- State Key Laboratory Breeding Base of Green Pesticide and Agricultural Bioengineering, Key Laboratory of Green Pesticide and Agricultural Bioengineering, Ministry of Education, State-Local Joint Laboratory for Comprehensive Utilization of Biomass, Center for R&D of Fine Chemicals, Guizhou University, Guiyang, China
Increasing fossil fuels consumption and global warming have driven the global revolution towards renewable energy sources. Lignocellulosic biomass is the main source of renewable carbon-based fuels. The abundant intermolecular linkages and high oxygen content between cellulose, hemicellulose, and lignin limit the use of traditional fuels. Therefore, it is a promising strategy to break the above linkages and remove oxygen by selective catalytic cracking of C–O bond to further transform the main components of biomass into small molecular products. This mini-review discusses the significance of selectivity control in C–O bond cleavage with well-tailored catalytic systems or strategies for furnishing biofuels and value-added chemicals of high efficiency from lignocellulosic biomass. The current challenges and future opportunities of converting lignocellulose biomass into high-value chemicals are also summarized and analyzed.
Introduction
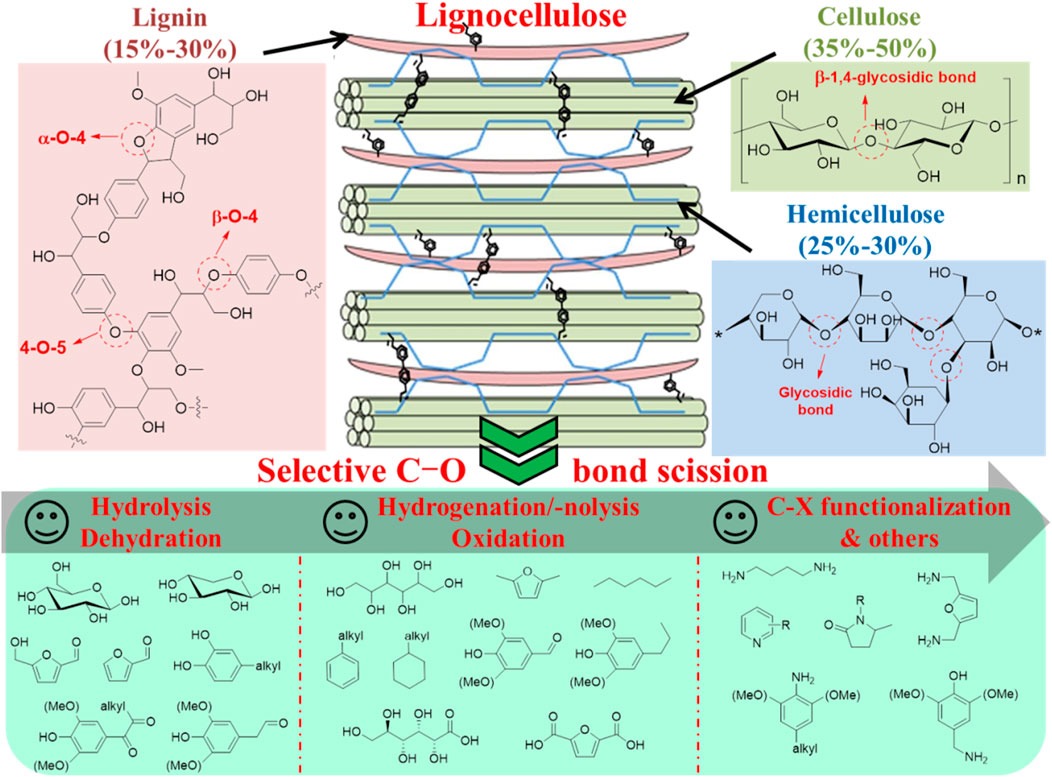
With the over-exploitation and utilization of non-renewable fossil fuels in the conventional chemical industry, the concomitant issues like energy depletion and environmental contamination stimulate the human to explore and develop renewable sources (Li et al., 2017; Wu H. et al., 2020). Lignocellulosic biomass is the most abundant organic carbon source on earth, with great potential to reduce the global reliance on fossil fuels by catalytic upgrading to obtain renewable energy, high-value chemicals, and functional materials (Liu et al., 2019; Wang et al., 2020). However, the oxygen content of virgin biomass is quite high (ca. 40%), mainly existing in the form of cellulose (35–50%), hemicellulose (25–30%), lignin (15–30%), and other extractives (1–5%), which limits its use as traditional fuels (Schutyser et al., 2018). To make better use of lignocellulosic biomass as energy, it is necessary to reduce the oxygen content not only by using the existing infrastructure, but by maximizing energy return (Deng et al., 2015a; Shivhare et al., 2021). To achieve this goal, it is necessary to develop a strategy for selectively controlling the C–O bond scission in biomass to remove oxygen (Krishna et al., 2018). Therefore, exploring new and advanced catalytic systems for selective catalytic cleavage of C–O bonds from lignocellulose biomass under mild conditions has attracted great interest in various fields.
