
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Energy Res. , 09 January 2023
Sec. Process and Energy Systems Engineering
Volume 10 - 2022 | https://doi.org/10.3389/fenrg.2022.1063315
This article is part of the Research Topic Recent Development in Energy Conversion Systems View all 11 articles
Infrastructure upgrades in the energy sector are encouraged to satisfy the expanding world’s energy needs, including innovation, consumption, production, and transportation. Thus, steel has been an extensively used construction material, particularly for pipelines and oil wells. However, in their application, every step of the production cycle results in the corrosion of metal parts. One of the simplest and most active ways to inhibit steel from corroding, especially in acidic situations, is to use a corrosion inhibitor. Synthetic organic compounds have been used successfully as corrosion inhibitors in the gas and oil industry. However, their use is today restricted and controlled due to their toxicity, environmental harm, and growing concern about the preservation of ecosystems. This has necessitated the present trend of searching for and developing green inhibitors that are environmentally benign, non-toxic, biodegradable, and low in cost. Corrosion inhibitor develops a shielding layer on the metal surface. Corrosion is avoided by a thin coating that has been adsorbed on the metal surface, which keeps the metal isolated from its surroundings. Several researchers have reported on the success of green inhibitors for steel corrosion protection, particularly in acidic environments. However, the use of green inhibitors still leaves several questions about inhibitor formulation, content, and adsorption mechanisms to be answered. Therefore, based on provided experimental results and an explanation of their inhibitory action, the use of green inhibitors (especially organic inhibitors) for the prevention of pipeline steel corrosion in various grades is studied in this review. Both the identified drawback and the projected future trend have also been highlighted.
Corrosion is a gradual deterioration of components caused by various environmental conditions. This scenario potentially damages the manufacturing and transportation of oil and gas facilities. Practically any environment with an aqueous solution can initiate corrosion, which happens under various difficult situations during the processing and production of oil and gas in pipeline systems. This mechanism entails three factors: an electrolyte, an anode, and a cathode (Dehghani et al., 2019a; Mobin et al., 2019a). The electrolyte is the corrosive medium that initiates the electron movement from the anode to the cathode, the anode is the area where the corroding metal is situated, and the cathode in the cell that produces the electrical conductor is herewith not used in the corrosion mechanism. Natural gas and crude oil can convey different high-impure substances which are intrinsically corrosive, and one of the vital materials used for industrial purposes is steel and its alloys. However, these materials experience huge corrosion deterioration when encountered by acidic media. In the case of gas and oil wells and pipelines, some extremely corrosive media, such as hydrogen sulfide (H2S), carbon dioxide (CO2), and water, can initiate corrosion (Oguntuyi et al., 2021a). The continuous flow of H2S, CO2, and water via gas and oil material can induce the internal surface of the material to be corroded. Hence, the lines and the material fittings would experience deterioration with the different situations of the well because of overtime well souring, alteration in fluid contents, and changes in operating temperatures and pressures. The material’s deterioration produces a decline in mechanical features such as impact strength, density, ductility, and hardness. Thus, the material reduces in thickness and sometimes causes total material failure during service. A stage will be attained where the material will entirely break down, and the assemblage will be required to be changed while the manufacturing is ceased (Villamizar et al., 2006; Subasree and Selvi, 2020).
Universally, the corrosion mechanism is a serious challenge to the energy industries. Therefore, industries have considered the impact of corrosion on the design and fabrication of their equipment. History shows that industries have lost billions of dollars due to corrosion (Shukla and Quraishi, 2009; Eliyan et al., 2013; Oguntuyi et al., 2021b; Oguntuyi et al., 2022a). Some reports in the world stated that some oil pipeline industries had their equipment destroyed because of corrosion, which consequently led to oil leakages that developed environmental contamination (ecological damage). Moreover, much capital is being consumed in rectifying the environmental nuisance. The occurrence of corrosion in an industrial plant has developed many challenges for chemical, mechanical, and petroleum engineers. Corrosion can influence a material’s procedural chemistry and the purity of the substance being conveyed (Quraishi et al., 2007; Raja and Sethuraman, 2008). The existence of oxidizing acids, such as HNO3, in reacting with steel forms a layer of oxide [Fe (NO3)3] on a metal surface. This formation (layer) first inhibits the contact metal acid to prevent further occurrence. Afterward, the brittle oxide layer continuously experiences exfoliation, thus redeveloping the contacted metal acid, prompting adverse metal surface dissolution and material corrosion. The contact of the non-oxidizing acids (HCl) with steel produces iron salts (FeCl2) in the aftermath of corrosion. Synonymously, the contact of strong sulphuric acid (H2SO4) with steel produces FeSO4.
There are diverse methods to shield steel from the attack of corrosion, such as changes in the environment, cathodic protection, material modification, and application of corrosion inhibitors. Some techniques depend on the elimination of oxygen moisture, while others apply permanent coating on the metal surface. Various mechanisms depend on the changes to a cathode from the anodic components. However, the present applied techniques are constrained by diverse restrictions. For example, the modification of materials is always difficult or expensive. Moreover, the process of environmental replacement is not the possible answer in various industrial practices because the metal may undergo a specific reactive solution. Applying some techniques, such as coating, could erupt CO2 above the standard levels. Likewise, some techniques, such as cathodic protection, need expensive devices, consequently increasing the general cost. However, the corrosion inhibitors are a user-friendly and less expensive solution as an alternative to the above challenges encountered by the other methods (Kiefner and Kolovich, 2007; Cheng, 2013; Tawancy et al., 2013; Oguntuyi et al., 2022b).
When introduced in minute quantity in a corrosive media, an inhibitor is intended to inhibit corrosion reaction by developing a shielding film. These inhibitors have various uses in many steel pipeline industries because they are active agents that prevent some in-service steel materials (heat exchangers, boilers and gas, oil containers, or pipelines) from corrosion. Usually, before performing coating or welding, metals and steels are opened to an acidic solution. Furthermore, to inhibit corrosion products, corroded structures (e.g., heat exchangers, oil wells, pipelines, and oil tankers) are made to undergo acidification. The application of these inhibitors for structural treatments has depicted capable outcomes in restricting the corrosion reactions and the resulting metal deterioration. Diverse important factors influence inhibitor selection. The major factor is inhibitor toxicity. Overall, harmful traditional inhibitors of high volatility, such as phosphates, chromates, and nitrates and toxic gases in the products, negatively influence the environment. Alternative green methods have emerged owing to the strict legislation on the environment over the past years for toxic inhibitors. This inhibitor operates on a green chemistry mechanism, which entails the importance of the atomic economy, waste prevention, derivatives minimization, energy efficiency, decrement in hazard chemical synthesis, pollution prevention, safer solvent development, and safer chemistry for accident prevention (Kiefner and Kolovich, 2007; Marc, 2013).
The green inhibitor is an effective eco-friendly method that has had more recognition in past years. Many prevalent bases of green inhibitors comprise ionic liquids, pharmaceutical drugs, plant extracts, and synthetic inhibitors, which are roots of ecological benign corrosion inhibitors. The important origin of green corrosion inhibitors is plants (e.g., oils and their derivatives). They can operate in diverse acidic solutions because they possess versatile chemical, physical, and biological features. Low cost, biodegradability, and availability are the other importance of plants being applied as the bases of corrosion inhibitors. Plants are considered a high natural origin of chemical compounds, which can be easily hauled out with less cost and low ecosystem pollution. Also known as green solvents are ionic liquids containing ions that can dissociate various organic and inorganic compounds. Ionic liquid application in practically all chemical engineering fields is due to their promising features, which have recognized them as environmentally friendly chemicals.
Furthermore, green corrosion inhibitor drugs have comparatively huge structures comprising synthetic or natural compositions. Drugs produced from natural bases have engrossed more interest in past years. Furthermore, outdated drugs can be applied as corrosion inhibitors, thus minimizing environmental pollution and disposal expenses. Gece (2011) studied the efficiency of drugs as corrosion shielding. Various eco-friendly inhibitors have been discovered and are being studied often time. Mobin et al. (2019a) examined the applicability of drugs (bronopol) on aluminum metal as a corrosion inhibitor in HCl media. The magnitude of its inhibition mechanism and shielding capacity was determined by an electrochemical test and weight loss. The outcome indicated that optimum inhibition of 93.89% was achieved at an inhibitor concentration of 4,000 ppm in 0.5 M HCl. Two other studies published in 2011 stated the significance of surfactants and biopolymers in diverse solutions (Mobin and Rizvi, 2017; Fawzy et al., 2018; Oguntuyi et al., 2021c). Rani and Basu (2012) successfully introduced natural substances as green corrosion shields in various media. Verma et al. (2018) highlighted the behavior of ionic liquids as corrosion protectors. Thakur et al. (2022a) revealed the capability of a weed known as Cnicus benedictus extract as a corrosion restrictor on mild steel. An inhibition efficiency (IE) of 92.45% was attained. Moreover, the increment in the inhibitor concentration in the solution significantly decreased the corrosion rate. The IE of 91.24% was attained at 298 K when a weed regarded as Vicia sativa was examined as an inhibitor in 0.5 M HCl for mild steel (Thakur et al., 2022b), hence demonstrating the protective ability of the inhibitor extract. Therefore, huge success has been recorded in different green inhibitor categories in the last 10 years.
This review emphasizes the formulation, content, and adsorption mechanisms of green inhibitors (especially organic inhibitors) as a prevention technique from corrosion for all grades of pipeline steel. Moreover, the identified drawbacks and the projected future trend have been highlighted. In addition, the effort aims to provide useful insight into future research in this area for precise measurement of inhibitory efficiency and the development of industrial applications.
One of the huge impacts of failures in the pipeline of gas and oil manufacturing systems due to corrosion is that it influences about one-quarter to two-thirds of the overall breakdown of service in the industry [25] (Da Rocha et al., 2010; Eduok et al., 2012). A massive amount of money is consumed each year on diverse categories of corrosion regulation to sustain pipeline integrity. However, challenges associated with acquiring, monitoring, specific designs and mitigation approaches employed portrays high huge sums of money consumed around the world to corrosion related with pipelines and other structural damage in the oil and gas industries (Bedairi et al., 2012; Ashassi-Sorkhabi et al., 2015). Studies have indicated that above 80% of pipeline failures due to corrosion are regulated from one system to the other (Al-Turkustani et al., 2011; Abdelaziz et al., 2021; Alao et al., 2022). In contrast, the total amount, especially 20%–65%, consumed on corrosion impairment could only be kept if there is an availability of outstanding knowledge on corrosion inhibitors, control methods, and protection (Brown, 2014). Pipeline corrosion can be related to different influences, which could be chemical or physical factors, environmental circumstances, or material features. Figure 1 displays the factors that influence pipeline corrosion.
The occurrence of inorganic and organic acids, CO2, and H2S in an operating pipeline surrounding may produce corrosion effects of diverse categories (Ossai et al., 2015). Li et al. (2014) observed the influence of the concentrated H2S on grade X60 pipe in a coexisting medium of CO2 and H2S. The corrosion rate of CO2 declined below 0.05 mmol/L of H2S, and at the concentration of H2S between 0.05 and 2 mmol/L, the CO2 corrosion rate revealed negligible variation. However, it rises at a concentration beyond 2 mmol/L. The development of sulfide layers on the steel surfaces produces some forms of crystal structures (e.g., pyrite, mackinawite, greigite, troilite, and pyrrhotite), which can be ascribed to the coexisting CO2 and H2S (Yin et al., 2008; Davoodi et al., 2011). Water slugs or splashes and the water-oil interface can influence corrosion propagation on pipelines. This corrosion propagation occurs when fluid flows in a multiphase from scattered flow to a constant water-in-oil movement. This only occurs when there is intense turbulence in the oil phase to produce water breakage to droplets. This droplet breakage flows to the pipeline’s walls when it reaches above a specific critical diameter and thus causes corrosion speedup. The effect of inclination angles on the pipeline interior with the multiphase flow was performed by Khaksarfard et al. (2013) using computational fluid dynamics. There was a wet-water surface in the pipeline when there was upward flow compared to when it was downward. This signifies that with the upward flow, more surfaces are susceptible to corrosion than in the situation where it is a downward flow. Thus, the application of corrosion-resistant alloys for making flexible pipes is highly needed for some energy (oil and gas) distribution operations, especially in gas lift lines, well tubing, and subsea risers, because they possess high resistance to corrosion (Guo et al., 2005).
