connected with the over-potential (η), can be decreased by utilizing optimal catalysts because the enthalpy change (equals to thermodynamic potential of 1.23 V) is intrinsic to the process (Zhang et al., 2022). In order to lessen the additional energy loss at high current density, extensive research has been done to enhance the bulk and surface characteristics of HER or OER catalysts. Since noble metals are expensive and require inadequate storage, it is best to use as many atoms as possible. Nevertheless, increasing the single metal atom loading content may lead to metal atom aggregation and decreased catalytic activity because to the high surface energy. Therefore, it is essential to develop the high loading concentration and efficient dispersion of isolated atomic catalysts for long-term electrocatalysis (Zhang and Guan, 2020). High activity, selectivity, and stability bifunctional catalysts for both OER and HER are expected to be produced. By making the equipment and operating procedure simpler, this will aid in the industrialization of water splitting in the future.
Over the last 10 years, significant progress has been made in Si-based LIBs. However, cycle instability has gained attention recently since it is a critical component of half/full-battery design and has a major impact on the weight of the constructed battery as well as the consumption of active components (Zhao H. et al., 2024).
Growing energy demands and environmental concerns from fossil fuel misuse are driving the search for alternative energy sources. LIBs and electro-catalytic water splitting have gained popularity as efficient energy conversion and storage using electrochemistry techniques. These devices are incredibly efficient, pollution-free, and sustainable. High energy density LIBs are widely employed in industry; electro-catalytic water splitting of electrical energy may provide green H2 energy. Hydrogen is used as fuel in fuel cell electric vehicles, which have zero emissions. Hydrogen is created during the water splitting process and is used as a long-term energy storage device. On the other hand, lithium-ion batteries provide incredibly effective temporary storage.
Sharp fluctuations in supply and demand can be mitigated using lithium-ion batteries, and during periods of low demand, water splitting can use additional renewable energy. By storing excess energy from sources like wind and sunshine and releasing it when demand is high or output is low, both technologies can help stabilise the grid.
Bifunctional electrocatalysts are necessary for water splitting due to their low price, high activity, and reasonably priced synthesis methodologies (Wang et al., 2020). These catalysts stabilise the hydrogen evolution reaction (HER) and the oxygen evolution reaction (OER) in the electrolyte over an extended period of time. To encourage the development of hydrogen fuel, scientists are producing a range of bifunctional electrocatalysts with different performance levels. Sulphides, nitrides, phosphides, transition metal oxides, and selenides are possible transition metal options for water electrolysis. For total water splitting, Ni foam-based Ni3S2/MnS–O nanosheets served as the anode and cathode, requiring a voltage of 1.54 V at a current density of 10 mA cm−2 (Meng et al., 2024). For long-term water splitting, metal-free electrocatalysts are a possible substitute for metal-based electrocatalysts due to their higher action, superior constancy, and cost effectiveness. Pulse laser technology has been extensively used in time-resolved characterization techniques, including transient absorption and fluorescence spectrum analysis (Mezzetti et al., 2022). Laser ablation (LAL) is a technique used to create nanomaterials like nanowires, nanoparticles, and clusters. However, LAL is costly and complex, making it less suitable for energy materials. Laser Ablation in air (LAA) is a quicker and easier method for direct fabrication of energy materials in ambient air. LAA treats finished products as unique modifications of the raw materials, making it a versatile material manipulator. LAA treatment can dramatically increase a material’s activity in just a few minutes, making it a viable technique for the energy-related sector. Recent studies using LAA in energy-related catalysis have demonstrated its enormous promise for regulating and managing catalytic materials (Lal and Sundara, 2022; Maragkaki et al., 2021). Because of the increased demand for electricity and decreased reliance on traditional energy supplies, the amount of Renewable Energy Sources (RES) in the electrical grid is growing at a rapid pace. Since the need for electrical energy has risen in industries such as manufacturing, transportation, and communication, micro grids may effectively combine renewable energy resources with Energy Storage Systems (ESS). Although RES are plentiful, environmentally benign, and scalable, they have poor load following and are not dispatchable. By offering storage solutions like batteries, flow batteries, and supercapacitors, ESS can get around these restrictions. By supporting various energy sources, addressing power intermittency problems, enhancing power quality, and offering frequency and voltage management, ESS contributes significantly to the production of electricity. Batteries, supercapacitors, flywheels, CAES (compressed-air energy storage), SMES (superconducting magnetic energy storage), hydrogen storage and pumped hydro, systems are just a few of the energy storage technologies that have been created. Systems for storing battery energy are seen as disruptive developments in the power industry that have the ability to alter the way electricity is provided in the future. If storage prices continue to decline with the help of state administrative actions, the potential market for storage might increase by 55,000 MW over the next 10 years.
In conclusion, two technologies that have demonstrated exceptional promise for improving our ability to produce and store energy are water splitting and lithium-ion batteries. The convergence of water splitting and LIB technologies represents a pioneering step in field of green energy. Both technologies exclusively have exhibited immense potential in handling the global energy crisis, and their integration could open the door to sustainable and high performing. Hydrogen, as a zero-emission fuel, can significantly reduce our dependency on oil, coal, and natural gas and mitigate GHG emissions. Its applications span various sectors, encompassing transportation, industry, and power generation. On the other hand, by enabling the energy storage which is generated from renewable sources, Li-ion batteries contribute to grid stability and ensure a consistent energy supply. Through the use of Transition Metal based catalysts, recent developments in water splitting technologies have increased catalytic efficiency. By using materials with nanostructures, these catalysts’ surface area and reactivity can be increased, improving their efficiency. Solid oxide electrolyzers (SOEs) and proton exchange membrane (PEM) electrolyzers are two more significant advancements in this field. They showed great strength and efficiency. PEM electrolyzers in particular have an advantage because they can generate high-purity hydrogen while operating at low temperatures. The performance and durability of electrolyzers have been further enhanced by advancements in membrane materials and electrode design. Higher solar-to-hydrogen conversion efficiencies are the result of advancements in photoelectrochemical cells (PECs) and semiconductor material optimisation. The development of co-catalysts and light-absorbing materials, along with photocatalytic water splitting, holds promise for a large-scale creation of hydrogen. Investigating new electrode materials is one of the main developments in LIBs. Graphite anodes are being replaced by materials based on silicon. Although they have a greater capacity, these have difficulties when it comes to volume growth. Utilising composite materials and nanostructures, this problem is being solved. Li-ion batteries’ energy density has increased on cathode side as an outcome of the switch from cobalt-based to nickel-rich cathodes. Cathodes rich in nickel offer greater stability and capacity. Additionally, the safety and cycle life of batteries have been enhanced by the development of lithium iron phosphate (LFP) cathodes, making them appropriate for uses such as grid storage. Li-ion battery electrolytes and separators are also essential parts. Solid-state electrolytes offer a promising way to enhance energy density and ensuring safety. Solid-state batteries allow use of lithium metal anodes, which have a higher capacity than graphite, and eradicate the danger of leakage and thermal runaway linked to liquid electrolytes. An energy system with greater coherence and efficiency could be achieved through the combination of Li-ion battery technology and water splitting. The ability to use excess renewable energy for water splitting to yield hydrogen, that can be stored and utilised in fuel cells or combined with Li-ion batteries for hybrid energy storage solutions, is one of the integration’s most alluring features. Hybrid systems that integrate Li-ion batteries and hydrogen fuel cells can benefit from each technology’s advantages.
