- State Key Laboratory of Oil and Gas Reservoir Geology and Development Engineering, Southwest Petroleum University, Chengdu, China
During shale gas reservoir development, obtaining actual formation pressure is challenging; therefore, it is challenging to obtain a single well production allocation using the current formation pressure based on a productivity equation. Different shale gas reservoirs with different rock adsorption properties and the traditional Langmuir isotherm adsorption equations are not accurate in describing the adsorption properties of shale gas reservoirs, causing significant errors. BET multimolecular adsorption, considering the shale gas surface fractal dimension theory to describe the adsorption properties, can describe the adsorption surface as a multimolecular layer and regard adsorption using a fractal dimension, describing the adsorption property of shale gas more accurately. According to the core adsorption test data and theory, the actual BET multimolecular adsorption is established by considering the shale gas surface fractal dimension. Therefore, the actual material balance equation was obtained using the theory, establishing the relationship between the formation pressure and cumulative shale gas production. A time-independent distribution and cumulative gas production chart were formed using the productivity equation. Consequently, the material balance equation, which takes advantage of the BET multimolecular fractal theory, was conducted. This allocation production method obtained from the material balance equation has significant importance in shale gas development.
Introduction
Shale gas reserves comprised free, adsorbed, and dissolved gases, of which free and adsorbed gases are the main parts (Taghavinejad et al., 2020); (Mengal and Wattenbarger, 2011); (Huang and Zhao, 2017); (Boadu, 2000); (Sanyal et al., 2006); (Curtis, 2002); (Jenkins and Boyer, 2008). In this regard, the study of shale gas adsorption behavior is significant to reservoir volume calculation and development (Huang and Zhao, 2017); (Shao et al., 2017); (Wu et al., 2015). Currently, the Langmuir isotherm, BET equations, and other modified Langmuir and BET equations predominantly describe the adsorption behavior (Li et al., 2020); (Jaroniec et al., 1989); (Zhang et al., 2015). The classic Langmuir equation assumes that methane is monolayer-adsorbed, and the surface of the adsorbent is homogeneous with constant adsorption heat (Langmuir, 1918); (Langmuir, 2015). The adsorption behavior of shale gas is complex, and Langmuir’s isotherm adsorption equation is too ideal and cannot describe it accurately. The BET adsorption theory considers the adsorption surface as a multilayer, and the adsorption capacity of every layer is the same (Myers, 1968); (Brunauer et al., 1940); (Zhou et al., 2019); (Myers and Prausnitz, 1965); (Ritter and Yang, 1987); (Dang et al., 2020). To simulate the enrichment and production of methane in shale gas reservoirs, an accurate gas adsorption model is critically required (Chareonsuppanimit et al., 2012), (Clarkson and Haghshenas, 2013), (Huang et al., 2018a). Fortunately, the BET multi-molecular fractal theory, considering the surface fractal dimension of shale gas, can describe adsorption more accurately by considering the multilayer and fractal dimension of shale gas adsorption (Brunauer et al., 1938), (Wang et al., 2022), (Chai et al., 2019), (Zheng et al., 2019), (Wang et al., 2016), (Fan and Liu, 2021). Therefore, the material balance equation can enhance the accuracy of future reservoir prediction about reserves and formation pressure based on the adsorption theory (Zhang et al., 2017), (Canel and Rosbaco, 2006), (Fianu et al., 2019).
Ambrose et al., (2012), (Xiong et al., 2021), and (Memon et al., 2020) combined the Langmuir adsorption isotherm with the volume for free gas and formulated a new gas-in-place equation accounting for the pore space occupied by the adsorbed phase; however, the adsorption surface layer and fractal dimension were not considered (Pang et al., 2019). The material balance equation proposed by Daniel et al. (Orozco and Aguilera, 2015) simultaneously considered stress-dependent porosity and permeability, free gas, adsorbed gas, and dissolved gas but also excluded the adsorption surface layer and fractal dimension. Usually, the material balance equation combined with the shale gas productivity equation is used to calculate the reservoir reserves and conduct the relevant model for predicting the production rate and formation pressure versus time (Sun et al., 2019), (Gu et al., 2014), (Hu et al., 2019). The shale gas well allocation is usually based on this method to carry out well productivity tests (Deng et al., 2014), (Wheaton, 2019). According to the test, the shale gas well binomial productivity formula is determined. If the present reservoir pressure is calculated by the material balance equation, then the actual open flow capacity of a single well can be calculated. The reasonable gas well allocation is also determined. Nevertheless, practical reservoir production requires convenient and efficient allocation progress. The aforementioned method aims at a certain moment, is complex, and cannot satisfy the practical production needs. Therefore, finding an efficient and convenient shale gas allocation method for the whole well life is necessary.
