- 1Institute of Chemistry, University of Sargodha, Sargodha, Pakistan
- 2Department of Chemistry, Division of Science and Technology, University of Education, Lahore, Pakistan
- 3K.A.CARE Energy Research and Innovation Center, King Abdulaziz University, Jeddah, Saudi Arabia
- 4Department of Chemical and Materials Engineering, Faculty of Engineering, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Centre for Applied Molecular Biology (CAMB), University of the Punjab, Lahore, Pakistan
The present work reveals variation in the nutritional and antioxidant profiles of Moringa oleifera leaves with regard to four maturity stages (early, mid, penultimate and late). The corresponding yield of 80% methanolic extract (14.21 and 9.69%) and total phenolic contents (TPC) of the extract (95.26 and 38.22 mg GAE/g) from M. oleifera leaves were found to be maximum at early stage and minimum at the later stage. Total flavonoids, ash, protein, vitamin C and β-carotene contents were found to be minimum at the early stage and maximum at later stage (12.26 ± 0.47 to 30.07 ± 1.09 mg/g, 10.36–15.92%,50.3 ± 0.15 to 56 ± 0.77 mg/100 g, 143.14 ± 0.74 to 164.81 ± 0.44 mg/100g, and 89 ± 0.28 to 112.8 ± 1.40 mg/100 g). Amino acids including valine, alanine, leucine and phenylalanine were identified with their least contents at early stages (90.87, 53.07, 55.21, and 48.65 µg/g) and maximum at later stages (197.66, 114.3, 114.2, and 104.5 µg/g, respectively). The levels of different minerals such as Cu, Fe, Mn in M. oleifera leaves at different maturity stages varied from 0.59 to 2.08, 21.96 to 58.68, and 5.56 to 13.84 mg/100 g, respectively. RP-HPLC analysis of the nutritionally rich later-stage leave samples revealed the presence of quercetin as a major component (21.64 mg/kg), followed by benzoic acid, ferulic acid, sinapic acid, gallic acid, and p-coumaric acid with contributions of 13.03, 8.85, 3.39, 2.88, and 1.59 mg/kg, respectively. Overall, a considerable variation in the profile of different nutrients and antioxidants was noted in M. oleifera leaves as maturity progressed. These results support the harvesting of M. oleifera leaves at an appropriate maturity stage to maximize the functional food and nutraceutical benefits of this valuable food commodity.
Introduction
Plants are a rich source of valuable nutrients and high-value components with nutraceutical and medicinal potential (Qadir et al., 2019a). Various nutrients (proteins, lipids, and carbohydrates) and phytochemicals (such as alkaloids, polyphenols, and steroids) are formed as a result of primary and secondary metabolisms and play a vital role in the growth and development of plants (Qadir et al., 2019b).
One of the traditionally important food plants, namely, Moringa oleifera, a member of the family Moringaceae, is currently grown in various tropical and subtropical areas of the world. M. oleifera possesses an impressive range of nutritional and physiological benefits (Anwar et al., 2007). In Pakistan, it is commonly known as “Sohanjna” and is cultivated everywhere in the country, especially in the plain areas of Punjab and Sindh. Different parts of this multipurpose tree are not only used for edible purposes but are also employed for the treatment of different ailments in the folk medicine systems. Different parts of M. oleiefra including the leaves, flowers, fruits and roots have wide array of nutritional, functional food and medicinal benefits. The seeds, being natural coagulant, are used for water purification as well as employed for high-oleic oil production. As a non-conventional energy crop, M. oleifera seed oil is a potential feedstock for biodiesel (Rashid et al., 2008).
Phytochemical investigations have demonstrated that M. oleifera leaves are a rich source of iron, phosphorous, calcium, potassium, fundamental amino acids, vitamin A and D, and anti-cancer agents such as phenolics, flavonoids, vitamin C, and β-carotene (Anwar et al., 2007; Abdull Razis et al., 2014). Moringa leaves are also a rich source of pro-vitamin A, proteins, nutrients, and antioxidants (Anwar et al., 2007). Moringa leaves are consumed naturally, cooked, and can be kept as dried powder for a long period without refrigeration. In particular, the leaves of M. oleifera are valued as the most important part and are used in both nutritional and therapeutic measures (Nouman et al., 2016).
The growth and development period of any plant species depends upon external and internal factors which further shape the morphological features and phytochemistry. In some of our previous studies, it was investigated that phenolic antioxidants, organic acids, and natural sugars in selected fruits varied significantly as a function of fruit maturity stages (Mahmood et al., 2012).