Fuel cells use hydrogen to produce energy continuously, whereas Li-ion batteries have a high power output and fast response times. Energy systems for electric cars and stationary storage applications may become more effective as a result of this collaboration. Furthermore, an inventive strategy is the creation of integrated systems that make use of the oxygen produced by water splitting in Li-ion battery chemistries. Lithium-air batteries, which have the potential to achieve higher energy densities than traditional Li-ion batteries, can use oxygen, according to research. These integrated systems have the potential to greatly improve energy generation and storage’s overall sustainability and efficiency. Although the combination of Li-ion battery technology and water splitting shows promise, there are still a number of obstacles to overcome. Further improvements in water splitting’s cost and efficiency are required to make hydrogen production more cost-effective. The optimisation of electrolyzer technologies is just as important as the development of robust and reasonably priced catalysts. It is imperative to tackle concerns pertaining to material accessibility, safety, and recycling in the context of Li-ion batteries. Li-ion battery technology will be greatly impacted by the switch to more abundant and sustainable anodes and cathodes made of silicon rather than cobalt. In order to guarantee the smooth functioning of fuel cells and batteries, the development of hybrid energy systems necessitates sophisticated control and management techniques. For these technologies to be widely adopted, it will also be essential to integrate them into the current energy infrastructures and to establish regulatory frameworks that support them. Finding sustainable energy solutions has advanced significantly with the combination of Li-ion battery and water splitting technologies. Both technologies have advanced remarkably on their own, and their combination provides a way to create energy systems that are more ecologically friendly and efficient. We are able to develop hybrid systems that improve renewable energy storage and utilisation by utilising the advantages of each technology. The prospect for these integrated systems to completely transform the energy landscape is becoming more and more real as long as research and development keep working to overcome the remaining obstacles. Water splitting in conjunction with Li-ion batteries is an example of next-generation technologies can collaborate to create sustainable energy in the future. By means of persistent innovation and cooperation, we can make progress towards a future in which everyone has access to clean and dependable energy.
SH: Data curation, Writing–original draft, Writing–review and editing. SN: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing–original draft, Writing–review and editing. WP: Formal analysis, Writing–review and editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acar, C., Dincer, I., and Naterer, G. F. (2016). Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 40 (11), 1449–1473. doi:10.1002/er.3549
Adams, M. J., Wadge, M. D., Sheppard, D., Stuart, A., and Grant, D. M. (2024). Review on onshore and offshore large-scale seasonal hydrogen storage for electricity generation: focusing on improving compression, storage, and roundtrip efficiency. Int. J. Hydrogen Energy 73, 95–111. doi:10.1016/j.ijhydene.2024.05.421
Ahsan, M. B. F., Mekhilef, S., Soon, T. K., Mubin, M. B., Shrivastava, P., and Seyedmahmoudian, M. (2022). Lithium-ion battery and supercapacitor-based hybrid energy storage system for electric vehicle applications: a review. Int. J. Energy Res. 46 (14), 19826–19854. doi:10.1002/er.8439
Ameen, M. T., Ma, Z., Smallbone, A., Norman, R., and Roskilly, A. P. (2023). Demonstration system of pumped heat energy storage (PHES) and its round-trip efficiency. Appl. Energy 333, 120580. doi:10.1016/j.apenergy.2022.120580
Ampah, J. D., Jin, C., Afrane, S., Yusuf, A. A., Liu, H., and Yao, M. (2024). Race towards net zero emissions (NZE) by 2050: reviewing a decade of research on hydrogen-fuelled internal combustion engines (ICE). Green Chem. 26, 9025–9047. doi:10.1039/d4gc00864b
Anantharaj, S., Noda, S., Jothi, V. R., Yi, S., Driess, M., and Menezes, P. W. (2021). Strategies and perspectives to catch the missing pieces in energy-efficient hydrogen evolution reaction in alkaline media. Angew. Chem. Int. Ed. 60 (35), 18981–19006. doi:10.1002/anie.202015738
Aravindan, M., and Kumar, P. (2023). Hydrogen towards sustainable transition: a review of production, economic, environmental impact and scaling factors. Results Eng. 20, 101456. doi:10.1016/j.rineng.2023.101456
Aravindan, M., V, M. K., Hariharan, V., Narahari, T., P, A. K., K, M., et al. (2023). Fuelling the future: a review of non-renewable hydrogen production and storage techniques. Renew. Sustain. Energy Rev. 188, 113791. doi:10.1016/j.rser.2023.113791
Arsad, A., Hannan, M., Al-Shetwi, A. Q., Begum, R., Hossain, M., Ker, P. J., et al. (2023a). Hydrogen electrolyser technologies and their modelling for sustainable energy production: a comprehensive review and suggestions. Int. J. Hydrogen Energy 48, 27841–27871. doi:10.1016/j.ijhydene.2023.04.014
Arsad, S., Ker, P. J., Hannan, M., Tang, S. G., R S, N., Chau, C., et al. (2023b). Patent landscape review of hydrogen production methods: assessing technological updates and innovations. Int. J. Hydrogen Energy 50, 447–472. doi:10.1016/j.ijhydene.2023.09.085
Arsalis, A., Papanastasiou, P., and Georghiou, G. E. (2022). A comparative review of lithium-ion battery and regenerative hydrogen fuel cell technologies for integration with photovoltaic applications. Renew. Energy 191, 943–960. doi:10.1016/j.renene.2022.04.075
Atkins, D., Ayerbe, E., Benayad, A., Capone, F. G., Capria, E., Castelli, I. E., et al. (2022). Understanding battery interfaces by combined characterization and simulation approaches: challenges and perspectives. Adv. Energy Mater. 12 (17), 2102687. doi:10.1002/aenm.202102687
Bashir, T., Ismail, S. A., Song, Y., Irfan, R. M., Yang, S., Zhou, S., et al. (2021). A review of the energy storage aspects of chemical elements for lithium-ion based batteries. Energy Mater 1 (2), 100019. doi:10.20517/energymater.2021.20
Bethoux, O. (2020). Hydrogen fuel cell road vehicles: state of the art and perspectives. Energies 13 (21), 5843. doi:10.3390/en13215843
Bhatt, M. D., and Lee, J. Y. (2020). Advancement of platinum (Pt)-free (non-Pt precious metals) and/or metal-free (non-precious-metals) electrocatalysts in energy applications: a review and perspectives. Energy and Fuels 34 (6), 6634–6695. doi:10.1021/acs.energyfuels.0c00953
