
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Energy Res. , 09 September 2022
Sec. Process and Energy Systems Engineering
Volume 10 - 2022 | https://doi.org/10.3389/fenrg.2022.979857
This article is part of the Research Topic Advanced Thermal Conduction, Thermal Insulation, Thermal Storage Materials and Technologies View all 7 articles
Working fluids, working as a medium for energy transportation, play an important role in both thermal energy transportation and thermal management. Traditional heat transfer media, such as water, organic solvents, and mineral oils, have been unable to meet the increasing efficiency demand for heat transfer (Zhang et al., 2021; Esfe et al., 2022; Kursus et al., 2022; Younes et al., 2022). Generally speaking, the thermal conductivity of a nanoparticle is usually higher than that of a liquid; out of this consideration, Eastman et al. (2001) improve the heat-transfer performance of the fluid by adding nanoparticles to the base liquid and named nanofluids for the first time. Nanofluids have been widely used in many fields due to their good thermal properties; many researchers study the application prospects of nanofluids in the field of solar heat collection by simulating the working state of nanofluids in the process of heat transfer (Wang et al., 2022) so as to improve the heat collection efficiency of the collector and the stability and durability of the overall system (Figure 1A). A deep eutectic solvent (DES) is generally a eutectic mixture prepared by two or three components through the interaction between hydrogen bonds and has a wide application prospect (Walvekar et al., 2021). The raw materials are cheap, the preparation process is simple, and the thermophysical properties are excellent. At the same time, the liquid range of DES is a key concern, and the wide liquid range can meet the working requirements of working fluids under various climatic conditions.
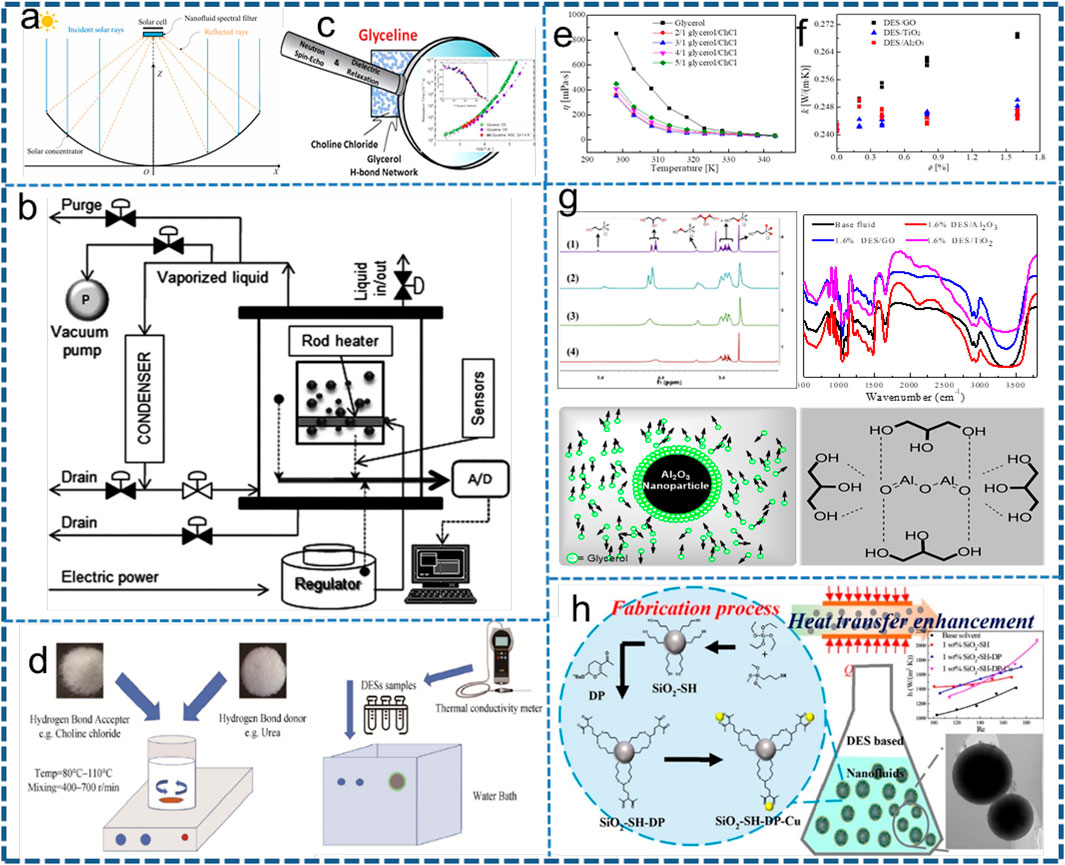
FIGURE 1. (A) Configuration of the PV/thermal system (Wang et al., 2022), (B) glycerol hydrogen-bonding network (Faraone et al., 2018), (C) temperature-dependent viscosity variation of glycerol/ChCl DESs, (D) thermal conductivity of the three nanofluids at 320 K as a function of volume fraction (Liu et al., 2019b), (E) scheme of the experimental apparatus (Fazel et al., 2013), (F) structure test and schematic of mechanism analysis (Liu C. et al., 2019b), (G) DES preparation and DES thermal conductivity measurements (Noor et al., 2021), (H) schematic of the DP and Cu nanoparticle-modified SiO2 nanoparticle and thermal conductivity enhancement in glycerol DESs (Liu C. et al., 2019a).
This opinion includes the study of glycerol and glycerol DES as the nanofluid base and their application to industrial processes in the field of heat transfer.
