
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Energy Res. , 13 September 2022
Sec. Nuclear Energy
Volume 10 - 2022 | https://doi.org/10.3389/fenrg.2022.996253
This article is part of the Research Topic Nuclear safety: Waste Remediation, Radiation Protection, and Health Assessment View all 5 articles
 Tarek E. Sayed*
Tarek E. Sayed* El-Sayed S. Ahmed
El-Sayed S. AhmedArtemisia plants process potential bioactive secondary metabolites such as artemisinin and essential oil. They are useful in controlling potential pests and microbes and have a therapeutic effect. The commercial production of artemisinin and essential oil is limited to regarding the worldwide demand. Urgent attempts must be undertaken to improve the production of bioactive secondary metabolites. The objectives of this experiment were to increase the production and improve the quality of bioactive secondary metabolites in order to limit the use of agrochemicals. Therefore, a field experiment was conducted during the 2018 and 2019 seasons. Treatments included three elicitations (gamma irradiation, nano-selenium, and chitosan) and three kinds of fertilizers (NPK, Moringa leaf extract, and humic acid). The experiment was conducted as a factorial with a completely randomized block. The designs and treatments were arranged in a split–split plot with three replicates. A single application of elicitors showed that chitosan > nano-selenium, chitosan > gamma irradiation, and Moringa > humic acid > NPK in plants’ artemisinin and essential oil content, while the interaction showed a significant synergistic relationship between elicitors and the fertilizers in enhancing the quantity and quality of artemisinin and essential oil of Artemisia plants. Without using any pesticides, there was no infection that appeared in Artemisia plants, this could be due to the enhancement of bioactive secondary metabolite production.
Currently, clean water and safe food for a healthy man is an important issue for scientists and researchers, to solve major environmental problems worldwide (Saleh et al., 2021; Saleh et al., 2022). The sustainable application of nuclear technology in agricultural and medicinal plants is currently a global issue that has provoked significant challenges for modern economic technologies that offer high productivity of food and human welfare. In addition to increasing applications of nuclear technologies, radioactive contamination has been considered a great threat that has to be treated with economic and green techniques (Saleh et al., 2019), (Saleh et al., 2020). Nuclear techniques are becoming more important in medicine and agriculture. However, nuclear technology increases agricultural productivity and plays an important role in improving global access to a safe, secure, high-quality food supply, cultivating crops, and raising livestock (Udalova, 2020). When it comes to agriculture, nuclear techniques can make a significant difference even before the seeds are planted. One such technique makes it easier to breed hardier plant varieties. This is accomplished by exposing them to radiation and selecting mutations that increase their chances of survival and flourishing (Oladosu et al., 2016). Medicinal plants are beneficial to humans not only as a primary source of medicines but also as phytochemical building blocks for the development of new drugs, while 67% of drugs used in chemotherapy are derived from natural products (Phumthum et al., 2019). Treatment with medicinal plants is thought to be very safe because there are no or few side effects; however, based on the World Health Organization, 80% of people around the world rely on herbal medicines for some aspect of their primary health care needs (Ekor, 2014).
Radioactivity is considered one of the most important characteristics of radioisotopes. In this way, radioactivity was utilized as gamma irradiation of medicinal plants to improve their activity and productivity (Nabi et al., 2022). Recently, gamma irradiation was used to increase the yield of vinblastine in fungal cultures. Furthermore, various fermentation media were tested in order to determine the best one for maximum vinblastine production (El-Sayed, 2021). In a recent study, elicitation coupled with organic fertilizer mediates biomass and bioactive secondary metabolite production and quality to promote the achievement of sustainable development for marjoram under organic agriculture (Sayed and Ahmed, 2022).
Artemisia (Artemisia annua), is an annual medicinal and aromatic plant belonging to the family Asteraceae (Ferreira and Janick, 1996). Its leaves contain potential bioactive secondary metabolites (BSMs) such as essential oil (EO) and artemisinin (ART) in low concentration (Acton and Klayman, 1985). These BSMs revealed therapeutic properties and may be useful for controlling insect pests and microbes agents (Stanković et al., 2015; Towler and Weathers, 2015). Artemisinin a sesquiterpene (Guerriero et al., 2018) and its analogous are naturally antimicrobial BSMs. Because of their low concentrations in the Artemisia plant, artemisinin and essential oil production do not satisfy the world demand (Malik et al., 2013). These natural secondary metabolites are environmentally friendly and safe to be used as bio-pesticides to protect plants (Sarkhosh et al., 2017).
Since the beginning of the chemical revolution, agriculture has changed by the excessive use of fertilizers, pesticides, and microbicides (Pereira et al., 2019) in order to increase plant productivity. However, agrochemical compounds, pesticides, and microbicides have a negative impact on the environment perverting sustainable development (Maas et al., 2020).