Bie, C., Wang, L., and Yu, J. (2022). Challenges for photocatalytic overall water splitting. Chem 8 (6), 1567–1574. doi:10.1016/j.chempr.2022.04.013
Budama, V. K., Rincon Duarte, J. P., Roeb, M., and Sattler, C. (2023). Potential of solar thermochemical water-splitting cycles: a review. Sol. Energy 249, 353–366. doi:10.1016/j.solener.2022.11.001
Chen, M., Zhang, Y., Xing, G., Chou, S. L., and Tang, Y. (2021b). Electrochemical energy storage devices working in extreme conditions. Energy and Environ. Sci. 14 (6), 3323–3351. doi:10.1039/d1ee00271f
Chen, T., Jin, Y., Lv, H., Yang, A., Liu, M., Chen, B., et al. (2020). Applications of lithium-ion batteries in grid-scale energy storage systems. Trans. Tianjin Univ. 26 (3), 208–217. doi:10.1007/s12209-020-00236-w
Chen, T.-W., Anushya, G., Chen, S. M., Kalimuthu, P., Mariyappan, V., Gajendran, P., et al. (2022). Recent advances in nanoscale based electrocatalysts for metal-air battery, fuel cell and water-splitting applications: an overview. Materials 15 (2), 458. doi:10.3390/ma15020458
Chen, Z., Wei, W., and Ni, B.-J. (2021a). Cost-effective catalysts for renewable hydrogen production via electrochemical water splitting: recent advances. Curr. Opin. Green Sustain. Chem. 27, 100398. doi:10.1016/j.cogsc.2020.100398
Costa, C. M., Barbosa, J., Gonçalves, R., Castro, H., Campo, F. D., and Lanceros-Méndez, S. (2021). Recycling and environmental issues of lithium-ion batteries: advances, challenges and opportunities. Energy Storage Mater. 37, 433–465. doi:10.1016/j.ensm.2021.02.032
Dai, Y., Yu, J., Cheng, C., Tan, P., and Ni, M. (2020). Engineering the interfaces in water-splitting photoelectrodes–an overview of the technique development. J. Mater. Chem. A 8 (15), 6984–7002. doi:10.1039/d0ta01670e
Das, C., Sinha, N., and Roy, P. (2022). Transition metal non-oxides as electrocatalysts: advantages and challenges. Small 18 (28), 2202033. doi:10.1002/smll.202202033
Das, D., Manna, S., and Puravankara, S. (2023). Electrolytes, additives and binders for NMC cathodes in Li-Ion batteries—a review. Batteries 9 (4), 193. doi:10.3390/batteries9040193
Datta, U., Kalam, A., and Shi, J. (2021). A review of key functionalities of battery energy storage system in renewable energy integrated power systems. Energy Storage 3 (5), e224. doi:10.1002/est2.224
Davis, K. M., Sullivan, B. T., Palenik, M. C., Yan, L., Purohit, V., Robison, G., et al. (2018). Rapid evolution of the photosystem II electronic structure during water splitting. Phys. Rev. X 8 (4), 041014. doi:10.1103/physrevx.8.041014
Deng, C., Shen, W., Li, M., and Zhang, T. (2019). A single-atom catalyst of cobalt supported on a defective two-dimensional boron nitride material as a promising electrocatalyst for the oxygen reduction reaction: a DFT study. Phys. Chem. Chem. Phys. 21 (13), 6900–6907. doi:10.1039/c9cp00452a
Deng, H., and Aifantis, K. E. (2023) “Applications of lithium batteries,” in Rechargeable ion batteries: materials, design and applications of Li-ion cells and beyond, 83–103.
Devadas, A., Baranton, S., and Coutanceau, C. (2020). Green synthesis and modification of RuO2 materials for the oxygen evolution reaction. Front. Energy Res. 8, 571704. doi:10.3389/fenrg.2020.571704
Dimitriou, P., and Tsujimura, T. (2017). A review of hydrogen as a compression ignition engine fuel. Int. J. Hydrogen Energy 42 (38), 24470–24486. doi:10.1016/j.ijhydene.2017.07.232
Du, L., Sun, Y., and You, B. (2021). Hybrid water electrolysis: replacing oxygen evolution reaction for energy-efficient hydrogen production and beyond. Mater. Rep. Energy 1 (1), 100004. doi:10.1016/j.matre.2020.12.001
Durga, G., Kalra, P., Kumar Verma, V., Wangdi, K., and Mishra, A. (2021). Ionic liquids: from a solvent for polymeric reactions to the monomers for poly (ionic liquids). J. Mol. Liq. 335, 116540. doi:10.1016/j.molliq.2021.116540
Durmus, Y. E., Zhang, H., Baakes, F., Desmaizieres, G., Hayun, H., Yang, L., et al. (2020). Side by side battery technologies with lithium-ion based batteries. Adv. energy Mater. 10 (24), 2000089. doi:10.1002/aenm.202000089
Eftekhari, A. (2019). Lithium batteries for electric vehicles: from economy to research strategy. Washington, DC: ACS Publications.
Escobar-Yonoff, R., Maestre-Cambronel, D., Charry, S., Rincón-Montenegro, A., and Portnoy, I. (2021). Performance assessment and economic perspectives of integrated PEM fuel cell and PEM electrolyzer for electric power generation. Heliyon 7 (3), e06506. doi:10.1016/j.heliyon.2021.e06506
Famprikis, T., Canepa, P., Dawson, J. A., Islam, M. S., and Masquelier, C. (2019). Fundamentals of inorganic solid-state electrolytes for batteries. Nat. Mater. 18 (12), 1278–1291. doi:10.1038/s41563-019-0431-3
Feng, Q., Yuan, X., Liu, G., Wei, B., Zhang, Z., Li, H., et al. (2017). A review of proton exchange membrane water electrolysis on degradation mechanisms and mitigation strategies. J. Power Sources 366, 33–55. doi:10.1016/j.jpowsour.2017.09.006
Fernandez-Ibanez, P., McMichael, S., Rioja Cabanillas, A., Alkharabsheh, S., Tolosana Moranchel, A., and Byrne, J. A. (2021). New trends on photoelectrocatalysis (PEC): nanomaterials, wastewater treatment and hydrogen generation. Curr. Opin. Chem. Eng. 34, 100725. doi:10.1016/j.coche.2021.100725
Fragiacomo, P., Piraino, F., Genovese, M., Corigliano, O., and Lorenzo, G. D. (2022). Strategic overview on fuel cell-based systems for mobility and electrolytic cells for hydrogen production. Procedia Comput. Sci. 200, 1254–1263. doi:10.1016/j.procs.2022.01.326
Francis, C. F., Kyratzis, I. L., and Best, A. S. (2020). Lithium-ion battery separators for ionic-liquid electrolytes: a review. Adv. Mater. 32 (18), 1904205. doi:10.1002/adma.201904205
Fu, X., Shi, R., Jiao, S., Li, M., and Li, Q. (2022). Structural design for electrocatalytic water splitting to realize industrial-scale deployment: strategies, advances, and perspectives. J. Energy Chem. 70, 129–153. doi:10.1016/j.jechem.2022.02.010
Galos, J., Pattarakunnan, K., Best, A. S., Kyratzis, I. L., Wang, C., and Mouritz, A. P. (2021). Energy storage structural composites with integrated lithium-ion batteries: a review. Adv. Mater. Technol. 6 (8), 2001059. doi:10.1002/admt.202001059
Gao, Y., Pan, Z., Sun, J., Liu, Z., and Wang, J. (2022). High-energy batteries: beyond lithium-ion and their long road to commercialisation. Nano-micro Lett. 14 (1), 94. doi:10.1007/s40820-022-00844-2
