
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Energy Res. , 12 October 2021
Sec. Bioenergy and Biofuels
Volume 9 - 2021 | https://doi.org/10.3389/fenrg.2021.728682
This article is part of the Research Topic Sustainable Aviation Fuels View all 26 articles
Converting biomass into jet fuel involves more than the core chemical process. The overall process includes the logistics of harvesting and transporting the biomass, handling and preparing the material for processing, and processing and disposal of waste. All of these activities contribute to cost. Controlling cost involves more than developing efficient process chemistry. Choice of feedstock also has a significant impact on process economics. We consider chemical conversion of paper from municipal solid waste as a feedstock for the production of jet fuel and diesel. Paper has a significantly higher cellulose content than raw lignocellulosic biomass such as corn stover, so it requires less pretreatment to convert it into hydrocarbons than lignocellulosic biomass. Our techno-economic analysis showed that the cost of converting paper waste into jet fuel is about $1.00/gal less than jet fuel produced from corn stover. Although the cost of recycling paper into jet fuel is less than producing it from corn stover, the process is not competitive with petroleum. We estimated a minimum selling price of $3.97/gal for paper-derived jet fuel. Our sensitivity studies indicated that the biggest economic obstacle is the cost of cellulose hydrolysis. Direct hydrogenation of paper to sugar alcohols combined with increased economy of scale could make recycling paper jet fuel competitive.
In 2019, the US aviation industry consumed 636 million barrels or 3.8 EJ of fuel (US Energy Information Agency, 2021a). Air travel accounts for about 12% of the fuel consumed by the US transportation sector and about 13% of the carbon dioxide emissions (US Energy Information Agency, 2021a). The International Air Transport Association is committed to carbon-neutral growth of their industry (Stalnaker et al., 2016). This goal will limit carbon dioxide emissions from air transportation to 2020 levels (International Air Transport Association, 2015). Strategies for meeting this goal include efficiency improvements, but efficiency improvements alone are not sufficient. A bio-based hydrocarbon fuel with low life-cycle carbon dioxide emissions is also needed.
Sustainability encompasses economic and social impacts as well as environmental impact. Use of lignocellulosic biomass addresses, in part, economic and social impacts by avoiding competition with food crops and the social impact of higher food prices. However, a sustainable fuel also must be cost competitive. The average US jet fuel price in 2019 was $1.97/gal (US Energy Information Agency, 2021a). The US Energy Information Agency projects only a moderate increase in price to $2.04/gal by 2030 (US Energy Information Agency, 2021b). The National Renewable Energy Laboratory (NREL) estimated the cost of producing hydrocarbon fuels from lignocellulosic biomass to be $4.05/gal in 2011 US dollars (USD) (Davis et al., 2015). Wang et al. (2016) report the cost of converting lignocellulosic biomass into jet fuel to be $4.00–$23.30/gal. Currently, producing jet fuel from lignocellulosic biomass is not sustainable because it is not economically competitive.
Producing jet fuel from biomass involves more than the chemistry. It includes the logistics of obtaining the biomass, preparation and pretreatment of the biomass, and processing and disposal of waste. All of these associated processing steps contribute to the overall capital investment and operating costs of the plant. Controlling cost requires more than efficient process chemistry. Choice of feedstock with its associated costs, availability, and logistics has a significant impact on process economics.
We investigated other sources of sustainable jet fuel and concluded that recycle paper is a promising alternative to raw lignocellulosic biomass. Paper is a refined product with significantly higher cellulose content than raw biomass (see Table 1); so it requires less handling and pretreatment. It also contains less waste materials than raw biomass. Therefore, producing hydrocarbon fuels from recycle paper instead of raw biomass reduces the capital investment and the operating costs. Cultivated forest biomass, such as the loblolly pine in the Southeastern US, is an important source of wood pulp (Gonzalez et al., 2011) and a possible source of lignocellulosic biomass for fuel production (US Department of Energy, 2016). Figure 1 shows that producing jet fuel from recycled paper is part of a closed carbon cycle similar to other biofuels. The biomass to paper to jet fuel cycle differs from other biofuel cycles in that it involves reuse of a commercially valuable intermediate product.
We considered whether using recycle paper to produce fuels has any economic advantages over processes based on raw lignocellulosic biomass. Therefore, we performed a techno-economic analysis of a process for converting recycle paper into jet fuel. Our conceptual design is based on a process that Blommel and Price (2017) patented for converting sugars into hydrocarbon fuels. The evaluation included the availability of paper for recycling, costs, and lifecycle carbon dioxide emissions and solid waste generation. The time needed to commercialize a new process for a commodity chemical is on the order of 10 years (Vogel 2005), so we have set our target production cost to the projected 2030 jet fuel price of $2.04/gal.
The goal is not to argue that recycling paper to jet fuel is the solution to sustainable air transportation. Instead, we want to show that using recycled paper as a feedstock could be a first step in commercializing technology for producing fuels from cellulosic materials.
Figure 2 shows US paper consumption for 2017 and its ultimate disposition. Overall paper usage in the US is decreasing; but wrapping, packaging, and board, which is the major use of paper products, is increasing as a result of increased e-commerce (Food and Agricultural Organization of the UN, 2015). About 83% of the paper consumed is suitable or available for recycling into paper products. The remaining 17% is used for books and other permanent records or it is contaminated with food and materials that make it unsuitable as a source of paper products. Currently, about 64% of the paper used in the US is collected for recycling (US Environmental Protection Agency, 2020). Of the paper collected, domestic recyclers use 59% and the remainder is exported. When paper is recycled, an average of 12% of the cellulose fibers are rejected because of degradation (European Integrated Pollution Prevention and Control Bureau, 2001).
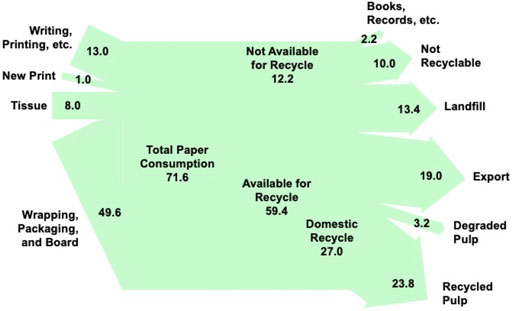
FIGURE 2. 2017 US paper consumption and ultimate disposition in million annual tonnes. The diagram is based on information from (European Integrated Pollution Prevention and Control Bureau, 2001), (Food and Agriculture Organization of the United Nations, 2015), and (US Environmental Protection Agency, 2020).
Scrap paper currently being exported, paper currently discarded to landfills, and degraded pulp from recycling plants could be used for jet fuel production without any impact on current domestic recyclers. Thus, the minimum amount of scrap paper available for fuel product would be 36 million tonnes/yr. We estimated that 1 tonne of mixed paper waste could produce about 2.4 bbl of jet fuel. (We will discuss the basis of this estimate in subsequent sections.) The minimum amount scrap paper available for fuel production could yield about 76–83 million bbl of jet fuel per year, which is 13–14% of the annual US demand. The maximum amount of paper that could be converted to jet fuel is the total amount of paper available for recycling plus part of the paper that is currently not recyclable, or 59 million tonne/yr. The maximum jet fuel production would be about 124–136 million bbl/yr or 21–22% of the US demand. We estimated that up to 3 million tonnes/yr of food contaminated waste that is currently considered not recyclable may be suitable for producing an additional 7 million bbl/yr of jet fuel. Although recycle paper cannot be used to replace all US jet fuel needs, the amount of fuel that could be produced from this raw material is not trivial.