A uniform corroded pipeline devoid of defects may be predicted directly with linear and non-linear models about the projected corrosion damage. However, the present defects, such as cracks, dents, buckles, and gouges around the corroded pipeline surfaces, could produce a more difficult estimation because these defects will have a greater stress load on the pipeline, leading to pressure burst at the corroded surfaces. The crack-in-corrosion defect is one of the defects that researchers have focused on. This defect is a hybrid one whose cracks are equally incident in a corroded surface, and the pipe wall thickness is consumed by 10% of this defect. To depict the influence of hybrid defects, Bedairi et al. (2012) examined corrosion, crack-in-corrosion, and cracking defects of pipelines with the aid of finite element analysis subjected to elastic–plastic fracture mechanics. This research was practically confirmed on 5.7 mm thickness and 508 mm diameter pipe. It was concluded that the corrosion defect was highly projected with 3.2% differences between FEA data and the practical outcome after examining the corroded depth percentages of 200 mm elongated defect and cracked wall thickness. This was tracked by crack-in-corrosion and crack defects, which have 17.4% and 12.4% differences between the practical outcome and FEA data, respectively. These results depict that the prediction accuracy of crack-in-corrosion defects should be enhanced so that the aging pipeline’s integrity can be well managed. Diverse research has shown that flow configuration can impact pipeline corrosion (Hernandez-Rodriguez et al., 2007; Ilman, 2014). Biomorgi et al. (2012) experimentally analyzed the differential impact of operating parameters on energy (oil and gas) pipeline corrosion. It was revealed that after 4 months of experimentation, the impacts of iron carbonate, sand, flow pattern, and sulfide scales were hugely produced under deposits in the examined 102 and 154 mm diameter pipelines. Furthermore, the flow of the slug and pipe configurationcontributed to the corrosion mechanism more than the gas bubbles in the pipeline, and pitting corrosion was affected by the interior diameter of the pipeline. Papavinasam et al. (2010) synonymously established that subcutaneous materials, such as sand, were added to pitting corrosion development in energy pipelines. Pitting corrosion rates differ from phase to phase because of the flow velocities and configuration. However, Mazumder et al. (2008) ascribed these differences to particle-to-fluid, particle-to-particle, and particle-to-wall relationships at such phases. Production techniques have likewise been discovered to influence pipelines’ vulnerability to corrosion, as revealed by Zhang et al. (2009) after examining the hydrostatic pressure impact on Fe-20Cr components. The outcome showed palpable pit development and growth rates and metastable pits enlargement into cavities.
The corrosion inhibitor operating system depends on the indirect or direct inhibitor molecules adsorbed on the metal surface, consequently shielding the contact of the harsh or corrosive medium from the metal surface. Common metallic substances are unstable naturally. For example, they are inclined to electrochemically react with destructive constituents of the medium (e.g., H+ and Cl−) to develop a steady corrosion product. Introducing corrosion inhibitors in the corrosive solution will yield adsorption on the metal surface in its active sites after protective film development, thus shielding it from corrosion. When inhibited by organic substances, metal surface passivation is highly important in contrast to inorganic inhibitors. For example, there is uniform passivation on the surface when organic inhibitors are applied, thus producing optimal feasible shielding. However, when inorganic inhibitors are applied, the passive film is very brittle, thus creating a metal surface liable to local corrosion occurrence (crevice, pitting) (Chadli et al., 2017).
Chemical or physical adsorption or the mixed mode of the two are the ways by which inhibitor adsorption may occur. The correlation between the inhibitor substances and a metal surface in any of the two methods described above relies on the substrate surface charge. The electrostatic relationship between the oppositely charged metal surface and the charged inhibitors produces inhibitor adsorption directly on the metal surface. However, the electrostatic correlation of previously adsorbed ions (e.g., ions of halide in amino acids) is the surface that is negatively charged, thus enhancing its efficiency to adsorbed proton inhibitors. This mechanism could particularly happen in acidic solutions. Hence,in the situation where the metal surface is of null charged, or no anions or cations which could undergo adsorption on the surface, inhibitor adsorption will happen via a chemical reaction between the inhibitor substance and the metal surface. Inhibitor adsorption will happen via a chemical reaction betwixt the inhibitor substances and the metal surface. Inhibitors are considered donor–acceptor reactants because they are electron donators in such a way that unpaired electrons of heteroatoms (e.g., S, N, and O) or the aromatic ring inhibitors of p-electrons react with a metal surface substrate of d-orbital to produce shielding layer. Some reactions of functional groups (−NH2, −OH) with metallic ions can also configure a defensive film (insoluble complexes) that shields the surface of the metal from corrosion. Through molecular adsorption by a shielding film formation, organic inhibitors can inhibit corrosion when adsorbed on the metal surface (Amitha and Bharathi, 2012; Popoola, 2019a; Popoola, 2019b). The existence of phytochemicals, especially in plant derivatives, is applied to corrosion restrictors because their protective influence is owed to the inhibitor molecule adsorption on the metal surface, thus initiating a shielding layer on the metal surface via active site blockage (as shown in Figure 2 and Figure 3). Therefore, this protection is less reactive than the surface without an inhibitor. The substitution of π electrons and aromatic rings with heteroatoms is applied to initiate the corrosion inhibitor. Moreover, the inhibitor’s solubility in a prevalent corrosive solution is due to the polar functional groups, which behave as the adsorption region for the inhibitor molecules (Anadebe et al., 2019; Abdelaziz et al., 2021).
Green corrosion inhibitors are hugely applied to regulate the various corrosion categories in diverse steel grades, especially in acidic mediums. These steel pipelines are needed to convey gas and oil in the energy industry. Green inhibitors can be categorized into two phases: organic and inorganic. High deliberation will only be channeled to organic green inhibitors for this research. Some diverse organic green inhibitors comprise ionic liquids, plants (oil and other derivatives), natural polymers, drugs, and biomass wastes. All these substances possess heteroatoms comprising elements with electron density, such as S, O, and N, acting as active phases for which they will be adsorbed on the metal area. Overall, organic inhibitors are more appropriate in an acidic solution; they have anodic or cathodic behavior and the two may coexist together. In contrast to inorganic inhibitors, organic green inhibitors display higher efficiency in corrosion inhibition. A huge amount of research in the past 6 years has concentrated on plant derivatives. Their outcomes depicted an outstanding capacity to inhibit steel corrosion in acidic environments. Synonymous results as regards amino acids and ionic liquids have been published (Nair, 2017; Rathi et al., 2017; Asmara et al., 2018; Verma et al., 2018; Popoola, 2019b; El Ibrahimi et al., 2020). Diverse organic inhibitors, such as drugs and natural polymers, have likewise revealed encouraging outcomes (Gece, 2011; Macedo et al., 2019; Shongwe, 2020). These organic inhibitors have functional groups in which heteroatoms are embedded (e.g., –NH2, −OH, −COOH, and −NO2) and/or molecular structures of aromatic rings, making them interact with metallic parts electrostatically or chemically. Therefore, this leads to the protective film development on the metal parts. Contrastingly, inorganic inhibitors are usually anodic, and thus, their bounded metal atoms’ layer can enhance substrate corrosion shielding. Due to their unfriendliness to the ecosystem, their research and work for corrosion protection in materials have begun to decline.
There has been some substantial recognition of eco-friendly green inhibitors as corrosion resistance due to the harmful influence of conventional corrosion inhibitors. The plant extracts or derivatives have depicted excellent outcomes. Owing to the high polarity of these derivatives prompted as a consequence of unshared electrons in their functional groups, they made excellent adsorption mechanism for the steelcomponents. The other importance comprises biocompatibility, reliability, less cost, ease of application, and renewable nature. The following post-work emphasizes how “plant derivatives” have been applied as corrosion inhibition for different categories of steel, which can be used for energy distribution in pipelines.
Haldhar et al. (2018) used a theoretical and empirical approach for mild steel’s IE with derivatives’ root of Valeriana wallichii as a green inhibitor. Due to the several existing phytochemicals comprising iridoid and naphthoic acid. Observation revealed that iridoid derivatives gave the optimal inhibition efficacy for mild steel in an environment comprising acid. Figure 4 reveals the SEM image of the mild steel surface when dipped in 0.5 M of sulphuric acid for 24 h at 298 K in the existence of an inhibitor and without an inhibitor. The sample without the inhibitor in the corrosive medium was detected to develop a porous surface of wide tracks and deep holes from metal surface dissociation. However, the solution with inhibitor has its surface quality improved. In the HCl solution, the leaf plant derivative of Aquilaria subintegra has been discovered as the most effective inhibitor, with an optimum 94% inhibition for mild steel. The inhibition method of this derivative was analyzed as physisorption aligning to Langmuir adsorption isotherm.
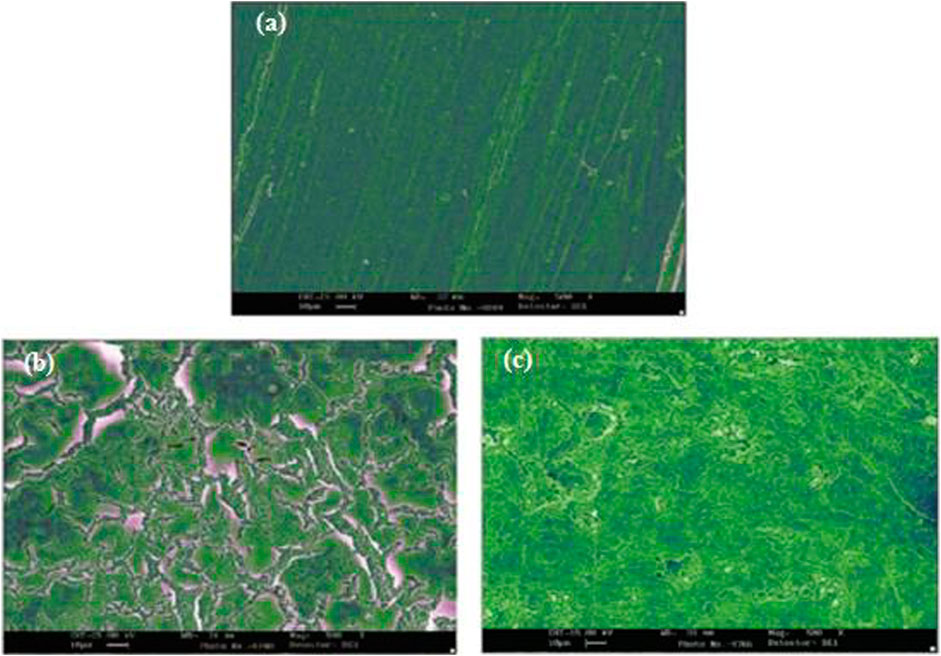
FIGURE 4. SEM image of a mild steel surface when submerged in 0.5 M of sulphuric acid for 24 h at 298 K, (A) polished sample (B) sample without inhibitor and (C) sample in presence of inhibitor.
Singh et al. (2013) introduced a theoretical route to examine plant derivatives such as Murraya koenigii, Andrographis paniculata, Terminalia arjuna, Citrus aurantium, Strychnos nux-vomica, Aegle marmelos, and Moringa oleifera, to ease the mild steel corrosion in the HCl solution. The abovementioned derivatives possess a set of effective phytochemical contents, such as brucine, andrographolide, mahabinine, sitosterol, arginine, pyrayafoline, skimmianine, brucine, and threonine. Diverse factors were examined in the experimental setup, such as dipole moment, lowest unoccupied molecular orbital, highest occupied molecular orbital, Mulliken charges on molecular capacity, and heteroatoms. The diverse plant derivatives showed suitable shielding efficiency because of the adherent inhibitor molecule adsorption on the mild steel surface. The outcomes demonstrated that the inhibitor adsorption features rely on diverse factors, such as molecular size, the number of adsorption phases, the development of metallic complexes, and interface nature.
Al-Turkustani et al. (2011) examined the protective behavior of alcohol derivatives of Medicago sativa plant and water to regulate the mild steel corrosion in a 2 M medium of sulphuric acid. Two chemical analysis methods were applied: the mass-loss technique and hydrogen evolution. The analysis behavior was performed in two processes: electrochemical impedance spectroscopy (EIS) and potentiodynamic polarization (PDP). It was discovered that the derivatives behave as a mixed inhibitor following the Langmuir adsorption isotherm. Moreover, the main corrosion defensive system was credited to the chemical adsorption via charge transference between the mild steel substrate and the inhibitor’s molecules.
Dehghani et al. (2019b) conducted a theoretical and experimental investigation on the fruit derivatives of aqueous Citrullus lanatus (CL) in HCl media for inhibition against corrosion of mild steel. The functional groups of O-H and C=C in the phytochemicals, especially riboflavin, citrulline, resveratrol, and hesperetin, were found to be the reason why the 91% IE was attained. The synchronized reaction was likewise discovered to be a fundamental protective factor. Figure 5 describes the shielding behavior of CL derivatives on mild steel. The achieved outcome from SEM (Figure 6) revealed that a smoother surface on mild steel was attained as the CL concentration was increased.