Ge, M., Lu, Y., Ercius, P., Rong, J., Fang, X., Mecklenburg, M., et al. (2014). Large-scale fabrication, 3D tomography, and lithium-ion battery application of porous silicon. Nano Lett. 14 (1), 261–268. doi:10.1021/nl403923s
Gopinath, M., and Marimuthu, R. (2022). A review on solar energy-based indirect water-splitting methods for hydrogen generation. Int. J. Hydrogen Energy 47 (89), 37742–37759. doi:10.1016/j.ijhydene.2022.08.297
Govind Rajan, A., Martirez, J. M. P., and Carter, E. A. (2020). Why do we use the materials and operating conditions we use for heterogeneous (photo) electrochemical water splitting? ACS Catal. 10 (19), 11177–11234. doi:10.1021/acscatal.0c01862
Grape, S., Jaunin, E., El-Boghdadly, K., Chan, V., and Albrecht, E. (2020). Analgesic efficacy of PECS and serratus plane blocks after breast surgery: a systematic review, meta-analysis and trial sequential analysis. J. Clin. Anesth. 63, 109744. doi:10.1016/j.jclinane.2020.109744
Grigoriev, S., Fateev, V., Bessarabov, D., and Millet, P. (2020). Current status, research trends, and challenges in water electrolysis science and technology. Int. J. Hydrogen Energy 45 (49), 26036–26058. doi:10.1016/j.ijhydene.2020.03.109
Guo, J., and Liu, J. (2019). A binder-free electrode architecture design for lithium–sulfur batteries: a review. Nanoscale Adv. 1 (6), 2104–2122. doi:10.1039/c9na00040b
Gutierrez-Pardo, A., Aguesse, F., Fernández-Carretero, F., Siriwardana, A. I., García-Luis, A., and Llordés, A. (2021). Improved electromechanical stability of the Li metal/garnet ceramic interface by a solvent-free deposited OIPC soft layer. ACS Appl. Energy Mater. 4 (3), 2388–2397. doi:10.1021/acsaem.0c02439
Hasan, M. K., Mahmud, M., Ahasan Habib, A., Motakabber, S., and Islam, S. (2021). Review of electric vehicle energy storage and management system: standards, issues, and challenges. J. energy storage 41, 102940. doi:10.1016/j.est.2021.102940
Hassan, I., Ramadan, H. S., Saleh, M. A., and Hissel, D. (2021). Hydrogen storage technologies for stationary and mobile applications: review, analysis and perspectives. Renew. Sustain. Energy Rev. 149, 111311. doi:10.1016/j.rser.2021.111311
Hassan, N., Jalil, A., Rajendran, S., Khusnun, N., Bahari, M., Johari, A., et al. (2023). Recent review and evaluation of green hydrogen production via water electrolysis for a sustainable and clean energy society. Int. J. Hydrogen Energy 52, 420–441. doi:10.1016/j.ijhydene.2023.09.068
Ikuerowo, T., Bade, S. O., Akinmoladun, A., and Oni, B. A. (2024). The integration of wind and solar power to water electrolyzer for green hydrogen production. Int. J. Hydrogen Energy 76, 75–96. doi:10.1016/j.ijhydene.2024.02.139
Ishag, A., and Sun, Y. (2021). Recent advances in two-dimensional MoS2 nanosheets for environmental application. Industrial and Eng. Chem. Res. 60 (22), 8007–8026. doi:10.1021/acs.iecr.1c01311
Jamesh, M.-I., and Harb, M. (2021). Tuning the electronic structure of the earth-abundant electrocatalysts for oxygen evolution reaction (OER) to achieve efficient alkaline water splitting–A review. J. Energy Chem. 56, 299–342. doi:10.1016/j.jechem.2020.08.001
Janani, G., Surendran, S., Choi, H., An, T.-Y., Han, M.-K., Song, S.-J., et al. (2021). Anchoring of Ni12P5 microbricks in nitrogen-and phosphorus-enriched carbon frameworks: engineering bifunctional active sites for efficient water-splitting systems. ACS Sustain. Chem. and Eng. 10 (3), 1182–1194. doi:10.1021/acssuschemeng.1c06514
Jeong, Y. T., Shin, H. R., Lee, J., Ryu, M. H., Choi, S., Kim, H., et al. (2023). Insight into pulse-charging for lithium plating-free fast-charging lithium-ion batteries. Electrochimica Acta 462, 142761. doi:10.1016/j.electacta.2023.142761
Jiang, M., Tan, Z., and Cao, M. (2021). A robust bifunctional electrocatalyst for rechargeable zinc-air batteries: NiFe nanoparticles encapsulated in nitrogen-doped carbon nanotubes. Int. J. Hydrogen Energy 46 (29), 15507–15516. doi:10.1016/j.ijhydene.2021.02.079
Jiang, X., Chen, Y., Meng, X., Cao, W., Liu, C., Huang, Q., et al. (2022). The impact of electrode with carbon materials on safety performance of lithium-ion batteries: a review. Carbon 191, 448–470. doi:10.1016/j.carbon.2022.02.011
Junaid, M., Batoo, K. M., Hussain, S. G., Khan, W. Q., and Hussain, S. (2023). Photodegradation of CdTe thin film via PVD for water splitting to generate hydrogen energy. Results Chem. 6, 101102. doi:10.1016/j.rechem.2023.101102
Kebede, A. A., Kalogiannis, T., Van Mierlo, J., and Berecibar, M. (2022). A comprehensive review of stationary energy storage devices for large scale renewable energy sources grid integration. Renew. Sustain. Energy Rev. 159, 112213. doi:10.1016/j.rser.2022.112213
Khakimov, R., Moskvin, A., and Zhdaneev, O. (2024). Hydrogen as a key technology for long-term and seasonal energy storage applications. Elsevier, 374–381.
Khan, F. N. U., Rasul, M. G., Sayem, A., and Mandal, N. (2023a). Maximizing energy density of lithium-ion batteries for electric vehicles: a critical review. Energy Rep. 9, 11–21. doi:10.1016/j.egyr.2023.08.069
Khan, F. N. U., Rasul, M. G., Sayem, A., and Mandal, N. K. (2023b). Design and optimization of lithium-ion battery as an efficient energy storage device for electric vehicles: a comprehensive review. J. Energy Storage 71, 108033. doi:10.1016/j.est.2023.108033
Khan, S. A. R., Yu, Z., Belhadi, A., and Mardani, A. (2020) “Flexible batteries,” in Rechargeable batteries: history, progress, and applications, 41–60.
Kim, H., Surendran, S., Chae, Y., Lee, H. Y., An, T. Y., Han, H. S., et al. (2020). Fabrication of an ingenious metallic asymmetric supercapacitor by the integration of anodic iron oxide and cathodic nickel phosphide. Appl. Surf. Sci. 511, 145424. doi:10.1016/j.apsusc.2020.145424
Kim, J. H., Jo, Y., Kim, J. H., Jang, J. W., Khan, H. J., Lee, Y. H., et al. (2015). Wireless solar water splitting device with robust cobalt-catalyzed, dual-doped BiVO4 photoanode and perovskite solar cell in tandem: a dual absorber artificial leaf. ACS Nano 9 (12), 11820–11829. doi:10.1021/acsnano.5b03859
Kim, S. Y., Cha, H., Kostecki, R., and Chen, G. (2022). Composite cathode design for high-energy all-solid-state lithium batteries with long cycle life. ACS Energy Lett. 8 (1), 521–528. doi:10.1021/acsenergylett.2c02414
Ko, M., Chae, S., and Cho, J. (2015). Challenges in accommodating volume change of Si anodes for Li-ion batteries. ChemElectroChem 2 (11), 1645–1651. doi:10.1002/celc.201500254
Kopasz, J., Dolan, C., Gangi, J., and Homann, Q. (2022). Hydrogen and fuel cell technologies market report. Argonne, IL United States: Argonne National Lab.ANL.