Elicitation is a process of inducing enhancement of the synthesis of secondary metabolites by plants to ensure their survival, enhancing competitiveness (Sharma and Zafar, 2016; Świeca, 2016). Secondary metabolites are compounds that are essential for plant growth and survival, and they contribute to the economic importance of the plants. These secondary metabolites play a major role in plant adaptation to biotic and abiotic environmental stresses (Jansen et al., 2008). Elicitation has been applied to stimulate medicinal plant production through organic and agrochemical management (Złotek, 2017).
Among the various chitosan applications, the stability of microparticulate-based delivery systems is critical because it is strongly dependent on the surface electrostatic charge, which changes during storage (Abdel Ghaffar et al., 2018). Various radiation processing techniques, including electron beam, gamma radiation, UV, and X-rays, have been shown to significantly improve the properties of chitosan. Chitosan’s biocompatibility is unaffected by a sterilizing irradiation dose of 25 kGy (Seif et al., 2022). Crosslinking was reported to be negligible in chitosan irradiated with up to 25 kGy, but scissions of the 1–4 glycosidic bonds caused a reduction in the polymer’s molecular weight (Wach et al., 2020). The irradiation spectrum of chitosan exposed to a high dose of 560 kGy in air revealed the formation of carbonyl and carboxyl groups, as well as the decay of the C1 O C4, OH, and NH2 groups (Lim et al., 1998).
Elicitation has played a distinct role in the regulation of plant and pathogen attacks that cause huge loss in yield under production of agrochemical traditional agriculture (Zheng et al., 2005).
Organic fertilizers enhance plant dry weight, and BSM production increases the biological and pharmaceutical activities in addition to overcoming biotic and abiotic environmental stresses (Banchio et al., 2008; del Rosario Cappellari et al., 2013). Humic acid has proven to be effective in increasing growth, yield, and physiological processes in plants (Charles et al., 1990). Chitosan is an exogenous biotic elicitor. It is a polysaccharide and composed of a 2-deoxy-2- (acetylamino) glucose unit (N-acetyl glucosamine). Chitosan is produced as processing waste from shellfish krill and oyster squid and fungal cell walls (Montesano et al., 2003). Ahamed and Ahamed (2018) found a significant increase in cress plant biomass and seed yield in response to low doses of gamma radiation (15, 20, and 30 Gy) while the interaction between gamma radiation and iron nanoparticles (20 Gy + NFe 30 ppb) achieved a significant synergistic increase in essential oil percentage, total phenolic content, and total flavonoid content. Dandelion (Taraxacum officinale) plant treated with gamma radiation and nano and micro-zinc showed a significant increase in biomass and BSM (phenol flavonoids) production and antioxidants (Ahamed and El-Sayed, 2018). However, the nano-zinc effect encoded both micro-zinc and gamma radiation, and the production of secondary metabolites in plants is very low (less than 1% dry weight) (Dixon, 2001; Oksman-Caldentey and Inzé, 2004). Therefore, improving their production through elicitation could be significant in the pharmaceutical and therapeutic industries. The specific objectives of the current research are 1) to investigate the effect of elicitation on biomass production of the Artemisia plant, 2) to determine the quantitative and qualitative improvement of BSMs (essential oil and artemisinin, and 3) to identify the most effective elicitor that is capable of inducing the highest BSM production. The ultimate goal was to develop alternative control strategies (elicitation under organic fertilizers) to reduce the use of synthetic agrochemicals (microbicides and pesticides) in order to promote the sustainable development of the Artemisia plant.
Field experiments were conducted in two subsequent seasons during 2018 and 2019 at the “Horticultural production Farm” El-Sharkeya Governorate–Egypt. Artemisia plants were grown in sandy soil under the drip irrigation system using brackish shallow well water (900 ppm). The experiment design used was a factorial completely randomized block design, with three replicates. Treatments were arranged as split–split plots. The main plots contain three elicitors and the control while the sub-plots contain three fertilizers. A graphic diagram summarizing the materials and methods is reported in Scheme 1.
a. Gamma irradiation (GI), physical elicitor, at 2.5 KGy dos.
b. Nano-selenium oxide (NSe), abiotic elicitor, at 30 ppb.
c. Chitosan (CH), biotic elicitor, at 250 ppm.
d. No elicitor (NE NPK, the control treatment).
a. Agrochemical fertilizer NPK (19:19:19).
b. Organic fertilizers Moringa (MO), dry leaves’ extract.
c. Organic fertilizers, humic acid (HA).
All fertilizers were applied at 20 g/m2 rate, nano-selenium and chitosan were applied as foliar spray and Tween-20 (0.1 v/v) was added to the solution as surfactant.
Twenty grams of Moringa-dried leaves were extracted in boiling water, filtered, and then volume was increased to 1 L (20 g/L).