Huang et al. (2018b) proposed a new method. Shale reservoirs were depicted by the De Swaan dual porosity model, where the secondary and hydraulic fractures were characterized by discrete units to conduct the shale gas well productivity equation. This method can calculate more efficiently than the Eclipse simulator. Furthermore, it can describe the complex fracture network more correctly even though the calculating process and single shale gas well allocation of every production moment are complex. Sang et al. (2014) used a numerical model considering desorption and adsorption processes to establish and solve, under polar coordinates and Laplace space, respectively, predicting the productivity of volume-fractured horizontal wells in shale gas reservoirs. This model can predict the production rate versus time, but the allocation production of the single well cannot be obtained. At present, the existing allocation methods combining the material balance equation with productivity formula cannot offer well the allocation of well life and satisfy the convenience of practical allocation apart from numeric simulation, decline analysis, and so on (Huang et al., 2018b); (Arps, 1945). As for the adsorption of shale gas reservoirs, we adopted the BET multi-molecular fractal theory. Creatively, we developed a material balance equation based on the BET multi-molecular fractal theory, considering the dissolved gas of the formation water. Furthermore, we combined this new material balance equation with the productivity equation to obtain single well allocation production versus cumulative gas production for the well life cycle. As a result, we can offer the shale gas development worker an allocation plate, and there is no need for paying attention to complex equations about the well and gas reservoir properties. In a word, this method can not only calculate the adsorption gas volume but also propose a convenient and fast life cycle allocation method for shale gas reservoirs.
Material Balance Equation of a Shale Gas Well Based on the BET Multi-Molecular Fractal Theory
The material balance equation of the confining shale gas reservoir was established by considering the reservoir as a fracture-matrix dual system and the dissolved gas in the formation water to obtain the chart of shale gas dynamic allocation production. Furthermore, the BET multimolecular and fractal adsorption theory described the gas adsorption behavior.
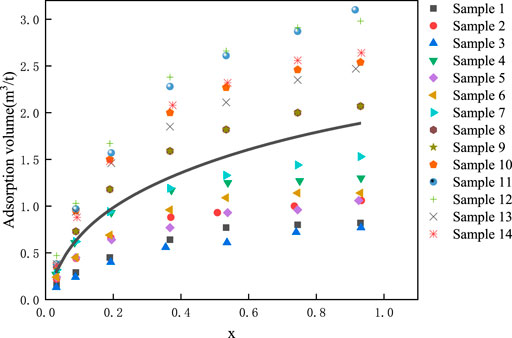
The Actual Isotherm Adsorption Curve of Shale Gas Block XX