Recyclable paper has some logistical advantages over lignocellulosic biomass. First, paper is not a seasonal crop. It is available continually throughout the year, which reduces storage requirements and costs. Second, the largest sources of recyclable paper are large metropolitan areas where the amount of paper available per hectare is much greater than lignocellulosic biomass derived from agricultural waste. If 75% of the rural land in a Midwestern state is available for corn production, and corn stover are harvested from 50% of the available land, the concentration of biomass would be 1.9 tonne/ha which yields 1.0 tonne/ha of sugar (Aden et al., 2002). Based on average US paper consumption per capita, average recycling rates, and population density, we estimated the concentration of recyclable paper in New York City to be 19 tonne/ha, which yields 16 tonne/ha of sugar. New York City is the most concentrated source of recyclable paper, but the concentration of recyclable paper in less densely populated cities is still greater than the concentration of corn stover in a Midwestern farming area (European Integrated Pollution Prevention and Control Bureau 2001). The amount of recyclable paper available in the 10 largest US metropolitan areas is sufficient to produce about 10% of the fuel consumed by the domestic air transport industry. Because of the high concentration of recyclable paper in cities, collection cost per tonne for recycle paper are less than harvesting agricultural waste. Also, plants for converting recycled paper into jet fuels would be best located near large cities serviced by one or more large airports. Thus, jet fuel production would be located near the largest consumers, which would reduce distribution costs.
Blommel and Price (2017) patented a process for converting corn syrup into a hydrocarbon mixture encompassing the boiling range of jet fuel and diesel. The National Renewable Energy Laboratory (NREL) developed conceptual design of a process for converting lignocellulosic biomass into naphtha and diesel fuel based on Blommel and Price’s patent and enzymatic hydrolysis of cellulose. We developed two concepts based on Blommel and Price’s patent for converting recycle paper into jet fuel. The first concept is an adaptation of NREL’s process with enzymatic hydrolysis of cellulose. The second concept uses acid hydrolysis to produce the sugar syrup. Both processes require hydrogen, which is assumed to be supplied by an on-site steam reforming planted located on site.
Figure 3 is a simplified block diagram showing the major steps of the paper to jet fuel process with enzymatic hydrolysis. This process is a version of the process developed by Davis et al. (2015) that has been modified to accept paper as the feedstock rather than corn stover. The process consists of eight major steps plus storage and utilities.
• Mechanical Repulping uses technology from the paper recycling industry to convert the recycled paper into a cellulose fiber slurry. The step includes creation of the fiber slurry, removal of filler materials, and deinking. Calcium carbonate is the major component of the filler, and it must be removed to reduce sulfuric acid consumption. The process differs from paper recycling because it does not include fractionation of the fibers or extensive dewatering steps. Because fiber quality is irrelevant, the process can accept paper contaiminate with food and other materials that make it unsuitable for paper products. The process is purely mechanical and uses no heat or chemicals.
• Pretreatment and Conditioning uses a dilute sulfuric acid to hydrolyze hemicellulose into its component sugars and organic acids. The sulfuric acid is neutralized with sodium hydroxide prior to Enzymatic Hydrolysis.
• Enzyme Production is a fermentation for production of the cellulase used for enzymatic hydrolysis of the cellulose fibers.
• Enzymatic Hydrolysis first converts the cellulose to dissolved glucose via an enzymatic hydrolysis. The hydrolysate is filtered to separate lignin and other solids from the aqueous solution.
• Concentration, Filtration, Ion Exchange evaporates excess water from the hydrolysate, it filters out any remaining solids, and it removes dissolved ionic species in a series of ion exchange columns. The product of this step is an aqueous solution that is nearly 50 wt% soluble sugars.
• Catalytic Conversion and Upgrading is based on (Blommel and Price, 2017) process chemistry. The sugars are first hydrogenated to produce sugar alcohols. A sequence of dehydration, hydrogenation, and condensation reactions to covert sugar alcohols into C1–C24+ hydrocarbons. This process step includes distillation to separate a light of hydrocarbons from the heavier distillate product.
• Wastewater Treatment is a combination of anaerobic and aerobic digestion to remove organic materials from the water. Anaerobic digestion produces a CH4/CO2 biogas that can be used as fuel in the boiler. Sludge for aerobic digestion is dewatered and used as fuel. The wastewater treatment process also removes dissolved solids making the treated water suitable for reuse in the process.
• Boiler/Turbogenerator burns biogas, off gas from Catalytic Conversion and Upgrading, solid waste and organic materials from Mechanical Repulping, dewatered sludge for Wastewater Treatment, lignin, and other combustible solids to produce steam. Steam is used for process heat and generating electricity.
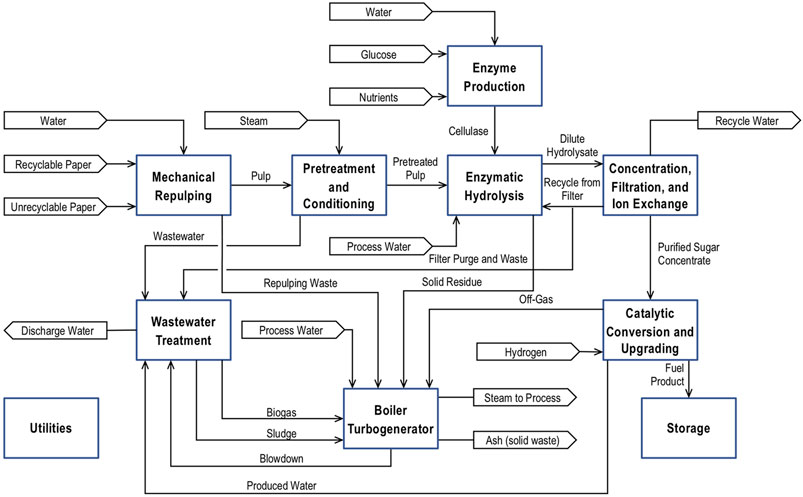
FIGURE 3. A block diagram of a process for converting recycle paper into jet fuel with enzymatic hydrolysis.
Our Catalytic Conversion and Upgrading process is nearly identical to NREL’s (Davis et al., 2015) realization of (Blommel and Price, 2017) process. This process consists of four reaction steps and a distillation. We used the same catalysts and operating conditions for the reactor in our design, but we modified the distillation to produce an off gas (C1–C7) and a fraction with the boiling range of jet fuel (C8+). Davis et al. (2015) give the details of the catalysts and operating conditions used for Catalytic Conversion and Upgrading process.
The first step is catalytic hydrogenation to reduce the sugars to sugar alcohols (e.g., sorbitol) or other polyols. The sugar alcohols then undergo catalytic aqueous-phase reforming (APR), which is a complex set of reactions that produce hydrogen, carbon dioxide, light alkanes, oxygenated compounds (Cortright et al., 2002). APRs tend to cleave C-C bonds and C-O bonds. Oxygenated products include alcohols, ketones, aldehydes, furans, diols, triols, and organic acids. Cleavage of aldehyde groups form hydrogen, carbon monoxide, and smaller polyols. In the water rich environment, carbon monoxide undergoes the water-gas shift reaction to form hydrogen and carbon dioxide. Carbon monoxide can also participate in the methanation and Fischer-Tropsch reactions to for light hydrocarbons.
The organic compounds in the APR product stream have an average carbon number less than six. In the condensation reactor, chain length increase to C8–C24. Multiple reactions occur in this step including dehydration, oligomerization, cyclization, aromatization, and hydrogenation producing normal and iso-paraffins, olefins, ketones, aromatics, and cycloparaffins (Blommel and Price, 2017; Cortright and Blommel, 2013) The organic products, which are insoluble in water, are separated from the aqueous phase and fed to hydrotreating reactor where hydrodeoxygenation reactions remove oxygen from condensation products while leaving the carbon chains intact.
Figure 4 is a block diagram of the process with acid hydrolysis. The process is similar in structure to the process with enzymatic hydrolysis. Acid hydrolysis performs the same function as the combined function Pretreatment and Conditioning, Enzyme Production, and Enzymatic Hydrolysis. The processing step takes the repulped fibers and hydrolyzes the cellulose and hemicellulose into simple sugars. Acid Recovery, Concentration, and Ion Exchange removes the sulfuric acid for the hydrolysate and concentrates it for recycling. This step also includes filtering out residual solids, concentrating the hydrolysate, and removing dissolved ionic species from the hydrolysate. The product of these two steps is a syrup containing about 50 wt% dissolve sugars. The sugar solution is converted into jet fuel using the same method as the process with enzymatic hydrolysis.