Kuai, L., Chen, Z., Liu, S., Kan, E., Yu, N., Ren, Y., et al. (2020). Titania supported synergistic palladium single atoms and nanoparticles for room temperature ketone and aldehydes hydrogenation. Nat. Commun. 11 (1), 48. doi:10.1038/s41467-019-13941-5
Kumar, S. S., and Lim, H. (2022). An overview of water electrolysis technologies for green hydrogen production. Energy Rep. 8, 13793–13813. doi:10.1016/j.egyr.2022.10.127
Lal, M. S., and Sundara, R. (2022). Multifunctional high entropy oxides incorporated functionalized biowaste derived activated carbon for electrochemical energy storage and desalination. Electrochimica Acta 405, 139828. doi:10.1016/j.electacta.2021.139828
Lamy, C., and Millet, P. (2020). A critical review on the definitions used to calculate the energy efficiency coefficients of water electrolysis cells working under near ambient temperature conditions. J. power sources 447, 227350. doi:10.1016/j.jpowsour.2019.227350
Lee, M., Haas, S., Smirnov, V., Merdzhanova, T., and Rau, U. (2022). Scalable photovoltaic-electrochemical cells for hydrogen production from water-recent advances. ChemElectroChem 9 (24), e202200838. doi:10.1002/celc.202200838
Lee, Y.-H., Zhang, X., Zhang, W., Chang, M., Lin, C., Chang, K., et al. (2012). Synthesis of large-area MoS2 atomic layers with chemical vapor deposition. arXiv Prepr. arXiv:1202.5458 24, 2320–2325. doi:10.1002/adma.201104798
Li, H., Yang, J., Chen, S., Xu, Z., Wang, J., Nuli, Y., et al. (2021). Inherently flame-retardant solid polymer electrolyte for safety-enhanced lithium metal battery. Chem. Eng. J. 410, 128415. doi:10.1016/j.cej.2021.128415
Li, J., Gao, C., Lu, X., and Hoseyni, A. (2022b). A combined energy system consisting of fuel cell, water electrolyzer and solar technologies to produce hydrogen fuel and electricity. Energy Sources, Part A Recovery, Util. Environ. Eff. 44 (1), 1173–1188. doi:10.1080/15567036.2022.2055680
Li, L., Wang, D., Xu, G., Zhou, Q., Ma, J., Zhang, J., et al. (2022a). Recent progress on electrolyte functional additives for protection of nickel-rich layered oxide cathode materials. J. Energy Chem. 65, 280–292. doi:10.1016/j.jechem.2021.05.049
Li, X., Zhao, L., Yu, J., Liu, X., Zhang, X., Liu, H., et al. (2020). Water splitting: from electrode to green energy system. Nano-Micro Lett. 12, 1–29. doi:10.1007/s40820-020-00469-3
Liang, Q., Brocks, G., and Bieberle-Hütter, A. (2021). Oxygen evolution reaction (OER) mechanism under alkaline and acidic conditions. J. Phys. Energy 3 (2), 026001. doi:10.1088/2515-7655/abdc85
Liang, Y., Zhao, C., Yuan, H., Chen, Y., Zhang, W., Huang, J., et al. (2019). A review of rechargeable batteries for portable electronic devices. InfoMat 1 (1), 6–32. doi:10.1002/inf2.12000
Liu, F., Shi, C., Guo, X., He, Z., Pan, L., Huang, Z., et al. (2022). Rational design of better hydrogen evolution electrocatalysts for water splitting: a review. Adv. Sci. 9 (18), 2200307. doi:10.1002/advs.202200307
Liu, J., Ma, J., Zhang, Z., Qin, Y., Wang, Y. J., Wang, Y., et al. (2021). 2021 Roadmap: electrocatalysts for green catalytic processes. J. Phys. Mater. 4 (2), 022004. doi:10.1088/2515-7639/abd596
Liu, K.-K., Zhang, W., Lee, Y. H., Lin, Y. C., Chang, M. T., Su, C. Y., et al. (2012). Growth of large-area and highly crystalline MoS2 thin layers on insulating substrates. Nano Lett. 12 (3), 1538–1544. doi:10.1021/nl2043612
Liu, L.-B., Yi, C., Mi, H.-C., Zhang, S. L., Fu, X.-Z., Luo, J. L., et al. (2024a). Perovskite oxides toward oxygen evolution reaction: intellectual design strategies, properties and perspectives. Electrochem. Energy Rev. 7 (1), 14–37. doi:10.1007/s41918-023-00209-2
Liu, L.-B., Yi, C., Mi, H.-C., Zhang, S. L., Fu, X.-Z., Luo, J. L., et al. (2024b). Perovskite oxides toward oxygen evolution reaction: intellectual design strategies, properties and perspectives. Electrochem. Energy Rev. 7 (1), 14. doi:10.1007/s41918-023-00209-2
Liu, R.-T., Xu, Z. L., Li, F. M., Chen, F. Y., Yu, J. Y., Yan, Y., et al. (2023). Recent advances in proton exchange membrane water electrolysis. Chem. Soc. Rev. 52, 5652–5683. doi:10.1039/d2cs00681b
Liu, Z., Tan, H., Liu, D., Liu, X., Xin, J., Xie, J., et al. (2019). Promotion of overall water splitting activity over a wide pH range by interfacial electrical effects of metallic NiCo-nitrides nanoparticle/NiCo2O4 nanoflake/graphite fibers. Adv. Sci. 6 (5), 1801829. doi:10.1002/advs.201801829
Lopez, V. M., Ziar, H., Haverkort, J., Zeman, M., and Isabella, O. (2023). Dynamic operation of water electrolyzers: a review for applications in photovoltaic systems integration. Renew. Sustain. Energy Rev. 182, 113407. doi:10.1016/j.rser.2023.113407
Luo, J., Li, Z., Nishiwaki, S., Schreier, M., Mayer, M. T., Cendula, P., et al. (2015). Targeting ideal dual-absorber tandem water splitting using perovskite photovoltaics and CuInxGaxSe2 photocathodes. Adv. Energy Mater. 5 (24), 1501520. doi:10.1002/aenm.201501520
Ma, D., Jin, T., Xie, K., and Huang, H. (2021). An overview of flow cell architecture design and optimization for electrochemical CO 2 reduction. J. Mater. Chem. A 9 (37), 20897–20918. doi:10.1039/d1ta06101a
Maiyalagan, T., and Elumalai, P. (2021). Rechargeable lithium-ion batteries: trends and progress in electric vehicles. Boca Raton: CRC Press.