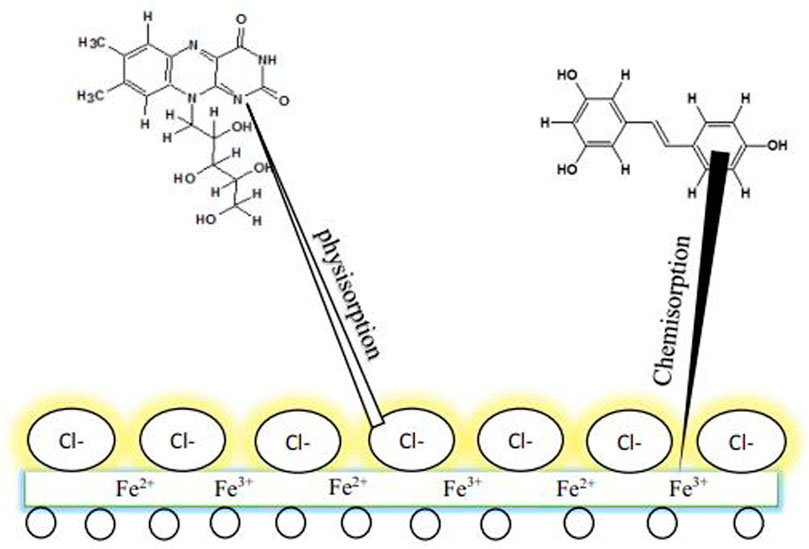
FIGURE 5. The adsorption system of CL extracts on mild steel in 1 M HCl medium (Dehghani et al., 2019b).
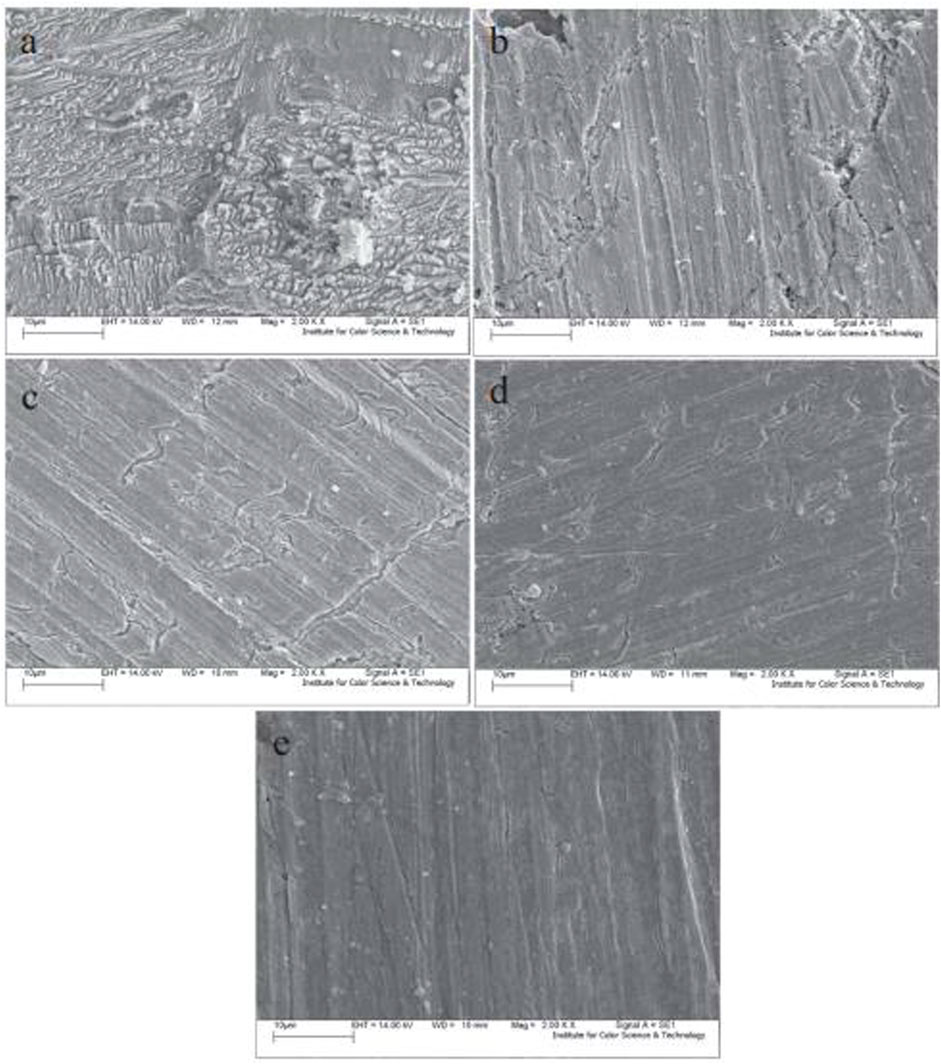
FIGURE 6. SEM image of mild steel for different concentration of Cl in 1M HCL—0 ppm (A) 200 ppm (B) 400 ppm (C) 600 ppm (D) and 800 ppm (E).
Wei et al. (2020) estimated various plant extract parts comprising Cassia occidentalis, Poinciana pulcherrima, papaya, Azadirachta indica, Calotropis Procera B, Cassia occidentalis, and Auforpio turkiale. In all the abovementioned derivatives, Poinciana pulcherrima Cassia and Azadirachta indica revealed the optimum efficiency of 96% and 98%, respectively. Regarding polarization curves, these derivatives behaved as mixed inhibitors, hence decreasing iron anodic reaction dissolution and the hydrogen cathodic reaction change. Because the major parts for photosynthesis are plant leaves, these parts possess huge phytochemicals. This reason gives “leaves” a special selection for effectual green corrosion inhibition. The behavior of plant leaves derivatives of Eriobotrya japonica Lin. in an aqueous sulphuric solution is worth stating. The outcome depicted that 96% efficiency could be attained for mild steel (Acharya et al., 2013). The evaluation, such as electrochemical measurements, weight-loss technique, and SEM, depicted that these derivatives were hugely cathodic inhibitors, thus satisfying the Langmuir adsorption isotherm. The combined influence of diverse phytochemicals in the derivatives was stated to be a major cause of their optimum IE.
Oguzie (2007) researched the shielding behavior of plant substances containing leaf derivatives such as Azadirachta indica (AI), Ocimum viridis (OV), Garcinia kola (GK), Hibiscus sabdariffa (HS), Telfairia occidentalis (TO), for mild steel in 1 M sulphuric acid and 2 M HCl. This experiment discovered that the plant derivatives possess adequate IE. The combined chemical adsorption with the halide ion’s reaction of protonated organic substances for physical adsorption produces the optimal behavior of these constituents as an effective corrosion protector in the arrangement Cl− < Br− < I−. Generally, peak inhibition efficiencies of 92.9% and 95% were achieved for GK and HS in 2 M HCl and 1 M sulphuric solutions, respectively.
Okafor et al. (2008) inspected the protective behavior of seeds (SD), leaves (LV), and the combined leave and seeds (LVSD) of Phyllanthus amarus (Pa) extracts in sulphuric and HCl media on mild steel corrosion. The behavior of the inhibition was examined with gasometric techniques and weight loss. The outcome revealed that the derivatives performed excellently as an excellent shield in both media, and the inhibition capacity improved with an upsurge in the extracts’ concentration (Figure 7). The temperature examinations exhibited that the rise in IE resulted from the temperature rise. However, this led to the activation energy decrement in the extract existence. The inhibition performance is due to the extracts’ chemical adsorption system on the metal surface. The adsorption inhibitor features were conformed to Temkin isotherm.
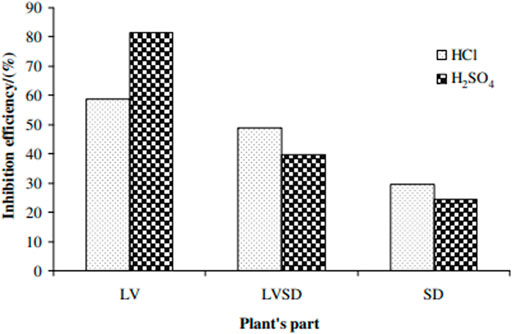
FIGURE 7. The optimum IE in 5 M acid solutions for mild steel sample containing 4 g/l PA at 30°C (Okafor et al., 2008).
Another research examined rubber leaf derivatives as corrosion protective in HCl medium for mild steel (Okewale and Olaitan, 2017). FTIR and the gravimetric system were applied to analyze the shielding behavior. The derivatives’ phytochemicals, such as flavonoids, saponins, tannins, and anthraquinone, produced 86% inhibition capacity. Moreover, the impulsive inhibitive adsorption conformed to the Langmuir isotherm. The plant IE relies on the quantity and the sorts of phytochemical changes in phases.
Eduok et al. (2012) assessed the inhibition capacity of Sida acuta plant stem and leaves for mild steel in a 1 M medium of sulphuric acid. Iodide ions were included in the medium to enhance their inhibition capacity. Weight loss and hydrogen evaluation measurements (HEMs) were the inhibition mechanism used at 30°C–60°C to determine shielding capacity. Although the inhibition followed similarly (Freundlich adsorption isotherm), the leaves were detected to possess a peak inhibition of 85.25%, which was attributed to the greater phytochemical percentage in the leaves.
Okafor and Ebenso (2007) juxtaposed the inhibitive behavior of diverse Carica papaya plant parts. They regarded leaves, bark (BK), seeds (SD), considered leaves (LV), and heartwood (HW) for the examination of mild steel in sulphuric acid. The plant derivatives are supplemented with organic compounds and nitrogenous substances such as saponins, choline, cardenolides, prunasin, anthraquinones, dehydrocarpaines, and pseudocarpaine. Gasometric and gravimetric systems were applied to determine the inhibition mechanism of the diverse components. All the derivatives were detected to restrict mild steel corrosion. Hence, the plant derivatives conform to the following arrangement with regard to the IE BK (85%) < HW (86%) < SD (94%) < LV (97.3%). The IE was detected proportionate to the concentration and inversely proportional to the temperature. Regarding the inhibition system, all the concerned derivatives involved phytochemicals’ physical adsorption on the metal surface. Furthermore, the Temkin and Langmuir isotherms aligned with the inhibitors.
Kumar and Mohana (2013) demonstrated that Achyranthes aspera L. plant derivatives could regulate mild steel corrosion in 1.0 M of HCl at normal temperature with 82.3% IE. Negative adsorption energy contributed to the instant adsorption actions, hence enhancing IE. The outcomes fitted perfectly with Flory–Huggins, Langmuir, and Frumkin adsorption isotherms. Furthermore, a spontaneous reaction was discovered between the molecules of the inhibitors, thus specifying an appreciable stability inhibitor in the acidic solution.
There are various findings on inhibitors, especially from a natural source that has appreciable efficiency for mild steel. They comprise pomegranate peel extract (Li et al., 2015), Psidium guajava seed extract (Kumar et al., 2011), Neolamarckia cadamba crude extract (bark, leaves) (Raja et al., 2013), panacea (Flacourtia jangomas) (Hasan and Sisodia, 2011), and Salvia officinalis oil (Chetouani and Hammouti, 2014). Some of these inhibitor mechanisms and efficiency are itemized in Table 1. These inhibitors align with chemical or physical adsorption. The main adsorption focus in organic compounds based on their reaction with steel surface is the oxygen-comprising hetero atoms in various derivatives phytoconstituents, such as phenolic compounds (specifically flavonoids). Derivatives such as fatty acids (linolenic acid), amino acids (methionine), tannins, steroids, antioxidant materials, and carbonyl groups are effective substances for the inhibition behavior of these derivatives.