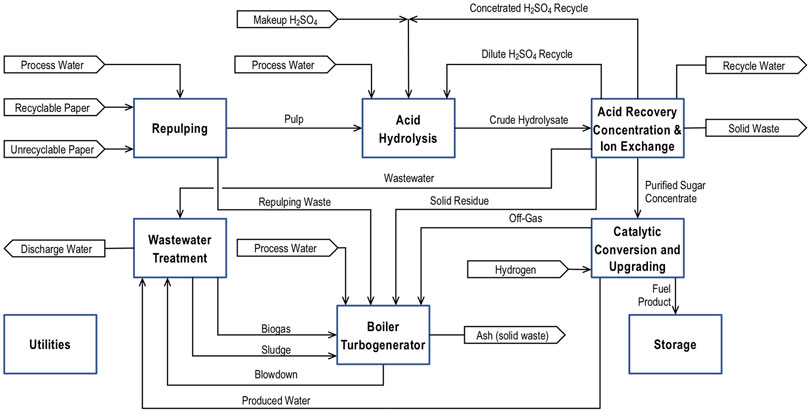
FIGURE 4. A block diagram of a process for converting recycle paper into jet fuel with acid hydrolysis.
Acid hydrolysis is based on what the technical literature refers to as the two-step process. It is called the two-step process because it consists of two hydrolysis steps–a dilute acid hydrolysis of hemicellulose followed by a concentrated acid hydrolysis of cellulose (Kosaric et al., 2011). The complete process includes the hydrolysis steps plus separation processes. The process begins with the dilute acid hydrolysis. Sulfuric acid is added to the pulp slurry creating a mixture containing 4.4 wt% sulfuric acid. Dilute acid hydrolysis occurs at 100°C. This step results in complete hydrolysis of the hemicellulose in the paper. The mild operating conditions minimize the conversion of pentoses into furfural and the production furfural oligomers and polymers. After dilute hydrolysis, the remaining solids are filtered out of the slurry, dried, and combined with 85 wt% sulfuric acid. After the solids are mixed with the acid, water is added to reduce the sulfuric acid concentration to 8 wt%. Concentrated acid hydrolysis occurs at 110°C and results in a 90% cellulose conversion. Residual solids are removed from the hydrolysate, and the hydrolysate is combined with the dilute acid hydrolysate.
The first step in purifying and concentrating the hydrolysate is removing the sulfuric acid using resin wafer electrodeionization (RW-EDI) (Datta et al., 2013). RW-EDI is a modified version of electrodialysis that incorporates ion exchange resin beads within the electrodialysis stack. RW-EDI removes 99% of the sulfuric acid from the hydrolysate and concentrates it to 25 wt%. The hydrolysate passes through an ultrafilter prior to RW-EDI to ensure that it contains no fine particles that could foul the membrane. Part of the sulfuric acid is distilled to produce 85 wt% sulfuric acid for concentrated acid hydrolysis. The remainder is recycled to dilute acid hydrolysis. After the sulfuric acid has been removed, the hydrolysate is concentrated using the same process as in the process with enzymatic hydrolysis, and dissolved anionic species are removed in an ion exchange column. The hydrolysate contains no cationic species other than hydrogen ions.
We determined the material balances for a 3,900 bbl/day jet fuel plant. We included a corn stover to jet fuel process based on the biomass to hydrocarbon process of Davis et al. (2015). The process was modified to produce a distillate consisting of hydrocarbons with chain-lengths in the jet fuel range. The corn stover composition for this process is given in Table 1. Material and energy balances for this process were obtained directly from Davis et al. (2015) with slight modifications. Table 2 contains a summary of the material and energy balances for the corn stover to jet fuel process.
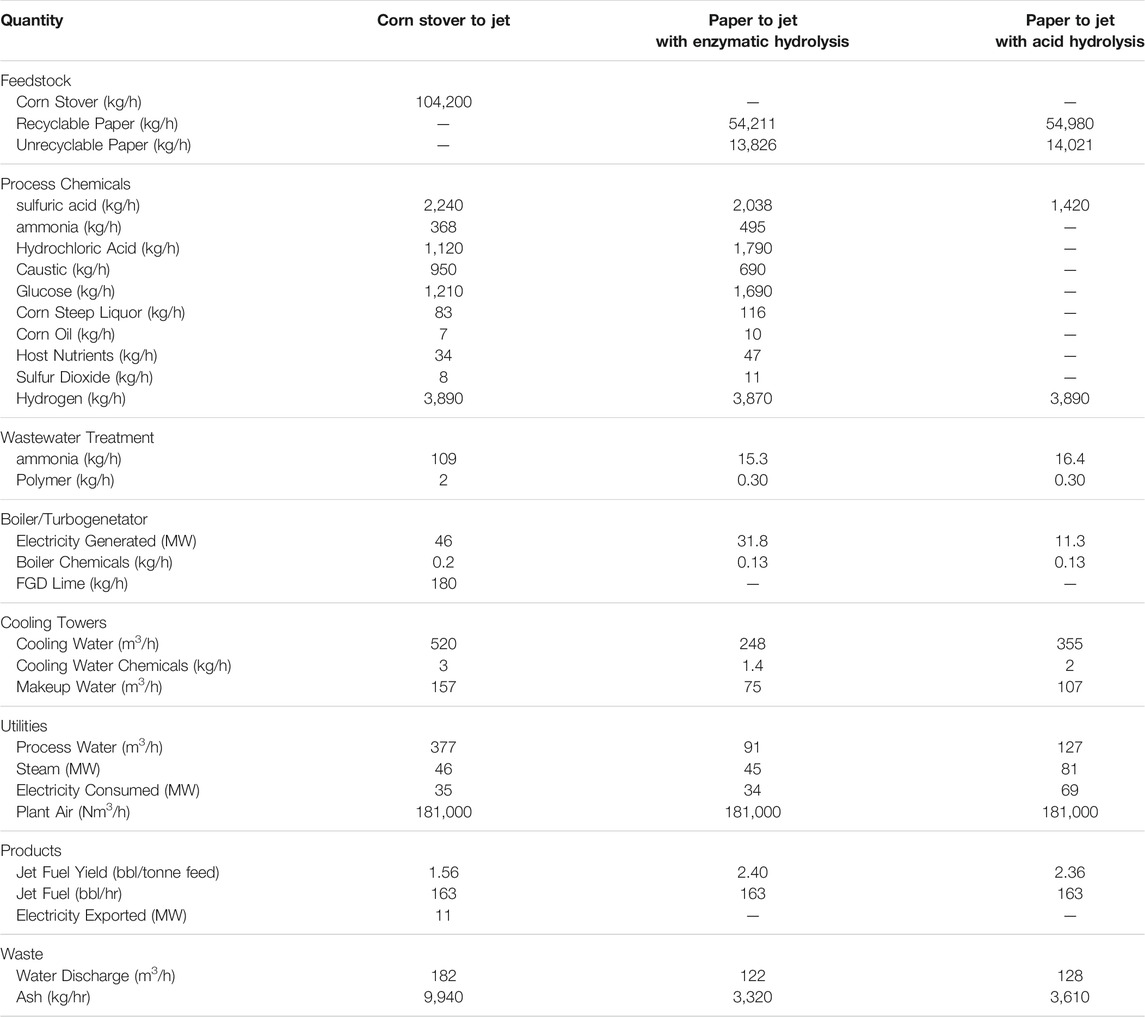
TABLE 2. Overall material and energy balances for corn stover to jet fuel, recycle paper to jet fuel with enzymatic hydrolysis, and recycle paper to jet fuel with acid hydrolysis.
The feedstock for the recycle paper to jet fuel process consists of 80% recyclable paper and 20% unrecyclable paper, which approximates typical municipal solid waste. Table 1 gives the composition of recyclable and unrecyclable paper. Food contamination of unrecyclable paper is represented by a high lipids content. The inorganic content of paper consists of whitening agents and filler. Calcium carbonate and talc constitute the vast majority of these inorganic materials. The metal content consists of staples, fastener, foil, and other metals that were not removed when the paper was discarded or segregated for recycling.