Cellulose is a macromolecular polymer of d-glucose, in which a single glucose unit is connected by β-1,4-glycosidic (C–O–C) bonds (Deng et al., 2015a). This connection causes the glucose units to be arranged side by side in a chain-like manner, resulting in a strong intramolecular hydrogen bond interaction between the hydroxyl groups near the sugar bond, which makes the crystal structure of cellulose very robust (Rinaldi and Schuth, 2009). Therefore, the glycosidic bond of cellulose is also difficult to access by a catalytic site for C–O bond cleavage. Compared with cellulose, hemicellulose is a polysaccharide composed of different sugar units (e.g., glucose, xylose, arabinose, and galactose). Although these basic sugar units are also linked by glycosidic bonds, the overall heterogeneity of hemicellulose structure makes it a highly branched polymer, which makes the crystallinity of hemicellulose much lower than that of cellulose (Maki-Arvela et al., 2011). Lignin is a three-dimensional amorphous and complex aromatic polymer mainly composed of phenylpropanoids (Delgado-Aguilar et al., 2016). The three main monomers in lignin have been identified as p-coumaric acid, coniferous alcohol, and mustard alcohol (Jampa et al., 2019). Different monomers are connected by various C–O (e.g., α-O–4, β-O–4 and 4-O–5) and C–C bonds (Figure 1) (Wu et al., 2021). Among these bonds, the β-O–4 bond is dominant (Enright et al., 2019; Huang et al., 2020).
In view of the different composition and structure of cellulose, hemicellulose, and lignin, it is of great significance to develop an efficient catalytic strategy for selectively converting each component into value-added chemicals, and the cleavage of C–O bonds is a common and important step in the transformation process to release their potential and value-added components (Deng et al., 2015a; Iravani and Varma, 2020). Acid-catalyzed hydrolysis of cellulose is an effective strategy for decomposing its glycosidic (C–O) bond to produce glucose as a primary product, and the further cleavage of glycosidic bonds can be converted into alkyl glucoside, gluconic acid, or hexanol (Li et al., 2018). Compared with cellulose, the glycosidic bond catalytic cracking of hemicellulose is easier to generate monosaccharides (e.g., xylose and arabinose), which is due to the higher reactivity of polysaccharides (Zakzeski et al., 2010). Lignin can decompose its C–O bond by hydrolysis and hydrogenolysis to obtain phenols and a series of other aromatic compounds (Subbotina et al., 2021). However, the selective and efficient cleavage of C–O bonds in lignocellulose biomass is still a major challenge, and the realization of this transformation is crucial for the bio-renewable industry at present.
In the past few years, some excellent reviews have also discussed the selective control of C–O bond cleavage in lignocellulosic biomass from the aspects of different reaction types using various catalysts (Lohr et al., 2016; Yoo et al., 2020). This mini-review summarizes the various high-value biological products available through the highly selective control cleavage pathways of C–O bonds. In addition, different conversion methods of cellulose, hemicellulose, lignin, and their model compounds controlled by solvents, catalysts, and temperature were discussed, and the challenges faced by selective C–O bond cleavage in biomass upgrading were also summarized.
The C-O Bond Scission Routes to High-Value Bioproducts
The composition and structure of cellulose, hemicellulose, and lignin are different. The intermolecular linkages among these three components are complicated and strong, in which cellulose is covered by the lignin shell (biomass outer cell wall) while hemicellulose is located around cellulose (Figure 1) (Rinaldi and Schuth, 2009; Laurichesse and Avérous, 2014). Ether bond, the most abundant intermolecular C–O linkages, exists between side-chain Cα of lignin and C6 of cellulose or C of hemicellulose (Grabber, 2005). Also, Cγ and Cβ in the lignin side-chain can partially connect with cellulose via glycosidic bonding, while ester and acetal/hemiacetal bonds can be formed between the hydroxyl species in the lignin side-chain and–COOH in low quantity or free hydroxyl species of polysaccharides, respectively (Jiang et al., 2018). In addition, the three components interact with each other through hydrogen bonding (Zhang et al., 2015). In this respect, severe mass and heat transfer hindrance are unavoidable in the thermal or catalytic treatment of solid lignocellulosic materials, due to their integral and recalcitrant structure resulting from various C–O binding (e.g., ether bond, glycosidic bond, ester bond, and acetal/hemiacetal bond) and hydrogen bonding modes, as well as low miscibility with water and organic solvents (Grabber, 2005; Hosoya et al., 2007).