Brunauer et al. (1940) proposed the BET multimolecular adsorption theory in 1940, and it was an addition to the Langmuir monolayer molecular adsorption equation. The assumption of the BET multimolecular adsorption theory is as follows:
1) The gas adsorption is multimolecular, and it does not have to cover the first layer completely and then the second.
2) The heat of adsorption (E1) of the first layer is a certain value; the other is the liquefaction heat (EL) of the adsorbate.
3) The adsorption and desorption of the adsorbate are exposed on the surface of the gas phase directly.
Consequently, the BET multimolecular adsorption expression is described in Eq. 1.
In the equation,
Eq. 2 shows the relationship of the adsorption capacity between layers
In Eq. 2,
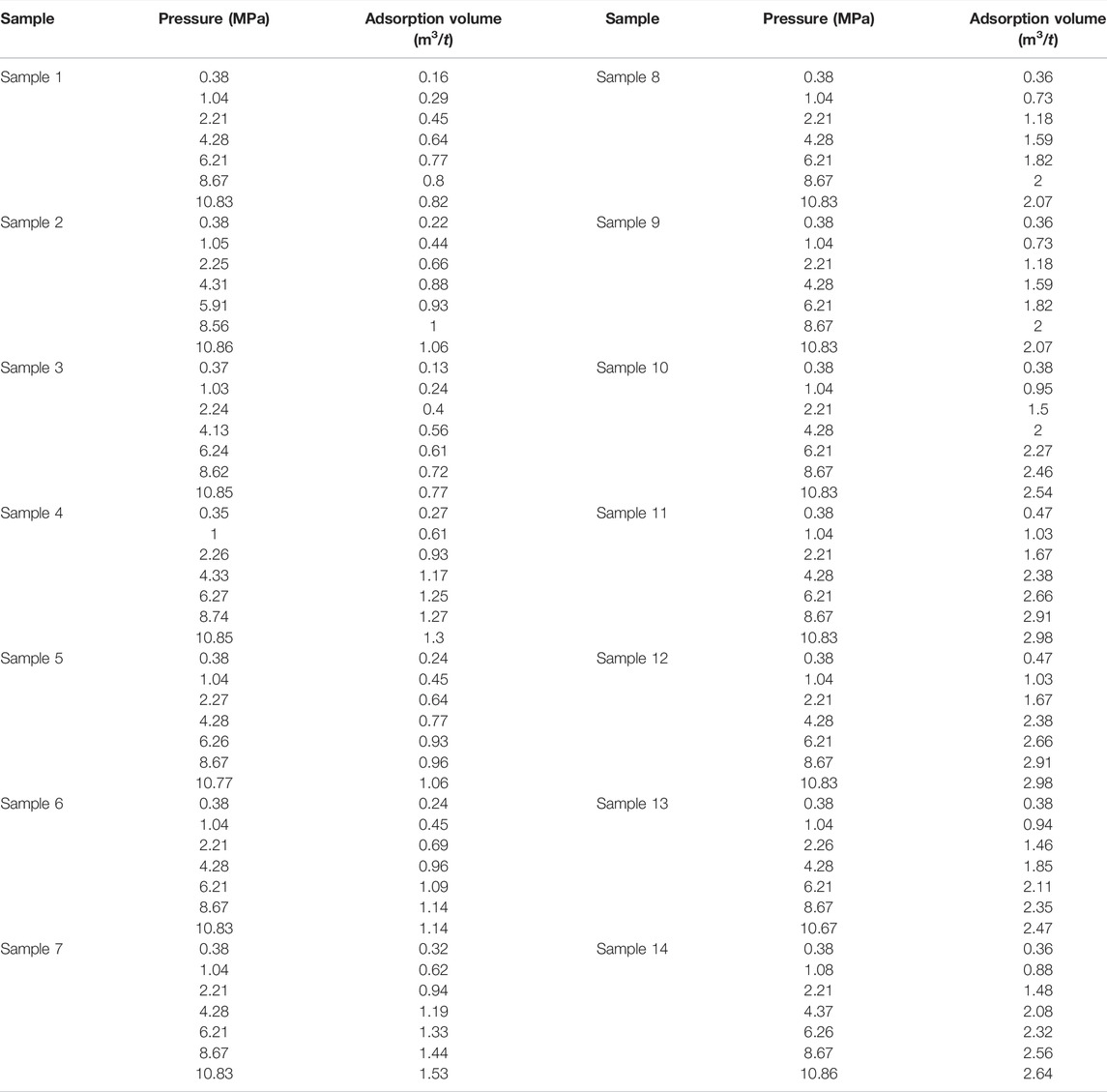
According to Eq. 3, the actual isotherm adsorption curve of shale gas Block XX can be obtained by regression, as shown in Eq. 4, and Figure 1 shows the curve. The gas adsorption test data is showed in Table 1. According to the regression results, we obtained the following conclusions. The adsorption volume of shale gas Block XX was 1.19924 m3/t, and the constant related to the energy of adsorption and liquefaction was 9.86193, based on the fractal dimension 2.5 and the layer of surface molecular adsorption 3 (Figure 1).
Material Balance Equation of Shale Gas Block XX
The assumption of the material balance equation of shale gas Block XX is as follows:
1) The shale gas reservoir is an isotherm system; in other words, the reservoir temperature is unchanged during exploitation.
2) The water saturation of the matrix and fracture system is different.
3) There is no formation of water to be produced.
According to the principle of the material balance equation, the underground volume of the produced shale gas comprised underground expansion, including free gas, formation water, rock, dissolved gas of formation water underground volume variation of the matrix, and fracture system. For the shale gas reservoir, it included the underground adsorption gas volume variation of the matrix system. When the shale gas reservoir proceeded to desorption, the material balance equation was as shown in Eq. 5.
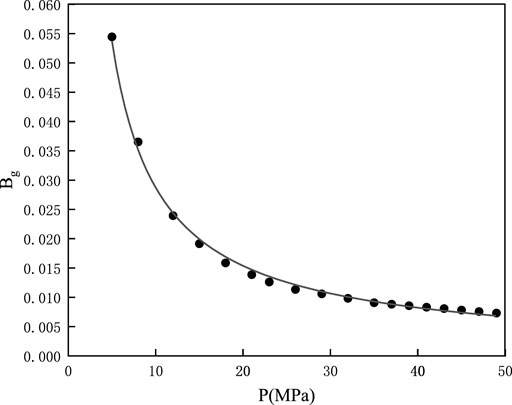
The high-pressure fluid parameter testing of shale gas Block XX regressed the relationship between the gas volume factor and formation pressure. Eq. 6 shows the function, and Figure 2 shows the curve of the gas volume factor versus the formation pressure.
The empirical formula of the formation water property is as suggested by Yuanqian (Chen, 1990) and (Myers and Prausnitz, 1965), as shown in Eq. 7.