Like in other plants, growth and maturity stages might affect the nutritional and phytochemical profile in M. oleifera leaves (Aissi et al., 2014). Thus it can be hypothesized that leaves of M. oleifera might have varied nutritional and bioactive profiles with respect to different maturity and development stages. Keeping this rational in mind, this research work was aimed at evaluating the variations in nutritional and antioxidant attributes of M. oleifera leaves harvested at different maturity stages.
Materials and Methods
Collection and Pre-Treatment of Leaves
The leaves of M. oleifera were harvested from Silanwali, district Sargodha, Punjab, Pakistan at four different stages /periods (15th October/ early stage, 15th November/mid stage, 15th December/penultimate stage and 15th January/late stage) with the gap of 1 month during the year 2017. Leaves were washed with water to remove any dusty particles, then shade-dried for 1 week and finally pulverized into a fine powder. The powdered sample was used for different experiments and analyses.
Preparation of Phenolic Crude Extracts (Orbital Shaking Technique)
Moringa leaf powder was weighed (20 g) and mixed with 200 ml of 80% methanol in a conical flask; shaking was carried out by using an orbital shaker for 8 h at 200 rpm. After shaking, the extracts were separated from the residue by filtering through Whatman filter paper No. 1. The residue was extracted again in a similar fashion, and both the filtrates recovered were combined. The extra solvent was distilled off under reduced pressure/vacuum using a rotary evaporator machine until a solvent-free extract was obtained. The extract was kept at −4°C for further studies.
Estimation of Total Phenolic Contents and Total Flavonoid Contents
Total phenolic contents (TPC) were analyzed by the Folin-Ciocalteu reagent method (Zahoor et al., 2016). A weighed amount of extract was placed in a test tube and mixed with Folin-Ciocalteu reagent (0.5 ml) and distilled water (7.5 ml). The mixture was kept at room temperature (22°C) for 5 min, and then 2 ml of 20% Na2CO3 was added. After that, the mixture was further incubated for 20 min at around 40°C and then absorbance was noted by a spectrophotometer at a wavelength of 700 nm. TPC was calculated using a gallic acid standard calibration curve.
For TFC determination, the weighed amount of aqueous extract (0.5 ml) was mixed with 0.3 ml of 5% NaNO2 in a test tube. After 5 min, 0.3 ml of 10% AlCl3 was added and the mixture incubated for 6 min, followed by the addition of 2 ml of 1.0 M NaOH. The absorbance of the final reaction mixture was measured at 510 nm using a spectrophotometer, and the amount of TF was calculated as catechin equivalents using a standard calibration curve (Aiyegoro and Okoh., 2010; Babbar et al., 2011).
Determination of Proteins by the Bradford Assay
For the preparation of Bradford reagent, 100 mg of Coomassie brilliant blue G250 was dissolved in 50 ml of ethanol. Then 100 ml of 85% phosphoric acid was added till the complete dissolution of the dye and filtered through the Whatman No.1 filter paper. Briefly, 1 ml of different concentrations of stock solutions like 20, 40, 60, 80, and 100 mg were taken and then in each of the test tubes, 5 ml of Bradford reagent was added. Similarly, 1 ml of each sample was taken in a labeled test tube and 5 ml of the Bradford reagent was added and the mixture incubated for 5 min. A blank, containing only water and 5 ml of Bradford reagent, was also prepared. The absorbance was measured at 595 nm against a blank solution and the amount of protein was calculated (Stoscheck, 1990).
Determination of Ash Contents
Five Gram of each sample was taken into porcelain crucible and heated on flame until turned black. This black ash was then placed into the muffle furnace for about 5 h until it turned into white or gray ash. The furnace temperature was slowly increased from room temperature to 500°C, and the percentage of ash content was determined gravimetrically (Turkekul et al., 2004; Farhoosh and Riazi., 2007).
Estimation of Minerals
The sample was ashed and then placed in Eppendorf tube for further analysis. For acidic digestion of ash, two drops of HNO3 and 3-4 drops of acidic water were added to the ash. The acidic water was prepared by adding a few drops of HCL to 200 ml of distilled water. This solution was then filtered into a volumetric flask and further diluted. Finally, the analysis was made by using an atomic absorption spectrophotometer using the respective minerals/metals’ hollow-cathode lamps. The mineral content was determined by preparing and analyzing a series of standard solutions of the minerals through the construction of calibration curves (Varadharaju et al., 2001).