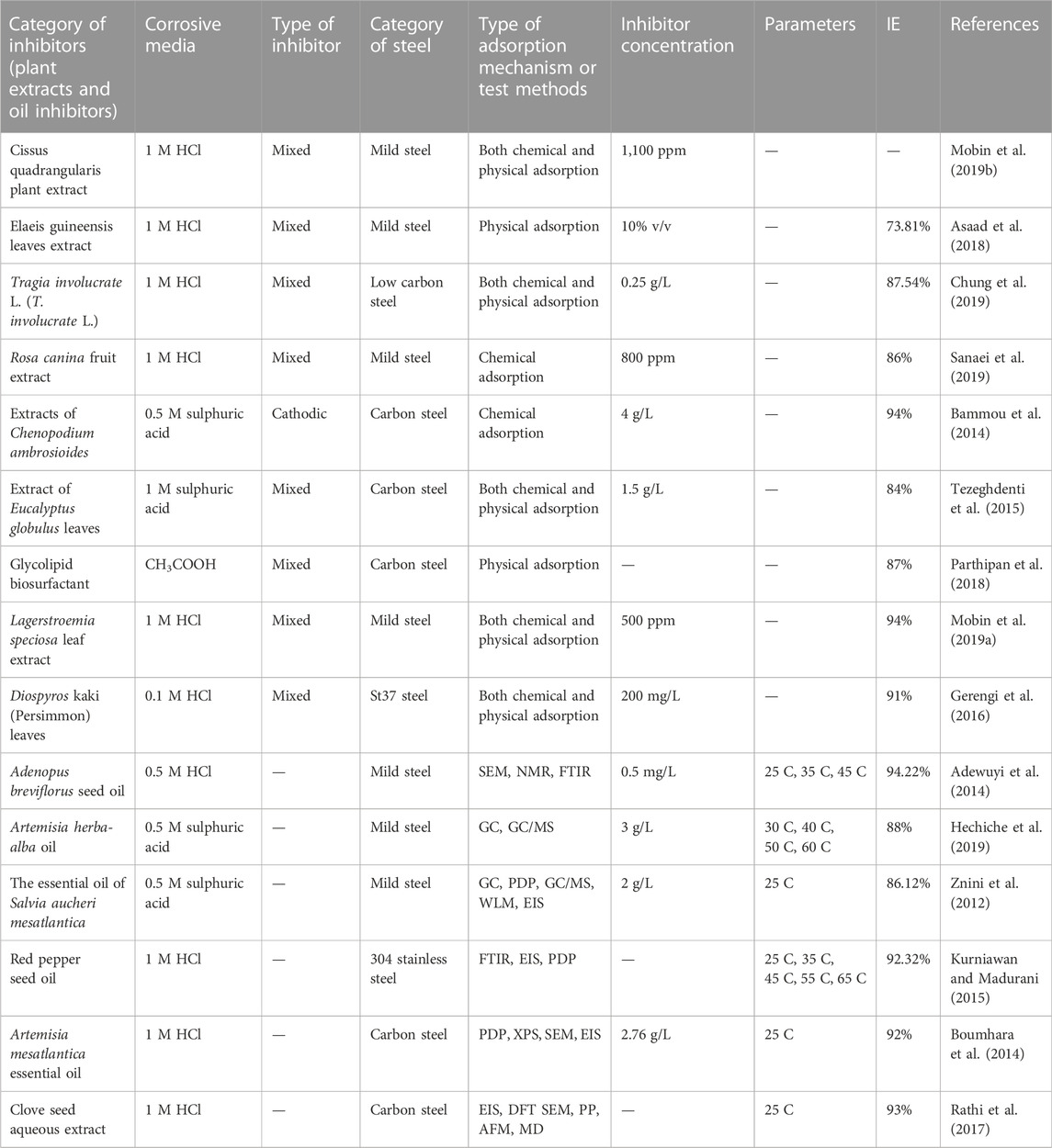
TABLE 1. Some plant extracts and essential oil inhibitors for steel against corrosion in an acidic medium.
Researchers have given plant oil derivatives huge recognition. Some important oils, which are also regarded as ethereal or volatile oils, are aggregated hydrophobic liquids that comprise sesquiterpene, monoterpene hydrocarbons, and oxygen-doped groups, such as acids, esters, ethers, alcohols, oxides, phenols, ketones, aldehydes, and lactones. Over the past ten years, various works have been conducted to determine IE for diverse metallic alloys using natural oils in alkaline, neutral, and acidic solutions. In the 1960s, the foremost effort to apply oil as a protective shield for the material was developed by Faraj and Khan (2015). They fruitfully applied molasses with vegetable oil in the acid pickling technique to prevent steel corrosion. Then, the utilization of natural oils as a material shield became common, hence providing other fruitful innovations, such as henna, ginger, artemisia, and jojoba oil (Buchweishaija, 2009; Azeiteiro et al., 2015; Nair, 2017). The adsorption on a metal surface by the mechanism of oil was simplified by Lahhit et al. (2011). Foeniculum vulgare was applied in this work as the examined oil, with steel applied as a substrate in an electrolyte of 1 M HCl. The Tafel polarization techniques, EIS, and weight loss characterizations were engaged to examine the protective mechanism. As vindicated via hydro-distillation, the oil comprised 17.8% pinene and 20.8% limonene. Through the reaction with the vacant iron atoms “d-orbitals,” the two molecules (pinene and limonene) are adsorbed on the steel surface, thus producing a combined impact. As the oil concentration got risen, the adsorption rate was detected (via EIS analysis) to rise with the charge-transmission resistance. The polarization curves depicted that the incorporated natural oil changes the anodic and cathodic branches to the lesser currents, hence showing that the inhibitor oil was a mixed category.
Velázquez-González et al. (2014) investigated the protective performance of methanol, hexane, and acetone derivatives from the oil of Rosmarinus officinalis. Their behavior was examined in 0.5 M sulphuric acid for 1,018 steel. Every one of the derivatives depicted considerable optimum corrosion inhibitive features, though the 96% IE was depicted by the hexane derivative. The flavonoids in the derivatives are the attributing feature to the inhibitive performance. The derivative conforms to the isotherm of Frumkin adsorption, and its inhibitive attributes were controlled by the chemisorption system established by the adsorption free-energy value (−37.4 kJ.mol−1).
Different modern green capacity oil-based inhibitors have been applied in the past years. Some discoveries entail red pepper seed oil, Nigella sativa L. seed oil, palm oil, and Artemisia mesatlantica essential oil (Da Rocha et al., 2010; Machado et al., 2019). These inhibitors have depicted exceptional efficiency in acidic media with above 90%, hence revealing their capacity for industrial use. Some other important plant derivatives and oil-based inhibitors are presented in Table 1.
In the exploration of environmentally friendly corrosion protectors, drugs epitomize another invention that sprung from wide research and efforts produced over a decade. Drugs possess an appropriate potential for inhibitive features against corrosion similar to other inhibitors with similar molecular structures. For instance, heterocyclic systems (e.g., isoxazoles, imidazoles, and furans) and carboxylic are numerously available in different categories of drugs and other various inhibitors. Synonymously, 5 or 6 participant rings with largely pseudo-aromatic or aromatic structures (e.g., benzene rings) are another similarity (Gece, 2011; Fouda et al., 2017; Singh et al., 2019a). Antibacterial drugs are among the various drug categories that have depicted excellent behavior in shielding steel corrosion in acidic environments. Various drugs, such as aminoglycosides, cephalosporins, and penicillin, have been stated to possess significant efficiency as green inhibitors. In the medicine industry, the highly applied drug is penicillin. It consists of heteroatoms in heterocycles regarded as b-lactam, which behave as an operative region for the adsorption mechanism. Moreover, adsorption happens because of the p-electrons interaction in the drugs with metal surface “d-electron” valency, thus producing protective layer formation. Cephalosporins are similar to penicillin in action system and molecular structure (e.g., the fungus Cephalosporin acremonium derivative). Cephalosporins can be applied in situations where prohibition is ascribed to the use of penicillin. However, their inhibitive capacity is relatively less because they have lower solubility.
Liang et al. (2015) scrutinized the capacity of three sorts of penicillin (i.e., amoxicillin, ampicillin, and penicillin G) for carbon steel corrosion protection in 1 M HCl. The behavior was examined via EIS, PDP, and electrochemical noise checks. The outcomes showed that the active region needed for drug adsorption on the steel surface is the b-lactam rings’ oxygen atom. Moreover, the measured drugs had high solubility, hence providing a good adsorption rate and inhibition capacity. In all the drugs, a chemisorption system was observed for penicillin G, whereas the physisorption mechanism followed the other two, thus producing 96% optimum efficiency for penicillin against 74.2% ampicillin and 61.2% amoxicillin.
Oguntuyi et al. (2021a) evaluated the drug (acarbose), with the molecular name of dideoxy-4-4,5,6-trihydroxy-3-(hydroxymethyl)cyclohexane-2-en-1-yl C7 cyclitol moiety, as a protective substance against corrosion for mild steel. Langmuir adsorption isotherm was confirmed owing to the inhibitor adsorption on the mild steel surface. The comprehensive theoretical study of the corrosion and inhibitor mechanism was achieved by computational evaluation, such as density functional theory and molecular dynamic simulations. From all the collective outcomes, significant IE was achieved. At the concentration of 4,000 ppm, optimum IE of 96% was noted. The microstructural evaluation via SEM (Figure 8B) revealed that the sample without acarbose as the inhibitor displayed a huge rough surface, which can be ascribed to the metal disintegration by the acidic media. However, a minute rough surface (Figure 8C) can be noticed for the sample immersed in the inhibited media (Marsoul et al., 2020), revealing the establishment of an efficient defensive layer on the sample’s surface.
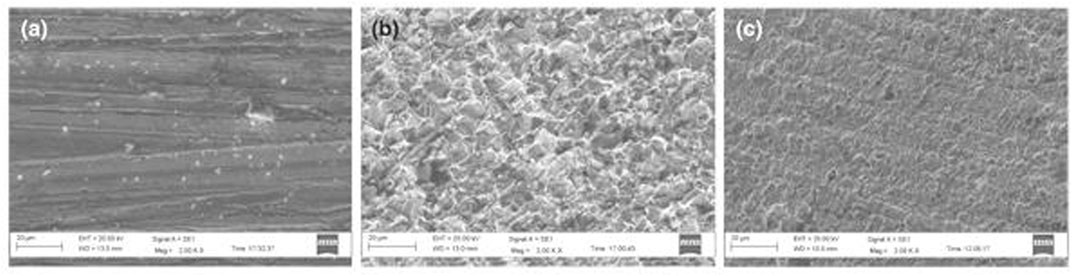
FIGURE 8. The SEM images of (A) polished sample, (B) sample submerged in a blank medium. and (C) sample submerged in an inhibited medium (Oguntuyi et al., 2021a).
Talari et al. (2019) demonstrated the behavior of twin imidazole-based drugs (namely, 2-methyl-1, 3-thiazole-4-carboxylic acid and thiazole-4-carboxylic acid) by applying HCl solution as an electrolyte for the electrochemical examinations. A higher IE of 90% was depicted by the drug: thiazole-4-carboxylic acid of 150 ppm concentration at 25°C. It was concluded that the adsorption rate of the two inhibitors conforms to the Langmuir isotherm with a quickened adsorption mechanism. The charges of the dipole moment revealed effective adherence of the inhibitors on the metal surface.
Singh et al. (2019a) suggested that an outdated drug can likewise be applied efficiently as corrosion shielding. Two outdated drugs (i.e., dapsone-salicylaldehyde and dapsone-benzaldehyde) were examined to determine their inhibitive capacity. Examinations showed that outdated drugs could produce high efficiency of 94.23%–95.67% between the two. Potassium iodide ions were included in the HCl media to corroborate the drug’s inhibitive capacity. They discovered that drug efficiency was enhanced to 97.98% and 99.03%, respectively.
In the area of green inhibitors when using drugs, the urispas drug is highly regarded as one of the efficient inhibitors. PDP, gravimetric measurements, and EIS tests were applied to determine the inhibitive performance. The drug was investigated on sulphuric acid using soft steel as the specimen. The IE reaches the optimal value of 97.85% when the inhibitor concentration rises to 1,000 ppm. The capacity of urispas to inhibit steel corrosion was deduced from its effective performance even at a lower concentration of 150 ppm (Anaee et al., 2019). Some other important drugs as green inhibitors are presented in Table 2.
Biomass waste is one of the organic inhibitors applied to inhibit corrosion. Numerous works have been conducted on organic inhibitors (plant derivatives, amino, and drugs). However, little research has been conducted to determine biomass waste’s efficiency as a corrosion inhibitor. Some studies experimenting with the inhibitive features of biomass waste are stated below.
Gapsari et al. (2019) applied the derivatives from the honeycomb waste as an inhibitor for stainless steel in the sulphuric medium. The inhibitor’s concentration (about 2,000 mg/L) initiated the increase in the stainless corrosion rate. However, it declined when the concentration was 3,000–4,000 mg/L. Hence, the 97.29% IE was attained when the 2,000 mg/L inhibitor concentration was used in 0.5 M sulphuric acid. The inhibitive feature is connected to the adsorption performance of the honeycomb derivative because it contains vitexin, pinobanksin, luteolin, apigenin, fisetin, isoferulic, quercetin, and isorhamnetin.
Cruz-Zabalegui et al. (2019) used a gemini surfactant extracted from avocado oil on API X-52 steel as a CO2 inhibitor. The examination depicted that at the inhibitor’s concentration of 10–50 ppm, for 24 h at the temperature of 50°C, the compound shielded the steel because of the physical adsorption. Liao et al. (2018) revealed that the waste achieved from peel and seed (lychee) is realistic to undergo recycling and applied as an ecologically friendly inhibitor in 0.5 M HCl media on mild steel, with the inhibitor’s concentration getting to 600 mg/L to attain the highest protection. The waste materials derived from eggshell was examined to hugely change the austenitic stainless steel corrosion inhibition (Sanni et al., 2018). It was further revealed that the inhibitor concentration increment restricted the passive film development, and the optimal shielding capacity of 94.74% was attained via weight loss scrutiny.
Montoya et al. (2019) applied the Pinus radiata bark as organic corrosion on 1020 steel. The biomass was extracted from the wastepaper and timber industries. The development of tannin-Fe acted as the shielding coatings.
One of the constant diets for humans is Prunus dulcis (almond), though its peel is regarded as waste. Pal et al. (2019) applied this waste substance for corrosion protection for mild steel. The inhibition capacities of 85% and 93% were attained, respectively, when aqueous and methanolic almond extracts shielded the mild steel. AFM and SEM examination revealed this greater efficiency of methanolic extract. It was vindicated that methanol comprises higher phytochemicals than water, such as chlorogenic acid, isorhamnetin-3-O-rutinoside, and catechin, which are accountable for mild steel protection. The extracts are categorized as a mixed type, where the adsorption is impure chemical or physical.