In the equation,
Practical Material Balance Equation of Shale Gas Block XX
Based on the BET multimolecular adsorption theory equation (Eq. 4) of shale gas Block XX, the practical material balance equation of the shale gas Block XX can be established by considering the dissolved gas of formation water and adsorption of shale gas. The practical material balance equation of Well #1 Block XX was obtained from Eq. 8 by substituting Eqs 6 and 7 into Eq. 5.
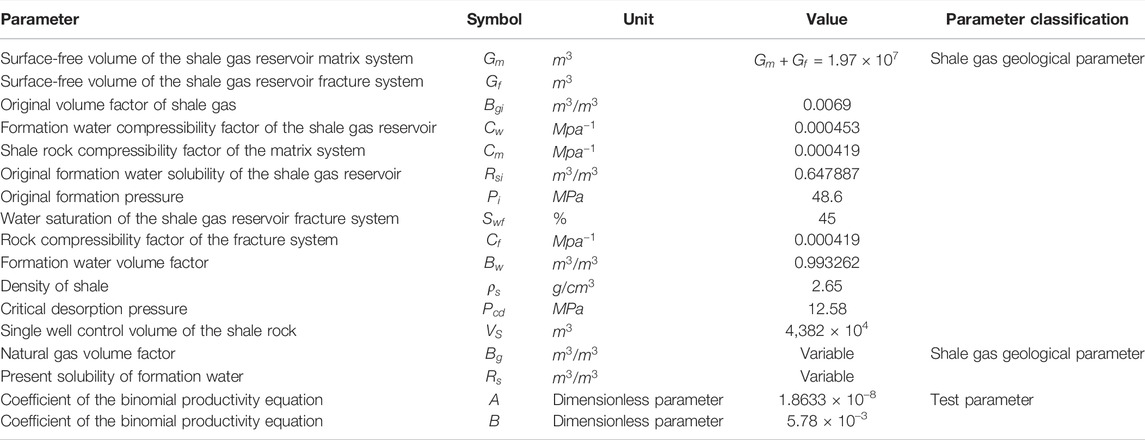
According to the formation of physics and fluid properties (Table 2), the practical material balance equation can be established, and a double logarithm chart (allocation versus cumulative gas production) can be obtained. Therefore, the allocation of a single shale gas well can be calculated according to the cumulative gas production using the chart in the progress of shale gas exploitation.
From the productivity test data, the gas well productivity equation was as in Eq. 9.
The 1/5 times of the gas well open flow was often used as the allocation in situ production; therefore, the production allocation formula was as in Eq. 10.
Case Study
As the description of the practical material balance equation of shale gas Block XX, at first, the shale gas material balance equation is conducted, considering multimolecular fractal adsorption about the single shale gas well. Second, the binomial productivity equation is obtained by regressing the productivity test data. Third, cumulative gas productivity is substituted into the first step regarding the material balance equation in order to obtain the present reservoir pressure. Fourth, the pressure is substituted into the second step productivity equation, and the single well allocation can be calculated. Finally, the abovementioned steps are repeated, and then, the allocation plate allocation production versus cumulative gas production double logarithm is drawn. According to the plate, the practical allocation process is guided quickly. The specific procedures are as follows.
Figures 3, 4 and Table 3 show the cumulative gas production chart versus formation pressure and single well production allocation chart taking advantage of Eqs 8–10.
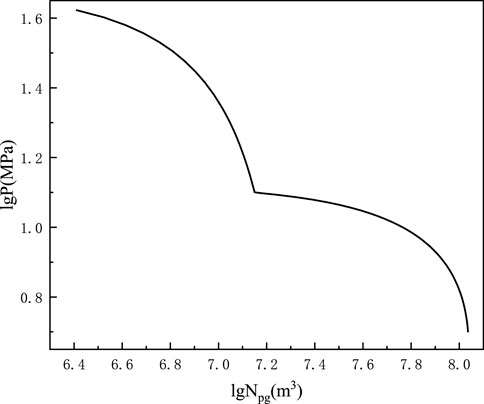
FIGURE 3. Formation pressure versus the cumulative gas production double logarithm chart of Well #1 shale gas Block XX.
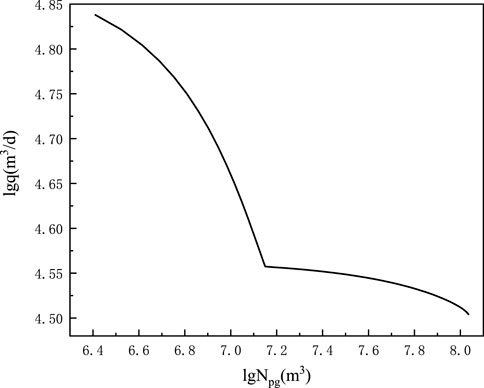
FIGURE 4. Allocation production versus the cumulative gas production double logarithm chart of Well #1 shale gas Block XX.