Estimation of Amino Acids by the Ninhydrin Method
Different standard amino acid solutions (0.1–1.0 ml) were pipetted into the respective marked test tubes and further diluted with distilled water. One mL of ninhydrin reagent was added to all the test tubes. All the test tubes were placed in a water bath for 15 min. Similarly, test samples were prepared/treated. After cooling the sample and standard test tubes, 1 ml of ethanol was added to each test tube and absorbance was recorded at 570 nm for each solution. The amount of amino acids were calculated following the standard calibration curve (Yemm et al., 1955).
Spectrophotometric Determination of Vitamin C
The determination of vitamin C was carried out spectrophotometrically using 2,4-dinitrophenylhydrazine reagent after pre-treatment of samples with trichloroacetic acid (TCA). The absorbance of the sample and standard solution was recorded at 540 nm (Tantray et al., 2017).
Determination of β-Carotene
A well-blended sample (5–25 g) containing 10–500 mg total carotenoids was taken in a conical flask. The carotene extraction was performed using a mixture of petroleum ether and distilled water via liquid–liquid partitioning. The absorbance of the test solution recovered along with the standard solutions was measured at 452 nm. The total carotenoid content was estimated using a standard curve, and the results were expressed as mg/100 g of sample (Mustapha and Babura., 2009).
Statistical Analysis
The data were expressed as mean values ± SD for triplicate measurements. A 2-way ANOVA was performed using SPSS version 22.0.0.0 software.
Results and Discussion
Plantation and Soil Conditions
The plantation of M. oleifera is considered suitable in regions where temperature ranges from 25–35°C. The soil should be loamy with a moderate acidic to alkaline pH. Most of the soils in Pakistan are calcareous in nature with a pH of 8. The pH of the soil in Silanwali is also reported to be 7.9–10.4. At this pH, the availability of P, K, Zn, Cu, Fe, Mn, and B is high, which is useful for the health and growth of most plants (Aslam et al., 2005).
Yield of Antioxidant Extracts
The percentage yield of phenolic bioactive extracted from M. oleifera leaves using 80% methanol ranged from 14.21 to 9.69% in relation to different maturity stages (Figure 1). Extraction yield of early, mid, penultimate, and late stages are 14.21, 13.66, 10.99, and 9.69%. There was no literature available regarding the effect of maturity stages on the extract yield of M. oleifera leaves. However in the studies conducted by Gull et al., 2012 on guava fruits, they found that the fully ripened fruits from the three selected regions exhibited the highest methanolic extract yields (18.92–24.91%), while those picked at the un-ripe stage offered the lowest (12.05–13.23%).
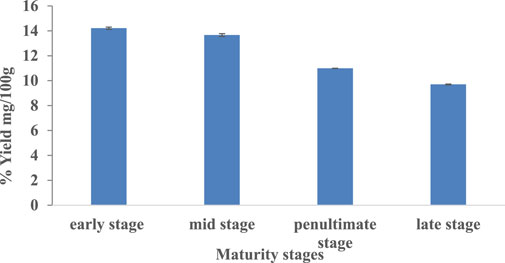
FIGURE 1. Percentage yield (g/100 g) of extracts from M. oleifera leaves at different maturity stages.
Total Phenolic and Flavonoid Contents
The total phenolic contents of the 80% methanol extract of M. oleifera leaves at different maturity stages are shown in Table 1. The total phenolic contents in M. oleifera leaves were found to vary from 95.26 to 38.22 mg GAE/g. The TPC in early, mid, penultimate and late stage samples was 95.26 ± 0.89, 60.36 ± 1.14, 46.85 ± 1.55, and 38.22 ± 1.61 mg/g, respectively. The highest contents of phenolics were recorded in young leaves, and the lowest contents were present in late stage leaves. In line with current results, Jahan et al., 2015 also reported that the TPC of the tender leaves (high chlorophyll) and mature leaves (low chlorophyll) were observed at 35.51 and 30.83 mg/g GAE, respectively.
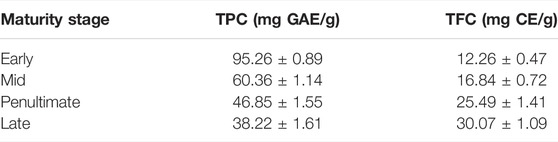
TABLE 1. Total phenolic content and total flavonoid content (mg/g) of Moringa oleifera leaf extract.