For the direct valorization of lignocellulosic biomass, gasification is able to produce syngas that can further undergo the Fischer-Tropsch synthesis to afford hydrocarbon fuels, while pyrolysis can be utilized to yield bio-oils in relatively low quality (Kamm, 2007; Deng et al., 2015a). Because of the relatively high temperature involved, the dominant shortages of these two thermal conversion processes are high energy consumption and low selectivity. It is thus desirable to develop mild and improved routes for the catalytic transformation of lignocellulose into terminal products in satisfactory selectivity. As a prerequisite process to undergo enhanced depolymerization of the three main components, the biomass intermolecular linkages need efficient cleavage to afford the corresponding fluids primarily via C–O bond scission (Zakzeski et al., 2010). The fractionation or pretreatment step coupled with downstream processes can further facilitate the breaking of the intramolecular linkages inside each component, and then simplify the starting materials for furnishing valuable chemicals with high selectivity (Wong et al., 2020).
Both water and organic solvents can destroy the intermolecular linkages among the biopolymeric mixtures under thermal conditions. Hydrothermal conversion of biomass under conventional, subcritical, and supercritical conditions is considered as one of the most economic and greenest processes, generally in need of relatively lower temperatures compared to pyrolysis and gasification (Xue et al., 2016; Jiang et al., 2018). There is an increase in the Kw value of H2O in the hydrothermal system, giving more active H+ and OH− ions that show enhanced capability in biomass depolymerization and hydrolysis by disrupting the intermolecular linkages of hemicellulose-cellulose and hemicellulose-lignin (Luo et al., 2017). In the organosolv process, hemicellulose and lignin can be dissolved into liquid organic solvents, while solid cellulose is recovered in high purity. The presence of acid catalysts can remarkably decrease the reaction temperature, and Brønsted and Lewis acids mainly help to break intermolecular linkages and undergo hydrolysis to yield carbohydrates-based chemicals and lignin-derived oligomers (Constant et al., 2015; Jiang et al., 2018). Base can efficiently catalyze lignin depolymerization to produce monophenols. Moreover, metal metals are beneficial for breaking inter- and intramolecular linkages in carbohydrates or lignin by hydrogenolysis using H2 or alcohols as hydrogen donor, affording polyols/alkanes or monophenols of high efficiency, respectively.
Cellulose is a crystalline macropolymer composed of glucose units linked by β-1,4-glycosidic (C-O-C) bonds, while hemicellulose in relatively lower crystallinity is a heteropolysaccharide consisting of C6 and C5 sugar units (e.g., glucose, mannose, xylose, arabinose and galactose) (Deng et al., 2015a; Deng et al., 2015b). Due to the formation of hydrogen bond network, glycosidic bonds are easier to be protonated and hydrolyzed to glucose in H2O. Acid catalysts can significantly promote the scission of glycosidic (C-O-C) bonds in cellulose to yield glucose via hydrolysis, while alkyl glucosides can be obtained in an alcohol solvent instead of H2O (Figure 1). These alkyl glycosides are more stable than glucose, which is an effective strategy to accelerate the selective activation of glycosidic bond (Almohalla et al., 2018). In addition, with breaking glycosidic bonds in combination with hydrogenation or oxidation, cellulose can be transformed into hexitols/alkanes or gluconic acid in high selectivity due to their relatively higher stability than glucose. In contrast, the glycosidic bonds of hemicellulose are more likely to cleave compared with cellulose due to the higher reactivity of the heteropolysaccharide, which can afford monosaccharides (e.g., xylose and arabinose) through dilute acid-catalyzed hydrolysis of hemicellulose (Deng et al., 2015a; Song et al., 2019). In both cases, to efficiently access the carbohydrates-derived chemicals such as furanic compounds, nitrogenous chemicals, organic acids, and polyols, well-tailored bifunctional catalysts are therefore required for realizing the occurrence of multiple reactions (Maki-Arvela et al., 2011).
Apart from sugar components, the C–O bonds (e.g., α-O-4, β-O-4 and 4-O-5 linkages) of lignin are often selectively disrupted by hydrolysis and hydrogenolysis, especially the β-O-4 bonds (representing 45–62% of the linkages in lignin), which can produce phenols via simultaneous extraction and conversion processes, but accompanying other aromatic compounds or low-molecular-mass products generated (Guo et al., 2016; Yoo et al., 2020). In addition to the one-step conversion approaches, the oxidative, reductive, and thermal depolymerization or chemical modification of lignin can initially afford platform monomeric products in the form of a complex mixture of oxygenated hydrocarbons or solid polymers, which can further undergo downstream processes such as deoxygenation, dealkylation, transalkylation, oxidation and polymerization to provide high-value chemicals, biofuels, and polymeric materials (Wong et al., 2020). Three strategies are typically adopted for lignin valorization (Figure 1) (Gazi, 2019). 1) Lignin directly undergoes gasification to syngas or pyrolysis to small molecules mixture. 2) Extensive removal of functional groups in lignin monomers gives simple aromatic compounds (e.g., benzene, toluene, xylene, and phenol) (Figure 1), followed by using subsequent commercial technology to yield bulk and fine chemicals. 3) Oriented or targeted conversion of lignin and its derivatives furnishes specific functionalized aromatic or aliphatic compounds using highly selective catalysts. Furthermore, the introduction of heteroatom (X = N, Si, Li, etc.) elements rather than C, H, and O in the lignin depolymerization processes permit the formation of C-X and O-X bond in the aromatic ring skeleton, further expanding the scope of lignin products (Li et al., 2020).