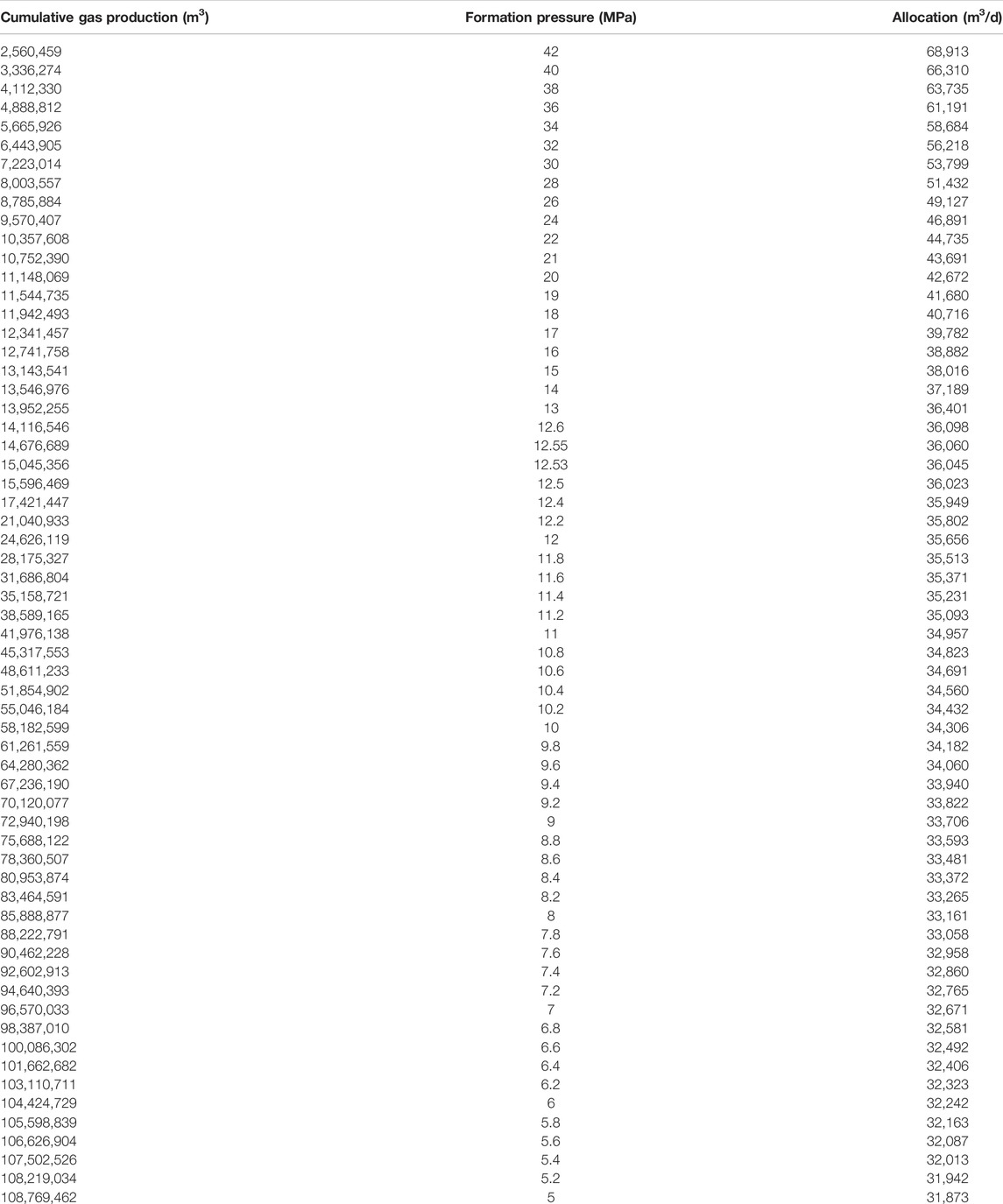
TABLE 3. Cumulative gas production versus the formation pressure and single well allocation production.
By viewing the cumulative gas production in Figure 4, the allocation production can be obtained. Through analysis, in Figure 3, the decrease in the formation pressure on the cumulative gas production was gentle, and the allocation on cumulative gas production decreased similar to that in Figure 4 when the shale gas reservoir entered desorption. Thus, the formation pressure can keep well after desorption, and shale gas had a long dependable crop time.