As a kind of biomass energy, biodiesel has similar physical properties to diesel and has good stability, excellent environmental protection properties, and combustion performance, and glycerol is an accompanying substance in its production process (i.e., over 10 wt%) (Liu D. et al., 2019). Glycerol has a low vapor temperature and a high boiling point due to its hydrogen bond network. Also, glycerol has good thermal stability, a wide working temperature range, and excellent environmental protection properties, so it is suitable for use in energy transportation. Due to the promotion of biomass energy, the production of glycerol has also increased, resulting in a situation of oversupply. In order to solve the problem of excess glycerol production capacity, many researchers have devoted themselves to the practical application of glycerol. At present, the heat exchanger can exchange heat directly and can also be made into mixed solvents or DES. For example, Moghaddam et al. (2016) checked the stability of dispersion of pure graphene nanosheets in glycerol by calculating the solubility parameter of pure graphene and pure glycerol (Figure 1D). It is shown that pure graphene with five layers can form a stable dispersion in glycerol. Fazel et al. (2013) studied the pool boiling heat-transfer characteristics of water/glycerol binary mixed solvents with different concentrations (0–35% mass glycerol) on horizontal rod heaters and found that when the concentration of glycerol was 0.02% mole fraction, the pool boiling heat-transfer coefficient can be increased by 15–20% (Figures 1E,F). However, the glycerol molecule has three hydroxyl groups, which lead to the formation of an intermolecular hydrogen bond network structure and make it have a high viscosity, which is the main problem limiting the application of glycerol-based nanofluids.
The DES prepared by glycerol as a hydrogen bond donor can effectively inhibit the hydrogen bond interaction between glycerol molecules and plays an important role in reducing the overall viscosity of the solvent. Faraone et al. (2018) of the National Institute of Standards and Technology of the United States systematically studied the relationship between hydrogen bonds and properties of glycerol/ChCl DES and concluded that choline ions were in the hydrogen bond network gaps constructed by glycerol molecules, and this “isolation effect” led to the reduction of glycerol molecular viscosity (Figure 1B). Dziubinska-Kühn et al. (2022) investigated the effect of hydrogen bond donor supersaturation on the properties of DES by titration analysis of glycerol/ChCl DES. Zhao et al. (2013) used glycerol/ChCl as a solvent and soybean oil as a raw material to enzymatically hydrolyze biodiesel, which could convert 88% of triglycerides by enzymatic hydrolysis within 1 day. In addition, Noor et al. (2021) studied the change of thermal conductivity of various glycerol-based DESs in the temperature range of 25–60°C and explored the application of fluids in heat transfer. It was found that the thermal conductivity decreased with the increase of temperature, and the solvent system with a high ChCl ratio had better thermal stability (Figures 1G,H). In addition, Noor et al. (2021) studied the thermal conductivity of glycerol-based DES with different collocations. It is shown from all results that the thermal conductivity decreases with the increase of temperature. This is because the temperature has a negative effect on electrons’ randomness and a positive effect on electrons’ free motion. Additionally, mixtures of a high ChCl ratio showed more stable values of thermal conductivity.