To calculated the material and energy balances for the paper to jet fuel process with enzymatic hydrolysis, we used the same assumptions and models used by Davis et al. (2015) for their biomass to hydrocarbon process. Most of the ink, inorganic filler materials, and metal are removed from the paper during the repulping process using a series of settling and flotation operations. Repulping consumed electricity to drive the mechanical repulping and physical separation processes. We obtained an estimate of the power consumption from an International Energy Agency publication (Börjessen and Ahlgren, 2015). Table 2 contains a summary of the material and energy for the paper to jet fuel process with enzymatic hydrolysis.
For the paper to jet fuel process with acid hydrolysis, we used a ChemCAD model to determine the material and energy balances for acid hydrolysis, acid recovery, hydrolysate concentration, and ion exchange. Power needed for the RW-EDI process was estimated from the current cell, voltage, and cell efficiency (Patel et al., 2020). The paper to jet fuel process with acid hydrolysis produces a concentrated hydrolysate containing about 50 wt% sugars, which is fed to Catalytic Conversion and Upgrading. Assumptions and models used for Chemical Conversion and Upgrading are the same as those used for the process with enzymatic hydrolysis. The assumptions and models for wastewater treatment and the boiler/turbogenerator are the same as used in the process based on corn stover (Davis et al., 2015). Table 2 contains a summary of the material and energy for the paper to jet fuel process with acid hydrolysis.
A key difference among the three processes is yield per tonne of feedstock. The yields of all three processes are about 80% of the theoretical maximum based on carbohydrate content (i.e. cellulose, hemicellulose, and starches), but the carbon hydrate content of corn stover is significantly less than paper. Corn stover contains about 47 wt% carbohydrates while paper contains 63–76 wt% carbohydrates. The lower carbohydrate content means that 1.4–1.5 tonnes of corns stover is required to produce the same volume of jet fuel as 1.0 tonne of recycle paper.
Another key difference is the fuel produced per tonne of feedstock. Corn stover contains more lignin and other organic matter that can be used as fuel than paper. The additional fuel means that the corn stover process is net producer of electricity while recycle paper processes are net electric consumers. Because of the greater volume processed and the chemical form of the inorganic material, burning the corn stover residue produces approximately 3 times the solid waste than burning the residue of a paper to jet fuel process.
A third key difference is the ratio of cellulose to hemicellulose. Paper is a refined bioproduct that contains nearly 5 times more cellulose than hemicellulose. The cellulose to hemicellulose ratio in corn stover is about 1.8. Because the glucose from cellulose constitutes a greater fraction of the total sugars produced, a jet fuel process with enzymatic hydrolysis of paper requires more cellulase than a process based on corn stover as well as the more if the chemical feedstocks needed to produce cellulase.
The hydrolysis process has a significant impact on material and energy balances for the paper to jet fuel processes. The overall yields of both processes are about the same. However, recovery and recycling of sulfuric acid is also energy intensive. A process with acid hydrolysis consumes about twice as much steam as a process with enzymatic hydrolysis. Increased process steam consumption in the process using acid hydrolysis result in approximately 64% less electrical power generation than a process with enzymatic hydrolysis. Use of RW-EDI in for sulfuric acid production results in a process that consumes about twice as much electricity as the process with enzymatic hydrolysis. As a consequence of lower power generation and higher power demand, the net electrical power consumption is about 12 times greater for the process with acid hydrolysis than the process with enzymatic hydrolysis.
Techno-economic analysis consists of three major parts–capital cost estimation to determine the investment required to build the process; operating costs estimate to determine the annual expenses of operating the plant; and a cash flow analysis, which combines capital and operating costs to determine the overall production costs. We use a methodology and assumptions that have been benchmarked against cellulosic ethanol production for the analysis (Kubic, 2019). Cost estimates are based on a US Midwest location. Estimates are in 2020 USD.
Capital cost estimates for the recycle paper to jet fuel were based on conceptual designs with a low level of maturity. Given the low level of process definition, the appropriate estimation method should be consistent with an Association for the Advancement of Cost Engineering International Class 5 (AACE International, 2011) estimate with an accuracy range of -20/+30% to 50/+100% or an American National Standards Institute order-of-magnitude estimate (Institute of Industrial Engineers, 2000) with an accuracy of −30/+50%. We used a factor method (Woods, 2007) to determine fixed capital investment (FCI) and total capital investment (TCI) from purchased equipment cost (FOB cost).
We determined FOB costs and installation factors from correlations in Woods (2007) and data in Davis et al. (2015). FOB costs were converted to 2020 USD using Chemical Engineering Plant Cost Index. Assumptions for estimating additional direct costs, indirect costs, and additional capital costs were based on the recommendations of (Kubic et al., 2019). Contingencies are added to the cost estimate to account for judgment errors in accumulation of the project scope (Page 1996). Contingencies can range from 10 to 80% of direct costs depending on the degree of project definition (Garrett, 1989; Woods, 2007). Cost estimates in this study are based on a conceptual design, so large contingencies are appropriate. We assumed a contingency of 30% of direct costs based on (US Department of Energy, 1997) guidance.
Table 3 is a summary of the capital cost estimates. The FCI for the corn stover to jet fuel is 5% greater than the paper to jet fuel with enzymatic hydrolysis. This difference is well withing the estimation errors for a Class 5 estimate. The FCI for the paper to jet fuel process with enzymatic hydrolysis is 10% greater than the process with acid hydrolysis. About 80% of this difference can be attributed to differences in the cost of the hydrolysis process. The installed equipment cost for enzymatic hydrolysis is more than 40% greater than the installed equipment cost for acid hydrolysis. Although the differences in FCI and installed equipment costs of hydrolysis equipment are within the uncertainty of Class 5 estimate, the count of major operations suggest that the difference may be real. Correlations for order-of-magnitude cost estimates have been developed that give FCI as a function of number of processing steps and plant capacity (Zhang and El-Halwagi, 2017). Enzymatic hydrolysis requires 12 processing steps while acid hydrolysis requires 9. Fewer processing steps suggest that the FCI for acid hydrolysis should be less than the FCI for enzymatic hydrolysis.
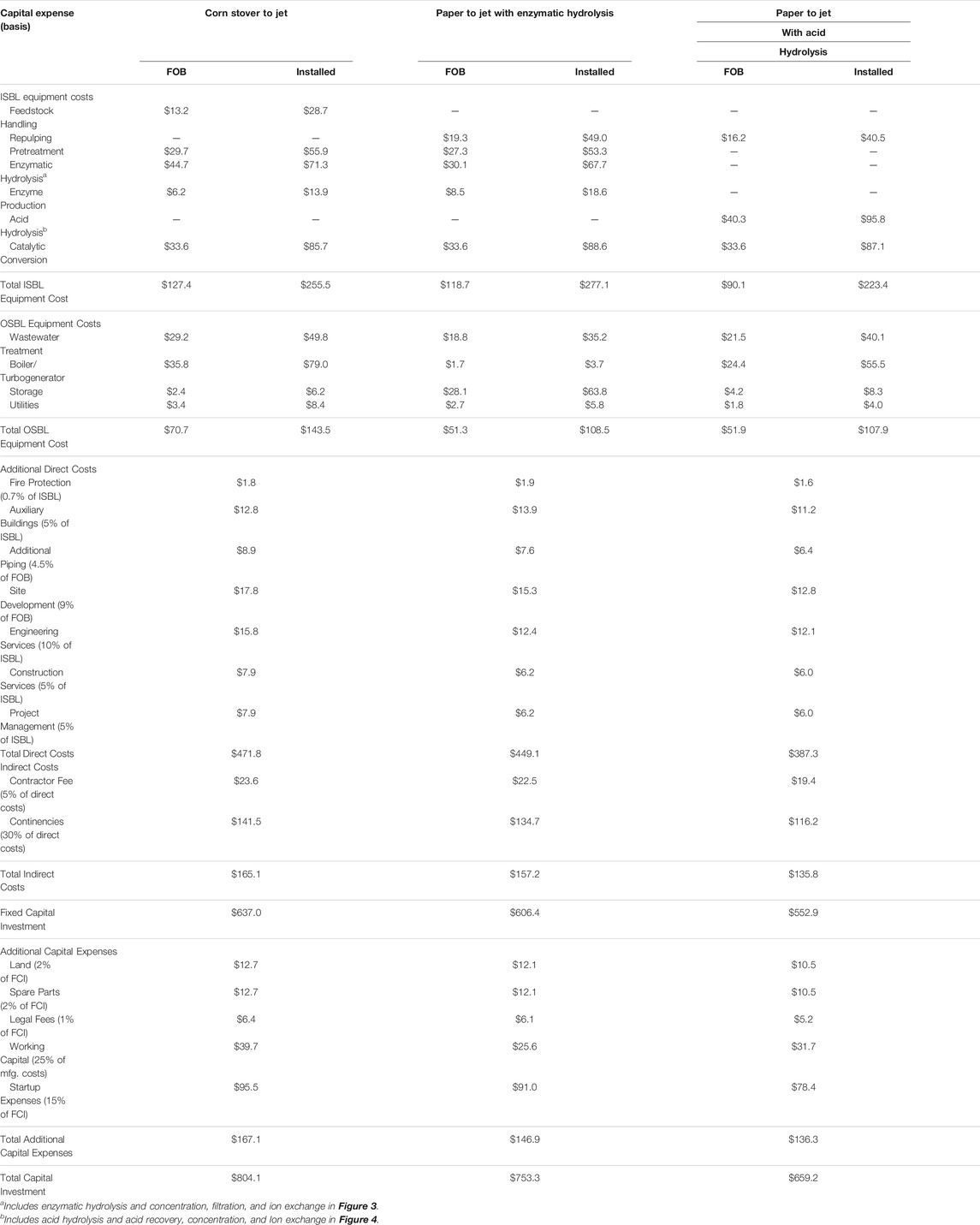
TABLE 3. Capital cost estimates for corn stover to jet fuel, recycle paper to jet fuel with enzymatic hydrolysis, and recycle paper to jet fuel with acid hydrolysis in million USD (2020).