Conclusion
Based on the practical BET multimolecular adsorption, considering the fractal dimension of the adsorption surface, a production allocation chart about Well #1 shale gas Block XX can be established using the material balance equation. The conclusions are as follows:
1) The adsorption volume of the shale gas Block XX was 1.19924 m3/t, and the constant related to the energy of adsorption and liquefaction was 9.86193, based on the fractal dimension 2.5 and the layer of surface molecular adsorption 3.
2) According to the production allocation chart of Well #1 shale gas Block XX, before the shale gas reservoir entered the desorption, the desorption pressure decreased faster, and the cumulative gas production increased slowly. However, when the reservoir entered the desorption stage, the decrease in the formation pressure was gentle, despite the low formation pressure. Furthermore, the shale gas production allocation can maintain a certain time, indicating that most shale gas of the production well was exploited during the desorption stage.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
TQ: responsible for the main writing of the thesis and the derivation of the formula. SH: responsible for guiding the author’s research and revision of the manuscript. JZ: responsible for the provision of experimental data and email communication.
Funding
This work was supported by the National Science and Technology Major Project “Zhaotong Shale Gas Exploration and Development Demonstration Project-Comprehensive Mountain Shale Gas Well Productivity Evaluation and Production System Optimization and Application” (Grant No. 2017ZX05063005-019) and The National Natural Science Fund (No. 51574199) administered by the National Natural Science Foundation of China.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
References
Ambrose, R. J., Hartman, R. C., Diaz-Campos, M., Akkutlu, I. Y., and Sondergeld, C. H. (2012). Shale Gas-In-Place Calculations Part I: New Pore-Scale Considerations. SPE J. 17, 219–229. doi:10.2118/131772-PA
Boadu, F. K. (2000). Predicting the Transport Properties of Fractured Rocks from Seismic Information: Numerical Experiments. J. Appl. Geophys. 44 (2-3), 103–113. doi:10.1016/S0926-9851(99)00020-8
Brunauer, S., Deming, L. S., Deming, W. E., and Teller, E. (1940). On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 62, 1723–1732. doi:10.1021/ja01864a025
Brunauer, S., Emmett, P. H., and Teller, E. (1938). Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 60 (2), 309–319. doi:10.1021/ja01269a023
Canel, C. A., and Rosbaco, J. (2006). “Compositional Material Balance: its Application to the Development of an Oil and Gas Field with Retrograde Condensation,” in Paper SPE 23647 presented at the Second Latin American Petroleum Engineering Conference, Caracas, Venezuela, January 01, 1992. 117–128 March. doi:10.2118/23647-MS
Chai, D., Yang, G., Fan, Z., and Li, X. (2019). Gas Transport in Shale Matrix Coupling Multilayer Adsorption and Pore Confinement Effect. Chem. Eng. J. 370, 1534–1549. doi:10.1016/j.cej.2019.03.276
Chareonsuppanimit, P., Mohammad, S. A., Robinson, R. L., and Gasem, K. A. M. (2012). High-pressure Adsorption of Gases on Shales: Measurements and Modeling. Int. J. Coal Geology. 95, 34–46. doi:10.1016/j.coal.2012.02.005
Chen, Y. (1990). The Property of Formation Water Relevant Empirical Equation. Well Test. Prod. Technology 11 (3), 31–33.
Clarkson, C. R., and Haghshenas, B. (2013). “Modeling of Supercritical Fluid Adsorption on Organic-Rich Shales and Coal,” in Paper SPE 164532 presented at Unconventional Resources Conference-USA held in The Woodlands, Texas, USA, April 2013. doi:10.2118/164532-ms
Curtis, J. B. (2002). Fractured Shale-Gas Systems. AAPG Bull. 86 (11), 1921–1938. doi:10.1306/61eeddbe-173e-11d7-8645000102c1865d