Nanofluids involving metal oxide. As an efficient heat-transfer medium, DESs have been widely used in catalysis, biomass degradation, materials chemistry, and other fields. Changhui Liu et al. (2019b) prepared glycerol/ChCl DES and filled it with TiO2 and Al2O3 nanoparticles to study their thermal properties such as thermal conductivity and specific heat. The results show that the nanofluid can maintain the liquid working state at −35 to 275°C, and the thermal conductivity is increased by 3–11.4%; after filling with nanoparticles, the specific heat capacity is partially decreased compared with the base liquid (Figure 1C).
Nanofluids involving non-metal oxide. In order to further study the heat transfer of glycerol-based DES nanofluids, Changhui Liu et al. (2019a) prepared a new type of Cu-SiO2 hybrid nanoparticle to enhance the stability and thermal conductivity of DES nanofluids. In this work, 2-butoxy-3,4-dihydropyran (DP) was employed as a metal nanoparticle anchor. It was found that DP was able to enhance the dispersing ability and thermal conductivity of SiO2 nanoparticle-filled DES-based nanofluids. The thermal conductivity of the Cu-SiO2-filled DES nanofluid was enhanced by a maximum of 13.6%, which indicated the positive effect of DP on the thermal conductivity improvement of the nanofluid.
With the application of nanofluids in the fields of flow heat transfer and energy transmission, the excellent thermal properties can significantly improve the energy transmission efficiency and save energy. However, the challenges of thermophysical properties of nanofluids, the mechanism of hydrogen bonding in DES, and the application of DES nanofluids as energy transmission media remain to be resolved. The following issues require further study:
1) Due to the easy agglomeration of nanoparticles, the stability of nanofluids is reduced, which makes it difficult for existing nanofluids to maintain stability for a long time, so glycerol-based DES nanofluids with high stability, a wide liquid range, high thermal conductivity, and low viscosity should be intensively investigated. In the meantime, compatibility between nanoparticles and the base solvent deserved to be further studied with the purpose to enhance the static stability.
2) The energy-transfer mechanism in the environment of the hydrogen bonding network in nanofluids prepared from glycerol or glycerol-based DES still needs further study since it is of great significance for understanding the improvement direction for the application of glycerol in the field of heat transfer, and its mechanism of action in nanofluids also needs to be further studied.
3) The research on glycerol-based DES nanofluids is still in the theoretical research stage, and it is necessary to study the stability and heat-transfer efficiency in different working environments to promote the application of nanofluids in the field of energy transportation.
CL proposed the opinion. WS drafted the original manuscript. WS and QL conducted research and collected background information. All authors check and revise the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Dziubinska‐Kühn, K., Pupier, M., Matysik, J., Viger‐Gravel, J., Karg, B., and Kowalska, M. (2022). Time‐dependent hydrogen bond network formation in glycerol‐based deep eutectic solvents. ChemPhysChem 23 (10), e202200283. doi:10.1002/cphc.202100806
Eastman, J. A., Choi, S. U. S., Li, S., Yu, W., and Thompson, L. J. (2001). Anomalously increased effective thermal conductivities of ethylene glycol-based nanofluids containing copper nanoparticles. Appl. Phys. Lett. 78 (6), 718–720. doi:10.1063/1.1341218