The total flavonoid content of an 80% methanolic extract of M. oleifera leaves at four different maturity stages ranged from 12.26 to 30.07 mg CE/g. Values of TFC in early to late stages ranged from 12.26 ± 0.47, 16.84 ± 0.72, 25.49 ± 1.41 and 30.07 ± 1.09 mg CE/g, respectively. In line with TPC results, Jahan et al., 2015 described in their work that the mature leaves (high chlorophyll) were found to have high flavonoids (98.67 mg CE/g) as compared with tender leaves (low chlorophyll), which had lower flavonoids (32.74 mg CE/g).
HPLC Analysis of Phenolics and Flavonoids
Phenolics and flavonoids of M. oleifera methanolic leaf extract were individually identified by using HPLC with a UV-visible detector. The signals were detected at 280 nm and the identification of unknown compounds was made by comparing the retention times with those of pure standards. The HPLC results revealed the presence of six phenolics and flavonoids such as quercetin, gallic acid, benzoic acid, sinapic acid, ferulic acid, and p-coumaric acid (Table 2). The major flavonoid was quercetin with a concentration of 21.64 ppm, followed by benzoic acid, ferulic acid, sinapic acid, gallic acid, and p-coumaric acid with concentrations such as 13.03, 8.85, 3.39, 2.88, and 1.59 ppm, respectively. The HPLC chromatogram of M. oleifera leaves is shown in Figure 2.
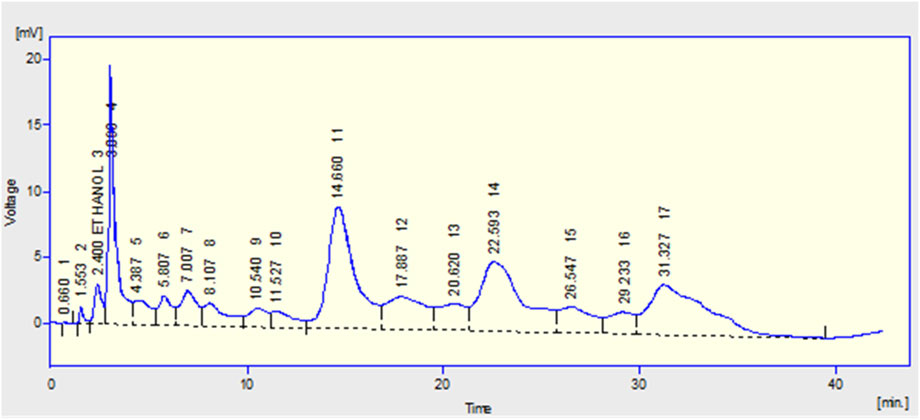
FIGURE 2. HPLC chromatogram showing separation of phenolics and flavonoids in the M. oleifera leaf extracts at a late stage.
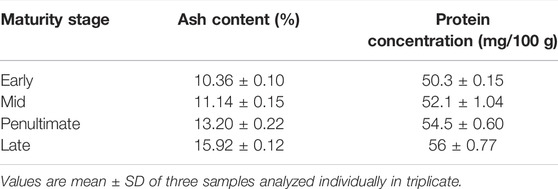
Ash and Protein Contents
The values of ash content in M. oleifera leaves were found at 10.36% at first stage, 11.14% at 2nd stage, 13.20% at 3rd stage and 15.9% at the late stage (Table 3). The lowest ash content was found in the early stage (10.36%) and the highest amount of ash was found in the late stage (15.9%). However, the studies conducted by Agamou et al., 2015 demonstrated that young and mature leaves had almost similar total ash contents, such as 8.90 ± 0.45 and 8.97 ± 0.50 g per 100 g, respectively. Likewise, they also observed no difference in the ash content of M. oleifera leaves collected from different localities.
The concentration of proteins in M. oleifera leaves at different maturity stages ranged from 50.3 to 56 mg/100 g. The protein concentrations in the early to late stage were 50.3, 52.1, 54.5, and 56 mg/100g, respectively. Maximum protein content was found in mature leaves, and minimum content was observed in tender leaves. Moyo et al. (2011) presented in studies that M. oleifera leaf powder had a considerable amount of proteins, such as 29 g/100. The protein content in moringa leaves was also affected (11.98%) by the stages of maturity of the plant.