All in all, it is still challenging to control the selectivity in cleavage of specific C–O linkages together with other bonds (especially C–C linkage) on lignocellulosic biomass, so as to exclusively afford the desired bioproducts. Developing more versatile and suitable techniques for biomass conversion that destroy the targeted C–O bonds while preserving the pivotal structure (e.g., carbon chain, furanic ring, and aromatic ring) may be one of the most essential objectives.
The Challenges Associated With the Selective C–O Bond Cleavage for Biomass Upgrading
Indeed, the oriented breakage of inter- and intramolecular C–O linkages along with C–C bonding in lignocellulosic biomass is crucial for the whole conversion process, which not only affects the distribution and selectivity of products obtained by downstream processing, but also determines the subsequent treatment parameters and reaction conditions (e.g., solvent, catalyst, and temperature with the heating method) (Hu et al., 2010; Wu X. et al., 2020). A variety of fractionation approaches have been explored to initially break the specific C–O linkages among the biopolymeric mixtures, followed by selective conversion of fractionated-derivatives to either terminal products or intermediates (Jiang et al., 2018). One or two component-first strategy has been adopted on the basis of the component contents and the bonding nature, while the product selectivity is still not satisfying over designed catalysts in optimal solvents, which is hence generally faced with high separation cost, and additional investigations on the fate of disrupting the involved inter- and intramolecular bonds to guide the design of appropriate solvent systems and renewed catalysts.
To selectively break inter- and intramolecular linkages of biomass in a simultaneous or consecutive manner, multifunctional or mixture catalysts able to modulate different reactions towards the desired direction can be envisioned but greatly challenging (Zheng et al., 2020). Instead, the initial production of building monomers (primarily monosaccharides and monophenols) in high purity using industrially available processes can significantly increase the flexibility of biomass being further converted to specific value-added products (Renders et al., 2017). However, the dominant challenge is to suppress the further degradation of the resulting small molecules, considering the difficulty in switching the catalyst reactivity. With a fortunate possibility, chemical funneling and functionalization of a mixture stream derived from biomass to a single product seems a promising strategy for improving the selectivity of the overall conversion process.
The focus of biorefinery in the early 21 century is predominantly on cellulose and hemicellulose. Substantial efforts have been devoted to hydrolytic cleavage of the polysaccharide glycosidic bonds catalyzed by an acid species, including intrinsic acid, and H2O- or H2-derived Brønsted acidic sites (Hilgert et al., 2013; Zhang et al., 2014; Zhou et al., 2015). A wide range of bioproducts like monosaccharides, polyols, alkanes, organic acids, 5-hydroxymethylfurfural/furfural, and their derivatives can be attained in the presence of acid and/or metal catalysts (Luo et al., 2016; Sweygers et al., 2020). However, the leaching issue of acidic or metallic species from the solid catalysts is significant and needs to solve properly.
For lignin valorization, inter- and intramolecular C–O linkages (α-O-4, β-O-4 and 4-O-5) can be efficiently destroyed by both hydrolysis with acid or base catalysts and hydrogenolysis with metal catalysts (e.g., Pt, Pd, Rh, Ru, and Ni) (Deng et al., 2015a). The recalcitrant lignin intramolecular linkages are required to be disrupted under more rigorous reaction conditions compared to those for the intermolecular linkages. High-pressure water or organic solvents (e.g., methanol, ethanol, and THF) are efficient for the degradation of lignin fragments, especially for breaking the ether linkages (Jasiukaityte-Grojzdek et al., 2020). Nevertheless, various cross-linking side reactions (predominantly Friedel-Crafts) take place to produce larger fragments, due to the high reactivity of phenolic intermediates and products at high temperatures (Zakzeski et al., 2010). Also, the appropriate design of metal catalysts with enhanced capability to destroy the C–O bonds by hydrogenolysis while inhibiting the hydrogenation of the aromatic rings is highly challenging and in need of solution (Wu et al., 2021). Developing novel strategies like pre-modification of active groups and stabilization of in situ formed active species to obstruct the repolymerization of lignin fragments and the occurrence of over-hydrogenation is essential for comprehensive utilization of lignocellulosic biomass.
As a simple and potentially low-cost strategy, simultaneous extraction and depolymerization of lignin directly from biomass to produce monophenols can be implemented over Ni or precious metal catalysts, which is attributed to the fact that nickel or precious metal catalysts can significantly increase the activation of phenoxy and reduce the reaction barrier (Jiang and Hu, 2016). However, the co-existent sugar-derived products markedly complicate the lignin conversion process, because the accompanying degradation of carbohydrates is easier to occur under thermal conditions (Luo et al., 2014; Jiang and Hu, 2016). In addition, the cleavage temperature for cellulose intermolecular and hemicellulose intramolecular linkages is greatly overlapped, which should be thoroughly selected to impede unwanted reactions in a one-pot conversion process. Alternatively, step-wise routes are often employed for the three components in actual biomass, in which lignin or saccharides are first separated selectively, followed by catalytic degradation to produce targeted small molecules.