Munawaroh et al. (2022) evaluated the inhibitive behavior of protoporphyrin. A biomass waste derived from animal blood hemin was applied to carbon steel in 0.5 M HCl. The protoporphyrin quantity was evaluated as 40–200 ppm at a temperature variation of 298–318 K. The IE of 46.2% was attained with a concentration of the inhibitor at 160 ppm in the acidic solution at 298 K. However, with further increment in the temperature, the IE declined, thus indicating the exothermic nature of the reaction that consequently weakened the adsorption of the inhibitors on the carbon steel surface. A mixed-type inhibition was observed for the inhibitor as detected by the potentiodynamic polarization test. Furthermore, Langmuir adsorption isotherm was obeyed due to inhibitor adsorption on the steel surface.
Dehghani et al. (2019a) investigated that the fruit shell of Chinese gooseberry contains maltose, sucrose, and folate aqueous derivatives. These derivatives were examined as corrosion inhibitors in HCl media on carbon steel. The steel insertion in 1,000 ppm concentration of the inhibitors developed 92% shielding capacity. The achieved outcome via EIS collaborates with molecular simulation discoveries, which revealed that amidst the three main organic compositions, folate has an optimum inhibition mechanism over maltose and sucrose. Furthermore, amine groups, carbonyl, hydroxyl, and imine, with nitrogen and oxygen centers, are liable for the folate’s higher inhibition system in contrast to the other two.
Fiori-Bimbi et al. (2015) discovered the derivation of pectin from the citrus peel as a corrosion inhibitor in HCl media for mild steel. The inhibitor concentration of about 2.0 g/L at 45°C produced 94.2% inhibition capacity via anodic and cathodic metallic surface shielding. Furthermore, Grassino et al. (2016) and Ninčević Grassino et al. (2020) depicted that pectin achieved from the waste of tomato peel can be applied as an environmental corrosion shield for tin. The corrosion examination mechanized in a citric acid/NaCl/acetic acid indicated that pectin produced 65.8% and 73% inhibition capacity for EIS and PDP, respectively, at less concentration of 4 g/L.
Adewuyi and Oderinde (2018) evaluated the inhibition capacity of Khaya senegalensis fatty hydroxylamine (KSFA) in an HCl solution for aluminum. The unused seed oil and its breakdowns, such as hydroxylation, esterification, amidation, and transesterification, were applied to create KSFA. The inhibition capacity of 90.43% was attained with 0.001 mg/L inhibitor concentration. This efficiency was associated with heteroatoms’ presence in the KSFA.
Tiwari et al. (2018) examined the Musa paradisiaca peels in sulphuric and HCl acids for corrosion protection on mild steel. Ethyl acetate was derived from the peel, and the electrochemical tests indicated that the peel derivatives prevent the mild steel from corrosion in both acidic solutions, owing to aromatic rings and heteroatoms abundance (nitrogen and oxygen), which are adsorbed on the surface via an electrostatic or chemical interface with iron atoms. However, the derivative of banana peel consists of various bioactive compounds. Hence, they could influence the overall protective behavior of the derivatives. The inhibition mechanism is ruled by the gallocatechin molecule.
Generally, the inhibitive capability of beneficial-added compounds from underused and abundant materials paves the way for manufacturing modern categories of inhibitors, which could be applied as a shield against corrosion after general quantitative and qualitative examinations. Some biomass wastes and their extracts for corrosion inhibitors are tabulated in Table 3.
The biodegradability and non-toxicity of amino acids have made them good corrosion inhibitors. Amino acids have molecules with at least an amino group (-NH2) and carbonyl group (-COOH) attached with a single carbon atom in their structure. Moreover, amino acids have good solubility in aqueous solutions. They can be produced easily with high purity and are comparatively cheap. Amino acids can be categorized into various classes depending on their molecular structure, such as sulfur-containing cationic/anionic, W-amides, linear aliphatic, aromatics, and heterocyclic. (El Ibrahimi et al., 2020). The appearance of heteroatoms (e.g., O, N, and S) and associated p-electrons in their structure make the acids possess excellent inhibitive capacity against corrosion.
Some green inhibitors, such as tryptophan, imidazolium zwitterions, and konjac glucomannan, are excellent among diverse amino acid-based agents because they have outstanding shielding properties (Abdel-Fatah et al., 2017; Srivastava et al., 2017). In the inhibitors mentioned earlier, imidazolium zwitterions have depicted 96.08% inhibition, whereas the others have also been examined to have above 90% inhibition. The effective inhibitor adsorption on the steel surface is attained via mixed adsorption, which obeys the Langmuir isotherm.
Gong et al. (2019a) evaluated the protective capacity of amino acids against corrosion. Three halogen-alternated extracts [2-amino-4-(4-chlorophenyl)-thiazole (CPT), 2-amino-4-(4-fluorophenyl)-thiazole (FPT), and 2-amino-(4-bromophenyl)-thiazole (BPT)] were examined by temperature variation of 30°C–60°C. The electrochemical analysis depicted that BPT and CPT produced 95.45% and 95.16%, respectively. However, these efficiencies were sustained at elevated temperatures up to 60°C. Hence, this was attributed to the elevated stability behavior in the binding energy between the metal surface and inhibitors. FPT has 95% inhibition, although it decreased at 60°C by 22.62%. Furthermore, the authors examined the IE of 2-amino-4-(4-methoxyphenyl)-thiazole (MPT) in 0.5 sulphuric acids for mild steel against corrosion. Figure 9 reveals the diagrammatical representation of the adsorption system. Synonymous properties of this corrosion inhibition system were also observed in other categories of inhibitors. The introduction of the mild steel specimen into the corrosive solution caused a positive charge to form on the metal surface. However, the adsorption in the next stage of the mechanism happened in a way that the electrostatic energy in the acid medium produced negatively charged sulfate ion adsorption on the metal surface. Hence protonated H + and MPT were adsorbed on the steel surface (negatively charged), and the hydrogen evolution decreased because of the adsorption opposition. After that, the adsorption process stability developed chemical adsorption via covalent bond formation between Fe atoms and MPT molecules. Furthermore, the relationship between chemical and physical adsorption produces defensive layers on the surface, thus shielding the mild steel in an acidic medium.
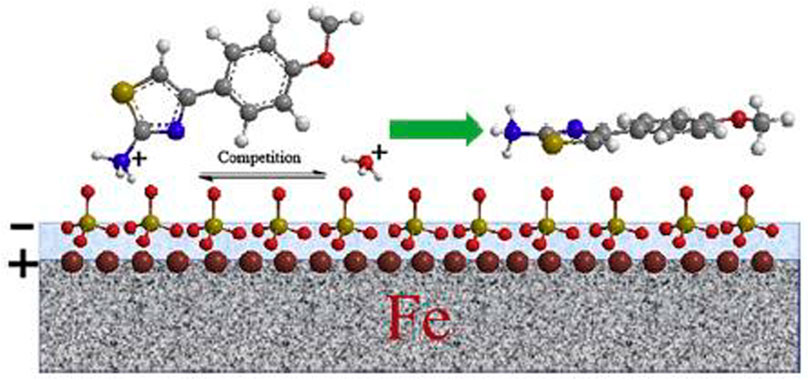
FIGURE 9. MPT adsorption system in a sulphuric solution on a mild steel surface (Gong et al., 2019a).
Verma and Ma (2014) demonstrated inhibitive extracts feature of 2-[(1H-indol-2-yl)thio]-6-amino-4-phenyl pyridine-3,5-dicarbonitriles (TAPD) in 1 M HCl for mild steel. Computational evaluation and diverse experimental analysis (PDP, WLM, EDX, and SEM) were all conducted. At least minimum efficiency of 90% was attained, showing that all extracts behaved well even at low concentrations of the inhibitors. It was observed that when the amino acid TAPD-III was substituted with –OH compound, a higher efficiency was developed than when replaced with NO2 compound. Furthermore, the mechanism behaves as mixed inhibitors, which align with the Langmuir adsorption isotherm.
Loto (2019) evaluated the corrosion inhibition of 2-amino-4-methylpentanoic acid on carbon steel in 1 M acid solution (HCl). The outcomes obtained from the electrochemical examination revealed that the compound produced 85.88% and 87.46% inhibition efficiencies at 1.88% and 0% of the inhibitor concentration, respectively. Thermodynamic evaluations via Frumkin isotherm and Langmuir models depicted the adsorption and interaction of chemisorption molecules on the carbon steel surface.
Amino acids often conform to a synergism mechanism, similar to other inhibitors. This synergism mechanism occurs when surfactants are introduced into amino acids, which consequently enhances their protective efficiency to a high level. The synergistic influence of L-cysteine (CYS) and imidazoline derivative (IM) on carbon steel was investigated by Zhang et al. (2018). Similarly, Srivastava et al. (2017) discovered that increase in efficiency up to 84.66% was observed when the surfactant of cetyltrimethylammonium bromide and surfactant sodium dodecyl sulfate (SDS) were included in amino acid L-methionine. Some other worthwhile inhibitive capacities of amino acids are presented in Table 4.
Green inhibitors have been employed extensively in managing the corrosion problems of steels, particularly in acidic conditions, according to the research articles listed here. Plants extracts (e.g., peel, root, flower, wood, fruit, seed, and bark) and oils are discovered to be the most numerous sources of green inhibitors among the various groups of inhibitors. They are widely available and comparatively inexpensive in contrast to other forms of green inhibitors. Additionally, they contain several active ingredients and can be obtained using straightforward extraction procedures. However, items extracted from naturally occurring consumable sources (e.g., fruits and vegetables) must be gathered over a long period because they are seasonal and takes time to harvest. This could decrease their large-scale manufacture and industrial applicability. Furthermore, some of the important resources that have been evaluated for the creation of green inhibitors have competitive uses in the pharmaceutical and medical industries, as well as in other human requirements. According to the literature review, researchers do not place a high value on biowaste extracts. As a result, a thorough study procedure that investigates the extract characterization and the key corrosion inhibition elements, as well as theoretical investigations, from widely available waste sources, could significantly contribute to this sector.
The efficiency of corrosion inhibition is governed by active compounds contained in green inhibitors, such as phenols, flavonoids, pectin, and aldehydes. The amount of such a chemical in a certain extract is expected to change depending on the weather and location of such material sources. Additionally, these have restricted the rationalization of laboratory findings for various inhibitors to justify their industrial implementation. Therefore, the recognition of this feature during characterization can result in inhibitor consistency in an industrial setting. Furthermore, a good understanding (insight) of molecular structure and its impact on corrosion inhibition might help with inhibition performance enhancement.
Despite the extensive experimental effort and several research articles published in the field, there remains a gap in the scientific method, with the results frequently phenomenological and not often theoretical and mechanistic. Corrosion inhibitor evaluation is typically done through electrochemical studies to directly determine its efficiency in the ultimate use as corrosion protecting substance instead of focusing on the precise adsorption process and associated mechanisms. Another significant limitation is the poor identification of the effective ingredient in the green extract, which is frequently a combination of unidentified compounds. The inability to correctly identify the inhibitive substances, whether they inhibit alone or synergistically, is the other adsorption mechanism that needs further clarification. The inhibitory effect could be due to a single molecule or a synergistic impact of the complete extract. This inefficient identification is an issue that is frequently overlooked in the literature, thus restricting the reproducibility of outcomes in this area.
Another weak point worth of note in utilizing green inhibitors is their inability to endure high temperatures and storage concerns. When it comes to extracting natural compounds, the temperature has a significant impact. At low temperatures, the solubility of phytochemical compounds in extraction solvents is diminished, although, at elevated temperatures, the phytochemicals may decompose. The ideal temperature for a successful extraction is 60°C–80°C. Oven drying is somewhat advised because drying at normal temperature can consume a huge duration. Additionally, the efficiency of green inhibitors declines as the operational temperature rises for industrial applications, and trying to store them for an extended period causes them to degrade. More studies are needed to understand the impacts and use of additives such as iron control agents, anti-sludge agents, surfactants, and stabilizers to green inhibitors, as well as the formulation of composites with high refractoriness. These elements may not be inhibitory, but they may add to the corrosion inhibition properties of green inhibitors and enhance the temperature challenges.
Green inhibitors are generally efficient corrosion inhibitors in a variety of corrosive conditions. The main benefits of these inhibitors are their non-toxicity and biodegradability. Future research should be conducted under the guiding principle of a huge economic perspective with a clear evaluation of the obtainability and price of the green inhibitor source and the manufacturing procedure for huge-scale usage. The use of green inhibitors is an advanced practice that is environmentally benign, long-lasting, and cost-effective for steel protection. These days, corrosion prevention applications from nanoparticles are being researched. The usage of green inhibitors, however, continues to be a far safer and more environmentally friendly choice. The application of green inhibitors is possibly going to expand in the future as constant additional discoveries are made in this area.