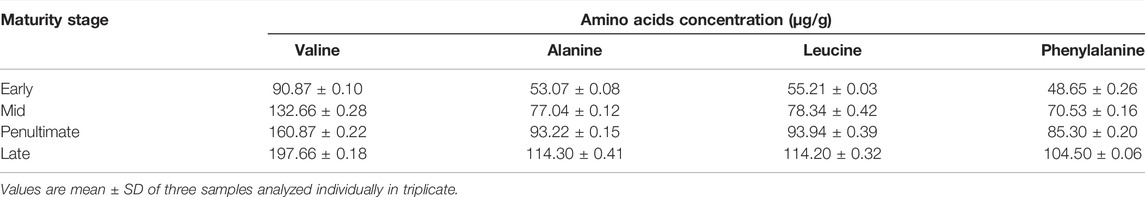
Amino Acid Content
In the current study, among the amino acids analyzed, valine content ranged from 90.87 to 197.66 μg/g (Table 4). The maximum content was noted at late stage (197.66 ± 0.18 μg/g) and minimum content was found in early stage (90.87 ± 0.10 μg/g). Alanine concentration was found to be in the range of 53.07 to 114.3 μg/g. The maximum content was exhibited in late stage samples (114.3 ± 0.41 μg/g) and minimum in early stage sample (53.07 ± 0.08 μg/g). Leucine content was found to be in the range of 55.21 to 114.2 μg/g. The maximum content was exhibited by late stage (114.2 ± 0.32 μg/g) and minimum content was observed in early stage (55.21 ± 0.03 μg/g). Phenylalanine levels were found to be in the range of 48.65 to 104.5 μg/g. The maximum content was shown in late stage sample (104.5 ± 0.06 μg/g) and minimum in early stage sample (48.65 ± 0.26 μg/g). The amino acid peak area of standards and samples was calculated to determinate the amino acid concentration.
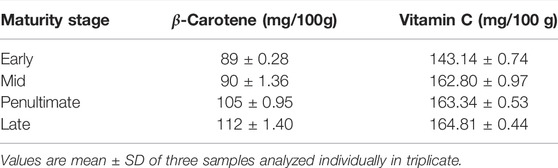
Vitamin A (β- Carotene) and Vitamin C (Ascorbic Acid)
β- Carotene concentration was found to be in the range of 89 to 112 mg/100 g (Table 5). The maximum content was shown in the late stage (112 ± 1.40) and the minimum content was found in the early stage (89 ± 0.28). The maximum content was shown in the late stage (112 ± 1.40) and the minimum content was found in the early stage (89 ± 0.28). Our results are contrary to the studies reported by Moran-Palacio et al. (2014), wherein mature leaves had low carotene content (122.46 ± 1.38) and tender leaves had more carotene content (92.77 ± 1.19). In another study, the concentration of carotenoids was appreciably higher in young leaves than in mature leaves and was also affected by the maturity stages. Other than carotenoids, ascorbic acid (271 mg/100 g) was also present in moringa leaves (Shih et al., 2011).
The concentration of vitamin C in M. oleifera leaves at different maturity stages ranged from 143.14 to 164.81 mg/100 g. Highest levels were observed in late stage and minimum in early maturity stage samples. The results agree with the literature, such as fresh M. oleifera samples showed vitamin C content of 62.66–143.58 mg/100 g for tender leaves, 51.22–150.15 mg/100 g for matured leaves. Kalappurayil and Joseph, 2016, demonstrated that the contents of vitamin C in moringa leaves were not affected by maturity stages, but a minor effect was observed by the locality. Whereas the young and mature moringa leaves had almost the same amount of vitamin C, such as 50.22 and 50.78 mg/100g, respectively. Fat-soluble vitamins such as vitamin-A (pre-cursor of beta-carotene) and vitamin-C were also present in M. oleifera (Mbikay., 2012). In another study conducted by Phullakhandam and Failla., 2007, the amounts of lutein and β-carotene were 418 and 272 mg/kg, respectively, in the fresh leaves of moringa and 472 and 166 mg/kg in dried powder.