The development of efficient biomass conversion routes is highly correlated with the understanding of the unconverted components. Notably, a big difference in the conversion performance is generally observed between lignin model molecules and real lignin in biomass (Jiang and Hu, 2016). It is a pity that the catalytic mechanism for natural lignin depolymerization remains blurred due to its recalcitrant structure, and most current investigations focus on the catalytic materials and systems explored for upgrading of lignin-derived oligomers and model compounds that mimic the C–O linkages in lignin (Deng et al., 2015a). In connection to this, scission and functionalization of C–O bonds triggered by heteroatoms (e.g., N, S, and P) in atypical ways have been exploited as competitive routes to utilize lignocellulose despite of unsatisfactory outcome obtained in most real biopolymeric feedstocks, which needs much further improvement (Liu et al., 2018; Li et al., 2020). Moreover, it would be another economical process to prepare artificial polymeric materials from lignocellulose such as lignin and cellulose through either modification of inherent polymeric frameworks or bio-derived specific monomers. These achievements are closely to rely on the development of innovative strategies that can effectively control the cleavage of designated linkages, especially C–O bonds that are rich and ubiquitous in the connections of actual lignocellulosic biomass.
Conclusion and Perspectives
In conclusion, it is crucial to develop efficient selective pyrolysis methods of C–O bonds in cellulose, hemicellulose, lignin, or their model compounds to convert them into value-added chemicals or fuels under mild conditions. This mini-review introduces the research progress of selective cleavage of C–O bonds in cellulose and its carbohydrate derivatives and hemicellulose, as well as lignin or its model compounds. In addition, it is still challenging to control the cleavage selectivity of specific C–O bonds and other bonds (especially C–C bonds) on lignocellulose biomass, which provides a reference for the development of more general and applicable biomass conversion technologies, the destruction of target C–O bonds and the retention of key structures (e.g., carbon-chain, furanic ring, and aromatic ring).
Hydrolytic cleavage of glycosidic bonds in cellulose leads to the formation of glucose or related oligomers. The acidic catalyst has high catalytic performance and can selectively hydrolyze glycosidic bonds to obtain glucose, which is attributed to the formation of hydrogen bonds between the functional groups of the acidic catalysts and cellulose. The hydrolysis of hemicellulose in the presence of acid catalyst can lead to the cleavage of glycosidic bonds. Xylose, arabinose and other monosaccharides can be obtained under mild conditions, but also can undergo further dehydration of monosaccharides to furfural and its derivatives. Apart from acid and alkali that can catalyze the hydrolysis of lignin and its model compounds, metal catalysts can also effectively hydrogenate C–O bonds in lignin to give corresponding aromatic compounds. In some cases, the cleavage of C–O bonds is accompanied by the hydrogenation of aromatic rings, thus providing a complex mixture of corresponding cyclohexane derivatives. Therefore, it is necessary to design efficient catalysts with enhanced hydrogenolysis ability but inhibiting hydrogenation ability to improve the selectivity in catalytic production of aromatic compounds.
To meet mankind’s demand for chemical products and fuels, efficient catalytic strategies should be developed to convert lignocellulose biomass fractions from low-quality, low-cost wastes into high-quality, high-value feedstocks. This shift is crucial because lignocellulose biomass is the only viable alternative source on which society depends today. Last but not the least, talented and dedicated efforts along with insights and holistic analyses to depreciate the “cost-determining” conversion processes would shift biomass valorization from “proof-of-concept” to “proof-of-value” stage and perpetuate its research liveliness.
Author Contributions
YJ organized and prepared all this manuscript; YM made preliminary revisions to the manuscript; HL contributed to writing and reviewing the part of the manuscript.