The challenges of corrosion in the energy (oil and gas) industry are an unavoidable experience. Thus, full inhibition of this scenario is not feasible. However, proffering a preventive mechanism is more proficient and economical. This can be actualized by inhibiting the surface of the metal from the corrosion medium. The system of conventional (organic) inhibitors against corrosion has been prominent in use for a long time. In contrast, commercial inhibitors (usually inorganic) have been limited over time because of their toxic nature, which contributes to environmental (habitat) annihilation. Hence, organic inhibitors are highly preferred to other inhibitors because of their availability, low cost, environmentally benign and renewable nature, biodegradability, optimal efficiency, and natural compatibility. Therefore, this review has elaborated on the inhibitory mechanism of organic inhibitors, especially plant extracts, amino acids, drugs, and biomass waste. The phytochemicals generated from plant derivatives, the amino group (NH2) present in the molecular structure of amino groups and drugs’ molecular weights, and the shielding effect of biomass wastes are the major inhibitory properties of these organic inhibitors against corrosion. From the various literature, it is well understood that the increment in the inhibitor concentration consequently enhances the sample’s resistivity to corrosion. Although some situations were observed where irrespective of the increment in the inhibitor’s concentration at some high temperature, there was no improvement in the samples’ IE. Hence, this evaluation revealed that some organic inhibitors could thrive under any circumstances (temperatures, pressure, and other parameters), whereas others may not sustain their inhibition at certain parameters. Therefore, judicious parameters should be considered before the selection and use of any organic inhibitor as a corrosion inhibitor for different pipeline steel.
AA: data curation, methodology, and original draft. AP: proofreading, editing, and supervision. DM: data analysis. OS: data analysis.
The authors would like to thank the Surface Engineering Research Laboratory (SERL), Tshwane University of Technology, Pretoria, South Africa for their assistance.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abdel-Fatah, H. T. M., Kamel, M. M., Hassan, A. A., Rashwan, S. A., Abd El Wahaab, S. M., and El-Sehiety, H. E. (2017). Adsorption and inhibitive properties of Tryptophan on low alloy steel corrosion in acidic media. Arabian J. Chem. 10, S1164–S1171. doi:10.1016/j.arabjc.2013.02.010
Abdelaziz, S., Benamira, M., Messaadia, L., Boughoues, Y., Lahmar, H., and Boudjerda, A. (2021). Green corrosion inhibition of mild steel in HCl medium using leaves extract of Arbutus unedo L. plant: An experimental and computational approach. Colloids Surfaces A Physicochem. Eng. Aspects 619, 126496. doi:10.1016/j.colsurfa.2021.126496
Acharya, M., Chouhan, J. S., Dixit, A., and Gupta, D. K. (2013). Green inhibitors for prevention of metal and alloys corrosion: An overview. Chem. Mater. Res. 3 (6), 16–24.
Adewuyi, A., Göpfert, A., and Wolff, T. (2014). Succinyl amide gemini surfactant from adenopus breviflorus seed oil: A potential corrosion inhibitor of mild steel in acidic medium. Industrial Crops Prod. 52, 439–449. doi:10.1016/j.indcrop.2013.10.045
Adewuyi, A., and Oderinde, R. A. (2018). Synthesis of hydroxylated fatty amide from underutilized seed oil of Khaya senegalensis: A potential green inhibitor of corrosion in aluminum. J. Anal. Sci. Technol. 9 (1), 26–13. doi:10.1186/s40543-018-0158-9
Al-Fahemi, J. H., Abdallah, M., Gad, E. A., and Jahdaly, B. (2016). Experimental and theoretical approach studies for melatonin drug as safely corrosion inhibitors for carbon steel using DFT. J. Mol. Liq. 222, 1157–1163. doi:10.1016/j.molliq.2016.07.085
Al-Turkustani, A. M., Arab, S. T., and Al-Qarni, L. S. S. (2011). Medicago Sative plant as safe inhibitor on the corrosion of steel in 2.0 M H2SO4 solution. J. Saudi Chem. Soc. 15 (1), 73–82. doi:10.1016/j.jscs.2010.10.008
Alao, A. O., Popoola, A. P., and Sanni, O. (2022). The influence of nanoparticle inhibitors on the corrosion protection of some industrial metals: A review. J. Bio-and Tribo-Corrosion 8 (3), 68–16. doi:10.1007/s40735-022-00665-1
Amitha, B. E., and Bharathi, B. J. (2012). Green inhibitors for corrosion protection of metals and alloys: An overview. Inter. J. Corros. 2012, 380217. doi:10.1155/2012/380217
Anadebe, V. C., Onukwuli, O., Omotioma, M., and Okafor, N. (2019). Experimental, theoretical modeling and optimization of inhibition efficiency of pigeon pea leaf extract as anti-corrosion agent of mild steel in acid environment. Mater. Chem. Phys. 233, 120–132. doi:10.1016/j.matchemphys.2019.05.033
Anaee, R. A., Tomi, I. H. R., Abdulmajeed, M. H., Naser, S. A., and Kathem, M. M. (2019). Expired Etoricoxib as a corrosion inhibitor for steel in acidic solution. J. Mol. Liq. 279, 594–602. doi:10.1016/j.molliq.2019.01.169
Asaad, M. A., Ismail, M., A. Khalid, N. H., Huseien, G. F., and Bothi Raja, P. (2018). Elaeis guineensis leaves extracts as eco-friendly corrosion inhibitor for mild steel in hydrochloric acid. J. Teknol. 80 (6). doi:10.11113/jt.v80.11191
Ashassi-Sorkhabi, H., Mirzaee, S., Rostamikia, T., and Bagheri, R. (2015). Pomegranate (Punica granatum) peel extract as a green corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Corros. 2015, 1–6. doi:10.1155/2015/197587
Asmara, Y. P., Kurniawan, T., Sutjipto, A. G. E., and Jafar, J. (2018). Application of plants extracts as green corrosion inhibitors for steel in concrete-A review. Indonesian J. Sci. Technol. 3 (2), 158–170. doi:10.17509/ijost.v3i2.12760
Ayoola, A. A., Fayomi, O. S. I., and Ogunkanmbi, S. O. (2018). Data on inhibitive performance of chloraphenicol drug on A315 mild steel in acidic medium. Data Brief 19, 804–809. doi:10.1016/j.dib.2018.05.108
Azeiteiro, U. M., Bacelar-Nicolau, P., Caetano, F. J., and Caeiro, S. (2015). Education for sustainable development through e-learning in higher education: Experiences from Portugal. J. Clean. Prod. 106, 308–319. doi:10.1016/j.jclepro.2014.11.056
Baig, N., Chauhan, D. S., Saleh, T. A., and Quraishi, M. A. (2019). Diethylenetriamine functionalized graphene oxide as a novel corrosion inhibitor for mild steel in hydrochloric acid solutions. New J. Chem. 43 (5), 2328–2337. doi:10.1039/c8nj04771e
Bammou, L., Belkhaouda, M., Salghi, R., Benali, O., Zarrouk, A., Zarrok, H., et al. (2014). Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium Ambrosioides Extracts. J. Assoc. Arab Univ. Basic Appl. Sci. 16, 83–90. doi:10.1016/j.jaubas.2013.11.001
Bedairi, B., Cronin, D., Hosseini, A., and Plumtree, A. (2012). Failure prediction for Crack-in-Corrosion defects in natural gas transmission pipelines. Int. J. Press. vessels Pip. 96, 90–99. doi:10.1016/j.ijpvp.2012.06.002
Biomorgi, J., Hernandez, S., Marin, J., Rodriguez, E., Lara, M., and Viloria, A. (2012). Internal corrosion studies in hydrocarbons production pipelines located at Venezuelan Northeastern. Chem. Eng. Res. Des. 90 (9), 1159–1167. doi:10.1016/j.cherd.2011.12.013
Boumhara, K., Bentiss, F., Tabyaoui, M. H., Costa, J., Desjobert, J. M., Bellaouchou, A., et al. (2014). Use of Artemisia mesatlantica essential oil as green corrosion inhibitor for mild steel in 1 M hydrochloric acid solution. Int. J. Electrochem. Sci. 9, 1187–1206.
Brown, G. K. (2014). Appendix–internal corrosion monitoring techniques for pipeline systems. Pipeline integrity handbook. Boston: Gulf Professional Publishing, 255–292.
Buchweishaija, J. (2009). Phytochemicals as green corrosion inhibitors in various corrosive media: A review. Tanzan. J. Sci. 35. doi:10.4314/tjs.v29i1.18370
Chadli, R., Elherri, A., Elmsellem, H., Elazzouzi, M., Merad, N., Aouniti, A., et al. (2017). Synthesis of aza-pseudopeptides and the evaluation of their inhibiting efficacy of mild steel corrosion in 1.0 M HCl. Prot. Metals Phys. Chem. Surfaces 53 (5), 928–936. doi:10.1134/s2070205117050033
Cheng, Y. F. (2013). Stress corrosion cracking of pipelines. Hoboken, New Jersey: John Wiley & Sons.
Chetouani, A., and Hammouti, B. (2014). Salvia officinalis essential oil and the extract as green corrosion inhibitor of mild steel in hydrochloric acid. J. Chem. Pharm. Res. 6 (7), 1401–1416.
Chung, I.-M., Kim, S. H., Hemapriya, V., Kalaiselvi, K., and Prabakaran, M. (2019). Inhibition behavior of Tragia involucrata L. phenolic compounds against acidic medium corrosion in low carbon steel surface. Chin. J. Chem. Eng. 27 (3), 717–725. doi:10.1016/j.cjche.2018.10.008
Cruz-Zabalegui, A., Vazquez-Velez, E., Galicia-Aguilar, G., Casales-Diaz, M., Lopez-Sesenes, R., Gonzalez-Rodriguez, J., et al. (2019). Use of a non-ionic gemini-surfactant synthesized from the wasted avocado oil as a CO2-corrosion inhibitor for X-52 steel. Industrial Crops Prod. 133, 203–211. doi:10.1016/j.indcrop.2019.03.011
Da Rocha, J. C., Gomes, J. A. d. C. P., and D’Elia, E. (2010). Corrosion inhibition of carbon steel in hydrochloric acid solution by fruit peel aqueous extracts. Corros. Sci. 52 (7), 2341–2348. doi:10.1016/j.corsci.2010.03.033
Davoodi, A., Pakshir, M., Babaiee, M., and Ebrahimi, G. (2011). A comparative H2S corrosion study of 304L and 316L stainless steels in acidic media. Corros. Sci. 53 (1), 399–408. doi:10.1016/j.corsci.2010.09.050
Dehghani, A., Bahlakeh, G., and Ramezanzadeh, B. (2019). A detailed electrochemical/theoretical exploration of the aqueous Chinese gooseberry fruit shell extract as a green and cheap corrosion inhibitor for mild steel in acidic solution. J. Mol. Liq. 282, 366–384. doi:10.1016/j.molliq.2019.03.011
Dehghani, A., Bahlakeh, G., Ramezanzadeh, B., and Ramezanzadeh, M. (2019). A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 279, 603–624. doi:10.1016/j.molliq.2019.02.010
Dehghani, A., Bahlakeh, G., Ramezanzadeh, B., and Ramezanzadeh, M. (2019). Electronic/atomic level fundamental theoretical evaluations combined with electrochemical/surface examinations of Tamarindus indiaca aqueous extract as a new green inhibitor for mild steel in acidic solution (HCl 1 M). J. Taiwan Inst. Chem. Eng. 102, 349–377. doi:10.1016/j.jtice.2019.05.006
Eduok, U. M., Umoren, S. A., and Udoh, A. P. (2012). Synergistic inhibition effects between leaves and stem extracts of Sida acuta and iodide ion for mild steel corrosion in 1 M H2SO4 solutions. Arabian J. Chem. 5 (3), 325–337. doi:10.1016/j.arabjc.2010.09.006
El Ibrahimi, B., Jmiai, A., Bazzi, L., and El Issami, S. (2020). Amino acids and their derivatives as corrosion inhibitors for metals and alloys. Arabian J. Chem. 13 (1), 740–771. doi:10.1016/j.arabjc.2017.07.013
Eliyan, F. F., Mahdi, E., Farhat, Z., and Alfantazi, A. (2013). Interpreting the passivation of HSLA steel from electrochemical corrosion investigations in bicarbonate-oil aqueous emulsions. Int. J. Electrochem. Sci. 8, 3026–3038.
Faraj, L., and Khan, G. M. (2015). Application of natural product extracts as green corrosion inhibitors for metals and alloys in acid pickling processes-A. Int. J. Electrochem. Sci. 10, 6120–6134.