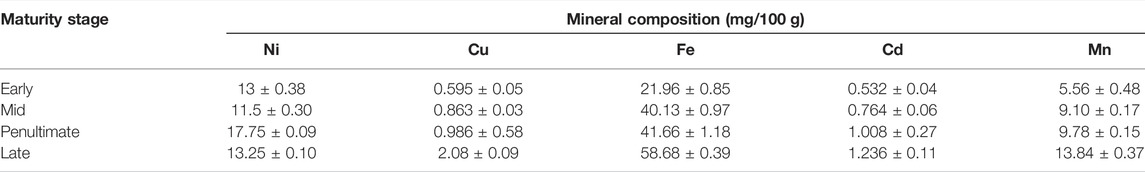
Mineral Composition
The composition of different minerals such as copper, nickel, manganese, iron, and manganese was also observed in the current work (Table 6) and found in confirmation with the literature (Castillo-López et al., 2017). The level of copper in the samples of M. oleifera leaves at different maturity stages ranged between 0.595 and 2.08 mg/100 g. The maximum Cu content was found in the late stage (2.08 ± 0.09 mg/100 g), and the minimum copper content was found in the early stage (0.595 ± 0.05 mg/100 g). The levels of nickel in the samples of M. oleifera leaves at different maturity stages ranged from 13.0 and 17.75 mg/100 g. The maximum Ni content was found to be in the penultimate stage (17.75 ± 0.09 mg/100 g), and the minimum Ni content was found to be in the early stage (13.0 ± 0.38 mg/100 g). The level of iron in the samples of M. oleifera leaves at different maturity stages ranged between 21.96 and 58.68 mg/100 g. The maximum Fe content was found to be in the late stage (58.68 ± 0.39 mg/100 g), and the minimum Fe content was observed in the early stage (21.96 ± 0.85 mg/100 g). The level of cadmium in the samples of M. oleifera leaves at different maturity stages ranged from 0.532 and 1.236 mg/100 g. The maximum Cd content was found to be in the late stage (1.236 ± 0.11 mg/100 g), and the minimum Cd content was found to be in the early stage (0.532 ± 0.04 mg/100 g). The level of manganese in the samples of M. oleifera leaves at different maturity stages ranged between 5.56 and 13.84 mg/100 g. The maximum Mn content was found to be in the late stage (13.84 ± 0.37 mg/100 g), and the minimum Mn content was found to be in the early stage (5.56 ± 0.48 mg/100 g). The moringa leaves are also rich in minerals such as potassium, zinc, magnesium, iron, and copper (Kasolo et al., 2010).
Conclusion
Nutritional and phenolic profiling of M. oleifera leaves along with assessment of antioxidant potential were made as a function of maturity stages. The maximum extract yield and TPC were noted for tender leaves and the minimum content in the mature leaves. In contrast, TFC, proteins, amino acids (alanine, valine, leucine, and phenylalanine), vitamins C, β-carotene, and mineral contents (Cu, Fe, Cd, Mn, and Ni) of M. oleifera leaves showed maximum contents in mature stages and minimum in tender leaves, except for Ni, which showed maximum contents in the third stage and minimum in the second stage among the four maturity stages. As far as composition of individual phenolics is concerned, HPLC results depicted the detection of six phenolics and flavonoids such as quercetin, gallic acid, benzoic acid, sinapic acid, ferulic acid and p-coumaric acid in the late stage M. oleifear leave samples. The findings of this study thus revealed considerable variations in the contents of analyzed nutrients and antioxidants with respect to different maturity stages. Overall, fully matured stage samples of leaves demonstrated higher contents of phenolic bioactives thus advocating the potential uses of mature leaves in nutra-pharmaceutical products.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material; further inquiries can be directed to the corresponding author.
Author Contributions
MT, FA, HA, and TM contributed in formulating the research work and manuscript, and KB and RQ worked on it.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors acknowledge the support provided by King Abdullah City for Atomic and Renewable Energy (K.A.CARE) under the K.A.CARE-King Abdulaziz University Collaboration Program.