Irradiated and non-irradiated seeds were planted on 1 August 2018 and 2019 in a sandy soil. The plot size was 4 × 3 m2, and the rows were 50 and 30 cm for intra and inter spacing, respectively, to achieve 6.6 plant/m2, when seedlings reached 4-week age, they were sprayed with micronutrient solution containing (80zn, 50Cu, 65Fe, 75B, and 15Mo mgl−1). The plants were subjected to foliar spray with (CH) or (NSe) elicitors three times at 60, 90, and 120 days. The control plants were sprayed with water. However, fertilizers (NPK, MO, and HA) were applied via fertigation plants harvested on 1 February 2019 and 2020.
Fresh and dry weights of leaves were determined per plot and per m2 for 2018 and 2019 seasons.
a. Artemisinin (%ART)
Artemisinin percentage in leaves were measured using HPLC (Agilent, 1,200 series, United States) according to Acton and Klayman (1985) and later modified by Charles et al. (1990). Artemisinin yield g/m2 was determined as follows:
ARTg./m2 = leaves dry weight g/m2)x % ART.
b. Essential oil (EO)
The essential oil was extracted and determined related to continuous extraction with acetone using the Soxhlet apparatus. The obtained EO solution was evaporated under reduced pressure, in a rotatory evaporator. The EOY, (g/m2) is calculated as follows:
The datasets were first tested for normality by the Anderson and Darling normality tests using a statistical analysis system (SAS, 2003). There were no significant differences between the data of the two seasons. Therefore, the pooled mean values of two seasons for all traits tested were subjected to statistical analysis of variance. The significant means were compared using LSD at 1% probability.
The results presented in Table 1 and Figure 1 demonstrate that the fresh and dry weights of leaves of the control treatment were 5 and 0.62 kg/m2, respectively. There is a significant increase in fresh and dry leaves’ yield in response to the individual elicitors (E) and fertilizers (F). However, the interaction between E and F showed a positive synergistic relationship (fertilizers increased the effect of elicitors). The percentage increase in leaves’ dry weight over the control is as follows:
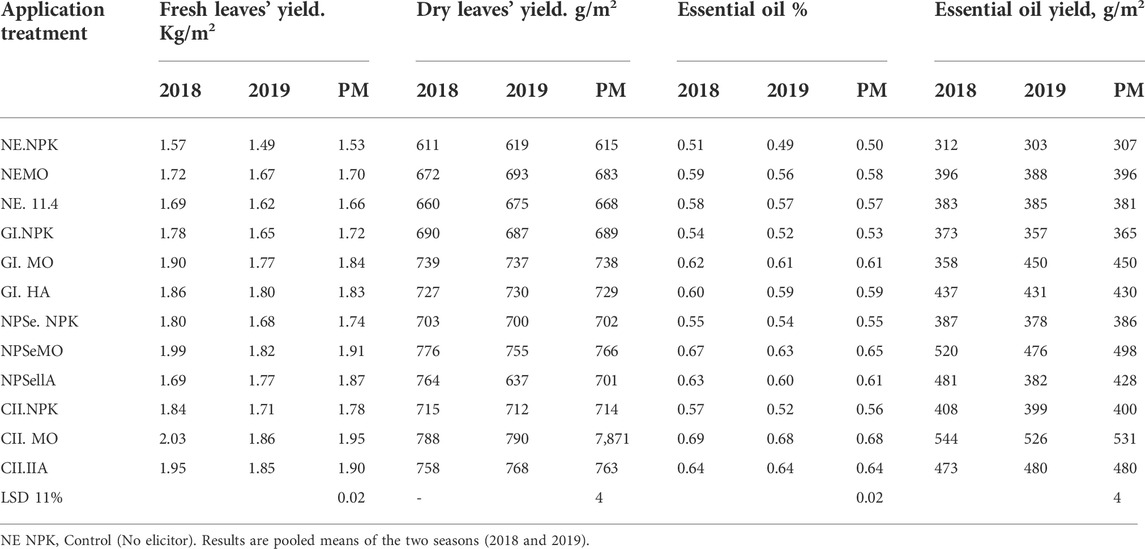
TABLE 1. Artemisia fresh and dry leaves’ yield, essential oil percentage, and essential oil yield in response to elicitors (GI, NSe, and CH) under (NPK),Moringa (MO), and humic acid (HA) fertilizers.
CH NPK, CH MO, and CH HA were 16, 28, and 24%, respectively. Moreover, NSe NPK, NP MO, and NP HA were 14, 25, and 22%, respectively, and GI NPK, GI MO, and GI HA were 12, 20, and 19%, respectively. In addition, EO NPK, EO MO, and EO HA were 0, 11, and 8%, respectively. The current results agree with those of other researchers (Caradonia et al., 2019; KarimzadehAsl and Hatami, 2019) who found that the elicitor application improves biomass production. They also agree with the results obtained by some workers (Ahamed and Ahamed, 2018), (Ahamed and El-Sayed, 2018) who found an increase in the biomass production of cress and dandelion plants in response to different elicitors (gamma radiation, nano-iron, and nano-zinc).