Maragkaki, S., Savva, K., and Stratakis, E. (2021). Advanced photonic processes for photovoltaic, energy storage, and environmental systems. Adv. Sustain. Syst. 5 (3), 2000237. doi:10.1002/adsu.202000237
Masias, A., Marcicki, J., and Paxton, W. A. (2021). Opportunities and challenges of lithium ion batteries in automotive applications. ACS energy Lett. 6 (2), 621–630. doi:10.1021/acsenergylett.0c02584
McCreary, K. M., Hanbicki, A. T., Robinson, J. T., Cobas, E., Culbertson, J. C., Friedman, A. L., et al. (2014). Large-area synthesis of continuous and uniform MoS2 monolayer films on graphene. Adv. Funct. Mater. 24 (41), 6449–6454. doi:10.1002/adfm.201401511
Mehrpooya, M., Raeesi, M., Pourfayaz, F., and Delpisheh, M. (2021). Investigation of a hybrid solar thermochemical water-splitting hydrogen production cycle and coal-fueled molten carbonate fuel cell power plant. Sustain. Energy Technol. Assessments 47, 101458. doi:10.1016/j.seta.2021.101458
Meng, W., Pang, R., Li, M., Han, L., Kong, X., Zhang, D., et al. (2024). Integrated catalyst-substrate electrodes for electrochemical water splitting: a review on dimensional engineering strategy. Small, 2310469. doi:10.1002/smll.202310469
Mezzetti, A., Schnee, J., Lapini, A., and Di Donato, M. (2022). Time-resolved infrared absorption spectroscopy applied to photoinduced reactions: how and why. Photochem. and Photobiological Sci. 21 (4), 557–584. doi:10.1007/s43630-022-00180-9
Mitchell, S., and Pérez-Ramírez, J. (2021). Atomically precise control in the design of low-nuclearity supported metal catalysts. Nat. Rev. Mater. 6 (11), 969–985. doi:10.1038/s41578-021-00360-6
Mlilo, N., Brown, J., and Ahfock, T. (2021). Impact of intermittent renewable energy generation penetration on the power system networks – a review. Energy 6 (1), 25. doi:10.1007/s40866-021-00123-w
Mollaei, A. (2020). Design and implementation of a PI-controlled lithium ion battery charger with protection and monitoring. East. Mediterr. Univ. (EMU)-Doğu Akdeniz Üniversitesi (DAÜ). doi:10.1007/s10895-024-03982-5
Navalón, S., Dhakshinamoorthy, A., Álvaro, M., Ferrer, B., and García, H. (2022). Metal–organic frameworks as photocatalysts for solar-driven overall water splitting. Chem. Rev. 123 (1), 445–490. doi:10.1021/acs.chemrev.2c00460
Nguyen, H. Q., and Shabani, B. (2021). Review of metal hydride hydrogen storage thermal management for use in the fuel cell systems. Int. J. Hydrogen Energy 46 (62), 31699–31726. doi:10.1016/j.ijhydene.2021.07.057
Nkembi, A. A., Simonazzi, M., Santoro, D., Cova, P., and Delmonte, N. (2024). Comprehensive review of energy storage systems characteristics and models for automotive applications. Batteries 10 (3), 88. doi:10.3390/batteries10030088
Noor, T., Yaqoob, L., and Iqbal, N. (2021). Recent advances in electrocatalysis of oxygen evolution reaction using noble-metal, transition-metal, and carbon-based materials. ChemElectroChem 8 (3), 447–483. doi:10.1002/celc.202001441
Ouyang, D., Weng, J., Chen, M., Wang, J., and Wang, Z. (2022). Sensitivities of lithium-ion batteries with different capacities to overcharge/over-discharge. J. Energy Storage 52, 104997. doi:10.1016/j.est.2022.104997
Pai, R., Singh, A., Tang, M. H., and Kalra, V. (2022). Stabilization of gamma sulfur at room temperature to enable the use of carbonate electrolyte in Li-S batteries. Commun. Chem. 5 (1), 17. doi:10.1038/s42004-022-00626-2
Park, H. G., Min, K., and Park, K. (2022). A synergistic effect of Na+ and Al3+ dual doping on electrochemical performance and structural stability of LiNi0. 88Co0. 08Mn0. 04O2 cathodes for Li-ion batteries. ACS Appl. Mater. and Interfaces 14 (4), 5168–5176. doi:10.1021/acsami.1c16042
Park, J., Lee, S. Y., Kim, J. Y., Surendran, S., Lee, D. K., Park, Y. I., et al. (2023). Boosting ion diffusion at Ni2P@ 3D nanostructure carbon network interface for superior and durable sodium-ion hybrid capacitor. Electrochimica Acta 453, 142363. doi:10.1016/j.electacta.2023.142363
Peng, L., and Wei, Z. (2020). Catalyst engineering for electrochemical energy conversion from water to water: water electrolysis and the hydrogen fuel cell. Engineering 6 (6), 653–679. doi:10.1016/j.eng.2019.07.028
Qadir, S. A., Ahmad, F., Mohsin A B Al-Wahedi, A., Iqbal, A., and Ali, A. (2024). Navigating the complex realities of electric vehicle adoption: a comprehensive study of government strategies, policies, and incentives. Energy Strategy Rev. 53, 101379. doi:10.1016/j.esr.2024.101379
Qin, R., Chen, G., Feng, X., Weng, J., and Han, Y. (2024). Ru/Ir-Based electrocatalysts for oxygen evolution reaction in acidic conditions: from mechanisms, optimizations to challenges. Adv. Sci. 11, 2309364. doi:10.1002/advs.202309364
Qiu, Y., Liu, W., Chen, W., Chen, W., Zhou, G., Hsu, P. C., et al. (2016). Efficient solar-driven water splitting by nanocone BiVO4-perovskite tandem cells. Sci. Adv. 2 (6), e1501764. doi:10.1126/sciadv.1501764
Quilty, C. D., Wu, D., Li, W., Bock, D. C., Wang, L., Housel, L. M., et al. (2023). Electron and ion transport in lithium and lithium-ion battery negative and positive composite electrodes. Chem. Rev. 123 (4), 1327–1363. doi:10.1021/acs.chemrev.2c00214
Radisavljevic, B., Radenovic, A., Brivio, J., Giacometti, V., and Kis, A. (2011). Single-layer MoS2 transistors. Nat. Nanotechnol. 6 (3), 147–150. doi:10.1038/nnano.2010.279
Rafique, M., Mubashar, R., Irshad, M., Gillani, S. S. A., Tahir, M. B., Khalid, N. R., et al. (2020). A comprehensive study on methods and materials for Photocatalytic water splitting and hydrogen production as a renewable energy resource. J. Inorg. Organomet. Polym. Mater. 30 (10), 3837–3861. doi:10.1007/s10904-020-01611-9
Rajagopal, V., Kathiresan, M., Manivel, P., Suryanarayanan, V., Velayutham, D., and Ho, K. C. (2020). Porous organic polymer derived metal-free carbon composite as an electrocatalyst for CO2 reduction and water splitting. J. Taiwan Inst. Chem. Eng. 106, 183–190. doi:10.1016/j.jtice.2019.10.016
Rana, M. M., Uddin, M., Sarkar, M. R., Meraj, S. T., Shafiullah, G., Muyeen, S., et al. (2023). Applications of energy storage systems in power grids with and without renewable energy integration—a comprehensive review. J. energy storage 68, 107811. doi:10.1016/j.est.2023.107811
Rashidi, S., Karimi, N., Sunden, B., Kim, K. C., Olabi, A. G., and Mahian, O. (2022). Progress and challenges on the thermal management of electrochemical energy conversion and storage technologies: fuel cells, electrolysers, and supercapacitors. Prog. Energy Combust. Sci. 88, 100966. doi:10.1016/j.pecs.2021.100966
Rasul, M., Hazrat, M., Sattar, M., Jahirul, M., and Shearer, M. (2022). The future of hydrogen: challenges on production, storage and applications. Energy Convers. Manag. 272, 116326. doi:10.1016/j.enconman.2022.116326
Riaz, A., Sarker, M. R., Saad, M. H. M., and Mohamed, R. (2021). Review on comparison of different energy storage technologies used in micro-energy harvesting, WSNs, low-cost microelectronic devices: challenges and recommendations. Sensors 21 (15), 5041. doi:10.3390/s21155041
Roth, T., Streck, L., Graule, A., Niehoff, P., and Jossen, A. (2023). Relaxation effects in self-discharge measurements of lithium-ion batteries. J. Electrochem. Soc. 170 (2), 020502. doi:10.1149/1945-7111/acb669
Rouhi, H., Karola, E., Serna-Guerrero, R., and Santasalo-Aarnio, A. (2021). Voltage behavior in lithium-ion batteries after electrochemical discharge and its implications on the safety of recycling processes. J. Energy Storage 35, 102323. doi:10.1016/j.est.2021.102323
Sarker, M. T., Haram, M. H. S. M., Shern, S. J., Ramasamy, G., and Al Farid, F. (2024). Second-life electric vehicle batteries for home photovoltaic systems: transforming energy storage and sustainability. Energies 17 (10), 2345. doi:10.3390/en17102345
Schrotenboer, A. H., Veenstra, A. A., uit het Broek, M. A., and Ursavas, E. (2022). A Green Hydrogen Energy System: optimal control strategies for integrated hydrogen storage and power generation with wind energy. Renew. Sustain. Energy Rev. 168, 112744. doi:10.1016/j.rser.2022.112744
Schuler, T., Kimura, T., Schmidt, T. J., and Büchi, F. N. (2020). Towards a generic understanding of oxygen evolution reaction kinetics in polymer electrolyte water electrolysis. Energy and Environ. Sci. 13 (7), 2153–2166. doi:10.1039/d0ee00673d
Seifert, P. E. (2022). The value of large-scale offshore distribution islands. Trondheim, Norway: NTNU.