Funding
The study was funded by the National Natural Science Foundation of China (21908033), and Fok Ying-Tong Education Foundation (161030).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Almohalla, M., Rodríguez-Ramos, I., Ribeiro, L. S., Órfão, J. J. M., Pereira, M. F. R., and Guerrero-Ruiz, A. (2018). Cooperative Action of Heteropolyacids and Carbon Supported Ru Catalysts for the Conversion of Cellulose. Catal. Today 301, 65–71. doi:10.1016/j.cattod.2017.05.023
Constant, S., Basset, C., Dumas, C., Di Renzo, F., Robitzer, M., Barakat, A., et al. (2015). Reactive Organosolv Lignin Extraction from Wheat Straw: Influence of Lewis Acid Catalysts on Structural and Chemical Properties of Lignins. Ind. Crops Prod. 65, 180–189. doi:10.1016/j.indcrop.2014.12.009
Delgado-Aguilar, M., González, I., Tarrés, Q., Pèlach, M. À., Alcalà, M., and Mutjé, P. (2016). The Key Role of Lignin in the Production of Low-Cost Lignocellulosic Nanofibres for Papermaking Applications. Ind. Crops Prod. 86, 295–300. doi:10.1016/j.indcrop.2016.04.010
Deng, W., Zhang, H., Xue, L., Zhang, Q., and Wang, Y. (2015a). Selective Activation of the C-O Bonds in Lignocellulosic Biomass for the Efficient Production of Chemicals. Chin. J. Catal. 36, 1440–1460. doi:10.1016/s1872-2067(15)60923-8
Deng, W., Zhang, Q., and Wang, Y. (2015b). Catalytic Transformation of Cellulose and its Derived Carbohydrates into Chemicals Involving C C Bond Cleavage. J. Energ. Chem. 24, 595–607. doi:10.1016/j.jechem.2015.08.016
Enright, M. J., Gilbert-Bass, K., Sarsito, H., and Cossairt, B. M. (2019). Photolytic C-O Bond Cleavage with Quantum Dots. Chem. Mater. 31, 2677–2682. doi:10.1021/acs.chemmater.9b00943
Gazi, S. (2019). Valorization of wood Biomass-Lignin via Selective Bond Scission: A Minireview. Appl. Catal. B: Environ. 257, 117936. doi:10.1016/j.apcatb.2019.117936
Grabber, J. H. (2005). How Do Lignin Composition, Structure, and Cross‐Linking Affect Degradability? A Review of Cell Wall Model Studies. Crop Sci. 45, 820–831. doi:10.2135/cropsci2004.0191
Guo, H., Zhang, B., Li, C., Peng, C., Dai, T., Xie, H., et al. (2016). Tungsten Carbide: A Remarkably Efficient Catalyst for the Selective Cleavage of Lignin C−O Bonds. ChemSusChem 9, 3220–3229. doi:10.1002/cssc.201600901
Hilgert, J., Meine, N., Rinaldi, R., and Schüth, F. (2013). Mechanocatalytic Depolymerization of Cellulose Combined with Hydrogenolysis as a Highly Efficient Pathway to Sugar Alcohols. Energy Environ. Sci. 6, 92–96. doi:10.1039/c2ee23057g
Hosoya, T., Kawamoto, H., and Saka, S. (2007). Influence of Inorganic Matter on wood Pyrolysis at Gasification Temperature. J. Wood Sci. 53, 351–357. doi:10.1007/s10086-006-0854-8
Hu, R., Lin, L., Liu, T., and Liu, S. (2010). Dilute Sulfuric Acid Hydrolysis of Sugar maple wood Extract at Atmospheric Pressure. Bioresour. Technol. 101, 3586–3594. doi:10.1016/j.biortech.2010.01.005
Huang, D., Li, R., Xu, P., Li, T., Deng, R., Chen, S., et al. (2020). The Cornerstone of Realizing Lignin Value-Addition: Exploiting the Native Structure and Properties of Lignin by Extraction Methods. Chem. Eng. J. 402, 126237. doi:10.1016/j.cej.2020.126237
Iravani, S., and Varma, R. S. (2020). Greener Synthesis of Lignin Nanoparticles and Their Applications. Green. Chem. 22, 612–636. doi:10.1039/c9gc02835h
Jampa, S., Puente-Urbina, A., Ma, Z., Wongkasemjit, S., Luterbacher, J. S., and van Bokhoven, J. A. (2019). Optimization of Lignin Extraction from Pine Wood for Fast Pyrolysis by Using a γ-Valerolactone-Based Binary Solvent System. ACS Sustain. Chem. Eng. 7, 4058–4068. doi:10.1021/acssuschemeng.8b05498
Jasiukaitytė-Grojzdek, E., Huš, M., Grilc, M., and Likozar, B. (2020). Acid-catalysed α-O-4 Aryl-Ether Bond Cleavage in Methanol/(aqueous) Ethanol: Understanding Depolymerisation of a Lignin Model Compound during Organosolv Pretreatment. Sci. Rep. 10, 11037. doi:10.1038/s41598-020-67787-9