1. Essential oil percentage
The results presented in Table 1 and Figure 1 show that the essential oil of the control treatment is 0.50%. Essential oil percentage increased significantly in response to individual elicitor or fertilizer application. The interaction between the elicitors and fertilizers achieved a significant synergistic relationship. The percentage increase in essential oil over the control is as follows:
CH NPK, CH MO, and CH HA were 13, 36, and 28%, respectively. Moreover, NSe NPK, NP MO, and NP HA were 9, 30, and 23%, respectively, and GI NPK, GI MO, and GI HA were 5, 21, and 18%, respectively. In addition, NE NPK, EO NE, and NE HA were 0, 15, and 14%, respectively.
2. Essential oil yield:
The results shown in Table 1 and Figure 1 show that the control treatment (EO and NPK) was (307 g/m2) individual elicitors (GI, CH, and NP) and those individual fertilizers (NPK, MO, and HA) achieved a significant increase in oil yield over the control. However, a synergistic positive relationship was observed between elicitors and fertilizers. The percentage increase in essential oil yield over the control is as follows: EOY, g/m2
CH NPK, CHMO, and CH HA were 30, 73, and 56%, respectively. Moreover, NSe NPK, NP MO, and NP HA were 26, 62, and 40%, respectively. In addition, GI NPK, GI MO, and GI HA were 19, 47, and 31%, respectively, and NE NPK, NE MO, and NE HA were 0, 29, and 24%, respectively.
The obtained results agree with those of Namdeo (Stanković et al., 2015) who found that elicitation induces or enhances the synthesis of the secondary metabolites (essential oil) by plants. They also agree with those obtained by other authors (Ahamed and Ahamed, 2018; Ahamed and El-Sayed, 2018) who found a significant synergistic increase in cress plant essential oil percentage in response to low doses of gamma radiation (15, 20, and 30 Gy) and iron nanoparticles (20 Gy + NFe 30 ppb).
1. Artemisinin percentage
The results shown in Table 2 and Figure 2 demonstrate that the artemisinin percentage of the control (NE and NPK) treatment is (0.12%). The artemisinin percentage significantly increased over the control in response to individual elicitors or fertilizers’ application. However, a significant synergistic relationship was observed between elicitors and fertilizers. The percentage increase in artemisinin over the control is as follows:
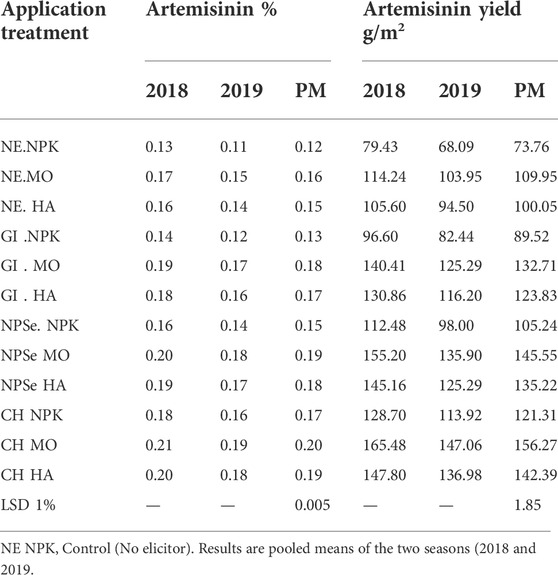
TABLE 2. ART % and artemisinin yield and their pooled means (PM) in response to CH, NSe, and GI elicitors under chitosan (CH) under (NPK), Moringa (MO and humic acid (HA) fertilizers.
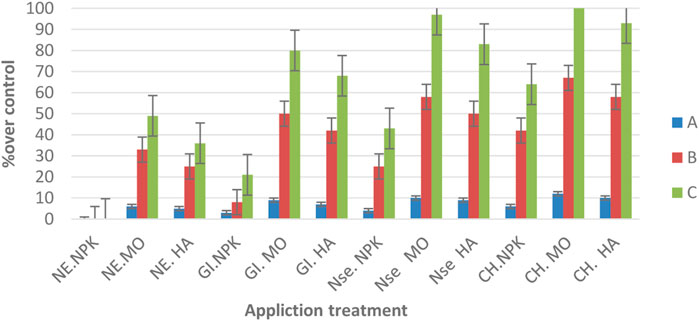
FIGURE 2. A: Total terpene NE constituents % over control, B: ART % constituents % over control, C: ARTY % over control.
CH NPK, CH MO, and CH HA were 42, 67, and 58%, respectively, and NSe NPK, NSe MO, and NSe HA were 25% 58%, and 50%, respectively. Moreover, GI NPK, GI MO, and GI HA were 8, 50, and 42%, respectively. In addition, NE NPK, NE MO, and NE HA were 0, 33, and 25%, respectively.