References
Abdull Razis, A. F., Ibrahim, M. D., and Kntayya, S. B. (2014). Health Benefits of Moringa Oleifera. Asian Pac. J. Cancer Prev. 15, 8571–8576. doi:10.7314/APJCP.2014.15.20.8571
Agamou, J. A. A., Fombang, E. N., Fombang, E. N., and Mbofung, C. M. F. (2015). Particular Benefits Can Be Attributed to Moringa Oleifera Lam Leaves Based on Origin and Stage of Maturity. Jebas 3, 541–555. doi:10.18006/2015.3(6).541.555
Aissi, A. K., Pazou, E. Y., Ahoyo, T. A., Fah, L., Fanou, B., Koumolou, L., et al. (2014). Evaluation of Toxicological Risk Related to Presence of Lead and Cadmium in <i>Moringa Oleifera</i> Lam. Leaves Powders Marketed in Cotonou (Benin). Fns 05, 770–778. doi:10.4236/fns.2014.59087
Aiyegoro, O. A., and Okoh, A. I. (2010). Preliminary Phytochemical Screening and In Vitro Antioxidant Activities of the Aqueous Extract of Helichrysum Longifolium DC. BMC Complement. Altern. Med. 10, 1–8. doi:10.1186/1472-6882-10-21
Anwar, F., Latif, S., Ashraf, M., and Gilani, A. H. (2007). Moringa Oleifera: a Food Plant with Multiple Medicinal Uses. Phytother. Res. 21, 17–25. doi:10.1002/ptr.2023
Aslam, M., Anwar, F., Nadeem, R., Rashid, U., Kazi, T. G., and Nadeem, M. (2005). Mineral Composition of Moringa Oleifera Leaves and Pods from Different Regions of Punjab, Pakistan. Asian J. Plant Sci. 4, 417–421. doi:10.3923/ajps.2005.417.421
Babbar, N., Oberoi, H. S., Uppal, D. S., and Patil, R. T. (2011). Total Phenolic Content and Antioxidant Capacity of Extracts Obtained from Six Important Fruit Residues. Food Res. Int. 44, 391–396. doi:10.1016/j.foodres.2010.10.001
Castillo-López, R. I., Leon-Felix, J., Angulo-Escalante, M., Gutiérrez-Dorado, R., Muy-Rangel, M. D., and Heredia, J. B. (2017). Nutritional and Phenolic Characterization of Moringa Oleifera Leaves Grown in Sinaloa, Mexico. Pak. J. Bot. 49, 161–168. doi:10.3390/foods11081107
Farhoosh, R., and Riazi, A. (2007). A Compositional Study on Two Current Types of Salep in Iran and Their Rheological Properties as a Function of Concentration and Temperature. Food Hydrocoll. 21, 660–666. doi:10.1016/j.foodhyd.2006.07.021
Gull, J., Sultana, B., Anwar, F., Naseer, R., Ashraf, M., and Ashrafuzzaman, M. (2012). Variation in Antioxidant Attributes at Three Ripening Stages of Guava (Psidium Guajava L.) Fruit from Different Geographical Regions of Pakistan. Molecules 17, 3165–3180. doi:10.3390/molecules17033165
Jahan, M. S., Zawawi, D. D., and Abdulkadir, A. R. (2015). Effect of Chlorophyll Content and Maturity on Total Phenolic, Total Flavonoid Contents and Antioxidant Activity of Moringa Oleifera Leaf (Miracle Tree). J. Chem. Pharm. Res. 7, 1147–1152.
Kalappurayil, T. M., and Joseph, B. P. (2016). A Review of Pharmacognostical Studies on Moringa Oleifera Lam. Flowers. Pj 9, 1–7. doi:10.5530/pj.2017.1.1
Kasolo, J. N., Bimenya, G. S., Ojok, L., Ochieng, J., and Ogwal-Okeng, J. W. (2010). Phytochemicals and Uses of Moringa Oleifera Leaves in Ugandan Rural Communities. J. Med. Plants Res. 4, 753–757. doi:10.5897/JMPR10.492
Mahmood, T., Anwar, F., Abbas, M., Boyce, M. C., and Saari, N. (2012). Compositional Variation in Sugars and Organic Acids at Different Maturity Stages in Selected Small Fruits from Pakistan. Ijms 13, 1380–1392. doi:10.3390/ijms13021380
Mbikay, M. (2012). Therapeutic Potential of Moringa Oleifera Leaves in Chronic Hyperglycemia and Dyslipidemia: a Review. Front. Pharmacol. 3, 24. doi:10.3389/fphar.2012.00024
Moran-Palacio, E., Tortoledo-Ortiz, O., Yañez-Farias, G., Zamora-Álvarez, L., Stephens-Camacho, N., Soñanez-Organis, J., et al. (2014). Determination of Amino Acids in Medicinal Plants from Southern Sonora, Mexico. Trop. J. Pharm. Res. 13, 601–606. doi:10.4314/tjpr.v13i4.17