Fawzy, A., Abdallah, M., Zaafarany, I., Ahmed, S., and Althagafi, I. (2018). Thermodynamic, kinetic and mechanistic approach to the corrosion inhibition of carbon steel by new synthesized amino acids-based surfactants as green inhibitors in neutral and alkaline aqueous media. J. Mol. Liq. 265, 276–291. doi:10.1016/j.molliq.2018.05.140
Fiori-Bimbi, M. V., Alvarez, P. E., Vaca, H., and Gervasi, C. A. (2015). Corrosion inhibition of mild steel in HCL solution by pectin. Corros. Sci. 92, 192–199. doi:10.1016/j.corsci.2014.12.002
Fouda, A. S., El Morsi, M. A., and El Mogy, T. (2017). Studies on the inhibition of carbon steel corrosion in hydrochloric acid solution by expired Carvedilol drug. Green Chem. Lett. Rev. 10 (4), 336–345. doi:10.1080/17518253.2017.1380236
Gapsari, F., Madurani, K. A., Simanjuntak, F. M., Andoko, A., Wijaya, H., and Kurniawan, F. (2019). Corrosion inhibition of honeycomb waste extracts for 304 stainless steel in sulfuric acid solution. Materials 12 (13), 2120. doi:10.3390/ma12132120
Gece, G., and Bilgiç, S. (2010). A theoretical study on the inhibition efficiencies of some amino acids as corrosion inhibitors of nickel. Corros. Sci. 52 (10), 3435–3443. doi:10.1016/j.corsci.2010.06.015
Gece, G. (2011). Drugs: A review of promising novel corrosion inhibitors. Corros. Sci. 53 (12), 3873–3898. doi:10.1016/j.corsci.2011.08.006
Gerengi, H., Uygur, I., Solomon, M., Yildiz, M., and Goksu, H. (2016). Evaluation of the inhibitive effect of Diospyros kaki (Persimmon) leaves extract on St37 steel corrosion in acid medium. Sustain. Chem. Pharm. 4, 57–66. doi:10.1016/j.scp.2016.10.003
Ghareba, S., and Omanovic, S. (2010). Interaction of 12-aminododecanoic acid with a carbon steel surface: Towards the development of ‘green’corrosion inhibitors. Corros. Sci. 52 (6), 2104–2113. doi:10.1016/j.corsci.2010.02.019
Gong, W., Xu, B., Yin, X., Liu, Y., Chen, Y., and Yang, W. (2019). Halogen-substituted thiazole derivatives as corrosion inhibitors for mild steel in 0.5 M sulfuric acid at high temperature. J. Taiwan Inst. Chem. Eng. 97, 466–479. doi:10.1016/j.jtice.2019.02.018
Gong, W., Yin, X., Liu, Y., Chen, Y., and Yang, W. (2019). 2-Amino-4-(4-methoxyphenyl)-thiazole as a novel corrosion inhibitor for mild steel in acidic medium. Prog. Org. Coatings 126, 150–161. doi:10.1016/j.porgcoat.2018.10.001
Grassino, A. N., Halambek, J., Djakovic, S., Rimac Brncic, S., Dent, M., and Grabaric, Z. (2016). Utilization of tomato peel waste from canning factory as a potential source for pectin production and application as tin corrosion inhibitor. Food Hydrocoll. 52, 265–274. doi:10.1016/j.foodhyd.2015.06.020
Guo, B., Song, S., Ghalambor, A., Lin, T., and Chacko, J. (2005). Offshore pipelines. Amsterdam, Netherlands: Elsevier.
Haldhar, R., Prasad, D., Saxena, A., and Singh, P. (2018). Valeriana wallichii root extract as a green & sustainable corrosion inhibitor for mild steel in acidic environments: Experimental and theoretical study. Mater. Chem. Front. 2 (6), 1225–1237. doi:10.1039/c8qm00120k
Hasan, S. K., and Sisodia, P. (2011). Paniala (Flacourtia Jangomas) plant extract as eco friendly inhibitor on the corrosion of mild steel in acidic media. Rasayan J. Chem. 4 (3), 548–553.
Hechiche, N., Kadri, A., and Boughrara, D. (2019). Artemisia herba alba essential oil as green corrosion inhibitor for aluminum in hydrochloric acid solution. Anal. Bioanal. Electrochem. 11, 1129–1147.
Hernandez-Rodriguez, M. A. L., Martinez-Delgado, D., Gonzalez, R., Perez Unzueta, A., Mercado-Solis, R., and Rodriguez, J. (2007). Corrosive wear failure analysis in a natural gas pipeline. Wear 263 (1-6), 567–571. doi:10.1016/j.wear.2007.01.123
Hussein, M. H. M., El-Hady, M. F., Shehata, H. A. H., Hegazy, M. A., and Hefni, H. H. H. (2013). Preparation of some eco-friendly corrosion inhibitors having antibacterial activity from sea food waste. J. surfactants Deterg. 16 (2), 233–242. doi:10.1007/s11743-012-1395-3
Ilman, M. N. (2014). Analysis of internal corrosion in subsea oil pipeline. Case Stud. Eng. Fail. Analysis 2 (1), 1–8. doi:10.1016/j.csefa.2013.12.003
Khaksarfard, R., Paraschivoiu, M., Zhu, Z., Tajallipour, N., and Teevens, P. J. (2013). CFD based analysis of multiphase flows in bends of large diameter pipelines. In CORROSION 2013.
Kiefner, J. F., and Kolovich, K. M. (2007). Calculation of a corrosion rate using Monte Carlo simulation. OnePetro. www.onepetro.org.
Kowsari, E., Arman, S., Shahini, M., Zandi, H., Ehsani, A., Naderi, R., et al. (2016). In situ synthesis, electrochemical and quantum chemical analysis of an amino acid-derived ionic liquid inhibitor for corrosion protection of mild steel in 1M HCl solution. Corros. Sci. 112, 73–85. doi:10.1016/j.corsci.2016.07.015
Kumar, C. B. P., and Mohana, K. N. (2013). The effect of Achyranthes aspera extracts on mild steel corrosion in industrial water medium. Int. Sch. Res. Notices 2013 (4), 261847. doi:10.1155/2013/261847
Kumar, K. P. V., Pillai, M. S. N., and Thusnavis, G. R. (2011). Seed extract of Psidium guajava as ecofriendly corrosion inhibitor for carbon steel in hydrochloric acid medium. J. Mater. Sci. Technol. 27 (12), 1143–1149. doi:10.1016/s1005-0302(12)60010-3
Kurniawan, F., and Madurani, K. A. (2015). Electrochemical and optical microscopy study of red pepper seed oil corrosion inhibition by self-assembled monolayers (SAM) on 304 SS. Prog. Org. Coatings 88, 256–262. doi:10.1016/j.porgcoat.2015.07.010
Lahhit, N., Bouyanzer, A., Desjobert, J. M., Hammouti, B., Salghi, R., Costa, J., et al. (2011). Fennel (Foeniculum vulgare) essential oil as green corrosion inhibitor of carbon steel in hydrochloric acid solution. Port. Electrochimica Acta 29 (2), 127–138. doi:10.4152/pea.201102127
Li, D.-p., Zhang, L., Yang, J. w., Lu, M. x., Ding, J. h., and Liu, M. l. (2014). Effect of H2S concentration on the corrosion behavior of pipeline steel under the coexistence of H2S and CO2. Int. J. Minerals, Metallurgy, Mater. 21 (4), 388–394. doi:10.1007/s12613-014-0920-y
Li, Y., Fan, J., Hu, Z., Shao, Q., Zhang, L., and Yu, H. (2015). Influence of land use patterns on evapotranspiration and its components in a temperate grassland ecosystem. Adv. Meteorology 2015, 1–12. doi:10.1155/2015/452603
Liang, Y., Wang, C., Li, J., and Wang, L. (2015). The penicillin derivatives as corrosion inhibitors for mild steel in hydrochloric acid solution: Experimental and theoretical studies. Int. J. Electrochem. Sci. 10, 8072–8086.
Liao, L. L., Mo, S., Luo, H. Q., and Li, N. B. (2018). Corrosion protection for mild steel by extract from the waste of lychee fruit in HCl solution: Experimental and theoretical studies. J. colloid interface Sci. 520, 41–49. doi:10.1016/j.jcis.2018.02.071
Loto, R. T. (2019). Corrosion inhibition effect of non-toxic α-amino acid compound on high carbon steel in low molar concentration of hydrochloric acid. J. Mater. Res. Technol. 8 (1), 484–493. doi:10.1016/j.jmrt.2017.09.005
Macedo, R. G. M. d. A., Marques, N. d. N., Paulucci, L. C., Cunha, J. V. M., Villetti, M. A., Castro, B. B., et al. (2019). Water-soluble carboxymethylchitosan as green scale inhibitor in oil wells. Carbohydr. Polym. 215, 137–142. doi:10.1016/j.carbpol.2019.03.082
Machado, C., Ferreira Fagundes, T. d. S., Escarpini dos Santos, N., Shewry de M. Rocha, T., Garrett, R., Borges, R. M., et al. (2019). Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. Electrochim. Acta 312, 137–148. doi:10.1016/j.electacta.2019.04.148
Marc, A. M. (2013). Corrosion growth models and ILI-based estimation procedures for reliability-based and deterministic pipeline integrity assessments. Houston, TX, United States: Pipeline Research Council International.
Marsoul, A., Ijjaali, M., Elhajjaji, F., Taleb, M., Salim, R., and Boukir, A. (2020). Phytochemical screening, total phenolic and flavonoid methanolic extract of pomegranate bark (Punica granatum L): Evaluation of the inhibitory effect in acidic medium 1 M HCl. Mater. Today Proc. 27, 3193–3198. doi:10.1016/j.matpr.2020.04.202
Mazumder, Q. H., Shirazi, S. A., and McLaury, B. (2008). Experimental investigation of the location of maximum erosive wear damage in elbows. J. Press. Vessel Technol. 130 (1). doi:10.1115/1.2826426
Mobin, M., Basik, M., and El Aoufir, Y. (2019). Corrosion mitigation of mild steel in acidic medium using lagerstroemia speciosa leaf extract: A combined experimental and theoretical approach. J. Mol. Liq. 286, 110890. doi:10.1016/j.molliq.2019.110890
Mobin, M., Basik, M., and Shoeb, M. (2019). A novel organic-inorganic hybrid complex based on Cissus quadrangularis plant extract and zirconium acetate as a green inhibitor for mild steel in 1 M HCl solution. Appl. Surf. Sci. 469, 387–403. doi:10.1016/j.apsusc.2018.11.008
Mobin, M., and Rizvi, M. (2017). Polysaccharide from Plantago as a green corrosion inhibitor for carbon steel in 1 M HCl solution. Carbohydr. Polym. 160, 172–183. doi:10.1016/j.carbpol.2016.12.056
Montoya, L. F., Contreras, D., Jaramillo, A., Carrasco, C., Fernandez, K., Schwederski, B., et al. (2019). Study of anticorrosive coatings based on high and low molecular weight polyphenols extracted from the Pine radiata bark. Prog. Org. Coatings 127, 100–109. doi:10.1016/j.porgcoat.2018.11.010
Munawaroh, H. S. H., Sunarya, Y., Anwar, B., Priatna, E., Risa, H., Koyande, A. K., et al. (2022). Protoporphyrin extracted from biomass waste as sustainable corrosion inhibitors of T22 carbon steel in acidic environments. Sustainability 14 (6), 3622. doi:10.3390/su14063622
Nair, R. N. (2017). Green Corrosion Inhibitors for mild steel in acidic medium. Int. J. Mod. trends Eng. Res. 4 (12), 216–221.
Naseri, E., Hajisafari, M., Kosari, A., Talari, M., Hosseinpour, S., and Davoodi, A. (2018). Inhibitive effect of Clopidogrel as a green corrosion inhibitor for mild steel; statistical modeling and quantum Monte Carlo simulation studies. J. Mol. Liq. 269, 193–202. doi:10.1016/j.molliq.2018.08.050
Ninčević Grassino, A., Djakovic, S., Bosiljkov, T., Halambek, J., Zoric, Z., Dragovic-Uzelac, V., et al. (2020). Valorisation of tomato peel waste as a sustainable source for pectin, polyphenols and fatty acids recovery using sequential extraction. Waste Biomass Valorization 11 (9), 4593–4611. doi:10.1007/s12649-019-00814-7
Oguntuyi, S. D., Johnson, O. T., and Shongwe, M. B. (2021). Spark plasma sintering of ceramic matrix composite of TiC: Microstructure, densification, and mechanical properties: A review. Int. J. Adv. Manuf. Technol. 116, 69–82. doi:10.1007/s00170-021-07471-y
Oguntuyi, S. D., Johnson, O. T., Shongwe, M. B., Jeje, S. O., and Rominiyi, A. L. (2021). The effects of sintering additives on the ceramic matrix composite of ZrO2: Microstructure, densification, and mechanical properties–a review. Adv. Appl. Ceram. 120 (5-8), 319–335. doi:10.1080/17436753.2021.1953845
Oguntuyi, S. D., Johnson, O. T., and Shongwe, M. B. (2021). Spark plasma sintering of ceramic matrix composite of ZrB2 and TiB2: Microstructure, densification, and mechanical properties—a review. Metals Mater. Int. 27 (7), 2146–2159. doi:10.1007/s12540-020-00874-8
Oguntuyi, S. D., Malatji, N., Shongwe, M., Johnson, O., Khoathane, C., and Tshabalala, L. (2022). The influence of Si3N4 on the microstructure, mechanical properties and the wear performance of TiB2–SiC synthesized via spark plasma sintering. Int. J. Lightweight Mater. Manuf. 5 (3), 326–338. doi:10.1016/j.ijlmm.2022.04.004
Oguntuyi, S. D., Shongwe, M. B., Tshabalala, L., Johnson, O. T., and Malatji, N. (2022). Effects of SiC on the microstructure, densification, hardness and wear performance of TiB2 ceramic matrix composite consolidated via spark plasma sintering. Arabian J. Sci. Eng. 2022, 1–15. doi:10.1007/s13369-022-07026-7
Oguzie, E. E. (2007). Corrosion inhibition of aluminium in acidic and alkaline media by Sansevieria trifasciata extract. Corros. Sci. 49 (3), 1527–1539. doi:10.1016/j.corsci.2006.08.009
Okafor, P. C., and Ebenso, E. E. (2007). Inhibitive action of Carica papaya extracts on the corrosion of mild steel in acidic media and their adsorption characteristics. Pigment Resin Technol. 36, 134–140. doi:10.1108/03699420710748992
Okafor, P. C., Ikpi, M., Uwah, I., Ebenso, E., Ekpe, U., and Umoren, S. (2008). Inhibitory action of Phyllanthus amarus extracts on the corrosion of mild steel in acidic media. Corros. Sci. 50 (8), 2310–2317. doi:10.1016/j.corsci.2008.05.009
Okewale, A. O., and Olaitan, A. (2017). The use of rubber leaf extract as a corrosion inhibitor for mild steel in acidic solution. Int. J. Mater. Chem. 7 (1), 5–13.