2. Artemisinin yield
The results shown in Table 2 and Figure 2 demonstrate that the artemisinin yield of the control (NE and NPK) treatment is 73.76 g/m2. It was significantly enhanced under individual elicitors or fertilizers’ application. However, the interaction between elicitors and fertilizers showed a synergistic relationship. The percentage increase in artemisinin yield over the control is as follows:
CH NPK, CH MO, and CH HA were 64, 112, and 93%, respectively. NSe NPK, NP MO, and NP HA were 43, 97, and 83%, respectively. Moreover, GI NPK, GI MO, and GI HA were 21, 80, and 68%, respectively, and NE NPK, NE MO, and NE HA were 0, 49, and 36%, respectively. Several researchers supported our results (Yadav and Sarkar, 2019; Mejdoub-Trabelsi et al., 2020). They declared that the application of elicitors in vitro and in vivo improves the bioactive secondary metabolite production and quality.
The main constituents of essential oil are presented in Table 3 and Figures 2, 3.
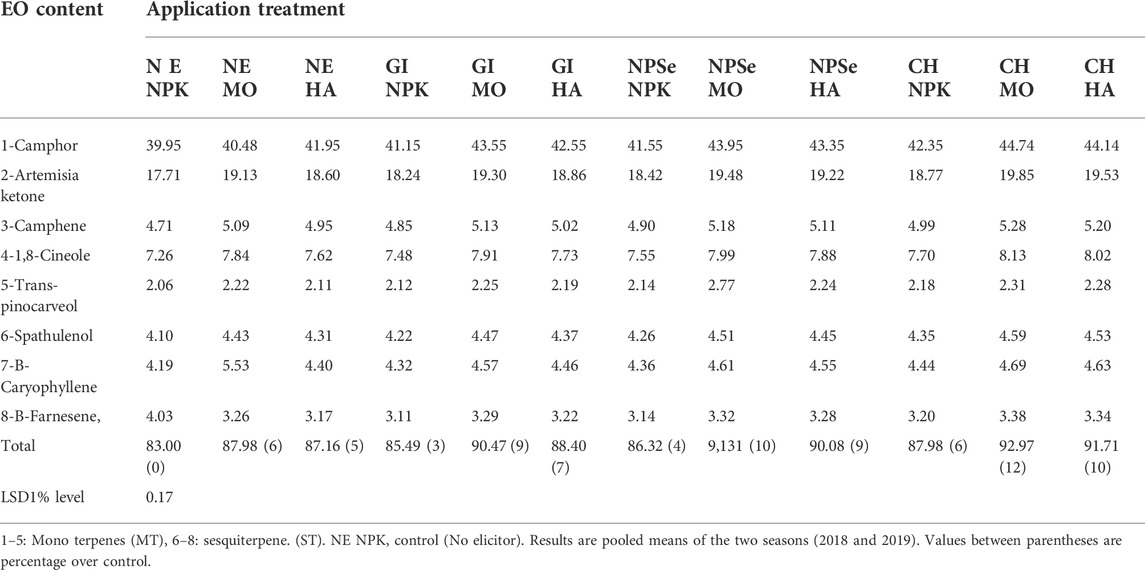
TABLE 3. Artemisia essential oil content, mono terpene (MT), and sesquiterpene (ST) in response to gamma irradiation (GI) nano-selenium oxide (NP), chitosan (CH) integrated with (NPK), Moringa (MO) and humic acid fertilizers.
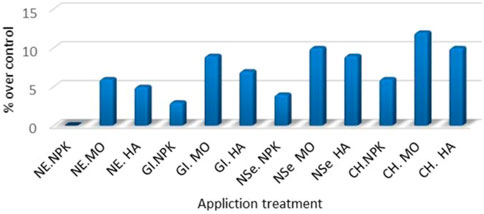
FIGURE 3. Total terpenoid content of essential oil (pooled mean for (2018 and 2019 seasons) as % of over control (NE NPK) under application treatment.
The results show the presence of 5-monoterpenes (camphor > Artemisia ketone > camphene >1.8- cineole > trans 5-pinocarveol) and 3 sesquiterpene (B-caryophyllene > spathulenol > B-farnesene). The total terpenoid content increased significantly over the control in response to elicitors and/or fertilizers application is as follows:
CH NPK, CH MO, and CH HA were 6, 12, and 10%, respectively. Furthermore, NSe NPK, NSe MO, and NSe HA were 4% 10%, and 9%, respectively. In addition, GI NPK, GI MO, and GI HA were 3, 9, and 7%, respectively, and NE NPK, NE MO, and NE HA were 0, 6, and 5%, respectively. The overall results manifest strong evidence that CH > NP > GI integrated with MO > HA > NPK could be considered a reliable technological strategy to improve the plant biomass and bioactive secondary metabolite (e.g., EO and ART) BM, production, and quality of Artemisia plant. This is due to the high production of BSMs (bio-microbicides and insecticides) as perceived by other investigators (Stanković et al., 2015). This is attributed to the following aspects: 1) elicitations enhance the bioactive secondary metabolite production that overcomes biotic and/or abiotic stresses (Valletta et al., 2016), 2) the presence of secondary metabolites (e.g., EO and ART) (El-Mohamedy and Mohamed, 2018; Trinh et al., 2018), 3) chitosan improves the tolerance of plants to biotic and abiotic stresses (KarimzadehAsl and Hatami, 2019), 4) nano-selenium reduces the environmental stress and is an eco- friendly alternative to chemical elicitors (Zhu et al., 2008; Usman et al., 2020), 5) Moringa suppressed plant diseases and induced significant resistance against pathogen growth and disease development (El-Mohamedy and Mohamed, 2018) and Moringa is considered to be an organic fertilizer and bio-pesticide (Yaseen and TAKÁCSNÉHÁJOS, 2020).