Sharma, V., Singh, K., and Krishnamurthy, N. (2024). A review on conventional to bipolar design of anode less all solid-state batteries. Energy Adv. doi:10.1016/j.envres.2023.117431
Shchurov, N. I., Dedov, S. I., Malozyomov, B. V., Shtang, A. A., Martyushev, N. V., Klyuev, R. V., et al. (2021). Degradation of lithium-ion batteries in an electric transport complex. Energies 14 (23), 8072. doi:10.3390/en14238072
Shi, X., Zhang, K., Shin, K., Ma, M., Kwon, J., Choi, I. T., et al. (2015). Unassisted photoelectrochemical water splitting beyond 5.7% solar-to-hydrogen conversion efficiency by a wireless monolithic photoanode/dye-sensitised solar cell tandem device. Nano Energy 13, 182–191. doi:10.1016/j.nanoen.2015.02.018
Song, B. F. (2020). Investigating and addressing the degradation mechanisms in silicon-based anodes for lithium-ion batteries. Houston, TX: Rice University.
Sorensen, J. J., Tieu, E., and Morse, M. D. (2020). Bond dissociation energies of the diatomic late transition metal sulfides: RuS, OsS, CoS, RhS, IrS, and PtS. J. Chem. Phys. 152 (24), 244305. doi:10.1063/5.0011754
Stančin, H., Mikulčić, H., Wang, X., and Duić, N. (2020). A review on alternative fuels in future energy system. Renew. Sustain. energy Rev. 128, 109927. doi:10.1016/j.rser.2020.109927
Sun, Y.-Y., Zhang, Q., Yan, L., Wang, T. B., and Hou, P. Y. (2022). A review of interfaces within solid-state electrolytes: fundamentals, issues and advancements. Chem. Eng. J. 437, 135179. doi:10.1016/j.cej.2022.135179
Tang, Z., Feng, D., Xu, Y., Chen, L., Zhang, X., and Ma, Q. (2023). Safety issues of layered nickel-based cathode materials for lithium-ion batteries: origin, strategies and prospects. Batteries 9 (3), 156. doi:10.3390/batteries9030156
Tao, X., Zhao, Y., Wang, S., and Li, C. (2022). Recent advances and perspectives for solar-driven water splitting using particulate photocatalysts. Chem. Soc. Rev. 51 (9), 3561–3608. doi:10.1039/d1cs01182k
Tashie-Lewis, B. C., and Nnabuife, S. G. (2021). Hydrogen production, distribution, storage and power conversion in a hydrogen economy-a technology review. Chem. Eng. J. Adv. 8, 100172. doi:10.1016/j.ceja.2021.100172
Tee, S. Y., Win, K. Y., Teo, W. S., Koh, L., Liu, S., Teng, C. P., et al. (2017). Recent progress in energy-driven water splitting. Adv. Sci. 4 (5), 1600337. doi:10.1002/advs.201600337
Theerthagiri, J., Lee, S. J., Shanmugam, P., and Choi, M. Y. (2020). “Basic principles in energy conversion and storage,” in Nanostructured, functional, and flexible materials for energy conversion and storage systems (Elsevier), 1–14.
Toki, G. F. I., Hossain, M. K., Rehman, W. U., Manj, R. Z. A., Wang, L., and Yang, J. (2024). Recent progress and challenges of silicon-based anode materials for lithium-ion batteries. Cambridge, United States: Industrial Chemistry and Materials.
Wang, F., Wang, L., Ou, Y., Lei, X., Yuan, J., Liu, X., et al. (2021d). Thermodynamic analysis of solid oxide electrolyzer integration with engine waste heat recovery for hydrogen production. Case Stud. Therm. Eng. 27, 101240. doi:10.1016/j.csite.2021.101240
Wang, H., Chen, Z. n., Wu, D., Cao, M., Sun, F., Zhang, H., et al. (2021a). Significantly enhanced overall water splitting performance by partial oxidation of Ir through Au modification in core–shell alloy structure. J. Am. Chem. Soc. 143 (12), 4639–4645. doi:10.1021/jacs.0c12740
Wang, J., Yue, X., Yang, Y., Sirisomboonchai, S., Wang, P., Ma, X., et al. (2020). Earth-abundant transition-metal-based bifunctional catalysts for overall electrochemical water splitting: a review. J. Alloys Compd. 819, 153346. doi:10.1016/j.jallcom.2019.153346
Wang, L., Wang, Y., and Xia, Y. (2015). A high performance lithium-ion sulfur battery based on a Li 2 S cathode using a dual-phase electrolyte. Energy and Environ. Sci. 8 (5), 1551–1558. doi:10.1039/c5ee00058k
Wang, S., Lu, A., and Zhong, C.-J. (2021b). Hydrogen production from water electrolysis: role of catalysts. Nano Converg. 8 (1), 4. doi:10.1186/s40580-021-00254-x
Wang, Z., Gu, Y., and Wang, L. (2021c). Revisiting solar hydrogen production through photovoltaic-electrocatalytic and photoelectrochemical water splitting. Front. Energy 15 (3), 596–599. doi:10.1007/s11708-021-0745-0
Wei, P., Sun, X., Liang, Q., Li, X., He, Z., Hu, X., et al. (2020). Enhanced oxygen evolution reaction activity by encapsulating NiFe alloy nanoparticles in nitrogen-doped carbon nanofibers. ACS Appl. Mater. and interfaces 12 (28), 31503–31513. doi:10.1021/acsami.0c08271
Wen, J., Zhao, D., and Zhang, C. (2020). An overview of electricity powered vehicles: lithium-ion battery energy storage density and energy conversion efficiency. Renew. Energy 162, 1629–1648. doi:10.1016/j.renene.2020.09.055
Wu, F., Maier, J., and Yu, Y. (2020). Guidelines and trends for next-generation rechargeable lithium and lithium-ion batteries. Chem. Soc. Rev. 49 (5), 1569–1614. doi:10.1039/c7cs00863e
Wu, S., Huang, C., Aivazian, G., Ross, J. S., Cobden, D. H., and Xu, X. (2013). Vapor–solid growth of high optical quality MoS2 monolayers with near-unity valley polarization. ACS Nano 7 (3), 2768–2772. doi:10.1021/nn4002038
Xie, X., Du, L., Yan, L., Park, S., Qiu, Y., Sokolowski, J., et al. (2022). Oxygen evolution reaction in alkaline environment: material challenges and solutions. Adv. Funct. Mater. 32 (21), 2110036. doi:10.1002/adfm.202110036
Xu, J., Cai, X., Cai, S., Shao, Y., Hu, C., Lu, S., et al. (2023). High-energy lithium-ion batteries: recent progress and a promising future in applications. Energy and Environ. Mater. 6 (5), e12450. doi:10.1002/eem2.12450
Yang, B., Qian, Y., Li, Q., Chen, Q., Wu, J., Luo, E., et al. (2024). Critical summary and perspectives on state-of-health of lithium-ion battery. Renew. Sustain. Energy Rev. 190, 114077. doi:10.1016/j.rser.2023.114077