Moyo, B., Masika, P. J., Hugo, A., and Muchenje, V. (2011). Nutritional Characterization of Moringa (Moringa Oleifera Lam.) Leaves. Afr. J. Biotechnol. 10, 12925–12933. doi:10.5897/AJB10.1599
Mustapha, Y., and Babura, S. (2009). Determination of Carbohydrate and β-carotene Content of Some Vegetables Consumed in Kano Metropolis, Nigeria. Bayero J. Pure App. Sci. 2, 119–121. doi:10.4314/bajopas.v2i1.58515
Nouman, W., Anwar, F., Gull, T., Newton, A., Rosa, E., and Domínguez-Perles, R. (2016). Profiling of Polyphenolics, Nutrients and Antioxidant Potential of Germplasm's Leaves from Seven Cultivars of Moringa Oleifera Lam. Industrial Crops Prod. 83, 166–176. doi:10.1016/j.indcrop.2015.12.032
Pullakhandam, R., and Failla, M. L. (2007). Micellarization and Intestinal Cell Uptake Ofβ-Carotene and Lutein from Drumstick (Moringa Oleifera) Leaves. J. Med. Food 10, 252–257. doi:10.1089/jmf.2006.250
Qadir, R., Anwar, F., Batool, F., Mushtaq, M., and Jabbar, A. (2019b). Enzyme-assisted Extraction of Momordica Balsamina L. Fruit Phenolics: Process Optimized by Response Surface Methodology. Food Meas. 13, 697–706. doi:10.1007/s11694-018-9982-2
Qadir, R., Anwar, F., Gilani, M. A., Zahoor, S., Misbah ur Rehman, M., and Mustaqeem, M. (2019a). RSM/ANN Based Optimized Recovery of Phenolics from Mulberry Leaves by Enzyme-Assisted Extraction. Czech J. Food Sci. 37, 99–105. doi:10.17221/147/2018-CJFS
Rashid, U., Anwar, F., Masore, B. R., and Knothe, G. (2008). Moringa oleifera Oil: A Possible Source of Biodiesel. Biores. Technol. 99, 8175–8179.
Shih, M.-C., Chang, C.-M., Kang, S.-M., and Tsai, M.-L. (2011). Effect of Different Parts (Leaf, Stem and Stalk) and Seasons (Summer and Winter) on the Chemical Compositions and Antioxidant Activity of Moringa Oleifera. Ijms 12, 6077–6088. doi:10.3390/ijms12096077
Stoscheck, C. M. (1990). Quantitation of Protein. Meth. Enzymol. 463, 50–68. doi:10.1016/0076-6879(90)82008-p
Tantray, A. K., Dar, S. A., Ahmad, S., and Bhat, S. A. (2017). Spectrophotometric and Titrimetric Analysis of Phytoascorbate. J. Pharmacogn. Phytochem. 6, 27–31.
Turkekul, I., Elmastas, M., and Tüzen, M. (2004). Determination of Iron, Copper, Manganese, Zinc, Lead, and Cadmium in Mushroom Samples from Tokat, Turkey. Food Chem. 84, 389–392. doi:10.1016/S0308-8146(03)00245-0
Varadharaju, N., Karunanidhi, C., and Kailappan, R. (2001). Coffee Cherry Drying: a Two-Layer Model. Dry. Technol. 19, 709–715. doi:10.1081/DRT-100103947
Yemm, E. W., Cocking, E. C., and Ricketts, R. E. (1955). The Determination of Amino-Acids with Ninhydrin. Analyst 80, 209–214. doi:10.1039/an9558000209
Keywords: moringa leaves, vitamins, amino acids, total phenolics, HPLC, quercetin, ferulic acid
Citation: Qadir R, Anwar F, Bashir K, Tahir MH, Alhumade H and Mehmood T (2022) Variation in Nutritional and Antioxidant Attributes of Moringa oleifera L. Leaves at Different Maturity Stages. Front. Energy Res. 10:888355. doi: 10.3389/fenrg.2022.888355
Received: 02 March 2022; Accepted: 16 June 2022;
Published: 09 August 2022.
Edited by:
Abdul-Sattar Nizami, Government College University, PakistanReviewed by:
Muhammad Ibrahim, Government College University, PakistanIrfan Rana, Sogang University, South Korea
Awais Bokhari, Brno University of Technology, Czechia
Copyright © 2022 Qadir, Anwar, Bashir, Tahir, Alhumade and Mehmood. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farooq Anwar, ZnFhbndhckB5YWhvby5jb20=
 Rahman Qadir
Rahman Qadir Farooq Anwar
Farooq Anwar Kiran Bashir1
Kiran Bashir1 Tahir Mehmood
Tahir Mehmood