The results show strong evidence for the potent of CH > NSe > GI coupled with MO > HA > NPK as reliable eco-friendly elicitors to enhance the synthesis of bioactive secondary metabolites which achieve biological control and crop protection. This will lead to a significant increase in artemisinin and essential oil from the Artemisia plant without using insecticides and/or microbicides. Chitosan is proven to be the best elicitor when compared with nano-selenium or gamma radiation. However, Moringa (organic fertilizer) was the best fertilizer compared with humic acid (organic fertilizer) or NPK (chemical fertilizer). The best treatment that achieved the highest value for all the tested parameters is (Chitosan + Moringa).
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel Ghaffar, A. M., Ali, H. E., Nasef, S. M., and El-Bialy, H. A. (2018). Effect of gamma radiation on the properties of crosslinked chitosan nano-composite film. J. Polym. Environ. 26, 3226–3236. doi:10.1007/s10924-018-1208-5
Acton, N., and Klayman, D. L. (1985). Artemisitene, a new sesquiterpene lactone endoperoxide from Artemisia annua. Planta Med. 51, 441–442. doi:10.1055/s-2007-969543
Ahamed, T. E. S., and Ahamed, E. S. S. (2018). Synergy prospect low gamma irradiation doses incorporating elicitation with iron nanoparticles to hyper production biomass yield and bioactive secondary metabolites for cress, medicinal plant. J. Plant Sci. 6, 157–163.
Ahamed, T. E. S., and El-Sayed, S. A. (2018). Verification and validation of dandelion (Taraxacum officinal) seeds-gamma irradiated under elicitation with nano-and micro-zinc for potential optimization biomass and ennghanci phenolics, flavonoids and antioxidant activity. Int. J. Innov. Sci. Res. Technol. 3, 398–403.
Banchio, E., Bogino, P. C., Zygadlo, J., and Giordano, W. (2008). Plant growth promoting rhizobacteria improve growth and essential oil yield in Origanum majorana L. Biochem. Syst. Ecol. 36, 766–771. doi:10.1016/j.bse.2008.08.006
Caradonia, F., Battaglia, V., Righi, L., Pascali, G., and La Torre, A. (2019). Plant biostimulant regulatory framework: Prospects in europe and current situation at international level. J. Plant Growth Regul. 38, 438–448. doi:10.1007/s00344-018-9853-4
Charles, D. J., Simon, J. E., Wood, K. V., and Heinstein, P. (1990). Germplasm variation in artemisinin content of Artemism annua using an alternative method of artemisinin analysis from crude plant extracts. J. Nat. Prod. 53, 157–160. doi:10.1021/np50067a021
del Rosario Cappellari, L., Santoro, M. V., Nievas, F., Giordano, W., and Banchio, E. (2013). Increase of secondary metabolite content in marigold by inoculation with plant growth-promoting rhizobacteria. Appl. soil Ecol. 70, 16–22. doi:10.1016/j.apsoil.2013.04.001
Dixon, R. A. (2001). Natural products and plant disease resistance. Nature 411, 843–847. doi:10.1038/35081178
Ekor, M. (2014). The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4, 177. doi:10.3389/fphar.2013.00177
El-Mohamedy, R. S. R., and Mohamed, S. K. (2018). Effect of moringa oleifera seed oil, root and leave extracts on growth of major pathogenic fungi of tomato, green bean and potato in vitro. Int. J. Agric. Technol. 14, 505–520.
El-Sayed, E. R. (2021). Discovery of the anticancer drug vinblastine from the endophytic Alternaria alternata and yield improvement by gamma irradiation mutagenesis. J. Appl. Microbiol. 131, 2886–2898. doi:10.1111/jam.15169
Ferreira, J. F. S., and Janick, J. (1996). Distribution of artemisinin in Artemisia annua. Prog. new Crop, 579–584.