Ossai, C. I., Boswell, B., and Davies, I. J. (2015). Pipeline failures in corrosive environments–A conceptual analysis of trends and effects. Eng. Fail. Anal. 53, 36–58. doi:10.1016/j.engfailanal.2015.03.004
Pal, S., Lgaz, H., Tiwari, P., Chung, I. M., Ji, G., and Prakash, R. (2019). Experimental and theoretical investigation of aqueous and methanolic extracts of Prunus dulcis peels as green corrosion inhibitors of mild steel in aggressive chloride media. J. Mol. Liq. 276, 347–361. doi:10.1016/j.molliq.2018.11.099
Papavinasam, S., Doiron, A., and Revie, R. W. (2010). Model to predict internal pitting corrosion of oil and gas pipelines. Corrosion 66 (3), 35006–35011. doi:10.5006/1.3359623
Parthipan, P., Sabarinathan, D., Angaiah, S., and Rajasekar, A. (2018). Glycolipid biosurfactant as an eco-friendly microbial inhibitor for the corrosion of carbon steel in vulnerable corrosive bacterial strains. J. Mol. Liq. 261, 473–479. doi:10.1016/j.molliq.2018.04.045
Popoola, L. T. (2019). Organic green corrosion inhibitors (OGCIs): A critical review. Corros. Rev. 37 (2), 71–102. doi:10.1515/corrrev-2018-0058
Popoola, L. T. (2019). Progress on pharmaceutical drugs, plant extracts and ionic liquids as corrosion inhibitors. Heliyon 5 (2), e01143. doi:10.1016/j.heliyon.2019.e01143
Quraishi, M. A., Rafiquee, M. Z. A., Khan, S., and Saxena, N. (2007). Corrosion inhibition of aluminium in acid solutions by some imidazoline derivatives. J. Appl. Electrochem. 37 (10), 1153–1162. doi:10.1007/s10800-007-9379-0
Raja, P. B., Qureshi, A. K., Abdul Rahim, A., Osman, H., and Awang, K. (2013). Neolamarckia cadamba alkaloids as eco-friendly corrosion inhibitors for mild steel in 1 M HCl media. Corros. Sci. 69, 292–301. doi:10.1016/j.corsci.2012.11.042
Raja, P. B., and Sethuraman, M. G. (2008). Inhibitive effect of black pepper extract on the sulphuric acid corrosion of mild steel. Mater. Lett. 62 (17-18), 2977–2979. doi:10.1016/j.matlet.2008.01.087
Rani, B. E., and Basu, B. B. J. (2012). Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012, 1–15. doi:10.1155/2012/380217
Rathi, P., Trikha, S., and Kumar, S. (2017). Plant extracts as green corrosion inhibitors in various corrosive media-a review. World J. Pharm. Pharm. Sci. 6 (4), 482–514. doi:10.20959/wjpps20174-8903
Rodrigues, L. S., do Valle, A. F., and D’Elia, E. (2018). Biomass of microalgae spirulina maxima as a corrosion inhibitor for 1020 carbon steel in acidic solution. Int. J. Electrochem. Sci. 13, 6169–6189. doi:10.20964/2018.07.11
Sanaei, Z., Ramezanzadeh, M., Bahlakeh, G., and Ramezanzadeh, B. (2019). Use of rosa canina fruit extract as a green corrosion inhibitor for mild steel in 1 M HCl solution: A complementary experimental, molecular dynamics and quantum mechanics investigation. J. industrial Eng. Chem. 69, 18–31. doi:10.1016/j.jiec.2018.09.013
Sanni, O., Popoola, A. P. I., and Fayomi, O. S. I. (2018). Enhanced corrosion resistance of stainless steel type 316 in sulphuric acid solution using eco-friendly waste product. Results Phys. 9, 225–230. doi:10.1016/j.rinp.2018.02.001
Shongwe, M. B. (2020). Spark plasma sintering of ceramic matrix composite of TiC: Microstructure, densification, and mechanical properties: A review. Adv. Material Sci. Eng. Sel. Articles ICMMPE 2020, 93.
Shukla, S. K., and Quraishi, M. A. (2009). 4-Substituted anilinomethylpropionate: New and efficient corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 51 (9), 1990–1997. doi:10.1016/j.corsci.2009.05.020
Singh, A., Adrianzen Herrera, D., Zhang, Y., Perez-Soler, R., and Cheng, H. (2018). Mouse models in squamous cell lung cancer: Impact for drug discovery. J. Alloys Compd. 762, 347–358. doi:10.1080/17460441.2018.1437137
Singh, A. K., Pandey, A. K., Banerjee, P., Saha, S. K., Chugh, B., Thakur, S., et al. (2019). Eco-friendly disposal of expired anti-tuberculosis drug isoniazid and its role in the protection of metal. J. Environ. Chem. Eng. 7 (2), 102971. doi:10.1016/j.jece.2019.102971
Singh, A., Kumar, A., and Pramanik, T. (2013). A theoretical approach to the study of some plant extracts as green corrosion inhibitor for mild steel in HCl solution. Orient. J. Chem. 29 (1), 277–283. doi:10.13005/ojc/290144
Singh, P., Chauhan, D., Chauhan, S., Singh, G., and Quraishi, M. (2019). Chemically modified expired Dapsone drug as environmentally benign corrosion inhibitor for mild steel in sulphuric acid useful for industrial pickling process. J. Mol. Liq. 286, 110903. doi:10.1016/j.molliq.2019.110903
Srivastava, V., Haque, J., Verma, C., Singh, P., Lgaz, H., Salghi, R., et al. (2017). Amino acid based imidazolium zwitterions as novel and green corrosion inhibitors for mild steel: Experimental, DFT and MD studies. J. Mol. Liq. 244, 340–352. doi:10.1016/j.molliq.2017.08.049
Subasree, N., and Selvi, J. A. (2020). Imidazolium based ionic liquid derivatives; synthesis and evaluation of inhibitory effect on mild steel corrosion in hydrochloric acid solution. Heliyon 6 (2), e03498. doi:10.1016/j.heliyon.2020.e03498
Talari, M., Mozafari Nezhad, S., Alavi, S. J., Mohtashamipour, M., Davoodi, A., and Hosseinpour, S. (2019). Experimental and computational chemistry studies of two imidazole-based compounds as corrosion inhibitors for mild steel in HCl solution. J. Mol. Liq. 286, 110915. doi:10.1016/j.molliq.2019.110915
Tawancy, H. M., Al-Hadhrami, L. M., and Al-Yousef, F. K. (2013). Analysis of corroded elbow section of carbon steel piping system of an oil–gas separator vessel. Case Stud. Eng. Fail. Analysis 1 (1), 6–14. doi:10.1016/j.csefa.2012.11.001
Tezeghdenti, M., Dhouibi, L., and Etteyeb, N. (2015). Corrosion inhibition of carbon steel in 1 M sulphuric acid solution by extract of eucalyptus globulus leaves cultivated in Tunisia arid zones. J. Bio-and Tribo-Corrosion 1 (3), 16–19. doi:10.1007/s40735-015-0016-x
Thakur, A., Kaya, S., Abousalem, A., Sharma, S., Ganjoo, R., Assad, H., et al. (2022). Computational and experimental studies on the corrosion inhibition performance of an aerial extract of Cnicus Benedictus weed on the acidic corrosion of mild steel. Process Saf. Environ. Prot. 161, 801–818. doi:10.1016/j.psep.2022.03.082
Thakur, A., Kaya, S., Abousalem, A. S., and Kumar, A. (2022). Experimental, DFT and MC simulation analysis of Vicia Sativa weed aerial extract as sustainable and eco-benign corrosion inhibitor for mild steel in acidic environment. Sustain. Chem. Pharm. 29, 100785. doi:10.1016/j.scp.2022.100785
Tiwari, P., Srivastava, M., Mishra, R., Ji, G., and Prakash, R. (2018). Economic use of waste Musa paradisica peels for effective control of mild steel loss in aggressive acid solutions. J. Environ. Chem. Eng. 6 (4), 4773–4783. doi:10.1016/j.jece.2018.07.016
Vaszilcsin, N., Ordodi, V., and Borza, A. (2012). Corrosion inhibitors from expired drugs. Int. J. Pharm. 431 (1-2), 241–244. doi:10.1016/j.ijpharm.2012.04.015
Velázquez-González, M. A., Gonzalez-Rodriguez, J. G., Valladares-Cisneros, M. G., and Hermoso-Diaz, I. A. (2014). Use of Rosmarinus officinalis as green corrosion inhibitor for carbon steel in acid medium. Am. J. Anal. Chem. 2014.
Vengatesh, G., Karthik, G., and Sundaravadivelu, M. (2017). A comprehensive study of ondansetron hydrochloride drug as a green corrosion inhibitor for mild steel in 1 M HCl medium. Egypt. J. petroleum 26 (3), 705–719. doi:10.1016/j.ejpe.2016.10.011
Verma, C. B., and Ma, Q. (2014). Schiff’s bases of glutamic acid and aldehydes as green corrosion inhibitor for mild steel: Weight-loss, electrochemical and surface analysis. Int. J. Innov. Res. Sci. Eng. Technol. 3, 14601–14613.
Verma, C., Ebenso, E. E., Bahadur, I., and Quraishi, M. (2018). An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. J. Mol. Liq. 266, 577–590. doi:10.1016/j.molliq.2018.06.110
Villamizar, W., Casales, M., Gonzales-Rodriguez, J. G., and Martinez, L. (2006). An EIS study of the effect of the pedant group in imidazolines as corrosion inhibitors for carbon steel in CO2 environments. Mater. Corros. 57 (9), 696–704. doi:10.1002/maco.200503957
Wei, H., Heidarshenas, B., Zhou, L., Hussain, G., Li, Q., and Ostrikov, K. K. (2020). Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 10, 100044. doi:10.1016/j.mtsust.2020.100044
Yin, Z. F., Zhao, W., Bai, Z., Feng, Y., and Zhou, W. (2008). Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2. Electrochimica Acta 53 (10), 3690–3700. doi:10.1016/j.electacta.2007.12.039
Zhang, K., Yang, W., Yin, X., Chen, Y., Liu, Y., Le, J., et al. (2018). Amino acids modified konjac glucomannan as green corrosion inhibitors for mild steel in HCl solution. Carbohydr. Polym. 181, 191–199. doi:10.1016/j.carbpol.2017.10.069
Zhang, T., Yang, Y., Shao, Y., Meng, G., and Wang, F. (2009). A stochastic analysis of the effect of hydrostatic pressure on the pit corrosion of Fe–20Cr alloy. Electrochimica Acta 54 (15), 3915–3922. doi:10.1016/j.electacta.2009.02.010
Keywords: corrosion, green inhibitors, environmentally friendly, sustainability, pipeline steel, adsorption
Citation: Alao AO, Popoola AP, Dada MO and Sanni O (2023) Utilization of green inhibitors as a sustainable corrosion control method for steel in petrochemical industries: A review. Front. Energy Res. 10:1063315. doi: 10.3389/fenrg.2022.1063315
Received: 06 October 2022; Accepted: 09 December 2022;
Published: 09 January 2023.
Edited by:
Chandrabhan Verma, King Fahd University of Petroleum and Minerals, Saudi ArabiaReviewed by:
Ashish Kumar, Government of Bihar, IndiaCopyright © 2023 Alao, Popoola, Dada and Sanni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alice Osheiza Alao, c3dlZXRkYW1zZWwwOEBnbWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.