Operating costs are generally divided into two categories–variable costs, which depend on production volume, and fixed costs, which are independent of production volume. Variable costs include feedstock costs, chemicals, utilities, and waste disposal. We obtained the price of delivered corn stover from Thompson and Tyner (2011). Price was adjusted for moisture content and converted to a 2020 price using the producer price index for hay from the US Bureau of Labor Statistics. The price of recycle paper depends on its classification, as shown in Table 4. We used a weighted average of mixed paper, old cardboard, and sorted residential paper for the price of recyclable paper for our cost estimates. Unrecyclable paper is currently sent to landfills for disposal. We assumed a credit for unrecyclable paper equal to the average landfill charge in the US Midwest.
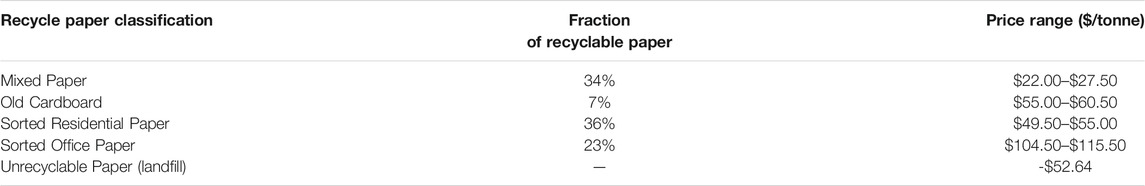
TABLE 4. Prices of recycle paper in the US Midwest in USD (2020) (Recycling Today, 2020).
We determined 2020 prices for chemicals, catalysts, and utilities from advertised prices, trade journals (e.g., ICIS Chemical Business), technical journals and reports, commodity trading data, and the US Energy Information Agency. If data was available, we used annual average values. If multiple sources of data were available, we used the median value. If prices for 2020 were not available, we estimated the price using the available data and the appropriate producer price index from the US Bureau of Labor Statistics. We assumed that an onsite natural gas steam reforming plant provides hydrogen, and we estimated the hydrogen prices based on the 2020 industrial natural gas price of $3.29/Mscf. Electricity is purchased at the average 2020 price for the industrial users in the Midwest, which is $66.60/MWh. Excess electricity is exported at the average wholesale price for the Midwest. Makeup water price is based on the 2020 price for the City of Chicago. The only waste produced is ash from the boiler. Table 5 lists the prices for chemicals, catalysts, utilities, and waste disposal.
To determine variable costs on an annual basis, we assumed a 90% process availability. Repulping is established and reliable technology, so an assumed availability of 90% is reasonable for the paper to jet fuel processes. Current technology for preprocessing corn stover is unreliable and, therefore, has a low availability. To determine the inherent price advantage of recycle paper as a feedstock, we assumed that reliable technology for preprocessing corn stover already exists; and the corn stover to jet fuel process also has a 90% availability.
The number of operators required per shift using Brown’s method (Brown, 2000). We assume that the plant employs five complete crews. Five complete crews provide a sufficient number of operators to ensure process is completely staffed at all times without the need for operators to work overtime. We assumed an operator wage of $26.50 per hour based on the average reported by the US Bureau of Labor Statistics for the Midwest in 2020.
The average maintenance cost for ethanol plants and biotechnology companies is less than the average for the chemical industry because materials tend to be less corrosive and operating conditions are milder than the chemical industry. Based on data from the ethanol industry and biotechnology companies, we estimated the average maintenance cost for a biorefinery to be 2.4% of FCI per year, which is less than average value of 6% for the chemical industry (Garrett, 1989). Corn stover to jet fuel and paper to jet fuel process have characteristics of biorefineries and ordinary chemical plants. For example, enzyme production is a biorefinery-like operation, which should have maintenance costs typical of a biorefinery. Catalytic conversion is petrochemical-like and should have maintenance costs typical of the chemical industry. To account for the mixed nature of the processes, we used a cost weight average to estimate maintenance cost.
We used the cost factors and methods recommended by Kubic et al. (2019) to estimate the remaining fixed operating costs. Table 6 contains a summary of variable and fixed operating cost estimates.
Inspection of Table 6 reveals three important differences among the three processes. First, the cost of corn stover is substantially higher than recycle paper. The cost of corn stover per tonne is greater than paper and more corn stover must be processed to produce volume of product because of its lower carbohydrate content. Second, chemical costs for the paper to jet fuel process with acid hydrolysis are less than the other two processes because acid hydrolysis requires no chemicals and nutrients for cellulase production. Electric costs for the paper to jet fuel process with acid hydrolysis are much greater than the other two processes because of the power consumption of RW-EDI.
We use a discount cash flow analysis to evaluate the minimum selling price for jet fuel. Minimum selling price is the product price needed to give a 10% real internal rate of return after taxes. The cash flow analysis begins with construction of the plant and continues through the life of the plant. Construction time can be estimated using the following correlation. (Kubic, 2014).
where θ is the construction time in years, α is a constant that depends on the type of project, and TCI is the total capital investment. For a chemical plant with TCI computed in million 2020 USD, α is 0.53. Construction spending as a function of time is approximated by a beta distribution. Working capital and start-up expenses are accrued during the final year of construction. Startup, which is the time from the introduction of feedstock until the process achieves some degree of steady operations, is assumed to be 3 months (Myers et al., 1986). Production is assumed to be zero during the startup period and 80% of nameplate capacity during the first year of operations.
Table 7 summarizes the financial parameters for the discount cash flow analysis. Plant life is measured from the end of start-up. It is not a true measure of plant life. Rather, it is a time horizon for the cash flow analysis. Depreciation in the analysis is only used to estimate corporate profit taxes. We use a modified accelerated cost recovery system with a 7 years depreciation time. The method and depreciation time are dictated by the US tax code. Both federal and state corporate taxes are included in the analysis. We use a state tax rate of 4.8%, which is the average state tax rate in the US.
The minimum selling prices for the three processes that we considered in this study are $5.14/gal for the corn stover to jet fuel process, $3.97/gal for the paper to jet fuel process with enzymatic hydrolysis, and $4.13/gal for the paper to jet fuel process based in acid hydrolysis. Figure 5 shows the cost breakdown for the three processes by expense category. The minimum selling price for jet fuel produced from corn stover is more than $1.00 higher than jet fuel produced from recycle paper. This difference is the result of the high cost of corn stover relative to recycle paper. The minimum selling price for the paper to jet fuel processes within the uncertainty limits of the analysis. Although the total capital investment is less for the process with acid hydrolysis, the operating cost are higher as a result of the high electrical power consumption by RW-EDI.