Guerriero, G., Berni, R., Muñoz-Sanchez, J. A., Apone, F., Abdel-Salam, E. M., Qahtan, A. A., et al. (2018). Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes (Basel). 9, 309. doi:10.3390/genes9060309
Jansen, M. A. K., Hectors, K., O’Brien, N. M., Guisez, Y., and Potters, G. (2008). Plant stress and human health: Do human consumers benefit from UV-B acclimated crops? Plant Sci. 175, 449–458. doi:10.1016/j.plantsci.2008.04.010
Karimzadeh Asl, K., and Hatami, M. (2019). Application of zeolite and bacterial fertilizers modulates physiological performance and essential oil production in dragonhead under different irrigation regimes. Acta Physiol. Plant. 41, 17–20. doi:10.1007/s11738-018-2801-x
Lim, L., Khor, E., and Koo, O. (1998). γ Irradiation of chitosan. J. Biomed. Mat. Res. 43, 282–290. doi:10.1002/(sici)1097-4636(199823)43:3<282::aid-jbm9>3.0.co;2-j
Maas, L., Malvestiti, R., and Gontijo, L. A. (2020). Work in organic farming: An overview. Cienc. Rural. 50. doi:10.1590/0103-8478cr20190458
Malik, A. A., Suryapani, S., Ahmad, J., Umar, S., Abdin, M. Z., and Mir, S. R. (2013). An attempt to enhance select secondary metabolite of Artemisia annua L. J. Biol. Sci. 13, 499–506. doi:10.3923/jbs.2013.499.506
Mejdoub-Trabelsi, B., Touihri, S., Ammar, N., Riahi, A., and Daami-Remadi, M. (2020). Effect of chitosan for the control of potato diseases caused by Fusarium species. J. Phytopathol. 168, 18–27. doi:10.1111/jph.12847
Montesano, M., Brader, G., and Palva, E. T. (2003). Pathogen derived elicitors: Searching for receptors in plants. Mol. Plant Pathol. 4, 73–79. doi:10.1046/j.1364-3703.2003.00150.x
Nabi, A., Mukarram, M., Aftab, T., Khan, M. M. A., and Naeem, M. (2022). “Acquisition of physiological modulations in medicinal plants through degraded natural polysaccharides under dynamic environment,” in Emerging plant growth regulators in agriculture (Amsterdam, Netherlands: Elsevier), 399–414.
Oksman-Caldentey, K.-M., and Inzé, D. (2004). Plant cell factories in the post-genomic era: New ways to produce designer secondary metabolites. Trends Plant Sci. 9, 433–440. doi:10.1016/j.tplants.2004.07.006
Oladosu, Y., Rafii, M. Y., Abdullah, N., Hussin, G., Ramli, A., Rahim, H. A., et al. (2016). Principle and application of plant mutagenesis in crop improvement: A review. Biotechnol. Biotechnol. Equip. 30, 1–16. doi:10.1080/13102818.2015.1087333
Pereira, M. M. A., Morais, L. C., Marques, E. A., Martins, A. D., Cavalcanti, V. P., Rodrigues, F. A., et al. (2019). Humic substances and efficient microorganisms: Elicitation of medicinal plants—a review. J. Agric. Sci. (Tor). 11, 268. doi:10.5539/jas.v11n7p268
Phumthum, M., Balslev, H., and Barfod, A. S. (2019). Important medicinal plant families in Thailand. Front. Pharmacol. 10, 1125. doi:10.3389/fphar.2019.01125
Saleh, H. M., Eskander, S. B., Mahmoud, H. H., and Abdou, M. I. (2022). Groundwater quality and health assessments based on heavy metals and trace elementscontent in DakhlaOasis, NewValley Governorate, Egypt. Water Sci. 36, 1–12. doi:10.1080/23570008.2021.2018540
Saleh, H. M., Mahmoud, H. H., Abdou, M. I., and Eskander, S. B. (2021). Health risk assessment based on metal analysis of soil and crops in Al-Dakhla Oasis. Arab. J. Geosci. 14, 260. doi:10.1007/s12517-021-06597-3
Saleh, H. M., Mahmoud, H. H., Aglan, R. F., and Bayoumi, T. A. (2019). Biological treatment of wastewater contaminated with Cu(II), Fe(II) and Mn(II) using Ludwigia stolonifera aquatic plant. Environ. Eng. Manag. J. 18, 1327–1336. doi:10.30638/eemj.2019.126
Saleh, H. M., Moussa, H. R., El-Saied, F. A., Dawoud, M., Nouh, E. S. A., and Abdel Wahed, R. S. (2020). Adsorption of cesium and cobalt onto dried Myriophyllum spicatum L. from radio-contaminated water: Experimental and theoretical study. Prog. Nucl. Energy 125, 103393. doi:10.1016/j.pnucene.2020.103393
Sarkhosh, A., Vargas, A. I., Schaffer, B., Palmateer, A. J., Lopez, P., Soleymani, A., et al. (2017). Postharvest management of anthracnose in avocado (Persea americana Mill.) fruit with plant-extracted oils. Food packag. shelf life 12, 16–22. doi:10.1016/j.fpsl.2017.02.001