Jiang, Z., and Hu, C. (2016). Selective Extraction and Conversion of Lignin in Actual Biomass to Monophenols: A Review. J. Energ. Chem. 25, 947–956. doi:10.1016/j.jechem.2016.10.008
Jiang, Z., Zhao, P., and Hu, C. (2018). Controlling the Cleavage of the Inter- and Intra-molecular Linkages in Lignocellulosic Biomass for Further Biorefining: A Review. Bioresour. Technol. 256, 466–477. doi:10.1016/j.biortech.2018.02.061
Kamm, B. (2007). Production of Platform Chemicals and Synthesis Gas from Biomass. Angew. Chem. Int. Ed. 46, 5056–5058. doi:10.1002/anie.200604514
Krishna, S. H., Huang, K., Barnett, K. J., He, J., Maravelias, C. T., Dumesic, J. A., et al. (2018). Oxygenated Commodity Chemicals from Chemo‐catalytic Conversion of Biomass Derived Heterocycles. Aiche J. 64, 1910–1922. doi:10.1002/aic.16172
Laurichesse, S., and Avérous, L. (2014). Chemical Modification of Lignins: Towards Biobased Polymers. Prog. Polym. Sci. 39, 1266–1290. doi:10.1016/j.progpolymsci.2013.11.004
Li, H., Bunrit, A., Li, N., and Wang, F. (2020). Heteroatom-participated Lignin Cleavage to Functionalized Aromatics. Chem. Soc. Rev. 49, 3748–3763. doi:10.1039/d0cs00078g
Li, H., Riisager, A., Saravanamurugan, S., Pandey, A., Sangwan, R. S., Yang, S., et al. (2017). Carbon-Increasing Catalytic Strategies for Upgrading Biomass into Energy-Intensive Fuels and Chemicals. ACS Catal. 8, 148–187. doi:10.1021/acscatal.7b02577
Li, S., Deng, W., Wang, S., Wang, P., An, D., Li, Y., et al. (2018). Catalytic Transformation of Cellulose and its Derivatives into Functionalized Organic Acids. ChemSusChem 11, 1995–2028. doi:10.1002/cssc.201800440
Liu, X., Zhang, H., Wu, C., Liu, Z., Chen, Y., Yu, B., et al. (2018). Copper-catalyzed Synthesis of Benzanilides from Lignin Model Substrates 2-phenoxyacetophenones under an Air Atmosphere. New J. Chem. 42, 1223–1227. doi:10.1039/c7nj02589k
Liu, Y., Nie, Y., Lu, X., Zhang, X., He, H., Pan, F., et al. (2019). Cascade Utilization of Lignocellulosic Biomass to High-Value Products. Green. Chem. 21, 3499–3535. doi:10.1039/c9gc00473d
Lohr, T. L., Li, Z., and Marks, T. J. (2016). Thermodynamic Strategies for C-O Bond Formation and Cleavage via Tandem Catalysis. Acc. Chem. Res. 49, 824–834. doi:10.1021/acs.accounts.6b00069
Luo, Y., Hu, L., Tong, D., and Hu, C. (2014). Selective Dissociation and Conversion of Hemicellulose in Phyllostachys Heterocycla Cv. Var. Pubescens to Value-Added Monomers via Solvent-thermal Methods Promoted by AlCl3. RSC Adv. 4, 24194–24206. doi:10.1039/c4ra02209b
Luo, Y., Li, Z., Zuo, Y., Su, Z., and Hu, C. (2017). A Simple Two-step Method for the Selective Conversion of Hemicellulose in Pubescens to Furfural. ACS Sustain. Chem. Eng. 5, 8137–8147. doi:10.1021/acssuschemeng.7b01766
Luo, Y., Yi, J., Tong, D., and Hu, C. (2016). Production of γ-valerolactone via Selective Catalytic Conversion of Hemicellulose in Pubescens without Addition of External Hydrogen. Green. Chem. 18, 848–857. doi:10.1039/c5gc01775k
Mäki-Arvela, P., Salmi, T., Holmbom, B., Willför, S., and Murzin, D. Y. (2011). Synthesis of Sugars by Hydrolysis of Hemicelluloses- A Review. Chem. Rev. 111, 5638–5666. doi:10.1021/cr2000042
Renders, T., Van den Bosch, S., Koelewijn, S.-F., Schutyser, W., and Sels, B. F. (2017). Lignin-first Biomass Fractionation: the Advent of Active Stabilisation Strategies. Energ. Environ. Sci. 10, 1551–1557. doi:10.1039/c7ee01298e
Rinaldi, R., and Schüth, F. (2009). Acid Hydrolysis of Cellulose as the Entry point into Biorefinery Schemes. ChemSusChem 2, 1096–1107. doi:10.1002/cssc.200900188
Schutyser, W., Renders, T., Van den Bosch, S., Koelewijn, S.-F., Beckham, G. T., and Sels, B. F. (2018). Chemicals from Lignin: an Interplay of Lignocellulose Fractionation, Depolymerisation, and Upgrading. Chem. Soc. Rev. 47, 852–908. doi:10.1039/c7cs00566k
Shivhare, A., Kumar, A., and Srivastava, R. (2021). Metal Phosphate Catalysts to Upgrade Lignocellulose Biomass into Value-Added Chemicals and Biofuels. Green. Chem. 23, 3818–3841. doi:10.1039/d1gc00376c