Yin, Z., Li, H., Li, H., Jiang, L., Shi, Y., Sun, Y., et al. (2012). Single-layer MoS2 phototransistors. ACS Nano 6 (1), 74–80. doi:10.1021/nn2024557
Yu, H., Ke, J., and Shao, Q. (2023). Two dimensional Ir-based catalysts for acidic OER. Small 19 (48), 2304307. doi:10.1002/smll.202304307
Yu, Z. Y., Duan, Y., Feng, X., Yu, X., Gao, M., and Yu, S. (2021). Clean and affordable hydrogen fuel from alkaline water splitting: past, recent progress, and future prospects. Adv. Mater. 33 (31), 2007100. doi:10.1002/adma.202007100
Yuan, X., Ma, F., Zuo, L., Wang, J., Yu, N., Chen, Y., et al. (2021b). Latest advances in high-voltage and high-energy-density aqueous rechargeable batteries. Electrochem. Energy Rev. 4, 1–34. doi:10.1007/s41918-020-00075-2
Yuan, Y., Guo, R. t., Hong, L. f., Ji, X. y., Li, Z. s., Lin, Z. d., et al. (2021a). Recent advances and perspectives of MoS2-based materials for photocatalytic dyes degradation: a review. Colloids Surfaces A Physicochem. Eng. Aspects 611, 125836. doi:10.1016/j.colsurfa.2020.125836
Zeng, Z., Yin, Z., Huang, X., Li, H., He, Q., Lu, G., et al. (2011). Single-layer semiconducting nanosheets: high-yield preparation and device fabrication. Angew. Chem. Int. Ed. 47 (50), 11093–11097. doi:10.1002/anie.201106004
Zhang, B., Wang, W., Liang, L., Xu, Z., Li, X., and Qiao, S. (2021a). Prevailing conjugated porous polymers for electrochemical energy storage and conversion: lithium-ion batteries, supercapacitors and water-splitting. Coord. Chem. Rev. 436, 213782. doi:10.1016/j.ccr.2021.213782
Zhang, C., Greenblatt, J. B., Wei, M., Eichman, J., Saxena, S., Muratori, M., et al. (2020b). Flexible grid-based electrolysis hydrogen production for fuel cell vehicles reduces costs and greenhouse gas emissions. Appl. Energy 278, 115651. doi:10.1016/j.apenergy.2020.115651
Zhang, C., Wang, F., Han, J., Bai, S., Tan, J., Liu, J., et al. (2021b). Challenges and recent progress on silicon-based anode materials for next-generation lithium-ion batteries. Small Struct. 2 (6), 2100009. doi:10.1002/sstr.202100009
Zhang, M., Li, X., Zhao, J., Han, X., Zhong, C., Hu, W., et al. (2020a). Surface/interface engineering of noble-metals and transition metal-based compounds for electrocatalytic applications. J. Mater. Sci. and Technol. 38, 221–236. doi:10.1016/j.jmst.2019.07.040
Zhang, M., Zhang, K., Ai, X., Liang, X., Zhang, Q., Chen, H., et al. (2022). Theory-guided electrocatalyst engineering: from mechanism analysis to structural design. Chin. J. Catal. 43 (12), 2987–3018. doi:10.1016/s1872-2067(22)64103-2
Zhang, Q., and Guan, J. (2020). Single-atom catalysts for electrocatalytic applications. Adv. Funct. Mater. 30 (31), 2000768. doi:10.1002/adfm.202000768
Zhang, Y., Fu, J., Zhao, H., Jiang, R., Tian, F., and Zhang, R. (2019). Tremella-like Ni3S2/MnS with ultrathin nanosheets and abundant oxygen vacancies directly used for high speed overall water splitting. Appl. Catal. B Environ. 257, 117899. doi:10.1016/j.apcatb.2019.117899
Zhang, Y., Lu, S., Wang, Z., Volkov, V., Lou, F., and Yu, Z. (2023). Recent technology development in solvent-free electrode fabrication for lithium-ion batteries. Renew. Sustain. Energy Rev. 183, 113515. doi:10.1016/j.rser.2023.113515
Zhao, H., Li, J., Zhao, Q., Huang, X., Jia, S., Ma, J., et al. (2024b). Si-based anodes: advances and challenges in Li-ion batteries for enhanced stability. Electrochem. Energy Rev. 7 (1), 11. doi:10.1007/s41918-024-00214-z
Zhao, Y., Pohl, O., Bhatt, A. I., Collis, G. E., Mahon, P. J., Rüther, T., et al. (2021). A review on battery market trends, second-life reuse, and recycling. Sustain. Chem. 2 (1), 167–205. doi:10.3390/suschem2010011
Zhao, Z., Sun, J., Li, X., Zhang, Z., and Meng, X. (2024a). Joule heating synthesis of NiFe alloy/MoO2 and in-situ transformed (Ni, Fe) OOH/MoO2 heterostructure as effective complementary electrocatalysts for overall splitting in alkaline seawater. Appl. Catal. B Environ. 340, 123277. doi:10.1016/j.apcatb.2023.123277
Zhong, H., Liu, D., Yuan, X., Xiong, X., and Han, K. (2024). Advanced micro/nanostructure silicon-based anode materials for high-energy lithium-ion batteries: from liquid-to solid-state batteries. Energy and Fuels 38 (9), 7693–7732. doi:10.1021/acs.energyfuels.4c00633
Zhou, X., Zhou, J., Huang, G., Fan, R., Ju, S., Mi, Z., et al. (2018). A bifunctional and stable Ni–Co–S/Ni–Co–P bistratal electrocatalyst for 10.8%-efficient overall solar water splitting. J. Mater. Chem. A 6 (41), 20297–20303. doi:10.1039/c8ta07197g
Zhu, R., Liu, S., and Yang, D. (2024). Precisely prelithiated polyacrylic acid binder improving electrochemical performance of micron-sized silicon anodes for lithium-ion batteries. ChemElectroChem 11, e202400326. doi:10.1002/celc.202400326
Keywords: energy storage, water splitting, lithium-ion batteries, hydrogen generation, electrolysis, electrode materials, electrolytes, battery architecture
Citation: Hashmi SM, Noor S and Parveen W (2025) Advances in water splitting and lithium-ion batteries: pioneering sustainable energy storage and conversion technologies. Front. Energy Res. 12:1465349. doi: 10.3389/fenrg.2024.1465349
Received: 24 July 2024; Accepted: 19 December 2024;
Published: 27 January 2025.
Edited by:
Rachel Carter, Naval Research Laboratory, United StatesReviewed by:
Uk Sim, Korea Institute of Energy Technology (KENTECH), Republic of KoreaCopyright © 2025 Hashmi, Noor and Parveen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shah Noor, c2hhaG5vb3J3YXppcjEwMEBnbWFpbC5jb20=, bm9vcnNoYWgyM0BtYWlscy5qbHUuZWR1LmNu
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.