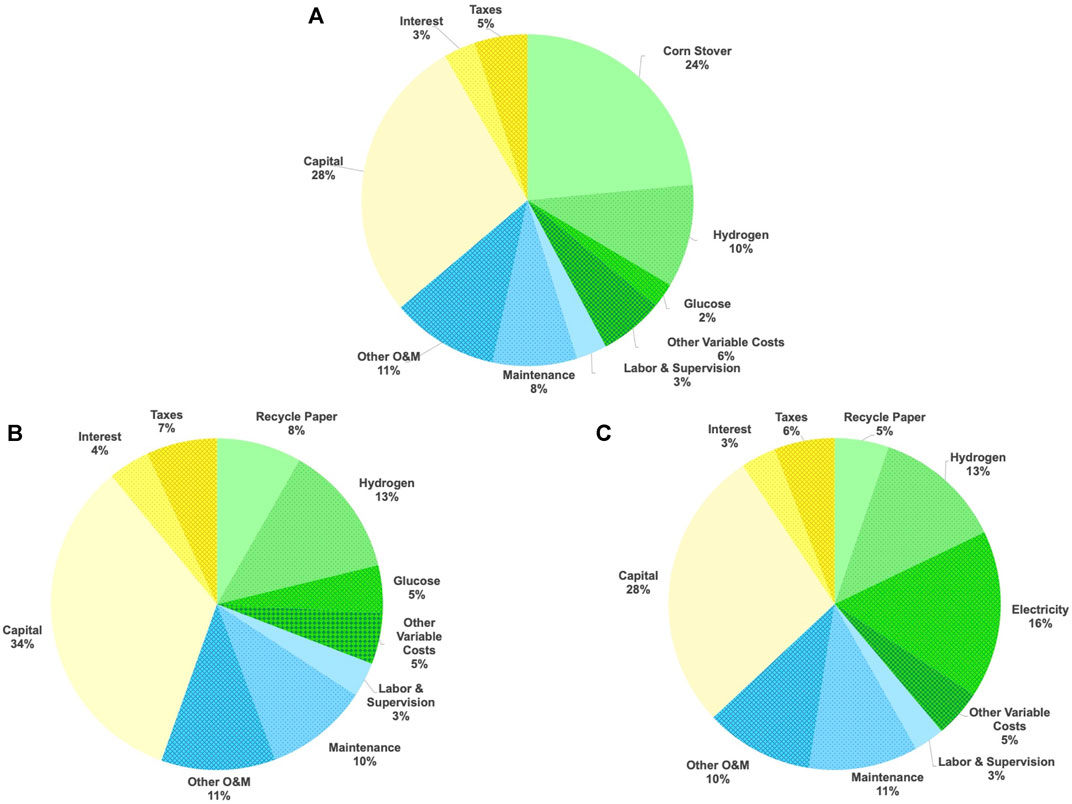
FIGURE 5. Cost breakdown by expense category for (A) Corn Stover to Jet Fuel Process, (B) Paper to Jet Fuel Process with Enzymatic Hydrolysis, and (C) Paper to Jet Fuel Process with Acid Hydrolysis.
The proposed paper to jet fuel processes do not meet the target production cost of $2.04/gal. Therefore, it is reasonable to consider possible technological improvements that could make the process competitive with petroleum-derived fuels. We identified six engineering improvements and technological advances that could improve process economics and determined their impact on cost.
• Increase Efficiency of RW-EDI–Figure 5 shows that electricity account for 16% of the cost for the paper to jet fuel process with acid hydrolysis, and RW-EDI accounts for over 60% of the energy consumption. Reducing RW-EDI power consumption and increasing the sulfuric acid concentration in the permeate would reduce power consumption and steam consumption as well as reduce capital costs. These savings would reduce the cost of producing jet fuel with the process with acid hydrolysis.
• Hydrogenation of Cellulose and Hemicellulose in Pulp–Pretreatment and hydrolysis account for over 25% of production costs for both paper to jet fuel processes. Direct hydrogenation of the pulp to produce sugar alcohols would eliminate the capital and operating costs associated with hydrolysis and reduce overall production costs.
• Hydrogenation of Cellulose and Hemicellulose in Paper–The cost of sorting paper in municipal solid waste is estimated to be about $75/tonne and the cost of repulping the paper is not negligible. Hydrogenating unsorted paper to produce sugar alcohols would reduce repulping costs and eliminate hydrolysis costs. By eliminating or substantially reducing sorting costs it could turn recycle paper costs into a credit.
• Increase Plant Capacity–Figure 5 shows that capital costs are the largest single factor in the overall cost of jet fuel. The paper to jet fuel processes scale with capacity to the 0.66 power. Doubling capacity will reduce capital costs relative to operating cost resulting in a reduction in product cost.
• Reduce Catalyst Cost–The catalysts for Catalytic Conversion and Upgrading are expensive. Reducing catalyst cost by a factor of 10 by finding less expensive options and improving catalyst life will reduce operating costs.
• Reduce Excess Hydrogen–In the current process design, about 22% more hydrogen is fed to Catalytic Conversion and Upgrading than is consumed by the process. Reducing excess hydrogen to less than 5%would reduce production costs.
We evaluated the possible cost saving for each of these scenarios considering reductions in capital costs as a result of eliminating processing steps, reduction in variable capital costs as a result of eliminating or reducing chemical feeds, and reducing the required number of operators. Only two of the perturbations could change overall process yields–direct hydrogenation of pulp and paper. For these two perturbations, we assumed yields were equal to those of the process based on acid hydrolysis.
Table 8 contains a summary of the results. The results show that no single innovation reduces the cost of converting recycled paper into jet fuel to the target value of $2.04/gal. The gains from incremental process improvements (i.e., increasing efficiency of RW-EDI, reducing catalyst cost, reducing excess hydrogen, and increasing plant capacity) are not large enough to meet the target price. A major technical innovation is needed.
Pretreatment and hydrolysis account for over 25% of production costs. Direct hydrogenation of cellulose is the subject of current research activities (Kobayashi et al., 2011; Jiang, 2014; Liao et al., 2014; Negoi et al., 2014). Using direct hydrogenation of cellulose and hemicellulose would eliminate the capital and operating costs associated with pretreatment and hydrolysis reducing production costs by $1.09/gal. Direct hydrogenation of pulp combined with a doubling of process capacity reduces costs to $2.44/gal, which still exceeds the target value of $2.04/gal. Additional cost savings could be achieved by developing a process for direct hydrogenation of unsorted paper. This innovation would eliminate repulping costs and sorting costs. Direct hydrogenation of unsorted paper would reduce production costs to $2.31/per gal, which is $1.80/gal reduction from the nominal value. Hydrogenation of unsorted paper combined with increased plant capacity could bring production costs down to $1.71.
We performed a lifecycle analysis to determine net carbon dioxide emissions and solid waste generation. The analysis was limited to the combined emissions from jet fuel and paper production assuming current levels of use. We based the analysis on the Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation (GREET) model (Energy Systems Division, 2014). Pathways not related to jet fuel production and use were eliminated from the GREET model; and pathways for paper production, use, and disposal were added. GREET was the primary data source supplemented with additional data for emissions and solid waste generation for the paper pathways (Suhr et al., 2010; Bajpai, 2014; US Environmental Protection Agency, 2018; Kinstrey and White, 2006) and solid waste from biomass (Lizotte et al., 2015). Emissions and solid waste associated with the conversion of biomass and recycled paper to jet fuel were based on the material and energy balances discussed in the previous section. Estimates of net solid waste production for the paper to jet processes account the reduction in paper waste currently been disposed of in landfills.