Song, H., Wang, P., Li, S., Deng, W., Li, Y., Zhang, Q., et al. (2019). Direct Conversion of Cellulose into Ethanol Catalysed by a Combination of Tungstic Acid and Zirconia-Supported Pt Nanoparticles. Chem. Commun. 55, 4303–4306. doi:10.1039/c9cc00619b
Subbotina, E., Rukkijakan, T., Marquez-Medina, M. D., Yu, X., Johnsson, M., and Samec, J. S. M. (2021). Oxidative Cleavage of C-C Bonds in Lignin. Nat. Chem. 13, 1118–1125. doi:10.1038/s41557-021-00783-2
Sweygers, N., Depuydt, D. E. C., Van Vuure, A. W., Degrève, J., Potters, G., Dewil, R., et al. (2020). Simultaneous Production of 5-hydroxymethylfurfural and Furfural from Bamboo (Phyllostachys Nigra “Boryana”) in a Biphasic Reaction System. Chem. Eng. J. 386, 123957. doi:10.1016/j.cej.2019.123957
Wang, H., Yang, B., Zhang, Q., and Zhu, W. (2020). Catalytic Routes for the Conversion of Lignocellulosic Biomass to Aviation Fuel Range Hydrocarbons. Renew. Sustain. Energ. Rev. 120, 109612. doi:10.1016/j.rser.2019.109612
Wong, S. S., Shu, R., Zhang, J., Liu, H., and Yan, N. (2020). Downstream Processing of Lignin Derived Feedstock into End Products. Chem. Soc. Rev. 49, 5510–5560. doi:10.1039/d0cs00134a
Wu, D., Wang, Q., Safonova, O. V., Peron, D. V., Zhou, W., Yan, Z., et al. (2021). Lignin Compounds to Monoaromatics: Selective Cleavage of C−O Bonds over a Brominated Ruthenium Catalyst. Angew. Chem. Int. Ed. 60, 12513–12523. doi:10.1002/anie.202101325
Wu, H., Dai, W., Saravanamurugan, S., Li, H., and Yang, S. (2020a). Endogenous X-C✓O Species Enable Catalyst-free Formylation Prerequisite for CO2 Reductive Upgrading. Green. Chem. 22, 5822–5832. doi:10.1039/d0gc02142c
Wu, X., Luo, N., Xie, S., Zhang, H., Zhang, Q., Wang, F., et al. (2020b). Photocatalytic Transformations of Lignocellulosic Biomass into Chemicals. Chem. Soc. Rev. 49, 6198–6223. doi:10.1039/d0cs00314j
Xue, Y., Chen, H., Zhao, W., Yang, C., Ma, P., and Han, S. (2016). A Review on the Operating Conditions of Producing Bio-Oil from Hydrothermal Liquefaction of Biomass. Int. J. Energ. Res. 40, 865–877. doi:10.1002/er.347310.1002/er
Yoo, C. G., Meng, X., Pu, Y., and Ragauskas, A. J. (2020). The Critical Role of Lignin in Lignocellulosic Biomass Conversion and Recent Pretreatment Strategies: A Comprehensive Review. Bioresour. Technol. 301, 122784. doi:10.1016/j.biortech.2020.122784
Zakzeski, J., Bruijnincx, P. C. A., Jongerius, A. L., and Weckhuysen, B. M. (2010). The Catalytic Valorization of Lignin for the Production of Renewable Chemicals. Chem. Rev. 110, 3552–3599. doi:10.1021/cr900354u
Zhang, J., Choi, Y. S., Yoo, C. G., Kim, T. H., Brown, R. C., and Shanks, B. H. (2015). Cellulose-Hemicellulose and Cellulose-Lignin Interactions during Fast Pyrolysis. ACS Sustain. Chem. Eng. 3, 293–301. doi:10.1021/sc500664h
Zhang, J., Teo, J., Chen, X., Asakura, H., Tanaka, T., Teramura, K., et al. (2014). A Series of NiM (M = Ru, Rh, and Pd) Bimetallic Catalysts for Effective Lignin Hydrogenolysis in Water. ACS Catal. 4, 1574–1583. doi:10.1021/cs401199f
Zheng, A., Huang, Z., Wei, G., Zhao, K., Jiang, L., Zhao, Z., et al. (2020). Controlling Deoxygenation Pathways in Catalytic Fast Pyrolysis of Biomass and its Components by Using Metal-Oxide Nanocomposites. iScience 23, 100814. doi:10.1016/j.isci.2019.100814
Keywords: high-value chemicals, biofuels, C-O bond, selectivity control, lignocellulosic biomass
Citation: Jian Y, Meng Y and Li H (2022) Selectivity Control of C-O Bond Cleavage for Catalytic Biomass Valorization. Front. Energy Res. 9:827680. doi: 10.3389/fenrg.2021.827680
Received: 02 December 2021; Accepted: 20 December 2021;
Published: 13 January 2022.
Edited by:
Xiaojun Shen, Dalian Institute of Chemical Physics (CAS), ChinaReviewed by:
Yaxuan JING, East China University of Science and Technology, ChinaQineng Xia, Jiaxing University, China
Copyright © 2022 Jian, Meng and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hu Li, aGxpMTNAZ3p1LmVkdS5jbg==
 Yumei Jian
Yumei Jian Hu Li
Hu Li