Esfe, M. H., Kamyab, M. H., and Toghraie, D. (2022). Statistical review of studies on the estimation of thermophysical properties of nanofluids using artificial neural network (ANN). Powder Technol. 400, 117210. doi:10.1016/j.powtec.2022.117210
Faraone, A., Wagel, D. V., Baker, G. A., Novak, E. C., Ohl, M., Reuter, D., et al. (2018). Glycerol hydrogen-bonding network dominates structure and collective dynamics in a deep eutectic solvent. J. Phys. Chem. B 122 (3), 1261–1267. doi:10.1021/acs.jpcb.7b11224
Fazel, S. A. A., Sarafraz, M., Shamsabadi, A. A., and Peyghambarzadeh, S. M. (2013). Pool boiling heat transfer in diluted water/glycerol binary solutions. Heat. Transf. Eng. 34 (10), 828–837. doi:10.1080/01457632.2012.746157
Kursus, M., Liew, P. J., Che Sidik, N. A., and Wang, J. (2022). Recent progress on the application of nanofluids and hybrid nanofluids in machining: A comprehensive review. Int. J. Adv. Manuf. Technol. 121 (3-4), 1455–1481. doi:10.1007/s00170-022-09409-4
Liu, C., Fang, H., Liu, X., Xu, B., and Rao, Z. (2019a). Novel silica filled deep eutectic solvent based nanofluids for energy transportation. ACS Sustain. Chem. Eng. 7 (24), 20159–20169. doi:10.1021/acssuschemeng.9b06179
Liu, C., Fang, H., Qiao, Y., Zhao, J., and Rao, Z. (2019b). Properties and heat transfer mechanistic study of glycerol/choline chloride deep eutectic solvents based nanofluids. Int. J. Heat Mass Transf. 138, 690–698. doi:10.1016/j.ijheatmasstransfer.2019.04.090
Liu, D., Liu, J.-C., Cai, W., Ma, J., Yang, H. B., Xiao, H., et al. (2019). Selective photoelectrochemical oxidation of glycerol to high value-added dihydroxyacetone. Nat. Commun. 10, 1779. doi:10.1038/s41467-019-09788-5
Moghaddam, M. B., Goharshadi, E. K., and Moosavi, F. (2016). Structural and transport properties and solubility parameter of graphene/glycerol nanofluids: A molecular dynamics simulation study. J. Mol. Liq. 222, 82–87. doi:10.1016/j.molliq.2016.07.014
Noor, A., Mohammed, K., Ghassan, A., and Suhaib, S. (2021). Thermal conductivity of room temperature deep eutectic solvents. J. Therm. Sci. 30 (6), 1960–1972. doi:10.1007/s11630-021-1428-1
Walvekar, R., Chen, Y. Y., Saputra, R., Khalid, M., Panchal, H., Chandran, D., et al. (2021). Deep eutectic solvents-based CNT nanofluid- A potential alternative to conventional heat transfer fluids. J. Taiwan Inst. Chem. Eng. 128, 314–326. doi:10.1016/j.jtice.2021.06.017
Wang, G., Zhang, Z., Jiang, T., Lin, J., and Chen, Z. (2022). Thermodynamic and optical analyses of a novel solar CPVT system based on parabolic trough concentrator and nanofluid spectral filter. Case Stud. Therm. Eng. 33, 101948. doi:10.1016/j.csite.2022.101948
Younes, H., Mao, M., Murshed, S. M. S., Lou, D., Hong, H., and Peterson, G. P. (2022). Nanofluids: Key parameters to enhance thermal conductivity and its applications. Appl. Therm. Eng. 207, 118202. doi:10.1016/j.applthermaleng.2022.118202
Zhang, T., Liu, C., Gu, Y., and Jerome, F. (2021). Glycerol in energy transportation: A state-of-the-art review. Green Chem. 23 (20), 7865–7889. doi:10.1039/d1gc02597j
Keywords: energy transportation, glycerol, deep eutectic solvent, nanofluids, thermophysical properties
Citation: Liu C, Sun W and Liu Q (2022) Opinions on glycerol-based deep eutectic solvent nanofluids for energy transportation. Front. Energy Res. 10:979857. doi: 10.3389/fenrg.2022.979857
Received: 28 June 2022; Accepted: 18 August 2022;
Published: 09 September 2022.
Edited by:
Iskander Tlili, National School of Engineers of Monastir, TunisiaReviewed by:
Yanxin Hu, Guangdong University of Technology, ChinaCopyright © 2022 Liu, Sun and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changhui Liu, bGl1Y2g5MTVAY3VtdC5lZHUuY24=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.