Table 9 gives the carbon dioxide emitted and solid waste generated from the three jet fuel processes evaluated in this study. The paper to jet fuel process with acid hydrolysis emits 2.4 times as much carbon dioxide as the process with enzymatic hydrolysis. The difference is the result of the high electrical power consumption of RW-EDI in the process with acid hydrolysis to recover and recycle sulfuric acid. Because the paper to jet fuel processes consume paper from municipal solid waste, net solid waste generation is negative.

TABLE 9. Carbon dioxide emissions and solid waste production for corn stover to jet fuel and paper to jet fuel processes.
We analyzed four scenarios to determine the combined carbon dioxide emissions and solid waste generation of commercial air transportation and the paper industry in the US.
• Baseline–The baseline scenario was the current case in which jet fuel is produced from petroleum and paper is produced from a combination of virgin and recycled pulp.
• Scenario 1–All recyclable paper not currently recycled domestically and degraded pulp from recycling plants are converted into jet fuel and the balance of the US jet fuel demand is obtained from petroleum. Paper is produced from the current combination of virgin paper and recycled pulp.
• Scenario 2–All discarded paper available for recycle is converted into jet fuel and the balance of the jet fuel demand is obtained from petroleum. All paper is produced in the US from virgin pulp.
• Scenario 3–The same volume of renewable jet fuel produced in this scenario as produced in Scenario 2, but only paper not recycled domestically is converted into jet fuel. The additional renewable jet fuel is produced from corn stover. The balance of the US jet fuel demand is obtained from petroleum. Paper is produced from the current combination of virgin paper and recycled pulp.
• Scenario 4–The same volume of renewable jet fuel produced in Scenario 2 is produced from the corn stover biomass. The balance of jet fuel needed domestic demand is obtained from petroleum. Paper is produced from the current combination of virgin and recycled pulp.
The results of the lifecycle analysis for these scenarios are summarized in Table 10. Because of the high carbon dioxide emissions from the paper to jet fuel process with acid hydrolysis, we only present results from the process with enzymatic hydrolysis. Scenario 4, in which all renewable jet fuel is produced from corn stover, results in the lowest level carbon dioxide emissions. As shown in Table 9, lifecycle carbon dioxide emissions jet fuel produced from corn stover are less than for jet fuel produced from recycle paper. US paper recycled in other countries also contributes to the reduction in emissions. However, considering the uncertainty in the analysis, carbon dioxide emissions for Scenarios 2, 3, and 4 are not significantly different. Scenario 1, in which domestic paper recycle is maintained at current levels, produces the minimum amount of solid waste. Producing virgin pulp generates significantly more solid waste than repulping recycled paper, and producing jet fuel from corn stover produces more solid waste than recycling paper into jet fuel. Scenarios 2 and 3 also produce significantly less solid waste than the baseline scenario.
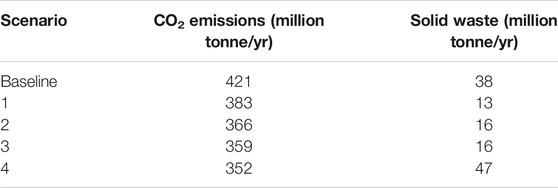
TABLE 10. Results for the lifecycle analysis of jet fuel and paper production and use. The analysis is based on the paper to jet fuel process with enzymatic hydrolysis.
Scenario 3, in which jet fuel is produced from recycled paper and corn stover, is probably the best from an environmental and social perspective. It reduces net carbon dioxide emissions from the US air transportation industry by 15% without increasing logging for virgin paper production or disrupting the domestic paper recycling industry. It also eliminates paper destined for landfills reducing total solid waste destine for landfills by 6%.
Recycle paper has advantages over agricultural residue, such as corn stover, as a cellulosic feedstock for fuel production. Efficient and reliable technology exists in the recycle paper industry for converting paper into fibers suitable for chemical conversion. Unlike equipment for handling and preprocessing of corn stover, industrial experience demonstrates that repulping equipment has high availability. The combination of proven repulping technology, the high cellulose content of paper, and existing supply network gives recycle paper a significant economic advantage over corn stover and other sources of lignocellulosic biomass. Net lifecycle carbon dioxide emissions of paper derived fuels are comparable to corn stover derived fuels, and paper generated significantly less solid waste.
A key disadvantage of producing jet fuel from paper is its limited supply, so it can only satisfy a fraction of the total demand. More importantly, the cost of producing jet fuel from recycle paper is not competitive with petroleum using current technology.
Our sensitivity studies have shown that the key to a competitive paper to jet fuel process is direct hydrogenation of cellulose and hemicellulose to sugar alcohols. Direct hydrogenation of cellulose would reduce capital and operating costs. Several researchers have explored direct catalytic hydrogenation of cellulose, but considerably more work is needed convert this idea into a practical industrial process. Direct hydrogenation of cellulose and hemicellulose combined with greater economy of scale could make paper to jet fuel comparative. In this study we have only considered the paper to jet fuel via sugar alcohols as an intermediate. Another possibility is a process with furfural and 5-methylfurfural or levulinic acid as intermediates. Such a process would eliminate hydrolysis as a separate processing step and reduce hydrogen consumption. This alternative route warrants consideration.
Perhaps the biggest value of developing a process to convert recycle paper into hydrocarbons is its use as a method of jumpstarting a cellulosic biofuels industry. The process chemistry for producing fuels from paper is the same as lignocellulosic biomass. Developing a paper to jet fuel process would provide an opportunity for demonstrating the process chemistry at an industrial scale without the need to develop a new supply chain for lignocellulosic biomass or solve all the current problems involved with handling and preprocessing lignocellulosic biomass. The process would also be useful for reducing municipal solid waste.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The concept of converting recycle paper into jet fuel and the technical approach to the problem were the result of discussions among WK, CM, TS, and AS. WK developed idea and executed the analysis including the material and energy balances, the techno-economic analysis, and the life cycle analysis.
This work was supported by the U.S. Department of Energy Office of Energy Efficiency and Renewable Energy Bioenergy Technologies Office (program Award Number NL0033622). Los Alamos National Laboratory is operated by Triad National Security, LLC, for the National Nuclear Security Administration of U.S. Department of Energy (Contract No. 89233218CNA000001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors thank members of the Los Alamos National Laboratory Biomass Conversion Team for their review and suggestion. The author also thanks Travis Moulton of the Process Modeling and Analysis Group at Los Alamos for his review of this manuscript.
AACE International (2011). AACE International Certified Cost Technician Primer. Morgantown, WV: Association for the Advancement of Cost Engineering International.
Aden, M. R., Ibsen, K., Jechura, J., Neeves, K., Sheehan, J., Wallace, B., et al. (2002). Lignocellulosic Biomass to Ethanol Process Design and Economic Utilization Co-current Dilute Acid Prehydrolysis and Enzymatic Hydrolysis for Corn Stover. Report No. NREL/TP-510-32438. Golden, CO: National Renewable Energy Laboratory.
Bajpai, P. (2014). Management of Pulp and Paper Mill Waste. Heidelberg, Germany: Springer International Publishing.
Blommel, P., and Price, R. (2017). Production of Alternative Gasoline Fuels. US Patent Application No. US 2017/0044443 A1. Washington, DC: US Patent and Trademark Office.
Börnjesson, M. H., and Ahlgren, E. O. (2015). Energy Technology Systems Analysis Programme. Paris, France: International Energy Agency.Pulp and Paper Industry. Technical Brief 107.
Cortright, R. D., and Blommel, P. G. (2013). Synthesis of Liquid Fuels and Chemicals from Oxygenated Hydrocarbons. Washington, DC: U.S. Patent No.US Patent and Trademark Office, 455.
Cortright, R. D., Davda, R. R., and Dumesic, J. A. (2002). Hydrogen from Catalytic Reforming of Biomass-Derived Hydrocarbons in Liquid Water. Nature 418 (6901), 964–967. doi:10.1038/nature01009
DattaLin, S. Y., Lin, Y. J., Schell, D. J., Millard, C. S., Ahmad, S. F., Henry, M. P., et al. (2013). Removal of Acidic Impurities from Corn Stover Hydrolysate Liquor by Resin Wafer Based Electrodeionization. Ind. Eng. Chem. Res. 52, 13777–13784. doi:10.1021/ie4017754
Davis, R., Tao, L., Scarlats, C., Tan, E., Ross, J., Lukas, J., et al. (2015). Process Design and Economics for Conversion of Lignocellulosic Biomass to Hydrocarbons: Dilute Acid and Enzymatic Deconstruction of Biomass to Sugars and Catalytic Conversion of Sugars to Hydrocarbons. Report No. NREL/TP-5100-62498. Golden, CO: National Renewable Energy Laboratory.