Sayed, T. E., and Ahmed, E.-S. S. (2022). Elicitation promoability with gamma irradiation, chitosan and yeast to perform sustainable and inclusive development for marjoram under organic agriculture. Sustainability 14, 9608. doi:10.3390/su14159608
Seif, M. B., Motawea, I. T., Shoreibah, E. A., El-Maghraby, E. M. F., and Shalaby, H. A. (2022). Evaluation of surface characteristic of Ti-implant coated with nano-ceramic and chitosan after sterilization by gamma irradiation. ADJ-for. Girls 9, 29–37. doi:10.21608/adjg.2021.50250.1322
Sharma, K., and Zafar, R. (2016). Optimization of methyl jasmonate and β-cyclodextrin for enhanced production of taraxerol and taraxasterol in (Taraxacum officinale Weber) cultures. Plant Physiol. biochem. 103, 24–30. doi:10.1016/j.plaphy.2016.02.029
Stanković, M. S., Petrović, M., Godjevac, D., and Stevanović, Z. D. (2015). Screening inland halophytes from the central Balkan for their antioxidant activity in relation to total phenolic compounds and flavonoids: Are there any prospective medicinal plants? J. Arid. Environ. 120, 26–32. doi:10.1016/j.jaridenv.2015.04.008
Świeca, M. (2016). Elicitation and treatment with precursors of phenolics synthesis improve low-molecular antioxidants and antioxidant capacity of buckwheat sprouts. Acta Sci. Pol. Technol. Aliment. 15, 17–28. doi:10.17306/j.afs.2016.1.2
Towler, M. J., and Weathers, P. J. (2015). Variations in key artemisinic and other metabolites throughout plant development in Artemisia annua L. for potential therapeutic use. Ind. Crops Prod. 67, 185–191. doi:10.1016/j.indcrop.2015.01.007
Trinh, H., Yoo, Y., Won, K.-H., Ngo, H. T. T., Yang, J.-E., Cho, J.-G., et al. (2018). Evaluation of in-vitro antimicrobial activity of Artemisia apiacea H. and Scutellaria baicalensis G. extracts. J. Med. Microbiol. 67, 489–495. doi:10.1099/jmm.0.000709
Udalova, A. A. (2020). “Nonpower applications of nuclear technology,” in Nuclear reactor technology development and utilization (Amsterdam, Netherlands: Elsevier), 319–341.
Usman, M., Farooq, M., Wakeel, A., Nawaz, A., Cheema, S. A., ur Rehman, H., et al. (2020). Nanotechnology in agriculture: Current status, challenges and future opportunities. Sci. Total Environ. 721, 137778. doi:10.1016/j.scitotenv.2020.137778
Valletta, A., De Angelis, G., Badiali, C., Brasili, E., Miccheli, A., Di Cocco, M. E., et al. (2016). Acetic acid acts as an elicitor exerting a chitosan-like effect on xanthone biosynthesis in Hypericum perforatum L. root cultures. Plant Cell Rep. 35, 1009–1020. doi:10.1007/s00299-016-1934-x
Wach, R. A., Adamus-Wlodarczyk, A., Olejnik, A. K., Matusiak, M., Tranquilan-Aranilla, C., and Ulanski, P. (2020). Carboxymethylchitosan hydrogel manufactured by radiation-induced crosslinking as potential nerve regeneration guide scaffold. React. Funct. Polym. 152, 104588. doi:10.1016/j.reactfunctpolym.2020.104588
Yadav, K. K., and Sarkar, S. (2019). Biofertilizers, impact on soil fertility and crop productivity under sustainable agriculture. Environ. Ecol. 37, 89–93.
Yaseen, A., and Takácsné Hájos, M. (2020). Study on moringa tree (moringa oleifera lam.) leaf extract in organic vegetable production: A review. Res. Crop. 21 (2), 402–414. doi:10.31830/2348-7542.2020.067
Zheng, L., Hong, F., Lu, S., and Liu, C. (2005). Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol. Trace Elem. Res. 104, 083–092. doi:10.1385/bter:104:1:083
Zhu, H., Han, J., Xiao, J. Q., and Jin, Y. (2008). Uptake, translocation, and accumulation of manufactured iron oxide nanoparticles by pumpkin plants. J. Environ. Monit. 10, 713–717. doi:10.1039/b805998e
Keywords: BSMs, chitosan, elicitors, essential oil, gamma irradiation, HPLC, nano-selenium
Citation: Sayed TE and Ahmed E-SS (2022) Improving artemisinin and essential oil production from Artemisia plant through in vivo elicitation with gamma irradiation nano-selenium and chitosan coupled with bio-organic fertilizers. Front. Energy Res. 10:996253. doi: 10.3389/fenrg.2022.996253
Received: 17 July 2022; Accepted: 17 August 2022;
Published: 13 September 2022.
Edited by:
Shripad T. Revankar, Purdue University, United StatesReviewed by:
Fadwa Fayad, ASE, United StatesCopyright © 2022 Sayed and Ahmed. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tarek E. Sayed, ZHIudGFyZWtlbHNheWVkNjRAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.