Dang, W., Zhang, J., Nie, H., Wang, F., Tang, X., Wu, N., et al. (2020). Isotherms, Thermodynamics and Kinetics of Methane-Shale Adsorption Pair under Supercritical Condition: Implications for Understanding the Nature of Shale Gas Adsorption Process. Chem. Eng. J. 383, 123191. doi:10.1016/j.cej.2019.123191
Deng, J., Zhu, W., and Ma, Q. (2014). A New Seepage Model for Shale Gas Reservoir and Productivity Analysis of Fractured Well. Fuel 124, 232–240. doi:10.1016/j.fuel.2014.02.001
Fan, L., and Liu, S. (2021). A Novel Experimental System for Accurate Gas Sorption and its Application to Various Shale Rocks. Chem. Eng. Res. Des. 165, 180–191. doi:10.1016/j.cherd.2020.10.034
Fianu, J., Gholinezhad, J., and Hassan, M. (2019). Application of Temperature-dependent Adsorption Models in Material Balance Calculations for Unconventional Gas Reservoirs. Heliyon 5 (5), e01721. doi:10.1016/j.heliyon.2019.e01721
Fripiat, J. J., Gatineau, L., and Van Damme, H. (1986). Multilayer Physical Adsorption on Fractal Surfaces. Langmuir 2 (5), 562–567. doi:10.1021/la00071a006
Hu, S., Hu, X., He, L., and Chen, W. (2019). A New Material Balance Equation for Dual-Porosity Media Shale Gas Reservoir. Energ. Proced. 158, 5994–6002. doi:10.1016/j.egypro.2019.01.520
Huang, H., Sun, W., Xiong, F., Chen, L., Li, X., Gao, T., et al. (2018). A Novel Method to Estimate Subsurface Shale Gas Capacities. Fuel 232, 341–350. doi:10.1016/j.fuel.2018.05.172
Huang, S., Ding, G., Wu, Y., Huang, H., Lan, X., and Zhang, J. (2018). A Semi-analytical Model to Evaluate Productivity of Shale Gas wells with Complex Fracture Networks. J. Nat. Gas Sci. Eng. 50, 374–383. doi:10.1016/j.jngse.2017.09.010
Huang, X., and Zhao, Y.-P. (2017). Characterization of Pore Structure, Gas Adsorption, and Spontaneous Imbibition in Shale Gas Reservoirs. J. Pet. Sci. Eng. 159 (159), 197–204. doi:10.1016/j.petrol.2017.09.010
Jaroniec, M., Lu, X., Madey, R., and Choma, J. (1989). Extension of the Langmuir Equation for Describing Gas Adsorption on Heterogeneous Microporous Solids. Langmuir 5 (3), 839–844. doi:10.1021/la00087a044
Jenkins, C. D., and Boyer, C. M. (2008). Coalbed- and Shale-Gas Reservoirs. J. Pet. Technology 60 (2), 92–99. doi:10.2118/103514-JPT
Langmuir, I. (1918). The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 40 (9), 1361–1403. doi:10.1063/1.492960910.1021/ja02242a004
Langmuir, I. (2015). The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 40 (12), 1361–1403.
Li, J., Lu, S., Zhang, P., Cai, J., Li, W., Wang, S., et al. (2020). Estimation of Gas-In-Place Content in Coal and Shale Reservoirs: A Process Analysis Method and its Preliminary Application. Fuel 259, 116266. doi:10.1016/j.fuel.2019.116266
Memon, A., Li, A., Jacqueline, N., Kashif, M., and Ma, M. (2020). Study of Gas Sorption, Stress Effects and Analysis of Effective Porosity and Permeability for Shale Gas Reservoirs. J. Pet. Sci. Eng. 193, 107370. doi:10.1016/j.petrol.2020.107370
Mengal, S. A., and Wattenbarger, R. A. (2011). “Accounting for Absorbed Gas in Shale Gas Reservoirs,” in Paper SPE 141085 presented at SPE Middle East Oil and Gas Show and Conference, Manama, Bahrain, 25–28 September (MS). doi:10.2118/141085
Myers, A. L. (1968). Adsorption of Gas Mixtures-A Thermodynamic Approach. Ind. Eng. Chem. 60 (5), 45–49. doi:10.1021/ie50701a007
Myers, A. L., and Prausnitz, J. M. (1965). Thermodynamics of Mixed-Gas Adsorption. Aiche J. 11 (1), 121–127. doi:10.1002/aic.690110125
Orozco, Daniel., and Aguilera, Roberto. (2015). “A Material Balance Equation for Stress-Sensitive Shale Gas Reservoirs Considering the Contribution of Free, Adsorbed and Dissolved Gas,” in Paper SPE-175964-MS presented at SPE/CSUR Unconventional Resources Conference held in Calgary, Alberta, Canada, October 2015. 10.2118/175964-MS. doi:10.2118/175964-ms
Pang, Y., Tian, Y., Soliman, M. Y., and Shen, Y. (2019). Experimental Measurement and Analytical Estimation of Methane Absorption in Shale Kerogen. Fuel 240, 192–205. doi:10.1016/j.fuel.2018.11.144
Ritter, J. A., and Yang, R. T. (1987). Equilibrium Adsorption of Multicomponent Gas Mixtures at Elevated Pressures. Ind. Eng. Chem. Res. 26 (8), 1679–1686. doi:10.1021/ie00068a032