Energy Systems Division (2014). GREET Life-Cycle Model: Model. Lemont, IL: Argonne National Laboratory.
European Integrated Pollution Prevention and Control Bureau (2001). Best Available Techniques in the Pulp and Paper Industry. Reference Document. Luxembourg, Luxembourg: European Commission, Integrated Pollution Prevention and Control Bureau.
Food and Agriculture Organization of the United Nations, (2015). Rome: Food and Agriculture Organization of the United Nations.Pulp and Paper Capacities.
Gonzalez, R., Phillips, R., Saloni, D., Jameel, H., Abt, R., Pirraglia, A., et al. (2011). Biomass to Energy in Sothern United States: Supply Chain and Delivered Cost. Bioresources 6, 2954–2976.
Institute of Industrial Engineers (2000). Industrial Engineering Terminology: A Revision of ANSI Z94.0-1989. Norcross, GA: Engineering and Management Press.
International Air Transport Association (2015). IATA Sustainable Aviation Fuel Roadmap. Montreal-Geneva: International Air Transport Association.
Jiang, C. (2014). Hydrolytic Hydrogenation of Cellulose to Sugar Alcohols by Nickel Salts. Cellulose Chem. Tech. 48, 75–78.
Kinstrey, R., and White, D. (2006). Pulp and Paper Industry – Energy Bandwidth Study. Greenville, SC: Jacobs: Project No. 16CX8700.
Kobayashi, H., Matsuhashi, H., Komanoya, T., Hara, K., and Fukuoka, A. (2011). Transfer Hydrogenation of Cellulose to Sugar Alcohols over Supported Ruthenium Catalysts. Chem. Commun. 47, 2366–2368. doi:10.1039/c0cc04311g
Kosaric, N., Duvnjak, Z., Farkas, A., Sahm, H., Bringer-Meyer, S., Goebel, O., et al. (2011). “Ethanol,” in Ullmann’s Encyclopedia of Chemical Technology (Weinheim: Wiley-VCH Verlag GmbH & Co), 1–72. doi:10.1002/14356007.a09_587.pub2
Kubic, W., Booth, S., Suton, A., and Moore, C. (2019). Evaluation and Benchmarking of Economic Analysis Methods for Biofuels. Report No. LA-UR-10-30785. Los Alamos, NM: Los Alamos National Laboratory.
Kubic, W. (2014). Construction Cost Growth for New Department of Energy Nuclear Facilities, Report No. LA-UR-14-23698. Los Alamos, NM: Los Alamos National Laboratory.
Liao, Y., Liu, Q., Wang, T., Long, J., Zhang, Q., Ma, L., et al. (2014). Promoting Hydrolytic Hydrogenation of Cellulose to Sugar Alcohols by Mixed Ball Milling of Cellulose and Solid Acid Catalyst. Energy Fuels 28, 5778–5784. doi:10.1021/ef500717p
Lizotte, P-L., Savoie, P., and de Champlain, A. (2015). Ash Content and Calorific Energy of Corn Stover Components in Eastern Canada. Energies 8, 3827–4838. doi:10.3390/en8064827
Myers, C. W., Shangraw, R. F., Devey, M. R., and Hayashi, T. (1986). Understanding Process Plant Schedule Slippage and Startup Costs. Report No. R-3215-PSSP/RC. Santa Monica, CA: Rand Corporation.
Negoi, A., Triantafyllidis, K., Parvulescu, V. I., and Coman, S. M. (2014). The Hydrolytic Hydrogenation of Cellulose to Sorbitol over M (Ru, Ir, Pd, Rh)-BEA-Zeolite Catalysts. Catal. Today 223, 122–128. doi:10.1016/j.cattod.2013.07.007
Patel, S. K., Qin, M., Walker, W. S., and Elimelech, M. (2020). Energy Efficiency of Electro-Driven Brackish Water Desalination: Electrodialysis Significantly Outperforms Membrane Capacitive Deionization. Environ. Sci. Technol. 54, 3663–3677. doi:10.1021/acs.est.9b07482
Recycling Today (2020). Weird Times for Recovered Paper. Recycling Today. Available at: https://www.recyclingtoday.com/article/recovered-paper-market-report-september-2020/(Accessed June 3, 2021).
Stalnaker, T., Usman, K., and Taylor, A. (2016). Airline Economic Analysis for the Raymond James Global Airline Book. New York: Oliver Wyman.
Suhr, M., Klein, G., Kourti, I., Rodrigo Gonzalo, M., Giner Santonjia, G., Roudier, S., et al. (2010). Best Available Techniques (BAT) Reference Document for Production of Pulp, Paper, and Board. Luxembourg: Publications Office of the European Union.
Thompson, S., and Tyner, W. (2011). Purdue Extension. West Layfayette, IN: Purdue University.Corn Stover for Bioenergy Production: Cost Estimates and Farmer Supply Response. Report No. RE-3-W.
US Department of Energy (1997). Cost Estimating Guide. Report No. DOE G430.1-1. Washington, DC: US Department of Energy.
US Energy Information Agency (2021b). Annual Energy Outlook 2021 with Projections to 2050. Washington, DC: US Department of Energy.
US Energy Information Agency (2021a). May 2021 Monthly Energy Review. Report No DOE/EIA-0035(2021/5). Washington, DC: US Department of Energy.
US Environmental Protection Agency (2018). 2011 – 2016 Greenhouse Gas Reporting Program Industrial Profile: Pulp and Paper. Available at: https://www.epa.gov/sites/production/files/2018-10/documents/pulp_and_paper_2016_industrial_profile.pdf (Accessed June 11, 2021).
US Environmental Protection Agency (2020). Facts and Figures about Materials, Waste and Recycling. Paper and Paperboard: Material-Specific Data. Available at: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/paper-and-paperboard-material-specific-data (Accessed May 28, 2021).
U.S. Department of Energy (2016). 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy, 1. Oak Ridge, TN: Economic Availability of FeedstocksOak Ridge National Laboratory. Report No. ORNL/TM-2016/160. doi:10.2172/1271651
Wang, W-C., Tao, L., Markham, J., Zhang, Y., Tan, E., Batan, L., et al. (2016). Review of Biojet Fuel Conversion Technologies. Report No. NREL/TP-5100-66291. Golden CO: National Renewable Energy Laboratory.
Woods, D. R. (2007). Rules of Thumb in Engineering Practice. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co.
Zhang, C., and El-Halwagi, M. (2017). Estimating the Capital Cost of Shale-Gas Monetization Projects. Chem. Eng. Prog. 113 (120), 28–32.
APR Aqueous-phase reforming
FCI Fixed capital investment
FOB Cost Purchased equipment cost–freight on board
GREET Greenhouse Gases, Regulated Emissions, and Energy Use in Transportation
NREL National Renewable Energy Laboratory
RW-EDI Resin Wafer Electrodeionization
TCI Total Capital Investment.
Keywords: recycled paper, municipal solid waste, cellulosic biomass, renewable jet fuel, renewable hydrocarbons, techno-economic analysis
Citation: Kubic W, Moore C, Semelsberger T and Sutton A (2021) Recycled Paper as a Source of Renewable Jet Fuel in the United States. Front. Energy Res. 9:728682. doi: 10.3389/fenrg.2021.728682
Received: 21 June 2021; Accepted: 23 September 2021;
Published: 12 October 2021.
Edited by:
Jalel Labidi, University of the Basque Country, SpainReviewed by:
Jianguo Zhang, University of Shanghai for Science and Technology, ChinaCopyright © 2021 Kubic, Moore, Semelsberger and Sutton. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: William L. Kubic Jr., d2t1YmljQGxhbmwuZ292
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.