Sang, Y., Chen, H., Yang, S., Guo, X., Zhou, C., Fang, B., et al. (2014). A New Mathematical Model Considering Adsorption and Desorption Process for Productivity Prediction of Volume Fractured Horizontal wells in Shale Gas Reservoirs. J. Nat. Gas Sci. Eng. 19, 228–236. doi:10.1016/j.jngse.2014.05.009
Sanyal, D., Ramachandrarao, P., and Gupta, O. P. (2006). A Fractal Description of Transport Phenomena in Dendritic Porous Network. Chem. Eng. Sci. 61 (2), 307–315. doi:10.1016/j.ces.2005.06.0010.1016/j.ces.2005.06.005
Shao, X., Pang, X., Li, Q., Wang, P., Chen, D., Shen, W., et al. (2017). Pore Structure and Fractal Characteristics of Organic-Rich Shales: A Case Study of the Lower Silurian Longmaxi Shales in the Sichuan Basin, SW China. Mar. Pet. Geology. 80, 192–202. doi:10.1016/j.marpetgeo.2016.11.025
Sun, H., Wang, H., Zhu, S., Nie, H., Liu, Y., Li, Y., et al. (2019). Reserve Evaluation of High Pressure and Ultra-high Pressure Reservoirs with Power Function Material Balance Method. Nat. Gas Industry B 6 (5), 509–516. doi:10.1016/j.ngib.2019.03.007
Taghavinejad, A., Sharifi, M., Heidaryan, E., Liu, K., and Ostadhassan, M. (2020). Flow Modeling in Shale Gas Reservoirs: a Comprehensive Review. J. Nat. Gas Sci. Eng. 83, 103535. doi:10.1016/j.jngse.2020.103535
Wang, P., Jiang, Z., Ji, W., Zhang, C., Yuan, Y., Chen, L., et al. (2016). Heterogeneity of Intergranular, Intraparticle and Organic Pores in Longmaxi Shale in Sichuan Basin, South China: Evidence from SEM Digital Images and Fractal and Multifractal Geometries. Mar. Pet. Geology. 72, 122–138. doi:10.1016/j.marpetgeo.2016.01.020
Wang, Y., Cheng, H., Hu, Q., Liu, L., Jia, L., Gao, S., et al. (2022). Pore Structure Heterogeneity of Wufeng-Longmaxi Shale, Sichuan Basin, China: Evidence from Gas Physisorption and Multifractal Geometries. J. Pet. Sci. Eng. 208, 109313. doi:10.1016/j.petrol.2021.109313
Wheaton, R. (2019). Modeling of Gas Flow in Fractured Shale. Upstream Oil Gas Technology 1, 100001. doi:10.1016/j.upstre.2020.100001
Wu, K., Chen, Z., and Li, X. (2015). Real Gas Transport through Nanopores of Varying Cross-Section Type and Shape in Shale Gas Reservoirs. Chem. Eng. J. 281, 813–825. doi:10.1016/j.cej.2015.07.012
Xiong, F., Rother, G., Gong, Y., and Moortgat, J. (2021). Reexamining Supercritical Gas Adsorption Theories in Nano-Porous Shales under Geological Conditions. Fuel 287, 119454. doi:10.1016/j.fuel.2020.119454
Zhang, C.-Y., Chai, X.-S., and Xiao, X.-M. (2015). A Simple Method for Correcting for the Presence of Minor Gases when Determining the Adsorbed Methane Content in Shale. Fuel 150, 334–338. doi:10.1016/j.fuel.2015.02.050
Zhang, W., Xu, J., Jiang, R., Cui, Y., Qiao, J., Kang, C., et al. (2017). Employing a Quad-Porosity Numerical Model to Analyze the Productivity of Shale Gas Reservoir. J. Pet. Sci. Eng. 157, 1046–1055. doi:10.1016/j.petrol.2017.07.031
Zheng, S., Yao, Y., Liu, D., Cai, Y., Liu, Y., and Li, X. (2019). Nuclear Magnetic Resonance T2 Cutoffs of Coals: A Novel Method by Multifractal Analysis Theory. Fuel 241, 715–724. doi:10.1016/j.fuel.2018.12.044
Keywords: Langmuir isotherm adsorption equation, BET multimolecular adsorption, fractal dimension, material balance equation, allocation production method
Citation: Qiu T, Hu S and Zhang J (2022) Application of the Material Balance Equation Based on the BET Multimolecular Fractal Theory in a Shale Gas Reservoir. Front. Energy Res. 10:829800. doi: 10.3389/fenrg.2022.829800
Received: 06 December 2021; Accepted: 21 March 2022;
Published: 26 April 2022.
Edited by:
Lin Teng, Fuzhou University, ChinaReviewed by:
Yang Xiao, Xi’an University of Science and Technology, ChinaMuhammad Wakil Shahzad, Northumbria University, United Kingdom
Copyright © 2022 Qiu, Hu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayi Zhang, eWl4aW5namlhdGllQDE2My5jb20=
 Tingting Qiu
Tingting Qiu Shuyong Hu
Shuyong Hu Jiayi Zhang
Jiayi Zhang